Abstract
Parkinson’s disease (PD) is a common neurodegenerative disorder, which involves degeneration of dopaminergic neurons that are present in the substantia nigra pars compacta (SNpc) region. Many factors have been identified that could lead to Parkinson’s disease; however, almost all of them are directly or indirectly dependent on Ca2+ signaling. Importantly, though disturbances in Ca2+ homeostasis have been implicated in Parkinson’s disease and other neuronal diseases, the identity of the calcium channel remains elusive. Members of the transient receptor potential canonical (TRPC) channel family have been identified as a new class of Ca2+ channels, and it could be anticipated that these channels could play important roles in neurodegenerative diseases, especially in PD. Thus, in this chapter we have entirely focused on TRPC channels and elucidated its role in PD.
Pramod Sukumaran and Yuyang Sun contributed equally
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
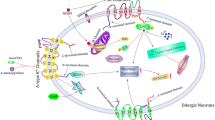
Calcium (Ca2+) is an important element that functions as a prominent regulator for processes such as gene regulation, neuronal cell growth and differentiation, motility and axonal development, and even neuronal cell death [6, 9, 65]. Thus, it is not surprising that disruption of Ca2+ homeostasis in neuronal cells results in decreased neuronal functions leading to neurodegenerative diseases such as Parkinson’s, Huntington’s, and Alzheimer’s [1, 10, 11, 46, 77, 78]. Due to these outcomes, Ca2+ homeostasis is strictly maintained in neuronal cells. Cells have evolved a multitude of mechanisms to regulate cellular Ca2+ levels and Ca2+ channels, and pumps play a key role in this regulation. Cumulative studies suggest that both excessive elevation and attenuation of intracellular Ca2+ will lead to neuronal degeneration through different mechanisms as suggested in this chapter (Fig. 8.1). Increased intracellular calcium [Ca2+]i concentrations mainly via the AMPA or NMDA channels lead to enhanced activation of Ca2+-dependent processes that are normally inert or functional at low Ca2+ levels, thereby causing metabolic imbalances which result in neuronal death [3, 13, 20]. In contrast, decreased [Ca2+]i, upon store depletion, could induce ER stress or inhibit activation of proteins that are essential for cell survival [48]. Thus, different actions of Ca2+ in neuronal cells could be dependent not only on its cellular concentration but also on the ion channels that modulate Ca2+ entry [44, 48, 74]. Ca2+ channels, mainly the transient receptor potential canonical (TRPC) channels, have recently emerged as a key regulator of Ca2+ homeostasis in neuronal cell function [9, 57]. Further, TRPC channel function and expression are altered in various neuronal diseases such as Parkinson’s disease (PD) [4, 57, 59]. PD is a neurodegenerative disorder, which is characterized with progressive degeneration of dopaminergic neurons and affects the aging population. Thus, in this chapter we will focus on the functional implication of TRPC channels in neuronal cell function and their roles in PD.
8.2 TRPC Functions in Neurons
Neuronal cells are heavily dependent on Ca2+ signaling for their function and survival [32]. Initiation of the action potential activates the voltage-gated calcium channels that modulate neurosecretion. Growing evidence suggests that this mode of Ca2+ entry is also essential in maintaining the cytosolic, ER, and mitochondrial Ca2+ levels. Importantly, as TRPC proteins have been identified as putative calcium channels that are activated by second messenger-mediated store depletion, they could play a critical role in neuronal survival, proliferation, and differentiation. There is evidence that TRPCs are highly expressed in all regions of the central nervous system (CNS) [24], but in some cases the expression of individual TRPC channels is altered during development, especially as observed with TRPC1 and TRPC3, which are more expressed in embryonic CNS than in adult neurons [62]. Furthermore, neuronal growth in the presence of growth factors, such as basic fibroblast growth factor (bFGF), was dependent on Ca2+ entry through TRPC channels, especially TRPC1, 4, but not TRPC5, suggesting their important roles in neuronal growth and survival [17]. Importantly, inhibiting the function of TRPC channels or even silencing of TRPC1 alone decreases bFGF-induced intracellular Ca2+ increase and proliferation of neuronal stem cells [36, 40, 63]. TRPC3 has also been shown to be associated with BDNF receptors stimulation of neuronal growth [21, 66] and Epo-persuade cell differentiation and proliferation [39]. Consistent with these results, expression of TRPC1, TRPC2, and TRPC4 is observed to be higher, whereas expression of TRPC6 is decreased in neuronal stem cells. This differential expression of various TRPC channels suggests that different TRPC channels play contrasting roles in neuronal cell proliferation and differentiation [63]. In addition, using hippocampal neuronal cells (H19-7), Wu and their colleagues showed that Ca2+ influx through TRPC1 and TRPC3 was essential in regulating the shift between proliferation and differentiation [71].
