Abstract
Emerging variants of known RNA viruses present an increasing threat to mankind worldwide through their enlarging impact on morbidity and mortality. One of them is the chikungunya disease, which becomes a major public health problem and economic threat. Current world has no approved antiviral drugs available against chikungunya infection. This Book Chapter mainly focuses on discussion of the antiviral compounds that have been reported to inhibit chikungunya virus replication. Various syntheses of antiviral agents, compounds isolated from natural sources, and some structure–activity relationships are illustrated.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Chikungunya virus (CHIKV) is an alphavirus and was first recognized as an epidemic form in East Africa in the early 1950s. Most patients of CHIKV infection suffer from severe persistent arthralgia [1]. Female mosquitoes of the species Aedes aegypti and Aedes albopictus are mainly responsible for its transmission. The dramatic turn of CHIKV history is its unexpected re-emergence in 2004, which was associated with mutations in the viral genome and a new epidemic strain emerged from the East, Central, South Africa enzootic linage [1, 2]. The outbreaks took place mainly around the Indian Ocean, in particular, the French Island of La Réunion (2005–2006), where about 300,000 cases were confirmed [1, 2]. Since then, thousands of infected travelers imported this virus to many countries of the world. As a result, it is endemic in northern Italy and southern France in 2007. Around the same timeframe, several CHIKV re-emerged incidents happened in Asia, including a local case in Singapore (2008) [3] and hundreds of cases in southern Thailand (2008–2009) [4]. In March 2011, autochthonous transmission of CHIKV was reported in New Caledonia (South Pacific Region), which is also the first report of CHIKV transmission in this region [5]. Another outbreak of autochthonous chikungunya fever with more than 10 cases occurred in Montpellier, France in October 2014 [6]. Beginning in late 2013, the virus started to spread to the Caribbean and into Central and South America, affecting people from 41 countries or more [7, 8]. According to the data of the Pan American Health Organization, about 1.3 million suspected and confirmed cases were reported in these regions by March 2015 [7]. Many factors like commercial transportation, urbanization, deforestation, climate change, have inadvertently formed environments, which brought emerging RNA virus pathogens increasing at an accelerating rate.
In 2008, chikungunya fever is listed as a category C priority pathogen by The U.S. National Institute of Allergy and Infectious Diseases [9]. Considering the global need of new antiviral therapeutics and responding to the health theme of European Union the 7th Framework Call, the Small-molecule Inhibitor Leads Versus Emerging and Neglected RNA Viruses (SILVER) project was conceived in 2010. The SILVER project, led by E. A. Gould and J.-L. Romette, include 24 international research teams and scientists from 12 countries of Europe and Asia. Furthermore, the “Global Virus Network” was initiated in 2011 to identify research gaps and opportunities, including models of infection and disease, epidemiology, candidate vaccines, vector control measures, and antivirals [7].
After being transmitted to the body, CHIKV circulates to the liver, muscle, joints, lymphoid tissue, and brain [9]. There are two phases of infections that were reported in the recent epidemic areas. The first is an acute phase, which lasts from a few days to several weeks. The symptoms include high fever, rigors, headache, photophobia, and petechial/maculopapular rash [9]. The second is a chronic phase, which shows symptoms of polyarthralgia. Although its mortality rate is low, the elderly or those with underlying chronic problems are most likely to have severe complications [9]. During the most recent epidemics in India and in Réunion Island, severe cases have been described involving encephalitis, myelopathy, peripheral neuropathy, myeloneuropathy, and myopathy [10]. Moreover, some cases of multiorgan failure and eye infections have also been reported [11].
The CHIKV belongs to the Togaviridae family and consists of a positive-sense single-stranded RNA genome of about 11.8 kb size. This genome has two open reading frames 5′ and 3′ ends. The 5′ end encodes nsP1, nsP2, nsP3, and nsP4 non-structural proteins; the 3′ end encodes the capsid (C), two glycoproteins E1, E2, and two small cleavage products (E3, 6 K) [11]. Keller et al. [12] present a detailed description of the CHIKV life cycle and identify the key viral target proteins for drug design in a perspective article.
