Abstract
The textile industry deals with the design and production of various fabrics with a web of processes intertwined to produce the final product. Among these, the dyeing and finishing processes in particular use large quantities of water and consequently lead to production of large volumes of wastewater. Colour, dissolved solids, toxic heavy metals, residual chlorine and other non-degradable organic materials are the pollutants of major concern present in effluent from textile industries. Advancements in nanotechnology have enabled us to explore the applications of nanochemicals for effluent treatment in textile industries. Nanochemicals have the desired properties required for pollutant and pathogen removal from wastewater by methods such as chemical oxidation, disinfection and photocatalysis. This chapter discusses the various pollutants present in wastewater effluent from textile industries and their sources, the present effluent standards and the application of nanochemicals for wastewater treatment.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Textile industry
- Wastewater treatment technologies
- Novel textile effluent treatment
- Organic and inorganic impurity removal
- Nanochemicals for wastewater treatment
1 Introduction
Textile industry is concerned with the design and production of textile fibres such as yarn, cotton, wool and silk. This industry plays a crucial part in providing the society with basic needs. It is also important in the economic perspective, providing employment and high industrial output. Especially developing countries have high economic contribution from textile industries.
The textile industry spans a spectrum of processes. It can range from the small-scale cottage industries and spinning looms with low economic output, usually established in rural areas which are of domestic value, to the large-scale industries which involve dyeing and designing. The present-day industries use integrated mills which are used to convert different raw materials such as cotton and silk to different products using a unified facility. They also use modern machines with advanced technology such as carding machines for producing spun yarn and draw frames for combining two fibres.
In India, the textile industry has been prevalent for several centuries and has also been among the important contributors to the economic development of the country. This industry alone accounted for earnings through export worth US$41.4 billion in the fiscal year 2014–2015, an annual growth of 5.4 %, as released by the Cotton Textiles Export Promotion Council.
The textiles industry in India is tremendously diverse, with hand-spun and hand-woven textiles sectors in one end of the spectrum, and the high capital sophisticated mills sector in the other end as shown in Table 1. The biggest component of the textile industry is formed mainly by the decentralized power looms/hosiery and knitting sector.
The Indian textile industry is unique when compared to those in other countries because of the intimate relation it shares with the Indian agriculture sector in the way of its dependence for raw materials such as cotton. It also caters to the global demand for a wide range of textile products as required by various market segments, making the Indian textile industry truly versatile.
The fundamental function of the textile industry is to convert various fibres into yarn and further into fabrics and other products. This is followed by the dyeing and finishing processes which could be done either during the various phases of production or separately in the end. Therefore, the textile industry depends on a string of operation’s to produce the final product.
The state-wise distribution of textile industries (Table 2) shows that Tamil Nadu, Andhra Pradesh, Maharashtra and Gujarat possess the maximum number of textile mills, accounting for more than 70 % of the total in the country as shown in Fig. 1.
It is during the processing stage of textile fibres that the operations use several chemicals such as dyes, auxiliary chemicals and sizing materials, to impart desired characters to fabrics. During the entire dyeing and printing process, large volumes of water are used for several processes such as wetting of fibres and dissolving dyes. This water is released in large amounts and causes most of the water pollution. The water released carries with it most of the toxic chemicals and therefore needs to be treated. The wastewater treatment is usually done through primary, secondary and tertiary stages. However, these treatment processes do not remove many of toxic materials such as dissolved solids and trace metals. This calls for advanced treatment technologies for the treatment of wastewater effluent from textile industries. Currently, studies are conducted to explore the possibilities of using nanomaterials for treating wastewater from textile industries. Some of the techniques include the use of nanochemicals for photocatalysis, nanosorbents for adsorption and zeolites for pollutants removal. Also, several treatment technologies are combined together to form the zero liquid discharge process to purify and recycle virtually all of the wastewater produced.
2 An Overview of the Textile Industry
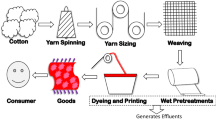
The textile industry can be subdivided into different sectors based on the raw material being processed: the cotton industry, woollen industries, synthetic fabric industries, etc. These industries consist of a string of elaborate processes to produce the final product as shown in Fig. 2. The major process among them is the textile printing and dyeing process. Some of the steps involved in these two processes are pre-treatment, the dyeing and printing processes, finishing.
Textile industry flow chart (Babu et al. 2007)
Pre-treatment is carried out by washing, desizing, scouring and other processes. These processes require large amounts of water. Dyeing is done to transfer the desired colour to the required fabric and produce the coloured fabric. For this purpose, the dye is dissolved in water. Printing on the other hand is a kind of dyeing which is concentrated on a single portion of the fabric that makes up the design. In dyeing, the colour is applied using a solution of the dye, where the dye is suspended in water. Printing on the other hand employs the dye in the form of a thick paste. After the dyeing or printing processes, it is important for the fabric to undergo the finishing processes. The finishing processes are necessary to impart specific properties to the fabric such as strength, softness and durability.
The finishing process is carried out using several finishing agents for softening, creating cross-links within the fabric and waterproofing. All these processes require some amount of water and therefore can lead to release of harmful chemicals and toxins into the effluent stream, essentially requiring effective water treatment. In some cases, mercerization, base reduction processes are carried out before dyeing and printing.
Bleaching is the process of removal of any undesired colouring from fabric before dyeing or printing. Bleaching uses three major chemical procedures, namely sodium chlorite, sodium hypochlorite or hydrogen peroxide bleaching, with the first two procedures being the most common ones. These bleaching agents are carried in with water usually to either dilute or for better reaction with fabric due to the ease of adsorption on fabric. Chlorine dioxide acts as the oxidizing agent. However, chlorine dioxide being a powerful oxidizing agent is corrosive and highly harmful in nature. Therefore, proper disposal and treatment of this effluent are highly necessary.
3 Major Pollutants
The different processes in the textile industry lead to discharge of various toxic pollutants into the water stream which can be harmful. It is important to understand the nature of these pollutants in order to ensure the proper removal and application of the right treatment method (Tüfekci et al. 2007). The major pollutants are the organic chemicals such as azo dyes, pulp, gum, cellulose, hemicellulose and alkali. Water from dyeing and printing process accounts for more than 50 % of the total effluent (Saxena and Kaushik 2011).
Pre-treatment of polyester fibres is done by reduction reaction between the polyester fibre and 8 % sodium hydroxide at 90° for duration of 45 min. This leads to decomposition of the fibre into terephthalic acid and ethylene glycol. The COD of the resulting effluent accounts for about 60 % of the effluent from dyeing and printing processes even though it accounts for only 5 % by volume of the total effluent (Razzak 2014).
Chromium is another kind of pollutant in wastewater which causes a lot of concerns. Chromium is usually used as a catalyst or as chromium dyes for wool industries or comes from potassium dichromate used for tanning. Depending on the type of dye and process being employed, the chromium content is 200–500 times more in effluent than earlier due to the dyeing rate being ceased after process (Wang et al. 2011).
pH is another factor which needs to be considered in wastewater from dyeing processes. During the processes of scouring, desizing and mercerization, which are carried out before printing and dyeing, the pH of effluent wastewater stays around 10–11 when treated with alkali at 90°. As already mentioned, reduction of polyester fibres uses sodium hydroxide base which has a pH ranging between 10 and 11 (Menezes and Choudhari 2011). Therefore, the effluent wastewater is usually alkaline in nature and the first treatment process includes adjusting the pH value of the dyeing wastewater.
Nitrogen in ammonia and urea is also major pollutants and harmful. Batik and other complicated techniques use urea. The total nitrogen content in urea is 300 mg/L, which can be difficult to treat. Phosphorus is also present in wastewater which usually comes from the phosphor-containing detergents and monosodium phosphate used as buffer. Phosphorous can lead to rapid eutrophication of water bodies and therefore must be kept under check. Sulphide mainly comes from the sulphur used in sulphur dyes which are preferred due to their low price. However, sulphur is highly toxic and its use is forbidden in a number of developed nations.
A list of the pollutants present in textile wastewater is given in Table 3.
3.1 Colour/Dyes
The presence of colour in the effluent from dyeing and printing processes is one of the main problems in textile industry. The colour of the dye comes from the chromophore in the dye. Colour in water is easily visible to the naked eye even at very low concentration. Hence, colour from textile wastes carries significant aesthetic importance. Most of the dyes are stable and is not degradable even under the effect of light or ozone. Another reason for concern is that the conventional treatment methods do not degrade the dyes completely. Due to this reason, removal of dyes from the wastewater effluent remains a major problem in most of textile industries. Aniline is one of the organic compounds which are mainly released from the dyes used. Dyes such as Congo red, amino or azo groups contain benzene rings which lead to increased carbon rings and nitrogen groups in the wastewater streams, which are extremely difficult to degrade.
3.2 Dissolved and Suspended Solids
Another critical parameter which has to be kept under constant check in effluent from textile industries is the amount of dissolved solids present. Common salt and Glauber salt, which are used for the recovery of dyes in textile industries, lead to a rapid increase in the total dissolved solids (TDS) of the effluent. However, just like dyes, it is difficult to treat TDS with conventional treatment systems. The presence of high TDS in the effluent can lead to an increase in TDS of surface water and groundwater sources which can be catastrophic as groundwater and surface water are subject to human consumption. The presence of dissolved solids in effluent may also be harmful to crops and in turn restrict the use of water for agricultural purposes.
The sources of suspended solids during the production process include undissolved raw materials, such as cellulose and pulp and fibre scrap. They can be removed by suitable mechanical separation methods. The secondary sedimentation tank outflow therefore contains large amount of suspended solids.
3.3 Toxic Metals
The effluent from textile industries contains metal ions as well. There are two main sources of metal ions. Firstly, the metals present in chemicals such as caustic soda, sodium carbonate and other salts which are used during alkali reduction or bleaching can be washed into the effluent as impurity. For instance, mercury can be present in caustic soda as impurity if it is produced using mercury cell processes (production of sodium hydroxide). Secondly, the source of metal could be from the dyes itself, i.e. the metalized mordent dyes. Most of the metal complex dyes contain a chromium base and as already discussed chromium can easily be washed into effluent from tanning industries.
3.4 Chlorine
The use of various chlorine carrying compounds such as sodium hypochlorite for bleaching in textile processing leads to the presence of residual chlorine in the effluent water stream. This chlorine-containing wastewater if released without treatment leads to reduction of dissolved oxygen from water bodies and reversely affects marine life. Chlorine in wastewater can also react with other substances in water to produce harmful and toxic materials.
