Abstract
Hepatitis B reactivation (HBR) occurs in patients with inactive or resolved hepatitis B virus (HBV) infection, in whom the host immune response is overtaken by the replicative drive of the virus. While HBR may be clinically silent, there is abrupt reappearance or rise of HBV DNA in the serum (usually > 2 log) with or without a flare of alanine aminotransferase (ALT) activities. HBR commonly occurs in the setting of B-cell depleting agents and corticosteroids although cytotoxic chemotherapeutic and other immunosuppressant and immunomodulant agents may precipitate it. The risk of HBR may be determined by host characteristics, viral factors, and the type of medical therapy. For an optimal outcome, screening for patients at risk of HBR is critical. For patients identified to be at risk, strategic utilization of antiviral prophylaxis has been shown to improve outcomes.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Hepatitis B reactivation (HBR) is characterized by an abrupt reappearance or rise of hepatitis B virus (HBV) DNA in the serum of a patient with previously inactive or apparently resolved HBV infection (Lok et al. 1991). Compared to their baseline status of minimal or inactive disease activity, patients experiencing HBR may end up with recrudescence or flare of hepatitis activity. The most severe end of the spectrum may be symptomatic acute hepatitis, which may progress to fulminant liver failure.
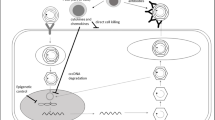
In the background of HBR is the balance between the replicative drive of the virus and the immune response from the host (Fig. 16.1) (Lok et al. 1991; Yeo et al. 2004a). In patients with inactive HBV infection, viral replication is inhibited as a result of the control by the host immune system. HBR occurs when this balance is perturbed by an environmental agent such as an immunosuppressive or cancer therapeutic compound which lifts the immune control allowing HBV replication to resume (Hoofnagle 2009; Keam et al. 2011). More recently, there may be another mechanism to HBR when there is a coexistent hepatotropic virus such hepatitis C virus (HCV). Successful eradication of HCV with recently available direct-acting antiviral agents has been associated with HBR.
Although HBR is often temporary and clinically silent, it may cause a symptomatic flare of hepatitis. While the flare in and of itself may evolve into a serious condition incurring morbidity and even mortality, another major clinical consequence of HBR is the need for interruption of immunosuppressive or chemotherapy. Moreover, in patients who are not suspected to have HBV infection, HBR may be a source of confusion and misdiagnosis, leading to a delay in appropriate clinical management. Hence, preventing HBR protects the patient from experiencing potentially dangerous flares and from failing to achieve the intended goals of the immunosuppressive therapy.
In this chapter, we will review the current literature on HBR and discuss its epidemiology, risk factors and mechanisms, manifestations and diagnosis, and clinical management in terms of prevention and therapy. We will also discuss challenges and future research direction and devote a section on the recent topic of HBR in the setting of HBV-HCV dual infection.
2 Mechanisms of HBR
HBV genome is able to persist in the liver for long periods of time, even when HBsAg is cleared in the serum. In patients in whom HBV infection is inactive, viral replication is suppressed by immune control of HBV infection, which is mediated through HBV-specific cytotoxic T cells (Rehermann et al. 1996; Zhang et al. 2012), while B cells also play a role in antigen presentation and viral clearance (Chang and Lewin 2007). Administration of an immunosuppressive drug to chronic HBV patients may potentially allow the virus to escape the immune control, leading to increased HBV replication and a marked increase in expression of HBV transcription intermediaries and products within hepatocytes (Keam et al. 2011).
2.1 Corticosteroids
Corticosteroids have a number of ways to promote HBV replication. The HBV genome has a glucocorticoid-responsive element which enhances replication of the virus (Tur-Kaspa et al. 1986; Calabrese et al. 2006; Tur-Kaspa et al. 1988). In a recent prospective study, half of the study patients had increased HBV DNA levels within 2 weeks of starting a corticosteroid-containing chemotherapy regimen. This occurred well before the development of neutropenia, suggesting a direct stimulatory effect of corticosteroids on HBV DNA transcription (Cheng et al. 2003). Indirectly, corticosteroids have a number of immunosuppressive effects including inhibition of cytotoxic T-cell function (Tur-Kaspa et al. 1988). The risk of HBR among those treated with corticosteroid varies by the dosages, duration of treatment, and HBV serologic status of the host.
2.2 B-Cell-Depleting Agents
Rituximab and ofatumumab are the two licensed monoclonal antibodies against CD20 of B cells in the USA, which possess potent immunosuppressive effects. They are used to treat hematologic malignancies and, less commonly, severe autoimmune diseases such as rheumatoid arthritis and vasculitis. While the mechanism of rituximab-/ofatumumab-associated HBV reactivation is not completely understood, the putative mechanisms are that depletion of B cells and the resulting disruption of antigen presentation impair CD8+ cytotoxic T cell’s ability to kill HBV-infected hepatocytes. Anti-CD20 antibodies reduce the number of CD4 memory T cells, increase Th1/Th2 and Tc1/Tc2 ratios, and upregulate Fas ligand on Th1 and Th2 cells, further impairing the host immune control against the virus (Evens et al. 2011; Misumi and Whitmire 2014; Tsutsumi et al. 2015). B-cell depletion may lead to loss of anti-HBs (Pei et al. 2012).
2.3 Cytotoxic Chemotherapeutic and Immunosuppressant Agents
Cytotoxic cancer chemotherapeutic agents disrupt cell cycles, leading to DNA destruction. Subsequent activation of chemotherapy-induced DNA repair mechanisms results in a cascade of responses including upregulation of promyelocytic leukemia protein (PML) and PML nuclear body (PML-NB), which have been linked with increased HBV pregenomic transcription, HBV-core expression, and HBV DNA replication (Chung and Tsai 2009). Traditional immunosuppressants such as methotrexate, azathioprine, and 6-mercaptopurine also disrupt DNA synthesis. However, these are apparently not as detrimental from the standpoint of HBR as other chemotherapeutic agents (Calabrese et al. 2006; Droz et al. 2013; Flowers et al. 1990).
2.4 Biological Immunomodulants
HBV-specific cytotoxic T cells inhibit hepatocellular HBV gene expression and replication through a noncytotoxic mechanism, which is mediated in part by TNF-α and interferon-α (Kasahara et al. 2003; Tzeng et al. 2014). Inhibition of TNF-α activity may lead to enhanced viral replication (Carroll and Forgione 2010). Currently, there are three commonly used TNF-α inhibitors, etanercept, infliximab, and adalimumab, all of which have been implicated in HBR.
Cytokine or integrin inhibitors have been introduced in practice only in the past few years, and reported experience of HBR related to these agents is limited. Abatacept blocks costimulation of T lymphocytes (Herrero-Beaumont et al. 2012). Imatinib and other tyrosine kinase inhibitors can inhibit T-cell activation and proliferation (Seggewiss et al. 2005; Dietz et al. 2004). Ustekinumab is a human monoclonal antibody that is directed to interleukin-12 and interleukin-23 (Cingoz 2009). Natalizumab and vedolizumab are recently developed inhibitors to the cell adhesion molecule α4 integrin found on lymphocytes (Hutchinson 2007; Rezaie 2014). While empirical data are lacking, the direct effects of these agents on T-cell immunity raise concerns that the risk of HBR may be significant (Perrillo et al. 2015).
3 Incidence of HBR
The accurate incidence of HBR among patients with immunosuppressive therapy is poorly defined for a number of reasons. First, the settings in which HBR occurs are heterogeneous depending upon host characteristics, baseline HBV status, types of immunosuppressive therapy, and the underlying disease that requires the immunosuppressive therapy. Second, most studies are conducted in retrospective fashion and would be enriched with patients with severe HBR requiring medical attention. Finally, the criteria to diagnose HBR are not uniformly defined, creating further heterogeneity in study results. With these caveats, we summarize data regarding the incidence of HBR according to the clinical scenario.
