Abstract
In mammals, the decision to become male or female is initiated in the gonad by the sex determination pathway, which drives and instructs the differentiation of the gonad into a testis or ovary. The gonad develops as an undifferentiated primordium that is initially indistinguishable between XX (female) and XY (male) embryos; the gonad is a uniquely bipotential organ in its ability to give rise to a testis or ovary. Prior to sex determination, the establishment of the gonadal anlage (i.e., the setup of genetic and cellular programs promoting its identity) by a set of specification factors is critical. The master switch of mammalian sex determination, the Sry gene on the Y chromosome, is the genetic trigger that sets sex determination in motion and launches the testis program in the gonad. Sry is necessary and sufficient for male development, while the ovarian pathway (under the control of a female-specific program) ensues in the absence of Sry expression during a critical developmental time window. In this chapter, we will cover the processes of gonad specification and sex determination, focusing on major factors and signaling pathways involved in the male-versus-female decision and the establishment of sexual dimorphism in the gonad. Additionally, we will briefly discuss evolutionarily conserved aspects of chromosomal sex determination mechanisms and environmental influences that potentially impact sex determination and sex ratio in mammals.
Sarah J. Potter and Deepti Lava Kumar contributed equally to this work.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Gonad specification
- Sex determination
- Genital ridge
- GATA4
- ZFPM2/FOG2
- WT1
- OSR1
- SIX1
- SIX4
- NR5A1/SF1
- INSR
- IGF1R
- TCF21
- CITED2
- CBX2
- LHX9
- PBX1
- EMX2
- MFGE8
- WNT4
- RSPO1
- Sex chromosomes
- XX/XY system
- ZZ/ZW system
- SRY
- DMRT1
- Doublesex
- MAB-3
- DM-domain proteins
- Haplodiploidy sex determination
- SOX9
- TGF-beta
- Sertoli cells
- MAP3K4
- GADD45g
- FGF9
- PTGDS
- NR0B1/DAX1
- Beta-catenin/CTNNB1
- FOXL2
Gonad Specification
Origins of the Bipotential Gonadal Primordium
Both the urinary and reproductive tract organs form from the same cellular source, the intermediate mesoderm. Within the intermediate mesoderm, the adreno-gonadal primordium (AGP) arises at 9.5 days post coitus (dpc) in the mouse; soon thereafter, the adrenal gland and gonad separate to form independent organs. At first the defined region of the gonad is referred to as the “genital ridge ,” which represents the gonad anlage, i.e., the newly formed layers of cells on the surface of the mesonephros. The process of gonad specification by targeted cellular recruitment and cell fate specification starts to occur by 10.0–10.5 dpc in mice (McLaren 2000; Swain and Lovell-Badge 1999). The gonad is comprised of both somatic cells and germ cells. Germ cells migrate from the allantois via the gut tube, along the dorsal mesentery, and through the mesonephros to colonize the forming gonad between 10.5 and 11.5 dpc (discussed in Chap. 6, “Male Sexual Differentiation”). Germ cell colonization is not absolutely essential for early gonad formation and somatic cell development; however, the somatic cells are important for germ cell survival and proliferation (Kimble and White 1981). This chapter will focus specifically on the development of the somatic cell lineage. The gonad is a unique organ in that its primordium is bipotential, with the capability to become either a testis or an ovary, dependent on the presence or absence of sex-specific factors. Section “Gonad Specification” focuses on the factors important for the initial formation of the genital ridge, while section “Sex Determination” will cover the factors involved in sex determination within the gonad.
Genital Ridge Formation and Specification Factors
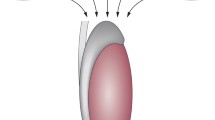
Both cell migration and cell proliferation contribute to the initial formation of the coelomic epithelium on the surface of the mesonephros. Subsequent proliferation of the coelomic epithelium promotes the formation of the gonad, and a host of factors are important for the specification of the gonad fate (Fig. 1). After cellular migration helps form the epithelium, proliferation of the epithelium and subsequent epithelial-to-mesenchymal transition (EMT) drive the expansion of the forming gonad. Cells arising from early epithelial proliferation have the potential to become supporting cells; however, later epithelial divisions give rise to interstitial cells (Karl and Capel 1998). The underlying mechanisms for these events will be discussed in more detail in Chap. 6, “Male Sexual Differentiation.”
Initial gonad specification and formation. (a) Initial signaling pathways involved in progenitor cell formation and gonad specification in mammals (10.0–10.4 dpc of mouse development). Diagram depicts expression of gonad specification factors involved in the development of the adreno-gonadal primordium and the formation of the early gonad. (b) Important signaling interactions for gonad formation, focusing on factors involved in the growth, proliferation, and migration of cells during gonadogenesis. Factors in red text are suppressors of proliferation or growth, whereas factors in green text promote proliferation or growth. Additionally, round circles specify factors involved in epithelial-to-mesenchymal transition (EMT). (c) Diagram of coelomic epithelial thickening and gonad formation in mammals. Schematic depicts three areas: the coelomic epithelium, gonad, and mesonephros between 10.5 and 11.5 dpc, when sex determination occurs. The gonad forms by the expansion of the coelomic epithelium and through epithelial-to-mesenchymal transition. Additionally, at that time germ cells begin to arrive and colonize the gonad
Many factors are important to the process of gonad specification and formation (Fig. 1); some of the major ones are included in this chapter, including GATA-binding protein 4 (GATA4); Wilms’ tumor 1 (WT1); nuclear receptor subfamily 5, group A, member 1 (NR5A1); sine oculis-related homeobox members SIX1/SIX4; odd-skipped related 1 (OSR1); chromobox 2 (CBX2); LIM homeobox protein 9 (LHX9); empty spiracles homeobox 2 (EMX2); pre-B-cell leukemia homeobox 1 (PBX1); insulin receptor (INSR) and insulin-like growth factor 1 receptor (IGF1R); milk fat globule-EGF factor 8 protein (MFGE8); wingless-type MMTV integration site family 4 (WNT4); and R-spondin 1 (RSPO1). Mutations in most of these genes usually result in a structure called a “streak gonad,” which fails to develop beyond the bipotential state and contains mostly fibrous, undifferentiated tissue; alternatively, they may alter the gonadal primordium such that the development of the testis or ovary is particularly hindered. This section will discuss each factor in detail and the known interactions between them.
GATA-Binding Protein 4 (GATA4)
GATA4, named after the GATA DNA sequence to which it binds, is an important zinc finger transcription factor for genital ridge development and is expressed in gonadal somatic cells regardless of later sexual fate (Heikinheimo et al. 1997; Viger et al. 1998). Mice with systemic mutations in Gata4 are embryonic lethal by 8.5–10.5 dpc, and, therefore, gonad development cannot be studied in these models; however, the conditional loss of Gata4 (through a tamoxifen-inducible Cre/LoxP system removing Gata4 after 8.75 dpc) results in the lack of gonadal ridge formation/thickening, demonstrating its functional importance in gonad specification (Molkentin et al. 1997; Hu et al. 2013). Gata family members are expressed at various stages of gonad development, such as Gata1, Gata4, and Gata6; however, only Gata4 is expressed during gonad formation (Heikinheimo et al. 1997; Viger et al. 1998; Ketola et al. 1999). GATA4-positive cells within the splanchnic mesoderm cells of the hindgut, a dorsal mesentery region continuous with the coelomic epithelium, migrate toward explanted gonads in culture (McCoard et al. 2001); therefore, it is presumed that a released gonadal factor creates a chemotactic gradient to recruit GATA4-positive cells to the forming gonad; however, the role of GATA4 in survival or proliferation cannot be discounted within this model (McCoard et al. 2001). Interestingly, no GATA4 was observed in the mesonephric structures, so GATA4 may be more specific for gonad specification, rather than overall urogenital development, at this stage. Upon the formation of the genital ridge at 9.25 dpc, GATA4 is initially expressed anteriorly, after which it expands in an anterior-to-posterior fashion within the epithelium of the gonad (Hu et al. 2013).
The function of GATA4 has been well characterized in the development of the heart. GATA4 regulates the cell cycle transition between G1 and S phases and proliferation through direct binding to the promoter regions of the cell cycle genes Cyclin D2 (Ccnd2) and Cyclin-dependent kinase 4 (Cdk4). Additionally, GATA4 has been shown to influence EMT through mitogen-activated protein kinase 1 (MAPK1) and Erb-b2 receptor tyrosine kinase 3 (ERBB3) signaling (Rojas et al. 2008; Rivera-Feliciano et al. 2006). The overall importance of GATA4 during gonad formation has been demonstrated through GATA4-mediated Nr5a1 and Lhx9 activation, in which GATA4 may directly or indirectly interact to influence coelomic epithelial thickening (Hu et al. 2013). Additionally, the Gata4 locus contains binding sites for the transcription factors WT1 and NR5A1, and its expression is localized within WT1- and NR5A1-expressing cells. Through elucidation of the interaction of Nr5a1 and GATA4, it has been determined that GATA4 can only transactivate Nr5a1 in some cell types, such alphaT3-1 and MSC-1 cell lines, but was very poor at transactivation in L, TM3, or Y-1 cells. This GATA4-dependent activation of Nr5a1 occurs through the GATA element and not the minimal promoter, implicating that the presence of cell-specific cofactors or posttranslational modifications might contribute to transcriptional activation differences in different tissues or cell lines. It could be speculated that these cofactors or posttranslational modifications could also occur during a particular developmental time window of activation; therefore, additional positive or negative regulatory elements may control this interaction (Tremblay and Viger 2001). Additionally, a feedback loop between NR5A1 and GATA4 may regulate Gata4 expression, as a binding site in the Gata4 promoter specific for NR5A1 has been identified (Tremblay and Viger 2001). GATA4 can autoregulate through the binding of itself on the Gata4 exon 1b (E1b) promoter (Mazaud-Guittot et al. 2014). The difference between the Gata4 E1b transcript, as compared to the transcript containing exon 1a (E1a), is that E1b allows regulation of GATA4 by itself, whereas E1a transcripts require other more ubiquitous factors binding to either the GC- or E-box motif through different promoter regions (Mazaud-Guittot et al. 2014). Gata4 E1b-based transcriptional autoregulation of itself is repressed by interaction of GATA4 with zinc finger protein, multitype 2 (ZFPM2, also known as FOG2) (Mazaud-Guittot et al. 2014). This interaction is most likely why Gata4 autoregulation occurs during initial gonadal formation, but not during later stages when ZFPM2 is present (Lakshmaiah et al. 2010; Tevosian et al. 2002), which will be discussed in more detail in a later section of this chapter .
Wilms’ Tumor 1 (WT1)
WT1 is an important transcription factor required for development of the urogenital tract, especially the gonad and kidney, as mice mutant for Wt1 lack both these organs (Kreidberg et al. 1993). Two known mutations in human WT1, one at 11p13 affecting structural integrity of the WT1 protein and the other a dominant point mutation disrupting its zinc finger domains, lead to Wilms’ tumor-aniridia-genital anomalies-retardation (WAGR) and Denys-Drash syndromes , respectively. Both syndromes have overlapping symptoms of abnormal genitalia and predisposition to Wilms’ tumors, which are characteristic of WT1 mutations (Glaser et al. 1989; Pelletier et al. 1991). Mice with a homozygous mutation for Wt1 are embryonic lethal (Kreidberg et al. 1993). Wt1 is expressed throughout early fetal development in the adreno-gonadal primordium starting at 9.0 dpc, and its expression continues into genital ridge formation. At early stages, such as 9.5 dpc, WT1 expression is throughout the urogenital ridge. Although initially also in the adrenal gland, adrenal WT1 expression is decreased after adrenal-gonad separation, while the gonad continues to express WT1 even at later stages, including 11.5–12.5 dpc (Bandiera et al. 2013). In the absence of Wt1 function, the thickening of the coelomic epithelium of the gonad is reduced by 11.0 dpc, and gonad formation is stunted and resultant apoptosis causes massive degeneration of the gonad by 14.0 dpc (Kreidberg et al. 1993). The impact of Wt1 deficiency was limited to somatic cells, as germ cell migration was normal in these mutants (Kreidberg et al. 1993).
WT1 can potentially exist as 36 different isoforms due to splice variations and differential start sites; however, there are two specific WT1 isoforms that are involved in gonad specification, which either contain (WT1+KTS) or lack (WT1-KTS) a tripeptide of lysine, threonine, and serine (Hohenstein and Hastie 2006). The presence of the KTS region alters WT1 function, as the insertion of these three amino acids is between the third and fourth zinc fingers and affects WT1’s ability to bind DNA. When present, the KTS sequence prevents zinc finger function due to increased flexibility of the linker between the third and fourth zinc fingers and abrogates the binding of the fourth zinc finger in the major groove of DNA; these changes drastically reduce DNA binding, so the WT1+KTS isoform instead preferentially binds to RNA and thus is involved in RNA metabolism (by shuttling between the nucleus and cytoplasm and associating with ribonucleoproteins and actively translating polysomes) (Caricasole et al. 1996; Kennedy et al. 1996; Niksic et al. 2004). In contrast, WT1-KTS regulates gene expression and chromatin architecture, as it has a high affinity for DNA. The ratio of these two isoforms is thought to be of importance because Frasier syndrome patients have a loss of the WT1+KTS isoform; however, this syndrome seems to be linked to sex determination-associated defects (rather than initial gonadal formation defects) due to its role in Sry and anti-Müllerian hormone (Amh) gene regulation. In addition, GATA4 cooperative binding with WT1 on the Amh promoter requires the WT1-KTS isoform (Miyamoto et al. 2008).
WT1-KTS binds to the same DNA sequence as early growth response 1 (EGR1), but, unlike EGR1, which activates insulin growth factor II and platelet-derived growth factor A chain, WT1-KTS represses the activity of these genes (Drummond et al. 1992; Wang et al. 1992). WT1-KTS also has a binding site for Nr5a1 (Wilhelm and Englert 2002), which is another gene crucial for gonad development (discussed in more detail later in this section). Additionally, WT1 can activate various genes, including but not limited to cell cycle and apoptosis genes, such as Cyclin-dependent kinase inhibitor 1A (also known as P21) and B-cell leukemia/lymphoma 2 (Bcl2); G-protein coupled receptor genes (Syndecan 1); genes encoding transcription factors, such as nuclear receptor subfamily 0, group B, member 1 (also known as Dax1) and Paired box 2 (Pax2); and genes encoding growth factors/mitogens (Amphiregulin) in other cellular systems, demonstrating its diverse role in cellular functions (Kreidberg et al. 1993; Cook et al. 1996; Englert et al. 1997; Kim et al. 1999; Lee et al. 1999; Mayo et al. 1999). One of the more important roles of WT1 in the gonad and adrenal gland, as demonstrated through ectopic expression of WT1-KTS, is that WT1 without KTS can prevent differentiation of AGP WT1-positive precursor cells into steroidogenic cells through its regulation of proposed Nr5a1 repressors, GLI-Kruppel family member GLI1 (Gli1) and transcription factor 21 (Tcf21), demonstrating the importance of the isoform in development (Bandiera et al. 2013). TCF21 has a known involvement in gonadal formation, as the lack of Tcf21 causes impaired gonadal growth (shortened gonadal length).
Odd-Skipped Related 1 (OSR1)
OSR1, also known as ODD1, is a transcription factor containing zinc finger motifs, whose mRNA is expressed starting as early as 7.5–8.5 dpc, during the earliest stage of intermediate mesoderm development (So and Danielian 1999). OSR1 plays a role in p53-mediated apoptosis in zebrafish, but increased apoptosis was restricted to specific regions of the embryo, rather than widespread apoptosis (Huang et al. 2004). This role in apoptosis is likely the reason for agenesis of the kidney and gonad in Osr1 knockout mice. Between 9.5 and 10.5 dpc, a massive Caspase3-driven apoptosis occurs in the gonad (Wang et al. 2005; Fernandez-Teran et al. 1997), resulting in gonadal loss. Interestingly, overexpression or continuous expression of Osr1 mRNA in the kidney leads to ectopic or expanded kidney formation in Danio rerio (zebrafish), Xenopus (frog), and Gallus (chicken) without nephrogenic differentiation. Although the gonad has not been analyzed as of yet, we speculate that similarly to the kidney that arises from all Osr1-positive cells, Osr1 may play a role in promoting the cellular expansion of gonadal progenitor cells (James et al. 2006; Tena et al. 2007). Osr1 and Wt1 knockout mice have similar defects in embryonic development (Kreidberg et al. 1993; Wang et al. 2005), including both heart and urogenital abnormalities (Wang et al. 2005). OSR1 may act upstream or in concert with WT1, as OSR1 is expressed earlier than WT1 during mesoderm differentiation; WT1 is downregulated in Osr1 mutants, and Osr1 knockouts produce less developed kidneys than Wt1 knockouts (Wang et al. 2005; Armstrong et al. 1993). Whether these factors are part of the same signaling pathway needs to be elucidated further .
S ine Oculis-Related Homeobox 1 (SIX) SIX1 and SIX4
There are six member genes of the Six family, all of which regulate cell fate. Two of these members, SIX1 and SIX4, are transcription factors that work in concert for gonad primordium formation and testicular differentiation by influencing two downstream targets, Nr5a1 and Zfpm2 (also known as Fog2), respectively (Fujimoto et al. 2013). Both SIX1 and SIX4 were expressed in the coelomic epithelium and co-localized in cells displaying NR5A1 expression (Fujimoto et al. 2013). Mice lacking Six1 die at birth due to multiple organ malformations (Xu et al. 2003; Ozaki et al. 2004; Laclef et al. 2003a, b); however, mice lacking Six4 do not show any major developmental deficiencies (Ozaki et al. 2004). Interestingly, mice lacking both Six1 and Six4 have more severe defects, including kidney agenesis (Kobayashi et al. 2007). Mice lacking both Six1 and Six4 have defective upregulation of NR5A1 as early as 9.5 dpc, which influences gonad primordium development and subsequently leads to decreased gonadal size (Fujimoto et al. 2013). Furthermore, decreased gonadal size (and possibly delayed development) was observed at later stages (such as 11.5 dpc) in Six1/Six4 double-knockout mice; therefore, the deficit of SIX1 and SIX4 goes uncompensated. SIX1 and SIX4 regulate Nr5a1 independently of the later sex differentiation factor, Zfpm2. Unchanged levels of Nr5a1 in Zfpm2-mutant mice further demonstrate that Nr5a1-independent signaling pathways that occur during gonadal formation are separated from later deficiencies (Tevosian et al. 2002). Furthermore, Six1 and Six4 knockout mice had normal expression of other important genital ridge formation genes, such as Lhx9, Emx2, Cbx2, Gata4, or Wt1-KTS, so this influence seems to be specific for Nr5a1 at 10.5 dpc (Fujimoto et al. 2013) .