3.5 Refractory Materials
Wastewater effluents from textile industries are often polluted with non-biodegradable organic materials which are also referred to as refractory materials. An example of refractory materials includes detergents. The presence of such refractory materials in turn increases the chemical oxygen demand (COD) of the effluent stream. Other organic contaminants, such as sizing materials, acids and enzymes are also present in the effluent. The level of these pollutants in wastewater is controlled by the use of suitable biological treatment processes.
4 Effluent Standards
4.1 Standards for Various Pollutants Present in Textile Effluent in India
Every country has a set of effluent standards for every industry, established by the pollution control board of the country. In India, the Central Pollution Control Board (CPCB), Ministry of Environment and Forests, Government of India, establishes the effluent standards for various industries. The effluent standards for effluent from cotton textile industries as per the Environment (Protection) Rules, 1986 set by the CPCB is given in Table 4.
The effluent hence released has to comply with these standards, and the treatment methods adopted have to ensure that they work around these standards. There are several specific standards depending on the final water body into which the effluent is discharged into. The trade effluent standards for being discharged into inland surface waters in the state of Tamil Nadu are given in Table 5.
4.2 Standards for Various Pollutants Present in Textile Effluent in Germany
The standards for various pollution parameters in textile industry effluent Germany is shown in Table 6.
4.3 Standards for Various Pollutants Present in Textile Effluent in the USA
The EPA publishes the effluent requirements using best practical control tech (BPT) in the USA, the details of which have been shown in Table 7.
5 Conventional Treatment Methods
The wastewater effluent from textile dyeing and printing process contains large concentration of organic matter, most of which is difficult to degrade. The released effluent has high BOD and COD content which can be perilous to the state of aquatic life and further to human health. For instance, the presence of coloured water does not allow penetration of light and therefore restrains growth of life and the presence of phosphorus can accelerate Eutrophication. In order to check the adverse effects of these toxic chemicals in the discharged effluent, it is important to establish suitable treatment methods for textile wastewater.
The conventional treatment techniques that have existed in the past few decades include physicochemical treatment methods, biological treatment and tertiary treatment methods which employ a combination of methods.
5.1 Physicochemical/Primary Treatment
5.1.1 Screening
Treatment of wastewater involves a series of unit processes some of which are physical, chemical and biological in nature. An industry releases different streams from different points at different times, and hence, the first step in treatment of wastewater is to mix and equalize these streams. Some industries use screening process and oil trap processes before mixing various streams. Screening involves the use of meshes and sieves of appropriate size to hold back the grit and the undissolved large particulate matter or debris. Oil trap follows the same function but with the purpose of adhesion separation and removal of oil and grease.
5.1.2 Equalization
Equalization is performed to ensure the uniform distribution of pH, pollution load and temperature. It is carried out by air agitation or mechanical mixing. It helps to fix a single separation process as conditions remain a constant. The hydraulic retention time for this is around 8 h.
5.1.3 Floatation
Equalization is followed by the floatation process. In this process three phases are established of liquid, gas and solid due to the production of microbubbles. Compressed air is usually passed in through the mixture which causes smaller particles to attach to it due to surface tension, buoyancy of the rising bubble hydrostatic pressure. Due to lower density compared to water, these bubbles rise, helping to get rid of tiny fibres and oil particles.
5.1.4 Coagulation, Flocculation and Settling
After all these processes are complete, coagulating chemicals such as alum, ferrous sulphate, ferric chloride and lime are added to the system in a process called flash mixing. The colloidal particles which were not separated during settling and floatation are separated during this process. Colloidal particles usually carry a charge, either positive or negative and the addition of a coagulating agent exposes the charged colloid to an oppositely charged agent which leads to coagulation and further settling due to increase in density. Formation of flocs (aggregates) increases the density further and helps in rapid settling.
This mixture is then processed through the flocculator and further the coagulated mass is separated by settling in settling tanks. A clariflocculator helps to remove sludge content by some amount of adsorption. Choosing the right coagulant depends on the kind of pollutants present in the effluent stream after equalization. Coagulation or flocculation helps in removal of suspended solids and to a certain extent helps in removal of colour from the exit steam as well. They also help reduce the BOD and COD (Verma et al. 2012).
5.2 Biological Treatment
Biological treatment helps remove dissolved matter and is more efficient than physicochemical treatment. The ratio between organic load and the biomass present in the reaction tank, the reactor temperature and oxygen concentration determines the removal efficiency. Aeration leads to a suspension effect, but it is important to ensure that a mixing energy that can destroy the flocks is not reached, because it can inhibit the settling.
The biomass concentration usually is between a range of 2500–4500 mg/l and oxygen content is found to be around 2 mg/l. Based on the oxygen availability, biological treatment can be classified into two main types: aerobic and anaerobic treatment methods. Aerobic biological treatment has higher efficiency and wider application compared to anaerobic treatment methods and therefore is more widely used (Rai et al. 2005).
5.2.1 Aerobic Biological Treatment
There are different kinds of bacteria which can be classified based on their oxygen requirements. These include aerobic, anaerobic and facultative bacteria. As the name suggests, aerobic biological treatment employs aerobic bacteria and some facultative bacteria. Aerobic biological treatment is usually of two types: activated sludge process and biofilm process.
5.2.1.1 Activated Sludge Process
Activated sludge is composed of an aggregate mixture of a number of micro-organisms and has the ability to decompose and adsorb organic matter (Pala and Tokat 2002). Once the activated sludge is formed, it can be removed and the remaining water further purified. The oxidation ditch process and sequencing batch reactor (SBR) process are types of activated sludge processes.
Oxidation ditch process
Oxidation ditch process is a type of activated sludge process which was first developed in the Netherlands. The components of an oxidation ditch process include an aeration equipment, an equipment for the water which has to be purified to come in or go out and mixing equipment. The ditch can be of different shapes which include a ring structure, L shape, rectangular or round shapes. Wastewater activated sludge and various microbes are mixed in a continuous loop ditch. This completes nitrification and denitrification of wastewater. The oxidation ditch resembles a plug flow, completely mixed oxidation tank in nature.
The advantages of this process include long hydraulic retention time (HRT), long sludge age and low organic loading which in turn lead to high degree of purification, high reliability, easier maintenance, high impact resistance, stability, lower investment, easy operation and management, and lower energy consumption. Due to the formation of discrete aerobic, anoxic and anaerobic zones, the oxidation ditch process performs denitrification efficiently.
Sequencing Batch Reactor Process
SBR treatment process leads to removal of COD and colour from effluent water. This process has good shock loading which means it can withstand good amount of organic matter and reserve water. Also, the total residence time, run time and gas supply of each stage can be altered based on the quantity of water coming in or going out. The original water contains large amount of organic matter, which provides good condition for the bacteria to grow. This activated sludge leads to low sludge production compared to the standby phase when the sludge is in the endogenous respiration phase, rendering low sludge yield. Other advantages of this process include less processing equipment, simple structure requirement and ease of operation.
5.2.1.2 Biofilm
In this process various micro-organisms are made to attach to a single support and the wastewater is purified when it flows on this surface by contact. The biofilm processes can further be divided into three categories which include biological fluidized beds, biological contact oxidation and rotating biological contactors.
Biological Fluidized Bed
The fluidized bed process is also referred to as the suspended carrier biofilm process (Chaohai et al. 1998). This is a new and highly efficient wastewater treatment process which uses the modern fluidization technology whose principle is to keep the whole system at a fluidized state and in turn enhance the contact time and area between solid and fluid particles. The substances usually used as carrier or filling in the system include carbonaceous materials such as sand, activated carbon, anthracite or other particles. The diameter of these particles needs to be less than 1 mm.
A pulse of the wastewater is allowed to pass through the carrier which leads to fluidization of the carrier particles and in turn leads to formation of biofilm on these particles. Smaller size of carrier particles is preferred as it leads to higher surface area (the preferred area is between 2000 and 3000 m2/m3), which in turn can hold higher amount of biomass. This process yields efficiency of 20–30 times higher than the conventional treatment processes and hence can be used for treating high consistency organic effluent from textile industries.
Biological Contact Oxidation
Biological contact oxidation is a process which is primarily used for treating wastewater from the dyeing process (Guosheng 2000). Fillers are set in tanks called aeration tanks and the set-up mimics that of the activated sludge process. A biofilm is formed on the fillers placed in the tank and the wastewater in the tank contains a certain quantity of activated sludge. Aerobic micro-organisms are present in the biofilm and these microbes degrade the organic matter present in wastewater when they come in contact with each other.
Rotating Biological Contactor
Rotating biological contactor is a wastewater treatment process which is modelled based on the conventional biological filter (Abraham et al. 2003). It consists of a series of discs which are usually composed of low weight materials such as plastic or glass platers. The diameter of the discs used ranges from 1 to 3 m. The disc is constructed such that half of the disc area is exposed to air while the other half is submerged in the supplied wastewater. The Rotating device makes the discs rotate slowly in the horizontal axis which in turn helps to completely mix the wastewater due to continuous rotational motion. The micro-organisms present in the wastewater form a biofilm on the disc when the disc is exposed to the wastewater. When the same disc is exposed to air, the biofilm comes in contact with oxygen and gets adsorbed. The micro-organisms oxidize and degrade the organic matter present in wastewater by catalysis reaction using enzymes and release the metabolite at the same time. Hence, an adsorption–oxidation–oxidative decomposition process which is followed continuously is used to treat the wastewater.
The rotating biological contactor has good power saving, has high shock load capability, produces very little sludge, is easy to maintain and manage and produces very little noise. The shortcomings of this equipment include the need for larger-area and well-maintained buildings. Main parameters which affect the performance of the RBC are speed of rotation, wastewater residence time, area of disc submerged and temperature.
5.2.2 Anaerobic Biological Treatment
The anaerobic biological treatment process is carried out in the absence of oxygen. Bacteria called anaerobic bacteria are used to degrade organic matter under anaerobic conditions. This process has been used for treatment of both high and low concentration organic wastewater such as textile dyeing wastewater (Delée et al. 1998). Effluent from textile industries contains organic matter with organic matter content as high as 1000 mg/L. Anaerobic biological treatment is usually adopted for the treatment of high concentration wastewater while aerobic treatment is used to treat wastewater with low organic matter.
Presently the most widely used technique for anaerobic biological treatment is the hydrolysis acidification process. In this process, the anaerobic and facultative bacteria decompose the biodegradable organic matter present in wastewater into small molecular organic matter. This principle can be used for biodegradation of coloured groups of dye molecules present in textile dyeing wastewater. The acids released by these bacteria can also be used to reduce the amount of alkalinity in textile effluent and in turn make the water more suitable for marine life. Other processes used for treatment of effluent from textile industries include upflow anaerobic fluidized bed (UABF), upflow anaerobic sludge bed reactor (UASB) and anaerobic biological filter.
