Abstract
By definition, cytokines are the first messengers of intercellular communications observed among leukocytes. Numerous cytokines control immune system and biological reactions thereof, but are functionally grouped into pro- and anti-inflammatory varieties (the latter are also involved in allergic reactions). The bulk of evidence points to substantial role played by cytokines in skeletal muscle growth and wasting. Cytokines and growth factors of immune origin affect skeletal muscle growth and organ formation, regeneration, and wasting but are also produced and secreted by muscle fibers as myokines. To orchestrate skeletal muscle growth, hepatocyte growth factor/scatter factor (HGF/SF) and insulin-like growth factors (IGF-I and IGF-II) play the primary physiological role being mediated through PI3-K/Akt signaling pathway. Skeletal muscle mass is in turn controlled negatively by myostatin, a member of transforming growth factor beta (TGF-β) superfamily. Following muscle injury, the immune-derived cytokines are most important in activation of muscle satellite cells: tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6); with IL-8 to arrange growth/regeneration; and IL-15 to control muscle hypertrophy. Some life-threatening diseases are associated with muscle wasting featured by accelerated muscle protein breakdown. In these catabolic states, cytokines such as TNF-α was often reported as causal factor. Moreover, cross talk between myokines (IL-6, IL-15) and adipokines (leptin) is vital for correct metabolic interorgan relations. Thus, cytokines and growth factors exist as basic chemical signals that orchestrate skeletal muscle fate in normal and diseased states.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Cytokines in Skeletal Muscle Myogenesis and Somatic Growth
In vertebrates skeletal muscles develop during embryonic life from paraxial mesoderm (dermomyotome, myotome) specified to the myogenic lineage by paracrine signals from surrounding tissues. Wingless-related integration sites (Wnts), sonic hedgehog (SHH), and Noggin (NOG) stimulate, whereas bone morphogenetic protein 4 (BMP4) inhibits the commitment to the myogenic lineage. Pax-3 and Pax-7 are paired box genes targeted and co-expressed in the myotomal cells (epithelial spheres of paraxial mesoderm) [166]. Next, sequential activation of basic helix-loop-helix transcription regulators known as myogenic regulatory factors (MRFs) determines muscle progenitors termed myoblasts. Initially, MyoD and/or Myf5 gene expressions are upregulated followed by Myogenin and/or MRF4 gene activation. The latter are essential for induction of muscle-specific genes and terminal differentiation of mononuclear myoblasts [16]. Some of muscle-specific gene expressions such as myosin heavy chain (MyHC) or muscle creatine kinase (MCK) are valuable skeletal muscle cell markers. Dividing myogenic cells express MyoD/Myf5 (myoblasts); subsequently they withdraw from the cell cycle to fuse upon Myogenin and/or MRF4 control. Replication of activated satellite cells is possible by elevated levels of cyclin D, whereas terminal differentiation is upregulated by Myogenin gene expression [144]. Finally multinucleated cell syncytium (myotubes) is formed before establishing the fully mature contracting skeletal muscle fiber. Some myoblasts do not differentiate and remain dormant (satellite cells) embedded to the plasma membrane of contracting muscle fibers. After asymmetric cell division, they form a subpopulation apt to regenerate damaged muscle [46].
The major role in postnatal skeletal muscle growth is played by the growth hormone (GH)-IGF axis. Receptors for GH are present in sarcolemma, although direct binding of GH to muscle cells has never been demonstrated. In contrary to GH, IGF-I and IGF-II are able to alter MRF expression and promote muscle growth by increasing the proliferation and the differentiation of myoblasts [4, 38]. IGFs augment muscle mass through elevated DNA and protein synthesis [121, 158]. Increased number (hyperplasia) and fiber size (hypertrophy) are attributed to activation of satellite cells that in turn supply myonuclei to existing or newly formed muscle fibers [18, 19]. The hypertrophic effect of IGF-I differs from the general meaning of hypertrophy, as it is not barely increase in cytoplasmic-to-DNA volume ratio. IGFs are upregulated in skeletal muscle undergoing regeneration [48, 102], and these cytokines ameliorate muscle progenitor cells’ (MPCs) viability and myogenic potential [95, 96]. Muscle growth and regeneration controlled by IGFs have distinct time windows, IGF-I being induced before IGF-II during muscle regeneration. In addition, IGFs’ activity brought about in target cells is altered by local growth factor availability modulated by IGF-binding proteins (IGFBPs) [207, 208]. Furthermore, by suppressing the expression of muscle-specific atrophy-related ubiquitin ligases, muscle RING-finger protein 1 (MuRF1) and muscle atrophy F-Box/atrogin 1 (MAFbx/atrogin 1), IGF-I inhibits protein degradation and protects skeletal muscle mass from loss. IGF-I differs from other growth factors as it stimulates both proliferation and differentiation of muscle cells [59]. Several existing isoforms of IGF-I (IGF-IEa, IGF-IEb, MGF) suggest distinct tasks played by each isoform and give possible explanation for apparently opposite roles played by IGF-I in muscle growth. Mechano growth factor (MGF) is believed to stimulate myogenic cell proliferation, while IGF-IEa seems to be decisive for terminal differentiation and muscle cell fusion [216]. It is less clear how IGF-II affects skeletal muscle growth, even though it is known that it acts on target cells through IGF-IR (no functional IGF-IIR was found) by autocrine and/or paracrine route. IGF-II was reported to accelerate terminal differentiation of C2C12 murine myoblasts in vitro and has some importance in prenatal skeletal muscle development [161]. Thus, IGF-I and IGF-II activate distinct signaling cascades, with IGF-II eliciting a stronger differentiation effect correlated with downregulation of Gαi2 protein [160]. Insulin, the main anabolic hormone in postnatal life, has numerous actions on target cells with one that mimics IGF-I-dependent skeletal muscle growth-promoting effect. Similar to IGF-I, insulin encourages skeletal myogenesis through activation of membrane receptor-associated phosphatidylinositol 3-kinase PI3-K/Akt signaling cascade that in turn inhibits glycogen synthase kinase 3-beta GSK-3β and forkhead box protein O1 (FoxO1) [104].
5.2 Skeletal Muscle Repair and Regeneration
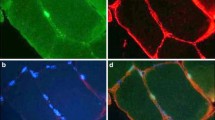
Regardless of injurious agent, any severe skeletal muscle damage starts the series of events that recapitulate myogenesis. Skeletal muscle regeneration is a highly coordinated process that absorbs adult muscle satellite cells to proliferate and differentiate. Following muscle injury, several biologically active substances are released, such as molecules from the injured fibers, soluble factors from connective tissue, and, finally, cytokines from infiltrating leukocytes. Some of them have been identified as trophic factors: fibroblast growth factor (FGF) and transforming growth factor beta (TGF-β) families, IGFs, hepatocyte growth factor/scatter factor (HGF/SF), tumor necrosis factor alpha (TNF-α), ciliary neurotrophic factor IL (interleukin)-6 (IL-6), and leukemia inhibitory factor (LIF). There are also neural-derived low molecular weight components including nitric oxide (NO) and ATP. Not only extracellularly released factors, but cell-cell and cell-extracellular matrix (ECM) communications are decisive to instigate muscle repair [49, 101, 182]. Disruption of muscle fiber integrity (necrosis) is critically important in the inflammatory response and recruitment of satellite cells to the site of injury (degeneration phase). Disturbed calcium homeostasis activates phospholipase A2 with arachidonic acid being converted to prostaglandins and thromboxanes. Sarcoplasmic proteins released through discontinued sarcolemma, as well as inflammatory mediators, are believed to be chemoattractants for resident-activated mononucleated inflammatory and muscle satellite cells (regeneration phase). Next, chemotactic signals attract circulating inflammatory cells. Reconstruction of injured muscle is a highly orchestrated process driven by chemical signals that grant the balance between pro- and anti-inflammatory factors. To fully resolve damage with newly formed or reconstituted muscle fibers, the kinetics of order of each phase (degeneration vs. regeneration) must be precisely synchronized. The magnitude of inflammatory reaction is highest within 1–4 days after muscle injury and lasts for another week or more [34]. Finally, after successive 3 weeks, skeletal muscle is fully restored morphologically and functionally. The exact timetable of regeneration is based on the observations obtained from laboratory animal experiments carried out after intramuscular myotoxin injections [42, 75, 76], crushing and/or freezing the muscle [98, 99], single muscle autologous transplantation, or after repeated bouts of eccentric exercise [15]. Alternatively, animal models of skeletal muscle dystrophies were in extensive use as they give opportunity to monitor the degeneration/regeneration process continuously. The mdx mouse line, i.e., the animal model of human Duchenne muscular dystrophy (DMD), is caused by deficiency of functional dystrophin protein, a fundamental component of dystrophin-glycoprotein complex (DGC). Truncated form of dystrophin renders skeletal muscle highly susceptible to contraction-induced injury as links between cytoskeleton and ECM are weak and during exercise sarcolemma is ruptured leading to myofiber necrosis [31]. From this and other mouse models established to study consequences of skeletal muscle damage, it is clear that, e.g., fibroblast growth factor (FGF) limits the efficiency of muscle regeneration [12]. Several FGFs have been shown as potent MPC mitogens and inhibitors of differentiation [4, 47, 70, 83, 98, 99, 112, 214]. Apparently, the main task of FGFs is to expand the MPC compartment. Among FGFs, the FGF-6 isoform is of particular interest, as this growth factor is specific for skeletal muscle and it is elevated during regeneration phase [43, 60, 83]. Similarly to FGF-6, the mitogenic effect was observed for FGF-2 and FGF-4 indicating possible redundancy among FGFs. Although FGFs stimulate satellite cell proliferation and muscle regeneration, the exact nature of FGF-induced skeletal muscle regeneration is still debated and some authors even point to improved revascularization as a cause, as FGFs are also highly angiogenic [100]. Physiological effects of FGFs are mediated through cognate transmembrane FGF receptors (FGFR 1–4) with the FGF-1R known to be the most prominent in skeletal muscle cells [162]. FGFRs possess intrinsic tyrosine kinase domain typical to mediate trophic actions of cytokines and growth factors. One has to admit that picture is more complex as ECM limits the access of several molecules to the plasma membrane receptors. Thus, it is obvious that availability of FGFs and other cytokines to regulate skeletal muscle growth and regeneration is altered by the composition of ECM. Heparan sulfate proteoglycans (HSPGs) are the most important for local modulation of signals from muscle and non-muscle cells. The synergy between FGFs and HGF/SF in MPC activation is believed to be dependent on HSPGs, as heparan sulfate assists in c-Met (tyrosine kinase domain) transmembrane receptor activation and amplifies downstream cellular signaling pathway [154]. For FGFRs the most potent HSPGs are syndecans because syndecan-3 and syndecan-4 were identified on membrane surfaces of dormant and stimulated satellite cells [39]. Thus, MPCs are prepared by ECM for early activation by FGFs and/or HGF/SF, and changes in ECM composition (especially protein sulfation) may considerably impair MPC activation. With regard to HGF/SF, this growth factor is known to play substantial role in primary and secondary organogenesis in prenatal as well as organ regeneration in postnatal life [122, 123, 219]. Injured or crushed muscle is a rich source of HGF/SF, as both HGF transcript and the peptide are elevated at the onset of muscle regeneration [185]. It is not only a potent mitogen related to serine protease plasminogen (precursor peptide has a catalytic inactive serine protease homology domain which is cleaved through proteases like plasminogen activator), but it also stimulates morphogenesis and cell migration (scattering) [163]. HGF/SF is the ligand for proto-oncogene c-Met, plasma transmembrane receptor that contains the tyrosine kinase domain liable for autophosphorylation on tyrosine residues [118]. It plays dual role in myogenesis, as it encourages muscle satellite cells to divide while it mitigates their subsequent differentiation and fusion [64]. MPCs sense HGF/SF merely at the initial phase of muscle regeneration as exogenous injection of this peptide do not improve muscle repair at later stages [118]. Similarly, HGF/SF immunostaining is reduced at specimens obtained from damaged muscle with time after injury [185]. c-Met exposed to HGF/SF is able to activate MPCs through several signaling pathways (Gab1/Shp2/Ras/Raf/Erk, Grb2/Sos/Ras/Raf/Erk, Gab1/PI3K/Akt, Gab1/Crk/C3G/Rap1) which regulate cell proliferation/morphogenesis, survival, and actin reorganization, respectively. Pleiotropic effects of HGF/SF lead to increased MPC population and backing up satellite cell migration that in overall provide optimal myoblast density to the site of muscle injury. Besides HGF/SF secreted by MPCs in an autocrine route [165], certain pool is available from the ECM, especially when basal lamina is disrupted [186] or NO is released by nNOS at the motor end plates [13]. Other organs can also contribute to skeletal muscle regeneration, e.g., muscle damage stimulates spleen to secrete HGF/SF in rats [183]. Altogether, HGF/SF comes out to be a primary mitogen and the most important growth factor involved in skeletal muscle regeneration (Fig. 5.1).
