Abstract
Background
The International association for the study of cancer (IASLC)/American thoracic society (ATS)/European respiratory society (ERS) has established a new subclassification of lung adenocarcinoma, especially for the lepidic pattern component, formerly called bronchioloalveolar adenocarcinoma (BAC). According to the new classification, BAC has been classified into the following 4 main subtypes: adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), invasive adenocarcinoma (IA), and variants of invasive adenocarcinoma (VIA). An observational study was conducted to validate this classification in patients with pathological stage IA pulmonary adenocarcinoma.
Patients and methods
147 patients treated for pathological stage IA lung adenocarcinoma by complete resection at Osaka University Medical Hospital from January 1993 to December 2002 were assessed. The tumor specimens of the cohort were classified into the 4 subgroups. In addition, these groups were compared for various prognostic factors.
Results
Adenocarcinoma in situ was observed in 30 patients, MIA in 8, IA in 104, and VIA in 5 patients, with 5-year survival rates of 100, 100, 85.5, and 60.0 %, respectively. The relationship between the histological classification and K-ras mutation was significant (p < 0.001), especially when comparing the VIA group with the others (p ≪ 0.001). Ki67-labeling indices were significantly different between the AIS and IA groups (p = 0.040).
Conclusions
This study validated the proposed IASLC/ATS/ERS classification for pulmonary adenocarcinoma in patients with pathological stage IA pulmonary adenocarcinoma. The difference between AIS and IA may depend on the proliferation of the carcinoma. In addition, the difference between VIA and the other adenocarcinoma types may depend on genetic factors, especially K-ras mutations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the most frequent cause of cancer death worldwide for both men and women [1]. Clinical stage IA lung cancer is seen in only 36.5 % of lung cancer patients who undergo surgery [2]. The 5-year survival rate is only 82.0 %, even in pathological stage IA pulmonary lung cancer [3]. The identification of prognostic factors is still required.
Recently, a new classification of pulmonary adenocarcinoma has been proposed especially to address the necessity to subclassify the lepidic pattern component, so-called bronchioloalveolar adenocarcinoma (BAC), by the International Association for the Study of Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) [4]. Before the classification, BAC contained a broad spectrum of tumors. According to the proposed new classification, BAC has been classified into 4 main subtypes: preinvasive lesion (adenocarcinoma in situ: AIS, ≤3 cm formerly BAC), minimally invasive adenocarcinoma (MIA, ≤3 cm lepidic predominant tumor with ≤5 mm invasion), invasive adenocarcinoma (IA: formerly BAC pattern, with >5 mm invasion), and variants of invasive adenocarcinoma (VIA, Fig. 1) [4].
To date, there has been no report of validation of the new classification in patients with pathological stage IA adenocarcinoma. The present study was an observational analysis to evaluate the new proposed classification in patients with pathological stage IA lung adenocarcinoma treated at our institute.
Patients and methods
From January 1993 to December 2002, 150 consecutive, surgically complete lung resections with lobectomy and lymph node dissection for pathological stage IA lung adenocarcinoma were performed at Osaka University Medical Hospital. Of these, 147 patients, excluding 3 patients whose tissue blocks were not available, were analyzed. Survival data were obtained by reviewing hospital records.
Classification of histological appearance
The pathologist (EM) re-diagnosed the tumor specimens of the cohort based on the IASLC/ATS/ERS classification of lung adenocarcinoma in a blind fashion. The tumor specimens were classified into the 4 groups (AIS, MIA, IA, and VIA).
EGFR and K-ras mutations
EGFR mutation was detected with fragment analysis for exon19 deletion and cycleave real-time polymerase chain reaction (PCR) for exon18 G719X, exon20 T790M, exon21 L858R, and exon21 L861Q mutations (SRL, Inc., Tokyo, Japan). K-ras mutation was detected with PCR-Direct sequencing for codons 12 and 13 (SRL, Inc.).
