Abstract
Over the last few years, a boom has been witnessed in the area of soil ecology which has produced numerous data on interactions between plant and rhizospheric microbes. The plant-microbe interactions in the rhizospheric niche have proved to be crucial for the advancement of sustainable farming practices which decrease the usage of chemical fertilizers and pesticides. Root exudates are substances released by plant roots that show a significant role in mediating the plant-microbe interactions in soil. These root exudates send chemical signals to microbes which in response are attracted towards the roots and influence growth of plants, soil properties, and microbial community. This chapter is focussed on recent advancements in the utilization of root exudates in plant-microbe interactions to enhance plant growth promotion. The plant-microbe interactions are categorized as beneficial or detrimental depending upon the characteristics of root exudates. This chapter also covers different types of root exudates and their function in modifying the exchanges between rhizospheric microbes and plants for the betterment of soil health and sustainable ecosystems.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
18.1 Introduction
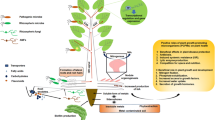
Soil, also known as a “black box,” inhabits diverse macro- and micro-community structures, and the rhizosphere is where contact between soil and roots is established. Biological, physical, and chemical activities are affected by the compounds that the root exudes and microbes feed on (Kamilova et al. 2006; Kumar et al. 2007).
There is a group of chemical substances and signaling compounds produced by plants that help the plant to impart defensive mechanisms against pathogens and attract beneficial microbes (Haichar et al. 2008). The rhizosphere is representative of an extremely lively base of communication between roots, soil microorganisms, invertebrates, and neighboring root systems of competitor plants (Hirsch et al. 2003). There are usually two sub-divisions to the rhizosphere: First is the endo-rhizosphere which contains the root cortex, epidermis, and root hairs. The second is the ecto-rhizosphere with root-connected soil compartments up to 5 mm in length (Fig. 18.1). Hence, this area has made an exciting zone for exploring associations between microorganisms and plants.
The prime role of the root is to provide anchorage and backing for the plant and to facilitate absorption and conduction of water and nutrients (Abbott and Murphy 2003). There is production of root hairs over epidermal cells in tap root and lateral root systems. They are specialized for absorbing water and nutrients from soil. Even though such root functions have been known for a long time, the diversity of root exudates present in the rhizosphere and their exact role in influencing microbial behavior is still unclear (Narula et al. 2009). Plant root exudates are essential parameters for structuring the bacterial community in the rhizosphere (Walker et al. 2003a), performing key roles such as defending against pathogens (Abbott and Murphy 2003), and forming a basis for chemotaxis to mediate attraction and repulsion among particular microbial species and communities (Kumar et al. 2007). Root exudates also maintain moisture and wetting of the soil, mobilize minerals and nutrients, change the chemical characteristics of the soil, provide stability to soil-aggregates surrounding the roots, and inhibit the development of neighboring competitor plants (Narula et al. 2009).
Plant roots continuously release a massive array of significantly beneficial low and high–molecular weight components in the rhizosphere like ions, oxygen, enzymes, water, mucilage, and a number of carbon-containing compounds as well as secondary metabolites (Nardi et al. 2000; Vishwakarma et al. 2016) which helps in complex biological and physico-chemical interactions occurring amongst plant roots and the near-by soil environment. The associations that involve rhizospheric roots consist of root-microbe, root-invertebrate, and root-root interactions (Bais et al. 2001; Gleba et al. 1999).
Root exudates are divided into two separate categories of compounds, namely, Low and High molecular weight compounds.
-
1.
Low–molecular weight exudates consists sugars, organic acids, secondary metabolites, phenols, and the amino acids. This category comprises most of the diversity of root exudates (Rougier 1981).
-
2.
High–molecular weight compounds include proteins and polysaccharides (mucilage) and form less molecular diversity than the low–molecular weight category but they make up a huge percentage of total exudates (Abbott and Murphy 2003; Walker et al. 2003b).
Numerous phytotoxic components exuded from roots have been characterised like 7,8-benzoflavone from Acroptilon repens (Russian knapweed) (Stermitz et al. 2003), (±)-catechin from Centaurea maculosa (spotted knapweed) (Bais et al. 2002a), DIMBOA and DIBOA from Triticum aestivum (wheat) (Wu et al. 2000), juglone from Juglans nigra (black walnut) (Jose and Gillespie 1998), 8- hydroxyquinoline from Centaurea diffusa (diffuse knapweed) (Vivanco et al. 2004), sorgoleone from Sorghum spp. (Nimbal et al. 1996), and 5,7,4′-trihydroxy-3′,5′- dimethoxyflavone from Oryza sativa (rice) (Kong et al. 2004). A study by Bertin et al. (2003) have shown that differential expression of proteins that are exuded from the roots of the cowpea is in response to environmental conditions with their composition varying with respect to pH and ionic level within the root environment. In this research, western blotting and specific enzyme tests have been used to investigate the presence of defense proteins chitinase, LTPs, and β-1,3-glucanase in imbibed root exudates.
The root exudation process was earlier considered a passive process, but evidence is now available for ATP-binding cassette (ABC) transporters forming the basis for phytochemical translocation in the rhizosphere. The evidence supports the fact that the plant is dynamically releasing metabolites in the surrounding soil (Loyola-Vargas et al. 2007; Badri et al. 2008). There are a number of factors on which constituents of root exudates depend; these include plant species and cultivar, phase of development, substrate to grow plant, and stress parameters (Uren 2000). For instance, root exudates of tomato, sweet pepper, and cucumber were shown to contain more organic acids (mainly succinic, citric, and malic acid) than sugars (fructose and glucose) when grown under gnotobiotic conditions (Kamilova et al. 2006). There is accumulation of ubiquitous phenylpropanoids together with phylogenetically restricted glucosinolates in A. thaliana roots (Bednarek et al. 2005). There is also root-derived secretion and accumulation of many flavonoids, triterpene saponins, and isoflavonoids in the cell cultures of M. trunculata (Farag et al. 2007; Huhman et al. 2005).
18.2 Importance and Function of Root Exudates
The major function that roots perform includes providing support and anchorage to the plant and facilitating conduction and absorption of water and nutrients (Abbott and Murphy 2003). There is production of root hairs over epidermal cells besides tap root and lateral root systems. They are specialized for absorbing water and nutrients from soil.
The rhizosphere is considered to be supportive for a wide range of bacteria capable of accelerating the plant growth. These plant growth-promoting rhizobacteria (PGPR) work through a number of mechanisms that have N2-fixation or phytohormone production (Barea et al. 2005).
18.2.1 Against Pathogenic Microorganisms
Plant-microbe communication is one of the most important interactions describing the below-ground zone. Root microbe interactions are influenced by some of the chemicals recognized in the root exudates; these include the flavonoid signals observed in the exudates of legumes, which trigger the genes of R. melitoti responsible for nodulation. Such compounds can also be accountable for foundation of vesicular arbuscular mycorrhizal (VAM) (Becard et al. 1995). For instance, extraction of the Phytophthora cinnamoni cell wall triggers the release of a multifunctional caffeic acid ester i.e. rosmarinic acid (RA), identified in the root exudates of sweet basil cultures (Bais et al. 2006). Similarly, in a report by Brigham et al. (1999), Lithospermum erythrorhizon was shown to produce cell-specific pigmented naphthoquinones.
The physiological state of plants is reflected by the constituents of their root exudates, which are further influenced by both abiotic and biotic parameters (Vishwakarma et al., 2017). For instance, energy is required for inducing expression of the plant defense. This demand for energy is well established for Arabidopsis, i.e., it requires substantial fitness costs to activate the defence responses (Van Hulten et al. 2006). As predicted by local reductions in photosynthetic activity, actuated plant defence need energy to form defence-related compounds (Berger et al. 2007; Bolton 2009). Salicylic acid treatment has been given to the roots of Arabidopsis, and a number of secondary metabolites were secreted, such as butanoic, ferulic and 3-indolepropanoic acid. All of these show antibacterial activity in vitro at the levels identified in exudates against pathogens like Erwinia spp., Xanthomonas campestris, and P. syringae (Walker et al. 2003a, b). On the other hand, P. fluorescens, which is non-pathogenic, was found to be less sensitive to those exudates.