At the present time, there is no vaccine against CHIKV infection licensed for human use. Most of the treatments are symptomatic [13]. Even worse is that the current world has no drug available against CHIKV. Four well-informative review articles covering structures and biological data have been published by Keller [12], Kaur and Chu [13], Neyts [14], Bhakat and Soliman [15], and respective co-authors. The former two are in 2013 and the latter two are in 2015. Moreover, recent review articles involving the discussion and analysis of epidemiology, pathogenesis, global virus network, or cellular mechanisms of action were published by Thiberville et al. [1], Weaver and Forrester [2], McSweegan et al. [7], Schwartz and Albert [9], Couderc and Lecuit [16], Singh and Unni [17], Birendra et al. [18], Parashar and Cherian [19], and Lum and Ng [20].
2 Compound Classes, Structures, Biological Activities, and Mechanisms of Action
In this review article, we illustrate antiviral agents on the basis of their classes of compounds, structures, synthetic routes, natural sources, biological activities, as well as structure–activity relationship (see Table 1). Emphasis will be placed on the newly developed agents reported after the year of 2013 and syntheses of artificially designed compounds. Efficacy in vivo of most of these compounds, however, has not yet been evaluated in animal models on the basis of the information reported in the original articles.
The established antiviral compounds towards CHIKV can mainly classified into five categories: purines/pyrimidines, nucleosides, alkaloids, terpenoids, and flavaglines. Their characteristics and biological data are illustrated as follows. The table contains information of newly developed antiviral agents reported during the past three years and some established compounds studied earlier for comparison.
-
A. Purines and Pyrimidines
D’hooghe, De Kimpe, and coworkers [21] synthesized a series of purine derivatives, of which antiviral activities were screened against nine different viruses including CHIKV. In 2012, they reported purine β–lactam 1 and purine–aminopropanol 2 with symptom against CHIKV. The key steps for their synthesis shown in Scheme 1 include a Staudinger [2 + 2] cyclocondensation between a Schiff base and a ketene (from PhOCH2COCl) to give a (ω-bromoalkyl)-cis-β-lactam with diastereoselectivity. Then N-alkylation of a purine derivative with this β-lactam intermediate gives the desired purine–β-lactam 1. Furthermore, a LiEt3BH-mediated β-lactam ring opening takes place to produce the target purine 2.
In 2015, Hwu, Tsay, Neyts, and co-workers [22] reported the design and synthesis of a new series of uracil–coumarin–arene conjugates against CHIKV. Five of 22 new hybrid conjugates can inhibit CHIKV in Vero cells with significant potency and low toxicity. As shown in Scheme 2, their synthesis includes a coupling reaction to form a coumarin derivative, its condensation with an organosulfonyl chloride to give sulfonylated intermediate, and a selective S-alkylation of 2-thiobenzouracil at the allylic position of (coumarinyl)chloride to yield the desired triply conjugated target 3. Its molecular framework is determined unambiguously by single X-ray diffraction analysis.
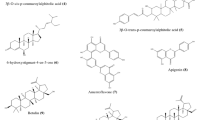
The coumarin moiety, –SCH2–, and –SO2– (but not –CH2–) joints in the conjugated compounds shown in Fig. 1 are essential to their antiviral activity. Use of either an Me or an NO2 group attached to the arene moiety brings enhanced activity to the target molecules. When coumarin–arenes are conjugated with benzouracil, the resultant hybrids (such as 3) exhibit better selectivity indexes than their kin with uracil or 5-methyluracil.
In 2014, Pérez–Pérez et al. [23] identified [1,2,3]triazolo[4,5-d]pyrimidin-7(6H)-ones as active inhibitors of CHIKV replication in the low micromolar range with no cytotoxicity detected up to 668 μM. The synthetic procedure for the most active compounds 4b as shown in Scheme 3 includes (3 + 2) cycloaddition between an arylazide and a cyanoacetamide to give a 5-aminotriazole amide. Subsequent condensation followed by ring formation produces the target triazolopyrimidine 4b.
