Abstract
Lakes Victoria, Kyoga, and Nabugabo (“the Lake Victoria region”) are remarkable for hosting one of the largest assemblages of cichlid fishes among the African inland lakes. Here, we review the role and severity of anthropogenic and environmental stressors on the cichlid communities in the Lake Victoria region to understand the mechanisms leading to the persistence and resurgence of some of the cichlid fishes. Our review suggests that (1) the native Oreochromis species populations primarily collapsed due to overfishing and that the introduced species and habitat change suppressed their ability to recover; (2) without primary triggers associated with change in the environment and habitat conditions, particularly eutrophication and associated anoxia and reduced water transparency, Nile perch (Lates niloticus) predation alone may not have caused the massive loss of species diversity; and (3) the resurgence of haplochromine cichlids is due to a combination of general improvement in the environment and reduction in L. niloticus abundance, with additionally possibly some rapid ecological adaptations. We conclude that environmental stressors will likely continue to shape the ecosystems in which the remaining endemic cichlid fish diversity continue to evolve, clearly involving genetic exchange between species. If water clarity can be improved again, it is possible to maintain a diverse assemblage of endemic species.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction: Cichlid Diversity and Adaptive Radiation
The family Cichlidae is the most species-rich , non-Ostariophysian freshwater fish family with 250 valid genera and 1723 valid species; when all the known species are included, cichlids constitute more than 2000 species (Fricke et al. 2020). Cichlid fishes have largely radiated into many species involving a combination of ecological opportunity, reproductive isolation (through sexual selection), and hybridization (Kocher 2004; Wagner et al. 2012; Brawand et al. 2014; Seehausen 2015; Meier et al. 2017), and geographical isolation, especially for the Amazon, the Congo, and Madagascar (Seehausen, 2015) . These fishes have evolved and adapted to specific habitat types (e.g. vegetated inshore areas, macrophyte fringes, rocky shores, sandy/muddy bottom, or open waters), trophic levels, and feeding types. This ecological specialization of cichlids has contributed to high levels of endemism, with most species restricted to specific aquatic systems (Fryer and Iles 1972).
The African Great Lakes (AGLs ), in particular, are remarkable for hosting the largest numbers of endemic cichlids: Lake Tanganyika has ca. 250 endemic cichlid species (Coulter 1991); Lake Victoria has ca. 550–700 cichlid species, most endemic to the lake or specific sections of the lake (Witte et al. 2007a); and Lake Malawi has up to 600 endemic cichlid species (Genner et al. 2003). The rates at which the cichlids have radiated and speciated in these lakes vary, with the cichlids of Lake Victoria representing the fastest large vertebrate adaptive radiation (McGee et al. 2020).
On the other hand, the high degree of ecological specialization of endemic African Great Lake cichlids means that only species that are able to adapt quickly are likely to survive under the rapidly changing environmental conditions, largely emanating from increased human activities . During the last half of the twentieth century, the AGLs endured multiple anthropogenic and environmental stressors related to predation and competition from introduced exotic species, habitat degradation (resulting from eutrophication and pollution), over exploitation, invasive plants, and climate variability and change. These stressors were manifested through rapid changes in the limnological conditions of lakes and fish communities (Hecky et al. 2010; Ogutu-Ohwayo et al. 2016), with cichlids being the most affected (Lowe-McConnell 2009). The effects of these stressors on cichlid diversity and abundance have been widely discussed in the scientific literature (see Lowe-McConnell 2003). However, there is still considerable debate among scientists as to which of these stressors were most responsible for the changes in cichlid communities (e.g. van Zwieten et al. 2016; Marshall 2018).
In this chapter, we review the chronology of events leading to the changes in the diversity and abundance of cichlid communities in three different water bodies within the Lake Victoria region (lakes Victoria, Kyoga system, and Nabugabo). The aim is to update the existing literature on the role and severity of each stressor on cichlid diversity and abundance in the Lake Victoria region, and to explore the mechanisms leading to the persistence and resurgence of some of the cichlid fishes. This information will guide discussions on future management and conservation of these fisheries, given that most of the stressors are intractable and may continue to intensify.
2 The Lake Victoria Region: Past Environment and Fish Fauna
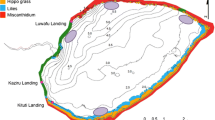
The Lake Victoria region comprises lakes Victoria and Kyoga and the associated satellite lakes (notably Lake Nabugabo) and rivers within their respective catchments (Fig. 1). Lakes Victoria and Kyoga are connected by the Victoria Nile, while Victoria and Nabugabo are separated by an extensive wetland and a sand bar. The three lakes differ in size, habitat types, and general environmental conditions; however, they are quite similar in their native fish fauna (Greenwood 1966; Witte et al. 2009).
Lake Victoria is the largest tropical lake in the world, with an area of 68,800 km2. However, it is relatively shallow (with an average depth of 40 m and maximum depth 80 m) compared to other AGLs, e.g. Lakes Malawi (704 m) and Tanganyika (1470 m) (Lowe-McConnell 2003). In addition, Lake Victoria (estimated to be 15,000 years old) is the youngest compared to the other major AGLs, e.g. Malawi (1–two million years) and Tanganyika (9–12 million years) (Fryer and Iles 1972; Cohen et al. 1993; Johnson et al. 1996). The lake bottom has numerous habitats types, characterized by muddy, sandy, or rocky substrates. The lake shoreline has numerous bays, with patchy swamp vegetation, much of which has been converted for agriculture and settlement by the rapidly growing human population.
During the first half of the twentieth century, the lake was well-mixed, with adequate oxygen to the bottom for most of the year (Talling 1966). The phytoplankton community was dominated by large diatoms, especially Aulocoseira and Stephanodiscus spp. (Mugidde 1993), while large calanoid and cyclopoid copepods and Chironomus spp. (notably Chironomus animalus ) dominated zooplankton and macroinvertebrates, respectively (Worthington 1932a). Fish stock assessments showed that haplochromine cichlids made up more than 80% of the demersal ichthyomass and were well distributed over the entire water column (Kudhongania and Cordone 1974).
The Lake Kyoga system (which is referred to as “Lake Kyoga” in this chapter) is primarily a flooded river valley downstream of Lake Victoria to which it is connected by the upper Victoria Nile. It consists of a system of interconnected lakes, with the main lake having two arms, Kyoga and Kwania, and over 30 smaller lakes that are separated from the main lake by swamps (van den Bossche and Bernacsek 1990; Ogutu-Ohwayo et al. 2013). The area of open water is about 2600 km2, and all the Kyoga lakes have a mean depth of 2–4 m, except the channel marking the course of the Nile River (7–9 m). At the time the first scientific survey of the lake was conducted in 1928, most of Lake Kyoga area was covered by aquatic macrophytes, with a limited portion of open, clear waters (Worthington 1929). The exceptionally heavy El Niño rains of 1961 raised the water level and submerged the open water macrophytes and marginal swamps, expanding the open water portions of the lake (Ogutu-Ohwayo 1995).
Lake Nabugabo is a small shallow open lake lying within an extensive swamp, which fills a former bay on the western shore of Lake Victoria. It is approximately 24 km2, with a mean depth of 4.5 m. It was once a bay of Lake Victoria, but was separated from the main lake by a sand bar about 5000 years ago (Greenwood 1965; Stager et al. 2005). The first detailed survey of Lake Nabugabo in 1962 showed that the lake was well-mixed and saturated with oxygen, although its conductivity was four times lower than that of Lake Victoria (Cambridge Nabugabo Biological Survey 1962).
2.1 Fish Fauna
The Lake Victoria region was originally dominated by two endemic oreochromine cichlids, Oreochromis esculentus (Graham, 1928) and O. variabilis (Boulenger, 1906), and hundreds of haplochromine cichlids the majority of which were endemic (Graham 1929, Worthington 1929, 1932b; Greenwood 1966). The two Oreochromis species were restricted to shallow inshore waters (<20 m deep), but were still segregated in terms of habitat type; O. esculentus was associated with more open waters, while O. variabilis was associated with vegetated inshore areas (Greenwood 1966 ). The same distribution pattern was observed in Lakes Kyoga and Nabugabo, except that the segregation was not obvious due to overlapping habitats (Worthington 1929; Ogutu-Ohwayo, 1995).
Haplochromine cichlids were the most abundant in all the three lakes except that species diversity varied substantially across individual lakes; in Lake Victoria haplochromines comprised over 80% of the bottom dwelling fish biomass (Kudhongania and Cordone 1974). Until 1980, Lake Victoria alone had an estimated 500+ haplochromine species (99% of them endemic) in 12 distinct trophic groups (including phytoplanktivores, detritivores, epilithic and epiphytic algal grazers, plant eaters, oral mollusc shellers, pharyngeal mollusc crushers, zooplanktivores, insectivores, piscivores, parasite eaters, paedophages, and scale eaters) (Witte et al. 1992a, b; Kaufman 1992; Fig. 2). Lake Kyoga had comparatively low diversity, with an estimated 100+ haplochromines species, the majority of which were undescribed (Turner et al. 2001; Ogutu-Ohwayo et al. 2013). Lake Nabugabo had at least eight haplochromines species, five of them endemic to the lake (Ogutu-Ohwayo 1993; Chapman et al. 2003).
