Abstract
Cell cycle progression and cell proliferation are under precise and orchestrated control in normal cells. However, uncontrolled cell proliferation caused by aberrant cell cycle progression is a crucial characteristic of cancer. Understanding cell cycle progression and its regulation sheds light on cancer treatment. Agents targeting cell cycle regulators (such as CDKs) have been considered as promising candidates in cancer treatment. Although the first-generation pan-CDK inhibitors failed in clinical trials because of their adverse events and low efficacy, new selective CDK 4/6 inhibitors showed potent efficacy with tolerable safety in preclinical and clinical studies. Here we will review the mechanisms of cell cycle regulation and targeting key cell cycle regulators (such as CDKs) in breast cancer treatment. Particularly, we will discuss the mechanism of CDK inhibitors in disrupting cell cycle progression, the use of selective CDK4/6 inhibitors in treatment of advanced, hormone receptor (HR)-positive postmenopausal breast cancer patients, and other clinical trials that aim to extend the utilization of these agents.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Introduction
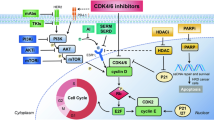
As the basic structural and functional unit of living organisms, cells reproduce themselves by means of cell cycle process, during which they duplicate their genetic materials and distribute their DNAs equally into two equal cells (also called daughter cells). In eukaryotic cells, cell cycle progression takes place in steps. The first step is called G1 phase, followed by the chromosomes replication in S phase. Then comes the G2 phase which is followed by chromosomes segregation in M phase [1]. Each step of cell cycle progresses in sequence, which is controlled by the actions of cyclins and their counterpart cyclin-dependent kinases (CDKs). Human cells contain a large family of CDKs and cyclins. However, only a few certain subsets of CDK-cyclin complexes are involved in cell cycle regulation [2]. The kinase activity of CDKs is controlled mainly by three different ways: the binding to their counterpart cyclins, the binding to negative regulators (CDK inhibitors, CKI), and phosphorylation/dephosphorylation of CDKs. The cell cycle is also supervised by checkpoints, which detect mistakes during DNA synthesis and chromosome segregation. CDKs activity interacts with checkpoints, which halts cell cycle progression and causes cell cycle arrest. This cell cycle progression brake enables cells to fix these mistakes, thus preventing defected DNA from transmitting to daughter cells [3]. Deregulation of CDKs leads to uncontrolled proliferation and increases genomic and chromosomal instability, which plays a significant role in carcinogenesis [4].
Deregulation of cell cycle, leading to aberrant cell proliferation, is a characteristic of cancer. Deranged CDK4/6 axis in the G1/S transition and perturbations in G2/M transition mediated by CDK1/2 are pivotal carcinogenesis events. Given their important role in cell cycle regulation, CDKs could be promising targets in cancer treatment. However, the first-generation pan-CDK inhibitors failed in preclinical/clinical trials because of the adverse events and low efficacy [3, 5]. In recent years, new selective CDK 4/6 inhibitors, including ribociclib, abemaciclib, and palbociclib, have been proved to be promising anticancer drugs with remarkable effects and manageable toxicity. Among these agents, palbociclib was the first CDK4/6 inhibitor that received FDA approval for treating postmenopausal women with estrogen receptor (ER)-positive, HER2-negative advanced breast cancer in combination with letrozole (February 2015) or with fulvestrant (February 2016) [6, 7].
In this review, we will introduce the mechanism of cell cycle progression, especially the aberrant cell cycle regulation in the development of breast cancer. We will also review the advantage and disadvantage of the first-generation pan-CDK inhibitors and the selective CDK4/6 inhibitors. Because of the high efficacy and tolerable adverse events of selective CDK4/6 inhibitors in treating advanced ER-positive breast cancer patients, we will also discuss the potential use of CDK4/6 inhibitors in treatment beyond current indication, with an aim to extend the utilization of these agents.
12.2 Cell Cycle and Its Regulation
Pioneer works by Lee Hartwell, Paul Nurse, and Tim Hunt demonstrated the mechanisms of mammalian cell division [1]. The well-established cell cycle regulation came from studies in yeast. Only one CDK (Cdc28 in Saccharomyces cerevisiae and Cdc2 in Schizosaccharomyces pombe) cooperated with its counterpart cyclins to regulate cell cycle progression in these simple cells. Although many new members of CDKs and cyclins have been identified in other species, only certain subsets of CDKs and cyclins are responsible for cell cycle regulation in human cells [2].
During cell cycle progression, each of the main events takes place sequentially. After cytokinesis is completed, daughter cells can either enter into the next stage of cell cycle or stay quiescence (also called G0). Cells initiate entry into cell cycle with the presence of extracellular signals such as growth factors. The cells that continue to divide need to go through the first stage (G1 phase) of the new cycle.
12.2.1 G1–S Phases
When cells enter into the cell cycle, mitogenic signals facilitate the synthesis of D-type cyclins (cyclin D1, D2, and D3) and relocation of CDK4/6 to nucleus, forming CDK4/6-cyclin D complexes. The interaction between CDK4/6 and cyclin D significantly enhances the kinase activity with a broader spectrum of substrate than other CDKs [8]. CDK4/6-cyclin D complexes phosphorylate retinoblastoma (Rb) protein family (including pRb, p107, and p130), which plays an important role in target gene suppression. Hypophosphorylated pRb prevents G1-S transition by blocking transcriptional activation of E2F and recruiting histone deacetylases to promoters of S-phase entry genes [9]. Once phosphorylated, inactivated pRb is released from E2F, which can then promote the transcription of E-type cyclins and other genes necessary for S-phase entry and DNA synthesis. Cyclin E binds to CDK2 and forms active CDK2-cyclin E complexes. At the end of G1, activated CDK2-cyclin E complexes facilitate Rb phosphorylation and cause the irreversible inactivation of Rb. This process, called the restriction point, is pivotal in carcinogenesis because alteration of the key regulators could lead to cell division without mitogenic stimuli [10]. In addition to Rb phosphorylation, CDK2-cyclin E complexes participate in phosphorylation of other substrates that involve DNA replication, histone modification, DNA repair, and centrosome duplication and maturation [11].
Inactivation of Rb also promotes expression of A-type and B-type cyclins. Once cells enter into S phase, cyclin E is rapidly degraded by SCF-Fbxw 7 ubiquitin ligase and then cleavage by proteasome, which leads to the inactivation of CDK2-cyclin E complexes [11]. With the accumulation of cyclin A during S phase, CDK2, detached from cyclin E, interacts with the newly synthesized cyclin A. CDK2-cyclin A complexes can phosphorylate numerous proteins necessary for finishing S phase, including transcription factors, proteins relevant to DNA synthesis, DNA repair, histone modification, and cell cycle checkpoints. After the completion of mitosis, CDK2 activity might still exist. Pre-mitotic levels of CDK2 and p21CIP1 activity partially predict whether the postmitotic daughter cells continue to divide or become quiescent [12].
Another kinase, CDK3, which binds to cyclin C, may also be involved in Rb phosphorylation during G0-G1 transition. Considering that cyclin C expression is prior to cyclin D, Rb phosphorylation could be initiated by CDK3-cyclin C. CDK3 also interacts with cyclin E and cyclin A, but the role of CDK3 and its counterparts remains unclear [13] .
Cyclin-dependent kinase inhibitors (CKIs) also play a pivotal role in G1-S transition. The inhibitor of CDK4 (INK4) includes four structurally related proteins, p16INK4A, p15INK4B, p18INK4C, and p19INK4D, which consist of numerous ankyrin repeats. The INK4 proteins exclusively bind to CDK4/6 rather than other CDKs or cyclin D [14,15,16,17,18]. The cyclin-dependent kinase inhibitor 1/kinase inhibitory proteins (CIP/KIP), including, p21CDKN1A/CIP1, p27CDKN1B/KIP1, and p57CDKN1C/KIP2, can bind to all CDKs in varying degree, which have an alternative positive or negative regulatory role.
The INK4 proteins disrupt the interaction between CDK4/6 and cyclin D, by binding to the catalytic domains of CDK4/6, which subsequently inhibits the kinase activity [19]. For example, diverse oncoproteins prevent neoplastic transformation by inducing p16INK4A, which results in G1 arrest of the cell cycle and facilitates oncogene-induced senescence [20]. Similarly, p15INK4B suppresses epithelial cell proliferation with the presence of transforming growth factor-B [21]. Therefore, in the development of cancer, cells must evade the oncogene-induced senescence, which may occur through the loss of p16INK4A or loss of Rb [22, 23]. The loss of p16INK4A releases the CDK4/6 and subsequently activates Rb phosphorylation, leading to oncogenic proliferation, whereas the loss of Rb causes dysregulation of downstream signaling in the cell cycle. Therefore, Rb is necessary for the p16INK4A-mediated cell cycle arrest, and Rb-negative cancer has intrinsic resistance to p16INK4A or the agents of CDK4/6 inhibitors [24].
In contrast to INK4 proteins in control of CDK4/6, the CIP/KIPs family binds to CDK2-cyclin E complexes, which potently inhibit kinase activity and thus stabilize cyclin E [11, 18]. p21CIP1, one of the most important target genes of p53, serves as a DNA damage checkpoint which blocks DNA synthesis, whereas p27KIP1 responses to mitogenic signaling and relates to deregulated proliferation [25, 26]. At the basal level, both p21CIP1and p27KIP1 can bind to and stabilize CDK4/6-cyclin D complexes without inhibiting their kinase activity. The sequestered p21CIP1and p27KIP1 released from CDK4/6-cylin D complexes indirectly inhibit CDK2-cyclin E complexes, which form an interaction network between the cyclins and CDK inhibitors [18, 27]. In addition, the inhibitory function of p27 was confirmed to rescue cyclin D1-null mice that displayed defects without p27 ablation [18]. However, different studies showed that p21CIP1and p27KIP1 proteins had no direct inhibitory effects on CDK4/6-cyclin D complexes. Instead, they were found to promote the assembly and proper nuclear translocation of the complexes [28, 29]. The role of p21CIP1and p27KIP1 in carcinogenesis remains elusive.
12.2.2 G2–M Phases
In late S phase, cyclin A binds to CDK1, forming CDK1-cyclin A complexes. Sharing similar substrates with CDK2-cyclin B, CDK1-cylin A phosphorylates numerous proteins involved in DNA synthesis and cell cycle regulators [30, 31]. The precise roles of CDK1-cyclin A and CDK2-cyclin B in S to G2 transition and their difference still need further study. After the nuclear envelope breakdown, cyclin A is degraded via ubiquitin-mediated proteolysis, whereas cyclin B becomes evident. The newly synthesized cyclin B binds to CDK1, forming CDK1-cyclin B complexes that may control G2-M transition and trigger mitosis [32]. CDK1-cyclin B is presumed to phosphorylate abundant substrates including microtubule-binding proteins, proteins relevant to translation, ubiquitin-dependent proteolysis, replication, and other mitosis regulators. Cytoplasmic CDK1-cyclin B complexes also facilitate centrosome segregation through the phosphorylation of the centrosome-associated motor protein Eg5 during prophase. In order to exit from mitosis, CDK1-cylin B complexes are decomposed by the degradation of cyclin B regulated by the anaphase-promoting complex [33, 34].