Nuclear Receptor Subfamily 5, Group A, Member 1 (NR5A1)
NR5A1 is an important nuclear receptor for development of all steroid-producing tissues, including the gonad and adrenal glands, as mice lacking Nr5a1 lack both organs (Luo et al. 1994). Nr5a1 is not expressed within the adjacent mesonephros and is restricted to the gonad. The homolog of NR5A1 in Bos taurus, named adrenal 4-binding protein (AD4BP) , has a functional role in the regulation of cytochrome P450 steroid hydroxylase genes, through a generally conserved sequence 5′-AGGTCA-3′ (with some variation) within the proximal promoter; therefore, NR5A1 is commonly known as its functional name, steroidogenic factor 1 (SF1) (Taketo et al. 1995; Morohashi et al. 1992).
Nr5a1 function may rely on the activation of its downstream targets, as it plays a role in activation of genes involved in steroidogenesis (Honda et al. 1993; Lala et al. 1992; Morohashi et al. 1993), proliferation (Nash et al. 1998; Wang et al. 2014), and differentiation (Combes et al. 2010; Tran et al. 2006). NR5A1 is already expressed by 9.0 dpc, separating into two populations with either low or high expression by 10.0 dpc; however, the number of NR5A1-high cells seemingly continues to increase during the development of the gonad and adrenal gland from 10.5 to 11.5 dpc. A homozygous deletion of the entire Nr5a1 gene leads to apoptosis of gonadal somatic cells by 12.5 dpc. These mice die by postnatal day 8, and it has been proposed that this lethality is due to adrenal defects (Luo et al. 1994). Rescue experiments with overexpressed Nr5a1 in Nr5a1-deficient animals further confirm that gonad development directly requires Nr5a1 (Fatchiyah et al. 2006). Interestingly, NR5A1 acts in a dose-dependent manner, as a heterozygous mutation of Nr5a1 results in reduced, but not absent, gonads (Bland et al. 2004). NR5A1 is a unique nuclear factor in that it binds monomerically, rather as a homo- or heterodimer. This unique aspect was demonstrated by the loss of 30–40% of NR5A1 DNA binding upon a homozygous point mutation (R92Q) in the A-box region of the DNA binding domain of human NR5A1, resulting in reduced transcriptional activity; however, individuals with a heterozygous point mutation remained phenotypically normal (Achermann et al. 2002). When compared to a P-box mutation, which yields sex reversal phenotypes upon a heterozygous mutation (G35E), the A-box mutation may disrupt a secondary DNA-binding domain and also reveals how dosage could be involved in NR5A1 functionality (Achermann et al. 2002). This region has been suggested to interact with the minor DNA groove to stabilize the interaction of NR5A1 with DNA (Achermann et al. 2002). As this reduction in binding is only partial, other transcription factors known to bind may further stabilize the NR5A1-DNA interaction.
I n the adrenal gland, as early as 11.5 dpc, pre-B-cell leukemia homeobox 1 (PBX1) (covered in more detail later in this chapter) is essential for Nr5a1 expression, as mice deficient in Pbx1 had reduced levels of Nr5a1, and both PBX1-homeobox superfamily (HOX) and PBX1-Pbx/knotted 1 homeobox (PKNOX1; also known as PREP1) complexes can bind the fetal adrenal enhancer (FAdE) to initiate Nr5a1 expression (Zubair et al. 2006). PBX1 and empty spiracles homeobox 2 (EMX2) work cooperatively to activate NR5A1 (their interaction will be discussed in further detail in the PBX1 section). Additionally, Nr5a1 is proposed to be initiated by GATA4. The maintenance of NR5A1 expression has been shown to be by SIX1 and SIX4 and also by NR5A1 autoregulation (through its FAdE). Six1 and Six4 double-deficient mice have a reduced number of NR5A1-positive cells as compared to GATA4-expressing cells (Fujimoto et al. 2013). This reduction in Nr5a1 was observed as early as 9.5 dpc in the coelomic epithelium, and both gene and protein expression were further reduced by 10.0 dpc through 11.5 dpc . Conversely, Six1/Six4 transgene overexpression induced Nr5a1 gene expression, further confirming the relationship between NR5A1 and SIX1/SIX4 (Fujimoto et al. 2013) .
Insulin Receptor (INSR) and Insulin-Like Growth Factor 1 Receptor (IGF1R)
INSR and IGF1R pathways are important signaling mechanisms for overall body growth, cellular proliferation, and differentiation. When analyzed at 10.5 dpc, mice deficient in both Insr and Igf1r had no considerable size defects, but over the next 2 days, progression of overall body size decreased. Due to their reduced size, mice lacking both Insr and Igf1r were analyzed to determine if their overall developmental programming was delayed. Interestingly, tail somites and other developmental structures, such as limbs, were normal in later developmental stages. Genes encoding receptor tyrosine kinases in the insulin pathway (INSR and IGF1R) are required for proliferation of the somatic progenitor cells within the gonad. Mice lacking Insr and Igfr lead to reduced NR5A1, WT1, and LHX9 expression (Pitetti et al. 2013), thereby influencing gonad development. Insr and Igf1r, in conjunction with insulin receptor-related receptor (Insrr, also known as Irr), have an additional critical role in later aspects of male sex determination and differentiation, such as regulating Sry expression (Nef et al. 2003); these functions will be discussed later in this chapter .
Cbp/p300-Interacting Transactivator, with Glu-/Asp-Rich Carboxy-Terminal Domain, 2 (CITED2)
Cited2 is expressed in the coelomic epithelium and adjacent mesenchyme at 10.0 dpc (Val et al. 2007). During the separation of the AGP at 10.5 dpc, Cited2 expression decreased in the coelomic epithelium and was barely detectable by 12.0 dpc. No changes in Cited2-deficient mice were observed in proliferation, apoptosis or in laminin expression at 10.5 or 11.5 dpc; however, there was an Nr5a1-specific transcriptional delay in gonad development at 11.5 dpc, which resulted in additional later disruptions of gonad morphology. Cited2-deficient mice have reduced expression of the gonadal formation gene Nr5a1, as well as a reduction in expression of a sex determination gene expressed in Sertoli cells , Sox9, at 11.5 dpc. Additionally, the expression of a Leydig cell-specific gene, cytochrome P450, family 11, subfamily a, polypeptide 1 (Cyp11a1), is also decreased at 13.5 dpc (Combes et al. 2010). However, transcription of Sox9, Cyp11a1, and Nr5a1 all recovered in Cited2-deficient gonads by 13.5 dpc, indicating that later differentiation and structure in both male and female gonads are normal (Combes et al. 2010).
Cited2-deficient mice have normal levels of WT1 and LHX9 ; therefore, CITED2 might be acting downstream of these two gonadogenesis factors. CITED2 is a non-DNA-binding cofactor for WT1 in the stimulation of Nr5a1 within the adreno-gonadal primordium (Val et al. 2007). The cooperation between WT1 and CITED2 leads to an expression increase of NR5A1 in the adreno-gonadal primordium, and this expression of NR5A1 over the required threshold allows for adrenal and gonad development (Val et al. 2007). Although CITED2 has been demonstrated to bind to both isoforms of WT1, the WT1-KTS has shown preferential binding (Val et al. 2007) .
C hromobox 2 (CBX2)
CBX2 (also known as M33; known in Drosophila melanogaster as Polycomb), a regulator of homeotic gene expression, is important for development of the gonad, adrenal, and spleen, as well as for sexual differentiation (Katoh-Fukui et al. 1998). CBX2 may regulate the differentiation of embryonic stem cells (ESCs), as increased CBX2 protein expression was observed in later differentiated stages of ESCs and retinoic acid-treated ESCs. Furthermore, ChIP sequencing and ESC teratoma formation experiments demonstrate a germ layer specification preference toward mesoderm and endoderm with the expression of CBX2 (Morey et al. 2012). Cbx2 and Polycomb ring finger oncogene (Bmi1) work synergistically to regulate mesodermal genes, as demonstrated by double-knockout experiments.
A Polycomb group (PcG) assembly of either PRC1 (polycomb repressive complex 1) or PRC2 (polycomb repressive complex 2) is crucial for developmental epigenetic regulation (via histone modifications) and for maintenance of gene repression. CBX2 directly recognizes modified histones, such as H3K27me3, through its chromodomain and recruits other members of the PRC1 complex. Phosphorylation of CBX2 provides its functionality, as phosphorylation is important for its nuclear translocation and interaction with H3K27me3 by increasing its affinity for H3K27me3 (Hatano et al. 2010). The interaction of CBX2 with the E3 ligase ring finger protein 2 (RNF2, also known as RING1B), which is another transcriptional repressor in the PRC1 complex, brings RNF2 in the proximity to H3K27me3, inducing chromatin modification through RNF2 ubiquitination of H2A at lysine 119, thereby furthering repression of the gene locus (Kaustov et al. 2011; van der Stoop et al. 2008). CBX2 is the only CBX family member known to induce chromatin compaction (Grau et al. 2011). Loss of Cbx2 results in reduced cellular proliferation through impaired regulation of H3K27me3. This effect is expected, as CBX family proteins have the shared function of repressing the INK4a-ARF locus, known to be an inhibitor of cell cycle, and, specifically, CBX2 in embryonic fibroblasts controls the entry into S phase during proliferation (as demonstrated by BrdU incorporation studies) (Core et al. 2004). In humans, two isoforms of CBX2, CBX2.1 and CBX2.2, have been observed, with different lengths (the latter being shorter than the former); both can functionally repress transcription. Both isoforms contain the chromodomain; however, only CBX2.1 contains the Polycomb box shown to directly bind to RNF2; therefore, the binding of the PRC1 complex is altered with CBX2.2 (Volkel et al. 2012). Similarly, zebrafish have Cbx loci coding for isoforms with and without the Polycomb box (Le Faou et al. 2011). Within the gonad, CBX2 expression is strong in epithelial cells (as defined by a single layer of cells lining the coelomic space), but is weak in gonadal mesenchymal cells (Katoh-Fukui et al. 2012).
The loss of Cbx2 results in underdeveloped and small gonads, with later sex differentiation defects. Defects in Cbx2-mutant gonads are most likely due to reduced mesenchymal proliferation, rather than migration defects, as Cbx2-deficient gonads demonstrated decreased mesenchymal proliferation (via BrdU incorporation assays), but exhibited no change in epithelial proliferation, overall apoptosis, or laminin expression (important for basement membrane formation) (Katoh-Fukui et al. 2012). Additional studies confirm CBX2 playing a role in the regulation of gonadal proliferation, as CBX2 accumulates and binds to an upstream promoter region of Nr5a1 in both mouse and human cells, and overexpression of NR5A1 has been demonstrated in chicken embryos to upregulate Cyclin D1, a known player involved in driving the G1/S phase transition (Ishimaru et al. 2008). CBX2 is an important nuclear receptor for genital ridge development and has been implicated in the regulation of NR5A1, as a human mutation in CBX2 failed to regulate NR5A1; decreased expression was observed in the gonad of a murine Cbx2 knockout, and CBX2 can accumulate and bind the promoter region of Nr5a1 (Katoh-Fukui et al. 1998, 2005; Biason-Lauber et al. 2009). Additional studies using DNA adenine methyltransferase identification (DamID) coupled to high-throughput sequencing (DamID-seq) demonstrated that overexpression or knocking down CBX2 via transfection methods in a NT-2D1 cell line resulted in increased or decreased NR5A1 expression, respectively (Eid et al. 2015). CBX2 is also known to influence other gonad formation-associated transcription factors, such as LHX9 and GATA4. LHX9, GATA4, and EMX2 are downregulated in Cbx2-deficient mouse gonads (Katoh-Fukui et al. 2012). However, other gonadal transcription factors, such as WT1 and CITED2, are unaffected in Cbx2-deficient gonads (Katoh-Fukui et al. 2012); therefore, CBX2 regulation of transcription factors may be selective for LHX9, GATA4, and EMX2 function .
L IM Homeobox Protein 9 (LHX9)
LHX9, part of the LIN11-ISLET1-MEC3 (LIM) homeodomain family, is an important transcription factor for genital ridge development, as Lhx9 knockout mice fail to develop bipotential gonads (Birk et al. 2000). LHX9, like most of the other family members, regulates transcription; they are characterized by their two LIM domains containing a total of four cysteine-rich zinc fingers that are important for protein-protein interactions and a homeobox domain crucial for DNA binding. Lhx9 is involved in proliferation of the gonad anlage; Lhx9 exon2-/exon3-deficient mice (removal of the first two LIM domains) have a reduced proliferation rate, but no changes in apoptosis, in LHX9-positive cells. LHX9 is expressed at 9.5 dpc in both the epithelial and mesenchymal cells (Birk et al. 2000). Lhx9 is subsequently (11.5 dpc) expressed at high levels in both the coelomic epithelium and the superficial mesenchyme that later becomes the tunica albuginea, but is expressed at lower levels within the deeper mesenchyme (Birk et al. 2000). LHX9 cooperates with WT1 to bind and transactivate Nr5a1 (Wilhelm and Englert 2002; Birk et al. 2000). Furthermore, the impact of LHX9 on Nr5a1 is demonstrated by the Lhx9-exon2-/exon3-deficient mouse displaying reduced levels of Nr5a1 (Birk et al. 2000); however, the reduction of proliferation of these Nr5a1-positive cells (as they overlap with Lhx9-positive cells) may be the indirect cause of LHX9 on these reduced levels. The GATA4/ZFPM2 complex has been shown to activate Lhx9 in the heart; however, as ZFPM2 is not required for Gata4 function in gonad formation, there is no effect of the mutant GATA4/ZFPM2 complex on Lhx9 expression in the gonad (Tevosian et al. 2002; Smagulova et al. 2008). In other embryonic tissues, such as the central nervous system and limbs, the expression of Lhx2, which has similar structure and overlaps with Lhx9 expression, may compensate for the lack of Lhx9 function in the Lhx9 exon2/exon3 mutant; however, since Lhx2 is not expressed within the gonad, that is likely why gonadal-specific defects are observed in Lhx9-mutant mice (Birk et al. 2000; Bertuzzi et al. 1999) .
Pre-B-cell Leukemia Transcription Factor1 (Pbx1)
The pre-B-cell leukemia transcription factor (PBX) family encodes three amino acid loop extension (TALE) homeodomain proteins , whose TALE domain allows them to form trimeric complexes with DNA (Burglin 1997; Ferretti et al. 2000; Jacobs et al. 1999). Of the four subclasses, only PBX1 (and weakly PBX3, which demonstrates an overlapping embryonic expression pattern with PBX1) is expressed in the gonad (Di Giacomo et al. 2006). PBX1 is a HOX cofactor (increasing HOX DNA-binding specificity/selectivity) that can be expressed as either of two splice variants (PBX1a and PBX1b) (Mann and Affolter 1998; Schnabel et al. 2001). PBX1b expression is localized to both nuclei and cytoplasm of gonadal cells (Ota et al. 2008). PBX1 is expressed in the adreno-gonadal primordium and the coelomic epithelium by 10.0 dpc, followed by later expression in the gonad interstitium after sex determination (Schnabel et al. 2003). Pbx1-knockout mice lack adrenal glands and Müllerian ducts, have problems with gonad development, have reduced kidney size, and are embryonic lethal by 15.5–16.5 dpc (Schnabel et al. 2001, 2003). PBX1 is not essential for the generation of mesoderm, but rather functions later in the development of the urogenital organs, likely through its involvement in cell cycle regulation (Schnabel et al. 2003; DiMartino et al. 2001; Kim et al. 2002; Selleri et al. 2001). The size of the genital ridge is severely decreased, mostly attributable to reduced adrenogenital precursor proliferation (as demonstrated by BrdU incorporation assays) in Pbx1-knockout mice as compared to control mice (Schnabel et al. 2003).
NR5A1 expression is reduced in Pbx1-deficient mice, resulting in only a few NR5A1-positive cells in the coelomic epithelium at 10.0 dpc, which persisted in some Sertoli and Leydig cells later at 13.0 dpc (Schnabel et al. 2003). This gonadal reduction in NR5A1 has been postulated to be caused by a reduction in gonadal proliferation (Schnabel et al. 2003). The role of PBX1 in modulating NR5A1 expression is more prominent in the adrenal gland, as Pbx1-deficient mice lack NR5A1-positive cells, although different mechanisms may occur in different tissues (Schnabel et al. 2003). Even though PBX1 may play a role in regulating NR5A1 expression, PBX1 does not seem to impact WT1 expression (Schnabel et al. 2003). Another interacting partner of PBX1 (although only analyzed in other tissue and cell systems) known to bind DNA as a cooperative partner with PBX1 is EMX2. In Pbx1/Pbx2/Pbx3 mutants, EMX2 is completely lost, and proliferation is reduced (Capellini et al. 2010); EMX2 is also important in genital ridge formation (see next section). EMX2 and PBX1 together in cell lines, such as COX and P13, have the ability to bind the DNA consensus sequence 5′-CTTTAATGAT-3′ as a heterodimer to activate transcription of genes; one example is the scapular patterning genes. Furthermore, the transcriptional activation of genes relied on cooperation between the two proteins, as separately neither PBX1 nor EMX2 could activate transcription (Capellini et al. 2010). Therefore, we speculate that Pbx1 is important in patterning and proliferation within the newly formed gonad .
E mpty Spiracles Homeobox 2 (EMX2)
EMX2 is an important transcription factor for development of the urogenital tract, including the gonad, kidneys, ureters, and genital tracts, as Emx2 mutant mice lack these organs (Miyamoto et al. 1997). EMX2 expression has been observed in the coelomic epithelium at 10.5–11.5 dpc. Mice lacking Emx2 exhibit defective gonad formation with sparse cells comprising the coelomic epithelium at 11.5 dpc. Through scanning electron microscopy, it was determined that Emx2-knockout gonads have irregular clustering of cells, rather than the smooth surface epithelium seen in controls (Kusaka et al. 2010). Later at 12.5 dpc, the gonad is lost through apoptosis in Emx2-deficient mice (Miyamoto et al. 1997).
Emx2-mutant gonads have ectopic tight junction formation, which inhibits EMT required during early gonad development, in which coelomic epithelial cells become the gonadal mesenchyme. EMT is normally controlled by epidermal growth factor receptor (EGFR) through the regulation of sarcoma viral oncogene homolog tyrosine (SRC) phosphorylation. Within Emx2-knockout gonads, phosphorylation of both SRC and EGFR increases, and subsequently EGFR expression is upregulated (Kusaka et al. 2010) .
Milk Fat Globule-EGF Factor 8 Protein (MFGE8)
A factor involved in genital ridge formation that is associated with epidermal growth factor signaling is MFGE8 (also known as lactadherin). Mfge8 encodes a soluble integrin-binding protein that mediates cellular interaction through two binding interactions: one interaction is through integrin beta 3, and the other is through either phosphatidylserine or phosphatidylethanolamine (Hanayama et al. 2002; Kanai et al. 2000). Similar to EGF-like repeats and discoidin I-like domain 3 (also known as Del1), the functionality of MFGE8 in cellular adhesion occurs through its binding to integrin beta 3 using the arginine-glycine-aspartic acid (RGD) motif of its second EGF domain; another region of MFGE8, the discoidin domain, is able to bind to phosphatidylserine and phosphatidylethanolamine. MFGE8 is expressed in both fetal and adult tissues and is known for mediating cellular adhesion during macrophage phagocytosis of apoptotic cells and maintaining cells within a niche location (Hanayama et al. 2002; Kanai et al. 2000). However, MFGE8 has also been known to play a role in a variety of other contexts, including mammary gland branching morphogenesis, sperm-oocyte adhesion, and angiogenesis (Hanayama et al. 2002; Ensslin and Shur 2007; Motegi et al. 2011; Uchiyama et al. 2014).