Importantly, Ca2+ entry is not always beneficial. Activation by massive glutamate increases [Ca2+]i mainly through TRPC1 channels, which leads to cell death as observed in hippocampal organotypic slice cultures. TRPC1 expression is enhanced after glutamate treatment, and both inhibition of TRPC channel by 2APB and knockdown of TRPC1 significantly reduce cell death, indicating that TRPC1 is involved in glutamate-induced cell death in the hippocampus. In contrast, studies have also shown that physiological activation of TRPC1 through other G-protein-coupled receptors could protect the neurons from several extracellular stimuli [15, 35, 37, 71]. Similarly, loss of Ca2+ entry has also been shown to decrease endoplasmic reticulum (ER) Ca2+ levels, induce ER stress, and promote cell death [58]. Together, these studies suggest that TRPC channels have a dual role where normal activation of TRPC channels regulates neuronal development, while excessive Ca2+ influx, as observed upon glutamate treatment, can induce neuronal damage (Fig. 8.2). In addition, there appears to be a set point for Ca2+ entry, where physiologic concentration of calcium is beneficial, but either excessive or decreased calcium entry is harmful. Consistent with this notion, studies show that equally high Ca2+ loads are toxic through the NMDA channels, compared to through the voltage-dependent Ca2+ channels [47, 61] suggesting that diverse Ca2+ channels have different roles in deciding the fate of the neuron. Another explanation could be that TRPC multimers that consist of different subunits could have completely different functions. Consistent with this notion, TRPC1, TRPC4, and TRPC5 are observed to be highly expressed in the pyramidal cell layer of the hippocampus, frontal cortex, and dentate gyrus, while TRPC6 is dispersedly expressed only at the molecular layer of the dentate gyrus [14, 61]. Furthermore, both TRPC1 and TRPC3 are observed to protect hippocampal cell lines, and silencing either of these channels inhibits cell development and proliferation [71]. In contrast, the expression of TRPC1 and TRPC6 is found in the substantia nigra region and colocalizes with tyrosine hydroxylase (TH), as well as with mGluR1, suggesting their role in modulating dopaminergic neuron function [9, 16]. Selective degeneration neuron is a common feature in several neurodegenerative diseases including Alzheimer’s disease, epilepsy, Huntington’s disease, stroke, and Parkinson’s disease. Accumulating evidence suggests that glutamate, an excitatory neurotransmitter, is involved in the neurodegeneration [50]. In addition, TRPC channels could also function as a scaffold protein to regulate other proteins. This function is independent of their traditional role of regulating Ca2+ influx, and most TRPC have been shown to form large multimers. Thus, exploring the mechanisms underlying expression regulation and Ca2+ entry modulation of TRPC channels would be helpful to clarify their roles in neurodegeneration.
Role of TRPC proteins in PD. Proposed model for MPP+/MPTP-induced DA loss which could lead to the onset/progression of PD. MPP+/MPTP attenuates the expression of TRPC1 and SOC-mediated Ca2+ influx, which leads to prolonged ER Ca2+ depletion and activation of the UPR pathways and subsequent ER stress-mediated neurodegeneration
8.3 Role of TRPC Channels in Parkinson’s Disease
Parkinson’s disease (PD) is the second most common neurodegenerative disorder, which is caused by progressive loss of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNpc). Degeneration of DA neurons in the SNpc region is the reason for the observed motor symptoms with this disease [26]. Although the mechanism underlying the selective degeneration of these neurons is largely unknown, a host of pathogenic factors have been suggested to be responsible for the degeneration of DA neurons in the SNpc. These factors include mitochondrial dysfunction, ER and oxidative stress, protein aggregation, excitotoxicity, and inflammation, and each factor could individually or collectively play a role in the pathogenesis of PD (Fig. 8.1). In addition, lysosomal-mediated degradation has recently been suggested to inhibit the protein degradation pathway that could eventually lead to protein aggregation as observed in PD. The role of Ca2+ regulation in PD has been of interest as it overlaps with several of the established pathways that lead to neurodegeneration. Importantly, as discussed above, changes in [Ca2+]i could mediate intracellular events that trigger or inhibit cell death process [7, 53]. Increases in [Ca2+]i via the Cav1.3 channels has been shown to be necessary to stimulate the release of dopamine (DA) from dopaminergic neurons of the SNpc [12, 49]. Interestingly, Cav1.3 channels are highly expressed in samples obtained from PD patients, indicating the importance of Ca2+ in PD. Furthermore, disturbances in Ca2+ homeostasis have been implicated in PD [1, 10, 11, 46, 77, 78]. As many factors involved in neuronal functions are dependent on Ca2+ signaling, it could be anticipated that loss of these critical functions could contribute to PD [7, 53]. In addition, [Ca2+]i is maintained by the removal of Ca2+ through plasma membrane Ca2+-ATPase pump and the Na+/Ca2+ exchanger or sequestration into intracellular organelle stores by the sarco-endoplasmic reticulum ATPase pump (SERCA), and most of these processes require ATP. However, ATP level is decreased in PD; thus, it can be speculated that the dysfunction of these Ca2+pump could be involved in PD pathogenesis. Moreover, decreases in ATP levels could decrease ER calcium levels that could stimulate store-operated Ca2+ entry which is dependent on TRPC and Orai channels. Thus, it is critical to establish their physiological functions as discussed in this chapter.
