Abstract
A predictive crude oil density model reliable over a wide range of temperature and pressure conditions is increasingly important for the safe production of oil and accurate estimation of oil reserves. While hydrocarbon density data at low-to-moderate temperatures and pressures are plentiful, data and validated models that have reasonable predictive capability for crude oil at extreme temperatures and pressures are limited. In this investigation, we present new experimental density data for crude oil sample obtained from the Gulf of Mexico region. Density data are measured at pressures to 270 MPa and temperatures to 524 K. These conditions simulate those encountered from ultra-deep formations to platforms. These density data points are then used to validate both empirical-based and molecular-based equations of state models. Results show that the molecular-based perturbed-chain statistical associating fluid theory (PC-SAFT) models, without the use of any fitting parameters, predict the crude oil density within 1% of the experimental data. These results are superior to the density predictions obtained with the high-temperature, high-pressure, volume-translated cubic equations of state.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Nomenclature
Latin symbols
- a i, b i :
-
constants in Tait equation
- B, P 0 :
-
pressure terms in Tait equation, MPa
- CN :
-
carbon number calculated using expression of Huang and Radosz
- CN p :
-
carbon number calculated assuming species of given M w has 100% paraffin character
- CN pna :
-
carbon number calculated assuming species of given M w has 100% polynuclear aromatic character
- H/C :
-
molecular hydrogen-to-carbon ratio
- m :
-
segment number parameter for PC-SAFT equation
- M w :
-
molecular weight, g/mol
- P :
-
pressure, MPa
- V :
-
molar volume, cm3/mol
- T :
-
temperature, K
- asph:
-
subscript to signify the asphaltene phase
- A + R:
-
subscript to signify the aromatics and resins
- w:
-
weight fraction
Greek symbols
- β :
-
isothermal compressibility, MPa−1
- δ :
-
mean absolute percent deviation for a set of numbers, %
- ε/k :
-
energy parameter for PC-SAFT equation, K
- λ :
-
standard deviation associated with a given value of δ
- ρ :
-
density, g/cm3
- σ :
-
size parameter for PC-SAFT equation, Å
- γ :
-
aromaticity parameter
1 Introduction
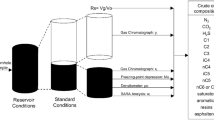
Rapid worldwide economic growth over the past century has led the petroleum industry to search for oil and gas in harsh conditions, including in ultra-deep reservoirs several miles beneath the deep seafloor where high temperature and pressure conditions prevail. While the first oil well drilled in the USA had a depth of approximately 100 feet, prospectors today must often drill several miles deep to tap oil reserves. The hydrocarbons within these wells are at increasingly under extreme conditions [1] of temperature and/or pressure (such as the oil located in deep wells in the Gulf of Mexico). These high-temperature, high-pressure (HTHP) wells can exhibit reservoir temperatures in excess of 423 K and/or reservoir pressures in excess of 10,000 psi (69 MPa). Between 1982 and 2012, there were 415 such wells established around the world [2]. This trend is expected to accelerate in the future as existing oil reserves are depleted [3]. From a thermodynamic and thermophysical property modeling perspective, the increased tendency for new reservoirs to exist at HTHP conditions is problematic as the predictive capability of many models at such conditions is unproven. Development of HTHP reservoirs is capital-intensive and therefore represents a significant financial risk, particularly when they are offshore. A proper evaluation of such reservoirs typically requires accurate pressure–volume–temperature (PVT) data. In addition, the safe transport of hydrocarbon liquids in pipelines from reservoir to processing facility requires knowledge of second derivative properties [4, 5], such as the isothermal compressibility β. Accurate prediction of β is also necessary to estimate the rate of production decline during the primary recovery phase. Accurate knowledge of bubble point and asphaltene onset pressures in oil wells and dew point pressures in gas wells is critical to the design of processes that maintain a steady flow of hydrocarbons from the well. Ideally, these properties are measured experimentally. However, often such experimentation is not feasible for technical and/or economic reasons. Therefore, the United States Department of Energy is interested in the identification and development of thermodynamic and thermophysical property models that give accurate predictions even at extreme conditions (defined in this work as temperatures to 533 K and pressures to 240 MPa).