A report by Mavrodi et al. (2012) shows the precise choice of plant growth protecting rhizobacteria under pathogen attack. It is supported by the results that DAPG-forming Pseudomonas were recruited by the wheat rhizosphere during irrigation, while dry conditions supported recruitment of phenazine producing Pseudomonas. G. graminis var. tritici is the chief soil-borne pathogen during irrigation conditions of wheat, while Rhizoctonia solani pose more threat during dry situations. Of note, G. graminis var. tritici shows more sensitivity to DAPG and R. solani has more sensitivity towards phenazines. Hence, in circumstances favoring particular pathogens, plants select those antagonists that show more efficiency against those pathogens (Walker et al. 2003b).
18.2.2 Remediation of Heavy Metals
A significant proportion of soil contamination is represented by heavy metals. The main cause of metal pollution in soil is due to anthropogenic activities such as the use of fertilizers and pesticides containing metal and accumulation of industrial waste (Vishwakarma et al. 2017). The microorganisms residing in rhizospheric niche possess high microbial activity and therefore their utilization of the transformation of organic pollutants or removal of contaminants from soil need to be considered (Kothe et al. 2005). The remediation process of pollutants in soil and water takes place exterior to the plant roots. Many organic pollutants are converted into nontoxic compounds by enzymatic actions of microorganisms, whereas several synthetic compounds known as being ”recalcitrant” are resistant to any kind of biological degradation.
Bioaccumulation of several metals have been studied. Reports are available on the accumulation of metals such as cadmium, copper, and nickel on Streptomycetes, which denotes a group of Gram-negative bacteria found predominantly in poor and contaminated soils (Albarracín et al. 2008; Schmidt et al. 2005; Sineriz et al. 2009).
It was previously reported that the plant-microbe interaction in soils is greatly influenced by the presence of secondary metabolites existing in the root exudates. These secondary metabolites categorize the association between the individual microbe and plant as mutualistic, associative, or pathogenic. For example, Rhizobium spp. form a symbiotic association with legumes and are responsible for symbiotic nitrogen fixation. The interaction between the two organisms is facilitated in part through root-secreted flavones (Redmond et al. 1986).
Plant root exudates have a variety of functions that influence plant growth and also enhance the degradation process of soil contaminants. Some of the important functions of root exudates in the soil include influence on the nutrient cycling processes, enhancement of the degradation organic matter, inhibition of the soil-nitrification process, and interference with the bacterial quorum-sensing response.
18.2.3 Availability of Soil Resources
Availability of phytosiderophores and plant nutrients: Some compounds that are found in the rhizosphere as a part of root exudates function as metal chelators and enhance the availability of metals such as iron, copper, zinc, and manganese for plant uptake (Lambers et al. 2009). It has also been shown that plants use metal chelators in root exudates to enhance nutrient availability for plant growth, for example, the function of graminoid phytosiderophores to transform Fe (III) to form Fe (III)-phytosiderophores, which is taken up by grasses more efficiently than other chelated forms of iron (Doornbos et al. 2012).
Organic acids and phosphorus availability: Organic acids for example citric, malic, and oxalic acid are an important part of root exudates, and also function as metal-chelators in the rhizosphere. More specifically, they are responsible for the solubilization of insoluble forms of phosphate rather than enhancing micronutrient availability (Bais et al. 2006). They form complexes with aluminum or iron in aluminum or ferric phosphates and release phosphates in the form that is taken up by plants (Dakora and Phillips 2002). The mechanism of phosphate solubilization is also reported where plants increase the secretion of carboxylate in the limiting condition of P to solubilize adsorbed phosphate (Vance et al. 2003). Root exudates also play an important role in enhancing the activity of phosphate-solubilizing bacteria to increase the supply of P to the plant (Richardson et al. 2009, 2011).
C- and N- Bio availability: Microbial population in the rhizosphere is highly influenced by those plants that release different types of nutrients from their roots, which are then utilized by the microbes for their growth (Haichar et al. 2012; Baetz and Martinoia 2014). A compound named aminocyclopropane-1-carboxylic acid (ACC) that is secreted by plants is a precursor of ethylene formation and consumed as a C and N source by rhizospheric bacteria (Haichar et al. 2014).
During nitrogen fixation, a less mobile NH4 + is transformed to the highly mobile NO3 − during the process of nitrification. It ultimately influences plant nitrogen uptake because the NO3 − is vulnerable to loss by the denitrification process from the root surface (Subbarao et al. 2007). To improve nitrogen recovery in soils, it is important to regulate the nitrification step. An example of a nitrification inhibitor was discovered in the root exudates of forage grass (Subbarao et al. 2009).
18.2.4 Regulation of Chemotaxis
Chemotaxis, a phenomenon possessed by most of the motile bacteria, is defined as the process where a microorganism moves in response to chemical gradients. Chemotaxis is a well-defined process in the application of plant-microbe interactions, where soil microbes get attracted towards plant roots (Kumar et al. 2007). The chemotactic response of microorganisms to root exudates performs a significant ecological function in plant-linked bacteria and constitutes the initiation of the interaction between roots and microorganisms. A study of Pseudomonas putida showing positive chemotaxis to maize-derived aromatic metabolites was also reported (Neal et al. 2012).
18.3 Plant Root-Microbe Interaction
Plant-microbe interactions show significant communications that characterize the different levels of soil. Root exudates represent an essential constituent mainly helps in maintaining communication between root-associated bacteria and plants. A broad range of chemical compounds and signaling molecules are produced by plants. Approximately 100,000 types of different substrates were produced by plants, which serve as chemotactic agents for microorganisms in plant systems (Bais et al. 2004b).
The unique bacterial communities in the rhizospheric region varies among plant species and through time (Baudoin et al. 2002). Separate root zones in a single plant can assist different bacterial populations, reflecting quantitative and qualitative variations in root exudation (Yang and Crowley 2000). Plants modify the rhizobacterial population by secreting different compounds, which range from single carbohydrate molecules to complex aromatic compounds (Kamilova et al. 2006; Cheng et al. 2014). The reaction of fungal communities to plants is not well documented, although many reports are available targeting mycorrhizal fungi. Similar to bacteria, root exudates are also utilized in maintaining fungal diversity and community structure (Innes et al. 2004). Therefore, it is concluded that the soil ecosystem helps in determining and modifying the rhizospheric microbiome (Garbeva et al. 2008; Lundberg et al. 2012).
A study presented by Broeckling et al. (2008) showed that root exudates from distinctive plant species such as arbuscular mycorrhizal fungi (AMF), nodulating legumes, and a nonmycorrhizal Brassicaceae may affect the diversity of fungal communities of various types within the intact root biomass. The results obtained from the experiment carried out by Broeckling et al. (2008) signify that the regulation of fungal communities by plant root exudates are completed through two different mechanisms that target specific fungal phylotypes. The first mechanism is involved in reducing the relative fungal quantity via an antifungal property of the exudate or a chemical signaling mechanism that restricts their growth. The second mechanism positively regulates abundance either by enhancing chemical signals or by supplying appropriate nutrients for their growth.
18.3.1 Positive Impact
Plant-microbe soil interaction processes are considered to have a significant effect on plant growth by enhancing the supply of nutrients, fixing atmospheric nitrogen, increasing tolerance towards stress conditions, and enhancing resistance against plant pathogens by different classes of endophytic microbes and PGPR (Gray and Smith 2005). Some bacteria can also produce antibiotics and form biofilms that protect them from potential phytopathogens, or degrade toxic compounds produced by either plants or microbes (Bais et al. 2004a).