6 Advanced Treatment Methods
6.1 Adsorption
Adsorption is a surface phenomenon which is used to remove suitable organic matter from wastewater effluents (Kumar et al. 2014; Oliveira et al. 2002). Chemicals such as cyanides and phenol derivatives can be removed using this process. This process is particularly useful as it can be used to remove compounds difficult to remove by conventional treatment methods. As the wastewater is passed through the adsorbent, the organic pollutants keep getting adsorbed. Activated carbon is the most widely used adsorbent. Activated carbon is usually produced by treatment of petroleum products or wood. The material is burned in the presence of air, creating a char. Oxidation of this charred substance at high temperature gives a porous substance which is activated carbon. An important characteristic of activated carbon is its high surface area which is important for adsorption of the organic matter onto the adsorbent.
The activated carbon once completely used up, i.e. when all its pores are clogged requires regeneration. Regeneration is usually done chemically or thermally; however, latest methods include solvent, electrochemical, microwave induced and supercritical regeneration. Suitable acids or chemicals can be passed to partially regenerate the adsorbent and this process leads to need for frequent regeneration (Leng and Pinto 1996). In case of thermal regeneration, the spent adsorbent (activated carbon) is transported in water slurry to a regeneration unit. In this unit, the adsorbent is dewatered by application of heat using a furnace and reheated under controlled conditions (Dabrowski et al. 2004). This complete process oxidizes the impurities and allows the volatile substances to escape leaving adsorbent with increased pore volume. In order to remove excess heat, the hot adsorbent is quenched with water and moved back to the column. This process has higher efficiency than chemical regeneration. Other materials used as possible adsorbents include clay, silica and fly ash.
6.2 Ion Exchange
The ion exchange process is another modern method of removal of inorganic salts, heavy metals and few specific organic anionic compounds from wastewater (Dabrowski et al. 2004). Salts are usually ionic compounds usually composed of a positive ion from an alkali and a negative ion from an acid. The ion exchange resins or membranes have the ability to exchange suitable ions present in electrolytic solutions, in this case wastewater with soluble ions. A cation exchanger when contacted with an electrolyte such as calcium chloride will scavenge the positively charged ion, in this case calcium, from the electrolyte and in turn replace it with the soluble ions which in this case is sodium. Hardness in water arises from salts of calcium and magnesium, and this method provides a convenient technique to reduce hardness in wastewater. Ion exchange resins can be made of different compounds such as phenolic compounds and sulphonic styrene’s.
6.3 Membrane Filtration
6.3.1 Reverse Osmosis
Reverse osmosis follows the principle that certain semipermeable membranes are selective in nature and allow the flow of only selective ions through them (Fritzmann et al. 2007). The commonly used membranes include nylon and cellulose membranes such as cellulose acetate. The wastewater to be treated is passed at high pressure through the semipermeable membrane as shown in Fig. 3.
Reverse osmosis (Wayman 2015)
The applied pressure has to be high enough to act as a driving force to overcome the overall osmotic pressure of the stream. When this criterion is met, water flows from the wastewater chamber to the clear water chamber through the semipermeable membrane. A reverse osmosis system consists of a pre-treatment section through which the wastewater is passed to remove suspended solids by mechanical separation methods and if necessary, ions such as iron and magnesium. This is necessary to ensure that these suspended solids and ions do not foul the semipermeable membrane. After pre-treatment the wastewater is pressurized and sent to the reverse osmosis chamber. When the applied pressure is greater than the osmotic pressure across the membrane, clear water passes from wastewater to the clear water chamber, emerging at atmospheric pressure.
Reverse osmosis can be used to treat effluent from primary, secondary and tertiary stages of wastewater treatment. The reverse osmosis models include spiral wound system, tubular systems, Hollow fibre membranes and the disc module. The main downfall of this process is fouling of the membranes in use due to organic materials, colloids and micro-organisms. Also scale formation can take place due to hardness-causing constituents (carbonates) in the effluent stream. Chlorine and ozone can oxidize the membrane and hence should not be present in wastewater to be treated.
6.3.2 Ultrafiltration
Ultrafiltration and reverse osmosis follow similar techniques. The difference between the two processes is primarily the retention properties of the membranes. Ultrafiltration membranes have higher pore size and can retain only macro-molecules and suspended solids. Reverse osmosis membranes on the other hand have smaller pore size and hence can be used for removal of all solutes including salts. The osmotic pressure difference across an ultrafiltration membrane is negligible as the salts pass through along with the permeate water. This makes it necessary for a much lesser pressure to be applied for the process to take place (Babu et al. 2007). Ultrafiltration membranes materials include cellulose derivatives such as cellulose acetate, polymers of nylon and other inert polymers. This makes it possible to treat acidic or caustic streams and not limiting the process unlike that for reverse osmosis due to chemical attack of the membranes.
6.3.3 Nanofiltration
When the salt concentration in the permeate water is not of prime importance as compared to the reverse osmosis operation, nanofiltration can be used for wastewater treatment (Nguyen et al. 2012). Hardness-causing elements in water are salts of calcium and magnesium, and nanofiltration can be used to remove these salts. It can also be used for the removal of micro-organisms such as bacteria and viruses. Nanofiltration is suitable to use when permeate with TDS, but without colour, COD and hardness is acceptable. It is a more cost-effective method. For higher efficiency, turbidity and the amount of colloidal content in wastewater should be low.
Fouling is a major problem whenever membrane separation techniques are used for purifying wastewater (Jiraratananon et al. 2000). Wastewater has dissolved elements such as silica, calcium, barium, iron and strontium which may precipitate on the surface of the membrane and in turn clog the pores of the membrane. This phenomenon is termed as fouling of the membrane. Bacteria present in wastewater which proliferates in warm environmental conditions can also cause fouling of membranes. This may cause increased pressure difference across the membranes and reduced flux leading to higher expenditure. Fouling can be avoided or reduced by selecting a suitable chemical dose to counteract the deposition and precipitation of organic matter. Antiscalents can be used for the removal of mineral scales. Back washing with acid also reduces fouling.
6.4 Ozonation
Ozone is known to be one of the strongest oxidizing agents commercially available and it can be used for disinfection purposes on wastewater. Ozonation can be used to break down complex large molecules such as phenols (Langlais et al. 1991). Oxidation of organic and inorganic materials, removal of odour and removal of colour are the main applications of ozone in industries. Naturally, ozone is present as an unstable gas; under normal conditions, it decomposes to give molecular oxygen and nascent oxygen. Due to this property, ozone has to be produced on the site for industrial applications. There are several methods of producing ozone, but the corona discharge method is the most widely used. An ozone generation unit usually consists of a series of electrodes. These electrodes are fit with cooling arrangements mounted in a gas tight container. The source gas use to produce ozone is usually air or oxygen. When this gas is passed between two electrodes which are separated by a narrow gap between them, the oxygen gets converted into ozone. The ozone released by the ozone generation unit is brought in contact with wastewater in the ozone contact basin by means of diffuser tubes or turbine mixers as shown in Fig. 4. Complete removal of colour and pollutants present in wastewater can be achieved by using 2 mg/l of ozone. The treated water is treated with sand (sand filtration) for the final cleaning.
6.5 Evaporation
6.5.1 Multiple-Effect Evaporation
In evaporation, the wastewater is heated in multiple-effect evaporators with the concentrate from one evaporator being fed to the next evaporator while the clear water is allowed to exit the system for further treatment. The fresh liquor and steam are added only to the first evaporator. The steam obtained by evaporation from the first stage acts as input steam for the next stage. The vapours and used steam are collected in the condenser.
Evaporators reduce the volume of the effluent and leads to an increase in concentration of salts in the stream. This makes their recovery more viable whenever intended. One problem of evaporation is the formation of scales on the walls and tubes of the evaporator due to the presence of salts of calcium and magnesium is wastewater. This leads to a reduction in rate of heat transfer and in turn the evaporation rate is also reduced. Cleaning is required for removal of these scales. Cleaning can be of both chemical and mechanical type. Chemical means involve acid treatment where acid is poured along the scale to loosen it from the surface of the tube. A scale cutter is a device mounted on a flexible shaft which is inserted from the top of the pipe. This device is moved up and down repeatedly to remove the scale in the entire length of tube. Once all the stages have been cleaned, the evaporator is closed and tested to check for any leaks. Proper seals are made to close these leaks.
6.5.2 Mechanical Vapour Compression
The mechanical vapour compression technique is used to concentrate the wastewater effluent. A heat exchanger is used which also acts as an evaporator or condenser. The heat is provided by the simultaneous condensation of the distillate. Latent heat is exchanged between hot and cool water in the evaporation–condensation process. A compressor which is present provides the energy required for separating the solution by heat transfer.
6.6 Crystallization
In crystallization, solid crystals are obtained from a homogeneous solution. It is a solid–liquid separation technique. Crystallization occurs only when a solution is supersaturated. Supersaturation of a solution is its state in which the solvent has higher concentration of solute than what can be dissolved in it at that temperature. By using this method, salts can be recovered from the mother liquor. Crystallizers are of two types, namely single-stage and multi-stage. The common chemicals removed from the effluent by crystallization include calcium sulphate, sodium chloride, calcium chloride and sodium sulphate. Therefore, the scale causing chemicals can be removed from wastewater and this can be used a pre-treatment method for wastewater being used for membrane purification and evaporation.
6.7 Zero Liquid Discharge (ZLD)
Zero liquid discharge is a modern separation technique which leads to a solid (or near solid) waste stream and purified water stream which can be treated further or used for domestic applications (Dalan 2000). Reverse osmosis and thermal methods can be used for concentrating the waste stream and formation of pure water. However, since reverse osmosis is more cost-effective, it is more widely used (Heijman et al. 2009). ZLD consists of a series of wastewater treatment techniques which include microfiltration (MF) for pre-treatment, reverse osmosis (RO) for pre-concentration of waste solutions followed by the application of a multiple-effect evaporator. The major objective is to produce a solid concentrated stream which can be sent for land-based disposal and a relatively clean water stream for domestic use or sewer discharge. This leaves a clean effluent stream without any dissolved solids for further treatment while the filtered or concentrated solids can be sent for suitable disposal.
7 Application of Nanochemicals for Effluent Treatment
Nanoscience is the field of science which deals with the study of materials at the nanoscale. The smaller size of nanomaterials highly influences their properties as compared to their bigger counterparts. Nanotechnology is an application of nanoscience and it deals with the control, integration and manipulation of various atoms and molecules to form different materials at the nanoscale (Hornyak et al. 2008, 2009). The past decade has been subjected to drastic increase in the application of nanotechnology in applied science, especially in the area of water purification, opening up a new potential alternative to treat wastewater more efficiently and cost-effectively (Baruah et al. 2012; Baruah and Dutta 2016) Some of the key advantages of nanochemicals include their small size, high reactivity, high accuracy and most importantly, their capability to be produced by environment-friendly techniques, most of which are potentially cost-effective as well. Two of the promising applications of nanochemicals for wastewater treatment in textile industries are:
-
Photocatalysis
-
Nanosorbents.