3.1 Patients Undergoing Cancer Chemotherapy
HBR was initially described in cancer patients undergoing chemotherapy. Today, the most common scenario for HBR to occur in cancer therapy is patients with hematological malignancies receiving anti-CD20 antibodies. The incidence of HBR is much lower with cytotoxic chemotherapy for solid tumors, with breast cancer being one of the most frequently tumors associated with HBR. The incidence of HBR in HBsAg+ patients being treated for cancer has been reported to be 14–72%, whereas it is much lower (<3%) among patients who are HBsAg−/anti-HBc+ (Lok et al. 1991; Kim et al. 2007; Kumagai et al. 1997; Yeo et al. 2000a, b; 2003; 2004b; Kusumoto et al. 2009; Vento et al. 2002). In patients receiving a regimen including anti-CD20 antibodies, the incidence of HBR may be as high as 60% in those with HBsAg+ (Abramson and Chung 2014; Mendez-Navarro et al. 2011; Mozessohn et al. 2015). Even in patients who are HBsAg−/anti-HBc+, HBR may be seen in 8–42%.
In addition to systemic chemotherapy, HBR may also occur in patients undergoing regional therapy such as transarterial chemoembolization (TACE) for hepatocellular carcinoma (HCC). Although the incidence of HBR in those settings has not been accurately defined, it may be as high as 34% (Jang et al. 2004).
3.2 Patients Undergoing Organ/Cell Transplantation
3.2.1 Hematopoietic Stem Cell Transplantation (HSCT)
Patients undergoing allogeneic HSCT tend to be heavily immunosuppressed, including immunoablative therapy applied prior to the infusion of the donor marrow. The incidence of HBR after HSCT is almost universal among HBsAg+ patients (Lalazar et al. 2007; Lau et al. 1997; Martin et al. 1995) and may be up to 50% in HBsAg−/anti-HBc+ patients (Hammond et al. 2009; Park et al. 2011; Seth et al. 2002; Vigano et al. 2011; Ramos et al. 2010; Knoll et al. 2004; Onozawa et al. 2005). Reverse HBsAg seroconversion describes the latter scenario in which a patient who initially lacked HBsAg becomes HBsAg positive. In HSCT patients, the risk of reverse seroconversion persists for many years because of the delay in reconstitution of the recipient’s immune response to HBV. Reverse seroconversion may occur in patients who are initially anti-HBs positive: in studies measuring the anti-HBs titer serially, HSCT recipients gradually lost anti-HBs to become undetectable 1–3 years after transplantation. Meanwhile, HBV DNA increased, and HBsAg reappeared in the serum. In one retrospective study of HBsAg−/anti-HBc+ HSCT recipients, the cumulative probability of reverse seroconversion was 9% at the end of 1st year, which more than quadrupled to 43% at the end of 4th year (Hoofnagle 2009; Hammond et al. 2009).
3.2.2 Solid Organ Transplantation (SOT)
The effect of HBV infection on the outcome of SOT has been studied most in kidney transplantation (KT). Patients with HBsAg+ have an increased risk of graft loss and mortality (Reddy et al. 2011; Fabrizi et al. 2005). The risk of HBR is higher in the HBsAg+ recipient, especially in those with detectable HBV DNA or HBeAg+ compared with HBsAg-recipients (Reddy et al. 2011). For the HBsAg+ recipients, Degos (Degos et al. 1988) demonstrated that HBR occurred in 11 of 12 (92%) initially HBsAg+ recipients. A study by Fornairon also described that 85% of HBsAg+ KT recipients developed histological progression, leading to cirrhosis and hepatocellular carcinoma in some patients (Fornairon et al. 1996). The reported HBR incidence among KT recipients with isolated anti-HBc+ was lower, varying from 0 to 6.5% (Chen et al. 2013; Kanaan et al. 2012) .
4 Risk Assessment for HBR
The risk of HBR depends on three important factors: host, baseline status of HBV infection and the type of treatment (Fig. 16.2). Clearly, presence of HBsAg is a predominant determinant of HBR. In a study by Lau, individuals who were HBsAg+ carried a greater risk for HBV reactivation compared with those who are HBsAg- (HR 33.3, 95%CI 7.4–142.9, p < 0.01). Among those with HBsAg+, the risk of HBR correlates with markers of viral replication status, namely, HBeAg and serum HBV DNA. In particular, HBV DNA levels exceeding 105 copies/mL were associated with the highest risk of HBR (Lau et al. 2002). Compared to undetectable HBV DNA, detectable viremia was associated with a HR of 9.35 (95%CI 1.65–52.6, p = 0.01). Another study by Yeo evaluated risk for HBR among cancer patients treated with chemotherapy. They found that HBeAg positivity (p < 0.01), male gender (p = 0.045), and diagnosis of lymphoma (p = 0.03) were associated with HBR (Yeo et al. 2000a). Other studies showed that among patients with solid organ tumors, HBR occurs more commonly in breast cancer patients (41%) compared with other organs (7–29%) (Yeo et al. 2003, 2004a). The presence of anti-HBs is in general protective, depending on the host and treatment factors. The role of quantitative anti-HBs titers in the prediction of HBR in these settings is not clear.
In addition to the viral characteristics, host and treatment factors play an important role in determining the risk of HBR. Figure 16.3 shows categories of patients requiring immunosuppressive treatment. Organ transplantation carries an immense risk of HBR, especially HSCT, which affects the host immune function most profoundly. The risk of HBR in patients receiving immunosuppression in settings other than transplantation correlates with the type and level of immunosuppression. For example, the risk is highest when the regimens contain rituximab or high-dose corticosteroid (Cheng et al. 2003; Abramson and Chung 2014; Mendez-Navarro et al. 2011; Mozessohn et al. 2015; Kim et al. 2010; Yeo et al. 2009).
Table 16.1 categorizes the risk of HBR based on the types of immunosuppressive agents and HBV serologic status of the patients. Three strata in HBR risk may be defined, high-, intermediate-, and low-risk groups, corresponding to anticipated incidence of >10%, 1–10%, and <1% of cases, respectively. These categories inform patient management, as described later in this chapter.
5 Diagnosis for HBR
Uniform, standardized nomenclature and definitions for HBR are unavailable. The most recent publication aimed toward a consensus was put forth as proceedings of the 2013 Emerging Trends Conference organized by the American Association for the Study of Liver Diseases (AASLD) on the topic of reactivation of hepatitis B (American Association for the Study of Liver Diseases 2013). Table 16.2 summarizes diagnostic criteria that we propose in part based on the AASLD report.
In patients with chronic HBV infection (HBsAg + and anti-HBc +), HBR is most commonly diagnosed by an increase in HBV DNA in the setting of immunosuppression. A consensus is lacking regarding the threshold in the rise of HBV DNA. In our view, an abrupt increase of ≥2 log in HBV DNA would be sufficient to diagnose HBR, regardless of changes in ALT. Similarly, in patients who were previously undetectable, a new appearance of HBV DNA at titers above 100 IU/ml would correspond to a 2 log rise. In patients with a clinically significant rise in ALT, a smaller degree of HBV DNA rise (≥ 1 log) should trigger management actions (Di Bisceglie et al. 2015; Hwang and Lok 2014).
There is even less clarity regarding what degree of ALT changes is needed to define HBR. Possible proposals have included (1) multiples of the baseline ALT (e.g., threefold increase), (2) multiples of the upper limit of normal (e.g., three times the upper limit of normal), or (3) an absolute cutoff (e.g., 100 IU/mL). The difficulty is compounded by the lack of uniform definition of normal ALT values and poor standardization across laboratories for ALT assays. In our practice, a twofold increase from the patient’s prior baseline is sufficient to prompt a diagnostic investigation.
Changes in the serological profile are easier to define and likely to connote more serious consequences of HBR. HBe seroreversion, namely, reappearance of HBeAg with or without the loss of anti-HBe, signals a significant shift in the immune status of the patient, corresponding to a diagnosis of HBR. HBs seroreversion is even more serious (Hwang and Lok 2014). Most patients experiencing HBs seroreversion have the baseline profile of HBsAg- and anti-HBc+; however, in patients receiving profound levels of immunosuppression (e.g., allogeneic HSCT), HBs seroreversion from anti-HBs-positive to HBsAg-positive status may occur and herald a severe degree of HBR.
