Abstract
Arbuscular mycorrhizal fungi (AMF) are obligate fungi (root symbionts) of the phylum of Glomeromycota that associated with 70–90% of land’s plants. AMF are found in many types of soils and ecosystems. AMF can colonize plant roots on serpentine soils, and 11 AMF genera and Glomeraceae as dominant family are found. Diversity of AMF on serpentine soil is influenced by soil chemical properties (metal content, Ni and Mg/Ca ratio), plant species, and vegetation types as well as AMF types. Inoculation of AMF improved growth, biomass, and nutrient uptake (especially P) for sensitive plant and nickel accumulators. Ni uptake by inoculated plants is inconsistent, showing that AMF reduced Ni in sensitive plant tissues. Otherwise, AMF increased Ni uptake in hyperaccumulator plants. Effectiveness of AMF is determined by plant species and AMF. AMF colonization is essential for vegetation successional acceleration and revegetation success in nickel post-mining land. AMF are potential to be developed as a biological fertilizer to support revegetation of nickel post-mining land on serpentine soil.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Introduction
Serpentine soil covers less than 3% of the earth’s surface and distributed in several regions in the world of California, Cuba, South Africa, South Europe, New Caledonia, Southeast Asia, and West Australia (Coleman and Jove 1992; Guillot and Hattori 2013). Lodging in Southeast Asia, serpentine soil spreads over the northern part of Borneo, Palawan, Mindanao, Sabah, and the majority of Sulawesi and Halmahera (Whitten et al. 1987; Proctor 2003; Ent et al. 2013, 2015). Serpentine soil has the following characteristics: high Mg/Ca ratio, heavy metal (Co, Cu, Cr, Mn, Ni) concentrations, and deficiencies of macronutrients (N, P, K) (Brooks 1987; Kruckeberg 1984; Proctor 2003). This is a characteristic of the syndrome known as serpentine. In addition to the phenomenon of serpentine syndrome, nickel mining activities also contribute to the degradation of serpentine soil and vegetation removal (Review of O’Dell and Claassen 2009). Conditioning the land can be toxic as well as restrict the growth and development of plants and soil microbial activity.
Each species has a specific mechanism for adaptation, tolerance, and survival on serpentine soil. One of the mechanisms of plant adaptation on serpentine soil may be symbiosed with arbuscular mycorrhizal fungi (AMF). AMF are an obligate fungi (root symbionts) of the phylum Glomeromicota with 80% of land plants (Smith and Read 2008). Southworth et al. (2014) reported that soil fertility was low in serpentine soil and stress that strong serpentine habitat was thought to stimulate the formation of mycorrhizae. AMF play an important role in supporting the growth and development of sensitive plants grown in serpentine soils through improved water and nutrient status and heavy metal stress tolerance. The presence of AMF on serpentine soil plays an important role in adaptation, distribution, and succession (community dynamics) in various types of vegetation in conditions of serpentine (Hopkins 1987; Castelli and Casper 2003; Perrier et al. 2006; Husna et al. 2016a). AMF influencing on crop tolerance to heavy metal toxicity is seriously affected by many factors such as soils, plant species, isolates, and type of symbiont and metal concentrations (Amir et al. 2013).
Various studies related to AMF symbiosis with plants in serpentine soil have been carried out. AMF ecological studies in the rhizosphere of plants on serpentine soil have been reviewed by Southworth et al. (2014). However, studies had examined the roles of AMF in the growth and uptake of unavailable plant nutrient. Therefore, this article is to review the AMF ecology, inoculated plant growth, and researches on the AMF symbiosis with plant species and planting practices in Indonesia.