8.3.1 TRPC1
TRPC1, the founding member of TRPCs, is ubiquitously expressed in high levels in all neuronal tissues [2, 62]. Alterations in intracellular Ca2+, especially in the storage organelles such as the ER and mitochondria, have also been shown to affect neuronal survival and are associated with PD [28]. Although several Ca2+ channels have been identified that bring Ca2+ into cells, Ca2+ entry through the store-operated calcium channels (SOC) could be the most important. Moreover, as Ca2+ entry through SOC has been shown to be essential for maintaining intracellular ER Ca2+ stores, along with regulating many cellular functions, they could play an important role in neuronal survival [8, 58, 64]. TRPC1 has been shown to be essential for the formation of the functional SOC as it is activated by store depletion per se in DA neurons [8, 58, 59, 64, 68].
One of the possible mechanisms that lead to neurodegeneration in PD could be the initiation of ER stress or the unfolded protein response (UPR) [22, 23]. Ca2+ in the ER is known to be important for protein synthesis and folding; thus, loss of this vital function could induce abnormal protein aggregation as well as ER stress that could activate cell death cascades [52, 73]. Ca2+ influx through the SOCE mechanism is essential for the refilling of the ER Ca2+ stores, which could prevent abnormal protein aggregation and ER stress. Recently published work [8, 58, 59] suggests that in DA neurons TRPC1 functions as the endogenous plasma membrane SOC Ca2+ channel. Importantly, the expression of TRPC1, but not that of other TRPCs as well as ORAI1 or SOCE modulator stromal interaction molecule 1 (STIM1), was decreased in cell and animal models as well as in PD patients. Interestingly, Ca2+ entry into DA neurons was also inhibited by neurotoxin that induced PD-like symptoms and decreased ER Ca2+ levels. Consistent with these results, overexpression of TRPC1 reduced neurotoxicity induced by MPP+ or salsolinol [8, 58]. In contrast, knockdown of TRPC1 or by addition of TRPC channel blockers inhibited DA neuron survival, indicating that the neuron protection role of TRPC1 might result from its Ca2+ influx ability. Similar results are also found in in vivo model and in tissue samples from human patients. Loss of TRPC1 shows a decrease in ER Ca2+ level and initiates the unfolded protein response. Moreover, overexpression of functional TRPC1 is protected against neurotoxin-induced loss of SOCE and the resultant ER stress response. In contrast, silencing of TRPC1 or its modulator STIM1 increased the UPR, which suggests that ER stress is induced in PD and TRPC1 attenuates ER stress [58] (Fig. 8.2). Furthermore, Ca2+ entry via TRPC1 also activates AKT/mTOR signaling and contributes to neuronal survival [58].
Mitochondrial dysfunction is another mechanism that has been demonstrated to have a role in the PD pathogenesis [41]. Importantly, calcium entry via SOC has been shown to modulate mitochondrial Ca2+ levels, and alterations in these Ca2+ levels could also lead to neurodegeneration. In addition, a strong correlation between SOCE and apoptosis has also been proposed, indicating that lack of Ca2+ entry would contribute to apoptosis [27]. Neurotoxins induce neuronal loss by decreasing TRPC1 levels, which could decrease mitochondrial Ca2+ levels necessary for ATP synthesis followed by disrupting mitochondrial membrane potential and initiation of apoptosis. Consistent with these results, activation of TRPC1 maintains mitochondrial membrane potential and inhibits Bax translocation to the mitochondria to prevent cytochrome c release and mitochondrial-mediated apoptosis. These results suggest that TRPC1 could prevent neurotoxin-induced cellular death by maintaining mitochondrial membrane potential, which prevents neurons from apoptosis [8, 10, 59]. Moreover, another study shows that downregulation of STIM1 expression, which is known to activate TRPC channels, inhibits cell apoptosis and reduces intracellular ROS production in PC12 cells by 6-hydroxydopamine [38].
8.3.2 TRPC2
Among the seven members of the TRPC, the TRPC2 channel, being a pseudogene in human, is the least investigated [33, 67]. Therefore, little is known about its physiological function. However, in rodents TRPC2 is highly expressed to the dendritic tip of the vomeronasal sensory neurons [30, 72] and plays an important role in pheromone sensing [18, 29], while its role in PD is not yet identified.
8.3.3 TRPC3
TRPC3 is highly expressed in the brain and oxidative stress has been shown to activate TRPC3 channels. The oxidant tertiary butyl hydroperoxide completely depolarized endothelial cells by activating TRPC3 [57], suggesting that TRPC3 determines endothelial redox sensitivity. In addition, overexpression of TRPC3 in HEK293T cells shows an increase in basal membrane conductance upon tertiary butyl hydroperoxide treatment, which is mainly due to the influx of Na+ [51]. In another study in primary rat cortical neurons and astrocytes, TRPC3 levels and TRPC3-mediated Ca2+ flux are dose-dependently decreased upon treatment with oxidative stressors [56]. In addition, in murine striatal astrocytes, additions of neurotoxins which mimic PD decrease ATP level and OAG-induced Ca2+ transients, [60]. Importantly, a slight increase in TRPC3 expression is observed in PD condition as well as neurotoxin models of PD [58]. Disruption of Ca2+ signaling especially in astrocytes significantly impairs neuronal function and survival in neurological injury and in disease conditions such as PD. Together, these studies suggest that TRPC3 dysfunction is involved in Ca2+ dyshomeostasis and oxidative stress signaling observed in PD.