Experimental data for crude oil systems at extreme HTHP conditions are necessary in order to properly validate model performance at such conditions. Limited high-pressure data are available for the density and isothermal compressibility of some crude oil systems. However, to our knowledge, no published data are available for crude oil systems at temperatures higher than ~400 K and 155 MPa [6]. Therefore, we present new experimental density data for a crude oil sample measured from ambient conditions to HTHP conditions at temperatures to 523 K and pressures to 270 MPa.
To date, several types of methods have been employed to describe hydrocarbon systems in petrochemical applications. A popular method involves a number of empirical correlations [4, 7,8,9,10,11,12,13,14,15,16] that are used in the estimation of crude oil properties, including PVT data, gas/oil ratio, and β. Such “black oil” correlations are typically only valid over a given range of temperature and pressure values; however, more HTHP wells are starting to exceed these ranges. Oftentimes, correlations developed for crude oil in one particular region of the world do not provide reliable predictions when applied to oil from another part of the world. The reason is mainly attributed to the significant compositional differences encountered between crude oils produced in various regions. For these reasons, investigators are increasingly turning to equation of states (EoSs) to obtain such HTHP crude oil properties.
While empirical “black oil” correlations are typically created for whole crude oil systems, EoSs rely on the use of suitable characterization procedures. A typical crude oil is comprised of numerous components, and it would not be computationally efficient to consider each component individually. However, characterization of the oil as a mixture of well-defined fractions greatly simplifies the computation. Several popular characterization procedures, based on different properties of the oil, have been reported in the literature [17,18,19] since the earliest studies reported by Katz and Firoozabadi [20], who used n-paraffin boiling point temperatures to represent the crude oil composition as separate carbon number fractions. The characterization procedure proposed by Whitson [19], widely used in upstream applications, also involves the subdivision of crude oil into single carbon number fractions. These methods are continually being applied and improved [21, 22].
Equation of state (EoS) methods, while more complex, are more versatile and can be divided into two general classes. Firstly, there are semiempirical methods based on cubic EoSs originating from the van der Waals EoS, such as the Peng–Robinson [23] (PR) and Soave–Redlich–Kwong (SRK) [24] equations. Cubic EoSs are often used in petrochemical applications and in the oil industry because of their relative simplicity and reliability at predicting the phase equilibrium of nonpolar hydrocarbon systems. However, their performance has been demonstrated to be poor for modeling thermophysical HTHP properties of asymmetric hydrocarbons [25] and their mixtures [26, 27].
Therefore, there is interest in a second, newer type of EoS method—the molecular-based statistical associating fluid theory (SAFT). These SAFT-based EoSs are typically constituted as a sum of residual Helmholtz energy terms, which account for hard-sphere repulsion between molecules, dispersion interactions between molecules, the acentric nature of a molecule as conceptualized by a chain comprising m number of hard-sphere segments, and associations between molecules (such as hydrogen bonding). For each component, three pure-component parameters must be defined: the segment number m, the segment size parameter σ, and the segment–segment energy parameter ε/k. Of the SAFT-based EoSs, the perturbed-chain SAFT (PC-SAFT) EoS has been widely evaluated for its ability to model hydrocarbon mixtures. The PC-SAFT EoS [28], in conjunction with an appropriate characterization method, is a promising modeling prospect for predicting crude oil properties.
Two general types of methods of crude oil characterization using PC-SAFT exist. The first such method of crude oil characterization is the paraffins–naphthenes–aromatics (PNA) method [29], which is based on refractive index data. The method used in this work is the second type of characterization method, which was used by Punnapala and Vargas [30]. This characterization method for crude oil is based on oil composition in terms of the saturate, aromatic, resin, and asphaltene (SARA) components. This method originates from the work proposed by Ting et al. [31], used to model crude oil bubble points, as well as asphaltene onset precipitation. In order to obtain PC-SAFT parameters for the SARA components, correlations have been derived using the parameter sets published by Gross and Sadowski [26].
2 Materials and Methods
2.1 Materials
A dead crude oil sample from the Gulf of Mexico was supplied by a company that has operations in the region. Carbon disulfide (CS2, HPLC grade, CAS number 75-15-0) was obtained from Aldrich and used in the preparation of the dead crude oil samples for GC analysis. Asphaltene precipitation measurements were performed by dissolving the dead crude oil sample in n-pentane (>99%, CAS 109-66-0), obtained from Sigma-Aldrich. For the solid-phase extraction (SPE) analysis of the crude oil sample, n-pentane, toluene (HPLC grade, CAS number 108-88-3), and ethyl acetate (LC-MS grade, CAS number 141-78-6) were used. Mallinckrodt Co. and Sigma-Aldrich supplied the toluene and ethyl acetate, respectively.
