Abstract
Classification of lung adenocarcinoma was largely revised in the 4th edition of WHO classification of tumors of the lung, pleura, thymus, and heart published in 2015. This chapter deals with the major changes in the adenocarcinoma classification, briefly describing the definition, gross and histopathological findings, genetic profiles and clinical features of each subtype, and variants of lung adenocarcinoma. Special reference was also made to the prognostic aspects. The new concepts of adenocarcinoma in situ and minimally invasive adenocarcinoma are especially important from the prognostic point of view because of their virtual connotation as 100% curable cancers if resected completely. Each subtype of invasive adenocarcinoma may be categorized into either good, intermediate, or poor prognostic group. Much progress has been made regarding the genetic profiles as well, such as the occurrence of EGFR and KRAS mutations, ALK fusion genes and recently discovered alterations, and NRG1 fusion genes in association with adenocarcinomas with certain characteristics. A brief overview of the major changes in the lung adenocarcinoma classification in this chapter will help physicians, radiologists, and pathologists grasp the significance and meaning of the histopathological diagnosis according to the new WHO classification.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Classification of Adenocarcinoma of the Lung in New WHO Classification
1.1 Introduction: Major Changes in the Classification
The 4th edition of WHO Classification of Tumours of the Lung, Pleura, Thymus, and Heart was published in 2015 [1]. In this new edition, the significant changes in the lung adenocarcinoma classification include (1) introduction of the new term “adenocarcinoma in situ (AIS)” as a preinvasive lesion in addition to atypical adenomatous hyperplasia (AAH), discarding the old and ambiguous term “bronchioloalveolar carcinoma (BAC)”; (2) introduction of the new term “minimally invasive adenocarcinoma (MIA)”; (3) classification of invasive adenocarcinomas according to the predominant subtype with additional description of minor subtypes; (4) introduction of the new term “invasive mucinous adenocarcinoma” (roughly corresponding to the former mucinous BAC) as a variant of adenocarcinoma; (5) refining the category of adenocarcinoma variants as including invasive mucinous adenocarcinoma, colloid adenocarcinoma, fetal adenocarcinoma (low- and high-grade), and enteric adenocarcinoma; (6) introduction of immunohistochememically defined “solid adenocarcinoma”, i.e., diagnosing the former large cell carcinoma as solid adenocarcinoma if tumor cells are immunopositive for pneumocyte markers (TTF1 and/or napsin A); and (7) avoiding the noncommittal diagnosis of non-small cell carcinoma in small biopsy/cytology samples as much as possible by introduction of the new immunohistochemically defined diagnostic category of “non-small cell carcinoma, favor adenocarcinoma” [1–4] (Table 1.1).
It should be emphasized that these major changes in adenocarcinoma classification are deeply related to the ever-growing recognition that a multidisciplinary approach is mandatory for the classification to be clinically relevant: (1) recent advance in molecular biology/oncology has led to the discovery of epidermal growth factor receptor (EGFR) mutations and ALK gene translocations almost exclusively in lung adenocarcinomas, and targeted therapy with tyrosine kinase inhibitors (TKIs) has become available for these tumors; (2) progress in treatment requires discrimination of squamous cell carcinoma from non-squamous, non-small cell carcinomas such as in application of certain drugs including pemetrexed and bevacizumab; and (3) advancement in knowledge of the intimate correlation between the developmental stages of adenocarcinoma and corresponding CT images has led to its utility in prediction of prognosis and choice of treatment in lung adenocarcinomas [3].
1.2 Preinvasive Lesions
1.2.1 Atypical Adenomatous Hyperplasia (AAH) (Fig. 1.1)
AAH, by definition, is a small, localized proliferation of mildly to moderately atypical type II pneumocytes and/or club cells (formerly named as Clara cells) lining alveolar walls and sometimes respiratory bronchioles [1]. This lesion is usually found incidentally in lung specimens resected for cancer or may incidentally be detected as a pure ground-glass nodule (GGN) on high-resolution CT scans during medical examination for some reasons.
Atypical adenomatous hyperplasia. (a) Low-power view. Note the slightly thickened alveolar septa with lining cells that show a sharp demarcation from the normal lung parenchyma occupying the lowermost quarter of the field. (b) High-power view. Cuboidal to somewhat flattened cells with mildly atypical nuclei and scant cytoplasm are growing along alveolar septa
In gross examination, AAH typically is a few millimeter-sized, barely discernable gray-white nodule in the peripheral lung. Histopathologically, the distinction between AAH and AIS is sometimes difficult because both show the lepidic pattern or growth along the alveolar wall throughout the lesion, but AAH typically is up to 5 mm in size, and the constituent cells show less nuclear atypism and are less densely populated along alveolar walls than those of AIS [5, 6]. Somewhat paradoxically, the cell shape in AAH is more various with cuboidal, pyramidal, or flat appearances than that of AIS.
AAH is considered to be a precursor lesion of peripheral lung adenocarcinoma. Clinicopathological and clonality/mutational studies have demonstrated that AAH is a clonal lesion with the potential for progression to adenocarcinoma [5–7], harboring KRAS and EGFR mutations in up to 33 % and 35 %, respectively [1, 8–11]. There is some evidence that KRAS-mutated AAH may not progress to AIS or invasive adenocarcinoma as frequently as EGFR-mutated AAH and that major driver genes (EGFR/KRAS/ALK/HER2) mutation-negative AAH/AIS may not progress to invasive adenocarcinoma so frequently [8, 12]. A recent genetic analysis of AAH/AIS/MIA utilizing next-generation sequencing (NGS) [13] showed an average mutation rate of 2.2 non-synonymous mutations (range 0–6 mutations) per one lesion among 25 AAHs, the most frequently mutated genes being BRAF and ARID1B. Genes associated with DNA repair and chromatin remodeling network such as ATM and ATRX were also mutated in multiple lesions, suggesting AAH may be predisposed to the acquisition of secondary genetic aberrations. Mutations in TP53, EGFR, and IGFR1 were noted in all developmental stages of AAH/AIS/MIA, but BRAF mutation was rarely found in MIA or invasive adenocarcinoma, again suggesting the inequity in the progression potential among various mutations.
The natural history of AAH is not fully elucidated, but a recent radiographic study [14] showed that solitary pure GGNs 5 mm or smaller in CT images, the majority of which presumably represented AAH, grew in 10 % of the cases and developed into MIA or invasive adenocarcinoma in 1 % with the mean period of 3.6 years. This observation appears to corroborate the aforementioned genetic inequity in the progression potential of AAH.
