Abstract
The execution of proper Ca2+ signaling requires close apposition between the endoplasmic reticulum (ER) and mitochondria. Hence, Ca2+ released from the ER is “quasi-synaptically” transferred to mitochondrial matrix, where Ca2+ stimulates mitochondrial ATP synthesis by activating the tricarboxylic acid (TCA) cycle. However, when the Ca2+ transfer is excessive and sustained, mitochondrial Ca2+ overload induces apoptosis by opening the mitochondrial permeability transition pore. A large number of regulatory proteins reside at mitochondria-associated ER membranes (MAMs) to maintain the optimal distance between the organelles and to coordinate the functionality of both ER and mitochondrial Ca2+ transporters or channels. In this chapter, we discuss the different pathways involved in the regulation of ER-mitochondria Ca2+ flux and describe the activities of the various Ca2+ players based on their primary intra-organelle localization.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Endoplasmic reticulum (ER)
- Mitochondria
- Mitochondria associated membranes (MAMs)
- Calcium
- ROS
- ER-mitochondria contact sites
- Cell death
- Apoptosis
- Autophagy
4.1 Introduction
The endoplasmic reticulum (ER) and the mitochondrion in living cells are two essential organelles with roles that are classically quite distinct. Evidence has been accruing over the years that points to a specific interplay and cooperation between these compartments that is essential for several cellular functions, such as Ca2+ signaling, lipid metabolism, autophagy, inflammation, cell survival, and cell death (Decuypere et al. 2011; Lamb et al. 2013; Marchi et al. 2014; Vance 2014; Patergnani et al. 2015). These close appositions between the ER and mitochondria represent a site where microdomains with a high Ca2+ concentration ([Ca2+]) are generated upon IP3-mediated Ca2+ release (Rizzuto et al. 1998). Ca2+ accumulation in the mitochondrial matrix occurs through the mitochondrial calcium uniporter (MCU) and by the membrane potential (ΔΨ m) that exists across the inner mitochondrial membrane (IMM) (Marchi and Pinton 2014). The MCU has a low Ca2+ affinity (K d approximately 10–20 μM), and thus, the Ca2+ uptake rate, particularly under resting conditions, is extremely slow (Gunter and Gunter 2001). Based on this consideration, it was long assumed that mitochondrial Ca2+ uptake was of little importance in cell physiology. Although initial experiments suggested that mitochondrial [Ca2+] ([Ca2+]m) could increase up to ∼10 μM, subsequent research revealed that [Ca2+]m may in fact transiently reach near mM levels in different systems (Montero et al. 2000). It is the ER-mitochondria connection that allows for fast uptake of a large amount of Ca2+ inside the mitochondrial matrix, where the rapid diffusion of ions within it permits the swift tuning of mitochondrial metabolism to address the needs of the cell (Rimessi et al. 2008). ER-mitochondria Ca2+ transfer is fundamental to ensure the cellular energy supply by modulating key enzymes involved in mitochondrial ATP production (Bonora et al. 2012). However, excessive transfer of ER Ca2+ to mitochondria is a pro-apoptotic signal with important consequences for cell fate (Giorgi et al. 2012). In other words, ER-mitochondria connections are relevant for cell survival, and the maintenance of the proper spacing between the ER and mitochondria appears critical for proper cell functioning (Naon and Scorrano 2014). Tomography analysis has shown that tethers of ∼10 nm or ∼25 nm adjoin the two organelles, depending on whether smooth ER and/or rough ER are implicated (Csordas et al. 2006). These sites of contact are typically termed mitochondria-associated ER membranes (MAMs) (Giorgi et al. 2015c).
The development and expression of ER-mitochondria artificial linkers revealed that the length of the tethers is strategic. Using rapamycin-inducible fluorescent inter-organelle linkers, Csordas et al. (Csordas et al. 2010) elegantly demonstrated that the spatial relationship between the ER and the outer mitochondrial membrane (OMM) is a critical factor in the efficient transfer of Ca2+ and is likely to affect the other functions of the junction in various ways. The distance between the ER and the OMM may vary in different pathophysiological situations. In response to apoptotic agents, the ER-mitochondria gap narrows (Csordas et al. 2006). Also, during the early phases of ER stress, the number of ER-mitochondria contacts increases and their Ca2+ transfer is enhanced, helping the cell to overcome this emergency state through the modulation of key mitochondrial metabolic events (Bravo et al. 2011, 2012). These observations reflect the extremely dynamic nature of MAMs, which is now generally assumed to be a strategic intracellular platform that employs Ca2+ flux to regulate a wide range of biological processes (Naon and Scorrano 2014). For example, ER-mitochondria connections are essential for modulating mitochondrial fission. In the close appositions between the ER and mitochondria, organelle constriction occurs via a Ca2+-dependent mechanism that involves recruitment of the cytosolic dynamin-related protein 1 (Drp1) and the mitochondria-associated membrane protein syntaxin17 (Friedman et al. 2011; Arasaki et al. 2015). Mitochondrial fission, in turn, is an essential event that is involved in mitochondrial network shaping, and it is required to generate small organelles to be transported or to facilitate the removal of damaged organelles by a selective form of autophagy, termed mitophagy (Haroon and Vermulst 2016). Bulk autophagy directly depends on ER-mitochondria juxtaposition. Not only does the ER membrane supply material for the formation of autophagosomes (Tooze et al. 2010), but the ER-mitochondria contacts may also be the specific regions where autophagosomes start to form, due to the MAM localization of the pre-autophagosomal ER protein Atg14 (Hamasaki et al. 2013; Lamb et al. 2013).
Therefore, the correct organization, the mutual interactions between the ER and mitochondria, and their Ca2+ crosstalk are linked events aimed at coordinating important functions of the two organelles, and these events determine key aspects of cell fate. For these reasons, a huge number of proteins are gathered at the ER-mitochondria interface to regulate MAM dynamics, thereby preserving the intracellular equilibrium. Indeed, alterations in both MAM architecture and composition lead to different pathological conditions, which in many cases are accompanied by a drastic dysregulation of the intracellular Ca2+ homeostasis and dynamics (Patergnani et al. 2011). In this chapter, we will discuss the different pathways controlling Ca2+ flux from the ER to the mitochondria and their impact on the physiological state of the cell, and the activities of the various Ca2+ players will be distinguished based on their primary intra-organelle localization.
4.2 Ca2+ Signaling on the ER Side
The ER is the largest store of Ca2+ inside the cell. In resting condition, the ER may contain hundreds of μM free Ca2+ (in order of magnitude, nearly three- to fourfold higher compared to the cytosol) (Hofer and Schulz 1996; Bonora et al. 2013). Specific Ca2+ pumps and channels operate to maintain the correct luminal [Ca2+] by executing the correct balance between ER Ca2+ uptake and release (Ashby and Tepikin 2001) (Fig. 4.1).
Ca2+ homeostasis at the ER-mitochondria interface. Overview on the multiple molecular pathways acting at the ER side (See Sect. 4.2 for details)
ER Ca2+ uptake is exclusively performed by the sarco-/endoplasmic reticulum Ca2+-ATPase (SERCA) pumps, which actively pump Ca2+ into the ER in an ATP-dependent manner (Vandecaetsbeek et al. 2011). Three SERCA genes (ATP2A1, ATPA2, and ATP2A3) are present in the human genome. They generate various splice variants that differ in their C-terminal regions, and their expression is dependent on tissue type and development stage (Papp et al. 2012). Generally, SERCA1a and 1b are widely present in adult and neonatal skeletal muscle. The isoform 2a is highly expressed in cardiomyocytes, while SERCA2b is ubiquitously expressed, functioning as the housekeeping isoform. Finally, SERCA3 is the least studied and gives rise to six isoforms. Among the different SERCAs, the 2b isoform displays the highest Ca2+ affinity and, thus, is the main isoform involved in Ca2+ uptake in the ER in virtually all cells, except skeletal and cardiac muscle. Because SERCAs are the only pumps regulating ER Ca2+ entry, it is not surprising that their activity mediates a wide range of cellular functions controlled by proper ER Ca2+ homeostasis, including protein folding, lipid and steroid synthesis, and cell death and survival processes like proliferation, apoptosis, growth, and differentiation (Vandecaetsbeek et al. 2011). These functions are regulated by the luminal ER [Ca2+] ([Ca2+]ER) and by intracellular Ca2+ peaks and oscillations. SERCAs influence the amplitude, the shape, and the frequency of these modulatory events. Their Ca2+-sequestering activity is regulated by several physiological actors, such as i) proteins, ii) posttranslational modifications, and iii) microRNAs (miRNAs) (Harada et al. 2014; Melo et al. 2015). Their activity can also be modulated by natural compounds and pharmacological tools that either inhibit SERCA like thapsigargin, BHQ, and CPAE (Lytton et al. 1991; Vangheluwe et al. 2009) or promote SERCA like CDN1163 (Kang et al. 2016). Important biological modulators of SERCA are phospholamban (PLN), sarcolipin (SLN), calreticulin, calnexin, TMX1, and ORMD1. Briefly, PLN regulates SERCA function through direct protein-protein interactions (Vittorini et al. 2007). Additionally, SLN directly interacts with SERCAs, and it has been demonstrated to modulate SERCA activity by lowering both the Ca2+ affinity and Ca2+ pumping rate (Asahi et al. 2003). Another protein that regulates SERCAs is selenoprotein N (SEPN1). This redox-sensitive protein is able to bind SERCAs and enhances their ER Ca2+ uptake activity. This feature was found sufficient to safeguard cells against reactive oxygen species (ROS) produced during oxidative protein folding (Marino et al. 2015). Also, chaperones like calreticulin and calnexin have been identified as functional SERCA interactors and modulators. Calreticulin and calnexin were proposed to inhibit SERCA based on their inhibitory impact on high-frequency Ca2+ waves in Xenopus oocytes (Camacho and Lechleiter 1995; Roderick et al. 2000). Further work however revealed that overexpression of calreticulin elevated steady-state [Ca2+]ER and increased the ER refilling rates (Arnaudeau et al. 2002). Finally, it has been reported recently that the ER luminal protein disulfide isomerase TMX1 is a strong SERCA inhibitor. In fact, a lack of TMX1 led to an increased ER Ca2+ uptake rate and an increase in ER Ca2+ storage due to enhanced ER Ca2+ uptake activities of SERCA2b (Krols et al. 2016; Raturi et al. 2016). Another SERCA interactor and inhibitor besides TMX1 is the ER-resident, transmembrane protein ORMDL3, an asthma-associated gene product (Cantero-Recasens et al. 2010). Modulation of its expression levels implicates steady-state ER and cytosolic Ca2+ levels and the activation of UPR components.
