Abstract
The visual ecology of cichlids has contributed greatly to our understanding of mechanisms driving spectacular, colorful cichlid radiations. Interactions between the underwater light environment, the transmission of visual signals, and the visual sensitivity of the signal receiver are integral to the processes driving this diversity. Researchers recognized the importance of vision early in the study of African cichlids, citing the diversity of habitats in which cichlids are found and brilliant male nuptial coloration as potential forces shaping visual differentiation. Later work focused more on visual systems, adapted to the local light environment, as drivers of color pattern diversification. Most recently, researchers have focused on the evolution of visual systems under both ecological and sexual selection and the mechanisms of spectral tuning by investigating opsin gene expression and co-expression across the cichlid phylogeny. In this chapter, I describe the historical context of cichlid vision research, the diversity in cichlid visual ecology, and the current state of our understanding of cichlid visual ecology. Additionally, I discuss the possible consequences of human-induced changes to the underwater visual environment for cichlid diversity and suggest avenues for future research.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Visual Ecology
Why is vision so important for cichlids? A dive into Lake Malawi, pulling a gill net on Lake Victoria, or a visit to your local aquarium will likely give you a clue given the extreme variation apparent in cichlid color and patterns (Fig. 1). Early research on cichlid vision focused on brilliant male nuptial coloration used in species recognition and mate choice decisions as a driver of visual diversity; more recently, focus has shifted to the evolution of visual systems under both ecological and sexual selection as integral drivers of adaptive cichlid evolution. Advances in our understanding of cichlid visual systems and the technology used to assess visual abilities have led to new hypotheses about the proximate mechanisms promoting the evolution of visual systems and discoveries of how spectral tuning functions via, for example, through co-expression and plasticity of opsin gene expression. It is crucial, therefore, to elucidate the complexity of visual processes in order to develop a framework for assessing the evolutionary ecology of cichlid vision. In this review, I lay out the foundations of a framework based on the basic biology of cichlid vision and the historical context in which current studies are based. Within this integrated framework, including visual systems, photic environment, and colorful signals (Fig. 2), recent research has focused on the regulation of opsin gene expression and the influence of spectral tuning of photopigments on the evolution of cichlid visual ecology. A number of excellent reviews have covered various aspects of the evolution of vertebrate vision (e.g., Bowmaker and Hunt 2006), photopigment evolution and speciation (Bowmaker 1995; Yokoyama 2000, 2002, 2008; Carleton 2014), the link between visual systems and colorful signals (e.g., Osorio and Vorobyev 2008), and more specifically spectral tuning and diversification in cichlids (Carleton 2009; Hofmann and Carleton 2009; Carleton et al. 2016). Therefore, I aim to give a broad overview of the current state of knowledge of cichlid visual ecology and highlight some of the most recent research contributing to our understanding of the evolutionary ecology of cichlid vision rather than provide an extensive review of the field. I also discuss the potential implications of human-altered visual environments and suggest directions for future research.
Simplified diagram of important components involved in cichlid visual ecology. In this example, (a) a female cichlid Pseudocrenilabrus multicolor (Seegers) on the left is viewing a larger and more colorful male P. multicolor on the right (photos by S.M.G.). (b) Visual sensitivity represented by absorbance spectra for three cone opsins typical for Lake Victoria haplochromines; (c) Irradiance spectrum of the transmission medium through which the visual signal is viewed; (d) Reflectance spectra from a yellow color pattern (yellow curve) component of the signaler and of the background plant (green curve) against which the signaler is viewed
2 Cichlid Vision
2.1 The Cichlid Eye
Vertebrate vision is highly conserved, and cichlid vision follows this plan. Cichlids, following the typical teleost eye plan, have a cup-shaped eye placed laterally and slightly forward on the head, a non-dilatory pupil, a spherical lens, retina, and pigment epithelium (Fig. 3a). Detailed descriptions of teleost eyes and vision can be found in various sources, including Lythgoe (1980), Fernald (1990), and Cronin et al. (2014). Here I give a brief description of cichlid visual structures to help contextualize this review. As in the typical vertebrate eye, light travels through the pupil and into the crystalline lens, which collects light, forms an image, and directs it onto the retina at the back of the eye. Two small muscles allow for slight axial adjustment of the lens position in relation to the retina to aid in focusing the image (i.e., accommodation). The gross morphology of the eye (e.g., eye size given by axial length, or the distance from the front to the back of the eye) helps to determine the visual abilities of an individual. In a simplified scenario, eyes with a longer axial length tend to have a longer focal length and thus project a larger image on the retina, providing more visual information to the brain (Howland et al. 2004). Fernald (1990) found that as Burton’s mouthbrooder, Astatotilapia burtoni , grows, visual abilities are not compromised despite considerable enlargement of the eye over time.
Diagram of a typical (a) cichlid eye, (b) retina, and (c) mosaic arrangement of cone photoreceptors in the retina. In this mosaic example, three cone classes are represented by colors with a single short-wavelength sensitive SWS cone (e.g., SWS2A) surrounded by four pairs of long-wavelength sensitive LWS cones (e.g., Rh2A, LWS)
The retina is composed of, from point of light entry to the back of the eye: the ganglion cell layer, inner plexiform layer, inner nuclear layer, outer nuclear layer, visual photoreceptors, and the pigment epithelium. The actual collection of photons and activation of visual processing occurs at the level of the visual photoreceptor, composed of an inner and outer segment. The inner segment contains the nucleus and other cellular functioning components (Fig. 3b) and sometimes oil droplets or carotenoid-derived pigments that can filter light of certain wavelengths (Bowmaker et al. 1997; Cronin et al. 2014; see Sect. 4.2 Spectral Tuning). The end of the inner segment forms a synapse that connects with other photoreceptor cells and neuronal cells, the axons of which are collected in the ganglion cell layer and leave the eye via the optic nerve.
The outer segment of the visual photoreceptor receives light travelling through the other components of the retina and is where the visual photopigments are found. Photopigments are composed of two components: an opsin protein, which is a membrane-bound G-protein coupling receptor, bound to an inactive form of a photosensitive vitamin A-based chromophore (Wald 1935). Rhodopsin and porphyropsin are the two types of visual pigments, respectively, found in marine and freshwater fishes (Toyama et al. 2008). They are distinguished by the retinal structure of the chromophores. A1 chromophores are composed of 11-cis-retinal whereas A2 chromophores are composed of 3,4-didehydroretinal. Most freshwater fishes, including most cichlids (Carleton et al. 2008), have predominantly A1 chromophores; however, many species exhibit a mix of A1 and A2 chromophores and the ratio of the two types can change throughout a fish’s life. For example, the A1/A2 ratio in Nile tilapia (Oreochromis niloticus ) varies between individuals and throughout development from the larval to adult stages (Carleton et al. 2008). A shift from A1- to A2-based chromophores alters the sensitivity of the photopigment toward longer wavelengths and broadens the bandwidth of spectral absorbance.
Detection of light is facilitated by isomerization of the chromophore (e.g., 11-cis retinal to all-trans retinal) upon absorption of a single photon, changing the molecular structure of the chromophore, thus activating the opsin and triggering an enzyme cascade that makes perception of light possible. Opsin proteins are variably sensitive to different wavelengths of light and can be characterized by the wavelength of maximal or peak absorbance (λmax). In most cases, one opsin class is expressed in a single photoreceptor. However, there is emerging evidence of two opsins being expressed simultaneously within a photoreceptor in some cichlids (i.e., co-expression and retinal specialization) resulting in shifts in spectral sensitivity (e.g., Dalton et al. 2014, 2016). Additionally, small changes in the opsin protein sequence can lead to changes in the λmax of a photoreceptor, which I discuss in detail in Sect. 3.3.
Vertebrates have two types of photoreceptor cells, each serving different functions. Rods, as the name suggests, are typically long and cylindrical (Fig. 3b) and function as highly sensitive receptors in low or dim light conditions (i.e., scotopic vision). Cones are typically shorter and cone-shaped (Fig. 3b) and are used in bright light conditions to facilitate visual acuity and color vision (i.e., photopic vision). One of the notable differences between rods and cones is the placement of the visual photopigments. In rods, photopigments are found in disc-like layers within the cell membrane; thus maximizing sensitivity by providing more surface area for more absorption of light by photopigments. Note that a single photon activates a single opsin thus more pigments allow for better detection of low light. However, this comes at the cost of reduced speed of photon detection (Cronin et al. 2014). In cone photoreceptor cells, the photopigments are embedded directly in the plasma membrane, allowing for faster signaling at the expense of sensitivity. The size of cones, rather than the size of the eye, determines the amount of light captured (Cronin et al. 2014).
Color vision is made possible by the presence of multiple cone photoreceptor classes with opsins tuned to different wavelengths of maximal absorbance, allowing a comparison between different colors. Thus, a minimum of two photoreceptors that absorb light maximally at different wavelengths are required for color vision with the appropriate neuronal connections (see Wandell 1995). Vertebrates possess four classes of opsins spanning the visible light spectrum: short-wavelength sensitive (SWS1; UV-Violet), SWS2 (Blue), Rhodopsin-like (RH2; green), and long-wavelength sensitive (LWS; yellow-red). African cichlids have an additional three opsin genes, a result of gene duplication leading to spectrally distinct cone classes (Hofman and Carleton 2009; see Table 1).
Cones are typically arranged within the retina in a specific mosaic pattern (Fig. 3c). Fernald (1981) described the retinal cone mosaic for Burton’s mouthbrooder as having a square pattern of four pairs of double cones with a central single cone, which has since been deemed the typical cichlid mosaic (Carleton and Kocher 2001). In cichlids, the central single cone is typically small and sensitive to short wavelengths. The surrounding four pairs of double cones are sensitive to middle to longer wavelengths of light, i.e., RH2 and LWS cone classes, and cones within a pair can either be identical or one each of RH2 and LWS (Fernald 1981; van der Meer et al. 1995; Carleton and Kocher 2001). Variation in the expression of cone pigments with different λmax and alterations in the protein coding sequence of opsin genes help to fine-tune the sensitivity of cone photoreceptors to match the background photic environment. Extensive research, especially over the past 40 years, has defined cichlids as a model for understanding the evolution of vertebrate visual systems and its role in adaptive radiation.
2.2 Groundbreaking Studies of Cichlid Vision
The extreme color variation observed among closely related cichlid species, combined with the wide variety of ecological niches they occupy, intrigued early visual scientists and prompted examination of the visual abilities of this diverse lineage of fishes (Eigenmann and Shafer 1900). With the advancement over time of techniques for quantifying variation in visual abilities, we have greatly increased our understanding not just of the visual processes involved in vision but also the underlying role of vision in adaptive divergence and speciation.
The late nineteenth and early twentieth centuries saw an upsurge in research pertaining to the morphology of the teleost retina (see Eigenmann and Shafer 1900). Morphological determination of visual acuity which is the ability of an animal to detect an object (e.g., food, predator, or mate), can be assessed by measuring gross sizes of the pupil, lens, and axial and eye diameters (Howland et al. 2004) as well as by investigating the detailed structure of the retina (Fernald 1981; van der Meer and Anker 1984; van der Meer et al. 1995). Vision depends upon the resolution of images, as determined by the density of photoreceptors, and sensitivity to different wavelengths and intensities of light as determined by the size of photoreceptors. Relatively larger eyes can accommodate either a larger number or larger sized photoreceptors; however, an increase in either variable might result in a trade-off between sensitivity and resolution (van der Meer and Anker 1984).
Early cichlid work also focused on understanding retinal morphology and the extent of color vision in cichlids. For example, Engström (1963), has reported on the lack of information for cichlids in the literature at that time, examined one specimen each of two Neotropical cichlid species, Apistogramma ramirezii , and the Convict cichlid (Archocentrus nigrofasciatus ) from the aquarium trade and gave brief descriptions of the cone mosaics. In both species, the cone mosaics were square with a central single cone surrounded by four pairs of double cones (Fig. 3c). The first report on the spectral sensitivity of retinal photopigments for Cichlasoma meeki and Aequidens portalegrensis followed shortly (Schwanzara 1967). At that time, a retinal pigment extraction process combined with bleaching of the extract and using various wavelengths of light showed that the λmax of the paired opsins was 500/522 nm. Fernald’s work in the 1980s, using Burton’s mouthbrooder as a model species (e.g., Fernald and Liebman 1980; Fernald 1984) helped to bridge the gap between reporting on retinal morphology and a better understanding of the links between visual pigment sensitivity and behavior. They reported a single, central cone in the retinal mosaic with λmax ~ 455 nm and mixed double cones having λmax ~ 523/562 nm in this African species. Fernald and Liebman (1980) discuss these findings in the context of the body color and patterns used in intraspecific signaling and the ambient photic environment, suggesting that the maximal absorbance of the three cone classes described likely allow for the detection of conspecifics under natural conditions.
