Abstract
Serine proteases, the largest human protease family, are found in many key developmental and physiological processes in the biological system. Protease signalling pathways are stringently controlled, and deregulation of proteolytic activity results in the degradation of extracellular matrix which plays a major role in cancer progression. The Type II transmembrane serine protease, hepsin, matriptase-2 and TMPRSS4, and secreted serine protease, urokinase plasminogen activator (uPA), kallikreins and HtrA, are closely related to cancer-associated proteases and also involved in perturbation of uPA plasminogen system, matrix metalloproteases (MMPs), upregulation of adhesion molecules like integrin family, activation of intracellular signalling cascade, inhibition of apoptosis pathway in various types of cancers which causes cell proliferation, invasion and metastasis. Serpin, an endogenous serine protease inhibitor, regulates the homeostasis by maintaining a delicate balance with the serine protease and prevents the process of invasion and metastasis of cancer cells thus inhibiting tumour growth. This chapter focuses on the role of serine proteases and their inhibitors in different types of tumours associated with cancer prognostication and therapy.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Type II transmembrane serine protease
- Extracellular matrix
- Matrix metalloproteases (MMPs)
- Secreted serine proteases
- Therapy
- Tumour
- Serpin
- Urokinase plasminogen activator (uPA)
- Plasminogen system
1 Introduction
Proteases occupy a pivotal position among biological molecules required for the physiological roles in living systems and commercial biotechnology markets and medical fields. They are called proteolytic enzymes or systemic enzymes, and their catalytic function is to hydrolyse the peptide bond that links amino acids together in a polypeptide chain. These are also called peptidase or proteinase (Fig. 12.1).
A large variety of proteases are found in intracellular or extracellular space in all eukaryotic and prokaryotic cells. They are mainly located in different organelles of eukaryotic cells such as the cytosol, mitochondria, vacuoles, lysosomes and endoplasmic reticulum. These intracellular proteases are involved in many important functions such as regulating synthesis, activation and proteolysis of proteins. The extracellular proteases are mostly secreted in the gastrointestinal tract of animals or involve in the blood coagulation and complement cascade events. Consequently, different organisms or different tissues have different sets of proteases.
1.1 Cellular and Physiological Functions of Protease and Their Industrial Applications
Proteases exhibit many important cascades such as homeostasis and inflammation which control the dynamics of protein turnover in various hierarchical levels of biological organisation. In thermodynamics, the hydrolysis of peptide bond is energetically favourable, for example, the equilibrium constant, K eq = 105, which indicates that proteolysis is irreversible and biological switches must be strictly controlled.
Proteases involve in different biological roles such as signal transduction through proteolysis of IkB-α: it is an inhibitory protein to release nuclear factor (NF-kB, a transcription factor) that enters from cytoplasm to nucleus [1], has defensive role in blood coagulation [2], displays the hydrolysed foreign proteins through major histocompatibility complex (MHC class I) in immune system [3], acts as a development process such as fertilisation [4] and, last but not the least, is useful for the proliferation programme in cell system with the help of cyclin degradation and programmed cell death and controls the homeostasis of biological system [5, 6].
Proteases have also been utilised in the field of food processing such as manufacturing of sauces, aroma formation for the milk products, tenderisation of meat and cold stabilisation of beer. These proteases are commonly used as a hypoallergenic food for digesting milk proteins into small peptides to protect the babies from developing milk allergies.
1.2 Classification of Protease
A well-known database, MEROPS (http://merops.sanger.ac.uk), was first developed by Barret et al. for the classification of proteases, their substrates and inhibitors on the basis of their homologies of their significant sequences and structures [7]. This database has hierarchical classification in which proteases are grouped into families and clans.
Furthermore, proteases can also be broadly categorised into two major types, exopeptidase and endopeptidases, characterised by their site of action of the peptide bond. Usually, exopeptidases break the peptide bond nearer to the amino- or carboxyl-termini of the substrate, while endopeptidases break peptide bonds distant from to the amino- or carboxyl-termini of the substrate (within a protein molecule).
On the basis of functional group/conserved amino acids found in the active site, proteases are also categorised into four major groups as shown in Table 12.1.
2 Serine Proteases
According to the MEROPS database, about 33% are serine proteases which are categorised into 40 families and 13 clans in both eukaryotes and prokaryotes [7, 8].
Usually, the family name is derived from the nucleophilic Ser present in the active site of the enzyme. The Ser amino acid of the active site cleaves the carbonyl terminus of the peptide bond to form an acyl-enzyme intermediate [9].
Thus, serine proteases (or serine endopeptidases) prefers serine at the active site for the hydrolysis of the peptide bond in proteins.
Usually, they are found in the form of zymogens (digestive enzymes are released in inactive forms) to regulate the enzyme activities by controlling the specific activation of proteolysis.
The main division of serine proteases is based on the site of cleavage of specific amino acids of the peptide bonds:
-
1.
Trypsin such as serine peptidases prefers to cleave the peptide bonds which have lysine and arginine at the cleavage sites.
-
2.
Chymotrypsin such as serine peptidases prefers aromatic amino acids (phenylalanine, tyrosine or tryptophan) at the cleavage site for the digestion of the peptide bond.
-
3.
Elastase such as serine peptidases prefers to cleave amino acids with short side chain groups such as alanine in their cleavage site.
2.1 The Catalytic Mechanism
The prime contributors of amino acids for the catalytic mechanism of serine protease classes such as chymotrypsin (in eukaryote) and subtilisin (in prokaryote) enzymes are their catalytic triad (Fig. 12.2). This triad is found in the active site of enzyme and conserved in all serine proteases. The triad comprises of three amino acids, namely, His57, Ser195 and Asp102, bonded in a network fashion (Fig. 12.2). The position of each amino acid of the triad is far from one another in the primary structure of the protein, but once folded, they will be in close proximity to the enzyme. This explicit tertiary structure of the triad members is vital for the specific catalysis of the enzyme.
Serine proteases follow ping-pong catalysis mechanism, and this involves formation of unstable enzyme-peptide intermediate by covalent catalysis mechanism, and finally the intermediate is stabilised, and consequently the peptide fragment is released [10].
The serine protease mechanism can be summarised in the following two steps: acylation followed by de-acylation process in which a nucleophilic attack takes place on the intermediate by water, which leads to the hydrolysis of the protein (Fig. 12.3). The overall process of the reaction mechanism utilises the catalytic triad (Asp-102-His-57-Ser-195) of serine protease. In this process, the serine-OH acts as a nucleophile, while histidine-NH acts as a base catalyst to activate the serine but later on it acts as an acid catalyst, whereas aspartate plays a supportive role by stabilising the histidine in the whole reactions.
Mechanism of a serine protease. In the acylation step, (a) substrate binds to active site of enzyme. (b) Tetrahedral intermediate is formed due to nucleophilic attack of serine on carbonyl part of peptide. (c) Acyl-enzyme intermediate is formed by breakage of peptide bond of the substrate. In the de-acylation step, (d–e) water acting as a nucleophile stabilises the cleavage peptide of carbonyl carbon and gives rise to a new tetrahedral intermediate with the nitrogen of the histidine. (f) Regeneration of the active site by releasing the product (Redrawn based on figure by Pratt CW, Cornely K (2012) Essential Biochemistry, 3rd edn. Wiley, New York, p 170)
The detailed process is given in the following steps:
-
1.
The peptide binds to the active site of the enzyme, in such a way that the sessile bond of the protein (indicated by –N - C-) is placed into the active site (catalytic triad) of the enzyme and the carbonyl-C part of peptide is present close to the nucleophilic serine [Fig. 12.3a].
-
2.
First, the electron-rich –N atom of the histidine activates the serine residue of the catalytic triad by extracting the –H atom from serine-OH and make it more nucleophilic, and thus the serine-OH is more likely to attack the electron sink of the carbonyl-C of the peptide. Consequently, a tetrahedral intermediate is generated in which a newly covalent bond is formed between the carbonyl-C of the peptide and –O atom of serine, whereas the hydrogen part of serine is covalently bond to –N atom of histidine as well as a pair of electrons from the double bond of the –C = O moves to the –O atom of carbonyl part of peptide, creating a negative charge on the –O atom, and this causes an unstable carbonyl anion of the peptide [Fig. 12.3b]. Moreover, the histidine residue carries a positive charge due to the newly covalent bond with the serine-OH which is stabilised by the hydrogen bond of aspartic acid of the catalytic triad.
-
3.
Because of the positive charge in the histidine, the histidine donate the –H atom to the –N atom of scissile bond of the peptide which results in the breaking of the sessile bond in which the sessile bond is now covalently bond with the –H atom of histidine. The negative charge on the oxygen atom that formed previously on the –C = O moves back to recreate a double bond. Thus, the peptide bond break results in the release of N-terminus part of the peptide, and C-terminus part of the peptide is covalently attached to serine residue, generating an acyl-enzyme intermediate. Now, the histidine residue of catalytic triad is back into the original form as an acid catalyst [Fig. 12.3c].
-
4.
After that, water comes to play an active role in this catalysis reaction. The electron-rich nitrogen atom of histidine residue acts as a nucleophile and extracts the proton of water, and this allows the –OH part of water to act as a nucleophile, and because of this, it attacks the electron sink of –C = O part of the peptide. This is exactly the same step as in 1. Now, the new -N-H bond is formed, and histidine again carries a positive charge which is stabilised by the hydrogen bond of aspartic acid of the catalytic triad. Once again, the electron pairs from the –C = O of the substrate move back to the oxygen making it negative charge, as the bond between the –OH of water and the carbonyl-C of the substrate is formed. Overall, this generates other tetrahedral intermediate results in an unstable carbonyl anion of peptide [Fig. 12.3d, e].
-
5.
Finally, in order to neutralise the positive charge of histidine, the covalent bond of -N-H of histidine residue is now breaking, and new covalent bond is formed between -H atom of -N-H bond of histidine and –O atom of serine residue by breaking the bond between the carbonyl-C of the peptide and oxygen atom of serine. Now, the electron-deficient carbonyl carbon of the peptide regains the previous double bond with the oxygen. Consequently the C-terminus of the peptide is now released along the formation of new –OH group of water [Fig. 12.3f]. In a nutshell, the peptide is hydrolysed with the help of protease by adding –H atom to N-terminus, and –OH atom is attached to C-terminus of the peptide bond of the substrate.
