Abstract
In this study we examined the role of chronic taurine supplementation on plasma glucose homeostasis and brain excitability through activation of the insulin receptor. FVB/NJ male mice were supplemented with taurine in drinking water (0.05% w/v) for 4 weeks and subjected to a glucose tolerance test (7.5 mg/kg BW) after 12 h fasting. We found that taurine-fed mice were slightly hypoglycemic prior to glucose injection and showed significantly reduced plasma glucose at 30 and 60 min post-glucose injection when compared to control mice. Previously, we reported that taurine supplementation induces biochemical changes that target the GABAergic system. Those studies show that taurine-fed mice are hyperexcitable, have reduced GABAA receptors expression and increased GAD and somatostatin expression in the brain. In this study, we found that taurine-fed mice had a significant increase in insulin receptor (IR) immuno-reactivity in the pancreas and all brain regions examined. At the mRNA level, we found that the IR showed differential regional expression. Surprisingly, we found that neurons express the gene for insulin and that taurine had a significant role in regulating insulin gene expression. We propose that increased insulin production and secretion in taurine-fed mice cause an increase activation of the central IR and may be partially responsible for the increased neuronal excitability observed in taurine supplemented mice. Furthermore, the high levels of neuronal insulin expression and its regulation by taurine implicates taurine in the regulation of metabolic homeostasis.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Taurine is a sulfur-containing semi-essential amino acid. Excitable tissues, including the brain, skeletal and cardiac muscles contain high levels of taurine. Taurine has been shown to play an important role in many physiological processes (Lambardini 1985; Solis et al. 1988; Saransaari and Oja 2000; Schaffer et al. 2000; Foos and Wu 2002). Additionally, taurine modulates both glutamate and GABA neurotransmission (Militante and Lombardini 1998; El Idrissi and Trenkner 1999, 2004). In the pancreas, taurine gestational taurine supplementation delays the onset of diabetes in non-obese diabetic mice (Arany et al. 2004). Furthermore, taurine has been shown to play a role in glucose homeostasis throughout life (Hansen 2001; Franconi et al. 2006).
Developing pancreas has been shown to undergo a significant level of remodeling, mediated by a balanced induction of cell proliferation and apoptotic cell death (Arany et al. 2004). Many factors have been shown to be implicated in this pancreatic remodeling, including IGF-II, inducible nitric oxide synthase (iNOS) and somatostatin (Scaglia et al. 1997; Liu et al. 1998; Petrik et al. 1998; El Idrissi et al. 2009). We have shown that taurine-fed mice have increased size and number of islets (El Idrissi et al. 2009). The role of taurine in pancreatic development has been postulated to be mediated by preventing or scavenging free radicals (Petrik et al. 1998), by inhibiting the expression of pro-inflammatory factors such as iNOS (Liu et al. 1998) and by promoting the expression of survival factors such as IGF-II and somatostatin (Scaglia et al. 1997; Petrik et al. 1998; El Idrissi et al. 2009).
We have shown that taurine supplementation increased islets size in the pancreas and insulin production by β cells (El Idrissi et al. 2009). These changes in pancreatic function are responsible for the increased resistance to glucose challenges in taurine-fed mice. Furthermore, circulating insulin crosses the blood brain barrier and activates IR expressed on neurons. We suggest that this activation of IR receptors may be an additional mechanism for increased excitability in taurine-fed mice. This is consistent with the effects of taurine on GABAergic synapses. We have shown that chronic interaction of taurine with GABAA receptors induces a variety of alterations to the GABAergic system. These include increased GAD expression, decreased expression of GABAA receptors (El Idrissi and Trenkner 2004), and increased number of somatostatin-positive neurons (El Idrissi et al. 2009; Levinskaya et al. 2006). The changes induced by taurine supplementation to the GABAergic system are consistent with increased neuronal excitability. Coupled with these changes in the GABAergic system, here we report an increased expression and activation of the insulin receptors which will further enhance neuronal excitability.
2 Methods
2.1 Animals
All mice used in this study were 2-month-old FVB/NJ males. For taurine-fed mice, taurine was dissolved in water at 0.05%, and this solution was made available to the mice for 4 weeks beginning at 4 weeks of age. All mice were housed in groups of three in a pathogen-free room maintained on a 12 h light/dark cycle and given food and water ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee of the College of Staten Island/CUNY, and were in conformity with National Institutes of Health Guidelines. The number of mice used in these studies was sufficient to provide statistically reliable results.