As reported above, the ER also works as the main source of releasable Ca2+ in the cells. Many stimuli induce Ca2+ release, but two channel families mainly control the ER Ca2+-release program: ryanodine receptors (RyRs) and inositol 1,4,5-trisphosphate (IP3) receptors (IP3Rs) (Foskett et al. 2007; Parys and De Smedt 2012; Amador et al. 2013; Van Petegem 2015). RyRs and IP3Rs form large tetrameric channels (2 MDa and 1.2 MDa in size, respectively) displaying structural and functional homology. RyRs and IP3Rs are each encoded by three different genes leading for each to the expression of three isoforms. RyR1 is predominantly expressed in skeletal muscle and RyR2 in cardiac muscle and brain, while RyR3 is expressed at low levels in various tissues. With respect to the IP3Rs, most cell types express a combination of IP3R1, IP3R2, and IP3R3 in various proportions (Vermassen et al. 2004; Ivanova et al. 2014). There is only one known biological/physiological activator of the IP3R, i.e., IP3 produced by phospholipase C after activation of G protein-coupled receptors or receptor tyrosine kinases by various stimuli, including growth factors and hormones (Foskett et al. 2007; Parys and De Smedt 2012). RyRs however can be activated through conformational coupling to voltage-operated Ca2+ channels, by direct activation by Ca2+ or in some cases by the second messengers cADPR and NAADP (Gerasimenko et al. 2006). The Ca2+ itself is for both the RyRs and the IP3Rs, a very important regulator which acts in a biphasic way, whereby a low [Ca2+] activates the channels while a high [Ca2+] has an inhibitory action. Finally, RyRs and IP3Rs are regulated by phosphorylation/dephosphorylation and by multiple regulatory proteins, some of them being further explicated below. These IP3R channels are implicated in a plethora of physiological processes, including fertilization, lymphocyte activation, brain rhythms and synaptic plasticity, memory formation, endocrine, and exocrine gland function, and their dysregulation underlies pathophysiological conditions, like neurodegenerative diseases like Alzheimer’s disease, Huntington’s disease and amyotrophic lateral sclerosis, autism spectrum disorders, bipolar disorder, epilepsy, schizophrenia, spinocerebellar ataxia, cancer, cardiac dysfunction, and hypertrophy (Berridge 2016). Also RyR channels control different physiological functions, mainly related to skeletal and cardiac muscle contraction features (Van Petegem 2012). However, RyR dysregulation has also been implicated in neurodegenerative diseases like Alzheimer’s disease (Briggs et al. 2017; Popugaeva et al. 2017) and malignancies like breast cancer (Zhang et al. 2011b).
Ca2+ influx into the cytosol also occurs through ORAI and TRP channels, present in the plasma membrane, in a mechanism dependent on IP3R opening. In fact, when [Ca2+]ER decreases during IP3-induced Ca2+ release, stromal interaction molecules 1 and 2 (STIM1 and 2) are activated and, in turn, induce opening of ORAIs and, finally, lead to the so-called capacitative or store-operated Ca2+ influx from the extracellular space (Prakriya and Lewis 2015). As a result of this increased intracellular [Ca2+], key Ca2+-dependent proteins (such as calpains and calmodulins) are activated, and various Ca2+-dependent cellular processes are initiated (Berridge 2016). Several proteins control the activity of the Ca2+-release mechanism mediated by IP3Rs (Fig. 4.1). For example, the ER-resident oxidoreductases Ero1α and ERp44 modulate ER Ca2+ release by direct interaction with IP3Rs in a redox-sensitive manner (Higo et al. 2005; Anelli et al. 2012). Specifically, the ERp44 chaperone, an ER luminal protein of the thioredoxin family, directly inhibits Ca2+ release (which reinforces Ca2+-dependent chaperones) by inactivating the channel activity of the IP3R in a pH-, redox-state-, and [Ca2+]ER-dependent manner (Higo et al. 2005). Furthermore, ERp44 mediates Ero1α localization through the formation of reversible mixed disulfides (Anelli et al. 2003). Ero1α is an ER-resident protein that localizes also at MAMs, and its siRNA-mediated downregulation slightly reduced the association between IP3R and ERp44, suggesting that Ero1α might have further roles in assembling and/or maintaining MAM integrity (Anelli et al. 2012).
It is very clear that a large number of proteins regulate SERCA pumps and IP3R channels to maintain the appropriate [Ca2+]ER. This is because Ca2+ signals originating from the ER are leading to Ca2+ oscillations, associated with several cellular processes. As mentioned in the “Introduction” section, Ca2+ release from the ER is one of the main determinants for mitochondrial homeostasis. In fact, basal Ca2+ oscillations modulate mitochondrial metabolism of ATP production, while sustained or excessive Ca2+ release may lead to cell death. As a demonstration of this feature, dysregulation of Ca2+ flux is involved in several human disorders (Patergnani et al. 2015).
The maintenance of proper ER Ca2+ homeostasis also controls an elaborate surveillance system called the ER quality control (ERQC) system. In fact, inside the ER lumen a series of chaperones exists, which are involved in ERQC for the correct folding of ER proteins, and their functions may vary depending on changes in Ca2+ concentration (Fig. 4.1). The Ca2+-binding proteins calreticulin, ERp57, and protein disulfide isomerase (PDI) are classical examples of this family. In fact, PDI directly interacts with calreticulin when the [Ca2+]ER is lower than 400 μM, whereas the complex dissociates upon higher [Ca2+] (Baksh et al. 1995). Conversely, ERp57-calreticulin is insensitive to variations in [Ca2+], but it is dependent on Ca2+ binding by calreticulin, showing a direct role for Ca2+ in the regulation and maintenance of structural and functional complexes involved in protein turnover and synthesis (Michalak et al. 2009). Interestingly, another member of the PDI family, calnexin, plays a dual role based on its localization. At resting conditions, calnexin is highly palmitoylated (“palm” in Figs.), which leads to an increase of its localization at MAMs and regulation of Ca2+ signaling through its interaction with SERCA2b causing its activation. During the early, adaptive phases of ER stress, calnexin becomes depalmitoylated, primarily acting at the rough ER and employing its quality control functions (Lynes et al. 2012, 2013). The MAM localization of calnexin however not only depends on a specific palmitoylation event but also on the phosphorylation state of its cytosolic domain, on its interaction with phosphofurin acidic cluster sorting protein 2 (PACS2) (Myhill et al. 2008), and on the activity of the ER Rab protein Rab32 (Bui et al. 2010). The GTPase Rab32 localizes to the ER and mitochondria, and it has been identified as a regulator of MAM properties that modulate ER Ca2+ handling and disrupt the specific enrichment of calnexin in MAMs. However, it does not affect the ER distribution of PDI and mitofusin-2 (Bui et al. 2010).
Ca2+ ions are also important for the correct maintenance of ER structure. This was first reported in starfish eggs and then confirmed in human cells, where the ER continuity was affected by elevation of intracellular Ca2+ levels (Terasaki et al. 1996). Interestingly, this feature seemed to be regulated by protein kinase C (Ribeiro et al. 2000), which was already found to be involved in Ca2+ homeostasis, regulation of cellular processes, and modulation of important proteins and kinases (Pinton et al. 2007). In addition, the authors found that the high [Ca2+] registered did not induce ER fragmentation, only rearrangements of the ER network (Ribeiro et al. 2000). Overall, these findings suggest that Ca2+ dynamics regulate ER function without promoting pathological effects.
Notably, the ER continuously exchanges protein and lipid components with the Golgi apparatus, which is also regulated by Ca2+. One example may be found in thyroglobulin protein that, once synthesized and modified in the ER, is exported to the Golgi apparatus (Di Jeso et al. 2003). By treating thyroid differentiated cells with Ca2+ ionophores or specific inhibitors of ER channels and pumps, transport between the ER and the Golgi apparatus is blocked (Di Jeso et al. 1998). Intriguingly, it is not only the transport from the ER to the Golgi that seems to be regulated by Ca2+ levels but also transport from the Golgi to the ER, and intra-Golgi transport is highly dependent on intracellular Ca2+ variations. Moreover, the existence of a local Ca2+ gradient between the ER, the cytoplasm, and the Golgi apparatus has been demonstrated (Wahl et al. 1992). These findings underline the important role of intracellular Ca2+ in the trafficking of material between the ER and the Golgi and suggest that the mitochondrial compartment is not the only organelle likely to receive Ca2+ signals from the ER.