7.1 Photocatalysis
Photocatalysis is a process which can be defined as a “change in the rate of a chemical reaction or its initiation under the action of ultraviolet, visible, or infrared radiation in the presence of a substance—the photo catalyst—that absorbs the light and is involved in the chemical transformation of the reaction partners” (McNaught and Wilkinson 1997). It is a process which is used to degrade pollutants in wastewater. A nanostructured catalyst medium which is sensitive to exposure to light is mainly employed.
The catalyst medium usually in use is a semiconductor material, which generates an electron–hole pair upon absorption of light with energy higher than its band gap energy. The electron–hole pair then reacts with water to produce highly reactive oxidizing and reducing radicals, such as super oxides, hydroxyl ions or other radicals. These radicals then degrade any organic/inorganic pollutant molecules present in the contaminated water through some secondary reactions. Photocatalysis is a surface phenomenon and its general mechanism is a complex process, which involves five basic steps (Pirkanniemi and Sillanpää 2002).
-
Reactants diffusing to the surface of the catalyst
-
Reactants getting adsorbed on the surface of the catalyst
-
Reaction taking place at the surface of the catalyst
-
Products undergoing desorption from the surface of the catalyst
-
Products diffusing away from the surface of the catalyst.
Nanostructured semiconductor materials possess higher surface area which enables them to have better photocatalytic properties compared to their larger bulk counterparts. This property makes it possible for the generated electrons and holes to be available at the surface of the nanophotocatalyst rather than to be available in the bulk of the material. An ideal photocatalyst should exhibit the following properties:
-
high photoactivity
-
biological and chemical inertness
-
photostability
-
non-toxicity
-
cost-effectiveness.
Some examples of typically used nanostructured semiconductor photocatalysts are zinc oxide (ZnO), titanium dioxide (TiO2), zinc sulphide (ZnS), ferric oxide (Fe2O3) and cadmium sulphide (CdS).
The wide band gap semiconductors absorb light in the UV region of the solar spectrum. However, using high-energy UV light sources to excite the photocatalysts may not be a cost-effective solution in all cases. Therefore, research is currently focusing on using visible portion of the solar spectrum for conducting photocatalysis experiments. The advantages of using solar light for photocatalysis are that solar energy is free and abundantly available. Some of the key areas in which photocatalysis can be used for the treatment of textile wastewater include removal of:
-
Organic contaminants.
-
Inorganic contaminants.
-
Heavy metals.
7.1.1 Removal of Organic Contaminants
Photocatalysis is widely used for the decomposition of harmful organic contaminants present in wastewater into harmless by-products, mostly carbon dioxide and water. The organic contaminants that can be removed from wastewater using photocatalysis include carboxylic acids, alcohols, phenolic derivatives and chlorinated aromatic compounds (Bhatkhande et al. 2002; Mills et al. 1993). Dyes being the major pollutants released into the effluent stream have to be treated and the nanophotocatalysts shown to be successful in this regard are semiconductor metal oxides such as TiO2 and ZnO.
Additionally, photocatalysis has been employed to degrade natural organic matter also called as humic substances. Humic substances are naturally occurring yellow–brown organic materials with high molecular weight. TiO2 nanoparticles have made it possible to achieve almost 50 % reduction in humic acid concentration in drinking water, observed under irradiation from a mercury lamp (Eggins et al. 1997).
7.1.2 Removal of Inorganic Contaminants
Inorganic contaminants include chemicals such as halide ions, cyanide, thiocyanate, ammonia, nitrates and nitrites that can be effectively decomposed by photocatalytic reaction (Hoffmann et al. 1995; Mills et al. 1996). The photocatalytic removal of toxic Hg(II) and CH3Hg(II) chlorides using TiO2 nanoparticles under simulated solar light (AM1.5) (Serpone et al. 1987) and the removal of toxic potassium cyanide and Cr(VI) ions from water using visible light and ZnO nanoparticles have been achieved. Recently it has been found that CdS/titanate nanotubes are useful in photocatalytic oxidation of ammonia in water (Lee et al. 2002).
7.1.3 Removal of Heavy Metals
Heavy metals present in wastewater streams is another area of concern as it directly affects human health and presents a challenge for treatment plants, since the amount of can vary, depending upon the type of industry. However, due to the rare availability and high cost of some metals, recovery of the metals is mostly preferred over removal of the metals itself. Photocatalysis has been shown to successfully remove heavy metals in several cases. TiO2 catalyst dispersion has been shown to help in the recovery of gold(III), platinum(IV), and rhodium(III), from a mixture of gold(III), platinum(IV), and rhodium(III) chloride salts (Borgarello et al. 1986). Recovery of gold from samples containing cyanide ions was also performed along with the degradation of CN− by employing two peroxides, H2O2 and S2O2. TiO2 has also been used for removing cadmium (Cd) from effluents by irradiating the stream with a source producing light rays of 253.7 nm wavelength. A combination of activated carbon obtained from sewage sludge along with TiO2 nanoparticles has been used to reduce Hg2+ ions (Asenjo et al. 2011) followed by recovery of metallic Hg(0).
7.2 Nanosorbents
Sorption is a process in which a material referred to as sorbate gets adsorbed on another substance, called sorbent, by some physical or chemical interaction. Sorbents are used extensively for the removal of organic pollutants from wastewater such as those from textile industries. The sorption process usually takes place in three steps:
-
Transport of the pollutant particle from water to the surface of the sorbent
-
Adsorption of pollutant at the surface of the sorbent
-
Transport within the sorbent.
Nanoparticles possess higher specific surface areas than the bulk particles. They also have the ability to functionalize easily with a variety of chemical groups and therefore improve their affinity towards the target contaminants. The aforementioned two properties make them very effective as sorbents. Moreover, nanosorbents possess nanosized pores that aid the sorption of contaminants. Nanosorbents have the ability to be used again through a regeneration operation where the absorbed pollutants are removed by suitable processes. Some of the nanosorbents are listed below.
7.2.1 Carbon Nanosorbents
Carbon-based nanomaterials are extensively used for the adsorption of various organic and inorganic pollutants in wastewater. Out of the carbon-based nanomaterials used, activated carbon is very popular because of its high adsorption capacity, high thermal stability, excellent resistance against attrition losses and low cost. Benzene and toluene are used as solvents for mixing dyes in the textile industry. Removal of benzene and toluene from the effluent stream is important as they add to the organic matter content and can be harmful. Adsorption of benzene and toluene from industrial wastewater on activated carbon has been carried out (Kadirvelu et al. 2001) and high adsorption capacity for benzene (∼400–500 mg/g) and toluene (∼700 mg/g) was noticed. Activated carbon has been proven effective for the removal of heavy metal ions, such as Hg(II), Ni(II), Co(II), Cd(II), Cu(II), Pb(II), Cr(III) and Cr(VI) (Kobya et al. 2005; Al-Omair and El-Sharkawy 2007; Zhong et al. 2006).
7.2.2 Metal Oxide Nanosorbents
The common metal oxides used as adsorbents are mostly oxides of iron (Fe), manganese (Mn), silicon (Si), titanium (Ti) and tungsten (W). As adsorbent materials, metal oxides have the advantages of being low-cost materials and can be functionalized easily to tune their adsorption capacity and selectivity. Fe oxides nanosorbents have recently been tested for the removal of several organic pollutants in wastewater (Luo et al. 2011; Zhang et al. 2010, 2013; Iram et al. 2010). The magnetic properties of Fe oxide nanosorbents allow them to be magnetically separated from water (Hu et al. 2010) The Fe oxides also showed excellent adsorption capacity for heavy metal ions (Nassar 2010; Jeon and Yong 2010). Nanostructured tungsten oxide (WO2) has also displayed very high adsorption capacity for organic dyes in water (Abdolmohammad-Zadeh et al. 2013). A zinc–aluminium layered double hydroxide nanosorbent has been developed and successfully applied for the removal of reactive yellow dyes from several textile wastewater streams (El-Safty et al. 2012). Applications of non-metallic oxide, such as silica (SiO2), as nanosorbents have also shown promising results in removing organic pollutants and heavy metals present in effluents (Yantasee et al. 2010; Zamzow et al. 1990; Maliou et al. 1992; Ouki and Kavannagh 1997; Ibrahim and Khoury 2002).
7.2.3 Zeolites as Sorbents
Zeolites have high specific surface area and high ion exchange capacity, making zeolites a sought after adsorbent for water treatment. Most of the zeolites occur naturally and can also be produced commercially. Zeolites are used widely for the adsorption of heavy metal ions (Pansini et al. 1991). The adsorption of lead and cadmium using two natural zeolites chabazite and clinoptilolite has been studied (Kesraoui-Ouki et al. 1993). Using the two natural zeolites pre-treated with NaOH, the authors demonstrated very high adsorption capacity for lead (Pb) and cadmium (Cd), with metal removal efficiency of more than 99 %. The high porosity of zeolite gives it a higher adsorption capacity, and the photocatalytic reduction ability of zeolite aids in reducing higher valence metal ions to the corresponding lower ones, thus decreasing their toxicity.
8 Nanochemicals Used for Textile Wastewater Treatment
Several commercial and advanced technological developments are employed for water treatment; however, nanotechnology has been established as one of the most advanced wastewater treatment techniques. Developments in nanoscale research has paved way for economically feasible and environmentally stable treatment technologies for effectively treating wastewater meeting the ever increasing water quality standards. Nanotechnology can possible address many of the water quality issues by using different types of nanoparticles. Nanotechnology uses materials of sizes smaller than 100 nm in at least one dimension meaning at the level of atoms and molecules as compared with other disciplines such as chemistry and materials science (Masciangioli and Zhang 2003; Eijkel and Van den Berg 2005).
At this scale, materials possess new and significantly changed physical, chemical and biological properties mainly due to their structural variation, higher surface area-to-volume ratio offering several uses for pollution control such as treatment, remediation and detection (Rickerby and Morrison 2007; Vaseashta et al. 2007). These unique properties of nanomaterials, for example, high reactivity and strong sorption, are explored for application in water/wastewater treatment based on their functions in unit.