2.2 Experimental Method
The high-pressure cell used in this study is based on the design of McHugh and colleagues [32], but with some alterations. Figure 1 shows a schematic of the high-pressure density cell used in this study. The body of the cell shown in Fig. 1 is constructed of a high-nickel content alloy, Inconel 625. The alteration involves the replacement of the elastomeric o-rings previously used for sealing the sapphire window to the front end of the view cell with a two-piece window holder made out of Inconel 625. As the name implies, the window holder encases the window and then mates with the front end of the view cell to provide a metal-to-metal seal with the view cell. In addition, the floating piston that also required the use of an elastomeric o-ring for sealing has been replaced with a bellows, which is also constructed from Inconel 625. The o-rings were replaced due to their potential degradation by any of the numerous components of crude oil.
The cell contents are compressed to the desired pressures by inflating the bellows (from BellowsTech) with water using a high-pressure syringe pump (Vinci Corp.) capable of generating pressures up to 40,000 psi (276 MPa). The cell pressure is measured using a Viatran pressure transducer (Viatran Corporation, model no. 245, pressure range 0–345 MPa and accurate to ±0.35 MPa) situated on the water side of the apparatus. The temperature of the cell contents is measured using a type-K thermocouple (Omega Corporation, calibrated at four temperatures from ambient to 528 K to within 0.2 K using methods traceable to NIST standards). Temperature variation for each experimental isotherm is within 0.2 K. The internal volume of the density cell is determined from positional information provided by a linear variable differential transformer (LVDT, Schaevitz Corp, Model 2000 HR). The LVDT positional information is correlated to the internal volume of the cell by calibrations against known n-decane density values obtained at 323, 423, and 523 K. The reference density values were obtained from NIST WebBook, which are available at pressures to 800 MPa and temperatures to 673 K for maximum density values to 770 kg·m−3 [33]. The estimated uncertainty of this volume calibration is within 0.7% of the calculated volume, while the accumulated uncertainty in the reported density data is estimated to be less than 1%.
Before loading the cell with the crude oil sample, air must be purged from the cell as it does not dissolve readily in hydrocarbons. Therefore, the cell is flushed three times with propane at pressures to about 0.5 MPa and then vacuumed. Following this, approximately 16 g of the crude oil sample is charged into the cell, and the cell is heated and allowed to stabilize at a desired temperature. For a particular isotherm, the cell contents are compressed to pressure points that are selected in a nonlinear fashion in order to minimize any experimental peculiarities.
3 Experimental Results and Correlations
For the dead crude oil studied, experimental data points are obtained for three isotherms and approximately two dozen pressures. If a suitable correlation is used to represent the experimental data points, then reliable reference density values can be interpolated for temperature and pressure conditions at intermediate conditions (e.g., the 373 K isotherm) for which experimental values were not recorded. Thus, the modified Tait equation [34] is fitted to experimental data measured from ambient conditions to 525 K and 270 MPa for the dead crude oil sample. Equation 1 gives the Tait equation where ρ 0 is the density at the reference pressure P 0 , which is chosen as 0.1 MPa, while B and C are adjustable parameters.
The Tait equation parameters are optimized by the minimization of the mean absolute percent deviation (δ, Eq. 2) while adjusting parameters ρ 0 , B, and C. A single value of C is used for the system, while ρ 0 and B are quadratic functions of temperature as given in Eqs. 3 and 4, respectively. Table 1 lists the parameters along with the δ and the standard deviation, λ, for the fit. The value of C is similar to what was obtained for the well-characterized hydrocarbon pure compounds and their binary mixtures previously investigated [23,24,25].
4 Modeling of Experimental Data
4.1 Volume-Translated Cubic EoS Modeling Methods for Crude Oil
Although cubic EoSs, such as the Peng–Robinson (PR) and Soave–Redlich–Kwong (SRK) equations, give accurate phase equilibrium predictions for hydrocarbon systems, they fail to give reliable density predictions. In an earlier study, promising results have been obtained with the model of Pedersen et al. [35] for the prediction of crude oil density at pressures to ~80 MPa. Pedersen et al. adapted the volume-translated SRK method of Peneloux and Rauzy [36] in order to model crude oil systems. Larsen et al. [6] used the model of Pedersen et al. [37] for predicting bottom-hole reservoir fluid density at temperatures to 400 K and pressures to 155 MPa that was within 3% of the experimental values. Therefore, in this work, we extended the capability of the model to reliably predict HTHP density of crude oils at pressures to 270 MPa and temperatures to 523 K.