Humoral mediators and associated pathways drive anabolic and catabolic responses in the skeletal muscle (Costamagna et al. [40])
5.3 Muscle Injury and Immune Cells
Even though molecular regulation of myogenesis and skeletal muscle regeneration are almost indistinguishable, the immune response is observed solely in harmed muscle. Immune cells are of myeloid origin and amount to high numbers (105/mm3) in damaged muscle site. Immune cells actively secrete numerous cytokines and growth factors in order to modulate own inflammatory activity, and they affect the viability and transcriptional activities of regenerating muscle cells [190]. Most of the biological effects observed in the immune system are mediated by cytokines divided into two groups: one represented by pro-inflammatory activity and second suited by anti-inflammatory action. Nowadays, there is a strong belief that the balance between pro- and anti-inflammatory cytokines is essential for accurate immune response to injury. Although expression of myogenic genes occurs in the absence of myeloid cells, the latter can control the extent and timing of expression. It is obvious that some cytokines endorse muscle satellite cell proliferation in vitro [29, 109].
T lymphocytes embody major source of cytokines with CD4-expressing T lymphocytes (CD4+) also known as helper cells, the most useful cytokine producers. CD4+ T lymphocytes could be further subdivided into Th1 and Th2, while the cytokines they secrete are termed Th1-type (pro-inflammatory such as interferon-γ, TNF-α, interleukin 1β) and Th2-type (anti-inflammatory such as interleukins 3, 5, 10, and 13), respectively. Inflammatory lesion in skeletal muscle begins the fast infiltration with Ly6C+/F4/80− neutrophils during acute phase of inflammation (the second hour) peaking between 6 and 24 h postinjury. This phase ends up with phagocytic CD68+ macrophages of M1 phenotype that rise in number between 24 and 48 h postinjury. M1 macrophages decline sharply 2 days postinjury, and this decline heralds the infiltration of non-phagocytic CD206-expressing macrophages of M2 phenotype (M2a, M2b, M2c) [170, 191]. The induction of MRF in skeletal muscle progenitors is in striking coincidence with the release of inflammatory and vascular mediators by immune cells. First, the degree of inflammatory reaction affects regenerating muscle, with greater injury observed in wide-ranging infiltration by neutrophils and M1 macrophages. Free radicals (oxygen, nitrogen, and chlorate species) released by neutrophils and M1 macrophages lead to plasma membrane degradation (lipid peroxidation) and an extracellular debris for phagocytosis [27]. Thus, reductions in phagocyte-mediated damage facilitate and accelerate muscle regeneration [220].
One must bear in mind that innate immune response begins with TNF-α- and interferon-γ-driven activation of macrophages. These cells acquire M1 pro-inflammatory phenotype with vast generation of nitric oxide (NO) by inducible form of nitric oxide synthase (iNOS). By the way, other destructive nitrogen species such as peroxynitrite could come out [201]. Importantly, M1 macrophages express CD68, the receptor for oxidized LDLs which in turn stimulates phagocytosis and secretion of pro-inflammatory cytokines [153].
TNF-α plays a critical role in inflammation as this is the first cytokine released by neutrophils and M1 macrophages to trigger production of other Th1 cytokines. Besides, TNF-α was frequently reported to encourage skeletal muscle regeneration at early stage following acute injury as TNF-α null mutants and TNF-α receptor mutants had lower expression levels of MyoD and MEF-2 than wild-type controls [35, 204]. In differentiating C2C12 myotubes, TNF-α administration augmented cell growth [103], whereas it inhibited MyHC IIa protein expression in differentiated myotubes [145, 146]. Apparently, muscle cell fusion is inhibited, whereas myoblasts proliferation is stimulated by TNF-α that might trigger network of complex intracellular signaling pathways engaging NF-κB, signal transducer and activator of transcription (STAT-1α) and other transcription factors [120, 145, 146]. NF-κB activation by alternative pathway plays important function in skeletal muscle, as it promotes the expression and stability of cyclin D1 leading to increased cell proliferation and inhibition of differentiation [73]. Another explanation of NF-κB-dependent skeletal muscle growth retardation comes from the observation that MyoD and MyoD mRNA template are NF-κB targets for degradation [91]. Finally, NF-κB was shown to bind skeletal muscle transcriptional repressor, namely, YY1 which depresses activation of muscle-specific genes [203]. Thus, the exact role of TNF-α in muscle growth and regeneration varies on the degree of muscle damage, particular phase (transition from early to terminal differentiation), and cellular target such as NF-κB [218]. Although TNF-α levels peak at 24 h postinjury, this cytokine levels remain elevated up to 2 weeks following acute muscle injury which advocates principal role of TNF-α played in skeletal muscle repair [204]. It also points to TNF-α targets other than NF-κB muscle regulatory pathways, possibly p38 MAP kinase signaling [221]. Blocking the p38 kinase pathway resulted in diminished myogenesis and increased NF-κB activity in C2C12 myotubes [90]. TNF-α-dependent effects on muscle growth/regeneration are also associated with its chemoattractant attribute to stimulate directional migration of myoblasts and satellite cells [195]. Presumably, when TNF-α is released from polymorphonuclear cells (PMCs) and M1 macrophages at the site of injury, it is chemical attractant for muscle stem and progenitor cells, by sensing this cytokine peel direction where the cell damage is to be compensated. Chemoattraction can be inhibited by deletion of TNF-α with specific antibodies or by the suppression of M1 macrophages [106, 115]. Elevated muscle protein loss is another important product of TNF-α activity regardless of circumstances. It is now well established that this effect of TNF-α is associated with induction of FBXO32/Atrogin1 gene and its expression product atrogin-1 protein [24, 145, 146] mediated by p38 MAPK [103]. Apparently, TNF-α plays two opposite roles in muscle repair, one related to degenerative phase where it stimulates proliferation and migration of muscle stem and progenitor cells and the second where it holds back regenerative phase by impaired muscle cell fusion and accelerated protein decay [68, 145, 146].
Immune, type II, or γ-interferon (IFN-γ) is secreted by thymus-derived (T) cells under certain conditions of activation and by natural killer (NK) cells. IFN-γ controls several aspects of the immune response, comprising stimulation of bactericidal activity of phagocytes, stimulation of antigen presentation through class I and class II major histocompatibility complex (MHC) molecules, or by orchestration of leukocyte-endothelium interactions linked to cell proliferation and apoptosis [25].
IFN-γ is also another pro-inflammatory cytokine of Th1 series widely known from its pleiotropic activity taking in muscle growth and repair [36, 61]. It is worth noting, IFN-γ cooperates with TNF-α in many aspects of initial adaptation to skeletal muscle injury [10, 145, 146, 194, 207, 208]. Furthermore, IFN-γ and TNF-α relations are also evident during muscle cell differentiation when muscle fibers grow up and develop [145, 146, 168]. While early stages of skeletal myogenesis are apparently stimulated by both cytokines (augmented proliferation), later phases are markedly hampered, with noticed ATP-dependent proteolysis associated with E3 ubiquitin ligases atrogin-1/MAFbx and MuRF1 [145, 146]. The latter muscle-specific RING-finger protein 1 ligase and MAFbx are indicated as important IFN-γ and TNF-α targets in cancer cachexia [1]. Transcriptome analysis of differentiating C2C12 muscle cells revealed that IFN-γ affects the expression of several genes. The cytokine promoted cytokine/growth factor expression, cell proliferation, and migration but impaired muscle cell differentiation [72]. Presumably, the implementation of such a variety of effects by a single cytokine is achieved by complex patterns of skeletal muscle cell-specific gene regulation with IFN-γ response regulated by interaction with responses to other cytokines including TNF-α.
IFN-γ blocks the secretion of Th2 cytokines bulk which inhibits the inflammatory response. Thus, IFN-γ probably upholds inflammatory response of injured muscle in order to postpone tissue repair unless the population of muscle progenitor cells is sufficient for muscle regeneration. Th2 cytokines represented by IL-4, IL-10, and IL-13 stimulate M2 macrophages to take over M1 macrophages [69]. There are several subcategories of M2 macrophages indicated by small letters (M2a, M2b, M2c) (Fig. 5.2).