Immunohistopathological staining
Formalin-fixed and paraffin-embedded blocks of the tumors were used for immunohistochemistry. Serial 4-μm-thick sections were sliced from the paraffin blocks and mounted on glass slides. The slides were then deparaffinized and rehydrated. For antigen retrieval, the samples were heated in a pressure chamber at 125 °C for 30 s and then at 90 °C for 10 s in pH 6.5 buffer, except for ALK staining. For ALK staining, the samples were heated in a pressure chamber at 97 °C for 20 min, and then at 90 °C for 10 s in pH 9.5 buffer for antigen retrieval. All slides were incubated with peroxidase blocking solution (Dako, Kyoto, Japan) for 10 min at room temperature. Each sample was incubated overnight at 4 °C with anti-human Ki-67 (SP6) rabbit monoclonal antibody (1:400; Thermo Scientific, Fremont, CA), or anti-human E-cadherin (HECD-1) monoclonal antibody (1:500; Takara Bio Inc. Shiga, Japan), or for 1 h at room temperature with anti-human ALK monoclonal antibody [5A4] (1:30; Abcam, Tokyo, Japan). In addition, each slide was incubated with Linker (Dako Autostainer/Autostainer Plus, Dako) for ALK staining. The samples were reacted with polymer solution (ChemMate EnVision, Dako) for 30 min at room temperature. After that, diaminobenzidine (DAB, Dako) was applied to the samples, and the slides were counterstained with hematoxylin.
E-cadherin score and Ki-67 labeling index
For E-cadherin, staining intensity was assessed using the Intensity Reactivity Score (IRS), where staining intensity (SI) was classified as negative (= 0), weak (= 1), moderate (= 2), or strong (= 3), and reactivity was determined by the percentage of positive cells (PP). IRS was calculated by multiplying SI with PP, resulting in a minimum score of 0 and a maximum score of 300. In addition to the IRS, expression patterns were assessed. Blinded to the patients’ information, 3 authors (TN, YS, and NS) assessed each sample at 4 randomly chosen points in the area of the tumors, and the average IRS was used for statistical analysis.
For the Ki-67 labeling index, at least 1000 nuclei were counted at high magnification (40× objective) without recounting the same areas, and the average was expressed as a percentage. Foci of necrosis were excluded. Three authors assessed each sample blinded to the patients’ information, and the average Ki-67 labeling index was used for statistical analysis.
Statistical analysis
A difference in incidence between two or more groups was compared by contingency table analysis with Pearson χ2 test. Kaplan–Meier analyses and the log-rank test were used for survival curves and their comparisons, respectively. Values of p ≤ 0.05 were considered significant.
Results
Patient age at the time of surgery ranged between 30 and 83 years with a mean of 62.9 ± 9.8 years. The mean observation period was 85.2 ± 38.8 months (median, 73.7 months; range 19.9–202.0 months; Table 1).
Histological classification of tumors and survival
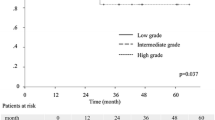
Overall 5-year survival was 88.6 % for all patients. Adenocarcinoma in situ was observed in 30 patients (20.4 %), MIA in 8 (5.4 %), IA in 104 (70.7 %), and VIA in 5 (3.4 %) in this cohort. Adenocarcinoma in situ included no cases of mucinous adenocarcinoma. In addition, VIA contained only mucinous lesions in this cohort. AIS, MIA, IA, and VIA showed 5-year survival rates of 100, 100, 85.5, and 60.0 %, respectively (IA vs. VIA: p = 0.26; Fig. 2).
Survival curve for patients according to the IASLC/ATS/ERS histological classification. Patients with adenocarcinoma in situ (AIS) (n = 30) have a 5-year survival rate (5YSR) of 100 %, whereas those with minimally invasive adenocarcinoma (MIA), invasive adenocarcinoma (IA), and variants of invasive adenocarcinoma (VIA) (n = 8, 104, and 5, respectively) have 5YSRs of 100, 85.5, and 60.0 %, respectively (IA versus VIA: p = 0.26)
Relationships between histological classification and mutations
EGFR and K-ras mutations were observed in 70 and 10 cases, respectively. ALK rearrangement was observed in 18 cases. Among the EGFR mutations, exon19 deletion was observed in 36 cases, exon18 G719X mutation was observed in 8, exon20 T790M mutation was observed in 3, exon21 L858R mutation was observed in 22, and exon21 L861Q mutation was observed in 1. In the AIS group, EGFR mutation was observed in 15 patients (50 %). Similarly, in the invasive MIA, IA, and VIA groups, EGFR mutation was observed in 5 (50 %), 52 (50 %), and 0 patients, respectively. There was no significant relationship between the histological classification and EGFR mutation (χ2 test, p = 0.48; Table 2). In addition, when the VIA group and the others were compared as in the IASLC/ATS/ERS classification article [4], there was also no significant difference between the groups (χ2 test, p = 0.12).