-
B. Nucleosides
During the past five decades, nucleosides have been used in clinics and, nowadays, become cornerstones of treatment for patients with viral infections. Two drugs in this family were reported with anti-CHIKV activity in 2004 [24]. Ribavirin (5), a “synthetic nucleoside” containing a 1H-1,2,4-triazole moiety, is effective against a variety of RNA viruses, especially in the genus Alphavirus. The combination of ribavirin and interferon-α shows a synergistic anti-chikungunya viral effect [24]. Several studies have elucidated the mechanisms of anti-viral action of ribavirin. They involve predominantly inhibition of inosine monophosphate dehydrogenase (IMPDH) activity, depletion of the intracellular guanosine triphosphate (GTP) pools, inhibition of viral RNA capping, and induction of an error catastrophe [14, 37–39].
6-Azauridine (6) [24], containing a 1,2,4-triazine-3,5(2H,4H)-dione moiety, is an another “synthetic nucleoside” with a broad-spectrum of anti-metabolite. It inhibits both DNA and RNA virus replication. In comparison with ribavirin (5), 6-azauridine (6) shows a greater potency against CHIKV with EC50 = 0.82 µM and SI = 254. Its activity might be through the inhibition of orotidine monophosphate decarboxylase activity and the depletion of uridine triphosphate pools [40].
-
C. Alkaloids
Favipiravir (7) is a pyrazinecarboxamide derivative, which was discovered and synthesized by Toyama Chemical Co. in Japan as a candidate antiviral drug [41]. It is active against many viruses, including influenza viruses, West Nile virus, yellow fever virus, foot-and-mouth disease virus, flaviviruses, arenaviruses, bunyaviruses, alphaviruses, picornavirus and norovirus [41, 42]. In 2014, favipiravir (7) was approved in Japan for the treatment of influenza virus disease. The mechanism of its action is related to the selective inhibition of viral RNA-dependent RNA polymerase. In the same year, Neyts et al. [25] disclosed that favipiravir (7) inhibits viral genome replication of laboratory strains and clinical isolates of CHIKV.
The antiviral drug umifenovir (i.e., arbidol, 8), an indole derivative, was originally developed at the Research Institute of Pharmaceutical Chemistry in Russia about three decades ago [43]. Since 1990, this drug has been used in Russia mainly for the intervention of prophylaxis and acute respiratory infections like influenza [44, 45]. In 2011, Pastorino et al. [26] reported that umifenovir (8) presents potent inhibitory activity against CHIKV. The significant anti-viral activity of this drug may be attributed to the diverse mechanisms of action, including interference with the early stages of CHIKV attachment or entry or the replication cycle, as well as alterations of cellular membranes [44, 46]. A synthetic procedure leading to umifenovir (8) as illustrated in Scheme 4 includes seven steps, for which the key steps are the Friedel–Crafts alkylation, reductive cyclization, and the Mannich condenation [43, 47]. On the other hand, this drug can also be produced through four steps as shown in the synthetic route in Scheme 5. They involve Nenitzescu indole synthesis, acylation/bromination, S-alkylation, and the Mannich condensation [48, 49].
Recently, de Lamballerie, Jayaprakash et al. [27] reported a series of aryl alkylidene alkaloids, among which 1,3-thiazolidin-4-ones 9 and 10 showed anti-chikungunya activity with IC50 values of 0.42 and 6.8 μM. These two compounds can be synthesized by simple steps shown in Scheme 6 [50, 51]. The key step is the Knoevenagel condensation. Moreover, the authors performed molecular docking simulation of the active compound 9 with the X-ray crystal structure of CHIKV nsP2 protease. As a result, the mechanism of action may come from the protease inhibition.
Harringtonine (11) is a natural alkaloid isolated from the Japanese plum yew, Cephalotaxus harringtonia in only 0.0064% yield [52]. In 2013, Chu et al. [28]. reported that it exhibits potent anti-CHIKV activity with an EC50 value of 0.24 μM with minimal cytotoxicity. Harringtonine inhibits an early stage of the CHIKV replication cycle, affects CHIKV RNA production, and interferes with viral protein expression.
-
D. Terpenoids
As a major class of natural compounds, terpenoids display a wide range of biological activity against a variety of infectious diseases. The four representatives shown in Table 1 contribute significantly to the anti-CHIKV development.
