Abstract
The sex of birds is determined by inheritance of sex chromosomes at fertilization. The embryo with two Z chromosomes (ZZ) develops into a male; by contrast, the embryo with Z and W chromosomes (ZW) becomes female. Two theories are hypothesized for the mechanisms of avian sex determination that explain how genes carried on sex chromosomes control gonadal differentiation and development during embryogenesis. One proposes that the dosage of genes on the Z chromosome determines the sexual differentiation of undifferentiated gonads, and the other proposes that W-linked genes dominantly determine ovary differentiation or inhibit testis differentiation. Z-linked DMRT1, which is a strong candidate avian sex-determining gene, supports the former hypothesis. Although no candidate W-linked gene has been identified, extensive evidence for spontaneous sex reversal in birds and aneuploid chimeric chickens with an abnormal sex chromosome constitution strongly supports the latter hypothesis. After the sex of gonad is determined by a gene(s) located on the sex chromosomes, gonadal differentiation is subsequently progressed by several genes. Developed gonads secrete sex hormones to masculinize or feminize the whole body of the embryo. In this section, the sex-determining mechanism as well as the genes and sex hormones mainly involved in gonadal differentiation and development of chicken are introduced.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 The Z and W Chromosomes in Birds
The chicken is the most useful experimental model for birds. Draft genome sequences of chicken have been available since 2004 (International Chicken Genome Sequencing Consortium 2004), and the estimated genome size is approximately 1 billion bp. The chromosomes of a female chicken (Gallus gallus domesticus) are shown in Fig. 2.1. The chromosome number of chickens is 2n = 78, and ten pairs of macrochromosomes (Chromosomes 1–9, Z, and W) and 29 pairs of microchromosomes can be observed. As with the chicken, the typical karyotype of birds consists of several pairs of macrochromosomes and many microchromosomes. The macrochromosomes are distinguishable by their size, morphology, and banding pattern, which can be obtained by treatment with enzymes or salt solutions. By contrast, microchromosomes are too small to distinguish individually.
The sex of birds is genetically determined. Females have the heterogametic sex chromosomes ZW, whereas males have the homogametic ZZ. This sex-determining mechanism is highly conserved in avians, which include nearly ten thousand species.
The Z chromosome is relatively large. The estimated size of the Z chromosome is 82 million bp, and the number of genes reported on the Z chromosome is 1137 (NCBI, Gnome: http://www.ncbi.nlm.nih.gov/genome/111?genome_assembly_id=22848). The W chromosome has degenerated during evolution, and its estimated size is 5.16 million bp. At least 50 genes are estimated on the W chromosome.
2.2 Gonadal Development in Chickens
Development of the chicken embryo takes 21 days. The developmental stage is described by 45 distinct stages under the Hamburger and Hamilton (HH) system (Hamburger and Hamilton 1951). The development of embryo can also be staged according to days of embryonic development post-lay (embryonic day [E]).
The developmental process of the urogenital system in chickens is similar to that in other amniotes. Urogenital tissues arise from the intermediate mesoderm, and the first evidence of gonadal development is observed at E3.5, characterized by a thickening of the coelomic epithelium ventral to the mesonephros. Until this embryonic stage, primordial germ cells (PGCs) of extragonadal origin migrate into the gonads through the blood stream.
The sex of birds is determined by genes located on the sex chromosome (see Sect. 2.3). In chickens, it is thought that sex determination occurs at E4.5. After sex determination, the gonads differentiate to testes or ovaries according to the sex chromosome constitution of cells, ZZ or ZW. However, until around E6.5, the gonads are considered “bipotential,” which means they are able to differentiate into either testes or ovaries. After E6.5, histological differentiation of the gonads can be observed between sexes. In ZZ embryos, the gonads are differentiated to bilateral testes (Fig. 2.2). The medulla is developed and is characterized by seminiferous tubules with Sertoli cells and prospermatogonia (Fig. 2.3). The pre-Sertoli cells produce anti-Müllerian hormone (AMH), which regresses embryonic oviducts (Müllerian ducts). Leydig cells adjacent to the seminiferous tubules in the testicle secrete testosterone to differentiate around cords (Wolffian ducts) to internal genitalia. The PGCs become enclosed in developing seminiferous cords, and undergo mitotic arrest in males. Meiosis only occurs after hatching.
Histological section of embryonic gonads with hematoxylin and eosin (HE)-staining. Left: a ZZ male gonad exhibits a developed medulla characterized by seminiferous tubules with Sertoli cells and prospermatogonia. Right: a left gonad of ZW female exhibits a diagnostic thickened cortex and lacunae in the cortex by becoming vacuolated. Scale bar means 100 μm
The right gonads are gradually depressed and fail to develop in ZW female embryos. The left gonads rapidly develop into ovaries with diagnostic thickened cortexes (Fig. 2.2). This asymmetric morphology observed between right and left gonads in females becomes apparent at E6.5 (Fig. 2.2). The proliferating germ cells exhibit a cortical distribution and begin to enter meiosis at E15.5 in the left gonad. PGCs in the right gonad undergo some proliferation, but do not enter meiosis (Ukeshima 1996). The Müllerian duct on the right side also regresses to form a dysfunctional vestige (Carlon and Stahl 1985). The medullary cords in the cortex of the female left gonad form lacunae by becoming vacuolated during development (Fig. 2.3). The left ovary finally develops into a functional organ, in which follicles are formed.