Behavioral proxies have also been used to assess cichlid visual abilities. Visual acuity, for example, can be estimated behaviorally (Browman et al. 1990; Wanzenböck et al. 1996) by assessing the distance from an object that the fish first reacts to the object (i.e., reaction distance) using a standard reaction distance protocol (e.g., Vogel and Beauchamp 1999). Visual sensitivity, the ability to detect an object under different light intensities and colors, can also be determined using behavioral experiments. For example, the optomotor response test has been successfully used in a number of studies on fishes to test for sensitivity or detection thresholds to varying light conditions (e.g., Kröger et al. 2003; Boughman 2001; Neumeyer 2003; Maan et al. 2006). Due to an innate optokinetic response (visually induced eye movements) or optomotor response (visually induced head or body movements), fish will follow a rotating grate, with their eyes or by swimming, as long as they can distinguish the light and dark bars of the grate, but will stop swimming when a visual sensitivity threshold is reached. As the light intensity is incrementally decreased, the detection threshold is assessed as the light level one step above when the fish ceases to follow the rotating screen. The optomotor response method was used by Maan et al. (2006) to investigate detection thresholds for two sympatric and closely related Lake Victoria cichlids, Pundamilia pundamilia and P. nyererei . While the two species are morphologically and ecologically very similar, males have divergent nuptial coloration: P. pundamilia males are blue and P. nyererei males are red (Seehausen et al. 1999; Maan et al. 2004, 2006), and are respectively found in shallow and deep areas surrounding islands. Due to the relatively turbid conditions of Lake Victoria, strong light gradients can exist with shorter-wavelength (UV-Blue) light attenuating faster than longer-wavelength light (Seehausen et al. 2008). Thus, each of the two species typically occupies different photic microhabitats, with P. pundamilia in shallower waters dominated by short-wavelength light and P. nyererei in red-shifted deeper waters. The optomotor tests revealed that under decreasing intensity of blue light, female P. pundamilia performed better than P. nyererei , while the opposite pattern was found when females were tested under red light, demonstrating an association between female visual sensitivity, male color pattern, and the photic environment.
The early method of measuring the absorbance of pigments in solution to describe the sensitivity of photopigments has been supplanted by microspectrophotometry (MSP). MSP, described by Liebman (1972), is a technique that targets the opsin-containing outer segment of individual photoreceptors by focusing a narrow beam of light, typically one nanometer wide, through the outer segment. The absorbance of light through the cell is measured as a motorized monochronometer changes the wavelength of the light beam from 300 to 750 nm typically at 1 nm intervals, thus producing a spectral absorbance curve. The peak of the curve represents the wavelength of maximal absorbance, λmax, for an individual photoreceptor cell. MSP results were first reported for cichlids by Levine and McNichol (1979) and Levine et al. (1979), and it is this method that Fernald and Liebman (1980) used to detect the spectral sensitivity of cones in Burton’s mouthbrooder. MSP was also used to compare photopigment sensitivity across African and Neotropical species (Levine et al. 1980). MSP, coupled with access to technology for more easily measuring solar irradiance under water, has allowed researchers to develop models for understanding the perceptual abilities of cichlids under different environmental conditions (see Sect. 3.2).
Recent advances in genetic analysis of opsin gene expression have provided greater insight into the evolution of cichlid vision. Researchers can reconstitute visual pigments using quantitative reverse-transcription polymerase chain reaction (qRT-PCR) and measure maximal absorbance (similar to MSP generating λmax) as well as the concentration of pigment types (e.g., Parry et al. 2005; Carleton et al. 2008; Carleton 2009). Whereas MSP is useful for determining if an animal is sensitive to particular wavelengths of light by assessing λmax of individual photoreceptor cells, it does not rule out the presence of rare photopigments and does not always provide a quantitative measure of the abundance of each type of opsin pigment present. Parry et al. (2005) demonstrated a good match between MSP and qRT-PCR maximum absorbance in several Lake Malawi cichlids. Within this framework, much recent research has focused on the regulation of opsin gene expression/coexpression and the influence of spectral tuning of retinal photopigments on cichlid vision and evolution, as described below (see Sect. 4.2).
3 Visual Ecology
Sensory ecology describes the study of how animals collect, interpret, and respond to information from their environment and thus necessarily requires an understanding of sensory abilities, transmission media, signal content (Endler 1992, 1993; Cronin et al. 2014), and the variation inherent in each of these components across time, space, and level of biological organization (Dangles et al. 2009; Ord et al. 2010). The visual environment is the medium through which signals are transmitted and the background (or “visual scene”) against which visual signals are viewed. Visual ecology, therefore, encompasses the integral link between the visual environment, the spectral properties of the visual signal, and the visual abilities of the signal receiver (Fig. 2). Thus, any treatment of visual ecology should incorporate these components, in addition to factors that might alter them, such as behavioral displays that can highlight certain components of a signal or environmental perturbation that might mask signals. In this section I discuss each of these components in turn.
3.1 Visual Environment: Heterogeneity and Water Clarity
The underwater visual environment is complex, more so than terrestrial environments (Lythgoe 1979; Levine et al. 1980). Cichlids are found across photic extremes, including the clear, broad-spectrum waters of Lake Malawi, the turbid waters of the white Amazon, and the tannin-stained, red-shifted waters of swamps and rivers high in dissolved organic matter. While the large-scale photic differences in bodies of water such as those found among the African rift lakes have exerted an influence on the evolution of cichlids (Hofmann et al. 2009), heterogeneity in the visual environment is also found at smaller scales within those systems. An excellent example of this are the sand and rock habitats of Lake Malawi (Fig. 4). Each of these environments provides light at different intensities and with different spectral properties (e.g., Sabbah et al. 2011) that are expected to favor different visual systems.
The photic environment influences both the ability of animals to detect objects, such as prey, predators, potential mates, and competitors, and the transmission of visual signals. Here I describe the important components of light that affect visual signals and their detection. First, light penetrating the water’s surface is absorbed and scattered such that it attenuates steeply with depth and attenuation is wavelength dependent (Levine and MacNichol 1982). In clear water, the extreme ends of the spectrum (i.e., UV and red) are attenuated fastest which is why blue or mid-wavelength light dominates deep water, giving clear open water the appearance of being blue. Alternatively, water containing high concentrations of dissolved organic material or suspended particulates (i.e., turbid water) will selectively absorb and scatter short-wavelength light, shifting the underwater visual environment to longer wavelengths, giving it a yellowish or reddish appearance. Second, spatial and temporal variations in the visual environment (at many scales, from the flicker imposed by surface waves to diurnal and seasonal shifts) will influence the perception of a signal by a receiver (Lythgoe 1979). For example, light entering the water at dusk and dawn contains substantially more long-wavelength light compared to the solar zenith at noon when the sun is overhead, providing intense, broad-spectrum light. Other spatial scales of photic variation include depth, substrate, and slope of the substrate (e.g., from shore). We also expect the complexity of the habitat (e.g., rock vs. sand vs. vegetation) to influence the photic environment by creating spectrally divergent and potentially complex viewing backgrounds (e.g., Endler 1992; Shumway et al. 2007). For example, Fig. 4 shows the obvious differences in habitat complexity between adjacent rock and sand patches in Lake Malawi. Therefore, in the context of the evolution of cichlids, and cichlid vision in particular, it is essential to know something about the photic environment.
Cichlids are found across a great number and complexity of underwater visual habitats. It is thought that cichlid ancestors inhabited rivers and floodplains, thus likely experiencing medium to long-wavelength shifted and turbid photic environments resulting from high dissolved organic material and sediment flux (Bowmaker 1995). Under such visual conditions, red nuptial coloration, as displayed by Nile tilapia (considered the phylogenetic outgroup to modern cichlids; Takahashi and Sota 2016) would be particularly conspicuous if the visual system was also medium- to long-wavelength shifted. On a broad scale, many extant African cichlids are found in historically relatively clear lakes, whereas Neotropical species are often found in more turbid and or tannin-stained waters (Sioli 1984; Duncan and Fernandes 2010; Cooke et al. 2012). Across the East African Great Lakes, Lakes Malawi and Tanganyika are relatively clearer than the shallower and more turbid waters of Lake Victoria. The differences in photic environment may have influenced contemporary visual sensitivity profiles in each clade such that in the broader spectrum waters of Lake Malawi and Tanganyika there is more diversity in opsin expression and hence visual systems, including sensitivity to short-wavelength light (Carleton et al. 2000; Hofmann et al. 2009). Within the East African Great Lakes, major habitats linked with the independent evolution of two lineages include rocky outcrops and sandy bottoms (Kocher 2004). The visual environment significantly differs between these two habitats (Fig. 4): sand habitats are less complex, almost featureless zones, while rocky outcrops are relatively more complex with three-dimensional structure and associated crevices and shadows (Shumway et al. 2007; Dalton et al. 2010; Sabbah et al. 2010). Clear, broad-spectrum environments may favor UV sensitivity, especially in planktivores because the space light (i.e., down-welling, side-welling, and up-welling light) in this environment is rich in short-wavelength light (Lythgoe 1979; Hofmann et al. 2009). A fish that is sensitive to short-wavelengths can take advantage of this because of increased contrast and hence detectability of zooplankton and algae against a predominantly short-wavelength background (Utne-Palm 2002; Hofmann et al. 2009). Hofmann et al. (2009) show an association between cichlids with UV sensitivity and planktivory. Similarly, attenuation of light with depth, modified by level of turbidity or suspended particulates, has played a role in visual divergence between cichlids and speciation (Seehausen et al. 2008).
Water clarity, or the amount of suspended and dissolved solids in the water column, significantly influences the availability and color of light underwater (Lythgoe 1979; Seehausen et al. 2003). Consider your ability to detect a friend standing on the beach on a clear, sunny day versus a very foggy day—the veiling light resulting from scattering and absorption by the water droplets constituting fog obscure your friend, making them more difficult to detect and resolve. Suspended particulates differentially scatter and absorb light of different wavelengths underwater. In turbid water, high in suspended particulates, short-wavelength, higher energy photons are scattered and absorbed more than longer-wavelength photons such that with depth, the water becomes dominated by longer wavelength (yellow-red) light (Levine and MacNichol 1982). Thus, the photic environment can vary drastically across gradients of depth and water clarity, and subsequently alter the strength and shape of selection on visual sensitivity and colorful signals. Work by Seehausen et al. (2008 ibid) assessed water clarity and the spectral composition of underwater light along transects in the Mwanza Gulf of Lake Victoria, with the aim of correlating the diversity of male color morphs and distinct species with water clarity and color (see Sect. 4.1 for details of that study). As water became more turbid further into the Gulf, the intensity of light decreased and the spectral content shifted to be dominated by long-wavelength (orange) light, subsequently altering the selective pressures on cichlid vision.
Characterization of the underwater photic environment at different spatial and temporal scales is essential if we are to fully integrate our understanding of mechanisms of driving diversification in cichlid visual systems and color patterns. However, such characterizations are logistically difficult and only a few studies have attempted thorough examinations (e.g., Seehausen et al. 2008; Hofmann et al. 2009; Sabbah et al. 2011; Maan et al. 2010). The work by Seehausen et al. (2008) as described above is one such study that explored large-scale variation in photic environment directly associated with cichlid visual sensitivity and male color patterns. Another broad-scale study that provides a general assessment of the photic environment is the work by Hofmann et al. (2009) in which large differences in the photic environment between clear, deep Lake Malawi and shallow, turbid Lake Victoria are studied in the context of cichlid vision. A more detailed and localized physical characterization of the light environment was performed by Sabbah et al. (2011). They measured the spectral irradiance, background radiance and the beam attenuation coefficient (i.e., the contrast of an object against the background) along depth and slope transects in one rocky and one sandy habitat near Cape Maclear, Lake Malawi. These three variables, in addition to the spectral reflectance of an object, are required to understand how detectable that object might be in a given underwater environment (i.e., radiance contrast). Sabbah et al. (2011) detected some variations in irradiance between sand and rock habitats: longer-shifted light was found at depth in the sand compared to the rocky site. Although no difference in radiance was detected, radiance contrast calculations suggested that at depth in the sandy site objects reflecting longer wavelength light would be more detectable and shorter-wavelength signals at depth in the rocky habitat, which could have implications for detection of visual signals in those locations. One of the challenges of these types of rigorous studies is ensuring that the measurements are taken at ecologically relevant locations (e.g., where fish are present) and times (e.g., whether fish are foraging and/or mating or avoiding predators). The depths at which major differences were detected by Sabbah et al. (2011) are not necessarily where cichlids are mating, so the influence on male nuptial coloration may not be large, but maybe important for other ecological functions.
The Neotropical cichlid lineage is not as large or diverse as the African cichlids (Genner et al. 2007; López-Fernández et al. 2010, 2013); however, they are also found in diverse and complex photic environments, especially among the tributaries of the Amazon. Three broad visual environments where riverine Neotropical cichlids are found are described as clear, black, and white, each providing substantially different photic environments (Sioli 1984; Duncan and Fernandes 2010; Cooke et al. 2012). Blackwaters are typically found in organic-rich swamps or rivers and may be very clear but the tannins (dissolved organic matter) absorb short-wavelength light, thereby decreasing the intensity of light underwater and shifting the spectral composition to increasingly long-wavelength light. White water is extremely turbid with suspended sediments, which also absorb and scatter light, and depending on the size, structure, and composition of sediments, will appear muddy yellow-brown to cloudy white (Lythgoe 1979). Nicaragua’s large lakes (Lakes Managua and Nicaragua) and crater lakes also harbor cichlid species complexes with parallel patterns of color diversification (Elmer et al. 2009, 2010); however, the large lakes are shallow, leading to resuspension of sediments via wind action and eutrophication, making the water very turbid, while the crater lakes are deep and clear. Characterization of the photic environment in the Neotropics has not been as rigorous as in African waters. Nor have the relationships between the photic environment, body coloration, and visual sensitivity been explored as thoroughly as in African waters to date (but see Härer et al. 2017; Hauser et al. 2017; Torres-Dowdall et al. 2017; see Torres-Dowdall and Meyer 2021).