In 2014, Litaudon et al. [29] reported their isolation of diterpene jatrophane ester (12) in 0.0006% yield from the whole plant of Euphorbia amygdaloides ssp. Semiperfoliata, an endemic plant of Corsica and Sardinia. Jatrophane ester (12) shows an EC50 value of 0.76 μM with a high SI value of 208.
In the same year, Chu et al. [30] disclosed their isolation of two aplysiatoxin-related compounds 13a,b from the marine cyanobacterium Trichodesmium erythraeum in ~0.0022% yield. These two 12-membered ring terpenes exhibit significant anti-CHIKV activity in post-treatment of infected SJCRH30 cells with EC50 values of 1.3 and 2.7 μM, respectively. Their potency is on the same order as that of the highly-oxygenated natural trigocherrin A (14) [31]. This chlorinated daphnane diterpene orthoester was isolated from the bark of Trigonostemon cherrieri in 0.00017% yield, an endemic plant of New Caledonia [53]. Nevertheless, they are much less potent in comparison with 12-O-tetradecanoylphorbol 13-acetate (15) [54], which was reported by Litaudon et al. in 2012 [32]. Tetradecanoyl phorbol acetate 15 presents an EC50 value of 2.9 nM with a very high SI value of 1965. The activation of the signal transduction enzyme protein kinase C could be the mechanism of action on its anti-CHIKV activity.
-
E. Flavagline
Plant natural products flavaglines are characterized with a unique cyclopenta [b]benzofuran nuclues. Fused benzofurans display an array of biological effects as insecticidal, antifungal, anti-inflammatory, and neuroprotective agents [55]. In 2015, Smith et al. [33] reported that flavaglines FL23 (16) and FL3 (17) inhibit the CHIKV by interaction with prohibitin-1, which is a receptor protein used by the virus to enter mammalian cells. The synthetic route to give flavaglines FL3 (±)-(17) is shown in Scheme 7. The key steps include the Friedel–Craft acylative cyclization, the Michael addition, and Evans–Saksena reduction reactions [56, 57].
-
Others
Apart from the above classes of compounds, Freitas–Junior et al. [34] screened a kinase inhibitor library of 4000 compounds against CHIKV infection by using high throughput screening. In 2013, they reported that four benzofuran derivatives were active as inhibitors associated with CHIKV cell death in a dose-dependent manner. Compounds with a scaffold as benzofuran 18 (CND0335) exhibit the EC50 values of between 2.2 and 7.1 μM.
Brancale et al. [35] reported their computer-aided identification and design of a series of (α-carbony)hydrazones with selective activity against CHIKV. They initially obtained the hit candidates from a virtual screening simulation of ~5 million compounds on the CHIKV nsP2. After investigation of their structure–activity relationship in silico and optimization of the candidate compounds, a simplified chemical structure (α-carbonyl)hydrazone 19 was attained. Synthesis of compound 19 is illustrated in Scheme 8, in which sequential Knoevenagel–Doebner reaction and condensation reactions are in operation. This compound indeed exhibits promising activity profile as shown in Table 1.
In 2015, van Hemert et al. [36] first reported that the approved anti-parasitic drug suramin (20b) inhibits CHIKV RNA synthesis with an IC50 value of ~5 μM. It also inhibits replication of various CHIKV isolates in cell culture with an EC50 of 79 μM and CC50 >800 µM. Furthermore, suramin can inhibit a post-attachment early step of the CHIKV replicative cycle and (re)initiation of CHIKV RNA synthesis by possibly interfering with binding of the template RNA. These findings are in agreement with the previous studies in vitro that suramin inhibits RNA viral polymerases and helicases [36]. Very soon, closely related results on inhibition of the same virus entry and transmission by suramin (20b) is reported by Kuo, Lin et al. [58].
A series of suramin derivatives (e.g., 20a) were synthesized by Bolognesi, Hwu et al. [59] based on the design of compounds with fewer sulfonate fingers, shorter arms, only one side, or no neck in comparison with suramin. As depicted in Scheme 9, the representative procedure includes amide formation, followed by reduction of nitro compounds and carbonylation. These suramin derivatives were also tested for their ability to inhibit CHIKV RNA synthesis in vitro. Unsymmetrical compounds possessing only one arm were inactive regardless of its length. It has been proved that compound with six sulfonate groups showed greater anti-CHIKV (EC50 = 79 µM) than tetrasulfonate (EC50 = 210 µM) in the cell culture (see Table 1 and Fig. 2).