During fetal stages, MFGE8 expression is restricted to the urogenital ridge, the nervous system, and the bone (Kanai et al. 2000). Mfge8 RNA is first observed in the coelomic epithelium at 10.0 dpc (protein expressed by 10.5 dpc); then by 10.5 dpc Mfge8 RNA is localized to the region below the coelomic epithelium containing mesenchymal cells (Kanai et al. 2000). By 11.5–12.5 dpc, the expression of Mfge8 is restricted to the border region between the gonad and the mesonephros and in stromal tissues that eventually develop into the tunica albuginea; however, by 15.5 dpc Mfge8 was no longer expressed within the developing gonad (Kanai et al. 2000).
Ishii et al. (2005) demonstrated that MFGE8 is important for gonadal cell-cell adhesion during the critical stages of gonad morphogenesis between 11.5 and 12.5 dpc, as higher binding activity is observed in alkaline phosphatase-positive germ cells, as well as both NR5A1-positive and NR5A1-negative somatic cells, as compared to other time ranges including 10.5 dpc or 15.5 dpc using ex vivo binding assays. These cell types can bind to both of MFGE8’s domains (the two EGF and the two discoidin regions) through a mechanism described above (Ishii et al. 2005). Interestingly, Mfge8 expression partially overlaps with that of Lhx9, but not Wt1 or Emx2, at 11.5 dpc (Kanai et al. 2000). Further elucidation of the interaction between LHX9 and MFGE8 is required to understand how these two factors may synergize for gonad development .
Win gless-Type MMTV Integration Site Family 4 (WNT4) and Roof Plate-Specific Spondin (RSPO1)
Although WNT4 and RSPO1 are associated with female sex differentiation (and shown to play a role in the same pathway), these factors also play a role in initial gonad formation. For more information regarding their roles in sexual differentiation of the gonad, please read section “Forkhead Box L2 (Foxl2)”. Wnt4 is important in kidney, adrenal gland, mammary gland, and reproductive tract morphogenesis by regulating endothelial and steroidogenic cell migration (Jeays-Ward et al. 2003). WNT4 is important in kidney formation, as it is involved in EMT; additionally, Wnt4-knockout mice lack kidneys and die shortly after birth (Kispert et al. 1998). RSPO1 has also been described in other systems to induce WNT/CTNNB1 (β-catenin) signaling, generally associated with increased proliferation (Kazanskaya et al. 2004). Wnt4 expression occurs as early as 9.5 dpc, and WNT4 has been observed in the forming gonad between 10 and 11.5 dpc, after which female-specific expression is observed (Vainio et al. 1999). In males, WNT4 is decreased after 11.5 dpc, whereas in females both RSPO1 and WNT4 are upregulated (Vainio et al. 1999; Barrionuevo et al. 2006). Both Wnt4 and Rspo1 lead to gonad cellular proliferation between 10.5 and 11.5 dpc (Chassot et al. 2012). The exact mechanism of interaction between WNT4 and RSPO1 is unclear both during early gonad formation and later in sex determination. Although Rspo1-knockout mice do not have any observable defects in initial gonad formation, there is a known influence of Rspo1 and CTNNB1 on later Wnt4 expression (11.5 dpc) (Chassot et al. 2008; Liu et al. 2009; Tomizuka et al. 2008).
Synergy between RSPO1 and WNT4 was observed in Rspo1/Wnt4 double-knockout mice, which have a more severe gonadal phenotype with a hypoplastic testis and a reduced number of Sertoli cells (and reduced number of seminiferous tubules), most likely due to a decreased proliferation of the coelomic epithelium which gives rise to Sertoli cells (Chassot et al. 2012). Insr/Igf1r double-knockout mutants demonstrate a decrease in Wnt4, and Wnt4/Rspo1 double-knockout mutants have decreased Igf1r levels; therefore, there is likely a mutual interaction or feedback loop between these two pathways within the forming gonad (Pitetti et al. 2013; Chassot et al. 2012). WNT4 and RSPO1 do not influence Nr5a1; therefore, WNT4/RSPO1 likely functions downstream of Nr5a1 (Chassot et al. 2012) .
Sex Determination
R ole of Chromosomes in Sex Determination
Ancient theories, such as those put forth by Aristotle, posited that the heat of the man’s sperm or the male’s “principle” drives sex determination (Haqq and Donahoe 1998). These ideas persisted for many centuries until modern science in the past two centuries revealed the role of chromosomes in heredity; of particular importance was the discovery of the role of chromosomes in sex determination by Clarence Erwin McClung (McClung 1918). As an expansion to this early work, genetic analyses have shown that a network of factors encoded on chromosomes (both sex chromosomes and autosomes) is important for sex determination. Sex determination occurs when cells are progressively restricted in their developmental potential and led down a particular lineage path to their resulting end fate, in this case male (testis) or female (ovary). Once the fate of a cell has been “determined,” it normally does not change, except if there are defects in the network of genes that maintain sexual fate or if there is an external influence by environmental factors (e.g., hormones in nonmammalian species).
Sexual development occurs at various steps: sex determination, which is the mechanism that triggers the male-versus-female choice and sets that pathway into motion (often, but not always, encoded genetically); and sexual differentiation (gonadal and extra-gonadal), which is downstream of sex determination and is the phenotypic manifestation of male and female identity. This chapter will cover sex determination, while sexual differentiation will be discussed in Chap. 6, “Male Sexual Differentiation”
Chromosomal Sex Determination Mechanisms
For centuries, the mechanisms that drove the decision in utero for the embryo to develop as a male or female were a source of great debate. Early ideas centered on the environment playing a major role in sex determination. During the nineteenth century, studies by Mendel and others put forth the idea that heritable factors are responsible for determining genetic traits of offspring, including possibly their sex. The discovery of chromosomes as the vessels for genetic material was a critical step in securing a model of chromosomal sex determination. In particular, the observation that the karyotype of males and females of certain species (those with heteromorphic sex chromosomes) was different sparked a new area of research focusing on uncovering the genetic mechanisms driving sex determination.
Sex-specific chromosomes were first described for their role in sex determination at the turn of the twentieth century, due in large part to research on insect model systems. Clarence Edward McClung, who was studying spermatogenesis in the grasshopper Xiphidium fasciatum, first proposed in 1901 that a particular “nuclear element” (which McClung demonstrated was in fact a chromosome) was responsible for sex determination (McClung 1918). While he was not the first to describe that the karyotypes of sperm were different from one another and one-half of sperm contained a unique chromosome, he was among the first to propose that chromosomal makeup was directly linked to sex determination. A few years later, while studying the fruit fly, Nettie Stevens and Edmund Beecher Wilson in 1905 confirmed this idea by defining sex-specific chromosomes through the observation that chromosomal karyotype correlated with the sex of the individual (Wilson 1905). These findings launched the idea that there was a chromosomal basis for sex determination .
C hromosomal Sex Determination in Mammals (XX/XY System)
Almost half a century after the work of Stevens and Wilson, the research of Alfred Jost pushed the field of sex determination even further by defining the gonad as the central factor which determines the sex of the embryo in mammals. In his groundbreaking experiments, Jost removed gonads from fetal rabbits in utero early during gestation (i.e., before sex determination took place) and found that gonadectomized embryos invariably developed as females in terms of reproductive tract and external genital development (Jost 1947). In his research, Jost showed that the testis was sufficient to induce male-specific development of the reproductive tract and external genitalia by secreting essential signals for sexual differentiation (later shown to be testosterone and anti-Müllerian hormone) (Jost 1953). Several years later, clinical studies revealed that sex chromosomal aberrations were likely the basis for Klinefelter’s (XXY males) and Turner’s (XO females) syndromes in humans (Ford et al. 1959; Jacobs and Strong 1959); soon thereafter, it was reported that partial deletions of the Y chromosome likely were responsible for sexual differentiation phenotypes in humans (Conen et al. 1961). These data led to and supported the hypothesis that the Y chromosome was the major determinant of sex determination in mammals.
It was only recently discovered in 1990 that the dominant factor in sex determination was a gene on the Y chromosome, named Sry (sex-determining region of chromosome Y) , which encodes a HMG-box transcription factor necessary and sufficient for male sex determination (Koopman et al. 1990, 1991; Sinclair et al. 1990) (see later in this chapter). This gene on the Y chromosome, when placed on an autosome in an XX mouse, was sufficient to endow a fully male phenotype (however, the XX/Sry mouse was sterile since the Y chromosome also contains other genes required for spermatogenesis and XX germ cells in a XY-like somatic environment have difficulty completing gametogenesis) (Koopman et al. 1991). Finally, the evidence was conceptualized in a model in which sex determination in mammals was a XX/XY system , where females are the homogametic sex (XX) and males are the heterogametic sex (XY), and, additionally, the Y chromosome is the major determining chromosomal component for male sex determination .
Other Chromosomal Sex Determination Mechanisms
W hile the most familiar system of chromosomal sex determination is the XY system, such as used by humans, there are a variety of genetic mechanisms that drive sex determination among animal species (Fig. 2). These systems vary not only in the identity and makeup of the sex chromosomes themselves but also the mechanisms downstream of the chromosomal trigger that drives the decision to undertake male-specific or female-specific development (described in further detail in the upcoming section).
Chromosomal sex determination pathways in diverse animal species. Cartoon representing key genetic triggers in sex determination of Caenorhabditis elegans, Drosophila melanogaster, the honeybee Apis mellifera, chickens, and mammals. Bars on top row represent chromosomes, in which female-specific sex chromosomes are red, male-specific sex chromosomes are blue, and autosomes are black. The ratio of X chromosomes to autosomes is the genetic trigger in C. elegans and Drosophila, which leads to the expression of xol-1 and Sxl, respectively, which subsequently drives sex-specific regulation of mab-3 and dsx, respectively. In honeybees, the haploid/diploid state and the hetero-/hemi-/homozygosity of Csd lead to sex-specific development. In birds and mammals, a master gene (DMRT1 or Sry, respectively) sets off the male pathway
In avian species, the males are the homogametic sex (ZZ) and females are the heterogametic sex (ZW) (Ayers et al. 2013). Therefore, this system has been termed ZZ/ZW , in contrast to XX/XY, to emphasize the consensus in the field that the mammalian X and Y sex chromosomes are unrelated to the avian Z and W sex chromosomes (Fridolfsson et al. 1998; Matsubara et al. 2006). Similarly to SRY in the XX/XY system, it has been suggested that transcription factor, DMRT1 (Doublesex- and MAB-3-related transcription factor 1) , acts as the master sex determination factor in birds (Smith et al. 2009); however, DMRT1 is different from Sry in that avian DMRT1 likely acts in a dosage-dependent manner in which two copies are required for male sex determination (since DMRT1 is located on the Z chromosome). The DMRT1 gene and its homologs are present in most vertebrate species examined and also have significant evolutionary conservation among invertebrates, such as Drosophila and C. elegans, suggesting that this gene family has a more ancestral role in this process (Raymond et al. 1998, 1999) (see more discussion below).
The molecular mechanism underlying the chromosomal-based system of Drosophila and C. elegans sex determination is a “counting mechanism” in which the ratio of X chromosomes to autosomes drives a regulatory gene cascade. The X:autosome ratio drives the expression (or repression) of the master regulator genes Sex lethal (Sxl) in Drosophila and XO lethal-1 (xol-1) in C. elegans, which ultimately leads to sex-specific expression of target genes responsible for sexual dimorphism, such as doublesex (dsx) in Drosophila and male abnormal-3 (mab-3) in C. elegans, both of which encode a DM-domain homolog of mammalian DMRT1 (reviewed in Salz and Erickson 2010; Zarkower 2006).
Lastly, in the haplodiploidy system of honeybees and some other insect species, sex chromosomes with unique genetic information are not required for sex determination; instead, sex-specific development is controlled via a dose-dependent signal from a single or two different alleles of the complementary sex determiner (Csd) gene, in which male or female sex determination depends on whether the embryo is homozygous (nonreproducing male), heterozygous (female), or hemizygous (male) at the Csd locus (Beye et al. 2003). Therefore, males develop from haploid unfertilized eggs and females develop from diploid fertilized eggs .
E volutionarily Conserved Aspects of Chromosomal Sex Determination
It is clear the upstream “trigger” that launches sex determination has rapidly evolved over the course of time; the labile nature of this process is demonstrated by the fact that certain mammalian species have lost Sry and that the closely related species Japanese medaka and Luzon ricefish (Oryzias latipes and Oryzias luzonensis, respectively) have different master sex determination triggers (a DMRT1-like transcription factor versus a transforming growth factor-beta (TGF-β) secreted factor) (Matsuda et al. 2002; Myosho et al. 2012). While the upstream mechanisms are widely diverged in the animal kingdom, many parts of the molecular machinery involved in sex determination are conserved among animals.
The DMRT1 family of transcription factors, as the name suggests (Doublesex- and MAB-3-related transcription factor 1), is a central part of sex determination mechanisms in a number of animal phyla (Matson and Zarkower 2012). This transcription factor is the homolog of two factors involved in sexual development in invertebrates: DSX in Drosophila and MAB-3 in C. elegans, all of which contain a DM (Doublesex and MAB-3) domain, which is a novel zinc finger DNA-binding motif (Zhu et al. 2000). Recently, DM-domain homologs of DMRT1 have been implicated as key sex determination factors in birds, the Japanese medaka, Chinese tongue sole, and frog (Xenopus) (Smith et al. 2009; Matsuda et al. 2002; Chen et al. 2014; Yoshimoto et al. 2008). Genomic deletion of the distal short arm of chromosome 9 (9p) in humans, a region which contains DMRT1, is associated with gonadal dysgenesis and XY sex reversal phenotypes (Onesimo et al. 2012), suggesting that human DMRT1 is critical for male sex determination. XY mice with mutations in Dmrt1 do not show a sex reversal phenotype and are born with normal testes (Raymond et al. 2000), indicating that Dmrt1 is not required for mouse primary sex determination; however, in postnatal stages Dmrt1 has been shown to be important for sexual differentiation and maintenance (discussed in the next chapter of this volume).
A factor central to mammalian sex determination is Sox9, which encodes a SoxE group transcription factor necessary and sufficient for male sex determination (Barrionuevo et al. 2006; Vidal et al. 2001). SOX9 protein and its homologs are specifically expressed within the testes (not ovaries) in a number of species, such as Gallus gallus (chicken), Danio rerio (zebrafish), and Drosophila (Chiang et al. 2001; DeFalco et al. 2003; Kent et al. 1996). Sox9 plays a key role in male development in mammals as it is a direct downstream molecular target of SRY (Sekido et al. 2004). In particular, as a determinant of Sertoli cells, Sox9 is important for forming testis cords, the embryonic precursors of the seminiferous tubules (the sites of spermatogenesis in the gonads of many vertebrate species); experiments in mice deleting Sox9 and its closely related gene family member Sox8 after sex determination has already occurred reveal that Sox gene function is required for maintenance of seminiferous tubule integrity (Barrionuevo et al. 2009). The Drosophila homolog of Sox9, Sox100B, is not required for primary sex determination in flies, but does play a role in adult testicular morphogenesis (Nanda et al. 2009), suggesting that there is broad evolutionary conservation of Sox9 function in male-specific development .
Throughout evolution, it is common to see the same extracellular signaling pathways used in different contexts. An example of one such pathway is TGF-β. While TGF-β is used in multiple developmental processes, it also plays a role in sex determination in different species. In particular, the involvement of homologs of the TGF-β factor anti-Müllerian hormone (Amh) is widespread in sex determination of various animal species, especially in fish. In the medaka, Oryzias latipes, the hotei mutation (in the Amh receptor type II) revealed that disruption of Amh signaling drives excessive germ cell proliferation and male-to-female sex reversal (Morinaga et al. 2007). Amh pathway member homologs are also sex-determining factors in tiger pufferfish (fugu; Takifugu rubripes), Luzon ricefish (Oryzias luzonensis; closely related to Japanese medaka), Patagonian pejerrey (Odontesthes hatcheri), and sablefish (Anoplopoma fimbria) (Myosho et al. 2012; Hattori et al. 2012; Kamiya et al. 2012; Rondeau et al. 2013). Interestingly, mammalian Amh is not involved in primary sex determination and instead is critical for sexual dimorphism of the reproductive tract and for ovarian follicular development (Behringer et al. 1994; Durlinger et al. 1999). However, this later role for Amh in mammals is likely an exception among animals, since in most other species examined, such as alligators and birds, Amh is expressed prior to Sox9 (Oreal et al. 1998; Western et al. 1999); therefore, Amh and related TGF-β factors likely play a more central, ancestral role in sex determination in some animal species (rather than in differentiation as in mammals).
Regardless of the chromosomal systems used to trigger testicular or ovarian development, many of the downstream factors are evolutionarily conserved. Further examination of these factors that drive gonad specification and formation should shed light on the basic mechanisms responsible for sex determination, as well as how perturbations in these pathways play a role in disorders of sexual development and other congenital conditions within the reproductive system .
Mammalian Testicular Sex Determination Genes
In mammals, sex determination is equivalent to gonad determination, in that the gonad is the initial and primary site where the male-versus-female decision is triggered, with profound downstream effects on the rest of the reproductive system and body. In the developing mouse embryo, the gonadal primordium arises at around 10.0 dpc (Kashimada and Koopman 2010). At this stage the genital ridges are morphologically indistinguishable between XX and XY embryos. The main trigger for sex determination is the expression of Sry in somatic cells of the XY gonadal ridge starting at 10.0–10.5 dpc (Hacker et al. 1995), which triggers male sex determination and directs the bipotential gonad toward testicular differentiation (Fig. 3); in the absence of Sry expression, the gonad develops into an ovary, under the influence of signaling by the WNT4/β-catenin pathway and NR0B1/DAX1. This section of the chapter discusses the major genes involved in both testicular and ovarian sex determination in mammals.