6 Clinical Manifestations of HBR
The clinical features of HBR vary from asymptomatic changes in the laboratories to fulminant hepatic failure leading to death. The majority of patients present with mild degree of HBR.
The course of HBR has been described in three phases (Fig. 16.4). The first phase is mainly a virological event, characterized by an abrupt increase in viral replication soon after immunosuppressive therapy is initiated. There are no apparent hepatitis symptoms, and serum aminotransferase levels are usually unchanged from baseline. HBV DNA levels continue to rise during the second phase and may be accompanied by elevation in serum aminotransferases with or without symptoms such as fatigue. In severe cases, hepatitis activities may be severe enough to result in liver failure. As expected, these poor outcomes tend to occur more frequently in cirrhotic patients. In the third phase, HBV DNA levels and serum aminotransferases levels start to decrease, and HBV markers may return to the baseline. Not all HBR patients go through these three phases.
7 Management Strategies for Hepatitis B Reactivation
The goal in the management of HBR is two-fold: (1) prevent liver-related morbidity and mortality and (2) allow the immunosuppressive therapy to continue unperturbed. In achieving these goals, the most effective strategy is to prevent HBR to begin with. This principle is best demonstrated in randomized controlled trials that compared prophylactic antiviral therapy in patients considered to be at high risk versus withholding antiviral treatment until a diagnosis of HBR is established. Figure 16.5 summarizes the results of trials in which lamivudine was used to prevent HBR (Lau et al. 2003; Hsu et al. 2008; Jang et al. 2006; Long et al. 2011). In patients who did not receive prophylactic antivirals, HBR occurred in 30–50%—more frequently in lymphoma patients. Prophylactic lamivudine was able to virtually eliminate HBR. While lamivudine may not be the ideal agent today, these data are convincing that in high-risk patients, prevention is a preferred strategy than reactive treatment of HBR once it has occurred.
Recently, American Gastroenterological Association (AGA) released a technical review and guideline on the prevention and treatment of HBR during immunosuppressive drug therapy (Perrillo et al. 2015; Reddy et al. 2015). Table 16.3 summarizes recommendations addressed in the document.
7.1 Screening and Risk Stratification
A crucial element in HBR management is to identify patients with HBV infection prior to initiation of immunosuppressive therapy. Various governmental and professional organizations have published guidelines about screening as shown in Table 16.4 (Reddy et al. 2015; Weinbaum et al. 2008; Lok and McMahon 2009; Baden et al. 2012; European Association for the Study of the Liver 2012; LeFevre 2014; Sarin et al. 2016; Hwang et al. 2015). Although these guidelines vary in some of the details in their recommendations, they all agree that initial screening should be performed with HBsAg and anti-HBc. Regarding anti-HBc testing, it can be either total anti-HBc or anti-HBc immunoglobulin G, but not immunoglobulin M.
An approach to diagnose all patients at risk of HBR would be universal screening—namely testing every patient for HBV infection before immunosuppressant therapy is instituted. For example, the US Food and Drug Administration (FDA) recommends all healthcare providers to routinely screen all patients for HBV infection prior to the initiation of chemotherapy or immunosuppressive therapy. A study by Hwang found that case identification was substantially improved through universal screening rather compared to the usual practice (Hwang et al. 2012). While it would detect the most number of patients with HBV infection and optimize patient management, this approach is not widely practiced because most oncologists do not perceive the benefit of universal screening to be large enough to justify the efforts and expenses needed, particularly in low HBV prevalence settings such as the US general practice. Presumably, if HBV prevalence is high enough, universal screening may prove to be cost-effective. To date, available cost-effectiveness analyses suggested that universal screening with HBsAg and anti-HBc is not cost-effective in palliative or adjuvant setting for solid tumors but it is cost-saving for lymphoma patients undergoing chemotherapy with a rituximab-containing regimen (Day et al. 2011; Zurawska et al. 2012).
An alternate strategy in screening for HBR prophylaxis candidates is to stratify individual patients according to their risk of HBR. The recommendations by AGA in Table 16.3 utilize such a risk stratification scheme. Based on the immunosuppressive regimen and the serologic profile (see Table 16.1), the patient may be classified as high, moderate, and low risk. High- and moderate-risk patients should receive HBV screening, whereas screening may be reserved for certain low-risk patients who meet the screening criteria recommended for the general population according to the CDC and the US Preventive Service Task Force. Patients are screened for HBsAg and anti-HBc, followed by HBV DNA, if either is positive. The advantage of this risk stratification strategy is that it is more likely to be cost-effective than universal screening and reduce the potential harm of false-positive results. However, it is limited by the complexity in its application. In clinical scenarios where care is being planned for a cancer patient requiring chemotherapy or a patient with an immunological disorder in need of immunosuppressive therapy, applying the HBR risk rules may not be considered high priority, and screening may not be performed at all.
Finally, the guideline addresses the utility of anti-HBs in the management of HBR. It is often believed that the presence of anti-HBs makes it less likely that the patient will experience HBR. However, HBR may occur despite anti-HBs, particularly in patients undergoing the deepest level of immunosuppression (e.g., HSCT), in whom HBR may occur in conjunction with HBs seroreversion. The guideline recommends against using anti-HBs status in determining the need for antiviral prophylaxis regardless of the risk level.
7.2 Antiviral Prophylaxis Algorithm
The next set of questions in the management of patients at risk of HBR addresses (1) who are candidates for antiviral prophylaxis and (2) what antiviral regimen should be used. Figure 16.6 represents an algorithm that we propose, based on the AGA guideline for the management of patients undergoing immunosuppressive therapy to optimize their outcomes with regard to HBR. Once a decision is made how screening is performed (universal versus risk-stratified), the patient will undergo testing for a minimum of HBsAg and anti-HBc. Depending on the patient’s risk profile, additional testing for hepatitis C, human immunodeficiency virus or hepatitis D (if HBsAg is positive) may also be considered.
In a patient who is negative for both HBsAg and anti-HBc, there is no need for antiviral prophylaxis. If, however, the patient is either HBsAg+ or anti-HBc+, the risk of HBR needs to be assessed. If the patient meets the high-risk criteria (Table 16.1), prophylactic antiviral is indicated, whereas in a patient who is at low risk, prophylaxis is not recommended. In patients who are at moderate risk, the guideline expresses preference for antiviral prophylaxis. However, the evidence to support the recommendation is not very robust, and an alternate approach may be to monitor HBV DNA levels for early detection and prompt treatment for HBR. However, there is no consensus about optimal ways to monitor for HBR both during and after cessation of immunosuppressive therapy, although some have suggested a monitoring interval of 3 months (Hwang and Lok 2014). In our opinion, upfront institution of antiviral prophylaxis obviates the cost and inconvenience of repeated HBV DNA testing, especially if the antiviral therapy can be delivered inexpensively. We do concur with the AGA guideline which recommended that in patients who place a higher value on avoiding any long-term use of antiviral therapy and costs associated with its use and consider avoiding the small risk of reactivation less important, it may be reasonable to choose no prophylaxis over antiviral prophylaxis, particularly if HBsAg is negative.
With regard to the choice of prophylactic antiviral agent, lamivudine has been most widely studied (Yeo et al. 2004b, c; Lau et al. 2003; Hsu et al. 2008; Jang et al. 2006; Loomba et al. 2008; Ahmed and Keeffe 1999; Kohrt et al. 2006; Li et al. 2006; Rossi et al. 2001; Nagamatsu et al. 2004; Dai et al. 2004). Those studies showed that lamivudine improved outcome of patients with respect to HBR, including the occurrence of HBR, the need to delay or interrupt chemotherapy, and ultimately the outcome of the cancer therapy. While lamivudine was effective in proving the concept of antiviral prophylaxis of HBR, it has fallen out of favor in the treatment of chronic HBV infection in general, due in part to its susceptibility to viral mutations that negate its efficacy and lower potency compared to more modern agents. The AGA guideline prefers a third-generation NA over lamivudine for HBR prophylaxis. A randomized controlled trial showed the superiority of entecavir over lamivudine in decreasing the risk of HBR, hepatitis B flare, and interruption of immunosuppressive therapy (Huang et al. 2013). There are other studies with a similar conclusion, although the quality of those studies is not as robust.