1.2.2 Adenocarcinoma In Situ (AIS) (Fig. 1.2)
AIS is a newly introduced entity in the current WHO classification [1]. It is a small (<=3 cm), localized adenocarcinoma with neoplastic cell growth restricted along alveolar walls (pure lepidic growth), lacking stromal, vascular, or pleural invasion. The constituent cells are mostly non-mucinous, but mucinous in rare cases as well. AIS is usually found incidentally as a pure GGN or part-solid nodule on CT scan [14, 15]. Mucinous AIS tends to present as a solid or part-solid nodule with air-containing spaces [16].
Grossly, AIS is an ill-defined, gray-white to tan-colored nodule with somewhat spongy consistency. Histopathologically, type II pneumocyte/club cell-like cuboidal to columnar cells with mild to moderately atypical nuclei are seen along alveolar walls. The alveolar walls are almost normal to moderately thickened with collapse-type fibroelastosis [17]. In the rare mucinous AIS, the lining cells have mucinous cytoplasm, resembling gastric foveolar epithelium or goblet cells. Non-mucinous AIS expresses TTF1 and napsin A, whereas mucinous AIS is often negative for these immunohistochemical markers of alveolar pneumocytes and positive for gastric epithelium-associated mucin such as MUC5AC and MUC6 [18, 19].
Genetically, non-mucinous AIS harbors EGFR mutations frequently (40–86 %), but KRAS mutations rarely (0–4 %) [12, 19–23]. A recent NGS analysis of AIS in five patients showed an average mutational rate of 6.2 non-synonymous mutations per patient; the mutational landscape varied widely, most mutations including EGFR and TP53 mutations found only in one patient [13]. The lower mutational rate of EGFR compared with those of the aforementioned studies [12, 19–23] may be related to different ethnic backgrounds of the cohorts. EGFR mutations are rare in mucinous AIS [19, 24].
The clinical significance of diagnosing AIS lies in its connotation as a neoplasm with 100 % disease-free survival if it is resected completely [1, 17, 19–24] (Fig. 1.3) (Table 1.2). It is noteworthy that most of these data are from Japan, where EGFR mutation-related adenocarcinomas are common and CT-based examination is part of routine clinical practice. The frequency of AIS among resected lung adenocarcinomas has been 4.5–8.4 % in Japanese cohorts [19–21, 23], whereas it has been less than 1 % in Western countries [24]. The clinical behavior of mucinous AIS is less well elucidated but may also be good [15, 19, 20, 24, 25]. Thus, the most recent article on the eighth TNM classification of lung cancer has proposed the code Tis in place of T1 for AIS [15].
Pulmonary adenocarcinoma subtypes and prognosis. Stage I (n = 514). (a) Disease-free survival (DFS) for all histological categories (P < 0.001). The favorable group includes adenocarcinoma in situ (AIS) and minimally invasive adenocarcinomas (MIA) with 100 % 5-year disease-free survival. Disease-free survival for the intermediate group was 90, 83, and 84 % for lepidic predominant, papillary (PAP) predominant and acinar predominant, and adenocarcinomas, respectively. Disease-free survival for the unfavorable group was 70, 67, 71, and 76 % for solid predominant, micropapillary (MPAP) predominant, colloid predominant, and mucinous and mixed adenocarcinomas, respectively. (b) Disease-free survival according to combined histological groupings according to low-, intermediate-, and high-grade clinical aggressiveness. (c) Overall survival (OS) according to combined histological groupings according to low-, intermediate-, and high-grade clinical aggressiveness (Adopted from Fig. 1.4 of reference [20]). (b) Stages I–III (n = 440). (A) Disease-free survival curves and (B) overall survival curves, for the groups, separated by the IASLC/ATS/ERS classification of lung adenocarcinomas (Adopted from Fig. 1.6 of reference [33]). AIS adenocarcinoma in situ, MIA minimally invasive adenocarcinoma, Lepidic lepidic predominant adenocarcinoma, Aci acinar predominant adenocarcinoma, Pap papillary predominant adenocarcinoma, Solid solid predominant adenocarcinoma, MP micropapillary predominant adenocarcinoma, IMA invasive mucinous adenocarcinoma, IASLC International Association for the Study of Lung Cancer, ATS American Thoracic Society, ERS European Respiratory Society
1.3 Minimally Invasive Adenocarcinoma (MIA) (Fig. 1.4)
MIA is another new entity incorporated into the current WHO classification. It defines the solitary adenocarcinoma (<=3 cm) with a predominantly lepidic pattern and <=5 mm invasion in greatest dimension [1]. MIA should lack lymphatic/vascular/pleural/air space invasion or spread. MIA is non-mucinous in most cases but may rarely be mucinous as well. This lesion is usually discovered incidentally as a part-solid nodule, pure GGN, or rarely as a solid nodule on CT [15].
Minimally invasive adenocarcinoma. (a) The left upper field shows the lepidic pattern of tumor growth with preserved alveolar framework, whereas the right lower field shows a fibrotic focus with an invasive growth of neoplastic cells. (b) Note an invasive neoplastic acinar structure within the fibrous stroma
Historically, the criteria for this entity were searched after the epoch-making publication of an article on AIS by Noguchi et al. in 1995 [17], and several pioneering studies contributed to its establishment [25–32]. Validation studies [19–23, 33–37] suggested the prognosis of MIA is virtually equal to that of AIS, supporting its recognition as a distinct entity (Fig. 1.3, Table 1.2). The code T1mi is proposed for MIA in the latest TNM system [15].
Histopathologically, the invasive focus may take one of the basic patterns of invasive adenocarcinoma, i.e., papillary, acinar, solid, or micropapillary pattern or tumor cells infiltrating myofibroblastic stroma [1].
Genetically, MIA shows high rates of EGFR mutation similar to AIS [20–23]. An NGA analysis of MIA in five patients revealed an average mutation rate of 10.8 non-synonymous mutation per patient with EGFR and TP53 being the most frequently mutated genes [13].
1.4 Invasive Adenocarcinoma (Fig. 1.3) (Table 1.2)
Invasive adenocarcinoma is a carcinoma with glandular differentiation, mucin production, or pneumocyte marker expression [1]. The growth pattern includes acinar, papillary, micropapillary, and solid. These patterns often appear admixed with and in transition to one another within the same tumor, and therefore the tumor is classified according to the predominant pattern in proportion with additional description of each component present in 5–10 % increment. Invasive adenocarcinoma is typically localized in the periphery of the lung. Pleural indentation is common due to the retraction caused by central collapse and fibrosis in the tumor. CT images of invasive non-mucinous adenocarcinoma appear solid or part solid depending on the proportion of lepidic growth vs. invasive growth as well as on the extent of alveolar collapse [1, 3].