Secreted molecules and paracrine effects from resident and circulating cells involved in skeletal muscle inflammation (Costamagna et al. [40])
5.4 Skeletal Muscle as an Important Source of IL-6
Myokines, a term used to describe a group of cytokines produced by skeletal muscle during and following exercise, point to a brand new role played by this organ [138]. Except MGF, remaining myokines are identical to previously described and identified leukocytes (IL-6, IL-1, interleukin 1 receptor antagonist (IL-1ra), IL-8, macrophage inflammatory protein α and β (MIP-1α and MIP-1β), TNF-α). Nowadays, it is widely recognized that strenuous prolonged exercise, e.g., marathon run, causes huge increase in the concentration of several pro- and anti-inflammatory cytokines in the peripheral blood [51, 52, 138]. Previously, it was well established that physical exercise stimulates the leukocytosis (increased number of leukocytes in peripheral blood). However, the origin of myokines is different from immune cells as both transcripts and proteins concentrate in skeletal muscles [66, 86, 176, 179]. The most prominent among myokines is IL-6 for the reason that following exercise this cytokine basal plasma levels may increase up to 100-fold [58, 132, 135, 140–142]. The peak IL-6 plasma concentration is observed at the end of the exercise or soon after [56, 57, 58, 132]. For a long time, IL-6 has been considered as classical inflammatory cytokine suggesting that muscle fiber injuries promote almost exponential raise of the cytokine plasma level during a bout of heavy exercise. This assumption has no evidence as increase in IL-6 is observed in both non-damaging (concentric) [211] and damaging (eccentric) skeletal muscle contractions [107]. What is the role of IL-6 and why this myokine is that much upregulated in exercised skeletal muscle? Since IL-6 release by skeletal muscle is proportional to the muscle mass, exercise intensity, and duration (twofold increase in IL-6 level was observed after 6 min, tenfold after 2 h, and 100-fold after 6 h of running), there is presumption that this myokine is of paramount importance for homeostasis in contracting muscles, as resting muscles exposed to identical hormonal milieu do not release IL-6 [78]. Fluorescence-activated cell sorting (FACS) of peripheral monocytes isolated from blood samples of cycling or running athletes did not demonstrate any significant changes in IL-6 even during prolonged exercise [173–175]. These and other experiments show that contracting skeletal muscle is the major source of IL-6 in the peripheral blood in response to exercise and that muscle fibers but not myoblasts, endothelial cells, fibroblasts, or smooth muscle cells account for the systemic increase of plasma IL-6 [77, 86, 108, 143]. Interestingly, exercise-induced increase in IL-6 release from the exercised legs (based on arteriovenous difference) could be blocked completely by the supplementation of vitamins C and E for 4 weeks prior to the challenge [56, 57]. It appears that IL-6 gene activation is redox mediated in skeletal muscles following exercise as antioxidants abolish this effect. Ablation of redox stimulus was also observed in differentiating myoblasts following vitamin C preincubation [131]. Given that IL-6 derived from skeletal muscle is the normal physiologic response to exercise, one may ask about its role and significance. IL-6 appears to be involved in glucose homeostasis [181, 66], and IL-6 response to exercise is dependent on pre-exercise skeletal muscle glycogen content [176]. Moreover, carbohydrate supplementation attenuates the increase of plasma IL-6 of skeletal muscle origin [174, 175]. In addition, IL-6 infusion is accompanied by extensive lipolysis and accelerated fat oxidation pointing to its metabolic rather than immunologic function [199]. Nowadays, the consistent findings indicating IL-6 secretion from exercised muscles in the absence of noticeable inflammatory markers designate this cytokine as a master switch that stimulates utilization of neutral fat when carbohydrates are in shortage. With respect to IL-6, skeletal muscle is prompted to transcriptional, translational, and secretory activities in reaction to rise in sarcoplasmic Ca2+ level during repetitive contractions. Importantly, the effect is calcineurin/nuclear factor of activated T-cell (NFAT) dependent (inhibited by cyclosporin A and potentiated by ionomycin). Sustained intracellular Ca2+ is a well-known calcineurin activator which is calcium- and calmodulin-dependent serine/threonine protein phosphatase upstream to NFAT [62]. Furthermore, IL-6 gene expression at transcriptional and translational levels is controlled by calcineurin/NFAT in cultured muscle cells [86]. NFAT may be rephosphorylated by NFAT kinases like glycogen synthase kinase (GSK-3β) which makes NFAT inactive and withdrawn from the nucleus [54]. We observed critical role played by GSK-3β in myogenesis, as both insulin and metabolic inhibitors of this kinase led to ameliorated muscle fiber formation [104]. Strict pro-inflammatory TNF-α is repressed in the same circumstances (exercised muscle) demonstrating that IL-6 and TNF-α are regulated differently in skeletal muscle cells by altered Ca2+ levels [84]. In addition, other possible signaling pathways explain IL-6 increase in exercised skeletal muscle. Abundantly expressed neuronal NO synthase isoform (nNOS) points toward NO production within contracting skeletal muscles with cGMP and/or nitrosative mechanisms leading to IL-6 production [93, 94, 156, 167]. Bulk of evidence collected from the experiments with NO donors showed increase in IL-6 mRNA content and IL-6 release from skeletal muscles challenged with NO, whereas opposite response was noted upon the use of nNOS inhibitors [178]. Nuclear factor kappa light chain enhancer of activated B cells (NF-κB) is another candidate as it is redox-sensitive transcription factor, while trained skeletal muscle is a rich source of reactive oxygen species (ROS) [167]. Antioxidants were mentioned as IL-6 repressors [189, 200]; thus it is very likely that ROS formation in skeletal muscle following exercise causes IL-6 release in a NF-κB-dependent manner. In order to position NF-κB in the redox-dependent cell signaling, nonsteroidal anti-inflammatory drugs (NSAIDs) were used as NF-κB inhibitors [88]. Actually, indomethacin diminishes the exercise-induced increase of IL-6 further supporting the notion of causal relationship between skeletal muscle contractions, NF-κB and IL-6 synthesis [155]. Moreover, basal IL-6 plasma concentrations are highly correlated with the skeletal muscle training, being lower in trained vs. non-trained individuals [32, 137]. Despite the marked rise in IL-6 mRNA during exercise, the long-term training (1 h, 5 times a week for 10 weeks) led to almost ten times lower IL-6 mRNA content in exercised skeletal muscles [56, 57]. Concomitantly IL-6R mRNA content was almost doubled in trained skeletal muscle [85]. It reveals the nature of feedback mechanism of IL-6 myokine; while plasma IL-6 is apparently downregulated by training, the muscular expression of the IL-6R is upregulated at the same time to augment IL-6 sensing by muscle fibers. The last but probably not the least, signaling pathway activated in skeletal muscle during contraction is p38 MAPK known as stress-activated protein kinase [21]. In fact, fatigued muscle with reduced glycogen content has been found to boost the p38 MAPK activity and its nuclear translocation [33]. Moreover, nuclear p38 MAPK phosphorylation through MAPK kinase (MKK-3 and MKK-6) is known to activate IL-6 gene since inhibition of p38 MAPK results in loss of the IL-6 mRNA transcriptional control [33]. For years, p38 MAPK was shown to be essential for muscle fiber formation in myogenesis; thus its additional control of IL-6 gene brings the role of the kinase updated [41, 90, 210]. It is very likely that the downstream targets of p38 MAPK, ATF-2, and Elk1 may participate in regulating the expression of IL-6 gene, owing to the fact that ATF-2 is a subunit of the AP-1 heterodimer (Jun:ATF) [198], while Elk1 is a member of the Ets superfamily of transcription factors [217].
5.5 Cytokines Negatively Targeting Skeletal Muscle
Chronic systemic inflammation (CSI) is a cause of cardiometabolic syndrome (CMS) associated with higher risk of cardiovascular disease, type 2 diabetes, and muscle cachexia (muscle wasting) [44, 53, 141, 142, 149, 157, 192, 209]. Two- to fourfold rise in plasma concentrations of pro- and anti-inflammatory cytokines and acute-phase proteins are regarded as a common attribute of CSI [28]. Regular exercise prevents CMS almost certainly through the elevated levels of anti-inflammatory cytokines [IL-1ra, soluble TNF-α receptors (sTNF-R), and IL-10] which in turn override inflammatory response [134, 136, 139]. Throughout CSI several pro-inflammatory cytokines are elevated in blood plasma, TNF-α, IL-1β, and IL-6, even though TNF-α plays the central role in insulin desensitization of skeletal muscle [147, 148, 196]. Insulin resistance results in marked decline of its anabolic effect and accelerated loss of skeletal muscle mass typical for type 2 diabetes [147, 148]. A bulk of evidence confirms that TNF-α-induced insulin resistance is associated with NF-κB and STAT-1α activation and inhibition of PI3-K/Akt signaling pathway [26, 145, 146]. Thus, TNF-α represents the causal factor of insulin resistance [147]. It appears that the role of IL-6 in muscle decays as pro- or anti-inflammatory cytokine is less clear as indicated by conflicting data [141, 142, 149, 172, 177]. Observations indicate that IL-6-deficient animals (knockout mice) have higher levels of circulating TNF-α [119], while IL-6 inhibits TNF-α formation [55, 110]. Additionally, IL-6 stimulates formation of anti-inflammatory cytokines IL-1ra and IL-10 [177]. IL-1ra is of particular importance as it weakens inflammatory response being the efficient blocker of IL-1α and IL-1β binding to cognate receptors [45]. Once it is established that IL-6 has anti-inflammatory rather than pro-inflammatory characteristics, the rationale for protective role of physical activity against muscle wasting is to be revealed. Nonetheless, there is no direct relationship between exercise and reduced CSI although several studies have reported improved status of patients with CMS [67, 180]. To decipher the physiological role of IL-6, further studies are needed, as IL-6 levels are augmented both in patients with metabolic disease featured by insulin resistance and hyperlipidemia [37, 129] and after strenuous exercise which improves insulin-stimulated glucose uptake and plasma lipid profile [212]. This actually contradicting observations might be explained by simultaneous alterations in TNF-α concentration as increased TNF-α and decreased IL-6 transcription lead to higher incidence of diabetes [89]; however, the exact etiology of the CMS has at least few scenarios including elevated IL-6 as a systemic feedback mechanism to adipocyte-secreted TNF-α or alternative route to sensitize skeletal muscle to insulin or perhaps it results from “IL-6 resistance.” None of these hypotheses have sufficient substantiation.
For decades TGF-β superfamily of cytokines (growth factors) has been acknowledged as inhibitors of both myoblasts proliferation and differentiation [3, 4, 23, 79]. Among several members myostatin (MSTN) also identified as growth and differentiation factor-8 (GDF-8) attracts special interest as MSTN knockout mice have unusually large shoulder and hip muscles [114, 184, 222]. “Loss of function” mutations in MSTN gene were demonstrated to enlarge skeletal muscles by increased number and size of muscle fibers in other species including human beings [71, 81, 82, 113, 164]. When secreted, MSTN protein decreases myoblast proliferation by stimulating p21 protein, a well-known cyclin-dependent kinase Cdk2 inhibitor [80, 187, 188]. Cdk2 in turn inhibits G1 to S phase transition. Molecular mechanism of MSTN activity seems to hamper satellite cell proliferation through activin type II receptor (Act RIIB) as dominant negative form of Act RIIB in transgenic mouse lines exhibited dramatic increases in muscle mass comparable to those seen in MSTN knockout mice [97]. MRF genes are transcriptionally repressed by MSTN because signal transduction from Act RIIB directly activates Smad2/3 proteins with resultant fall in MyoD and myogenin gene expression [92, 105]. Moreover, MSTN is associated with increased Smad2/3 phosphorylation levels and decline in MyoD and myogenin gene transcription [206]. Furthermore, satellite cell proliferation is noticeably elevated in MSTN-null mice [111]. MSTN is produced by skeletal muscle and adipose tissue [6], even though its expression levels are subject to regulation following muscle damage [215]. Fasting and glucocorticoids were indicated as possible MSTN gene incentives [11, 159], and both C/EBP-δ and fewer miR-27a/b seem to mediate the respective muscle wasting effects [5, 8]. Additionally, the FoxO1 and TGF-β/Smad3, MyoD, and JNK/p38 kinase pathways are involved in MSTN secretion [9, 74, 169]. The former, FoxO1-dependent muscle atrophy results from insulin resistance, as insulin inhibits FoxO1 in skeletal muscle cells [104]. Additionally, constitutive FoxO1 activity causes MSTN gene disinhibition [9] in insulin-resistant states being followed by elevated MSTN expression [7]. In contrast, MSTN is downregulated during muscle regeneration and its levels are returned to normal in mature fibers [116, 193, 197, 222]. To some extent, there is also contribution of MSTN to aberrant regeneration in mdx mice (animal model of Duchenne muscle dystrophy) as improved muscle regeneration was observed in mdx:MSTN −/− mice [202].