Parkinsonian movement disorders are also associated with abnormalities in SN pars reticulata (SNr) [34, 45, 54, 69]. TRPC3 channels are expressed in SNr GABA projection neurons, where TRPC3 channels are tonically active and mediate a voltage-independent inward current, leading to a substantial depolarization in these neurons [76]. Inhibition of TRPC3 channels induces hyperpolarization, decreases firing frequency, and increases firing irregularity, suggesting that TRPC3 channels play critical roles in maintaining the depolarized membrane potential, high firing frequency, and firing regularity in these basal ganglia output neurons crucial to Parkinsonian movement disorders [76]. In addition, dopamine released via the dopamine receptors from the dendrites activates TRPC3 channels in SNr GABA neurons and mediates an inward, Na+-dependent current, leading to a substantial depolarization and ensuring appropriate firing intensity and pattern in SNr GABA projection neurons [75]. TRPC3 channels have also been shown to modulate motor coordination [5, 19]. In an ataxic mouse mutant (moonwalker, Mwk mice) that displays motor and coordination defects, a gain-of-function mutation (T635A) in TRPC3 channels is observed. Sustained activation of TRPC3 channels is observed to be associated with diminished dendritic arborization and progressive loss of Purkinje neurons. Similarly, another study also shows that loss of TRPC3 exhibits atrophy and progressive paralysis [55].
8.3.4 TRPC4
TRPC4α/β isoforms are the most abundantly expressed and functionally characterized in brain. TRPC4 and TRPC5 are the major TRPC subtypes in the adult rat brain because both are expressed highly in the pyramidal cell layer of the hippocampus, frontal cortex, and dentate gyrus [14, 61]. TRPC4 is specifically detected throughout the layers (2–6) of the prefrontal cortex or the motor cortex [25, 42]. Although the role of TRPC4 in PD is not yet defined, its role in axonal regeneration in adult rat dorsal root ganglia (DRG) has been reported [70]. The expression of TRPC4 is enhanced, whereas TRPC1, TRPC3, TRPC6, and TRPC7 expression remains unchanged after nerve injury persuaded by either sciatic nerve transection or intra-ganglionic microinjection of dibutyryl cAMP [57, 70]. In addition, TRPC4 expression in various neuronal cells has been shown to be increased upon addition of NGF and dibutyryl cAMP that induced differentiation [57, 70]. Suppression of TRPC4 by specific small interfering RNA significantly reduced the length of neuritis in cultured DRG neurons [70]. Taken together, these results suggest that TRPC4 contributes to axonal regeneration especially after nerve injury. If these findings have generality, TRPC4 could be an important molecular target for potential regeneration therapies in patients suffering from neuronal injury. In contrast, by using whole-genome sequencing, a recent report showed that gain-of-function mutations in TRPC4 gene induce cell death in DA neurons through a defined, calcium-related downstream pathway [43]. High expression of TRPC4 is also found in the PM of soma and proximal dendrites of lateral septal neurons, colocalizing with mGlu receptors, which could also contribute to cell death. Studies also show that TRPC4 is expressed in cells in the ventral tegmental area, a region with extensive inputs from dopamine neurons which are important in regulating the animal behavior [24]. Importantly, a recent report has shown that self-administration of cocaine was significantly less in the TRPC4 KO group than WT controls [31]. Also, spontaneous DA neuronal activity in the ventral tegmental area revealed fewer cells with high-frequency firing rate in rats that lack TRPC4. Together these studies show the roles of TRPC4 channels in various functions of the CNS making them a potential target, especially for neurological diseases associated with excitotoxicity like PD or drug addiction that are also dependent on DA neurons.
8.4 Conclusion
Plenty of research in the past decades has explored the role of TRPC channels in neuronal survival, differentiation, and neurodegeneration as observed in Parkinson’s disease. It could be suggested that Ca2+ influx via the TRPC channels has an important role in the neurodegenerative diseases such as Parkinson’s, although further studies are needed. Therefore, targeting Ca2+ entry (both inhibition and activation) through TRPC channels could be critical for maintaining normal physiological function in dopaminergic neurons. Moreover, recent findings have also implicated STIM1 as a regulator for SOCE, making STIM1 a potential target, as they may be involved in neurological diseases such as Parkinson’s.
