Abstract
In the present study, a more potential and economical method is described to reutilize a waste adsorbent as the waste Cu2+-loaded poly(AAc/AM/SH) SAHs were not undergone any regeneration process and directly applied to adsorb phosphate ion from another waste solution. The SAHs poly(AAc-AM-SH) was anionic in nature, thus show higher affinity toward Cu2+ ions, but it hardly adsorb anions due to its characteristics of negative charge existing on the polymeric surface. The adsorption of Cu2+ makes it positively charged moiety and so it is being capable of anions/anionic dye adsorption. The various factors affecting the phosphate adsorption including pH, contact time, initial concentration of the phosphate were systematically investigated. The maximum phosphate adsorption was obtained 87.62 mg/g. The adsorption data fitted the Langmuir adsorption isotherm. The desorption studies showed that the regeneration of the poly(AAc/AM/SH)–Cu SAHs adsorbent can be easily achieved. The results confirmed that poly (AAc/AM/SH) superabsorbent hydrogels loaded with Cu2+ ion can be applied as effective solid adsorbent for the removal of phosphate ions from waste water and aqueous effluents.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Introduction
Over the recent years, the rapid development of modern industries has increased the threat of water pollution to the environment. The chief water pollutant consist non-biodegradable matter, dyes, metal ions, hydrocarbons, anions etc. that has been rapidly increased in water. Therefore, different efficient treatment methods (such as chemical precipitation, cross flow filtration, chelating ion exchange, crystallization, reverse osmosis, electrochemical treatment, neutralization, and adsorption) have been developed. However, among these methods, the adsorption process is found to be a highly efficient, regenerable, economical, and promising method. Superabsorbent hydrogels (SAHs) are a special class of adsorbents which are lightly crosslinked three dimensional macromolecular polymeric networks with the ability to absorb huge amounts of water and the absorbed water is hard to remove even under pressure.
Development of SAHs adsorbents is based on the interaction of adsorbate with the active chelating functional groups such as sulfonic acid, amine group, carboxylic acid, amine hydroxyl etc. and the selectivity, effectiveness, and reusability of these SAHs can be determined by these functional groups. These active functional groups adsorb, trap and bind the adsorbate molecules, and so make these SAHs as more effective adsorbent (Karadag et al. 1995). With the development and optimization of these adsorbents, production of waste products also occurs in industrial sectors. These products mainly include the already used adsorbents, which were applied for the adsorption process. These waste adsorbents can be (i) recycled for again adsorption process by regenerating them in acidic/basic elution medium for next cycle (Kara et al. 2004), (ii) disposed or cremated. For any adsorption process as the adsorption proceeded reaches to equilibrium, the original polymeric backbone structure of adsorbent SAHs changed and they can function as a new type of the adsorbent.
In the past few decades, phosphate has been recognized as a substantial non-point-source pollutant because of over application of animal-based and manures synthetic fertilizers (Arai and Sparks 2001) the electronic industry, pigment formulation, water treatment, detergents, as well as mineral processing. The existence of trace concentration of phosphate ion in the wastewater from municipalities and industries is responsible for eutrophication problems that lead to growth of the aquatic plants, and depletion of dissolved oxygen in coastal areas, lakes, and other water bodies. Therefore, industrial and municipal wastewater having phosphate must be treated before discharging.
Acrylic acid is cheap, highly hydrophilic and acts as a good chelating agent. Polyacrylamide has high hydrophilicity, excellent resilience, and its pendant amide group acts as efficient chelating group for the ionic and polar species. Humic acid, a principal component of humic substances, is found in many places in nature. It consists of large number of functional hydrophilic groups (including carboxylates and phenolic hydroxyls, NH2 groups and oxygen and nitrogen as bridge units) (Wang and Wang 2009). It is accepted that the removal capacity of an adsorbent is highly influenced by the adsorption conditions (such as pH, temperature and ionic strength). In our previous report, we synthesized a biodegradable multifunctional SAHs based on acrylic acid and acrylamide monomers modified with sodium humate and used this as an adsorbent for removal of Cu2+ (Singh and Singhal 2012). After the adsorption of Cu2+, it became positively charged moiety and being capable of phosphate ion removal. Thus, this Cu2+-loaded gel without any prior treatment serves as a new kind of adsorbent, i.e., metal loaded adsorbents.