A counterargument in favor of lamivudine is that most patients receiving antiviral prophylaxis have low or undetectable levels of HBV DNA at baseline and lamivudine failure is expected to be infrequent. The AGA guideline acknowledges this trade-off: it suggests that in patients who put a higher value on cost of antiviral therapy and a lower value on avoiding the potentially small risk of resistance development, it may be reasonable to select the least expensive anti-HBV medication over more expensive antiviral drugs with a higher barrier to resistance. In patients with undetectable viral load with expected duration of prophylaxis for 6 months or less, lamivudine may be acceptable.
Data are sparse as to the optimal timing of the initiation and discontinuation of antiviral prophylaxis. In our practice, we try to start HBR prophylaxis as soon as the need is determined. For patients with low or undetectable viremia, prophylaxis initiation concurrent to immunosuppressive therapy would be sufficient. In patients with higher levels of HBV DNA, we expect it to be advantageous if HBR prophylaxis can precede the onset of immunosuppression. However, immunosuppressive therapy, especially cancer chemotherapy, should not be delayed on account of achieving viral suppression. With regard to the duration of therapy, the AGA guideline recommends the prophylaxis to continue for at least 6 months after discontinuation of immunosuppressive therapy, with the exception of patients receiving B-cell-depleting agents, in whom prophylaxis is extended to 12 months .
7.3 Treatment of Established HBR
Any abnormalities in liver test of patients undergoing immunosuppressive therapy or chemotherapy need to be carefully investigated. It is necessary to differentiate HBR from various potential causes including infections from other hepatitis virus (A, C, D, and E), opportunistic pathogens (e.g., cytomegalovirus), drug-induced liver injury, or other causes (e.g., graft-versus-host disease). In patients who have been screened for HBV infection and deemed to be at moderate risk for HBR and elect to be monitored without prophylactic antiviral therapy, HBR may be diagnosed early by rising HBV DNA levels before biochemical or clinical evidence of hepatitis activities emerges. Whether employing the so-called on-demand rescue therapy in that setting is inferior to upfront prophylaxis remains uncertain, and the AGA guideline makes no recommendation about such a strategy. In patients who were not screened initially and develop active hepatitis B, HBR may be misdiagnosed as acute HBV infection since anti-HBc IgM may be detected in severe hepatitis B flare (Law et al. 2016).
Once the diagnosis of HBR is established, the treatment goal is to prevent severe hepatitis and hepatic failure. This may be achieved by (1) effective and expeditious viral control and (2) monitoring and supportive treatment for hepatic insufficiency. To achieve viral control, potent oral NAs must be initiated as soon as possible, although high-quality evidence demonstrating the efficacy of antiviral therapy in reducing morbidity and mortality in patients with HBR is lacking (Liao et al. 2002). Delay in institution of antiviral therapy may lead to hepatic failure, liver transplantation, and death, and interferon-based therapy is inappropriate in this setting (Lok et al. 1991; Lau et al. 2003; Jindal et al. 2013; Hsu et al. 2014).
With regard to the choice of antiviral agents, there have been no randomized studies of the clinical effectiveness comparing third-generation NAs with earlier generation agents. In part based on data in immunocompetent patients, the AGA guideline recommends entecavir or tenofovir in this setting (Perrillo et al. 2015). There are little data to define the optimal duration of therapy—it may take patients with established HBR longer to bring HBV replication under control compared to patients with low viral burden undergoing antiviral prophylaxis (Hwang and Lok 2014). In patients with satisfactory viral control, we believe it would be reasonable to apply the same rule for therapy discontinuation as that for prophylaxis. In most patients, antiviral therapy should be continued for at least 6 months after discontinuation of immunosuppressive therapy. In population treated with a B-cell-depleting regimen, consideration should be given to continue the therapy for at least 12 months after discontinuation of immunosuppressant.
In patients whose HBR progresses to symptomatic hepatitis and develop signs of hepatic insufficiency, rapid cessation of ongoing necro-inflammation and loss of functioning hepatocyte mass is even more important. While the definitive treatment for liver failure would be liver transplantation, rarely patients with HBR are candidates for liver transplantation because of their underlying disease (Noterdaeme et al. 2011). However, we believe that these patients should be cared for by a team of healthcare providers with hepatology expertise to maximize support and afford a chance for recovery.
7.4 Management of Transplant Recipients
In addition to being subjected to immunosuppression, organ transplant recipients may develop HBR as a result of transmission of donor-derived HBV. The risk of HBV transmission is highest in liver transplantation, since hepatocytes are the primary site of HBV infection. However, recipients of other organs may also be at risk for HBV infection. Several management guidelines have been published in order to enhance the quality of care and improve the efficiency of HBR prevention in transplanted patients (Tomblyn et al. 2009; Kasiske et al. 2010). Care of liver transplantation patients is discussed elsewhere.
In general, both donors and recipients should be tested for HBsAg, anti-HBs, and anti-HBc. If the recipient is either HBsAg+ or anti-HBc+, he/she should be tested for HBV DNA. Non-HSCT candidates who are HBsAg+ or HBV DNA+ should receive antiviral prophylaxis. Whenever possible, HBsAg- candidates should be immunized against HBV and the response to vaccination be confirmed. Transplant recipients who are HBsAg−/anti-HBc+ may be managed in a similar fashion as immunosuppressed patients at moderate risk—they may be given antiviral prophylaxis or monitored for HBV DNA level for early detection of HBR followed by preemptive treatment. All candidates with evidence of active HBV DNA replication (either HBsAg+ or detectable HBV DNA) should be evaluated for the degree of liver fibrosis, preferably by a liver biopsy, prior to the transplantation, since advanced fibrosis/cirrhosis can increase treatment-related morbidity and mortality.
A special consideration is given for HSCT patients. HSCT candidates should be immunized prior to chemotherapy with the initial two doses given 3–4 weeks apart, followed by the third dose 6 months later. If this schedule cannot be met, the third dose may be administered a few months after completion of chemotherapy. If anti-HBs titer after vaccination is <10 IU/L or pre-transplant vaccination is impractical, hepatitis B immune globulins (HBIg) at a dose of 0.06 ml/kg should be administered immediately prior to infusion of stem cells.
HSCT donors with detectable HBV DNA should be treated with antivirals for at least 4 weeks or until HBV DNA becomes undetectable. The cell volume from HBsAg+ and/or anti-HBc+ should be minimized and all cell products be tested for HBV DNA at the time of harvest. If HBV DNA is detectable at harvest either in the donor or harvested cells, the recipients should receive antiviral prophylaxis and optionally HBIg for 4 weeks after transplantation. If HBV is undetectable in the donor and harvested cells, recipients may be monitored with monthly ALT for the first 6 months. If ALT increases, HBV DNA and HBsAg should be tested. If there is detectable HBV DNA or HBsAg+, preemptive therapy is needed (Tomblyn et al. 2009).
With regard to kidney transplantation, a kidney from an HBsAg+ donor should not be transplanted into an HBsAg- recipient as there is a significantly high risk for HBV transmission. A kidney transplant from a donor with isolated anti-HBc+ possesses a relatively low risk of transmission and may be considered for candidates with anti-HBs+ (Abrao et al. 2014).
According to the 2009 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline on the monitoring, management, and treatment of kidney transplant recipients, if KT candidates have postvaccination anti-HBs titer <10 IU/L, booster vaccination should be administered in an attempt to raise the titer to ≥100 IU/L. In those with ongoing HBV infection, adequate suppression of HBV must precede the transplant. Prior to the availability of oral antiviral agents, recipients with HBsAg+ had 2.5-fold increased risk of death and 1.4-fold increased risk of allograft loss compared to HBsAg- recipients (Fabrizi et al. 2005). More recent data in the era of oral antiviral agents indicate improved survival of KT recipients with HBV infection. Five-year survival rates of KT recipients with and without HBV infection were 85% versus 86%, respectively. Graft survival was also similar approximately at 75% (Reddy et al. 2011). These support KDIGO recommendations that recipients with HBsAg+ must be given antiviral therapy. For recipients with isolated anti-HBc+, there is insufficient evidence to recommend routine antiviral prophylaxis. However, we suggest to follow AGA guideline to judge the need for prophylactic treatment. Regarding the choice of antivirals, entecavir is a preferable choice for KT recipients due to its high barrier of resistance and non-nephrotoxic property. Interferon should be avoided as it is associated with the increased risk of acute rejection (Kasiske et al. 2010).