Plant root exudates perform several functions and play a significant role in root-microbe interactions. Activation of Rhizobium meliloti genes initiates nodulation process with the help of compounds like, flavonoids present in exudates of leguminous plants. The colonization of VAM is also influenced by the composition of root exudates (Becard et al. 1995). The root exudates contain several phytoalexins and many defense proteins, which always protect the survival and growth of the delicate and unprotected root cells from pathogenic microorganisms (Flores et al. 1999).
18.3.1.1 Nitrogen Fixation
Gram-negative nitrogen-fixing bacteria have important plant-microbe interactions with leguminous plants (Morgan et al. 2005). Symbiotic relationships between rod shaped proteobacteria like Rhizobium, Bradyrhizobium, Sinorhizobium, Mesorhizobium, Azorhizobium, and Photorhizobium and infected legume plants are prominent in nature (Bloemberg and Lugtenberg 2001; Madigan and Martinko 2006). Root nodules are formed when a suitable species of one of these rhizobia infects roots (Heidstra and Bisseling 1996), which is why leguminous crops around the world are being treated by these biofertilizers (Deaker et al. 2004). Nod factor, which is a bacterial substance, induces the penetration of rhizobia into root hairs (Perret et al. 2000). These bacteria follow root hair curlings to enter the plant root hairs and then trigger formation by the plant of a cellulosic tube known as an infection thread. These infection threads are then used as a way to spread all over the root hairs and infect adjacent root cells, which helps in triggering plant cell division. This continuous plant cell division later forms root nodules.
This rhizobia-legume communication is very specific, allowing only particular rhizobia strains to nodulate with particular host legumes. By using the lacz reporter gene, Zhu et al. (1997) have shown in their study that Sinorhizobium meliloti effectively nodulated species of the Trigonella, Medicago and Melilotus genera, while Rhizobium leguminosarum bv viciae produces nodulation in the Lathyrus, Lens, Vicia and Pisum genera via border cells.
In a study on non-legume Parasponia andersonii, a elm family member was nodulated by Rhizobium strain NGR234, which also nodulates 232 species of legumes (Pueppke and Broughton 1999). However, not every member of the legume family forms root nodules. For example, Caesalpinoideae are one of three non-nodulating legume sub-families: Caesalpinoideae, Mimosoideae, and Papilionoideae. Nod genes are induced in Mesorhizobium loti by aldonic, erythronic, and tetronic acid, which are all exuded by Lotus corniculatus (Morgan et al. 2005).
Studies have shown that bacteria have the potential to recognize plant-derived compounds (flavonoids) that help in rhizobia-legume interactions through many molecular signaling pathways (Nagahashi and Douds 2003). Flavonoids can be both antagonistic and agonistic for rhizobia species (Cooper 2007). For example, Bradyrhizobium japonicum nod genes are induced by daidzein and genistein, isoflavonoids produced by soybean (Glycine max), but inhibit S. meliloti nod gene expression, whereas luteolin is an inducing agent in the case of S. meliloti nod genes (Peters et al. 1986).
These specificity powers allow rhizobia to differentiate other legumes from their hosts. Rhizobial chemotaxis as well as nod gene expression are induced by a specific flavonoid (Peck et al. 2006; Wang et al. 2012). RNA interference was used by Wasson et al. (2006) to silence chalcone synthase (CHS), which then inhibited nodule formation. In M. truncatula, this enzyme catalyzes the initial dedicated step of the flavonoid pathway, which activates nod genes in S. meliloti. To achieve flavonoid accumulation and nodule development in plants, a supplementation of the flavonoid precursors liquiritigenin and naringenin could be used as an inducer.
Another nitrogen-fixing association is found with tree roots and the Gram-positive, filamentous actinobacterial genus Frankia, which forms an intracellular nitrogen-fixing symbiosis with over 200 angiosperm species belonging to eight families. The best-known association of Frankia is with Alnus (Daniel et al. 2007).
This interaction between plants that belong to eight dicotyledonous families and the actinobacterium Frankia is known as “actinorhizal symbiosis,” collectively called “actinorhizal” (Wall 2000). Frankia was inoculated in A. glutinosa to check the expression of genes coding for chalcone synthase (chs) and phenylammonia lyase (pal), which are involved in the biosynthesis of flavonoids (Hammad et al. 2003; Kim et al. 2003). A study analyzing the expressed sequence tag (EST) database of a nodule and a Casuarina glauca root led to the detection of eight genes responsible for enzymes involved in the biosynthesis of the flavonoid pathway (Auguy et al. 2011). Further study is needed to enhance the understanding of actinorhizal symbiosis and its relationship with Frankia cultivability. Such a study may provide important data on developmental biology and plant-microbe interactions.
18.3.1.2 Mycorrhizal Interactions
The symbiotic relationship between plant roots, fungi and soil is known as “mycorrhizae.” Greater than 80% of terrestrial plants form an association with AMF. AMF are obligate symbionts, which means they are incapable of finishing their life cycle with a lack of host roots. Highly crossed web-like structures are formed by fungi after penetrating plant roots. These branching structures are known as “arbuscules,” and they are thought to be the primary sites of nutrient interchange between roots and fungi (Akiyama and Hayashi 2006).
This branch formation by fungus is due to an inducing factor, which is a plant-signaling molecule that activates hyphal morphogenesis leading to successful root colonization (Buee et al. 2000; Giovannetti et al. 1996). Studies have shown that all the mycotrophic plants have this branch-inducing factor present in their root exudates, but it was absent in non-host plants. A study on root exudate sesquiterpene identified from Lotus japonicas, a mycotrophic plant, has confirmed its role in activating hyphal branching in dormant mycorrhizal fungi (Akiyama et al. 2005). Ecto and endomycorrhiza are differentiated by their expansion around or inside root cells. Endomycorrhizal AMF are constantly found in relationship with roots and are therefore considered as obligate parasites. Unlike AMF, ectomycorrhiza belonging mainly to the basidiomycetes and not often to asco- or zygomycetes, are not obligate biotrophs but can live saprophytically in soil.
VAM forms a tight and communally useful association between mycorrhizal fungus and plant roots. Metabolic changes are undergone by both host and AMF to meet each other’s needs. As a result, root exudation induced by VAM will enhance microbial community growth in the rhizosphere and have growth-promoting effects on plants. Mycorrhizal fungi get carbon from the host root while inorganic nutrients are taken up from the soil around the plant root, creating one of the best examples of a plant-microbe symbiotic relationship where both benefit. The fungus benefits through the continuous supply of organic nutrients from the plant while the plant functions physiologically well and competes effectively with other plant communities (Bago et al. 2003).
This fungus-plant association has some other advantages such as a phosphate solubilization property that makes phosphorous available to plants. This is why VAM is widely used as a biofertilizer in the field (Behl et al. 2007). AMF may distinguish the occurence of a well-matched host through their root exudates, similar to the method of recognition used by rhizobia (Nagahashi and Douds 2003). Also, AMF can provide phytohormones, which enhance plant growth. The main step in AMF maturation is the development of extraradical hyphae induced by signal molecules secreted by plants. These lead to the beginning of AMF-induced symbiosis (De Carvalho-Niebel et al. 2002). These signal molecules are known as “strigolactones” (SLs), which acts as plant hormones (Koltai 2013). Currently, it is known that carotenoids are used to derive terpenid lactones like SLs (Matusova et al. 2005). Studies have shown that SLs are found in a broad different type of plant species, including primitive plants, dicots and monocots (Liu et al. 2009; Xie et al. 2010; Proust et al. 2011).
18.3.1.3 Plant Growth Promotion
Number of studies are focussed on a new cluster of microbes due to their involvement in increasing the productivity and health of crops. These microbes are called the PGPRs, they and affect crop yield and growth by releasing necessary compounds that stimulate growth (Bloemberg and Lugtenberg 2001). The establishment of bacteria in the roots of plants takes place due to signals given by root exudates. These exudates, for example- sugars, amino acids, etc., further rouse PGPR chemotaxis over the surface of roots and impact the motility of flagella in some bacteria (Somers et al. 2004).