Overview of the mammalian sex determination pathway. In mice, the bipotential gonad (gray) arises by 10.5 dpc and is morphologically indistinguishable between XX and XY embryos. Sry expression (triggered by Wt1, Gatat4, and Zfpm2) at 10.5 dpc (filled blue box) in the somatic cells of the XY gonad triggers male-specific gonad development (right side of the figure). SRY, along with SF1, upregulates its downstream target Sox9 (schematic in the left bottom inset) in pre-Sertoli cells to trigger testis development. SOX9 expression is sustained by positive feedback loops involving Fgf9, Ptgds, and SOX9 itself (schematic in the right bottom inset). This feedback loop ensures continued Sox9 expression which is required to activate male-specific genes during development and sustains expression of Sox9 in the postnatal and adult testis. In the absence of Sry (left side of the figure), expression of female-specific genes such as Nr0b1, Wnt4, and Foxl2 ensures proper ovarian development. Abbreviations: Fgfr2 fibroblast growth factor receptor 2, Esr1 estrogen receptor 1
Sex-Determining Region of Chromosome Y (Sry)
Sry, the master gene in the mammalian sex determination pathway, encodes a protein that belongs to the SOX (SRY-related HMG box) family of transcription factors. Evolutionary studies have revealed that Sry is a recently arisen mammal-specific gene, which likely evolved from the Sox3 gene currently on the mammalian X chromosome (Foster and Graves 1994), and Sry does not exist in other animal classes such as birds. Consistent with the idea that Sry function evolved from Sox3 (and potentially replaced Sry as a central sex-determining gene), it was shown that ectopic expression or genomic duplication of Sox3 results in XX male sex reversal in mice and humans (Haines et al. 2015; Moalem et al. 2012; Sutton et al. 2011), while mutation of Sox3 alone (in mice) does not affect sex determination (Weiss et al. 2003).
The discovery of two human disorders of sexual development (DSDs), namely Turner’s syndrome (XO females) and Klinefelter’s syndrome (XXY males) (Ford et al. 1959; Jacobs and Strong 1959), led to the identification of a sex-determining region on the Y chromosome in humans. This region was thought to carry a gene that determines maleness. Almost 30 years later, the Sry gene was discovered. The human SRY gene was discovered while searching for conserved regions in translocated Y chromosomal DNA from XX male patients (Sinclair et al. 1990). Subsequently, the homologous Y chromosome sequence from mouse was cloned, leading to the discovery of Sry as the testis-determining gene in mice (Koopman et al. 1990). The role of Sry as a master regulator in male sex determination in mammals was determined using sex reversal experiments in which XX mice developed as males following ectopic expression of Sry (Koopman et al. 1991). Additionally, loss of Sry function in mice and humans led to XY gonadal dysgenesis or Turner’s syndrome with XO/XY mosaicism (Zhao and Koopman 2012), confirming the essential role of Sry in mammalian sex determination.
The expression of Sry in mice is tightly regulated during gonad development. Its expression begins at approximately 10.5 dpc in the supporting cells (pre-Sertoli cells) of the XY genital ridges, peaks at around 11.5 dpc, and then gradually declines by 12.5 dpc (Koopman et al. 1990; Hacker et al. 1995; Bullejos et al. 2001; Jeske et al. 1995; Wilhelm et al. 2005). The expression pattern of Sry is unique, with its expression initiating in pre-Sertoli cells at the center of the genital ridge and gradually expanding to the poles of the gonad (not via migration, but rather via new expression in pre-Sertoli cells), eventually occupying the entire length of the gonad over a period of several hours. This center-to-pole expansion of Sry expression in the pre-Sertoli cells of the gonad was shown to be essential for proper center-to-pole expansion of testicular development (Hiramatsu et al. 2010). Furthermore, using partition culture assays (where the genital ridge was partitioned into anterior and posterior domains), it was shown that center-to-pole progression of testis cord formation was mediated by FGF signaling (Hiramatsu et al. 2010). In addition to its unique expression pattern, a critical threshold of Sry expression is required for Sertoli cell specification (Kashimada and Koopman 2010). As the expression of Sry is highly dynamic and transient, it was suggested that Sry functions during a critical time window limited to 6 h (which corresponds to 11.0–11.25 dpc) in the developing gonad (Hiramatsu et al. 2009). Using transgenic mice in which the Sry expression was driven by a heat shock protein 70.3 (Hsp70.3; official name is Hspa1a) promoter that allows for experimental induction of Sry expression at different time points, a delay of 6 h in Sry expression in XX embryos resulted in failure to initiate the testis development pathway and shifted the balance toward the female pathway, as visualized by a lack of testis cord formation, low levels of proliferation in the coelomic epithelium, reduced male-specific gene expression, and induction of female-specific genes such as Wnt4 (Hiramatsu et al. 2009). The significance for a critical window during which Sry expression occurs could be to suppress the ovarian pathway and activate the testicular development pathway.
Although the regulation of Sry expression is not well understood, several genes have been implicated in its regulation; some of these genes include Wt1, Cbx2, Gata4, Zfpm2, mitogen-activated protein kinase kinase kinase 4 (Map3k4), Insrr, Irr, and Igf1r (Tevosian et al. 2002; Nef et al. 2003; Katoh-Fukui et al. 1998; Biason-Lauber et al. 2009; Barbaux et al. 1997; Bogani et al. 2009; Hammes et al. 2001). Targeted deletion of these genes in mice resulted in reduced expression of Sry and XY sex reversal phenotypes, in which testis cord formation does not take place and female-specific gene expression is observed. The precise mechanism of how loss of function of these genes results in reduced Sry expression is unclear, as some of these genes (such as Cbx2, Gadd45γ, Map3k4, and insulin receptors) do not encode for transcription factors and therefore mostly act indirectly to affect Sry expression (Kashimada and Koopman 2010). Of all the genes listed above, much of the work has been focused on WT1+KTS as the main regulator of Sry expression. In humans, reduced expression of WT1+KTS causes XY sex reversal accompanied by a condition called Frasier syndrome (Barbaux et al. 1997). In vitro studies using luciferase reporter assays have shown that the WT1+KTS isoform, along with GATA4, cooperatively activates transcription from the mouse Sry promoter (Miyamoto et al. 2008; Hossain and Saunders 2001; Shimamura et al. 1997). Interestingly, studies have shown that WT1+KTS preferentially binds to mRNA targets during mRNA processing, thereby regulating gene expression at the RNA level (Morrison et al. 2008). Whether WT1+KTS regulates Sry expression at the RNA level is still unclear and requires further investigation.
In addition to WT1+KTS, studies have shown that Map3k4 and Gadd45γ also regulate Sry expression in the early mouse gonad (Warr et al. 2012). MAP3K4 is a mitogen-activated protein kinase that is involved in p38 MAPK and JNK signaling pathways to regulate a number of cellular processes such as proliferation, differentiation, apoptosis, and inflammatory response (Gerwins et al. 1997; Takekawa et al. 1997). XY embryos lacking functional MAP3K4 have reduced levels of Sry expression in pre-Sertoli cells, absence of Sertoli cell differentiation, and defective testis cord formation (Bogani et al. 2009). Furthermore, the mutant gonads developed ovarian morphology at stage 14.5 dpc and exhibited very low levels of Sox9 (Sertoli cell marker) expression and high levels of Stra8 and Wnt4 expression. High levels of Stra8 and Wnt4 expression are indicative of germ cell entry into meiosis and activation of the ovarian pathway in the absence of functional MAP3K4 (Bogani et al. 2009). In all, these data indicate that MAP3K4 signaling is essential for sex determination in mice.
MAP3K4 is known to interact with a number of proteins, one of them being GADD45, a growth arrest and DNA damage response protein family member (Takekawa and Saito 1998). Studies have shown that GADD45 activates MAP3K4 by disrupting the autoinhibitory domain of the MAP3K4 protein (Miyake et al. 2007). This interaction leads to the formation of an active dimer and induces autophosphorylation of MAP3K4 (Miyake et al. 2007). Of the three related proteins (namely, GADD45α, GADD45β, and GADD45γ) in the GADD45 family, GADD45γ is known to activate p38 MAPK and JNK pathways in T-cells (Lu et al. 2001) and interacts with MAP3K4 to regulate the production of the cytokine interferon gamma (IFNγ) in T-cells in vitro (Chi et al. 2004). GADD45γ is also known to be required for testis determination, as mice lacking functional GADD45γ displayed reduced levels of Sry expression and XY gonadal sex reversal (Warr et al. 2012). Furthermore, it was shown that Gadd45γ and Map3k4 interact to regulate Sry expression through a p38- and MAPK-mediated pathway and thereby regulate testis determination (Warr et al. 2012) .
Sex-Determining Region Y (SRY)-Box 9 (Sox9)
SOX9 also belongs to the SOX family of transcription factors. It is widely expressed in the developing heart, kidney skeleton, brain, and gonads (Wright et al. 1995). It is thought to be the major downstream target of SRY during mammalian sex determination. In the mouse XY gonad, the expression of Sox9 is initiated in the bipotential genital ridge at 11.5 dpc and is upregulated in the pre-Sertoli cells immediately after initiation of Sry expression. The expression of Sox9 within pre-Sertoli cells initiates at the center of the gonad and gradually expands toward the poles of the gonad, mimicking the Sry expression pattern (Kent et al. 1996; Sekido et al. 2004; Wilhelm et al. 2005; Bullejos and Koopman 2005; Morais da Silva et al. 1996). Despite their similar expression, Sox9 expression, unlike Sry, is maintained in the gonad beyond fetal stages and throughout postnatal and adult life (Kent et al. 1996; Morais da Silva et al. 1996). This sustained expression of Sox9 might be associated with maintenance of Sertoli cell fate or identity in the gonad (DiNapoli and Capel 2008).
The role of Sox9 in sex determination was revealed when loss-of-function mutation in human SOX9 was shown to cause a male-to-female sex reversal phenotype in 75% of males, accompanied by a skeletal defect called campomelic dysplasia (CD) (Foster et al. 1994; Wagner et al. 1994). Sex reversal phenotypes of XY Sox9-null mice and XX Sox9-overexpressing mice have confirmed the essential role of Sox9 in testis determination (Barrionuevo et al. 2006; Vidal et al. 2001; Bishop et al. 2000). Furthermore, Sox9-overexpressing XX gonads have a similar phenotype as Sry-overexpressing gonads (Kashimada and Koopman 2010), indicating that Sox9 is the major target of Sry that is required for activation of the downstream testicular program in Sertoli cells. Despite a long-standing speculation that SRY regulates the expression of Sox9, it took more than a decade to demonstrate definitively that SRY regulates Sox9 expression. The discovery of a gonad-specific enhancer of mouse Sox9 called TESCO (testis-specific enhancer of mouse Sox9 core) was crucial in the quest to determine how SRY upregulates Sox9 expression (Sekido and Lovell-Badge 2008). TESCO is a 1.4-kb (kilobase) sequence that lies 11–13 kb upstream of the Sox9 transcription start site and is highly conserved in mammals (Sekido and Lovell-Badge 2008). Sekido and Lovell-Badge (2008) showed that SRY and SF1 directly bind to TESCO and act synergistically to upregulate Sox9 expression (Fig. 3). This was the first report demonstrating that SRY acts as a transcriptional activator in vivo. After Sry expression starts to decrease at 12.5 dpc, SOX9 itself is able to recognize and bind to sites previously bound by SRY in TESCO along with NR5A1, ensuring its continued expression in a positive autoregulatory feedback loop (Sekido and Lovell-Badge 2008) (Fig. 3). Thus, the positive feedback loop not only ensures that Sox9 expression is sustained long after Sry expression ceases but also ensures that Sry signal is amplified in each of the developing Sertoli cells. Other positive feedback loops known to maintain Sox9 expression in the gonad involve FGF9 signaling and PGD2 signaling from the Sertoli cells (discussed below) .
F ibroblast Growth Factor 9 (Fgf9)
FGF9 is one of the members of the fibroblast growth factor (FGF) family that plays essential roles in growth, morphogenesis, and differentiation during development. Fgf9 is widely expressed in the mouse embryo and is initially expressed in both XY and XX gonads (Colvin et al. 2001). However, following Sry expression, Fgf9 expression becomes male specific and is expressed in the Sertoli cells of the developing testis. In order to determine the in vivo role of Fgf9, Colvin and colleagues generated a deletion of Fgf9 in mice. They reported an overrepresentation of phenotypically female embryos in mice lacking functional Fgf9, which ultimately led to the identification of a novel role for Fgf9 in sex determination and testis development (Colvin et al. 2001). Loss of Fgf9 led to male-to-female sex reversal in 18.5 dpc embryos, accompanied by disruption of male-specific events, including cell proliferation, mesonephric cell migration, differentiation of Sertoli cells, and testis cord formation (Colvin et al. 2001). Further studies revealed that Fgf9 acts downstream of Sry and is required for promoting proliferation of Sertoli cell precursors. Following up on this study, it was shown that loss of Fgf9 did not affect the expression of Sry or the initial upregulation of Sox9 expression (Kim et al. 2006). However, Fgf9-null gonads did not maintain Sox9 expression, indicating that FGF9 signaling is required for maintaining Sox9 expression in the fetal gonad. The authors suggested that a feedback loop involving FGF9 is important in upregulating Sox9 expression and that Sox9 expression in turn is required for upregulation of FGF9 in the XY gonad (Fig. 3) (Kim et al. 2006). Recently, it was reported that aberrant expression of testicular FGF9 is associated with Sertoli cell-only (SCO) syndrome (patients with SCO are azoospermic and have atrophic testis and hypogonadism), indicating that Fgf9 may play an important role in male factor infertility (Chung et al. 2013) .
P rostaglandin D2 Synthase (Ptgds)
The Ptgds gene encodes for an enzyme that is involved in the synthesis of prostaglandin D2 (PGD2), which is critical for various physiological processes including male sex determination, platelet aggregation, bronchoconstriction, allergy, and inflammation (Moniot et al. 2011). Studies have reported the presence of a male-specific Ptgds gene called lipocalin-type Ptgds (L-Ptgds) (Malki et al. 2005). Its expression begins in the developing gonad between 11.5 and 12.5 dpc (Adams and McLaren 2002) and is mainly restricted to Sertoli cells and germ cells (Adams and McLaren 2002). PGD2 signaling is involved in a positive feedback loop with SOX9 (Fig. 3). It has been shown that SOX9 initiates the transcriptional activation of Ptgds, which results in production of PGD2 that then activates PKA (cAMP-dependent protein kinase A). PKA then phosphorylates SOX9; phosphorylation of SOX9 facilitates its nuclear localization where the SOX9 binds to its own promoter, thereby maintaining its expression (Sekido and Lovell-Badge 2008; Malki et al. 2005). Targeted deletion of L-Ptdgs results in abnormal SOX9 protein localization and reduced Sox9 gene expression and, hence, delayed testis cord formation until 14.5 dpc (She and Yang 2014). Additionally, Sox9-null gonads have reduced levels of L-PTGDS, indicating that SOX9 is required to maintain the expression of L-Ptgds (She and Yang 2014). L-Ptgds in turn functions in a positive feedback loop to maintain SOX9 expression in the developing testis .
G a ta4 and Zinc Finger Protein, Multitype 2 (Zfpm2/Fog2)
GATA4 belongs to the zinc finger family of transcription factors that recognizes the consensus sequence (T/A)GATA(A/G) (Tevosian et al. 2002). The GATA zinc finger transcription factors are known to play critical roles during development, including differentiation of hematopoietic cells, cardiac and coronary vascular development, and morphogenesis of various tissues like the liver, lung, and gut (Tevosian et al. 2002). In the developing gonadal ridge, GATA4 expression is restricted to somatic cells only. At 11.5 dpc both XX and XY gonads express GATA4. By 13.5 dpc Gata4 is expressed at high levels in the Sertoli cells and low levels in the interstitial cells of the XY gonads; however in XX gonads, its expression is low in all cells (Tevosian et al. 2002). Studies have shown that GATA4, along with its cofactor ZFPM2, is required for normal gonad differentiation, as homozygous Zfpm2-null mice show failure of testis differentiation, lack of testis cord development, and significantly reduced gonad size (Tevosian et al. 2002). Furthermore, it was shown that Sry expression was significantly downregulated in Zfpm2-null gonads at 11.5 dpc, the time point when Sry expression normally peaks (Tevosian et al. 2002). Additionally, homozygous targeted mutation in Gata4 (Gata4 ki) that abolishes the interaction between GATA4 and its cofactor ZFPM2 also resulted in loss of Sertoli cell differentiation and abnormal testis development (Tevosian et al. 2002; Manuylov et al. 2011). Detailed analysis of Zfpm2-null XY gonads and Gata4 ki XY gonads revealed that expression of genes involved in Sertoli cell differentiation (such as Sox9, Amh, and Dhh) and genes involved in androgen biosynthesis (Cyp11a1, Hsd3b1, and Cyp17a1) was absent. These results indicate that the interaction between GATA4 and ZFPM2 is essential for normal Sertoli cell development and Leydig cell development (Tevosian et al. 2002). Recently, it was reported that a disruption of the human ZFPM2 protein resulted in failure of direct ZFPM2 and GATA4 interaction, which led to abnormal sex determination and gonadal dysgenesis in humans (Bashamboo et al. 2014). In all, GATA4 and ZFPM2 are essential for male gonadogenesis, and their direct physical interaction is essential for maintaining normal Sry expression during testis differentiation (Fig. 3) .
Mammalian Ovarian Sex Determination Genes
Wingless-Type MMTV Integration Site Family, Member 4 (Wnt4)
WNT4 is a growth factor that plays essential roles during mammalian embryogenesis. In mice, Wnt4 is expressed in the mesonephros of the early gonad (9.5–10.5 dpc) of both sexes. By 12.5 dpc, its expression becomes restricted to the mesenchyme cells surrounding the Müllerian duct epithelium in the mesonephros (Binnerts et al. 2007). Once sex determination events are initiated, the expression of Wnt4 is restricted to the female gonad. Loss of Wnt4 in mice results in a partial sex reversal phenotype in the female reproductive system, indicating that Wnt4 is a female determinant. Wnt4 is essential for the development of the Müllerian duct, inhibition of steroidogenic cell differentiation, inhibition of testis-specific vascular remodeling, and development of normal oocytes (Jeays-Ward et al. 2003; Vainio et al. 1999). Furthermore, loss of Wnt4 also resulted in a transient increase in the expression of both Sox9 and Fgf9 in the absence of Sry, thus indicating that FGF9 and WNT4 function as antagonistic signals that regulate mammalian sex determination. Fgf9 downregulates WNT4 signaling to support testis development, while Wnt4 downregulates FGF9 signaling to support normal ovarian development (Kim et al. 2006). In addition, targeted deletion of Wnt4 in the granulosa cells of the ovary revealed that Wnt4 is required for normal follicular development and regulation of steroidogenesis in granulosa cells (Boyer et al. 2010).