12.2 Ecology of AMF on Serpentine Soils
AMF symbiosis with plants in serpentine soil has been reported in both temperate and tropical regions (Southworth et al. 2014; Husna et al. 2015). AMF have been associated with 27 species of herbaceous plants colonized in serpentine grassland in California (Hopkins 1987). On serpentine soils in Italy, four plant species, namely, Centaurea paniculata, Genista salzmannii, Helichrysum italicum, and Thymus striatus, were colonized by native AMF (Lioi and Giovannetti 1989). There is also a symbiosis between AMF plant species in grassland of Pennsylvania dominated by Andropogon gerardii, Schizachyrium scoparium, Sorghastrum nutans, and Sporobolus heterolepis (Castelli and Casper 2003). Perrier et al. (2006) found a symbiosis between AMF and 10 endemic species of plants that grow on ultramafic lands in Koniambo Massif, New Caledonia. Cumming and Kelly (2007) also reported six AMF species in the rhizosphere of three vegetation types in Maryland, USA, site, while the abundance and sporulation of AMF were more in grassland site. According to Lagrange et al. (2011), roots of nine types of Cyperaceae homegrown ultramaphic New Caledonia were colonized by AMF. AMF had also been found in serpentine revegetated lands of Southeast and South Sulawesi, Indonesia (Setiadi and Setiawan 2011; Husna et al. 2014, 2015, 2016a).
In addition to ultramaphic adaptive and tolerant plants, the presence of AMF also reported in symbiosis with plants hyperaccumulator of Ni. AMF colonization was found to be high on hyperaccumulator of Ni Berkheya coddii and three other types of family Asteraceae, namely, Senecio anomalochrous, S. coronatus, and B. zeyherri in South Africa (Turnau and Mesjasz-Przbylowicz 2003). There are variations in mycorrhizal colonization on plant hyperaccumulation on nickel site of Phyllanthus favieri in humid forest dominated by Nothofagus. The Koniambo Massif, New Caledonia, and plants that are not colonized tend to have high nickel content in leaves (Perrier et al. 2006). According to Amir et al. (2007), nine endemic species of New Caledonia were symbiosed with the AMF, while Sebertia acuminata, Psychotria douarrei, and Phyallanthus favieri plants represented lower root AMF colonization than the others. Amir et al. (2013) have again reported that AMF colonized all types of hyperaccumulators of nickel and AMF colonization was relatively low on conditions of strong hyperaccumulator while weaker than hyperacumulators. Rhizosphere of AMF of strong nickel hyperaccumulators of plant has a high tolerance to the high Ni and is able to colonize the roots (Amir et al. 2007).
AMF genera found in the root zone of plants in the serpentine soil were Archaespora, Acaulopsora, Diversispora, Scutellospora, Pacispora, Glomus, Sclerocystis, Funneliformis, Rhizophagus, Claroideoglomus, and Paraglomus (Table 12.1). Glomeraceae is the largest family. F. mosseae, C. etunicatum, and R. fasciculatum are types of dominant AMF and widespread (Hopkins 1987; Lioi and Giovannetti 1989; Castelli and Casper 2003; Gustafson and Casper 2004; Lagrange et al. 2011; Orlowska et al. 2011; Husna et al. 2015). In addition to the AMF, the ectomycorrhiza type has also been found to associate with plants in serpentine soil, in Japan (Kayama et al. 2006), Portugal (Gonçalves et al. 2009), New Caledonia (Perrier et al. 2006; Jourand et al. 2010a, b), and the USA (Gladish et al. 2010).
Various factors that affect colonization, spore abundance, and species richness AMF in serpentine soil include soil chemical properties (metal content, Ni and Mg/Ca ratio), plant species, and vegetation types as well as type of AMF. Ni content can lower sporulation and AMF species richness (Perrier et al. 2006; Cumming and Kelly 2007; Amir et al. 2007) and root colonization (Lagrange et al. 2011; Amir et al. 2013). Nonetheless, Doubková et al. (2011) found a low AMF colonization in rooting types of Knautia sparsiflora on serpentine than non-serpentine, and there is a positive correlation between colonization of AMF and soil pH, Ca, and K or Ca/Mg ratio. Colonization level and diversity of AMF are reported to be relatively similar between on and off serpentine soil (Schechter and Bruns 2008). Perrier et al. (2006) reported that there was a positive relationship between the availability of metals (Ni and Co) and abundance of black spore. Relationships of fungi and plants in serpentine grassland in Pensylvannia were heavily influenced by the performance of the plant or regulatory diversity (Castelli and Casper 2003). Moreover, there was a reciprocal relationship between plants and fungal-specific serpentine soil, where Gigaspora gigantea increased plant biomass of Schizachyrium and Andropogon, but a decline in biomass of Andropogon inoculated with Glomus etunicatum.