The molecular weight distribution of the crude oils is obtained from the results of gas chromatography (GC) analyses. Specific single carbon number (SCN) fraction molecular weights are defined from either the GC chromatogram or in the manner put forth by Katz and Firoozabadi [20]. Predictions obtained with the model are within 3% of the experimental density data. However, as shown in Fig. 2a, a noticeable drop-off in performance is seen at pressures above ~70 MPa. Therefore, we investigated the performance of the volume-translated method of Baled et al. [38], which was specifically developed to be applicable within the full temperature and pressure range of interest—that is, to 533 K and 276 MPa. While density predictions in Fig. 2b indicate that this model yields improved density predictions at higher pressures, poorer predictions are obtained at pressures below 150 MPa. Therefore, on average, there is no significant difference between the volume translation methods of both Baled et al. [36] and Pedersen et al. [33].
4.2 PC-SAFT EoS Modeling Methods for Crude Oil
The perturbed-chain statistical associating fluid theory (PC-SAFT) EoS has been widely evaluated for its ability to describe hydrocarbon mixtures. Most previous PC-SAFT crude oil modeling studies have focused on modeling of asphaltene precipitation, but PC-SAFT has also been used to model reservoir hydrocarbon density [6]. In recent PC-SAFT modeling studies of crude oils, it has been established that dispersion force and not polarity is the major intermolecular force that dominates the phase behavior calculations [28, 39, 40]. Thus, for nonpolar molecules, only three characteristic parameters are required when using PC-SAFT: the number of segments, m; the temperature-independent segment diameter, σ; and the segment–segment dispersion interaction energy, ε/k. The parameters are obtained for pure compounds by fitting the PC-SAFT EoS to experimental vapor pressure and density data, and these parameters are reported for n-alkanes by Gross and Sadowski [26]. However, crude oils contain numerous compounds, and it is not feasible to define pure-component parameters for all the compounds. Therefore, the crude oil is divided into saturate, aromatic, and resin (SARA) pseudocomponents. The PC-SAFT EoS parameters have been demonstrated to be a smooth function of molecular weight for several chemical families [26]. Researchers have obtained the PC-SAFT parameters m, σ, and ε/k for SARA components from such correlations [28, 29, 41,42,43,44,45,46]. Several sets of correlations are available in the literature, from which we chose the correlations recently proposed by Punnapala and Vargas [28] that are listed in Table 2. Note that the γ parameter listed in this table represents the aromaticity of a SARA pseudocomponent.
This parameter, first proposed by Ting et al. [29], has been employed in a number of PC-SAFT modeling studies concerning asphaltene precipitation [29, 40, 41, 43, 44]. In this work, we define γ in the manner of Punnapala and Vargas [28], who assumed that a value of γ = 1 represents components having 100% character of a polynuclear aromatic (PNA) such as naphthalene or anthracene. Conversely, a value of γ = 0 means that the component has 100% saturate character (100% alkane behavior). We chose to use the definition of Punnapala and Vargas because it greatly facilitates the development of a predictive method for the γ values of the aromatics + resins and asphaltenes pseudocomponents.
The organic contents of crude oils are predominantly present as saturated and unsaturated hydrocarbons. Therefore, the dead oils used in this study can be characterized based on the SARA composition of the dead oil. The SARA method has been applied on several occasions to facilitate PC-SAFT modeling of asphaltene precipitation in crude oil systems [28, 39, 41,42,43,44]. The saturate fraction contains linear, branched, and cyclic hydrocarbons. Aromatics comprise any molecule that contains one or more aromatic rings. Resins, or polar aromatics that contain at least one polar heteroatom such as oxygen, comprise the fraction of the stock tank oil that is insoluble in propane yet soluble in n-pentane, n-heptane, and toluene. Asphaltenes are defined as the portion of the oil that is soluble in aromatic solvents such as toluene but insoluble in an excess of light alkane solvents such as n-pentane [28].