2.3 The Two Hypotheses for Sex Determination
Two hypotheses have been proposed for the mechanism of avian sex determination (Fig. 2.4). One of them is called the “Z dosage” model. This model explains that the dosage of a Z-linked gene mediate sex determination, whereby two copies are required for male development (ZZ). This model is supported by the observation that birds have no dosage compensation system for the Z chromosome, such as X inactivation observed in mammals (McQueen et al. 2001; Kuroda et al. 2001; Kuroiwa et al. 2002; Itoh et al. 2010). Therefore, it is thought that a high expression level of a Z-linked gene in the gonads of ZZ embryo triggers testis development. A strong candidate gene for sex determination under this hypothesis is the Z-linked DMRT1 (doublesex and mab-3-related transcription factor 1) gene (see Fig. 2.5).
The other hypothesis is the “W dominant” model. According to this model, the W chromosome carries a dominant-acting ovary determinant or an inhibitor of testis differentiation. Two W-linked genes have been reported as candidate sex-determining genes in chickens.
HINTW (histidine triad nucleotide-binding protein W) was reported as the best candidate W-linked ovary-determining gene (also known as WPKCI and ASW) at approximately the same time by two research groups (Hori et al. 2000; O’Neill et al. 2000). This gene is an ortholog of HINT1 (histidine triad nucleotide binding protein 1) on autosome in mammals. The HINT gene encodes an aberrant form of a nucleotide hydrolase enzyme (HINT). HINT proteins generally have endogenous adenosine 5′ monophosphoramidate enzyme activity. HINTZ which is a Z homologue of chicken HINTW, has a functional catalytic domain, the HIT motif, like other HINT proteins. By contrast, this motif is absent in HINTW. Several in-vitro biochemical experiments have shown that HINTZ function can be inhibited via the formation of HINTZ/HINTW heterodimers (Pace and Brenner 2003). However, ZZ embryos that overexpress HINTW develop to normal males with bilateral testes (Smith et al. 2009a). This provides genetic evidence against a role for HINTW in avian sex determination.
FET1 (female-expressed transcript 1) is another candidate W-linked ovary determinant (Reed and Sinclair 2002). This gene is found only in chicken genome, thus there are no orthologs in another bird species. The expression was almost exclusively observed in the female urogenital system. In particular, it is strongly expressed in female left gonads leading up to sexual differentiation, at E4.5–E6.5. However, genome sequencing analyses revealed that the gene is located on chromosome 4. Therefore, there are no candidate W-linked ovary-determining genes at present.
2.4 ZO and ZZW Chickens
Abnormal sex chromosome constitutions are useful to understand the sex-determining mechanism of a species. Very nice examples are mammals and fruit flies (Drosophila melanogaster). They have XX/XY sex chromosome constitutions; however, their mechanism for sex determination differs. To distinguish between these, sex chromosome aneuploids could be particularly helpful. Mammals have a male-dominant Y chromosome, whereas the sex of fruit flies is determined by X chromosome dosage. Therefore, XO individuals are female in mammals, but male in fruit flies. By contrast, XXY animals are male in mammals, but female in fruit flies. From these findings, distinguishing between the mechanisms of sex determination in birds would be straightforward if sex chromosome aneuploids were available (Graves 2003).
Unfortunately, despite intensive studies of chickens with aberrant sex chromosome constitutions, there are no reports of ZO chickens (Graves 2003). This means that ZO chromosomes may indeed be embryonic lethal. By contrast, several reports have described ZZW triploid chickens. Thorne and Sheldon (1991) reported these chickens were sterile intersex with both ovarian and testicular tissues. Furthermore, Lin et al. (1995) gave a detailed description of the gonads of 63 ZZW triploid chickens ranging in age from 1 day to 4.5 years. ZZW triploid fowls developed similarly to normal ZW hens until about 20 weeks of age, when their plumage, comb, and wattles developed like a male (Lin et al. 1995). Both left and right gonads were found in 59 chickens, and only a left gonad was found in the remaining four chickens. The development of right and left gonads was separately described by Lin et al. (1995), because they developed differently.
In the right gonads of 1-day-old chickens (chicks), seminiferous tubules were well developed, as observed in normal diploid ZZ males. After 3 months, the development of seminiferous tubules was retarded in the right gonads. The slow growth in the diameter of seminiferous tubules up to 7 weeks of age was associated with a twofold increase in the number of Sertoli cells from hatching (Lin et al. 1995). In 6-month-old chickens, few spermatozoa among a large number of round, condensed spermatid nuclei in the seminiferous tubules were observed. In 9-month-old chickens, the seminiferous tubules were degenerated. Primary spermatocytes were found in some tubules by 9 weeks of age, their formation being retarded by about 2 weeks by comparison with ZZ males. By 15 weeks of age, the most advanced germ cells were primary spermatocytes, whereas spermatozoa were present in ZZ males (Lin et al. 1995).
