Abstract
Obtaining of good-quality image is essential to make accurate measurements. The image should be correctly calibrated for z-offset, the zero-point setting of the system before measurements. The definition of lesion, reference, and stented segment from the Journal of American College of Cardiology intravascular ultrasound (IVUS) consensus document has been adopted for optical coherence tomography (OCT) [1]. For standardization of OCT measurement, expert review documents and consensus standards have been published previously [2–4]. Studies regarding the accuracy and the reproducibility of qualitative and quantitative OCT measurements have been published previously [5–7].
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Obtaining of good-quality image is essential to make accurate measurements. The image should be correctly calibrated for z-offset, the zero-point setting of the system before measurements. The definition of lesion, reference, and stented segment from the Journal of American College of Cardiology intravascular ultrasound (IVUS) consensus document has been adopted for optical coherence tomography (OCT) [1]. For standardization of OCT measurement, expert review documents and consensus standards have been published previously [2,3,4]. Studies regarding the accuracy and the reproducibility of qualitative and quantitative OCT measurements have been published previously [5,6,7].
1 Border Identification
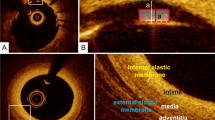
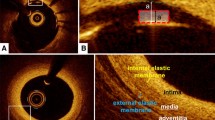
The borders of the lumen, external elastic membrane (EEM) , internal elastic membrane (IEM), plaque, and stent could be demarcated in OCT cross-sectional images similar to IVUS. In normal vessel without any plaque, OCT may discriminate IEM which is defined as the border between the intima and media and EEM which is defined as the border between the media and the adventitia. Measurements that EEM uses are likely closer to those of IVUS, whereas IEM measurements that use the IEM more closely approximate the pathologic definition of atherosclerosis as a disease of the intima. However, because of low penetration depth and rapid attenuation of its signal, OCT could not visualize IEM or EEM border in most diseased segments. The border measurements should not be made in cross-sectional images that contain artifacts that obscure a significant portion (>90°) of the image or over regions that contain side branches. The differences between OCT and IVUS -->measurements were demonstrated in Table 12.1 and Fig. 12.1.
Comparision of border detection between optical coherence tomography (OCT) and intravascular ultrasound (IVUS). Normal artery wall shows a 3-layered architecture, comprising a high backscattering, thin intima, a low backscattering media, a heterogeneous and/or high backscattering adventitia in both OCT (a) and IVUS (b). OCT could visualize internal elastic membrane (IEM) and external elastic membrane (EEM) (bold arrow heads) (inset, x3). The OCT-derived EEM or IEM measurement could not be made in cross-sectional image that contains diseased vessel (c) whereas IVUS demonstrate EEM border well (d). * represents wire artifact. CSA cross sectional area; PB plaque burden
2 Lesion Assessment
2.1 Reference Segment
Reference Assessment
Proximal or distal reference is defined as the sites with the largest lumen proximal or distal to a stenosis within the same segment with no major intervening branches (usually within 10 mm of the stenosis).
Reference Lumen and EEM Assessment
Proximal or distal mean reference lumen diameter is the mean value of the shortest and the longest lumen diameter through the center of mass of the lumen at proximal or distal reference site. Proximal or distal mean reference EEM diameter is the mean value of the shortest and the longest EEM diameter through the center of mass of the lumen at proximal or distal reference site.
Average reference lumen diameter is the average value of mean lumen diameter at the proximal and distal reference sites. Average reference -->EEM diameter is the average value of mean EEM diameter at the proximal and distal reference sites. Both average reference lumen diameter and average reference EEM diameter are useful parameters for stent sizing during PCI.
Average reference EEM CSA , which is a useful parameter for evaluation of lesion severity in terms of stenosis, is the average value of EEM CSA at the proximal and distal reference sites.
