Abstract
The spectrum of gastric pathologies involves heterogeneity with respect to biochemical mechanisms and clinical outcome and is globally common. Each year, 5–6 million people worldwide are affected by gastric ulcer, gastric cancer and inflammatory bowel diseases, and mortality rate being >50% shows steep increase in incidence. Hence, understanding the underlying pathogenesis and better therapeutic strategies remain the major challenges in gastroenterology field. Current knowledge of gastric pathology reveals that extracellular proteases vastly influence functional irregularities of cells along with their responses to microenvironment. Based on studies on metalloproteinases and their inhibitors, it is well accepted about their important roles in physiological developmental processes as well as pathological conditions. From past several years of extensive research on matrix, metalloproteinases (MMPs) establish their critical role in several cellular functions including proliferation, apoptosis and angiogenesis. MMPs are a family of “molecular scissors” with ambivalent actions and ability to cleave extracellular matrix (ECM) proteins that in turn facilitate tissue remodelling. Approximately, 27 subtypes of MMPs are there having mutual interaction among each of them in gastrointestinal disorders. Functional overlap between the MMPs leads to non-specificity, which makes designing MMP inhibitors more difficult. Thus, specific MMP inhibitors would be promising therapeutic tool against inflammatory diseases including gastric diseases. This chapter illustrates the new insights into mechanism of MMP regulation in gastrointestinal inflammatory disorders encompassing clinical trials for MMP inhibitors and new therapeutic strategies by targeting specific MMP(s) to control gastrointestinal pathologies.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Metalloproteinases degrade extracellular matrix (ECM) proteins and regulate both cell–cell and cell–ECM interactions, which influence cell differentiation, migration, proliferation and survival. They belong to metzincin group of proteases, characterized by the presence of zinc in the catalytic domain, that includes bacterial serralysins and astacins, adamalysins (a disintegrin and metalloproteinase domain or ADAMs) and matrixins (matrix metalloproteinases or MMPs) [1,2,3]. Metzincins use three histidine (H) residues to bind the zinc ion at their active site [4, 5]. A water molecule that is essential for hydrolysis of the peptide bond also coordinates with the metal ion as a fourth ligand in the active form of metallopeptidase. Members of this superfamily of enzymes are involved in diverse physiological processes as embryonic development, morphogenesis, bone formation, reproduction, cell adhesion and migration. Aberrant activities of metalloproteases have been implicated in various pathological conditions like arthritis, cancer, cardiovascular diseases, nephritis, central nervous system disorders and fibrosis [2, 6, 7]. There are ample literatures which state that ECM degradation plays pivotal role in gastrointestinal diseases, thus role of MMPs are evident [2, 3, 8]. Collectively, these enzymes are capable of degrading collagens, elastins, gelatin, matrix glycoproteins and proteoglycan as well as number of bioactive molecules.
According to the classification of proteases, based on their 3-D structure in the MEROPS database (http://merops.sanger.ac.uk), metallopeptidases may be classified into forty-six families. The families are further grouped into fourteen different clans based on metal ion binding motifs and 3-D structure similarities [9, 10]. MMPs are calcium-dependent, zinc containing endopeptidases [11]. The name is derived from consensus sequence and structural features, specifically a “HExxH” zinc-binding motif (zincin) and a C-terminal conserved methionine residue, which forms a conserved structure, called “met turn” [8]. MMP family comprises ∼27 member proteases characterized in humans, rodents and amphibians [12,13,14]. They were first described in vertebrates (1962), including humans, but are also found in invertebrates and plants. MMPs are secreted by a variety of connective tissues and pro-inflammatory cells including fibroblasts, osteoblasts, endothelial cells, macrophages, neutrophils and lymphocytes. These enzymes are expressed as zymogens, which are subsequently processed by other proteolytic enzymes (such as serine proteases, furin, plasmin and others) to generate the active forms through a cysteine switch mechanism.
MMPs are classified into collagenases, gelatinases, stromelysins and matrilysins depending on their specificity as depicted in Fig. 1. Another subclass of MMPs is membrane-type MMPs (MT-MMPs) that additionally contain a transmembrane and cytoplasmic domain [12]. The activities of most MMPs are very low or negligible in the normal steady-state tissues, and their expression is transcriptionally controlled by inflammatory cytokines, growth factors, hormones, cell–cell and cell–matrix interactions [15] (Fig. 2).
Generalized domain structure and amino acid length of mammalian MMPs. MMPs contain a signal peptide followed by a propeptide which constitutes the pro-domain. It contains the conserved cysteine switch sequence, which makes a complex with the Zn2+ ion in the zymogen form of it. In case of only gelatinases (MMP-2 and MMP-9), the catalytic domain has a gelatin binding domain. Except for few MMPs (MMP-9, MMP-26, MMP-7), all other members contain a proline-rich hinge region followed by a hemopexin-like C-terminal domain, which helps in substrate recognition and its interaction with endogenous inhibitors. A major difference between secreted and cell surface anchored MMPs (MMP-14, MMP-15, MMP-16 and MMP-24) consists of intrinsic motif called transmembrane region and cytoplasmic tail
From the structural point of view, a typical MMP consists of approximately 80 amino acid long propeptide, about 170 amino acids catalytic metalloproteinase domain, followed by a linker peptide of variable length and a 200 amino acid long hemopexin (Hpx) domain (Fig. 3).
Schematic diagram of intercellular signalling events that drive secretion of MMPs towards ECM during physiological and pathological conditions. Infiltrating immune cells secrete cytokines and MMPs simultaneously, which either activate other cells or degrade the ECM. These fragments of ECM components can mount the inflammatory response by a variety of events including immune cell chemotaxis, activation of receptors or chemokine ligands
Among all the members of MMP family, MMP-7, MMP-26 and MMP-23 are the exceptions as they lack the Hpx domain along with the linker peptide, and MMP-23 has an additional cysteine-rich domain followed by an immunoglobulin-like domain after the metalloproteinase domain [16,17,18,19]. The signal peptide is removed during translation, and proMMPs are generated [20].
The activity of MMPs is very tightly regulated in the cell under normal physiological conditions. This regulation occurs at different levels; gene expression, proteolytic cleavage of the zymogens, transcription and inhibition of the active forms by various non-specific endogenous inhibitors such as α2-macroglobulin and specific tissue inhibitors of metalloproteinases (TIMPs) [1, 12, 13]. TIMPs inhibit active MMPs by forming 1:1 stoichiometric enzyme-inhibitor complexes leading to inhibition of their proteolytic activity [14, 15, 21]. TIMP-1, -2 and -4 are secreted, while TIMP-3 is sequestered to the ECM. The substrate specificity of TIMPs varies. A critical balance between MMPs and their endogenous inhibitors plays a pivotal role in vivo. Similar to MMPs, the proteolytic ADAM and ADAMTS family members are inhibited by specific TIMPs [18, 22, 23].
Reactive oxygen species (ROS) produced at the site of inflammation produced by activated neutrophils and macrophages has also a great influence on the function of MMPs [24]. These oxidants initially activate MMPs via oxidation of the pro-domain cysteine [25]. Eventually, MMPs may be inactivated by the enzyme myeloperoxidase secreted from inflammatory cells or by modification of catalytic domain amino acids by hypochlorous acid [26].
Detailed genetic and proteomic studies in experimental animals as well as in humans have provided insights into the involvement of MMPs in various disorders. The first human degenerative disease identified where MMPs were found to be linked was Sorsby’s fundus dystrophy [27]. Stromelysin-1 knockout mice showed increased occurrence of collagen-induced arthritis. Several studies on MMP-null mice demonstrated impaired responses to pathological conditions. MMP-2, -7, -9 and -11 showed considerable [28, 29] influences on tumour progression and carcinogenesis in null mice. High expression levels of several MMPs have been correlated with tumour aggressiveness, stage and poor prognosis of various human cancers, but not always [29, 30]. MMPs are known to contribute to angiogenesis by degrading basement membranes, allowing for endothelial cell invasion, thus metastasis [25, 31, 32]. Abnormalities in ECM glycosaminoglycans and loss of glycosaminoglycans in epithelial basal lamina are detected in gastrointestinal inflammation like ulcerative colitis, peptic ulcers and Crohn disease [33, 34]. There is also increased expression of stromelysins, matrilysins and collagenases, which suggests a strong correlation among inflammation and tissue injury. Literatures also suggest that mucosal immune system triggers the response through MMP-dependent pathways [35]. The extent of damage in gastric tissues due to breakdown of ECM by MMPs not only depends on the high expression of MMPs but also on the relative ratio of MMPs and TIMPs [23]. However, in some diseases like inflammatory bowel disease (IBD), there is evidence for overproduction of few MMPs [35].