In mammals, serine proteases take part in multiple functions of living organisms such as protein digestion, blood coagulation, complement system, differentiation and development [11, 12]. Serine protease can be broadly classified into the following two broad classes based on their localisation within the extracellular space:
-
1.
Secreted type
-
2.
Membrane-anchored type
The secreted serine proteases are the well-characterised members of S1 family of serine proteases which are produced from secretory vesicles into the extracellular environment. Chymotrypsin, trypsin and thrombin are the prototype members of the S1 family (Fig. 12.4). Other examples of secreted serine proteases such as uPA and kallikrein involve in pericellular proteolysis by either activating zymogen forms of other substrates or binding with the co-receptors (Fig. 12.4). These secreted serine proteases exhibit various biological events such as tissue repair, immunity and nutrient uptake [11].
Classification of serine proteases: (1) secreted serine protease type ((i) chymotrypsin type, (ii) role of uPA enzyme in pericellular proteolysis by binding to specific cell-surface receptors uPAR (GPI-anchored type)) and (2) membrane-anchored type: (i) The human GPI-anchored serine proteases, prostasin and testisin, (ii) Type 1 transmembrane serine protease, Tryptase γ1, (iii) The Type II transmembrane serine proteases (TTSPs): (a) the human airway trypsin-like protease expressed in squamous cell carcinoma (HAT/DESC) subfamily; (b) hepsin/TMPRSS subfamily which consists of SR, SEA and LDLA domains; (c) Matriptase subfamily, particularly, Matriptase-2, which consists of SEA, two CUB and three LDLA domains; and (d) the corin subfamily which consists of two FD domains, eight LDRA domains and one SR domain (abbreviations: SR scavenger receptor domain (group A), SPD serine protease domain consist of three catalytic residues histidine, aspartate and serine, FD Frizzled domain, LDLA LDL receptor class A)
In a recent decade, a structurally and functionally unique subgroup of S1 serine proteases, termed broadly as the membrane-anchored serine proteases, has been reported which are found to be directly anchored to the plasma membrane through its amino- or carboxy-terminal domains [13] (Fig. 12.4). In compare with secreted serine proteases, the membrane-anchored serine proteases are involved in a diverse array of physiological functions such as epithelial barrier, fertilisation, cell signalling, embryo development and tissue morphogenesis [11].
On the basis of their structural features, they can be divided into subgroups and are anchored to the membrane by three ways: (1) a carboxyl-terminal transmembrane domain through a GPI (glycosyl phosphatidylinositol) linkage which is added post-translationally, (2) a carboxy-terminal transmembrane domain (Type I) and (3) an amino-terminal transmembrane domain with a cytoplasmic extension (Type II transmembrane serine proteases – TTSPs) [13, 14]. Type I serine proteases, Tryptase γ1 and the GPI-anchored serine proteases, prostasin and testisin contain a carboxy-terminal hydrophobic extension that serves as a transmembrane domain (ranging from 310 to 370 amino acids). GPI anchors have been known to modify C-terminal domain of prostatin and testisin post-transcriptionally [15,16,17] (Fig. 12.4).
TTSPs are the group of membrane-anchored serine proteases with 17 members and 19 members of humans and mice, respectively. TTSPs are trypsin-like (family S1) proteases and have the potential to be linked to cellular membranes via a hydrophobic stretch at their amino terminus. These proteases have two parts, one with a cytoplasmic amino-terminal signal anchor of variable length (20 to 160 amino acids) and the other with a catalytic serine protease domain at the carboxyl-terminus. All the membrane-anchored serine proteases are structurally conserved catalytic domains and belong to S1 peptidase family. These serine proteases usually exist as zymogens (inactive form); and their autoactivation cleavage occurs after a basic amino acid residues present in a highly conserved activation motif producing a two-chain form with their chains bonded by a disulphide bridge, eventually, separating the pro- and catalytic domains with the catalytic domain remaining membrane bound. Some examples of TTSPs are TMPRSS2, matriptase, hepsin and TMPRSS4 [18]. Therefore, they represent enzymes whose peptide bond cleaving activities are specifically targeted to cellular membranes. They have been phylogenetically categorised into four subfamilies on the basis of C-terminal transmembrane domain: (1) the human airway trypsin-like (HAT)/differentially expressed in squamous cell carcinoma gene (DESC) subfamily, (2) the hepsin/transmembrane protease serine (TMPRSS) subfamily, (3) the matriptase subfamily, and (4) the corin subfamily (Fig. 12.4). In compare with the GPI-anchored and Type I serine proteases, which consist of SPD (serine protease domain) and membrane anchor, the TTSPs possess a stem region which is C-terminal to SPD having a variety of modular structural accessory domains (SEA, CUB, FD, SR) that are involved in protease activation, localisation and substrate recognition to maintain the homeostasis of pericellular microenvironment (Fig. 12.4).
Dysregulation of pericellular and extracellular proteolysis that involve the membrane-anchored and secreted serine proteases, respectively, are the hallmarks in various clinical disorders. Reports have shown that proteolytic breakdown of the extracellular matrix (ECM) is the key step in spreading tumour cell [19, 20]. The series of activities of proteases involved in tumour progression is collectively called as the cancer ‘degredom’. A positive cooperativity between the aggressiveness of tumour and the overexpression of many proteases has been detected [20]. In the series of events in cancer progression, serine proteases may be involved in any of the fundamental processes of tumorigenesis with unique specifications [13]. In normal physiological conditions, an endogenous anti-serine protease system known as serpins regulate the serine protease activity and maintain the balance between proteases and their inhibitors in the organism. An imbalance between the proteolytic and antiproteolytic may be of major significance in the cancer development. For example, hepsin, a cell surface serine protease, and maspin, a serine protease inhibitor, are both showing highly upregulated and downregulated, respectively, in prostate cancer, and this causes an imbalance in cellular homeostasis which are believed to promote tumour growth, invasion and metastasis. This shows that the improper function of serine protease leads to cancer which will suggest the need of therapeutic agents against the serine protease to prevent tumour progression and metastasis. As a tumour biomarker, serine proteases are important in detecting certain cancers at an earlier stage. For example, determination of coagulation factor levels and serum prostate-specific antigen can be used for detecting thrombotic and prostate cancer patients. Furthermore, targeting and modulation of overexpressed proteases are the efficient selective approaches for the development of antitumour therapies [21]. Due to the ever-increasing, newly found roles of serine proteases in cancer, there has been increasing attention in the specific roles of members of serine proteases and their inhibitors in array of diverse cancer progression. In this chapter, a review of the role of these members of serine proteases (secreted type and membrane anchored of TTSPs family) and their inhibitors in tumour progression has been discussed in order to understand their therapeutic applications.
2.2 Secreted Serine Proteases and Its Role in Cancer
There are about 175 predicted serine proteases in humans. Most of them are found to be secretory in nature that has major roles in a multiple metabolic functions in maintaining the tissues homeostasis. For example, the uPA (urokinase plasminogen activator) and kallikrein system participate in a range of physiological function from cell growth, cell signalling to tissue remodelling process. However, dysregulation expression of serine proteases leads to tumour invasion and cancer. In this section, the main focus is on particular secreted serine proteases, which are reported to be the vital causes of cancer progression and metastasis.
2.2.1 Urokinase Plasminogen Activator
At physiological condition, the uPA system is linked with various tissues remodelling processes, immune system and inflammation, fibrinolysis, embryogenesis, angiogenesis, cell migration and activation and differentiation of white blood cells. The active form of uPA is mostly synthesised by cells, in tissues and extracellular fluids with mild intrinsic activity [22].
The uPA system belongs to a serine protease family, playing an important function in tumour invasion and metastasis in cancer. The plasminogen activator (PA) system comprises the two serine proteases, uPA and tissue plasminogen activator (tPA), the two serpin inhibitors, plasminogen activator inhibitor-1 (PAI-1) and plasminogen activator inhibitor-2 (PAI-2) and the glycolipid-anchored uPA receptor (uPAR). Both uPA and tPA catalyse the formation of active protease plasmin from the inactive zymogen, plasminogen, which can break down most extracellular proteins. However, tPA mainly acts as a fibrin-dependent pathway for blood clot dissolution process [23]. While uPA performs through fibrin-independent pathway and largely acts on the cell surface receptor-bound plasminogen activator like uPAR which controls the pericellular proteolysis of the system, this involves in the degradation of ECM and causes invasion and cancer metastasis [24] (Fig. 12.4). uPA and uPAR are observed to be highly expressed in various human cancers in contrast to the corresponding normal tissue. In this regard, uPAR is a highly glycosylated cell surface protein which do not contain transmembrane and intracellular domains but are attached to the cell membrane by a GPI anchor (Fig. 12.4).
uPA is a small trypsin-like protease having molecular weight of 53 kDa. It performs the catalysis of zymogen, plasminogen, into its active form plasmin that facilitates the degradation of various ECM proteins such as fibronectin (FN), vitronectin (VN) and fibrin which results in the loss of interactions between cells, leading to the invasion of cancer cells [25]. In addition, it is also able to activate the inactivated forms of various metalloproteases (MMPs) [26]. In this regard, uPA in combination with uPAR plays a pivotal role in inducing the proteolytic cascade reactions that promote tumour growth through the process of metastasis of cancer cells [27]. The effect of uPAR on cancer cell migration is characterised on the basis of whether it is protease dependent or not. The protease-dependent function is catalysed by uPAR-bound uPA. Since uPAR has no transmembrane structure, its non-protease function depends on the interaction with VN, integrin family, G-protein-coupled receptors and growth factor receptors to relay its downstream signals [28]. Signalling through uPAR activates Tyr kinases, Src, the serine kinase Raf, FAK and extracellular signal-regulated kinase (ERK)/mitogen-activated protein kinase (MAPK) pathway which results in the broad modulation of cell proliferation, metastasis and cell-cell interactions [29]. The universal functions of uPAR are proteolytic extracellular matrix degradation for the progression of cancer, angiogenesis, modulation of cAMP levels for downstream signalling and cell interaction with integrins, tyrosine kinases and serine/threonine kinases [30].
uPA, uPAR and also PAI-1 are constitutively expressed in human breast cancer. In most of the cancers especially breast cancer, high levels of uPA and uPAR are overexpressed, proposing the enhanced role in ECM degradation, migration and adhesion and cancer invasion [31]. PAI-1 and uPA are the first novel tumour biological predictive factors found, in evidence with their clinical utility for breast cancer [32]. uPA has also been observed to be a predictive marker in many types of organ cancers such as cancers of the lung [33], bladder [34], stomach [35], etc.