Phytostimulators are secreted by some rhizobacteria that directly add to the growth of the plant. Azospirillum sp., despite its nitrogen-fixing properties, also releases phyto-hormones like auxins, gibberellins, and cytokinins (Steenhoudt and Vanderleyden 2000). Root exudates help PGPRs by supplying the precursors for biotransformation. For example, the root exudate tryptophan is a precursor for an important auxin, i.e., IAA (indole acetic acid), which is exploited by rhizobacteria (Cooke et al. 2002). A study also reported the presence of sugars and amino acids in Avena barbata root exudates.
There also exists an indirect mechanism for plant growth through suppression of the capacity of phyto-pathogens. This mechanism involves the capability of bacteria to produce siderophores (Parez-Miranda et al. 2007). Siderophores chelate iron hence making it unavailable to pathogens. Other mechanisms include production of anti-fungal metabolites, enzymes to degrade the cell wall, and hydrogen cyanide (HCN) to retard the growth of fungal pathogens.
Apart from indirect mechanisms, there are also certain direct ones. These include atmospheric nitrogen fixing to make it available to plants, formation of siderophores, phosphate solubilization and mineralization, and production of phyto-hormones (Page 1987; Guan and Kamino 2001).
18.3.2 Negative Impact
A number of secondary metabolites have been identified, and their properties with respect to the rhizosphere have been elucidated.
18.3.2.1 Inhibition of Pathogenicity by Secreting Antimicrobials
Soil microbial communities including pathogens are thought to be attracted by plants when compounds are exuded from plant roots. There is a wide variety of chemo-diversity in root exudates and a continuous search is on for the identification of suitable antimicrobials.
For instance, an extract of the cell wall from Phytophthora cinnamoni leads to the precipitation of release of rosmarinic acid (RA), a multifunctional caffeic acid ester, identified in the root exudates of sweet basil cultures (Bais et al. 2006). Root cultures of basil were also shown to exude RA when challenged in situ with Pythium ultimum, which further demonstrates antimicrobial activity against a number of soil-borne pathogenic microbes like Pseudomonas aeruginosa (Bais et al. 2002b). A similar study carried out by Brigham et al. (1999) showed that upon elicitation, pigmented naphthoquinones were released by hairy root cultures of Lithospermum erythrorhizon followed by many other biological activities against pathogens. Knowing the demonstrated antimicrobial activities of RA and naphthoquinones, root exudates can signify their importance in protecting the rhizosphere from pathogenic microbes.
18.3.2.2 Antimicrobials
There are certain compounds to which the bacterial pathogenic microbes causing disease and infection in roots were known to be resistant. Such compounds can find their role in providing defense against non-host pathogens (Haichar et al. 2012). For instance, phenyl propanoid is one such compound that has been shown to be considerably higher in roots challenged by non-host-pathogenic bacteria, i.e., non-host Pseudomonas syringae strains, in comparison to host-pathogenic bacteria, i.e., P. syringae pv. tomatoDC3000. It is released in reaction to attack by pathogens.
Lanoue et al. (2010) observed that the root system of Barley (Hordeum vulgare) released some phenol compounds that have antimicrobial activity when subjected to infection with Fusarium graminearum. Earlier, in a report by Vaughan et al. (2013), it was observed that uninfected A. thaliana roots were constitutively producing and releasing a diterpene called “rhizathalene A.” It was also shown that plants that are not producing this compound are found to be more vulnerable to be attacked by herbivorous insects.
The synthetic analogue of strigolactone, i.e. GR24, has been found to inhibit the growth of a wide varieties of phytopathogenic fungi in the growth medium (Dor et al. 2011). This indicates that released strigolactones can have either direct or indirect effects on their natural enemies by altering hormonal defense pathways along with contributing to the below-ground biotic stress response to plants (Dor et al. 2011; Torres-Vera et al. 2013; Baetz and Martinoia 2014).
Another set of compounds that can show antimicrobial activity towards a number of organisms are biosurfactants. They show antimicrobial activity against pathogenic oomycetes Pythium and Phytophthora, the fungus Rhizoctonia, in addition to various Gram-positive and Gram-negative bacteria that pose pathogenicity to humans like Staphylococcus aureus and Proteus vulgaris (Raaijmakers et al. 2006; Das et al. 2008). There are a number of secondary metabolites secreted by the roots of Arabidopsis when given treatment with salicylic acid (Walker et al. 2003a, b). Such compounds involve butanoic acid, ferulic acid, and 3-indolepropanoic acid. All of them were shown to display in vitro antibacterial activity against the pathogens Erwinia, Xanthomonas campestris, and P. syringae in the amounts found in root exudates.
18.3.2.3 Quorum Sensing
Quorum sensing (QS) is the capability of bacteria to develop communication and coordination of behavior through signaling molecules. It is a controlling process through which bacteria examine their growth. During growth, signal molecules are subsequently released by bacteria (Xuesong et al. 2003). Quorum-sensing systems are possessed by both Gram-negative and Gram-positive bacteria. They comprise significant plant pathogenic bacteria, namely Erwinia spp., Pseudomonas spp., and Agrobacterium spp., and control the expression of the number of genes that are necessary for creating pathogenesis (Fray 2002). QS is the phenomena by which production and release of the virulence parameters are regulated in several bacterial pathogens.
There is a difference in the detection of chemical signals in Gram-positive and Gram-negative bacteria, i.e. α-homoserine lactones (AHLs) are for Gram–negative bacteria whereas peptide auto-inducers are for Gram–positive bacteria. The mechanism of QS was first defined in Vibrio fischeri (an aquatic bacteria) through the signal-regulated induction of lux genes responsible for bioluminescence. This process is dependent on density. Normally there is constitutive synthesis of a basic level of AHLs until the levels reach a threshold value. This threshold is a point at which such molecules start acting as ligands for the global transcription regulator LuxR/LuxR-like proteins. These proteins are thought to activate many genes controlled by QS involving virulence factors. The rhizosphere has high levels of AHL-forming bacteria in comparison to bulk soil, showing their important role in colonization (Elasri et al. 2001).
The very first defined examples of QS mimicking plant-secreted compounds were halogenated furanones produced by Delisea pulchra (marine red algae) (Givskov et al. 1996). Such compounds are shown to be structurally similar to α-homoserine lactones. Certain bioactive components were found to be present in the root exudates of Pisum sativum (pea) that mimic AHL signaling in fully characterized reporter strains of bacteria. Through the regulation of AHLs these components can help with stimulation of behaviors in some strains and cause the inhibition of behaviors in others (Teplitski et al. 2000).
In a study carried out by Fray (2002), it was demonstrated that pathogenicity was re-established in AHL-producing transgenic tobacco plants to an avirulent AHL-deficient Erwinia carotovora mutant. In addition to the release of AHL-mimicking compounds, Rasmussen et al. (2005) revealed certain quorum sensing inhibitors (QSIs) in garlic extracts that are specific to the QS-controlled virulent genes in P. aeruginosa analyzed by gene chip-based transcriptomics.
Hence, it is likely that roots have the capability to develop defense strategies with the help of several secreted molecules in the rhizosphere. These secreted molecules obstruct the QS responses of bacteria like mimicking, blocking the signals, and secreti ng enzymes to degrade signals and thus induce chemical-attenuation of pathogens (Rasmussen and Givskov 2006; Defoirdt et al. 2010).
18.4 Root–Invertebrate (Nematode) Interaction
Root exudates are considered to be a well-known source of carbon for microorganisms residing in soil allowing profound populations to exist in the rhizosphere. The value and amount of carbon and other nutrients released in the rhizosphere, the microbial community structure surrounding the roots, and the effects on microorganism-nematode association, all are significantly influenced by the species of plant and the environmental conditions. The interaction between plants and invertebrates as facilitated by chemical signals has mainly been studied in leaves and stems, whereas the interaction between roots and invertebrates has just begun to be explored.