2 Various Gastrointestinal Pathologies and Role of MMPs Therein
Gastrointestinal pathology is the subspecialty of surgical pathology that deals with the diagnosis and characterization of malignant, non-malignant, acute and chronic diseases of the digestive tract along with the accessory organs such as the pancreas, gallbladder, liver and intestine. MMPs play pivotal role in many gastrointestinal ailments like gastrointestinal mucositis, gastric ulcer, gastric cancer, colon cancer, pancreatic cancer, gallbladder cancer, hepatic cancer, colorectal cancer, etc. Detailed discussions have been provided in the following section.
2.1 Involvement of MMPs in Gastric Ulcer
Non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, stress, alcohol consumption, smoking and family history are considered as risk factors in the pathogenesis of gastric ulcer [36]. Mucosal tissue injury may lead to gastric ulcer, which is triggered primarily by ischaemia, along with depletion of nutrient delivery [37]. There are many endogenous aggressive factors (gastric hydrochloric acid, pepsin, reactive free radicals and oxidants, leukotrienes, refluxed bile and endothelins) which actually counterbalanced by the protective factors like gastric mucosal barrier, bicarbonate, mucosal blood flow, surface active phospholipids, prostaglandins (PG), nitric oxide (NO) and antioxidants [38, 39].
MMPs, especially proMMP-2 (72-kDa gelatinase A) and proMMP-9 (92-kDa gelatinase B) as well as their active forms, are associated with gastric injury [40]. Although MMP-2 appears to be constitutively expressed by many cell types in culture, MMP-9 expression is induced during gastric ulcer development [41]. In addition, MMP-1 and 3 are also upregulated in gastric and duodenal ulcers [42]. Other studies reported that NSAIDs increase MMP-9 activity and suppress MMP-2 activity during gastric ulcer. In chronic conditions, MMP-2 activity also gets upregulated with MMP-9. This suggests that MMP-9 expression is crucial for the development of gastric ulcers, but MMP-2 may be involved in the turnover of gastric ECM. Menges et al. reported the upregulation of MMP-1 and MMP-9 during Helicobacter pylori (H. pylori) infection in cultured cells [43]. Acetic acid-induced experimental ulcer also showed the upregulation of MMP-9, but there were no significant change in the expression of MMP-2 [44]. In addition, infection can also influence the upregulation of MMP-1, -2, -3 and -7, but the mechanisms and pathways are not yet well understood. In contrast, in H2O2-mediated ulcers, MMP-2 activity and expression get downregulated [45]. Witzum et al. demonstrated that H2O2 alters the structure of MMP-2 by oxidation and catalytic domain inhibition [46, 47]. Singh et al. demonstrated that proMMP-9 along with pro and active MMP-2 gets upregulated in ethanol-induced gastric ulcer in experimental rats [48, 49]. Thus, the critical balance of MMP-9 and MMP-2 activities may be a determinant in the- progression as well as healing of gastric ulcer.
2.2 Role of MMPs in Gastric Cancer
Cancer is a multistage process, which requires various genetic and epigenetic changes in the tissue microenvironment. Alterations that occur during the malignant transformation are regulated by MMPs and their endogenous inhibitors TIMPs. Gastric cancer is the fourth most common cancer and the second leading cause of cancer-related death worldwide [50].
Different factors are having role and influence gastric cancer development and progression. Chronic inflammation or ulcer might be a linker for gastric cancer to many other types of malignancies for the future [51]. Studies of different surgical specimens showed chronic gastritis were more advanced in individuals with gastric cancer than in individuals with duodenal ulceration. It is now known that H. pylori is a major factor in both the induction of atrophic gastritis and histological progression to gastric cancer [52]. Literatures suggest that MMPs modulate the function of cytokines and chemokines and the consequences of functions of immunoregulatory cells during progression of gastrointestinal cancers.
Several knockout animal-based studies and case-control studies have confirmed that MMP-7 is an important member of MMP family that is upregulated in gastric carcinoma. Histology and immunohistochemistry revealed that it promotes tissue invasion and metastasis. Studies from our laboratory showed that single nucleotide polymorphism (SNP) in MMP-1 promoter at -519 A/G and MMP-3 promoter -375 C/G increases the risk of gastric cancer in Indian population [53]. Recently described downstream signalling molecules of MMP-7 include E-cadherin, Fas ligand and pro TNFα. E-cadherin is a cell adhesion molecule, which is responsible for the epithelial to mesenchymal (EMT) transition during cancer progression. Witty et al. reported that the colon cancer cells gained significant invasive potential when MMP-7 was transfected to them. Studies also emphasized the involvement of MMP-7 to the tumourigenicity and disease progression in malignant colorectal tumours [54]. Reverse transcription-polymerase chain reaction (RT-PCR) data revealed high expression of MMP-7 mRNA in the sentinel node lesions in patients with gastric carcinoma. In addition, SNP in MMP-7 promoter at -181 A/G increases the gastric cancer risk as reported from our laboratory [53, 55, 56].
In vitro studies on gastric cancer, cell lines demonstrated that the gene and protein levels of human epidermal growth factor 2 (HER 2) and MMP-9 are very tightly associated in the pathogenesis of gastric cancer [57]. Knocking down of HER 2 gene by shRNA significantly inhibited the invasion and metastasis of gastric cancer by downregulating the expression of MMP-9 while HER 2 overexpression again improved the MMP-9 transcription.
Yoo et al. found that signalling through sonic hedgehog pathway promotes the invasiveness of gastric tumours through activation of PI3 k/Akt pathway leading to EMT followed by MMP-9 activation [58]. Alakus et al. found that expression of MMP-2 was linked with the clinicopathological parameters in gastric cancer. High expression of MMP-2 from epithelial cells was associated with tumour stage and poor survival [59].
2.3 Specific Role of MMPs in Colorectal Cancer
Colorectal cancer (CRC) is a complex, multistage process, starts from neoplasia, followed by tissue invasion, vascular intra and extravasation and distant metastasis. The stromal cells of colon interact with the ECM and breakdown of ECM components is important for a cell to migrate from the primary site of tumour. Research on CRC has elucidated the role of distinct immune cells, cytokines and other immune mediators in virtually all steps of colon tumourigenesis, including initiation, promotion, progression and metastasis [60].
All groups of MMPs play role in the development as well as progression of CRC. The collagenases, i.e. MMP-1 and MMP-13 expressions were observed in the advanced stages of CRC with the lymph node involvement and poor prognosis. Huang et al. reported an approximately eightfold increased risk of post-operative recurrence in those patients who had MMP-13 overexpression [61]. There are studies on correlation of MMP-2 and -9 expressions with CRC and worse outcome. Patients having lymph node metastasis with CRC had an elevated level of plasma MMP-2 compared to the patients of early stage. Some reports also suggested serum MMPs as candidate biomarkers for CRC metastasis, as researchers have found higher ratio of expressions of MMP-2 and MMP-9 in CRC patients compared to normal subjects, and TGFβ is the key transcription factor responsible for MMP-9 expression [62, 63]. Elevated level of p38 gamma MAPK induces c-Jun synthesis, which in turn, increases the transcription of MMP-9, thus invasion in CRC [64]. TGFβ receptor kinase blocker was found effective to reduce MMP-9 expression and block CRC metastasis [65, 66]. In addition, MMP-7 was also found to activate proMMP-2 and proMMP-9 to promote lymph node metastasis, and upregulation of MMP-7 was found in ~80% of advanced stage of CRC [64]. MMP-7 knockout mice models of CRC demonstrated decreased tumour burden and reduced colon cancer multiplicity. Interestingly, MMP-12, also called the metalloelastase, was found to be protective in CRC and its inhibition was lethal in experimental animal models. Higher expression of MMP-12 inhibits distant metastasis by downregulating VEGF expression and angiogenesis in CRC [64].