3.2 Visual Signals
The visual signals of cichlids, males in particular, are what literally caught the eye of evolutionary ecologists and spurred the field to use cichlids as a model for understanding adaptive radiation. Visual signals are generated by reflection of light from an object through a medium. Signals directed toward a receiver are considered communication traits and are most effective when they provide contrast for efficient detection; whereas some patterns are meant to prevent detection by masking or camouflaging the object. Highly contrasting signals are considered conspicuous. Conspicuousness can be achieved either by contrasting with the background against which the object is viewed, or by contrasting elements within the object. For example, the dark vertical bars typical of many cichlids are viewed against a lighter background (e.g., Jordan et al. 2004), or the highly saturated and long-wavelength yellow-red egg spots of haplochromine cichlids are often circled by white rings thus increasing the contrast of the signal.
The visual signals of cichlids cover the entire visible spectrum, and often extend into the ultraviolet. Fish color patterns can be expressed in a number of ways: through pigment-containing chromatophores in fish skin or through structural components that refract and reflect light depending on the viewing angle (Grether et al. 2004; Leclercq et al. 2010). Chromatophores containing different pigments often overlay each other, and the pigment granules can be aggregated or dispersed to affect changes in color pattern. Some cichlids are known for their ability to change color rapidly (e.g., in seconds to minutes) in response to social or external cues (e.g., Muske and Fernald 1987; Nelissen 1991; Jordan et al. 2004). Jordan et al. (2004) showed that males of three Lake Malawi mbuna (rock-dwelling) cichlids, Metriaclima zebra , M. benetos , and M. barlowi , reflect strongly in the UV end of the spectrum, and will alter the intensity of their UV signal depending on the social context (e.g., presence of a conspecific female). Males in these species can alter their color pattern within minutes, suggesting a physiological mechanism of color change, such as expansion or contraction of pigments within the chromatophores of the skin (Grether et al. 2004). In a study investigating neuronal control of expression of the facial “eyebar” in Burton’s mouthbrooder, Muske and Fernald (1987) demonstrated that melanin pigments can be aggregated in a matter of seconds upon electrical stimulation, thus dimming the eyebar signal that is used in aggressive male–male encounters.
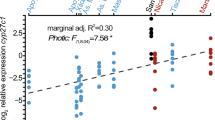
Longer-term, morphological, or developmental changes in color are often a result of genetic and environmental variations. For example, the acquisition and utilization of pigments such as carotenoids will alter the expression of long-wavelength yellow and red color pattern elements (Grether et al. 2004). In a recent study, among population male color differences have been reported for the haplochromine Egyptian mouthbrooder Pseudocrenilabrus multicolor victoriae found in a variety of habitats in the Nile River basin (McNeil et al. 2016; Fig. 2a). Males from long-wavelength shifted (but clear) swamp sites expressed more red coloration compared to males from turbid river sites that expressed more yellow coloration. Investigations into the mechanisms associated with color divergence across extreme environments (Chapman 2015) in this species are ongoing due to the complexity of the system; however, we do know that male color is at least partly controlled by dietary carotenoid uptake (McNeil et al. 2016). Vertebrates cannot synthesize carotenoids and therefore rely on acquiring it from their diet for expression of certain long-wavelength reflecting pigments (Olson and Owens 1998; Leclercq et al. 2010).
Colorful signals can additionally be enhanced by body positioning and the frequency of behavioral displays. Baerends and Baerends-van Roon (1950) outlined the first ethogram of cichlid behaviors, indicating the ways in which males will position and display their bodies toward females. As an example, in Lake Malawi I observed Metriaclima aurora males with intensely yellow-colored throats and abdomens (Dalton et al. 2010) swim upward from their rocky territory and directly toward a potential mate, and then immediately turn to perform a lateral display toward the female. While this behavior was observed repeatedly, formal investigation is required to determine if this upward display is perceived as more conspicuous by females and thus favored. Additionally, altering the photic environment can result in changes in the frequency of courtship displays, as first described in Threespine stickleback from the Baltic Sea. Under conditions of high eutrophication (e.g., green-shifted watercolor ), male sticklebacks significantly increased the frequency of courtship displays, presumably to better attract a female when the red nuptial throat patch would be masked against the green background. The result of this more frenetic behavior did not result in higher reproductive success (Heuschele et al. 2009). We have also recently shown, using a split-brood rearing experiment with Egyptian mouthbrooder broods from one swamp (clear, red-shifted) and one river (turbid, yellow-shifted) population and a male–male competition experiment that when in turbid water males perform more aggressive behaviors toward the other males than when the pair is tested in turbid water (Gray et al. 2012). This was true for fish from both parental populations and regardless of whether they were reared under clear or turbid conditions, suggesting that P. multicolor can alter behavior to compensate for an altered photic environment.
The heterogeneity of photic environments occupied by cichlids, and the various ways in which signals can be expressed or altered, have contributed to the extreme diversity of color patterns observed in cichlids (e.g., Baerends and Baerends-van Roon 1950; Fryer and Iles 1972; Konings 1990; McElroy and Kornfield 1990). Yet, there are some distinct and likely convergent patterns that emerge. Among African cichlids, sister species tend to share similar morphological and behavioral traits, with the major differences linked to reproductive isolation in the form of male nuptial coloration (Seehausen 1996; Albertson et al. 1999; Danley and Kocher 2001; Allender et al. 2003; Konings 2007). For example, there is a repeated pattern of intraspecific red and blue male color morphs and red and blue sister species pairs found throughout Lake Victoria that has been shown to be related to the steepness of light and depth gradients (Seehausen et al. 1997, 2008). In the mbuna, or rock-dwelling, cichlids of Lake Malawi, parallel evolution of color patterns has also occurred (Allender et al. 2003). In the relatively clear, broad-spectrum waters of Lake Malawi, species pairs with blue or yellow males dominate more so than the red-blue pairs of Lake Victoria. Pauers et al. (2016) found independently originating stripe, bar, and solid patterns among the Lake Malawi radiation. In this case, horizontal stripes are typically associated with reducing conspicuousness to predators, whereas bars tend to be involved in communication (e.g., species or mate recognition). The Midas cichlid (Amphilophus ) species complex of Nicaragua also shows parallel evolution of color patterns, though they exist as color polymorphisms within species (Elmer et al. 2010): “dark,” the most common morph, is greyish with various melanic patches, bars, and stripes; and the “gold” morph is yellow, orange, or white (Barlow 1983). Morphs are genetically determined in this case, with some within-morph modification of color intensity via carotenoid uptake (Henning et al. 2010).
In many cichlids, sexual dichromatism suggests that color patterns play an integral role in sexual selection (e.g., Kocher 2004) and that visual systems must be linked somehow to signals in order for the signals to be effective (Endler 1992). So, if a male expresses bright red coloration in a reproductive context, we expect that females (and conspecific male competitors) should be able to detect that signal (Gray and McKinnon 2007). The efficiency of a signal depends upon transmission of the signal through the medium, and this will change with the medium. As an example, a fish viewed in clear water will likely be much more detectable than if viewed by the same receiver from the same distance in turbid water. The same would hold true as one moves from shallow to deeper waters. This has been shown to be true for a number of cichlids (e.g., Seehausen et al. 2008) and the diversity of photic habitats has contributed to both the adaptive radiation of African cichlids and the loss of biodiversity when the photic environment is disrupted (Seehausen et al. 1997). A question of interest among evolutionary biologists is if this link between environment, signal, and visual system stem from ecological selection on visual systems with subsequent sexual selection for male color patterns that are conspicuous to females in a given photic environment (i.e., sensory drive hypothesis; discussed in detail in 4.1).
3.3 Visual Sensitivity
Detection of visual signals requires appropriate sensory structures for capturing light, thus the visual sensory system needs to respond to the spectrum of light available (Lythgoe 1979). The term visual sensitivity is used in several contexts: photoreceptor spectral sensitivity describes the spectral absorbance properties of retinal photopigments. Or, in a more holistic sense, the term is used to describe the overall spectral sensitivity of the visual system to light, including through interactions between cones and retinal neurons and further processing of a visual signal in the brain. Evidence of spectral sensitivity is much more prevalent than evidence for information processing, largely due to the ways in which each is measured. To determine photoreceptor sensitivity, either MSP of individual photoreceptor cells or reconstitution of opsin proteins is used. These techniques capture the different classes of photopigments expressed and the genetic potential (i.e., complement of opsin genes present) an individual has for spectral sensitivity. Both can also reveal the presence of rare cone photoreceptor types (e.g., Parry et al. 2005; Carleton et al. 2008), but cannot describe the mechanism of visual information processing. Determination of the spectral sensitivity of the visual system as a whole requires whole retina physiological techniques, such as electroretinograms (ERGs) or optic nerve compound action potential (CAP) recordings. ERGs measure the response to various wavelengths of light at the level of the retina (i.e., an electrode is placed near the vitreus humor of the eyecup near the retina) and CAPs measure the response at the level of the optic nerve. Behavioral tests, such as the optomotor response, can also provide information about visual sensitivity but at an even higher level of organization (i.e., post-processing of the visual stimulus). Thus, photoreceptor sensitivity provides information about the cone classes present and their λmax, whereas ERG and CAP tests provide information about the processing of visual information, and behavioral tests are helpful for understanding post-processing responses. Ideally, a combination of tests would be used to infer perceptual abilities; however, this would be logistically difficult for the large number of species and specimens needed to characterize and compare visual system diversity across the Cichlidae.
For a growing number of African cichlids, and several Neotropical species, we know the spectral sensitivity of photoreceptors combined with their morphological arrangement found in the retina, which can infer the colors of maximal sensitivity for an individual. Fernald and Liebman (1980) provided the first documentation of retinal sensitivity for a cichlid, the Lake Tanganyikan Burton’s mouthbrooder. As mentioned above, we now know that African cichlids have a suite of seven distinct cone opsin genes (SWS1 ~ UV, SWS2A ~ blue, SWS2b ~ blue, RH2B ~ blue-green, RH2Aβ ~ green, RH2Aα ~ green, and LWS ~ yellow-red) coding for distinct cone classes maximally sensitive to a particular wavelength of light, λmax (Table 1). However, African cichlids typically express only three cone classes at a given time (Parry et al. 2005; Carleton et al. 2008), which also appears to be true for Neotropical cichlids (Escobar-Camacho et al. 2017). With the advancement of methodologies for measuring photoreceptor sensitivity, researchers were able to ask if there were differences in visual sensitivity between fish found in divergent ecological niches. For example, MSP was used to investigate differences in visual sensitivity between sand- and rock-dwelling Lake Malawi species, finding that species have divergent cone spectral sensitivities from each of these distinct habitats with distinct photic properties (Levine and McNichol 1979; Carleton and Kocher 2001). By combining data from MSP, opsin gene sequencing, and spectral analysis of reconstituted visual pigments, Parry et al. (2005) confirmed differences in the three cone classes expressed by several sand- vs. rock-dwelling cichlids from the Lake Malawi. For example, they found that the sand-dweller, Tramitichromis intermedius expressed three opsin pigments with the following λmax: SWS2A = 455 nm, RH2Aα = 532 nm, LWS = 569 nm, while the rock-dweller M. zebra , expressed a different complement of cone opsins and associated λmax: SWS1 = 368 nm, RH2B = 488 nm, RH2Aα = 533 nm. These differences in opsin expression would result in T. intermedius having a long-wavelength shifted spectral sensitivity compared to more short-wavelength sensitive vision in M. zebra . A pattern has emerged among African cichlids of specific subsets of three dominant cone classes being expressed together and further work has revealed that these subsets, or visual “palettes,” can vary between species, sexes, and ontogenetically (Parry et al. 2005; Carleton 2009; Hofmann et al. 2009; O’Quin et al. 2010; Smith et al. 2011; Table 2).
Among Neotropical cichlids, the cone opsin gene complement has been characterized for only a few species to date: Trinidadian Pike cichlids (Crenicichla frenata ) by Weadick et al. (2012); convict cichlid by Fisher et al. (2015); angelfish (Pterophyllum scalare ), discus (Symphysodon discus ), and the oscar (Astrotnotus ocellatus ) by Escobar-Camacho et al. (2017). Among the Pike and Convict cichlids, these two species possess only a subset of the opsin genes found in African cichlids: SWS2A, SWS2B, RH2b, RH2a, and LWS (see Table 1 in Fisher et al. 2015). Fisher et al. (2015) determined that the Convict cichlid expresses a three-cone complement including SWS2A, SWS2B, and LWS opsins, which is a unique combination in comparison with the palettes typically expressed by African rift lake cichlids (Table 2). It also suggests long-wavelength sensitivity. A similar long-wavelength shifted palette (SWS2A, RH2a, and LWS) was discovered among the three Amazonian species examined by Escobar-Camacho et al. (2017). Interestingly, Härer et al. (2017) found evidence of shifts to more short-wavelength palettes in the Nicaraguan Midas cichlids that within the last few thousand years have colonized several clear crater lakes from the very turbid Lake Nicaragua. This limited data set suggests a reduced number of opsin genes in Neotropical cichlids (Fisher et al. 2015; Weadick et al. 2012). However, given the diversity of visual sensitivities found among cichlids as a whole, many more Neotropical species should be sampled before conclusions are drawn.