12.2.3 Biological Function of Other CDKs
CDK5
Primarily active in postmitotic neurons, CDK5 interacts with p35 and p39, which are specific in brain tissue. CDK5-p35 and CDK5-p29 complexes can phosphorylate numerous substrates, which are relevant to neuronal cell cycle arrest and differentiation and apoptosis in neuronal diseases. These substrates are involved in transcription, neuronal function, migration, and synaptic transmission [35,36,37,38].
CDK7
As a component of the CDK-activating kinase (CAK), CDK7 interacts with cyclin H, forming CDK7-cyclin H complexes. The CDK7-cyclin H complexes are presumed to phosphorylate and facilitate all cell cycle CDKs. Given its interaction with TFIIH and RNA polymerase III, CDK7-cyclin H may also function in the regulation of transcription [39, 41].
CDK8 and CDK9
CDK8 and CDK9 cooperate with cyclin C and cyclin T, respectively. CDK8-cyclin C and CDK9-cyclin T complexes regulate transcription by phosphorylating the large subunit of RNA polymerase II. CDK8-cyclin C complexes can also inhibit CAK activity by phosphorylating cyclin H. Increased CDK8 kinase activity is relevant to expression of β-catenin transcriptional targets and the inhibition of E2F1 targets apoptotic genes [41]. On the other hand, CDK9 interacts with cyclin H and cyclin K, forming P-TEFb transcription factors that regulate transcriptional elongation [42].
CDK10 and CDK11
Although its cyclin partner has not been identified yet, CDK10 may function in the regulation of G2-M transition. CDK10 also modulates the trans-activation activity of Ets2 transcription factors, a regulator of CDK1 expression [43]. CDK11 binds to cyclin L and interacts with the general precursor mRNA splicing factors RNAPS1 and 9G8 and RNA polymerase II [44]. In addition to RNA process regulation, CDK11 is relevant to the duplication and maturation of centrosome, the assembly of spindle, the binding of chromatid, and the division of the cytoplasm at the end of mitosis [45,46,47,48].
CDK12 and CDK13
CDK12 (also called Crkrs) and CDK13 (also called CDC2L5) are involved in alternative splicing regulation by binding to cyclin L [45, 46].
12.3 Cell Cycle Dysregulation in Breast Cancer
Breast cancer is a heterogeneous disease generated from various genetic and epigenetic mutations of oncogenes and tumor suppressor genes that ruin homeostasis maintenance of proliferation, differentiation, and apoptosis in mammary epithelial cells. Under cell cycle dysregulation, decreased CDKs activities result in defective homeostasis, whereas hyperactivation of CDKs favors carcinogenesis by inducing uncontrolled cell division with subsequent development of malignant phenotypes. The mutations in CDKs and their regulators have been under extensive study. Dysregulation of the CDK4/6 axis and CDK2 has been emphasized in many human cancers including breast cancer due to its distinct mechanisms [49].
12.3.1 Cyclin D1 in ER-Positive Breast Cancer
Cyclin D1, encoded by CCND1 gene, was first described in carcinogenesis due to gene rearrangement—the chromosome 11p15:q13 inversion in parathyroid adenoma [50]. Overexpression of cyclin D1, with an incidence of 45–50% in primary ductal carcinomas, is one of the most common oncogenic events in breast cancer [51]. In patients with luminal estrogen receptor(ER)-positive breast cancer, activated ER signaling boosts the CCND1 transcription and leads to cyclin D1 overexpression [52]. In breast cancer cells, cyclin D1 is a direct target of estrogen signaling and enhances cell proliferation [53]. Cyclin D1 can also bind to ER and enhance transcriptional activity of ER through its CDK-independent function, which probably reinforces the interaction of cyclin D1 and ER signaling in ER-positive luminal breast cancer [54]. Additional dysregulation in ER-positive breast cancers includes cyclin D1 gene amplification and gene translocation [5, 55]. In patients with primary breast cancer, cyclin D1 overexpression is restricted to specific pathological subtypes. For example, cyclin D1 overexpression exists in almost exclusively estrogen receptor-positive ductal carcinoma and in vast majority of lobular carcinoma [56, 57].
In mouse mammary tumor virus (MMTV)-cyclin D1 transgenic mice model, overexpression of cyclin D1 results in mammary hyperplasia and development of mammary adenocarcinomas, implicating that cyclin D1 plays an important role in the development of breast cancer [58]. The distinction of cyclin D1 mRNA expression levels between benign and malignant lesion indicates that cyclin D1 overexpression is pivotal in the transition from ductal carcinoma in situ to invasive breast cancer [59]. Cyclin D1 protein overexpression in mammary hyperplasia and intraductal breast carcinoma suggests that cyclin D1 protein is important at the very early stage of breast carcinogenesis and continues to have a crucial role throughout the development of malignancy [60]. In human breast cancer cells, induction of cyclin D1 accelerates G1 phase, which makes it possible for the arrested cells to complete the cell cycle [61]. Cyclin D1 knockout mice are protected from breast cancer induced by Ras or Neu oncogenes, rather than c-myc or Wnt-1 oncogenes, revealing that cyclin D1 is a mediator in carcinogenesis [62]. The oncogenic action of Neu oncogenes seems to reflect a requirement for the cyclin D1-CDK4/6 interactions, since overexpression of p16 blocks carcinogenesis by Neu [63]. Taking together, cyclin D1 overexpression plays a critical role in evolution of breast cancer, and targeting cyclin D1 may be a feasible strategy in breast cancer treatment, specifically in patients with activated Neu-Ras pathways.
In addition to the CDK4/6-dependent activities, cyclin D1 has non-cell cycle-associated CDK-independent function, acting as transcriptional regulator in ER-positive breast cancer [64]. Cyclin D1 binds to the hormone-binding domain of ER and subsequently facilitates the interaction between ER and its coactivators, leading to upregulation of ER-mediated transcriptional activity through a CDK4/6-independent mechanism [65, 66].
12.3.2 Cyclin E in HER2-Positive Breast Cancer
In ER-positive breast cancer, cyclin E expression is at a low level. On the contrary, HER2-positive breast cancer is characterized by overexpression of cyclin E [67, 68]. Cyclin E overexpression also associates with poor differentiation [69], poor endocrine response [70], poor prognosis [71], and predicting sensitivity to cisplatin/Taxol chemotherapy and trastuzumab [72, 73]. In mouse model, cyclin E overexpression results in mammary hyperplasia and tumor formation at low incidence after long latency [74]. In breast cancer cell line, amplification of cyclin E results in a 64-fold increase of cyclin E mRNAs that express cyclin E throughout all stages of cell cycle [75]. In addition to the overexpression of full-length 50kD cyclin E, these cell lines overexpress other low molecular weight isoforms of cyclin E. These isoforms, lacking the amino terminus, are hyperactive in activating substrates and accelerating the cell cycle progression through G1/S phase. The level of cyclin E and the summation level of cyclin E isoforms are shown to be strongly associated with breast cancer patient survival [71]. Cyclin E overexpression coexists with HER2 gene amplification in some patients with HER2-positive breast cancers, which is generally associated with poor survival and probably trastuzumab resistance [76]. Previous studies showed contradictory prognostic effects of cyclin E in breast cancer patients, which was possibly due to the use of varying breast cancer phenotypes, different methods, and threshold values to evaluate the expression of cyclin E [77]. A recent study of 2494 patients with breast cancer shows that cytoplasmic cyclin E is a predictor of recurrence with the highest likelihood consistently across different patient cohort and subtypes, suggesting cyclin E as a critical target in breast cancer treatment [78].
Cyclin E and HER2 interact with each other by various mechanisms in patients with HER2-positive breast cancer. HER2 receptor-mediated carcinogenesis was shown to shorten G1 phase, resulting in aberrant cell cycle and subsequently uncontrolled proliferation, probably through upregulation of CDK2 activity [79]. Other studies demonstrated that HER2 straightly enhanced cyclin E activity since decreased HER2 signaling resulted in lower cyclin E expression, particularly the low molecular weight (LMW) isoforms, which in turn had prognostic and predictive roles in HER2-overexpressing breast cancer [80]. LMW-cyclin E binds to and activates CDK2 more strongly, leading to increased CDK2 activity and decreased sensitivity of the LMW-cyclinE-CDK2 complexes to inhibition by p21 and p27 [81]. The mammary tumorigenesis caused by LMW-cyclin E requires CDK2 activity, indicating that anti-CDK2 therapy may have potential role in LMW-overexpressing human breast tumors [82].
12.3.3 CKIs in Breast Cancer
Cyclin-dependent kinase inhibitors (CKIs) function as tumor suppressors predominately in the end of G1 phase, which trigger DNA damage checkpoint to block impaired cells and initiate repair progression or apoptosis. Despite distinct mechanisms of tumor suppressor genes, the interferences of these genes lead to accumulation of mutation and eventually cause carcinogenesis. The INK4 proteins play a pivotal role in carcinogenesis for the high incidence of p16INK4A and/or p15INK4B inactivation in various human cancers, including breast cancer [83].
p16INK4A
In normal breast tissue, the absence of p16INK4A is associated with hyper-methylation of p16INK4A gene, whereas hypo-methylation of p16INK4A is associated with expression of p16INK4A mRNA in breast cancer [84]. Overexpression of p16INK4A occurs in both grade 1 and grade 2 breast carcinomas with a marked decline in grade 3 tumors [85]. A study in 14 breast cell lines showed that p16INK4A defect existed in 4 (29%) breast cell lines, 2 (14%) of which had p16INK4A gene methylation [86].These data suggest the role of p16INK4A is much more complex than previously hypothesized.
p15INK4B
In 14 breast cancer cell lines, 3 (21%) have p15INK4B gene mutation, whereas no methylated one is found in primary breast carcinomas [86, 87]. Although the methylation of p15INK4B gene is common in leukemia and glioma, this mutant was rare in breast cancer, which suggests that the mechanism of p15INK4B gene inactivation may be more complicated in different organs [88].
p21CIP1
p21CIP1 has been long considered as a potential tumor suppressor gene, because p21CIP1-null mice develop mammary tumor with the presence of Ras expression [89]. Nevertheless, the expression of p21CIP1 is suppressed in normal breast tissue, whereas the accumulation of p21CIP1 is observed in breast tumor tissues [85]. Clinical study implicates that the cytoplasmic localization of p21CIP1 is relevant to HER2-overexpression, both of which predict poor prognosis in breast cancer patients [90].
p27KIP1
The p27KIP1 acts as another important tumor suppressor gene for mice with deficient p27KIP1 generated pituitary adenomas and displays higher risk of carcinoma when exposed to carcinogens [91]. The expression of p27KIP1 is at relatively high levels in normal breast, whereas the expression of p27KIP1 is decreased in tumor tissues, particularly in high-grade tumors [85, 92].