References
Albers DS, Beal MF (2000) Mitochondrial dysfunction and oxidative stress in aging and neurodegenerative disease. J Neural Transm Suppl 59:133–154
Ambudkar IS, Bandyopadhyay BC, Liu X, Lockwich TP, Paria B, Ong HL (2006) Functional organization of TRPC-Ca2+ channels and regulation of calcium microdomains. Cell Calcium 40(5–6):495–504. doi:10.1016/j.ceca.2006.08.011
Arundine M, Tymianski M (2003) Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium 34(4–5):325–337
Badger JL, Cordero-Llana O, Hartfield EM, Wade-Martins R (2014) Parkinson’s disease in a dish – using stem cells as a molecular tool. Neuropharmacology 76(Pt A):88–96. doi:10.1016/j.neuropharm.2013.08.035
Becker EB, Oliver PL, Glitsch MD, Banks GT, Achilli F, Hardy A, Nolan PM, Fisher EM, Davies KE (2009) A point mutation in TRPC3 causes abnormal Purkinje cell development and cerebellar ataxia in moonwalker mice. Proc Natl Acad Sci U S A 106(16):6706–6711. doi:10.1073/pnas.0810599106
Berridge MJ, Bootman MD, Roderick HL (2003) Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4(7):517–529. doi:10.1038/nrm1155
Berridge MJ, Lipp P, Bootman MD (2000) The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1(1):11–21. doi:10.1038/35036035. 35036035 [pii]
Bollimuntha S, Ebadi M, Singh BB (2006) TRPC1 protects human SH-SY5Y cells against salsolinol-induced cytotoxicity by inhibiting apoptosis. Brain Res 1099(1):141–149. doi:10.1016/j.brainres.2006.04.104
Bollimuntha S, Selvaraj S, Singh BB (2011) Emerging roles of canonical TRP channels in neuronal function. Adv Exp Med Biol 704:573–593. doi:10.1007/978-94-007-0265-3_31
Bollimuntha S, Singh BB, Shavali S, Sharma SK, Ebadi M (2005) TRPC1-mediated inhibition of 1-methyl-4-phenylpyridinium ion neurotoxicity in human SH-SY5Y neuroblastoma cells. J Biol Chem 280(3):2132–2140. doi:10.1074/jbc.M407384200
Cali T, Ottolini D, Brini M (2011) Mitochondria, calcium, and endoplasmic reticulum stress in Parkinson’s disease. Biofactors 37(3):228–240. doi:10.1002/biof.159
Chen BT, Rice ME (2001) Novel Ca2+ dependence and time course of somatodendritic dopamine release: substantia nigra versus striatum. J Neurosci 21(19):7841–7847
Choi DW (1988) Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci 11(10):465–469
Chung YH, Sun Ahn H, Kim D, Hoon Shin D, Su Kim S, Yong Kim K, Bok Lee W, Ik Cha C (2006) Immunohistochemical study on the distribution of TRPC channels in the rat hippocampus. Brain Res 1085(1):132–137. doi:10.1016/j.brainres.2006.02.087
Dhar M, Wayman GA, Zhu M, Lambert TJ, Davare MA, Appleyard SM (2014) Leptin-induced spine formation requires TrpC channels and the CaM kinase cascade in the hippocampus. J Neurosci 34(30):10022–10033. doi:10.1523/JNEUROSCI.2868-13.2014
Fendt M, Schmid S, Thakker DR, Jacobson LH, Yamamoto R, Mitsukawa K, Maier R, Natt F, Husken D, Kelly PH, McAllister KH, Hoyer D, van der Putten H, Cryan JF, Flor PJ (2008) mGluR7 facilitates extinction of aversive memories and controls amygdala plasticity. Mol Psychiatry 13(10):970–979. doi:10.1038/sj.mp.4002073
Fiorio Pla A, Maric D, Brazer S-C, Giacobini P, Liu X, Chang YH, Ambudkar IS, Barker JL (2005) Canonical transient receptor potential 1 plays a role in basic fibroblast growth factor (bFGF)/FGF receptor-1-induced Ca2+ entry and embryonic rat neural stem cell proliferation. J Neurosci 25(10):2687–2701. doi:10.1523/JNEUROSCI.0951-04.2005
Flanagan KA, Webb W, Stowers L (2011) Analysis of male pheromones that accelerate female reproductive organ development. PLoS ONE 6(2):e16660. doi:10.1371/journal.pone.0016660
Hartmann J, Dragicevic E, Adelsberger H, Henning HA, Sumser M, Abramowitz J, Blum R, Dietrich A, Freichel M, Flockerzi V, Birnbaumer L, Konnerth A (2008) TRPC3 channels are required for synaptic transmission and motor coordination. Neuron 59(3):392–398. doi:10.1016/j.neuron.2008.06.009
Herman B, Gores GJ, Nieminen AL, Kawanishi T, Harman A, Lemasters JJ (1990) Calcium and pH in anoxic and toxic injury. Crit Rev Toxicol 21(2):127–148. doi:10.3109/10408449009089876