2.4 MMPs in Inflammatory Bowel Disease
Ulcerative colitis and Crohn’s disease both together called as inflammatory bowel disease IBD, which can affect any segment of the gastrointestinal tract. From the epidemiological survey, it was seen that genetic predisposition for IBD might play a role towards development of malignancy from IBD [67]. Epidemiological studies estimated the occurrence of IBD is 1 in 1000 individuals in western countries, but the rate is rising globally because of the lifestyle and diet [68]. Histologically, the disease is characterized by presence of granulomas, fibrosis in the tissue space along with fistulae [69].
The levels of several MMPs including MMP-1, MMP-2, MMP-3, MMP-7, MMP-9, MMP-10, MMP-12, MMP-13 are modulated during IBD pathogenesis in the inflamed colon mucosa or serum [70, 71]. Gene expression profiling demonstrated the transcriptional upregulation of MMP-1 with the severity of the disease and is linked with hypoxia inducing factor-1. Most importantly, the critical ratio of MMP-1: TIMP-1 gets altered with the severity of the disease [70]. The secretory MMP-9 mucosal expression level as well as serum antigen level was found significantly higher in ulcerative colitis patients compared to healthy subjects [72]. In vivo gelatinases double knockout mice model showed resistance from DSS, TNBS and Salmonella typhimurium-induced colitis [73]. In addition, MMP-12−/− mice were protected from TNBS-induced colitis [74]. Beside gelatinases, stromelysins (MMP-3 and MMP-10) were also found upregulated in the inflamed areas of IBD patients. SiRNA mediated silencing of MMP-3 confers protection from DSS-induced colitis [75]. A study in New Zealand patient pool on SNP showed that genes of MMP-3, MMP-8, MMP-10 and MMP-14 were associated with IBD [76].
Accumulating data from several studies indicated that IL-17A and IL-17F can act as inducers for the secretion of MMP- 1 and -3 in subepithelial myofibroblasts and also promote the actions of IL-1 and TNF- on these MMPs via MAPK mediated pathway [77]. The disruption in the protease–antiprotease balance of MMP: TIMP may also promote fibrosis in the intestine during the disease progression. In humans, the fibrosis in the gut is inhibited by TGFβ/Smad pathway where MMPs are downregulated and TIMP expression gets upregulated. MMPs regulate both pro- and anti-angiogenic factors which may contribute to the pathogenesis of IBD or mucosal healing. While new mechanisms are emerging for the IBD pathogenesis, it is crucial to understand the scenario where MMPs play significant role in mucosal healing, ECM remodelling, regulation of angiogenesis or immune response during disease pathogenesis.
2.5 Implication of MMPs in Crohn’s Disease
Crohn’s disease is a chronic inflammatory disease of the digestive tract and also falls under IBD. It affects the end of the small intestine, i.e. the ileum, but it may also affect other parts of the gastrointestinal tract and the entire thickness of the intestinal wall [78]. Epidemiology states that Crohn’s disease affects approximately 3 per 1000 individual in western countries, and it is less common in Asia and Africa [68].
MMPs have been strongly implicated in the tissue injury in Crohn’s disease. Recently, elevated expressions of MMPs have been found in the inflamed tissues of patients having Crohn’s disease [79], which implies that there is a role of MMPs in the increased proteolysis in the mucosa, ulceration followed by inflammation and fistula formation. MMP-9 gets upregulated in the inflamed tissues, and MMP-9 transcripts were found only in the highly inflamed regions of the tissues [80]. MMP-3 levels were also found elevated in mononuclear macrophage-like cells and fibroblasts in patients [81]. There were no significant differences in MMP-2 expression reported. Downregulation of TIMPs is also very significant as TIMP-1, TIMP-2 and TIMP-3 level goes down during acute stage of the disease [82]; thus, disrupts the protease–antiprotease homeostasis. In addition, high MMP-3 expression was consistently found in fistulae in patients suffering from Crohn’s disease. Microarray analysis of the inflamed tissue lysates showed that MMP-3 transcripts and proteins were localised particularly in large mononuclear cells as well as macrophages. Although MMP-10 falls under the same stromelysin group of MMP family with MMP-3, transcripts as well as expression of MMP-10 were found negative. Moreover, SNP in the promoter region of MMP-3 gene in 5A/6A position confers higher rate of promoter activity and increases the susceptibility of the disease [83] (Table 1).
3 Role of Microbiome in Gastrointestinal Ailments
Human beings are inhabited by a complex array of microorganisms that interact with each other and with the host. They, as a whole, represent an integrated and functional ecosystem (microbiota) that have important role in human health and disease. The composition and diversity of the microbiota vary among different normal individuals [84]. Herein, the latest findings on gastrointestinal microbiota, in relation to their composition and prevalence in the presence or absence of H. pylori infection are highlighted. It was a notion that stomach is a sterile organ due to several innate defences including acid secretion, migrating motor complexes, enterosalivary circulation of nitrate. However, there is a significant influence of microorganisms on stomach and intestinal microenvironment, both in physiological and pathological conditions (Fig. 4).
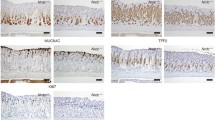
Microbiome in human gut and MMPs expressed. Histology of the epithelium of stomach infested with pathogenic bacteria shows denudation and exfoliation of the layers in comparison with the control. Infiltration of inflammatory cells in the connective tissue is the benchmark of inflammation due to infection. The bacterial population and MMP expressed in presence and absence of H. pylori are shown in tables, but there is a gap in the information about the MMPs expressed in particular infection of the gastric system
3.1 Helicobacter pylori Mediated Gastric Ulcer and Involvement of MMPs
Nobel laureate Robin Warren and Barry Marshall discovered the role of H. pylori in gastric ulcers in the year 1982 and considerable literature documented the presence of many acid-resistant strains, such as Streptococcus, Neisseria, Lactobacillus etc. [85,86,87]. H. pylori has been recognised as a Class I carcinogen [88]. There is enormous heterogeneity in the consequences of H. pylori infections. People acquire the infection early in life and are followed by a long quiescent phase when there is a chronic gastritis of variable intensity but with minimal symptoms [89]. Infection with H. pylori is not sufficient to induce gastric cancer, some other factors, i.e. bacterial and host cofactors are required to establish the disease [90, 91]. Only 10–15% of individuals infected with H. pylori develop ulcerative lesions in stomach, and the risk of gastric cancer is estimated to be approximately 1–3% [85, 92, 93].
Vitro studies suggest that H. pylori induce apoptosis of gastric epithelial cells and stimulate epithelial cells to secrete several chemoattractants [94]. Moreover, there is a marked increase in Th1-type cytokines, including IFN-γ, IL-12 and TNF-α in H. pylori-infected mucosa, all of which have been reported to be involved in tissue degradation in other systems [95, 96].
H. pylori infection induces the secretion of MMPs from a variety of gastric cells in vivo as well as in cultured cells, which in turn contribute to the pathogenesis of gastric ulcer and gastric cancer [97,98,99]. Gastric epithelial cells appear to be the major source of MMPs in H. Pylori-infected gastric tissues [100, 101]. A recent hospital-based study on gastric cancer patients with H. pylori infection revealed that the infection upregulated the expression of MMP-1 and MMP-10 [102, 103]. MMP-1 predominantly degrades the stroma, which is linked with invasion and metastasis [23]. In addition, microarray analysis of uninfected human gastric epithelial cell line (AGS) and H. Pylori-infected co-cultures demonstrated that along with MMP-10, several other MMPs, such as MMP-1, MMP-7, MMP-25 genes were also upregulated [104, 105]. Among them, MMP-10 showed significant increase in expression in comparison to other MMPs [103].