4.3 Ca2+ Signaling on the Mitochondrial Side
Mitochondria play a key role in many cell functions through the regulation of Ca2+ signaling. The increase in mitochondrial Ca2+ uptake activates several dehydrogenases and carriers, inducing an increase in the respiratory rate, H+ extrusion, and ATP production necessary for the proper energy state of the cell (Rizzuto et al. 2012). As a matter of fact, overexpression of isoforms of the mitochondrial aspartate/glutamate carrier (AGC) promotes ATP production during agonist-triggered Ca2+ increases, revealing that AGC plays an important role in decoding Ca2+ signals in the activation of mitochondrial oxidative metabolism (Lasorsa et al. 2003).
However, prolonged increase in [Ca2+]m leads to opening of the mitochondrial permeability transition pore (PTP) (Halestrap 2014; Jonas et al. 2015; Morciano et al. 2015), a critical event that leads to cell death by apoptosis (Rizzuto et al. 2012; Bonora et al. 2015).
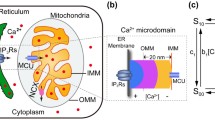
As stated previously, mitochondria can rapidly achieve a high [Ca2+]m due to (1) the presence of a driving force for Ca2+ generated by a ΔΨm of −180 mV under physiological conditions, (2) the formation of a large number of Ca2+ microdomains at the ER-mitochondria interface, and (3) the existence of a Ca2+-selective channel, termed the MCU complex, that is able to receive the Ca2+ signals originating from the ER (Fig. 4.2). Electrophysiological studies have shown that the uniporter is an ion channel with strikingly high conductance and selectivity (Kirichok et al. 2004). MCU is part of the uniporter holocomplex, which is also composed of two membrane proteins, MCUb and EMRE, which is regulated by MICU1 and MICU2 (Foskett and Philipson 2015; Kamer and Mootha 2015; Raffaello et al. 2016). Overexpression of MCUb reduces the amplitude of the transient mitochondrial Ca2+ response evoked by agonist stimulation, whereas MCUb silencing achieves the opposite effect, indicating that it acts as a dominant-negative subunit that reduces the uniporter channel activity (Raffaello et al. 2013). The role of EMRE in the regulation of MCU activity is dual: (1) it was suggested to be necessary for MCU channel activity, and indeed, its silencing abrogates Ca2+ entry into mitochondria (Sancak et al. 2013), and (2) it was required for the interaction of MCU with the regulatory subunits MICU1 and MICU2 (Tsai et al. 2016). Moreover, MCU complex is regulated by miRNAs (Marchi et al. 2013; Pan et al. 2015; Hong et al. 2017), underlining its role in multiple physiopathological contexts, or could be subjected to posttranslational modifications, such as phosphorylations (Joiner et al. 2012; O-Uchi et al. 2014) or methylations (Madreiter-Sokolowski et al. 2016). These recent observations confirm that mitochondrial Ca2+ homeostasis could be shaped by the wide molecular panel of intracellular transducers (Pinton et al. 2004).
Ca2+ homeostasis at the ER-mitochondria interface. Overview on the multiple molecular pathways acting at the mitochondrial side (See Sect. 4.3 for details)
The composition of the MCU complex has not yet been fully defined. Several proteins have been proposed to be part of it. One of the most characterized is an IMM integral protein, named MCUR1. Indeed, MCUR1 was initially shown as a regulator of the MCU complex (Mallilankaraman et al. 2012a), interacting with MCU and EMRE but not with MICU1 or MICU2, thereby functioning as a scaffolding factor (Vais et al. 2015; Tomar et al. 2016). However, MCUR1 was not identified by mass spectrometry of affinity-purified MCU complexes (Sancak et al. 2013), and it has been proposed as a cytochrome c oxidase assembly factor (Paupe et al. 2015).
The very low affinity of the MCU complex for Ca2+ depends on the activity of the MICU1 and MICU2 subunits, which localize at the mitochondrial intermembrane space and sense the cytoplasmic Ca2+ through their EF-hand domains, thus regulating the open/closed state of the whole uniporter complex (Patron et al. 2014). At resting conditions (cytoplasmic [Ca2+]<500 nM), the MICU1-2 dimer maintains the complex in a closed state, preserving it from the continuous accumulation of Ca2+ inside the matrix and thus avoiding Ca2+-mediated detrimental effects, such as ROS production and PTP opening (Mallilankaraman et al. 2012b; Csordas et al. 2013). However, at high cytoplasmic Ca2+ levels ([Ca2+]>1–2 μM) or during agonist stimulation, the MICU1–2 dimer undergoes conformational changes that allow opening of the channel, ensuring a prompt and complete mitochondrial Ca2+ response. The “high [Ca2+] microdomains theory” implies that the MCU complexes along the IMM should distribute at ER-mitochondria associations to promote effective Ca2+ transfer. Indeed, De La Fuente et al. recently showed that in cardiac mitochondria the MCU complexes were enriched in the IMM-OMM contact sites, positioned more to the mitochondrial periphery than inside the cristae, indicating high accessibility to cytoplasm-derived Ca2+ inputs (De La Fuente et al. 2016). In other words, mitochondrial Ca2+ channels are close to IP3Rs and RYRs on the ER or sarcoplasmic reticulum. Therefore, high [Ca2+] “hotspots” ([Ca2+]>10 μM) can be formed transiently in these regions of close apposition between the two organelles.
The conformational coupling between the Ca2+ channels of the two organelles highlights the importance of the macromolecular complexes located in the MAMs for their functional interaction (Fig. 4.2). In this respect, the OMM, although traditionally considered freely permeable, is a critical determinant of mitochondrial Ca2+ accumulation (Rapizzi et al. 2002). Ca2+ import across the OMM occurs through the voltage-dependent anion channel (VDAC), the most abundant protein of the OMM. The VDAC protein family consists of three isoforms (VDAC1–3), sharing a 75% sequence similarity. VDACs are expressed in almost all mammalian tissues, but recent studies indicate their nonredundant role in a plethora of cell functions (Naghdi and Hajnoczky 2016). Silencing of either of the VDAC isoforms limits mitochondrial Ca2+ uptake, but only VDAC1 was found to mediate pro-apoptotic Ca2+ transfer to mitochondria with consequent cell death (De Stefani et al. 2012; Ben-Hail and Shoshan-Barmatz 2016). In contrast, VDAC2 appears to be the pivotal isoform to locally couple mitochondrial Ca2+ uptake with RyR-mediated Ca2+ release in cardiac cells (Shimizu et al. 2015). Further evidence of the role of VDACs was supported by the demonstration of the physical link between VDACs and the IP3R. Indeed, the molecular chaperone glucose-regulated protein 75 (GRP75) was demonstrated to mediate the molecular interaction between VDACs and IP3Rs, allowing a positive regulation of mitochondrial Ca2+ uptake (Szabadkai et al. 2006). Small interfering RNA (siRNA) silencing of GRP75 abolishes the functional coupling between IP3Rs and VDACs, thereby reducing mitochondrial Ca2+ uptake in response to agonist stimulation (Szabadkai et al. 2006). Interestingly, thymocyte-expressed, positive selection-associated gene 1 (Tespa1) has been demonstrated to mediate Ca2+ transfer from mitochondria-associated ER to mitochondria interacting with GRP75 (Matsuzaki et al. 2013).
A crucial implication of a microdomain-based signaling mechanism is that the positioning and the shape of mitochondria within the cell become critical determinants of their responsiveness to Ca2+ inputs. Because of this, several mitochondrial proteins involved in the regulation of mitochondrial movement and morphology have also been considered key regulators of MAM integrity and functionality.
Mitochondrial trafficking is regulated by a subfamily of the Ras GTPases, the proteins Miro 1 and 2, which are located at the OMM through a short C-terminal anchor domain and have two EF-hand Ca2+-binding domains through which they are able to sense high levels of Ca2+ (Liu and Hajnoczky 2009). Miro proteins have an important role in tethering the mitochondria to the cytoskeleton by binding a cytoplasmic factor, Milton, which binds the kinesin 1 heavy chain on microtubules (Glater et al. 2006). Miro is proposed to be a Ca2+ sensor that stops mitochondrial movement in response to increasing Ca2+ levels. In fact, increased cytoplasmic Ca2+ levels stop mitochondrial movement, and this effect is suppressed when Miro is depleted or a Miro EF-hand is mutated (Fransson et al. 2003; Saotome et al. 2008).
In addition to the positioning of mitochondria, fusion and fission events regulating the shape of the organelles drastically influenced the mitochondrial Ca2+ responses (Patron et al. 2013). Recent studies suggest a link between components of mitochondrial dynamics and Ca2+ signaling. Mitochondrial fission is primarily driven by Drp1, a cytoplasmic protein that is recruited to the mitochondrial membrane, where it circumscribes the OMM as a helical oligomer (Smirnova et al. 2001; Rowland and Voeltz 2012). It is also interesting that high Ca2+ levels lead to activation of Drp1, which increases mitochondrial fission, cooperating with Miro (Saotome et al. 2008).