Studies have shown that Wnt4 plays an essential role during various aspects of both male and female sexual development (Boyer et al. 2010; Coveney et al. 2008; Jeays-Ward et al. 2004). Wnt4-null testes show defects in Sertoli cell differentiation, and these defects were shown to occur downstream of Sry but upstream of Sox9 and Dhh. Additionally, Wnt4-null testes were smaller in size and had disorganized testis cords. These studies indicate that Wnt4 is required for normal testis development (Jeays-Ward et al. 2004). Genome-wide microarray analysis of Wnt4-null XX gonads led to the identification of several candidate genes (Tcf21, Igfbp7, Cbln1, Sostdc1, Gpc3, and Sulf2) that may be involved in testis vascular development (Coveney et al. 2008). Additionally, loss of Wnt4 affected the expression of genes expressed in testis cords (Robo1, Pitx2, and Spp1) or related to germ cell development (Coveney et al. 2008). In all, these data suggest that WNT signaling is essential for testis differentiation. Recently, Wnt4-null mice were used to identify several candidate Wnt4 downstream target genes that might be important for both female and male gonadogenesis (Naillat et al. 2015). Notum, Phlda2, Runx1, and Msx1 genes were identified as candidate target genes for female reproductive development. In addition, the authors reported that WNT4 signaling might also regulate genes associated with the male testicular developmental pathway. These genes include Osr2, Dach2, Pitx2, and Tacr3 (Naillat et al. 2015). These studies have identified a number of potential target genes that may be subject to regulation by WNT4 signaling during mammalian sex determination and development.
W NT4 likely acts through the canonical β-catenin signaling pathway. The disruption of β-catenin signaling in the XX gonad leads to a partial female-to-male sex reversal (Liu et al. 2009), similar to Wnt4 mutants (Jeays-Ward et al. 2003; Vainio et al. 1999), while ectopic β-catenin signaling in XY gonads results in male-to-female sex reversal (Maatouk et al. 2008). Mutants for Rspo1, another positive effector of β-catenin signaling, also show a partial female-to-male sex reversal phenotype reminiscent of Wnt4 and β-catenin mutants (Chassot et al. 2008). These findings demonstrate that β-catenin signaling, downstream of WNT4 and RSPO1 activity, is required for ovarian determination and differentiation .
F orkhead Box L2 (Foxl2)
FOXL2 belongs to the forkhead/winged-helix family of transcription factors. Studies have shown that FOXL2 is essential for granulosa cell differentiation and normal ovarian development in mice, but is not required for primary sex determination (Schmidt et al. 2004; Uda et al. 2004). Its expression begins at 12.5 dpc in the pre-granulosa cells of the developing gonad. In humans, loss-of-function mutation in the FOXL2 gene results in the autosomal disorder blepharophimosis/ptosis/epicanthus inversus syndrome (BPES) and premature ovarian failure in adults (Uhlenhaut and Treier 2006). Foxl2 has also been implicated in the polled intersex syndrome (PIS) of goats, and a recent study revealed that Foxl2 is required for primary sex determination in that species (Boulanger et al. 2014). Female mice with targeted deletion of Foxl2 displayed premature disruption of the primordial follicular pool, inhibition of granulosa cell differentiation, and complete absence of secondary follicles (Ottolenghi et al. 2007). Furthermore, it was shown that male-specific genes involved in male sex determination (such as Sox9, Fgf9, Fgfr2, Nr5a1, and Gata4) were upregulated in Foxl2-null ovaries. This indicates that Foxl2 functions to repress the pathway that regulates male sex determination in mammals (Ottolenghi et al. 2007). Conditional deletion of Foxl2 in the adult ovarian follicles has shown that Foxl2 is required to prevent the transdifferentiation of adult ovary granulosa and theca cells into their testicular counterparts (Uhlenhaut et al. 2009). In vitro studies have shown that FOXL2, together with estrogen receptor 1 (ESR1), negatively regulates the expression of SOX9 (Fig. 3). The exact mechanism of Foxl2 in this transdifferentiation process is discussed in greater detail in the subsequent chapter of this volume .
Nuclear Receptor Subfamily 0, Group B, Member 1 (Nr0b1/Dax1)
N r0b1, also known as Dax1, encodes an orphan nuclear receptor that is expressed in the developing gonad, adrenal gland, pituitary, and hypothalamus (Luo et al. 1994; Shinoda et al. 1995). In both mouse and human, DAX1 is coexpressed with NR5A1/SF1 in male and female gonads prior to gonad differentiation. In mice Dax1 expression is upregulated in the Sertoli cells of the XY gonad at 12.5 dpc (Ikeda et al. 2001). Its expression declines soon after in the Sertoli cells but increases in the interstitial cells between 13.5 and 17.5 dpc. In XX gonads Dax1 is expressed between 12.5 and 14.5 dpc. DAX1 was originally thought to act like an “anti-testis” factor (Swain et al. 1998); however, it was later reported that Dax1 plays an essential role in testis differentiation (Meeks et al. 2003). Human XY individuals with DAX1 duplications exhibited male-to-female sex reversal (dosage-sensitive sex reversal). Transgenic mice carrying duplication of the Dax1 gene exhibited decreased Sry expression and delayed testis development, resulting in a XY sex reversal phenotype (Ludbrook and Harley 2004). Hence, it was concluded that Dax1 antagonizes Sry expression to regulate sex determination (Swain et al. 1998). Targeted deletion of Dax1 in mice resulted in disorganized testis structure, although Sertoli cells and fetal Leydig cells were present and appeared to function normally. Additionally, mutation in the Dax1 gene was associated with testicular dysgenesis and abnormal peritubular myoid cell differentiation (Meeks et al. 2003) .
Environmental Influences in Mammalian Sex Determination/Sex Ratio
While mammals utilize sex determination pathways and machinery outlined in previous sections of this chapter, other organisms that lack sex chromosomes or a genetic sex determination mechanism rely on the environment to determine the sex of an organism. Additionally, there are organisms that have a combination of both environmental and genetic components driving their sex determination (Yamamoto et al. 2014). Sry (a major male-determining gene, described above) is present in virtually all mammals; however, it is not found in nonmammalian species (such as birds, fish, or reptiles), whose sex determination is sensitive to environmental cues rather than solely relying on genetic information. In some cases, even though Sry is absent, the balance of sex-specific downstream signaling, such as FGF-versus-WNT signaling, still regulates sex determination. Some species use environmental sex determination, rather than chromosomal methods, while others utilize external influences to override or coincide with chromosomal sex determination (Quinn et al. 2007; Radder et al. 2008), relying on environmental, hormonal, and/or behavioral switches. Genetic factors play an essential role in sex determination in mammals (described in previous sections); however, the maternal environment may also be important for sex determination and/or sex ratio. This section will highlight examples of how certain environmental cues, such as maternal influence on the pre-conception environment and other external factors, can influence sex ratio and/or determination in mammals (Fig. 4).
P re-conception Maternal Environment (pH)
The uterus is a controlled environment that allows the fetus to develop and was generally thought to be a passive player in embryonic sexual development; however, it has been demonstrated that the maternal environment before, during, and after conception can influence both long-term health and the sex (and sex ratio) of the offspring. The Trivers-Willard hypothesis of sex ratio is based on the idea that pregnant females can alter the sex of their offspring based on their health condition in terms of nutrition, body size, as well as dominance characteristics (covered later in section) and their parental ability to invest in their offspring to outcompete others for increased offspring in the following generation (also termed the maternal dominance hypothesis). This theory states if the maternal condition is poor, mothers tend to produce a higher ratio of females to males (Trivers and Willard 1973). This sex skewing due to maternal condition was found to be a potential consequence of maternal glucose levels, which differentially supports male-versus-female blastocysts (Larson et al. 2001). This principle was subsequently built upon by a controversial concept that human couples can influence the sex of their offspring, first introduced by Kleegman, but then popularized as Shettles’ method in the 1970s (Shettles 1973).
Shettles’ concept is based on the timing of coitus in proximity to ovulation, in addition to sexual position during intercourse, as the X- and the Y-spermatozoa have different speeds and sensitivities to acidity (e.g., within the vaginal/cervical environment). His theory states that for male offspring to be created, intercourse must be performed closest (<2 days) to ovulation, compared to female offspring (optimal at 2.5–3 days); this idea is based on the ability of Y-spermatozoa to swim faster than X-spermatozoa due to their physical differences (X-spermatozoa have smaller heads and longer tails) and that Y-sperm are more sensitive to acidic environments. Therefore, this increased time in the cervix will result in removal of male-determining sperm due to the acidity (Shettles 1973). However, this theory is still controversial as others have not been able to determine any significant differences, besides DNA content, between X- and Y-bearing spermatozoa.
The cervix is the major passageway for sperm to move from the vagina to the uterine cavity; therefore, differences in the mucous composition or pH can “select” or control the sperm that travel through it. The cervix is most well known as a barrier against pathogens and also prevents entry of abnormal sperm. The cervical mucus exits into the vaginal cavity, and the whole process of entry into the uterine cavity is influenced by this mucus environment. Since the pH of the cervix changes according to the stages of ovulation due to reproduction-associated hormones (including estrogen, progesterone, and luteinizing hormone), the different time windows relative to ovulation, according to his theory, may allow the pH of the woman’s cervical mucus to play a role in sex ratios (Bott et al. 2006). During pre-ovulation/midcycle, the levels of estradiol are high, physically changing the size and decreasing the firmness of the cervix itself and increasing water content, which leads to decreased mucus viscosity and alkaline pH, thereby becoming more hospitable to sperm (Bigelow et al. 2004; Kopito et al. 1973). The chemical composition of the mucus, including the ratios and amounts of specific electrolytes, such as sodium, changes over the course of the cycle (Gould and Ansari 1981), which can influence the overall pH of the cervix .
Maternal Diet
Both the number and sex ratio of offspring produced can be influenced by overall food intake and/or the presence of nutrients during both preconception and early pregnancy (Schmidt and Hood 2012). Studies have shown that in humans, the sex ratio of the fetus can be influenced by the maternal diet at the time of conception (Mathews et al. 2008). The nutrition of the mother greatly influences sex ratio of her offspring, as mothers with malnutrition produce increased numbers of female offspring, whereas higher caloric intake resulted in male-biased offspring. In Western countries, the human male-female offspring ratio is already skewed slightly toward males, possibly caused by population-wide obesity in these cultures (the influence of fat on sex ratios will be discussed later in this chapter). Human male offspring have higher in utero caloric demands than females (Tamimi et al. 2003). Although this skewing may be due to energy requirements for each sex (causing sex-selective spontaneous abortion or miscarriage), it also can be related to sex determination (genetic machinery discussed in early sections of this chapter). In humans, analysis of maternal caloric intake around the time of conception revealed 56% of the highest energy intake group had sons, compared with 45% in the lowest energy intake group (Mathews et al. 2008). This study has been similarly replicated in ruminants and rodents with increased skewed ratios. Diets high in lipids, fats (e.g., lard), and dietary fatty acids (including foods high in omega-3 (n-3) polyunsaturated essential fatty acids) are linked to increases in male births in mammalian species, whereas a carbohydrate-based diet (high in sugars and complex carbohydrates) or diets rich in omega-6 (n-6) fatty acids increased female births (Austad and Sunquist 1986; Crawford et al. 1987; Fountain et al. 2008; Gulliver et al. 2013a, b; Rosenfeld and Roberts 2004). As many of these diet-based studies did not result in any malnutrition or decreased litter size, it demonstrates that diet can skew sex ratios without sex-selective miscarriage being the major cause in this shift. A few of these studies noted that older/more mature mothers showed a greater response to changes in the diet than younger mothers (Rosenfeld et al. 2003). Other studies investigating low-fat diets still had skewing toward females, but in addition resulted in smaller litter sizes; therefore, male-selective losses may also play a role in cases of malnutrition and/or lower caloric intake (Meikle and Drickamer 1986; Rivers and Crawford 1974). Interestingly, mouse placentas associated with female embryos demonstrate a higher sensitivity to the maternal diet, by changes in gene expression, compared to their male counterparts (Mao et al. 2010).
In humans and other mammals (such as roe deer, reindeer, mature ewes, Barbary sheep, domestic pigs, and other species), greater maternal body mass and a particular maternal fat distribution (an android pattern in which fat is mainly in the upper trunk portion of the body) are associated with increased male offspring (Cassinello 1996; Kent 1995; Kojola and Eloranta 1989; Meikle et al. 1996; Wauters et al. 1995; Singh and Zambarano 1997). As adipose tissue is a major location for hormone biosynthesis and metabolism, these changes may have hormonal origins. Interestingly, a diet free of fatty acids resulted in a more acidic vaginal environment (Whyte et al. 2007). These studies further highlight how the maternal diet impacts the overall cervical/vaginal environment, thereby influencing the selectivity of the sperm and overall sex ratio of the offspring.
Minerals in the maternal diet are essential for production of offspring and for normal sex ratios. One example of this is that female white-footed mice fed on a low-calcium diet from reproductive age until senescence had a poor skeletal condition and produced smaller female-biased litters (Schmidt and Hood 2012), demonstrating that calcium intake influences reproductive output and sex ratios. The mechanism underlying the relationship between calcium in the maternal diet and skewed sex ratios in the offspring is unclear, but as calcium has been found to be important for sperm motility, fertilization, and oocyte activation (Homa et al. 1993; Hong et al. 1984). Additionally, it has been speculated that the female reproductive tract milieu influences the functionality of the sperm (in terms of motility and fertilization capacity), perhaps in a sex-specific manner, leading to a biased sex ratio (see earlier in this section). Interestingly, the ratio of monovalent ions (potassium/sodium) to divalent ions (calcium/magnesium) plays a role in sex ratios, whereby higher calcium/magnesium intake is associated with an increase in female offspring, whereas increased potassium/sodium corresponds to more male offspring (Mathews et al. 2008; Bolet et al. 1982; Oun et al. 2016; Stolkowski and Choukroun 1981; Stolkowski and Lorrain 1980; Vahidi and Sheikhha 2007). In all, a wide range of dietary factors work together to regulate sex determination in a species-specific manner .
M aternal Menstrual Cycle
A study in humans controlling the timing between coitus and ovulation, in combination with maintaining a strict mineral-containing diet (low in sodium, high in calcium), demonstrated that the time window 3–4 days before ovulation skews the offspring toward female, while any variation/shift in this coitus time window (<2 days or >5 days prior to ovulation) while still on the diet resulted in a decreased female offspring ratio (Noorlander et al. 2010). This study had stringent compliance efforts; therefore, those that did not comply were compared for their contribution to the sex ratio based on their noncompliant status. Previous to this study, many studies were performed providing results either stating there was a difference based on ovulation or there was simply no difference; however, this data aligns with other studies that also report a higher likelihood of male offspring if copulation occurs closer to ovulation, whereas there will be an increased likelihood of female offspring if copulation occurs 3 days prior to ovulation, which is consistent with this time window .
Maternal Dominance and Sex Hormones
The theory of maternal dominance (in which a dominant female is defined as an authoritative and influential female, not to be confused with other personality traits, such as aggressive or domineering) states that a maternal masculine phenotype before conception causes sex skewing toward male offspring (Grant 1996; Sadalla et al. 1987). For example, in social mammalian species, females displaying dominant personalities produce more male offspring as compared to subordinate females. Interestingly, this trend disappeared at higher population densities (and for animals in captivity) as the number of males born each year is inversely correlated with the population density and can be attributed to the amount of winter rainfall (Kruuk et al. 1999). These changes could be linked to stress caused by competition for resources and/or nutrition (discussed in other sections of this chapter). In another case, some species of temperature-dependent sex-determining fish born earlier in the birthing season in the northern latitudes have a strong female bias to allow females access to a longer feeding season, permitting females to become larger and grants them a competitive edge (via dominance) that promotes both survival and fecundity (Conover 1984). Both these scenarios represent types of environmental variables that are associated with nutritional stress and fecundity that leads to changes in sex ratios.
The basis for maternal dominance sex skewing is that the levels of sex hormones at the time of conception are correlated with sex-specific skewing of births. For example, a mother who has high estrogen and androgen levels may display masculine-like behavioral characteristics, both of which are correlated to a higher production of male offspring (James 1990). Interestingly, under conditions of chronic stress, females respond differently than males, by increasing their testosterone levels, rather than decreasing them (Gray 1992). The cause of this difference is that the adrenal glands normally produce small amounts of androgens (including the precursor to testosterone, androstenedione), but in response to stress only the female adrenal gland increases its production, in addition to cortisol (Mazur and Petrenko 1997), thereby leading to increased maternal testosterone levels.
Stress causes the mother’s adrenal glands to release high levels of stress hormones into the blood, which can cross the placental barrier and can interfere with the fetus’s production of sex hormones temporarily. One sex hormone most notably influenced by stress hormone levels is testosterone (Ellis and Cole-Harding 2001). Later during testicular development, testosterone is required for masculinization, demonstrated by problems with secondary sex characteristics in testosterone-deficient or androgen receptor-deficient models. Interestingly, maternal levels of testosterone during the time of conception can also further skew the sex bias, by selectively tilting the sex ratio. Female testosterone can also be produced intrafollicularly within the ovary, and these local levels of testosterone have been linked to sex ratios, thought to influence fertilization by X- versus Y-bearing spermatozoa (Grant and Irwin 2005; Grant et al. 2008). The mechanism behind this selection may be due to the influence of maternal hormones on uterine responsiveness, which influences the survival of the fertilized blastocyst, leading to sex-specific resorption, mortality, and, ultimately, sex skewing of offspring (Krackow 1995) .
O ther External Factors
In addition to the maternal pre-conception environment, other external factors, such as external stress and geographic location (e.g., latitude), have been reported to affect sex ratio. Stress in humans influences sex ratio such that more male offspring are produced in the absence of stress (Masukume and Grech 2015). An example of this is the increased number of male newborns in South Africa 9 months after the 2010 FIFA World Cup soccer tournament was held in that country. The authors reasoned that the increased male sex ratio was most likely due to increased sexual intercourse during the tournament (Masukume and Grech 2015). Additional causes for the skewed ratio toward males may also be associated with decreased male fetal deaths and the quality of sperm (Masukume and Grech 2015). During negatively stressful situations, however, such as after an earthquake, the inverse sex ratio skewing occurs, tilting toward more female births. An example of this is 9 months following a devastating earthquake in Kobe, Japan, in which more females were born. Acute stress may decrease sperm motility, reduce fertility, and thus reduce male sex ratios (Fukuda et al. 1998).
Other conditions of stress, such as war, lead to small but significant increases in male offspring. More male births were reported during and after wars (including World War I and II and the Korean and Vietnam wars) (Ellis and Bonin 2004; Hesketh and Xing 2006). The reasoning for the skewed sex ratio is that the physiological state of mothers during war caused the release of stress hormones, such as cortisol and adrenaline, potentially inducing a higher rate of miscarriages; however, male fetuses are more prone to miscarriages than female fetuses, which contradicts this argument (Ellis and Bonin 2004).
Another factor potentially influencing sex ratio in humans is geographical latitude, with more female births occurring at tropical latitudes than at temperate and subarctic latitudes (Navara 2009). The relationship between sex allocation and latitude was attributed to latitude-dependent factors, such as length of the day and temperature; a study reported that human sex ratio may be influenced by these factors (Navara 2009). The authors suggested that the quality of the semen and miscarriage rates may vary with latitude and, hence, indirectly affect sex ratio. Confirming that both temperature and day length are important for sex ratios in mammals, other species (such as hamsters, mice, and meadow voles) are also influenced by length of the day and temperature, with more male offspring produced during shorter days or colder weather (Drickamer 1990; Mcshea and Madison 1986). In all, a wide range of environmental factors work together to regulate sex determination and sex ratios in a species-specific manner .