Among the K-ras mutations, codon 12 mutation was observed in 10 patients, and codon 13 mutation was observed in 1. In the AIS group, K-ras mutation was observed in 3 patients (10 %). In the invasive MIA, IA, and VIA groups, K-ras mutation was observed in 1, 2, and 3 patients (10, 2, 60 %), respectively. There was a significant relationship between the histological classification and K-ras mutation (χ2 test, p < 0.001; Table 2), and there was also a significant difference between the VIA group and the other groups (χ2 test, p ≪ 0.0001). In addition, there were no significant relationships for the histological characteristics in the 6 cases with K-ras mutation-positive adenocarcinoma in the groups other than the VIA group.
ALK rearrangement was observed in 6 patients (33.3 %) in the AIS group and in 12 (66.6 %) in the IA group. There was no significant relationship between the histological classification and ALK rearrangement (χ2 test, p = 0.32; Table 2).
Relationships between histological classification and the Ki67-labeling index
In the AIS group, the average ± standard deviation (SD) Ki67-labeling index was 10.15 ± 15.3. In the invasive MIA, IA, and VIA groups, they were 10.1 ± 13.0, 18.0 ± 19.0, and 10.2 ± 10.7, respectively. There was a significant difference between the AIS and IA groups (AIS versus IA, p = 0.040; Fig. 3), but no significant differences among the others (AIS versus MIA, p = 0.495; MIA versus IA, p = 0.105; IA versus VIA, p = 0.168; AIS versus VIA, p = 0.496; and MIA versus VIA, p = 0.493).
The Ki67-labeling index of adenocarcinoma in situ (AIS) and invasive adenocarcinoma (IA). There is a significant difference in the Ki67-labeling index between AIS and IA (p = 0.040), but no significant differences among the others (AIS versus minimally invasive adenocarcinoma (MIA), p = 0.495; MIA versus IA, p = 0.105; IA versus variants of invasive adenocarcinoma (VIA), p = 0.168; AIS versus VIA, p = 0.496; and MIA versus VIA, p = 0.493)
Relationships between histological classification and E-cadherin and the E-cadherin score
The appearances of E-cadherin staining are presented in Fig. 4. The expression of E-cadherin in AIS was rare or localized at the intercellular junctions, while in MIA, it was uniformly localized at the junction. However, the expression pattern in IA varied: no staining, less frequently, or uneven. In addition, no expression was seen in VIA. It seems that there was a tendency of the expression pattern to change with differentiation of the cancer. However, for the E-cadherin score, there were no significant differences (AIS versus IA, p = 0.496; AIS versus MIA, p = 0.234; MIA versus IA, p = 0.193; IA versus VIA, p = 0.333; AIS versus VIA, p = 0.336; and MIA versus VIA, p = 0.224).
The E-cadherin expression patterns in pulmonary adenocarcinomas. The expression of E-cadherin in AIS is rare or localized at the intercellular junctions (a), while in MIA it is uniformly localized at the junction (b). However, the expression pattern in IA is various: no staining, less frequently, or uneven (c and c’). In addition, no expression is seen in VIA (d)
Discussion
Recently, Travis et al. [4] proposed a new classification for pulmonary adenocarcinoma (IASLC/ATS/ERS classification) based on the histological appearance, especially the lepidic pattern, and central fibrosis. With respect to the lepidic pattern, a small (≤3 cm) lepidic pattern tumor, including mucinous and non-mucinous components, is classified as AIS. Many previous reports have documented that patients with AIS have 100 % disease-free survival after resection [5–7] Moreover, the new classification contains a new entity, MIA, formerly known as BAC with ≤5 mm invasion, which usually contains fibrous stroma. The 5-year survival of patients with MIA is also expected to be 100 %.