The present report deals with an economical and potential method to treat a waste adsorbent. So, the novelty of the present work is to identify applicability of waste adsorbent poly(AAc/AM/SH)–Cu hydrogels for the adsorption of phosphate ions so that to provide a new potential way to use an already used adsorbent without any regeneration or purification process. The experiments were performed as a function of different pH, contact time and various initial concentrations of phosphate ion to determine the optimum conditions for the adsorption of phosphate ion from aqueous solution. Langmuir and Freundlich adsorption isotherm models were applied to the experimental isotherms and isotherms constants to validate the usefulness of this waste hydrogel in the field of wastewater treatment.
Materials and Method
Materials
Acrylic acid ((AAc), analytical grade), acrylamide ((AM), analytical grade), ammonium per sulfate ((APS), analytical grade), sodium hydroxide ((NaOH), analytical grade), N, N-methylene bisacrylamide ((NMBA), analytical grade), copper sulfate ((CuSO4), analytical grade), potassium dihydrogen phosphate (KH2PO4), hydrochloric acid (HCl) were purchased from CDH New Delhi, India. Methanol (analytical grade) was purchased from Qualikems, New Delhi. Acrylamide was recrystallized from methanol before use. Sodium humate ((SH), analytical grade), (supplied from Aldrich) was used as received. Double distilled water was used throughout the experiments.
Synthesis of Poly(AAc/AM/SH) Superabsorbent Hydrogels
AAc (7 g) and AM (7 g) were dissolved in 30 ml distilled water and after neutralizing the reaction mixture with NaOH solution, the solution was poured in a 250-ml three-neck round bottom flask which is equipped with a stirring rod, a nitrogen inlet and a reflux condenser. After that (0.20 wt% of total monomer), NMBA was added to the monomer solution, subsequently dispersed SH (0.35 g) into mixed solution. Then, reaction mixture was stirred under nitrogen atmosphere for 30 min to remove the dissolved oxygen, and after that the mixed reaction solution was heated in a thermostat oil bath for 1 h at 60 °C; then, the initiator, APS (0.40 wt% of total monomer), was introduced into the flask. The reaction solution was again stirred under nitrogen atmosphere for 2 h at 60 °C for homogeneity. The resulting reaction solution was poured into Petri-dishes and kept in a hot air oven at 60 °C for 2 h to complete polymerization and subsequent cross-linking process. After the polymerization (completion of reaction) in 2 h, the firm, SAHs, was carefully removed from the Petri-dish surface and cut into small pieces (0.1–0.5 cm in thickness).
Separation of unreacted monomers was done by washing with methanol, followed by swelling in distilled water for 4 h and then dried in an oven at 60 °C up to the constant weight. The dried hydrogels were stored in desiccators.
To prepare the Cu2+-loaded SAHs poly(AAc/AM/SH), 50 mg of dry SAHs was introduced in 100 ml of Cu2+ ions salt solution (initial ion concentration 1 g/L) at pH 5.0 and was left in the solution for 24 h. The SAH samples were withdrawn at different time intervals (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 and 24 h) and analyzed for Cu2+ content left in solution. The adsorption capacity of the hydrogel for Cu2+ was evaluated through the following equation:
where q e is the amount of Cu2+ adsorbed at equilibrium, C o is the initial concentration of Cu2+, C e is the equilibrium concentration of Cu2+ ions, V is the volume of the Cu2+ ions solution, and m is the mass of SAHs sample.