5.6 Myogenic Myokines
Different cytokines are produced and released by skeletal muscle, while the exact role played by each is not fully elucidated. Despite IL-6 which is fairly well described in the context of skeletal muscle as endocrine organ, IL-8 is acknowledged as potent chemoattractant for neutrophils during acute phase of inflammatory reaction [17]. IL-8 belongs to distinct family of CXC chemokines where the acronym’s C stands for cysteine while X is any amino acid that separates two cysteine residues at the NH2 terminus. Interestingly, IL-8 plasma concentration is altered by physical activity but barely moderate-to-exhaustive eccentric exercise rouses IL-8 gene expression in skeletal muscle and its blood plasma levels [125, 126]. A number of reports corroborated elevated IL-8 plasma levels following training during and after marathon run [127, 128, 133]. While accurate physiological IL-8 function in skeletal muscle is not known, some authors ponder IL-8 as locally acting and associated with mild inflammatory response associated with single muscle fiber damage. This assumption is substantiated by the miniature IL-8 observed during and following concentric muscle contractions that in general are not injurious to skeletal muscle. Some studies indicate that IL-8 is angiogenic. Summing up, IL-8 by binding to its receptor CXCR1 induces chemotactic effect, whereas it stimulates endothelial epithelium for neovascularization through CXCR2 [20, 87,130].
Besides IL-6 and IL-8, also IL-15 gene was shown to be induced in skeletal muscle during strength training [124]. The IL-15 signaling peptide acts on target cells by attaching to heterotrimeric membrane receptors (IL-15Rαβγ) with Janus kinases (JAK1 and 3) and STAT-3 and STAT-5 [65]. Since IL-15 cytokine is present as intracellular and secretory form, its detection is not easy carrying inconsistent reports [124, 125, 132, 135]. Anyway, several “in vitro” studies carried out on myogenic cells revealed IL-15 as anabolic factor capable to increase MyHC in differentiating muscle cells autonomously from IGFs [63, 151]. Similarly, this cytokine was demonstrated to have anabolic effects on skeletal muscle “in vivo” [150]. Furthermore, IL-15 participates in cross talk between muscle fibers and adipocytes [14]. It reduces adipose tissue mass with concurrent skeletal muscle overgrowth [30], and this is a unique muscle-to-fat endocrine axis with high therapeutic potential to combat muscle wasting in cachexia. The distinctive function of IL-15 is manifested in fully differentiated muscle cells where this cytokine represses proteolysis in muscle cachexia [150]. Accordingly, the IL-15 mRNA was markedly upregulated in differentiated skeletal myotubes but not undifferentiated myoblasts [152]. Summing up, IL-15 could be regarded as skeletal muscle growth-promoting myokine underestimated in prevention of muscle wasting.
5.7 Adipokines in Skeletal Muscle Growth
There is more than 30 different adipokines produced by adipocytes, making adipose tissue a significant source of regulatory factors with some being essential for fat-to-muscle communication. Leptin peptide, a “satiety hormone” is probably the most interesting amongst adipokines. It acts on target cells similarly to IL-6 as both leptin receptor (LRb) and IL-6 receptor (gp130Rβ) share a sequence homology liable for JAK recruitment and STAT phosphorylation needed for gene regulation although LRb and gp130Rβ are ligand specific without cross-reactivity [50]. Can a common signaling pathway for leptin and IL-6 evoke distinct cellular responses? The answer is yes, as IL-6 binds initially to IL-6Rα and as multifunctional cytokine exerts its biological activities through two molecules: IL-6Rα (IL-6 receptor) and gp130Rβ. When IL-6 binds to mIL-6Rα (membrane-bound form of IL-6R), homodimerization of gp130β is induced and a high-affinity functional receptor complex of IL-6, IL-6Rα, and gp130β is formed. Interestingly, sIL-6R (soluble form of IL-6R) also binds with IL-6, and the IL-6–sIL-6R complex can then form a complex with gp130β [117]. The complex activates JAKs that then phosphorylate tyrosine residues in the cytoplasmic domain of gp130β. The gp130β-mediated JAK activation by IL-6 triggers two main signaling pathways: the gp130β Tyr759-derived SHP-2 (Src homology 2 domain-containing protein tyrosine phosphatase-2)/ERK (extracellular signal-regulated kinase) MAPK (mitogen-activated protein kinase) pathway and the gp130β YXXQ-mediated JAK/STAT pathway [171]. In contrast to LRb, gp130β has more tyrosines for phosphorylation (Y767, 814, 905, 915) which is functionally important to negate feedback mechanism mediated by suppressor of cytokine signaling (SOCS) proteins [205]. SOCS3 binds to phosphorylated LRb through its SH2 domain to stop JAK-mediated phosphorylations [22] and additionally recruits ubiquitin-transferases for JAK/STAT degradation [2]. Consequently, leptin-dependent regulation of skeletal muscle is transient, whereas IL-6 seems to induce long-term effects. It was noticed that both cytokines possibly stimulate AMP-activated protein kinase (AMPK) by T172 phosphorylation mediated by AMPK kinase (LKB1) [213]. Alternatively, by increased cellular ATP turnover, leptin and IL-6 may activate AMPK with elevated levels of allosteric activator AMP. This system is fundamental for energy sensing and fatty acid oxidation in skeletal muscle, so leptin and IL-6 are mighty directors in delivery of substrates for energy production (acetyl-CoA). Recently, we observed other metabolic effects of leptin. In cultured C2C12 mouse myogenic cell line, this cytokine stimulated myoblast mitogenicity and inhibited muscle cell differentiation through JAK/STAT and MEK signaling pathway where MEK is an upstream ERK kinase (MAPKK) [145, 146]. Leptin through MEK-dependent manner caused GSK-3β phosphorylation (Y216-GSK-3β) with resultant drop in myoblast viability and fusion. Overall, more research on adipokines is required in order to shed light on fat-to-muscle influences.
5.8 Conclusions and Perspectives
Skeletal muscle fibers as permanent cells do not divide, so upon injury this organ regeneration is entirely reliant on stem cell subpopulations including satellite cells which are activated by a myriad of signals where cytokines play the prime role. Nowadays, it is widely accepted that cytokines which were initially specific growth/differentiation factors of leukocyte origin are also synthesized and secreted by other tissues including skeletal muscle (myokines). It appears, that myokine profile is dependent on the physiologic (type and intensity of exercise, muscle fiber type) or pathologic (the magnitude of injury) conditions. Thus, it is obvious the pattern of myokines controls skeletal muscle growth and decline in health and disease. Skeletal muscle growth is regulated by mitogenic and survival signals (HGF/SF, FGF, IGFs, LIF), whereas damaged skeletal muscle is characterized by necrosis and activation of pro-inflammatory (TNF-α, IFN-γ, IL-1β, IL-6) followed by anti-inflammatory cytokines (IL-1ra, soluble TNF-α receptors (sTNF-R), and IL-10) targeting muscle satellite cells and controlling skeletal muscle degeneration and regeneration phase, respectively. Finally, TGF-β superfamily of cytokines, greatly MSTN, stops further growth and muscle tissue regeneration. As skeletal muscle satellite cells are decisive for skeletal muscle regeneration, understanding the molecular mechanisms of their activation by myokines seems to be essential to combat aberrant muscle growth and repair.