In the present analysis, the 5-year survivals of patients with AIS and with MIA were also 100 %. In addition, patients with VIA, which included only a mucinous lesion, had the lowest 5-year survival (60.0 %).
EGFR contributes to signal transduction related to cell proliferation and cell maintenance [8]. When exon 18, 19, 20, or 21 has a mutation, EGFR activation occurs without a ligand, and cell tumorigenesis occurs [9, 10]. EGFR mutation can be seen in 40 % of adenocarcinomas in Japanese cases [11]. The present proportions of EGFR mutation subsets (exon18: exon19: exon20: exon21 = 8: 36: 3: 22), even in pathological stage I adenocarcinoma, were similar to those of a previous study reported in Japanese patients that also included other stages, stages IB, II, and III (exon18: exon19: exon20: exon21 = 3: 48: 3: 36, p = 0.20) [12], which can mean that the present cohort had a common proportion of EGFR mutations. The proportions of EGFR mutations in the present cohort were not significantly different among the histological classifications. In addition, when the VIA and the other groups were selected, as in the previous report [4], there were also no significant differences between them. However, comparing with the previous report [4], the percentage of EGFR mutation in invasive mucinous adenocarcinoma was almost zero. In addition, that of non-mucinous lesions was about 45 %. Both rates were similar to the present results.
The proportion of K-ras mutation is about 10 % in Japan [13]. The mutation can occur at codon 12, 13, or 61, and the most frequent mutation occurs at codon 12 (>90 %) [14]. The present cohort had a similar proportion of K-ras mutations (codon 12: codon 13 = 9: 1). In addition, there were significant differences among all groups and also between the two groups based on VIA. The results were also similar to the IASLC/ATS/ERS proposed classification report [4]. In addition, there were no significant differences for the histological characteristic of the 6 cases with K-ras-positive adenocarcinoma in the groups other than the VIA group, though all cases had a mucinous part in the lesion. However, there were no significant relationships. Further investigation is required on this point.
Rearrangements of the ALK gene were first identified in non-small cell lung cancer in 2007 [15]. ALK gene rearrangement was observed in approximately 5 %, which was detected with gene analysis [15–17] In the present cohort, ALK rearrangement was observed in 18 cases (12.2 %). The frequency seemed higher than in a previous study. However, Shaw et al. [18] reported that the frequency was 13 % in lung adenocarcinoma, which was detected with immunohistochemistry, and the frequency was similar to the present result. The difference in the frequencies could come from the difference in detection methods. In addition, a previous study reported that ALK rearrangement was more frequently observed in solid-type lung adenocarcinoma [19]. However, the dispersion was not different between the ALK rearrangement-positive and -negative groups in the present cohort. The difference might also come from the difference in the detection methods. Further investigation of this point is also required.
As expected, there was a significant difference in the Ki67-labeling index between AIS and IA. Interestingly, there were no significant differences between MIA and IA. The reason for the good outcome of patients with AIS could be the slow proliferation of the cells. However, the reasons for the good outcome of the MIA group may include other reasons.
The E-cadherin score inversely reflects the epithelial to mesenchymal transition (EMT), which has a profound impact on cancer progression [20]. Shintani et al. [21, 22] reported that collagen, which is made by fibroblasts, promotes EMT in lung cancer. In addition, Nozawa et al. [22] previously reported that high E-cadherin expression was related to a low Ki67 index and good histological differentiation in pulmonary adenocarcinoma. From these reports, we expected that early stage pulmonary adenocarcinoma was also related to high E-cadherin expression, and the central fibrosis of MIA and IA could cause EMT, which means that the E-cadherin score decreases in these groups. Unfortunately, there was no difference in E-cadherin scores among the groups in the present study. However, the expression patterns seemed to have a tendency to be related to histological differentiation. The paper also reported that the expression of E-cadherin is an important “phenotypical” marker for the histological differentiation of human lung adenocarcinoma [22]. In addition, the previous report also mentioned that the E-cadherin-mediated adhesion system may participate more in the maintenance of structural polarity than in the cell–cell adhesiveness of cancer cells in all stages of lung adenocarcinoma [22 ]. In the present study, the IA group had various E-cadherin expression patterns, which may have depended on the variety of sub-classifications of pulmonary adenocarcinoma. The present result may also lead to a similar conclusion to that of the previous report in early stage adenocarcinoma.