ZZW triploid chickens differed with respect to the development of the left gonads. In 1-day-old chickens, the left gonads showed ovotestis features: oocytes in the cortex and seminiferous tubules in the medulla. The cortex was less developed in ZZW chickens than in ZW chickens. After 1 week, more than 50% of oocytes in cortical cords degenerated and contained no nucleus or a poorly defined nucleus. Seminiferous tubules were present in the medullae of all left gonads. After 3 weeks old, the cortex was structurally distinct from the medulla. Seminiferous tubules were developing in the medulla, whereas the development remained less advanced than that of those in the right gonads. The cortex continued to degenerate, and was infiltrated by leukocytes that were mainly small lymphocytes in 5-week-old fowls. By 6 months of age, leukocytic infiltration of the cortical region stopped, and there were no ovarian components in the gonads. By contrast, the structure of seminiferous tubules in the medulla was similar to that of those in the right gonads. A second phase of gonadal degeneration began at 9 months old, and all left gonads degenerated, leaving a large portion of parenchyma composed of loose connective tissue. In chickens older than 1 year, seminiferous tubules could not be observed in the left gonads.
These observations in ZZW triploid chickens, which are complete triploids with all autosomes present in triplicate, indicated that the W chromosome is associated with ovarian development to some degree; however, ovarian features are ultimately overridden by two Z chromosomes (reviewed in Lambeth and Smith 2012). Interestingly, chimeric chickens with a mixture of diploid and triploid cells are also informative. Although the estimated ratio of 2AZZ/3AZZW chimeras (2A: two sets of autosome, 3A: three sets of autosome) is only 5%, the left gonads consistently develop to an ovary (Thorne and Sheldon 1993). This means that the ovary-determining gene is located on the W chromosome, because a small number of cells including the W chromosome is sufficient to induce ovarian development (reviewed in Lambeth and Smith 2012). This observation supports the “W dominant” model.
2.5 DMRT1: A Z-Linked Candidate for Sex Determination in Avians
In birds and lower vertebrates, DM domain genes that encodes transcription factors with a zinc-finger-like DM domain are highly conserved. One of these genes, DMRT1 located on the Z chromosome in birds, is a strong candidate avian sex determinant under the “Z dosage” model. This gene is highly conserved in vertebrate and non-vertebrate species, and is involved in the development of male reproductive organs (Raymond et al. 1999). In vertebrates, DMRT1 expression is essential for testis differentiation. The overexpression of DMRT1 in XX mouse fetal gonads drives the development of testes and represses the expression of key markers of ovarian development (Zhao et al. 2015). DMRT1 paralogs were identified as sex-determining genes in medaka (Oryzias latipes, DMY/Dmrt1bY in the Y chromosome) (Matsuda et al. 2002, 2003; Nanda et al. 2002) and African clawed frogs (Xenopus laevis, DMW in the W chromosome) (Yoshimoto et al. 2008). These DM domain genes have acquired new roles in gonadal sex differentiation via gene duplication and translocation (medaka), duplication and truncation (African clawed frogs), or loss of function of one allele (birds) (Cutting et al. 2013).
The chicken homolog of DMRT1 is located on the Z chromosome. It is expressed more highly in male undifferentiated gonads than in females (Smith et al. 1999). DMRT1 knockdown via RNA interference results in the feminization of embryonic gonads in genetically male (ZZ) embryos (Smith et al. 2009b). The feminized left gonad shows female-like histology, disorganized testis cords, and a decline in the testicular marker SOX9 (SRY-box 9). The ovarian marker aromatase is ectopically activated. The feminized right gonad shows a more variable loss of DMRT1 and ectopic aromatase activation, suggesting differential sensitivity to DMRT1 between the left and right gonads. Germ cells also show a female pattern of distribution in feminized male gonads. Furthermore, over-expressing of DMRT1 by infection of retroviruses in the left and right gonads of ZW embryo induces masculinization, characterized by increased expression of male marker genes and reduced expression of female marker genes (Lambeth et al. 2014). These reports indicate that DMRT1 is a master regulator for testis determination in the chicken and also support the “Z dosage” model for avian sex determination.
2.6 Genes Involved in Male Sexual Differentiation
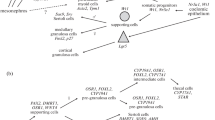
DMRT1 expression begins at ~E3.5. The expression is observed in the medulla of gonads and is higher in ZZ males than in ZW females. After high DMRT1 expression, SOX9 functions in testis development in ZZ chicken embryos (Fig. 2.5). In placental mammals, the sex determination gene SRY (sex-determining region Y) directly activates SOX9 expression by binding to the SOX9 enhancer together with the NR5A1 (nuclear receptor subfamily 5, group A, member 1) protein in the undifferentiated gonads of XY embryos (Sekido and Lovell-Badge 2008). However, in chickens, there is a time-lag between the initial expression of DMRT1 and SOX9, which occur at days 3.5 and 6.5 respectively. Therefore, other factors, which are probably chicken-specific, must be components of the molecular cascade between DMRT1 and SOX9.