3 Concluding Remarks
Development of new antiviral compounds for chikungunya fever meets an urgent need of global societies. It is due to the re-emerged outbreaks occurring in 2004 and recent spreading to the Americas in late 2013. A limited number of natural products exhibit great potency with an appealing selective index value. Unfortunately, their isolation yields are often very low, as represented by the naturally occurring 12-O-tetradecanoylphorbol 13-acetate (15) obtained in 0.00017% yield. Complex structures associated with these natural products with multiple stereogenic centers, various functional groups, and several rings make their total synthesis very challenging.
As a result, medicinal chemists, biologists, and virologists with interdisciplinary expertise have been seeking for unnatural targets that can be obtained in a large quantity by chemical synthesis. By far, various types of compounds with anti-CHIKV activity have been obtained; among which the ones reported recently are listed in the Table 1. These compounds belong to purine/pyrimidine, nucleoside, alkaloid, flavagline, etc. Nevertheless, none of them has been yet approved as a drug to serve the purpose. The opportunity remains high for scientists to devote their efforts to design and synthesize small molecules to fight for the human battle against CHIKV with success.
Moreover, some molecules in the compound libraries of the Table 1, such as benzouracil–coumarin–arene conjugates, suramin derivatives et al. may be suitable for their development to become potential new drugs for the treatment of neurodegenerative disorders. Multi-functional design, in silico computational screening, in vitro/ex vivo/in vivo experiments, and organic syntheses of these novel candidate compounds are in progress.
References
Thiberville S-D, Moyen N, Dupuis–Maguiraga L, Nougairede A, Gould EA, Roques P, de Lamballerie X (2013) Chikungunya fever: epidemiology, clinical syndrome, pathogenesis and therapy. Antiviral Res 99:345–370.
Weaver SC, Forrester NL (2015) Chikungunya: evolutionary history and recent epidemic spread. Antiviral Res 120:32–39.
Leo YS, Chow ALP, Tan LK, Lye DC, Lin L, Ng LC (2009) Chikungunya outbreak, Singapore, 2008. Emerg Infect Dis 15:836–837.
Rianthavorn P, Prianantathavorn K, Wuttirattanakowit N, Theamboonlers A, Poovorawan Y (2010) An outbreak of chikungunya in southern Thailand from 2008 to 2009 caused by African strains with A226 V mutation. Int J Infect Dis 14S:e161–e165.
Dupont–Rouzeyrol M, Caro V, Guillaumot L, Vazeille M, D’Ortenzio E, Thiberge J-M, Baroux N, Gourinat A-C, Grandadam M, Failloux A-B (2012) Chikungunya virus and the mosquito vector Aedes aegypti in New Caledonia (South Pacific region). Vector Borne Zoonotic Dis 12:1036–1041.
Delisle E, Rousseau C, Broche B, Leparc–Goffart I, L’Ambert G, Cochet A, Prat C, Foulongne V, Ferré JB, Catelinois O, Flusin O, Tchernonog E, Moussion IE, Wiegandt A, Septfons A, Mendy A, Moyano MB, Laporte L, Maurel J, Jourdain F, Reynes J, Paty MC, Golliot F (2015) Chikungunya outbreak in Montpellier, France, September to October 2014. Euro Surveill 20: pii=21108.
McSweegan E, Weaver SC, Lecuit M, Frieman M, Morrison TE, Hrynkow S (2015) The global virus network: challenging chikungunya. Antiviral Res 120:147–152.
Scholte FEM, Tas A, Albulescu IC, Žusinaite E, Merits A, Snijder EJ, van Hemert MJ (2015) Stress granule components G3BP1 and G3BP2 play a proviral role early in chikungunya virus replication. J Virol 89: 4457–4469.
Schwartz O, Albert ML (2010) Biology and pathogenesis of chikungunya virus. Nat Rev Microbiol 8: 491–500.
Chandak NH, Kashyap RS, Kabra D, Karandikar P, Saha SS, Morey SH, Purohit HJ, Taori GM, Daginawala HF (2009) Neurological complications of chikungunya virus infection. Neurol India 57: 177–180.