HBsAg-positive KTx recipients receiving prophylaxis must be monitored closely, although the optimal monitoring strategy to detect HBR remains to be defined. For patient with high viral load prior to the prophylactic treatment, HBV DNA may be checked every 1–3 months until it becomes undetectable before initiation of immunosuppression. During the period of immunosuppression, HBV DNA should be checked every 3–6 months to ensure viral suppression. For those who are not on prophylactic antiviral therapy, ALT may be checked every other week for the first 16 weeks then every 3–4 weeks for the first years. HBV DNA should be monitored every month for the first year. If there is an increase in ALT level, evaluation of HBR should be performed, and preemptive therapy is needed when HBR is established (Chan and Lok 2016).
8 Emerging Trend: HBV Reactivation After Successful Treatment of Hepatitis C
HBV/HCV dual infection is not uncommon especially in the endemic areas of HBV and among high-risk population as these two viruses share similar routes of transmission. The prevalence of dual infection with HBV has been reported from 5% to 20% of individuals with HCV infection (Chu and Lee 2008). In addition, occult HBV infection, defined by the presence of HBV DNA in the absence of HBsAg, may be found in 12–44% of HCV-infected patients (Fukuda et al. 1999). As commonly seen in patients with infection with multiple hepatotropic viruses, in HBV/HCV dual infection, one of the viruses predominates (as measured by the viral load), which tends to be HCV.
Direct-acting antivirals against HCV available today afford high rates of cure. Recently, the US FDA has warned of the risk of HBR in patients with HBV/HCV dual infection treated with DAAs. Although HBR has been previously reported with interferon and ribavirin, HBR may occur much more rapidly during DAA treatment. The mechanism behind HBR after DAA therapy remains unclear. In experimental conditions, HBV and HCV could replicate in the same hepatocyte without evidence of interference, suggesting that HCV may suppress HBV replication via an indirect mechanism (Yang et al. 2014)—relatively abrupt elimination of HCV from hepatocytes would disinhibit HBV replication. It has also been postulated that the clearance of HCV may mitigate the immune control (e.g., downregulation of previously overexpressed IFN-stimulated genes).
To date, there have been 24 cases with HBR identified in the FDA Adverse Event Reporting System (FAERS ) database and/or reported in the published medical literature. HBR has been reported in various DAA regimens including simeprevir and sofosbuvir (Collins et al. 2015), daclatasvir and asunaprevir (Hayashi et al. 2016; Takayama et al. 2016), and ledipasvir and sofosbuvir (De Monte et al. 2016). As expected, HBR has been reported more commonly in patients with HBsAg-positive dual infection compared to those with isolated anti-HBc positivity. In an observational study in Chinese patients treated with DAAs, 3/10 HBsAg+ patients developed HBR, compared to none of 124 who were HBsAg negative and anti-HBc positive. In most cases, HBR occurred within 4–8 weeks after starting DAA therapy. These patients were heterogeneous in terms of HCV genotype and baseline HBV status. The severity of HBR in those patients varied from no symptoms to severe hepatic failure or death (U.S. Food and Drug Administration 2016).
There is insufficient data based on which to incorporate HBV/HCV dual infection patients into our management algorithm (Fig. 16.6). Table 16.5 describes our proposed approach to these patients. Patients undergoing DAA therapy must be screened for HBsAg, anti-HBc, and anti-HBs, followed by HBV DNA in patients who are either HBsAg negative or anti-HBc positive. We propose that anti-HBV prophylaxis be started concurrent with the DAA therapy in patients who are HBsAg positive or HBV DNA detectable, whereas in patients who are anti-HBc positive and HBV DNA undetectable, prophylaxis may not be necessary. In the former category of patients, it is important to determine whether the patient would have been a candidate for HBV therapy regardless of the HCV dual infection and define the HBV treatment endpoint, such as HBe seroconversion. We believe it is also important to assess their liver fibrosis status, which is commonly performed in preparation of the HCV therapy. For patients with isolated anti-HBc without detectable HBV DNA, monitoring for a rise in HBV DNA may be reasonable, perhaps at week 4 of therapy, as patients are often tested for HCV RNA at the same time.
There is no data available to inform the optimal duration of HBV therapy in dual infection patients being treated for HCV. To the degree that there may be host immunological shift that underlie the development of HBR, in our practice, we continue the prophylaxis for 3 months after discontinuation of DAA. These patients are monitored for another 3 months to ensure absence of HBR off anti-HBV prophylaxis. Obviously, in patients determined to be candidates for HBV therapy independent of HCV, therapy should be continued until the planned endpoint is met. Care must be taken in discontinuing anti-HBV prophylaxis in patients with cirrhosis, which may precipitate hepatic decompensation.
9 Current Challenges and Future Directions
HBR leading to a poor patient outcome such as liver failure or disruption of cancer chemotherapy represents an unnecessary clinical tragedy, which is eminently preventable by appropriate screening and prophylaxis. Despite a multitude of guidelines to inform clinicians caring for patients undergoing cancer treatment, transplantation, and immunomodulatory therapy, HBR continues to occur (Patel et al. 2016; Yuen 2016). Survey studies conducted in practicing physicians indicate that adherence to routine HBV screening prior to the immunosuppressive therapy is unacceptably low—approximately 20–40% of oncologists, 40% of dermatologists, and 70% of rheumatologists follow a guideline in some fashion (Hwang et al. 2012; Stine et al. 2011, 2010; Tran et al. 2010; Kawsar et al. 2012).
This problem may be partly attributable to the inconsistency among the guidelines. Clearly, multi-society collaboration to develop a broadly applicable consensus is an essential step. Secondarily, efforts to disseminate the consensus guideline to all practitioners are needed. For healthcare providers that are not routinely involved in the care of patients at risk of HBR, electronic mechanisms may be helpful to alert them of candidates for screening and to guide them to initiate appropriate prophylaxis. Such a proactive measure may be even more important in the future, as increasingly more complex and potent immunosuppressive and chemotherapeutic regiments are being developed.
Finally, as investigators strive toward gaining more biological insight and immunopathogenetical knowledge of HBV infection, deeper understanding of the basic mechanisms of HBV reactivation may help better inform clinical decisions for HBR. This is particularly true of the HBV/HCV dual infection cases. In addition, the effect of new therapeutic agents that interact with the immune system in a nonconventional manner on the occurrence and course of HBR remains to be studied. Finally, as new diagnostic biomarkers and therapeutic agents are being actively developed for the goal of “cure” of HBV, additional tools may become available to provide more accurate risk stratification and then inactivate, if not cure, HBV in a sustainable fashion in patients undergoing increasingly sophisticated regimens that have a diverse effect on the immune system.
References
Abramson JS, Chung RT. Optimal antiviral prophylaxis against hepatitis B reactivation in patients receiving rituximab-based chemotherapy for lymphoma. JAMA. 2014;312(23):2505–7.
Abrao JM, Carvalho MF, Garcia PD, Contti MM, Andrade LG. Safety of kidney transplantation using anti-HBc-positive donors. Transplant Proc. 2014;46(10):3408–11.
Ahmed A, Keeffe EB. Lamivudine therapy for chemotherapy-induced reactivation of hepatitis B virus infection. Am J Gastroenterol. 1999;94(1):249–51.
American Association for the Study of Liver Diseases Emergin Trends Conference. Reactivation of Hepatitis B. March 21–22, 2013; Arlington, VA, 2013.