AMF isolated from the roots of hyperaccumulator ultramaphic Ni in soil are more tolerant than nonhyperaccumulators isolated from ultramaphic Ni in soil (Amir et al. 2008). Amir et al. (2008) revealed that five isolates of Glomus spp. in ultramaphic soil were capable of germination under the condition of up to 30 μg/g Ni, but the spores of non-ultramaphic overall stunted at 15 μg/g Ni. According to Doubková et al. (2012), a high tolerance on populations of plants and fungi on serpentine was recorded.
12.3 Plant Growth Affected by AMF on Serpentine Soils
AMF presence on serpentine soil conditions can contribute to tolerance of crops to Ni, decreasing/increasing the uptake and accumulation of Ni. AMF is also suitable for phytoremediation activities with phytoextraction mechanism and phytostabilization (reducing nickel translocation root shoots) and phyto-mining of nickel, as well as local AMF isolated from ultramaphic soil has the potential to support the success of ecological restoration in degraded ecosystems.
Inoculation with indigenous AMF strains significantly enhanced the growth, biomass, and survival of Berkheya coddii than non-mycorrhizal plants (Orlowska et al. 2011). Colonization by Glomus intraradices increased the weight of dry matter sunflower on level 0 and 100 Ni mg/kg treatment, compared to non-mycorrhizal control (Ker and Christine 2009). Glomus sp. and a mixture of AMF improved plant growth and P uptake in Knautia arvensis with increasing intensity of drought (Doubkova et al. 2013). Under greenhouse, native AMF inoculation increased biomass of hyperaccumulator plant shoots in Ni. Berkheya coddii in South Africa had a positive correlation between AMF colonization and shoot height (Turnau and Mesjasz-Przybylowics 2003). Gustafson and Casper (2004) reported that G. intraradices promoted plant growth in Andropogon gerardii on serpentine soil and sand mixture.
Two types of ultramaphic endemic in New Caledonia, Alphitonia neocaledonica, and Cloezia artensis inoculated with Glomus etunicatum at the age of 12 months accumulated dry matter and high P (Amir et al. 2013). Boulet and Lambers (2005) reported that inoculation with AMF induced lower plant growth but increased levels of P and K in the shoot. The concentration of P, Ca, Zn, and Cu in mycorrhizal Berkheya coddii plants was higher than in non-mycorrhizal plants, while an increase in the levels of P in inoculated plant was ten times, compared to the control (Orlowska et al. 2008). AMF inoculation with Glomus sp. increased the biomass of shoots and roots in Costularia comosa by 124% and 246%, respectively (Lagrange et al. 2011). Inoculation with local AMF improved P uptake of the Knautia arvensis plant (Doubková et al. 2011). Doubková et al. (2012) reported that isolates of AMF on serpentine soil not only had high root colonization but also efficiently supported plant growth and P uptake in Knautia arvensis.
Ni uptake by inoculated plants is inconsistent. Inoculation with AMF increased Ni content types such as nickel hyperaccumulator in Berkheya coddii (Turnau and Mesjasz-Przybylowics 2003; Orlowska et al. 2011) and sunflower (Ker and Christine 2009). Inoculation with AMF reduced the Ni content at the top of Phaseolus vulgaris (Guo et al. 1996), shoots of Trifolium repens (Vivas et al. 2006), the root of Costularia comosa (Lagrange et al. 2011, 2013), Knautia arvensis (Doubková et al. 2011), Alphitonia neocaledonica, and Cloezia artensis (Amir et al. 2013) compared to the control. Ni content in leaves was not influenced by the presence of the AMF (Boulet and Lambers 2005). High Ni accumulation in plants is allegedly due to AMF which enhances the activity of glutamine synthesis (chelate nickel) in the root (Ker and Christine 2009).