Conclusions and Future Areas of Research in the Field
Sex determination is critical for reproduction and for the survival of sexually reproducing species. While virtually all mammals, such as humans, use the Y chromosome-encoded gene Sry as the genetic trigger for sex determination, the molecular mechanism by which sex is determined is highly variable across different animal classes. For those species whose sex determination is sensitive to external influences, such as exogenous hormones, elucidating how environmental contaminants impact reproductive fitness is of major interest for the fields of ecology and reproductive biology. Studying these model systems has the additional potential to inform us of the imminent consequences of endocrine-disrupting compounds on human health and sexual development.
In mammals, Sry is the master switch gene that launches the undifferentiated bipotential gonad into the testis program. Although much is known about Sry, several things are still not completely understood, such as the mechanism of its regulation, the proteins with which SRY interacts, and all the downstream genes it directly or indirectly targets. Additionally, genome-wide studies and genetic analyses have led to the identification of several other genes with sex-specific expression patterns that play important roles in sex determination. How these genes function in a network to regulate sex determination is still unclear and is a matter for further investigation. Only about 20% of human cases of reproductive system abnormalities have a defined molecular diagnosis (i.e., a known causative genetic factor) (Hughes et al. 2006), so it is clear that more research is needed into defining the mechanisms that promote the development of the fetal testis and ovary. Understanding the molecular pathways regulating sex determination is essential to gaining insight into some of the yet unidentified causes of human sexual disorders of development, such as gonadal dysgenesis and malformation, ambiguous genitalia, and sex reversal.
References
Achermann JC, Ozisik G, Ito M, Orun UA, Harmanci K, Gurakan B, et al. Gonadal determination and adrenal development are regulated by the orphan nuclear receptor steroidogenic factor-1, in a dose-dependent manner. J Clin Endocrinol Metab. 2002;87(4):1829–33.
Adams IR, McLaren A. Sexually dimorphic development of mouse primordial germ cells: switching from oogenesis to spermatogenesis. Development. 2002;129(5):1155–64.
Armstrong JF, Pritchard-Jones K, Bickmore WA, Hastie ND, Bard JB. The expression of the Wilms’ tumour gene, WT1, in the developing mammalian embryo. Mech Dev. 1993;40(1–2):85–97.
Austad S, Sunquist M. Sex-ratio manipulation in the common opossum. Nature. 1986;324:58–60.
Ayers KL, Smith CA, Lambeth LS. The molecular genetics of avian sex determination and its manipulation. Genesis. 2013;51(5):325–36.
Bandiera R, Vidal VP, Motamedi FJ, Clarkson M, Sahut-Barnola I, von Gise A, et al. WT1 maintains adrenal-gonadal primordium identity and marks a population of AGP-like progenitors within the adrenal gland. Dev Cell. 2013;27(1):5–18.
Barbaux S, Niaudet P, Gubler MC, Grunfeld JP, Jaubert F, Kuttenn F, et al. Donor splice-site mutations in WT1 are responsible for Frasier syndrome. Nat Genet. 1997;17(4):467–70.
Barrionuevo F, Bagheri-Fam S, Klattig J, Kist R, Taketo MM, Englert C, et al. Homozygous inactivation of Sox9 causes complete XY sex reversal in mice. Biol Reprod. 2006;74(1):195–201.
Barrionuevo F, Georg I, Scherthan H, Lecureuil C, Guillou F, Wegner M, et al. Testis cord differentiation after the sex determination stage is independent of Sox9 but fails in the combined absence of Sox9 and Sox8. Dev Biol. 2009;327(2):301–12.
Bashamboo A, Brauner R, Bignon-Topalovic J, Lortat-Jacob S, Karageorgou V, Lourenco D, et al. Mutations in the FOG2/ZFPM2 gene are associated with anomalies of human testis determination. Hum Mol Genet. 2014;23(14):3657–65.
Behringer RR, Finegold MJ, Cate RL. Mullerian-inhibiting substance function during mammalian sexual development. Cell. 1994;79(3):415–25.
Bertuzzi S, Porter FD, Pitts A, Kumar M, Agulnick A, Wassif C, et al. Characterization of Lhx9, a novel LIM/homeobox gene expressed by the pioneer neurons in the mouse cerebral cortex. Mech Dev. 1999;81(1–2):193–8.
Beye M, Hasselmann M, Fondrk MK, Page RE, Omholt SW. The gene csd is the primary signal for sexual development in the honeybee and encodes an SR-type protein. Cell. 2003;114(4):419–29.
Biason-Lauber A, Konrad D, Meyer M, DeBeaufort C, Schoenle EJ. Ovaries and female phenotype in a girl with 46, XY karyotype and mutations in the CBX2 gene. Am J Hum Genet. 2009;84(5):658–63.
Bigelow JL, Dunson DB, Stanford JB, Ecochard R, Gnoth C, Colombo B. Mucus observations in the fertile window: a better predictor of conception than timing of intercourse. Hum Reprod. 2004;19(4):889–92.
Binnerts ME, Kim KA, Bright JM, Patel SM, Tran K, Zhou M, et al. R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc Natl Acad Sci U S A. 2007;104(37):14700–5.
Birk OS, Casiano DE, Wassif CA, Cogliati T, Zhao L, Zhao Y, et al. The LIM homeobox gene Lhx9 is essential for mouse gonad formation. Nature. 2000;403(6772):909–13.
Bishop CE, Whitworth DJ, Qin Y, Agoulnik AI, Agoulnik IU, Harrison WR, et al. A transgenic insertion upstream of sox9 is associated with dominant XX sex reversal in the mouse. Nat Genet. 2000;26(4):490–4.
Bland ML, Fowkes RC, Ingraham HA. Differential requirement for steroidogenic factor-1 gene dosage in adrenal development versus endocrine function. Mol Endocrinol. 2004;18(4):941–52.
Bogani D, Siggers P, Brixey R, Warr N, Beddow S, Edwards J, et al. Loss of mitogen-activated protein kinase kinase kinase 4 (MAP3K4) reveals a requirement for MAPK signalling in mouse sex determination. PLoS Biol. 2009;7(9):e1000196.
Bolet G, Gueguen L, Dando P, Ollivier L. Influence of mineral diet of the sow on the sex ratio of the newborn. Reprod Nutr Dev. 1982;22(6):1073–81.
Bott EM, Young IR, Jenkin G, McLaren WJ. Detection of morphological changes of the ovine cervix in response to sex steroids using a fluorescence confocal endomicroscope. Am J Obstet Gynecol. 2006;194(1):105–12.
Boulanger L, Pannetier M, Gall L, Allais-Bonnet A, Elzaiat M, Le Bourhis D, et al. FOXL2 is a female sex-determining gene in the goat. Curr Biol. 2014;24(4):404–8.
Boyer A, Goff AK, Boerboom D. WNT signaling in ovarian follicle biology and tumorigenesis. Trends Endocrinol Metab. 2010;21(1):25–32.
Bullejos M, Koopman P. Delayed Sry and Sox9 expression in developing mouse gonads underlies B6-Y(DOM) sex reversal. Dev Biol. 2005;278(2):473–81.
Bullejos M, Bowles J, Koopman P. Searching for missing pieces of the sex-determination puzzle. J Exp Zool. 2001;290(5):517–22.
Burglin TR. Analysis of TALE superclass homeobox genes (MEIS, PBC, KNOX, Iroquois, TGIF) reveals a novel domain conserved between plants and animals. Nucleic Acids Res. 1997;25(21):4173–80.
Capellini TD, Vaccari G, Ferretti E, Fantini S, He M, Pellegrini M, et al. Scapula development is governed by genetic interactions of Pbx1 with its family members and with Emx2 via their cooperative control of Alx1. Development. 2010;137(15):2559–69.
Caricasole A, Duarte A, Larsson SH, Hastie ND, Little M, Holmes G, et al. RNA binding by the Wilms tumor suppressor zinc finger proteins. Proc Natl Acad Sci U S A. 1996;93(15):7562–6.
Cassinello J. High-ranking females bias their investment in favour of male calves in captive Ammotragus lervia. Behav Ecol Sociobiol. 1996;38(6):417–24.
Chassot AA, Ranc F, Gregoire EP, Roepers-Gajadien HL, Taketo MM, Camerino G, et al. Activation of beta-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Hum Mol Genet. 2008;17(9):1264–77.
Chassot AA, Bradford ST, Auguste A, Gregoire EP, Pailhoux E, de Rooij DG, et al. WNT4 and RSPO1 together are required for cell proliferation in the early mouse gonad. Development. 2012;139(23):4461–72.
Chen S, Zhang G, Shao C, Huang Q, Liu G, Zhang P, et al. Whole-genome sequence of a flatfish provides insights into ZW sex chromosome evolution and adaptation to a benthic lifestyle. Nat Genet. 2014;46(3):253–60.
Chi H, Lu B, Takekawa M, Davis RJ, Flavell RA. GADD45beta/GADD45gamma and MEKK4 comprise a genetic pathway mediating STAT4-independent IFN gamma production in T cells. EMBO J. 2004;23(7):1576–86.
Chiang EF, Pai CI, Wyatt M, Yan YL, Postlethwait J, Chung B. Two sox9 genes on duplicated zebrafish chromosomes: expression of similar transcription activators in distinct sites. Dev Biol. 2001;231(1):149–63.
Chung CL, Lu CW, Cheng YS, Lin CY, Sun HS, Lin YM. Association of aberrant expression of sex-determining gene fibroblast growth factor 9 with Sertoli cell-only syndrome. Fertil Steril. 2013;100(6):1547–54 .e1–4
Colvin JS, Green RP, Schmahl J, Capel B, Ornitz DM. Male-to-female sex reversal in mice lacking fibroblast growth factor 9. Cell. 2001;104(6):875–89.
Combes AN, Spiller CM, Harley VR, Sinclair AH, Dunwoodie SL, Wilhelm D, et al. Gonadal defects in Cited 2-mutant mice indicate a role for SF1 in both testis and ovary differentiation. Int J Dev Biol. 2010;54(4):683–9.
Conen PE, Bailey JD, Allemang WH, Thompson DW, Ezrin C. A probable partial deletion of the Y chromosome in an intersex patient. Lancet. 1961;2(7197):294–5.
Conover DO. Adaptive significance of temperature-dependent sex determination in a fish. Am Nat. 1984;123(3):297–313.
Cook DM, Hinkes MT, Bernfield M, Rauscher 3rd FJ. Transcriptional activation of the syndecan-1 promoter by the Wilms’ tumor protein WT1. Oncogene. 1996;13(8):1789–99.
Core N, Joly F, Boned A, Djabali M. Disruption of E2F signaling suppresses the INK4a-induced proliferative defect in M33-deficient mice. Oncogene. 2004;23(46):7660–8.
Coveney D, Ross AJ, Slone JD, Capel B. A microarray analysis of the XX Wnt4 mutant gonad targeted at the identification of genes involved in testis vascular differentiation. Gene Expr Patterns. 2008;8(7–8):529–37.
Crawford MA, Doyle W, Meadows N. Gender differences at birth and differences in fetal growth. Hum Reprod. 1987;2(6):517–20.
DeFalco TJ, Verney G, Jenkins AB, McCaffery JM, Russell S, Van Doren M. Sex-specific apoptosis regulates sexual dimorphism in the Drosophila embryonic gonad. Dev Cell. 2003;5(2):205–16.
Di Giacomo G, Koss M, Capellini TD, Brendolan A, Popperl H, Selleri L. Spatio-temporal expression of Pbx3 during mouse organogenesis. Gene Expr Patterns. 2006;6(7):747–57.
DiMartino JF, Selleri L, Traver D, Firpo MT, Rhee J, Warnke R, et al. The Hox cofactor and proto-oncogene Pbx1 is required for maintenance of definitive hematopoiesis in the fetal liver. Blood. 2001;98(3):618–26.
DiNapoli L, Capel B. SRY and the standoff in sex determination. Mol Endocrinol. 2008;22(1):1–9.
Drickamer LC. Seasonal-variation in fertility, fecundity and litter sex-ratio in laboratory and wild stocks of house mice (Mus-Domesticus). Lab Anim Sci. 1990;40(3):284–8.
Drummond IA, Madden SL, Rohwer-Nutter P, Bell GI, Sukhatme VP, Rauscher 3rd FJ. Repression of the insulin-like growth factor II gene by the Wilms tumor suppressor WT1. Science. 1992;257(5070):674–8.
Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, et al. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology. 1999;140(12):5789–96.
Eid W, Opitz L, Biason-Lauber A. Genome-wide identification of CBX2 targets: insights in the human sex development network. Mol Endocrinol. 2015;29(2):247–57.
Ellis L, Bonin S. War and the secondary sex ratio: are they related? Soc Sci Inf. 2004;43(1):115–22.
Ellis L, Cole-Harding S. The effects of prenatal stress, and of prenatal alcohol and nicotine exposure, on human sexual orientation. Physiol Behav. 2001;74(1–2):213–26.
Englert C, Maheswaran S, Garvin AJ, Kreidberg J, Haber DA. Induction of p21 by the Wilms’ tumor suppressor gene WT1. Cancer Res. 1997;57(8):1429–34.
Ensslin MA, Shur BD. The EGF repeat and discoidin domain protein, SED1/MFG-E8, is required for mammary gland branching morphogenesis. Proc Natl Acad Sci U S A. 2007;104(8):2715–20.
Fatchiyah, Zubair M, Shima Y, Oka S, Ishihara S, Fukui-Katoh Y, et al. Differential gene dosage effects of Ad4BP/SF-1 on target tissue development. Biochem Biophys Res Commun. 2006;341(4):1036–45.
Fernandez-Teran M, Piedra ME, Simandl BK, Fallon JF, Ros MA. Limb initiation and development is normal in the absence of the mesonephros. Dev Biol. 1997;189(2):246–55.
Ferretti E, Marshall H, Popperl H, Maconochie M, Krumlauf R, Blasi F. Segmental expression of Hoxb 2 in r4 requires two separate sites that integrate cooperative interactions between Prep 1, Pbx and Hox proteins. Development. 2000;127(1):155–66.
Ford CE, Jones KW, Polani PE, De Almeida JC, Briggs JH. A sex-chromosome anomaly in a case of gonadal dysgenesis (Turner’s syndrome). Lancet. 1959;1(7075):711–3.
Foster JW, Graves JA. An SRY-related sequence on the marsupial X chromosome: implications for the evolution of the mammalian testis-determining gene. Proc Natl Acad Sci U S A. 1994;91(5):1927–31.
Foster JW, Dominguez-Steglich MA, Guioli S, Kwok C, Weller PA, Stevanovic M, et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372(6506):525–30.
Fountain ED, Mao J, Whyte JJ, Mueller KE, Ellersieck MR, Will MJ, et al. Effects of diets enriched in omega-3 and omega-6 polyunsaturated fatty acids on offspring sex-ratio and maternal behavior in mice. Biol Reprod. 2008;78(2):211–7.
Fridolfsson AK, Cheng H, Copeland NG, Jenkins NA, Liu HC, Raudsepp T, et al. Evolution of the avian sex chromosomes from an ancestral pair of autosomes. Proc Natl Acad Sci U S A. 1998;95(14):8147–52.
Fujimoto Y, Tanaka SS, Yamaguchi YL, Kobayashi H, Kuroki S, Tachibana M, et al. Homeoproteins Six1 and Six4 regulate male sex determination and mouse gonad development. Dev Cell. 2013;26(4):416–30.
Fukuda M, Fukuda K, Shimizu T, Moller H. Decline in sex ratio at birth after Kobe earthquake. Hum Reprod. 1998;13(8):2321–2.
Gerwins P, Blank JL, Johnson GL. Cloning of a novel mitogen-activated protein kinase kinase kinase, MEKK4, that selectively regulates the c-Jun amino terminal kinase pathway. J Biol Chem. 1997;272(13):8288–95.
Glaser T, Driscoll DJ, Antonarakis S, Valle D, Housman D. A highly polymorphic locus cloned from the breakpoint of a chromosome 11p13 deletion associated with the WAGR syndrome. Genomics. 1989;5(4):880–93.
Gould KG, Ansari AH. Electrolyte interactions in cervical mucus and their relationship to circulating hormone levels. Contraception. 1981;23(5):507–16.
Grant VJ. Sex determination and the maternal dominance hypothesis. Hum Reprod. 1996;11(11):2371–5.
Grant VJ, Irwin RJ. Follicular fluid steroid levels and subsequent sex of bovine embryos. J Exp Zool A Comp Exp Biol. 2005;303(12):1120–5.
Grant VJ, Irwin RJ, Standley NT, Shelling AN, Chamley LW. Sex of bovine embryos may be related to mothers’ preovulatory follicular testosterone. Biol Reprod. 2008;78(5):812–5.
Grau DJ, Chapman BA, Garlick JD, Borowsky M, Francis NJ, Kingston RE. Compaction of chromatin by diverse polycomb group proteins requires localized regions of high charge. Genes Dev. 2011;25(20):2210–21.
Gray RH. In: Sheppard KE, Boublik JH, Funder JW, editors. Stress and reproduction: an epidemiological perspective. New York: Raven Press; 1992.
Gulliver CE, Friend MA, King BJ, Robertson SM, Wilkins JF, Clayton EH. Increased prostaglandin response to oxytocin in ewes fed a diet high in omega-6 polyunsaturated fatty acids. Lipids. 2013a;48(2):177–83.
Gulliver CE, Friend MA, King BJ, Clayton EH. A higher proportion of female lambs when ewes were fed oats and cottonseed meal prior to and following conception. Anim Prod Sci. 2013b;53(5):464–71.
Hacker A, Capel B, Goodfellow P, Lovell-Badge R. Expression of Sry, the mouse sex determining gene. Development. 1995;121(6):1603–14.
Haines B, Hughes J, Corbett M, Shaw M, Innes J, Patel L, et al. Interchromosomal insertional translocation at Xq26.3 alters SOX3 expression in an individual with XX male sex reversal. J Clin Endocrinol Metab. 2015;100(5):E815–20.
Hammes A, Guo JK, Lutsch G, Leheste JR, Landrock D, Ziegler U, et al. Two splice variants of the Wilms’ tumor 1 gene have distinct functions during sex determination and nephron formation. Cell. 2001;106(3):319–29.
Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417(6885):182–7.
Haqq CM, Donahoe PK. Regulation of sexual dimorphism in mammals. Physiol Rev. 1998;78(1):1–33.
Hatano A, Matsumoto M, Higashinakagawa T, Nakayama KI. Phosphorylation of the chromodomain changes the binding specificity of Cbx2 for methylated histone H3. Biochem Biophys Res Commun. 2010;397(1):93–9.
Hattori RS, Murai Y, Oura M, Masuda S, Majhi SK, Sakamoto T, et al. A Y-linked anti-Mullerian hormone duplication takes over a critical role in sex determination. Proc Natl Acad Sci U S A. 2012;109(8):2955–9.