Recent in the OPINION study , which had a randomized controlled design to compare the benefit of OCT guidance with IVUS guidance during percutaneous coronary intervention (PCI), OCT reference site was defined as the most normal-looking site with free of lipidic plaque (defined as signal-poor region with diffuse border) at a cross-section adjacent to the target lesion [8]. In other randomized controlled study, the ILUMIEN III: OPTIMIZE PCI study comparing OCT guidance, IVUS guidance, or angiography-guided stent implantation, proximal and distal reference mean EEM diameters and the smaller of these diameters to determine stent diameter or the proximal and distal lumen diameters were used if the EEM could not be visualized [9].
2.2 Lesion Segment
Lumen Measurements
Lumen CSA is the area bounded by the luminal border. Minimum lumen diameter is the shortest diameter through the center of mass of the lumen. Maximum lumen diameter is the longest diameter through the center of mass of the lumen. Lumen eccentricity is calculated as (maximum lumen diameter minus minimum lumen diameter) divided by maximum lumen diameter.
OCT-measured lumen CSA is well correlated with IVUS-measured lumen CSA. In both phantom models and in vivo study comparing quantitative coronary analysis (QCA) for angiography vs IVUS vs OCT measurements, OCT was most precise to the real value, and IVUS measurement was 8% larger than OCT measurement [6]. The mean minimum lumen diameter (MLD) measured by QCA was 5% smaller than that measured by OCT, and the minimum lumen diameter measured by IVUS was 9% greater than that measured by FD-OCT [6].
Previously several studies regarding IVUS criteria for defining the functional significance evaluated with fractional flow reserve (FFR) demonstrated that MLD had a good correlation with the FFR values, but the utility of IVUS MLA as an alternative to FFR to guide intervention in intermediate lesions may be limited in accuracy and vessel dependent [10,11,12,13]. Anatomical measurements of coronary stenosis obtained by OCT show significant correlation with FFR. OCT-derived parameters were smaller than those reported in previous IVUS studies (Table 12.2) [14, 15]. Recent study assessing computational fractional flow reserve from OCT in patient with intermediate stenosis showed promising approach of it in assessment not only of anatomic information but also of the functional significance of intermediate stenosis [16].
EEM Measurements
EEM CSA is the area bounded by EEM border as a surrogated parameter for vessel area. A discrete interface at the border between the media and the adventitia is almost invariably present within OCT images and corresponds closely to the location of the EEM. Because of low penetration depth of OCT signal and rapid OCT signal attenuation within plaque, EEM circumference and area mostly cannot be measured reliably especially in lesion segment. If low signal involves a relatively small arc (<90°), planimetry of the circumference can be performed by extrapolation from the closest identifiable EEM borders, although measurement accuracy and reproducibility will be reduced.
Plaque (or Atheroma) Measurement
Plaque (or atheroma) CSA is the EEM CSA minus the lumen CSA. Maximum plaque (or atheroma) thickness is the largest distance from the intimal leading edge to the EEM along any line passing through the center of mass of the lumen. Minimum plaque (or atheroma) thickness is the shortest distance from the intimal leading edge to the EEM along any line passing through the center of mass of the lumen. Plaque (or atheroma) eccentricity is calculated as (maximum plaque thickness minus minimum plaque thickness) divided by maximum plaque thickness. If EEM area could not be obtained, plaque measurement is not available.
Plaque Burden
Plaque (or atheroma) burden is assessed as plaque CSA divided by the EEM CSA. This parameter can only be defined when the EEM can be demonstrated. The plaque burden is distinct from the luminal area stenosis. The former represents the area within the EEM occupied by atheroma regardless of lumen compromise. The latter is a measure of luminal compromise relative to a reference lumen analogous to the angiographic diameter stenosis. If EEM area cannot be obtained, plaque burden cannot be assessed.
Lumen Area Stenosis
Lumen area stenosis is assessed as reference lumen CSA minus minimum lumen CSA divided by reference lumen CSA.