By utilizing a 14C pulse-labelling practice, Yeates (1999) observed that there was a substantial upsurge in fixing of label C via photosynthesis in the soil microbial biomass after infecting the roots of white clover (Trifolium repens) with Heterodera trifolii and numerous other nematodes (Nobili et al. 2001). This outcome suggests that infection by parasitic nematodes in white clover plant liberates additional organic compounds in the rhizosphere in general. Similarly, M. incognita infection in the roots of tomatoes led to an increased amount of water-soluble 14C and metal ions in its exudates as compared to healthy plants.
The majority of the information about communication between microorganisms and nematodes was the result of research done on rhizobia, mycorrhizal fungi, and plant pathogens (Baetz and Martinoia 2014). Such studies evidently demonstrate the complex tri-trophic webs wherein competition, addition, and synergistic interactions take place between nematodes and microbes in order to affect the plant host.
18.5 Root- Root Interaction
Even though there have been significant improvements in the understanding of root functions in the past decade, the complex associations arising at the interface of root and soil involving root exudation are still beginning to be investigated.
There are three modes of interference,namely resource competition, chemical interference, and/or parasitism, and the root exudates have the ability to affect all three of them. Root exudates have been shown to exhibit the properties of phytotoxins in order to mediate chemical interference for several species of plant. Natural compounds derived from plants to facilitate plant defense are termed allelochemicals; allelopathy is described as the phenomena in which bioactive secondary compounds are produced and released by the plants in order to affect the development of neighboring plant species (Weston et al. 2012). Allelochemicals that are secreted as root exudates are shown to penetrate the rhizosphere just after their secretion (Inderjit 2001). These chemicals are thought to be liberated in bulk but are subjected to sorption (physical), metal oxidation (chemical), and microbial degradation (biological) within the rhizosphere (Narula et al. 2009).
The allelochemicals released by roots are shown to reduce the growth of neighboring plants as well as suppress pathogenic microbes, insects, and herbivores. Nowadays, it is possible to characterize very small quantities of secondary bioactive compounds in the rhizosphere as well as study their metabolism and secretion in the soil (Mohney et al. 2009).
In one study, Sorghum spp., including johnson grass (Sorghum halpense L. Pers.) and sorghum sudan grass hybrid (Sorghum bicolor × Sorghum sudanese) produced ample amounts of potential allelochemicals in their exudates. Sorghum exudates when chemically characterized reveal many related long-chain hydroquinones that include sorgoleone with its resorcinol-analogue. These compounds are found to deter the growth of neighboring plants by inhibiting processes like photosynthesis and respiration (Czarnota et al. 2003; Dayan et al. 2009). Similarly, limited growth of weeds was observed in agricultural systems with Triticum aestivum (Wu et al. 2000) and Oryza sativa (Kong et al. 2004) in the presence of DIBOA and 5,7,4′-trihydroxy-3′,5′-dimethoxyflavone, respectively. In the maize rhizosphere, there was enhancement in the population of P. putida with valuable and positive characteristics gained as a result of exudation of benzoxazinone DIMBOA (2,4-dihydroxy-7-methoxy-1,4- benzoxazin-3-one) (Neal et al. 2012).
A secondary metabolite released from the roots of Centaurea maculosa (knapweed) sets up a standard example of an exudate compound displaying negative root-root interaction in the rhizosphere. The intrusive behavior of knapweed into the rhizosphere was due to the phytotoxin released by roots, i.e. (±)-catechin. Interestingly, (−)-catechin was found to show allelochemical activity, while (+)-catechin was shown to inhibit soil-borne bacteria (Perry et al. 2005). This study clearly shows that one root exudate can exhibit different properties such as autotoxicity and allelopathy in a plant species.
There are a few plants that prevent the inhibition of phytotoxin by changing their structure chemically. For instance, N-glucosylation is the pathway on which Zea mays (corn) depends in order to nullify the influence of DIMBOA, DIBOA, and BOA, phytotoxins that are released in the rhizosphere by Triticum aestivum (wheat) and many other grasses.
Root exudates are crucial in advancing the interaction between a parasitic plant and its host, where the interaction is considered negative for host and positive for parasite (Weston et al. 2012). The secondary metabolites released by the roots act as chemical messengers and are often utilized by plants for initiating the growth of invasive organs like haustoria needed for heterotrophic progression (Walker et al. 2003a).
Well-established physical associations between host and parasite are known for many obligate parasites such as Striga spp., witchweed, Orobanche spp., and broomrape (Palmer et al. 2004). A number of major food crops are parasitized by plants belonging to Scrophulariaceae, for example maize (Zea mays), sorghum (Sorghum bicolor), millet (Panicum milaceum), rice (Oryza sativa), and legumes. This family is known for invading the roots of surrounding plants in order to parasitize them for water, minerals, and other important nutrients (Yoder 2001).
Many exudates show positive responses in defense of neighboring plants in order to diminish the population of herbivores by drawing them indirectly towards aberrant plants. For example, infection of V. faba plants led to secretion of root exudates that are shown to regulate the green-leafy volatile formation in uninfected V. faba plants, thereby attracting the aphid parasitoids to the already infested V. faba (Du et al. 1998).
18.6 Conclusion and Future Prospects
Current studies are still at the learning phase about the multifaceted communication between root of the plants and their extremely varied and active micro-flora. It’s capability that enables plants to respond differently to pathogenic and beneficial microbes is of utmost importance for their survival. Sorting out the molecular chemistry of plant and microbial relations will not only give the opportunity to make changes in plant defense for human advantage, but also to promote establishment of helpful rhizospheric microbes. Therefore, modelling novel techniques and procedures in order to investigate rhizospheric ecological parameters under inherent conditions is urgently needed. Finally, capturing knowledge about plant growth-promoting rhizobacteria and root exudation, from genetic to the ecosystem level, will actually help in improvement of plants in terms of absorption of nutrients, detoxification of soils, and protection against invasive weeds and microbial pathogens.