Nanoparticles can penetrate deeper and thus can treat water/wastewater which is generally not possible by conventional technologies. Their higher surface area-to-volume ratio enhances the reactivity with environmental contaminants. Nanotechnology has the potential to provide both water quality and quantity in the long run through the use of, for example, membranes enabling water reuse, desalination. In addition, it yields low-cost and real-time measurements through the development of continuous monitoring devices (Riu et al. 2006; Theron et al. 2008). Nanoparticles, having high absorption, interaction and reaction capabilities, can behave as colloid by mixing mixed with aqueous suspensions and they can also display quantum size effects (Alivisatos 1996). Energy conservation leading to cost savings is possible due to their small sizes; however, overall usage cost of the technology should be compared with other techniques in the market (Crane and Scott 2012).
Membrane technology, considered as one of the advanced water/wastewater treatment processes due to its efficient and low-cost filtration technique (Allabashi et al. 2007), has been developed to be even more efficient using nanomaterials. Nanoparticles have been frequently used in the manufacturing of membranes, allowing permeability control and fouling resistance in various structures and relevant functionalities (Li et al. 2009; Kim et al. 2008). Both polymeric and inorganic membranes are manufactured by either assembling nanoparticles into porous membranes or blending process. The examples of nanomaterials used in this formation include, for example, metal oxide nanoparticles such as TiO2. CNTs have resulted in desired outputs of improved permeability, inactivation of bacteria and so forth (Chae et al. 2009; Barhate and Ramakrishna 2007).
Finally, nanofibrous media have also been used to improve the filtration systems because of the high permeability and small pore size properties they possess (Escobar et al. 2001). They are synthesized by a novel fabrication technique called electrospinning. Depending on the polymers selected, they exhibit different properties. In short, the development of different nanomaterials such as nanosorbents, nanocatalysts, zeolites, dendrimers and nanostructured catalytic membranes has made it possible to disinfect disease-causing microbes, removing toxic metals, and organic and inorganic solutes from water/wastewater. An attempt is made to highlight the factors that may influence the efficiency of the removal processes based on the available literature in the following section.
8.1 Disinfection
Biological contaminants are generally classified under three categories, namely micro-organisms, natural organic matter (NOM) and biological toxins. Microbial contaminants include human pathogens and free living microbes (Majsterek et al. 2004; Berry et al. 2006; Dugan and Williams 2006; Srinivasan et al. 2008; Jain and Pradeep 2005). Adsorbents such as activated carbon have reasonably good removal efficiencies and again a number of factors influence the removal process.
Groundwater can get easily polluted by bacteria and protozoa. The easiest method for treating micro-organisms is using oxidizing agents such as chlorine and ozone. However, chlorine dioxide is expensive and results in the production of hazardous substances such as chlorite and chlorate in manufacturing process. Ozone, on the other hand, has no residual effects but produces unknown organic reaction products. For UV disinfection, longer exposure time is required for effectiveness and also there is no residual effect. So, advanced disinfection technologies must, at least, eliminate the emerging pathogens, in addition to their suitability for large-scale adoption. There are many different types of nanomaterials such as Ag, titanium and zinc capable of disinfecting waterborne disease-causing microbes present in effluent. Their antibacterial properties arise from their charge. TiO2 photocatalysts and metallic and metal oxide nanoparticles are a set of promising nanomaterials that exhibit antimicrobial properties. The ability of metal ions to produce the desired results in water disinfection has been highlighted by many researchers (Sondi and Salopek-Sondi 2004). The possible nanomaterials that can be utilized as disinfectants are listed.
8.1.1 Silver Nanoparticles
Silver has low toxicity and microbial inactivation and hence is the most widely used nanomaterial. Silver nanoparticles can be derived from its salts such as silver nitrate and silver chloride, and their effectiveness as biocides is widely known (Baker et al. 2005; Panáček et al. 2006; Makhluf et al. 2005). Though the antibacterial effect is size dependent, smaller Ag nanoparticles (8 nm) were most effective, while larger particle size (11–23 nm) resulted in lower bactericidal activity (Xiu et al. 2011). There are several mechanisms which have been found to explain the bactericidal effects of Ag nanoparticles such as, the damaging of the bacterial membranes due to the formation of free radicals (Xiu et al. 2012; Esteban-Cubillo et al. 2006) interactions with DNA, alteration of membrane properties due to adhesion on cell surface and enzyme damage.
Immobilized nanoparticles possess high antimicrobial activity (Balogh et al. 2001). In a study, cellulose acetate fibres embedded with Ag nanoparticles by direct electro spinning method were shown effective against both Gram-positive and Gram-negative bacteria. Ag nanoparticles are also incorporated into different types of polymers for the production of antimicrobial nanofibres (Chen et al. 2003; Botes and Cloete 2010; Chou et al. 2005). Water filters prepared using polyurethane foam coated with Ag nanofibres have shown good antibacterial properties against Escherichia coli (E. coli) (Lee et al. 2007).
Ag nanoparticles also find their applications in construction of filtration membranes, for example, in polysulphone membranes, for biofouling reduction. These Ag nanoparticles laden membranes had good antimicrobial activities against E. coli, Pseudomonas and so forth (Adesina 2004; Li et al. 2008). Although Ag nanoparticles have been used efficiently for inactivating bacteria and viruses as well as reducing membrane biofouling, in the long term their capacity against membrane biofouling decreased considerably due to the loss of silver ions with time. Therefore, for long-term control of membrane biofouling, further work to reduce this loss of silver ions is required. Alternatively, doping of Ag nanoparticles with other metallic nanoparticles or its composites with metal oxide nanoparticles can solve the issue and this could also lead to the parallel removal of inorganic/organic compounds from wastewater.
8.1.2 TiO2 Nanoparticles
TiO2 nanoparticles are among the emerging and most promising photocatalysts for water purification. TiO2 exposed to 8 h of simulated solar light, has been reported to reduce the viability of aqueous microbial pathogens, mainly protozoa and fungi. Nitrogen-doped TiO2 nanoparticles catalysts have proved their efficiency for reduction in microbial contaminants in wastewater (Chaturvedi et al. 2012). Nanostructured TiO2 films and membranes are capable of both disinfecting micro-organisms and decomposing organic pollutants under UV and visible light irradiation (Choi et al. 2009). Due to its stability in water, TiO2 can be incorporated in thin films or merged onto membrane filters.
8.2 Removal of Heavy Metals Ions
The major heavy metals and metal ions present in wastewater from textile industries include copper, arsenic, lead, cadmium, mercury and chromium. Different types of nanomaterials have been introduced for removal of heavy metal ions from wastewater such as nanosorbents including CNTs, zeolites and dendrimers, and they have exceptional adsorption properties (Savage and Diallo 2005) The ability of CNTs to adsorb heavy metals such as Cd2+ (Li et al. 2003), Cr3+ (Di et al. 2006), Pb2+ (Li et al. 2005), and Zn2+ (Lu et al. 2006) and metalloids such as arsenic (As) compounds (Peng et al. 2005) has been well documented. CNT composites with iron and cerium oxide (CeO2) have been used for the removal of heavy metal ions in few studies (Lu et al. 2005). CeO2 nanoparticles supported with CNTs are used effectively to adsorb arsenic. Fast adsorption kinetics of CNTs is predominantly to the easily accessible adsorption sites and small intraparticle diffusion distance.
Metal-based nanomaterials are better in removing heavy metals than activated carbon (Sharma et al. 2009), for example, adsorption of arsenic by using TiO2 nanoparticles and nanosized magnetite. Also, a nanocomposite of TiO2 nanoparticles anchored on graphene sheet was used to reduce Cr(VI) to Cr(III) in sunlight (Zhang et al. 2012). Similar Cr treatment was carried out by using palladium nanoparticles in another experiment (Omole et al. 2009). The capability of removing heavy metals such as As has also been investigated by using iron oxide nanomaterials (Fe2O3 and Fe3O4) as cost-effective adsorbents.
Iron nanoparticles (Fe0) are very effective in reducing heavy metals such as As(III), As(V), Pb(II), Cu(II), Ni(II) and Cr(VI) (Ponder et al. 2000; Kanel et al. 2005, 2006; Yang and Lee 2005; Li and Zhang 2006). Novel self-assembled iron oxide nanostructures were also used to successfully adsorb both As(V) and Cr(VI) (Zhong et al. 2006). The 3D nanostructures of CeO2 are used as good adsorbents for both As and Cr. Finally, there are commercial products for efficient removal of arsenic and these include FeO2 nanoparticles and polymers and nanocrystalline TiO2 medium as beads (Sylvester et al. 2007).
8.3 Removal of Organic Contaminants
Natural organic matter (NOM) constitutes a diverse group of hydrophobic (humic and fulvic acids) and hydrophilic organic compounds and it contributes significantly towards water contamination. Adsorption is the major principle used for removal of NOM from wastewater effluent and carbon-based adsorbents have been widely used for the same.
8.3.1 CNTs
Nanosorption has been carried out using nanosorbents, namely zeolites, polymeric materials such as dendrimers and carbon nanotubes (CNTs). These nanosorbents have exceptional adsorption properties and are used for removing organic content from wastewater (Savage and Diallo 2005). CNTs have exceptional water treatment capabilities due to their ability to adsorb high amount of organics on their surface (Rao et al. 2007) The removal of NOM by CNTs is higher in comparison with other carbon-based adsorbents because of the provision of large surface area in CNTs (Saleh et al. 2008). CNTs are also effective in removing polycyclic aromatic compounds (Di et al. 2006) and hence find special application in the textile industry as the dyeing process releases maximum amount of aromatic compounds into the effluent stream. Activated carbon fibres prepared by electrospinning of CNTs which are nanoporous in nature showed much greater organic sorption for benzene, toluene, xylene and ethyl benzene (which are used as solvents in the textile industry) than granular activated carbon (Mangun et al. 2001). Multi-walled CNTs functionalized with Fe nanoparticles can be used as effective sorbents for aromatic compounds such as benzene and toluene (Jin et al. 2007).
8.3.2 TiO2 Nanoparticles
Nanomaterials of metal oxides such as TiO2 in addition to CeO2 have also been used as catalysts for quick and relatively higher degree of degradation of organic contaminants in ozonation processes (Nawrocki and Kasprzyk-Hordern 2010; Orge et al. 2011). Polychlorinated biphenyls (PCBs) which are highly toxic to the environment are majorly used in nylon industries and can enter the effluent stream during discharge. Photocatalysts such as TiO2 nanoparticles are used effectively for treating wastewater polluted by organic contaminants such as polychlorinated biphenyls (PCBs), benzenes and chlorinated alkanes (Kabra et al. 2004). Removal of total organic carbon from wastewater has been enhanced by the use of TiO2.