12.4 Targeting CDKs in Breast Cancer Treatment
Breast cancer treatment is a combination of surgery, chemotherapy, endocrine therapy, radiotherapy, targeted therapy, and other therapies [93]. The critical role of CDKs and their counterparts in cell cycle regulation and carcinogenesis raises the possibility of targeting these molecules. The therapeutic value of targeting CDKs has been intensively investigated, especially the interphase CDKs (CDK1, CDK2, CDK4, CDK5, and CDK6). Nevertheless, their usages in breast cancer treatment as pharmaceutical targets still need further study [93]. The ideal CDK-targeted therapy requires interruption of specific CDKs signaling in malignant cells but spares other CDKs activities that are critical in normal cell cycle progress to achieve high efficacy and low toxicity. As mentioned above, dysregulation of the cyclin D-CDK4/6-Rb pathways may lead to acceleration of G1-S progression and uncontrolled proliferation. These observations enable the development of CDK4/6 inhibitors for specific transformed cells. Feasible CDK4/6 inhibitors are supposed to decrease Rb phosphorylation and block cell cycle progression in cells with Rb persistent activation. In cells that lose Rb function, these agents may be ineffective. Thus, selection of appropriate patients for specific anti-CDK4/6 therapy depends on whether the cancer mainly relies on CDK4/6 axis dysregulation to accelerate G1/S transition. Luminal ER-positive breast cancer, but not basal-like ER-negative breast cancer, is the subtype with amplication/overexpression of cyclin D1 and is suitable for anti-CDK4/6 therapy. Even for women with advanced ER-positive breast cancer who have developed resistance to endocrine therapy, most of them still rely on cyclin D1-CDK4/6 complexes to initiate the G1/S transition.
12.4.1 The First-Generation Pan-CDK Inhibitors
In the past two decades, numerous CDK inhibitors have been discovered as potential therapeutic agents and evaluated in preclinical/clinical trials in different tumor models. However, none of the first-generation pan-CDK inhibitors, including flavopiridol, olomoucine, and roscovitine, achieved permission in clinical application. These agents fail to meet the expectation in preclinical/clinical studies, exhibiting limited activity and severe toxicity.
Among these first-generation inhibitors, flavopiridol, also known as alvocidib, has been extensively investigated in more than 60 clinical trials up to now [49]. Flavopiridol derived from chromone alkaloid inhibits kinase activities of several CDKs (CDK1, CDK2, CDK4, CDK6, CDK7, and CDK9). Although flavopiridol has limited clinical effects in patients with hematological malignancies, including chronic lymphocytic leukemia, adverse events come out when the dose increases [94, 95]. Previous studies about flavopiridol showed disappointing results for the treatment of breast cancer. No evident antitumor response was observed in two patients (6%) with advance breast cancer in a phase I trial [96]. Another phase I trial showed that only one patient (5%) with breast cancer might benefit from the combination of flavopiridol with 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX) [97].The most common adverse events, including hypotension, neutropenia, fatigue, diarrhea, and nausea, often lead to discontinuation of the trials. Since flavopiridol did not achieve expected success as an ideal CDK inhibitor, no phase III trial was carried out, and the development of flavopiridol was given up.
In parallel with flavopiridol, a phase I trial was carried out to evaluate roscovitine (also called R-roscovitine, CY-202, or seliciclib), which had an inhibitory effect on CDK1, CDK2, CDK5, and CDK7. Of the 56 patients receiving roscovitine treatment, only one patient with hepatocellular carcinoma achieved a partial response and sustained tumor stabilization [98]. In breast cancer cell line MCF-7, roscovitine was shown to suppress cell proliferation and reduce cell survival of endocrine-resistant breast cancer cells [99]. In vivo model of MCF-7 cell line, roscovitine can synergize with the antitumor effect of doxorubicin without increasing toxicity [100]. These results reveal the potential therapeutic role of CDK2 inhibition in abrogating growth of endocrine-resistant breast cancer cells.
SNS032 (also called BMS-387032), with an inhibitory effect on CDK2, CDK7, and CDK9, has been shown to sensitize hypoxic and quiescent non-small cell lung cancer cells to radiation therapy. The inhibitory activity may rely on cell cycle independent of CDKs, including CDK7 and CDK9, which are presumed to modulate DNA double-strand break repair [101]. Other studies show that AML cells treated with SNS-032 are more susceptible to the cytotoxic effects of Ara-C, whereas SNS-032 fails to achieve expected clinical outcomes in patients with chronic lymphocytic leukemia and multiple myeloma [102, 103]. In a phase I trial of 21 patients with metastatic solid tumors, only 3 patients (15%) achieved a response of stable disease, while the results of 2 patients (10%) with advanced breast cancer were not published [104].
Dinaciclib (also called MK-7965 and SCH727965) is a potent pan-CDK inhibitor, with higher inhibitory effect on CDK1, CDK2, CDK5, and CDK9. It exhibits better inhibition of Rb phosphorylation, compared with flavopiridol [105]. Dinaciclib has been well tolerated in initial trials, and patients with advanced solid malignancies, myeloma, and chronic lymphocytic leukemia have received profitable clinical efficacy [105,106,107].However, a few studies on patients with advanced breast cancers showed disappointing outcomes. For example, a phase I trial in patients with metastatic triple-negative breast cancer revealed that the combination of dinaciclib and epirubicin might result in massive adverse events and failed to be an effective therapy for metastatic triple-negative breast cancer [108]. Randomized phase II trial also received disappointing results, which compared the therapeutic efficacy of dinaciclib with the chemotherapy drug capecitabine in patients with advanced breast cancer. Although dinaciclib monotherapy suppressed tumor progression with generally tolerated adverse events, its efficacy was inferior to capecitabine [109].
The reasons why the first-generation pan-CDK inhibitors fail in clinical trials may be explained by the following. Firstly, the first-generation pan-CDK inhibitors, with low specificity, may influence cell cycle progression in different aspects. It remains unknown what kind of CDKs are actually blocked in vivo and whether one may interfere with another. Secondly, the biomarkers for anti-CDKs therapy are unclear. Because of the inter- and intra-tumor heterogeneity of breast cancer, different subpopulations may respond totally differently to an identical agent. Therefore, the identification of sensitive subpopulations and the selection for appropriate agents need to be further optimized. Thirdly, some of these CDKs inhibitors can also target proteins (such CDK9) that are crucial in cellular transcription and thus influence cell proliferation and apoptosis in both cancer cell and normal cells. The inhibition of transcriptional CDKs may prevent carcinogenesis by inducing apoptosis of cancer cells. However, it limits the therapeutic dose of these nonselective agents because they fail to distinguish transformed cells from normal cells. As a result, severe adverse effects arise, such as hypotension, neutropenia, fatigue, diarrhea, and nausea [49, 93].
12.4.2 The Selective CDK4/6 Inhibitors
Since cyclin D-CDK4/6 pathways alteration provides a proliferative and survival advantage to various cancers, including breast cancer, targeting CDK4/6 may achieve more therapeutic benefits than targeting other CDKs. For example, CDK4/6 gene amplification and cyclin D1 gene amplification/translocation mainly exist in ER-positive breast cancer. Estrogen-mediated signaling can also lead to cyclin D1 overexpression. Preclinical studies in cell lines and xenografts have revealed that selective CDK4/6 inhibitors have potent inhibitory effects on malignancies with limited cytotoxicity [110].
Understanding the molecular structure of CDKs leads to the development of more selective CDK4/6 inhibitors [55].And up to now, three selective CDK4/6 inhibitors (palbociclib, ribociclib, and abemaciclib) have been widely studied in preclinical and clinical trials, with promising efficiency and manageable adverse events.
12.4.2.1 Palbociclib
Palbociclib (PD0332991) is one of the most well-known selective CDK4/6 inhibitors discovered from a subset of pyridopyrimidine compounds according to its unprecedented levels of selectivity for CDK4 as well as its superior physical and pharmaceutical properties. In vitro, it has a prior selectivity for CDK4 and CDK6 (IC50 = 0.011 μmol/L, 0.016 μmol/L, respectively) but has limited activity against other CDKs or tyrosine kinases. In preclinical studies, palbociclib was shown to arrest cells exclusively in G1, decrease phospho-Rb and Ki-67, and reduce expression of E2F target genes in Rb-positive tumors. Consistent with its mechanism of action, palbociclib failed to inhibit the growth of triple-negative breast cancer cell line with the feature of Rb deficiency [111]. Later study found that palbociclib might have inhibitory activity in Rb-deficient cells, probably because of the supplementary function of other phosphorylate retinoblastoma (Rb) proteins like p107 or p130 [112].
Two phase I studies investigating the dose-limiting toxicities (DLTs) and the maximum tolerated dose (MTD) of palbociclib were conducted in patients with relapsed or refractory cancer, including Rb-positive advanced solid tumors and non-Hodgkin’s lymphoma [113, 114]. A phase I study of 33 patients, who received palbociclib in 2/1 schedule (palbociclib once daily for 2 weeks on treatment; 1 week rest), gained therapeutic benefits. A case of partial response was reported in the patient with testicular cancer, who received palbociclib 200 mg/d. Additional nine cases were reported to achieve stable disease for more than two cycles, and three cases maintained stable disease for more than ten cycles. Although treatment-related adverse events happened in 29 cases (88%), most of them were manageable [114]. Another study of 41 patients that administered palbociclib once daily for 21 of 28 days (3/1 schedule) revealed that 10 (27%) patients maintained stable disease for more than 4 cycles and 6 of them achieved prolonged benefit for more than 10 cycles with tolerated toxicities [113]. Similar dose-limiting adverse events were observed, and the most common adverse event was neutropenia. Based on these studies, the MTD, 200 mg/d and 125 mg/d, respectively, was recommended for phase II study.