References
Acharyya S, Ladner KJ, Nelsen LL, Damrauer J, Reiser PJ, Swoap S, Guttridge DC (2004) Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J Clin Invest 114:370–378
Alexander WS (2002) Suppressors of cytokine signalling (SOCS) in the immune system. Nat Rev Immunol 2:410–416
Allen RE, Boxhorn LK (1987) Inhibition of skeletal muscle satellite cell differentiation by transforming growth factor-beta. J Cell Physiol 133:567–572
Allen RE, Boxhorn LK (1989) Regulation of skeletal muscle satellite cell proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor I, and fibroblast growth factor. J Cell Physiol 138:311–315
Allen DL, Cleary AS, Hanson AM, Lindsay SF, Reed JM (2010) CCAAT/enhancer binding protein-delta expression is increased in fast skeletal muscle by food deprivation and regulates myostatin transcription in vitro. Am J Phys Regul Integr Comp Phys 299:R1592–R1601. doi:10.1152/ajpregu.00247.2010
Allen DL, Cleary AS, Speaker KJ, Lindsay SF, Uyenishi J, Reed JM, Madden MC, Mehan RS (2008) Myostatin, activin receptor IIb, and follistatin-like-3 gene expression are altered in adipose tissue and skeletal muscle of obese mice. Am J Physiol Endocrinol Metab 294:E918–E927. doi:10.1152/ajpendo.00798.2007
Allen DL, Hittel DS, McPherron AC (2011) Expression and function of myostatin in obesity, diabetes, and exercise adaptation. Med Sci Sports Exerc 43:1828–1835. doi:10.1249/MSS.0b013e3182178bb4
Allen DL, Loh AS (2011) Posttranscriptional mechanisms involving microRNA-27a and b contribute to fast-specific and glucocorticoid-mediated myostatin expression in skeletal muscle. Am J Phys Cell Phys 300:C124–C137. doi:10.1152/ajpcell.00142.2010
Allen DL, Unterman TG (2007) Regulation of myostatin expression and myoblast differentiation by FoxO and SMAD transcription factors. Am J Phys Cell Phys 292:C188–C199. doi:10.1152/ajpcell.00542.2005
Alvarez B, Quinn LS, Busquets S, Lopez-Soriano FJ, Argiles JM (2002) TNF-alpha modulates cytokine and cytokine receptors in C2C12 myotubes. Cancer Lett 175:181–185
Anagnostis P, Athyros VG, Tziomalos K, Karagiannis A, Mikhailidis DP (2009) Clinical review: the pathogenetic role of cortisol in the metabolic syndrome: a hypothesis. J Clin Endocrinol Metab 94:2692–2701. doi:10.1210/jc.2009-0370
Anderson JE, Mitchell CM, McGeachie JK, Grounds MD (1995) The time course of basic fibroblast growth factor expression in crush-injured skeletal muscles of SJL/J and BALB/c mice. Exp Cell Res 216:325–334
Anderson JE (2000) A role for nitric oxide in muscle repair: nitric oxide-mediated activation of muscle satellite cells. Mol Biol Cell 11:1859–1874
Argiles JM, Lopez-Soriano J, Almendro V, Busquets S, Lopez-Soriano FJ (2005) Cross-talk between skeletal muscle and adipose tissue: a link with obesity? Med Res Rev 25:49–65
Armstrong RB, Warren GL, Warren JA (1991) Mechanisms of exercise-induced muscle fibre injury. Sports Med 12:184–207
Asakura A, Rudnicki MA (2002) Cellular and molecular mechanisms regulating skeletal muscle development. In: Mouse development. Academic, Orlando, FL, pp 253–278
Baggiolini M (2001) Chemokines in pathology and medicine. J Intern Med 250:91–104
Bark TH, McNurlan MA, Lang CH, Garlick PJ (1998) Increased protein synthesis after acute IGF-I or insulin infusion is localized to muscle in mice. Am J Physiol Endocrinol Metab 275:E118–E123
Barton-Davis ER, Shoturma DI, Sweeney HL (1999) Contribution of satellite cells to IGF-I induced hypertrophy of skeletal muscle. Acta Physiol Scand 167:301–305
Bek EL, McMillen MA, Scott P, Angus LD, Shaftan GW (2002) The effect of diabetes on endothelin, interleukin-8 and vascular endothelial growth factor-mediated angiogenesis in rats. Clin Sci 103(Suppl 48):424S–429S
Ben-Levy R, Hooper S, Wilson R, Paterson HF, Marshall CJ (1998) Nuclear export of the stress-activated protein kinase p38 mediated by its substrate MAPKAP kinase-2. Curr Biol 8:1049–1057
Bjorbaek C, Buchholz RM, Davis SM, Bates SH, Pierroz DD, Gu H, Neel BG, Myers MG Jr, Flier JS (2001) Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem 276:4747–4755
Blachowski S, Motyl T, Orzechowski A, Grzelkowska K, Interewicz B (1993) Comparison of metabolic effects of EGF, TGF-a, and TGF-b1 in primary culture of fetal bovine myoblasts and rat L6 myoblasts. Int J Biochem 25:1571–1577
Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ (2001) Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294:1704–1708
Boehm U, Klamp T, Groot M, Howard JC (1997) Cellular responses to interferon-γ. Annu Rev Immunol 15:749–795
Bouzakri K, Zierath JR (2007) MAP4K4 gene silencing in human skeletal muscle prevents tumor necrosis factor-alpha-induced insulin resistance. J Biol Chem 282:7783–7789
Brickson S, Ji LL, Olabisi R, Schneider BSP, Best TM (2003) M1/70 attenuates blood-borne neutrophil oxidants, activation, and myofiber damage following stretch injury. J Appl Physiol 95:969–976
Bruunsgaard H, Pedersen BK (2003) Age-related inflammatory cytokines and disease. Immunol Allergy Clin N Am 23:15–39
Cantini M, Carraro U (1995) Macrophage-released factor stimulates selectively myogenic cells in primary muscle culture. J Neuropathol Exp Neurol 54:121–128
Carbo N, Lopez-Soriano J, Costelli P, Alvarez B, Busquets S, Baccino FM, Quinn LS, Lopez-Soriano FJ, Argiles JM (2001) Interleukin-15 mediates reciprocal regulation of adipose and muscle mass: a potential role in body weight control. Biochim Biophys Acta 1526:17–24
Carnwath JW, Shotton DM (1987) Muscular dystrophy in the mdx mouse: histopathology of the soleus and extensor digitorum longus muscles. J Neurol Sci 80:39–54
Cesari M, Penninx BW, Pahor M, Lauretani F, Corsi AM, Rhys WG, Guralnik JM, Ferrucci L (2004) Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci 59:242–248
Chan MH, McGee SL, Watt MJ, Hargreaves M, Febbraio MA (2004) Altering dietary nutrient intake that reduces glycogen content leads to phosphorylation of nuclear p38 MAP kinase in human skeletal muscle: association with IL-6 gene transcription during contraction. FASEB J 18:1785–1787
Charge´ SBP, Rudnicki MA (2004) Cellular and molecular regulation of muscle regeneration. Physiol Rev 84: 209–238
Chen SE, Gerken E, Zhang Y, Zhan M, Mohan RK, Li AS, Reid MB, Li YP (2005) Role of TNF-α signaling in regeneration of cardiotoxin-injured muscle. Am J Phys Cell Phys 289:C1179–C1187
Cheng M, Nguyen MH, Fantuzzi G, Koh TJ (2008) Endogenous interferon-γ is required for efficient skeletal muscle regeneration. Am J Phys 294:1183–1191
Choy EH, Isenberg DA, Garrood T, Farrow S, Ioannou Y, Bird H, Cheung N, Williams B, Hazleman B, Price R, Yoshizaki K, Nishimoto N, Kishimoto T, Panayi GS (2002) Therapeutic benefit of blocking interleukin-6 activity with an anti-interleukin-6 receptor with an anti-interleukin-6 receptor monoclonal antibody in rheumatoid arthritis: a randomized, double-blind, placebo-controlled, dose-escalation trial. Arthritis Rheum 46:3143–3150
Coleman ME, Demayo F, Yin KC, Lee HM, Geske R, Montgomery C, Schwartz RJ (1995) Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J Biol Chem 270:12109–12116
Cornelison DD, Filla MS, Stanley HM, Rapraeger AC, Olwin BB (2001) Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev Biol 239:79–94
Costamagna D, Costelli P, Sampaolesi M, Penna F (2015)Role of inflammation in muscle homeostasis and myogenesis. Mediat Inflamm 2015 (Artcile ID 805172): 14, http://dx.doi.org/10.1155/2015/805172
Craig R, Larkin A, Mingo AM, Thuerauf DJ, Andrews C, Mc-Donough PM, Glembotski CC (2000) p38 MAPK and NF-kappa B collaborate to induce interleukin-6 gene expression and release. Evidence for a cytoprotective autocrine signaling pathway in a cardiac myocyte model system. J Biol Chem 275:23814–23824
D’Albis A, Couteaux R, Janmot C, Roulet A, Mira JC (1988) Regeneration after cardiotoxin injury of innervated and denervated slow and fast muscles of mammals. Myosin isoform analysis. Eur J Biochem 174:103–110
Delapeyriere O, Ollendorff V, Planche J, Ott MO, Pizette S, Coulier F, Birnbaum D (1993) Expression of the Fgf6 gene is restricted to developing skeletal muscle in the mouse embryo. Development 118:601–611
Di FM, Barbier D, Mege JL, Orehek J (1994) Tumor necrosis factor-alpha levels and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 150:1453–1455
Dinarello CA (2000) The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. N Engl J Med 343:732–734
Dumont NA, Wang YX, Rudnicki MA (2015) Intrinsic and extrinsic mechanisms regulating satellite function. Development 142:1572–1581
Dusterhoft S, Pette D (1999) Evidence that acidic fibroblast growth factor promotes maturation of rat satellite-cell-derived myotubes in vitro. Differentiation 65:161–169
Edwall D, Schalling M, Jennische E, Norstedt G (1989) Induction of insulin-like growth factor I messenger ribonucleic acid during regeneration of rat skeletal muscle. Endocrinology 124:820–825
El Fahime E, Mills P, Lafreniere JF, Torrente Y, Tremblay JP (2002) The urokinase plasminogen activator: an interesting way to improve myoblast migration following their transplantation. Exp Cell Res 280:169–178
Ernst M, Jenkins BJ (2004) Acquiring signalling specificity from the cytokine receptor gp130. Trends Genet 20:23–32
Febbraio MA, Pedersen BK (2005) Contraction-induced myokine production and release: is skeletal muscle an endocrine organ? Exerc Sport Sci Rev 33:114–119
Febbraio MA, Pedersen BK (2002) Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J 16:1335–1347
Ferrucci L, Penninx BW, Volpato S, Harris TB, Bandeen-Roche K, Balfour J, Leveille SG, Fried LP, Md JM (2002) Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc 50:1947–1954
Fiedler B, Wollert KC (2004) Interference of antihypertrophic molecules and signaling pathways with the Ca2+-calcineurin-NFAT cascade in cardiac myocytes. Cardiovasc Res 63:450–457
Fiers W (1991) Tumor necrosis factor. Characterization at the molecular, cellular and in vivo level. FEBS Lett 285:199–212
Fischer CP, Hiscock N, Basu S, Vessby B, Kallner A, Sjoberg LB, Febbraio MA, Pedersen BK (2004a) Supplementation with vitamins C and E inhibits the release of interleukin-6 from contracting human skeletal muscle. J Physiol 558:633–645
Fischer CP, Plomgaard P, Hansen AK, Pilegaard H, Saltin B, Pedersen BK (2004b) Endurance training reduces the contraction-induced interleukin-6 mRNA expression in human skeletal muscle. Am J Physiol Endocrinol Metab 287:E1189–E1194