Decomposition of organic compounds can be enhanced by noble metal doping into TiO2 due to enhanced hydroxyl radical production and so forth. For example, the doping of Si into TiO2 nanoparticles was proved to be effectual in improving its efficiency due to the increase in surface area and crystallinity (Iwamoto et al. 2000, 2005). TiO2 nanocrystals modified with noble metal deposits and so forth were used for degrading methylene blue dye in the visible light range (Liu and Gao 2005; Wu et al. 2006). Nitrogen- and Fe(III)-doped TiO2 nanoparticles are useful in degrading azo dyes and phenol, respectively, than commercially available TiO2 nanoparticles. TiO2 nanotubes have been effectively used to degrade organic compounds and were found to be more efficient than TiO2 nanoparticles (Macak et al. 2007).
8.3.3 Zero-Valent Iron
Azo dyes are among the pollutants of significant concern that are being let out by most of the textile industries. Nanocatalysts including semiconductors, zero-valent metals, and bimetallic nanoparticles have been used for the removal of several organic pollutants, namely PCBs, pesticides and azo dyes. The main property that enables them to be effective is their greater surface area and shape specific properties (Zhao et al. 2011).
Other class of chlorinated organic compounds and PCBs have been transformed successfully using nanoscale zero-valent iron (nZVI) (Wang and Zhang 1997) and inorganic ions such as nitrate and perchlorate (Choe et al. 2000; Cao et al. 2005). The stabilized nZVI particles could also be an efficacious way for in situ treatment of industrial effluents such as the textile industry (Xu and Zhao 2007). The nZVI and bimetallic nZVI can be used as effective redox reagents for reducing a variety of organic pollutants released by textile industries such as PCBs and organic dyes owing to larger surface areas and higher reactivity (Schrick et al. 2002; Nurmi et al. 2005).
8.4 Other Nanomaterials
The nanocatalyst of Ag and amidoxime fibres was used efficiently for the degradation of organic dyes useful for treating effluent from textile industries (Wu et al. 2010). Manganese dioxide (MnO2) films have been used for the mineralization of organic dyes (Espinal et al. 2004). Similarly, MnO2 hierarchical hollow nanostructures have been put to use for the removal of organic pollutant in wastewater (Fei et al. 2008). Several millions of dollars are invested into the material research of such useful nanochemicals.
9 Challenges and Limitations of Using Nanochemicals
The uses of nanochemicals for wastewater treatment remains to be relatively less explored. Several academic breakthroughs and research are yet to be translated to benefit the industry and their allied applications. The use of nanochemicals for photocatalysis and nanosorption for the wastewater treatment in textile industries requires skilled personnel with particular expertise in the area. Multiple modifications in the existing treatment equipment are also necessary for the application of nanochemicals to be functional and efficient. The lack of knowledge on the fate of nanochemicals in water and the difficulty to predict their properties and behaviour is of concern. Unintended pollution–catalytic effects on the environment through reactions in macro-interfacial processes accounts as a potential hazard (Yao et al. 2013).
10 Conclusion
The textile industry consists of a series of processes which lead to discharge of harmful pollutants into the effluent stream. These pollutants if released unchecked and untreated can cause adverse effects to the environment and aquatic life. Hence, the effluents from textile industries need to be properly treated and discharged. Present techniques are finding it difficult to keep up with effluent standards and volumes of pollutants being released. Novel techniques are being developed and one of the most promising solutions could be the use of nanochemicals for the treatment of wastewater from textile industries.
Nanochemicals are capable of removal of pollutants by photocatalysis or nanosorption. They can also be moulded to form nanofiltration membranes. Nanochemicals can be used for the removal of organic dyes released throughout the dyeing process, solvents released during the scouring process, heavy metals and ions released during both the dyeing and printing process. TiO2, nZVI, zeolites are some of the nanochemicals which are of great use and can be used practically for the treatment of wastewater effluent from textile industries.
References
Abdolmohammad-Zadeh, H., Ghorbani, E., & Talleb, Z. (2013). Zinc–aluminum layered double hydroxide as a nano-sorbent for removal of Reactive Yellow 84 dye from textile wastewater effluents. Journal of the Iranian Chemical Society, 10, 1103–1112.
Abraham, T. E., Senan, R. C., Shaffiqu, T. S., Roy, J. J., Poulose, T. P., & Thomas, P. P. (2003). Bioremediation of textile azo dyes by an aerobic bacterial consortium using a rotating biological contactor. Biotechnology Progress, 19, 1372–1376.
Adesina, A. A. (2004). Industrial exploitation of photocatalysis: Progress, perspectives and prospects. Catalysis Surveys from Asia, 10, 265–273.
Alivisatos, A. P. (1996). Perspectives on the physical chemistry of semiconductor nanocrystals. The Journal of Physical Chemistry, 100, 13226–13239.
Allabashi, R., Arkas, M., Hörmann, G., & Tsiourvas, D. (2007). Removal of some organic pollutants in water employing ceramic membranes impregnated with cross-linked silylated dendritic and cyclodextrin polymers. Water Research, 41, 476–486.
Al-Omair, M. A., & El-Sharkawy, E. A. (2007). Removal of heavy metals via adsorption on activated carbon synthesized from solid wastes. Environmental Technology, 28, 443–451.
Asenjo, N. G., Álvarez, P., Granda, M., Blanco, C., Santamaría, R., & Menéndez, R. (2011). High performance activated carbon for benzene/toluene adsorption from industrial wastewater. Journal of Hazardous Materials, 192, 1525–1532.
Babu, B. R., Parande, A. K., & Raghu, S. (2007). Cotton textile processing: Waste generation and effluent treatment. Journal of cotton science, 11, 141–153.
Baker, C., Pradhan, A., Pakstis, L., Pochan, D. J., & Shah, S. I. (2005). Synthesis and antibacterial properties of silver nanoparticles. Journal of Nanoscience and Nanotechnology, 5, 244–249.
Balogh, L., Swanson, D. R., Tomalia, D. A., Hagnauer, G. L., & McManus, A. T. (2001). Dendrimer-silver complexes and nanocomposites as antimicrobial agents. Nano Letters, 1, 18–21.
Barhate, R. S., & Ramakrishna, S. (2007). Nanofibrous filtering media: Filtration problems and solutions from tiny materials. Journal of Membrane Science, 296, 1–8.
Baruah, S., & Dutta, J. (2016). Hydrothermal growth of ZnO nanostructures. Science and Technology of Advanced Materials.
Baruah, S., K Pal, S., & Dutta, J. (2012). Nanostructured zinc oxide for water treatment. Nanoscience & Nanotechnology-Asia, 2, 90–102.
Berry, D., Xi, C., & Raskin, L. (2006). Microbial ecology of drinking water distribution systems. Current Opinion in Biotechnology, 17, 297–302.
Bhatkhande, D. S., Pangarkar, V. G., & Beenackers, A. A. (2002). Photocatalytic degradation for environmental applications–a review. Journal of Chemical Technology and Biotechnology, 77, 102–116.
Borgarello, E., Serpone, N., Emo, G., Harris, R., Pelizzetti, E., & Minero, C. (1986). Inorganic Chemistry, 25, 4499.
Botes, M., & Cloete, T. E. (2010). The potential of nanofibers and nanobiocides in water purification. Critical Reviews in Microbiology, 36, 68–81.
Cao, J., Elliott, D., & Zhang, W. X. (2005). Perchlorate reduction by nanoscale iron particles. Journal of Nanoparticle Research, 7, 499–506.
Chae, S. R., Wang, S., Hendren, Z. D., Wiesner, M. R., Watanabe, Y., & Gunsch, C. K. (2009). Effects of fullerene nanoparticles on Escherichia coli K12 respiratory activity in aqueous suspension and potential use for membrane biofouling control. Journal of Membrane Science, 329, 68–74.
Chaohai, W., Xiangdong, J., & Huanqin, C. (1998). Advances in the technology of wastewater treatment by aerobic biological fluidized bed. Journal of Environmental Science and Technology, 4, 001.
Chaturvedi, S., Dave, P. N., & Shah, N. K. (2012). Applications of nano-catalyst in new era. Journal of Saudi Chemical Society, 16, 307–325.
Chen, Y., Wang, L., Jiang, S., & Yu, H. (2003). Study on novel antibacterial polymer materials (I) preparation of zeolite antibacterial agents and antibacterial polymer composite and their antibacterial properties. Journal of Polymer Materials, 20, 279–284.
Choe, S., Chang, Y. Y., Hwang, K. Y., & Khim, J. (2000). Kinetics of reductive denitrification by nanoscale zero-valent iron. Chemosphere, 41, 1307–1311.
Choi, H., Al-Abed, S. R., & Dionysiou, D. D. (2009). Nanostructured titanium oxide film- and membrane-based photocatalysis for water treatment. Nanotechnology Applications for Clean Water, 3, 39–46.
Chou, G. E. W. L., Yu, D. G., & Yang, M. C. (2005). The preparation and characterization of silver-loading cellulose acetate hollow fibre membrane for water treatment. Polymers for Advanced Technologies, 16, 600–607.
Crane, R. A., & Scott, T. B. (2012). Nanoscale zero-valent iron: Future prospects for an emerging water treatment technology. Journal of Hazardous Materials, 211, 112–125.
Dabrowski, A., Hubicki, Z., Podkościelny, P., & Robens, E. (2004). Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere, 56, 91–106.
Dalan, J. A. (2000). 9 things to know about zero liquid discharge. Chemical Engineering Progress, 96, 71–76.
Delée, W., O’Neill, C., Hawkes, F. R., & Pinheiro, H. M. (1998). Anaerobic treatment of textile effluents: A review. Journal of Chemical Technology and Biotechnology, 73, 323–335.
Di, Z. C., Ding, J., Peng, X. J., Li, Y. H., Luan, Z. K., & Liang, J. (2006). Chromium adsorption by aligned carbon nanotubes supported ceria nanoparticles. Chemosphere, 62, 861–865.
Dugan, N. R., & Williams, D. J. (2006). Cyanobacteria passage through drinking water filters during perturbation episodes as a function of cell morphology, coagulant and initial filter loading rate. Harmful Algae, 5, 26–35.
Eggins, B. R., Palmer, F. L., & Byrne, J. A. (1997). Photocatalytic treatment of humic substances in drinking water. Water Research, 31, 1223–1226.
Eijkel, J. C. T., & Van den Berg, A. (2005). Nanofluidics: What is it and what can we expect from it? Microfluidics and Nanofluidics, 1, 249–267.
El-Safty, S. A., Shahat, A., & Ismael, M. (2012). Mesoporous aluminosilica monoliths for the adsorptive removal of small organic pollutants. Journal of Hazardous Materials, 201, 23–32.