A phase II study of palbociclib for monotherapy (125 mg/d; 3/1 schedule) was performed in 37 patients with Rb-positive advanced breast cancer [115]. Most of these patients had hormone receptor (HR)-positive disease and were pretreated with two or more prior hormonal therapy or chemotherapy. The overall median progression-free survival was 3.7 months, which was significantly related to HR level as well as progression on prior hormone treatment. Patients with HR-positive tumor had significantly longer progression-free survival than those with HR-negative tumors (4.5 months versus 1.5 months). The progression-free survival in those with progression disease was associated with the number of previous prior hormone treatment. As for the overall response rate, partial response (PR) was reported in two cases, and stable disease (SD) for more than 6 months existed in five cases. The clinical benefit rate (CBR = PR + 6 months SD) was 19% overall, 21% in HR positive, and 29% in patients with progress disease who previously received more than two prior hormone treatments. Notably, none of the markers (including Rb in nuclear, Ki67, p16 defect, and cyclin D1 overexpression) was relevant to either clinical benefit rate or progression-free survival. As for the safety and tolerability, 59 grade 3/4 adverse events were observed because of myelotoxicity. In addition, grade 3/4 neutropenia and leukopenia were observed in 19 cases (51%), grade 3/4 lymphopenia in 11 cases (30%), grade 3/4 thrombocytopenia in 7 cases (19%), and grade 3/4 anemia in 2 cases (5%). Nine patients (24%) suspended treatment, and 19 (51%) reduced drug dose due to cytopenias. Only one patient (3%) quitted the treatment after two cycles due to a moderate fatigue [115]. Taking together, this study has revealed that single-agent palbociclib is potent in patients with Rb-positive advanced breast cancer, particularly in HR-positive and endocrine-resistant patients, with manageable adverse events.
An open-label randomized phase I/II study (NCT00721409, also known as PALOMA-1/TRIO-18) aimed to verify the effect and safety of the combination of palbociclib and letrozole in women with estrogen receptor (ER)-positive and HER2-negative breast cancer [116]. Previous phase I study demonstrated tolerable treatment-related adverse events and no significant drug-drug interaction, suggesting a dose of oral palbociclib 125 mg/d on 3/1 schedule in combination with letrozole 2.5 mg/d orally [117]. The phase II study included 165 patients from 50 sites in 12 countries, who were divided into two separate cohorts. In cohort I, 66 women were recruited according to their estrogen receptor-positive and HER2-negative biomarker status alone. They received the combination of palbociclib (125 mg/d; 3/1 schedule) and letrozole (2.5 mg/d; continuously), compared with letrozole monotherapy. Meanwhile, in cohort II, 99 women with CCND1 amplification, loss of p16, or both were selected to receive the same treatment allocations. The primary intention of this study was to explore and analyze progression-free survival in cohort I. Unexpectedly, a remarkable improvement of progression-free survival was shown in cohort I with no evident association between prognosis and status of CCND1 or p16, leading to a combined analysis for both cohorts. Final analysis showed that median progression-free survival in combination therapy group versus monotherapy group was 20.2 months versus 10.2 months. In concert with previous studies, neutropenia, leucopenia, and fatigue had higher incidence in combination therapy group. Although slight increased incidence of adverse events was reported in combination therapy group, most of them were low grade [116]. The promising results from PLAOMA-1/TRIO 18 study allow FDA to speed up palbociclib approval. The combination therapy of palbociclib and letrozole is recommended as the prior endocrine-based therapy in postmenopausal women with estrogen receptor-positive, HER2-negative advanced breast cancer [115].
A randomized, multicenter phase III study PALOMA-2 (NTC01740427) was carried out to validate the results in a larger population. In this double-blinded study, 666 patients with estrogen receptor-positive, HER2-negative breast cancer, who had not received prior treatment, were recruited. These patients were randomly divided into two groups in a 2:1 ratio, with 444 patients to receive palbociclib plus letrozole and 222 patients to receive placebo plus letrozole for the same treatment allocations as PLAOMA-1/TRIO18. The progression-free survival was assessed, as well as other indexes such as overall survival and clinical benefit response. In combination therapy group (palbociclib plus letrozole), the median progression-free survival was 24.8 months comparing with 14.5 months in the monotherapy group (placebo plus letrozole). Neutropenia, leukopenia, and anemia mainly occur in palbociclib plus letrozole group with a higher incidence. Results from PALOMA-2 verified that in postmenopausal patients with ER-positive and HER2-negative advanced breast cancer, the combination therapy of palbociclib and letrozole significantly improved progression-free survival when compared with letrozole monotherapy. These findings indicate that selective CDK4/CDK6 inhibitor can be used as first-line treatment for the above patient group [118].
Another double-blinded, randomized phase III study PALOMA-3 (NCT01942135) confirmed the safety and efficacy of the combination of palbociclib and fulvestrant (a selective estrogen receptor degrader) in women with hormone receptor-positive, HER2-negative advanced breast cancer who were relapsed or refractory. Five hundred twenty-one patients were randomly divided in a 2:1 ratio into the combination therapy group of palbociclib plus fulvestrant and the fulvestrant monotherapy group. The median progression-free survival was 9.2 months in the combination therapy group versus 3.8 months in the fulvestrant monotherapy group. The most common adverse events in the combination therapy group were neutropenia, leukopenia, and anemia, with a much higher incidence than placebo plus fulvestrant group. PALOMA-3 was the first large trial to testify efficacy and safety of a selective CDK4/6 inhibitor in endocrine-resistant breast cancer [119, 120].
An increasing number of trials are assessing the safety and efficacy of palbociclib in different clinical conditions (including adjuvant therapy and neoadjuvant chemotherapy) and combining other drugs like trastuzumab with palbociclib in breast cancer treatment [121]. Given that palbociclib was synergistic with trastuzumab in HER2-positive breast cancer cell, addition of palbociclib to HER2 targeted therapy has raised great interest [122]. Preclinical breast cancer models revealed that CDK4/6 controlling downstream of HER2 served as a feasible therapeutic target in HER2-positive breast cancer. Selective CDK4/6 inhibitor palbociclib was synergistic with multiple HER2-targeted agents, which provided an additional mechanism to potently suppress the propagation of T-DM1-resistant HER2-positive cancer cells [123]. A phase 1b trial is ongoing to evaluate the combination therapy of palbociclib plus T-DM1.
12.4.2.2 Ribociclib
Ribociclib (LEE011) is another orally administered small molecular with high selectivity to inhibit CDK4/6 at nanomolar concentrations, which reduces Rb phosphorylation, blocks cell cycle progression, and induces G1 arrest. In preclinical studies, ribociclib was shown to have inhibitory activity in cancer cell lines and xenograft models of neuroblastoma, liposarcoma, and ER-positive breast cancer [124, 125].
In a phase I trial, ribociclib was tested as a monotherapy in 132 patients with Rb-positive malignancies, including 20 patients with breast cancer. This trial explored the maximum tolerated dose (MTD), recommended dose for expansion (RDE), and safety of ribociclib. The maximum tolerated dose (MTD) and the recommended phase 2 dose (RP2D) were 900 and 600 mg/day, respectively, on a 3/1 schedule. Among the 70 patients evaluated for MTD/RDE determination, 9 DLTs were observed during cycle 1. The most common DLTs were neutropenia and thrombocytopenia. The Ki67 levels of skin and tumor tissues were decreased due to the ribociclib-mediated antiproliferative activity. Stable disease was reported in 43 cases (including 8 progression-free cases for more than 6 months). Partial response was observed in three cases (including one patient with ER-positive, PIK3CA-mutant, and CCND1-amplified breast cancer) [126].
In a recent phase Ib/II study involving patients with ER-positive, HER2-negative advanced breast cancer, ribociclib (600 mg/d;3/1 schedule) in combination with letrozole showed an acceptable safety profile and exhibited promising clinical activity, particularly in patients who had never received previous systemic treatment for advanced disease. Between previously untreated patients and previously treated patients, the overall response rate (ORR) was 83% versus 5%, while the clinical benefit rate (CBR) was 73% versus 32% [127]. Since preclinical studies showed that ribociclib and the alpha-specific PI3K inhibitor alpelisib (BYL719) had synergistic activity in PIK3CA-mutant breast cancer, a phase 1b/2 study was carried out to access the safety and efficacy of the combination with ribociclib and PI3K inhibitors [128]. A triplet combination with ribociclib, letrozole, and alpelisib was administered in patients with HR-positive, HER2-negative advanced breast cancer (NCT01872260). There were 41 patients receiving ribociclib (300–500 mg QD in 3/1 schedule) plus letrozole (2.5 mg QD continuous), 21 patients receiving alpelisib (200–250 mg QD continuous) plus letrozole (2.5 mg QD continuous), and 36 patients receiving ribociclib (300–500 mg QD in 3/1 schedule) plus alpelisib (200–250 mg QD continuous) plus letrozole (2.5 mg QD continuous). Of the 27 patients evaluated for response, 2 (7%) patients had confirmed partial response, 4 (15%) patients had unconfirmed partial response, and 6 (22%) patients had stable disease with response of mild adverse events [129]. Another phase II study evaluated the biological activity of ribociclib plus letrozole compared with single-agent letrozole in the presurgical condition of breast cancer (NCT01919229). The combination of ribociclib and letrozole reduced Ki67-positive cell fraction potently with tolerate adverse event [130]. To explore potential inhibitory effect of CDK4/6 inhibitors in HER2-positive breast cancer, an ongoing open-label, phase 1b/2 clinical trial accesses the safety and efficacy of the combination of ribociclib and trastuzumab in comparison with T-DM1 monotherapy for patients with HER2-positive advanced or metastatic breast cancer (NCT02657343).
Three large, international, double-blinded, placebo-controlled phase III trials are evaluating the addition of ribociclib to endocrine therapy in patients with HR-positive, HER2-negative breast cancer. The MONALEESA-2 confirmed the combination therapy (ribociclib plus letrozole) as the prior treatment in patients with previously untreated HR-positive, HER2-negative advanced breast cancer (NCT01958021). A total of 668 patients were randomly divided in a 1:1 ratio into the combination therapy group (ribociclib plus letrozole) and the letrozole monotherapy group. The trial met its primary end point, with the median duration of progression-free survival not reached in the combination therapy group versus 14.7 months in the monotherapy group. The overall response rates were 40.7% in the combination therapy group and 27.5% in the monotherapy group, including 9 (2.7%) complete response versus 7 (2.1%) and 127 (38%) partial response versus 85 (25.4%). The clinical benefit rates were 79.6% in the combination therapy group versus 72.8% in the monotherapy group. As for safety, more grade 3/4 adverse events arise in the combination therapy group (81.2%) than in the monotherapy group (32.7%). The most common adverse events were neutropenia (74.3% versus 5.2%), nausea (51.5% versus 28.5%), infections (50.3% versus 42.4%), fatigue (36.5% versus 30.0%), and diarrhea (35.0% versus 22.1%). The MONALEESA-3 (NCT02422615) is another ongoing phase III trial, which accesses the efficacy and safety of the combination therapy of ribociclib plus fulvestrant for treatment of patients with untreated HR-positive, HER2-negative advanced breast cancer. The primary end point of the study is progression-free survival, and the secondary end points include overall survival, overall response rate, and safety. The MONALEESA-7 (NCT02278120) is another ongoing phase III trial, which aims to assess the safety and efficacy of ribociclib or placebo in combination with tamoxifen and goserelin or a nonsteroidal aromatase inhibitor (NSAI) and goserelin for the treatment of premenopausal women with HR-positive, HER2-negative advanced breast cancer.