3.2 Human Gastric Microbiota and Gastric Disorders
The gastrointestinal tract is the most populated organ in human body. Different sites of the GI tract are inhabited by different microbiota, including the stomach [106]. The very low pH value (median pH 1.4) of stomach makes it a very harsh and hostile environment for bacterial growth. Thus, the microbial colonization in stomach is very less (102–104 colony forming units (CFU)/g) compared to colon (1010–1012 colony forming units (CFU)/g) [107]. The major constituent of human gastric microbiota is the Proteobacteria H. pylori although many other bacteria can also survive in this hostile environment, making it more diverse and complex. Along with the host physiology various other factors, including diet, H. pylori infection, enteral feeding, proton pump inhibitors, antibiotics and diseases shown to contribute in shaping the gastric microbiota [108]. With the advances of the DNA-based sequencing technologies, the culture-independent survey of human gastric microbiota is possible based on the analysis of a gastric biopsy sample. In 2013, Sheh and Fox summarized in their study that in stomach the most commonly found phyla are Proteobacteria, Firmicutes, Bacteroidetes, Actinobacteria and Fusobacteria. The most abundant phyla are Proteobacteria, Firmicutes and Actinobacteria for H. pylori positive samples, while Firmicutes, Bacteroidetes and Actinobacteria are most abundant phyla for H. pylori-negative samples [109]. Interestingly, H. pylori being the most dominant bacteria comprise of 72–99% of sequencing reads in the stomach [110, 111]. Other commonly identified genera are of Streptococcus, Prevotella, Veillonella and Rothia species [112]. Surprisingly, the correlation was found only between the presence of Streptococci and peptic ulcer disease [113]. Another study compared gastric microbiota profile according to H. pylori status in chronic gastritis patients using high-throughput 16S rRNA sequencing. The microbiota of H. Pylori-negative patient sample was represented by the member of bacterial class alpha-, beta-, gamma-proteobacteria, bacilli, bacteroidia, clostridia, flavobacteria and fusobacteria [114].
The model proposed by Correa postulated that the chronic H. pylori infection of the gastric mucosa progresses through different stages like chronic active gastritis, intestinal metaplasia, dysplasia to subsequent development of gastric cancer [115]. Nonetheless, it has been clearly identified that H. pylori is the major risk factor in gastric cancer development [116, 117].
Other studies suggested that there is a gradual shift in the composition of gastric microbiota, which might plays a key role in the progression of pre-malignant lesions to gastric carcinoma. Additionally, gastric cancer samples showed decrease in the relative abundances in bacteria belonging to the phyla Proteobacteria, namely Neisseria spp, Haemophilus spp, Bergeriella denitrificans, Epsilonproteobacteria and Helicobacteriaceae [114, 115] as well as Bacteroidetes (Porphyromonas spp and Prevotella pallens). The bacteria within the phyla Firmicutes are increased (Streptococcaceae, Lachnospiraceae and Lactobacillus coleohominis) [114, 115] or decreased Streptococcus sinensis [115].
4 Targeting MMPs as Therapeutic Strategy
Protease inhibitors are essential tools for the investigations of MMPs activities. They are useful not only for assessing the activity but also for inhibition of unwanted proteolysis in an experimental system. People started working on MMPs after proving its role on cancer stage, patient prognosis and death. Almost every pharmaceutical company started manufacturing MMP inhibitor (MMPI) to block MMP-mediated angiogenesis and metastasis. The programme started about 25 years ago and led to a number of small-molecule inhibitors in phase III clinical trials [25, 118, 119].
Chelating agents such as EDTA and 1,10-phenanthroline are routinely used in the laboratories to block MMP activities in in vitro experiments. Synthetic inhibitors commonly contain a chelating moiety, such as a carboxyl, a thiol, a phosphorous or a hydroxamic acid group. The chelating group is attached to a series of other groups that fit the specificity pocket of a particular metallopeptidase [120].
4.1 Endogenous MMP Inhibitors: TIMPs
TIMPs contain an N- and C-terminal domain of ~125 and 65 amino acids, respectively, with each containing three conserved disulfide bonds. The N-terminal domain folds as a separate unit and is capable of inhibiting MMPs. However, their range of activities is broader as it inhibits several disintegrin-metalloproteinases, namely ADAMs and ADAMTSs. Pathological conditions are associated with imbalanced MMP activities due to altered TIMP levels as important factor. Structural studies of TIMP-MMP complexes have allowed the generation of TIMP variants that selectively inhibit different groups of metalloproteinases. Engineering such variants is complicated by the fact that TIMPs can undergo changes in molecular dynamics induced by their interactions with MMPs. TIMPs are involved in cell growth and differentiation, cell migration, anti-angiogenesis, anti- and pro-apoptosis and synaptic plasticity [120, 121].
4.2 Antibody-Based Inhibition Targeting Catalytic Domain
Reports are available on the use of functional blocking antibodies, which have high potency for MMPs. Several functional blocking antibodies have been developed that selectively target the membrane-anchored MMPs. Combining a human antibody phage display library with automated selection and screening strategies resulted in the identification of a highly selective antibody-based MMP-14 inhibitor called DX-2400. It displayed anti-invasive, antitumour and anti-angiogenic properties and blocked MMP-14 mediated pro-MMP-2 processing [122]. To date, at least two monoclonal antibodies have been tested which bind to the catalytic domain, without interacting with the catalytic zinc. DX-2400 is a MMP-14 specific inhibitor, which binds to the catalytic domain with a K i in the sub-nanomolar range.
Thus far, preclinical studies of DX-2400 indicated that the antibody is capable of inhibiting all of these activities while there was no measurable effect on other MMPs. In mouse studies, the drug was observed to decrease tumour burden significantly and decreased metastases in lung and liver. Further, DX-2400 was effective against HER2-positive xenografts both when used as a single agent or in combination with paclitaxel. This marks DX-2400 as an attractive candidate for patients diagnosed with triple-negative breast cancer, although clinical trials for this therapeutic have not yet been initiated [118, 122].
Other groups developed selective MMP-14 inhibitory antibodies that were successfully tested in vitro and in vivo. The neutralizing monoclonal antibody REGA-3G12 acts as a selective inhibitor of MMP-9 by binding against catalytic domain but not against the fibronectin or zinc-binding domains. A murine monoclonal antibody, termed REGA-3G12, has also been generated by hybridoma technology against the catalytic domain of human MMP-9. This inhibits MMP-9 without affecting activity of MMP-2, which shares high homology. Therefore, a therapeutic that can differentiate between these two highly similar enzymes may prove very useful [120]. In this regard, blocking antibodies were found to act on specific functions of the MMP rather than the general proteolytic activity. For example, 9E8 monoclonal antibody targets the MMP-2 activation capacity of MT1–MMP rather than the general proteolytic activity [118, 121].
The mechanism for TIMPs to inhibit MMPs can be utilized to develop different antibody-based strategies for effectively targeting the in vivo activity of MMP. A neutralizing antibody was developed based on the three-dimensional structure and amino acid sequence of MMP-13, which bind only to the active form of MMP-13. Monoclonal antibody against MMP-2 exhibited inhibition over MMP-2 activity but did not affect the structurally similar MMP-9 activity [121].
4.2.1 Hemopexin Domain Inhibitor and Small Molecule Inhibitor of MMPs
The interaction of inhibitor with the hemopexin-like domain prevents binding of endogenous partners that can promote angiogenesis or cancer cell migration. The hemopexin domain among different MMPs exhibits significantly less sequence and structural homology compared to the catalytic domain. This domain comprises a succession of four structurally similar hemopexin-like repeats to create a central funnel-like tunnel. Each hemopexin-like repeat is made up of four β-strands; the first three β-strands bear the highest homology across the MMP family whereas the b4 strands bear the least. As many as four structural ions have been found to be coordinated within this tunnel, and it has been proposed that these ions confer a stabilizing function for the whole domain. MMP-9 has only a sodium ion and displays a flexible architecture and considerable deviation from the structure of hemopexin domains reported for other MMPs. The first ion binding position, which is closest to the linker region connecting the hemopexin domain to the catalytic domain, is generally either a sodium or calcium ion [122].