Collecting evidence for visual system sensitivity at the level of the retina or whole eye versus the photoreceptor is much more difficult and logistically limiting for large comparative studies, as it requires physiological tests of the response of photopigment cells to varying intensities and wavelengths of light in situ, using ERGs and CAPs. However, a few examples have helped us to better understand the processing of visual information in the cichlid eye. Lisney et al. (2010) used both of these methods to determine spectral sensitivity in Nile tilapia and compared their results to the MSP findings of Parry et al. (2005) and Carleton et al. (2008) for the same species. The electrophysiological studies largely matched the MSP findings (i.e., three dominant cone classes corresponding to SWS2, RH2, and LWS), although the ERG sensitivity peaks were somewhat long-wavelength shifted compared to earlier results, possibly due to differences in A1/A2 ratios in the specimens used for each study. The evidence for rare SWS cones was also stronger than from MSP studies, which found SWS cones to represent <10% of retinal photoreceptors (Parry et al. 2005). Sabbah et al. (2010) used data from whole organism ERG and opsin gene expression experiments that provided evidence of a pentachromatic visual system in cichlids (i.e., up to five cone classes interacting to determine color sensitivity). This was accomplished by modeling the results of each method for three Lake Malawi mbuna cichlids, M. zebra , Melanochromis auratus , and Protomelas taeniolatus . While the results of this study at first seem to suggest greater variation in visual sensitivity than previously discovered for cichlids, most earlier studies report the presence of more than three cone photoreceptors, but indicate these are typically rare, representing <10% of the total number of cone opsins expressed in the retina at a given time. The fact that those rare cones may contribute to visual sensitivity, as shown by the limited electrophysiological results (e.g., Lisney et al. 2010; Sabbah et al. 2010), is intriguing and requires further investigation. Additionally, evidence in M. zebra of coexpression of opsin genes within individual cones (Dalton et al. 2015, 2016), coincident with topographical variation in coexpression across the retina seems to support a more complex mechanism of visual sensitivity (Dalton et al. 2016).
Importantly, discovering the spectral sensitivity for a large number of cichlids has allowed us to specifically address questions about relationships between vision, the photic environment, and visual signals. We expect variation in sensory systems in animals found across divergent habitats, to accommodate predator avoidance, mating, and foraging (Endler 1992). Numerous studies have found that the sensitivity of vertebrate double cones, composed of mid-to-long-wavelength sensitive pigments in cichlids, tend to match the background photic environment (McFarland and Munz 1975; Loew and Lythgoe 1978; Lythgoe 1980; Loew and McFarland 1990; Cummings 2004; Dalton et al. 2014). In cichlids specifically, there is good evidence of visual sensitivity matching the photic environment and male nuptial coloration for a number of species (Carleton et al. 2006). For example, Seehausen et al. (2008) show a close correlation between visual sensitivities tuned via changes in amino acid sequences in opsin genes and male mating color in Lake Victoria cichlids; species with long-wavelength shifted sensitivity have red male nuptial coloration reflecting a longer wavelength signal. Fisher et al. (2015) also suggest that the LWS opsin found in Convict cichlids is tuned to the orange ventral patch found on females; however, further assessment of peak absorbance is still needed to confirm this (e.g., MSP, optomotor response). Additionally, UV sensitivity is important for discriminating zooplankton against light space (Utne-Palm 2002) and behavioral studies have corroborated this for some zooplanktivorous cichlids (Jordan et al. 2004; Hofmann et al. 2009).
Cichlids display the largest observed shifts in spectral sensitivity of any vertebrates, due largely to differential opsin gene expression and tuning of spectral sensitivity via changes in the amino acid sequences of opsin genes (Carleton 2009; Hofmann and Carleton 2009; Hofmann et al. 2009). Sensitivity of individual photoreceptors may be further tuned by coexpression of multiple opsin pigments within a single cell (Dalton et al. 2014, 2016). The extent of variation and lability in the visual sensitivity of cichlids has likely contributed to the adaptive radiation of cichlids.
4 Evolution of Cichlid Visual Systems
The recent and rapid radiation of cichlids in the African rift lakes is often linked directly with visual system diversity within this group (e.g., Terai et al. 2006; Seehausen et al. 2008; Brawand et al. 2014). The relative roles of natural versus sexual selection in shaping cichlid visual diversity are an area of intense research with many unanswered questions. It is likely that natural selection on visual sensitivity has played a large role in cichlid diversification, with sexual selection on male coloration leading to further speciation. Thus, recent work investigating sensory drive, or the process by which sensory systems and communication traits evolve for effective perception and appropriate responses (Endler 1992, 1993; Boughman 2002; Maan et al. 2006; Fuller et al. 2010), underscores the relevance of the light environment for effective visual communication. In this section, I review recent advances in our understanding of the contribution of sensory drive and spectral tuning to cichlid speciation.
4.1 Sensory Drive and Speciation
Sensory drive is a hypothesis for the evolution of divergent sensory traits that may contribute to speciation (e.g., Endler and Basolo 1998; Boughman 2002; Seehausen et al. 2008). Using vision as an example for this hypothesis, across different light environments (e.g., clear water vs. tannin-stained water) visual systems and signaling cues are expected to diverge to maximize efficient communication, potentially leading to premating isolation if the two become linked. In one of the most complete examples of sensory drive promoting speciation in sympatry, Seehausen et al. (2008) demonstrated links between shifts in the λmax of LWS opsins, ambient light, male nuptial color, and female preference for male nuptial coloration. They used a pair of sympatric sister species, Pundamilia pundamilia and P. nyererei , that express blue and red male nuptial color patterns, respectively, and are found at multiple islands in the Lake Victoria that vary with respect to shore slope and turbidity . As described above (Sect. 2.2), in clear water longer wavelengths of light attenuate with depth faster than short-wavelengths; however, under conditions of turbidity in which the photic zone is much more shallow, light underwater becomes red-shifted with depth as the higher energy photons of short-wavelength light (UV-blue) are scattered more by suspended particulates. Therefore, the combination of turbidity and depth creates gradients of red-shifted light at depth and more blue-shifted light along shallower portions of the slope. P. pundamilia , having blue-gray males, is largely distributed in the more blue-shifted shallow zones and P. nyererei , with yellow-red males, is predominantly found along deeper portions of the rocky slope in red-shifted light (Maan et al. 2006). From extensive previous research, incorporated with new work, Seehausen et al. (2008) established a suite of evidence pointing toward sensory drive as a likely hypothesis for speciation among these closely related species.
First, water clarity and hence ambient light varied across islands and with depth, creating photic slopes that differed in steepness (Seehausen et al. 1997; Seehausen et al. 2008). Second, among Lake Victoria cichlids there is typically only a single visual palette expressed, the long-wavelength palette of cone opsins (Table 2; λmax for Pundamilia spp.: SWS2A ~455 nm, RH2A ~ 528 nm, LWS ~ 565 nm; Carleton et al. 2005); however, there is considerable variation in the coding sequence of the LWS gene among Lake Victoria cichlids (Terai et al. 2002, Spady et al. 2005). In fact, among Lake Victoria blue-red species pairs, the species with red male nuptial coloration have LWS λmax values higher than those species with blue-gray males as would be predicted by a relationship between visual sensitivity and the photic environment (van der Meer et al. 1995; Carleton et al. 2005). Third, behavioral optomotor response tests demonstrated that P. pundamilia had lower detection thresholds in red-shifted water and vice versa for P. nyererei under blue-shifted light, suggesting that each species has a visual system tuned to the ambient light environment they inhabit (Maan et al. 2006). Finally, females were shown to prefer to mate with males with the same color as conspecific males. This suite of evidence points to sensory drive as the mechanism of speciation between sister species P. pundamilia and P. nyererei such that natural selection favors tuning of the visual systems to divergent light environments and sexual selection favors male nuptial coloration that takes advantage of the predominant ambient light. Further, among the islands where P. pundamilia and P. nyererei are found, there are different degrees of distinctness between them with respect to male nuptial coloration. Seehausen et al. (2008) therefore evaluated if the steepness of the environmental gradient, mediated here by water clarity and steepness of the rocky slope, contributed to divergence in the sensory and signaling systems of these fish, with the expectation that an intermediate slope would provide enough distinction between light environments for altered visual systems to improve visual detection thresholds and enough connection between populations for low migration to produce intermediate forms that are selected against (i.e., disruptive selection) (e.g., Schluter and Nagel 1995). Thus, Seehausen et al. (2008) demonstrate a case for speciation driven by sensory drive among Lake Victoria cichlids.
In Lake Malawi, the evidence for sensory drive as a mechanism for speciation is less clear despite (1) the relative clarity of the lake, (2) the diversity in visual palettes expressed among species, and (3) repeated evolution of color patterns throughout the lake. However, a phylogenetic analysis by Pauers et al. (2016), in which they compiled visual sensitivity, color pattern, and photic environment data for 39 Lake Malawi species, found a relationship between UV color pattern elements and UV sensitivity. Of the three typical color patterns (stripe, bar, solid), they found that UV reflective patterns in barred and solid-color species were correlated with short-wavelength sensitivity (i.e., expression of SWS1). In the species with horizontal stripes, there was little to no UV reflectivity and expression of SWS2 was most common. They did not, however, find a relationship between visual sensitivity and the photic environment, similar to the findings of Hofmann et al. (2009) and Dalton et al. (2010). This suggests that the barred color pattern with UV elements and UV sensitivity co-evolved, with UV patterning potentially evolving as a means of “private” communication for mating or species identification.
With the visual sensitivity of only a few Neotropical species characterized, there has not been a comprehensive study investigating a possible role for sensory drive in the relatively ancient radiations of Neotropical cichlids in Amazonian rivers (López-Fernández et al. 2010, 2013; López-Fernández 2021) nor the more recent crater lake radiations (Barluenga et al. 2006). Hauser et al. (2017) further found that among 101 Neotropical species examined for variation in the rhodopsin gene (functioning in dim or low light environments), those species from Central America appear to have accelerated rates of evolution compared to South American species. Given the parallel evolution of color morphs within the Nicaraguan crater lakes, and the recent colonization of these clear lakes, this may be an area ripe for further study. Additionally, Weadick et al. (2012) have found molecular evidence of positive selection on the SWS2B and RH1 (rod) opsins in the Trinidadian Pike cichlid; however, studies to determine which forces might shape selection have yet to be performed.
4.2 Spectral Tuning and Adaptive Radiation
Animals that rely on vision have visual systems that are tuned to the ambient light environment and background against which objects are viewed to optimize detection (Cronin et al. 2014). This can be accomplished in a number of ways including: (1) the presence of structures such as the tapetum lucidum within the eyes of some fish and other vertebrates that reflects light back into the retina under low light conditions; (2) differential topographic distribution of cone photoreceptors throughout the retina to take advantage of heterogeneous light space and for different ecological tasks (Temple 2011; Dalton et al. 2016); (3) pre-photoreceptor filtering of light; and, (4) tuning the specific spectral sensitivity of photoreceptors (i.e., spectral tuning) through molecular and differential expression mechanisms (Carleton 2009). Cichlids of the African Great Lakes have served as a model for understanding the underlying mechanisms generating visual system diversity across vertebrates.
Pre-Receptor Tuning
Several pre-receptor tuning mechanisms are found in cichlids. These can take the form differential refraction by crystalline structures or pigments in the lens or through oil droplets or carotenoid-based pigments in the inner segment of the photoreceptor cell (Cronin et al. 2014). In Lake Malawi mbuna, Dalton et al. (2010) report species-specific variation in lens transmission (i.e., T50: the wavelength at which transmission of light through the lens reaches 50%). For example, solid blue Metriaclima callainos have T50 = 360 nm whereas Melanochromis heterochromis , with a horizontally striped color pattern, have T50 = 400 nm, thereby blocking most short-wavelength UV light from reaching the retina. Interestingly, Met. callainos expresses the SWS1 opsin while Mel. heterochromis expresses the longer wavelength SWS2B opsin, suggesting a link between pre-filtering media, photoreceptor sensitivity, and male nuptial coloration as described in Pauers et al. (2016). The relationship between lens transmission and opsin expression was further confirmed in a much larger sample size of 65 Lake Malawi species, with SWS1 expression corresponding with lens transmittance of UV light, SWS2B (violet) expression having a mixed result of lens transmittance between 360 and 400 nm, and those species expressing SWS2A (blue) generally displaying T50 = 400 nm (Hofmann et al. 2010a). In general, the presence of amino acids found in the lens typically filters short-wavelength light (Thorpe et al. 1993; Hofmann et al. 2010a). The patterns emerging with respect to the adaptive capacity of Lake Malawi cichlid vision is intriguing in that there appear to be strong links between UV-sensitivity coupled with lenses that transmit UV light, foraging behavior such as planktivory that is optimized by having UV sensitivity, and male nuptial coloration that uses UV reflective patterns to signal privately to conspecifics (Dalton et al. 2010; Hofmann et al. 2010a, b; Pauers et al. 2016). In this case, natural selection appears to be driving visual sensitivity, with male sexual communication taking advantage of this trait.
Spectral Tuning
Spectral tuning of visual sensitivity is the process by which sensitivity of photoreceptors is tuned to particular wavelengths (i.e., shifts in peak wavelength of maximum absorbance). There are several known mechanisms by which animals can tune spectral sensitivity to optimize vision in a given environment. The major mechanisms for tuning spectral sensitivity take place at the photopigment level, either through coding sequence changes in the opsin gene that alter the function of the protein, or via regulatory factors that alter opsin gene expression (Carleton 2009).