12.4.2.3 Abemaciclib
Abemaciclib (LY283521), another oral selective CDK4/6 inhibitor characterized with its clinical safety profile, is currently in clinical development. At low nanomolar, abemaciclib strongly inhibits CDK4 and CDK6 and therefore reduces Rb phosphorylation, leading to cell cycle arrest in G1 and proliferation suppression, particularly in Rb-proficient breast cancer cell lines. Oral administration of abemaciclib suppressed tumor growth in human tumor xenografts including various tumor subtypes in tumor-bearing mice [131, 132].
The first-in-human phase I study evaluated the safety and efficacy of abemaciclib for the treatment of patients with solid tumors including breast cancer. In this trial, abemaciclib demonstrated promising single-agent activity, and limited toxicities occurred with the increase of drug dose. A total of 225 patients were recruited, including 33 patients in dose escalation and 192 patients in tumor-specific cohorts. The median progression-free survival was 8.8 months in HR-positive patients versus 1.1 months in HR-negative ones. Similarly, disease control rate could be associated with the HR status in patients who had been previously treated (HR positive, 29 in 36 cases (81%) versus HR negative, 3 in 9 cases (33%)). Based on the Rb inhibition and cell cycle arrest in normal cells and tumor cells, the maximum tolerated dose was 200 mg every 12 hours. Among the most common treatment-related toxicities, fatigue was manageable. Meanwhile, other toxicities occurred in gastrointestinal system, renal system, and hematopoietic system. A subgroup of 19 patients with HR-positive breast cancer received the combination therapy of abemaciclib plus fulvestrant. Partial responses were observed in four patients (21%) with no different adverse events compared to single-agent cohorts. The antitumor activity of abemaciclib in patients with HR-positive breast cancer was probably associated with TP53 rather than PIK3CA [133]. These results inspired the idea to test the combination of different therapies (letrozole, anastrozole, tamoxifen, exemestane, exemestane plus everolimus, trastuzumab) for patients with metastatic breast cancer in a phase 1b multiple cohorts study (NCT02057133). A total of 65 patients were assigned into 6 cohorts to receive the combination therapy of abemaciclib and other drugs (such as letrozole, anastrozole, tamoxifen, and trastuzumab). This study indicates that the combination of abemaciclib and different therapies is promising for patients with metastatic breast cancer [134].
The phase II study MONARCH-1 (NCT02102490) evaluated the safety and efficacy of abemaciclib as monotherapy for patients with previously treated, advanced, or metastatic HR-positive/HER2-negative breast cancer who had progressive disease on or after endocrine therapy and chemotherapy. In 132 eligible patients, the confirmed overall response rate was 19.7%, the clinical benefit rate was 42.4%, and the median PFS was 6.0 months, with a higher response rate than other CDK4/6 inhibitors [135]. Considering that abemaciclib can cross the blood-brain barrier, abemaciclib is supposed to have potential antitumor activity in patients with central nervous system metastases [136]. A currently ongoing phase II study (NCT02308020) is evaluating the efficacy of abemaciclib in patients with brain metastases from different solid primary tumors including HR-positive breast cancer. Another ongoing phase II study (NCT02675231) is exploring the efficacy of abemaciclib plus trastuzumab with or without fulvestrant or chemotherapy in patients with HR-positive, HER2-positive locally advanced or metastatic breast cancer. NeoMONARCH (NCT02441946) is a randomized, multicenter, open-label phase II neoadjuvant study comparing the biological effects of abemaciclib plus anastrozole, abemaciclib monotherapy, and anastrozole monotherapy in patients with early-stage HR-positive/HER2-negative breast cancer. Two hundred twenty-three patients were stratified by progesterone receptor status and tumor size and randomized into three groups at a ratio of 1:1:1 to receive abemaciclib (150 mg orally Q12H) plus anastrozole (1 mg orally QD), abemaciclib (150 mg orally Q12H), and anastrozole (1 mg orally QD) for 2 weeks followed by administration of abemaciclib (150 mg orally Q12H) plus anastrozole (1 mg QD) for the next 14 weeks. In a 9-month interim analysis, a single agent of abemaciclib or in combination with anastrozole exhibited significantly greater suppression of Ki67 after 2 weeks of dosing than anastrozole alone. Further results including safety, clinical efficacy, final Ki67, and RNA expression at surgery are not reported yet [137].
Two large, randomized, double-blinded, placebo-controlled, phase III studies are currently ongoing to confirm the effects of adding abemaciclib to fulvestrant and aromatase inhibitors, respectively. MONARCH-2 (NCT02107703) aims to compare progression-free survival for women with HR-positive (HR+)/HER2-negative advanced breast cancer who are randomized in a 2:1 ratio to receive either abemaciclib plus fulvestrant or fulvestrant alone. Another trial MONARCH-3 (NCT02246621) is to evaluate the effect of nonsteroidal aromatase inhibitors (anastrozole or letrozole) plus abemaciclib or placebo in postmenopausal women with breast cancer. Both trials use progression-free survival as primary end point and overall survival/ objective response rate as secondary end points.
12.5 Future Direction
Cell cycle dysregulation has been one of the most important therapeutic targets in cancer for many years. Selective CDK4/6 inhibitors have been recently approved by FDA to treat ER-positive advanced breast cancer, which takes more than two decades after the discovery of cyclin D1-CDK4/6 interaction. Many questions remain to be answered, including the biomarker, indication, and drug combination of anti-CDK4/6 therapy. Anti-CDK4/6 therapy could be a promising strategy for treating high-risk early breast cancer patients, HER2-positive patients, or even triple-negative breast cancer patients with functional Rb. More selective CDK2 inhibitors may also be useful in disrupting cyclin E-CDK2 function and treating a broader spectrum of cancer than CDK4/6 inhibitors.
References
Nurse PM (2002) Cyclin dependent kinases and cell cycle control. Bioscience Rep 22(5-6):487–499. doi:10.1023/A:1022017701871
Malumbres M, Barbacid M (2005) Mammalian cyclin-dependent kinases. Trends Biochem Sci 30(11):630–641. doi:10.1016/j.tibs.2005.09.005
Malumbres M, Barbacid M (2009) Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer 9(3):153–166. doi:10.1038/nrc2602
Kastan MB, Bartek J (2004) Cell-cycle checkpoints and cancer. Nature 432(7015):316–323. doi:10.1038/nature03097
Shapiro GI (2006) Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol 24(11):1770–1783. doi:10.1200/Jco.2005.03.7689
Beaver JA, Amiri-Kordestani L, Charlab R, Chen W, Palmby T, Tilley A, Zirkelbach JF, Yu J, Liu Q, Zhao L, Crich J, Chen XH, Hughes M, Bloomquist E, Tang S, Sridhara R, Kluetz PG, Kim G, Ibrahim A, Pazdur R, Cortazar P (2015) FDA approval: Palbociclib for the treatment of postmenopausal patients with estrogen receptor-positive, HER2-negative metastatic breast cancer. Clin Cancer Res 21(21):4760–4766. doi:10.1158/1078-0432.CCR-15-1185
Walker AJ, Wedam S, Amiri-Kordestani L, Bloomquist E, Tang S, Sridhara R, Chen W, Palmby TR, Fourie Zirkelbach J, Fu W, Liu Q, Tilley A, Kim G, Kluetz PG, McKee AE, Pazdur R (2016) FDA approval of Palbociclib in combination with Fulvestrant for the treatment of hormone receptor-positive, HER2-negative metastatic breast cancer. Clin Cancer Res 22(20):4968–4972. doi:10.1158/1078-0432.CCR-16-0493
Anders L, Ke N, Hydbring P, Choi YJ, Widlund HR, Chick JM, Zhai H, Vidal M, Gygi SP, Braun P, Sicinski P (2011) A systematic screen for CDK4/6 substrates links FOXM1 phosphorylation to senescence suppression in cancer cells. Cancer Cell 20(5):620–634. doi:10.1016/j.ccr.2011.10.001
Harbour JW, Dean DC (2000) The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev 14(19):2393–2409
Malumbres M, Barbacid M (2001) To cycle or not to cycle: a critical decision in cancer. Nat Rev Cancer 1(3):222–231. doi:10.1038/35106065
Hwang HC, Clurman BE (2005) Cyclin E in normal and neoplastic cell cycles. Oncogene 24(17):2776–2786. doi:10.1038/sj.onc.1208613
Spencer SL, Cappell SD, Tsai FC, Overton KW, Wang CL, Meyer T (2013) The proliferation-quiescence decision is controlled by a bifurcation in CDK2 activity at mitotic exit. Cell 155(2):369–383. doi:10.1016/j.cell.2013.08.062
Ren S, Rollins BJ (2004) Cyclin C/cdk3 promotes Rb-dependent G0 exit. Cell 117(2):239–251
Serrano M, Hannon GJ, Beach D (1993) A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature 366(6456):704–707. doi:10.1038/366704a0
Hannon GJ, Beach D (1994) p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature 371(6494):257–261. doi:10.1038/371257a0
Chan FK, Zhang J, Cheng L, Shapiro DN, Winoto A (1995) Identification of human and mouse p19, a novel CDK4 and CDK6 inhibitor with homology to p16ink4. Mol Cell Biol 15(5):2682–2688
Hirai H, Roussel MF, Kato JY, Ashmun RA, Sherr CJ (1995) Novel INK4 proteins, p19 and p18, are specific inhibitors of the cyclin D-dependent kinases CDK4 and CDK6. Mol Cell Biol 15(5):2672–2681