References
Abbott L, Murphy D (2003) Soil biology fertility: a key to sustainable land use in agriculture. Kluwer Academic Publishers, Dordrecht, pp 187–203
Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435:824–827
Akiyama K, Hayashi H (2006) Strigolactones: chemical signals for fungal symbionts and parasitic weeds in plant roots. Ann Bot Lond 97:925–931
Albarracín VH, Winik B, Kothe E, Amoroso MJ, Abate CM (2008) Copper bioaccumulation by the actinobacterium Amycolatopsis sp. AB0. J Basic Microbiol 48:323–330
Auguy F, Abdel-Lateif K, Doumas P, Badin P, Guerin V, Bogusz D, Hocher V (2011) Activation of the isoflavonoid pathway in actinorhizal symbioses. Funct Plant Biol 38:690–696
Badri DV, Loyola-Vargas VM, Broeckling CD, De-la-Pena C, Jasinski M, Santelia D, Martinoia E, Sumner LW, Banta LM, Stermitz F, Vivanco JM (2008) Altered profile of secondary metabolites in the root exudates of Arabidopsis ATP-binding cassette transporter mutants. Plant Physiol 146:762–771
Baetz U, Martinoia E (2014) Root exudates: the hidden part of plant defense. Trends Plant Sci 19:90–98
Bago NB, Pfeffer PE, Abubakar J, Jun J, Allen JW (2003) Carbon export from arbuscular mycorrizal roots involves the translocation of carbohydrate as well as lipid. Plant Physiol 13:1496–1507
Bais HP, Fall R, Vivanco JM (2004a) Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol 134:307–319
Bais HP, Loyola-Vargas VM, Flores HE, Vivanco JM (2001) Root-specific metabolism: the biology and biochemistry of underground organs. In Vitro Cell Dev Biol Plant 37:730–741
Bais HP, Park SW, Weir TL, Callaway RM, Vivanco JM (2004b) How plants communicate using the underground information superhighway. Trends Plant Sci 9:26–32
Bais HP, Walker TS, Schweizer HP, Vivanco JM (2002a) Root specific elicitation and antimicrobial activity of rosmarinic acid in hairy root cultures of sweet basil (Ocimum basilicum L.) Plant Physiol Biochem 40:983–995
Bais HP, Walker TS, Stermitz FR, Hufbauer RA, Vivanco JM (2002b) Enantiomeric dependent phytotoxic and antimicrobial activity of (±)-catechin. A rhizosecreted racemic mixture from spotted knapweed. Plant Physiol 128:1173–1179
Bais HP, Tiffony L, Weir LT, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plant and other organisms. Annu Rev Plant Biol 57:233–266
Barea JM, Pozo MJ, Azcon R, Azcon-Aguilar C (2005) Microbial cooperation in the rhizosphere. J Exp Bot 56:1761–1778
Baudoin E, Benizri E, Guckert AV (2002) Impact of growth stage on the bacterial community structure along maize roots, as determined by metabolic and genetic fingerprinting. Appl Soil Ecol 19:135–145
Becard G, Taylor LP, Douds DD, Pfeffer PE, Doner LW (1995) Flavonoids are not necessary plant signal compounds in arbuscular mycorrhizal symbiosis. Mol Plant-Microbe Interact 8:252–258
Bednarek P, Schneider B, Svatos A, Oldham NJ, Hahlbrock K (2005) Structural complexity, differential response to infection, and tissue specificity of indolic and phenylpropanoid secondary metabolism in Arabidopsis roots. Plant Physiol 138:1058–1070
Behl RK, Ruppel S, Kothe E, Narula N (2007) Wheat × Azotobacter × VA mycorrhiza interactions towards plant nutrition and growth – a review. J Appl Bot Food Qual 81:95–109
Berger S, Sinha AK, Roitsch T (2007) Plant physiology meets phytopathology: plant primary metabolism and plant-pathogen interactions. J Exp Bot 58:4019–4026
Bertin C, Yang X, Weston LA (2003) The role of root exudates and allelochemicals in the rhizosphere. Plant Soil 256:67–83
Bloemberg GV, Lugtenberg BJ (2001) Molecular basis of plant growth promotion and biocontrol by rhizobacteria. Curr Opin Plant Biol 4:343–350
Bolton MD (2009) Primary metabolism and plant defense—fuel for the fire. Mol Plant-Microbe Interact 22:487–497
Brigham LA, Michaels PJ, Flores HE (1999) Cell-specific production and antimicrobial activity of naphthoquinones in roots of Lithospermum erythrorhizon. Plant Physiol 119:417–428
Broeckling CD, Broz AK, Bergelson J, Manter DK, Vivanco JM (2008) Root exudates regulate soil fungal community composition and diversity. Appl Environ Microbiol 74:738–744
Buee M, Rossignol M, Jauneau A, Ranjeva R, Becard G (2000) The pre-symbiotic growth of arbuscular mycorrhizal fungi is induced by a branching factor partially purified from plant root exudates. Mol Plant-Microbe Interact 13:693–698
Cheng W, Parton WJ, Gonzalez-Meler MA, Phillips R, Asao S, McNickle GG, Brzostek E, Jastrow JD (2014) Synthesis and modelling perspectives of rhizosphere priming. New Phytol 201:31–44
Cooke TJ, Poli D, Sztein AE, Cohen JD (2002) Evolutionary patterns in auxin action. Plant Mol Biol 49:319–338
Cooper JE (2007) Early interactions between legumes and rhizobia: disclosing complexity in a molecular dialogue. J Appl Microbiol 103:1355–1365
Czarnota MA, Paul RN, Weston LA, Duke SO (2003) Anatomy of sorgoleone-secreting root hairs of Sorghum species. Int J Plant Sci 164:861–866
Dakora FD, Phillips DA (2002) Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 245:35–47
Daniel G, Jaffre T, Prin Y (2007) Abundance of Frankia from Gymnostoma spp. in the rhizosphere of Alphitonia neocaledonica, a nonnodulated Rhamnaceae endemicto New Caledonia. Eur J Soil Biol 36:169–175
Das P, Mukherjee S, Sen R (2008) Antimicrobial potential of a lipopeptide biosurfactant derived from a marine Bacillus circulans. J Appl Microbiol 104:1675–1684
Dayan FE, Howell JE, Weidenhamer JD (2009) Dynamic root exudation of sorgoleone and its in planta mechanism of action. J Exp Bot 60:2107–2117
De Carvalho-Niebel F, Timmers AC, Chabaud M, Defaux P, Abarkar DG (2002) The nod factor-elicited annexin MtAnn1 is preferentially localized at the nuclear periphery in symbiotically activated root tissues of Medicago truncatula. Plant J 32:343–352
Deaker R, Roughley RJ, Kennedy IR (2004) Legume seed inoculation technology – a review. Soil Biol Biochem 36:1275–1288
Defoirdt T, Boon N, Bossier P (2010) Can bacteria evolve resistance to quorum sensing disruption? PLoS Pathog 6:e1000989
Doornbos RF, van Loon LC, Bakker PAHM (2012) Impact of root exudates and plant defense signalling on bacterial communities in the rhizosphere. A review. Agron Sustain Dev 32:227–243
Dor E, Joel DM, Kapulnik Y, Koltai H, Hershenhorn J (2011) The synthetic strigolactone GR24 influences the growth pattern of phytopathogenic fungi. Planta 234:419–427
Du YJ, Poppy GM, Powell W, Pickett JA, Wadhams LJ, Woodcock CM (1998) Identification of semiochemicals released during aphid feeding that attract parasitoid Aphidius ervi. J Chem Ecol 24:1355–1368
Elasri M, Delorme S, Lemanceau P, Stewart G, Laue B et al (2001) Acyl-homoserine lactone production is more common among plant-associated Pseudomonas spp. than among soil borne Pseudomonas spp. Appl Environ Microbiol 67:1198–1209
Farag MA, Huhman DV, Lei ZT, Sumner LW (2007) Metabolic profiling and systematic identification of flavonoids and isoflavonoids inroots and cell suspension cultures of Medicago truncatula using HPLC-UVESI- MS and GC-MS. Phytochemistry 68:342–354
Flores HE, Vivanco JM, Loyola-Vargas VM (1999) “Radicle” biochemistry: the biology of root-specific metabolism. Trends Plant Sci 4:220–226
Fray RG (2002) Altering plant microbe interaction through artificially manipulating bacterial quorum sensing. Ann Bot 89:245–253
Garbeva PV, Van Elsas JD, Van Veen JA (2008) Rhizosphere microbial community and its response to plant species and soil history. Plant Soil 302:19–32