Research in Karen Carleton’s lab (University of Maryland, College Park) has spearheaded much of the most recent advances in understanding the mechanisms underlying tuning of spectral sensitivity and correlating these processes with functional diversification, adaptive evolution, and speciation (Carleton 2009; Carleton et al. 2016). As an example, Hofmann et al. (2009) examined approximately 60 cichlid species from Lake Malawi (n = 54 wild-caught) and Lake Victoria (n = 11 lab reared) to test if changes in opsin gene coding sequences, differential opsin gene expression, and correlations between spectral sensitivity and various ecological parameters such as trophic position and ambient light environment can help explain the proximate mechanisms driving adaptive radiation in African cichlids. This work was prompted by a large suite of evidence from cichlids that directly links differences in opsin gene coding sequences with visual sensitivity tuned to match the ambient light environment (e.g., Lake Victoria: Terai et al. 2002, 2006; Carleton et al. 2005; Seehausen et al. 2008; Lakes Malawi and Tanganyika: Sugawara et al. 2005) and the fact that African cichlids have a complement of seven cone opsin genes that can be differentially expressed (e.g., Carleton and Kocher 2001; Spady et al. 2006; Carleton et al. 2008; Shand et al. 2008; Hofmann and Carleton 2009; Table 1). Hofmann et al. (2009) found that cichlids from deeper and relatively clear Lake Malawi had a higher diversity of visual profiles, or palettes, compared to a single expression profile in shallow, turbid Lake Victoria (Table 2). Lake Malawi cichlids tended to express three distinct visual palettes that span the spectrum from a short wavelength-sensitive palette, a medium (or middle) wavelength sensitive palette, and a third visual profile with expression of the longer-wavelength sensitive opsins. Lake Victoria cichlids, on the other-hand only expressed a single visual palette in the long-wavelength end of the spectrum. Additionally, they showed that Lake Malawi species with high SWS1 (UV) opsin expression were more likely to be planktivores and algae eaters and that Lake Victoria species had LWS opsins tuned to longer-wavelength as expected in the shallow and turbid waters of that lake. Importantly, their work showed that functional changes in the amino acid sequence of opsin genes operated in fine-scale tuning (shift in λmax of 5–10 nm) at the extreme ends of the visual spectrum (e.g., SWS1 and LWS genes) where gene expression is bounded by available genes. Tuning by differential opsin expression, on the other hand, operates at a coarser scale among the middle wavelength opsins, creating 30–100 nm shifts in peak absorbance. Hofmann et al. (2009) comprehensive study demonstrated that both sequence variations in the opsin gene and differential cone opsin expressions are adaptive and play a role in generating the incredible diversity of visual systems within these two cichlid radiations (also see Hofmann and Carleton 2009). Evidence from multiple studies suggests natural selection has acted on cichlid opsin genes in all three African great lakes (Sugawara et al. 2002; Spady et al. 2005). Lake Tanganyika is similar to Lake Malawi in that it is relatively deep and clear with a generally broad-spectrum photic environment and contains the most diverse group of cichlids among the three African Great Lakes (e.g., Salzburger et al. 2002). O’Quin et al. (2010) discovered that Lake Tanganyikan cichlids also express the three common visual palettes found in Lake Malawi cichlids (Table 2) and suggest that the long-wavelength visual palette is likely the ancestral state, with repeated evolution of factors controlling the expression of the short- and middle-wavelength sensitive palettes.
Carleton et al. (2010) tested the mechanistic basis of spectral tuning using a rearing experiment. Two intergeneric crosses (Dimidiochromis compressiceps × Copadichromis eucinostomus , Tramitichromis intermedius × Aulonocara baenschi ) between species that express different subsets of opsins were reared under controlled laboratory conditions to determine if opsin gene expression in cichlids is controlled by one or two genes. If only a few genes are involved in regulating opsin expression, thus providing some ease of lability, cichlids may be able to rapidly tune spectral sensitivity if the light environment shifts. Both cis- and trans-regulatory elements were involved in the regulation of cone opsin expression, with high correlation between some single and double cone opsin genes (Carleton and Kocher 2001), providing a possible explanation for why the three visual palettes typically observed are quite common (Hofmann et al. 2009). Furthermore, Schulte et al. (2014) have discovered a specific transcription factor (RX1) that controls SWS2A expression. They found a 413 base pair deletion in the RX1 gene of species that do not express SWS2A. Of the three SWS opsins, SWS2A has the longest λmax and is found in all three of the African Great Lakes.
Ontogenetic Shifts in Sensitivity
There is also evidence in cichlids that expression of cone opsins can change throughout ontogeny and that ambient light may influence this process. For example, Nile tilapia shifts from having short-wavelength sensitive opsin palettes to long-wavelength sensitive palettes through development (Spady et al. 2006; Carleton et al. 2008). In another rearing study, Hofmann et al. (2010b) tested for plasticity in opsin expression by comparing across five Lake Malawi species reared in the laboratory under an UV-absent photic rearing environment with the known cone expression of adults from the wild. They found species-specific responses to being reared in an UV-absent environment, with expression of SWS1 downregulated (but still present) in some species, and with no response in other species, confirming a major role for genetic control of cone opsin expression (see Carleton et al. 2010) and some evidence for a plastic component. Another experiment suggests that the spectral sensitivity of adult African cichlids can change within as little as 3 days (Nandamuri et al. 2017). In this study, the authors’ exposed wild-caught adults to UV deficient laboratory lighting, and lab-reared fish to high-UV lights, finding rapid shifts in SWS1 expression, suggesting that rapid, plastic responses to variation in the lighting environment are possible. Coexpression of opsin genes may also contribute to spectral tuning. Opsin coexpression can occur either by having two or more opsin proteins expressed within a single photoreceptor cell or by having multiple cones expressing different opsins (Dalton et al. 2014, 2016). The effects of coexpression on visual perception (i.e., neural processing and behavioral response to visual stimuli) remain to be tested, although advances are being made (Dalton et al. 2016).
4.2.1 Adaptive Radiation
If spectral sensitivity is adaptive, then there should be a direct link between shifts in peak absorption and behavioral detection thresholds to different photic environments. This link has been established in a number of cichlid species, including Pundamilia spp. from Lake Victoria as described above (Maan et al. 2006), and more recently by Smith et al. (2011) for Lake Malawi cichlids. In the latter study, Smith and coauthors tested the optomotor response of two Lake Malawi species, Met. lombardo i and Mel. auratus , that were each reared under different photic environments. They found that rearing environment influenced cone opsin expression and behavioral detection thresholds; specifically, fish that expressed relatively more LWS opsin had higher sensitivity to red (long-wavelength) light and fish that expressed relatively more RH2A and RH2B opsin were more sensitive to green (mid-wavelength) light. These tests, across multiple species from different lakes and lineages, provide a better understanding of the evolution of spectral tuning by differential opsin expression and sensory processing.
Given the diversity of available opsin genes, and evidence supporting directional selection on opsins, why do cichlids retain so many opsin genes? The answer may lie in a combination of spectral tuning across heterogeneous photic environments, and also in ontogenetic shifts in visual sensitivity. For example, Nile tilapia expresses all seven opsins throughout ontogeny, while only four opsin genes are expressed in adults. This suggests that subfunctionalization of opsin genes from the juvenile to adult state could help to maintain genetic diversity (Spady et al. 2006). The Neotropical cichlid, Aequidens pulcher , had reduced number of SWS photoreceptors under blue light, suggesting that excess light in one region of the spectrum can lead to reduced sensitivity in that area of the spectrum (Kröger et al. 1999; Wagner and Kröger 2005). This finding is counter to the findings from Lake Victoria cichlids that have higher long-wavelength sensitivity in red-shifted environments, thus maximizing sensitivity in the predominant range of photic environment, highlighting the variability in sensory systems among cichlids and the need for more comparative work between Neotropical and African lineages. An extreme case of reduction in visual sensitivity comes from the blind cichlid fish, Lamprologus lethops, of the Congo River (Schobert et al. 2013). In this species, the eyecups are present but found underneath the layers of skin and connective tissue and are much reduced in size compared to a sympatric and putative sister species, L. tigripictilis . While Schobert et al. (2013) were able to distinguish rod photoreceptor cells in the retina of L. lethops , their methodology did not provide information about cone photoreceptor cells, restricting interpretation at this time to L. lethops having light sensitivity and further investigation to determine if they have color and image-forming vision.
Despite the extreme variation in photic environment in South and Central American riverine habitats where cichlids are ubiquitous (e.g., “black,” “white,” and clear rivers), there has been little to no work done to examine the links between spectral sensitivity, photic environment, and behavior in the Neotropical lineage. There is great potential both in Amazonian rivers and in the crater lakes of Central America for this kind of work. In one direct comparison of African and Neotropical cichlid vision evolution, Schott et al. (2014) examined the evolution of rhodopsin (responsible for dim light, scotopic vision) among cichlid lineages, examining both ecological (riverine, lacustrine) and biogeographical (Neotropical, African) factors as drivers of RH1 diversification. A follow-up study by Torres-Dowdall et al. (2015) incorporated lacustrine Neotropical cichlids to the Schott et al. (2014) analysis, both studies finding evidence of positive divergent selection in both radiations, with stronger selection in lacustrine than riverine cichlids, and stronger selection in African than Neotropical cichlid radiations. Additionally, Escobar-Camacho et al. (2017) suggest that their finding of a dominant long-wavelength opsin palette expressed by cichlids found in the more turbid waters of the Amazon supports the role of visual environment in selecting for spectral tuning.
We now have multiple lines of evidence that help us to better understand the proximate mechanisms driving the evolution of visual diversity among cichlid fishes: (1) tuning of spectral sensitivity by both amino acid substitutions and differential opsin gene expressions, each tuning to different parts of the visible spectrum and at different scales, and coexpression of opsins in the retina and within photoreceptor cells (Dalton et al. 2016); (2) genetic and plastic control of opsin expression; (3) only a few genes involved in regulating opsin expression; (4) at least one gene (Rx1) identified as controlling the expression of a short-wavelength sensitive opsin (SWS2A); and, (5) evidence of functionality of shifted spectral sensitivity selected by behavioral detection thresholds (Maan et al. 2006; Smith et al. 2011) as well as by sensory-driven mate choice preferences (Seehausen et al. 1997, 2008). This evidence stems largely from studies on African rift lakes studies, highlighting the need for more research on the visual ecology of other cichlid lineages.
5 Cichlid Vision and Human-Induced Environmental Change
The classic work by Seehausen et al. (1997) describes how an altered visual environment contributed to the loss of potentially hundreds of endemic Lake Victoria cichlids. Increased turbidity, either through eutrophication or increased sedimentary inputs, is cited as one of the most detrimental forms of habitat degradation leading to loss of aquatic biodiversity globally (Kemp et al. 2011; Gray 2016). It is important to note that many cichlids, especially those Neotropical species found in the extremely turbid waters of parts of the Amazon River, have likely evolved under high turbidity and/or tannin-stained conditions. However, it is the increase over natural levels induced by human activities in and around water that is concerning, especially for cichlids that have evolved and rapidly speciated in relatively clear water.
5.1 Lake Victoria Eutrophication
While the direct physiological effects of increased turbidity, such as reduced aerobic capacity due to clogged or abraded respiratory structures (e.g., Sutherland and Meyer 2007; Gray et al. 2016), can negatively impact survival of fishes, the alteration to the visual environment has greatly impacted cichlid diversity. The rapid loss of cichlid diversity in Lake Victoria is the most famous example. Eutrophication of the southern basin of Lake Victoria starting in the ~1920s led to an approximately 50–60% decrease in water clarity in parts of the lake (Hecky 1993) and continues unchecked today with further deforestation, agriculture, and urbanization surrounding the lake. Eutrophication altered the underwater spectral composition such that less light and a narrower spectrum of light that is long-wavelength shifted dominated the visual scene. While eutrophication coincided with the introduction of the predatory Nile perch (Lates niloticus ), and perch are likely responsible for the loss of a large number of Lake Victoria cichlids (Ogutu-Ohwayo 1990; Kaufman 1992; Witte et al. 1992; Kaufman and Ochumba 1993), their invasion does not completely explain the disappearance in a 10-year period of many of the ~200 rock-dwelling species that are rarely eaten by Nile perch. Seehausen et al. (1997) compiled a suite of evidence from mate-choice experiments in the laboratory, laboratory-rearing studies, surveys of cichlid species diversity and color differentiation across a transect of rocky islands accompanied by characterization of the underwater light environment to test the hypothesis that decreased availability of light and a shift in the underwater photic environment to longer-wavelength light due to eutrophication contributed to the loss of cichlid species. As mentioned above, sister species and morphs in Lake Victoria tend to have male nuptial color patterns at opposite ends of the visible spectrum, with pairs of species/morphs having a blue-gray male and a yellow-red male . In brief, the authors found that under broad-spectrum lighting, females prefer to mate with conspecific males; but under monochromatic light that masks male color, females mate indiscriminately. In many Lake Victoria insipient species pairs, pre-copulatory mate choice is the only form of reproductive isolation, therefore indiscriminate mating in the absence of appropriate color clues would lead to the breakdown of isolation. Indeed, Seehausen et al. (1997) found a positive correlation between the number of cichlid species and the distinctness of color morphs with water clarity and breadth of the light spectrum, suggesting that as water clarity decreases (and light becomes more monochromatic) so does the sexual selection pressure maintain differences between morphs and species pairs. The multiple lines of evidence exposed in this study demonstrate a strong relationship between human-induced disturbance of the visual environment and loss of species diversity. Since the decline in Lake Victoria Nile perch abundance in the 1990s, resurgence in haplochromine populations has been observed (see Natugonza et al. 2021). Interestingly, several studies investigating recovering cichlids have found correlations between turbidity and less colorful hybrids (e.g., Mrosso et al. 2004; Witte et al. 2013), confirming that even without being exacerbated by the Nile perch introduction, eutrophication would likely have resulted in great diversity loss.
5.2 Compensatory Responses
Organisms facing human-induced rapid environmental change are often lost, while others are able to adapt and so persist (Sih et al. 2011). Further work on Lake Victoria cichlids has demonstrated morphological adaptation of visual sensory structures since the eutrophication of the lake. Using historical (1978) and contemporary (2001) collections of two zooplanktivorous haplochromines (Haplochromis pyrrhocephalus , H. tanaos ), van der Meer et al. (2012) assessed visual resolution and sensitivity based on relative lens and photoreceptor size and distribution of cones in the retina, respectively. Both species had reduced eye size in contemporary relative to historical populations likely resulting in reduced resolution, but with retinal adaptations that preserved photon capture and thus sensitivity. They also found a reduction in the density (in some cases complete loss) of single cones, suggesting a loss of sensitivity to shorter wavelengths (recalling that single cones in African cichlids are always from the SWS class of cone photoreceptors). In a darker and long-wavelength shifted environment, the benefit of short-wavelength sensitivity for detecting zooplankton against a broader spectrum background space light would be lost.