HEMGN (hemogen) was firstly reported as a chicken-specific factor for testis differentiation that mediates SOX9 regulation under DMRT1 (Nakata et al. 2013, Fig. 2.5). In mice, Hemgn (also known as EDAG in humans) is a recently characterized hematopoietic tissue-specific gene encoding a nuclear protein (Yang et al. 2001). Hemgn expression is restricted to the blood islands of the yolk sac and the fetal liver during embryogenesis, the adult spleen, and bone marrow. EDAG shows a similar expression pattern in humans. High EDAG expression is observed in bone-marrow cells in acute myeloid leukemia, suggesting that EDAG plays a regulatory role in acute myeloid leukemia (An et al. 2005). However, the gene is not expressed in the gonads during embryogenesis in mammals. In chickens, HEMGN is located on the Z chromosome and expressed not only in hematopoietic tissues, but also in the early embryonic gonads of male chickens (Nakata et al. 2013). Male-specific expression has been observed in the nuclei of (pre-)Sertoli cells after the sex determination period and prior to the expression of SOX9. In ZW embryonic gonads masculinized by aromatase inhibitor treatment (see Sect. 2.8), the expression of HEMGN was induced. ZW embryos overexpressing HEMGN, generated by infection with a retrovirus carrying HEMGN, had masculinized gonads: the expressions of male marker genes, DMRT1 and SOX9, are increased, whereas female marker genes, aromatase and FOXL2 (forkhead box L2) (see Sect. 2.7) are decreased. Furthermore, distribution of germ cells showed a testis-like pattern. These findings suggest that HEMGN is a transcription factor that is specifically involved in chicken sex determination.
AMH is a glycoprotein belonging to the transforming growth factor-β (TGF-β) superfamily. This hormone is secreted by the gonads, and plays a role in sexual differentiation of reproductive organs. AMH is synthesized and secreted by Sertoli cells of the embryonic testis, and directly acts to regress the paired Müllerian ducts of males (Josso and Picard 1986; Josso et al. 1993; Vigier et al. 1983). In mammals, SOX9 directly regulates Amh transcription together with NR5A1, GATA4 (GATA binding protein 4), and WT1 (Wilms tumor protein homolog). However, in chickens, AMH mRNA expression is expressed prior to SOX9 mRNA (Fig. 2.5). AMH mRNA is also present in the female gonads of embryonic chickens, and acts to regress the right female Müllerian ducts (Hutson et al. 1981). It is thought that estrogens protect the left duct from regression by AMH (Josso et al. 2001; Hutson et al. 1982; Tran and Josso 1977). However, the exact mechanism is not known.
AMH is widely conserved in vertebrates. Its function is primarily related to Müllerian duct regression, whereas Y-linked duplicated AMH functions in male sex differentiation in the Patagonia pejerrey (Odontesthes hatcheri). In chickens, AMH expression precedes that of SOX9, indicating that AMH plays a more central role in avian testis development, similar to fish species. Lambeth et al. (2015) suppressed AMH expression in embryonic gonads of chickens using RNA interference, and did not observe an effect on the expression of the key testis pathway genes DMRT1 and SOX9; and male embryos exhibited normal testicular structure. However, the sizes of the mesonephros and gonads were reduced, with normal phenotypes in male and female embryos. These findings indicate that AMH is required for proper cell proliferation and urogenital system growth, irrespective of sex, whereas AMH does not directly contribute to testicular or ovarian differentiation (Lambeth et al. 2015). The same research group continuously characterized embryos overexpressing AMH generated by infection with a retrovirus carrying AMH (Lambeth et al. 2016). The embryos overexpressing AMH showed small and undeveloped structures in gonads of both sexes at embryonic and adult stages. ZW female gonads developed to testis-like cords lacking Sertoli cells, and were incapable of steroidogenesis. In ZZ males, a similar phenotype is observed: complete loss of both Sertoli cells and gonadal steroidogenesis. These observations suggest that AMH does not operate as an early testis activator in the chicken but can affect sex hormone production (Lambeth et al. 2016).
2.7 Genes Involved in Female Differentiation
In most vertebrates including birds, gonadal sex differentiation in females is sensitive to the sex steroid hormone estradiol. This hormone is only detected in female embryonic gonads, and is required and sufficient for ovarian development (Elbrecht and Smith 1992). The enzyme aromatase is responsible for converting androgens to estradiol. The aromatase protein is expressed in the medullae of female gonads from E6.5 onwards, and its expression increases during ovarian development (Smith et al. 2005).
Unfortunately, there are no candidates for W-linked ovary-determining genes. However, several genes of autosomes have been known to be involved in female sex determination. FOXL2 is an essential factor that is widely conserved in vertebrates, including chickens (Loffler et al. 2003; Wang et al. 2007; Pisarska et al. 2011, Fig. 2.5). The expression patterns of FOXL2 and aromatase transcripts are highly correlated in the developing ovary at E4.7–12.7 (Govoroun et al. 2004). The proteins encoded by both genes are colocalized in the nuclei of medullar cord cells, and FOXL2 is mainly expressed in the granulosa cells of developing follicles. The expression timing of FOXL2 is just prior to that of aromatase, suggesting that FOXL2 directly or indirectly regulates aromatase transcription. The FOXL2 expression is reduced by aromatase inhibitor treatment in vivo, suggesting that there is a feedback regulator loop between FOXL2 and aromatase (Hudson et al. 2005).
RSPO1 (R-spondin 1) and WNT4 (wingless-type MMTV integration site family, member 4) activation of β-catenin signaling plays an important role in the developing ovary in several vertebrate species (Biason-Lauber and Konrad 2008; Liu et al. 2010; Chue and Smith 2011), including chickens (Fig. 2.5). RSPO1 mRNA is expressed in the left and right gonads of ZW chicken embryos from E4.5, and increases strongly from E8.5 onward (Smith et al. 2008). By contrast, its expression remains low in the gonads of ZZ embryos. WNT4 expression is observed in the bipotential gonads of ZZ and ZW embryos at E4.5. However, in the left gonads of ZW embryos, it is upregulated during sexual differentiation, at E6.5–8.5. RSPO1 and WNT4 are strongly expressed in the cortex of the developing ovary (Ayers et al. 2013). They may act synergistically to activate β-catenin.