Species photos that represent a trophic group for each time period on the research transect (station E-J) in the Mwanza Gulf. Representative species were selected in such a way that, when possible, each time period shows a different species per trophic group. Photos were selected, when possible, based on being representative for the species, the location (Mwanza Gulf), and the time period. When photos for a specific time period were not available or representative, a photo from a different time period was chosen. The year when the photo was taken is indicated in the photo subscript. We are unsure whether the photo of H. ‘lividus-like’ represents H. lividus as described by Greenwood correctly. Photos made by HEST, O. Seehausen, M. A. Kishe, F.N. Moser, and J. C. van Rijssel
2.2 Exposure and Sensitivity to Multiple Anthropogenic and Environmental Stressors
The Lake Victoria region is one of the most densely populated areas not only in Africa but globally, with a population density of more than 500 persons/km2 (Kolding et al. 2014). The effects of high human population on aquatic living resources are well-known: increased demand on aquatic living resources, leading to (1) overexploitation of indigenous fisheries, (2) introduction of non-indigenous species to cope with declining catches, and (3) alteration of habitat quality through land use change in the catchment basin. Most urban areas are also located in close proximity to these water bodies, where increased loss of vegetation cover and riparian wetlands has exposed the lakes to both municipal and industrial waste pollution (Seto et al. 2012).
Cichlids, especially haplochromines, depend on light for foraging and for communication, including the visual signals used for mate choice; for example, males have diverse breeding colours which are used in female mate choice and male competition over territories (Selz et al. 2014). Changes in habitat conditions, especially when accompanied with reduction in water clarity, can affect reproductive isolation and ecological differentiation, with negative effects on overall cichlid diversity (Seehausen et al. 1997, 2008).
3 Relationship Between Multiple Stressors and Cichlid Diversity and Abundance in the Lake Victoria Region
The chronology of events in the course of the 20th and 21st centuries, leading to the changes in abundance and diversity of cichlids in the Lake Victoria region is summarized in Table 1. The main stressors can, therefore, be summarized in five major categories: (1) overfishing, (2) non-native fish species, (3) habitat degradation (eutrophication), (4) invasive weeds, and (5) climate variability and change.
3.1 Overfishing
Intensive fishing was the first major threat that contributed to the decline and complete disappearance of native Oreochromis species in the Lake Victoria region (Graham 1929; Worthington 1929). Some researchers have suggested fishing also contributed to the decline of large piscivorous haplochromine cichlids in Lake Victoria, paving the way for establishment of L. niloticus (Goudswaard et al. 2008; van Zwieten et al. 2016), although this hypothesis has been questioned (see Marshall, 2018). Unlike other stressors that intensified after the 1970s, the effects of intensive fishing on oreochromine cichlid species became manifest in the early years of the twentieth century, following improvements in fishing technology and transport communication to the urban markets (Table 1). Prior to 1910, the native Oreochromis species were exploited using simple traditional fishing gears, consisting of basket traps, hooks, and papyrus seines, to meet the small local market demand (Graham 1929; Jackson 1971). These fishing methods were less efficient and had little impact on the stocks (Ogutu-Ohwayo 1990). The arrival of the railway in Kisumu, Kenya, in 1908, which improved access to urban markets, concomitant with the introduction of more efficient flax gillnets, substantially increased the fishing effort. O. esculentus was the largest and the most abundant and commercially important among the native oreochromines; its stocks decreased rapidly, with the catch rates declining from about 78 fish per net in 1910 to fewer than 2 fish per net by the 1950s (Fig. 3). Continuous improvement in fishing technology, e.g. the shift from flax to synthetic gillnets in the 1950s and a shift to smaller mesh size nets (3.5-4 inches) that harvested immature fish, further depressed the native fisheries (Jackson 1971; Ogutu-Ohwayo 1990).
In Lake Kyoga, native Oreochromis species also contributed more than 90% of commercial catches during the 1940s and early 1950s, but the stocks declined rapidly during the 1960s and collapsed in the subsequent decade (Ogutu-Ohwayo 1990; Ogutu-Ohwayo et al. 2013). Catch rates, for instance, decreased from about 30 fish per net per night in the 1940s to about 7 fish per net per night in the 1960s, after which the fishery collapsed and never recovered. As observed in Lake Victoria, the decline in the stocks of native oreochromines in Lake Kyoga coincided with the introduction of synthetic gillnets in 1951 and 1952, which were more efficient than the indigenous fishing gears that consisted of locally made papyrus traps (Table 1).
Why did the native oreochromine cichlids disappear very quickly? The events outlined in Table 1 show that the native Oreochromis species collapsed well before the introduced species became established and that overfishing was primarily responsible, while competition for resources and/or hybridization with the introduced species, particularly O. niloticus (Linnaeus, 1758), only suppressed their ability to recover. Yet, the present-day fishing effort in these lakes is higher by orders of magnitude compared to the effort in the 1950s and 1960s (Fig. 4c; Fig. 5d), and fishing technology has improved by several folds, but the introduced Oreochromis species (O. niloticus , O. leucostictus Trewavas 1933) and Coptodon species (Coptodon zilli i (Gervais, 1848), C. rendalli Boulenger, 1897) have not collapsed. It is likely that the native fisheries were inherently less productive and less adaptable, with no capacity to sustain a commercial fishery of any nature. The native oreochromine cichlids had a narrow habitat range and were restricted to shallow inshore waters, although in the shallow lakes such as Kyoga and Nabugabo, they were found over the entire lake. First, this narrow habitat range means that the fishes had limited capacity to compete with the versatilie introduced cichlids once they became established. Secondly, these shallow areas, where the native oreochromine cichlids were concentrated, are easily accessed by fishermen, even with the most primitive parachute or dugout canoes, making the fish more susceptible to overexploitation. The introduced cichlids (both Oreochromis and Coptodon spp.) also have a narrow habitat range (the fishes are restricted to areas <20 m deep, NaFIRRI unpublished trawl survey data 2003–2017). These areas are also easily accessed by fishermen; however, the species, particularly O. niloticus, have adaptable life history parameters (growth rates, size at maturity, and fecundity) that enable the species to withstand high fishing pressure and changes in the environment (Bwanika et al. 2004; Njiru et al. 2007; Natugonza et al. 2015).
Limited resilience to exploitation has also been observed among haplochromine cichlids. Once the stocks of the larger native oreochromine cichlids were depleted, especially in Lake Victoria, fishing shifted to the smaller haplochromine cichlids, which were harvested using small mesh seines and trawl nets. Data from the Tanzanian waters show that the overall catch rates of haplochromines rapidly declined from 1753 kg h−1 in 1976 to 680 kg h−1 in 1982 (Goudswaard and Ligtvoet 1988). During this period, non-human predation was minimal because the native predator populations (especially the catfishes) were already collapsed due to overfishing (Goudswaard and Witte 1997), while the introduced Nile perch, Lates niloticus (Linnaeus, 1758), was not yet abundant; eutrophication, especially in the offshore waters, only became pronounced beginning in the mid-1980s (Hecky et al. 2010). It seems possible, therefore, that haplochromines were already exhibiting signs of distress from fishing even before the impacts of L. niloticus and eutrophication became apparent. Limited resilience of haplochromine cichlids to fishing has also been observed in other cichlid-dominated lakes such as Lake Malawi (Turner 1977a, b).
3.2 Non-native Fish Species
Although non-native species introductions can have positive outcomes, e.g. trophic subsidy, competitive release, or predatory release, in many instances, when they become invasive, they exert negative ecological and evolutionary impacts ranging from behavioural shifts of native species in the presence of invaders to complete restructuring of food webs (Rodriguez 2006; Schlaepfer et al. 2010). The events in the Lake Victoria region represent classical examples of both positive impacts (e.g. increased fishery production and transformed livelihoods of fishers and riparian communities) and negative impacts (e.g. biodiversity loss) of the introduced fish species (Witte et al. 1992a, b; Ogutu-Ohwayo 1990, 1993).