Several studies suggest that uPA binds with uPAR that facilitate the cell migration process through diverse cell signalling pathways. In this regard, integrins are crucial uPAR signalling co-receptors, and activation of integrin stimulates the focal adhesion kinase (FAK) and, thereby also, activates Src/MEK/ERK-dependent signalling pathways, resulting in transcriptional activation of the uPA promoter, which promotes tumour cell proliferation and tumour invasion (Fig. 12.5). Similarly, the p38 MAPK and myosin light-chain kinase (MLCK) pathways are involved in uPA-promoted cell migration through MEK/ERK, PI3K/AKT and Ras/ERK signalling pathways, respectively [36] (Fig. 12.5).
uPA system in cancer malignancy. In the plasma membrane, uPA binds to uPAR which promotes uPA activation resulting in the catalysis of plasminogen into active form, plasmin. Plasmin can subsequently activate MMPs in the pericellular environment that results in invasion and metastasis via ECM degradation. Intracellular activation of uPA/uPAR along with integrins activates FAK, PI3/AKT and p38-MAPK signalling pathways, which finally leads to pathophysiological events, such as metastasis and inhibition of apoptosis pathway
2.2.2 Kallikreins
In 1930, Kraut and colleagues coined the term kallikrein (kallikreas is the Greek word for pancreas) from an identified substance (human kallikrein 1) that was present at significant concentration in the pancreas. Human tissue kallikreins (hKs) are secreted serine proteases that convert high molecular weight proteins into biologically active peptides known as kinins. There are two families of kallikreins, the tissue and plasma kallikreins. Human plasma kallikrein cleaves high molecular weight kininogen into a bradykinin which is a potent vasodilator nonapeptide (Fig. 12.6). The only enzyme which has been found with appreciable kallikrein activity is kallikrein I (hK1, pancreatic-renal kallikrein) in human tissue kallikreins. There are a total of 15 tissue kallikreins genes named as KLK1 to KLK15 encoding hK1 to hK15 which are mostly regulated by steroid hormones [37]. Most of the kallikreins are expressed in endocrine-related organs, such as the breast, ovary, prostrate and testis. These are secreted by extracellular matrix. It has been known for decades that the ECM degradation performs a crucial function in tumour metastasis through extracellular proteolytic activity. The ECM maintains its own structural integrity which involves various growth factors and signalling molecules. Therefore, imbalances created by the activity of extracellular proteases modify the microenvironment of the ECM which either directly or indirectly poses an impact on the number of cell activity processes such as apoptosis, angiogenesis and metastasis via the breakdown of ECM and non-ECM components [38]. This breakdown of ECM results in the alteration of cell-cell and cell-ECM interactions which in turn perturb the activity of growth factors and growth factor receptors and finally leading to either tumour promoting or tumour-suppressive effects. This shows that it is a very complex process and contains many factors. For example, the proteolytic activity of kallikrein is found to be deregulated in tumours such as adenocarcinomas, and it is also used in patient prognosis [38]. Similarly, several studies reported the overexpression of 12 KLK genes in ovarian carcinoma associated with steroid-hormone-regulated cancer [39]. Interestingly, kallikreins are normally found to be downregulated in breast, prostate and testicular tumours. Apart from steroid-hormone-regulated cancers, kallikreins are deregulated in various tumour types such as lung adenocarcinomas, pancreatic cancer and acute lymphoblastic leukaemia [38].
Kallikrein’s signalling pathway system. Kallikreins activate the uPA system resulting in the catalysis of plasminogen into active plasmin which in turn activates the pro-KLK proteins leading to the breakdown of various downstream targets, such as latent MMPs. Kallikreins take part in ECM remodelling directly and/or indirectly via activation of pro-MMPs. Active kallikrein is involved in the conversion of kininogen into kinin fragment which induces angiogenesis and other pathological processes via activation of the cAMP, Akt/PKB and VEGF pathway
The current perception is that pericellular cascade is not only regulated by the serine protease system of uPA, uPAR and plasminogen, but it has adverse impacts on activation of MMPs, which is associated with the extracellular proteolysis in tumorigenesis. Despite it, the activation of the uPA-uPAR-MMP proteolytic cascade by various hK-family members further widens their various routes in cancer progression. Thus, many members of proteolytic network especially kallikrein family is involved in cascading reactions of tumour progression [38].
Angiogenesis is a process of differentiation of new capillary blood vessels from pre-existing vessels. Angiogenesis is mainly controlled by the ratio of pro- and anti-angiogenic growth factors exist in the blood. The increased ratio of pro-angiogenic stimuli and inhibitory regulators activates or switches on the angiogenic process.
Human tissue kallikrein possesses potent angiogenic effects by processing many elements of the extracellular matrix. It has been classified as a pleiotropic angiogenic agent which catalyses the inactive form of kininogen into active form of kinin peptides which in turn activate cAMP, Akt/PKB and VEGF (vascular endothelial growth factor) pathways, and this promotes the process of angiogenesis [40] (Fig. 12.6). KLKs may also participate in remodelling of ECM indirectly through the MMPs, uPA and kinin signalling pathways [41,42,43]. The kallikrein family such as KLK2, KLK4 and KLK12 activates the uPA system, resulting in plasmin formation, and this activated plasmin causes the breakdown of a number of ECM proteins, for example, fibronectin, proteoglycans and fibrin [28, 44,45,46] (Fig. 12.6). Similarly, KLK1 and KLK12 catalyse the conversion of kininogen to active kinin peptides and bradykinin, and this promotes angiogenesis and metastasis through the activation of downstream signalling pathways, for example, basic fibroblast growth factor (bFGF) cAMP, Akt/PKB and VEGF pathways [45, 47, 48] (Fig. 12.6).
2.2.3 PSA/hK3 (Prostate-Specific Antigen)
PSA consists of a 240-amino acids long glycoprotein, and it comes under the category of human glandular kallikrein family (hK3, a 33 kDa serine protease) [49]. PSA is mainly synthesised in prostrate ductal and acinal epithelium and is secreted into seminal plasma. PSA plays an important role in semen liquefaction by hydrolysing semenogelin I and II in the seminal coagulum [50]. It has chymotrypsin-like activity, does not hydrolyse synthetic substrates for plasmin and displays a weak interaction with aprotinin, a plasmin inhibitor [51]. This suggests that PSA primarily acts independently as a protease in protein degradation, and not via plasmin, like uPA. PSA is organ-specific and is characteristically expressed in prostatic epithelial cells, and its expression is regulated by androgens [52]. It has been observed that proteolytic cascade pathways may also exist in the absence of MMPs but through plasmin-dependent pathway which involves the degradation of type IV collagen, an essential part of a basement membrane [53]. In this regard, urokinase performs a pivotal role in the proteolytic cascade pathway in prostate cancer invasion. Furthermore, kallikreins such as PSA can activate pro-urokinase to its active form, and subsequently, uPA activates plasmin and, which in turn, can recruit collagenases from pro-collagenases which can cause massive degradation of ECM [54]. It has been shown that the dissolution of ECM involve direct degradation of fibronectin by uPA and degradation of fibronectin and laminin by plasmin before the degradation of collagen matrix [54].
2.2.4 HtrA1 (Prss11 or IGFBP-5)
HtrA1 (also known as Prss11 or IGFBP-5 or DegP) belongs to a family of high-temperature requirement factor A (HtrA) of oxidative stress-response proteases. It is a heat shock-induced envelope-associated serine protease and performs as a chaperone which is crucial for the survival of bacteria at elevated temperature [55, 56]. They are widely distributed from prokaryotes to eukaryotes. Evolutionarily, these serine proteases have independent ATP conserved sequences and are believed to act as a defence mechanism against cellular stresses including the proteolysis of the misfolded proteins to maintain the homeostasis of the cell [57]. There are four human HtrAs: HtrA1 [58], HtrA2 [59], HtrA3 (pregnancy-related serine protease, PRSP) [60] and HtrA4 [61]. They carry out a number of biological functions such as mitochondrial homeostasis, apoptosis and cell signalling, and their improper functions lead to various clinical disorders [62, 63].
HtrA1 is the first reported member of the human HtrA protein family isolated from a normal fibroblast cell [64]. HtrA1 is downregulated in a variety of cancers such as melanoma [65], glioma [66], ovarian tumours [67, 68], endometrial cancer [68, 69], lung cancers [70], etc. Interestingly, studies have reported that overexpression of HtrA1 functions as a tumour suppressor either by inhibiting the cancer cells or through the apoptosis of cancer cells [65]. Despite it, the mechanism of HtrA1 involved in cancer is still unexplored [71]. In cancer development, it was also proven that HtrA1 and HtrA3 are the inhibitors of growth factor systems such as transforming growth factor β (Tgfβ) family members which are the key regulators for cell growth and differentiation in different tissues [72].
One of the most promising approaches in the discovery of drug cancer is to rationally identify such type of therapeutic agent which regulates the apoptotic process [73]. The biochemical events in apoptosis is regulated by pro-apoptotic and anti-apoptotic proteins which come under the category of Bcl-2 family anti-apoptotic survival proteins such as inhibitors of apoptosis protein (IAP) family and caspases. It was found that HtrA2/Omi functions as a promoter in apoptotic cell death [74]. The mature HtrA2/Omi is capable of inducing apoptosis in human cells and functions as a caspase-independent system through its proteolytic activity and in a caspase-dependent manner through the degradation of IAPs [75]. The function of HtrA2 in tumorigenesis is not yet fully understood; however, its increased levels in the cell upon apoptotic stimuli might prevent the cells from apoptosis which act as a defence mechanism to malignancy. This suggested that HtrA proteins can be used as a novel approach in cancer therapy.