Baden LR, Bensinger W, Angarone M, Casper C, Dubberke ER, Freifeld AG, et al. Prevention and treatment of cancer-related infections. J Natl Compr Cancer Netw. 2012;10(11):1412–45.
Calabrese LH, Zein NN, Vassilopoulos D. Hepatitis B virus (HBV) reactivation with immunosuppressive therapy in rheumatic diseases: assessment and preventive strategies. Ann Rheum Dis. 2006;65(8):983–9.
Carroll MB, Forgione MA. Use of tumor necrosis factor alpha inhibitors in hepatitis B surface antigen-positive patients: a literature review and potential mechanisms of action. Clin Rheumatol. 2010;29(9):1021–9.
Chan TM, Lok AS. Hepatitis B virus infection in renal transplant recipients. Accessed from https://www.uptodate.com/contents/hepatitis-b-virus-infection-in-renal-transplant-recipients on 6 Nov. 2016.
Chang JJ, Lewin SR. Immunopathogenesis of hepatitis B virus infection. Immunol Cell Biol. 2007;85(1):16–23.
Chen GD, Gu JL, Qiu J, Chen LZ. Outcomes and risk factors for hepatitis B virus (HBV) reactivation after kidney transplantation in occult HBV carriers. Transpl Infect Dis. 2013;15(3):300–5.
Cheng AL, Hsiung CA, Su IJ, Chen PJ, Chang MC, Tsao CJ, et al. Steroid-free chemotherapy decreases risk of hepatitis B virus (HBV) reactivation in HBV-carriers with lymphoma. Hepatology. 2003;37(6):1320–8.
Chu CJ, Lee SD. Hepatitis B virus/hepatitis C virus coinfection: epidemiology, clinical features, viral interactions and treatment. J Gastroenterol Hepatol. 2008;23(4):512–20.
Chung YL, Tsai TY. Promyelocytic leukemia nuclear bodies link the DNA damage repair pathway with hepatitis B virus replication: implications for hepatitis B virus exacerbation during chemotherapy and radiotherapy. Mol Cancer Res. 2009;7(10):1672–85.
Cingoz O. Ustekinumab MAbs. 2009;1(3):216–21.
Collins JM, Raphael KL, Terry C, Cartwright EJ, Pillai A, Anania FA, et al. Hepatitis B virus reactivation during successful treatment of hepatitis C virus with Sofosbuvir and Simeprevir. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;61(8):1304–6.
Dai MS, Wu PF, Lu JJ, Shyu RY, Chao TY. Preemptive use of lamivudine in breast cancer patients carrying hepatitis B virus undergoing cytotoxic chemotherapy: a longitudinal study. Support Care Cancer. 2004;12(3):191–6.
Day FL, Karnon J, Rischin D. Cost-effectiveness of universal hepatitis B virus screening in patients beginning chemotherapy for solid tumors. J Clin Oncol. 2011;29(24):3270–7.
De Monte A, Courjon J, Anty R, Cua E, Naqvi A, Mondain V, et al. Direct-acting antiviral treatment in adults infected with hepatitis C virus: reactivation of hepatitis B virus coinfection as a further challenge. J Clin Virol. 2016;78:27–30.
Degos F, Lugassy C, Degott C, Debure A, Carnot F, Theirs V, et al. Hepatitis B virus and hepatitis B-related viral infection in renal transplant recipients. A prospective study of 90 patients. Gastroenterology. 1988;94(1):151–6.
Di Bisceglie AM, Lok AS, Martin P, Terrault N, Perrillo RP, Hoofnagle JH. Recent US Food and Drug Administration warnings on hepatitis B reactivation with immune-suppressing and anticancer drugs: just the tip of the iceberg? Hepatology. 2015;61(2):703–11.
Dietz AB, Souan L, Knutson GJ, Bulur PA, Litzow MR, Vuk-Pavlovic S. Imatinib mesylate inhibits T-cell proliferation in vitro and delayed-type hypersensitivity in vivo. Blood. 2004;104(4):1094–9.
Droz N, Gilardin L, Cacoub P, Berenbaum F, Wendling D, Godeau B, et al. Kinetic profiles and management of hepatitis B virus reactivation in patients with immune-mediated inflammatory diseases. Arthritis Care Res (Hoboken). 2013;65(9):1504–14.
European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 2012;57(1):167–85.
Evens AM, Jovanovic BD, Su YC, Raisch DW, Ganger D, Belknap SM, et al. Rituximab-associated hepatitis B virus (HBV) reactivation in lymphoproliferative diseases: meta-analysis and examination of FDA safety reports. Ann Oncol. 2011;22(5):1170–80.
Fabrizi F, Martin P, Dixit V, Kanwal F, Dulai G. HBsAg seropositive status and survival after renal transplantation: meta-analysis of observational studies. Am J Transplant. 2005;5(12):2913–21.
Flowers MA, Heathcote J, Wanless IR, Sherman M, Reynolds WJ, Cameron RG, et al. Fulminant hepatitis as a consequence of reactivation of hepatitis B virus infection after discontinuation of low-dose methotrexate therapy. Ann Intern Med. 1990;112(5):381–2.
Fornairon S, Pol S, Legendre C, Carnot F, Mamzer-Bruneel MF, Brechot C, et al. The long-term virologic and pathologic impact of renal transplantation on chronic hepatitis B virus infection. Transplantation. 1996;62(2):297–9.
Fukuda R, Ishimura N, Niigaki M, Hamamoto S, Satoh S, Tanaka S, et al. Serologically silent hepatitis B virus coinfection in patients with hepatitis C virus-associated chronic liver disease: clinical and virological significance. J Med Virol. 1999;58(3):201–7.
Hammond SP, Borchelt AM, Ukomadu C, Ho VT, Baden LR, Marty FM. Hepatitis B virus reactivation following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15(9):1049–59.
Hayashi K, Ishigami M, Ishizu Y, Kuzuya T, Honda T, Nishimura D, et al. A case of acute hepatitis B in a chronic hepatitis C patient after daclatasvir and asunaprevir combination therapy: hepatitis B virus reactivation or acute self-limited hepatitis? Clin J Gastroenterol. 2016;9(4):252–6.
Herrero-Beaumont G, Martinez Calatrava MJ, Castaneda S. Abatacept mechanism of action: concordance with its clinical profile. Reumatol Clin. 2012;8(2):78–83.
Hoofnagle JH. Reactivation of hepatitis B. Hepatology. 2009;49(5 Suppl):S156–65.
Hsu C, Hsiung CA, Su IJ, Hwang WS, Wang MC, Lin SF, et al. A revisit of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in non-Hodgkin’s lymphoma: a randomized trial. Hepatology. 2008;47(3):844–53.
Hsu C, Tsou HH, Lin SJ, Wang MC, Yao M, Hwang WL, et al. Chemotherapy-induced hepatitis B reactivation in lymphoma patients with resolved HBV infection: a prospective study. Hepatology. 2014;59(6):2092–100.
Huang YH, Hsiao LT, Hong YC, Chiou TJ, Yu YB, Gau JP, et al. Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol. 2013;31(22):2765–72.
Hutchinson M. Natalizumab: a new treatment for relapsing remitting multiple sclerosis. Ther Clin Risk Manag. 2007;3(2):259–68.
Hwang JP, Lok AS. Management of patients with hepatitis B who require immunosuppressive therapy. Nat Rev Gastroenterol Hepatol. 2014;11(4):209–19.
Hwang JP, Fisch MJ, Zhang H, Kallen MA, Routbort MJ, Lal LS, et al. Low rates of hepatitis B virus screening at the onset of chemotherapy. J Oncol Pract. 2012;8(4):e32–9.
Hwang JP, Somerfield MR, Alston-Johnson DE, Cryer DR, Feld JJ, Kramer BS, et al. Hepatitis B virus screening for patients with cancer before therapy: American Society of Clinical Oncology provisional clinical opinion update. J Clin Oncol. 2015;33(19):2212–20.
Jang JW, Choi JY, Bae SH, Kim CW, Yoon SK, Cho SH, et al. Transarterial chemo-lipiodolization can reactivate hepatitis B virus replication in patients with hepatocellular carcinoma. J Hepatol. 2004;41(3):427–35.