1.4.1 Lepidic Adenocarcinoma (Fig. 1.5)
In this tumor, the predominant pattern is lepidic with type II pneumocyte/club cell-like atypical cells growing along alveolar walls, but also present is an invasive component of various patterns such as papillary and acinar larger than 5 mm in greatest dimension. Grossly, part of the tumor, often centrally located, is grayish white in color with carbon dust deposition and solid in consistency, whereas the peripheral portion is somewhat ill defined, tan in color, and soft in consistency (Fig. 1.5). The former roughly corresponds to the invasive component with fibrosis and the latter the lepidic component with preserved airspace. This feature is usually reflected as a part solid image at CT scan. The frequency of this subtype among invasive adenocarcinomas varies from 5 % [34] to 18.3 % [23], probably reflecting different ethnic and clinical backgrounds of these cohorts.
Genetically, EGFR mutation is frequent [20, 21, 23]. Adenocarcinoma of lepidic pattern with type II pneumocyte/ club cell-like cells (bronchioloalveolar features) has been termed terminal respiratory unit (TRU)-type adenocarcinoma and known to be intimately associated with EGFR mutation [38].
Prognostically, this tumor lies intermediate between the good prognostic group of AIS/MIA and the poor prognostic group of micropapillary adenocarcinoma/solid adenocarcinoma [20, 21, 23, 33, 35] (Fig. 1.3, Table 1.2). The prognosis of lepidic adenocarcinoma is related to the proportion of the lepidic growth within the entire tumor, tumors with >50 % to >75 % lepidic pattern showing good prognosis similar to those of AIS/MIA [24, 27]. Adenocarcinomas even with a non-predominant lepidic component show a better outcome than adenocarcinomas without the component [39]. This tendency in prognosis will be more accurately reflected in the 8th edition of the TNM classification of lung cancer in which the invasive tumor size, excluding the lepidic growth, will be used as the T descriptor size [15]. Risk factors for recurrence in lepidic adenocarcinoma may include limited resection with a close margin, lymphovascular invasion, and a substantial component of high-grade pattern such as micropapillary [24].
1.4.2 Acinar Adenocarcinoma (Fig. 1.6)
Acinar adenocarcinoma is composed predominantly of acinar or glandular structures with cuboidal to columnar neoplastic cells forming central lumina of various size. Of all subtypes of pulmonary adenocarcinoma, acinar adenocarcinoma is less common (10.8–20.4 %) in Japan [20, 21, 23] than in Western countries (40–45.1 %) [33, 34, 40].
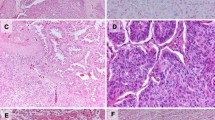
Acinar adenocarcinoma. (a) Neoplastic cells are arranged in acinar or tubular structures. (b) ALK-rearranged adenocarcinoma. The neoplastic cells are arranged in a so-called mucinous cribriform pattern. (c) ALK-rearranged adenocarcinoma. The luminal space and cytoplasmic vacuoles of tumor cells are abundant in mucin. (PAS reaction). (d) ALK-rearranged adenocarcinoma. The neoplastic cells are diffusely positive for ALK protein (immunostaining)
Genetically, acinar adenocarcinoma shows EGFR mutation less frequently and ALK rearrangement more frequently than AIS/MIA and lepidic and papillary adenocarcinomas [20, 21, 41]. Mucinous cribriform pattern has been reported as a variant of acinar pattern intimately associated with ALK-rearranged pulmonary adenocarcinoma (Fig. 1.6) [41, 42].
Prognostically, acinar adenocarcinoma together with lepidic adenocarcinoma and papillary adenocarcinoma belongs to the intermediate group between the groups of AIS/MIA and micropapillary/solid adenocarcinoma [20, 21, 23, 33, 35] (Fig. 1.3, Table 1.2). In a study of stage I pulmonary adenocarcinomas [43], however, a cribriform pattern-predominant adenocarcinoma has been proposed as a distinct subtype of acinar adenocarcinoma with a poor prognosis compatible to those of the high-grade adenocarcinomas. This needs further validation.
1.4.3 Papillary Adenocarcinoma (Fig. 1.7)
Papillary adenocarcinoma shows a predominant papillary pattern with neoplastic cuboidal to columnar cells growing along fibrovascular cores in papillary configuration.
Genetically, papillary adenocarcinoma is among the subtypes with most frequent EGFR mutations, revealing the mutation in 50–68.5 % of cases [20, 21, 23]. This corroborates with the observation that this subtype is quite frequent (28–40.7 %) among various subtypes of adenocarcinoma in Japan [20, 21, 23], where EGFR mutation-related adenocarcinoma is prevalent, but is less common (12–27.8 %) in Western countries [33, 34, 44].
Prognostically, most studies placed papillary adenocarcinoma in the intermediate prognostic group [20, 21, 23, 33, 35] (Fig. 1.3, Table 1.2), but papillary adenocarcinoma belonged to the poor survival group together with micropapillary and solid adenocarcinomas in a study on a German cohort [40]. The reason for this discrepancy appears to be the presence of a range of papillary growth from the type architecturally close to lepidic pattern (type I) to the type showing the highest degree of architectural aberrations (type III) [44]: any presence of the type III papillary pattern was associated with poor overall and disease-free survivals, the aforementioned study having applied the most strict criteria (type III) to the recognition of the papillary pattern [41]. Tumors with any type I papillary growth were significantly more likely to harbor EGFR mutations than cases with any type II or type III papillary growth [44].
1.4.4 Micropapillary Adenocarcinoma (Fig. 1.8)
This is a newly introduced subtype in the current WHO classification [1]. This adenocarcinoma shows the predominant growth of neoplastic cells in micropapillary configuration, i.e., cells forming florets that lack fibrovascular cores, either connected to or detached from alveolar walls. This subtype frequently shows lymphatic permeation and spread through air spaces (STAS) [1, 45]. Micropapillary adenocarcinoma is relatively uncommon, constituting 2.3–19.5 % of all resected pulmonary adenocarcinomas [20, 21, 23, 24, 34–37, 40], most of the cohorts showing the frequency of less than 10 % [20, 21, 23, 24, 34, 37, 40]. However, the presence of micropapillary component itself is not uncommon, any presence (=>1 %) and =>5 % of this component representing 43.6 % and 21.7 % of 525 resected invasive adenocarcinomas, respectively, in one study [46].
Genetically, micropapillary adenocarcinoma shows relatively high rates of EGFR mutation (39.7–43 %) next to the adenocarcinomas with predominant lepidic and papillary patterns [20, 21, 23].