Fischer CP (2006) Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev 12:6–33
Florini JR, Ewton DZ, Coolican SA (1996) Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev 17:481–517
Floss T, Arnold HH, Braun T (1997) A role for FGF-6 in skeletal muscle regeneration. Genes Dev 11:2040–2051
Foster W, Li Y, Usas A, Somogyi G, Huard J (2003) Gamma interferon as an antifibrosis agent in skeletal muscle. J Orthop Reprod 21:4–12
Frost RA, Nystrom GJ, Lang CH (2002) Lipopolysaccharide regulates proinflammatory cytokine expression in mouse myoblasts and skeletal muscle. Am J Phys Regul Integr Comp Phys 283:R698–R709
Furmanczyk PS, Quinn LS (2003) Interleukin-15 increases myosin accretion in human skeletal myogenic cultures. Cell Biol Int 27:845–851
Gal-Levi R, Leshem Y, Aoki S, Nakamura T, Halevy O (1998) Hepatocyte growth factor plays a dual role in regulating skeletal muscle satellite cell proliferation and differentiation. Biochim Biophys Acta 1402:39–51
Giri JG, Kumaki S, Ahdieh M, Friend DJ, Loomis A, Shanebeck K, DuBose R, Cosman D, Park LS, Anderson DM (1995) Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J 14:3654–3663
Gleeson M (2000) Interleukins and exercise. J Physiol 529:1
Goldhammer E, Tanchilevitch A, Maor I, Beniamini Y, Rosenschein U, Sagiv M (2005) Exercise training modulates cytokines activity in coronary heart disease patients. Int J Cardiol 100:93–99
Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL (2001) Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A 98:14440–14445
Gordon S (2003) Alternative activation of macrophages. Nat Rev Immunol 3:23–35
Greene EA, Allen RE (1991) Growth factor regulation of bovine satellite cell growth in vitro. J Anim Sci 69:146–152
Grobet L, Martin LJ, Poncelet D, Pirottin D, Brouwers B, Riquet J, Schoeberlein A, Dunner S, Menissier F, Massabanda J, Fries R, Hanset R, Georges M (1997) A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nat Genet 17:71–74
Grzelkowska-Kowalczyk K, Wicik Z, Majewska A, Tokarska J, Grabiec K, Kozlowski M, Milewska M, Baszczyk M (2015) Transcriptional regulation of important cellular processes in skeletal myogenesis through interferon-γ. J Interf Cytokine Res 35:89–99
Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS Jr (1999) NF-κB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol 19:5785–5799
Han DS, Huang HP, Wang TG, Hung MY, Ke JY, Chang KT (2010) Transcription activation of myostatin by trichostatin A in differentiated C2C12 myocytes via ASK1-MKK3/4/6-JNK and p38 mitogen-activated protein kinase pathways. J Cell Biochem 111:564–573. doi:10.1002/jcb.22740
Hall-Craggs EC (1974) Rapid degeneration and regeneration of a whole skeletal muscle following treatment with bupivacaine (Marcaine). Exp Neurol 43:349–358
Harris JB, Johnson MA (1978) Further observations on the pathological responses of rat skeletal muscle to toxins isolated from the venom of the Australian tiger snake Notechis scutatus scutatus. Clin Exp Pharmacol Physiol 5:587–600
Hiscock N, Chan MH, Bisucci T, Darby IA, Febbraio MA (2004) Skeletal myocytes are a source of interleukin-6 mRNA expression and protein release during contraction: evidence of fiber type specificity. FASEB J 18:992–994
Jonsdottir IH, Schjerling P, Ostrowski K, Asp S, Richter EA, Pedersen BK (2000) Muscle contractions induce interleukin-6 mRNA production in rat skeletal muscles. J Physiol 528:157–163
Joulia-Ekaza D, Cabello G (2006) Myostatin regulation of muscle development: molecular basis, natural mutations, physiopathological aspects. Exp Cell Res 312:2401–2414. doi:10.1016/j.yexcr.2006.04.012
Joulia D, Bernardi H, Garandel V, Rabenoelina F, Vernus B, Cabello G (2003) Mechanisms involved in the inhibition of myoblast proliferation and differentiation by myostatin. Exp Cell Res 286:263–275. doi:10.1016/S0014- 4827(03)00074-0
Kambadur R, Sharma M, Smith TP, Bass JJ (1997) Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res 7:910–916
Karim L, Coppieters W, Grobet L, Valentini A, Georges M (2000) Convenient genotyping of six myostatin mutations causing double-muscling in cattle using a multiplex oligonucleotide ligation assay. Anim Genet 31:396–399
Kastner S, Elias MC, Rivera AJ, Yablonka-Reuveni Z (2000) Gene expression patterns of the fibroblast growth factors and their receptors during myogenesis of rat satellite cells. J Histochem Cytochem 48:1079–1096
Keller C, Hellsten Y, Steensberg A, Pedersen BK (2006) Differential regulation of IL-6 and TNF-alpha via calcineurin in human skeletal muscle cells. Cytokine 36:141–147
Keller C, Steensberg A, Hansen AK, Fischer CP, Plomgaard P, Pedersen BK (2005) The effect of exercise, training, glycogen availability on IL-6 receptor expression in human skeletal muscle. J Appl Physiol 99:2075–2079
Keller C, Steensberg A, Pilegaard H, Osada T, Saltin B, Pedersen BK, Neufer PD (2001) Transcriptional activation of the IL-6 gene in human contracting skeletal muscle: influence of muscle glycogen content. FASEB J 15:2748–2750
Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM (1992) Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 258:1798–1801
Kopp E, Ghosh S (1994) Inhibition of NF-kappa B by sodium salicylate and aspirin. Science 265:956–959
Kubaszek A, Pihlajamaki J, Komarovski V, Lindi V, Lindstrom J, Eriksson J, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Tuomilehto J, Uusitupa M, Laakso M (2003) Promoter polymorphisms of the TNF-alpha (G-308A) and IL-6 (C-174G) genes predict the conversion from impaired glucose tolerance to type 2 diabetes: the Finnish Diabetes Prevention Study. Diabetes 52:1872–1876
Kwiecińska P, Roszkiewicz B, Łokociejewska M, Orzechowski A (2005) Elevated expression of NF-κB and Bcl-2 proteins in C2C12 myocytes during myogenesis is affected by PD98059, LY294002 and SB203580. Cell Biol Int 29:319–331
Langen RC, Van der Velden JL, Schols AM, Kelders MC, Wouters EF, Janssen-Heininger YM (2004) Tumor necrosis factor-α inhibits myogenic differentiation through MyoD protein destabilization. FASEB J 18:227–237
Langley B, Thomas M, Bishop A, Sharma M, Gilmour S, Kambadur R (2002) Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J Biol Chem 277:49831–49840. doi:10.1074/jbc.M2042 91200
Lau KS, Grange RW, Chang WJ, Kamm KE, Sarelius I, Stull JT (1998) Skeletal muscle contractions stimulate cGMP formation and attenuate vascular smooth muscle myosin phosphorylation via nitric oxide. FEBS Lett 431:71–74
Lau KS, Grange RW, Isotani EIJI, Sarelius IH, Kamm KE, Huang PL, Stull JT (2000) nNOS and eNOS modulate cGMP formation and vascular response in contracting fast-twitch skeletal muscle. Physiol Genomics 2:21–27
Lawlor MA, Rotwein P (2000) Insulin-like growth factor-mediated muscle cell survival: central roles for Akt and cyclin-dependent kinase inhibitor p21. Mol Cell Biol 20:8983–8995
Lawlor MA, Feng X, Everding DR, Sieger K, Stewart CE, Rotwein P (2000) Dual control of muscle cell survival by distinct growth factor-regulated signaling pathways. Mol Cell Biol 20:3256–3265
Lee SJ, McPherron AC (2001) Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci U S A 98:9306–9311
Lefaucheur J, Sebille A (1995a) Basic fibroblast growth factor promotes in vivo muscle regeneration in murine muscular dystrophy. Neurosci Lett 202:121–124
Lefaucheur J, Sebille A (1995b) The cellular events of injured muscle regeneration depend on the nature of the injury. Neuromuscul Disord 5:501–509
Lefaucheur JP, Gjata B, Lafont H, Sebille A (1996) Angiogenic and inflammatory responses following skeletal muscle injury are altered by immune neutralization of endogenous basic fibroblast growth factor, insulin-like growth factor-1 and transforming growth factor-beta 1. J Neuroimmunol 70:37–44
Lescaudron L, Peltekian E, Fontaine-Perus J, Paulin D, Zampieri M, Garcia L, Parrish E (1999) Blood borne macrophages are essential for the triggering of muscle regeneration following muscle transplant. Neuromuscul Disord 9:72–80
Levinovitz A, Jennische E, Oldfors A, Edwall D, Norstedt G (1992) Activation of insulin-like growth factor II expression during skeletal muscle regeneration in the rat: correlation with myotube formation. Mol Endocrinol 6:1227–1234
Li YP (2003) TNF-α is a mitogen in skeletal muscle. Am J Phys Cell Phys 285:C370–C376
Litwiniuk A, Pijet B, Pijet-Kucicka M, Gajewska M, Pająk B, Orzechowski A (2016) FOXO1 and GSK-3β are main targets of insulin-mediated myogenesis in C2C12 muscle cells. PLoS One 11:e0146726. doi:10.1371/journal.pone.0146726
Liu D, Black BL, Derynck R (2001) TGF-beta inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3. Genes Dev 15:2950–2966. doi:10.1101/gad.925901
Lolmede K, Campana L, Vezzoli M, Bosurgi L, Tonlorenzi R, Clementi E, Bianchi ME, Cossu G, Manfredi AA, Brunelli S, Rovere-Querini P (2009) Inflammatory and alternatively activated human macrophages attract vessel-associated stem cells, relying on separate HMGB1- and MMP-9-dependent pathways. J Leukoc Biol 85:779–787
MacIntyre DL, Sorichter S, Mair J, Berg A, McKenzie DC (2001) Markers of inflammation and myofibrillar proteins following eccentric exercise in humans. Eur J Appl Physiol 84:180–186
Malm C, Nyberg P, Engstrom M, Sjodin B, Lenkei R, Ekblom B (2000) Immunological changes in human skeletal muscle and blood after eccentric exercise and multiple biopsies. J Physiol 529:243–262
Massimino M, Rapizzi E, Cantini M, Libera L, Mazzoeni F, Arsian P, Carraro U (1997) ED2+ macrophages increase selectively myoblast proliferation in muscle cultures. Biochem Biophys Res Commun 235:754–759
Matthys P, Mitera T, Heremans H, Van Damme J, Billiau A (1995) Anti-gamma interferon and anti-interleukin-6 antibodies affect staphylococcal enterotoxin B-induced weight loss, hypoglycemia, cytokine release in D-galactosamine-sensitized and unsensitized mice. Infect Immun 63:1158–1164
McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R (2003) Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol 162:1135–1147. doi:10.1083/jcb.200207056
McFarland DC, Pesall JE, Gilkerson KK (1993) The influence of growth factors on turkey embryonic myoblasts and satellite cells in vitro. Gen Comp Endocrinol 89:415–424
McPherron AC, Lee SJ (1997) Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci U S A 94:12457–12461
McPherron AC, Lawler AM, Lee SJ (1997) Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387:83–90
Mendias CL, Tatsumi R, Allen RE (2004) Role of cyclooxygenase-1 and -2 in satellite cell proliferation, differentiation, and fusion. Muscle Nerve 30:497–500