2.2 Immunohistochemistry and Confocal Microscopy
Frozen sections were made as previously described (Levinskaya et al. 2006). Briefly, primary antibodies (Chemicon International) used were directed against insulin receptor (mouse host) and insulin (rabbit) diluted 1:500 and incubated overnight at 4 °C. Secondary antibodies were all raised in goat and conjugated to Alexa Fluor 488 or Cy5 (Invitrogen/Molecular probes). Images were obtained by confocal microscopy (Leica SP2 AOBS). Nuclei were counterstained with SlowFade with DAPI (Invitrogen). Immunoreactivity was quantified using Imaris ×64 (Bitplane).
2.3 Intraperitoneal Glucose Tolerance Test
Glucose test was performed as previously reported (El Idrissi et al. 2009). Briefly, mice were fasted overnight (12 h) and then injected intraperitoneally with 0.02 mL/g of body weight D-glucose (7.5% stock solution in saline). Blood samples were taken by tail venesection at the indicated times
2.4 RNA Preparation, cDNA Preparation and Real-Time PCR Analysis
RNA was prepared from tissue samples as described previously (Zhang et al. 2009). Equal amounts of RNA (0.5 μg) were used to prepare cDNA using the SYBR GreenER Two-Step qRT-PCR kit (Invitrogen 11,748–100) and analyzed by real-time PCR in a 7500-sequence detection system (Applied Biosystems). All experiments were repeated twice and, in each experiment, real-time PCR reactions were done in triplicate. Target DNA quantities were calculated as described previously (Zhang et al. 2009). Statistical significance was determined by Student’s t-test. Each value was expressed as the mean ± SEM. Differences were considered statistically significant when the calculated P value was less than 0.05.
2.5 Statistical Analysis
Statistical significance was determined by Student’s t-test. Each value was expressed as the mean ± SEM. Differences were considered statistically significant when the calculated P value was less than 0.05.
3 Results
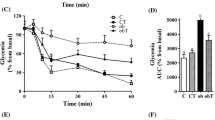
3.1 Taurine-Fed Mice Exhibit Tolerance to Glucose Challenges
We have shown that taurine supplementation increased islets size in the pancreas and insulin production by β cells (El Idrissi et al. 2009). These changes in pancreatic function are responsible for the increased resistance to glucose challenges in taurine-fed mice. Control mice showed a significant increase in plasma glucose concentration 30 min after glucose injection with a gradual decrease thereafter. By 120 min, mice were slightly hypoglycemic relative to baseline (Fig. 1). In contrast, taurine-fed mice showed a drastically different response to glucose injection. There was a delayed peak of plasma glucose at 60 min post injection and the plasma glucose in these mice was significantly lower than controls at all times measured (p < 0.001).
3.2 Taurine Supplementation Increases the Expression of Insulin Receptors in the Brain and Pancreas
Insulin is primarily a metabolic hormone functioning on muscle, fat and liver via activation of IR receptor. Insulin also function on other non-metabolic tissues such as the brain. Once insulin is secreted it crosses the blood-brain barrier by a transporter-mediated saturable mechanism. The IR is widely expressed in the brain at various levels (Unger et al. 1991). This regional specify implicates insulin, through activation of its receptor, in various brain function that are mediated by these brain structures. In this study, we examined the levels of IR expression in the pancreas and brain and found that taurine-fed mice have a significant increase in IR expression in all brain regions and pancreas compared to controls (Fig. 2).
Effect of taurine supplementation on IR expression in the brain and pancreas. Images depict Imaris reconstruction of z-stacks of confocal images obtained from 30 μm cryosections. Upper panel representative images showing insulin receptor (green) immunoreactivity in CA3 region of the hippocampus from control (a) and taurine-fed mouse (b). (c, d) are representative images obtained from the pancreas of controls and taurine-fed mice, respectively. Red is insulin, green is IR immunoreactivity. Hippocampi and pancreas from taurine-fed mice show a significant increase in immunoreactivity for insulin receptor. Scale bar 15 μm
3.3 Taurine Supplementation Alters Insulin and Insulin Receptor Gene Expression
To further investigate the functional significance increased insulin receptor expression in the brain, we examined mRNA levels of the IR and insulin. We found that the insulin receptor gene was differentially expressed in various brain regions and affected by taurine supplementation. In the cerebellum, taurine caused a significant decrease in IR gene expression, where as in the brain stem and hippocampus, there was a significant increase in IR expression. Interestingly, we found that taurine supplementation had a significant role in the regulation of insulin gene in the brain. Taurine caused a downregulation of insulin gene expression in all brain regions examined, except the diencephalon where taurine caused an increased in insulin gene expression.