The large piscivorous L. niloticus and four other species, O. niloticus , O. leucostictus , C. zillii , and C. rendalli , were introduced into the Lake Victoria region in the 1950s and 1960s (Ogutu-Ohwayo and Hecky 1991; Pringle 2005). Introduction of L. niloticus was primarily intended to feed on haplochromine cichlids, which were abundant in this lake, and convert them into a suitable commercially important table and recreational fish. The introductions in Lakes Kyoga and Nabugabo were possibly experimental, though conducted late, following the recommendation by Worthington (1932b). Worthington (1932b) suggested that the introduction of L. niloticus into Lake Victoria would be considered if experimental introduction in Lake Nabugabo could be effected and there was evidence of a natural balance between L. niloticus and other fishes in the lake. In contrast, both Oreochromis and Coptodon species were introduced to compensate for the diminishing catches of the native oreochromine cichlids (O. esculentus and O. variabilis ). However, only O. niloticus contributed substantially to the commercial fisheries (Ogutu-Ohwayo 1990).
There were time variations in the establishment of introduced species in different lakes, although the pattern of establishment was generally consistent. In Lake Kyoga, both L. niloticus and O. niloticus took approximately 10 years before they contributed substantially to the fisheries. Landings increased eightfold between 1960 and 1980, but later declined and stabilized at low numbers, especially beginning in the mid-1980s (Fig. 4). The reduction in landings coincided with the increase in fishing effort as manifested by the twofold increase in the number of fishing boats (Fig. 4). In Lake Victoria, however, the establishment of introduced species took a longer period (approximately 25 years), but after establishment, the fishery dynamics were similar to those of Lake Kyoga. Landings of L. niloticus also rapidly increased from almost nothing to 330,000 tons between 1980 and 1990, but declined afterwards and stabilized at lower levels following the rapid rise in fishing effort (Fig. 5). Although the introduced Oreochromis and Coptodon species started appearing in the catches at the same time in both Lake Victoria and Lake Kyoga (i.e. in the mid-1960s), the contribution to the commercial fisheries in Lake Victoria was only realized in the late 1990s (Fig. 5). The parallel success of O. niloticus alongside the piscivorous L. niloticus may be related to their origin: both species are native to the Nile below the Murchison Falls, Lake Albert, Lake Turkana, the Chad basin, and rivers of West Africa (Lowe-McConnell 1987). Therefore, O. niloticus may have already evolved behavioural responses to overcome predation by L. niloticus .
Annual catches of (a) L. niloticus and (b) introduced cichlids, mainly O. niloticus, and (c) fishing effort on Lake Kyoga (Data from Ogutu-Ohwayo et al. 2013)
3.2.1 Impact on Native Oreochromine Cichlids
The introduced cichlids may not have directly contributed to the collapse of native oreochromine cichlids, but rather exacerbated the effects of intensive fishing and habitat modification through direct competition and/or hybridization. The cichlids that were introduced in the Lake Victoria region have overlapping ecological requirements as the native oreochromines. In lakes Victoria and Kyoga, the native O. esculentus and O. variabilis were ecollogically segregated, where the smaller O. variabilis were common in areas covered by aquatic vegetation closer to the shore, while O. esculentus were found in more open waters. The introduced O. leucostictus and C. zillii occupied the same vegetated inshore habitat as O. variabilis, which may have created competition for space. Oreochromis niloticus , on the other hand, occupied more open waters, directly competing with O. esculentus .
The disappearance of native oreochromine cichlids has also been observed in other lakes where O. niloticus has been introduced; O. spirulus (Günther, 1894) and O. macrochir (Boulenger, 1912), for instance, have disappeared from lakes Naivasha (Kenya) and Itasy (Madagascar), respectively, following the introduction of O. niloticus (Siddiqui 1977; Welcomme 1984).
3.2.2 Impact on Haplochromine Cichlids
The role of L. niloticus in the massive loss of haplochromine cichlid diversity has been widely discussed (van Zwieten et al. 2016; Marshall 2018). Generally, the increase in L. niloticus biomass in all the lakes where the species was introduced was accompanied by a rapid decline in haplochromines as manifested in both the landings (Fig. 5) and standing stock biomass (Fig. 6). Whereas landings may be influenced by other factors, particularly changes in fishing behaviour and catchability of the target species, the concomitant reduction in standing stock biomass shows that abundance of haplochromines was greatly diminished. An estimated 40% of the 500+ haplochromine species disappeared from Lake Victoria alone, which has been attributed to several factors: (1) predation by L. niloticus (Witte et al. 1992a, b; Marshall 2018) and (2) a combination of stressors, including fishing, eutrophication, and predation by L. niloticus (Witte et al. 2007a, b), and competition (McGee et al. 2015).
Different haplochromine trophic groups declined at different rates. The large piscivorous haplochromines disappeared first in both lakes Victoria and Nabugabo, possibly due to competition with L. niloticus for the same food resources (McGee et al. 2015) and/or the reduced ability to avoid L. niloticus predation due to increased blooms and reduction in visibility (Witte et al. 2007b; also see Sect. 3.3). The smaller species of the detritivore and zooplanktivore trophic guilds declined slowly, especially the latter group (Witte et al. 2007b). During the 1990s, however, some haplochromines started recovering. Data on the past and the present trends in biomass and spatial distribution of haplochromines suggest there were multiple causes for both the decline and resurgence. In the northern part of Lake Victoria, for instance, haplochromines declined drastically between 1982 and 1986, coinciding with the upsurge in L. niloticus biomass, and started recovering during the late 1990s following a reduction in the predator biomass (Fig. 6). However, the timing of haplochromine decline and resurgence is also consistent with the period of severe eutrophication and anoxia (Hecky et al. 1994) and the general improvement in water quality (higher oxygen levels, higher visibility, and weaker stratification; Sitoki et al. 2010; Marshall et al. 2013; van Rijssel et al. 2016), respectively. It is difficult to attribute the changes in haplochromines to a single cause, but the trends in Lakes Nabugabo and Kyoga, which are clearly associated with L. niloticus dynamics (Chapman et al. 2003; Ogutu-Ohwayo et al. 2013), suggest that predation played a major role in the northern portion of the lake.
Data from the southern part of Lake Victoria also show the timing of haplochromine declines to be consistent with the L. niloticus upsurge; however, the resurgence is inconsistent with the L. niloticus dynamics. In the Mwanza Gulf, haplochromines started recovering between 1990 and 1992 at the peak biomass of L. niloticus (Seehausen et al. 1997; Witte et al. 2007a, b). In addition, despite the low diversity, the current biomass of haplochromines, especially in the zooplanktivore and detritivore guilds, is comparable to the pre-collapse levels, and they occupy demersal open waters (Witte et al. 2007b; Kishe-Machumu et al. 2015), where L. niloticus densities are highest (Taabu-Munyaho et al. 2014). Therefore, predation by L. niloticus may have exacerbated effects of the trawl fishery (Witte et al. 2007b) and environmental change (Kudhongania and Chitamwebwa 1995; van Zwieten et al. 2016), while the improvement in limnological conditions (Sitoki et al. 2010; Marshall et al. 2013) and adaptation to the changed environment (van der Meer et al. 2012; van Rijssel and Witte 2013; van Rijssel et al. 2015, 2017) may have facilitated the recovery. In summary, it seems there are multiple causes for both the decline and resurgence of the haplochromines in different parts of the lake, but it remains difficult to pinpoint which of these contributed most to either the decline or resurgence.
3.3 Habitat Degradation
Lake Victoria changed abruptly from an oligotrophic–mesotrophic system to a eutrophic one during the early 1980s, a period when the introduced L. niloticus was beginning to expand. The emergence of these stressors at the same time has made the separation of primary and secondary drivers of changes in cichlid assemblages, especially haplochromines, a challenging task. Nutrient loading in Lake Victoria has been ongoing since the 1920s, primarily from land use changes in the catchment (Hecky 1993; Hecky et al. 1994). However, the impacts of eutrophication became manifest especially after 1980 with the onset of blue-green algal blooms, anoxia, reduced water transparency, and even fish kills (Hecky 1993; Mugidde 1993; Sitoki et al. 2010; Hecky et al. 2010; van Rijssel et al. 2016). The concentration of total phosphorus (TP) in the lake doubled; dissolved silicon decreased by one order of magnitude; the concentration of chlorophyll-a increased fivefold; water transparency diminished; and deep water anoxia intensified. The algal communities shifted from the dominance of diatoms to blue-green algae; the larger diatom genera, e.g. Aulocoseira , were replaced by the smaller thinly silicified genera, especially Nitzschia , which were adapted to low silicon concentrations (Hecky et al. 2010). The zooplankton community changed from dominance of the large-bodied herbivorous calanoid copepods and cladocerans to the small-bodied predatory cyclopoid copepods (Mwebaza-Ndawula 1994; Wanink et al. 2002). Midge larvae, particularly Chironomus and Chaoborus spp. and the detritivorous shrimp, Caridina nilotica , which were tolerant to anoxia, increased (Sekiranda et al. 2004; Goudswaard et al. 2006); C. nilotica subsequently became an important prey item in the diet of L. niloticus (Budeba and Cowx 2007).