Desorption Study of Cu2+ from Poly(AAc/AM/SH)–Cu SAHs
To evaluate the stability of Cu2+ into poly(AAc/AM/SH)-Cu SAHs at adsorption process, 50 mg of poly(AAc/AM/SH)–Cu SAHs was added to twelve conical flasks with 100 mL of deionized water. The pH of solution of each conical flask was adjusted to 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 7.0, 8.0, 9.0, 10.0, 11.0 and 12.0, respectively. The final Cu2+ concentration in the aqueous phase was determined by above given procedure. The desorption percentage was calculated by the amount of Cu2+ ions adsorbed on the SAHs surface and final Cu2+ concentration in the desorption medium. Desorption percentage was calculated using the following expression:
Adsorption Analysis of Phosphate Ions
For the determination of phosphate adsorption experiments, 50 mg of the adsorbent poly(AAc/AM/SH)–Cu hydrogel was loaded into a conical flask with 100 ml of phosphate solution in various initial concentrations. Following the adsorption, the samples were withdrawn at different time intervals (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 and 24 h) and analyzed for phosphate content left in solution. The capacity of phosphate adsorption was also calculated using Eq. (1). To evaluate the influence of pH on phosphate ion adsorption, experiments were performed at different initial pH, ranging between 1.0 and 12.0. Initial phosphate ion concentration of 180 mg/L and 50 mg of adsorbent was used. The solutions were shaken for 24 h at 30 ± 0.5 °C. The capacity of phosphate adsorption was also calculated using Eq. (1).
Desorption and Regeneration
In order to explore the potential of reusability of adsorbent and recovery of phosphate ions, consecutive adsorption–desorption cycles were repeated five times using the adsorbent prepared following the described procedure in adsorption experiments. Desorption of phosphate ions from the SAHs poly(AAc/AM/SH)–Cu (having 87.62 mg/g) was carried out in batch mode using 50 mg adsorbent in 25 ml of 0.1 M NaOH solution (elution medium) for 48 h. The SAHs was taken out and then washed several times with distilled water followed by methanol and then dried at 60 °C for 24 h. The regenerated SAHs were used for another adsorption. Desorption ratio was calculated by applying the Eq. (2).
Results and Discussion
Effect of PH on the Desorption Behavior of Cu2+ from Poly(AAc/AM/SH)–Cu SAHs
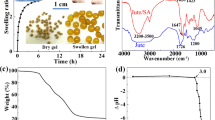
The release behavior of Cu2+ from poly(AAc/AM/SH)-Cu SAHs was investigated at various pHs solutions under the identical conditions as the adsorption process for phosphate ion-containing water. Figure 1 shows the percent released of Cu2+ from poly(AAc/AM/SH)-Cu SAHs at various pH range.
It can be observed that at lower pH (pH < 3.5) the desorption of Cu2+ occurs extensively (>98%), due to competitive adsorption of protons for adsorption sites by substituting the Cu2+ from the Cu loaded hydrogel surfaces. On further increasing the pH (pH > 4.2), desorption of Cu2+ decreased sharply and the sample shows very little desorption (because of the chemical bonding formed due to chelation of the –NH2, –COOH, and –OH groups with the Cu2+).
Effect of Contact Time on Phosphate Adsorption
Figure 2 shows the influence of contact time on the phosphate uptake. As clear from Fig. 2, the adsorption of phosphate ion increases rapidly with increase of contact time initially and then reaches at a constant value beyond which no phosphate ion was further adsorbed from the solutions
This phenomenon can be explained by the fact that, initially adsorption binding sites were void and phosphate ions may easily interact with these sites. The phosphate ion uptake almost remained constant after 12 h, and with prolonged time, the removal capacity hardly increased, so 12 h could be considered as the equilibrium contact time. The results suggest that the phosphate ion adsorption was fast at the initial part of the contact time, and thereafter it goes slower at near the equilibrium point.