Mendler L, Zador E, Ver Heyen M, Dux L, Wuytack F (2000) Myostatin levels in regenerating rat muscles and in myogenic cell cultures. J Muscle Res Cell Motil 21:551–563
Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M (2012) IL-6/IL6 receptor system and its role in physiological and pathological conditions. Clin Sci 122:143–159
Miller KJ, Thaloor D, Matteson S, Pavlath GK (2000) Hepatocyte growth factor affects satellite cell activation and differentiation in regenerating skeletal muscle. Am J Phys Cell Phys 278:C174–C181
Mizuhara H, O’Neill E, Seki N, Ogawa T, Kusunoki C, Otsuka K, Satoh S, Niwa M, Senoh H, Fujiwara H (1994) T cell activation associated hepatic injury: mediation by tumor necrosis factors and protection by interleukin 6. J Exp Med 179:1529–1537
Mourkioti F, Rosenthal N (2008) NF-B signaling in skeletal muscle: prospects for intervention in muscle diseases. J Mol Med 86:747–759
Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N (2001) Localized IGF-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet 27:195–200
Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, Tashiro K, Shimizu S (1989) Molecular cloning and expression of human hepatocyte growth factor. Nature 342:440–443
Nakamura T, Teramoto H, Ichihara A (1986) Purification and characterization of a growth factor from rat platelets for mature parenchymal hepatocytes in primary cultures. Proc Natl Acad Sci 83:6489–6493
Nielsen AR, Mounier R, Plomgaard P, Mortensen OH, Penkowa M, Speerschneider T, Pilegaard H, Pedersen BK (2007a) Expression of interleukin-15 in human skeletal muscle: effect of exercise and muscle fibre type composition. J Physiol 584:305–312
Nieman DC, Davis JM, Brown VA, Henson DA, Dumke CL, Utter AC, Vinci DM, Downs MF, Smith JC, Carson J, Brown A, McAnulty SR, McAnulty LS (2004) Influence of carbohydrate ingestion on immune changes after 2 h of intensive resistance training. J Appl Physiol 96:1292–1298
Nieman DC, Davis JM, Henson DA, Walberg-Rankin J, Shute M, Dumke CL, Utter AC, Vinci DM, Carson JA, Brown A, Lee WJ, McAnulty SR, McAnulty LS (2003) Carbohydrate ingestion influences skeletal muscle cytokine mRNA and plasma cytokine levels after a 3-h run. J Appl Physiol 94:1917–1925
Nieman DC, Henson DA, McAnulty SR, McAnulty L, Swick NS, Utter AC, Vinci DM, Opiela SJ, Morrow JD (2002) Influence of vitamin C supplementation on oxidative and immune changes after an ultramarathon. J Appl Physiol 92:1970–1977
Nieman DC, Henson DA, Smith LL, Utter AC, Vinci DM, Davis JM, Kaminsky DE, Shute M (2001) Cytokine changes after a marathon race. J Appl Physiol 91:109–114
Nishimoto N, Yoshizaki K, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, Hashimoto J, Azuma J, Kishimoto T (2004) Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum 50:1761–1769
Norrby K (1996) Interleukin-8 and de novo mammalian angiogenesis. Cell Prolif 29:315–323
Orzechowski A, Łokociejewska M, Pawlikowska P, Kruszewski A (2005) Preincubation with sodium ascorbate potentiates insulin-dependent PKB/Akt and c-Jun phosphorylation in L6 rat myoblasts challenged with reactive oxygen/nitrogen species. Life Sci 77:496–511
Ostrowski K, Hermann C, Bangash A, Schjerling P, Nielsen JN, Pedersen BK (1998a) A trauma-like elevation of plasma cytokines in humans in response to treadmill running. J Physiol 513:889–894
Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK (2001) Chemokines are elevated in plasma after strenuous exercise in humans. Eur J Appl Physiol 84:244–245
Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK (1999) Pro and anti-inflammatory cytokine balance in strenuous exercise in humans. J Physiol 515:287–291
Ostrowski K, Rohde T, Zacho M, Asp S, Pedersen BK (1998b) Evidence that IL-6 is produced in skeletal muscle during prolonged running. J Physiol 508:949–953
Ostrowski K, Schjerling P, Pedersen BK (2000) Physical activity and plasma interleukin-6 in humans: effect of intensity of exercise. Eur J Appl Physiol 83:512–515
Panagiotakos DB, Pitsavos C, Chrysohoou C, Kavouras S, Pedersen BK, Febbraio MA (2008) Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 88:1379–1406
Pedersen BK, Febbraio MA (2008) Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 88:1379–1406
Pedersen BK, Saltin B (2006) Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports 16(Suppl 1):3–63
Pedersen BK, Steensberg A, Fischer C, Keller C, Keller P, Plomgaard P, Wolsk-Petersen E, Febbraio M (2004) The metabolic role of IL-6 produced during exercise: is IL-6 an exercise factor? Proc Nutr Soc 63:263–267
Pedersen BK, Steensberg A, Keller P, Keller C, Fischer C, Hiscock N (2003b) Hall Gv Plomgaard P and Febbraio MA. Muscle-derived interleukin-6: lipolytic, anti-inflammatory and immune regulatory effects. Pflugers Arch 446:9–16
Pedersen M, Bruunsgaard H, Weis N, Hendel HW, Andreassen BU, Eldrup E, Dela F, Pedersen BK (2003a) Circulating levels of TNF-alpha and IL-6-relation to truncal fat mass and muscle mass in healthy elderly individuals and in patients with type-2 diabetes. Mech Ageing Dev 124:495–502
Penkowa M, Keller C, Keller P, Jauffred S, Pedersen BK (2003) Immunohistochemical detection of interleukin-6 in human skeletal muscle fibers following exercise. FASEB J 17:2166–2168
Philippou A, Halapas A, Maridaki M, Koutsilieris M (2004) Type I insulin-like growth factor receptor signaling in skeletal muscle regeneration and hypertrophy. J Musculoskelet Neuronal Physiol 96:1292–1298
Pijet B, Pijet M, Litwiniuk A, Gajewska M, Pajak B, Orzechowski A (2013a) TNF- and IFN-s-Dependent Muscle Decay Is Linked to NF- B- and STAT-1 -Stimulated Atrogin1 and MuRF1 Genes in C2C12 Myotubes. Mediators Inflamm 2013 (171437): 18 http://dx.doi.org/10.1155/2013/171437
Pijet M, Pijet B, Litwiniuk A, Pajak B, Gajkowska B, Orzechowski A (2013b) Leptin impairs myogenesis in C2C12 cells through JAK/STAT and MEK signaling pathways. Cytokine 61:445–454
Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK (2005) TNF-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes 54:2939–2945
Plomgaard P, Nielsen AR, Fischer CP, Mortensen OH, Broholm C, Penkowa M, Krogh-Madsen R, Erikstrup C, Lindegaard B, Petersen AM, Taudorf S, Pedersen BK (2007) Associations between insulin resistance and TNF-alpha in plasma, skeletal muscle and adipose tissue in humans with and without type 2 diabetes. Diabetologia 50:2562–2571
Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM (2001) C-reactive protein, interleukin 6, risk of developing type 2 diabetes mellitus. JAMA 286:327–334
Quinn LS, Anderson BG, Drivdahl RH, Alvarez B, Argiles JM (2002) Overexpression of interleukin-15 induces skeletal muscle hypertrophy in vitro: implications for treatment of muscle wasting disorders. Exp Cell Res 280:55–63
Quinn LS, Haugk KL, Damon SE (1997) Interleukin-15 stimulates C2 skeletal myoblast differentiation. Biochem Biophys Res Commun 239:6–10
Quinn LS, Strait-Bodey L, Anderson BG, Argiles JM, Havel PJ (2005) Interleukin-15 stimulates adiponectin secretion by 3 T3–L1 adipocytes: evidence for a skeletal muscle-to-fat signaling pathway. Cell Biol Int 29:449–457
Ramprasad MP, Fischer W, Witztum JL, Sambrano GR, Quehenberger O, Steinberg D (1995) The 94- to 97-kDa mouse macrophage membrane protein that recognizes oxidized low density lipoprotein and phosphatidylserine-rich liposomes is identical to macrosialin, the mouse homologue of human CD68. Proc Natl Acad Sci U S A 92:9580–9584
Rapraeger AC (2000) Syndecan-regulated receptor signaling. J Cell Biol 149:995–998
Rhind SG, Gannon GA, Shephard RJ, Shek PN (2002) Indomethacin modulates circulating cytokine responses to strenuous exercise in humans. Cytokine 19:153–158
Roberts CK, Barnard RJ, Jasman A, Balon TW (1999) Acute exercise increases nitric oxide synthase activity in skeletal muscle. Am J Physiol Endocrinol Metab 277:E390–E394
Roubenoff R, Roubenoff RA, Cannon JG, Kehayias JJ, Zhuang H, Lawson-Hughes B, Dinarello CA, Rosenberg IH (1994) Rheumatoid cachexia: cytokine-driven hypermetabolism accompanying reduced body cell mass in chronic inflammation. J Clin Invest 93:2379–2386
Sacheck JM, Ohtsuka A, McLary SC, Goldberg AL (2004) IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am J Physiol Endocrinol Metab 287:E591–E601
Salehian B, Mahabadi V, Bilas J, Taylor WE, Ma K (2006) The effect of glutamine on prevention of glucocorticoid-induced skeletal muscle atrophy is associated with myostatin suppression. Metabolism 55:1239–1247. doi:10.1016/j.metabol.2006.05.009
Sarbassov D, Jones LG, Peterson CA (1997) Extracellular Signal-Regulated Kinase-1 and -2 Respond Differently to Mitogenic and Differentiative Signaling Pathways in Myoblasts. Mol Endocrinol 11:2038–2047
Sarbassov D, Stefanova R, Grigoriev VG, Peterson CA (1995) Role of insulin-like growth factors and myogenin in the altered program of proliferation and differentiation in the NFB4 mutant muscle cell line. Proc Natl Acad Sci U S A 92:10874–10878
Scata KA, Bernard DW, Fox J, Swain JL (1999) FGF receptor availability regulates skeletal myogenesis. Exp Cell Res 250:10–21
Schaeper U, Birchmeier W (2004) Cell migration in development and disease. HGF/SF c-Met signaling in the epithelial-mesenchymal transition and migration of muscle progenitor cells. Ed. Doris Wedlich. Wiley-VCH 11:191–202
Schuelke M, Wagner KR, Stolz LE, Hubner C, Riebel T, Komen W, Braun T, Tobin JF, Lee S-J (2004) Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med 350:2682–2688
Sheehan SM, Tatsumi R, Temm-Grove CJ, Allen RE (2000) HGF is an autocrine growth factor for skeletal muscle satellite cells in vitro. Muscle Nerve 23:239–245
Shi X, Garry DJ (2006) Muscle stem cells in development, regeneration, and disease. Genes Dev 20:1692–1708
Silveira LR, Pereira-Da-Silva L, Juel C, Hellsten Y (2003) Formation of hydrogen peroxide and nitric oxide in rat skeletal muscle cells during contractions. Free Radic Biol Med 35:455–464
Smith MA, Moylan JS, Smith JD, Li W, Reid MB (2007) IFN-gamma does not mimic the catabolic effects of TNF-alpha. Am J Phys Cell Phys 293:C1947–C1952
Spiller MP, Kambadur R, Jeanplong F, Thomas M, Martyn JK, Bass JJ, Sharma M (2002) The myostatin gene is a downstream target gene of basic helix-loop-helix transcription factor MyoD. Mol Cell Biol 22:7066–7082. doi:10.1128/MCB.22.20.7066-7082.2002