These changes affected cichlid fish communities differently. The disappearance of large diatoms, Aulocoseira , which were the main food for O. esculentus , affected remnant stocks of this species. On the other hand, the increase in abundance of blue-green algae improved food availability for O. niloticus , which had the capacity to digest the algae (Moriarty and Moriarty 1973). This may also explain why O. niloticus , besides out-competing O. esculentus , was also able to dominate introduced Oreochromis and Coptodon species.
The blooms created by dense mats of blue-green algae decreased light penetration, but also decomposition enhanced turbidity and decreased overall water transparency. Light is important in foraging, mate recognition, and predator avoidance among cichlids, especially haplochromines (Seehausen et al. 1997, 2003). Poor vision can affect feeding specialization, and predatory species are expected to suffer most due to increased competition amongst each other and with L. niloticus. It is, therefore, not surprising that the large piscivorous haplochromines disappeared first in both lakes Victoria and Nabugabo, possibly because of increased competition with the introduced L. niloticus for prey and also the reduced ability to evade predation (Witte et al. 1992a, b; Ogutu-Ohwayo 1993; McGee et al. 2015).
The rate at which haplochromines declined between 1980 and 1985 was rapid, reflecting more of an abrupt recruitment failure, although predation by L. niloticus could have contributed to the decline. Detritivorous haplochromines were originally the most abundant among the 11 major trophic groups and formed approximately 60% of the total haplochromine biomass in the sublittoral and demersal waters of the Mwanza Gulf (Witte et al. 1992a, 2007b). Ogutu-Ohwayo and Hecky (1991) suggested that the development of the deep water anoxia changed habitat conditions for these detritivores and exposed them to extreme predation by L. niloticus . The development of deep water anoxia is not doubted (Hecky et al. 1994); however, the conditions may not have favoured L. niloticus , considering that the species is quite sensitive to hypoxia (Schofield and Chapman 2000). Although Wanink et al. (2001) found some L. niloticus in deep waters with dissolved oxygen (DO) concentration less than 2 mgL−1, including areas where DO was almost zero, their numbers were comparatively lower. Under such stressful anoxic conditions, L. niloticus may not have had either ecological or numerical advantage to decimate detritivorous haplochromines. Instead, eutrophication and reduced water transparency could have severely interfered with haplochromine reproduction and feeding (Seehausen et al. 1997, 2003; Seehausen and van Alphen 1998; van Zwieten et al. 2016; van Rijssel et al. 2016) and caused steep decline in their populations and loss of species diversity.
The effect of habitat degradation can also be seen in the decline of native oreochromine cichlids in both lakes Victoria and Kyoga, although the effect was secondary (exacerbating the effects of overfishing). The increase in lake levels, following the El Niño rains of 1961/1962, and the subsequent submersion of open water macrophytes and marginal wetlands of Lake Kyoga as well as the inshore wetlands of Lake Victoria (Ogutu-Ohwayo et al. 2013) greatly modified the inshore habitat, which benefited the introduced species but led to the collapse of the less adaptive native oreochromine cichlids. The preferred habitat for the inshore vegetation-dwelling O. variabilis , for instance, was lost, and the species was later displaced from by the introduced O. leucostictus , which occupied the same habitat. For the open water native O. esculentus , which were more abundant and preferred by fishermen, the submersion of macrophytes presented two fundamental risks: (1) the fish was exposed to more fishing pressure as more open water fishing grounds were opened; and (2) the rise in lake levels also opened up more areas for O. niloticus spawning, allowing its proliferation.
In Lake Kyoga, at the time Worthington conducted the first survey in Lake Kyoga in 1928, most of the small fishes were found in aquatic macrophyte beds, which provided refugia from the large native predators, e.g. catfishes and lungfish (Worthington 1929). The submersion of macrophyte cover by the El Niño opened up the lake and exposed these small fishes, notably haplochromines, to increased predation pressure. Additionally, although there has not been any systematic collection of environmental data from Lake Kyoga on the nutrient status over time, the concentration of total phosphorus was three times higher soon after the El Niño rains in early 1960s compared to 1990/2000 (Evans 1962; Ogutu-Ohwayo et al. 2013). It is, therefore, likely that the submersion and decay of aquatic vegetation was also accompanied by increase in nutrients and productivity in Lake Kyoga, as well as reduced water clarity.
There is no doubt that L. niloticus predation contributed to the decline of haplochromines in the Lake Victoria region. However, the events leading to the disappearance and resurgence of haplochromines suggest that in the absence of primary environmental triggers, which either affected recruitment directly or compromised the ability of haplochromines to evade predators, L. niloticus alone may not have caused such a mass destruction of haplochromine cichlids and especially not the massive loss of species diversity. The spontaneous decline and gradual recovery of haplochromines following deterioration and general improvement in water quality, respectively, may not be a mere coincidence. Zooplanktivorous haplochromines started recovering before the detritivores, despite higher abundance of the latter before the decline in the mid-1980s (Witte et al. 2007b), and are abundant in offshore coastal demersal waters where L. niloticus densities are highest (Taabu-Munyaho et al. 2014). In addition, some surviving detritivores that were previously restricted to the littoral zones (0–6 m depth) have also extended their habitat and are now also present in the sublittoral zones (6–20 m depth; Seehausen et al. 1997; Kishe-Machumu et al. 2015) where L. niloticus is also common.
3.4 Invasive Plants
There are two aquatic weeds, water hyacinth (Eichhornia crassipes ) and Kariba weed (Salvinia molesta ), both native to South America, which have had substantial ecological impact in the Lake Victoria region although information on Kariba weed is scanty. Whereas the water hyacinth may have been in the Nile basin and Lake Victoria catchment since the 1950s, it was first sighted in Lake Kyoga in 1988 and in Lake Victoria in 1989 (Twongo 1991). By the mid-1990s, the weed covered 60% of the shoreline (ca. 570 ha) of the length of Lake Kyoga (Twongo 1996) and approximately 18,000 ha of the inshore areas of Lake Victoria (Albright et al. 2004). In both lakes, the weed formed extensive mats, with the zones below extensive water hyacinth mats anoxic, which changed the composition of the biota. For instance, the invertebrate communities under the water hyacinth mats were dominated by low oxygen-tolerant types such as chironomids (Wanda et al. 2001), while the fish community was dominated a few hypoxia-tolerant species, e.g. marbled lungfish Protopterus aethiopicus Heckel, 1851. However, the dense water hyacinth mats may have also provided refugia for the littoral, vegetation-dwelling, haplochromines, especially Astatotilapia species against L. niloticus predation (Ogutu-Ohwayo et al. 2013).
The water hyacinth was brought under control in the late 1990s by introduction of a weevil (Neochetina sp.) (Wilson et al. 2007), although the heavy storms of 1997 could have also enhanced their mortality by physically dislodging their mats (Williams et al. 2007). The weed is still abundant and forms dense mats (Fig. 7) in bays adjacent to the major towns and cities, and continued nutrient enrichment through discharge of municipal and industrial wastes could lead to its full-scale resurgence.
3.5 Climate Variability and Change
Climate variability and change contribute to changes in hydrology, stratification and circulation dynamics, and composition of aquatic organisms, especially to those that have the capacity to adapt fast to the changed conditions (Barange and Perry 2009; Ogutu-Ohwayo et al. 2016). Generally, climate-induced changes in fish species diversity and abundance have been most prevalent in relatively shallow systems, e.g. lakes Chad (FAO 2012), Ngami and Liambezi (Moyle et al. 2009), Rukwa (Mbungu 2015), Chilwa (Njaya et al. 2011), and Wamala (Natugonza et al. 2015), although changes have also been reported in relatively larger lakes such as Victoria (Birkett et al. 1999; Hecky et al. 2010; van Zwieten et al. 2016; van Rijssel et al. 2016) and Tanganyika (Verburg et al. 2003; Cohen et al. 2016). Warming of Lake Victoria generally begun in the early twentieth century, but the impacts on the fisheries became manifest beginning in the 1980s through eutrophication (and associated anoxia) and dramatic shifts in fish communities (Hecky et al. 2010).
The effects of warming on the physical properties of African Great Lakes have been widely discussed (O’Reilly et al. 2003; Verburg et al. 2003; MacIntyre 2012). The periodic decreases in vertical mixing observed during conditions of higher rainfall, reduced winds, and higher relative humidity can substantially affect the productivity of the lake. Vertical mixing is an important driver of the lakes’ physico-chemical processes, including nutrient circulation, phytoplankton abundance, water transparency, and dissolved oxygen (DO) levels. An increase in physical stability and shallower mixing depths due to warming may intensify anoxia, and this has been observed in Lake Victoria (Hecky et al. 1994). The predicted increase in climate variability and warming (IPCC 2014) will likely affect these processes further , and fisheries will likely be dominated by species that can persist and adapt fast to the changed conditions . Data consistent with this come from work on some of the resurgent haplochromine cichlids in Lake Victoria that have undergone ecological changes of habitat choice (Seehausen et al. 1997; Kishe-Machumu et al. 2015) and diet (Katunzi et al. 2003; Kishe-Machumu et al. 2008, 2017; van Rijssel et al. 2015, 2017) and also structural modifications that may be adaptations to the changing environment and prey (van der Meer et al. 2012; van Rijssel and Witte 2013; van Rijssel et al. 2015, 2016).