Limitations
This was a retrospective, single-institution study. In addition, the number of patients was not large enough to investigate all items in this study design. Further investigations are required in this respect.
Conclusion
This study evaluated the new proposed classification for small pulmonary adenocarcinoma in one institutional cohort of pathologically stage IA adenocarcinoma. The histological subtypes of the IASLC/ATS/ERS classification appear to be useful even among pathological stage IA adenocarcinoma in our institution.
References
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108.
Goya T, Asamura H, Yoshimura H, Kato H, Shimokata K, Tsuchiya R, Sohara Y, Miya T, Miyaoka E. Japanese joint committee of lung cancer registry. Prognosis of 6644 resected non-small cell lung cancers in Japan: a Japanese lung cancer registry study. Lung Cancer. 2005;50:227–34.
Sawabata N, Miyaoka E, Asamura H, Nakanishi Y, Eguchi K, Mori M, Nomori H, Fujii Y, Okumura M, Yokoi K, For the Japanese joint committee for lung cancer registration. Japanese lung cancer registry study of 11,663 surgical cases in demographic and prognosis changes over decade. J Thorac Oncol. 2004;2011(6):1229–35.
Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/American thoracic society/European respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–85.
Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung histologic characteristics and prognosis. Cancer. 1995;75:2844–52.
Watanabe S, Watanabe T, Arai K, et al. Results of wedge resection for focal bronchioloalveolar carcinoma showing pure ground-glass attenuation on computed tomography. Ann Thorac Surg. 2002;73:1071–5.
Sakurai H, Dobashi Y, Mizutani E, et al. Bronchioloalveolar carcinoma of the lung 3 centimeters or less in diameter: a prognostic assessment. Ann Thorac Surg. 2004;78:1728–33.
Yarden Y. The EGFR family and its ligands in human cancer. Signalling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37(Suppl 4):S3–8.
Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500.
Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39.
Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–46.
Yoshizawa A, Sumiyoshi S, Sonobe M, et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol. 2013;8:52–61.
Shigematsu H, Gazdar AF. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int J Cancer. 2006;118:257–62.
Mitsudomi T, Viallet J, Mulshine JL, et al. Mutations of ras genes distinguish a subset of non-small-cell lung cancer cell lines from small-cell lung cancer cell lines. Oncogene. 1991;6:1353–62.
Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–6.
Wong DW, Leung EL, So KK, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer. 2009;115:1723–33.
Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK lung cancer are characterized by rare other mutations, a TTF-a cell lineage, an cinar histology, and young onset. Mod Pathol. 2009;22:508–15.
Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27:4247–53.
Shintani Y, Okimura A, Sato K, et al. Epithelial to mesenchymal transition is a determinant of sensitivity to chemoradiotherapy in non-small cell lung cancer. Ann Thorac Surg. 2011;92:1794–804.
Shintani Y, Maeda M, Chaika N, Johnson KR, Wheelock MJ. Collagen I promotes epithelial-to-mesenchymal transition in lung cancer cells via transforming growth factor-beta signaling. Am J Respir Cell Mol Biol. 2008;38:95–104.
Shintani Y, Hollingsworth MA, Wheelock MJ, Johnson KR. Collagen I promotes metastasis in pancreatic cancer by activating c-Jun NH(2)-terminal kinase 1 and up-regulating N-cadherin expression. Cancer Res. 2006;66:11745–53.
Nozawa N, Hashimoto S, Nakashima Y, et al. Immunohistochemical α- and β-catenin and E-cadherin expression and their clinicopathological significance in human lung adenocarcinoma. Pathol Res Pract. 2006;202:639–50.
Conflict of interest
All the authors have declared no competing interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakagiri, T., Sawabata, N., Morii, E. et al. Evaluation of the new IASLC/ATS/ERS proposed classification of adenocarcinoma based on lepidic pattern in patients with pathological stage IA pulmonary adenocarcinoma. Gen Thorac Cardiovasc Surg 62, 671–677 (2014). https://doi.org/10.1007/s11748-014-0429-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11748-014-0429-3