St. Pierre BA, Tidball JG (1994) Differential response of macrophage subpopulations to soleus muscle reloading after rat hindlimb suspension. J Appl Physiol 77:290–297
Stahl N, Farruggella TJ, Boulton TG, Zhong Z, Darnell JE Jr, Yancopoulos GD (1995) Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science 267:1349–1353
Starkie RL, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK (2003) Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J 17:884–886
Starkie RL, Angus DJ, Rolland J, Hargreaves M, Febbraio M (2000) Effect of prolonged submaximal exercise and carbohydrate ingestion on monocyte intracellular cytokine production in humans. J Physiol 528:647–655
Starkie RL, Arkinstall MJ, Koukoulas I, Hawley JA, Febbraio MA (2001a) Carbohydrate ingestion attenuates the increase in plasma interleukin-6, but not skeletal muscle interleukin-6 mRNA, during exercise in humans. J Physiol 533:585–591
Starkie RL, Rolland J, Angus DJ, Anderson MJ, Febbraio MA (2001b) Circulating monocytes are not the source of elevations in plasma IL-6 and TNF-alpha levels after prolonged running. Am J Phys Cell Phys 280:C769–C774
Steensberg A, Febbraio MA, Osada T, Schjerling P, van Hall G, Saltin B, Pedersen BK (2001a) Interleukin-6 production in contracting human skeletal muscle is influenced by pre-exercise muscle glycogen content. J Physiol 537:633–639
Steensberg A, Fischer CP, Keller C, Moller K, Pedersen BK (2003) IL-6 enhances plasma IL-1ra, IL-10, cortisol in humans. Am J Physiol Endocrinol Metab 285:E433–E437
Steensberg A, Keller C, Hillig T, Frosig C, Wojtaszewski JF, Pedersen BK, Pilegaard H, Sander M (2007) Nitric oxide production is a proximal signaling event controlling exercise-induced mRNA expression in human skeletal muscle. FASEB J 21:2683–2694
Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B, Klarlund PB (2000) Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J Physiol 529:237–242
Stefanadis C (2005) The associations between leisure-time physical activity and inflammatory and coagulation markers related to cardiovascular disease: the ATTICA Study. Prev Med 40:432–437
Stouthard JM, van der Romijn JAPT, Endert E, Klein S, Bakker PJ, Veenhof CH, Sauerwein HP (1995) Endocrinologic and metabolic effects of interleukin-6 in humans. Am J Phys 268:E813–E819
Suelves M, Lopez-Alemany R, Lluis F, Aniorte G, Serrano E, Parra M, Carmeliet P, Munoz-Canoves P (2002) Plasmin activity is required for myogenesis in vitro and skeletal muscle regeneration in vivo. Blood 99:2835–2844
Suzuki S, Yamanouchi K, Soeta C, Katakai Y, Harada R, Naito K, Tojo H (2002) Skeletal muscle injury induces hepatocyte growth factor expression in spleen. Biochem Biophys Res Commun 292:709–714
Szabo G, Dallmann G, Muller G, Patthy L, Soller M, Varga L (1998) A deletion in the myostatin gene causes the compact (Cmpt) hypermuscular mutation in mice. Mamm Genome 9:671–672
Tatsumi R, Anderson JE, Nevoret CJ, Halevy O, Allen RE (1998) HGF/SF is present in normal adult skeletal muscle and is capable of activating satellite cells. Dev Biol 194:114–128
Tatsumi R, Hattori A, Ikeuchi Y, Anderson JE, Allen RE (2002) Release of hepatocyte growth factor from mechanically stretched skeletal muscle satellite cells and role of pH and nitric oxide. Mol Biol Cell 13:2909–2918
Taylor WE, Bhasin S, Artaza J, Byhower F, Azam M, Willard DH Jr, Kull FC Jr, Gonzalez-Cadavid N (2001) Myostatin inhibits cell proliferation and protein synthesis in C2C12 muscle cells. Am J Physiol Endocrinol Metab 280:E221–E228
Thomas M, Langley B, Berry C, Sharma M, Kirk S, Bass J, Kambadur R (2000) Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem 275:40235–40243. doi:10.1074/jbc. M004356200
Thompson D, Williams C, McGregor SJ, Nicholas CW, McArdle F, Jackson MJ, Powell JR (2001) Prolonged vitamin C supplementation and recovery from demanding exercise. Int J SportNutr Exerc Metab 11:466–481
Tidball JG, Villalta SA (2010) Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Phys Regul Integr Comp Phys 298:R1173–R1187
Tidball JG, Berchenko E, Frenette J (1999) Macrophage invasion does not contribute to muscle membrane injury during inflammation. J Leukoc Biol 65:492–498
Tisdale MJ (1999) Wasting in cancer. J Nutr 129:243S–246S
Tkatchenko AV, Le Cam G, Leger JJ, Dechesne CA (2000) Largescale analysis of differential gene expression in the hindlimb muscles and diaphragm of mdx mouse. Biochim Biophys Acta 1500:17–30
Tolosa L, Morla M, Iglesias A, Busquets X, Llado J, Olmos G (2005) IFN-gamma prevents TNF-alpha-induced apoptosis in C2C12 myotubes through down-regulation of TNF-R2 and increased NF-kappaB activity. Cell Signal 17:1333–1342
Torrente Y, El Fahime E, Caron NJ, Del Bo R, Belicchi M, Pisati F, Tremblay JP, Bresolin N (2003) Tumor necrosis factor-alpha (TNF- ) stimulates chemotactic response in mouse myogenic cells. Cell Transplant 12:91–100
Torres SH, De Sanctis JB, De Briceño L, Hernandez N, Finol HJ (2004) Inflammation and nitric oxide production in skeletal muscle of type 2 diabetic patients. J Endocrinol 181:419–427
Tseng BS, Zhao P, Pattison JS, Gordon SE, Granchelli JA, Madsen RW, Folk LC, Hoffman EP, Booth FW (2002) Regenerated mdx mouse skeletal muscle shows differential mRNA expression. J Appl Physiol 93:537–545
Van Dam H, Castellazzi M (2001) Distinct roles of Jun:Fos and Jun:ATF dimers in oncogenesis. Oncogene 20:2453–2464
Van Hall G, Steensberg A, Sacchetti M, Fischer C, Keller C, Schjerling P, Hiscock N, Moller K, Saltin B, Febbraio MA, Pedersen BK (2003) Interleukin-6 stimulates lipolysis and fat oxidation in humans. J Clin Endocrinol Metab 88:3005–3010
Vassilakopoulos T, Karatza MH, Katsaounou P, Kollintza A, Zakynthinos S, Roussos C (2003) Antioxidants attenuate the plasma cytokine response to exercise in humans. J Appl Physiol 94:1025–1032
Villalta SA, Nguyen HX, Deng B, Gotoh T, Tidball JG (2009) Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum Mol Genet 18:482–496
Wagner KR, McPherron AC, Winik N, Lee SJ (2002) Loss of myostatin attenuates severity of muscular dystrophy in mdx mice. Ann Neurol 52:832–836
Wang H, Hertlein E, Bakkar N, Sun H, Acharyya S, Wang J, Carathers M, Davuluri R, Guttridge DC (2007) NF-κB regulation of YY1 inhibits skeletal myogenesis through transcriptional silencing of myofibrillar genes. Mol Cell Biol 27:4374–4387
Warren GL, Hulderman T, Jensen N, McKinstry M, Mishra M, Luster MI, Simeonova PP (2002) Physiological role of tumor necrosis factor α in traumatic muscle injury. FASEB J 16:1630–1632
Watt MJ, Dzamko N, Thomas WG, Rose-John S, Ernst M, Carling D, Kemp BE, Febbraio MA, Steinberg GR (2006) CNTF reverses obesity-induced insulin resistance by activating skeletal muscle AMPK. Nat Med 12:541–548
Watts R, McAinch AJ, Dixon JB, O’Brien PE, Cameron-Smith D (2013) Increased Smad signaling and reduced MRF expression in skeletal muscle from obese subjects. Obesity (SilverSpring) 21:525–528. doi:10.1002/oby.20070
Wieteska-Skrzeczyńska W, Grzelkowska-Kowalczyk K, Rejmak E (2011a) Growth factor and cytokine interactions in myogenesis. Part II. Expression of IGF binding proteins and protein kinases essential for myogenesis in mouse C2C12 myogenic cells exposed to TNF-α and IFN-γ. Pol J Vet Sci 14:425–431
Wieteska-Skrzeczyńska W, Grzelkowska-Kowalczyk K, Tokarska J, Grabiec K (2011b) Growth factor and cytokine interactions in myogenesis. Part I. The effect of TNF-α and IFN-γ on IGF-I-dependent differentiation in mouse C2C12 myogenic cells. Pol J Vet Sci 14:417–424
Willerson JT, Ridker PM (2004) Inflammation as a cardiovascular risk factor. Circulation 109:II2–I10
Williamson D, Gallagher P, Harber M, Hollon C, Trappe S (2003) Mitogen-activated protein kinase (MAPK) pathway activation: effects of age and acute exercise on human skeletal muscle. J Physiol 547:977–987
Willoughby DS, McFarlin B, Bois C (2003) Interleukin-6 expression after repeated bouts of eccentric exercise. Int J Sports Med 24:15–21
Wojtaszewski JF, Jorgensen SB, Frosig C, Macdonald C, Birk JB, Richter EA (2003) Insulin signalling: effects of prior exercise. Acta Physiol Scand 178:321–328
Woods A, zzout-Marniche D, Foretz M, Stein SC, Lemarchand P, Ferre P, Foufelle F, Carling D (2000) Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol Cell Biol 20:6704–6711
Yablonka-Reuveni Z, Seger R, Rivera AJ (1999) Fibroblast growth factor promotes recruitment of skeletal muscle satellite cells in young and old rats. J Histochem Cytochem 47:23–42
Yamanouchi K, Soeta C, Naito K, Tojo H (2000) Expression of myostatin gene in regenerating skeletal muscle of the rat and its localization. Biochem Biophys Res Commun 270:510–516
Yang SY, Goldspink G (2002) Different roles of the IGF-I Ec peptide (MGF) and mature IGF-I in myoblast proliferation and differentiation. FEBS Lett 522:156–160
Yordy JS, Muise-Helmericks RC (2000) Signal transduction and the Ets family of transcription factors. Oncogene 19:6503–6513
Zádor E, Mendler L, Takács V, de Bleecker J, Wuytack F (2001) Regenerating soleus and extensor digitorum longus muscles of the rat show elevated levels of TNF-α and its receptors, TNFR-60 and TNFR-80. Muscle Nerve 24:1058–1067
Zarnegar R, Michalopoulos GK (1995) The many faces of hepatocyte growth factor: from hepatopoiesis to hematopoiesis. J Cell Biol 129:1177–1180
Zerria K, Jerbi E, Hammami S, Maaroufi A, Boubaker S, Xiong JP, Arnaout MA, Fathallah DM (2006) Recombinant integrin CD11b A-domain blocks polymorphonuclear cells recruitment and protects against skeletal muscle inflammatory injury in the rat. Immunology 1194:431–440
Zetser A, Gredinger E, Bengal E (1999) p38 mitogen-activated protein kinase pathway promotes skeletal muscle differentiation. Participation of the Mef2c transcription factor. J Biol Chem 274:5193–5200
Zhu X, Hadhazy M, Wehling M, Tidball JG, McNally EM (2000) Dominant negative myostatin produces hypertrophy without hyperplasia in muscle. FEBS Lett 474:71–75
Acknowledgements
Support for this work was provided by grant No UMO-2013/11/B/NZ5/03106 from the National Science Centre in Poland.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Orzechowski, A. (2017). Cytokines in Skeletal Muscle Growth and Decay. In: Sakuma, K. (eds) The Plasticity of Skeletal Muscle. Springer, Singapore. https://doi.org/10.1007/978-981-10-3292-9_5
Download citation
DOI: https://doi.org/10.1007/978-981-10-3292-9_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-3291-2
Online ISBN: 978-981-10-3292-9
eBook Packages: MedicineMedicine (R0)