4 Persistence of Cichlid Fishes Under Multiple Stressors
The ability to adapt quickly to the changed habitat conditions and to the pressures from exploitation, predation, competition, and warming climate is central to the persistence of fish under multiple stressors (Petitjean et al. 2019). The disappearance of the native Oreochromis and many haplochromine species from lakes Victoria, Kyoga, and Nabugabo as the stressors intensified suggest limited resilience of the species or of the species-rich communities to cope with the changed conditions. On the other hand, some of the cichlids that have persisted, and whose stocks have increased, have undergone life history modifications which may be adaptations to the new environments. The ability to adapt quickly to changing conditions may determine the future diversity and abundance of cichlid fishes more generally, not only in the Lake Victoria region but also in other lakes.
Among the oreochromine and tilapiine cichlids, only O. niloticus has increased and become abundant in lakes Victoria, Kyoga, and Nabugabo (Ogutu-Ohwayo 1990). The success of O. niloticus is associated with its life history attributes, including early maturation, a broad diet, and a wide habitat range (Fryer and Iles 1972; Martin et al. 2010). The species grows and matures faster, can mature at a wide range of body sizes, has a wider food spectrum and is less habitat restricted than any of the other Oreochromis or Coptodon species in the system, and can alter its fecundity and size of eggas in response to stressful conditions, including exploitation, habitat change, and predation (Njiru et al. 2007; Bwanika et al. 2004; Natugonza et al. 2015). Further, O. niloticus is one of the few oreochromine cichlids with the capacity to digest blue-green algae (Moriarty and Moriarty 1973), which have become dominant in most lakes following eutrophication. It is likely that these life history attributes have contributed to the success of O. niloticus , becoming now one of the most important commercial fish species in capture fisheries in the African Great Lakes region despite heavy exploitation and environmental changes (Ogutu-Ohwayo et al. 2016).
Some haplochromine species persisted and increased in numbers, especially after the 2000s, despite the diminished diversity (Chapman et al. 2003; Seehausen et al. 1997; Ogutu-Ohwayo et al. 2013; Kishe-Machumu et al. 2015). A few species previously considered lost from the main lake have been recorded in marginal habitats in the periphery of the main lake and in the small satellite water bodies in the Lake Victoria basin (Mwanja et al. 2001; Katunzi and Kishe-Machumu 2004; Katunzi et al. 2003, 2010; Seehausen et al. 2015). Rocky shore habitats within the main lake also still support a great diversity and concentration of haplochromines (Seehausen 1996; Seehausen et al. 1997). Recent surveys of deep water sections of Lake Victoria also suggest the reappearance of deep water species, e.g. Gaurochromis spp. and Labrochromis spp., and even large piscivores of the genera Harpagochromis and Prognathochromis (O. Seehausen pers. comm.).
The recovery of haplochromine biomass after its nearly complete collapse, especially in the southern part of the lake, is remarkable, but its species diversity is only a fraction of what it was before the collapse. Prior to 1980, detritivorous haplochromines formed about 60% of total haplochromine biomass in Mwanza Gulf of Lake Victoria, while zooplanktivorous haplochromines constituted about 30% (Witte et al. 1992b). However, zooplanktivorous haplochromines recovered first, starting in 1991 (Seehausen et al. 1997). By 2006, this trophic group constituted 70% of the biomass in open waters despite low species diversity, although detritivores overtook them in abundance afterwards (Fig. 8).
Catch rates of Lates niloticus and haplochromine cichlids (in different trophic groups) in the Mwanza Gulf from 1979 to 2008. Haplochromine catch rates are based on 10 minutes of fishing using a small bottom trawler (Data for haplochromines from Witte et al. 2007a, for the period 1979–2005, and Kishe-Machumu et al. 2015, for the period 2006 and 2008, and that of L. niloticus from Goudswaard et al. 2006)
Despite the increased abundance in the Mwanza Gulf over the past two decades, the species diversity has not recovered to its former state. Witte et al. (1992a) reported 72 different species belonging to 12 trophic groups on the research transect in the Mwanza Gulf during the period 1979–1982 based on trawl catches. This number dropped to only 12 species belonging to 5 trophic groups during the period 1987–1990, but has since been slowly increasing (i.e. 17 species in 5 trophic groups for the period 1991–1995 (Seehausen et al. 1997), 19 species in 5 trophic groups for the period 2001–2005, 27 species in 9 trophic groups for the period 2006–2008, and 26 species in 6 trophic groups during 2014 (Fig. 9); also, see Fig. 2 for the trophic groups) despite the diversity remaining below 50% of the pre-1980 period. However, it should be noted that the sampling effort in 2014 was for only six sampling days over a 2-month period (September–October) and was less intense compared to the one reported in Kishe-Machumu et al. (2015) for the period 2006–2008, which might have underestimated the species diversity for 2014.
Haplochromine species richness (numbers observed on the same research transect) in the Mwanza Gulf from 1979 to 2014. Species numbers are based on collection periods of 1-4 years and are depicted as species number per year. (Sources of data: Witte et al. (1992a), Seehausen et al. (1997), Witte et al. (2007a), and Kishe-Machumu et al. (2015))
The resurging haplochromine cichlids exhibit both morphological and ecological modifications, reflecting possible adaptation to the changing environment (Seehausen et al. 1997; Witte et al. 2008; van der Meer et al. 2012; van Rijssel and Witte 2013; van Rijssel et al. 2015, 2016, 2017; Kishe-Machumu et al. 2017). The zooplanktivores constitute majorly three species; Haplochromis (Yssichromis) laparogramma Greenwood and Gee 1969, “Haplochromis” tanaos van Oijen and Witte 1996, and Haplochromis (Yssichromis) pyrrhocephalus Witte and Witte-Maas, 1987 (Kishe-Machumu et al. 2015). These species have also extended their distribution from deeper (8–14 m) (Witte et al. 1992a, b) to shallow waters or vice versa (for “H”. tanaos from shallow sand bottom to deep mud bottoms, Seehausen et al. 1997) in response to the changed environment and also developed a new feeding strategy by shifting their diet from zooplankton to insects and other larger preys (Katunzi et al. 2003; Kishe-Machumu et al. 2015, 2017; van Rijssel et al. 2017). These dietary shifts are consistent with several anatomical changes in Y. pyrrhocephalus : the cheek depth has increased by 9% (Witte et al. 2008); the premaxilla in the upper jaw and the muscle responsible for pharyngeal biting has changed, providing greater biting force (e.g. 6% larger dentigerous arm of the premaxilla and a 64% larger musculus levator posterior depth) (van Rijssel et al. 2015; Witte et al. 2008). The ratio of the length of the intestine to standard length has also decreased (i.e., by 29–35% for the detritivorous H. ‘paropius-like’, and 35-44% for the “pooled detritivores”, including Haplochromis (Enterochromis) antleter , Haplochromis (E.) cinctus , Haplochromis (E.) coprologus , Haplochromis (E.) katunzii , Haplochromis (E.) ‘purple head), associated with the change in diet (Kishe-Machumu et al. 2008). The head surface–caudal peduncle area ratio has also decreased by 4% in Y. pyrrhocephalus , possibly as an adaptive response to L. niloticus predation (van Rijssel and Witte 2013). The gill surface area in Y. pyrrhocephalus has increased by 50–64% (van Rijssel et al. 2016; Witte et al. 2008) over the period, which may be a response to the reduced dissolved oxygen levels (Wanink et al. 2001). It is not clear whether the observed behavioural and morphological changes in the resurging zooplanktivorous haplochromine species are heritable and hence a product of evolution or merely phenotypic plasticity. Given that several of these species hybridized with other species in the recent decades, it is also possible that rapid evolution was facilitated by such exchange of genetic material between species (Marques et al. 2019). During the 2000s, environmental variables, including wind speed, oxygen levels, water transparency, and water temperature increased again, and the gill surface area of this species correspondingly decreased (van Rijssel et al. 2016). This observation suggests that these fishes have rapid adaptive responses to environmental changes, which may have contributed to the fast recovery of Y. pyrrhocephalus . The genetic or developmental basis of putatively adaptive responses of recovering cichlid species is a larger and important area of future investigation (see van Rijssel et al. 2021).