Plaque Component and Other Measurements
The presence of specific component within the plaque or over the plaque, such as calcium, lipid, or thrombus, could be assessed as quantitative measurements like angle, depth, thickness, or area. Angle or arc could be measured using the center of mass of the lumen as the angle point. Depth is the distance between the lumen and the leading edge of the plaque feature. Thickness is usually assessed as the thickest distance between the inner and outer surfaces of the plaque component (valid only if the deep boundary can be identified). Area of some component could be described as the CSA of the plaque component (valid only if the deep boundary can be identified).
Fibrous cap thickness can be measured by the thickness of a cap present over OCT-delineated lipid or necrotic core either at the single cross-section where the fibrous cap thickness is considered minimal or from multiple samples (three or more). Although studies have been performed to compare the OCT measurement of fibrous cap thickness with histologic measurements of cap thickness, it was generally considered that this area needs further validation, as the boundary between the cap and the necrotic core is not always straightforward to precisely determine.
Remodeling
An index of remodeling can be assessed as lesion EEM CSA/reference EEM CSA, if the EEM CSA is identified in OCT image.
Because of its limited tissue penetration, OCT does not appear to be suited to study vessel remodeling.
3 Stent Measurements
OCT has been considered as an useful intracoronary imaging modality for the lesion assessment, stent sizing, and stent optimization during PCI (Figs. 12.2 and 12.3). The Clinical usefulness of OCT-guided PCI will be discussed in next chapter (Chap. 13).
Pre-percutaneous coronary intervention optical coherence tomography measurements. Both proximal and distal reference was obtained at normal looking segments from longitudinal and cross-sectional images. Mean reference lumen diameter (ø) was assessed from the each cross-sectional images (a and c) and the lesion length was the distance between proximal and distal reference segments. Minimum lumen area (MLA) was measured by detection of lumen border at the most narrowest site (b)
OCT is capable of visualizing the vascular response between stent strut and vessel wall, and identifying tissues surrounding stent struts. Most metallic stent struts have strong reflection to optic signal creating a bright hyperintense signal at the surface of strut (blooming appearance) with a shadow that obscures deeper structure within the vessel. The polymeric struts of bioabsorbable vascular scaffolds are transparent to the optic signal, allowing visualization of the vascular wall structure behind the struts without shadowing (Fig. 12.4).
Comparison of stent struts detection between metal stent and bioabsorbable vascular scaffolds (BVS) assessed by optical coherence tomography.Metallic drug eluting stent struts have strong reflection to optic signal creating a bright hyper-intense signal at the surface of strut (blooming appearance) with a shadow that obscures deeper structure within the vessel (a). BVS are transparent to the optic signal, allowing visualization of the vascular wall structure behind the struts without shadowing (b)
Strut assessment is limited by the axial resolution of the OCT system, and OCT could not allow the visualization of a single layer of endothelial cells. Furthermore the biological and clinical significance of some OCT-derived stent measurements within stent segment has not been fully understood. Recently a retrospective data evaluating OCT measurements to predict very late stent thrombosis demonstrated that malapposition, neoatherosclerosis, uncovered struts, and stent underexpansion, without differences between patients treated with early- and new-generation drug-eluting stents, were leading OCT findings associated with very late stent thrombosis in descending order [17].
3.1 Stented Segment
Stent Area Measurements
Stent CSA is the area bounded by the stent border. Minimum stent diameters are the shortest diameter through the center point of the stent. Maximum stent diameters are the shortest and the longest diameter through the center point of the stent. Stent eccentricity (symmetry) is calculated as (maximum stent diameter minus minimum stent diameter) divided by maximum stent diameter. Stent expansion is calculated as the minimum stent CSA compared with the predefined reference area which can be the average reference lumen area or EEL area if possible. An underexpanded stent has an in-stent minimal lumen area less than 90% of the average reference lumen area. In the CLI-THRO study, which compared OCT parameters between in patient with subacute stent thrombosis and in those without, stent thrombus group had smaller OCT stent CSA (5.6 ± 2.6 vs 6.8 ± 1.7 mm2, p = 0.03) and higher incidence of stent underexpansion (42.8% vs 16.7%, p = 0.05) when compared with control group [18].