Pialoux G, Gaüzère BA, Jauréguiberry S, Strobel M (2007) Chikungunya, an epidemic arbovirosis. Lancet Infect Dis 7: 319–327.
Rashad AA, Mahalingam S, Keller PA (2014) Chikungunya virus: emerging targets and new opportunities for medicinal chemistry. J Med Chem 57: 1147–1166.
Kaur P, Chu JJH (2013) Chikungunya virus: an update on antiviral development and challenges. Drug Discov Today 18: 969–983.
Abdelnabi R, Neyts J, Delang L (2015) Towards antivirals against chikungunya virus. Antiviral Res 121: 59–68.
Bhakat S, Soliman MES (2015) Chikungunya virus (CHIKV) inhibitors from natural sources:a medicinal chemistry perspective. J Nat Med 69: 451–462.
Couderc T, Lecuit M (2015) Chikungunya virus pathogenesis: from bedside to bench. Antiviral Res 121: 120–131.
Singh SK, Unni SK (2011) Chikungunya virus: host pathogen interaction. Rev Med Virol 21: 78–88.
Birendra VK, Vishwabhan S, Uttam J, Vishal S (2012) A review on chikungunya virus. IRJP 3: 58–60.
Parashar D, Cherian S (2014) Antiviral perspectives for chikungunya virus. BioMed Research International http://dx.doi.org/10.1155/2014/631642.
Lum FM, Ng LFP (2015) Cellular and molecular mechanisms of chikungunya pathogenesis. Antiviral Res 120: 165–174.
D’hooghe M, Mollet K, Vreese RD, Jonckers THM, Dams G, De Kimpe N (2012) Design, synthesis, and antiviral evaluation of purine–β-lactam and purine–aminopropanol hybrids. J Med Chem 55: 5637–5641.
Hwu JR, Kapoor M, Tsay S-C, Lin C-C, Hwang KC, Horng J-C, Chen I-C, Shieh F-K, Leyssen P, Neyts J (2015) Benzouracil–coumarin–arene conjugates as inhibiting agents for chikungunya virus. Antiviral Res 118: 103–109.
Gigante A, Canela M-D, Delang L, Priego E-M, Camarasa M-J, Querat G, Neyts J, Leyssen P, Pérez–Pérez M-J (2014) Identification of [1,2,3]Triazolo[4,5-d]pyrimidin-7(6H)-ones as novel inhibitors of chikungunya virus replication. J Med Chem 57: 4000–4008.
Briolant S, Garin D, Scaramozzino N, Jouan A, Crance JM (2004) In vitro inhibition of Chikungunya and Semliki Forest viruses replication by antiviral compounds: synergistic effect of interferon-α and ribavirin combination. Antiviral Res 61: 111–117.
Delang L, Guerrero NS, Tas A, Quérat G, Pastorino B, Froeyen M, Dallmeier K, Jochmans D, Herdewijn P, Bello F, Snijder EJ, de Lamballerie X, Martina B, Neyts J, van Hemert MJ, Leyssen P (2014) Mutations in the chikungunya virus non-structural proteins cause resistance to favipiravir (T-705), a broad-spectrum antiviral. J Antimicrob Chemother 69: 2770–2784.
Delogu I, Pastorino B, Baronti C, Nougairède A, Bonnet E, de Lamballerie X (2011) In vitro antiviral activity of Arbidol against chikungunya virus and characteristics of a selected resistant mutant. Antiviral Res 90: 99–107.
Jadav SS, Sinha BN, Hilgenfeld R, Pastorino B, de Lamballerie X, Jayaprakash V (2015) Thiazolidone derivatives as inhibitors of chikungunya virus. Eur J Med Chem 89: 172–178.
Kaur P, Thiruchelvan M, Lee RCH, Chen H, Chen KC, Ng ML, Chu JJH (2013) Inhibition of chikungunya virus replication by harringtonine, a novel antiviral that suppresses viral protein expression. Antimicrob Agents Chemother 57: 155–167.