It has also been shown that the mitochondrial fission protein fission 1 homolog (Fis1) conveys an apoptotic signal from the mitochondria to the ER by interacting with Bap31 at the ER. Therefore, Fis1 facilitates the cleavage of Bap31 to its pro-apoptotic form, p20Bap31, promoting the recruitment of procaspase-8. Moreover, this signaling pathway establishes a feedback loop by releasing Ca2+ from the ER and, consequently, results in Ca2+ accumulation in mitochondria, amplifying cell death by activating the apoptotic pathway in many mitochondria that are in close proximity to the ER (Iwasawa et al. 2011).
Other mitochondrial dynamin-related GTPases involved in mitochondrial Ca2+ regulation include mitofusin 1 and 2. In particular, mitofusin 2 (MFN2) is a critical component of the mitochondrial fusion/fission machinery. This OMM profusion protein is also observed in the MAMs where it couples to MFN1 or MFN2 on the mitochondria to physically tether the organelles. Indeed, in 2008 de Brito and Scorrano showed that MFN2 is enriched at contact sites between the ER and mitochondria, regulating ER morphology and directly tethering the two organelles (de Brito and Scorrano 2008). Moreover, the distance between the ER and mitochondria increases in cells lacking MFN2, and this leads to impaired mitochondrial Ca2+ uptake, further verifying the validity of the Ca2+ microdomains theory.
The tethering role of MFN2 was confirmed by other laboratories (Chen et al. 2012; Sebastian et al. 2012; Schneeberger et al. 2013), but its function was recently challenged by different experimental approaches. Contrary to previous studies, electron microscopy analyses suggested that loss of MFN2 increased, rather than reduced, ER-mitochondria juxtaposition (Cosson et al. 2012; Filadi et al. 2015). Moreover, it was demonstrated that reduced Ca2+ transfer in MFN2-knockout cells is the result of a lower expression of MCU and is independent of ER-mitochondria juxtapositions (Filadi et al. 2015). Therefore, they proposed a different role for MFN2 in ER-mitochondria coupling, in which the protein, rather than being a component of the tethering complex, acts as a negative regulator of organelle apposition. However, very recently a critical reappraisal of MFN2’s role in the ER-mitochondria connection was published, supporting previous results and identifying MFN2 as a physical tether between the two organelles in multiple tissues (Naon et al. 2016).
The activity of MFN2 at the ER-mitochondria interface is regulated by a mitochondrial ubiquitin ligase called MITOL (Sugiura et al. 2013). MITOL interacts with mitochondrial MFN2, but not with ER MFN2, and mediates the addition of lysine 63-linked polyubiquitin chains to MFN2 but not its proteasomal degradation. This polyubiquitination event induces MFN2 oligomerization, allowing ER-mitochondria tethering and Ca2+ uptake in the mitochondria upon stimulation with histamine. The reduction in mitochondrial Ca2+ uptake that occurs in MITOL-deficient cells highlights its key role as a MAM regulator and confirms the idea that the distance between the ER and mitochondria is crucial for proper Ca2+ transfer. However, the role of MFN2 at MAM is still highly debated, and new experimental evidence is required for definitively establish its anti- or pro-tethering functions.
4.4 Ca2+ Signaling at the MAM Interface
MAMs represent the physical association between the ER and mitochondria, an entity with a defined structure and architecture with distinct biochemical properties and a characteristic set of proteins. The MAM fraction was first separated and characterized by J. E. Vance (Vance 1990), who described the isolation from rat liver of a unique membrane, initially termed “fraction X,” that was associated with mitochondria and had a high specific activity for several proteins attributed to the ER. After this seminal observation, several biochemical protocols have been described to isolate the MAMs fraction, both from organs and cells, and these studies confirmed that MAMs are composed of membrane fragments from both the ER and the OMM (Wieckowski et al. 2009).
In recent years, different proteomics studies identified the molecular components of the MAMs fraction, starting from human fibroblasts (Zhang et al. 2011a) and mouse brain (Poston et al. 2013), demonstrating that more than 1000 “MAM proteins” reside in this fraction. More recently, Sala-Vila et al. performed a very rigorous high-throughput mass spectrometry-based proteomics characterization of MAMs from mouse liver, identifying 1052 MAM-enriched proteins, which included several Ca2+ players, such as SERCA2, IP3R, and the Ca2+-binding mitochondrial carrier SLC25A12 (Sala-Vila et al. 2016). Interestingly, they observed the MAM localization of caveolin 1 (CAV1), and CAV1-deficient cells displayed ER and mitochondrial aberrations, as well as reduced contact sites between the two organelles (Sala-Vila et al. 2016). In line with this evidence, our group showed that in transformed cells, H-RAS12V expression was associated with CAV1 downregulation and a drastic alteration in Ca2+ homeostasis (Rimessi et al. 2014).
Other central players in the ER-mitochondria Ca2+ flux include a series of chaperones and oxidoreductases, which also localize to the ER/MAMs compartment (Fig. 4.3). In addition to the previously cited Ca2+-binding chaperone calnexin (see Sect. 4.2), sigma-1 receptor (Sig-1R) is one of the pivotal Ca2+ regulators residing at MAMs (Hayashi and Su 2007). Under normal conditions, Sig-1R resides specifically at MAMs and forms a complex with BiP (also named grp78) when the ER Ca2+ level is 0.5–1 mM. However, when the IP3R is activated, the subsequent drop of the [Ca2+]ER causes the dissociation of Sig-1R from BiP, unleashing the chaperone activity of the receptor. Interestingly, IP3R3 seems to be enriched at MAMs (Mendes et al. 2005), and its stabilization by Sig-1R ensures proper Ca2+ influx into mitochondria.
Ca2+ homeostasis at the ER-mitochondria interface. Overview on the multiple molecular pathways acting at the MAMs side (See Sect. 4.4 for further details)
A link between MAMs and Ca2+ signaling also appears in the context of ER stress-mediated apoptosis. The RNA-dependent protein kinase (PKR)-like ER kinase (PERK), a key ER stress sensor of the unfolded protein response, is uniquely enriched at MAMs (Verfaillie et al. 2012). PERK-knockout cells display an aberrant ER morphology, disturbed Ca2+ signaling, and weaker contact sites between ER and mitochondria; consequently, PERK likely serves as a structural tether at the ER-mitochondria interface. Collectively, these data highlight that a conserved MAM structure is indispensable for transmitting Ca2+ signaling as well as ROS-mediated signals to the mitochondria after ROS-based ER stress.
Ca2+ homeostasis is fundamental for numerous cellular mechanisms, including cell death; thus, it is not surprising that several oncogenes and tumor suppressors localize at the MAMs, where they play a crucial role in the control of ER-mitochondria Ca2+ flux, favoring either survival or cell death (Bittremieux et al. 2016) (Fig. 4.3).
A few years ago, our group showed that the promyelocytic leukemia protein (PML), a tumor suppressor known as an essential component of nuclear structures termed PML nuclear bodies, was localized to the ER and MAMs. PML regulates apoptosis by modulating ER Ca2+ release (Giorgi et al. 2010). In MAMs, PML was found to coordinate a complex that includes IP3R3, Akt kinase, and phosphatase PP2A. In the absence of PML, Akt phosphorylation and activity was increased at the ER due to impaired PP2A activity, which resulted in impaired Ca2+ flux through the IP3R because of its Akt-mediated hyperphosphorylated state. Indeed, IP3R is a target of Akt kinase activity (Khan et al. 2006; Szado et al. 2008), and Akt activation drastically reduces IP3R-dependent ER Ca2+ release (Marchi et al. 2008; Szado et al. 2008), especially through IP3R3 phosphorylation (Marchi et al. 2012). These data have been confirmed by an independent study, which reported the localization of mechanistic target of rapamycin (mTOR) complex 2 (mTORC2) at the MAMs (Betz et al. 2013). The serine/threonine kinase (mTOR) is a pivotal regulator of autophagy and exists in two protein complexes, mTORC1 and mTORC2. The latter complex phosphorylates and activates Akt, which phosphorylates MAMs-resident proteins PACS2, IP3R, and hexokinase 2 (HK2) to regulate MAMs integrity, Ca2+ flux, and energy metabolism, respectively (Betz et al. 2013).
More recently, we demonstrated that MAMs-enriched PML also exerts an important Ca2+-dependent role in the autophagic process, through the AMPK/mTOR/Ulk1 pathway (Missiroli et al. 2016). We overexpressed MCU in PML-KO cells to verify whether downregulated ER-mitochondria Ca2+ transfer is important for the induction of autophagy. We demonstrated that increasing the ability of mitochondria to accumulate Ca2+ in PML-KO cells suppressed AMPK activity, thereby repressing autophagic flux. These data suggest that PML controls autophagy at MAMs through its effects on Ca2+ homeostasis and that the loss of PML from MAMs results in autophagy activation, a feature that promotes cell survival under stress conditions and thus facilitates malignant cell growth.