Giovannetti M, Sbrana C, Silvia A, Avio L (1996) Analysis of factors involved in fungal recognition response to host-derived signals by arbuscular mycorrhizal fungi. New Phytol 133:65–71
Givskov M, Nys RD, Manefield M, Gram L, Maximilien R, Eberl L et al (1996) Eukaryotic interference with homoserine lactone-mediated prokaryotic signaling. J Bacteriol 178:6618–6622
Gleba D, Borisjuk NV, Borisjuk LG, Kneer R, Poulev A, Skarzhinskaya M et al (1999) Use of plant roots for phytoremediation and molecular farming. Proc Natl Acad Sci U S A 25:5973–5977
Gray EJ, Smith DL (2005) Intracellular and extracellular PGPR: commonalities and distinctions in the plant-bacterium signaling processes. Soil Biol Biochem 37:395–410
Guan LL, Kamino K (2001) Bacterial response to siderophore and quorum sensing chemical signals in the seawater microbial community. BMC Microbiol 1:27
Haichar FZ, Santaella C, Heulin T, Achouak W (2014) Root exudates mediate interactions belowground. Soil Biol Biochem 77:69–80
Haichar FZ, Marol C, Berge O, Rangel-Castro J, Prosser JI, Balesdent J, Heulin T, Achouak W (2008) Plant host habitat and root exudates shape soil bacterial community structure. ISME J 2:1221–1230
Haichar FZ, Roncato MA, Achouak W (2012) Stable isotope probing of bacterial community structure and gene expression in the rhizosphere of Arabidopsis thaliana. FEMS Microbiol Ecol 81:291–302
Hammad Y, Nalin R, Marechal K, Fiasson K, Pepin R, Berry AM, Normand P, Domenach AM (2003) A possible role for phenylacetic acid (PAA) in Alnus glutinosa nodulation by Frankia. Plant Soil 254:193–205
Heidstra R, Bisseling T (1996) Nod factor induced host responses and mechanisms of Nod factor perception. New Phytol 133:25–43
Hirsch AM, Bauer WD, Bird DM, Cullimore J, Tyler B, Yoder JI (2003) Molecular signals and receptors: controlling rhizosphere interactions between plants and other organisms. Ecology 84:858–868
Huhman DV, Berhow MA, Sumner LW (2005) Quantification of saponins in aerial and subterranean tissues of Medicago truncatula. J Agric Food Chem 53:1914–1920
Inderjit (2001) Soil: environmental effect on allelochemical activity. Agron J 93:79–84
Innes L, Hobbs PJ, Bardgett RD (2004) The impacts of individual plant species on rhizosphere microbial communities in soils of different fertility. Biol Fertil Soils 40:7–13
Jose S, Gillespie AR (1998) Allelopathy in black walnut (Juglans nigra L.) alley cropping. I. Spatio-temporal variation in soil juglone in a black walnut-corn (Zea mays L.) alley cropping system in the midwestern USA. Plant Soil 203:191–197
Kamilova F, Kravchenko LV, Shaposhnikov AI, Azarova T, Makarova N, Lugtenberg B (2006) Organic acids, sugars, and Ltryptophane in exudates of vegetables growing on stonewool and their effects on activities of rhizosphere bacteria. Mol Plant-Microbe Interact 19:250–256
Kanchan Vishwakarma, Neha Upadhyay, Nitin Kumar, Gaurav Yadav, Jaspreet Singh, Rohit K. Mishra, Vivek Kumar, Rishi Verma, R. G. Upadhyay, Mayank Pandey, Shivesh Sharma, (2017) Abscisic Acid Signaling and Abiotic Stress Tolerance in Plants: A Review on Current Knowledge and Future Prospects. Frontiers in Plant Science 08
Kim HB, Oh CJ, Lee H, Sun An C (2003) A type-i chalcone lsomerase mRNA is highly expressed in the root nodules of Elaeagnus umbella. J Plant Biol 46:263–270
Koltai H (2013) Strigolactones’ ability to regulate root development may be executed by induction of the ethylene pathway. Plant Signal Behav 6:1004–1005
Kong CH, Liang WJ, Xu XH, Hu F, Wang P, Jiang Y (2004) Release and activity of allelochemicals from allelopathic rice seedlings. J Agric Food Chem 52:2861–2865
Kothe E, Bergmann H, Büchel G (2005) Molecular mechanisms in bio-geo-interactions. Chem Erde 65(S1):7–27
Kumar R, Bhatia R, Kukreja K, Behl RK, Dudeja SS, Narula N (2007) Establishment of Azotobacter on plant roots: chemotactic response, development and analysis of root exudates of cotton (G. hirusitum L.) and wheat (T. aestivum L.) J Basic Microbiol 47:436–439
Lambers H, Mougel C, Jaillard B, Hinsinger P (2009) Plant-microbe-soil interactions in the rhizosphere: an evolutionary perspective. Plant Soil 321:83–115
Lanoue A, Burlat V, Henkes GJ, Koch I, Schurr U, Rose USR (2010) De novo biosynthesis of defense root exudates in response to Fusarium attack in barley. New Phytol 185:577–558
Liu J, Magalhaes JV, Shaff J, Kochian LV (2009) Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J 57:389–399
Loyola-Vargas VM, Broeckling CD, Badri D, Vivanco JM (2007) Effect of transporters on the secretion of phytochemicals by the roots of Arabidopsis thaliana. Planta 225:301–310
Lundberg DS, Lebeis SL, Herrera Paredes S, Yourstone S, Gehring J, Malfatti S et al (2012) Defining the core Arabidopsis thaliana root microbiome. Nature 488:86–90
Madigan MT, Martinko JM (2006) Brock: biology of microorganisms, 655–667. Pearson Prentice Hall, New Jersey
Matusova R, Rani K, Verstappen FWA, Franssen MCR, Beale MH, Bouwmeester HJ (2005) The strigolactone germination stimulants of the plant parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiol 139:920–934
Mavrodi OV, Mavrodi DV, Parejko JA, Thomashow LS, Weller DM (2012) Irrigation differentially impacts populations of indigenous antibiotic-producing Pseudomonas spp. in the rhizosphere of wheat. Appl Environ Microbiol 78:3214–3220
Mohney BK, Matz T, LaMoreaux J, Wilcox DS, Gimsing AL, Mayer P, Weidenhamer JD (2009) In situ silicone tube microextraction: a new method for undisturbed sampling of root exuded thiophenes from marigold (Tagetes erecta L.) in soil. J Chem Ecol 35:1279–1287
Morgan JA, Bending W, White PJ (2005) Biological costs and benefits to plant microbe interactions in the rhizosphere. J Exp Bot 56:1729–1739
Nagahashi G, Douds DD Jr (2003) Action spectrum for the induction of hyphal branches of an arbuscular mycorrhizal fungus: exposure sites versus branching sites. Mycol Res 107:1075–1082
Nardi S, Concheri G, Pizzeghello D, Sturaro A, Rella R, Parvoli G (2000) Soil organic matter mobilization by root exudates. Chemosphere 5:653–658
Neal AL, Ahmad S, Gordon-Weeks R, Ton J (2012) Benzoxazinoids in root exudates of maize attract Pseudomonas putida to the rhizosphere. PLoS One 7:e35498
Narula N, Kothe E, Behl RK (2009) Role of root exudates in plant-microbe interactions. J Appl Bot Food Qual 82:122–130
Nimbal CI, Pedersen JF, Yerkes CN, Weston LA, Weller SC (1996) Phytotoxicity and distribution of sorgoleone in grain sorghum germplasm. J Agric Food Chem 44:1343–1347
Nobili M, Contin M, Mondini C, Brookes PC (2001) Soil microbial biomass is triggered into activity by trace amounts of substrate. Soil Biol Biochem 33:1163–1170
Page WJ (1987) Iron dependent production of hydroxamate by sodium dependent Azotobacter chroococcum. Appl Environ Microbiol 53:1418–1424
Palmer AG, Gao R, Maresh J, Erbil WK, Lynn DG (2004) Chemical biology of multihost/ pathogen interactions: chemical perception and metabolic complementation. Annu Rev Phytopathol 42:439–464
Parez-Miranda S, Cabirol N, George-Tellez R, Zamudio-Rivera LS, Fernandez FJ (2007) O-CAS, a fast and universal method for siderophore detection. J Microbiol Meth 70:127–131
Peck MC, Fisher RF, Long SR (2006) Diverse flavonoids stimulate NodD1 binding to nod gene promoters in Sinorhizobium meliloti. J Bacteriol 188:5417–5427
Perret X, Staehelin C, Broughton WJ (2000) Molecular basis of symbiotic promiscuity. Microbiol Mol Biol Rev 64:180–201
Perry LG, Thelen GC, Ridenour WM, Weir TL, Callaway RM et al (2005) Dual role for an allelochemical: (±)-catechin from Centaurea maculosa root exudates regulates conspecific seedling establishment. J Ecol 93:1125–1136