3.2 Stent Strut Measurements
OCT has been considered as the most useful intracoronary imaging modality to assess immediate- and long- term vascular response after stent implantation. Stent strut measurements can be obtained at a cross-section level or can be evaluated at the strut level analysis. (Fig. 12.5). The assessment of stent struts requires strict interval ranging from every 0.5 mm to 1 mm to obtain high rate of reproducibility. Stent strut maps can be computed with the x axis representing the length of the stent (millimeters) and the y axis representing the circumference (0–360°). A contour plot optical coherence tomography analysis for evaluating stent strut may provide more useful information to understanding the serial changes in strut coverage [19].
Stent Apposition
Incomplete stent apposition or malapposition occurs if there is a separation of a stent strut from the vessel wall. Malapposition is defined as a measured distance greater than the strut thickness for stent materials (metal or metal plus polymer). Malapposition distance could be measured as the distance between the luminal surface of the covering tissue and the luminal surface of the strut. The area between the endoluminal midpoint of the struts and the vessel wall was measured as malapposition area.
Acute, late-persistent, and late-acquired stent malapposition assessed by OCT has relatively high incidences, but their clinical importance and the mechanism have been shown different. The clinical outcome of acute malapposition is favorable, but late malapposition has been considered as a predictor of stent thrombosis [20].
Strut Coverage and Neointima Measurements
Strut coverage thickness is the distance between the luminal surface of the covering tissue and the luminal surface of the strut. Percentage of uncovered stent struts is calculated as the number of struts without distinct overlying tissue, in which the luminal reflection of the strut surface is directly interfacing with the lumen, divided by total number of analyzable struts.
Variable thickness of stent struts which consisted of metal and polymer should be considered to determine whether struts are “covered” or “uncovered.” OCT cannot visualize a single layer of endothelium over the strut, or it does not demonstrate accurate nature of tissue. In a case-controlled study, the presence of uncovered stent struts assessed by OCT was associated with late stent thrombosis after DES implantation [21]. Won et al. showed that the best cutoff value of percentage of uncovered struts for predicting major safety events (a composite occurrence of cardiovascular death, myocardial infarction, and stent thrombosis) was 5.9% using the maximal χ [2] method (area under the receiver-operating characteristic curve, 0.779; 95% confidence interval, 0.648–0.910; p = 0.019, a sensitivity of 83.3% and a specificity of 70.3%) [22].
Neointima area is defined as stent CSA minus lumen CSA. Percent neointima area is defined as (neointima area divided by stent CSA) X 100. The qualitative assessment of the neointima pattern is assessed at the site of the largest cross-sectional area of neointima within stent. OCT has been considered as the best tool to evaluate tissue characterization of neointima, and it also could discriminate neoatherosclerosis from intimal hyperplasia by OCT qualitative measurements.
The rates of stent strut coverage or the characteristics of neointima assessed by OCT surveillance differed according to stent type. The clinical implications of these differences require further study but may imply on the differences in rates of stent thrombosis observed in clinical trials with different stent types [23,24,25,26].
4 Length and Volume Measurements
OCT image acquisition is performed using motorized transducer pullback commonly at 100 frames/s with an automatic pullback speed of 20 mm/s. Longitudinal view is obtained automatically, and length measurements can be assessed from longitudinal view or calculated as the number of seconds by the pullback speed. This approach can be used to determine the length of a lesion, stenosis, stent, or any other longitudinal features (calcium, lipid, thrombus, etc.). OCT offered more accuracy than IVUS in longitudinal geometric measurement of coronary artery [27].
Lesion length is determined as the distance from distal to proximal reference site using the OCT automated lumen detection feature. Stent length is determined as the distance from distal to proximal edge of stent using the OCT automated lumen detection feature. Length measurements of any longitudinal features can be performed using motorized transducer pullback (number of seconds x pullback speed).
Volume measurements are calculated by Simpson’s rule and area measurements from every single frame usually at 0.5–1 mm.