Hirschler-Laszkiewicz I, Tong Q, Conrad K, Zhang W, Flint WW, Barber AJ, Barber DL, Cheung JY, Miller BA (2009) TRPC3 activation by erythropoietin is modulated by TRPC6. J Biol Chem 284(7):4567–4581. doi:10.1074/jbc.M804734200
Hoozemans JJ, van Haastert ES, Eikelenboom P, de Vos RA, Rozemuller JM, Scheper W (2007) Activation of the unfolded protein response in Parkinson’s disease. Biochem Biophys Res Commun 354(3):707–711. doi:10.1016/j.bbrc.2007.01.043
Hoozemans JJ, van Haastert ES, Nijholt DA, Rozemuller AJ, Scheper W (2012) Activation of the unfolded protein response is an early event in Alzheimer’s and Parkinson’s disease. Neurodegener Dis 10(1–4):212–215. doi:10.1159/000334536
Huang J, Du W, Yao H, Wang Y (2011) TRPC channels in neuronal survival. In: Zhu MX (ed) TRP channels. CRC Press, Boca Raton
Huang WC, Young JS, Glitsch MD (2007) Changes in TRPC channel expression during postnatal development of cerebellar neurons. Cell Calcium 42(1):1–10. doi:10.1016/j.ceca.2006.11.002
Javitch JA, D'Amato RJ, Strittmatter SM, Snyder SH (1985) Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6 -tetrahydropyridine: uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc Natl Acad Sci U S A 82(7):2173–2177
Jayadev S, Petranka JG, Cheran SK, Biermann JA, Barrett JC, Murphy E (1999) Reduced capacitative calcium entry correlates with vesicle accumulation and apoptosis. J Biol Chem 274(12):8261–8268
Kazuno AA, Munakata K, Nagai T, Shimozono S, Tanaka M, Yoneda M, Kato N, Miyawaki A, Kato T (2006) Identification of mitochondrial DNA polymorphisms that alter mitochondrial matrix pH and intracellular calcium dynamics. PLoS Genet 2(8):e128. doi:10.1371/journal.pgen.0020128
Kimchi T, Xu J, Dulac C (2007) A functional circuit underlying male sexual behaviour in the female mouse brain. Nature 448(7157):1009–1014. doi:10.1038/nature06089
Kiselyov K, van Rossum DB, Patterson RL (2010) TRPC channels in pheromone sensing. Vitam Horm 83:197–213. doi:10.1016/S0083-6729(10)83008-0
Klipec WD, Burrow KR, O’Neill C, Cao JL, Lawyer CR et al (2016) Loss of the trpc4 gene is associated with a reduction in cocaine self-administration and reduced spontaneous ventral tegmental area dopamine neuronal activity, without deficits in learning for natural rewards. Behav Brain Res 306:117–127
Komuro H, Kumada T (2005) Ca2+ transients control CNS neuronal migration. Cell Calcium 37:387–393
Löf C, Viitanen T, Sukumaran P, Törnquist K (2011) TRPC2: of mice but not men. Adv Exp Med Biol 704:125–134. doi:10.1007/978-94-007-0265-3_6
Lee JI, Verhagen Metman L, Ohara S, Dougherty PM, Kim JH, Lenz FA (2007) Internal pallidal neuronal activity during mild drug-related dyskinesias in Parkinson’s disease: decreased firing rates and altered firing patterns. J Neurophysiol 97(4):2627–2641. doi:10.1152/jn.00443.2006
Li H, Huang J, Du W, Jia C, Yao H, Wang Y (2012) TRPC6 inhibited NMDA receptor activities and protected neurons from ischemic excitotoxicity. J Neurochem 123(6):1010–1018. doi:10.1111/jnc.12045
Li HS, Xu XZ, Montell C (1999) Activation of a TRPC3-dependent cation current through the neurotrophin BDNF. Neuron 24(1):261–273
Li M, Chen C, Zhou Z, Xu S, Yu Z (2012) A TRPC1-mediated increase in store-operated Ca2+ entry is required for the proliferation of adult hippocampal neural progenitor cells. Cell Calcium 51(6):486–496. doi:10.1016/j.ceca.2012.04.014
Li Y, Guo Y, Tang J, Jiang J, Chen Z (2014) New insights into the roles of CHOP-induced apoptosis in ER stress. Acta Biochim Biophys Sin 46:629–640
Mattson MP, Guthrie PB, Kater SB (1989) Intrinsic factors in the selective vulnerability of hippocampal pyramidal neurons. Prog Clin Biol Res 317:333–351
Montecinos-Oliva C, Schuller A, Parodi J, Melo F, Inestrosa NC (2014) Effects of tetrahydrohyperforin in mouse hippocampal slices: neuroprotection, long-term potentiation and TRPC channels. Curr Med Chem 21(30):3494–3506
Moon HE, Paek SH (2015) Mitochondrial dysfunction in Parkinson’s disease. Exp Neurobiol 24(2):103–116. doi:10.5607/en.2015.24.2.103
Morelli MB, Amantini C, Liberati S, Santoni M, Nabissi M (2013) TRP channels: new potential therapeutic approaches in CNS neuropathies. CNS Neurol Disord Drug Targets 12(2):274–293