2.3 Membrane-Anchored Serine Proteases
The membrane-anchored Type II serine proteases are identified as essential part of the human degradome, and they function in the conversion of precursor molecules into active molecules in the pericellular microenvironment, playing absolute functions in tissues homeostasis and cancer [76]. The TTSP family is the recently known protease family, and much is still to be explored. TTSPs have diverse roles in mammalian system, and their structural homology does not linked to a common biochemical function. They are mostly participating in either hormone or growth factor activation or in the initiation of proteolytic cascades. This suggests that they are maintaining basic homeostasis by activating or deactivating the signalling molecules involve in the biochemical reactions.
Recently, great attention has been paid to the members of TTSP family such as hepsin, matriptase-2 and TMPRSS4 for their vital physiological roles, role in tumourigenic activity and distinctive regulatory mechanisms. These TTSPs are being increasingly documented for their important roles in regulating the pericellular microenvironment and thus providing new insights of their mechanisms of mammalian health and diseases.
2.3.1 Membrane-Anchored Type II Serine Proteases (TTSP) and Its Role in Cancer
2.3.1.1 Hepsin
In the United States, about 2 lakhs of new cases of prostate cancer of adult men and 40,000 deaths were observed in 1995. The occurrence of prostate cancer is more prevalent in the later age of 60 years and above, and about 80% of prostate cancers are diagnosed of this age group [77].
Hepsin (TMPRSS1) is one of the members of Type II transmembrane serine proteases and is expressed in prostate cancer [78]. Hepsin is mostly present in the liver but is also found at trace amount in tissues of the stomach, kidney, prostate, thyroid and inner ear. This subfamily is composed of seven members in human and mice. It possesses only one additional domain in its stem region, a group A scavenger receptor domain (SR) in addition to serine protease domain (SPD) (Fig. 12.4).
It can cleave and activate pro-uPA, pro-HGF, Laminin332 and pro-MSP [78]. It activates pro-hepatocyte growth factor and is inhibited by hepatocyte growth factor activator inhibitor-1B (HAI-1B) and hepatocyte growth factor activator inhibitor-2 (HAI-2) [79]. It is involved in the activation of various proteolytic cascades especially the activation of non-active proteases which leads to breakdown of the extracellular matrix proteins. Moreover, this membrane-associated serine protease helps in the blood coagulation pathway by converting factor VII to VIIa resulting in the formation of thrombin, deposition of pericellular fibrin as well as the activation of PAR-1 (protease activated receptor) [80]. Hepsin was also to be highly expressed in ovarian cancers [81,82,83].
The TMPRSS2 and TMPRSS4 are the Type II serine proteases that are highly expressed in prostate and pancreatic cancers, respectively, and activate proteolytic cascades which lead to metastasis events [84].
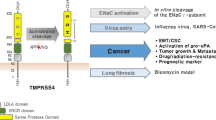
2.3.1.2 TMPRSS4
TMPRSS4 is a member of Type II transmembrane serine protease and is found to be overexpressed mostly in the pancreas, the thyroid and cancer tissues. As compared to hepsin, it possesses an additional low-density lipoprotein receptor class A (LDLA) domain which is N-terminal to the SR and SPD domains (Fig. 12.4). The molecular mechanism of TMPRSS4 for metastasis of cancer cells is still unclear. However, it promotes the cancer progression by activating the loss of E-cadherin-mediated cell-cell adhesion and facilitating the epithelial-mesenchymal transition (EMT). The EMT is a process of conversion of epithelial cells into motile mesenchymal cells characterised by the change in the polarity of epithelial cells, cell-cell adhesion, enhanced proteolytic activity, migratory capacity and invasiveness resulting in increased production of ECM components [85].
One of the factors that contribute to metastasis is the downregulation of E-cadherin through E-cadherin transcriptional repressors/EMT-inducing transcription factors, including the snail superfamily consisting snail and slug factors, and this leads to EMT events in human epithelial cancer cells. In colon cancer, TMPRSS4 significantly promoted FAK signalling pathway activation that includes FAK, ERK1/2, Akt, Src and Rac1 activation which in turn stimulate the transcription factors SIP1/ZEB2, resulting in E-cadherin loss, a major event found in EMT (Fig. 12.7) [86,87,88]. Furthermore, interestingly, TMPRSS4 downregulates the expression of RECK, an inhibitor of tumour angiogenesis, via the activation of ERK1/2 pathway [89,90,91]. The overexpression of TMPRSS4 is the major event in hepatocellular carcinoma (HCC) progression and can be used as a good predictive biomarker for HCC.
Effect of overexpression of TMPRSS4 in cancer malignancy. It helps in the activation of the intracellular pathways through phosphorylation of ERK, JNK, Akt, Src, FAK and Rac1 which in turn upregulates integrin (ITG-α5) and transcription factors such as Sip1/Zeb2 (a repressor of E-cadherin) resulting in invasiveness and EMT. In the cell membrane, TMPRSS4 converts the precursor of uPA (pro-uPA) to its active form which accelerate the invasiveness
TMPRSS4 activates uPA by two ways, one through increased gene expression (JNK and transcription factors Sp1 and Sp3 and AP-1 pathway) and another by activating pro-uPA, and this leads to enhanced invasion [92] (Fig. 12.7).
2.3.1.3 Matriptase-2
Matriptase-2 or TMPRSS6 (80–90-kDa cell surface glycoprotein) belongs to a Type II transmembrane serine protease family [93, 94]. Matriptase-2 comprises of a short N-terminal cytoplasmic tail, a transmembrane domain, an extracellular stem region containing a SEA domain (a single sea urchin sperm protein), two CUB domains (urchin embryonic growth factor), three LDLA repeats and a C-terminal trypsin-like SPD domain [93, 94] (Fig. 12.4). It was mostly found in breast and prostate cancers [95, 96].
Matriptase-2 is a hepatic membrane serine protease and is expressed as zymogen on the cell surface, and this inactive proenzyme undergoes shedding to a single chain form followed by autoactivation by cleavage at conserved site represented as RIVGG between the pro-domain and the catalytic domain, and the activated protease domain fragment remains on the membrane via a single disulphide bond linking the pro- and catalytic domains [97, 98]. Matriptase-2 shows high homology in terms of structure as well as its function with matriptase-1 [99], which is found to be overexpressed in epithelial cells, and in various cancers [100]. Matriptase-2 is primarily found to be expressed in human liver that shows connection with the dissolving of extracellular matrix proteins including laminin and fibronectin [94]. It was established that the degradome components such as hepsin, MTSP1, MMP26, plasminogen activator inhibitor-1 (PAI-1), uPAR, MMP15, TIMP3, TIMP4, maspin and RECK are associated with cancer progression in human prostatic tissues [101].
The protease activity can be controlled by its pericellular environment in various ways. In our living system, it was found that several proteases can be activated in an acidic environment such as cathepsins in lysosomes and pepsinogen in the stomach [102,103,104]. It was also observed that the activity of matriptase is firmly controlled by the chemical environment of the cell [105]. Like other secreted or lysosomal proteases which are activated by an acidic pH, matriptase is also activated in the same way but is unique in the sense that it is attached onto the surface of cells [106]. Matriptase is released as a zymogen and its autoactivation activity depends on intrinsic activity of matriptase zymogen, non-catalytic domains of the enzyme and post-translational modifications [107, 108].
This protease is mainly co-expressed with hepatocyte growth factor activator inhibitor-1 (HAI-1) in the normal epithelial components of tissues, suggesting that the protease activity of matriptase is tightly regulated [100, 109, 110].
Reports suggested that an imbalance of matriptase and HAI-1 ratio is the key factor for the indication of a cancer-related proteolytic events. It is being shown that the ratio of matriptase and HAI-1 has been increased in many cancers such as in breast and prostate [111, 112]. Although the proper mechanism of the dysregulation of matriptase activity is still not known, it may directly affect the cellular microenvironment via the activation or inactivation of downstream signalling molecules leading to the breakdown of ECM components and cell-cell adhesion [113].
3 Serpins for Diagnosis and Therapy in Cancer
The significance of regulated membrane-anchored and secreted serine proteases to maintain homeostasis and its relation with these enzymes and cancer reflects that these enzymes must be strictly controlled in normal physiological conditions. Therefore, enzymatic breakdown of serine proteases is considered to be one of the important regulators for maintaining cellular homeostasis. However, excessive enzymatic activity is often an adverse effect on the cellular processes, and this can also be associated with cancer. In conjunction with evolutionary development of proteases, regulators for proteases have also been developed. These anti-regulators of cellular serine protease are known as serpin. Selective serpins which are thought to be correlated with progression or remission of selected cancers have been selected for the critical reviews so that they can be used for diagnosis and therapy in cancer.
3.1 Serpin
SERPIN (an acronym of SERine Protease INhibitors) is a protein superfamily representing a core structure of 370–390 conserved amino acids residues with three β sheets (A, B, C) and seven to nine α-helixes. In humans, plasma serpins comprise 2%–10% of all proteins in the blood circulation and perform a crucial role in regulation of a various types of biological functions.
In the serpin, a reactive centre loop (RCL) is found to be involved in the inhibition of proteases target. This RCL is about 20–24 residues long and is present in the extended conformation above the body of the serpin scaffold. Serpins use S (stressed) which are in the native to R (relaxed) transition forms for inhibition of serine proteases. During this transition, the long, flexible RCL of serpin interacts with target protease by inserting itself into the centre of β-sheet A to form an extra strand that locks it into a canonical (key-like) conformation via a non-covalent, reversible mechanism [114]. This results into the distortion of the active site of protease which causes an irreversible covalent serpin-enzyme complex formation. This mechanism is also known as suicide substrate mechanism.
The serpin suicide inhibitors such as α-antitrypsin, α-antichymotrypsin, antithrombin and PAI-1 regulate coagulation pathway, neurotrophic factors, hormone transport, inflammation, angiogenesis, hormone transport, blood pressure and various biological processes. Surprisingly, not all the serpins are acting as protease inhibitors but few of them are found to inhibit other types of proteases whereas others are found to be non-inhibitors. For example, antigen-1 (SCCA-1) inhibits cysteinyl proteases of the papain family. Non-inhibitory serpins exhibit various important functions, including roles as chaperones, for example, the 47-kD heat shock protein (HSP47) and hormone transportation like cortisol-binding globulin [115]. Serpins such as PAI-1, maspin, neuroserpin, PEDF and SPINK1 have been selected to understand further of their antitumour mechanisms in various type of cancers.