Sherr CJ, Roberts JM (1999) CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 13(12):1501–1512
Pavletich NP (1999) Mechanisms of cyclin-dependent kinase regulation: structures of Cdks, their cyclin activators, and Cip and INK4 inhibitors. J Mol Biol 287(5):821–828. doi:10.1006/jmbi.1999.2640
Serrano M, Blasco MA (2001) Putting the stress on senescence. Curr Opin Cell Biol 13(6):748–753
Reynisdottir I, Polyak K, Iavarone A, Massague J (1995) Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev 9(15):1831–1845
Witkiewicz AK, Knudsen KE, Dicker AP, Knudsen ES (2011) The meaning of p16(ink4a) expression in tumors: functional significance, clinical associations and future developments. Cell Cycle 10(15):2497–2503. doi:10.4161/cc.10.15.16776
LaPak KM, Burd CE (2014) The molecular balancing act of p16(INK4a) in cancer and aging. Mol Cancer Res 12(2):167–183. doi:10.1158/1541-7786.MCR-13-0350
Lukas J, Parry D, Aagaard L, Mann DJ, Bartkova J, Strauss M, Peters G, Bartek J (1995) Retinoblastoma-protein-dependent cell-cycle inhibition by the tumour suppressor p16. Nature 375(6531):503–506. doi:10.1038/375503a0
van den Heuvel S, Harlow E (1993) Distinct roles for cyclin-dependent kinases in cell cycle control. Science 262(5142):2050–2054
Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massague J (1994) Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 78(1):59–66
Coqueret O (2003) New roles for p21 and p27 cell-cycle inhibitors: a function for each cell compartment? Trends Cell Biol 13(2):65–70
LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, Fattaey A, Harlow E (1997) New functional activities for the p21 family of CDK inhibitors. Genes Dev 11(7):847–862
Cheng M, Olivier P, Diehl JA, Fero M, Roussel MF, Roberts JM, Sherr CJ (1999) The p21(Cip1) and p27(Kip1) CDK ‘inhibitors’ are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J 18(6):1571–1583. doi:10.1093/emboj/18.6.1571
Frank CJ, Hyde M, Greider CW (2006) Regulation of telomere elongation by the cyclin-dependent kinase CDK1. Mol Cell 24(3):423–432. doi:10.1016/j.molcel.2006.10.020
Pagano M (2004) Control of DNA synthesis and mitosis by the Skp2-p27-Cdk1/2 axis. Mol Cell 14(4):414–416
Nigg EA (2001) Mitotic kinases as regulators of cell division and its checkpoints. Nat Rev Mol Cell Biol 2(1):21–32. doi:10.1038/35048096
Harper JW, Burton JL, Solomon MJ (2002) The anaphase-promoting complex: it’s not just for mitosis any more. Genes Dev 16(17):2179–2206. doi:10.1101/gad.1013102
Ubersax JA, Woodbury EL, Quang PN, Paraz M, Blethrow JD, Shah K, Shokat KM, Morgan DO (2003) Targets of the cyclin-dependent kinase Cdk1. Nature 425(6960):859–864. doi:10.1038/nature02062
Maestre C, Delgado-Esteban M, Gomez-Sanchez JC, Bolanos JP, Almeida A (2008) Cdk5 phosphorylates Cdh1 and modulates cyclin B1 stability in excitotoxicity. EMBO J 27(20):2736–2745. doi:10.1038/emboj.2008.195
Zhang J, Cicero SA, Wang L, Romito-Digiacomo RR, Yang Y, Herrup K (2008) Nuclear localization of Cdk5 is a key determinant in the postmitotic state of neurons. Proc Natl Acad Sci U S A 105(25):8772–8777. doi:10.1073/pnas.0711355105
Cruz JC, Tsai LH (2004) A Jekyll and Hyde kinase: roles for Cdk5 in brain development and disease. Curr Opin Neurobiol 14(3):390–394. doi:10.1016/j.conb.2004.05.002
Kesavapany S, Li BS, Amin N, Zheng YL, Grant P, Pant HC (2004) Neuronal cyclin-dependent kinase 5: role in nervous system function and its specific inhibition by the Cdk5 inhibitory peptide. Biochim Biophys Acta 1697(1–2):143–153. doi:10.1016/j.bbapap.2003.11.020
Fisher RP (2005) Secrets of a double agent: CDK7 in cell-cycle control and transcription. J Cell Sci 118(Pt 22):5171–5180. doi:10.1242/jcs.02718
Lolli G, Johnson LN (2005) CAK-Cyclin-dependent activating kinase: a key kinase in cell cycle control and a target for drugs? Cell Cycle 4(4):572–577
Morris EJ, Ji JY, Yang F, Di Stefano L, Herr A, Moon NS, Kwon EJ, Haigis KM, Naar AM, Dyson NJ (2008) E2F1 represses beta-catenin transcription and is antagonized by both pRB and CDK8. Nature 455(7212):552–556. doi:10.1038/nature07310
Garriga J, Grana X (2004) Cellular control of gene expression by T-type cyclin/CDK9 complexes. Gene 337:15–23. doi:10.1016/j.gene.2004.05.007
Kasten M, Giordano A (2001) Cdk10, a Cdc2-related kinase, associates with the Ets2 transcription factor and modulates its transactivation activity. Oncogene 20(15):1832–1838. doi:10.1038/sj.onc.1204295
Loyer P, Trembley JH, Katona R, Kidd VJ, Lahti JM (2005) Role of CDK/cyclin complexes in transcription and RNA splicing. Cell Signal 17(9):1033–1051. doi:10.1016/j.cellsig.2005.02.005
Yokoyama H, Gruss OJ, Rybina S, Caudron M, Schelder M, Wilm M, Mattaj IW, Karsenti E (2008) Cdk11 is a RanGTP-dependent microtubule stabilization factor that regulates spindle assembly rate. J Cell Biol 180(5):867–875. doi:10.1083/jcb.200706189
Hu D, Valentine M, Kidd VJ, Lahti JM (2007) CDK11(p58) is required for the maintenance of sister chromatid cohesion. J Cell Sci 120(Pt 14):2424–2434. doi:10.1242/jcs.007963
Wilker EW, van Vugt MA, Artim SA, Huang PH, Petersen CP, Reinhardt HC, Feng Y, Sharp PA, Sonenberg N, White FM, Yaffe MB (2007) 14-3-3sigma controls mitotic translation to facilitate cytokinesis. Nature 446(7133):329–332. doi:10.1038/nature05584
Petretti C, Savoian M, Montembault E, Glover DM, Prigent C, Giet R (2006) The PITSLRE/CDK11p58 protein kinase promotes centrosome maturation and bipolar spindle formation. EMBO Rep 7(4):418–424. doi:10.1038/sj.embor.7400639
Asghar U, Witkiewicz AK, Turner NC, Knudsen ES (2015) The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov 14(2):130–146. doi:10.1038/nrd4504
Motokura T, Bloom T, Kim HG, Juppner H, Ruderman JV, Kronenberg HM, Arnold A (1991) A novel cyclin encoded by a bcl1-linked candidate oncogene. Nature 350(6318):512–515. doi:10.1038/350512a0
Sutherland RL, Musgrove EA (2002) Cyclin D1 and mammary carcinoma: new insights from transgenic mouse models. Breast Cancer Res 4(1):14–17
Gillett C, Fantl V, Smith R, Fisher C, Bartek J, Dickson C, Barnes D, Peters G (1994) Amplification and overexpression of cyclin D1 in breast cancer detected by immunohistochemical staining. Cancer Res 54(7):1812–1817
Foster JS, Henley DC, Ahamed S, Wimalasena J (2001) Estrogens and cell-cycle regulation in breast cancer. Trends Endocrinol Metab 12(7):320–327
Musgrove EA, Caldon CE, Barraclough J, Stone A, Sutherland RL (2011) Cyclin D as a therapeutic target in cancer. Nat Rev Cancer 11(8):558–572. doi:10.1038/nrc3090
Choi YJ, Anders L (2014) Signaling through cyclin D-dependent kinases. Oncogene 33(15):1890–1903. doi:10.1038/onc.2013.137
Herschkowitz JI, He X, Fan C, Perou CM (2008) The functional loss of the retinoblastoma tumour suppressor is a common event in basal-like and luminal B breast carcinomas. Breast Cancer Res 10(5):R75. doi:10.1186/bcr2142
Buckley MF, Sweeney KJ, Hamilton JA, Sini RL, Manning DL, Nicholson RI, deFazio A, Watts CK, Musgrove EA, Sutherland RL (1993) Expression and amplification of cyclin genes in human breast cancer. Oncogene 8(8):2127–2133
Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, Schmidt EV (1994) Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature 369(6482):669–671. doi:10.1038/369669a0
Weinstat-Saslow D, Merino MJ, Manrow RE, Lawrence JA, Bluth RF, Wittenbel KD, Simpson JF, Page DL, Steeg PS (1995) Overexpression of cyclin D mRNA distinguishes invasive and in situ breast carcinomas from non-malignant lesions. Nat Med 1(12):1257–1260
Alle KM, Henshall SM, Field AS, Sutherland RL (1998) Cyclin D1 protein is overexpressed in hyperplasia and intraductal carcinoma of the breast. Clin Cancer Res 4(4):847–854
Musgrove EA, Lee CS, Buckley MF, Sutherland RL (1994) Cyclin D1 induction in breast cancer cells shortens G1 and is sufficient for cells arrested in G1 to complete the cell cycle. Proc Natl Acad Sci U S A 91(17):8022–8026
Yu Q, Geng Y, Sicinski P (2001) Specific protection against breast cancers by cyclin D1 ablation. Nature 411(6841):1017–1021. doi:10.1038/35082500
Yang C, Ionescu-Tiba V, Burns K, Gadd M, Zukerberg L, Louis DN, Sgroi D, Schmidt EV (2004) The role of the cyclin D1-dependent kinases in ErbB2-mediated breast cancer. Am J Pathol 164(3):1031–1038. doi:10.1016/S0002-9440(10)63190-2
Roy PG, Thompson AM (2006) Cyclin D1 and breast cancer. Breast 15(6):718–727. doi:10.1016/j.breast.2006.02.005
Neuman E, Ladha MH, Lin N, Upton TM, Miller SJ, DiRenzo J, Pestell RG, Hinds PW, Dowdy SF, Brown M, Ewen ME (1997) Cyclin D1 stimulation of estrogen receptor transcriptional activity independent of cdk4. Mol Cell Biol 17(9):5338–5347
Zwijsen RM, Wientjens E, Klompmaker R, van der Sman J, Bernards R, Michalides RJ (1997) CDK-independent activation of estrogen receptor by cyclin D1. Cell 88(3):405–415
Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lonning PE, Brown PO, Borresen-Dale AL, Botstein D (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A 100(14):8418–8423. doi:10.1073/pnas.0932692100
Nielsen NH, Arnerlov C, Emdin SO, Landberg G (1996) Cyclin E overexpression, a negative prognostic factor in breast cancer with strong correlation to oestrogen receptor status. Br J Cancer 74(6):874–880