Nagarajan A, Ning Y, Reisner K, Buraei Z, Larsen JP et al (2014) Progressive degeneration of dopaminergic neurons through TRP channel-induced cell death. J Neurosci 34:5738–5746
Naziroglu M (2011) TRPM2 cation channels, oxidative stress and neurological diseases: where are we now? Neurochem Res 36(3):355–366. doi:10.1007/s11064-010-0347-4
Nevet A, Morris G, Saban G, Fainstein N, Bergman H (2004) Discharge rate of substantia nigra pars reticulata neurons is reduced in non-parkinsonian monkeys with apomorphine-induced orofacial dyskinesia. J Neurophysiol 92(4):1973–1981. doi:10.1152/jn.01036.2003
O’Bryant SE, Hobson V, Hall JR, Waring SC, Chan W, Massman P, Lacritz L, Cullum CM, Diaz-Arrastia R (2009) Brain-derived neurotrophic factor levels in Alzheimer’s disease. J Alzheimers Dis 17(2):337–341. doi:10.3233/JAD-2009-1051doi:052G70T078857553 [pii]
Ovey IS, Naziroglu M (2015) Homocysteine and cytosolic GSH depletion induce apoptosis and oxidative toxicity through cytosolic calcium overload in the hippocampus of aged mice: involvement of TRPM2 and TRPV1 channels. Neuroscience 284:225–233. doi:10.1016/j.neuroscience.2014.09.078
Paschen W (2000) Role of calcium in neuronal cell injury: which subcellular compartment is involved? Brain Res Bull 53(4):409–413
Patel JC, Witkovsky P, Avshalumov MV, Rice ME (2009) Mobilization of calcium from intracellular stores facilitates somatodendritic dopamine release. J Neurosci 29(20):6568–6579. doi:10.1523/JNEUROSCI.0181-09.2009
Phelan KD, Shwe UT, Abramowitz J, Wu H, Rhee SW, Howell MD, Gottschall PE, Freichel M, Flockerzi V, Birnbaumer L, Zheng F (2013) Canonical transient receptor channel 5 (TRPC5) and TRPC1/4 contribute to seizure and excitotoxicity by distinct cellular mechanisms. Mol Pharmacol 83(2):429–438. doi:10.1124/mol.112.082271
Poteser M, Graziani A, Rosker C, Eder P, Derler I, Kahr H, Zhu MX, Romanin C, Groschner K (2006) TRPC3 and TRPC4 associate to form a redox-sensitive cation channel. Evidence for expression of native TRPC3-TRPC4 heteromeric channels in endothelial cells. J Biol Chem 281(19):13588–13595. doi:10.1074/jbc.M512205200
Putney JW (2005) Physiological mechanisms of TRPC activation. Pflugers Arch Eur J Physiol 451(1):29–34. doi:10.1007/s00424-005-1416-4
Putney JW Jr (2003) Capacitative calcium entry in the nervous system. Cell Calcium 34(4–5):339–344. doi:S014341600300143X [pii]
Rivlin-Etzion M, Marmor O, Heimer G, Raz A, Nini A, Bergman H (2006) Basal ganglia oscillations and pathophysiology of movement disorders. Curr Opin Neurobiol 16(6):629–637. doi:10.1016/j.conb.2006.10.002
Rodriguez-Santiago M, Mendoza-Torres M, Jimenez-Bremont JF, Lopez-Revilla R (2007) Knockout of the trcp3 gene causes a recessive neuromotor disease in mice. Biochem Biophys Res Commun 360(4):874–879. doi:10.1016/j.bbrc.2007.06.150
Roedding AS, Tong SY, Au-Yeung W, Li PP, Warsh JJ (2013) Chronic oxidative stress modulates TRPC3 and TRPM2 channel expression and function in rat primary cortical neurons: relevance to the pathophysiology of bipolar disorder. Brain Res 1517:16–27. doi:10.1016/j.brainres.2013.04.025
Selvaraj S, Sun Y, Singh BB (2010) TRPC channels and their implication in neurological diseases. CNS Neurol Disord Drug Targets 9(1):94–104
Selvaraj S, Sun Y, Watt JA, Wang S, Lei S, Birnbaumer L, Singh BB (2012) Neurotoxin-induced ER stress in mouse dopaminergic neurons involves downregulation of TRPC1 and inhibition of AKT/mTOR signaling. J Clin Invest 122(4):1354–1367. doi:10.1172/JCI61332
Selvaraj S, Watt JA, Singh BB (2009) TRPC1 inhibits apoptotic cell degeneration induced by dopaminergic neurotoxin MPTP/MPP(+). Cell Calcium 46(3):209–218. doi:10.1016/j.ceca.2009.07.008
Streifel KM, Gonzales AL, De Miranda B, Mouneimne R, Earley S, Tjalkens R (2014) Dopaminergic neurotoxicants cause biphasic inhibition of purinergic calcium signaling in astrocytes. PLoS ONE 9:e110996
Strübing C, Krapivinsky G, Krapivinsky L, Clapham DE (2001) TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron 29(3):645–655
Strübing C, Krapivinsky G, Krapivinsky L, Clapham DE (2003) Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J Biol Chem 278(40):39014–39019. doi:10.1074/jbc.M306705200