Asphaltenes are precipitated from the dead crude oil by mixing the oil with n-pentane on a 1 g oil to 40 mL n-pentane basis. Fractional SARA composition results for the crude oil are obtained via solid-phase extraction technique once the asphaltene components are filtered off. The saturate contents are extracted with pentane, followed by the extraction of the aromatic contents with toluene. Lastly, the resin fractions are obtained by extraction with ethyl acetate. Table 3 shows the SARA analysis results as relative mass distribution of saturates, aromatic and resin, and asphaltene fractions for the dead crude oil sample.
The molecular weight distribution of the crude oils is obtained from the results of gas chromatography (GC) analyses. Specific single carbon number (SCN) fraction molecular weights are defined from either the GC chromatogram (carbon number 6 or lower) or in the manner put forth by Katz and Firoozabadi [20].
As discussed previously, the aromaticity γ is needed for the estimation of PC-SAFT parameters for the SARA constituents of crude oil. In this work, γ is estimated by obtaining molecular hydrogen-to-carbon ratios (H/C). Elemental analyses of the dead crude oil and its associated asphaltene fraction are obtained using a Perkin Elmer Series II CHNS/O Analyzer (model 2400). The molecular H/C ratio results are presented in Table 4 along with the calculated aromaticity values, γ, for the crude oil as well as its asphaltene fraction.
The values for γ are calculated using the correlations proposed by Huang and Radosz [45]. Strictly speaking, the correlations are defined for use with hydrocarbons only containing hydrogen and carbon atoms. However, crude oils do contain a small amount of sulfur, oxygen, nitrogen, and other heteroatoms. These heteroatoms are not deemed to be present in sufficient amount to significantly alter the γ calculations and are, thus, neglected. The γ values are calculated using Eq. 5 where CN, defined in Eq. 6, is the carbon number of the hydrocarbon in question. CN p is the carbon number that a sample of that molecular weight would possess if it were 100% saturate character, and CN PNA is the carbon number that it would possess if it had 100% PNA character. CN, CN p , and CN PNA are calculated as indicated by Huang and Radosz [45].
The aromaticity of the aromatic + resin fraction is calculated by taking a weighted average over the dead oil and using the values obtained for the dead oil and the asphaltene fraction according to Eq. 7. Note that in Eq. 7, the aromaticity value for the saturate fraction does not appear as it is defined to be equal to zero as per the definition of Punnapala and Vargas [28].
4.3 PC-SAFT Density Predictions
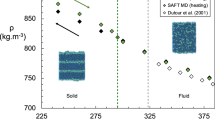
Once the characterization results are obtained, the PC-SAFT EoS can now be employed to model the crude oil density reported in this study. The PC-SAFT EoS is used with the correlations for the pure-component PC-SAFT parameters as defined in Sect. 4.2 and Table 2. A computer program, written in Visual BASIC, was used to perform all calculations. Necessary inputs to this model include the molecular weight distribution (molecular weight and mole fraction), which is resolved into a manageable number of pseudocomponents using a technique such as the one proposed by Whitson [19]. Other necessary inputs include the mass fractions of saturates, aromatics + resins, and asphaltenes pseudocomponents along with the aromaticity values for the asphaltene, γ asph , and the aromatics + resins, γ A+R , pseudocomponents. The user then specifies the temperature and pressure conditions at which experimental density predictions are desired. The PC-SAFT density modeling results for the crude oil, characterized by the mean absolute percent deviation, δ, and the standard deviation, λ, are presented in Table 5 and in Fig. 3.
As shown in Fig. 3, the PC-SAFT EoS under-predicts the density at low pressures, although the difference between calculated and experimental data decreases as the pressures increase. In fact, at high pressures, the model starts to over-predict the density data. Interestingly, for well-characterized systems, the PC-SAFT EoS typically over-predicts the density at high pressures by 5% or more [23,24,25, 30]. The deviation values obtained with PC-SAFT EoS are all within 1% and show an improvement on the performance of the volume-translated cubic EoS.
5 Conclusion
In order to meet the increasing world energy demand, the search for newer sources for petroleum has led to the necessity of pursuing and recovering petroleum from HTHP ultra-deep reservoirs that are several miles beneath the earth’s surface. In light of this fact, HTHP densities are measured at temperatures to 523 K and pressures to 270 MPa for a dead crude oil sample supplied by a company with operations in the Gulf of Mexico. This study covers a significant gap found in the literature by providing crude oil density at temperatures to 523 K and pressures to 275 MPa for the first time. Experimental data are measured with a variable-volume high-pressure view cell that is coupled to a linear variable differential transformer.