Compensatory behavior to counteract reduced visual range is also possible. For example, Gray et al. (2011) used a split-brood rearing experiment wherein fish from two divergent populations (one clear and one turbid) were reared under clear and turbid conditions, to determine if the Egyptian mouthbrooder, a widespread haplochromine cichlid of the Nile River Basin, responds to changes in water clarity. We found an increased rate of aggressive displays toward a conspecific competitor under increased turbidity, regardless of population of origin or rearing environment. In another study, we found repeated divergence in male nuptial coloration of wild-caught P. multicolor across habitats that vary in turbidity (McNeil et al. 2016). Together these data suggest a labile behavioral response to rapid changes in the visual environment and potential color matching associated with development under different photic environments; however, visual sensitivity and the long-term consequences of exposure to increased turbidity remain to be tested in this species. As another example, using a manipulative field experiment in southern Lake Malawi, Gray et al. (2011) showed immediate responses of the mbuna community to artificially increased turbidity. In this experiment, fish shifted behavior from territorial defense to foraging in a matter of minutes upon addition of a turbidity slurry from the surface (mimicking potential runoff, for example, during heavy rain). Again, the long-term consequences of increased turbidity in Lake Malawi and other relatively clear lakes are not certain, though the story of Lake Victoria is a likely scenario should, for example, Lake Malawi suffer more severe land-use changes and urbanization in the future.
Cichlids, and many other fishes, rely primarily on vision for those activities (e.g., mate choice and foraging) that promote reproduction and survival; however, multimodal communication is often an integral part of these processes. We may, therefore, expect that when faced with human-induced environmental change of the photic environment, reliance on visual cues may decrease while other sensory modes compensate (van der Sluijs et al. 2011). For example, sound production and audition may be used to choose mates in turbid waters when visual color cues are compromised. African cichlids from Lakes Malawi, Tanganyika, and Victoria have been shown to use sound production in several social contexts, including dominance hierarchies and courtship (e.g., Amorim et al. 2004, 2008; Smith and van Staaden 2009; Verzijden et al. 2010; Maruska et al. 2012; see Lobel et al. 2021). Smith and van Staaden (2009) studied the combined use of visual and auditory cues in several Lake Malawi mbuna, finding intra- and interspecific variations in the use of both modes simultaneously (multimodal) vs. in isolation (unimodal). Several Lake Victoria species (P. nyererei , P. pundamilia , and Neochromis onmicaeruleus ) also have males that produce species-specific sounds during courtship and aggressive displays (Verzijden et al. 2010). In the blind Congo River cichlid, Lamprologus lethops , diminished and covered eyes are accompanied by significantly enlarged lateral line pores, possibly indicating a sensory adaption to low or no light conditions (Schobert et al. 2013). We are only beginning to understand the role of multimodal signaling in a reproductive context in cichlids under natural conditions, and work is therefore required to determine how this might influence the persistence of species in human-altered environments (van der Sluijs et al. 2011). Evidence from other lineages suggests that fish can compensate for altered visual regimes but with unclear consequences. As an example, in threespine stickleback from eutrophied areas of the Baltic Sea, olfactory compensation in visually compromised environments provides an alternative indicator of male quality for females choosing mates (Heuschele et al. 2009). However, choice experiments revealed that the direction of sexual selection changes when visual cues are diminished and females use olfactory cues to choose a mate, thus potentially shifting the evolutionary trajectory of the population.
Compensatory behaviors, the use of alternate sensory modes, or plastic spectral tuning may provide means for cichlids to cope with alterations to the underwater visual environment; however, the long-term consequences for diversity are not yet understood, especially with more rapid, severe, and long-lasting changes to the environment. The stability of environmental change may be an important consideration for future research. If cichlids can compensate in the short term, will they have the adaptive capacity to persist in the long term? Evidence here, for example, from the extreme diversity loss with increased algal turbidity in Lake Victoria, is not promising; however, perhaps the ability of some species to alter coexpression of cone opsin genes dependent on the light environment during development (Dalton et al. 2015) and use behavioral compensation (Gray et al. 2012) suggests that some species may have the adaptive capacity to deal with rapid alterations to the light regime and persist in colorful forms.
6 Future Research
In this review, I have only presented illustrative studies rather than fully reviewed the cichlid visual ecology literature in its entirety. Research seems to be published frequently regarding cichlid fishes, their vision, and evolution; however, as the work synthesized above demonstrates, the potential for further investigation using this system is enormous. Here, I elucidate some of the possible avenues for future research that have emerged from this review.
-
1.
Comparative analyses of African and Neotropical cichlids: One of the most striking areas lacking research on the evolution of visual ecology across the Cichlidae is the geographic disparity in research between African cichlids and Neotropical cichlids. I could find only a few studies that fully examined the opsin gene complement of Neotropical cichlids. The rhodopsin studies by Schott et al. (2014), Torres-Dowdall et al. (2015, 2017), and Hauser et al. (2017), and the examination of opsin gene assemblages and expression in Amazonian species (Escobar-Camacho et al. (2017), are among the first in making broader comparative analyses exploring visual system evolution across the Cichlidae. More comparative work of this kind on the expression of cone opsins and associations with light environment and communication signals could benefit the field by generating new hypotheses about adaptive divergence under different visual backgrounds.
-
2.
The impact of rare cone photoreceptors: What contribution do rare cone photoreceptor classes in the retina make to visual sensitivity? Small differences in the output from MSP and rtQPCR studies and electrophysiology studies suggest the possibility that rare cone types might play an important role in cichlid vision. However, we need to know more about these rare cones, for example, if they are transient with respect to social status, sex, or developmental stage? Or are they distributed in such a way within the retina that enhances color discrimination from a particular direction (see Dalton et al. 2016)?
-
3.
How fast can spectral tuning take place? Color change in cichlid males switching dominance ranks can occur in a matter of seconds and is a physiological color change, whereas longer lasting, morphological alterations to body color that are controlled by a few (e.g., 4–7) genes in cichlids, are therefore likely less labile than opsin gene expression and can result in large (30–100 nm) shifts in peak sensitivity. It would be interesting to know how quickly shifts in spectral sensitivity via changes in cone opsin expression can occur within an individual, especially in light of rapidly changing light environments. Additionally, further understanding of the role of coexpression of opsins as a mechanism for rapid alteration to visual sensitivity is needed.
-
4.
What role does plasticity play in coping with altered photic environments? Plastic responses to altered environments are known to contribute to the adaptive capacity of species. There is some evidence of plasticity in spectral tuning during development (Härer et al. 2017) and adult cichlids (Nandamuri et al. 2017), but future investigations focused on rapidly changing environments would be interesting for evolutionary studies and especially important for making species conservation projections given the changes to the underwater visual environment wrought by human activities in and around freshwater ecosystems.
-
5.
Multimodal communication in altered environments: We know that some cichlids have the adaptive capacity to respond to human-induced alteration of the visual environment. Van der Sluijs et al. (2011) elucidated a number of questions that remain largely unanswered with respect to how sensory and communication systems will adapt to environmental change. They suggested a need to better understand how multiple sensory systems interact under altered conditions and if different systems are as labile as others. One avenue for broadscale investigation would be to examine the diversity of opsin and olfactory genes (which is very large in cichlids; Nikaido et al. 2013) across environmental gradients. Simultaneous evaluation of different sensory modalities at the individual level may also be an area ripe for future research (Escobar-Camacho and Carleton 2015). Since current human-driven environmental change could shift environments out of the detection range for some senses and species, this is a critical area for further investigation (van der Sluijs et al. 2011) for species conservation.
7 Summary
Our understanding of the evolutionary ecology of cichlid vision has increased greatly over the last two decades. Roles of ecological and sexual selection in driving variation in opsin gene expression associated with different light environments and visual signals have been explored, with work on cichlids providing a model for sensory ecologists in general. However, research exploring the links between signal reception, processing, and behavioral response is still needed to fully understand the mechanistic basis for compensatory responses to altered visual environments. Given the extent to which freshwater systems are being altered by human activity, in particular increases in turbidity above natural levels from biotic and abiotic sources that alter the underwater photic environment, our improved understanding of the visual ecology of cichlids is essential for promoting the conservation of natural cichlid populations.
References
Albertson RC, Markert JA, Danley PD, Kocher TD (1999) Phylogeny of a rapidly evolving clade: the cichlid fishes of Lake Malawi, East Africa. Proc Natl Acad Sci U S A 96:5107–5110
Allender CJ, Seehausen O, Knight ME, Turner GF, Maclean N (2003) Divergent selection during speciation of Lake Malawi cichlid fishes inferred from parallel radiations in nuptial coloration. Proc Natl Acad Sci U S A 100:14074–14079
Amorim MCP, Knight ME, Stratoudakis Y, Turner GF (2004) Differences in sounds made by courting males of three closely related Lake Malawi cichlid species. J Fish Biol 65:1358–1371
Amorim MCP, Simoes JM, Fonseca PJ, Turner GF (2008) Species differences in courtship acoustic signals among five Lake Malawi cichlid species (Pseudotropheus spp.). J Fish Biol 72:1355–1368
Baerends GP, Baerends-van Roon JM (1950) An introduction to the study of the ethology of cichlid fishes. E.J. Brill, Leiden
Barlow GW (1983) The benefits of being gold: behavioral consequences of polychromatism in the Midas cichlid, Cichlasoma citrinellum. Environ Biol Fish 8:235–247
Barluenga M, Stolting KN, Salzburger W, Muschick M, Meyer A (2006) Sympatric speciation in Nicaraguan crater lake cichlid fish. Nature 439:719–723
Boughman JW (2001) Divergent sexual selection enhances reproductive isolation in sticklebacks. Nature 411:944–948
Boughman JW (2002) How sensory drive can promote speciation. Trends Ecol Evol 17:571–576
Bowmaker JK (1995) The visual pigments of fish. Prog Retin Eye Res 15:1–31
Bowmaker JK, Hunt DM (2006) Evolution of vertebrate visual pigments. Curr Biol 16:R484–R489
Bowmaker JK, Heath LA, Wilkie SE, Hunt DM (1997) Visual pigments and oil droplets from six classes of photoreceptor in the retinas of birds. Vis Res 37:2183–2194
Brawand D, Wagner CE, Li YI et al (2014) The genomic substrate for adaptive radiation in African cichlid fish. Nature 513:375–381
Browman HI, Gordon WC, Evans BI, O'Brien WJ (1990) Correlation between histological and behavioral measures of visual acuity in a zooplanktivorous fish, the white crappie (Pomoxis annularis). Brain Behav Evol 35:85–97
Carleton KL (2009) Cichlid fish visual systems: mechanisms of spectral tuning. Integr Zool 4:75–86
Carleton KL (2014) Visual photopigment evolution in speciation. In: Hunt DM, Hankins M (eds) The evolution of visual and non-visual pigments. Springer, New York, pp 241–267