References
Mintz GS, Nissen SE, Anderson WD, Rosenfield K, Bailey SR, Siegel RJ, et al. American College of Cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (IVUS): a report of the American College of Cardiology task force on clinical expert consensus documents developed in collaboration with the European Society of Cardiology endorsed by the Society of Cardiac Angiography and Interventions. J Am Coll Cardiol. 2001;37:1478–92.
Prati F, Regar E, Mintz GS, Arbustini E, Di Mario C, Jang IK, et al. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J. 2010;31:401–15.
Prati F, Guagliumi G, Mintz GS, Costa M, Regar E, Akasaka T, et al. Expert review document part 2: methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures. Eur Heart J. 2012;33:2513–20.
Tearney GJ, Regar E, Akasaka T, Adroaemssems T, Barlis P, Bezerra HG, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the international working group for intravascular optical coherence tomography standardization and validation. J Am Coll Cardiol. 2012;59:1058–72.
Gerbaud E, Weisz G, Tanaka A, Kashiwagi M, Shimizu T, Wang L, et al. Multi-laboratory inter-institute reproducibility study of IVOCT and IVUS assessments using published consensus document definitions. Eur Heart J Cardiovasc Imaging. 2016;17:756–64.
Kubo T, Akasaka T, Shite J, Suzuki T, Uemura S, Yu B, et al. OCT compared with IVUS in a coronary lesion assessment: the OPUS-CLASS study. JACC Cardiovasc Imaging. 2013;6:1095–104.
Tanimoto S, Rodriguez-Granillo G, Barlis P, de Winter S, Bruining N, Hamers R, et al. A novel approach for quantitative analysis of intracoronary optical coherence tomography: high inter-observer agreement with computer-assisted contour detection. Catheter Cardiovasc Interv. 2008;72:228–35.
Kubo T, Shinke T, Okamura T, Hibi K, Nakazawa G, Morino Y, et al. Optical frequency domain imaging vs. intravascular ultrasound in percutaneous coronary intervention (OPINION trial): study protocol for a randomized controlled trial. J Cardiol. 2016;68:455–60.
Ali ZA, Maehara A, Généreux P, Shlofmitz RA, Fabbiocchi F, Nazif TM, et al. Optical coherence tomography compared with intravascular ultrasound and with angiography to guide coronary stent implantation (ILUMIEN III: OPTIMIZE PCI): a randomised controlled trial. Lancet. 2016;388:2618–28.
Ben-Dor I, Torguson R, Deksissa T, Bui AB, Xue Z, Satler LF, et al. Intravascular ultrasound lumen area parameters for assessment of physiological ischemia by fractional flow reserve in intermediate coronary artery stenosis. Cardiovasc Revasc Med. 2012;13:177–82.
Kang SJ, Lee JY, Ahn JM, Mintz GS, Kim WJ, Park DW, et al. Validation of intravascular ultrasound-derived parameters with fractional flow reserve for assessment of coronary stenosis severity. Circ Cardiovasc Interv. 2011;4:65–71.
Koo BK, Yang HM, Doh JH, Choe J, Lee SY, Yoon CH, et al. Optimal intravascular ultrasound criteria and their accuracy for defining the functional significance of intermediate coronary stenoses of different locations. JACC Cardiovasc Interv. 2011;4:803–11.
Waksman R, Legutko J, Singh J, Orlando Q, Marso S, Schloss T, et al. FIRST: fractional flow reserve and intravascular ultrasound relationship study. J Am Coll Cardiol. 2013;61:917–23.
Gonzalo N, Escaned J, Alfonso F, Nolte C, Rodriques V, Jimenez-Quevedo P, et al. Morphometric assessment of coronary stenosis relevance with optical coherence tomography: a comparison with fractional flow reserve and intravascular ultrasound. J Am Coll Cardiol. 2012;59:1080–9.
Shiono Y, Kitabata H, Kubo T, Masuno T, Ohta S, Ozaki Y, et al. Optical coherence tomography-derived anatomical criteria for functionally significant coronary stenosis assessed by fractional flow reserve. Circ J. 2012;76:2218–25.