Jang JW, Choi JY, Bae SH, Yoon SK, Chang UI, Kim CW, et al. A randomized controlled study of preemptive lamivudine in patients receiving transarterial chemo-lipiodolization. Hepatology. 2006;43(2):233–40.
Jindal A, Kumar M, Sarin SK. Management of acute hepatitis B and reactivation of hepatitis B. Liver Int. 2013;33(Suppl 1):164–75.
Kanaan N, Kabamba B, Marechal C, Pirson Y, Beguin C, Goffin E, et al. Significant rate of hepatitis B reactivation following kidney transplantation in patients with resolved infection. J Clin Virol. 2012;55(3):233–8.
Kasahara S, Ando K, Saito K, Sekikawa K, Ito H, Ishikawa T, et al. Lack of tumor necrosis factor alpha induces impaired proliferation of hepatitis B virus-specific cytotoxic T lymphocytes. J Virol. 2003;77(4):2469–76.
Kasiske BL, Zeier MG, Chapman JR, Craig JC, Ekberg H, Garvey CA, et al. KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int. 2010;77(4):299–311.
Kawsar HI, Shahnewaz J, Gopalakrishna KV, Spiro TP, Daw HA. Hepatitis B reactivation in cancer patients: role of prechemotherapy screening and antiviral prophylaxis. Clin Adv Hematol Oncol. 2012;10(6):370–8.
Keam B, Lee JH, Im SA, Yoon JH. Why, when, and how to prevent hepatitis B virus reactivation in cancer patients undergoing chemotherapy. J Natl Compr Cancer Netw. 2011;9(5):465–77.
Kim MK, Ahn JH, Kim SB, Im YS, Lee SI, Ahn SH, et al. Hepatitis B reactivation during adjuvant anthracycline-based chemotherapy in patients with breast cancer: a single institution’s experience. Korean J Intern Med. 2007;22(4):237–43.
Kim TW, Kim MN, Kwon JW, Kim KM, Kim SH, Kim W, et al. Risk of hepatitis B virus reactivation in patients with asthma or chronic obstructive pulmonary disease treated with corticosteroids. Respirology. 2010;15(7):1092–7.
Knoll A, Boehm S, Hahn J, Holler E, Jilg W. Reactivation of resolved hepatitis B virus infection after allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant. 2004;33(9):925–9.
Kohrt HE, Ouyang DL, Keeffe EB. Systematic review: lamivudine prophylaxis for chemotherapy-induced reactivation of chronic hepatitis B virus infection. Aliment Pharmacol Ther. 2006;24(7):1003–16.
Kumagai K, Takagi T, Nakamura S, Sawada U, Kura Y, Kodama F, et al. Hepatitis B virus carriers in the treatment of malignant lymphoma: an epidemiological study in Japan. Ann Oncol. 1997;8(Suppl 1):107–9.
Kusumoto S, Tanaka Y, Mizokami M, Ueda R. Reactivation of hepatitis B virus following systemic chemotherapy for malignant lymphoma. Int J Hematol. 2009;90(1):13–23.
Lalazar G, Rund D, Shouval D. Screening, prevention and treatment of viral hepatitis B reactivation in patients with haematological malignancies. Br J Haematol. 2007;136(5):699–712.
Lau GK, Liang R, Chiu EK, Lee CK, Lam SK. Hepatic events after bone marrow transplantation in patients with hepatitis B infection: a case controlled study. Bone Marrow Transplant. 1997;19(8):795–9.
Lau GK, Leung YH, Fong DY, Au WY, Kwong YL, Lie A, et al. High hepatitis B virus (HBV) DNA viral load as the most important risk factor for HBV reactivation in patients positive for HBV surface antigen undergoing autologous hematopoietic cell transplantation. Blood. 2002;99(7):2324–30.
Lau GK, Yiu HH, Fong DY, Cheng HC, Au WY, Lai LS, et al. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology. 2003;125(6):1742–9.
Law MF, Ho R, Cheung CK, Tam LH, Ma K, So KC, et al. Prevention and management of hepatitis B virus reactivation in patients with hematological malignancies treated with anticancer therapy. World J Gastroenterol. 2016;22(28):6484–500.
LeFevre ML. USPSTF. Screening for hepatitis B virus infection in nonpregnant adolescents and adults: U.S. preventive services task force recommendation statement. Ann Intern Med. 2014;161(1):58–66.
Li YH, He YF, Jiang WQ, Wang FH, Lin XB, Zhang L, et al. Lamivudine prophylaxis reduces the incidence and severity of hepatitis in hepatitis B virus carriers who receive chemotherapy for lymphoma. Cancer. 2006;106(6):1320–5.
Liao CA, Lee CM, Wu HC, Wang MC, Lu SN, Eng HL. Lamivudine for the treatment of hepatitis B virus reactivation following chemotherapy for non-Hodgkin’s lymphoma. Br J Haematol. 2002;116(1):166–9.
Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661–2.
Lok AS, Liang RH, Chiu EK, Wong KL, Chan TK, Todd D. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy. Report of a prospective study. Gastroenterology. 1991;100(1):182–8.
Long M, Jia W, Li S, Jin L, Wu J, Rao N, et al. A single-center, prospective and randomized controlled study: can the prophylactic use of lamivudine prevent hepatitis B virus reactivation in hepatitis B s-antigen seropositive breast cancer patients during chemotherapy? Breast Cancer Res Treat. 2011;127(3):705–12.
Loomba R, Rowley A, Wesley R, Liang TJ, Hoofnagle JH, Pucino F, et al. Systematic review: the effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med. 2008;148(7):519–28.
Martin BA, Rowe JM, Kouides PA, DiPersio JF. Hepatitis B reactivation following allogeneic bone marrow transplantation: case report and review of the literature. Bone Marrow Transplant. 1995;15(1):145–8.
Mendez-Navarro J, Corey KE, Zheng H, Barlow LL, Jang JY, Lin W, et al. Hepatitis B screening, prophylaxis and re-activation in the era of rituximab-based chemotherapy. Liver Int. 2011;31(3):330–9.
Misumi I, Whitmire JK. B cell depletion curtails CD4+ T cell memory and reduces protection against disseminating virus infection. J Immunol. 2014;192(4):1597–608.
Mozessohn L, Chan KK, Feld JJ, Hicks LK. Hepatitis B reactivation in HBsAg-negative/HBcAb-positive patients receiving rituximab for lymphoma: a meta-analysis. J Viral Hepat. 2015;22(10):842–9.
Nagamatsu H, Itano S, Nagaoka S, Akiyoshi J, Matsugaki S, Kurogi J, et al. Prophylactic lamivudine administration prevents exacerbation of liver damage in HBe antigen positive patients with hepatocellular carcinoma undergoing transhepatic arterial infusion chemotherapy. Am J Gastroenterol. 2004;99(12):2369–75.
Noterdaeme T, Longree L, Bataille C, Deroover A, Lamproye A, Delwaide J, et al. Liver transplantation for acute hepatic failure due to chemotherapy-induced HBV reactivation in lymphoma patients. World J Gastroenterol. 2011;17(25):3069–72.
Onozawa M, Hashino S, Izumiyama K, Kahata K, Chuma M, Mori A, et al. Progressive disappearance of anti-hepatitis B surface antigen antibody and reverse seroconversion after allogeneic hematopoietic stem cell transplantation in patients with previous hepatitis B virus infection. Transplantation. 2005;79(5):616–9.
Park S, Kim K, Kim DH, Jang JH, Kim SJ, Kim WS, et al. Changes of hepatitis B virus serologic status after allogeneic hematopoietic stem cell transplantation and impact of donor immunity on hepatitis B virus. Biol Blood Marrow Transplant. 2011;17(11):1630–7.
Patel A, Yapali S, Lok AS. Admissions for hepatitis B reactivation in patients receiving immunosuppressive therapy remain unchanged from 1999 to 2014. Hepatol Int. 2016;10(1):139–46.