Escobar, I. C., Randall, A. A., & Taylor, J. S. (2001). Bacterial growth in distribution systems: Effect of assimilable organic carbon and biodegradable dissolved organic carbon. Environmental Science and Technology, 35, 3442–3447.
Espinal, L., Suib, S. L., & Rusling, J. F. (2004). Electrochemical catalysis of styrene epoxidation with films of MnO2 nanoparticles and H2O2. Journal of the American Chemical Society, 126, 7676–7682.
Esteban-Cubillo, A., Pecharromán, C., Aguilar, E., Santarén, J., & Moya, J. S. (2006). Antibacterial activity of copper monodispersed nanoparticles into sepiolite. Journal of Materials Science, 41, 5208–5212.
Fei, J., Cui, Y., Yan, X., et al. (2008). Controlled preparation of MnO2 hierarchical hollow nanostructures and their application in water treatment. Advanced Materials, 20, 452–456.
Fritzmann, C., Löwenberg, J., Wintgens, T., & Melin, T. (2007). State-of-the-art of reverse osmosis desalination. Desalination, 216, 1–76.
Guosheng, C. H. S. (2000). Research progress and application on biological contact oxidation of micropolluted source water. Techniques and Equipment for Environmental Pollution, 3, 10.
Heijman, S. G. J., Guo, H., Li, S., Van Dijk, J. C., & Wessels, L. P. (2009). Zero liquid discharge: Heading for 99 % recovery in nanofiltration and reverse osmosis. Desalination, 236, 357–362.
Hoffmann, M. R., Martin, S. T., Choi, W., & Bahnemann, D. W. (1995). Chemical Reviews, 95, 69.
Hornyak, G. L., Dutta, J., Tibbals, H. F., & Rao, A. (2008). Introduction to nanoscience. Boca Raton: CRC Press.
Hornyak, G. L., Moore, J. J., Tibbals, H. F., & Dutta, J. (2009). Fundamentals of nanotechnology. Boca Raton: CRC Press.
Hu, H., Wang, Z., & Pan, L. (2010). Synthesis of monodisperse Fe3O4@ silica core–shell microspheres and their application for removal of heavy metal ions from water. Journal of Alloys and Compounds, 492, 656–661.
Ibrahim, K., & Khoury, H. (2002). Use of natural chabazite–phillipsite tuff in wastewater treatment from electroplating factories in Jordan. Environmental Geology, 41, 547–551.
Iram, M., Guo, C., Guan, Y., Ishfaq, A., & Liu, H. (2010). Adsorption and magnetic removal of neutral red dye from aqueous solution using Fe3O4 hollow nanospheres. Journal of Hazardous Materials, 181, 1039–1050.
Iwamoto, S., Iwamoto, S., Inoue, M., Yoshida, H., Tanaka, T., & Kagawa, K. (2005). XANES and XPS study of silica-modified titanias prepared by the glycothermal method. Chemistry of Materials, 17, 650–655.
Iwamoto, S., Tanakulrungsank, W., Inoue, M., Kagawa, K., & Praserthdam, P. (2000). Synthesis of large-surface area silica-modified Titania ultrafine particles by the glycothermal method. Journal of Materials Science Letters, 19, 1439–1443.
Jain, P., & Pradeep, T. (2005). Potential of silver nanoparticle-coated polyurethane foam as an antibacterial water filter. Biotechnology and Bioengineering, 90, 59–63.
Jeon, S., & Yong, K. (2010). Morphology-controlled synthesis of highly adsorptive tungsten oxide nanostructures and their application to water treatment. Journal of Materials Chemistry, 20, 10146–10151.
Jin, J., Li, R., Wang, H., Chen, H., Liang, K., & Ma, J. (2007). Magnetic Fe nanoparticle functionalized water-soluble multi-walled carbon nanotubules towards the preparation of sorbent for aromatic compounds removal. Chemical Communications, 4, 386–388.
Jiraratananon, R., Sungpet, A., & Luangsowan, P. (2000). Performance evaluation of nanofiltration membranes for treatment of effluents containing reactive dye and salt. Desalination, 130, 177–183.
Kabra, K., Chaudhary, R., & Sawhney, R. L. (2004). Treatment of hazardous organic and inorganic compounds through aqueous-phase photocatalysis: A review. Industrial and Engineering Chemistry Research, 43, 7683–7696.
Kadirvelu, K., Thamaraiselvi, K., & Namasivayam, C. (2001). Removal of heavy metals from industrial wastewaters by adsorption onto activated carbon prepared from an agricultural solid waste. Bioresource Technology, 76, 63–65.
Kanel, S. R., Greneche, J. M., & Choi, H. (2006). Arsenic(V) removal from groundwater using nano scale zero-valent iron as a colloidal reactive barrier material. Environmental Science and Technology, 40, 2045–2050.
Kanel, S. R., Manning, B., Charlet, L., & Choi, H. (2005). Removal of arsenic(III) from groundwater by nanoscale zero-valent iron. Environmental Science and Technology, 39, 1291–1298.
Kesraoui-Ouki, S., Cheeseman, C., & Perry, R. (1993). Effects of conditioning and treatment of chabazite and clinoptilolite prior to lead and cadmium removal. Environmental Science and Technology, 27, 1108–1116.
Kim, J., Davies, S. H. R., Baumann, M. J., Tarabara, V. V., & Masten, S. J. (2008). Effect of ozone dosage and hydrodynamic conditions on the permeate flux in a hybrid ozonation-ceramic ultrafiltration system treating natural waters. Journal of Membrane Science, 311, 165–172.
Kobya, M., Demirbas, E., Senturk, E., & Ince, M. (2005). Adsorption of heavy metal ions from aqueous solutions by activated carbon prepared from apricot stone. Bioresource Technology, 96, 1518–1521.
Kumar, P. S., Vijayakumar Abhinaya, R., Arthi, V., GayathriLashmi, K., Priyadharshini, M. & Sivanesan, S. (2014). Adsorption of methylene blue dye onto surface modified cashew nut shell. Environmental Engineering & Management Journal (EEMJ), 13.
Langlais, B., Reckhow, D. A., & Brink, D. R. (1991). Ozone in water treatment: Application and engineering. Boca Raton: CRC Press.
Lee, S. Y., Kim, H. J., Patel, R., Im, S. J., Kim, J. H., & Min, B. R. (2007). Silver nanoparticles immobilized on thin film composite polyamide membrane: Characterization, nanofiltration, antifouling properties. Polymers for Advanced Technologies, 18, 562–568.
Lee, J., Park, H., & Choi, W. (2002). Selective photocatalytic oxidation of NH3 to N2 on platinized TiO2 in water. Environmental Science and Technology, 36, 5462–5468.
Leng, C. C., & Pinto, N. G. (1996). An investigation of the mechanisms of chemical regeneration of activated carbon. Industrial and Engineering Chemistry Research, 35, 2024–2031.
Li, Y. H., Di, Z., Ding, J., Wu, D., Luan, Z., & Zhu, Y. (2005). Adsorption thermodynamic, kinetic and desorption studies of Pb2+ on carbon nanotubes. Water Research, 39, 605–609.
Li, Y. H., Ding, J., Luan, Z., et al. (2003). Competitive adsorption of Pb2+, Cu2+ and Cd2+ ions from aqueous solutions by multiwalled carbon nanotubes. Carbon, 41, 2787–2792.
Li, Q., Mahendra, S., Lyon, D. Y., et al. (2008). Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Research, 42, 4591–4602.
Li, J. F., Xu, Z. L., Yang, H., Yu, L. Y., & Liu, M. (2009). Effect of TiO2 nanoparticles on the surface morphology and performance of microporous PES membrane. Applied Surface Science, 255, 4725–4732.
Li, X. Q., & Zhang, W. X. (2006). Iron nanoparticles: The core-shell structure and unique properties for Ni(II) sequestration. Langmuir, 22, 4638–4642.
Liu, H., & Gao, L. (2005). Synthesis and properties of CdSe-sensitized rutile TiO2 nanocrystals as a visible light-responsive photocatalyst. Journal of the American Ceramic Society, 88, 1020–1022.
Lu, C., Chiu, H., & Liu, C. (2006). Removal of zinc(II) from aqueous solution by purified carbon nanotubes: Kinetics and equilibrium studies. Industrial and Engineering Chemistry Research, 45, 2850–2855.
Lu, C., Chung, Y. L., & Chang, K. F. (2005). Adsorption of trihalomethanes from water with carbon nanotubes. Water Research, 39, 1183–1189.
Luo, L. H., Feng, Q. M., Wang, W. Q., & Zhang, B. L. (2011). Fe3O4/Rectorite composite: Preparation, characterization and absorption properties from contaminant contained in aqueous solution. Advanced Materials Research, 287, 592–598.
Macak, J. M., Zlamal, M., Krysa, J., & Schmuki, P. (2007). Self-organized TiO2 nanotube layers as highly efficient photocatalysts. Small (Weinheim an der Bergstrasse, Germany), 3, 300–304.
Majsterek, I., Sicinska, P., Tarczynska, M., Zalewski, M., & Walter, M. (2004). Toxicity of microcystin from cyanobacteria growing in a source of drinking water. Comparative Biochemistry and Physiology—C Toxicology and Pharmacology, 139, 175–179.
Makhluf, S., Dror, R., Nitzan, Y., Abramovich, Y., Jelinek, R., & Gedanken, A. (2005). Microwave-assisted synthesis of nanocrystalline MgO and its use as a bacteriocide. Advanced Functional Materials, 15, 1708–1715.
Maliou, E., Malamis, M., & Sakellarides, P. O. (1992). Lead and cadmium removal by ion exchange. Water Science and Technology, 25, 133–138.
Mangun, C. L., Yue, Z., Economy, J., Maloney, S., Kemme, P., & Cropek, D. (2001). Adsorption of organic contaminants from water using tailored ACFs. Chemistry of Materials, 13, 2356–2360.
Masciangioli, J., & Zhang, W. X. (2003). Peer reviewed: Environmental technologies at the nanoscale. Environmental Science and Technology, 37, 102–108.
McNaught, A. D., & Wilkinson, A. (1997). IUPAC. Compendium of chemical terminology (“gold book”) (2nd ed.). Oxford: Blackwell Scientific Publications. (XML on-line corrected version created by Nic M, Jirat J, Kosata B).
Menezes, E., & Choudhari, M. (2011). Pre-treatment of textiles prior to dyeing.
Mills, A., Belghazi, A., & Rodman, D. (1996). Bromate removal from drinking water by semiconductor photocatalysis. Water Research, 30, 1973–1978.
Mills, A., Davies, R. H., & Worsley, D. (1993). Water purification by semiconductor photocatalysis. Chemical Society Reviews, 22, 417–425.