Donnellan R, Kleinschmidt I, Chetty R (2001) Cyclin E immunoexpression in breast ductal carcinoma: pathologic correlations and prognostic implications. Hum Pathol 32(1):89–94. doi:10.1053/hupa.2001.21141
Span PN, Tjan-Heijnen VC, Manders P, Beex LV, Sweep CG (2003) Cyclin-E is a strong predictor of endocrine therapy failure in human breast cancer. Oncogene 22(31):4898–4904. doi:10.1038/sj.onc.1206818
Keyomarsi K, Tucker SL, Buchholz TA, Callister M, Ding Y, Hortobagyi GN, Bedrosian I, Knickerbocker C, Toyofuku W, Lowe M, Herliczek TW, Bacus SS (2002) Cyclin E and survival in patients with breast cancer. N Engl J Med 347(20):1566–1575. doi:10.1056/NEJMoa021153
Scaltriti M, Eichhorn PJ, Cortes J, Prudkin L, Aura C, Jimenez J, Chandarlapaty S, Serra V, Prat A, Ibrahim YH, Guzman M, Gili M, Rodriguez O, Rodriguez S, Perez J, Green SR, Mai S, Rosen N, Hudis C, Baselga J (2011) Cyclin E amplification/overexpression is a mechanism of trastuzumab resistance in HER2+ breast cancer patients. Proc Natl Acad Sci U S A 108(9):3761–3766. doi:10.1073/pnas.1014835108
Smith ML, Seo YR (2000) Sensitivity of cyclin E-overexpressing cells to cisplatin/taxol combinations. Anticancer Res 20(4):2537–2539
Sutherland RL, Musgrove EA (2004) Cyclins and breast cancer. J Mammary Gland Biol Neoplasia 9(1):95–104. doi:10.1023/B:JOMG.0000023591.45568.77
Barton MC, Akli S, Keyomarsi K (2006) Deregulation of cyclin E meets dysfunction in p53: closing the escape hatch on breast cancer. J Cell Physiol 209(3):686–694. doi:10.1002/jcp.20818
Luhtala S, Staff S, Tanner M, Isola J (2016) Cyclin E amplification, over-expression, and relapse-free survival in HER-2-positive primary breast cancer. Tumour Biol 37(7):9813–9823. doi:10.1007/s13277-016-4870-z
Gao S, Ma JJ, Lu C (2013) Prognostic value of cyclin E expression in breast cancer: a meta-analysis. Tumour Biol 34(6):3423–3430. doi:10.1007/s13277-013-0915-8
Hunt KK, Karakas C, Ha MJ, Biernacka A, Yi M, Sahin A, Adjapong O, Hortobogyi GN, Bondy ML, Thompson PA, Cheung KL, Ellis IO, Bacus S, Symmans WF, Do KA, Keyomarsi K (2016) Cytoplasmic Cyclin E predicts recurrence in patients with breast cancer. Clin Cancer Res. doi:10.1158/1078-0432.CCR-16-2217
Timms JF, White SL, O’Hare MJ, Waterfield MD (2002) Effects of ErbB-2 overexpression on mitogenic signalling and cell cycle progression in human breast luminal epithelial cells. Oncogene 21(43):6573–6586. doi:10.1038/sj.onc.1205847
Mittendorf EA, Liu Y, Tucker SL, McKenzie T, Qiao N, Akli S, Biernacka A, Meijer L, Keyomarsi K, Hunt KK (2010) A novel interaction between HER2/neu and cyclin E in breast cancer. Oncogene 29(27):3896–3907. doi:10.1038/onc.2010.151
Akli S, Zheng PJ, Multani AS, Wingate HF, Pathak S, Zhang N, Tucker SL, Chang S, Keyomarsi K (2004) Tumor-specific low molecular weight forms of cyclin E induce genomic instability and resistance to p21, p27, and antiestrogens in breast cancer. Cancer Res 64(9):3198–3208
Akli S, Van Pelt CS, Bui T, Meijer L, Keyomarsi K (2011) Cdk2 is required for breast cancer mediated by the low-molecular-weight isoform of cyclin E. Cancer Res 71(9):3377–3386. doi:10.1158/0008-5472.CAN-10-4086
Robinson WA, Elefanty AG, Hersey P (1996) Expression of the tumour suppressor genes p15 and p16 in malignant melanoma. Melanoma Res 6(4):285–289
Van Zee KJ, Calvano JE, Bisogna M (1998) Hypomethylation and increased gene expression of p16INK4a in primary and metastatic breast carcinoma as compared to normal breast tissue. Oncogene 16(21):2723–2727. doi:10.1038/sj.onc.1201794
Wong SC, Chan JK, Lee KC, Hsiao WL (2001) Differential expression of p16/p21/p27 and cyclin D1/D3, and their relationships to cell proliferation, apoptosis, and tumour progression in invasive ductal carcinoma of the breast. J Pathol 194(1):35–42. doi:10.1002/path.838
Bisogna M, Calvano JE, Ho GH, Orlow I, Cordon-Cardo C, Borgen PI, Van Zee KJ (2001) Molecular analysis of the INK4A and INK4B gene loci in human breast cancer cell lines and primary carcinomas. Cancer Genet Cytogenet 125(2):131–138
Zariwala M, Liu E, Xiong Y (1996) Mutational analysis of the p16 family cyclin-dependent kinase inhibitors p15INK4b and p18INK4c in tumor-derived cell lines and primary tumors. Oncogene 12(2):451–455
Herman JG, Jen J, Merlo A, Baylin SB (1996) Hypermethylation-associated inactivation indicates a tumor suppressor role for p15INK4B. Cancer Res 56(4):722–727
Gartel AL, Radhakrishnan SK (2005) Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res 65(10):3980–3985. doi:10.1158/0008-5472.CAN-04-3995
Walsh A, Cook RS, Rexer B, Arteaga CL, Skala MC (2012) Optical imaging of metabolism in HER2 overexpressing breast cancer cells. Biomed Opt Express 3(1):75–85. doi:10.1364/BOE.3.000075
Musgrove EA, Davison EA, Ormandy CJ (2004) Role of the CDK inhibitor p27 (Kip1) in mammary development and carcinogenesis: insights from knockout mice. J Mammary Gland Biol Neoplasia 9(1):55–66. doi:10.1023/B:JOMG.0000023588.55733.84
Katayose Y, Kim M, Rakkar AN, Li Z, Cowan KH, Seth P (1997) Promoting apoptosis: a novel activity associated with the cyclin-dependent kinase inhibitor p27. Cancer Res 57(24):5441–5445
Lapenna S, Giordano A (2009) Cell cycle kinases as therapeutic targets for cancer. Nat Rev Drug Discov 8(7):547–566. doi:10.1038/nrd2907
Bose P, Simmons GL, Grant S (2013) Cyclin-dependent kinase inhibitor therapy for hematologic malignancies. Expert Opin Investig Drugs 22(6):723–738. doi:10.1517/13543784.2013.789859
Lin TS, Blum KA, Fischer DB, Mitchell SM, Ruppert AS, Porcu P, Kraut EH, Baiocchi RA, Moran ME, Johnson AJ, Schaaf LJ, Grever MR, Byrd JC (2010) Flavopiridol, fludarabine, and rituximab in mantle cell lymphoma and indolent B-cell lymphoproliferative disorders. J Clin Oncol 28(3):418–423. doi:10.1200/JCO.2009.24.1570
Ramaswamy B, Phelps MA, Baiocchi R, Bekaii-Saab T, Ni W, Lai JP, Wolfson A, Lustberg ME, Wei L, Wilkins D, Campbell A, Arbogast D, Doyle A, Byrd JC, Grever MR, Shah MH (2012) A dose-finding, pharmacokinetic and pharmacodynamic study of a novel schedule of flavopiridol in patients with advanced solid tumors. Investig New Drugs 30(2):629–638. doi:10.1007/s10637-010-9563-7
Hegeman RB, Mulkerin D, Thomas J, Alberti D, Binger K, Marnocha R, Kolesar J, Wilding G (2005) Phase I study of oxaliplatin in combination with 5-fluorouracil (5-FU), leucovorin (LV) and capecitabine (ORAL FOLFOX-6) in patients with advanced or metastatic solid tumors. J Clin Oncol 23(16):149s–149s
Le Tourneau C, Faivre S, Laurence V, Delbaldo C, Vera K, Girre V, Chiao J, Armour S, Frame S, Green SR, Gianella-Borradori A, Dieras V, Raymond E (2010) Phase I evaluation of seliciclib (R-roscovitine), a novel oral cyclin-dependent kinase inhibitor, in patients with advanced malignancies. Eur J Cancer 46(18):3243–3250. doi:10.1016/j.ejca.2010.08.001
Nair BC, Vallabhaneni S, Tekmal RR, Vadlamudi RK (2011) Roscovitine confers tumor suppressive effect on therapy-resistant breast tumor cells. Breast Cancer Res 13(3):R80. doi:10.1186/bcr2929
Appleyard MV, O’Neill MA, Murray KE, Paulin FE, Bray SE, Kernohan NM, Levison DA, Lane DP, Thompson AM (2009) Seliciclib (CYC202, R-roscovitine) enhances the antitumor effect of doxorubicin in vivo in a breast cancer xenograft model. Int J Cancer 124(2):465–472. doi:10.1002/ijc.23938
Kodym E, Kodym R, Reis AE, Habib AA, Story MD, Saha D (2009) The small-molecule CDK inhibitor, SNS-032, enhances cellular radiosensitivity in quiescent and hypoxic non-small cell lung cancer cells. Lung Cancer 66(1):37–47. doi:10.1016/j.lungcan.2008.12.026
Walsby E, Lazenby M, Pepper C, Burnett AK (2011) The cyclin-dependent kinase inhibitor SNS-032 has single agent activity in AML cells and is highly synergistic with cytarabine. Leukemia 25(3):411–419. doi:10.1038/leu.2010.290
Tong WG, Chen R, Plunkett W, Siegel D, Sinha R, Harvey RD, Badros AZ, Popplewell L, Coutre S, Fox JA, Mahadocon K, Chen T, Kegley P, Hoch U, Wierda WG (2010) Phase I and pharmacologic study of SNS-032, a potent and selective Cdk2, 7, and 9 inhibitor, in patients with advanced chronic lymphocytic leukemia and multiple myeloma. J Clin Oncol 28(18):3015–3022. doi:10.1200/JCO.2009.26.1347
Heath EI, Bible K, Martell RE, Adelman DC, Lorusso PM (2008) A phase 1 study of SNS-032 (formerly BMS-387032), a potent inhibitor of cyclin-dependent kinases 2, 7 and 9 administered as a single oral dose and weekly infusion in patients with metastatic refractory solid tumors. Investig New Drugs 26(1):59–65. doi:10.1007/s10637-007-9090-3
Kumar SK, LaPlant B, Chng WJ, Zonder J, Callander N, Fonseca R, Fruth B, Roy V, Erlichman C, Stewart AK (2015) Dinaciclib, a novel CDK inhibitor, demonstrates encouraging single-agent activity in patients with relapsed multiple myeloma. Blood 125(3):443–448. doi:10.1182/blood-2014-05-573741
Flynn J, Jones J, Johnson AJ, Andritsos L, Maddocks K, Jaglowski S, Hessler J, Grever MR, Im E, Zhou H, Zhu Y, Zhang D, Small K, Bannerji R, Byrd JC (2015) Dinaciclib is a novel cyclin-dependent kinase inhibitor with significant clinical activity in relapsed and refractory chronic lymphocytic leukemia. Leukemia 29(7):1524–1529. doi:10.1038/leu.2015.31
Nemunaitis JJ, Small KA, Kirschmeier P, Zhang D, Zhu Y, Jou YM, Statkevich P, Yao SL, Bannerji R (2013) A first-in-human, phase 1, dose-escalation study of dinaciclib, a novel cyclin-dependent kinase inhibitor, administered weekly in subjects with advanced malignancies. J Transl Med 11:259. doi:10.1186/1479-5876-11-259