Data collected from Lake Nabugabo show that haplochromines that reappeared in the open part of this lake are those that had persisted in marginal macrophytes from where they spread into open waters following the decline in L. niloticus stocks (Schofield and Chapman 2000; Chapman et al. 2003). These wetlands provided structural refugia, but only favoured a few haplochromines with the capacity to survive low oxygen conditions (especially in the non-endemic genera Astatoreochromis, Pseudocrenilabrus , and Astatotilapia ). Unfortunately, most of these wetland habitats, which, apart from being structural refugia, play a critical role in purifying water and trapping organic wastes, are increasingly being degraded, exposing the lake to land-based siltation which in turn interferes with feeding and mate choice. Similarly, haplochromine resurgence in Kyoga began following the decrease of L. niloticus stocks after 1990 and an increase in vegetation cover associated with water hyacinth infestation. Nonetheless, the species diversity has also remained low, and the taxa that reappeared are dominated by the insectivorous Astatotilapia spp., which are adapted to inhabiting marginal macrophyte beds (Ogutu-Ohwayo 1995; Ogutu-Ohwayo et al. 2013).
5 Conclusions
In this chapter, we have reviewed the past changes in the diversity and abundance of cichlid fish in three different water bodies within the Lake Victoria region to further understand the role and severity of multiple stressors on the cichlid fish communities and to explore the mechanisms leading to the persistence and/or resurgence of some of the cichlid species. We conclude that (1) native oreochromines were primarily collapsed by overfishing and that the introduced oreochromines and Coptodon ("tilapias") and habitat change suppressed their ability to recover once fishing shifted to the introduced species; (2) without primary environmental triggers, particularly eutrophication and reduced water transparency, which either interfered with haplochromine reproduction and feeding or compromised their ability to evade predators, L. niloticus predation alone may not have caused the massive collapse of haplochromine biomass and especially not the massive loss of species diversity; (3) haplochromine resurgence is linked to a combination of general improvement in the environment, eco-morphological changes that may include adaptations , and the reduction in L. niloticus abundance; and (4) environmental stressors are difficult to predict, but will likely continue to intensify and to shape the ecosystems in which cichlid fishes evolve, clearly involving genetic exchange between species. Cichlid fishes, therefore, may increasingly become dominated by species by capacity to adjust and adapt fast to the changing environmental conditions. If water clarity can be improved again, however, it is possible to maintain a diverse assemblage of endemic species. Research should, therefore, aim to provide information for management of highly species-diverse fisheries for resilience as multiple stressors continue to intensify.
References
Albright TP, Moorhouse TG, McNabb TJ (2004) The rise and fall of water hyacinth in Lake Victoria and the Kagera river basin, 1989–2001. J Aquat Plant Manag 42:73–84
Barange M, Perry RI (2009) Physical and ecological impacts of climate change relevant to marine and inland capture fisheries and aquaculture. In: Cochrane K, De Young C, Soto D, Bahri T (eds) Climate change implications for fisheries and aquaculture: overview of current scientific knowledge. FAO fisheries and aquaculture technical paper, vol 530. FAO, Rome, pp 7–106
Birkett C, Murtgudde R, Allan T (1999) Indian Ocean climate event brings floods to East Africa’s lake and the Sudd marsh. Geophys Res Lett 26:1031–1034
Brawand D, Wagner CE, Li YI et al (2014) The genomic substrate for adaptive radiation in African cichlid fish. Nature 513:375–381
Budeba YL, Cowx IG (2007) The role of the freshwater shrimp Caridina nilotica (Roux) in the diet of the major commercial fish species in Lake Victoria, Tanzania. Aquat Ecosyst Health Manage 10:368–380
Bwanika GN, Makanga B, Kizito Y, Balirwa, J (2004) Observations on the biology of Nile tilapia, Oreochromis niloticus L., in two Ugandan crater lakes. Afr J Ecol 42:93–101
Bwanika GN, Chapman LJ, Kizito Y, Balirwa, J (2006) Cascading effects of introduced Nile perch (Lates niloticus) on the foraging ecology of Nile tilapia (Oreochromis niloticus). Ecol Freshw Fish 15:470–481
Cambridge Nabugabo Biological Expedition (1962) Preliminary report (mimeographed). Makerere University, Kampala
Chapman LJ, Chapman CA, Shofield PJ et al. (2003) Fish faunal resurgence in Lake Nabugabo, East Africa. Conserv Biol 17:500–511
Cohen AS, Bills R, Cocquyt CZ, Caljon AG (1993) The impact of sediment pollution on biodiversity in Lake Tanganyika. Conserv Biol 7:667–677
Cohen AS, Gergurich EL, Kraemer BM et al (2016) Climate warming reduces fish production and benthic habitat in Lake Tanganyika, one of the most biodiverse freshwater ecosystems. Proc Nat Acad Sci 113:9563
Coulter GW (1991) Lake Tanganyika and its life. Oxford University Press, New York
Fricke R, Eschmeyer WN, Fong JD (2020) Eschmeyer's catalogue of fishes: genera/species by family/subfamily. http://researcharchivecalacademyorg/research/ichthyology/catalog/SpeciesByFamilyasp#Cichlidae
Evans JH (1962) The distribution of phytoplankton in some central east African waters. Hydrobiologia 19:299–315
FAO (2012) Climate change implications for fishing communities in the Lake Chad basin. What have we learnt and what can we do better? FAO/Lake Chad Basin Commission Workshop 18–20 November 2011, N′Djamena, Chad. FAO Fisheries and Aquaculture proceedings 25, Rome
Fryer G, Iles TD (1972) The cichlid fishes of the Great Lakes of Africa: their biology and evolution. Oliver and Boyd, London
Genner MJ, Seehausen O, Cleary DFR, Knight ME, Michel E, Turner GF (2003) How does the taxonomic status of allopatric populations influence species richness within African cichlid fish assemblages? J Biogeogr 31(1):93–102
Goudswaard PC, Ligtvoet W (1988). Recent developments in the fishery for haplochromines (Pisces: Cichlidae) and Nile perch (L.) (Pisces: Centropomidae) in Lake Victoria. In: CIFA, Report of the 4th session of the subcommittee for the development and management of the fisheries in Lake Victoria, Kisumu, Kenya, 6–10 April 1987, FAO Fish. Rep, pp 101–112
Goudswaard PC, Witte F (1997) The catfish fauna of Lake Victoria after the Nile perch upsurge. Environ Biol Fish 49:210–243
Goudswaard KPC, Witte F, Wanink JH (2006) The shrimp Caridina nilotica in Lake Victoria (East Africa), before and after the Nile perch increase. Hydrobiologia 563:31–44
Goudswaard PC, Witte F, Katunzi EFB (2008) The invasion of an introduced predator, Nile perch (Lates niloticus, L.) in Lake Victoria (East Africa): chronology and causes. Environ Biol Fish 81:127–139
Graham M (1929) The Victoria Nyanza and its fisheries. A report on the survey of Lake Victoria 1927-1928 and appendices. Crown Agents for Colonies, London
Greenwood PH (1965) The fishes of Lake Nabugabo, Uganda. Bull Br Mus Nat Hist 12:313–357
Greenwood PH (1966) The fishes of Uganda. The Uganda Society, Kampala
Hecky RE (1993) The eutrophication of Lake Victoria. Verh Int Verein Limnol 25:39–48
Hecky RE, Bugenyi FWB, Ochumba PBO (1994) De-oxygenation of the deep waters of Lake Victoria, East Africa. Limnol Oceanogr 39:1476–1481
Hecky RE, Mugidde R, Ramlal P (2010) Multiple stressors cause rapid ecosystem change in Lake Victoria. Freshw Biol 55:19–42
IPCC (2014) Climate change: the physical science basis. IPCC, WG1
Jackson PBN (1971) The African Great Lakes fisheries: past, present and future. Afr J Trop Hydrobiol Fish 1:35–49
Johnson TC, Scholz CA, Talbot MR et al (1996) Late Pleistocene desiccation of Lake Victoria and rapid evolution of cichlids fishes. Science 273:1091–1093
Katunzi EB, Kishe-Machumu MA (2004) Changes in population structures of the major species in selected satellite lakes around Lake Victoria following changes in fishing effort. Tanzan J Sci 30:53–64