In silico analysis of the MMP-14 hemopexin domain identified a druggable pocket-like site in the centre of the hemopexin structure. Binding of small molecule compounds in this site should, in theory, allosterically block dimerization. Compounds which bind to the hemopexin domains and prevent dimerization have been shown to significantly decrease tumour size, reduce MMP-mediated cell scattering/invasion, angiogenesis, and tumour metastasis both in vitro and in animal models. A subsequent docking study of small-molecule compounds led to identification of a compound which is selective for MMP-14 compared to MMP-2 and was not cytotoxic and did not affect catalytic activity (including MMP-14-mediated activation of MMP-2). Report is there for inhibition of hemopexin domain that was effective in attenuating cancer cell migration and in vivo-reduced tumour volume. In another study, an inhibitor was made with no proteolytic or cytotoxic effects while significantly decreased cancer cell migration and invasion and significantly decreased tumour size as well as the number of metastases in vivo [120, 123, 124].
Bimodal approach confers increased selectivity for MMP-2 as compared to individual subunit. A fusion protein has been designed which links the ten amino acid sequences of a MMP-2 selective inhibitory peptide (APP-IP, a β-amyloid precursor protein) to the N-terminus of TIMP-2. This macromolecular protein, which binds with a K i in the sub-picomolar range, is designed to interact with both the active site and the hemopexin-like domain of MMP-2 [125,126,127].
In addition, small peptides have been used successfully to block dimer-induced functions of MMPs. Owing to induced intracellular cytoskeleton rearrangements necessary for processing of migration and invasion machinery, MMP-14 homodimerizes and also heterodimerizes with CD44. This includes proteolysis of proMMP-2 by MMP-14. A number of peptides generated that mimic the sequence of the residues of hemopexin-like domains required for dimerization were able to reduce tumourigenic effects in vitro and in vivo [128] (Table 2).
5 Limitations/Challenges for MMPs Inhibition
Although several preclinical research that supported the importance of MMPs in cancer, all Phase III cancer trials using different inhibitors of MMPs are failed unfortunately. The major reason is lack of specificity of inhibitors and insufficient knowledge on the complexity of cancers [118]. The information of preclinical studies in the mouse models and the clinical trials in patients varied much that might be the reason behind the failure of the clinical trials as well as adverse effect on patients. The adverse effects were mostly due to their broad-spectrum inhibition of MMPs and the cross-inhibition among other family of proteins, e.g. ADAM (a disintegrin and metalloproteinase) and ADAMTS (ADAMs with thrombospondin motifs). In addition, several MMP inhibitors were neither metabolically stable, nor orally bio-available and toxic as well. In mammals, MMP genes are conserved indeed and are essential for normal functioning of the organism. It is worth mentioning that MMPs activities are not always harmful but becomes detrimental with its anti-targets actions in other physiological conditions. In contrast to other proteases (like caspases), most MMPs contain conserved amino acid sequences having high homology in the substrate binding domain which hinders the fabrication of specific substrate-based inhibitors. Interestingly, the evolution of many MMPs occurred by gene duplication in the mammalian genome, that leads to the formation of MMP genes clusters on particular chromosomes (for example, the chromosome-9 in the proximal mouse harbours ten MMP genes in less than 500 kb) and possess widespread homology in their amino acid sequence. As a consequence, translating in vitro research work with in vivo applications remains a difficult task. In vitro studies with active MMP and any protein are resulted in cleavage at particular sites. However, this cleavage might not essentially occur in an in vivo condition in physiological system. Scientists had attempted in knocking out many MMP-coding genes in mouse models (as in vivo systems) to investigate the consequences of the absence of these genes. However, not much has been evaluated in tissue-specific knockout mice as there is no obvious phenotypic abnormality in unstimulated conditions in most MMP-deficient mice (except for MMP-14 and MMP-20 deficient mice). Moreover, insufficient knowledge on the spatiotemporal activities of MMPs in pathological conditions adds to the unsuccessful attempts for clinical trial of MMP inhibitors.
6 Future Directions
A plethora of literature as well as supporting data revealed that MMPs play crucial roles in both physiological and pathological processes. They could be exploited as independent prognostic factors in gastrointestinal inflammation and malignancies. MMPs are associated with multiple diseases; hence they can be considered as drug targets to treat those diseases. A number of studies from knockout mice and in vitro cultured cells have shown that their involvement as integral part in acute as well as chronic inflammation. The major task for the future is to design specific MMP inhibitors and to elucidate the crosstalk among the members of MMP family. Newer activity-based imaging probes specific for MMPs will facilitate the elucidation of the structural role of inhibitors in gastrointestinal disorders. Although, clinical trials with the therapeutic MMP inhibitors encountered several challenges, studies in both in vivo and in vitro are in progress to target the specific MMP in gastrointestinal pathologies. Interaction between different transcription factors and different MMP promoters provides valuable insights into the mechanism of disease progression. Inhibition to specific MMP in gastrointestinal disorders and its effect at multiple cellular pathways become formidable task for therapeutic use. Although, monoclonal antibody-based therapy is promising for the prognosis and therapy of gastrointestinal cancers, however, its validation in experimental knockout animals and cancer models is prerequisite. Tailor-made therapies and drugs based on set of specific MMP in gastric disorders of different individual could be useful to develop good quality drug. Moreover, development of assay tool against a set of MMPs may lead to formulate commercially viable kits for early prognosis of gastrointestinal diseases using patient serum. Nonetheless, a role for MMPs in pathology of gastrointestinal tract could be related to tissue-specific expression and function of MMPs and be exploited as target for therapies.
References
Verma RP, Hansch C (2007) Matrix metalloproteinases (MMPs): chemical-biological functions and (Q)SARs. Bioorg Med Chem 15:2223–2268
Lu P, Takai K, Weaver VM, Werb Z (2011) Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 3:a005058
Bonnans C, Chou J, Werb Z (2014) Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 15:786–801
Gomis-Rüth FX (2009) Catalytic domain architecture of metzincin metalloproteases. J Biol Chem 284:15353–15357
Lund J, Olsen OH, Sørensen ES, Stennicke HR, Petersen HH, Overgaard MT (2013) ADAMDEC1 is a metzincin metalloprotease with dampened proteolytic activity. J Biol Chem 288:21367–21375
Ikonomidou C (2014) Matrix metalloproteinases and epileptogenesis. Mol Cell Pediatr 1:6
Mizoguchi H, Yamada K (2013) Roles of matrix metalloproteinases and their targets in epileptogenesis and seizures. Clin Psychopharmacol Neurosci 11:45–52