3.1.1 Plasminogen Activator Inhibitor-1
PAI-1 consists of 400 amino acid residues long glycoprotein, with molecular weight varying from 38 to 70 kDa, on the basis of their degree of glycosylation and functions in a wide variety of clinical and non-clinical conditions [116].
PAI-1 has a dual role in biological system. It inhibits uPA and tPA to prevent plasminogen cleavage into active plasmin, and this results in the inhibition of the process of carcinogenesis [117]. PAI-1 binding to the uPA/uPAR complex triggers the internalisation of uPA/uPAR through low-density receptor-related protein-1 (LRP-1) via endocytosis, and this results in de-adhesion of plasma membrane matrix which facilitate tumour growth and dissemination [118]. All forms of PAI (activated, latent and cleaved) interact directly with LRP1 and enhance cell motility via activation of the JAK/Stat 1 pathway. Studies have shown that in many cancer patients, there were contradictory reports of having positive association between high levels of PAI-1 in tumours and blood with poor clinical outcome. This contradictory effect of PAI-1 has been elucidated by its pro-angiogenic activity (angiogenic activity at low concentration and anti-angiogenic activity at high concentration) and its anti-apoptotic of cells. The pro-angiogenesis activity of PAI-1 is postulated to be associated with PAI-1 inhibition of plasmin-mediated cleavage of FAS-ligand preventing the apoptosis of the endothelial cells [119]. Similarly, reports have shown that a PAI-1 deficiency in mice and cancer cells has the ability to promote the apoptosis process and also inhibit angiogenesis [120]. Nishioka et al. reported that the deletion of PAI-1 in gastric cancer cells decreased down the tumourigenicity [121]. These results revealed that PAI-1 can be used as a good therapeutic agent for cancer.
3.1.2 Maspin (SERPINB5)
Maspin, a 42 kDa mammary serpin, was first reported as a class II tumour suppressor in human breast cancer. It comes under the category of non-inhibitory serpin that promotes the tumour cell towards apoptosis and inhibits invasion and metastasis, and thus, maspin plays a vital role against tumour growth [122]. It is located in the cytoplasm but is also secreted to the cell surface, where it has been postulated to prevent angiogenesis and reduce the migration of many cell types in different experimental models [123, 124]. Maspin in contrast to PAI-1 consists of a relatively short, non-conserved, hydrophobic RCL, and therefore it is incapable of conversion of stressed to relaxed transition form for inhibition. Furthermore, it is incapable to inhibit either tPA or uPA as their postulated targets [125]. Because of these properties, maspin is considered as non-inhibitory category of serpin superfamily. However, recently, it was shown that maspin has inhibitory effect against plasminogen activators uPA and tPA, but they work only when these proteases are bound to macromolecular cofactors, that is, tPA bound to fibrin and uPA on the cell surface [126,127,128]. The expression of maspin gene is controlled at the transcription level and is found downregulated with the degree of malignancy. For example, the concentration levels of maspin are relatively very low in breast and prostate cancer cells as compared to normal cells [129]. Many cancer studies have shown that the involvement of cytosine methylation and chromatin condensation are associated with the deregulation of maspin expression during cancer progression [130]. This suggested that an epigenetic mechanism which is involved in cytosine methylation, histone, deacetylation and chromatin condensation inhibits and thus regulating the expression of maspin. Since, maspin is an inhibitor of angiogenesis, it regulates adhesion-mediated cell signalling pathway through extracellular and cell-cell contact adhesion molecules. For example, Maspin enhances the endothelial cell adhesion to FN, laminin, collagen and vitronectin, leading to the activation of integrin family and FAK signal transduction pathway. These cause the modulation of focal adhesion and cytoskeleton reorganisation which finally prevent the degradation of EC components and migration of tumorigenic cells [131, 132].
Numerous studies have reported that maspin suppresses tumour cells through induction of apoptosis pathway. For example, mammary carcinoma cells transfected with maspin gene provide the evidence of inhibition of invasion and metastasis in nude mice [133]. Maspin was hypothesised to induce tumour cell apoptosis by modulating mitochondrial permeability transition and initiating apoptotic death [134]. Thus, such discoveries of molecular mechanisms regarding maspin-mediated apoptosis paved a new pathway for the treatment of cancer.
Reports have shown that maspin expression and ubiquitin-proteasome pathway are inversely correlated with each other, where expression of maspin reduces with the increase in chymotrypsin-like activity of the proteasome [135]. As the ubiquitin-proteasome pathway modulates several biochemical events through protein regulation, it is postulated that deregulation of proteasome function is an important factor responsible for the malignancy of tumours [136]. Thus, the establishment of a new distinct relationship between maspin and the ubiquitin-proteasome pathway also provides an important clue for the suppression of a multitude of processes of tumour and metastasis.
Recently, the use of maspin alone or in association with mammaglobin B (a secretoglobin) is exploited as two biomarkers at different stages (cell proliferation and pathological stage) of the detection of the breast cancer [137]. In context with the epigenetic regulation of maspin, it was observed that in a pregnant woman, the maspin gene promoter was unmethylated in foetus with respect to maternal blood cell, and this opened a new avenue for developing further biomarkers for prenatal diagnosis [138]. The established anti-tumorigenic/anti-metastatic characteristic of maspin in cancer provides useful information regarding the development of therapeutic agents [139, 140]. In a nutshell, maspin can be exploited as an antitumour agent in different cellular events such as actin cytoskeleton, apoptosis, proteasome function, oxidative stress for the inhibition of cell invasion and angiogenesis.
3.1.3 Neuroserpin (SERPINI1)
Neuroserpin (NSP), a protease inhibitor of 46–55 kDa glycoprotein, was first recognised as a secreted protein from cultured chicken neuronal axons and is predominantly present in both central and peripheral nervous systems [141, 142]. Neuroserpin is a trypsin-type protease, preferentially inhibits tPA and to a minor extent uPA plasmin, but shows no inhibition towards thrombin [143].
Ischemic stroke is a single largest cause of stroke and accounts to be the second largest contributor to mortality in the world [144]. It is due to the obstruction of a certain cerebral artery resulting in an absence of blood flow to artery and brain tissue, and this could induce an energy metabolism disorder which in turn perturbs the ion gradients and an excessive release of excitotoxic neurotransmitters such as dopamine and glutamate which ultimately leads to neuronal death [78, 145]. The effective treatment for acute ischemic stroke is the administration of tPA within 3 hours on the onset of stroke. Meanwhile, tPA is capable of activating matrix MMPs and converting plasminogen to plasmin which results in the blood brain degradation [145, 146]. This extra administration of extravascular tPA beyond its therapeutic window (hours) causes a more deleterious effect on the brain. However, the adjuvant treatment with neuroserpin along with tPA is found to increase the therapeutic window, and this could have better treatment for cerebral ischemia. Thus, the neuroprotective effect of neuroserpin for the treatment of cerebral ischemia is dependent on the balanced expression of tPA which in turn regulates the recanalisation of the occluded vessel [147]. It was observed that neuroserpin gene is a cancer-associated gene and acts as a tissue-specific tumour suppressor gene in the brain [148]. Like other members of serpin family such as maspin and pancpin, this tissue-specific tumour-suppressive gene is found to be downregulated in brain tumour and even absent in brain cancer cells. As mentioned before, tPA converts inactive plasminogen into active plasmin which results in the breakdown of the extracellular matrix that facilitates the invasion of cancer cells and enables tumour migration. It was observed that neuroserpin functions in the inhibitory process of tPA, and its absence in the CNS causes brain tumorigenesis. Based on this finding, it may be suggested that neuroserpin may represent a new approach for cancer therapy. Additionally, neuroserpin is a tPA-independent mediator of neurite such as outgrowth, cell-cell adhesion and N-cadherin and NFκB expression. The tPA-independent regulatory effect of neuroserpin participates in tumorigenesis as well as emotional and cognitive processes. These new finding of the multifaceted roles of neuroserpin and its polymers will be helpful in designing better methods for treating cancer-like diseases.
3.1.4 PEDF (SERPINF1)
Pigment epithelium-derived factor (PEDF) is a 50 kDa secreted glycoprotein consist of 418 amino acids, and this was first described and purified from cultured human foetal retinal pigment epithelium cells. It comes under the category of a non-inhibitory member of the superfamily of serpin [149]. PEDF is a multifunctional member and is widely present in foetal and adult tissues. It is a well-known protein and plays important functions in many physiological and pathological processes [150, 151]. PEDF exhibits a protective mechanism against tumour and represents as a biomarker for prostate cancer patients. For example, it was recently found that the PEDF level in the venous blood patients was significantly high. PEDF can decrease tumour growth either indirectly through the inhibition of angiogenesis or directly through the activation of cell apoptosis and/or differentiation process which inhibits the process of invasion and metastasis. PEDF also works as a protective factor for neuronal components of the eye as well as an important inhibitor of the growth of ocular blood vessels. It is a very selective inhibitor of angiogenesis, and it works only on new blood-forming vessels, while the old cells have no such inhibitory effect. It is also found to be a reversible process [152]. Dawson et al. were the first who proposed that PEDF can regulate the blood vessel growth for angiogenesis at hypoxia condition as it was found in tumours [153]. PEDF mediate the anti-angiogenesis activity by effectively blocking the VEGF-driven vascular permeability through internalisation and degradation of VEGF receptors, VEGFR-1 or VEGFR-2, in VEGF-stimulated endothelial cells [154]. This shows that PEDF prevents the angiogenesis process by nullifying the VEGF activity and inhibits the tumour growth from becoming more malignant.
The PEDF acts as a regulation of cell proliferation, and invasion is gradually lost with the aggressiveness of metastatic melanoma. It has been found that the expression of PEDF is reduced in many tumour grade of various forms of cancers such as prostate adenocarcinoma, pancreatic adenocarcinoma and hepatocellular carcinoma [155]. The molecular mechanism of PEDF to regulate the metastasis of cancer is yet not fully understood, but few studies suggest that the VEGF/PEDF ratio and regulation of MMPs function by PEDF are the key events that prevent cell invasion and tumour dissemination.