Ha J, Kim JS, Lim J, Kim G, Lee S, Lee JS, et al. Assessing computational fractional flow reserve from optical coherence tomography in patients with intermediate coronary stenosis in the left anterior descending artery. Cir Cardiovasc Interv. 2016;9(8):e003613. doi:10.1161/CIRCINTERVENTIONS.116.003613.
Taniwaki M, Radu MD, Zaugg S, Amabile N, Carcia-Carcia HM, Yamaji K, et al. Mechanisms of very late drug-eluting stent thrombosis assessed by optical coherence tomography. Circulation. 2016;133:650–60.
Prati F, Kodama T, Romagnoli E, Gatto L, Di Vito L, Ramazzotti V, et al. Suboptimal stent deployment is associated with subacute stent thrombosis: optical coherence tomography insights from a multicenter matched study. From the CLI foundation investigators: the CLI-THRO study. Am Heart J. 2015;169:249–56.
Kim JS, Ha J, Kim BK, Shin DH, Ko YG, Choi D, et al. The relationship between post-stent strut apposition and follow-up strut coverage assessed by a contour plot optical coherence tomography analysis. JACC Cardiovasc Interv. 2014;7:641–51.
Im E, Kim BK, Ko YG, Shin DH, Kim JS, Choi D, et al. Incidences, predictors, and clinical outcomes of acute and late stent malapposition detected by optical coherence tomography after drug-eluting stent implantation. Circ Cardiovasc Interv. 2014;7:88–96.
Guagliumi G, Sirbu V, Musumeci G, Gerber R, Biondi-Zoccai G, Ikejima H, et al. Examination of the in vivo mechanisms of late drug-eluting stent thrombosis: findings from optical coherence tomography and intravascular ultrasound imaging. JACC Cardiovasc Interv. 2012;5:12–20.
Won H, Shin DH, Kim BK, Mintz GS, Kim JS, Ko YG, et al. Optical coherence tomography derived cut-off value of uncovered stent struts to predict adverse clinical outcomes after drug-eluting stent implantation. Int J Cardiovasc Imaging. 2013;29:1255–63.
Kim JS, Jang IK, Fan C, Kim TH, Kim JS, Park SM, et al. Evaluation in 3 months duration of neointimal coverage after zotarolimus-eluting stent implantation by optical coherence tomography: the ENDEAVOR OCT trial. JACC Cardiovasc Interv. 2009;2:1240–7.
Lee KS, Lee JZ, Hsu CH, Husnain M, Riaz H, Riaz IB, et al. Temporal trends in strut-level optical coherence tomography evaluation of coronary stent coverage: a systematic review and meta-analysis. Catheter Cardiovasc Interv. 2016;88:1083–93.
Matsumoto D, Shite J, Shinke T, Otake H, Tanino Y, Ogasawara D, et al. Neointimal coverage of sirolimus-eluting stents at 6-month follow-up: evaluated by optical coherence tomography. Eur Heart J. 2007;28:961–7.
Toledano Delgado FJ, Alvarez-Ossorio MP, de Lezo Cruz-Conde JS, Bellido FM, Romero Moreno MA, Femandez-Aceytuno AM, et al. Optical coherence tomography evaluation of late strut coverage patterns between first-generation drug-eluting stents and everolimus-eluting stent. Catheter Cardiovasc Interv. 2014;84:720–6.
Liu Y, Shimamura K, Kubo T, Tanaka A, Kitabata H, Ino Y, et al. Comparison of longitudinal geometric measurement in human coronary arteries between frequency-domain optical coherence tomography and intravascular ultrasound. Int J Cardiovasc Imaging. 2014;30:271–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Choi, SY. (2018). Interpretation of Optical Coherence Tomography: Quantitative Measurement. In: Hong, MK. (eds) Coronary Imaging and Physiology. Springer, Singapore. https://doi.org/10.1007/978-981-10-2787-1_12
Download citation
DOI: https://doi.org/10.1007/978-981-10-2787-1_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-2786-4
Online ISBN: 978-981-10-2787-1
eBook Packages: MedicineMedicine (R0)