Nothias–Scaglia L-F, Retailleau P, Paolini J, Pannecouque C, Neyts J, Dumontet V, Roussi F, Leyssen P, Costa J, Litaudon M (2014) Jatrophane diterpenes as inhibitors of chikungunya virus replication: structure–activity relationship and discovery of a potent lead. J Nat Prod 77: 1505–1512.
Gupta DK, Kaur P, Leong ST, Tan LT, Prinsep MR, Chu JJH (2014) Anti-chikungunya viral activities of aplysiatoxin-related compounds from the marine cyanobacterium trichodesmium erythraeum. Mar Drugs 12: 115–127.
Allard P-M, Leyssen P, Martin M-T, Bourjot M, Dumontet V, Eydoux C, Guillemot J-C, Canard B, Poullain C, Guéritte F, Litaudon M (2012) Antiviral chlorinated daphnane diterpenoid orthoesters from the bark and wood of Trigonostemon cherrieri. Phytochemistry 84: 160–168.
Bourjot M, Delang L, Nguyen VH, Neyts J, Guéritte F, Leyssen P, Litaudon M (2012) Prostratin and 12-O-tetradecanoylphorbol 13-acetate are potent and selective inhibitors of chikungunya virus replication. J Nat Prod 75: 2183–2187.
Wintachai P, Thuaud F, Basmadjian C, Roytrakul S, Ubol S, Désaubry L, Smith DR (2015) Assessment of flavaglines as potential chikungunya virus entry inhibitors. Microbiol Immunol 59: 129–141.
Cruz DJM, Bonotto RM, Gomes RGB, da Silva CT, Taniguchi JB, No JH, Lombardot B, Schwartz O, Hansen MAE, Freitas-Junior LH (2013) Identification of novel compounds inhibiting chikungunya virus–induced cell death by high throughput screening of a kinase inhibitor library. PLoS Negl Trop Dis 7: e2471.
Bassetto M, Burghgraeve TD, Delang L, Massarotti A, Coluccia A, Zonta N, Gatti V, Colombano G, Sorba G, Silvestri R, Tron GC, Neyts J, Leyssen P, Brancale A (2013) Computer-aided identification, design and synthesis of a novel series of compounds with selective antiviral activity against chikungunya virus. Antiviral Res 98: 12–18.
Albulescu IC, van Hoolwerff M, Wolters LA, Bottaro E, Nastruzzi C, Yang SC, Tsay S-C, Hwu JR, Snijder EJ, van Hemert MJ (2015) Suramin inhibits chikungunya virus replication through multiple mechanisms. Antiviral Res 121:39–46.
Leyssen P, De Clercq E, Neyts J (2006) The anti-yellow fever virus activity of ribavirin is independent of error-prone replication. Mol Pharmacol 69: 1461–1467.
Paeshuyse J, Dallmeier K, Neyts J (2011) Ribavirin for the treatment of chronic hepatitis C virus infection: a review of the proposed mechanisms of action. Curr Opin Virol 1: 590–598.
Rothan HA, Bahrani H, Mohamed Z, Teoh TC, Shankar EM, Rahman NA, Yusof R, (2015) A combination of doxycycline and ribavirin alleviated chikungunya infection. PLoS ONE 10: e0126360.
Rada B, Dragun M (1977) Antiviral action and selectivity of 6-azauridine. Ann NY Acad Sci 284: 410–417.
Furuta Y, Takahashi K, Shiraki K, Sakamoto K, Smee DF, Barnard DL, Gowen BB, Julander JG, Morrey JD (2009) T-705 (favipiravir) and related compounds: novel broad-spectrum inhibitors of RNA viral infections. Antiviral Res 82: 95–102.
Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL (2013) Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res 100: 446–454.
Panisheva EK, Nikolaeva IS, Galenko-Yaroshevskii PA, Bartashevich VV, Cherkasova AA, Linchenko SN, Egik’yan AL, Golovanova EA, Pushkina TV (1988) Synthesis and biological activity of substituted 5-hydroxy-6-bromoindoles. Khim-Farm Zh 22: 565–569.
Boriskin YS, Leneva IA, Pécheur E-I, Polyak SJ (2008) Arbidol: a broad-spectrum antiviral compound that blocks viral fusion. Curr Med Chem 15: 997–1005.