Recent studies have shown that PEDF can also manifest its anti-angiogenesis activity by selectively inducing the apoptosis of endothelial cell. In this regard, Guan et al. observed that apoptotic cells are much higher in overexpressed PEDF in prostate cancer cells as referenced to control [156]. PEDF induces apoptosis of endothelial and tumour cells mainly by extrinsic and intrinsic pathways. The extrinsic pathway which is a cell surface death receptor-mediated pathway depends on the activation of Fas/FasL death pathway, whereas in the intrinsic pathway, also called as mitochondrial pathway, the apoptosis of cells is governed by mitochondrial permeability, manifested by Bcl-2 family proteins and caspases.
Another facet of PEDF is that it may exhibit antitumour activity by its ability to promote tumour cell differentiation. Crawford et al. showed that the intratumoural injection of rPEDF in primitive neuroblastomas which were grown in athymic mice results in tumour cell differentiation, evidenced by less malignant appearing cells histologically and immunohistochemical staining for neurofilament [157]. Filleur et al. suggested that PEDF functions in prostate neuroendocrine differentiation through feedforward mechanism [158]. Although very few studies have been done on this aspect, this added ability to prevent tumour cell growth by differentiation of malignant cells into normal phenotype is indeed promising and warrants further investigation.
Nowadays, PEDF has gained wide attention for the approach of making a potential endogenous agent for treating cancers. Therefore, drug delivery systems system such as gene therapy route in the form of a viral vector, systemic administration of naked PEDF (free, unmodified) or using nanoparticles for controlled release of drugs are needed for the smart delivery of PEDF, to neoplastic sites which not only results in tumour regression but also protects from any side effect [159]. For example, a new approach was used in enhancing PEDF expression through specific platinum-based chemotherapeutic phosphaplatin drugs [160].
3.1.5 SPINK1 (Kazal Type 1)
The serine protease inhibitor Kazal type 1 (SPINK1) was first purified from bovine pancreas as a pancreatic secretory trypsin inhibitor (PSTI) in 1948 by Kazal and colleagues [161]. Similarly, Stenman et al. (1982) also isolated SPINK1 from the urine of patients which were diagnosed with ovarian cancer, but they described it as a trypsin inhibitor (TATI) which is known to be associated with tumour [162]. This inhibitor consists of 56 amino acid residues containing three disulphide bonds and a trypsin-specific binding site formed by Lys-Ile. The primary function of SPINK1 is to inhibit pancreatic and small intestinal serine proteases. SPINK1 is produced in the acinal cells of pancreas where it prevents from autophagy of pancreas cells by inhibiting the trypsin activity in acinar cells. This is because trypsinogen, a precursor of trypsin, is also produced in the same cells and packed together with SPINK1 in zymogen granule [163]. Under normal physiological condition, the conversion of trypsinogen to trypsin is under strict control, and a balanced level of trypsinogen and trypsin is needed in acinar cells. So, it serves as a significant role in preventing the onset of pancreatitis [164].
SPINK1 is overexpressed in various human organ cancers found in the colon, breast, liver and urinary bladder [165, 166]. It has been reported that SPINK1 is found to be overexpressed in the prostrate, and its expression is directly proportional to the tumour grade [162]. The mechanism of SPINK1 involved in prostate cancer and tumour progression is not yet clear. It was observed that tumours producing SPINK1 also produce trypsin, and this trypsin can activate MMPs of the matrix leading metastasis of cancer. This shows that the imbalance secretion of SPINK1 and trypsin is linked with the adverse prognosis in cancer [167]. Thus, SPINK1 can be used as a good target for prostate cancer treatment.
It was proposed that SPINK1 can act as a growth factor because there are a lot of structural similarities, and 50% amino acid homology was found in between SPINK1 and epidermal growth factor (EGF). Thus, SPINK1 is also thought to be involved as a growth factor for tissue repair in inflammatory sites, and if it was prolonged, then it acts as a booster for cancerous cell [168]. Recently, it was reported that SPINK1 induces EMT through activating epidermal growth factor receptor (EGFR), causing proliferation of pancreatic and breast cancer cells [169].
For the specific therapy of cancer patients, it is necessary that specific or activated pathway, for that specific tumour should be targeted. Accordingly, small-molecule inhibitors should be discovered which interfere with specific signalling networks inside the cells. In this way, SPINK1 can be an excellent ‘druggable’ target [167, 170].
References
Lin YC et al (1995) Activation of NF-kappa B requires proteolysis of the inhibitor I kappa B-alpha: signal-induced phosphorylation of I kappa B-alpha alone does not release active NF-kappa B. Proc Natl Acad Sci U S A 92:552–556
Walsh PN, Ahmad SS (2002) Proteases in blood clotting. Essays Biochem 38:95–111
Borissenko L, Groll M (2007) Diversity of proteasomal missions: fine tuning of the immune response. Biol Chem 388:947–955
Roth S (2003) The origin of dorsoventral polarity in drosophila. Philos Trans R Soc Lond Ser B Biol Sci 358:1317–1329. discussion 1329
Bastians H et al (1999) Cell cycle-regulated proteolysis of mitotic target proteins. Mol Biol Cell 10:3927–3941
Turk B, Stoka V (2007) Protease signalling in cell death: caspases versus cysteine cathepsins. FEBS Lett 581:2761–2767
Rawlings ND et al (2008) MEROPS: the peptidase database. Nucleic Acids Res 36:D320–D325
Di Cera E (2009) Serine proteases. IUBMB Life 61:510–515
Hedstrom L (2002) Serine protease mechanism and specificity. Chem Rev 102:4501–4524
Fastrez J, Fersht AR (1973) Demonstration of the acyl-enzyme mechanism for the hydrolysis of peptides and anilides by chymotrypsin. Biochemistry 12:2025–2034
Puente XS et al (2005) A genomic view of the complexity of mammalian proteolytic systems. Biochem Soc Trans 33:331–334
Stroud RM (1974) A family of protein-cutting proteins. Sci Am 231:74–88
Hooper JD et al (2001) Type II transmembrane serine proteases. Insights into an emerging class of cell surface proteolytic enzymes J Biol Chem 276:857–860
Netzel-Arnett S et al (2003) Membrane anchored serine proteases: a rapidly expanding group of cell surface proteolytic enzymes with potential roles in cancer. Cancer Metastasis Rev 22:237–258
Chen LM et al (2001) Prostasin is a glycosylphosphatidylinositol-anchored active serine protease. J Biol Chem 276:21434–21442
Verghese GM et al (2006) Prostasin regulates epithelial monolayer function: cell-specific Gpld1-mediated secretion and functional role for GPI anchor. Am J Physiol Cell Physiol 291:C1258–C1270
Hooper JD et al (1999) Testisin, a new human serine proteinase expressed by premeiotic testicular germ cells and lost in testicular germ cell tumors. Cancer Res 59:3199–3205
Szabo R, Bugge TH (2008) Type II transmembrane serine proteases in development and disease. Int J Biochem Cell Biol 40:1297–1316
Lu P et al. (2011) Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 3: pii:a005058
Lopez-Otin C, Matrisian LM (2007) Emerging roles of proteases in tumour suppression. Nat Rev Cancer 7:800–808
Choi KY et al (2012) Protease-activated drug development. Theranostics 2:156–178
Hildenbrand R et al (2008) The urokinase-system--role of cell proliferation and apoptosis. Histol Histopathol 23:227–236
Collen D, Lijnen HR (1991) Basic and clinical aspects of fibrinolysis and thrombolysis. Blood 78:3114–3124
Blasi F (1997) uPA, uPAR, PAI-1: key intersection of proteolytic, adhesive and chemotactic highways? Immunol Today 18:415–417
Gondi CS et al (2007) Down-regulation of uPAR and uPA activates caspase-mediated apoptosis and inhibits the PI3K/AKT pathway. Int J Oncol 31:19–27
Andreasen PA et al (1997) The urokinase-type plasminogen activator system in cancer metastasis: a review. Int J Cancer 72:1–22
Fisher JL et al (2001) The expression of the urokinase plasminogen activator system in metastatic murine osteosarcoma: an in vivo mouse model. Clin Cancer Res 7:1654–1660
Sidenius N, Blasi F (2003) The urokinase plasminogen activator system in cancer: recent advances and implication for prognosis and therapy. Cancer Metastasis Rev 22:205–222
Aguirre Ghiso JA et al (1999) Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. J Cell Biol 147:89–104
Preissner KT et al (2000) Urokinase receptor: a molecular organizer in cellular communication. Curr Opin Cell Biol 12:621–628
Stillfried GE et al (2007) Plasminogen binding and activation at the breast cancer cell surface: the integral role of urokinase activity. Breast Cancer Res 9:R14
Harbeck N et al (2002) Clinical utility of urokinase-type plasminogen activator and plasminogen activator inhibitor-1 determination in primary breast cancer tissue for individualized therapy concepts. Clin Breast Cancer 3:196–200
Oka T et al (1991) Immunohistochemical evidence of urokinase-type plasminogen activator in primary and metastatic tumors of pulmonary adenocarcinoma. Cancer Res 51:3522–3525
Hasui Y et al (1992) The content of urokinase-type plasminogen activator antigen as a prognostic factor in urinary bladder cancer. Int J Cancer 50:871–873
Nekarda H et al (1994) Tumour-associated proteolytic factors uPA and PAI-1 and survival in totally resected gastric cancer. Lancet 343:117
Han Q et al (2002) Rac1-MKK3-p38-MAPKAPK2 pathway promotes urokinase plasminogen activator mRNA stability in invasive breast cancer cells. J Biol Chem 277:48379–48385
Borgono CA et al (2004) Human tissue kallikreins: physiologic roles and applications in cancer. Mol Cancer Res 2:257–280
Borgono CA, Diamandis EP (2004) The emerging roles of human tissue kallikreins in cancer. Nat Rev Cancer 4:876–890