12.4 Plant-AMF Symbiosis on Serpentine Soil in Indonesia
Indonesia is one of the regions in the world which has fairly large ultramaphic bedrock scattered on the island of Sulawesi and Halmahera (Whitten et al. 1987; Proctor 2003; Ent et al. 2013). Ultramaphic bedrock in Indonesia has productive potential for nickel mining operations. Nickel mining operations in Sulawesi have been conducted by PT. INCO (known as PT. Vale Indonesia) in South Sulawesi, Central and Southeast, as well as PT. ANTAM, Tbk in Pomalaa, Southeast Sulawesi, and ± 200an mining business license (IUP) has also been operating in Southeast Sulawesi. Nickel exploitation activities are generally carried out using opencast mining. This method usually ruins the landscape and leaving land nutrient deficiency and toxic heavy metals and reduces the biological activity, waterlogging, etc. (Review O’Dell and Claassen 2009). These lands should be restored with revegetation techniques. Selection of seed types and supplying seedling with soil microbes (Mycorrhizae and Rhizobium) are very important to support the success of the nickel post-mining site revegetation (Marpaung et al. 1994; Ambodo 2002; Mansur 2010; Husna et al. 2012).
Exploration, extraction, identification, and propagation of AMF on serpentine soils in Sulawesi began in the mid-1990s. Based on the identification of spore morphology, we discovered the eight AMF genera, including Glomus, Septoglomus, Claroideoglomus, Rhizopagus, Acaulospora, Gigaspora, Racocetra, and Scutellospora, in the rhizosphere of plant revegetation and vegetation succession on nickel post-mining lands (Review Husna et al. 2012; Husna et al. 2014, 2015; Husna et al. 2016a; Setiadi and Setiawan 2011). Husna et al. (2014, 2015) found seven kinds of AMF in revegetation serpentine lands in PT. Vale Indonesia, Southeast Sulawesi Pomalaa, namely, Glomus canadense, G. boreale, Rhizopagus diaphanous, Septoglomus constrictum, Claroideoglomus etunicatum, Racocetra gregaria, and Scutellospora auriglobosa. According to Husna et al. (2016a), AMF colonized 15 pioneer species nickel post-mining land in Pomalaa, Southeast Sulawesi. Plant types Cynodon dactylon, Ipomoea sp., and Sarcotheca celebica have the highest root mycorrhizal colonization (≥70%). Information on AMF colonization in rooting in hyperaccumulator of nickel (S. celebica) enriches knowledge of AMF symbiosis with hyperaccumulator types of nickel across the world. AMF colonization in all 15 types of pioneer speciesis also indicated that the AMF alleged role accelerating vegetation succession in post-mining land nickel.
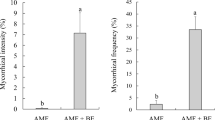
On a nursery and greenhouse scale, inoculation with exotic (GMRT-17 and ET-17) and local isolates (INCO-12) effectively increased the growth and improvement of seed quality of Paraserianthes falcataria, Acacia mangium, and Trichospermum buretti (Marpaung et al. 1994). According to Husna (2010), seedling of Pericopsis mooniana inoculated with 5 g of AMF Mycofer inocula (mix Glomus manihotis, Glomus etunicatum, Acaulospora tuberculata, Gigaspora margarita) and the addition of 20 g of pulp sago had high growth, dry weight, nodule, and the uptake of K and Ca with an increase in each of the controls 51.4%, 41.3%, 19.7%, 8.8%, and 25.6% and reduced by 32% of Ni. Inoculation with AMF Mycofer gave a high boost of nodules and biomass of Albizia saponaria plant age of 3 months with an increase over the control by 76%, 447%, and 309% (Tuheteru et al. 2011). In the serpentine soil media, P. mooniana desperately need mycorrhizal (MIE> 75%) to support growth (Husna et al. 2016b). Local AMF isolated from the roots of P. mooniana of CA Lamedai (non-serpentine) and PT. Vale Indonesia (serpentine) better increased the biomass of P. mooniana at 3 months in the media serpentine soil, which increased 442–472% over the controls and 64% and 73% over the commercial Mycofer inoculum (Fig. 12.1).
Growth performance of P. mooniana at 3 months of age inoculated with local AMF on serpentine soil media (Husna et al. 2016b)
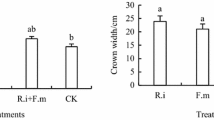
On the field scale, P. mooniana inoculated with AMF (CA Lamedai and PT. Vale Indonesia) planted in post-mining land of nickel (Mg/Ca ratio of 3.55 and a concentration of 2.1 ppm Ni) 3 months after implantation has the tolerance for survival, growth, biomass, and accumulation of N, P, and K, which is higher than the control and low Ni content (Husna et al. 2015). At the age of 24 months after planting, inoculation with local AMF also increased the height and diameter growth of P. mooniana on the field over the control (Fig. 12.2).