PC-SAFT EoS modeling methodology obtained from the literature is applied to model the crude oil density reported here without the use of any fitting parameters, which are typically required when using PC-SAFT to model crude oil properties. Fractional composition information for the saturate, aromatic + resin, and asphaltene fractions is obtained from the SARA analysis of the crude oil. The aromaticity parameters for both the aromatic + resin and the asphaltene fractions, γ A+R and γ ASPH , are estimated from the results of the elemental analysis of the crude oil. Using the results from these analyses, along with information from the molecular weight distribution, the PC-SAFT EoS parameters are calculated from correlations provided in the literature. PC-SAFT predictions obtained from these calculated parameter sets are then compared to the HTHP crude oil density data obtained in this study.
The PC-SAFT EoS provided better HTHP density predictions for the crude oil investigated in this study as compared to the volume-translated cubic EoS predictions. However, PC-SAFT under-predicts the density data at low pressures but over-predicts the data at high pressures, indicating that the slopes of the predicted density versus pressure curves are wrong. Therefore, predictions obtained by the model for the isothermal compressibility and other derivative properties, which depend on the derivative (dV/dP) T , are expected to be erroneous, and a more reliable model should be utilized for their predictions. Further work is required in the development and improvement of EoS models that can accurately capture the thermophysical properties of crude oil and its constituents at various conditions. One possibility is the use of correlations for the pure-component PC-SAFT parameters that are specifically designed for the one-phase, HTHP region.
References
Baird, T., Fields, T., Drummond, R., Mathison, D., Langseth, B., Martin, A., & Silipigno, L. (1998). High-pressure, high-temperature well logging, perforating and testing. Oilfield Review, 10(2), 50–67.
Avant, C., Daungkaew, S., Befera, B., Danpanich, S., Laprabang, W., De Santo, I., Heath, G., Osman, K., Khan, Z. A., Russell, J., Sims, P., Slapal, M., & Tevis, C. (2012). Testing the limits in extreme well conditions. Oilfield Review, 24(3), 4–19.
De Bruijn, G., Skeates, C., Greenaway, R., Harrison, D., Parris, M., James, S., Mueller, F., Ray, S., Riding, M., Temple, L., & Wutherich, K. (2008). High-pressure, high-temperature technologies. Oilfield Review, 20(3), 46–60.
Brelvi, S. W. (1998). Generalized correlations for the isothermal compressibility of reservoir fluids and crude oils. Saudi Aramco Journal of Technology, Spring, 30–34.
Ramage, W. E, Castanier, L. M., & Ramey, H. J. (1987). The comparative economics of thermal recovery projects. DOE/SF/11564-22, SUPRI TR-56, July 1987.
Larsen, J., Sorensen, H., Yang, T., & Pedersen, K. S. (2011). EOS and viscosity modeling for highly undersaturated Gulf of Mexico reservoir fluids. In SPE Annual Technical Conference and Exhibition, Society of Petroleum Engineers.
Petrosky, G. E., & Farshad, F. F. (1993) Pressure-volume-temperature correlations for Gulf of Mexico crude oils. In SPE 26644, Presented at the 68th Annual Technical Conference and Exhibition of the Society of Petroleum Engineers, Houston, Texas, October 3–6, 1993.
Petrosky, G. E., & Farshad, F. F. (1998). Pressure-volume-temperature correlations for Gulf of Mexico crude oils. SPEREE, 1(6), 416–420.
Elsharkawy, A. M., & Alikhan, A. A. (1997). Correlations for predicting solution gas/oil ratio, oil formation volume factor, and undersaturated oil compressibility. Journal of Petroleum Science and Engineering, 17, 291–302.
Dindoruk, B., & Christman, P. G. (2004). PVT properties and viscosity correlations for Gulf of Mexico oils. SPEREE, 7(6), 427–437.
Farshad, F., LeBlanc, J. L., Garber, J. D., & Osorio, J. G. (1996). Empirical PVT correlations for colombian crude oils. In SPE 36105, Presented at the 4th Latin American and Caribbean Petroleum Engineering Conference, Port-of-Spain, Trinidad and Tobago April 23–26, 1996.