Carleton KL, Kocher TD (2001) Cone opsin genes of African cichlid fishes: tuning spectral sensitivity by differential gene expression. Mol Biol Evol 18:1540–1550
Carleton KL, Harosi FI, Kocher TD (2000) Visual pigments of African cichlid fishes: evidence for ultraviolet vision from microspectrophotometry and DNA sequences. Vis Res 40:879–890
Carleton KL, Parry JWL, Bowmaker JK, Hunt DM, Seehausen O (2005) Colour vision and speciation in Lake Victoria cichlids of the genus Pundamilia. Mol Ecol 14:4341–4353
Carleton KL, Spady TC, Kocher TD (2006) Visual communication in east African cichlid fishes: diversity in a phylogenetic context. In: Ladich F, Collin SP, Moller P, Kapoor BG (eds) Communication in fishes. Science Publishers, Enfield, NH, pp 485–515
Carleton KL, Spady TC, Streelman JT, Kidd MR, McFarland WN, Loew ER (2008) Visual sensitivities tuned by heterochronic shifts in opsin gene expression. BMC Biol 6:22
Carleton KL, Hofmann CM, Klisz C, Patel Z, Chircus LM, Simenauer LH, Soodoo N, Albertson RC, Ser JR (2010) Genetic basis of differential opsin gene expression in cichlid fishes. J Evolution Biol 23:840–853
Carleton KL, Dalton BE, Escobar-Camacho D, Nandamuri SP (2016) Proximate and ultimate causes of variable visual sensitivities: insights from cichlid fish radiations. Genesis 54:299–325
Chapman LJ (2015) Low-oxygen lifestyles in extremophilic fishes. In: Reisch R, Plath M, Tobler M (eds) Extremophile fishes - ecology and evolution of teleosts in extreme environments. Springer, Heidelberg, pp 9–31
Cooke GM, Chao NL, Beheregaray LB (2012) Divergent natural selection with gene flow along major environmental gradients in Amazonia: insights from genome scans, population genetics and phylogeography of the characin fish Triportheus albus. Mol Ecol 21:2410–2427
Cronin TW, Johnsen S, Marshall NJ, Warrant EJ (2014) Visual ecology. Princeton University Press, Princeton, NJ
Cummings ME (2004) Modelling divergence in luminance and chromatic detection performance across measured divergence in surfperch (Embiotocidae) habitats. Vis Res 44:1127–1145
Dalton BE, Cronin TW, Marshall NJ, Carleton KL (2010) The fish eye view: are cichlids conspicuous? J Exp Biol 213:2243–2255
Dalton BE, Loew ER, Cronin TW, Carleton KL (2014) Spectral tuning by opsin coexpression in retinal regions that view different parts of the visual field. Proc Roy Soc B 281:20141980
Dalton BE, Lu J, Leips J, Cronin TW, Carleton KL (2015) Variable light environments induce plastic spectral tuning by regional opsin coexpression in the African cichlid fish, Metriaclima zebra. Mol Ecol 24:4193–4204
Dalton BE, de Busserolles F, Marshall NJ, Carleton KL (2016) Retinal specialization through spatially varying cell densities and opsin coexpression in cichlid fish. J Exp Biol 220:266. https://doi.org/10.1242/jeb.149211
Dangles O, Irschick D, Chittka L, Casas J (2009) Variability in sensory ecology: expanding the bridge between physiology and evolutionary biology. Q Rev Biol 84:51–74
Danley DD, Kocher TD (2001) Speciation in rapidly diverging systems: lessons from Lake Malawi. Mol Ecol 10:1075–1086
Duncan WP, Fernandes MN (2010) Physicochemical characterization of the white, black, and Clearwater rivers of the Amazon Basin and its implications on the distribution of freshwater stingrays (Chondrichthyes, Potamotrygonidae). Pan-Am J Aquat Sci 5:454–464
Eigenmann CH, Shafer GD (1900) The mosaic of single and twin cones in the retina of fishes. Am Nat 34:109–118
Elmer KR, Lehtonen TK, Meyer A (2009) Color assortative mating contributes to sympatric divergence of neotropical cichlid fish. Evolution 63:2750–2757
Elmer KR, Kusche H, Lehtonen TK, Meyer A (2010) Local variation and parallel evolution: morphological and genetic diversity across a species complex of Neotropical crater lake cichlid fishes. Phil Trans R Soc B 365:1769–1782
Endler JA (1992) Signals, signal conditions and the direction of evolution. Am Nat 139:s125–s153
Endler JA (1993) The color of light in forests and its implications. Ecol Monogr 63:1–27
Endler JA, Basolo AL (1998) Sensory ecology, receiver biases and sexual selection. Trends Ecol Evol 13:415–420
Engström K (1963) Cone types and cone arrangements in teleost retinae. Acta Zool 44:179–243
Escobar-Camacho D, Carleton KL (2015) Sensory modalities in cichlid fish behavior. Current Opin Behav Sci 6:115–124
Escobar-Camacho D, Ramos E, Martins C, Carleton KL (2017) The opsin genes of Amazonian cichlids. Mol Ecol 26:1343–1356
Fernald RD (1981) Chromatic organization of a cichlid fish retina. Vis Res 21:1749–1751
Fernald RD (1984) Vision and behavior in an African cichlid fish: combining behavioral and physiological analyses reveals how good vision is maintained during rapid growth of the eyes. Am Sci 72:58–65
Fernald RD (1990) Teleost vision: seeing while growing. J Exp Zool 256:167–180
Fernald RD, Liebman PA (1980) Visual receptor pigments in the African cichlid fish, Haplochromis burtoni. Vis Res 20:857–864
Fisher KJ, Recupero DL, Schrey AW, Draud MJ (2015) Molecular evidence of long wavelength spectral sensitivity in the reverse sexually dichromatic convict cichlid (Amatitlania nigrofasciata). Copeia 103:546–551
Fryer G, Iles TD (1972) The cichlid fishes of the Great Lakes of Africa: their biology and evolution. Oliver and Boyd, Edinburgh
Fuller RC, Noa LA, Strellner RS (2010) Teasing apart the many effects of lighting environment on Opsin expression and foraging preference in Bluefin killifish. Am Nat 176:1–13
Genner MJ, Seehausen O, Lunt DH, Joyce DA, Shaw PW, Carvalho GR, Turner GF (2007) Age of cichlids: new dates for ancient lake fish radiations. Mol Biol Evol 24:1269–1282
Gray SM (2016) Muddy waters: the influence of soil and sediment on aquatic life. In: Lal R (ed) Encyclopedia of soil science. Taylor & Francis, Boca Raton, FL. https://doi.org/10.1081/E-ESS3-120052911
Gray SM, McKinnon JS (2007) Linking color polymorphism maintenance and speciation. Trends Ecol Evol 22:71–79
Gray SM, Sabbah S, Hawryshyn CW (2011) Experimentally increased turbidity causes behavioural shifts in Lake Malawi cichlids. Ecol Fresh Fish 20:529–536
Gray SM, McDonnell LH, Cinquemani FG, Chapman LJ (2012) As clear as mud: turbidity induces behavioral changes in the African cichlid Pseudocrenilabrus multicolor. Curr Zool 58:146–157
Gray SM, McDonnell LH, Mandrak NE, Chapman LJ (2016) Species-specific effects of turbidity on the physiology of imperiled blackline shiners (Notropis spp.) in the Laurentian Great Lakes. Endanger Spec Res 31:271–277. https://doi.org/10.3354/esr00774
Grether GF, Kolluru GR, Nersissian K (2004) Individual colour patches as multicomponent signals. Biol Rev 79:583–610
Härer A, Torres-Dowdall J, Meyer A (2017) Rapid adaptation to a novel light environment: the importance of ontogeny and phenotypic plasticity in shaping the visual system of Nicaraguan Midas cichlid fish (Amphilophus citrinellus spp.). Mol Ecol 26:5582–5593
Hauser FE, Ilves KL, Schott RK, Castiglione GM, López-Fernández H, Chang BSW (2017) Accelerated evolution and functional divergence of the dim light visual pigment accompanies cichlid colonization of Central America. Mol Biol Evol 34:2650–2664
Hecky RE (1993) The eutrophication of Lake Victoria. Verh Internat Verein Limnol 25:39–48
Henning F, Renz AJ, Fukamachi S, Meyer A (2010) Genetic, comparative genomic, and expression analyses of the Mc1r locus in the polychromatic Midas cichlid fish (Teleostei, Cichlidae Amphilophus sp.) species group. J Mol Evol 70:405–412
Heuschele J, Mannerla M, Gienapp P, Candolin U (2009) Environment-dependent use of mate choice cues in sticklebacks. Behav Ecol 20:1223–1227
Hofmann CM, Carleton KL (2009) Gene duplication and differential gene expression play an important role in the diversification of visual pigments in fish. Integr Comp Biol 49:630–643
Hofmann CM, O’Quin KE, Marshall NJ, Cronin TW, Seehausen O, Carleton KL (2009) The eyes have it: regulatory and structural changes both underlie cichlid visual pigment diversity. PLoS Biol 7:13
Hofmann CM, O’Quin KE, Marshall NJ, Carleton KL (2010a) The relationship between lens transmission and opsin gene expression in cichlids from Lake Malawi. Vis Res 50:357–363
Hofmann CM, O’Quin KE, Smith AR, Carleton KL (2010b) Plasticity of opsin gene expression in cichlids from Lake Malawi. Mol Ecol 19:2064–2074
Howland HC, Merola S, Basarab JR (2004) The allometry and scaling of the size of vertebrate eyes. Vis Res 44:2043–2065
Jordan R, Kellogg K, Juanes F, Howe D, Stauffer J, Loew ER, Losey G (2004) Ultraviolet reflectivity in three species of Lake Malawi rock-dwelling cichlids. J Fish Biol 65:876–882
Kaufman L (1992) Catastrophic change in species-rich freshwater ecosystems: the lessons of Lake Victoria. Bioscience 42:846–858
Kaufman L, Ochumba P (1993) Evolutionary and conservation biology of cichlid fishes as revealed by faunal remnants in northern Lake Victoria. Cons Biol 7:719–730
Kemp P, Sear D, Collins A, Naden P, Jones I (2011) The impacts of fine sediment on riverine fish. Hydrol Process 25:1800–1821
Kocher TD (2004) Adaptive evolution and explosive speciation: the cichlid fish model. Nat Genet 5:288–298
Konings A (1990) Ad Konings’s book of cichlids and all the other fishes of Lake Malawi. TFH Publishing, New Jersey
Konings A (2007) Malawi cichlids in their natural habitat, 4th edn. Cichlid Press, El Paso, TX
Kröger RHH, Bowmaker JK, Wagner H-J (1999) Morphological changes in the retina of A. pulcher (Cichlidae) after rearing in monochromatic light. Vis Res 39:2441–2448
Kröger RHH, Knoblauch B, Wagner H-J (2003) Rearing in different photic and spectral environments changes the optomotor response to chromatic stimuli in the cichlid fish Aequidens pulcher. J Exp Biol 206:1643–1648
Leclercq E, Taylor JF, Migaud H (2010) Morphological skin colour changes in teleosts. Fish 11:159–193
Levine JS, MacNichol EF Jr (1979) Visual pigments in teleost fishes: effects of habitat, microhabitat, and behavior on visual system evolution. Sens Processes 3:95–131
Levine JS, MacNichol EF Jr (1982) Color vision in fishes. Sci Am 246:108–117
Levine JS, MacNichol EF Jr, Kraft T, Collins BA (1979) Intra-retinal distribution of cone pigments in certain teleost fishes. Science 204:523–526
Levine JS, Lobel PS, MacNichol EF (1980) Visual communication in fishes. In: Ali MA (ed) Environmental physiology of fishes. Springer US, Boston, MA, pp 447–475
Liebman PA (1972) Microspectrophotometry of photoreceptors. In: Dartnall HJA (ed) Handbook of sensory physiology Vol VII/1. Photochemistry of Vision. Springer Verlag, New York, pp 482–528
Lisney TJ, Studd E, Hawryshyn CW (2010) Electrophysiological assessment of spectral sensitivity in adult Nile tilapia Oreochromis niloticus: evidence for violet sensitivity. J Exp Biol 213:1453–1463
Lobel PS, Garner JG, Kaatz IM, Rice AN (2021) Sonic cichlids. In: Abate ME, Noakes DLG (eds) The behavior, ecology and evolution of cichlid fishes. Springer Nature, Dordrecht, pp 443–502. https://doi.org/10.1007/978-94-024-2080-7_13
Loew ER, Lythgoe JN (1978) The ecology of cone pigments in teleost fishes. Vis Res 18:715–722
Loew ER, McFarland WN (1990) The underwater visual environment. In: Douglas R, Djamgoz M (eds) The visual system of fish. Chapman Hall, London, pp 1–44
López-Fernández H (2021) Neotropical riverine cichlids: adaptive radiation and macroevolution at continental scales. In: Abate ME, Noakes DLG (eds) The behavior, ecology and evolution of cichlid fishes. Springer Nature, Dordrecht, pp 135–173. https://doi.org/10.1007/978-94-024-2080-7_5
López-Fernández H, Winemiller KO, Honeycutt RL (2010) Multilocus phylogeny and rapid radiations in Neotropical cichlid fishes (Perciformes: Cichlidae: Cichlinae). Mol Phylogenet Evol 55:1070–1086
López-Fernández H, Arbour JH, Winemiller KO, Kirk O, Honeycutt RL (2013) Testing for ancient adaptive radiations in Neotropical cichlid fishes. Evolution 67:1321–1337
Lythgoe JN (1979) The ecology of vision. Clarendon Press, Oxford
Lythgoe JN (1980) Vision in fishes: ecological adaptations. In: Ali MA (ed) Environmental physiology of fishes. Springer US, Boston, MA, pp 431–445
Maan ME, Seehausen O, Soderberg L, Johnson L, Ripmeester EAP, Mrosso HDJ, Taylor MI, van Dooren TJM, van Alphen JJM (2004) Intraspecific sexual selection on a speciation trait, male coloration, in the Lake Victoria cichlid Pundamilia nyererei. Proc Roy Soc Lond B 271:2445–2452
Maan ME, Hofker KD, van Alphen JJM, Seehausen O (2006) Sensory drive in cichlid speciation. Am Nat 167:947–954
Maan ME, Seehausen O, van Alphen JJM (2010) Female mating preferences and male coloration covary with water transparency in a Lake Victoria cichlid fish. Biol J Linn Soc 99:398–406
Maruska KP, Ung US, Fernald RD (2012) The African cichlid fish Astatotilapia burtoni uses acoustic communication for reproduction: sound production, hearing, and behavioral significance. PLoS One 7:e37612
McElroy DM, Kornfield I (1990) Sexual selection, reproductive behavior, and speciation in the mbuna species flock of Lake Malawi (Pisces: Cichlidae). Environ Biol Fish 28:273–284