Gong Y, Chippada-Venkata UD, Oh WK (2014) Roles of matrix metalloproteinases and their natural inhibitors in prostate cancer progression. Cancers 6:1298–1327
Massova I, Kotra LP, Fridman R, Maboshery S (1998) Matrix metalloproteinases: structures, evolution and diversification. FASEB J 12:1075–1095
Nagase H, Woessner JF Jr (1999) Matrix metalloproteinases. J Biol Chem 274:21491–21494
Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17:463–516
Harper E, Bloch KJ, Gross J (1971) The zymogen of tadpole collagenase. Biochemistry 10(16):3035–3041
Ra H-J, Parks WC (2007) Control of matrix metalloproteinase catalytic activity. Matrix Biol 26:587–596
Löffek S, Schilling O, Franzke C-W (2011) Biological role of matrix metalloproteinases: a critical balance. Eur Respir J 38:191–208
Andrian E, Mostefaoui Y, Rouabhia M, Grenier D (2007) Regulation of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases by Porphyromonas gingivalis in an engineered human oral mucosa model. J Cell Physiol 211:56–62
Carvalho HF, Roque ACA, Iranzo O, Branco RJF (2015) Comparison of the internal dynamics of metalloproteases provides new insights on their function and evolution. PLoS ONE 10:e0138118
Gomis-Rüth FX (2003) Structural aspects of the metzincin clan of metalloendopeptidases. Mol Biotechnol 24:157–202
Tallant C, Marrero A, Gomis-Rüth FX (2010) Matrix metalloproteinases: fold and function of their catalytic domains. Biochimica Biophysica Acta Mol Cell Res 1803:20–28
Fridman R (2003) Surface association of secreted metalloproteinases. Curr Top Dev Biol Elsevier Sci. 54:75–100
Cerdà-Costa N, Gomis-Rüth FX (2014) Architecture and function of metallopeptidase catalytic domains. Protein Sci 23:123–144
Duan JX, Rapti M, Tsigkou A, Lee MH (2015) Expanding the activity of tissue inhibitors of metalloproteinase (TIMP)-1 against surface-anchored metalloproteinases by the replacement of its C-terminal domain: implications for anti-cancer effects. PLoS ONE 10(8):e0136384
Visse R, Nagase H (2003) Matrix metalloproteinases and tissue inhibitors of metalloproteinases structure, function, and biochemistry. Circ Res 92:827–839
Brew K, Nagase H (2010) The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta 1803:55–71
Nita M, Grzybowski A (2016) The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid Med Cell Longev 3164734:1–23
Kessenbrock K, Plaks V, Werb Z (2010) Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141:52–67
Fu X, Kassim SY, Parks WC, Heinecke JW (2003) Hypochlorous acid generated by myeloperoxidase modifies adjacent tryptophan and glycine residues in the catalytic domain of matrix metalloproteinase-7 (matrilysin): an oxidative mechanism for restraining proteolytic activity during inflammation. J Biol Chem 278:28403–28409
Langton KP, McKie N, Smith BM, Brown NJ, Barker MD (2005) Sorsby’s fundus dystrophy mutations impair turnover of TIMP-3 by retinal pigment epithelial cells. Hum Mol Genet 14(23):3579–3586
Page-McCaw A, Ewald AJ, Werb Z (2007) Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 8(3):221–233
Egeblad M, Werb Z (2002) New functions for matrix metalloproteinases in cancer progression. Nat Rev Cancer 2:161–174
Martin TA, Ye L, Slanders AJ, Lane J, Jiang WG (2013) Cancer invasion and metastasis: molecular and cellular perspective. In: Jandial R Metastatic cancer: clinical and biological perspectives. Landes Bioscience
Rundhaug JE (2003) Matrix metalloproteinases, angiogenesis, cancer. Clin Cancer Res 9:551–554
Sang QXA (1998) Complex role of matrix metalloproteinases in angiogenesis. Cell Res 8:171–177
Murch SH, MacDonald TT, Walker-Smith JA, Lionetti P, Levin M, Klein NJ (1993) Disruption of sulphated glycosaminoglycans in intestinal inflammation. Lancet 341:711–714
O’Sullivan S, Gilmer JF, Medina C (2015) Matrix metalloproteinases in inflammatory bowel disease: an update. Med Inflamm 964131:1–19
Shihab PK, Al-Roub A, Al-Ghanim M, Al-Mass A, Behbehani K, Ahmad R (2015) TLR2 and AP-1/NF-kappaB are involved in the regulation of MMP-9 elicited by heat killed Listeria monocytogenes in human monocytic THP-1 cells. J Inflamm 12:32–40
Hansen JM, Hallas J, Lauritsen JM, Bytzer P (1996) Non-steroidal anti-inflammatory drugs and ulcer complications: a risk factor analysis for clinical decision-making. Scand J Gastroenterol 31:126–130
Matsui H, Shimokawa O, Kanekon T, Nagano Y, Rai K, Hyodo I (2011) The pathophysiology of non-steroidal anti-inflammatory drug (NSAID) induced mucosal injuries in stomach and small intestine. J Clin Biochem Nutr 48(2):107–111
Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, Dhama K (2014) Oxidative stress, prooxidants, and antioxidants: the interplay. BioMed Res Int ID 761264:19 p
Musumba C, Pritchard DM, Pirmohamed M (2009) Cellular and molecular mechanisms of NSAID-induced peptic ulcers. Aliment Pharmacol Ther 30(6):517–531
Frankowski H, Gu YH, Heo JH, Milner R, del Zoppo GJ (2012) Use of gel zymography to examine matrix metalloproteinase (gelatinase) expression in brain tissue or in primary glial cultures. Methods Mol Biol 814:221–233
Verma S, Kesh K, Ganguly N, Jana S, Swarnakar S (2014) Matrix metalloproteinases and gastrointestinal cancers: impacts of dietary antioxidants. World J Biol Chem 26:355–376
Wroblewski LE, Peek M, Wilson KT (2010) Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev 23:713–739
Cheng HC, Yang HB, Chang WL, Chen WY, Yeh YC, Sheu BS (2012) Expressions of MMPs and TIMP-1 in gastric ulcers may differentiate H. pylori infected from NSAID-related ulcers. Sci World J ID 539316:9
Cheng CL, Guo JS, Luk J, Koo MWL (2004) The healing effects of Centella and asiaticoside on acetic acid induced gastric ulcers in rats. Life Sci 74(18):2237–2249
Ganguly K, Kundu P, Banerjee A, Reiter RJ, Swarnakar S (2006) Hydrogen peroxide-mediated downregulation of matrix metalloproteinase-2 in indomethacin-induced acute gastric ulceration is blocked by melatonin and other antioxidants. Free Rad Biol Med 41:911–925
Witztum JL, Steinberg D (1991) Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest 88:1785–1792
Kar S, Subbaram S, Carrico PM, Melendez JA (2010) Redox-control of matrix metalloproteinase-1: a critical link between free radicals, matrix remodeling and degenerative disease. Respir Physiol Neurobiol 31:299–306
Singh LP, Kundu P, Ganguly K, Mishra A, Swarnakar S (2007) Novel role of famotidine in downregulation of matrix metalloproteinase-9 during protection of ethanol-induced acute gastric ulcer. Free Rad Biol Med 43:289–299
Chakraborty S, Stalin S, Das N, Choudhury ST, Swarnakar Ghosh S S (2012) The use of nano-quercetin to arrest mitochondrial damage and MMP-9 upregulation during prevention of gastric inflammation induced by ethanol in rat. Biomaterials 33:2991–3001
Rahman R, Asombang AW, Ibdah JA (2014) Characteristics of gastric cancer in Asia. World J Gastroenterol 20:4483–4490
Fox JG, Wang TC (2007) Inflammation, atrophy, and gastric cancer. J Clin Invest 117:60–69
Correa P, Piazuelo MB (2011) Helicobacter pylori infection and gastric adenocarcinoma. US Gastroenterol Hepatol Rev 7(1):59–64
Dey S, Ghosh N, Saha D, Kesh K, Gupta A, Swarnakar S (2014) Matrix metalloproteinase-1 (MMP-1) promoter polymorphisms are well linked with lower stomach tumor formation in eastern Indian Population. PLoS ONE 9:e88040
Witty JP, McDonnell S, Newell KJ, Cannon P, Navre M, Tressler RJ, Matrisian LM (1994) Modulation of matrilysin levels in colon carcinoma cell lines affects tumorigenicity in vivo. Cancer Res 54:4805–4812