Prognostically, there is an agreement that this subtype belongs to the poor prognostic group together with solid adenocarcinoma [20, 21, 23, 24, 33–37, 40] (Fig. 1.3, Table 1.2). The presence of a micropapillary component of 5 % or greater may be significantly associated with increased risk of local recurrence in patients treated with limited resection [47]. A recent study demonstrated overall survival was significantly better in patients without the micropapillary pattern (<1 %) than in those with the micropapillary pattern (<5 % of the entire tumor), emphasizing the recognition and description of this pattern even in a smallest proportion (=>1 %) [46].
1.4.5 Solid Adenocarcinoma (Fig. 1.9)
-
1.
Solid adenocarcinoma shows the predominant growth of neoplastic polygonal cells in a sheetlike arrangement without any recognized pattern of adenocarcinoma described above. In tumors entirely with the solid pattern, intracellular mucin should be present in =>5 tumor cells in each of two high-power fields histochemically, or tumor cells should be positive for pneumocyte markers, i.e., TTF1 and/or napsin A immunohistochemically [1]. The latter immunohistochemically defined solid adenocarcinoma is a newly introduced entity in the current WHO classification. This represents the incorporation of a subset of former large cell carcinomas, the rationale for which is that these immunomarker-defined large cell carcinomas had a distinct adenocarcinoma-related spectrum of therapeutically relevant-driver mutations, including EGFR, KRAS, and ALK [48–50]. The frequency of solid adenocarcinoma among resected lung adenocarcinomas (based on the 2011 IASLC/ATS/ERS international lung adenocarcinoma classification) varies widely from 13 to 37.6 % [21, 22, 34–37, 40].
Genetically, the frequency of KRAS mutation is especially high in solid adenocarcinoma, which parallels the observation that KRAS mutations are enriched in poorly differentiated adenocarcinomas with a solid component [20, 48–53].
Prognostically, solid adenocarcinoma belongs to the poor prognostic group [20, 21, 23, 33–37, 40] (Fig. 1.3, Table 1.2). Patients who had adenocarcinomas with a solid component had significantly lower overall survival and recurrence-free survival rates than patients who had adenocarcinomas with nonsolid components [53]. In patients with stage I pulmonary adenocarcinomas, solid adenocarcinoma recurred significantly earlier than nonsolid adenocarcinomas and was associated with worse post recurrence survival [54].
1.5 Variants of Adenocarcinoma
The new WHO classification lists invasive mucinous adenocarcinoma, fetal adenocarcinoma, colloid carcinoma, and enteric adenocarcinoma as variants of pulmonary adenocarcinoma [1]. These variants are all rare but should always be kept in mind as differential diagnoses for appropriate treatment of the patients.
1.5.1 Invasive Mucinous Adenocarcinoma (IMA) (Fig. 1.10)
IMA shows growth of neoplastic columnar cells with goblet cell-like or gastric foveolar epithelium-like morphology. The growth pattern can be various but predominantly lepidic in most cases. Tumors solely with the lepidic growth pattern, however, are rare and diagnosed as mucinous AIS. Most of the tumors formerly diagnosed as mucinous bronchioloalveolar carcinoma fall into the category of IMA in the current classification. The CT findings of IMA are variable, including consolidations, air bronchograms, and multifocal and sometimes multilobar solid or subsolid nodules or masses [56]. The frequency of IMA in resected lung adenocarcinomas ranges from 2.2 to 5 % [21, 22, 24, 33–37, 40].
In gross examination, IMA typically displays a somewhat ill-defined, mucinous grayish-white nodule. It may sometimes show a multinodular pattern or a broad lobar consolidation [1]. Histopathologically, the neoplastic columnar cells have basally situated, relatively small and round to oval nuclei with mild atypism. Alveolar spaces within and surrounding the tumor area are often filled with mucin.
Various growth patterns such as papillary and acinar can be seen in addition to the lepidic growth. Frankly invasive areas may show desmoplastic fibrosis.
Immunohistochemically, IMA cells express CK7 and MUC5AC in most cases, and sometimes CK20 as well, but TTF1 only in 11–27.5 % of the cases [18, 56]. Recently, HNF4α was reported as a new immunohistochemical marker for IMA, which was expressed in 92 % of IMA but was negative in normal lung tissues [57]. This transcription factor, however, is expressed in all gastrointestinal adenocarcinomas, pancreatic adenocarcinomas, and mucinous adenocarcinomas of the ovary and uterine cervix, precluding its utility for differentiating lung metastases of these tumors from IMA, which is a major challenge in the histopathological diagnosis of this variant [57].
Genetically, IMA is intimately associated with KRAS mutation, disclosing the gene mutation 40–86 % of the examined cases [21, 58–65]. The distribution of KRAS amino acid changes more resembled that of colorectal and pancreatobiliary adenocarcinomas than that of pulmonary non-mucinous adenocarcinomas [58, 60, 63, 65]. Smoking status may not be related to KRAS mutations in IMA [63]. In addition, NRG1 fusion genes were recently discovered as novel driver mutations in 6.7–27 % of IMA [62–64]. Interestingly, NRG1is known as a regulator of goblet cell formation with MUC5AC/ MUC5B expression in primary cultures of bronchial epithelial cells, suggesting a possible relationship between NRG1 gene mutation and goblet cell-like morphology/phenotype of IMA [64, 66]. EGFR mutations are rare in IMA, ranging 0–22 % in reported studies [21, 22, 24, 60–65]. KRAS and EGFR mutations are mutually exclusive in IMA but for a few exceptional cases [61, 65]. Rarity of TP53 mutations in IMA was noted in one study [63].
Prognosis of IMA is somewhat controversial. Some studies found IMA in the poor prognostic group [33, 34], others in the intermediate group [20, 21, 23], while another in the good prognostic group [40] (Fig. 1.3, Table 1.2). Some recent studies show there is no statistically significant difference in prognosis between IMA and non-mucinous invasive adenocarcinomas [58, 63]. Recurrence of IMA after surgical resection was limited to the lungs in one study, suggesting a nonaggressive nature of IMA [63].
1.5.2 Colloid Adenocarcinoma (Fig. 1.11)
Colloid adenocarcinoma is an adenocarcinoma in which abundant mucin pools replace air spaces, destroying alveolar framework [1]. This variant may be seen in a pure form or in association with conventional adenocarcinomas.