Mitri Z, Karakas C, Wei C, Briones B, Simmons H, Ibrahim N, Alvarez R, Murray JL, Keyomarsi K, Moulder S (2015) A phase 1 study with dose expansion of the CDK inhibitor dinaciclib (SCH 727965) in combination with epirubicin in patients with metastatic triple negative breast cancer. Investig New Drugs 33(4):890–894. doi:10.1007/s10637-015-0244-4
Mita MM, Joy AA, Mita A, Sankhala K, Jou YM, Zhang D, Statkevich P, Zhu Y, Yao SL, Small K, Bannerji R, Shapiro CL (2014) Randomized phase II trial of the cyclin-dependent kinase inhibitor dinaciclib (MK-7965) versus capecitabine in patients with advanced breast cancer. Clin Breast Cancer 14(3):169–176. doi:10.1016/j.clbc.2013.10.016
O’Leary B, Finn RS, Turner NC (2016) Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol 13(7):417–430. doi:10.1038/nrclinonc.2016.26
Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, Albassam M, Zheng X, Leopold WR, Pryer NK, Toogood PL (2004) Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther 3(11):1427–1438
Rivadeneira DB, Mayhew CN, Thangavel C, Sotillo E, Reed CA, Grana X, Knudsen ES (2010) Proliferative suppression by CDK4/6 inhibition: complex function of the retinoblastoma pathway in liver tissue and hepatoma cells. Gastroenterology 138(5):1920–1930. doi:10.1053/j.gastro.2010.01.007
Flaherty KT, Lorusso PM, Demichele A, Abramson VG, Courtney R, Randolph SS, Shaik MN, Wilner KD, O’Dwyer PJ, Schwartz GK (2012) Phase I, dose-escalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced cancer. Clin Cancer Res 18(2):568–576. doi:10.1158/1078-0432.CCR-11-0509
Schwartz GK, LoRusso PM, Dickson MA, Randolph SS, Shaik MN, Wilner KD, Courtney R, O’Dwyer PJ (2011) Phase I study of PD 0332991, a cyclin-dependent kinase inhibitor, administered in 3-week cycles (schedule 2/1). Br J Cancer 104(12):1862–1868. doi:10.1038/bjc.2011.177
DeMichele A, Clark AS, Tan KS, Heitjan DF, Gramlich K, Gallagher M, Lal P, Feldman M, Zhang P, Colameco C, Lewis D, Langer M, Goodman N, Domchek S, Gogineni K, Rosen M, Fox K, O’Dwyer P (2015) CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clin Cancer Res 21(5):995–1001. doi:10.1158/1078-0432.CCR-14-2258
Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, Ettl J, Patel R, Pinter T, Schmidt M, Shparyk Y, Thummala AR, Voytko NL, Fowst C, Huang X, Kim ST, Randolph S, Slamon DJ (2015) The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol 16(1):25–35. doi:10.1016/S1470-2045(14)71159-3
Finn RS, Hurvitz SA, Allison MA, Applebaum S, Glaspy J, DiCarlo B, Courtney R, Shaik N, Kim ST, Fowst C, Slamon DJ (2009) Phase I study of PD 0332991, a novel, oral, Cyclin-D kinase (CDK) 4/6 inhibitor in combination with Letrozole, for first-line treatment of metastatic post-menopausal, estrogen receptor-positive (ER plus ), human epidermal growth factor receptor 2 (HER2)-negative breast cancer. Cancer Res 69(24):788s–788s
Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S, Gauthier E, Lu DR, Randolph S, Dieras V, Slamon DJ (2016) Palbociclib and Letrozole in advanced breast cancer. N Engl J Med 375(20):1925–1936. doi:10.1056/NEJMoa1607303
Cristofanilli M, Turner NC, Bondarenko I (2016) Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial (vol 17, pg 431, 2016). Lancet Oncol 17(7):E270–E270
Turner NC, Huang Bartlett C, Cristofanilli M (2015) Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med 373(17):1672–1673. doi:10.1056/NEJMc1510345
Murphy CG, Dickler MN (2015) The role of CDK4/6 inhibition in breast cancer. Oncologist 20(5):483–490. doi:10.1634/theoncologist.2014-0443
Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, Ginther C, Atefi M, Chen I, Fowst C, Los G, Slamon DJ (2009) PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res 11(5):R77. doi:10.1186/bcr2419
Witkiewicz AK, Cox D, Knudsen ES (2014) CDK4/6 inhibition provides a potent adjunct to Her2-targeted therapies in preclinical breast cancer models. Genes Cancer 5(7-8):261–272. doi:10.18632/genesandcancer.24
Rader J, Russell MR, Hart LS, Nakazawa MS, Belcastro LT, Martinez D, Li Y, Carpenter EL, Attiyeh EF, Diskin SJ, Kim S, Parasuraman S, Caponigro G, Schnepp RW, Wood AC, Pawel B, Cole KA, Maris JM (2013) Dual CDK4/CDK6 inhibition induces cell-cycle arrest and senescence in neuroblastoma. Clin Cancer Res 19(22):6173–6182. doi:10.1158/1078-0432.CCR-13-1675
O’Brien NA, Tomaso ED, Ayala R, Tong L, Issakhanian S, Linnartz R, Finn RS, Hirawat S, Slamon DJ (2014) In vivo efficacy of combined targeting of CDK4/6, ER and PI3K signaling in ER plus breast cancer. Cancer Res 74(19). doi:10.1158/1538-7445.AM2014-4756
Infante JR, Cassier PA, Gerecitano JF, Witteveen PO, Chugh R, Ribrag V, Chakraborty A, Matano A, Dobson JR, Crystal AS, Parasuraman S, Shapiro GI (2016) A phase I study of the Cyclin-dependent kinase 4/6 inhibitor Ribociclib (LEE011) in patients with advanced solid tumors and lymphomas. Clin Cancer Res 22(23):5696–5705. doi:10.1158/1078-0432.CCR-16-1248
Juric DMP, Campone M et al (2016) Ribociclib (LEE011) and letrozole in estrogen receptor-positive (ER+), HER2-negative (HER2–) advanced breast cancer (aBC): phase Ib safety, preliminary efficacy and molecular analysis. Presented at the 2016 annual meeting of the American Society of Clinical Oncology, Chicago
Vora SR, Juric D, Kim N, Mino-Kenudson M, Huynh T, Costa C, Lockerman EL, Pollack SF, Liu M, Li X, Lehar J, Wiesmann M, Wartmann M, Chen Y, Cao ZA, Pinzon-Ortiz M, Kim S, Schlegel R, Huang A, Engelman JA (2014) CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell 26(1):136–149. doi:10.1016/j.ccr.2014.05.020
Juric D, Ismail-Khan R, Campone M, Garcia-Estevez L, Becerra C, De Boer R, Hamilton E, Mayer IA, Hui R, Lathrop KI, Pagani O, Asano S, Bhansali SG, Zhang V, Hewes B, Munster P (2016) Phase Ib/Il study of ribociclib and alpelisib and letrozole in ER+, HER2-breast cancer: safety, preliminary efficacy and molecular analysis. Cancer Res 76. doi:10.1158/1538-7445.SABCS15-P3-14-01
Curigliano G, Gomez Pardo P, Meric-Bernstam F, Conte P, Lolkema MP, Beck JT, Bardia A, Martinez Garcia M, Penault-Llorca F, Dhuria S, Tang Z, Solovieff N, Miller M, Di Tomaso E, Hurvitz SA (2016) Ribociclib plus letrozole in early breast cancer: a presurgical, window-of-opportunity study. Breast 28:191–198. doi:10.1016/j.breast.2016.06.008
Gelbert LM, Cai S, Lin X, Sanchez-Martinez C, Del Prado M, Lallena MJ, Torres R, Ajamie RT, Wishart GN, Flack RS, Neubauer BL, Young J, Chan EM, Iversen P, Cronier D, Kreklau E, de Dios A (2014) Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Investig New Drugs 32(5):825–837. doi:10.1007/s10637-014-0120-7
Tate SC, Cai S, Ajamie RT, Burke T, Beckmann RP, Chan EM, De Dios A, Wishart GN, Gelbert LM, Cronier DM (2014) Semi-mechanistic pharmacokinetic/pharmacodynamic modeling of the antitumor activity of LY2835219, a new cyclin-dependent kinase 4/6 inhibitor, in mice bearing human tumor xenografts. Clin Cancer Res 20(14):3763–3774. doi:10.1158/1078-0432.CCR-13-2846
Patnaik A, Rosen LS, Tolaney SM, Tolcher AW, Goldman JW, Gandhi L, Papadopoulos KP, Beeram M, Rasco DW, Hilton JF, Nasir A, Beckmann RP, Schade AE, Fulford AD, Nguyen TS, Martinez R, Kulanthaivel P, Li LQ, Frenzel M, Cronier DM, Chan EM, Flaherty KT, Wen PY, Shapiro GI (2016) Efficacy and safety of Abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov 6(7):740–753. doi:10.1158/2159-8290.CD-16-0095
Goetz MP, Beeram M, Beck T, Conlin AK, Dees EC, Dickler MN, Helsten TL, Conkling PR, Edenfield WJ, Richards DA, Turner PK, Cai N, Chan EM, Pant S, Becerra CH, Kalinsky K, Puhalla SL, Rexer BN, Burris HA, Tolaney SM (2016) Abemaciclib, an inhibitor of CDK4 and CDK6, combined with endocrine and HER2-targeted therapies for women with metastatic breast cancer. Cancer Res 76. doi:10.1158/1538-7445.SABCS15-P4-13-25
Dickler MN TS, Rugo HS et al (2016) MONARCH1: results from a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as monotherapy, in patients with HR+/HER2- breast cancer, after chemotherapy for advanced disease. J Clin Oncol 34(Suppl, abstr 510)
Raub TJ, Wishart GN, Kulanthaivel P, Staton BA, Ajamie RT, Sawada GA, Gelbert LM, Shannon HE, Sanchez-Martinez C, De Dios A (2015) Brain exposure of two selective dual CDK4 and CDK6 inhibitors and the antitumor activity of CDK4 and CDK6 inhibition in combination with Temozolomide in an intracranial glioblastoma Xenograft. Drug Metab Dispos 43(9):1360–1371. doi:10.1124/dmd.114.062745
Hurvitz S MM, Fernández Abad M, Chan D, Rostorfer R, Petru E, Barriga S, Costigan TM, Caldwell CW, Nguyen T, Press M, Slamon D (2016) Biological effects of abemaciclib in a phase 2 neoadjuvant study for postmenopausal patients with HR+, HER2- breast cancer. Presented at the 2016 San Antonio breast cancer symposium
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Cai, Z., Liu, Q. (2017). Cell Cycle Regulation in Treatment of Breast Cancer. In: Song, E., Hu, H. (eds) Translational Research in Breast Cancer. Advances in Experimental Medicine and Biology, vol 1026. Springer, Singapore. https://doi.org/10.1007/978-981-10-6020-5_12
Download citation
DOI: https://doi.org/10.1007/978-981-10-6020-5_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-6019-9
Online ISBN: 978-981-10-6020-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)