McFarland WN, Munz FW (1975) Part III: the evolution of photopic visual pigments in fishes. Vis Res 15:1071–1080
McNeil GV, Friesen CN, Gray SM, Aldredge A, Chapman LJ (2016) Male colour variation in a eurytopic African cichlid: the role of diet and hypoxia. Biol J Linn Soc 118:551–568
Mrosso HDJ, Msuku BS, Seehausen O (2004) Relationship between water transparency and species richness of surviving haplochromines in selected habitats in Mwanza gulf—Lake Victoria. Tanz J Sci 30:101–108
Muske LE, Fernald RD (1987) Control of a teleost social signal: I. Neural basis for differential expression of a color pattern. J Comp Physiol A 160:89–97
Nandamuri SP, Yourick MR, Carleton KL (2017) Adult plasticity in African cichlids: rapid changes in opsin expression in response to environmental light differences. Mol Ecol 26:6036–6052
Natugonza V, Musinguzi L, Kishe MA, van Rijssel JC, Seehausen O, Oguto-Ohwayo R (2021) The consequences of anthropogenic stressors on cichlid fish communities: revisiting Lakes Victoria, Kyoga, and Nabugabo. In: Abate ME, Noakes DLG (eds) The behavior, ecology and evolution of cichlid fishes. Springer Nature, Dordrecht, pp 217–246. https://doi.org/10.1007/978-94-024-2080-7_7
Nelissen MHJ (1991) Communication. In: Keenleyside MHA (ed) Cichlid fishes: behavior, ecology and evolution. Chapman and Hall, London, pp 225–240
Neumeyer C (2003) Wavelength dependence of visual acuity in goldfish. J Comp Physiol A 189:811–821
Nikaido M, Suzuki H, Toyoda A, Fujiyama A, Hagino-Yamagishi K, Kocher TD, Carleton K, Okada N (2013) Lineage-specific expansion of vomeronasal type 2 receptor-like (OlfC) genes in cichlids may contribute to diversification of amino acid detection systems. Genome Biol Evol 5:711–722
Ogutu-Ohwayo R (1990) The decline of the native fishes of lakes Victoria and Kyoga (East Africa) and the impact of introduced species, especially the Nile perch, Lates niloticus, and the Nile tilapia, Oreochromis niloticus. Environ Biol Fish 27:81–96
Olson VA, Owens IPF (1998) Costly sexual signals: are carotenoids rare, risky or required? Trends Ecol Evol 13:510–514
O'Quin KE, Hofmann CM, Hofmann HA, Carleton KL (2010) Parallel evolution of opsin gene expression in African cichlid fishes. Mol Biol Evol 27:2839–2854
O'Quin KE, Smith AR, Sharma A, Carleton KL (2011) New evidence for the role of heterochrony in the repeated evolution of cichlid opsin expression. Evol Dev 13:193–203
Ord TJ, Stamps JA, Losos JB (2010) Adaptation and plasticity of animal communication in fluctuating environments. Evolution 64:3134–3148
Osorio D, Vorobyev M (2008) A review of the evolution of animal colour vision and visual communication signals. Vis Res 48:2042–2051
Parry JW, Carleton KL, Spady TC, Carboo A, Hunt DM, Bowmaker JK (2005) Mix and match color vision: tuning spectral sensitivity by differential opsin gene expression in Lake Malawi cichlids. Curr Biol 15:1734–1739
Pauers MJ, Kuchenbecker JA, Joneson SL, Neitz J (2016) Correlated evolution of short wavelength sensitive photoreceptor sensitivity and color pattern in Lake Malawi cichlids. Front Ecol Evol 4:12
Sabbah S, Laria RL, Gray SM, Hawryshyn CW (2010) Functional diversity in the color vision of cichlid fishes. BMC Biol 8:133
Sabbah S, Gray SM, Boss ES, Fraser JM, Zatha R, Hawryshyn CW (2011) The underwater photic environment of Cape Maclear, Lake Malawi: comparison between rock- and sand-bottom habitats and implications for cichlid fish vision. J Exp Biol 214:487–500
Salzburger W, Meyer A, Baric S, Verheyen E, Sturmbauer C (2002) Phylogeny of the Lake Tanganyika cichlid species flock and its relationship to the central and east African Haplochromine cichlid fish faunas. Syst Biol 51:113–135
Schluter D, Nagel LM (1995) Parallel speciation by natural selection. Am Nat 146:292–301
Schobert CS, Stiassny MLJ, Schwab IR, Zeiss C, Schelly RC, Dubielzig RR (2013) Comparative ocular anatomy in a blind African cichlid fish, Lamprologus lethops. Vet Ophthalmol 16:359–364
Schott RK, Refvik SP, Hauser FE, López-Fernández H, Chang BS (2014) Divergent positive selection in rhodopsin from lake and riverine cichlid fishes. Mol Biol Evol 31:1149–1165
Schulte JE, O'Brien CS, Conte MA, O'Quin KE, Carleton KL (2014) Interspecific variation in Rx1 expression controls opsin expression and causes visual system diversity in African cichlid fishes. Mol Biol Evol 31:2297–2308
Schwanzara SA (1967) The visual pigments of freshwater fishes. Vis Res 7:121–148
Seehausen O (1996) Distribution of and reproductive isolation among color morphs of a rock-dwelling Lake Victoria cichlid (Haplochromis nyererei). Ecol Fresh Fish 6:59–66
Seehausen O, van Alphen JJM, Witte F (1997) Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science 277:1808–1811
Seehausen O, van Alphen JJM, Lande R (1999) Color polymorphism and sex ratio distortion in a cichlid fish as an incipient stage in sympatric speciation by sexual selection. Ecol Lett 2:367–378
Seehausen O, van Alphen JJM, Witte F (2003) Implications of eutrophication for fish vision, behavioural ecology, and species coexistence. In: Crisman TL, Chapman LJ, Chapman CA, Kaufman L (eds) Conservation, ecology and management of African freshwaters. University Press of Florida, Gainesville, pp 268–287
Seehausen O, Terai Y, Magalhaes IS, Carleton KL, Mrosso HDJ, Miyagi R, van der Sluijs I, Schneider MV, Maan ME, Tachida H, Imai H, Okada N (2008) Speciation through sensory drive in cichlid fish. Nature 455:620–626
Shand J, Davies WL, Thomas N, Balmer L, Cowing JA, Pointer M, Carvalho LS, Trezise AE, Collin SP, Beazley LD (2008) The influence of ontogeny and light environment on the expression of visual pigment opsins in the retina of the black bream, Acanthopagrus butcheri. J Exp Biol 211:1495–1503
Shumway CA, Hofmann HA, Dobberfuhl AP (2007) Quantifying habitat complexity in aquatic ecosystems. Fresh Biol 52:1065–1076
Sih A, Ferrari MCO, Harris DJ (2011) Evolution and behavioural responses to human-induced rapid environmental change. Evol Appl 4:367–387
Sioli H (1984) The Amazon and its main affluents: hydrography, morphology of the river courses, and river types. In: Sioli H (ed) the Amazon: limnology and landscape ecology of a mighty tropical river and its basin. Dr. W. Junk Publishers, Dordrecht, pp 127–165
Smith AR, van Staaden MJ (2009) The association of visual and acoustic courtship behaviors in African cichlid fishes. Mar Fresh Behav Phys 42:211–216
Smith AR, Ma K, Soares D, Carleton KL (2011) Relative LWS cone opsin expression determines optomotor thresholds in Malawi cichlid fish. Genes, Brain and Behavior 11:185–192
Spady TC, Seehausen O, Loew ER, Jordan RC, Kocher TD, Carleton KL (2005) Adaptive molecular evolution in the opsin genes of rapidly speciating cichlid species. Mol Biol Evol 22:1412–1422
Spady TC, Parry JWL, Robinson PR, Hunt DM, Bowmaker JK, Carleton KL (2006) Evolution of the cichlid visual palette through ontogenetic subfunctionalization of the opsin gene arrays. Mol Biol Evol 23:1538–1547
Sugawara T, Terai Y, Okada N (2002) Natural selection of the Rhodopsin gene during the adaptive radiation of East African Great Lakes cichlid fishes. Mol Biol Evol 19:1807–1811
Sugawara T, Terai Y, Imai H, Turner GF, Kobmuller S, Sturmbauer C, Shichida Y, Okada N (2005) Parallelism of amino acid changes at the RH1 affecting spectral sensitivity among deep-water cichlids from Lakes Tanganyika and Malawi. Proc Nat Acad Sci USA 102:5448–5453
Sutherland AB, Meyer JL (2007) Effects of increased suspended sediment on growth rate and gill condition of two southern Appalachian minnows. Environ Biol Fish 80:389–403
Takahashi T, Sota T (2016) A robust phylogeny among major lineages of the East African cichlids. Mol Phylogenet Evol 100:234–242
Temple SE (2011) Why different regions of the retina have different spectral sensitivities: a review of mechanisms and functional significance of intraretinal variability in spectral sensitivity in vertebrates. Vis Neurosci 28:281–293
Terai Y, Mayer WE, Klein J, Tichy H, Okada N (2002) The effect of selection on long wavelength-sensitive (LWS) opsin gene of Lake Victoria cichlid fishes. Proc Nat Acad Sci USA 99:15501–15,506
Terai Y, Seehausen O, Sasaki T, Takahashi K, Mizoiri S, Sugawara T, Sato T, Watanabe M, Konijnendijk N, Mrosso HDJ, Tachida H, Imai H, Shichida Y, Okada N (2006) Divergent selection on opsins drives incipient speciation in Lake Victoria cichlids. PLoS Biol 4:e433
Thorpe A, Douglas R, Truscott R (1993) Spectral transmission and short-wave absorbing pigments in the fish lens—I. Phylogenetic distribution and identity. Vision res 33:289–300
Torres-Dowdall J, Meyer A (2021) Sympatric and allopatric diversification in the adaptive radiation of Midas cichlids in Nicaraguan lakes. In: Abate ME, Noakes DLG (eds) The behavior, ecology and evolution of cichlid fishes. Springer Nature, Dordrecht, pp 175–216. https://doi.org/10.1007/978-94-024-2080-7_6
Torres-Dowdall J, Henning F, Elmer KR, Meyer A (2015) Ecological and lineage-specific factors drive the molecular evolution of Rhodopsin in cichlid fishes. Mol Biol Evol 32:2876–2882
Torres-Dowdall J, Pierotti MER, Härer A, Karagic N, Woltering JM, Henning F, Elmer KR, Meyer A (2017) Rapid and parallel adaptive evolution of the visual system of neotropical midas cichlid fishes. Mol Biol Evol 34:2469–2485
Toyama M, Hironaka M, Yamahama Y, Horiguchi H, Tsukada O, Uto N, Ueno Y, Tokunaga F, Seno K, Hariyama T (2008) Presence of Rhodopsin and Porphyropsin in the eyes of 164 fishes, representing marine, diadromous, coastal and freshwater species—A Qualitative and comparative study. Photochem Photobiol 84:996–1002
Utne-Palm AC (2002) Visual feeding of fish in a turbid environment: Physical and behavioural aspects. Mar Freshw Behav Physiol 35:111–128
van der Meer HJ, Anker GC (1984) Retinal resolving power and sensitivity of the photopic system in seven haplochromine species (Teleostei, Cichlidae). Neth J Zool 34:197–209
van der Meer HJ, Anker GC, Barel CDN (1995) Ecomorphology of retinal structures in zooplanktivorous haplochromine cichlids (Pisces) from Lake Victoria. Environ Biol Fishes 44:115–132
van der Meer HJ, van Rijssel JC, Wagenaar LC, Witte F (2012) Photopic adaptations to a changing environment in two Lake Victoria cichlids. Biol J Linn Soc 106:328–341
van der Sluijs I, Gray SM, Amorim MCP, Barber I, Candolin U, Hendry AP, Krahe R, Maan ME, Utne-Palm AC, Wagner H-J, Wong BBM (2011) Communication in troubled waters: the evolutionary implications of changing environments on fish communication systems. Evol Ecol 25:623–640
Verzijden MN, Ripmeester EA, Ohms VR, Snelderwaard P, Slabbekoorn H (2010) Immediate spectral flexibility in singing chiffchaffs during experimental exposure to highway noise. J Exp Biol 213:2575–2581
Vogel JL, Beauchamp DA (1999) Effects of light, prey size, and turbidity on reaction distances of lake trout (Salvelinus namaycush) to salmonid prey. Can J Fish Aquat Sci 56:1293–1297
Wagner H-J, Kröger RHH (2005) Adaptive plasticity during the development of colour vision. Prog Retin Eye Res 24:521–536
Wald G (1935) Pigments of the bullfrog retina. Nature 136:832
Wandell B (1995) Foundations of vision. Sinauer Associates, Sunderland, MA
Wanzenböck J, Zaunreiter M, Wahl CM, Noakes DLG (1996) Comparison of behavioural and morphological measures of visual resolution during ontogeny of roach (Rutilus rutilus) and yellow perch (Perca flavescens). Can J Fish Aquat Sci 53:1506–1512
Weadick CJ, Loew ER, Rodd FH, Chang BSW (2012) Visual pigment molecular evolution in the Trinidadian Pike Cichlid (Crenicichla frenata): A less colorful world for Neotropical cichlids? Mol Biol Evol 29:3045–3060
Witte F, Goldschmidt T, Wanink J, van Oijen M, Goudswaard K, Witte-Maas E, Bouton N (1992) The destruction of an endemic species flock: quantitative data on the decline of the haplochromine cichlids of Lake Victoria. Environ Biol Fishes 34:1–28
Witte F, Seehausen O, Wanink JH, Kishe-Machumu MA, Rensing M, Goldschmidt T (2013) Cichlid species diversity in naturally and anthropogenically turbid habitats of Lake Victoria, East Africa. Aquat Sci 75:169–183
Yokoyama S (2000) Molecular evolution of vertebrate visual pigments. Prog Retin Eye Res 19:385–419
Yokoyama S (2002) Molecular evolution of color vision in vertebrates. Gene 300:69–78
Yokoyama S (2008) Evolution of dim-light and color vision pigments. Annu Rev. Genom Hum Genet 9:259–282
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature B.V.
About this chapter
Cite this chapter
Gray, S.M. (2021). The Evolutionary Ecology of Cichlid Vision. In: Abate, M.E., Noakes, D.L. (eds) The Behavior, Ecology and Evolution of Cichlid Fishes. Fish & Fisheries Series, vol 40. Springer, Dordrecht. https://doi.org/10.1007/978-94-024-2080-7_11
Download citation
DOI: https://doi.org/10.1007/978-94-024-2080-7_11
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-024-2078-4
Online ISBN: 978-94-024-2080-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)