Su B, Ji Y-S, X-l S, Liu X-H, Chen Z-Y (2014) Brain-derived neurotrophic factor (BDNF)-induced mitochondrial motility arrest and presynaptic docking contribute to BDNF-enhanced synaptic transmission. J Biol Chem 289(3):1213–1226. doi:10.1074/jbc.M113.526129
Sun Y, Sukumaran P, Bandyopadhyay BC, Singh BB (2014) Physiological function and characterization of TRPCs in neurons. Cell 3(2):455–475. doi:10.3390/cells3020455
Sun Y, Sukumaran P, Schaar A, Singh BB (2015) TRPM7 and its role in neurodegenerative diseases. Channels (Austin) 9(5):253–261. doi:10.1080/19336950.2015.1075675
Tong Q, Hirschler-Laszkiewicz I, Zhang W, Conrad K, Neagley DW, Barber DL, Cheung JY, Miller BA (2008) TRPC3 is the erythropoietin-regulated calcium channel in human erythroid cells. J Biol Chem 283(16):10385–10395. doi:10.1074/jbc.M710231200
Tornquist K, Sukumaran P, Kemppainen K, Lof C, Viitanen T (2014) Canonical transient receptor potential channel 2 (TRPC2): old name-new games. Importance in regulating of rat thyroid cell physiology. Pflugers Arch Eur J Physiol 466(11):2025–2034. doi:10.1007/s00424-014-1509-z
Wang GX, Poo MM (2005) Requirement of TRPC channels in netrin-1-induced chemotropic turning of nerve growth cones. Nature 434(7035):898–904. doi:10.1038/nature03478
Wichmann T, Soares J (2006) Neuronal firing before and after burst discharges in the monkey basal ganglia is predictably patterned in the normal state and altered in parkinsonism. J Neurophysiol 95(4):2120–2133. doi:10.1152/jn.01013.2005
Wu D, Huang W, Richardson PM, Priestley JV, Liu M (2008) TRPC4 in rat dorsal root ganglion neurons is increased after nerve injury and is necessary for neurite outgrowth. J Biol Chem 283(1):416–426. doi:10.1074/jbc.M703177200
Wu X, Zagranichnaya TK, Gurda GT, Eves EM, Villereal ML (2004) A TRPC1/TRPC3-mediated increase in store-operated calcium entry is required for differentiation of H19-7 hippocampal neuronal cells. J Biol Chem 279(42):43392–43402. doi:10.1074/jbc.M408959200
Yildirim E, Birnbaumer L (2007) TRPC2: molecular biology and functional importance. Handb Exp Pharmacol 179:53–75. doi:10.1007/978-3-540-34891-7_3
Yoshida I, Monji A, Tashiro K, Nakamura K, Inoue R, Kanba S (2006) Depletion of intracellular Ca2+ store itself may be a major factor in thapsigargin-induced ER stress and apoptosis in PC12 cells. Neurochem Int 48(8):696–702. doi:10.1016/j.neuint.2005.12.012
Yuruker V, Naziroglu M, Senol N (2015) Reduction in traumatic brain injury-induced oxidative stress, apoptosis, and calcium entry in rat hippocampus by melatonin: possible involvement of TRPM2 channels. Metab Brain Dis 30(1):223–231. doi:10.1007/s11011-014-9623-3
Zhou FM (2010) A transient receptor potential channel regulates basal ganglia output. Rev Neurosci 21:95–118
Zhou FW, Matta SG, Zhou FM (2008) Constitutively active TRPC3 channels regulate basal ganglia output neurons. J Neurosci 28(2):473–482. doi:10.1523/JNEUROSCI.3978-07.2008
Zuccato C, Cattaneo E (2007) Role of brain-derived neurotrophic factor in Huntington’s disease. Prog Neurobiol 81(5–6):294–330 . doi:10.1016/j.pneurobio.2007.01.003doi:S0301-0082(07)00015-9 [pii]
Zuccato C, Cattaneo E (2009) Brain-derived neurotrophic factor in neurodegenerative diseases. Nat Rev Neurol 5(6):311–322 . doi:10.1038/nrneurol.2009.54doi:nrneurol.2009.54 [pii]
Acknowledgments
We thank the grant support from the NIH (DE017102; DE024300; GM113123) awarded to B.B.S, and the assistance of John Lee, School of Medicine and Health Sciences, in making the figures.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Sukumaran, P., Sun, Y., Schaar, A., Selvaraj, S., Singh, B.B. (2017). TRPC Channels and Parkinson’s Disease. In: Wang, Y. (eds) Transient Receptor Potential Canonical Channels and Brain Diseases. Advances in Experimental Medicine and Biology, vol 976. Springer, Dordrecht. https://doi.org/10.1007/978-94-024-1088-4_8
Download citation
DOI: https://doi.org/10.1007/978-94-024-1088-4_8
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-024-1086-0
Online ISBN: 978-94-024-1088-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)