Katunzi EFB, Zoutendijk J, Goldschmidt T et al (2003) Lost zooplanktivorous cichlid from Lake Victoria reappears with a new trade. Ecol Freshwater Fish 12:237–240
Katunzi EFB, Mbonde A, Waya R, Mrosso HDJ (2010) Minor water bodies around southern Lake Victoria-a replica of lost biodiversity. Aquat Ecosyst Health Manag 13:277–283
Kaufman L (1992) Catastrophic change in species rich freshwater ecosystems. Bioscience 42:846–858
Kishe-Machumu MA, Witte F, Wanink JH (2008) Dietary shift in benthivorous cichlids after the ecological changes in Lake Victoria. Anim Biol 58(4):401–417
Kishe-Machumu MA, van Rijssel JC, Wanink J, et al (2015) Differential recovery and spatial distribution pattern of haplochromine cichlids in the Mwanza gulf of Lake Victoria. J Great Lakes Res 41:454–462
Kishe-Machumu MA, van Rijssel JC, Poste A, Hecky RE, Witte F (2017) Stable isotope evidence from formalin-ethanol-preserved specimens indicates dietary shifts and increasing diet overlap in Lake Victoria cichlids. Hydrobiologia 791:155–173
Kocher TD (2004) Adaptive evolution and explosive speciation: the cichlid fish model. Nat Rev Genet 5:288–298
Kolding J, Medard M, Mkumbo O, van Zwieten P (2014) Status, trends and management of the Lake Victoria fisheries. In: Welcomme R, Valbo-Jorgensen J, Halls A (eds) Inland fisheries evolution: case studies from four continents. FAO, Rome, pp 49–62
Kudhongania AW, Chitamwebwa DBR (1995) Impact of environmental change, species introductions and ecological interactions on the fish stocks of Lake Victoria. In: Pitcher TJ, Hart PJB (eds) The impact of species changes in African lakes. Chapman and Hall, London, pp 19–32
Kudhongania AW, Cordone AJ (1974) Batho-spatial distribution patterns and biomass estimate of the major demersal fishes in Lake Victoria. Afr J Trop Hydro biol Fish 3:15–31
Lowe-McConnell RH (1987) Ecological studies in tropical fish communities. Cambridge University Press, Cambridge
Lowe-McConnell RH (2003) Recent research in the African Great Lakes: fisheries, biodiversity and cichlid evolution. Freshwater Forum 20:4–64
Lowe-McConnell RH (2009) Fisheries and cichlid evolution in the African Great Lakes: progress and problems. Freshwater Rev 2:131–151
LVFO (2016) Regional status report on Lake Victoria biennial frame surveys between 2000 and 2016. Lake Victoria fisheries organisation (LVFO). Jinja, Uganda
MacIntyre S (2012) Climate variability, mixing dynamics, and ecological consequences in the African great lakes. In: Goldman CR, Kamagai M, Robarts RD (eds) Climatic change and global warming of Inland waters: impacts and mitigations for ecosystems and societies. Wiley, New York, pp 311–336
Marques DA, Meier JI, Seehausen O (2019) A combinatorial view on speciation and adaptive radiation. TREE 34:531–544
Marshall B (2018) Guilty as charged. Nile perch caused the decline of haplochromines in Lake Victoria. Can J Fish Aquat Sci 75:1542–1559
Marshall BE, Ezekiel CN, Gichuki J et al (2013) Has climate change disrupted stratification patterns in Lake Victoria, East Africa? Afr J Aquat Sci 38:249–253
Martin WC, Valentine MM, Valentine JF (2010) Competitive interactions between invasive Nile tilapia and native fish: the potential for altered trophic exchange and modification of food webs. PLoS One 5(12):e14395. https://doi.org/10.1371/journal.pone.0014395
Mbungu W (2015) Climate change vulnerability assessment in the Lake Rukwa Basin. Lake Rukwa Basin water board. Mbeya, Tanzania
McGee MD, Borstein SR, Neches RY et al (2015) A pharyngeal jaw evolutionary innovation facilitated extinction in Lake Victoria cichlids. Science 350:1077–1079
McGee MD, Borstein SR, Meier JI et al (2020) The ecological and genomic basis of explosive adaptive radiation. Nature 586(7827):75–79. https://doi.org/10.1038/s41586-020-2652-7
Meier JI, Marques DA, Mwaiko S et al. (2017) Ancient hybridization fuels rapid cichlid fish adaptive radiations. Nat Commun 8:14363
Moriarty DJW, Moriarty CM (1973) The assimilation of carbon from phytoplankton by two herbivorous fishes: Tilapia nilotica and Haplochromis nigripinnis. J Zool 171:41–56
Moyle PB, Purkey DR, Mosepele K et al (2009) Fish, floods, and ecosystem engineers: aquatic conservation in the Okavango Delta, Botswana. Bioscience 59:53–64
Mugidde R (1993) The increase in phytoplankton primary productivity and biomass in Lake Victoria (Uganda). Verh Int Verein Limnol 25:846–849
Mwanja WW, Armoudlian AS, Wandera SB et al (2001) The bounty of minor lakes: the role of small satellite water bodies in evolution and conservation of fishes in the Lake Victoria region, East Africa. Hydrobiologia 458:55–62
Mwebaza-Ndawula L (1994) Changes in relative abundance of zooplankton in northern Lake Victoria, East Africa. Hydrobiology 272:259–264
Natugonza V, Ogutu-Ohwayo R, Efitre J et al (2015) The responses of Nile tilapia, Oreochromis niloticus (Linnaeus, 1758) in Lake Wamala (Uganda) to changing climatic conditions. Lakes Reserv Res Manag 20:101–119
Njaya F, Snyder KA, Jamu D et al (2011) The natural history and fisheries ecology of Lake Chilwa, southern Malawi. J Great Lakes Res 37:15–25
Njiru M, Okeyo-Owuor JE, Muchiri M et al (2007) Changes in population characteristics and diet of Nile tilapia Oreochromis niloticus (L.) from Nyanza gulf of Lake Victoria, Kenya: what are the management options? Aquat Ecosyst Health Manage 10:434–442
O’Reilly CM, Alin SR, Pilsnier PD, Cohen AS, McKee BA (2003) Climate change decreases aquatic ecosystem productivity of Lake Tanganyika, Africa. Nature 424:766–768
Ogutu-Ohwayo R (1990) The decline of the native fishes of lakes Victoria and Kyoga (East Africa) and the impact of introduced fish species, especially Nile perch, Lates niloticus, and Nile tilapia, Oreochromis niloticus. Environ Biol Fish 27:81–96
Ogutu-Ohwayo R (1993) The effects of predation by the Nile perch, Lates niloticus L., on the fishes of Lake Nabugabo, with suggestions for conservation of endangered endemic cichlids. Conserv Biol 7:701–711
Ogutu-Ohwayo R (1995) Diversity and stability of fish stocks in lakes Victoria, Kyoga, and Nabugabo after establishment of introduced species. In: Pitcher TJ, Hart PJB (eds) The impact of species changes in African lakes. Chapman and Hall, London, pp 59–81
Ogutu-Ohwayo R, Hecky RE (1991) Fish introductions in Africa and some of their implications. Can J Fish Aquat Sci 48:8–12
Ogutu-Ohwayo R, Odongkara K, Okello W et al (2013) Variations and changes in habitat, productivity, composition of aquatic biota and fisheries of the Kyoga lake system: lessons for management. Afr J Aquat Sci 38:1–14
Ogutu-Ohwayo R, Natugonza V, Musinguzi L et al (2016) Implications of climate variability and change for African lake ecosystems, fisheries productivity, and livelihoods. J Great Lakes Res 42:498–510
Petitjean Q, Jean S, Gandar A, Cote J, Laffaille P, Jacquin L (2019) Stress responses in fish: from molecular to evolutionary processes. Sci Total Environ 684(20):371–380
Pringle RM (2005) The origins of the Nile perch in Lake Victoria. BioScience 55:80–787
Rodriguez LF (2006) Can invasive species facilitate native species? Evidence of how, when, and why these impacts occur. Biol Invasions 8:927–939
Schlaepfer MA, Sax DF, Olden JD (2010) The potential conservation value of non-native species. Conserv Biol 3:428–437
Schofield PJ, Chapman LJ (2000) Hypoxia tolerance of introduced Nile perch: implications for survival of indigenous fishes in the Lake Victoria basin. Afr Zool 35:35–42
Seehausen O (1996) Distribution of and reproductive isolation among color morphs of a rock-dwelling Lake Victoria cichlid (Haplochromis nyererei). Ecol Freshw Fish 5:195–202
Seehausen O (2015) Process and pattern in cichlid radiations – inferences for understanding unusually high rates of evolutionary diversification. New Phytol 207:304–312
Seehausen O, van Alphen JJM (1998) The effect of male coloration on female mate choice in closely related Lake Victoria cichlids (Haplochromis nyererei complex). Behav Ecol Sociobiol 42:1–8
Seehausen O, van Alphen JJM, Witte F (1997) Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science 277:1908–1911