Dey S, Stalin S, Gupta A, Saha D, Kesh K, Swarnakar S (2012) Matrix metalloproteinase-3 gene promoter polymorphisms and their haplotypes are associated with gastric cancer risk in eastern Indian population. Mol Carcinog 51:E42–E53
Kesh K, Subramanian L, Ghosh N, Gupta V, Gupta A, Bhattacharya S, Mahapatra NR, Swarnakar S (2015) Association of MMP7-181A → G promoter polymorphism with gastric cancer risk. J Biol Chem 290:14391–14406
Shan YQ, Ying RC, Zhou CH, Zhu AK, Ye J, Zhu W et al (2015) MMP-9 is increased in the pathogenesis of gastric cancer by the mediation of HER2. Cancer Gene Ther 22:101–107
Yoo YA, Kang MH, Lee HJ, Kim BH, Park JK, Kim HK, Kim JS, Oh SC (2011) Sonic hedgehog pathway promotes metastasis and lymphangiogenesis via activation of Akt, EMT, and MMP-9 pathway in gastric cancer. Cancer Res 71:61–69
Alakus H, Grass AG, Hennecken JK, Bollschweiler E, Schulte C, Drebber U, Baldus SE, Metzger R, Hölscher AH, Mönig SP (2008) Clinicopathological significance of MMP-2 and its specific inhibitor TIMP-2 in gastric cancer. Histol Histopathol 23:917–923
Markman JL, Shiao SL (2015) Impact of the immune system and immunotherapy in colorectal cancer. J Gastrointest Oncol 6:208–223
Huang Z, Huang D, Ni S, Peng Z, Sheng W, Du X (2010) Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancers. Int J Cancer 127(1):118–126
Fanjul-Fernández M, Folgueras AR, Cabrera S, López-Otín C (2010) Matrix metalloproteinases: evolution, gene regulation and functional analysis in mouse models. Biochim Biophys Acta Mol Cell Res 1803:3–19
Grivennikov SI (2013) Inflammation and colorectal cancer: colitis-associated neoplasia. Semin Immunopathol 35(2):229–244
Said AH, Raufman JP, Xie G (2014) The role of matrix metalloproteinases in colorectal cancer. Cancers 6(1):366–375
Cherukua HR, Mohamedalib A, Cantora DI, Tanc SH, Niced EC, Baker MS (2015) Transforming growth factor-b, MAPK and Wnt signaling interactions in colorectal cancer. EuPa Open Proteom 8:104–115
Iiizumi M, Liu W, Pai SK, Furuta E, Watabe K (2008) Drug development against metastasis-related genes and their pathways: a rationale for cancer therapy. Biochim Biophys Acta 1786(2):87–104
Zhiqin W, Palaniappan S, Ali R, Affendi R (2014) Inflammatory bowel disease-related colorectal cancer in the Asia-Pacific region: past, present, and future. Intest Res 12:194–204
M’Koma AE (2013) Inflammatory bowel disease: an expanding global health problem. Clin Med Insights Gastroenterol 6:33–47
Medina C, Radomski MW (2006) Role of matrix metalloproteinases in intestinal inflammation. J Pharmacol 318(3):933–938
O’Sullivan S, Gilmer JF, Medina C (2015) Matrix metalloproteinases in inflammatory bowel disease: an update. Mediators Inflamm ID 964131:19
Deban L, Correale C, Vetrano S, Malesci A, Danese S (2008) Multiple pathogenic roles of microvasculature in inflammatory bowel disease: a jack of all trades. Am J Pathol 172(6):1457–1466
Lakatos G, Sipos F, Miheller P, Hritz I, Varga MZ, Juhasz M et al (2011) The behavior of matrix metalloproteinase-9 in lymphocytic colitis, collagenous colitis and ulcerative colitis. Pathol Oncol Rep 18(1):85–91
Shimoda M, Horiuchi K, Sasaki A et al (2016) Epithelial cell-derived a disintegrin and metalloproteinase-17 confers resistance to colonic inflammation through EGFR activation. EBioMedicine 5:114–124
Walter L, Harper C, Garg P (2013) Role of matrix metalloproteinases in inflammation/colitis-associated colon cancer. Immuno-Gastroenterol 2:22–28
Laroui H, Geem D, Xiao B et al (2014) Targeting intestinal inflammation with CD98 siRNA/PEI—loaded nanoparticles. Mol Ther 22(1):69–80
Godoy-Santos AL, Trevisan R, Fernandes TD, dos Santos MCLG (2011) Association of MMP-8 polymorphisms with tendinopathy of the primary posterior tibial tendon: a pilot study. Clinics 66(9):1641–1643
Li D-Q, Luo L, Chen Z, Kim H-S, Song XJ, Pflugfelder SC (2006) JNK and ERK MAP kinases mediate induction of IL-1β, TNF-α and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp Eye Res 82(4):588–596
Moon CM, Jung S-A, Kim S-E, Song HJ, Jung Y, Ye BD et al (2015) Clinical factors and disease course related to diagnostic delay in Korean Crohn’s disease patients: results from the connect study. PLoS ONE 10(12):e0144390. doi:10.1371/journal.pone.0144390
Sullivan SO’, Gilmer JF, Medina C (2015) Matrix metalloproteinases in inflammatory bowel disease: an update. Mediators Inflamm ID 964131:19 p
Pedersen G, Saermark T, Kirkegaard T, Brynskov J (2009) Spontaneous and cytokine induced expression and activity of matrix metalloproteinases in human colonic epithelium. Clin Exp Immunol 155(2):257–265
García MF, González-Reyes S, González LO et al (2010) Comparative study of the expression of metalloproteases and their inhibitors in different localizations within primary tumours and in metastatic lymph nodes of breast cancer. Int J Exp Pathol 91(4):324–334
Chang Y, Chiu Y, Cheng H et al (2015) Down-regulation of TIMP-1 inhibits cell migration, invasion, and metastatic colonization in lung adenocarcinoma. Tumor Biol 36:3957. doi:10.1007/s13277-015-3039-5
Pereira AC, Dias do Carmo E, Dias da Silva MA, Blumer Rosa LE (2012) Matrix metalloproteinase gene polymorphisms and oral cancer. J Clin Exp Dent 4(5):e297–e301
Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Reddy DN (2015) Role of the normal gut microbiota. World J Gastroenterol 21(29):8787–8803. doi:10.3748/wjg.v21.i29.8787
Kusters JG, van Vliet AHM, Kuipers EJ (2006) Pathogenesis of Helicobacter pylori infection. Clin Microbiol Rev 19(3):449–490
Nardone G, Compare D (2015) The human gastric microbiota: is it time to rethink the pathogenesis of stomach diseases? United Eur Gastroenterol J 3(3):255–260
Wang Z-K, Yang Y-S (2013) Upper gastrointestinal microbiota and digestive diseases. World J Gastroenterol 19(10):1541–1550
Wroblewski LE, Peek RM, Wilson KT (2010) Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev 23(4):713–739
Salih BA (2009) Helicobacter pylori infection in developing countries: the burden for how long? Saudi J Gastroenterol 15(3):201–207
Amsterdam KV, Van Vliet AHM, Kusters JG, Ende AVD (2006) Of microbe and man: determinants of H. pylori related diseases. Microbiol Rev 30(1):131–156
Fox JG, Wang TC (2007) Inflammation, atrophy and gastric cancer. J Clin Invest 117(1):60–69
Fitzgerald RC, Caldas C (2004) Clinical implications of E-cadherin associated hereditary diffuse gastric cancer. Gut 53(6):775–778
Hu B, El Hajj N, Sittler S, Lammert N, Barnes R, Meloni-Ehrig A (2012) Gastric cancer: classification, histology and application of molecular pathology. J Gastrointest Oncol 3(3):251–261
Alzahrani S, Lina TT, Gonzalez J, Pinchuk IV, Beswick EJ, Reyes VE (2014) Effect of Helicobacter pylori on gastric epithelial cells. World J Gastroenterol 20(36):12767–12780
Peek RM, Fiske C, Wilson KT (2010) Role of innate immunity in Helicobacter pylori-induced gastric malignancy. Physiol revi 90(3):831–858
White JR, Winter JA, Robinson K (2015) Differential inflammatory response to Helicobacter pylori infection: etiology and clinical outcomes. J Inflamm Res 8:137–147
Gööz M, Shaker M, Gööz P, Smolka AJ (2003) Interleukin 1β induces gastric epithelial cell matrix metalloproteinase secretion and activation during Helicobacter pylori infection. Gut 52(9):1250–1256
Pillinger MH, Marjanovic N, Kim SY, Lee YC, Scher JU, Roper J et al (2007) Helicobacter pylori stimulates gastric epithelial cell MMP-1 secretion via CagA-dependent and -independent ERK activation. J Biol Chem 282(26):18722–18731