Schmitt M et al (2013) Emerging clinical importance of the cancer biomarkers kallikrein-related peptidases (KLK) in female and male reproductive organ malignancies. Radiol Oncol 47:319–329
Milkiewicz M et al (2006) Regulators of angiogenesis and strategies for their therapeutic manipulation. Int J Biochem Cell Biol 38:333–357
Desrivieres S et al (1993) Activation of the 92 kDa type IV collagenase by tissue kallikrein. J Cell Physiol 157:587–593
Menashi S et al (1994) Regulation of 92-kDa gelatinase B activity in the extracellular matrix by tissue kallikrein. Ann N Y Acad Sci 732:466–468
Saunders WB et al (2005) MMP-1 activation by serine proteases and MMP-10 induces human capillary tubular network collapse and regression in 3D collagen matrices. J Cell Sci 118:2325–2340
Takayama TK et al (2001) Characterization of hK4 (prostase), a prostate-specific serine protease: activation of the precursor of prostate specific antigen (pro-PSA) and single-chain urokinase-type plasminogen activator and degradation of prostatic acid phosphatase. Biochemistry 40:15341–15348
Giusti B et al (2005) The antiangiogenic tissue kallikrein pattern of endothelial cells in systemic sclerosis. Arthritis Rheum 52:3618–3628
Frenette G et al (1997) Prostatic kallikrein hK2, but not prostate-specific antigen (hK3), activates single-chain urokinase-type plasminogen activator. Int J Cancer 71:897–899
Colman RW (2006) Regulation of angiogenesis by the kallikrein-kinin system. Curr Pharm Des 12:2599–2607
Emanueli C, Madeddu P (2001) Targeting kinin receptors for the treatment of tissue ischaemia. Trends Pharmacol Sci 22:478–484
Watt KW et al (1986) Human prostate-specific antigen: structural and functional similarity with serine proteases. Proc Natl Acad Sci U S A 83:3166–3170
Peter A et al (1998) Semenogelin I and semenogelin II, the major gel-forming proteins in human semen, are substrates for transglutaminase. Eur J Biochem 252:216–221
Christensson A et al (1990) Enzymatic activity of prostate-specific antigen and its reactions with extracellular serine proteinase inhibitors. Eur J Biochem 194:755–763
Christensson A, Lilja H (1994) Complex formation between protein C inhibitor and prostate-specific antigen in vitro and in human semen. Eur J Biochem 220:45–53
Mackay AR et al (1990) Basement membrane type IV collagen degradation: evidence for the involvement of a proteolytic cascade independent of metalloproteinases. Cancer Res 50:5997–6001
Webber MM, Waghray A (1995) Urokinase-mediated extracellular matrix degradation by human prostatic carcinoma cells and its inhibition by retinoic acid. Clin Cancer Res 1:755–761
Lipinska B et al (1990) The HtrA (DegP) protein, essential for Escherichia coli survival at high temperatures, is an endopeptidase. J Bacteriol 172:1791–1797
Spiess C et al (1999) A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell 97:339–347
Clausen T et al (2002) The HtrA family of proteases: implications for protein composition and cell fate. Mol Cell 10:443–455
Hu SI et al (1998) Human HtrA, an evolutionarily conserved serine protease identified as a differentially expressed gene product in osteoarthritic cartilage. J Biol Chem 273:34406–34412
Gray CW et al (2000) Characterization of human HtrA2, a novel serine protease involved in the mammalian cellular stress response. Eur J Biochem 267:5699–5710
Nie G et al (2006) Serine peptidase HTRA3 is closely associated with human placental development and is elevated in pregnancy serum. Biol Reprod 74:366–374
Inagaki A et al (2012) Upregulation of HtrA4 in the placentas of patients with severe pre-eclampsia. Placenta 33:919–926
Zurawa-Janicka D et al (2013) Temperature-induced changes of HtrA2(Omi) protease activity and structure. Cell Stress Chaperones 18:35–51
Canfield AE et al (2007) HtrA1: a novel regulator of physiological and pathological matrix mineralization? Biochem Soc Trans 35:669–671
Zumbrunn J, Trueb B (1996) Primary structure of a putative serine protease specific for IGF-binding proteins. FEBS Lett 398:187–192
Baldi A et al (2002) The HtrA1 serine protease is down-regulated during human melanoma progression and represses growth of metastatic melanoma cells. Oncogene 21:6684–6688
Kotliarov Y et al (2006) High-resolution global genomic survey of 178 gliomas reveals novel regions of copy number alteration and allelic imbalances. Cancer Res 66:9428–9436
Chien J et al (2004) A candidate tumor suppressor HtrA1 is downregulated in ovarian cancer. Oncogene 23:1636–1644
Narkiewicz J et al (2009) Expression of human HtrA1, HtrA2, HtrA3 and TGF-beta1 genes in primary endometrial cancer. Oncol Rep 21:1529–1537
Bowden MA et al (2006) Serine proteases HTRA1 and HTRA3 are down-regulated with increasing grades of human endometrial cancer. Gynecol Oncol 103:253–260
Esposito V et al (2006) Analysis of HtrA1 serine protease expression in human lung cancer. Anticancer Res 26:3455–3459
Chien J et al (2006) Serine protease HtrA1 modulates chemotherapy-induced cytotoxicity. J Clin Invest 116:1994–2004
Oka C et al (2004) HtrA1 serine protease inhibits signaling mediated by Tgfbeta family proteins. Development 131:1041–1053
Fesik SW (2005) Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer 5:876–885
Verhagen AM et al (2002) HtrA2 promotes cell death through its serine protease activity and its ability to antagonize inhibitor of apoptosis proteins. J Biol Chem 277:445–454
Suzuki Y et al (2001) A serine protease, HtrA2, is released from the mitochondria and interacts with XIAP, inducing cell death. Mol Cell 8:613–621
Antalis TM et al (2010) The cutting edge: membrane-anchored serine protease activities in the pericellular microenvironment. Biochem J 428:325–346
Carter BS et al (1990) Epidemiologic evidence regarding predisposing factors to prostate cancer. Prostate 16:187–197
Moran P et al (2006) Pro-urokinase-type plasminogen activator is a substrate for hepsin. J Biol Chem 281:30439–30446
Kirchhofer D et al (2005) Hepsin activates pro-hepatocyte growth factor and is inhibited by hepatocyte growth factor activator inhibitor-1B (HAI-1B) and HAI-2. FEBS Lett 579:1945–1950
Kazama Y et al (1995) Hepsin, a putative membrane-associated serine protease, activates human factor VII and initiates a pathway of blood coagulation on the cell surface leading to thrombin formation. J Biol Chem 270:66–72
Chen Z et al (2003) Hepsin and maspin are inversely expressed in laser capture microdissectioned prostate cancer. J Urol 169:1316–1319
Magee JA et al (2001) Expression profiling reveals hepsin overexpression in prostate cancer. Cancer Res 61:5692–5696
Tanimoto H et al (1997) Hepsin, a cell surface serine protease identified in hepatoma cells, is overexpressed in ovarian cancer. Cancer Res 57:2884–2887
Lin B et al (1999) Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2. Cancer Res 59:4180–4184
Thiery JP, Sleeman JP (2006) Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 7:131–142
Peinado H et al (2007) Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 7:415–428
Kim S et al (2010) TMPRSS4 induces invasion and epithelial-mesenchymal transition through upregulation of integrin alpha5 and its signaling pathways. Carcinogenesis 31:597–606
Jung H et al (2008) TMPRSS4 promotes invasion, migration and metastasis of human tumor cells by facilitating an epithelial-mesenchymal transition. Oncogene 27:2635–2647
Cheng H et al (2009) Hepatocyte growth factor activator inhibitor type 1 regulates epithelial to mesenchymal transition through membrane-bound serine proteinases. Cancer Res 69:1828–1835
Min HJ et al (2014) TMPRSS4 upregulates uPA gene expression through JNK signaling activation to induce cancer cell invasion. Cell Signal 26:398–408
Wang CH et al (2015) TMPRSS4 facilitates epithelial-mesenchymal transition of hepatocellular carcinoma and is a predictive marker for poor prognosis of patients after curative resection. Sci Rep 5:12366
Min HJ et al (2014) TMPRSS4 induces cancer cell invasion through pro-uPA processing. Biochem Biophys Res Commun 446:1–7
Ramsay AJ et al (2008) The type II transmembrane serine protease matriptase-2--identification, structural features, enzymology, expression pattern and potential roles. Front Biosci 13:569–579
Velasco G et al (2002) Matriptase-2, a membrane-bound mosaic serine proteinase predominantly expressed in human liver and showing degrading activity against extracellular matrix proteins. J Biol Chem 277:37637–37646
Webb SL et al (2012) The influence of matriptase-2 on prostate cancer in vitro: a possible role for beta-catenin. Oncol Rep 28:1491–1497
Shi YE et al (1993) Identification and characterization of a novel matrix-degrading protease from hormone-dependent human breast cancer cells. Cancer Res 53:1409–1415
Ramsay AJ et al (2009) Matriptase-2 (TMPRSS6): a proteolytic regulator of iron homeostasis. Haematologica 94:840–849
Ramsay AJ et al (2009) Matriptase-2 mutations in iron-refractory iron deficiency anemia patients provide new insights into protease activation mechanisms. Hum Mol Genet 18:3673–3683
Sanders AJ et al (2010) The type II transmembrane serine protease, matriptase-2: possible links to cancer? Anti Cancer Agents Med Chem 10:64–69
Oberst M et al (2001) Matriptase and HAI-1 are expressed by normal and malignant epithelial cells in vitro and in vivo. Am J Pathol 158:1301–1311
Riddick AC et al (2005) Identification of degradome components associated with prostate cancer progression by expression analysis of human prostatic tissues. Br J Cancer 92:2171–2180
Tannock IF, Rotin D (1989) Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res 49:4373–4384
McQueney MS et al (1997) Autocatalytic activation of human cathepsin K. J Biol Chem 272:13955–13960
Richter C et al (1998) Mechanism of activation of the gastric aspartic proteinases: pepsinogen, progastricsin and prochymosin. Biochem J 335(Pt 3):481–490
Tseng IC et al (2010) Matriptase activation, an early cellular response to acidosis. J Biol Chem 285:3261–3270
Lin CY et al (1997) Characterization of a novel, membrane-bound, 80-kDa matrix-degrading protease from human breast cancer cells. Monoclonal antibody production, isolation, and localization. J Biol Chem 272:9147–9152