Effect of PH and Ionic Strength of Solution on Adsorption of Phosphate
The pH of aqueous solution is one of the most important factors influencing the adsorption amount of cations and anions (Huang et al. 2008). In this study, influence of pHs on removal amount of phosphate ion was examined at various pHs levels namely between 2.00 and 12. At lower pHs (<2.15), the predominant species in solution is the neutral H3PO4, between pHs range of 2.15–7.20, the main species in solution is H2PO4 −, and at pHs values between 7.2 and 12.33 the predominant species is HPO4 2− (Lee and Davis 2001). Figure 3 shows the variation in removal capacity of phosphate ion on the poly(AAc/AM/SH) SAHs and poly(AAc/AM/SH)–Cu SAHs from aqueous solution with respect to pH (Mahadavinia et al. 2004).
It was observed from the Fig. 3 that poly(AAc/AM/SH)–Cu hydrogel shows higher adsorption capacity than the poly(AAc/AM/SH) hydrogel, which shows negligible adsorption in all measured pH ranges. The phosphate ion uptake increases sharply with an increase in initial pH (1–4) and then increased slowly until reaching a maximum 87.62 mg/g at pHs 6.1. After that on further increasing the pH, it goes down (Wang and Lin 2008). It was practically independent on pH values in the range of 5.0–6.1. When pH value was lower than 3.8 and higher than 6.1, a remarkable decrement in adsorption was observed.
What About Effect of Ionic Strength
Effect of Initial Concentration of Phosphate on Adsorption Capacity of the Poly(AAc/AM/SH)–Cu SAHs
Initial ion concentration is an important parameter so the effect of initial concentration on adsorption capacity was investigated. Figure 4 shows the adsorption capacity of the poly(AAc/AM/SH)–Cu SAHs for the phosphate ions as a function of various initial ion solution concentrations ranging from 100 to 200 mg/L (Perrin et al. 1974).
It can be observed from the Fig. 4 that adsorption amount of phosphate increased (from 72.66 to 87.62 mg/g) with increase in initial concentration of phosphate ions solution if the amount of adsorbent was constant. After reaching a maximum (87.62 mg/g) at 180 mg/L, it remained nearly constant. It can also be observed that in the initial stage the adsorption is rapid and increases gradually with progress of adsorption or with the increasing concentration of phosphate amount of removed phosphate ions increased but its adsorption percentage decreased.
Adsorption Isotherms
The most widely used isotherm for modeling of the adsorption data is the Langmuir adsorption isotherm. The Langmuir adsorption isotherm equation may be described as (Langmuir 1918).
The Freundlich model is an empirical equation and applied to describe heterogeneous surface system by a heterogeneity factor of 1/n. The Freundlich model in linear form may be expressed as follows (Jaroniec 1983);
where K e (sorption equilibrium constant (L/mg)) and q max (maximum amount of adsorption (mg/g), C e is the equilibrium concentration of the metal ion in the solution (mg/L), q e is the amount adsorbed at equilibrium mg/g. K f (L/g) and 1/n (dimensionless) are the Freundlich constants related to the adsorption capacity and the degree of heterogeneity, respectively (Matti et al. 2007). The Langmuir and Freundlich constants and regression coefficients were calculated from the linear plots of C e /q e versus C e and log(q e ) versus log(C e ) respectively (Ho 1999). The q max values of the superabsorbent hydrogels obtained by Langmuir equation were quite consistent with the experimental one (Table 1).
Desorption/Regeneration of Adsorbent
A good solid adsorbent in addition to its high adsorption capacity should also exhibit a good regeneration capacity for potential application. The poly(AAc/AM/SH)–Cu SAHs that were applied for the adsorption of phosphate ions were placed in 0.1 M NaOH solution for 48 h and the amount of phosphate desorbed to the elution medium was measured. Figure 5 displays the desorption ratio of poly(AAc/AM/SH)–Cu hydrogels (having 87.62 mg/g phosphate) as a function of time.
The desorption process reached equilibrium at about 29 h, and the desorption ratio was approximately 90%. Table 2 shows the experimental results for the adsorption capacity and times for reuse for the sample.