Oliveira MJ, Costa AC, Costa AM, Henriques L, Suriano G, Atherton JC (2006) Helicobacter pylori induces gastric epithelial cell invasion in a c Met and type IV secretion system-dependent manner. J Biol Chem 281(46):34888–34896
Kundu P, De R, Pal I, Mukhopadhyay AK, Saha DR, Swarnakar S (2011) Curcumin alleviates matrix metalloproteinase-3 and -9 activities during eradication of Helicobacter pylori infection in cultured cells and mice. PLoS ONE 6(1):e16306
Stein M, Ruggiero P, Rappuoli R, Bagnoli F (2013) Helicobacter pylori CagA: from pathogenic mechanisms to its use as an anti-cancer vaccine. Front Immunol 4:328
Jiang H, Zhou Y, Liao Q, Ouyang H (2014) Helicobacter pylori infection promotes the invasion and metastasis of gastric cancer through increasing the expression of matrix metalloproteinase-1 and matrix metalloproteinase-10. Exp Ther Med 8(3):769–774
Costa AM, Ferreira RM, Pinto-Ribeiro I, Sougleri IS, Oliveira MJ, Carreto L et al (2016) Helicobacter pylori activates matrix metalloproteinase-10 in gastric epithelial cells via EGFR and ERK-mediated pathways. J Infect Dis 214(4)
Bebb JR, Letley DP, Thomas RJ, Aviles F, Collins HM, Watson SA et al (2003) Helicobacter pylori upregulates matrilysin (MMP-7) in epithelial cells in vivo and in vitro in a Cag dependent manner. Gut 52(10):1408–1413
Nam YH, Ryu E, Lee D, Shim HJ, Lee YC, Lee ST (2011) Cag-A phosphorylation dependent MMP-9 expression in gastric epithelial cells. Helicobacter 16(4):276–283
Dethlefsen L, Mcfall-Ngai M, Relman DA (2007) An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature 449:811–818
Delgado S, Cabrera-Rubio R, Mira A, Suarez A, Mayo B (2013) Microbiological survey of the human gastric ecosystem using culturing an pyrosequencing methods. Microb Ecol 65:763–772
Wu WM, Yang YS, Peng LH (2014) Microbiota in the stomach: new insights. J Dig Dis 15:54–61
Sheh A, Fox JG (2013) The role of the gastrointestinal microbiome in Helicobacter pylori pathogenesis. Gut Microbes 4:505–531
Bik EM, Eckburg PB, Gill SR, Nelson KE, Purdom EA, Francois F, Perez-Perez E, Blaser MJ, Relman DA (2006) Molecular analysis of bacterial microbiota in human stomach. Proc Natl Acad Sci U S A 103:732–737
Andersson AF, Lindberg M, Jakobsson H, Bäckhed F, Nyrén P, Engstrand L (2008) Comparative analysis of human gut microbiota by barcoded pyrosequencing. PLoS ONE 3(7):e2836
Khosravi Y, Dieye Y, Poh BH, Ng CG, Loke MF, Goh KL, Vadivelu J (2014) Culturable bacterial microbiota of the stomach of helicobacter pylori positive and negative gastric disease patients. Sci World J 2014:610421
Eun CS, Kim BK, Han DS, Kim SY, Kim KM, Choi BY, Song KS, Kim YS, Kim JF (2014) Differences in gastric mucosal microbiota profiling in patients with chronic gastritis, intestinal metaplasia, and gastric cancer using pyrosequencing methods. Helicobacter 19:407–416
Correa P (1992) Human gastric carcinogenesis: a multistep and multifactorial process—first American cancer society award lecture on cancer epidemiology and prevention. Cancer Res 52:6735–6740
Polk DB, Peek RM Jr (2010) Helicobacter pylori: gastric cancer and beyond. Nat Rev Caner 10:403–414
Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ (2001) Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 345:784–789
Aviles-Jimenez F, Vazquez-Jimenez F, Medrano-Guzman R, Mantilla A, Torres J (2014) Stomach microbiota composition varies between patients with non-atrophic gastritis and patients with intestinal type of gastric cancer. Sci Rep 4:4202
Vandenbroucke RE, Libert C (2014) Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat Rev Drug Discov 13:904–927
Page-McCaw A, Ewald AJ, Werb Z (2007) Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 8:221–233
Cathcart J, Pulkoski-Gross A, Cao J (2015) Targeting matrix metalloproteinases in cancer: bringing new life to old ideas. Genes Dis 2:26–34
Devy L, Dransfield DT (2011) New strategies for the next generation of matrix-metalloproteinase inhibitors: selectively targeting membrane-anchored MMPs with therapeutic antibodies. Biochem Res Int 2011:1–11
Remacle AG, Golubkov VS, Shiryaev SA, Dahl R, Stebbins JL, Chernov AV, Cheltsov AV, Pellecchia M, Strongin AY (2012) Novel MT1-MMP small-molecule inhibitors based on insights into hemopexin domain function in tumor growth. Cancer Res 72:2339–2349
Coppola JM, Bhojani MS, Ross BD, Rehemtulla A (2008) A small-molecule furin inhibitor inhibits cancer cell motility and invasiveness. Neoplasia 10:363–370
Albini A, Tosetti F, Li VW, Noonan DM, Li WW (2012) Cancer prevention by targeting angiogenesis. Nat Rev Clin Oncol 9:498–509
Dormán G, Cseh S, Hajdú I, Barna L, Kónya D, Kupai K, Kovács L, Ferdinandy P (2010) Matrix metalloproteinase inhibitors: a critical appraisal of design principles and proposed therapeutic utility. Drugs 70:949–964
García-Pardo A, Opdenakker G (2015) Nonproteolytic functions of matrix metalloproteinases in pathology and insights for the development of novel therapeutic inhibitors. Metalloproteinases Med 2:19–28
Hu J, Van den Steen PE, Sang Q-XA, Opdenakker G (2007) Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug discov 6:480–498
Cathcart J, Pulkoski-Gross A, Cao J (2015) Targeting matrix metalloproteinases in cancer: bringing new life to old ideas. Genes Dis 2(1):26–34
Wojtowicz-Praga S, Low J, Marshall J, Ness E, Dickson R, Barter J, Sale M, McCann P, Moore J, Cole A (1996) Phase I trial of a novel matrix metalloproteinase inhibitor batimastat (BB-94) in patients with advanced cancer. Invest New Drugs 14:193–202
Lu C, Lee JJ, Komaki R et al (2010) Chemoradiotherapy with or without AE-941 in stage III non-small cell lung cancer: a randomized phase III trial. J Natl Cancer Inst 102(12):859–865
Bissett D, O’Byrne KJ, Von Pawel J, Gatzemeier U, Price A, Nicolson M, Mercier R, Mazabel E, Penning C, Zhang MH (2005) Phase III study of matrix metalloproteinase inhibitor prinomastat in non-small-cell lung cancer. J Clin Oncol 23:842–849
Leighl NB, Paz-Ares L, Douillard J-Y, Peschel C, Arnold A, Depierre A, Santoro A, Betticher DC, Gatzemeier U, Jassem J (2005) Randomized phase III study of matrix metalloproteinase inhibitor BMS-275291 in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: National Cancer Institute of Canada-Clinical Trials Group Study BR. 18. J Clin Oncol 23:2831–2839
Sparano JA, Bernardo P, Stephenson P, Gradishar WJ, Ingle JN, Zucker S, Davidson NE (2004) Randomized phase III trial of marimastat versus placebo in patients with metastatic breast cancer who have responding or stable disease after first-line chemotherapy: Eastern Cooperative Oncology Group Trial E2196. J Clin Oncol 22:4683–4690
Le Quement C, Guenon I, Gillon JY, Valenca S, Cayron-Elizondo V, Lagente V, Boichot E (2008) The selective MMP-12 inhibitor, AS111793 reduces airway inflammation in mice exposed to cigarette smoke. Br J Pharmacol 154:1206–1215
Bonnans C, Chou J, Werb Z (2014) Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 15(12):786–801. doi:10.1038/nrm3904
Acknowledgements
The financial assistance from CSIR network projects HUM (BSC 0119) and INDEPTH (BSC 0111) is thankfully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Swarnakar, S., Roy, A., Ghosh, S., Majumder, R., Paul, S. (2017). Gastric Pathology and Metalloproteinases. In: Chakraborti, S., Dhalla, N. (eds) Pathophysiological Aspects of Proteases. Springer, Singapore. https://doi.org/10.1007/978-981-10-6141-7_19
Download citation
DOI: https://doi.org/10.1007/978-981-10-6141-7_19
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-6140-0
Online ISBN: 978-981-10-6141-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)