Hanafy, H. H., Macary, S. M., ElNady, Y. M., Bayomi, A. A., & El Batanony, M. H. (1997). A new approach for predicting the crude oil properties. In SPE 37439, Presented at the 1997 SPE Production Operations Symposium, Oklahoma City, Oklahoma, March 9–11, 1997.
Almehaideb, R. A. (1997). Improved PVT correlations for UAE crude oils. In SPE 37691, Presented at the 1997 Middle East Oil Conference and Exhibition held in Manama, Bahrain, March 17–20, 1997.
Al-Marhoun, M. A. A New correlation for undersaturated isothermal oil compressibility. SPE 81432-SUM.
Vazquez, M., & Beggs, H. D. (1980). Correlations for fluid physical property prediction. Journal of Petroleum Technology, 32(6), 968–970.
Brelvi, S. W., & O’Connell, J. P. (1972). Corresponding states correlations for liquid compressibility and partial molal volumes of gases at infinite dilution in liquids. AIChE Journal, 18(6), 1239–1243.
Leelavanichkul, P., Deo, M. D., & Hanson, F. V. (2004). Crude oil characterization and regular solution approach to thermodynamic modeling of solid precipitation at low pressure. Petroleum Science and Technology, 22, 973–990.
Riazi, M. R. (1997). A continuous model for C7+ fraction characterization of petroleum fluids. Industrial & Engineering Chemistry Research, 36, 4299–4307.
Whitson, C. (1983). Characterizing hydrocarbon plus fractions. SPE J., 23, 683–694.
Katz, D. L., & Firoozabadi, A. (1978). Predicting phase behavior of condensate/crude oil systems using methane interaction coefficients. Journal of Petroleum Technology, 30, 1649–1655.
AlHammadi, A. A., Vargas, F. M., & Chapman, W. G. (2015). Comparison of cubic-plus-association and perturbed-chain statistical associating fluid theory methods for modeling asphaltene phase behavior and pressure-volume-temperature properties. Energy & Fuels, 29, 2864–2875.
He, P., & Ghoniem, A. F. (2015). A group contribution pseudocomponent method for phase equilibrium modeling of mixtures of petroleum fluids and a solvent. Industrial & Engineering Chemistry Research, 54, 8809–8820.
Peng, D.-Y., & Robinson, D. B. (1976). A new two-constant equation of state. Industrial & Engineering Chemistry Fundamentals, 15, 59–64.
Soave, G. (1972). Equilibrium constants from a modified Redlich-Kwong equation of state. Chemical Engineering Science, 27, 1197–1203.
Wu, Y., Bamgbade, B., Liu, K., McHugh, M. A., Baled, H., Enick, R. M., Burgess, W. A., Tapriyal, D., & Morreale, B. D. (2011). Experimental measurements and equation of state modeling of liquid densities for long-chain n-Alkanes at pressures to 265 MPa and temperatures to 523 K. Fluid Phase Equilibria, 311, 17–24.
Bamgbade, B. A., Wu, Y., Burgess, W. A., Tapriyal, D., Gamwo, I. K., Baled, H. O., Enick, R. M., & McHugh, M. A. (2015). Measurements and modeling of high-temperature, high-pressure density for binary mixtures of propane with n-Decane and propane with n-Eicosane. The Journal of Chemical Thermodynamics, 84, 108–117.
Bamgbade, B. A., Wu, Y., Burgess, W. A., Tapriyal, D., Gamwo, I. K., Baled, H. O., Enick, R. M., & McHugh, M. A. (2015). High-temperature, high-pressure volumetric properties of propane, squalane and their mixtures: Measurement and PC-SAFT modeling. Industrial & Engineering Chemistry Research, 54, 6804–6811.
Gross, J., & Sadowski, G. (2001). Perturbed-chain SAFT: An equation of state based on a perturbation theory for chain molecules. Industrial & Engineering Chemistry Research, 40, 1244–1260.
Pedersen, K. S., & Sorensen, C. H. (2007). PC-SAFT equation of state applied to petroleum reservoir fluids. SPE 110483.
Punnapala, S., & Vargas, F. M. (2013). Revisiting the PC-SAFT characterization procedure for an improved asphaltene precipitation prediction. Fuel, 108, 417–429.