Pei SN, Ma MC, Wang MC, Kuo CY, Rau KM, Su CY, et al. Analysis of hepatitis B surface antibody titers in B cell lymphoma patients after rituximab therapy. Ann Hematol. 2012;91(7):1007–12.
Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148(1):221–44. e3
Ramos CA, Saliba RM, de Padua SL, Khorshid O, Shpall EJ, Giralt S, et al. Resolved hepatitis B virus infection is not associated with worse outcome after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16(5):686–94.
Reddy PN, Sampaio MS, Kuo HT, Martin P, Bunnapradist S. Impact of pre-existing hepatitis B infection on the outcomes of kidney transplant recipients in the United States. Clin J Am Soc Nephrol. 2011;6(6):1481–7.
Reddy KR, Beavers KL, Hammond SP, Lim JK, Falck-Ytter YT, American Gastroenterological Association I. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148(1):215–9. quiz e16-7
Rehermann B, Ferrari C, Pasquinelli C, Chisari FV. The hepatitis B virus persists for decades after patients’ recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med. 1996;2(10):1104–8.
Rezaie A. Vedolizumab, a gut-specific monoclonal antibody, renews hope for an alternative to anti-TNF therapy in inflammatory bowel diseases. Ann Gastroenterol. 2014;27(2):179–80.
Rossi G, Pelizzari A, Motta M, Puoti M. Primary prophylaxis with lamivudine of hepatitis B virus reactivation in chronic HbsAg carriers with lymphoid malignancies treated with chemotherapy. Br J Haematol. 2001;115(1):58–62.
Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98.
Seggewiss R, Lore K, Greiner E, Magnusson MK, Price DA, Douek DC, et al. Imatinib inhibits T-cell receptor-mediated T-cell proliferation and activation in a dose-dependent manner. Blood. 2005;105(6):2473–9.
Seth P, Alrajhi AA, Kagevi I, Chaudhary MA, Colcol E, Sahovic E, et al. Hepatitis B virus reactivation with clinical flare in allogeneic stem cell transplants with chronic graft-versus-host disease. Bone Marrow Transplant. 2002;30(3):189–94.
Stine JG, Khokhar OS, Charalambopoulos J, Shanmugam VK, Lewis JH. Rheumatologists’ awareness of and screening practices for hepatitis B virus infection prior to initiating immunomodulatory therapy. Arthritis Care Res (Hoboken). 2010;62(5):704–11.
Stine JG, Bass M, Ibrahim D, Khokhar OS, Lewis JH. Dermatologists’ awareness of and screening practices for hepatitis B virus infection before initiating tumor necrosis factor-alpha inhibitor therapy. South Med J. 2011;104(12):781–8.
Takayama H, Sato T, Ikeda F, Fujiki S. Reactivation of hepatitis B virus during interferon-free therapy with daclatasvir and asunaprevir in patient with hepatitis B virus/hepatitis C virus co-infection. Hepatol Res. 2016;46(5):489–91.
Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15(10):1143–238.
Tran TT, Rakoski MO, Martin P, Poordad F. Screening for hepatitis B in chemotherapy patients: survey of current oncology practices. Aliment Pharmacol Ther. 2010;31(2):240–6.
Tsutsumi Y, Yamamoto Y, Ito S, Ohigashi H, Shiratori S, Naruse H, et al. Hepatitis B virus reactivation with a rituximab-containing regimen. World J Hepatol. 2015;7(21):2344–51.
Tur-Kaspa R, Burk RD, Shaul Y, Shafritz DA. Hepatitis B virus DNA contains a glucocorticoid-responsive element. Proc Natl Acad Sci U S A. 1986;83(6):1627–31.
Tur-Kaspa R, Shaul Y, Moore DD, Burk RD, Okret S, Poellinger L, et al. The glucocorticoid receptor recognizes a specific nucleotide sequence in hepatitis B virus DNA causing increased activity of the HBV enhancer. Virology. 1988;167(2):630–3.
Tzeng HT, Tsai HF, Chyuan IT, Liao HJ, Chen CJ, Chen PJ, et al. Tumor necrosis factor-alpha induced by hepatitis B virus core mediating the immune response for hepatitis B viral clearance in mice model. PLoS One. 2014;9(7):e103008.
U.S. Food and Drug Administration. FDA drug safety communication: FDA warns about the risk of hepatitis B reactivating in some patients treated with direct-acting antivirals for hepatitis C. Drug Safety Commun. 2016.
Vento S, Cainelli F, Longhi MS. Reactivation of replication of hepatitis B and C viruses after immunosuppressive therapy: an unresolved issue. Lancet Oncol. 2002;3(6):333–40.
Vigano M, Vener C, Lampertico P, Annaloro C, Pichoud C, Zoulim F, et al. Risk of hepatitis B surface antigen seroreversion after allogeneic hematopoietic SCT. Bone Marrow Transplant. 2011;46(1):125–31.
Weinbaum CM, Williams I, Mast EE, Wang SA, Finelli L, Wasley A, et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recommend Rep. 2008;57(RR-8):1–20.
Yang D, Zuo C, Wang X, Meng X, Xue B, Liu N, et al. Complete replication of hepatitis B virus and hepatitis C virus in a newly developed hepatoma cell line. Proc Natl Acad Sci U S A. 2014;111(13):E1264–73.
Yeo W, Chan PK, Zhong S, Ho WM, Steinberg JL, Tam JS, et al. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol. 2000a;62(3):299–307.
Yeo W, Zhong S, Chan PK, Ho WM, Wong HT, Chan AS, et al. Sequence variations of precore/core and precore promoter regions of hepatitis B virus in patients with or without viral reactivation during cytotoxic chemotherapy. J Viral Hepat. 2000b;7(6):448–58.
Yeo W, Chan PK, Hui P, Ho WM, Lam KC, Kwan WH, et al. Hepatitis B virus reactivation in breast cancer patients receiving cytotoxic chemotherapy: a prospective study. J Med Virol. 2003;70(4):553–61.
Yeo W, Zee B, Zhong S, Chan PK, Wong WL, Ho WM, et al. Comprehensive analysis of risk factors associating with hepatitis B virus (HBV) reactivation in cancer patients undergoing cytotoxic chemotherapy. Br J Cancer. 2004a;90(7):1306–11.
Yeo W, Chan PK, Ho WM, Zee B, Lam KC, Lei KI, et al. Lamivudine for the prevention of hepatitis B virus reactivation in hepatitis B s-antigen seropositive cancer patients undergoing cytotoxic chemotherapy. J Clin Oncol. 2004b;22(5):927–34.
Yeo W, Ho WM, Hui P, Chan PK, Lam KC, Lee JJ, et al. Use of lamivudine to prevent hepatitis B virus reactivation during chemotherapy in breast cancer patients. Breast Cancer Res Treat. 2004c;88(3):209–15.
Yeo W, Chan TC, Leung NW, Lam WY, Mo FK, Chu MT, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol. 2009;27(4):605–11.
Yuen MF. Need to improve awareness and management of hepatitis B reactivation in patients receiving immunosuppressive therapy. Hepatol Int. 2016;10(1):102–5.
Zhang Z, Zhang JY, Wang LF, Wang FS. Immunopathogenesis and prognostic immune markers of chronic hepatitis B virus infection. J Gastroenterol Hepatol. 2012;27(2):223–30.
Zurawska U, Hicks LK, Woo G, Bell CM, Krahn M, Chan KK, et al. Hepatitis B virus screening before chemotherapy for lymphoma: a cost-effectiveness analysis. J Clin Oncol. 2012;30(26):3167–73.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Udompap, P., Kim, W.R. (2018). Hepatitis B Virus Reactivation and Management of Patients Undergoing Immunosuppression. In: Kao, JH., Chen, DS. (eds) Hepatitis B Virus and Liver Disease. Springer, Singapore. https://doi.org/10.1007/978-981-10-4843-2_16
Download citation
DOI: https://doi.org/10.1007/978-981-10-4843-2_16
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-4842-5
Online ISBN: 978-981-10-4843-2
eBook Packages: MedicineMedicine (R0)