Based on the important role of the AMF, PT. Vale Indonesia (PT.) Sorowako South Sulawesi has been producing AMF-inoculated seeds on nursery scale for revegetation purposes (Ambodo 2002; Mansur 2010).
12.5 Conclusion Remarks
AMF, assosiated with plant root on serpentine soil, have important roles to support growth and improved nutrient status on serpentine soil media. Furthermore, AMF are important components in ecological restoration for revegetation degraded land (nickel post-mining land). AMF diversity study has been done in various countries (the center of serpentine soil) using morphology and DNA molecular approach, details the response of non-mycorrhizal and mycorrhizal plants on serpentine soil conditions, and includes adaptation mechanisms of anatomy, physiology, molecular and signaling pathways, as well as the response of AMF spores (serpentine and non-serpentine isolates) to heavy metal stress, especially Ni.
References
Ambodo AP (2002) Mine reclamation – the PT Inco experience. Proceedings of the 26th Annual British Columbia Mine Reclamation Symposium in Dawson Creek, BC
Amir H, Parrier N, Riagault F et al (2007) Relationships between Ni-hyperaccumulation and mycorrhizal status of different endemic plant species from New Caledonian ultramafic soils. Plant Soil 293:23–35
Amir H, Jasper DA, Abbott LK (2008) Tolerance and induction of tolerance to Ni of arbuscular mycorrhizal fungi from New Caledonian ultramafic soils. Mycorrhiza 19:1–6
Amir H, Lagrange A, Hassaïne N et al (2013) Arbuscular mycorrhizal fungi from New Caledonian ultramafic soils improve tolerance to nickel of endemic plant species. Mycorrhiza 23:585–595
Boulet FM, Lambers H (2005) Characterisation of arbuscular mycorrhizal fungi colonisation in cluster roots of Hakea verrucosa F. M. (Proteaceae), and its effect on growth and nutrient acquisition in ultramafic soil. Plant Soil 269:357–367
Brooks RR (1987) Serpentine and its vegetation. A multidisciplinary approach. Dioscorides Press, Portland
Castelli JP, Casper BB (2003) Intraspecific AM fungal variation contributes to plant-fungal feedback in a serpentine grassland. Ecology 84:323–336
Coleman R, Jove C (1992) Geological origin of serpentinites. In: Baker AJM, Proctor J, Reeves RD (eds) The vegetation ofultramafic (serpentine) soils. Intercept Ltd, Andover, pp 1–18
Cumming JR, Kelly CN (2007) Pinus virginiana invasion influences soils and arbuscular mycorrhizae of a serpentine grassland. J Torrey Bot Soc 134(1):63–73
Doubková P, Suda J, Sudová R (2011) Arbuscular mycorrhizal symbiosis on serpentine soils: the effect of native fungal communities on different Knautia arvensis ecotypes. Plant Soil 345:325–338
Doubková P, Suda J, Sudová R (2012) The symbiosis with arbuscular mycorrhizal fungi contributes to plant tolerance to serpentine edaphic stress. Soil Biol Biochem 44:56–64
Doubkova P, Vlasakova E, Sudova R (2013) Arbuscular mycorrhizal symbiosis alleviates drought stress imposed on Knautia arvensis plants in serpentine soil. Plant Soil 370:149–161
Ent AVD, Baker AJM, Van Balgooy MMJ et al (2013) Ultramafic nickel laterites in Indonesia (Sulawesi, Halmahera): mining nickel hyperaccumulators and opportinities for phytomining. University Of Queensland. J Chem Explor 128:72–79
Ent AVD, Erskine P, Sumail S (2015) Ecology of nickel hyperaccumulator plants from ultramafic soils in Sabah (Malaysia). Chemoecology 25:243–259
Gladish S, Frank JL, Southworth D (2010) The serpentine syndrome belowground: ectomycorrhizas and hypogeous fungi associated with conifers. Can J For Res 40:1671–1679