Heikinheimo M, Ermolaeva M, Bielinska M, Rahman NA, Narita N, Huhtaniemi IT, et al. Expression and hormonal regulation of transcription factors GATA-4 and GATA-6 in the mouse ovary. Endocrinology. 1997;138(8):3505–14.
Hesketh T, Xing ZW. Abnormal sex ratios in human populations: causes and consequences. Proc Natl Acad Sci U S A. 2006;103(36):13271–5.
Hiramatsu R, Matoba S, Kanai-Azuma M, Tsunekawa N, Katoh-Fukui Y, Kurohmaru M, et al. A critical time window of Sry action in gonadal sex determination in mice. Development. 2009;136(1):129–38.
Hiramatsu R, Harikae K, Tsunekawa N, Kurohmaru M, Matsuo I, Kanai Y. FGF signaling directs a center-to-pole expansion of tubulogenesis in mouse testis differentiation. Development. 2010;137(2):303–12.
Hohenstein P, Hastie ND. The many facets of the Wilms’ tumour gene, WT1. Hum Mol Genet. 2006;15(Spec No 2):R196–201.
Homa ST, Carroll J, Swann K. The role of calcium in mammalian oocyte maturation and egg activation. Hum Reprod. 1993;8(8):1274–81.
Honda S, Morohashi K, Nomura M, Takeya H, Kitajima M, Omura T. Ad4BP regulating steroidogenic P-450 gene is a member of steroid hormone receptor superfamily. J Biol Chem. 1993;268(10):7494–502.
Hong CY, Chiang BN, Turner P. Calcium ion is the key regulator of human sperm function. Lancet. 1984;2(8417–8418):1449–51.
Hossain A, Saunders GF. The human sex-determining gene SRY is a direct target of WT1. J Biol Chem. 2001;276(20):16817–23.
Hu YC, Okumura LM, Page DC. Gata4 is required for formation of the genital ridge in mice. PLoS Genet. 2013;9(7):e1003629.
Huang Q, Raya A, DeJesus P, Chao SH, Quon KC, Caldwell JS, et al. Identification of p53 regulators by genome-wide functional analysis. Proc Natl Acad Sci U S A. 2004;101(10):3456–61.
Hughes IA, Houk C, Ahmed SF, Lee PA. Consensus statement on management of intersex disorders. Arch Dis Child. 2006;91(7):554–63.
Ikeda Y, Takeda Y, Shikayama T, Mukai T, Hisano S, Morohashi KI. Comparative localization of Dax-1 and Ad4BP/SF-1 during development of the hypothalamic-pituitary-gonadal axis suggests their closely related and distinct functions. Dev Dyn. 2001;220(4):363–76.
Ishii M, Kanai Y, Kanai-Azuma M, Tajima Y, Wei TT, Kidokoro T, et al. Adhesion activity of fetal gonadal cells to EGF and discoidin domains of milk fat globule-EGF factor 8 (MFG-E8), a secreted integrin-binding protein which is transiently expressed in mouse early gonadogenesis. Anat Embryol (Berl). 2005;209(6):485–94.
Ishimaru Y, Komatsu T, Kasahara M, Katoh-Fukui Y, Ogawa H, Toyama Y, et al. Mechanism of asymmetric ovarian development in chick embryos. Development. 2008;135(4):677–85.
Jacobs PA, Strong JA. A case of human intersexuality having a possible XXY sex-determining mechanism. Nature. 1959;183(4657):302–3.
Jacobs Y, Schnabel CA, Cleary ML. Trimeric association of Hox and TALE homeodomain proteins mediates Hoxb2 hindbrain enhancer activity. Mol Cell Biol. 1999;19(7):5134–42.
James WH. The hypothesized hormonal control of human sex ratio at birth – an update. J Theor Biol. 1990;143(4):555–64.
James RG, Kamei CN, Wang Q, Jiang R, Schultheiss TM. Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation of kidney precursor cells. Development. 2006;133(15):2995–3004.
Jeays-Ward K, Hoyle C, Brennan J, Dandonneau M, Alldus G, Capel B, et al. Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development. 2003;130(16):3663–70.
Jeays-Ward K, Dandonneau M, Swain A. Wnt4 is required for proper male as well as female sexual development. Dev Biol. 2004;276(2):431–40.
Jeske YW, Bowles J, Greenfield A, Koopman P. Expression of a linear Sry transcript in the mouse genital ridge. Nat Genet. 1995;10(4):480–2.
Jost A. Recherches sur la différenciation sexuelle de l’embryon de lapin. Arch Anat Microsc Morph Exp. 1947;36:271–315.
Jost A. Problems of fetal endocrinology: the gonadal and hypophyseal hormones. Recent Prog Horm Res. 1953;8:379–418.
Kamiya T, Kai W, Tasumi S, Oka A, Matsunaga T, Mizuno N, et al. A trans-species missense SNP in Amhr2 is associated with sex determination in the tiger pufferfish, Takifugu rubripes (fugu). PLoS Genet. 2012;8(7):e1002798.
Kanai Y, Kanai-Azuma M, Tajima Y, Birk OS, Hayashi Y, Sanai Y. Identification of a stromal cell type characterized by the secretion of a soluble integrin-binding protein, MFG-E8, in mouse early gonadogenesis. Mech Dev. 2000;96(2):223–7.
Karl J, Capel B. Sertoli cells of the mouse testis originate from the coelomic epithelium. Dev Biol. 1998;203(2):323–33.
Kashimada K, Koopman P. Sry: the master switch in mammalian sex determination. Development. 2010;137(23):3921–30.
Katoh-Fukui Y, Tsuchiya R, Shiroishi T, Nakahara Y, Hashimoto N, Noguchi K, et al. Male-to-female sex reversal in M33 mutant mice. Nature. 1998;393(6686):688–92.
Katoh-Fukui Y, Owaki A, Toyama Y, Kusaka M, Shinohara Y, Maekawa M, et al. Mouse Polycomb M33 is required for splenic vascular and adrenal gland formation through regulating Ad4BP/SF1 expression. Blood. 2005;106(5):1612–20.
Katoh-Fukui Y, Miyabayashi K, Komatsu T, Owaki A, Baba T, Shima Y, et al. Cbx2, a polycomb group gene, is required for Sry gene expression in mice. Endocrinology. 2012;153(2):913–24.
Kaustov L, Ouyang H, Amaya M, Lemak A, Nady N, Duan S, et al. Recognition and specificity determinants of the human cbx chromodomains. J Biol Chem. 2011;286(1):521–9.
Kazanskaya O, Glinka A, del Barco Barrantes I, Stannek P, Niehrs C, Wu W. R-Spondin2 is a secreted activator of Wnt/beta-catenin signaling and is required for Xenopus myogenesis. Dev Cell. 2004;7(4):525–34.
Kennedy D, Ramsdale T, Mattick J, Little M. An RNA recognition motif in Wilms’ tumour protein (WT1) revealed by structural modelling. Nat Genet. 1996;12(3):329–31.
Kent JP. Birth sex-ratios in sheep over 9 lambing seasons – years 7–9 and the effects of aging. Behav Ecol Sociobiol. 1995;36(2):101–4.
Kent J, Wheatley SC, Andrews JE, Sinclair AH, Koopman P. A male-specific role for SOX9 in vertebrate sex determination. Development. 1996;122(9):2813–22.
Ketola I, Rahman N, Toppari J, Bielinska M, Porter-Tinge SB, Tapanainen JS, et al. Expression and regulation of transcription factors GATA-4 and GATA-6 in developing mouse testis. Endocrinology. 1999;140(3):1470–80.
Kim J, Prawitt D, Bardeesy N, Torban E, Vicaner C, Goodyer P, et al. The Wilms’ tumor suppressor gene (wt1) product regulates Dax-1 gene expression during gonadal differentiation. Mol Cell Biol. 1999;19(3):2289–99.
Kim SK, Selleri L, Lee JS, Zhang AY, Gu X, Jacobs Y, et al. Pbx1 inactivation disrupts pancreas development and in Ipf1-deficient mice promotes diabetes mellitus. Nat Genet. 2002;30(4):430–5.
Kim Y, Kobayashi A, Sekido R, DiNapoli L, Brennan J, Chaboissier MC, et al. Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination. PLoS Biol. 2006;4(6):1000–9.
Kimble JE, White JG. On the control of germ cell development in Caenorhabditis elegans. Dev Biol. 1981;81(2):208–19.
Kispert A, Vainio S, McMahon AP. Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development. 1998;125(21):4225–34.
Kobayashi H, Kawakami K, Asashima M, Nishinakamura R. Six1 and Six4 are essential for Gdnf expression in the metanephric mesenchyme and ureteric bud formation, while Six1 deficiency alone causes mesonephric-tubule defects. Mech Dev. 2007;124(4):290–303.
Kojola I, Eloranta E. Influences of maternal body-weight, age, and parity on sex-ratio in semidomesticated reindeer (Rangifer-Tarandus-Tarandus). Evolution. 1989;43(6):1331–6.
Koopman P, Munsterberg A, Capel B, Vivian N, Lovell-Badge R. Expression of a candidate sex-determining gene during mouse testis differentiation. Nature. 1990;348(6300):450–2.
Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351(6322):117–21.
Kopito LE, Kosasky HJ, Sturgis SH, Lieberman BL, Shwachman H. Water and electrolytes in human cervical mucus. Fertil Steril. 1973;24(7):499–506.
Krackow S. The developmental asynchrony hypothesis for sex ratio manipulation. J Theor Biol. 1995;176(2):273–80.
Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, et al. WT-1 is required for early kidney development. Cell. 1993;74(4):679–91.
Kruuk LEB, Clutton-Brock TH, Albon SD, Pemberton JM, Guinness FE. Population density affects sex ratio variation in red deer. Nature. 1999;399(6735):459–61.
Kusaka M, Katoh-Fukui Y, Ogawa H, Miyabayashi K, Baba T, Shima Y, et al. Abnormal epithelial cell polarity and ectopic epidermal growth factor receptor (EGFR) expression induced in Emx2 KO embryonic gonads. Endocrinology. 2010;151(12):5893–904.
Laclef C, Souil E, Demignon J, Maire P. Thymus, kidney and craniofacial abnormalities in Six 1 deficient mice. Mech Dev. 2003a;120(6):669–79.
Laclef C, Hamard G, Demignon J, Souil E, Houbron C, Maire P. Altered myogenesis in Six1-deficient mice. Development. 2003b;130(10):2239–52.
Lakshmaiah KC, Saini KS, Singh T, Jain A, Kumar RV, SK V, et al. Primary synovial sarcoma of kidney-a report of 2 cases and review of literature. J Egypt Natl Cancer Inst. 2010;22(3):149–53.
Lala DS, Rice DA, Parker KL. Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. Mol Endocrinol. 1992;6(8):1249–58.
Larson MA, Kimura K, Kubisch HM, Roberts RM. Sexual dimorphism among bovine embryos in their ability to make the transition to expanded blastocyst and in the expression of the signaling molecule IFN-tau. Proc Natl Acad Sci U S A. 2001;98(17):9677–82.
Le Faou P, Volkel P, Angrand PO. The zebrafish genes encoding the Polycomb repressive complex (PRC) 1. Gene. 2011;475(1):10–21.
Lee SB, Huang K, Palmer R, Truong VB, Herzlinger D, Kolquist KA, et al. The Wilms tumor suppressor WT1 encodes a transcriptional activator of amphiregulin. Cell. 1999;98(5):663–73.
Liu CF, Bingham N, Parker K, Yao HH. Sex-specific roles of beta-catenin in mouse gonad development. Hum Mol Genet. 2009;18(3):405–17.
Lu B, Yu H, Chow C, Li B, Zheng W, Davis RJ, et al. GADD45gamma mediates the activation of the p38 and JNK MAP kinase pathways and cytokine production in effector TH1 cells. Immunity. 2001;14(5):583–90.
Ludbrook LM, Harley VR. Sex determination: a ‘window’ of DAX1 activity. Trends Endocrinol Metab. 2004;15(3):116–21.
Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonad development and sexual differentiation. Cell. 1994;77(4):481–90.
Maatouk DM, DiNapoli L, Alvers A, Parker KL, Taketo MM, Capel B. Stabilization of beta-catenin in XY gonads causes male-to-female sex-reversal. Hum Mol Genet. 2008;17(19):2949–55.
Malki S, Nef S, Notarnicola C, Thevenet L, Gasca S, Mejean C, et al. Prostaglandin D2 induces nuclear import of the sex-determining factor SOX9 via its cAMP-PKA phosphorylation. EMBO J. 2005;24(10):1798–809.
Mann RS, Affolter M. Hox proteins meet more partners. Curr Opin Genet Dev. 1998;8(4):423–9.
Manuylov NL, Zhou B, Ma Q, Fox SC, Pu WT, Tevosian SG. Conditional ablation of Gata4 and Fog2 genes in mice reveals their distinct roles in mammalian sexual differentiation. Dev Biol. 2011;353(2):229–41.
Mao J, Zhang X, Sieli PT, Falduto MT, Torres KE, Rosenfeld CS. Contrasting effects of different maternal diets on sexually dimorphic gene expression in the murine placenta. Proc Natl Acad Sci U S A. 2010;107(12):5557–62.
Masukume G, Grech V. The sex ratio at birth in South Africa increased 9 months after the 2010 FIFA World Cup. Early Hum Dev. 2015;91(12):807–9.
Mathews F, Johnson PJ, Neil A. You are what your mother eats: evidence for maternal preconception diet influencing foetal sex in humans. Proc Biol Sci. 2008;275(1643):1661–8.
Matson CK, Zarkower D. Sex and the singular DM domain: insights into sexual regulation, evolution and plasticity. Nat Rev Genet. 2012;13(3):163–74.
Matsubara K, Tarui H, Toriba M, Yamada K, Nishida-Umehara C, Agata K, et al. Evidence for different origin of sex chromosomes in snakes, birds, and mammals and step-wise differentiation of snake sex chromosomes. Proc Natl Acad Sci U S A. 2006;103(48):18190–5.
Matsuda M, Nagahama Y, Shinomiya A, Sato T, Matsuda C, Kobayashi T, et al. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature. 2002;417(6888):559–63.
Mayo MW, Wang CY, Drouin SS, Madrid LV, Marshall AF, Reed JC, et al. WT1 modulates apoptosis by transcriptionally upregulating the bcl-2 proto-oncogene. EMBO J. 1999;18(14):3990–4003.
Mazaud-Guittot S, Prud’homme B, Bouchard MF, Bergeron F, Daems C, Tevosian SG, et al. GATA4 autoregulates its own expression in mouse gonadal cells via its distal 1b promoter. Biol Reprod. 2014;90(2):25.
Mazur SP, Petrenko A. Sex differences in biotransformation of the xenobiotic p-nitroanisole in isolated rat hepatocyte under the influence of phenobarbital. Biochemistry (Mosc). 1997;62(4):362–3.
McClung CE. Possible action of the sex-determining mechanism. Proc Natl Acad Sci U S A. 1918;4(6):160–3.
McCoard SA, Wise TH, Fahrenkrug SC, Ford JJ. Temporal and spatial localization patterns of Gata4 during porcine gonadogenesis. Biol Reprod. 2001;65(2):366–74.
McLaren A. Germ and somatic cell lineages in the developing gonad. Mol Cell Endocrinol. 2000;163(1–2):3–9.
Mcshea WJ, Madison DM. Sex-ratio shifts within litters of meadow voles (Microtus-Pennsylvanicus). Behav Ecol Sociobiol. 1986;18(6):431–6.
Meeks JJ, Crawford SE, Russell TA, Morohashi K, Weiss J, Jameson JL. Dax1 regulates testis cord organization during gonadal differentiation. Development. 2003;130(5):1029–36.
Meikle DB, Drickamer LC. Food availability and secondary sex ratio variation in wild and laboratory house mice (Mus musculus). J Reprod Fertil. 1986;78(2):587–91.
Meikle DB, Drickamer LC, Vessey SH, Arthur RD, Rosenthal TL. Dominance rank and parental investment in swine (Sus scrofa domesticus). Ethology. 1996;102(12):969–78.
Miyake Z, Takekawa M, Ge Q, Saito H. Activation of MTK1/MEKK4 by GADD45 through induced N-C dissociation and dimerization-mediated trans autophosphorylation of the MTK1 kinase domain. Mol Cell Biol. 2007;27(7):2765–76.
Miyamoto N, Yoshida M, Kuratani S, Matsuo I, Aizawa S. Defects of urogenital development in mice lacking Emx2. Development. 1997;124(9):1653–64.
Miyamoto Y, Taniguchi H, Hamel F, Silversides DW, Viger RS. A GATA4/WT1 cooperation regulates transcription of genes required for mammalian sex determination and differentiation. BMC Mol Biol. 2008;9:44.
Moalem S, Babul-Hirji R, Stavropolous DJ, Wherrett D, Bagli DJ, Thomas P, et al. XX male sex reversal with genital abnormalities associated with a de novo SOX3 gene duplication. Am J Med Genet A. 2012;158A(7):1759–64.
Molkentin JD, Lin Q, Duncan SA, Olson EN. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 1997;11(8):1061–72.
Moniot B, Farhat A, Aritake K, Declosmenil F, Nef S, Eguchi N, et al. Hematopoietic prostaglandin D synthase (H-Pgds) is expressed in the early embryonic gonad and participates to the initial nuclear translocation of the SOX9 protein. Dev Dyn. 2011;240(10):2335–43.
Morais da Silva S, Hacker A, Harley V, Goodfellow P, Swain A, Lovell-Badge R. Sox9 expression during gonad development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat Genet. 1996;14(1):62–8.
Morey L, Pascual G, Cozzuto L, Roma G, Wutz A, Benitah SA, et al. Nonoverlapping functions of the Polycomb group Cbx family of proteins in embryonic stem cells. Cell Stem Cell. 2012;10(1):47–62.
Morinaga C, Saito D, Nakamura S, Sasaki T, Asakawa S, Shimizu N, et al. The hotei mutation of medaka in the anti-Mullerian hormone receptor causes the dysregulation of germ cell and sexual development. Proc Natl Acad Sci U S A. 2007;104(23):9691–6.
Morohashi K, Honda S, Inomata Y, Handa H, Omura T. A common trans-acting factor, Ad4-binding protein, to the promoters of steroidogenic P-450s. J Biol Chem. 1992;267(25):17913–9.
Morohashi K, Zanger UM, Honda S, Hara M, Waterman MR, Omura T. Activation of CYP11A and CYP11B gene promoters by the steroidogenic cell-specific transcription factor, Ad4BP. Mol Endocrinol. 1993;7(9):1196–204.
Morrison AA, Viney RL, Ladomery MR. The post-transcriptional roles of WT1, a multifunctional zinc-finger protein. Biochim Biophys Acta. 2008;1785(1):55–62.