Among tumor suppressors, phosphatase and tensin homolog deleted on chromosome 10 (PTEN) was found to be localized at the ER and MAMs, where it modulates Ca2+ transfer from ER to mitochondria in a protein phosphatase-dependent manner that counteracts the Akt-mediated reduction in Ca2+ release via IP3Rs (Bononi et al. 2013). Moreover, the tumor suppressor p53 also resides at ER and MAMs, modulating ER Ca2+ efflux to the mitochondria through modulation of the oxidative state of SERCA pumps (Giorgi et al. 2015b; Giorgi et al. 2015a).
Very recently FATE1, a cancer-testis antigen, has been implicated in the regulation of ER-mitochondria distance and Ca2+ uptake by mitochondria (Doghman-Bouguerra et al. 2016). FATE1 is localized at the interface between the ER and mitochondria and decreases sensitivity to mitochondrial Ca2+-dependent pro-apoptotic stimuli and to chemotherapeutic drugs. This study emphasized how the ER-mitochondria uncoupling activity of FATE1 is harnessed by cancer cells to escape apoptotic death and resist the action of chemotherapeutic drugs.
Taken together, these observations highlight the role of ER-dependent Ca2+ release as a general mediator in many cell deaths or cell survival scenarios and reinforce the importance of MAMs in Ca2+ handling.
4.5 Conclusions
The importance of MAMs in the control of various cellular processes and its relevance for human health is underpinned by the disease that has linked to dysregulation and dysfunction of the ER-mitochondria interface and architecture. These diseases include obesity (Arruda et al. 2014) and type II diabetes (Tubbs et al. 2014), as well as Parkinson’s and Alzheimer’s diseases (Paillusson et al. 2016). MAM disorganization results in abnormal ER-mitochondria Ca2+ flux, which contributes to the formation of aberrant mitochondrial structures and deep metabolic alterations that are typical features of these pathological conditions. Based on the recent observations suggesting an optimal distance of 30–85 nm between IP3R and the MCU complex to achieve effective Ca2+ transfer and generation of Ca2+ inputs (Qi et al. 2015), we need novel technologies that enable an accurate and highly precise measurement of the functional changes occurring at MAMs to elucidate the local Ca2+ transport and signaling mechanisms.
References
Amador FJ, Stathopulos PB, Enomoto M, Ikura M (2013) Ryanodine receptor calcium release channels: lessons from structure-function studies. FEBS J 280:5456–5470
Anelli T, Alessio M, Bachi A, Bergamelli L, Bertoli G, Camerini S, Mezghrani A, Ruffato E, Simmen T, Sitia R (2003) Thiol-mediated protein retention in the endoplasmic reticulum: the role of ERp44. EMBO J 22:5015–5022
Anelli T, Bergamelli L, Margittai E, Rimessi A, Fagioli C, Malgaroli A, Pinton P, Ripamonti M, Rizzuto R, Sitia R (2012) Ero1α regulates Ca2+ fluxes at the endoplasmic reticulum-mitochondria interface (MAM). Antioxid Redox Signal 16:1077–1087
Arasaki K, Shimizu H, Mogari H, Nishida N, Hirota N, Furuno A, Kudo Y, Baba M, Baba N, Cheng J et al (2015) A role for the ancient SNARE syntaxin 17 in regulating mitochondrial division. Dev Cell 32:304–317
Arnaudeau S, Frieden M, Nakamura K, Castelbou C, Michalak M, Demaurex N (2002) Calreticulin differentially modulates calcium uptake and release in the endoplasmic reticulum and mitochondria. J Biol Chem 277:46696–46705
Arruda AP, Pers BM, Parlakgul G, Guney E, Inouye K, Hotamisligil GS (2014) Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat Med 20:1427–1435
Asahi M, Nakayama H, Tada M, Otsu K (2003) Regulation of sarco(endo)plasmic reticulum Ca2+ adenosine triphosphatase by phospholamban and sarcolipin: implication for cardiac hypertrophy and failure. Trends Cardiovasc Med 13:152–157
Ashby MC, Tepikin AV (2001) ER calcium and the functions of intracellular organelles. Semin Cell Dev Biol 12:11–17
Baksh S, Burns K, Andrin C, Michalak M (1995) Interaction of calreticulin with protein disulfide isomerase. J Biol Chem 270:31338–31344
Ben-Hail D, Shoshan-Barmatz V (2016) VDAC1-interacting anion transport inhibitors inhibit VDAC1 oligomerization and apoptosis. Biochim Biophys Acta 1863:1612–1623
Berridge MJ (2016) The Inositol trisphosphate/calcium signaling pathway in health and disease. Physiol Rev 96:1261–1296
Betz C, Stracka D, Prescianotto-Baschong C, Frieden M, Demaurex N, Hall MN (2013) Feature Article: mTOR complex 2-Akt signaling at mitochondria-associated endoplasmic reticulum membranes (MAM) regulates mitochondrial physiology. Proc Natl Acad Sci USA 110:12526–12534
Bittremieux M, Parys JB, Pinton P, Bultynck G (2016) ER functions of oncogenes and tumor suppressors: Modulators of intracellular Ca2+ signaling. Biochim Biophys Acta 1863:1364–1378
Bononi A, Bonora M, Marchi S, Missiroli S, Poletti F, Giorgi C, Pandolfi PP, Pinton P (2013) Identification of PTEN at the ER and MAMs and its regulation of Ca2+ signaling and apoptosis in a protein phosphatase-dependent manner. Cell Death Differ 20:1631–1643
Bonora M, Patergnani S, Rimessi A, De Marchi E, Suski JM, Bononi A, Giorgi C, Marchi S, Missiroli S, Poletti F et al (2012) ATP synthesis and storage. Purinergic Signal 8:343–357
Bonora M, Giorgi C, Bononi A, Marchi S, Patergnani S, Rimessi A, Rizzuto R, Pinton P (2013) Subcellular calcium measurements in mammalian cells using jellyfish photoprotein aequorin-based probes. Nat Protoc 8:2105–2118
Bonora M, Wieckowski MR, Chinopoulos C, Kepp O, Kroemer G, Galluzzi L, Pinton P (2015) Molecular mechanisms of cell death: central implication of ATP synthase in mitochondrial permeability transition. Oncogene 34:1475–1486
Bravo R, Vicencio JM, Parra V, Troncoso R, Munoz JP, Bui M, Quiroga C, Rodriguez AE, Verdejo HE, Ferreira J et al (2011) Increased ER-mitochondrial coupling promotes mitochondrial respiration and bioenergetics during early phases of ER stress. J Cell Sci 124:2143–2152
Bravo R, Gutierrez T, Paredes F, Gatica D, Rodriguez AE, Pedrozo Z, Chiong M, Parra V, Quest AF, Rothermel BA et al (2012) Endoplasmic reticulum: ER stress regulates mitochondrial bioenergetics. Int J Biochem Cell Biol 44:16–20
Briggs CA, Chakroborty S, Stutzmann GE (2017) Emerging pathways driving early synaptic pathology in Alzheimer’s disease. Biochem Biophys Res Commun 483(4):988–997
Bui M, Gilady SY, Fitzsimmons RE, Benson MD, Lynes EM, Gesson K, Alto NM, Strack S, Scott JD, Simmen T (2010) Rab32 modulates apoptosis onset and mitochondria-associated membrane (MAM) properties. J Biol Chem 285:31590–31602
Camacho P, Lechleiter JD (1995) Calreticulin inhibits repetitive intracellular Ca2+ waves. Cell 82:765–771
Cantero-Recasens G, Fandos C, Rubio-Moscardo F, Valverde MA, Vicente R (2010) The asthma-associated ORMDL3 gene product regulates endoplasmic reticulum-mediated calcium signaling and cellular stress. Hum Mol Genet 19:111–121
Chen Y, Csordas G, Jowdy C, Schneider TG, Csordas N, Wang W, Liu Y, Kohlhaas M, Meiser M, Bergem S et al (2012) Mitofusin 2-containing mitochondrial-reticular microdomains direct rapid cardiomyocyte bioenergetic responses via interorganelle Ca2+ crosstalk. Circ Res 111:863–875
Cosson P, Marchetti A, Ravazzola M, Orci L (2012) Mitofusin-2 independent juxtaposition of endoplasmic reticulum and mitochondria: an ultrastructural study. PLoS One 7:e46293
Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G (2006) Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol 174:915–921
Csordas G, Varnai P, Golenar T, Roy S, Purkins G, Schneider TG, Balla T, Hajnoczky G (2010) Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol Cell 39:121–132
Csordas G, Golenar T, Seifert EL, Kamer KJ, Sancak Y, Perocchi F, Moffat C, Weaver D, de la Fuente PS, Bogorad R et al (2013) MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca2+ uniporter. Cell Metab 17:976–987
de Brito OM, Scorrano L (2008) Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456:605–610
De La Fuente S, Fernandez-Sanz C, Vail C, Agra EJ, Holmstrom K, Sun J, Mishra J, Williams D, Finkel T, Murphy E et al (2016) Strategic positioning and biased activity of the mitochondrial calcium uniporter in cardiac muscle. J Biol Chem 291:23343–23362