Ting, P. D., Hirasaki, G. J., & Chapman, W. G. (2003). Modeling of asphaltene phase behavior with the SAFT equation of state. Petroleum Science and Technology, 21, 647–661.
Liu, K., Wu, Y., McHugh, M. A., Baled, H., Enick, R. M., & Morreale, B. D. (2010). Equation of state modeling of high-pressure, high-temperature hydrocarbon density data. The Journal of Supercritical Fluids, 55, 701–711.
Linstrom, P. J., & Mallard, W. G. (Eds.). (2005). NIST Chemistry WebBook, NIST Standard Reference Database Number 69, June 2005, National Institute of Standards and Technology, Gaithersburg MD, 20899. http://webbook.nist.gov/chemistry/fluid/.
Dymond, J., & Malhotra, R. (1988). International Journal of Thermophysics, 9, 941–951.
Pedersen, K. S., Milter, J., & Sorensen, H. (2004). Cubic equations of state applied to HT/HP and highly aromatic fluids. SPE J, 9, 186–192.
Peneloux, A., & Rauzy, E. (1982). A consistent correction for Redlich-Kwong-Soave volumes. Fluid phase equilibria, 8, 7–23.
Pedersen, K. S., Milter, J., & Sorensen, H. (2004). Cubic equations of state applied to HT/HP and highly aromatic fluids. SPE J, 9, 186–192.
Baled, H., Enick, R. M., Wu, Y., McHugh, M. A., Burgess, W., Tapriyal, D., & Morreale, B. D. (2012). Prediction of hydrocarbon densities using volume-translated SRK and PR equations of state fit to high temperature, high pressure PVT data. Fluid Phase Equilibria, 317, 65–76.
Buckley, J. S. (1999). Asphaltene precipitation and solvent properties of crude oils. Energy & Fuels, 13, 328–332.
Buckley, J. S, Hirasaki, G. J., Liu, Y., Von Drasek, S., Wang, J. X., & Gil, B. S. (1998). Asphaltene precipitation and solvent properties of crude oils. Petroleum Science and Technology, 16, 251–285.
Panuganti, S. R., Vargas, F. M., Gonzalez, D. L., Kurup, A. S., & Chapman, W. G. (2012). PC-SAFT characterization of crude oils and modeling of asphaltene phase behavior. Fuel, 93, 658–669.
Gonzalez, D. L., Ting, P. D., Hirasaki, G. J., & Chapman, W. G. (2005). Prediction of asphaltene instability under gas injection with PC-SAFT equation of state. Energy & Fuels, 19, 1230–1234.
Gonzalez, D. L., Hirasaki, G. J., Creek, J., & Chapman, W. G. (2007). Modeling of asphaltene precipitation due to changes in composition using the perturbed chain statistical associating fluid theory equation of state. Energy & Fuels, 21, 1231–1242.
Vargas, F. M., Gonzalez, D. L., Hirasaki, G. J., & Chapman, W. G. (2009). Modeling asphaltene phase behavior in crude oil systems using the perturbed chain form of the statistical associating fluid theory (PC-SAFT) equation of state. Energy & Fuels, 23, 1140–1146.
Gonzalez, D. L., Vargas, F. M., Hirasaki, G. J., & Chapman, W. G. (2008). Modeling study of CO2-induced asphaltene precipitation. Energy & Fuels, 22, 757–762.
Gonzalez, D. L. (2008). Modeling of asphaltene precipitation and deposition tendency using the PC-SAFT equation of state. Doctoral dissertation. Retrieved from ProQuest Dissertations and Theses. (Accession Order No. 3309879).
Huang, S., & Radosz, M. (1991). Phase behavior of reservoir fluids V: SAFT model of CO, and bitumen systems. Fluid Phase Equilibria, 70, 33–54.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Gamwo, I.K., Bamgbade, B.A., Burgess, W.A. (2018). Experimental Investigation and Molecular-Based Modeling of Crude Oil Density at Pressures to 270 MPa and Temperatures to 524 K. In: Khan, M., Chowdhury, A., Hassan, N. (eds) Application of Thermo-fluid Processes in Energy Systems. Green Energy and Technology. Springer, Singapore. https://doi.org/10.1007/978-981-10-0697-5_6
Download citation
DOI: https://doi.org/10.1007/978-981-10-0697-5_6
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-10-0695-1
Online ISBN: 978-981-10-0697-5
eBook Packages: EnergyEnergy (R0)