2.8 Sex Reversal in Birds
Sex-reversed animals have been particularly found in many species of fishes. Both cases, that of male-to-female and female-to-male, have been reported in wild fish species. The phenomenon of sex-reversal had also often observed in birds. Aristotle (384–322 BC) seems to have been the first to record the phenomenon of abnormal sex development in poultry. He observed hens that changed into cockerels (reviewed in Taber 1964). Examples of spontaneous sex reversal in chickens and many other bird species have been reported. Interestingly, only the masculinization of female birds (i.e., female-to-male) has been documented, and there are no reports of male-to-female sex reversal. Aristotle recorded the reciprocal event, cockerel to feminine behavior, but this has not been confirmed (Taber 1964).
Sex-reversed chickens can be generated experimentally. ZZ embryos treated with exogenous estrogen prior to gonadal differentiation show feminization of the left gonads, resulting in ovaries or ovotestes. Additionally, the administration of the anti-estrogen tamoxifen disturbs normal female development (Scheib 1983). Aromatase inhibitors, such as fadrozole, can effectually lead to feminization in ZW embryos (Elbrech and Smith 1992). These inhibitors induce female-to-male sex reversal in ZW females when applied between E0 and E7.5. Bilateral gonads develop, and a testis-like structure with a thick cortex and dense medulla is observed, although the embryo is genetically female (ZW) (Fig. 2.6). Furthermore, genes involved in testis differentiation are unregulated; by contrast, female marker genes are downregulated.
The gonads of ZW female embryo, ZZ male embryo, and masculinized ZW embryo induced by aromatase inhibitor treatment. Upper: gonads on top of the mesonephros of female, male, and masculinized ZW embryos at E10.5. The gonads of masculinized ZW embryos showed bilateral development, similar to male gonads. Dashed lines indicate gonads. Scale bar means 1 mm. Middle: HE-staining of gonad sections from female, male, and masculinized ZW embryos. The left masculinized ZW gonad has a testis-like phenotype with a dense medulla and thin cortex, although a slightly fragmented medulla was observed. The dashed line indicates the border between the cortex and medulla in the female gonad. Scale bar means 100 μm. Lower: aromatase and SOX9 in situ hybridization in male, female, and masculinized ZW gonad frozen sections at day 10.5. Aromatase is detected in female gonads, but no expression is observed in male or masculinized ZW gonads. By contrast, SOX9 expression is not detected in female
Evidence obtained from experimental sex-reversed chickens suggests that sex hormones and their enzymatic pathway are very important for ovary development in chickens, and potentially in all bird species. Furthermore, there are no examples of male-to-female sex reversal in birds under natural conditions, indicating that birds cannot be female without the W chromosome. This inference strongly supports the “W dominant” hypothesis, which maintains that W-linked genes dominantly determine ovary differentiation (or inhibit testis differentiation).
2.9 Sex Chimera Birds: Gynandromorphs—Genes or Hormones?
Gynandromorphs have been generally observed in insects and crustaceans. The individuals have the physical characteristics of both genders, usually displaying a bilateral difference. Interestingly, gynandromorphs are found only in birds in vertebrates; one side of the animal appears male and the other female. These birds are rare, but researchers have focused on this interesting phenomenon as a “genetic mosaicism” (Hollander 1944). Gynandromorph birds have been observed in pigeons, zebra finches, and especially domestic fowls (Hollander 1975; Agate et al. 2003; Cock 1955), among other species. A long-standing theory on the etiology of gynandromorphs proposes that a single sex chromosome is lost at the two-cell stage on one side of the animal (Cock 1955). However, it is now understood that gynandromorph birds arise as a result of a failure in the extrusion of a polar body during meiosis and subsequent fertilization of both a Z- and W-bearing female pronucleus (Hollander 1975; Zhao et al. 2010).
Gynandromorphs are spontaneously generated, namely the experimental samples are rare. Zhao et al. (2010) reported valuable data examined three lateral gynandromorph chickens. All chickens were ISA brown commercial hybrids with sex-linked coloration in which males show white plumage and females show brown plumage. Gynandromorph chickens show a marked bilateral asymmetry: half of the body is phenotypically female and the other side is phenotypically male. The female side with brown plumage appears a small wattle and small leg spur. By contrast, the male side, which is white, shows like a typical cockerel with a large wattle, a large leg spur, a heavier leg structure, and an obviously greater mass of breast muscle (Zhao et al. 2010).
In their report, to identify the sex chromosome constitutions of somatic cells, fluorescence in-situ hybridization using Z and W chromosome probes was performed using chromosome preparations obtained from cells in blood and skin samples from both sides of three gynandromorph birds. All three birds were composed of a mixture of normal diploid male and female cells. Tissues of the male side were mainly composed of ZZ (male) cells, whereas tissues of the female side mainly contained ZW (female) cells.