Gonçalves SC, Martins-Loução MA, Freitas H (2009) Evidence of adaptive tolerance to nickel in isolates of Cenococcum geophilum from serpentine soils. Mycorrhiza 19:221–230
Guillot S, Hattori K (2013) Serpentinites: essential roles in geodynamics, arc volcanism, sustainable development, and the origin of life. Elements 9(2):95–98
Guo Y, George E, Marschner H (1996) Contribution of an arbuscular mycorrhizal fungus to the uptake of cadmium and nickel in bean and maize plants. Plant Soil 184:195–205
Gustafson DJ, Casper BB (2004) Nutrient addition affects AM fungal performance and expression of plant/fungal feedback in three serpentine grasses. Plant Soil 259:9–17
Hopkins NA (1987) Mycorrhizae in a California serpentine grassland community. Can J Bot 65:484–487
Husna (2010) Pertumbuhan bibit kayu kuku (Pericopsis mooniana THW) melalui aplikasi fungi mikoriza arbuskula (FMA) dan ampas sagu pada media tanah bekas tambang nikel [thesis]. Univeristas Halu Oleo, Kendari
Husna, Tuheteru FD, Arif A (2012) Post-mine land re-vegetation in Southeast Sulawesi biotechnology-based Mycorrhizal Fungi. Proceeding of International Conference on Perspectives of Tropical Forest Rehabilitation Better Forest Functions and Management. Faculty of Forestry UGM, Yogyakarta, pp 186–190
Husna, Budi SWR, Mansur I, Kusmana C, Kramadibrata K (2014) Arbuscular Mycorrhizal Fungi from Rhizosphere of Pericopsis mooniana (Thw.) Thw. in South-East Sulawesi. Berita Biologi 13(3):263–273
Husna, Budi SWR, Mansur I, Kusmana C (2015) Diversity of arbuscular mycorrhizal fungi in the growth habitat of kayu kuku (Pericopsis mooniana Thw.) in Southeast Sulawesi. Pak J Biol Sci 18(1):1–10
Husna, Tuheteru FD, Khalifah N (2016a)Symbiosis arbuscular mycorrhizal fungi with pioneer plants on nickel post mining land. Presented paper on national seminar of Silviculture IV.Faculty of Forestry, Mulawarman University, Balikpapan (Indonesia) 19–20 Juli 2016
Husna, Sri Wilarso Budi R, Mansur I, Kusmana C (2016b) Growth and nutrient status of kayu kuku (Pericopsis mooniana Thw.) with mycorrhiza in soil media of nickel post mining. Pak J Biol Sci 19:158–170
Ji BM, Bentivenga SP, Casper BB (2010) Evidence for ecological matching of whole AM fungal communities to the local plant–soil environment. Ecology 91:3037–3046
Jourand P, Ducousso M, Loulergue-Majorel C et al (2010a) Ultramafic soils from New Caledonia structure Pisolithus albus in ecotype. FEMS Microbiol Ecol 72:238–249
Jourand P, Ducousso M, Reid R et al (2010b) Nickel-tolerant ectomycorrhizal Pisolithus albus ultramafic ecotype isolated from nickel mines in New Caledonia strongly enhance growth of a host plant at toxic nickel concentrations. Tree Physiol 30:1311–1319
Kayama M, Choi D, Tobita H et al (2006) Comparison of growth characteristics and tolerance to serpentine soil of three ectomycorrhizal spruce seedlings in northern Japan. Trees 20:430–440
Ker K, Christine C (2009) Nickel remediation by AM-colonized Sunflower. Mycorrhiza 20:399–406
Kruckeberg AR (1984) California serpentines: flora, vegetation,geology, soils and management problems. University of California Press, Berkeley
Lagrange A, Ducousso M, Jourand P et al (2011) New insights into the mycorrhizal status of Cyperaceae from ultramafic soils in New Caledonia. Can J Microbiol 57:21–28
Lagrange A, L’Huillier L, Amir H (2013) Mycorrhizal status of Cyperaceae from new Caledonian ultramafic soils: effects of phosphorus availability on arbuscular mycorrhizal colonization of Costularia comosa under field conditions. Mycorrhiza 23:655–661
Lioi L, Giovannetti M (1989) Vesicular-arbuscular mycorrhizae and species of the Endogonaceae in an Italian serpentine soil. G Bot Ital 123:1–8