Seehausen O, van Alphen JJM, Witte F (2003) Implications of eutrophication for fish vision, behavioral ecology and species coexistence. In: Crisman TL, Chapman LJ, Chapman CA, Kaufman LS (eds) Conservation ecology, and management of African freshwaters. University Press of Florida, Gainesville, pp 268–287
Seehausen O, Terai Y, Magalhaes IS et al (2008) Speciation through sensory drive in cichlid fish. Nature 455:620–626
Seehausen O, Kishe-Machumu MA, Mwaiko S (2015) Lake Victoria freshwater biodiversity assessment: a field and taxonomic survey of the fish diversity and their habitats in the upper Kagera satellite lakes, Tanzania. Technical report, IUCN
Selz OM, Thommen R, Maan ME, Seehausen O (2014) Behavioural isolation may facilitate homoploid hybrid speciation in cichlid fish. J Evol Biol 27(2):275–289
Sekiranda SBK, Okot-Okumu J, Bugenyi FWB et al (2004) Variations in composition of macro-benthic invertebrates as an indication of water quality status in three bays in Lake Victoria. UJAS 9:396–411
Seto CK, Guneralp B, Hutyra RL (2012) Global forecasts of urban expansion to 2030 and direct impacts on biodiversity and carbon pools. PNAS 109:16083–16088
Siddiqui AQ (1977) Lake Naivasha fishery and its management together with a note on the food habits of fishes. Biol Conserv 12:217–227
Sitoki L, Gichuki J, Ezekiel C et al (2010) The environment of Lake Victoria (East Africa): current status and historical changes. Int Rev Hydrobiol 95:209–223
Stager JC, Westwood J, Grzesik DA, Cumming B (2005) A 5500-year environmental history of Lake Nabugabo, Uganda. Palaeogeogr Palaeoclimatol Palaeoecol 218(3–4):347–354
Taabu-Munyaho A, Nyamweya CS, Sitoki L et al (2014) Spatial and temporal variation in the distribution and density of pelagic fish species in Lake Victoria, East Africa. Aquat Ecosyst Health Manage 17:52–61
Talling JF (1966) The annual cycle of stratification and phytoplankton growth in Lake Victoria (East Africa). Int Rev Hydrobiol 51:545–621
Turner GF (1977a) Changes in the size structure of cichlid populations in Lake Malawi resulting from bottom trawling. Can J Fish Aquat Sci 34(2):232–238
Turner GF (1977b) Some effects of demersal trawling in Lake Malawi (Nyasa) from 1968 to 1974. J Fish Biol 10:261–273
Turner GF, Seehausen O, Knight ME, Allender CJ, Robinson RL (2001) How many species of cichlids are there in African Lakes? Mol Ecol 10:793–806
Twongo T (1991) Status of water hyacinth in Uganda. In: Greathead A, de Groot P (eds) Control of Africa’s floating water weeds. Commonwealth Science Council, Zimbabwe, pp 55–57
Twongo T (1996) The impact of water hyacinth on near-shore environment of Lake Victoria and Kyoga (East Africa). In: Thomas C, Johnshon T, Odada EO (eds) The limnology, climatology and paleoclimatology of the east African Great Lakes. Gordon and Breach Publishers, Philadelphia, pp 633–642
van den Bossche JP, Bernacsek GM (1990) Source book for the inland fishery resources of Africa. FAO, Rome
van der Meer HJ, van Rijssel JC, De Wagenaar LC, Witte F (2012) Photopic adaptations to a changing environment in two Lake Victoria cichlids. Biol J Linn Soc 106:328–341
van Rijssel JC, Witte F (2013) Adaptive responses in resurgent Lake Victoria cichlids over the past 30 years. Evol Ecol 27:253–267
van Rijssel JC, Hoogwater ES, Kishe-Machumu MA et al (2015) Fast adaptive responses in the oral jaw of Lake Victoria cichlids. Evolution 69:179–189
van Rijssel JC, Hecky RE, Kishe-Machumu MA (2016) Climate variability in combination with eutrophication drives adaptive responses in the gills of Lake Victoria cichlids. Oecologia 182:1187–1201
van Rijssel JC, Hecky RE, Kishe-Machumu MA, Witte F (2017) Changing ecology of Lake Victoria cichlids and their environment: evidence from C13 and N15 analyses. Hydrobiologia 791:175–191
van Rijssel JC, de Jong RCM, Kishe MA, Witte F (2021) Rapid evolutionary responses in cichlids: genetics of adaptation, morphology and taxonomic implications. In: Abate ME, Noakes DLG (eds) The behavior, ecology and evolution of cichlid fishes. Springer Nature, Dordrecht, pp 247–283. https://doi.org/10.1007/978-94-024-2080-7_8
van Zwieten PAM, Kolding J, Plank M et al. (2016) The Nile perch invasion in Lake Victoria: cause or consequence of the haplochromine biomass decline. Can J Fish Aquat Sci 73:622–643
Verburg P, Hecky RE, Kling H (2003) Ecological consequences of warming in Lake Tanganyika. Science 301:505–507
Wagner CE, Harmon LJ, Seehausen O (2012) Ecological opportunity and sexual selection together predict adaptive radiation. Nature 487:366–369
Wanda FM, Twongo T, Denny P (2001) The impact of water hyacinth, Eichhornia crassipes (Mart) Solm on the abundance and diversity of aquatic macro-invertebrates along the shores of northern Lake Victoria, Uganda. Hydrobiologia 452:79–88
Wanink JH, Kashindye JJ, Goudswaard PCK, Witte F (2001) Dwelling at the oxycline: does increased stratification provide a predation refugium for the Lake Victoria sardine Rastrineobola argentea? Freshw Biol 46:75–85
Wanink JH, Katunzi EFB, Goudswaard KPC et al (2002) The shift to smaller zooplankton in Lake Victoria cannot be attributed to the ‘sardine’ Rastrineobola argentea (Cyprinidae). Aquat Living Res 15:37–43
Welcomme RL (1984) International transfers of inland fish species. In: Courteney WR, Stauffer JF (eds) Distribution, biology and management of exotic fishes. John Hopkins Press, Baltimore, pp 22–40
Williams ED, Hecky ER, Duthie HC (2007) Water hyacinth decline across Lake Victoria—was it caused by climatic perturbation or biological control? A reply. Aquat Bot 87:94–96
Wilson JRU, Ajuonu O, Center TD et al (2007) The decline of water hyacinth on Lake Victoria was due to biological control by Neochetina spp. Aquat Bot 87:90–93
Witte F, Goldschmidt T, Wanink J et al (1992a) The destruction of an endemic species flock: quantitative data on the decline of haplochromine cichlids of Lake Victoria. Environ Biol Fish 34:1–28
Witte F, Goldschmidt T, Goudswaard PC et al (1992b) Species extinction and concomitant ecological changes in Lake Victoria. Neth J Zool 42:214–232
Witte F, Wanink JH, Kishe-Machumu M (2007a) Species distinction and the biodiversity crisis in Lake Victoria. Am Fish Soc 136:1146–1159
Witte F, Wanink JH, Kishe-Machumu M et al (2007b) Differential decline and recovery of haplochromine trophic groups in the Mwanza gulf of Lake Victoria. Aquat Ecosyst Health Manage 10:416–433
Witte F, Welten M, Heemskerk M et al (2008) Major morphological changes in a Lake Victoria cichlid fish within two decades. Biol J Linn Soc 94:41–52
Witte F, van Oijen MJP, Sibbing FA (2009) Fish fauna of the Nile. In: Dumont HJ (ed) The Nile: origin, environments, limnology and human use. Springer Science, New York, pp 647–675
Worthington EB (1929) A report of the fishing survey of lakes Albert and Kyoga, March–July 1929. Crown agents for Colonies, London
Worthington EB (1932a) Scientific results of the Cambridge expedition to East African Lakes 1930-31. I. General introduction and station list. Zool J Linnean Soc 38:99–119
Worthington EB (1932b) A report on the fisheries of Uganda investigated by the Cambridge expedition to the East African Lakes, 1931–1932. Crown Agents for Colonies, London
Acknowledgement
We like to thank the scientists, technicians, and crew at the three research institutes (NaFIRRI, KMFRI, and TaFIRRI) for their effort in collecting the data presented in this chapter. We also thank Martien van Oijen for help with identifying haplochromine species in Fig. 2.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature B.V.
About this chapter
Cite this chapter
Natugonza, V., Musinguzi, L., Kishe, M.A., van Rijssel, J.C., Seehausen, O., Ogutu-Ohwayo, R. (2021). The Consequences of Anthropogenic Stressors on Cichlid Fish Communities: Revisiting Lakes Victoria, Kyoga, and Nabugabo. In: Abate, M.E., Noakes, D.L. (eds) The Behavior, Ecology and Evolution of Cichlid Fishes. Fish & Fisheries Series, vol 40. Springer, Dordrecht. https://doi.org/10.1007/978-94-024-2080-7_7
Download citation
DOI: https://doi.org/10.1007/978-94-024-2080-7_7
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-024-2078-4
Online ISBN: 978-94-024-2080-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)