In gross examination, this variant typically shows a well-demarcated solid or cystic tumor filled with abundant gelatinous material. Histopathologically, the neoplastic cells constitute a relatively small portion of the tumor, columnar cells growing along incompletely developed fibrous tissue septa or small neoplastic cell clusters floating within mucinous pool. Immunohistochemically, the neoplastic cells, especially of goblet cell morphology, often express intestinal markers such as CDX2, MUC2, and CK20, whereas pneumocyte markers such as TTF1and napsin A are variably expressed [56, 67, 68]. Expression of CK7 is usually retained [67, 68].
The genetic profile of colloid adenocarcinoma is not well known. KRAS mutations were identified in a few cases, while EGFR mutation and ALK fusion genes were so far not found [68]. Prognostically, a few recent studies suggest this variant may belong to the poor prognostic group [33, 34] in contrast to the previous notion of a relatively favorable prognosis for this tumor [67] (Fig. 1.3a, Table 1.2).
1.5.3 Fetal Adenocarcinoma (Fig. 1.12)
Fetal adenocarcinoma is an adenocarcinoma resembling fetal lung [1]. Low-grade and high-grade tumors exist, and they are considered histogenetically different despite their morphologic similarities [69, 70]. Low-grade fetal adenocarcinoma is considered as the epithelial prototype of pulmonary blastoma and occurs in a pure form, whereas high-grade fetal adenocarcinoma frequently coexists with other conventional adenocarcinomas and requires at least 50 % fetal morphology for its diagnosis.
Fetal adenocarcinoma. (a) Low-grade fetal adenocarcinoma. Note complex glandular structures lined by columnar cells with relatively small and regular nuclei and supra- and subnuclear vacuoles resembling fetal airway epithelium. A characteristic morular formation is also seen. (b) High-grade fetal adenocarcinoma. The histology resembles the low-grade form, but nuclear atypism is more obvious and morular formation is absent. (c) Low-grade fetal adenocarcinoma. The neoplastic cells show aberrant nuclear/cytoplasmic localization of β-catenin, especially in the morular area (immunostaining). (d) High-grade fetal adenocarcinoma. The localization of β-catenin is predominantly membranous (immunostaining)
Clinically, low-grade fetal adenocarcinoma occurs in relatively young population with a peak incidence in the fourth decade of life and with a slight female preponderance, whereas high-grade fetal adenocarcinoma occurs predominantly in male heavy smokers [69–71]. High-grade fetal pattern as a minor component of a tumor, however, can be seen more widely in age and gender [72].
Histopathologically, both low-grade and high-grade tumors are characterized by neoplastic columnar cells with glycogen-rich clear cytoplasm in complex papillotubular structures. Low-grade tumors have characteristically small and round nuclei of mild atypia and show morules or cell balls in most cases, whereas high-grade tumors show more obvious nuclear atypia and lack morular formation. Neuroendocrine cells are often admixed with the glandular component. Other types of carcinoma such as large cell neuroendocrine carcinoma, hepatoid adenocarcinoma, and choriocarcinoma may be seen in association with high-grade fetal adenocarcinoma [71, 72]. TTF1 is expressed in low-grade tumors, whereas its expression is often diminished or absent in high-grade tumors [71, 72].
Genetically, low-grade fetal adenocarcinoma is characterized by frequent β-catenin gene mutations with aberrant nuclear/cytoplasmic localization of the protein [68, 73], whereas high-grade fetal adenocarcinoma lacks the mutation, rarely showing major driver mutations of conventional pulmonary adenocarcinomas such as EGFR, KRAS, and PIK3CA mutations [71–73]. Somewhat surprisingly, DICER 1 mutation, which is a characteristic genetic feature of pleuropulmonary blastoma, has recently been reported in a case of low-grade fetal adenocarcinoma occurring in a patient with DICER1 syndrome [74, 75].
The prognosis of fetal adenocarcinoma is not fully elucidated because of the rarity of the tumors. Low-grade fetal adenocarcinomas are usually found at stage I and show an indolent behavior with approximately 10 % tumor death rate [67], whereas high-grade fetal adenocarcinomas are often found at more advanced stages and show much higher mortality rates [69, 71, 72] .
1.5.4 Enteric Adenocarcinoma (Fig. 1.13)
This variant is simply defined as an adenocarcinoma that resembles colorectal adenocarcinomas [1]. Adenocarcinomas may partially take this form, and tumors that show this component at least 50 % of the whole are diagnosed as this variant. This is a very rare tumor; all previous studies on this tumor have been based on a single case or a series of less than ten cases [76–86]. Clinically, this tumor occurs in both sexes almost equally with a median age of 66 [81]. Smoking may be related to the development of this variant [81, 82].
Histopathologically, enteric adenocarcinoma shows acinar, cribriform, or papillotubular structures lined by columnar cells with eosinophilic cytoplasm and brush borders just like conventional colorectal adenocarcinomas [1]. Central necrosis is common. Thus, it is mandatory to rule out the possibility of a metastasis of colorectal origin, especially if the tumor is entirely enteric in morphology. Immunohistochemically, the expression of CK7 is retained in the majority of the reported cases, and TTF1 over half of the cases, but the expression of intestinal markers such as CK20 and CDX2 is also noted approximately in one third and a half of the cases, respectively [79, 80]. Rare cases have also been reported in which tumor cells revealed a completely intestinal immunophenotype, i.e., CK7–, TTF1–, CK20+, and CDX2+ [80, 82, 83].
The genetic profile of this variant is not well known. A few cases revealed KRAS mutations [83, 85, 86] and EGFR mutation [83]. A rare KRAS Q22K mutation with concomitant KRAS polysomy was noted in one case, which could be related to the aggressive clinical course [85]. A recent MicroRNA profiling of this tumor disclosed similarities to non-small cell lung carcinoma and some overlap with pancreatic ductal adenocarcinoma [86].
Prognostically, it is not certain if this variant behaves differently from conventional invasive adenocarcinomas of the lung [82, 84].