De Stefani D, Bononi A, Romagnoli A, Messina A, De Pinto V, Pinton P, Rizzuto R (2012) VDAC1 selectively transfers apoptotic Ca2+ signals to mitochondria. Cell Death Differ 19:267–273
Decuypere JP, Monaco G, Bultynck G, Missiaen L, De Smedt H, Parys JB (2011) The IP3 receptor-mitochondria connection in apoptosis and autophagy. Biochim Biophys Acta 1813:1003–1013
Di Jeso B, Pereira R, Consiglio E, Formisano S, Satrustegui J, Sandoval IV (1998) Demonstration of a Ca2+ requirement for thyroglobulin dimerization and export to the golgi complex. Eur J Biochem 252:583–590
Di Jeso B, Ulianich L, Pacifico F, Leonardi A, Vito P, Consiglio E, Formisano S, Arvan P (2003) Folding of thyroglobulin in the calnexin/calreticulin pathway and its alteration by loss of Ca2+ from the endoplasmic reticulum. Biochem J 370:449–458
Doghman-Bouguerra M, Granatiero V, Sbiera S, Sbiera I, Lacas-Gervais S, Brau F, Fassnacht M, Rizzuto R, Lalli E (2016) FATE1 antagonizes calcium- and drug-induced apoptosis by uncoupling ER and mitochondria. EMBO Rep 17:1264–1280
Filadi R, Greotti E, Turacchio G, Luini A, Pozzan T, Pizzo P (2015) Mitofusin 2 ablation increases endoplasmic reticulum-mitochondria coupling. Proc Natl Acad Sci USA 112:E2174–E2181
Foskett JK, Philipson B (2015) The mitochondrial Ca2+ uniporter complex. J Mol Cell Cardiol 78:3–8
Foskett JK, White C, Cheung KH, Mak DO (2007) Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev 87:593–658
Fransson A, Ruusala A, Aspenstrom P (2003) Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis. J Biol Chem 278:6495–6502
Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK (2011) ER tubules mark sites of mitochondrial division. Science 334:358–362
Gerasimenko JV, Sherwood M, Tepikin AV, Petersen OH, Gerasimenko OV (2006) NAADP, cADPR and IP3 all release Ca2+ from the endoplasmic reticulum and an acidic store in the secretory granule area. J Cell Sci 119:226–238
Giorgi C, Ito K, Lin HK, Santangelo C, Wieckowski MR, Lebiedzinska M, Bononi A, Bonora M, Duszynski J, Bernardi R et al (2010) PML regulates apoptosis at endoplasmic reticulum by modulating calcium release. Science 330:1247–1251
Giorgi C, Baldassari F, Bononi A, Bonora M, De Marchi E, Marchi S, Missiroli S, Patergnani S, Rimessi A, Suski JM et al (2012) Mitochondrial Ca2+ and apoptosis. Cell Calcium 52:36–43
Giorgi C, Bonora M, Missiroli S, Poletti F, Ramirez FG, Morciano G, Morganti C, Pandolfi PP, Mammano F, Pinton P (2015a) Intravital imaging reveals p53-dependent cancer cell death induced by phototherapy via calcium signaling. Oncotarget 6:1435–1445
Giorgi C, Bonora M, Sorrentino G, Missiroli S, Poletti F, Suski JM, Galindo Ramirez F, Rizzuto R, Di Virgilio F, Zito E et al (2015b) p53 at the endoplasmic reticulum regulates apoptosis in a Ca2+-dependent manner. Proc Natl Acad Sci USA 112:1779–1784
Giorgi C, Missiroli S, Patergnani S, Duszynski J, Wieckowski MR, Pinton P (2015c) Mitochondria-associated membranes: composition, molecular mechanisms, and physiopathological implications. Antioxid Redox Signal 22:995–1019
Glater EE, Megeath LJ, Stowers RS, Schwarz TL (2006) Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol 173:545–557
Gunter TE, Gunter KK (2001) Uptake of calcium by mitochondria: transport and possible function. IUBMB Life 52:197–204
Halestrap AP (2014) The C ring of the F1Fo ATP synthase forms the mitochondrial permeability transition pore: a critical appraisal. Front Oncol 4:234
Hamasaki M, Furuta N, Matsuda A, Nezu A, Yamamoto A, Fujita N, Oomori H, Noda T, Haraguchi T, Hiraoka Y et al (2013) Autophagosomes form at ER-mitochondria contact sites. Nature 495:389–393
Harada M, Luo X, Murohara T, Yang B, Dobrev D, Nattel S (2014) MicroRNA regulation and cardiac calcium signaling: role in cardiac disease and therapeutic potential. Circ Res 114:689–705
Haroon S, Vermulst M (2016) Linking mitochondrial dynamics to mitochondrial protein quality control. Curr Opin Genet Dev 38:68–74
Hayashi T, Su TP (2007) Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell 131:596–610
Higo T, Hattori M, Nakamura T, Natsume T, Michikawa T, Mikoshiba K (2005) Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell 120:85–98
Hofer AM, Schulz I (1996) Quantification of intraluminal free [Ca] in the agonist-sensitive internal calcium store using compartmentalized fluorescent indicators: some considerations. Cell Calcium 20:235–242
Hong Z, Chen KH, Dasgupta A, Potus F, Dunham-Snary K, Bonnet S, Tian L, Fu J, Breuils-Bonnet S, Provencher S et al (2017) miR-138 and miR-25 Downregulate MCU, causing pulmonary arterial Hypertension’s cancer phenotype. Am J Respir Crit Care Med 195(4):515–529
Ivanova H, Vervliet T, Missiaen L, Parys JB, De Smedt H, Bultynck G (2014) Inositol 1,4,5-trisphosphate receptor-isoform diversity in cell death and survival. Biochim Biophys Acta 1843:2164–2183
Iwasawa R, Mahul-Mellier AL, Datler C, Pazarentzos E, Grimm S (2011) Fis1 and Bap31 bridge the mitochondria-ER interface to establish a platform for apoptosis induction. EMBO J 30:556–568
Joiner ML, Koval OM, Li J, He BJ, Allamargot C, Gao Z, Luczak ED, Hall DD, Fink BD, Chen B et al (2012) CaMKII determines mitochondrial stress responses in heart. Nature 491:269–273
Jonas EA, Porter GA Jr, Beutner G, Mnatsakanyan N, Alavian KN (2015) Cell death disguised: The mitochondrial permeability transition pore as the c-subunit of the F1Fo ATP synthase. Pharmacol Res 99:382–392
Kamer KJ, Mootha VK (2015) The molecular era of the mitochondrial calcium uniporter. Nat Rev Mol Cell Biol 16:545–553
Kang S, Dahl R, Hsieh W, Shin A, Zsebo KM, Buettner C, Hajjar RJ, Lebeche D (2016) Small molecular allosteric activator of the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) attenuates diabetes and metabolic disorders. J Biol Chem 291:5185–5198
Khan MT, Wagner L 2nd, Yule DI, Bhanumathy C, Joseph SK (2006) Akt kinase phosphorylation of inositol 1,4,5-trisphosphate receptors. J Biol Chem 281:3731–3737
Kirichok Y, Krapivinsky G, Clapham DE (2004) The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427:360–364
Krols M, Bultynck G, Janssens S (2016) ER-Mitochondria contact sites: A new regulator of cellular calcium flux comes into play. J Cell Biol 214:367–370
Lamb CA, Yoshimori T, Tooze SA (2013) The autophagosome: origins unknown, biogenesis complex. Nat Rev Mol Cell Biol 14:759–774
Lasorsa FM, Pinton P, Palmieri L, Fiermonte G, Rizzuto R, Palmieri F (2003) Recombinant expression of the Ca2+-sensitive aspartate/glutamate carrier increases mitochondrial ATP production in agonist-stimulated Chinese hamster ovary cells. J Biol Chem 278:38686–38692
Liu X, Hajnoczky G (2009) Ca2+-dependent regulation of mitochondrial dynamics by the Miro-Milton complex. Int J Biochem Cell Biol 41:1972–1976
Lynes EM, Bui M, Yap MC, Benson MD, Schneider B, Ellgaard L, Berthiaume LG, Simmen T (2012) Palmitoylated TMX and calnexin target to the mitochondria-associated membrane. EMBO J 31:457–470
Lynes EM, Raturi A, Shenkman M, Ortiz Sandoval C, Yap MC, Wu J, Janowicz A, Myhill N, Benson MD, Campbell RE et al (2013) Palmitoylation is the switch that assigns calnexin to quality control or ER Ca2+ signaling. J Cell Sci 126:3893–3903
Lytton J, Westlin M, Hanley MR (1991) Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem 266:17067–17071
Madreiter-Sokolowski CT, Klec C, Parichatikanond W, Stryeck S, Gottschalk B, Pulido S, Rost R, Eroglu E, Hofmann NA, Bondarenko AI et al (2016) PRMT1-mediated methylation of MICU1 determines the UCP2/3 dependency of mitochondrial Ca2+ uptake in immortalized cells. Nat Commun 7:12897
Mallilankaraman K, Cardenas C, Doonan PJ, Chandramoorthy HC, Irrinki KM, Golenar T, Csordas G, Madireddi P, Yang J, Muller M et al (2012a) MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat Cell Biol 14:1336–1343