Nassar, N. N. (2010). Rapid removal and recovery of Pb (II) from wastewater by magnetic nanoadsorbents. Journal of Hazardous Materials, 184, 538–546.
Nawrocki, J., & Kasprzyk-Hordern, B. (2010). The efficiency and mechanisms of catalytic Ozonation. Applied Catalysis, B: Environmental, 99, 27–42.
Nguyen, T., Roddick, F. A., & Fan, L. (2012). Biofouling of water treatment membranes: A review of the underlying causes, monitoring techniques and control measures. Membranes, 2, 804–840.
Nurmi, J. T., Tratnyek, P. G., Sarathy, V., et al. (2005). Characterization and properties of metallic iron nanoparticles: Spectroscopy, electrochemistry, and kinetics. Environmental Science and Technology, 39, 1221–1230.
Oliveira, L. C. A, Rachel Rios, V. R. A., Jose Fabris, D., Garg, V., Karim Sapag, & Rochel Lago, M. (2002). Activated carbon/iron oxide magnetic composites for the adsorption of contaminants in water. Carbon, 40, 2177–2183.
Omole, M. A., K’Owino, I., & Sadik, O. A. (2009). Nanostructured materials for improving water quality: Potentials and risks. Nanotechnology Applications for Clean Water, 17, 233–247.
Orge, C. A., Órfão, J. J. M., Pereira, M. F. R., Duarte de Farias, A. M., Neto, R. C. R., & Fraga, M. A. (2011). Ozonation of model organic compounds catalysed by nanostructured cerium oxides. Applied Catalysis, B: Environmental, 103, 190–199.
Ouki, S. K., & Kavannagh, M. (1997). Performance of natural zeolites for the treatment of mixed metal-contaminated effluents. Waste Management and Research, 15, 383–394.
Pala, A., & Tokat, E. (2002). Color removal from cotton textile industry wastewater in an activated sludge system with various additives. Water Research, 36, 2920–2925.
Panáček, A., Kvítek, L., Prucek, R., et al. (2006). Silver colloid nanoparticles: Synthesis, characterization, and their antibacterial activity. The Journal of Physical Chemistry B, 110, 16248–16253.
Pansini, M., Colella, C., & De Gennaro, M. (1991). Chromium removal from water by ion exchange using zeolite. Desalination, 83, 145–157.
Peng, X., Luan, Z., Ding, J., Di, Z., Li, Y., & Tian, B. (2005). Ceria nanoparticles supported on carbon nanotubes for the removal of arsenate from water. Materials Letters, 59, 399–403.
Pirkanniemi, K., & Sillanpää, M. (2002). Heterogeneous water phase catalysis as an environmental application: A review. Chemosphere, 48, 1047–1060.
Ponder, S. M., Darab, J. G., & Mallouk, T. E. (2000). Remediation of Cr(VI) and Pb(II) aqueous solutions using supported, nanoscale zero-valent iron. Environmental Science and Technology, 34, 2564–2569.
Rai, H. S., Bhattacharyya, M. S., Singh, J., Bansal, T. K., Vats, P., & Banerjee, U. C. (2005). Removal of dyes from the effluent of textile and dyestuff manufacturing industry: A review of emerging techniques with reference to biological treatment. Critical reviews in environmental science and technology, 35, 219–238.
Rao, G. P., Lu, C., & Su, F. (2007). Sorption of divalent metal ions from aqueous solution by carbon nanotubes: A review. Separation and Purification Technology, 58, 224–231.
Razzak, N. R. B. (2014). Effectiveness of fenton’s reagent in the treatment of textile effluent.
Rickerby, D. G., & Morrison, M. (2007). Nanotechnology and the environment: A European perspective. Science and Technology of Advanced Materials, 8, 19–24.
Riu, J., Maroto, A., & Rius, F. X. (2006). Nanosensors in environmental analysis. Talanta, 69, 288–301.
Saleh, N. B., Pfefferle, L. D., & Elimelech, M. (2008). Aggregation kinetics of multiwalled carbon nanotubes in aquatic systems: Measurements and environmental implications. Environmental Science and Technology, 42, 7963–7969.
Savage, N., & Diallo, M. S. (2005). Nanomaterials and water purification: Opportunities and challenges. Journal of Nanoparticle Research, 7, 331–342.
Saxena, S., & Kaushik, S. (2011). Effluent treatment in textile industries.
Schrick, B., Blough, J. L., Jones, A. D., & Mallouk, T. E. (2002). Hydrodechlorination of trichloroethylene to hydrocarbons using bimetallic nickel-iron nanoparticles. Chemistry of Materials, 14, 5140–5147.
Serpone, N., Ah-You, Y. K., Tran, T. P., Harris, R., Pelizzetti, E., & Hidaka, H. (1987). AM1 simulated sunlight photoreduction and elimination of Hg (II) and CH3 Hg (II) chloride salts from aqueous suspensions of titanium dioxide. Solar Energy, 39, 491–498.
Sharma, Y. C., Srivastava, V., Singh, V. K., Kaul, S. N., & Weng, C. H. (2009). Nano-adsorbents for the removal of metallic pollutants from water and wastewater. Environmental Technology, 30, 583–609.
Sondi, I., & Salopek-Sondi, B. (2004). Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. Journal of Colloid and Interface Science, 275, 177–182.
Srinivasan, S., Harrington, G. W., Xagoraraki, I., & Goel, R. (2008). Factors affecting bulk to total bacteria ratio in drinking water distribution systems. Water Research, 42, 3393–3404.
Sylvester, P., Westerhoff, P., Möller, T., Badruzzaman, M., & Boyd, O. (2007). A hybrid sorbent utilizing nanoparticles of hydrous iron oxide for arsenic removal from drinking water. Environmental Engineering Science, 24, 104–112.
Theron, J., Walker, W. A., & Cloete, T. E. (2008). Nanotechnology and water treatment: Applications and emerging opportunities. Critical Reviews in Microbiology, 34, 43–69.
Tüfekci, Neşe, Sivri, Nüket, & Toroz, İsmail. (2007). Pollutants of textile industry wastewater and assessment of its discharge limits by water quality standards. Turkish Journal of Fisheries and Aquatic Sciences, 7, 97–103.
Vaseashta, A., Vaclavikova, M., Vaseashta, S., Gallios, G., Roy, P., & Pummakarnchana, O. (2007). Nanostructures in environmental pollution detection, monitoring, and remediation. Science and Technology of Advanced Materials, 8, 47–59.
Verma, A. K., Dash, R. R., & Bhunia, P. (2012). A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. Journal of Environmental Management, 93, 154–168.
Wang, Z., Huang, K., Xue, M., & Liu, Z. (2011). Textile dyeing wastewater treatment, 91–116.
Wang, C. B., & Zhang, W. X. (1997). Synthesizing nanoscale iron particles for rapid and complete dechlorination of TCE and PCBs. Environmental Science and Technology, 31, 2154–2156.
Wayman, J. (2015). Brackish ground water desalination using solar reverse osmosis.
Wu, L., Yu, J. C., & Fu, X. (2006). Characterization and photocatalytic mechanism of nanosized CdS coupled TiO2 nanocrystals under visible light irradiation. Journal of Molecular Catalysis A: Chemical, 244, 25–32.
Wu, Z. C., Zhang, Y., Tao, T. X., Zhang, L., & Fong, H. (2010). Silver nanoparticles on amidoxime fibres for photo-catalytic degradation of organic dyes in waste water. Applied Surface Science, 257, 1092–1097.
Xiu, Z. M., Ma, J., & Alvarez, P. J. J. (2011). Differential effect of common ligands and molecular oxygen on antimicrobial activity of silver nanoparticles versus silver ions. Environmental Science and Technology, 45, 9003–9008.
Xiu, Z. M., Zhang, Q. B., Puppala, H. L., Colvin, V. L., & Alvarez, P. J. J. (2012). Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Letters, 12, 4271–4275.
Xu, Y., & Zhao, D. (2007). Reductive immobilization of chromate in water and soil using stabilized iron nanoparticles. Water Research, 41, 2101–2108.
Yang, G. C. C., & Lee, H. L. (2005). Chemical reduction of nitrate by nanosized iron: Kinetics and pathways. Water Research, 39, 884–894.
Yantasee, W., Rutledge, R. D., Chouyyok, W., Sukwarotwat, V., Orr, G., Warner, C. L., et al. (2010). Functionalized nanoporous silica for the removal of heavy metals from biological systems: Adsorption and application. ACS Applied Materials & Interfaces, 2, 2749–2758.
Yao, D., Chen, Z., Zhao, K., Yang, Q., & Zhang, W. (2013). Limitation and challenge faced to the researches on environmental risk of nanotechnology. Procedia Environmental Sciences, 18, 149–156.
Zamzow, M. J., Eichbaum, B. R., Sandgren, K. R., & Shanks, D. E. (1990). Removal of heavy metals and other cations from wastewater using zeolites. Separation Science and Technology, 25, 1555–1569.
Zhang, K., Kemp, K. C., & Chandra, V. (2012). Homogeneous anchoring of TiO2 nanoparticles on graphene sheets for waste water treatment. Materials Letters, 81, 127–130.
Zhang, S., Niu, H., Hu, Z., Cai, Y., & Shi, Y. (2010). Preparation of carbon coated Fe3O4 nanoparticles and their application for solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. Journal of Chromatography A, 1217, 4757–4764.
Zhang, S., Xu, W., Zeng, M., Li, J., Li, J., Xu, J., et al. (2013). Superior adsorption capacity of hierarchical iron oxide @ magnesium silicate magnetic nanorods for fast removal of organic pollutants from aqueous solution. Journal of Materials Chemistry A, 1, 11691–11697.
Zhao, X., Lv, L., Pan, B., Zhang, W., Zhang, S., & Zhang, Q. (2011). Polymer-supported nanocomposites for environmental application: A review. Chemical Engineering Journal, 170, 381–394.
Zhong, L. S., Hu, J. S., Liang, H. M., Cao, A. M., Song, W. G., & Wan, L. J. (2006). Self-assembled 3D flowerlike iron oxide nanostructures and their application in water treatment. Advanced Materials, 18, 2426–2431.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media Singapore
About this chapter
Cite this chapter
Senthil Kumar, P., Narayan, A.S., Dutta, A. (2017). Nanochemicals and Effluent Treatment in Textile Industries. In: Muthu, S. (eds) Textiles and Clothing Sustainability. Textile Science and Clothing Technology. Springer, Singapore. https://doi.org/10.1007/978-981-10-2188-6_2
Download citation
DOI: https://doi.org/10.1007/978-981-10-2188-6_2
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-2187-9
Online ISBN: 978-981-10-2188-6
eBook Packages: EngineeringEngineering (R0)