Peters NK, Frost JW, Long SR (1986) A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science 233:977–980
Proust H, Hoffmann B, Xie X, Yoneyama K, Schaefer DG, Yoneyama K, Nogue F, Rameau C (2011) Strigolactones regulate protonema branching and act as a quorum sensing-like signal in the moss Physcomitrella patens. Development 138:1531–1539
Pueppke SG, Broughton WJ (1999) Rhizobium sp. strain NGR234 and R. fredii USDA257 share exceptionally broad, nested host ranges. Mol Plant-Microbe Interact 12:293–318
Raaijmakers JM, De Bruijn I, De Kock MJD (2006) Cyclic lipopeptide production by plant-associated pseudomonas spp.: diversity, activity, biosynthesis, and regulation. Mol Plant-Microbe Interact 19:699–710
Rasmussen TB, Givskov M (2006) Quorum-sensing inhibitors as anti-pathogenic drugs. Int J Med Microbiol 296:149–161
Rasmussen TB, Skindersoe ME, Bjarnsholt T, Phipps RK, Christensen BK, Jensen PO (2005) Identity and effects of quorum-sensing inhibitors produced by Penicillium species. Microbiology 151:1325–1340
Redmond JW, Batley M, Djordjevic MA, Innes RW, Kuempel PL, Rolfe BG (1986) Flavones induce expression of nodulation genes in Rhizobium. Nature 323:632–635
Richardson A, Barea JM, McNeill A, Prigent-Combaret C (2009) Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 321:305–339
Richardson AE, Lynch JP, Ryan PR, Delhaize E, Smith FA, Smith SE, Harvey PR, Ryan MH, Veneklaas JE, Lambers H, Oberson A, Culvenor RA, Simpson RJ (2011) Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 4:59–61
Rougier M (1981) Secretory activity at the root cap. In: Tanner W, Loews FA (eds) Encyclopidia of plant physiology, B plant carbohydrates II, vol 13. Springer, Berlin, pp 542–574
Schmidt A, Haferburg G, Sineriz M, Merten D, Büchel G, Kothe E (2005) Heavy metal resistance mechanisms in actinobacteria for survival in AMD contaminated soils. Chem Erde 65:131–144
Sineriz ML, Kothe E, Abate CM (2009) Cadmium biosorption by Streptomyces sp. F4 isolated from former uranium mine. J Basic Microbiol 1:55–62
Somers E, Vanderleyden J, Srinivasan M (2004) Rhizosphere bacterial signalling: a love parade beneath our feet. Crit Rev Microbiol 30:205–235
Steenhoudt O, Vanderleyden J (2000) Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: genetic, biochemical and ecological aspects. FEMS Microbiol Rev 24:487–506
Stermitz FR, Bais HP, Foderaro TA, Vivanco JM (2003) 7, 8-benzoflavone: a phytotoxin from root exudates of invasive Russian knapweed. Phytochemistry 64:493–497
Subbarao GV, Nakahara K, Hurtado MP, Ono H, Moreta DE, Salcedo AF, Yoshihashi AT, Ishikawa T, Ishitani M, Ohnishi-Kameyama M, Yoshida M, Rondon M, Rao IM, Lascano CE, Berry WL, Ito O (2009) Evidence for biological nitrification inhibition in Brachiaria pastures. Proc Natl Acad Sci U S A 106:17302–17307
Subbarao GV, Rondon M, Ito O, Ishikawa T, Rao IM, Nakahara K, Lascano C, Berry WL (2007) Biological nitrification inhibition (BNI) – is it a widespread phenomenon? Plant Soil 294:5–18
Teplitski M, Robinson JB, Bauer WD (2000) Plants secrete substances that mimic bacterial N-acyl homoserine lactone signal activities and affect population density-dependent behaviors in associated bacteria. Mol Plant-Microbe Interact 13:637–648
Torres-Vera R, Garcia JM, Pozo MJ, Lopez-Raez JA (2013) Do strigolactones contribute to plant defence? Mol Plant Pathol 15:211–216
Uren NC (2000) Types, amounts and possible functions of compounds released into the rhizosphere by soil grown plants. In: Pinton R, Varanini Z, Nannipieri P (eds) The rhizosphere: biochemistry and organic substances at the soil interface. Marcel Dekker, New York, pp 19–40
Vance CP, Uhde-Stone C, Allen DL (2003) Phosphorus acquisition and use: critical adaptations by plants for securing a non-renewable source. New Phytol 157:423–447
Van Hulten M, Pelser M, Van Loon LC, Pieterse CMJ, Ton J (2006) Costs and benefits of priming for defense in Arabidopsis. Proc Natl Acad Sci U S A 103:5602–5607
Vaughan MM, Wang Q, Webster FX, Kiemle D, Hong YJ, Tantillo DJ, Coates RM, Wray AT, Askew W, O’Donnell C, Tokuhisa JG, Tholl D (2013) Formation of the unusual semivolatile diterpene rhizathalene by the Arabidopsis class I Terpene synthase TPS08 in the root stele is involved in defense against belowground herbivory. Plant Cell 25:1108–1125
Vishwakarma K, Sharma S, Kumar N, Upadhyay N, Devi S, Tiwari A (2016) Contribution of Microbial Inoculants to Soil Carbon Sequestration and Sustainable Agriculture. In: Microbial Inoculants in Sustainable Agricultural Productivity. Springer India, pp. 101–112.
Vivanco JM, Bais HP, Stermitz FR, Thelen GC, Callaway RM (2004) Biogeographical variation in community response to root allelochemistry: novel weapons and exotic invasion. Ecol Lett 7:285–292
Walker TS, Bais HP, Halligan KM, Stermitz FR, Vivanco JM (2003a) Metabolic profiling of root exudates of Arabidopsis thaliana. J Agric Food Chem 51:2548–2554
Walker TS, Bais HP, Grotewold E, Vivanco JM (2003b) Root exudation and rhizosphere biology. Plant Physiol 132:44–51
Wall LG (2000) The actinorhizal symbiosis. J Plant Growth Regul 19:167–182
Wang E, Schornack S, Marsh JF, Gobbato E, Schwessinger B, Eastmond P, Schultze M, Kamoun S, Oldroyd GE (2012) A common signaling proces that promotes mycorrhizal and oomycete colonization of plants. Curr Biol 22:2242–2246
Wasson AP, Pellerone FI, Mathesius U (2006) Silencing the flavonoid pathway in Medicago truncatula inhibits root nodule formation and prevents auxin transport regulation by rhizobia. Plant Cell 18:1617–1629
Weston LA, Ryan PR, Watt M (2012) Mechanism for cellular transport and release of allelochemicals from plant roots into rhizosphere. J Exp Bot 63:1–10
Wu HW, Haig T, Pratley J, Lemerle D, An M (2000) Allelochemicals in wheat (Triticum aestivum L.): variation of phenolic acids in root tissues. J Agric Food Chem 48:5321–5325
Xie X, Yoneyama K, Yoneyama K (2010) The strigolactone story. Annu Rev Phytopathol 48:93–117
Xuesong H, Williams C, Deanne LP, Laura OS, Wagner J, Clay F (2003) Quorum sensing in Rhizobium sp. strain NGR234 regulates conjugal transfer (tra) gene expression and influences growth rate. J Bacteriol 185:809–822
Yang CH, Crowley DE (2000) Rhizosphere microbial community structure in relation to root location and plant iron nutritional status. Appl Environ Microbiol 66:345–351
Yeates GW (1999) Effects of plants on nematode community structure. Annu Rev Phytopathol 37:127–149
Yoder JI (2001) Host-plant recognition by parasitic Scrophulariaceae. Curr Opin Plant Biol 4:359–365
Zhu Y, Pierson LS, Hawes MC (1997) Induction of microbial genes for pathogenesis and symbiosis by chemicals from root border cells. Plant Physiol 115:1691–1698
Acknowledgement
The authors are thankful to Director MNNIT Allahabad for providing necessary facilities and “Design and Innovation Centre” a project sponsored by Ministry of Human Resource Development, Government of India, for supporting the execution of the study.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Vishwakarma, K. et al. (2017). Current Scenario of Root Exudate–Mediated Plant-Microbe Interaction and Promotion of Plant Growth. In: Kumar, V., Kumar, M., Sharma, S., Prasad, R. (eds) Probiotics in Agroecosystem. Springer, Singapore. https://doi.org/10.1007/978-981-10-4059-7_18
Download citation
DOI: https://doi.org/10.1007/978-981-10-4059-7_18
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-4058-0
Online ISBN: 978-981-10-4059-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)