Mallilankaraman K, Doonan P, Cardenas C, Chandramoorthy HC, Muller M, Miller R, Hoffman NE, Gandhirajan RK, Molgo J, Birnbaum MJ et al (2012b) MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca2+ uptake that regulates cell survival. Cell 151:630–644
Marchi S, Pinton P (2014) The mitochondrial calcium uniporter complex: molecular components, structure and physiopathological implications. J Physiol 592:829–839
Marchi S, Rimessi A, Giorgi C, Baldini C, Ferroni L, Rizzuto R, Pinton P (2008) Akt kinase reducing endoplasmic reticulum Ca2+ release protects cells from Ca2+-dependent apoptotic stimuli. Biochem Biophys Res Commun 375:501–505
Marchi S, Marinello M, Bononi A, Bonora M, Giorgi C, Rimessi A, Pinton P (2012) Selective modulation of subtype III IP3R by Akt regulates ER Ca2+ release and apoptosis. Cell Death Dis 3:e304
Marchi S, Lupini L, Patergnani S, Rimessi A, Missiroli S, Bonora M, Bononi A, Corra F, Giorgi C, De Marchi E et al (2013) Downregulation of the mitochondrial calcium uniporter by cancer-related miR-25. Curr Biol 23:58–63
Marchi S, Patergnani S, Pinton P (2014) The endoplasmic reticulum-mitochondria connection: one touch, multiple functions. Biochimica et biophysica acta 1837:461–469
Marino M, Stoilova T, Giorgi C, Bachi A, Cattaneo A, Auricchio A, Pinton P, Zito E (2015) SEPN1, an endoplasmic reticulum-localized selenoprotein linked to skeletal muscle pathology, counteracts hyperoxidation by means of redox-regulating SERCA2 pump activity. Hum Mol Genet 24:1843–1855
Matsuzaki H, Fujimoto T, Tanaka M, Shirasawa S (2013) Tespa1 is a novel component of mitochondria-associated endoplasmic reticulum membranes and affects mitochondrial calcium flux. Biochem Biophys Res Commun 433:322–326
Melo SF, Barauna VG, Neves VJ, Fernandes T, Lara Lda S, Mazzotti DR, Oliveira EM (2015) Exercise training restores the cardiac microRNA-1 and -214 levels regulating Ca2+ handling after myocardial infarction. BMC Cardiovasc Disord 15:166
Mendes CC, Gomes DA, Thompson M, Souto NC, Goes TS, Goes AM, Rodrigues MA, Gomez MV, Nathanson MH, Leite MF (2005) The type III inositol 1,4,5-trisphosphate receptor preferentially transmits apoptotic Ca2+ signals into mitochondria. J Biol Chem 280:40892–40900
Michalak M, Groenendyk J, Szabo E, Gold LI, Opas M (2009) Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem J 417:651–666
Missiroli S, Bonora M, Patergnani S, Poletti F, Perrone M, Gafa R, Magri E, Raimondi A, Lanza G, Tacchetti C et al (2016) PML at mitochondria-associated membranes is critical for the repression of autophagy and cancer development. Cell Rep 16:2415–2427
Montero M, Alonso MT, Carnicero E, Cuchillo-Ibanez I, Albillos A, Garcia AG, Garcia-Sancho J, Alvarez J (2000) Chromaffin-cell stimulation triggers fast millimolar mitochondrial Ca2+ transients that modulate secretion. Nat Cell Biol 2:57–61
Morciano G, Giorgi C, Bonora M, Punzetti S, Pavasini R, Wieckowski MR, Campo G, Pinton P (2015) Molecular identity of the mitochondrial permeability transition pore and its role in ischemia-reperfusion injury. J Mol Cell Cardiol 78:142–153
Myhill N, Lynes EM, Nanji JA, Blagoveshchenskaya AD, Fei H, Carmine Simmen K, Cooper TJ, Thomas G, Simmen T (2008) The subcellular distribution of calnexin is mediated by PACS-2. Mol Biol Cell 19:2777–2788
Naghdi S, Hajnoczky G (2016) VDAC2-specific cellular functions and the underlying structure. Biochim Biophys Acta 1863:2503–2514
Naon D, Scorrano L (2014) At the right distance: ER-mitochondria juxtaposition in cell life and death. Biochim Biophys Acta 1843:2184–2194
Naon D, Zaninello M, Giacomello M, Varanita T, Grespi F, Lakshminaranayan S, Serafini A, Semenzato M, Herkenne S, Hernandez-Alvarez MI et al (2016) Critical reappraisal confirms that Mitofusin 2 is an endoplasmic reticulum-mitochondria tether. Proc Natl Acad Sci USA 113:11249–11254
O-Uchi J, Jhun BS, Xu S, Hurst S, Raffaello A, Liu X, Yi B, Zhang H, Gross P, Mishra J et al (2014) Adrenergic signaling regulates mitochondrial Ca2+ uptake through Pyk2-dependent tyrosine phosphorylation of the mitochondrial Ca2+ uniporter. Antioxid Redox Signal 21:863–879
Paillusson S, Stoica R, Gomez-Suaga P, Lau DH, Mueller S, Miller T, Miller CC (2016) There’s something wrong with my MAM; the ER-mitochondria axis and neurodegenerative diseases. Trends Neurosci 39:146–157
Pan L, Huang BJ, Ma XE, Wang SY, Feng J, Lv F, Liu Y, Li CM, Liang DD, Li J et al (2015) MiR-25 protects cardiomyocytes against oxidative damage by targeting the mitochondrial calcium uniporter. Int J Mol Sci 16:5420–5433
Papp B, Brouland JP, Arbabian A, Gelebart P, Kovacs T, Bobe R, Enouf J, Varin-Blank N, Apati A (2012) Endoplasmic reticulum calcium pumps and cancer cell differentiation. Biomolecules 2:165–186
Parys JB, De Smedt H (2012) Inositol 1,4,5-trisphosphate and its receptors. Adv Exp Med Biol 740:255–279
Patergnani S, Suski JM, Agnoletto C, Bononi A, Bonora M, De Marchi E, Giorgi C, Marchi S, Missiroli S, Poletti F et al (2011) Calcium signaling around Mitochondria Associated Membranes (MAMs). Cell Commun Signal 9:19
Patergnani S, Missiroli S, Marchi S, Giorgi C (2015) Mitochondria-associated endoplasmic reticulum membranes microenvironment: targeting autophagic and apoptotic pathways in cancer therapy. Frontiers in oncology 5:173
Patron M, Raffaello A, Granatiero V, Tosatto A, Merli G, De Stefani D, Wright L, Pallafacchina G, Terrin A, Mammucari C et al (2013) The mitochondrial calcium uniporter (MCU): molecular identity and physiological roles. J Biol Chem 288:10750–10758
Patron M, Checchetto V, Raffaello A, Teardo E, Vecellio Reane D, Mantoan M, Granatiero V, Szabo I, De Stefani D, Rizzuto R (2014) MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity. Mol Cell 53:726–737
Paupe V, Prudent J, Dassa EP, Rendon OZ, Shoubridge EA (2015) CCDC90A (MCUR1) is a cytochrome c oxidase assembly factor and not a regulator of the mitochondrial calcium uniporter. Cell Metab 21:109–116
Pinton P, Leo S, Wieckowski MR, Di Benedetto G, Rizzuto R (2004) Long-term modulation of mitochondrial Ca2+ signals by protein kinase C isozymes. J Cell Biol 165:223–232
Pinton P, Rimessi A, Marchi S, Orsini F, Migliaccio E, Giorgio M, Contursi C, Minucci S, Mantovani F, Wieckowski MR et al (2007) Protein kinase C β and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science 315:659–663
Popugaeva E, Pchitskaya E, Bezprozvanny I (2017) Dysregulation of neuronal calcium homeostasis in Alzheimer’s disease – A therapeutic opportunity? Biochem Biophys Res Commun 483(4):998–1004
Poston CN, Krishnan SC, Bazemore-Walker CR (2013) In-depth proteomic analysis of mammalian mitochondria-associated membranes (MAM). J Proteomics 79:219–230
Prakriya M, Lewis RS (2015) Store-Operated Calcium Channels. Physiol Rev 95:1383–1436
Qi H, Li L, Shuai J (2015) Optimal microdomain crosstalk between endoplasmic reticulum and mitochondria for Ca2+ oscillations. Sci Rep 5:7984
Raffaello A, De Stefani D, Sabbadin D, Teardo E, Merli G, Picard A, Checchetto V, Moro S, Szabo I, Rizzuto R (2013) The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J 32:2362–2376
Raffaello A, Mammucari C, Gherardi G, Rizzuto R (2016) Calcium at the center of cell signaling: interplay between endoplasmic reticulum, mitochondria, and lysosomes. Trends Biochem Sci 41(12):1035–1049
Rapizzi E, Pinton P, Szabadkai G, Wieckowski MR, Vandecasteele G, Baird G, Tuft RA, Fogarty KE, Rizzuto R (2002) Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J Cell Biol 159:613–624
Raturi A, Gutierrez T, Ortiz-Sandoval C, Ruangkittisakul A, Herrera-Cruz MS, Rockley JP, Gesson K, Ourdev D, Lou PH, Lucchinetti E et al (2016) TMX1 determines cancer cell metabolism as a thiol-based modulator of ER-mitochondria Ca2+ flux. J Cell Biol 214:433–444
Ribeiro CM, McKay RR, Hosoki E, Bird GS, Putney JW Jr (2000) Effects of elevated cytoplasmic calcium and protein kinase C on endoplasmic reticulum structure and function in HEK293 cells. Cell Calcium 27:175–185
Rimessi A, Giorgi C, Pinton P, Rizzuto R (2008) The versatility of mitochondrial calcium signals: from stimulation of cell metabolism to induction of cell death. Biochim Biophys Acta 1777:808–816