References
Travis WD, Brambilla E, Burke AP et al (2015) WHO classification of tumours of the lung, pleura, thymus and heart, 4th edn. International Agency for Research on Cancer Press, Lyon
Travis WD, Brambilla E, Nicholson AG (2015) The 2015 World health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 10:1243–1260. doi:10.1097/JTO.0000000000000630
Travis WD, Brambilla E, Noguchi M et al (2011) The new IASLC/ATS/ERS international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol 6:244–285
Travis WD, Brambilla E, Noguchi M et al (2013) Diagnosis of lung cancer in small biopsies and cytology: implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/ European Respiratory Society classification. Arch Pathol Lab Med 137:668–684
Nakayama H, Noguchi M, Tsuchiya R et al (1990) Clonal growth of atypical adenomatous hyperplasia of the lung: cytofluorometric analysis of nuclear DNA content. Mod Pathol 3:314–320
Kitamura H, Kameda Y, Ito T et al (1990) Atypical adenomatous hyperplasia of the lung. Implications for the pathogenesis of peripheral lung adenocarcinoma. Am J Clin Pathol 111:610–622
Takamochi K, Ogura T, Suzuki K et al (2001) Loss of heterozygosity on chromosomes 9q and 16p in atypical adenomatous hyperplasia concomitant with adenocarcinoma of the lung. Am J Pathol 159:1941–1948
Sakamoto H, Shimizu J, Horio Y et al (2007) Disproportionate representation of KRAS gene mutation in atypical adenomatous hyperplasia, but even distribution of EGFR gene mutation from preinvasive to invasive adenocarcinomas. J Pathol 212:287–294
Soh J, Toyooka S, Ichihara S et al (2008) Sequential molecular changes during multistage pathogenesis of small peripheral adenocarcinomas of the lung. J Thorac Oncol 3:340–347. doi:10.1097/JTO.0b013e318168d20a
Yoo SB, Chung JH, Lee HJ, Lee CT et al (2010) Epidermal growth factor receptor mutation and p53 overexpression during the multistage progression of small adenocarcinoma of the lung. J Thorac Oncol 5:964–969. doi:10.1097/JTO.0b013e3181dd15c0
Yoshida Y, Shibata T, Kokubu A et al (2005) Mutations of the epidermal growth factor receptor gene in atypical adenomatous hyperplasia and bronchioloalveolar carcinoma of the lung. Lung Cancer 50:1–8
Kobayashi Y, Mitsudomi T, Sakao Y et al (2015) Genetic features of pulmonary adenocarcinoma presenting with ground-glass nodules: the differences between nodules with and without growth. Ann Oncol 26:156–161. doi:10.1093/annonc/mdu505
Izumchenko E, Chang X, Brait M et al (2015) Targeted sequencing reveals clonal genetic changes in the progression of early lung neoplasms and paired circulating DNA. Nat Commun 16:8258. doi:10.1038/ncomms9258
Kakinuma R, Muramatsu Y, Kusumoto M et al (2015) Solitary pure ground-glass nodules 5 mm or smaller: frequency of growth. Radiology 276:873–882. doi:10.1148/radiol.2015141071
Travis WD, Asamura H, Bankier AA et al (2016) The IASLC Lung Cancer Staging Project: proposals for coding T categories for subsolid nodules and assessment of tumor size in part-solid tumors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol 11:1204–1223. doi:10.1016/j.jtho.2016.03.025
Miyata N, Endo M, Nakajima T et al (2015) High-resolution computed tomography findings of early mucinous adenocarcinomas and their pathologic characteristics in 22 surgically resected cases. Eur J Radiol 84:993–997. doi:10.1016/j.ejrad.2015.01.014
Noguchi M, Morikawa A, Kawasaki M et al (1995) Small adenocarcinoma of the lung. Histological characteristics and prognosis. Cancer 75:2844–2852
Tsuta K, Ishii G, Nitadori J et al (2006) Comparison of the immunophenotypes of signet-ring cell carcinoma, solid adenocarcinoma with mucin production, and mucinous bronchioloalveolar carcinoma of the lung characterized by the presence of cytoplasmic mucin. J Pathol 209:78–87
Sato S, Motoi N, Hiramatsu M et al (2015) Pulmonary adenocarcinoma in situ: analyses of a large series with reference to smoking, driver mutations, and receptor tyrosine kinase pathway activation. Am J Surg Pathol 39:912–921. doi:10.1097/PAS.0000000000000458
Yoshizawa A, Sumiyoshi S, Sonobe M et al (2013) Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol 8:52–61
Tsuta K, Kawago M, Inoue E et al (2013) The utility of the proposed IASLC/ATS/ERS lung adenocarcinoma subtypes for disease prognosis and correlation of driver gene alterations. Lung Cancer 81:371–376
Nakagiri T, Sawabata N, Morii E et al (2014) Evaluation of the new IASLC/ATS/ERS proposed classification of adenocarcinoma based on lepidic pattern in patients with pathological stage IA pulmonary adenocarcinoma. Gen Thorac Cardiovasc Surg 62:671–677. doi:10.1007/s11748-014-0429-3
Yanagawa N, Shiono S, Abiko M et al (2014) The correlation of the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) classification with prognosis and EGFR mutation in lung adenocarcinoma. Ann Thorac Surg 98:453–458. doi:10.1016/j.athoracsur.2014.04.108
Kadota K, Villena-Vargas J, Yoshizawa A et al (2014) Prognostic significance of adenocarcinoma in situ, minimally invasive adenocarcinoma, and nonmucinous lepidic predominant invasive adenocarcinoma of the lung in patients with stage I disease. Am J Surg Pathol 38:448–460
Eto T, Suzuki H, Honda A et al (1996) The changes of the stromal elastotic framework in the growth of peripheral lung adenocarcinomas. Cancer 77:646–656
Suzuki K, Yokose T, Yoshida J et al (2000) Prognostic significance of the size of central fibrosis in peripheral adenocarcinoma of the lung. Ann Thorac Surg 69:893–897
Yokose T, Suzuki K, Nagai K et al (2000) Favorable and unfavorable morphological prognostic factors in peripheral adenocarcinoma of the lung 3 cm or less in diameter. Lung Cancer 29:179–188
Terasaki H, Niki T, Matsuno Y et al (2003) Lung adenocarcinoma with mixed bronchioloalveolar and invasive components: clinicopathological features, subclassification by extent of invasive foci, and immunohistochemical characterization. Am J Surg Pathol 27:937–951
Sakurai H, Maeshima A, Watanabe S et al (2004) Grade of stromal invasion in small adenocarcinoma of the lung: histopathological minimal invasion and prognosis. Am J Surg Pathol 28:198–206
Minami Y, Matsuno Y, Iijima T et al (2005) Prognostication of small-sized primary pulmonary adenocarcinomas by histopathological and karyometric analysis. Lung Cancer 48:339–348
Kurokawa T, Matsuno Y, Noguchi M et al (1994) Surgically curable “early” adenocarcinoma in the periphery of the lung. Am J Surg Pathol 18:431–438
Maeshima AM, Niki T, Maeshima A et al (2002) Modified scar grade: a prognostic indicator in small peripheral lung adenocarcinoma. Cancer 95:2546–2554
Yoshizawa A, Motoi N, Riely GJ et al (2011) Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 24:653–664
Russell PA, Wainer Z, Wright GM et al (2011) Does lung adenocarcinoma subtype predict patient survival?: a clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol 6:1496–1504. doi:10.1097/JTO.0b013e318221f701