Oberst MD et al (2003) The activation of matriptase requires its noncatalytic domains, serine protease domain, and its cognate inhibitor. J Biol Chem 278:26773–26779
Xu H et al (2012) Mechanisms for the control of matriptase activity in the absence of sufficient HAI-1. Am J Physiol Cell Physiol 302:C453–C462
Lee MS et al (2005) Simultaneous activation and hepatocyte growth factor activator inhibitor 1-mediated inhibition of matriptase induced at activation foci in human mammary epithelial cells. Am J Physiol Cell Physiol 288:C932–C941
Szabo R et al (2007) Matriptase inhibition by hepatocyte growth factor activator inhibitor-1 is essential for placental development. Oncogene 26:1546–1556
Kang JY et al (2003) Tissue microarray analysis of hepatocyte growth factor/met pathway components reveals a role for met, matriptase, and hepatocyte growth factor activator inhibitor 1 in the progression of node-negative breast cancer. Cancer Res 63:1101–1105
Saleem M et al (2006) A novel biomarker for staging human prostate adenocarcinoma: overexpression of matriptase with concomitant loss of its inhibitor, hepatocyte growth factor activator inhibitor-1. Cancer Epidemiol Biomark Prev 15:217–227
Bhatt AS et al (2005) Adhesion signaling by a novel mitotic substrate of src kinases. Oncogene 24:5333–5343
Engh RA et al (1995) Divining the serpin inhibition mechanism: a suicide substrate ‘springe’? Trends Biotechnol 13:503–510
Irving JA et al (2000) Phylogeny of the serpin superfamily: implications of patterns of amino acid conservation for structure and function. Genome Res 10:1845–1864
Declerck PJ, Gils A (2013) Three decades of research on plasminogen activator inhibitor-1: a multifaceted serpin. Semin Thromb Hemost 39:356–364
Dass K et al (2008) Evolving role of uPA/uPAR system in human cancers. Cancer Treat Rev 34:122–136
Duffy MJ (2002) Urokinase plasminogen activator and its inhibitor, PAI-1, as prognostic markers in breast cancer: from pilot to level 1 evidence studies. Clin Chem 48:1194–1197
Bajou K et al (2008) Plasminogen activator inhibitor-1 protects endothelial cells from FasL-mediated apoptosis. Cancer Cell 14:324–334
Bajou K et al (1998) Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat Med 4:923–928
Nishioka N et al (2012) Plasminogen activator inhibitor 1 RNAi suppresses gastric cancer metastasis in vivo. Cancer Sci 103:228–232
Zou Z et al (1994) Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science 263:526–529
Sheng S et al (1994) Production, purification, and characterization of recombinant maspin proteins. J Biol Chem 269:30988–30993
Pemberton PA et al (1997) Maspin is an intracellular serpin that partitions into secretory vesicles and is present at the cell surface. J Histochem Cytochem 45:1697–1706
Bass R et al (2002) Maspin inhibits cell migration in the absence of protease inhibitory activity. J Biol Chem 277:46845–46848
McGowen R et al (2000) The surface of prostate carcinoma DU145 cells mediates the inhibition of urokinase-type plasminogen activator by maspin. Cancer Res 60:4771–4778
Biliran H Jr, Sheng S (2001) Pleiotrophic inhibition of pericellular urokinase-type plasminogen activator system by endogenous tumor suppressive maspin. Cancer Res 61:8676–8682
Sheng S et al (1998) Tissue-type plasminogen activator is a target of the tumor suppressor gene maspin. Proc Natl Acad Sci U S A 95:499–504
Zhang M et al (1997) Transactivation through Ets and Ap1 transcription sites determines the expression of the tumor-suppressing gene maspin. Cell Growth Differ 8:179–186
Domann FE et al (2000) Epigenetic silencing of maspin gene expression in human breast cancers. Int J Cancer 85:805–810
Qin L, Zhang M (2010) Maspin regulates endothelial cell adhesion and migration through an integrin signaling pathway. J Biol Chem 285:32360–32369
Zhang M et al (2000) Maspin is an angiogenesis inhibitor. Nat Med 6:196–199
Wang MC et al (2004) Maspin expression and its clinicopathological significance in tumorigenesis and progression of gastric cancer. World J Gastroenterol 10:634–637
Latha K et al (2005) Maspin mediates increased tumor cell apoptosis upon induction of the mitochondrial permeability transition. Mol Cell Biol 25:1737–1748
Chen EI et al (2005) Maspin alters the carcinoma proteome. FASEB J 19:1123–1124
Schwartz AL, Ciechanover A (1999) The ubiquitin-proteasome pathway and pathogenesis of human diseases. Annu Rev Med 50:57–74
Mercatali L et al (2006) RT-PCR determination of maspin and mammaglobin B in peripheral blood of healthy donors and breast cancer patients. Ann Oncol 17:424–428
Chim SS et al (2005) Detection of the placental epigenetic signature of the maspin gene in maternal plasma. Proc Natl Acad Sci U S A 102:14753–14758
Shi HY et al (2003) Modeling human breast cancer metastasis in mice: maspin as a paradigm. Histol Histopathol 18:201–206
Li Z et al (2005) Targeted expression of maspin in tumor vasculatures induces endothelial cell apoptosis. Oncogene 24:2008–2019
Ruegg MA et al (1989) Purification of axonin-1, a protein that is secreted from axons during neurogenesis. EMBO J 8:55–63
Hastings GA et al (1997) Neuroserpin, a brain-associated inhibitor of tissue plasminogen activator is localized primarily in neurons. Implications for the regulation of motor learning and neuronal survival J Biol Chem 272:33062–33067
Kaiserman D et al (2006) Mechanisms of serpin dysfunction in disease. Expert Rev Mol Med 8:1–19
Lloyd-Jones D et al (2009) Heart disease and stroke statistics--2009 update: a report from the American Heart Association statistics committee and stroke statistics subcommittee. Circulation 119:e21–181
Adibhatla RM, Hatcher JF (2008) Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: therapeutic strategies. CNS Neurol Disord Drug Targets 7:243–253
Lebeurrier N et al (2005) The brain-specific tissue-type plasminogen activator inhibitor, neuroserpin, protects neurons against excitotoxicity both in vitro and in vivo. Mol Cell Neurosci 30:552–558
Yepes M et al (2002) Regulation of seizure spreading by neuroserpin and tissue-type plasminogen activator is plasminogen-independent. J Clin Invest 109:1571–1578
Chang WS et al (2000) Tissue-specific cancer-related serpin gene cluster at human chromosome band 3q26. Genes Chromosomes Cancer 29:240–255
Steele FR et al (1993) Pigment epithelium-derived factor: neurotrophic activity and identification as a member of the serine protease inhibitor gene family. Proc Natl Acad Sci U S A 90:1526–1530
He X et al (2015) PEDF and its roles in physiological and pathological conditions: implication in diabetic and hypoxia-induced angiogenic diseases. Clin Sci (Lond) 128:805–823
Ide H et al (2015) Circulating pigment epithelium-derived factor (PEDF) is associated with pathological grade of prostate cancer. Anticancer Res 35:1703–1708
Seruga B et al (2011) Drug resistance in metastatic castration-resistant prostate cancer. Nat Rev Clin Oncol 8:12–23
Dawson DW et al (1999) Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science 285:245–248
Johnston EK et al (2015) Recombinant pigment epithelium-derived factor PEDF binds vascular endothelial growth factor receptors 1 and 2. In Vitro Cell Dev Biol Anim 51:730–738
Belkacemi L, Zhang SX (2016) Anti-tumor effects of pigment epithelium-derived factor (PEDF): implication for cancer therapy. A mini-review. J Exp Clin Cancer Res 35:4
Guan M et al (2007) Adenovirus-mediated PEDF expression inhibits prostate cancer cell growth and results in augmented expression of PAI-2. Cancer Biol Ther 6:419–425
Crawford SE et al (2001) Pigment epithelium-derived factor (PEDF) in neuroblastoma: a multifunctional mediator of Schwann cell antitumor activity. J Cell Sci 114:4421–4428
Filleur S et al (2005) Two functional epitopes of pigment epithelial-derived factor block angiogenesis and induce differentiation in prostate cancer. Cancer Res 65:5144–5152
Abramson LP et al (2003) Wilms’ tumor growth is suppressed by antiangiogenic pigment epithelium-derived factor in a xenograft model. J Pediatr Surg 38:336–342
Mishur RJ et al (2008) Synthesis, X-ray crystallographic, and NMR characterizations of platinum(II) and platinum(IV) pyrophosphato complexes. Inorg Chem 47:7972–7982
Kazal LA et al (1948) Isolation of a crystalline trypsin inhibitor-anticoagulant protein from pancreas. J Am Chem Soc 70:3034–3040
Stenman UH et al (1982) Immunochemical demonstration of an ovarian cancer-associated urinary peptide. Int J Cancer 30:53–57
Kuwata K et al (2002) Functional analysis of recombinant pancreatic secretory trypsin inhibitor protein with amino-acid substitution. J Gastroenterol 37:928–934
Hirota M et al (2006) Genetic background of pancreatitis. Postgrad Med J 82:775–778
Gaber A et al (2009) High expression of tumour-associated trypsin inhibitor correlates with liver metastasis and poor prognosis in colorectal cancer. Br J Cancer 100:1540–1548
Soon WW et al (2011) Combined genomic and phenotype screening reveals secretory factor SPINK1 as an invasion and survival factor associated with patient prognosis in breast cancer. EMBO Mol Med 3:451–464
Ateeq B et al. (2011) Therapeutic targeting of SPINK1-positive prostate cancer. Sci Transl Med 3: 72ra17
McKeehan WL et al (1986) Two apparent human endothelial cell growth factors from human hepatoma cells are tumor-associated proteinase inhibitors. J Biol Chem 261:5378–5383
Ozaki N et al (2009) Serine protease inhibitor Kazal type 1 promotes proliferation of pancreatic cancer cells through the epidermal growth factor receptor. Mol Cancer Res 7:1572–1581
Stenman UH (2011) SPINK1: a new therapeutic target in cancer? Clin Chem 57:1474–1475
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Poddar, N.K., Maurya, S.K., Saxena, V. (2017). Role of Serine Proteases and Inhibitors in Cancer. In: Chakraborti, S., Dhalla, N. (eds) Proteases in Physiology and Pathology. Springer, Singapore. https://doi.org/10.1007/978-981-10-2513-6_12
Download citation
DOI: https://doi.org/10.1007/978-981-10-2513-6_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-2512-9
Online ISBN: 978-981-10-2513-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)