Mansur I (2010) Teknik Silvikultur untuk Reklamasi Lahan Bekas Tambang. SEAMEO BIOTROP, Bogor. (in Indonesia)
Marpaung P, Setiadi Y, Tobing B (1994) Revegetation development and progress in nickel mine sites at PT. International Nickel Indonesia. In: Simatupang M, Wahju BN (eds) Mineral development in Asia Pasific into the year 2000. Proceeding of the 4th Asia Pacific mining Conference, Jakarta 26–29 Oktober 1994. Asean Federation of Mining Association, Jakarta
O’Dell RE, Claassen VP (2009) Serpentine revegetation: a review. Soil and biota of serpentine: a world view 2009. Northeast Nat 16:253–271
Orlowska E, Mesjasz-Przybylowicz J, Przybylowicz W et al (2008) Nuclear microprobe studies of elemental distribution in mycorrhizal and non-mycorrhizal roots of ni-hyperaccumulator Berkheya coddii. X-Ray Spectrom 37:129–132
Orlowska E, Przybylowicz W, Orlowski D et al (2011) The effect of mycorrhiza on the growth and elemental composition of ni-hyperaccumulating plant Berkheya coddii roessler. Environ Pollut 159:3730–3738
Perrier N, Amir H, Colin F (2006) Occurrence of mycorrhizal symbioses in the metal-rich lateritic soils of the Koniambo massif, New Caledonia. Mycorrhiza 16:449–458
Proctor J (2003) Vegetation and soil and plant chemistry on ultramic rocks in the tropical far east. Perspect Plant Ecol Evol Syst 6(1,2):105–124
Schechter SP, Bruns TD (2008) Serpentine and non-serpentine ecotypes of Collinsia sparsiflora associate with distinct arbuscular mycorrhizal fungal assemblages. Mol Ecol 17:3198–3210
Setiadi Y, Setiawan A (2011) Study of arbuscular mycorrhizal fungi status at rehabilitation post-nickel mining area (Case study at PT INCO Tbk. Sorowako, South Sulawesi). J Silvikultur Tropika 3(1):88–95
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic Press, USA
Southworth D, Tackaberry LE, Massicotte HB (2014) Mycorrhizal ecology on serpentine soils. Plant Ecol Divers 7(3):445–455
Tuheteru FD, Husna, Arif A (2011) Response of growth and dependency of Albizia saponaria (Lour.) Miq on local arbuscular mycorrhizae fungi from Southeast Sulawesi in post-nickel mining soil. Berita Biologi 5:605–611
Turnau K, Mesjasz-Przybylowicz J (2003) Arbuscular mycorrhiza of Berkheya coddii and other ni-hyperaccumulating members of Asteraceae from ultramafic soils in South Africa. Mycorrhiza 13:185–190
Vivas A, Biró B, Németh T et al (2006) Nickel-tolerant Brevibacillus brevis and arbuscular mycorrhizal fungus can reduce metal acquisition and nickel toxicity effects in plant growing in nickel supplemented soil. Soil Biol Biochem 38:2694–2704
Whitten AJ, Mustafa M, Henderson GS (1987) Ekologi Sulawesi. Gadjah Mada University Press, Yogyakarta
Acknowledgments
This research was supported by a competitive grant (No. 228c.1/UN29.20/PP/2016) and a fundamental grant (No. 229.a.1/UN.29.20/PPM/2016) of the Ministry of Research, Technology and Higher Education. The author would like to thank the leadership of the PT. Vale Indonesia (PT.) Pomalaa Region, Kolaka, and PT. Stargate Pacific Resources, North Konawe, Southeast Sulawesi.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Husna, Tuheteru, F.D., Arif, A. (2017). Arbuscular Mycorrhizal Fungi and Plant Growth on Serpentine Soils. In: Wu, QS. (eds) Arbuscular Mycorrhizas and Stress Tolerance of Plants. Springer, Singapore. https://doi.org/10.1007/978-981-10-4115-0_12
Download citation
DOI: https://doi.org/10.1007/978-981-10-4115-0_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-4114-3
Online ISBN: 978-981-10-4115-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)