Rimessi A, Marchi S, Patergnani S, Pinton P (2014) H-Ras-driven tumoral maintenance is sustained through caveolin-1-dependent alterations in calcium signaling. Oncogene 33:2329–2340
Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T (1998) Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280:1763–1766
Rizzuto R, De Stefani D, Raffaello A, Mammucari C (2012) Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 13:566–578
Roderick HL, Lechleiter JD, Camacho P (2000) Cytosolic phosphorylation of calnexin controls intracellular Ca2+ oscillations via an interaction with SERCA2b. J Cell Biol 149:1235–1248
Rowland AA, Voeltz GK (2012) Endoplasmic reticulum-mitochondria contacts: function of the junction. Nat Rev Mol Cell Biol 13:607–625
Sala-Vila A, Navarro-Lerida I, Sanchez-Alvarez M, Bosch M, Calvo C, Lopez JA, Calvo E, Ferguson C, Giacomello M, Serafini A et al (2016) Interplay between hepatic mitochondria-associated membranes, lipid metabolism and caveolin-1 in mice. Sci Rep 6:27351
Sancak Y, Markhard AL, Kitami T, Kovacs-Bogdan E, Kamer KJ, Udeshi ND, Carr SA, Chaudhuri D, Clapham DE, Li AA et al (2013) EMRE is an essential component of the mitochondrial calcium uniporter complex. Science 342:1379–1382
Saotome M, Safiulina D, Szabadkai G, Das S, Fransson A, Aspenstrom P, Rizzuto R, Hajnoczky G (2008) Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc Natl Acad Sci USA 105:20728–20733
Schneeberger M, Dietrich MO, Sebastian D, Imbernon M, Castano C, Garcia A, Esteban Y, Gonzalez-Franquesa A, Rodriguez IC, Bortolozzi A et al (2013) Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell 155:172–187
Sebastian D, Hernandez-Alvarez MI, Segales J, Sorianello E, Munoz JP, Sala D, Waget A, Liesa M, Paz JC, Gopalacharyulu P et al (2012) Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc Natl Acad Sci USA 109:5523–5528
Shimizu H, Schredelseker J, Huang J, Lu K, Naghdi S, Lu F, Franklin S, Fiji HD, Wang K, Zhu H et al (2015) Mitochondrial Ca2+ uptake by the voltage-dependent anion channel 2 regulates cardiac rhythmicity. Elife 4:4e0481
Smirnova E, Griparic L, Shurland DL, van der Bliek AM (2001) Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 12:2245–2256
Sugiura A, Nagashima S, Tokuyama T, Amo T, Matsuki Y, Ishido S, Kudo Y, McBride HM, Fukuda T, Matsushita N et al (2013) MITOL regulates endoplasmic reticulum-mitochondria contacts via Mitofusin2. Mol Cell 51:20–34
Szabadkai G, Bianchi K, Varnai P, De Stefani D, Wieckowski MR, Cavagna D, Nagy AI, Balla T, Rizzuto R (2006) Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol 175:901–911
Szado T, Vanderheyden V, Parys JB, De Smedt H, Rietdorf K, Kotelevets L, Chastre E, Khan F, Landegren U, Soderberg O et al (2008) Phosphorylation of inositol 1,4,5-trisphosphate receptors by protein kinase B/Akt inhibits Ca2+ release and apoptosis. Proc Natl Acad Sci USA 105:2427–2432
Terasaki M, Jaffe LA, Hunnicutt GR, Hammer JA 3rd (1996) Structural change of the endoplasmic reticulum during fertilization: evidence for loss of membrane continuity using the green fluorescent protein. Dev Biol 179:320–328
Tomar D, Dong Z, Shanmughapriya S, Koch DA, Thomas T, Hoffman NE, Timbalia SA, Goldman SJ, Breves SL, Corbally DP et al (2016) MCUR1 Is a scaffold factor for the MCU complex function and promotes mitochondrial bioenergetics. Cell Rep 15:1673–1685
Tooze SA, Jefferies HB, Kalie E, Longatti A, McAlpine FE, McKnight NC, Orsi A, Polson HE, Razi M, Robinson DJ et al (2010) Trafficking and signaling in mammalian autophagy. IUBMB Life 62:503–508
Tsai MF, Phillips CB, Ranaghan M, Tsai CW, Wu Y, Willliams C, Miller C (2016) Dual functions of a small regulatory subunit in the mitochondrial calcium uniporter complex. Elife 5:e15545
Tubbs E, Theurey P, Vial G, Bendridi N, Bravard A, Chauvin MA, Ji-Cao J, Zoulim F, Bartosch B, Ovize M et al (2014) Mitochondria-associated endoplasmic reticulum membrane (MAM) integrity is required for insulin signaling and is implicated in hepatic insulin resistance. Diabetes 63:3279–3294
Vais H, Tanis JE, Muller M, Payne R, Mallilankaraman K, Foskett JK (2015) MCUR1, CCDC90A, is a regulator of the mitochondrial calcium uniporter. Cell Metab 22:533–535
Van Petegem F (2012) Ryanodine receptors: structure and function. J Biol Chem 287:31624–31632
Van Petegem F (2015) Ryanodine receptors: allosteric ion channel giants. J Mol Biol 427:31–53
Vance JE (1990) Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem 265:7248–7256
Vance JE (2014) MAM (mitochondria-associated membranes) in mammalian cells: lipids and beyond. Biochimica et biophysica acta 1841:595–609
Vandecaetsbeek I, Vangheluwe P, Raeymaekers L, Wuytack F, Vanoevelen J (2011) The Ca2+ pumps of the endoplasmic reticulum and Golgi apparatus. Cold Spring Harb Perspect Biol 3
Vangheluwe P, Sepulveda MR, Missiaen L, Raeymaekers L, Wuytack F, Vanoevelen J (2009) Intracellular Ca2+- and Mn2+-transport ATPases. Chem Rev 109:4733–4759
Verfaillie T, Rubio N, Garg AD, Bultynck G, Rizzuto R, Decuypere JP, Piette J, Linehan C, Gupta S, Samali A et al (2012) PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ 19:1880–1891
Vermassen E, Parys JB, Mauger JP (2004) Subcellular distribution of the inositol 1,4,5-trisphosphate receptors: functional relevance and molecular determinants. Biol Cell 96:3–17
Vittorini S, Storti S, Parri MS, Cerillo AG, Clerico A (2007) SERCA2a, phospholamban, sarcolipin, and ryanodine receptors gene expression in children with congenital heart defects. Mol Med 13:105–111
Wahl M, Sleight RG, Gruenstein E (1992) Association of cytoplasmic free Ca2+ gradients with subcellular organelles. J Cell Physiol 150:593–609
Wieckowski MR, Giorgi C, Lebiedzinska M, Duszynski J, Pinton P (2009) Isolation of mitochondria-associated membranes and mitochondria from animal tissues and cells. Nat Protoc 4:1582–1590
Zhang A, Williamson CD, Wong DS, Bullough MD, Brown KJ, Hathout Y, Colberg-Poley AM (2011a) Quantitative proteomic analyses of human cytomegalovirus-induced restructuring of endoplasmic reticulum-mitochondrial contacts at late times of infection. Mol Cell Proteomics 10(M111):009936
Zhang L, Liu Y, Song F, Zheng H, Hu L, Lu H, Liu P, Hao X, Zhang W, Chen K (2011b) Functional SNP in the microRNA-367 binding site in the 3’UTR of the calcium channel ryanodine receptor gene 3 (RYR3) affects breast cancer risk and calcification. Proc Natl Acad Sci USA 108:13653–13658
Acknowledgments
P.P. is grateful to Camilla degli Scrovegni for continuous support. P.P. is supported by: Telethon (GGP15219/B), the Italian Association for Cancer Research (AIRC) (IG-18624), the Italian Cystic Fibrosis Research Foundation (19/2014), the Italian Ministry of Education, University and Research (COFIN no. 20129JLHSY_002, FIRB no. RBAP11FXBC_002, and Futuro in Ricerca no. RBFR10EGVP_001), local funds from the University of Ferrara and the Italian Ministry of Health. C.G is supported by AIRC (MFAG-13521), the Italian Ministry of Health, Cariplo and local funds from the University of Ferrara. S.P. is supported by a research fellowship FISM—Fondazione Italiana Sclerosi Multipla—cod. 2014/B/3. GB and JBP are supported by grants from the Research Foundation - Flanders (FWO) (grants G.0571.12N, G.0819.13N, G.0C91.14N, G.0927.15N and G.0A34.16N) and the Research Council - KU Leuven (OT14/101). MB and MK are holders of a Ph.D. Fellowship from the FWO.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Marchi, S. et al. (2017). Endoplasmic Reticulum-Mitochondria Communication Through Ca2+ Signaling: The Importance of Mitochondria-Associated Membranes (MAMs). In: Tagaya, M., Simmen, T. (eds) Organelle Contact Sites. Advances in Experimental Medicine and Biology, vol 997. Springer, Singapore. https://doi.org/10.1007/978-981-10-4567-7_4
Download citation
DOI: https://doi.org/10.1007/978-981-10-4567-7_4
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-4566-0
Online ISBN: 978-981-10-4567-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)