The adsorption capacities did not show any significant decrease after the fourth reuse cycle. After five cycles of adsorption–desorption operations, adsorption capacity of phosphate ions were around 83.14 mg/g. Therefore, it can be concluded that poly(AAc/AM/SH)–Cu hydrogel showed stable phosphate ions removal capacities after repeated regeneration and thus qualified for multiple practical application.
Conclusion
In the present study, it is attempted to provide a cost-efficient and effective method to reuse a waste adsorbent poly(AAc/AM/SH)–Cu SAHs for the phosphate ion adsorption from aqueous solution. The concentration of Cu2+ ions released from poly(AAc/AM/SH)–Cu SAHs decreased gradually on increasing the pH and almost stopped at pH higher than 4.5. Thus, the poly(AAc/AM/SH) SAHs after the adsorption of Cu2+ ions could be used directly for the removal of phosphate ion at pH > 4.5. Adsorption capacity was found to be maximum (87.62 mg/g) at pH 6.1. The adsorption isotherm agrees well with the Langmuir model as confirmed by linear fit of the plots to Langmuir equation. The results of five time consecutive adsorption–desorption cycle indicate that the poly(AAc/AM/SH)–Cu SAHs have high adsorption and desorption efficiency for the phosphate ions. The results confirmed that poly (AAc/AM/SH)–Cu SAHs can be used as effective solid adsorbent for the removal of phosphate ions from waste water and aqueous effluents, which seems the most efficient method to treat an already used adsorbent.
References
Arai Y, Sparks DL (2001) J of Colliod Interface Sci 241:317
Ho YS, Huang CIT, Huang HW (1999) Process Bio Chem 37:1421
Huang W, Wang S, Zhu Z, li L, Yao X, Rudolph V, Haghseresht F (2008). J of Hazard Mater 158: 35
Jaroniec M (1983) Physical adsorption on heterogeneous solids. Adv Colloid Interface Sci 18:149
Kara L, Uzun N, Denizli A (2004) Poly(ethylene glycol dimethacrylate-n-vinyl imidazole) beads for heavy metal removal. J Mater Hazard B106:93
Karadag E, Saraydin D, Guven O (1995) Behaviors of acrylamide itaconic acid hydrogels in uptake of uranyl ions from aqueous solutions. Sep Sci Technol 30:3287
Langmuir IJ (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361
Lee SM, Davis AP (2001) Water Res 35:534
Mahadavinia GR, Pourjavadi A, Hosseinzadeh H, Zohuriaan MJ (2004) Modified chitosan 4 Superabsorbent hydrogels from acrylic acid -co-acrylamide) grafted chitosan with salt and pH-responsive prperties. Eur Polymer J 40:1399–1407
Mattigod SV, Fryxell GE, Parker K (2007) Inorg Chem Mun 10: 646
Perrin DD, Dempsey B (1974) Chapman and Hall, London
Singh T, Singhal R (2012) Poly (acrylic acid/acrylamide/sodium humate) superabsorbent hydrogels for metal ion/dye adsorption: effect of sodium humate concentration. J Appl Polym Sci 125:1267
Wang J, Lin W (2008) J Appl Polym Sci 109:318
Wang W, Wang A (2009) Synthesis, swelling behaviors, and slow-release characteristics of a Guar Gum-g-Poly(sodium acrylate)/Sodium humate superabsorbent. J Appl Polym Sci 112:2102
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Singh, T., Singhal, R. (2018). Efficient and Economical Application of a Spent Waste Adsorbent Cu2+-Loaded Poly (AAc-AM-SH) Superabsorbent Hydrogels by Reusing It for Adsorption of Phosphate Ion. In: Singh, V., Yadav, S., Yadava, R. (eds) Water Quality Management. Water Science and Technology Library, vol 79. Springer, Singapore. https://doi.org/10.1007/978-981-10-5795-3_22
Download citation
DOI: https://doi.org/10.1007/978-981-10-5795-3_22
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-5794-6
Online ISBN: 978-981-10-5795-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)