Gu J, Lu C, Guo J et al (2013) Prognostic significance of the IASLC/ATS/ERS classification in Chinese patients-a single institution retrospective study of 292 lung adenocarcinoma. J Surg Oncol 107:474–480
Hung JJ, Yeh YC, Jeng WJ et al (2014) Predictive value of the international association for the study of lung cancer/American Thoracic Society/European Respiratory Society classification of lung adenocarcinoma in tumor recurrence and patient survival. J Clin Oncol 32:2357–2364
Cha MJ, Lee HY, Lee KS et al (2014) Micropapillary and solid subtypes of invasive lung adenocarcinoma: clinical predictors of histopathology and outcome. J Thorac Cardiovasc Surg 147:921–928
Yatabe Y, Kosaka T, Takahashi T et al (2005) EGFR mutation is specific for terminal respiratory unit type adenocarcinoma. Am J Surg Pathol 29:633–639
Mäkinen JM, Laitakari K, Johnson S (2015) Nonpredominant lepidic pattern correlates with better outcome in invasive lung adenocarcinoma. Lung Cancer 90:568–574. doi:10.1016/j.lungcan.2015.10.014
Warth A, Muley T, Meister M et al (2012) The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol 30:1438–1446
Inamura K, Takeuchi K, Togashi Y (2009) EML4-ALK lung cancers are characterized by rare other mutations, a TTF-1 cell lineage, an acinar histology, and young onset. Mod Pathol 22:508–515. doi:10.1038/modpathol.2009.2
Yoshida A, Tsuta K, Nakamura H et al (2011) A comprehensive histologic analysis of ALK-rearranged lung carcinomas. Am J Surg Pathol 35:1226–1234. doi:10.1097/PAS.0b013e3182233e06
Kadota K, Yeh YC, Sima CS et al (2014) The cribriform pattern identifies a subset of acinar predominant tumors with poor prognosis in patients with stage I lung adenocarcinoma: a conceptual proposal to classify cribriform predominant tumors as a distinct histologic subtype. Mod Pathol 27:690–700
Warth A, Muley T, Harms A et al (2016) Clinical relevance of different papillary growth patterns of pulmonary adenocarcinoma. Am J Surg Pathol 40:818–826. doi:10.1097/PAS.0000000000000622
Kadota K, Nitadori J, Sima CS et al (2015) Tumor spread through air spaces is an important pattern of invasion and impacts the frequency and location of recurrences after limited resection for small stage I lung adenocarcinomas. J Thorac Oncol 10:806–814
Lee G, Lee HY, Jeong JY et al (2015) Clinical impact on minimal micropapillary pattern in invasive lung adenocarcinoma: prognostic significance and survival outcomes. Am J Surg Pathol 39:660–666
Nitadori J, Bograd AJ, Kadota K et al (2013) Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Inst 105:1212–1220
Rekhtman N, Tafe LJ, Chaft JE et al (2013) Distinct profile of driver mutations and clinical features in immunomarker-defined subsets of pulmonary large-cell carcinoma. Mod Pathol 26:511–522. doi:10.1038/modpathol.2012.195
Rossi G, Mengoli MC, Cavazza A et al (2014) Large cell carcinoma of the lung: clinically oriented classification integrating immunohistochemistry and molecular biology. Virchows Arch 464:61–68. doi:10.1007/s00428-013-1501-6
Hwang DH, Szeto DP, Perry AS et al (2014) Pulmonary large cell carcinoma lacking squamous differentiation is clinicopathologically indistinguishable from solid-subtype adenocarcinoma. Arch Pathol Lab Med 138(5):626–635. doi:10.5858/arpa.2013-0179-OA
Rekhtman N, Ang DC, Riely GJ et al (2013) KRAS mutations are associated with solid growth pattern and tumor-infiltrating leukocytes in lung adenocarcinoma. Mod Pathol 26(10):1307–1319. doi:10.1038/modpathol.2013.74
Driver BR, Portier BP, Mody DR et al (2016) Next-generation sequencing of a cohort of pulmonary large cell carcinomas reclassified by World Health Organization 2015 Criteria. Arch Pathol Lab Med 140(4):312–317. doi:10.5858/arpa.2015-0361-OA
Solis LM, Behrens C, Raso MG et al (2012) Histologic patterns and molecular characteristics of lung adenocarcinoma associated with clinical outcome. Cancer 118:2889–2899
Ujiie H, Kadota K, Chaft JE et al (2015) Solid predominant histologic subtype in resected stage I lung adenocarcinoma is an independent predictor of early, extrathoracic, multisite recurrence and of poor postrecurrence survival. J Clin Oncol 33:2877–2884
Austin JH, Garg K, Aberle D et al (2013) Radiologic implications of the 2011 classification of adenocarcinoma of the lung. Radiology 266:62–71. doi:10.1148/radiol.12120240
Wu J, Chu PG, Jiang Z et al (2013) Napsin a expression in primary mucin-producing adenocarcinomas of the lung: an immunohistochemical study. J Clin Pathol 139(2):160–166. doi:10.1309/AJCP62WJUAMSZCOM
Sugano M, Nagasaka T, Sasaki E et al (2013) HNF4α as a marker for invasive mucinous adenocarcinoma of the lung. Am J Surg Pathol 37:211–218. doi:10.1097/PAS.0b013e31826be303
Geles A, Gruber-Moesenbacher U, Quehenberger F et al (2015) Pulmonary mucinous adenocarcinomas: architectural patterns in correlation with genetic changes, prognosis and survival. Virchows Arch 467(6):675–686
Kakegawa S, Shimizu K, Sugano M et al (2011) Clinicopathological features of lung adenocarcinoma with KRAS mutations. Cancer 15(117):4257–4266. doi:10.1002/cncr.26010
Hata A, Katakami N, Fujita S et al (2010) Frequency of EGFR and KRAS mutations in Japanese patients with lung adenocarcinoma with features of the mucinous subtype of bronchioloalveolar carcinoma. J Thorac Oncol 5:1197–1200. doi:10.1097/JTO.0b013e3181e2a2bc
Finberg KE, Sequist LV, Joshi VA et al (2007) Mucinous differentiation correlates with absence of EGFR mutation and presence of KRAS mutation in lung adenocarcinomas with bronchioloalveolar features. J Mol Diagn 2007(9):320–326
Fernandez-Cuesta L, Plenker D, Osada H et al (2014) CD74-NRG1 fusions in lung adenocarcinoma. Cancer Discov 4:415–422. doi:10.1158/2159-8290.CD-13-0633
Shim HS, Kenudson M, Zheng Z et al (2015) Unique genetic and survival characteristics of invasive mucinous adenocarcinoma of the lung. J Thorac Oncol 10:1156–1162. doi:10.1097/JTO.0000000000000579
Nakaoku T, Tsuta K, Ichikawa H et al (2014) Druggable oncogene fusions in invasive mucinous lung adenocarcinoma. Clin Cancer Res 20:3087–3093. doi:10.1158/1078-0432.CCR-14-0107
Ichinokawa H, Ishii G, Nagai K et al (2013) Distinct clinicopathologic characteristics of lung mucinous adenocarcinoma with KRAS mutation. Hum Pathol 44:2636–2642. doi:10.1016/j.humpath.2013.05.026
Kettle R, Simmons J, Schindler F et al (2010) Regulation of neuregulin 1beta1-induced MUC5AC and MUC5B expression in human airway epithelium. Am J Respir Cell Mol Biol 42:472–481. doi:10.1165/rcmb.2009-0018OC
Rossi G, Murer B, Cavazza et al (2004) Primary mucinous (so-called colloid) carcinomas of the lung: a clinicopathologic and immunohistochemical study with special reference to CDX-2 homeobox gene and MUC2 expression. Am J Surg Pathol 28(4):442–452.