Understanding gonadal differentiation in gynandromorph chickens is highly complicated, because gonadal structure does not correspond to external appearance (Zhao et al. 2010). Two gynandromorph chickens (G1 and G2) appeared female on the left side and male on the right, whereas the remained one, G3, showed the reverse external appearance, left is male and right is female. The left gonad differed in appearance between these three gynandromorph chickens. G1 contained a testis-like gonad composed primarily of sperm containing seminiferous tubules. G2 had an ovary-like gonad composed predominantly of large and small follicles. G3 showed an ovotestis comprised of a mixture of empty tubules and small follicular-like structures. The morphological appearance of the gonads reflected the cellular composition (ZZ or ZW) of the individual organs. Testis-like and ovary-like gonads were composed principally of ZZ- and ZW-containing cells respectively, whereas the ovotestis comprised a mixture of ZZ- and ZW-containing cells.
In the traditional view of sexual development in vertebrates including birds, the gonads develop into either ovaries or testis during the embryonic stage, and then release sex hormones to masculinize or feminize the rest of the body. However, the phenomenon of gynandromorphy cannot be explained by this process, because hormones are expected to flow equally to both sides of the body. Nevertheless, the organs exhibit male or female phenotypes depending on the cellular composition, ZZ or ZW.
Gonadal chimeras generated by transplantation of presumptive mesoderm exhibit a similar pattern. Zhao et al. (2010) transplanted sections of presumptive mesoderm from green fluorescent protein (GFP)-expressing embryos at developmental stage 12 to the equivalent tissue on non-GFP embryos at the same stage of development between sexes. The transplanted embryos were allowed to develop until stage 35, and the expression patterns of the male marker AMH and female marker aromatase were examined. Interestingly, donor male cells expressed AMH and donor female cells expressed aromatase in mixed-sex chimeras. Donor cells appear to be incapable of contributing to specialized compartments of the host gonad. Female donor cells in the testis of a male host cannot be recruited into the functional male Sertoli cell compartment, and male donor cells in the ovary of a female host are excluded from the functionally female compartment.
These observations seem paradoxical. Hormones must play a role in early sexual development because genetically female chicken embryos develop as males with testes when treated with an aromatase inhibitor (see Sect. 2.8). However, studies on gynandromorphy and gonadal chimeras provide evidence that all somatic cells recognize their sex, ZZ male or ZW female. This observation led to the idea that male and female chicken somatic cells possess a cell-autonomous sex identity (CASI) (Zhao et al. 2010; Clinton et al. 2012). Based on gynandromorph chicken studies, Zhao et al. (2010) proposed that Z-linked genes underlie sex determination throughout the avian body. This idea is supported by the fact that birds have no chromosome-wide dosage compensation mechanism (see Fig. 2.3). The dosage of most Z-linked genes is twofold, higher in male than in female cells, and this might determine the sex identities of each cell.
Conclusion
The sex-determining process is essential for reproduction. In recent years, understanding the molecular mechanism of sex-determination in birds has been progressed, however, it is lagging behind that of mammals and fishes. Although genes and regulatory networks that govern the fate of gonads were recently identified, many gaps in knowledge remain. There is conflicting evidence concerning the importance of sex hormones in sex differentiation. Additional investigations of the role of genes involved in sex determination and differentiation, and the relative contribution of the genetic sex of each somatic cell and hormones to sexual differentiation, are expected in the future.
References
Agate RJ, Grisham W, Wade J, Mann S, Wingfield J, Schanen C, Palotie A, Arnold AP. Neural, not gonadal, origin of brain sex differences in a gynandromorphic finch. Proc Natl Acad Sci U S A. 2003;100:4873–8.
An LL, Li G, KF W, Ma XT, Zheng GG, Qiu LG, Song YH. High expression of EDAG and its significance in AML. Leukemia. 2005;19:1499–502.
Ayers KL, Sinclair AH, Smith CA. The molecular genetics of ovarian differentiation in the avian model. Sex Dev. 2013;7:80–94.
Biason-Lauber A, Konrad D. WNT4 and sex development. Sex Dev. 2008;2:210–8.
Carlon N, Stahl A. Origin of the somatic components in chick embryonic gonads. Arch Anat Microsc Morphol Exp. 1985;74:52–9.
Chue J, Smith CA. Sex determination and sexual differentiation in the avian model. FEBS J. 2011;278:1027–34.
Clinton M, Zhao D, McBride D. Evidence for avian cell autonomous sex identity (CASI) and implications for the sex-determination process? Chromosome Res. 2012;20:177–90.
Cock AG. Half-and-half mosaics in the fowl. J Genet. 1955;53:49–80.
Cutting A, Chue J, Smith CA. Just how conserved is vertebrate sex determination? Dev Dyn. 2013;242:380–7.
Elbrecht A, Smith RG. Aromatase enzyme activity and sex determination in chickens. Science. 1992;255:467–70.
Govoroun MS, Pannetier M, Pailhoux E, Cocquet J, Brillard JP, Couty I, Batellier F, Cotinot C. Isolation of chicken homolog of the FOXL2 gene and comparison of its expression patterns with those of aromatase during ovarian development. Dev Dyn. 2004;231:859–70.
Graves JAM. Sex and death in birds: a model of dosage compensation that predicts lethality of sex chromosome aneuploids. Cytogenet Genome Res. 2003;101:278–82.
Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92.
Hollander WF. Mosaic effects in domestic birds. Q Rev Biol. 1944;19:285–307.
Hollander WF. Sectorial mosaics in the domestic pigeon: 25 more years. J Hered. 1975;66:197–202.
Hori T, Asakawa S, Itoh Y, Shimizu N, Mizuno S. Wpkci, encoding an altered form of PKCI, is conserved widely on the avian W chromosome and expressed in early female embryos: implication of its role in female sex determination. Mol Biol Cell. 2000;11:3645–60.
Hudson QJ, Smith CA, Sinclair AH. Aromatase inhibition method reduces expression of FOXL2 in the embryonic chicken ovary. Dev Dyn. 2005;233:1052–5.
Hutson J, Ikawa H, Donahoe PK. The ontogeny of Müllerian inhibiting substance in the gonads of the chicken. J Pediatr Surg. 1981;16:822–7.
Hutson JM, Ikawa H, Donahoe PK. Estrogen inhibition of Müllerian inhibiting substance in the chick embryo. J Pediatr Surg. 1982;17:953–9.
International Chicken Genome Sequencing Consortium. Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature. 2004;432(7018):695–716.
Itoh Y, Replogle K, Kim YH, Wade J, Clayton DF, Arnold AP. Sex bias and dosage compensation in the zebra finch versus chicken genomes: general and specialized patterns among birds. Genome Res. 2010;20:512–8.
Josso N, Cate RL, Picard JY, Vigier B, di Clemente N, Wilson C, Imbeaud S, Pepinsky RB, Guerrier D, Boussin L, et al. Anti-Müllerian hormone: the Jost factor. Recent Prog Horm Res. 1993;48:1–59.
Josso N, di Clemente N, Gouédard L. Anti-Müllerian hormone and its receptors. Mol Cell Endocrinol. 2001;179:25–32.
Josso N, Picard JY. Anti-Müllerian hormone. Physiol Rev. 1986;66:1038–90.
Kuroda Y, Arai N, Arita M, Teranishi M, Hori T, Harata M, Mizuno S. Absence of Z-chromosome inactivation for five genes in male chickens. Chromosome Res. 2001;9:457–68.
Kuroiwa A, Yokomine T, Sasaki H, Tsudzuki M, Tanaka K, Namikawa T, Matsuda Y. Biallelic expression of Z-linked genes in male chickens. Cytogenet Genome Res. 2002;99:310–4.
Lambeth LS, Ayers KL, Cutting AD, Doran TJ, Sinclair AH, Smith CA. Anti-Müllerian hormone is required for chicken embryonic urogenital system growth but not sexual differentiation. Biol Reprod. 2015; doi:10.1095/biolreprod.115.131664. [Epub ahead of print].
Lambeth LS, Morris K, Ayers KL, Wise TG, O’Neil T, Wilson S, Cao Y, Sinclair AH, Cutting AD, Doran TJ, Smith CA. Overexpression of anti-Müllerian hormone disrupts gonadal sex differentiation, blocks sex hormone synthesis, and supports cell autonomous sex development in the chicken. Endocrinology. 2016;157:1258–75.
Lambeth LS, Raymond CS, Roeszler KN, Kuroiwa A, Nakata T, Zarkower D, Smith CA. Over-expression of DMRT1 induces the male pathway in embryonic chicken gonads. Dev Biol. 2014;389:160–72.
Lambeth LS, Smith CA. Disorder of sexual development in poultry. Sex Dev. 2012;6:96–103.
Lin M, Thorne MH, Martin IC, Sheldon BL, Jones RC. Development of the gonads in the triploid (ZZW and ZZZ) fowl, Gallus domesticus, and comparison with normal diploid males (ZZ) and females (ZW). Reprod Fertil Dev. 1995;7:1185–97.
Liu CF, Liu C, Yao HH. Building pathways for ovary organogenesis in the mouse embryo. Curr Top Dev Biol. 2010;90:263–90.
Loffler KA, Zarkower D, Koopman P. Etiology of ovarian failure in blepharophimosis ptosis epicanthus inversus syndrome: FOXL2 is a conserved, early-acting gene in vertebrate ovarian development. Endocrinology. 2003;144:3237–43.
Matsuda M, Nagahama Y, Shinomiya A, Sato T, Matsuda C, Kobayashi T, Morrey CE, Shibata N, Asakawa S, Shimizu N, Hori H, Hamaguchi S, Sakaizumi M. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature. 2002;417:559–63.
Matsuda M, Sato T, Toyazaki Y, Nagahama Y, Hamaguchi S, Sakaizumi M. Oryzias curvinotus has DMY, a gene that is required for male development in the medaka, O. latipes. Zool Sci. 2003;20:159–61.
McQueen HA, McBride D, Miele G, Bird AP, Clinton M. Dosage compensation in birds. Curr Biol. 2001;11:253–7.
Nakata T, Ishiguro M, Aduma N, Izumi H, Kuroiwa A. Chicken hemogen homolog is involved in the chicken-specific sex-determining mechanism. Proc Natl Acad Sci U S A. 2013;110:3417–22.
Nanda I, Kondo M, Hornung U, Asakawa S, Winkler C, Shimizu A, Shan Z, Haaf T, Shimizu N, Shima A, Schmid M, Schartl M. A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes. Proc Natl Acad Sci U S A. 2002;99:11778–83.