Leneva IA, Russell RJ, Boriskin YS, Hay AJ (2009) Characteristics of Arbidol-resistant mutants of influenza virus: implications for the mechanism of anti-influenza action of Arbidol. Antiviral Res 81: 132–140.
Blaising J, Polyak SJ, Pécheur E-I (2014) Arbidol as a broad-spectrum antiviral:an update. Antiviral Res 107: 84–94.
Trofimov FA, Tsyshkova NG, Zotova SA, Grinev AN (1993) Synthesis of a new antiviral agent, Arbidole. Khim-farm Zh 27: 70–71.
Barraja P, Diana P, Montalbano A, Martorana A, Carbone A, Cirrincione G (2009) Synthesis of the new ring system 2-oxo-[1,4]oxazino[3,2-e]indole, heteroanalogue of Angelicin. Tetrahedron Lett 50: 4182–4184.
Chai H, Zhao Y, Zhao C, Gong P (2006) Synthesis and in vitro anti-hepatitis B virus activities of some ethyl 6-bromo-5-hydroxy-1H-indole-3-carboxylates. Bioorg Med Chem 14: 911–917.
Harada K, Kubo H, Abe J, Haneta M, Conception A, Inoue S, Okada S, Nishioka K (2012) Discovery of potent and orally bioavailable 17β-hydroxysteroid dehydrogenase type 3 inhibitors. Bioorg Med Chem 20: 3242–3254.
Al-Ansary GH, Ismail MAH, Ella DAAE, Eid S, Abouzid KAM (2013) Molecular design and synthesis of HCV inhibitors based on thiazolone scaffold. Eur J Med Chem 68: 19–32.
Powell RG (1972) Structures of homoerythrina alkaloids from cephalotaxus harringtonia. Phytochemistry 11: 1467–1472.
Allard P-M, Martin M-T, Tran Huu Dau M-E, Leyssen P, Guéritte F, Litaudon M (2012) Trigocherrin A, the first natural chlorinated daphnane diterpene orthoester from Trigonostemon cherrieri. Org Lett 14: 342–345.
El-Mekkawy S, Meselhy MR, Nakamura N, Hattori M, Kawahata T, Otake T (2000) Anti-HIV-1 phorbol esters from the seeds of Croton tiglium. Phytochemistry 53: 457–464.
Ribeiro N, Thuaud F, Nebigil C, Désaubry L (2012) Recent advances in the biology and chemistry of the flavaglines. Bioorg Med Chem 20: 1857–1864.
Bruce I, Cederbaum F, Cooke NG, Diorazio LJ, Dobler M, Hall RG, Szczepanski H (2000) Cyclopentabenzofuran-derivatives. Ger Offen DE 199 34 952 A 1 3 Feb 2000.
Thuaud F, Bernard Y, Turkeri G, Dirr R, eve Aubert G, Cresteil T, Baguet A, Tomasetto C, Svitkin Y, Sonenberg N, Nebigil CG, Désaubry L (2009) Synthetic analogue of rocaglaol displays a potent and selective cytotoxicity in cancer cells: involvement of apoptosis inducing factor and caspase-12. J Med Chem 52: 5176–5187.
Ho YJ, Wang YM, Lu JW, Wu TY, Lin LI, Kuo SC, Lin CC (2015) Suramin inhibits chikungunya virus entry and transmission. PLoS ONE 10: e0133511.
Croci R, Pezzullo M, Tarantino D, Milani M, Tsay S-C, Sureshbabu R, Tsai YJ, Mastrangelo E, Rohayem J, Bolognesi M, Hwu JR (2014) Structural bases of norovirus RNA dependent RNA polymerase inhibition by novel suramin-related compounds. PLoS ONE 9: e91765.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Hwu, J.R. et al. (2017). Antiviral Agents Towards Chikungunya Virus: Structures, Syntheses, and Isolation from Natural Sources. In: Tomioka, K., Shioiri, T., Sajiki, H. (eds) New Horizons of Process Chemistry. Springer, Singapore. https://doi.org/10.1007/978-981-10-3421-3_19
Download citation
DOI: https://doi.org/10.1007/978-981-10-3421-3_19
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-3420-6
Online ISBN: 978-981-10-3421-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)