Zenali MJ, Weissferdt A, Solis LM et al (2015) An update on clinicopathological, immunohistochemical, and molecular profiles of colloid carcinoma of the lung. Hum Pathol 46:836–842. doi:10.1016/j.humpath.2014.10.032
Nakatani Y, Kitamura H, Inayama Y et al (1998) Pulmonary adenocarcinomas of the fetal lung type: a clinicopathologic study indicating differences in histology, epidemiology, and natural history of low-grade and high-grade forms. Am J Surg Pathol 22:399–411
Nakatani Y, Masudo K, Miyagi Y et al (2002) Aberrant nuclear localization and gene mutation of beta-catenin in low-grade adenocarcinoma offetal lung type: up-regulation of the Wnt signaling pathway may be a common denominator for the development of tumors that form morules. Mod Pathol 15:617–624
Morita, AJSP, 2013
Suzuki M, Yazawa T, Ota S et al (2015) High-grade fetal adenocarcinoma of the lung is a tumour with a fetal phenotype that shows diverse differentiation, including high-grade neuroendocrine carcinoma: a clinicopathological, immunohistochemical mutational study of 20 cases. Histopathology 67:806–816
Sekine S, Shibata T, Matsuno Y et al (2003) Beta-catenin mutations in pulmonary blastomas: association with morule formation. J Pathol 200:214–221
Wu Y, Chen D, Li Y et al (2014) DICER1 mutations in a patient with an ovarian Sertoli-Leydig tumor, well-differentiated fetal adenocarcinoma of the lung, and familial multinodular goiter. Eur J Med Genet 57:621–625
Kock L, Bah I, Wu Y et al (2016) Germline and somatic DICER1 mutations in a well-differentiated fetal adenocarcinoma of the lung. J Thorac Oncol 11(3):e31–e33. doi:10.1016/j.jtho.2015.09.012
Inamura K, Satoh Y, Okumura S et al (2005) Pulmonary adenocarcinomas with enteric differentiation: histologic and immunohistochemical characteristics compared with metastatic colorectal cancers and usual pulmonary adenocarcinomas. Am J Surg Pathol 29(5):660–665
Yousem SA (2005) Pulmonary intestinal-type adenocarcinoma does not show enteric differentiation by immunohistochemical study. Mod Pathol 18:816–821
Maeda R, Isowa N, Onuma H et al (2008) Pulmonary intestinal-type adenocarcinoma. Interact Cardiovasc Thorac Surg 7:349–351. doi:10.1510/icvts.2007.168716
Li HC, Schmidt L, Greenson JK et al (2009) Primary pulmonary adenocarcinoma with intestinal differentiation mimicking metastatic colorectal carcinoma: case report and review of literature. Am J Clin Pathol 131:129–133. doi:10.1309/AJCPB04XWICTFERL
Hatanaka K, Tsuta K, Watanabe K et al (2011) Primary pulmonary adenocarcinoma with enteric differentiation resembling metastatic colorectal carcinoma: a report of the second case negative for cytokeratin 7. Pathol Res Pract 207:188–191. doi:10.1016/j.prp.2010.07.005
Lin D, Zhao Y, Li H et al (2013) Pulmonary enteric adenocarcinoma with villin brush border immunoreactivity: a case report and literature review. J Thorac Dis 5(1):E17–E20. doi:10.3978/j.issn.2072-1439.2012.06.06
Wang CX, Liu B, Wang YF et al (2014) Pulmonary enteric adenocarcinoma: a study of the clinicopathologic and molecular status of nine cases. Int J Clin Exp Pathol 7:1266–1274
László T, Lacza A, Tóth D et al (2014) Pulmonary enteric adenocarcinoma indistinguishable morphologically and immunohistologically from metastatic colorectal carcinoma. Histopathology 65:283–287. doi:10.1111/his.12403
Handa Y, Kai Y, Ikeda T et al (2015) Pulmonary enteric adenocarcinoma. Gen Thorac Cardiovasc Surg Jul 3. [Epub ahead of print]
Metro G, Valtorta E, Siggillino A et al (2015) Enteric-type adenocarcinoma of the lung harbouring a novel KRAS Q22K mutation with concomitant KRAS polysomy: a case report. Ecancermedicalscience 9:559. doi:10.3332/ecancer.2015.559
Garajová I, Funel N, Fiorentino M et al (2015) MicroRNA profiling of primary pulmonary enteric adenocarcinoma in members from the same family reveals some similarities to pancreatic adenocarcinoma-a step towards personalized therapy. Clin Epigenetics 7:129. doi:10.1186/s13148-015-0162-5
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media Singapore
About this chapter
Cite this chapter
Nakatani, Y., Yonemori, Y., Matsushima, J., Yazawa, T. (2017). Classification of Adenocarcinoma of the Lung, with a Special Reference to Prognosis. In: Takiguchi, Y. (eds) Molecular Targeted Therapy of Lung Cancer. Springer, Singapore. https://doi.org/10.1007/978-981-10-2002-5_1
Download citation
DOI: https://doi.org/10.1007/978-981-10-2002-5_1
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-2000-1
Online ISBN: 978-981-10-2002-5
eBook Packages: MedicineMedicine (R0)