O’Neill M, Binder M, Smith C, Andrews J, Reed K, Smith M, Millar C, Lambert D, Sinclair A. ASW: a gene with conserved avian W-linkage and female specific expression in chick embryonic gonad. Dev Genes Evol. 2000;210:243–9.
Pace HC, Brenner C. Feminizing chicks: a model for avian sex determination based on titration of Hint enzyme activity and the predicted structure of an Asw–Hint heterodimer. Genome Biol. 2003;4:R18.
Pisarska MD, Barlow G, Kuo FT. Mini review: roles of the forkhead transcription factor FOXL2 in granulosa cell biology and pathology. Endocrinology. 2011;152:1199–208.
Raymond CS, Kettlewell JR, Hirsch B, Bardwell VJ, Zarkower D. Expression of Dmrt1 in the genital ridge of mouse and chicken embryos suggests a role in vertebrate sexual development. Dev Biol. 1999;215:208–20.
Reed KJ, Sinclair AH. FET-1: a novel W-linked, female specific gene up-regulated in the embryonic chicken ovary. Mech Dev. 2002;119:S87–90.
Scheib D. Effects and role of estrogens in avian gonadal differentiation. Differentiation. 1983;23(Suppl):S87–92.
Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453:930–4.
Smith CA, McClive PJ, Hudson Q, Sinclair AH. Male-specific cell migration into the developing gonad is a conserved process involving PDGF signalling. Dev Biol. 2005;284:337–50.
Smith CA, McClive PJ, Western PS, Reed KJ, Sinclair AH. Conservation of a sex-determining gene. Nature. 1999;402:601–2.
Smith CA, Roeszler KN, Sinclair AH. Genetic evidence against a role for W-linked histidine triad nucleotide binding protein (HINTW) in avian sex determination. Int J Dev Biol. 2009a;53:59–67.
Smith CA, Roeszler KN, Ohnesorg T, Cummins DM, Farlie PG, Doran TJ, Sinclair AH. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature. 2009b;461:267–71.
Smith CA, Shoemaker CM, Roeszler KN, Queen J, Crews D, Sinclair AH. Cloning and expression of R-Spondin1 in different vertebrates suggests a conserved role in ovarian development. BMC Dev Biol. 2008;8:72.
Taber E. Intersexuality in birds. In: Armstrong CN, Marshall AJ, editors. Intersexuality of vertebrates including man. New York: Academic Press; 1964. p. 285–310.
Thorne MH, Sheldon BL. Cytological evidence of maternal meiotic errors in a line of chickens with a high incidence of triploidy. Cytogenet Cell Genet. 1991;57:206–10.
Thorne MH, Sheldon BL. Triploid intersex and chimeric chickens: useful models for studies of avian sex determination. In: Reed KC, Graves JAM, editors. Sex chromosomes and sex-determining genes. Chur: Harwood Academic Publishers; 1993. p. 199–205.
Tran D, Josso N. Relationship between avian and mammalian anti-Müllerian hormones. Biol Reprod. 1977;16:267–73.
Ukeshima A. Germ cell death in the degenerating right ovary of the chick embryo. Zool Sci. 1996;13:559–63.
Yang LV, Nicholson RH, Kaplan J, Galy A, Li L. Hemogen is a novel nuclear factor specifically expressed in mouse hematopoietic development and its human homologue EDAG maps to chromosome 9q22, a region containing breakpoints of hematological neoplasms. Mech Dev. 2001;104:105–11.
Vigier B, Tran D, du Mesnil du Buisson F, Heyman Y, Josso N. Use of monoclonal antibody techniques to study the ontogeny of bovine anti-Müllerian hormone. J Reprod Fertil. 1983;69:207–14.
Wang DS, Kobayashi T, Zhou LY, Paul-Prasanth B, Ijiri S, Sakai F, Okubo K, Morohashi K, Nagahama Y. Foxl2 up-regulates aromatase gene transcription in a female-specific manner by binding to the promoter as well as interacting with ad4 binding protein/steroidogenic factor 1. Mol Endocrinol. 2007;21:712–25.
Yoshimoto S, Okada E, Umemoto H, Tamura K, Uno Y, Nishida-Umehara C, Matsuda Y, Takamatsu N, Shiba T, Ito M. A W-linked DM-domain gene, DM-W, participates in primary ovary development in Xenopus laevis. Proc Natl Acad Sci U S A. 2008;105:2469–74.
Zhao D, McBride D, Nandi S, McQueen HA, McGrew MJ, Hocking PM, Lewis PD, Sang HM, Clinton M. Somatic sex identity is cell autonomous in the chicken. Nature. 2010;464:237–42.
Zhao L, Svingen T, Ng ET, Koopman P. Female-to-male sex reversal in mice caused by transgenic overexpression of Dmrt1. Development. 2015;142:1083–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Kuroiwa, A. (2017). Sex-Determining Mechanism in Avians. In: Sasanami, T. (eds) Avian Reproduction. Advances in Experimental Medicine and Biology, vol 1001. Springer, Singapore. https://doi.org/10.1007/978-981-10-3975-1_2
Download citation
DOI: https://doi.org/10.1007/978-981-10-3975-1_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-3974-4
Online ISBN: 978-981-10-3975-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)