Motegi S, Leitner WW, Lu M, Tada Y, Sardy M, Wu C, et al. Pericyte-derived MFG-E8 regulates pathologic angiogenesis. Arterioscler Thromb Vasc Biol. 2011;31(9):2024–34.
Myosho T, Otake H, Masuyama H, Matsuda M, Kuroki Y, Fujiyama A, et al. Tracing the emergence of a novel sex-determining gene in medaka, Oryzias luzonensis. Genetics. 2012;191(1):163–70.
Naillat F, Yan W, Karjalainen R, Liakhovitskaia A, Samoylenko A, Xu Q, et al. Identification of the genes regulated by Wnt-4, a critical signal for commitment of the ovary. Exp Cell Res. 2015;332(2):163–78.
Nanda S, DeFalco TJ, Loh SH, Phochanukul N, Camara N, Van Doren M, et al. Sox100B, a Drosophila group E Sox-domain gene, is required for somatic testis differentiation. Sex Dev. 2009;3(1):26–37.
Nash DM, Hess SA, White BA, Peluso JJ. Steroidogenic factor-1 regulates the rate of proliferation of normal and neoplastic rat ovarian surface epithelial cells in vitro. Endocrinology. 1998;139(11):4663–71.
Navara KJ. Humans at tropical latitudes produce more females. Biol Lett. 2009;5(4):524–7.
Nef S, Verma-Kurvari S, Merenmies J, Vassalli JD, Efstratiadis A, Accili D, et al. Testis determination requires insulin receptor family function in mice. Nature. 2003;426(6964):291–5.
Niksic M, Slight J, Sanford JR, Caceres JF, Hastie ND. The Wilms’ tumour protein (WT1) shuttles between nucleus and cytoplasm and is present in functional polysomes. Hum Mol Genet. 2004;13(4):463–71.
Noorlander AM, Geraedts JP, Melissen JB. Female gender pre-selection by maternal diet in combination with timing of sexual intercourse – a prospective study. Reprod BioMed Online. 2010;21(6):794–802.
Onesimo R, Orteschi D, Scalzone M, Rossodivita A, Nanni L, Zannoni GF, et al. Chromosome 9p deletion syndrome and sex reversal: novel findings and redefinition of the critically deleted regions. Am J Med Genet A. 2012;158A(9):2266–71.
Oreal E, Pieau C, Mattei MG, Josso N, Picard JY, Carre-Eusebe D, et al. Early expression of AMH in chicken embryonic gonads precedes testicular SOX9 expression. Dev Dyn. 1998;212(4):522–32.
Ota T, Asahina H, Park SH, Huang Q, Minegishi T, Auersperg N, et al. HOX cofactors expression and regulation in the human ovary. Reprod Biol Endocrinol. 2008;6:49.
Ottolenghi C, Pelosi E, Tran J, Colombino M, Douglass E, Nedorezov T, et al. Loss of Wnt4 and Foxl2 leads to female-to-male sex reversal extending to germ cells. Hum Mol Genet. 2007;16(23):2795–804.
Oun AE, Barkry S, Soltan S, Taha A, Kadry E. Preconceptional minerals administration skewd sex ration in rat offspring. Res Obstet Gynecol. 2016;4(1):11–5.
Ozaki H, Nakamura K, Funahashi J, Ikeda K, Yamada G, Tokano H, et al. Six1 controls patterning of the mouse otic vesicle. Development. 2004;131(3):551–62.
Pelletier J, Bruening W, Kashtan CE, Mauer SM, Manivel JC, Striegel JE, et al. Germline mutations in the Wilms’ tumor suppressor gene are associated with abnormal urogenital development in Denys-Drash syndrome. Cell. 1991;67(2):437–47.
Pitetti JL, Calvel P, Romero Y, Conne B, Truong V, Papaioannou MD, et al. Insulin and IGF1 receptors are essential for XX and XY gonadal differentiation and adrenal development in mice. PLoS Genet. 2013;9(1):e1003160.
Quinn AE, Georges A, Sarre SD, Guarino F, Ezaz T, Graves JA. Temperature sex reversal implies sex gene dosage in a reptile. Science. 2007;316(5823):411.
Radder RS, Quinn AE, Georges A, Sarre SD, Shine R. Genetic evidence for co-occurrence of chromosomal and thermal sex-determining systems in a lizard. Biol Lett. 2008;4(2):176–8.
Raymond CS, Shamu CE, Shen MM, Seifert KJ, Hirsch B, Hodgkin J, et al. Evidence for evolutionary conservation of sex-determining genes. Nature. 1998;391(6668):691–5.
Raymond CS, Kettlewell JR, Hirsch B, Bardwell VJ, Zarkower D. Expression of Dmrt1 in the genital ridge of mouse and chicken embryos suggests a role in vertebrate sexual development. Dev Biol. 1999;215(2):208–20.
Raymond CS, Murphy MW, O’Sullivan MG, Bardwell VJ, Zarkower D. Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes Dev. 2000;14(20):2587–95.
Rivera-Feliciano J, Lee KH, Kong SW, Rajagopal S, Ma Q, Springer Z, et al. Development of heart valves requires Gata4 expression in endothelial-derived cells. Development. 2006;133(18):3607–18.
Rivers JP, Crawford MA. Maternal nutrition and the sex ratio at birth. Nature. 1974;252(5481):297–8.
Rojas A, Kong SW, Agarwal P, Gilliss B, Pu WT, Black BL. GATA4 is a direct transcriptional activator of cyclin D2 and Cdk4 and is required for cardiomyocyte proliferation in anterior heart field-derived myocardium. Mol Cell Biol. 2008;28(17):5420–31.
Rondeau EB, Messmer AM, Sanderson DS, Jantzen SG, von Schalburg KR, Minkley DR, et al. Genomics of sablefish (Anoplopoma fimbria): expressed genes, mitochondrial phylogeny, linkage map and identification of a putative sex gene. BMC Genomics. 2013;14:452.
Rosenfeld CS, Roberts RM. Maternal diet and other factors affecting offspring sex ratio: a review. Biol Reprod. 2004;71(4):1063–70.
Rosenfeld CS, Grimm KM, Livingston KA, Brokman AM, Lamberson WE, Roberts RM. Striking variation in the sex ratio of pups born to mice according to whether maternal diet is high in fat or carbohydrate. Proc Natl Acad Sci U S A. 2003;100(8):4628–32.
Sadalla EK, Kenrich DT, Vershure B. Dominance and heterosexual attraction. J Pers Soc Psychol. 1987;52:730–8.
Salz HK, Erickson JW. Sex determination in Drosophila: the view from the top. Fly (Austin). 2010;4(1):60–70.
Schmidt CM, Hood WR. Calcium availability influences litter size and sex ratio in white-footed mice (Peromyscus leucopus). PLoS ONE. 2012;7(8):e41402.
Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier AC, et al. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development. 2004;131(4):933–42.
Schnabel CA, Selleri L, Jacobs Y, Warnke R, Cleary ML. Expression of Pbx1b during mammalian organogenesis. Mech Dev. 2001;100(1):131–5.
Schnabel CA, Selleri L, Cleary ML. Pbx1 is essential for adrenal development and urogenital differentiation. Genesis. 2003;37(3):123–30.
Sekido R, Lovell-Badge R. Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature. 2008;453(7197):930–4.
Sekido R, Bar I, Narvaez V, Penny G, Lovell-Badge R. SOX9 is up-regulated by the transient expression of SRY specifically in Sertoli cell precursors. Dev Biol. 2004;274(2):271–9.
Selleri L, Depew MJ, Jacobs Y, Chanda SK, Tsang KY, Cheah KS, et al. Requirement for Pbx1 in skeletal patterning and programming chondrocyte proliferation and differentiation. Development. 2001;128(18):3543–57.
She ZY, Yang WX. Molecular mechanisms involved in mammalian primary sex determination. J Mol Endocrinol. 2014;53(1):R21–37.
Shettles LB. Sperm morphology, cervical milieu, time of insemination and sex ratios (author’s transl). Andrologie. 1973;5(3):227–30.
Shimamura R, Fraizer GC, Trapman J, Lau Yf C, Saunders GF. The Wilms’ tumor gene WT1 can regulate genes involved in sex determination and differentiation: SRY, Mullerian-inhibiting substance, and the androgen receptor. Clin Cancer Res. 1997;3(12 Pt 2):2571–80.
Shinoda K, Lei H, Yoshii H, Nomura M, Nagano M, Shiba H, et al. Developmental defects of the ventromedial hypothalamic nucleus and pituitary gonadotroph in the Ftz-F1 disrupted mice. Dev Dyn. 1995;204(1):22–9.
Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, Smith MJ, et al. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990;346(6281):240–4.
Singh D, Zambarano RJ. Offspring sex ratio in women with android body fat distribution. Hum Biol. 1997;69(4):545–56.
Smagulova FO, Manuylov NL, Leach LL, Tevosian SG. GATA4/FOG2 transcriptional complex regulates Lhx9 gene expression in murine heart development. BMC Dev Biol. 2008;8:67.
Smith CA, Roeszler KN, Ohnesorg T, Cummins DM, Farlie PG, Doran TJ, et al. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature. 2009;461(7261):267–71.
So PL, Danielian PS. Cloning and expression analysis of a mouse gene related to Drosophila odd-skipped. Mech Dev. 1999;84(1–2):157–60.
Stolkowski J, Choukroun J. Preconception selection of sex in man. Isr J Med Sci. 1981;17(11):1061–7.
Stolkowski J, Lorrain J. Preconceptional selection of fetal sex. Int J Gynaecol Obstet. 1980;18(6):440–3.
Sutton E, Hughes J, White S, Sekido R, Tan J, Arboleda V, et al. Identification of SOX3 as an XX male sex reversal gene in mice and humans. J Clin Invest. 2011;121(1):328–41.
Swain A, Lovell-Badge R. Mammalian sex determination: a molecular drama. Genes Dev. 1999;13(7):755–67.
Swain A, Narvaez V, Burgoyne P, Camerino G, Lovell-Badge R. Dax1 antagonizes Sry action in mammalian sex determination. Nature. 1998;391(6669):761–7.
Takekawa M, Saito H. A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell. 1998;95(4):521–30.
Takekawa M, Posas F, Saito H. A human homolog of the yeast Ssk2/Ssk22 MAP kinase kinase kinases, MTK1, mediates stress-induced activation of the p38 and JNK pathways. EMBO J. 1997;16(16):4973–82.
Taketo M, Parker KL, Howard TA, Tsukiyama T, Wong M, Niwa O, et al. Homologs of Drosophila fushi-tarazu factor 1 map to mouse chromosome 2 and human chromosome 9q33. Genomics. 1995;25(2):565–7.
Tamimi RM, Lagiou P, Mucci LA, Hsieh CC, Adami HO, Trichopoulos D. Average energy intake among pregnant women carrying a boy compared with a girl. BMJ. 2003;326(7401):1245–6.
Tena JJ, Neto A, de la Calle-Mustienes E, Bras-Pereira C, Casares F, Gomez-Skarmeta JL. Odd-skipped genes encode repressors that control kidney development. Dev Biol. 2007;301(2):518–31.
Tevosian SG, Albrecht KH, Crispino JD, Fujiwara Y, Eicher EM, Orkin SH. Gonadal differentiation, sex determination and normal Sry expression in mice require direct interaction between transcription partners GATA4 and FOG2. Development. 2002;129(19):4627–34.
Tomizuka K, Horikoshi K, Kitada R, Sugawara Y, Iba Y, Kojima A, et al. R-spondin1 plays an essential role in ovarian development through positively regulating Wnt-4 signaling. Hum Mol Genet. 2008;17(9):1278–91.
Tran PV, Akana SF, Malkovska I, Dallman MF, Parada LF, Ingraham HA. Diminished hypothalamic bdnf expression and impaired VMH function are associated with reduced SF-1 gene dosage. J Comp Neurol. 2006;498(5):637–48.
Tremblay JJ, Viger RS. GATA factors differentially activate multiple gonadal promoters through conserved GATA regulatory elements. Endocrinology. 2001;142(3):977–86.
Trivers RL, Willard DE. Natural selection of parental ability to vary the sex ratio of offspring. Science. 1973;179(4068):90–2.
Uchiyama A, Yamada K, Ogino S, Yokoyama Y, Takeuchi Y, Udey MC, et al. MFG-E8 regulates angiogenesis in cutaneous wound healing. Am J Pathol. 2014;184(7):1981–90.
Uda M, Ottolenghi C, Crisponi L, Garcia JE, Deiana M, Kimber W, et al. Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum Mol Genet. 2004;13(11):1171–81.
Uhlenhaut NH, Treier M. Foxl2 function in ovarian development. Mol Genet Metab. 2006;88(3):225–34.
Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139(6):1130–42.
Vahidi AR, Sheikhha MH. Comparing the effects of sodium and potassium diet with calcium and magnesium diet on sex ratio of rats’ offspring. Asian Netw Sci Inf. 2007;6(1):44–8.
Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397(6718):405–9.
Val P, Martinez-Barbera JP, Swain A. Adrenal development is initiated by Cited 2 and Wt1 through modulation of Sf-1 dosage. Development. 2007;134(12):2349–58.
van der Stoop P, Boutsma EA, Hulsman D, Noback S, Heimerikx M, Kerkhoven RM, et al. Ubiquitin E3 ligase ring 1b/Rnf2 of polycomb repressive complex 1 contributes to stable maintenance of mouse embryonic stem cells. PLoS ONE. 2008;3(5):e 2235.
Vidal VP, Chaboissier MC, de Rooij DG, Schedl A. Sox9 induces testis development in XX transgenic mice. Nat Genet. 2001;28(3):216–7.
Viger RS, Mertineit C, Trasler JM, Nemer M. Transcription factor GATA-4 is expressed in a sexually dimorphic pattern during mouse gonad development and is a potent activator of the Mullerian inhibiting substance promoter. Development. 1998;125(14):2665–75.
Volkel P, Le Faou P, Vandamme J, Pira D, Angrand PO. A human Polycomb isoform lacking the Pc box does not participate to PRC1 complexes but forms protein assemblies and represses transcription. Epigenetics. 2012;7(5):482–91.
Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, et al. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994;79(6):1111–20.
Wang ZY, Madden SL, Deuel TF, Rauscher 3rd FJ. The Wilms’ tumor gene product, WT1, represses transcription of the platelet-derived growth factor A-chain gene. J Biol Chem. 1992;267(31):21999–2002.
Wang Q, Lan Y, Cho ES, Maltby KM, Jiang R. Odd-skipped related 1 (Odd 1) is an essential regulator of heart and urogenital development. Dev Biol. 2005;288(2):582–94.
Wang CY, Lai PY, Chen TY, Chung BC. NR5A1 prevents centriole splitting by inhibiting centrosomal DNA-PK activation and beta-catenin accumulation. Cell Commun Signal. 2014;12:55.
Warr N, Carre GA, Siggers P, Faleato JV, Brixey R, Pope M, et al. Gadd45gamma and Map3k4 interactions regulate mouse testis determination via p38 MAPK-mediated control of Sry expression. Dev Cell. 2012;23(5):1020–31.
Wauters LA, Decrombrugghe SA, Nour N, Matthysen E. Do female roe deer in good condition produce more sons than daughters. Behav Ecol Sociobiol. 1995;37(3):189–93.
Weiss J, Meeks JJ, Hurley L, Raverot G, Frassetto A, Jameson JL. Sox3 is required for gonadal function, but not sex determination, in males and females. Mol Cell Biol. 2003;23(22):8084–91.
Western PS, Harry JL, Graves JA, Sinclair AH. Temperature-dependent sex determination in the American alligator: AMH precedes SOX9 expression. Dev Dyn. 1999;216(4–5):411–9.
Whyte JJ, Alexenko AP, Davis AM, Ellersieck MR, Fountain ED, Rosenfeld CS. Maternal diet composition alters serum steroid and free fatty acid concentrations and vaginal pH in mice. J Endocrinol. 2007;192(1):75–81.
Wilhelm D, Englert C. The Wilms tumor suppressor WT1 regulates early gonad development by activation of Sf1. Genes Dev. 2002;16(14):1839–51.
Wilhelm D, Martinson F, Bradford S, Wilson MJ, Combes AN, Beverdam A, et al. Sertoli cell differentiation is induced both cell-autonomously and through prostaglandin signaling during mammalian sex determination. Dev Biol. 2005;287(1):111–24.
Wilson EB. The chromosomes in relation to the determination of sex in insects. Science. 1905;22(564):500–2.
Wright E, Hargrave MR, Christiansen J, Cooper L, Kun J, Evans T, et al. The Sry-related gene Sox9 is expressed during chondrogenesis in mouse embryos. Nat Genet. 1995;9(1):15–20.
Xu PX, Zheng W, Huang L, Maire P, Laclef C, Silvius D. Six1 is required for the early organogenesis of mammalian kidney. Development. 2003;130(14):3085–94.
Yamamoto Y, Zhang Y, Sarida M, Hattori RS, Strussmann CA. Coexistence of genotypic and temperature-dependent sex determination in pejerrey Odontesthes bonariensis. PLoS ONE. 2014;9(7):e102574.
Yoshimoto S, Okada E, Umemoto H, Tamura K, Uno Y, Nishida-Umehara C, et al. A W-linked DM-domain gene, DM-W, participates in primary ovary development in Xenopus laevis. Proc Natl Acad Sci U S A. 2008;105(7):2469–74.
Zarkower D. Somatic sex determination (February 10, 2006), WormBook, ed. The C. elegans Research Community, WormBook, doi:10.1895/wormbook.1.84.1, http://www.wormbook.org.
Zhao L, Koopman P. SRY protein function in sex determination: thinking outside the box. Chromosom Res. 2012;20(1):153–62.
Zhu L, Wilken J, Phillips NB, Narendra U, Chan G, Stratton SM, et al. Sexual dimorphism in diverse metazoans is regulated by a novel class of intertwined zinc fingers. Genes Dev. 2000;14(14):1750–64.
Zubair M, Ishihara S, Oka S, Okumura K, Morohashi K. Two-step regulation of Ad4BP/SF-1 gene transcription during fetal adrenal development: initiation by a Hox-Pbx1-Prep 1 complex and maintenance via autoregulation by Ad4BP/SF-1. Mol Cell Biol. 2006;26(11):4111–21.
Acknowledgments
This work was supported by Cincinnati Children’s Hospital Medical Center (CCHMC) developmental funds and a March of Dimes Basil O’Connor Starter Scholar Award to T.D.; S.J.P. and D.L.K. were also supported by a CCHMC Research Innovation and Pilot Funding Award.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this entry
Cite this entry
Potter, S.J., Kumar, D.L., DeFalco, T. (2017). Sex Determination. In: Simoni, M., Huhtaniemi, I. (eds) Endocrinology of the Testis and Male Reproduction. Endocrinology. Springer, Cham. https://doi.org/10.1007/978-3-319-44441-3_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-44441-3_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-44440-6
Online ISBN: 978-3-319-44441-3
eBook Packages: MedicineReference Module Medicine