4 Discussion
In this study we show that taurine, through supplementation drinking water, plays an important role in the function of the pancreas and neuronal excitability. Previously, we showed that taurine-fed mice have enlarged islets and increased insulin synthesis and secretion by β cells (El Idrissi et al. 2009). Additionally, here we show taurine regulates the expression of IR in both the brain and the pancreas (Figs. 2 and 3). We found that taurine supplementation caused an increase in IR expression notably in the brain stem and hippocampus (Fig. 3) with a decrease expression in the cerebellum. IR is widely expressed in the brain and the expression pattern shows regional specificity (Havrankova et al. 1978; Unger et al. 1991). Interestingly, we found that the gene for insulin is also widely expressed in brain (Fig. 3). This is consistent with previous finding suggesting that insulin gene is expressed in certain regions of the brain (Devaskar et al. 1993). Here we show that insulin gene is expressed in neurons and its expression is regulated by taurine supplementation.
There are numerous studies demonstrating that IR signaling plays a role in both excitatory and inhibitory neurotransmission and that the expression of IR in the hippocampus is activity-dependent (Plum et al. 2005). The expression of potassium ion channel Kv1.3 in the olfactory bulb is increased in response to intranasal insulin delivery to mice (Marks et al. 2009). These changes led to increased cognitive function as measured by short- and long-term object recognition, suggesting the insulin modulates neuronal activity and improves memory through changes in Kv1.3 expression levels. Furthermore, insulin has been shown to promote neuronal survival in the brain (Mielke et al. 2006) and prevent hippocampal cell death in response to glucose deprivation in vitro (Díaz et al. 1999).
Here, we showed that the expression of both insulin and its receptor are regulated by taurine supplementation. These effects are observed both in the brain and pancreas, suggesting a role for taurine in both regulation of glucose homeostasis and neuronal excitability.
5 Conclusion
In summary, taurine supplementation to mice in drinking water has a beneficial role on the function of the pancreas by increasing insulin production and secretion. Concomitant with this increased insulin secretion there is an increase in IR expression in both the brain and pancreas. Activation of IR on neurons would increase neuronal excitability. This is consistent with the increased excitability observed with taurine treatment. IRs are widely expressed in both the brain and the periphery. The regulation of IR expression by taurine may help explain the wide range of behavioral and physiological effects regulated by taurine.
Abbreviations
- GAD:
-
Glutamic acid decarboxylase
- IGF:
-
Insulin-like growth factor
- IR:
-
Insulin receptor
- Tau:
-
Taurine
- WT:
-
Wild type controls
References
Arany E, Strutt B, Romanus P, Remacle C, Reusens B, Hill DJ (2004) Taurine supplement in early life altered islet morphology, decreased insulitis and de-layed the onset of diabetes in non-obese diabetic mice. Diabetologia 47:1831–1837
Devaskar BS, Singh LR, Carnaghi PA, Rajakumar SJ (1993) Giddings Insulin II gene expression in rat central nervous system. Regul Pept 48:55–63
Díaz B, Pimentel B, de Pablo F, de La Rosa EJ (1999) Apoptotic cell death of proliferating neuroepithelial cells in the embryonic retina is prevented by insulin. Eur J Neurosci 11:1624–1632
El Idrissi A, Trenkner E (1999) Growth factors and taurine protect against exci-totoxicity by stabilizing calcium homeostasis and energy metabolism. J Neurosci 19:9459–9468
El Idrissi A, Trenkner E (2004) Taurine as a modulator of excitatory and inhibito-ry neurotransmission. Neurochem Res 1:189–197
El Idrissi A, Boukarrou L, L’Amoreaux WJ (2009) Taurine supplementation and pancreatic remodeling. Adv Exp Med Biol 643:353–358
Foos T, Wu JY (2002) The role of Taurine in the central nervous system and the modulation of intracellular calcium homeostasis. Neurochem Res 27:21–26
Franconi F, Loizzo A, Ghirlanda G, Seghieri G (2006) Taurine supplementation and diabetes mellitus. Curr Opin Clin Nutr Metab Care 9:32–36
Hansen SH (2001) The role of taurine in diabetes and the development of diabetic complications. Diabetes Metab Res Rev 17:330–346
Havrankova J, Roth J, Brownstein M (1978) Insulin receptors are widely distributed in the central nervous system of the rat. Nature 272:827–829
Lambardini JB (1985) Effects of taurine on calcium ion uptake and protein phos-phorylation in rat retinal membrane preparations. J Neurochem 45:268–275
Levinskaya N, Trenkner E, El Idrissi A (2006) Increased GAD-positive neurons in the cortex of taurine-fed mice. Adv Exp Med Biol 583:411–417
Liu Y, Tonna-DeMasi M, Park E, Schuller-Levis G, Quinn MR (1998) Taurine chloramine inhibits production of nitric oxide and prostaglandin E2 in activated C6 glioma cells by suppressing inducible nitric oxide synthase and cyclooxygenase-2 expression. Brain Res Mol Brain Res 59:189–195
Marks DR, Tucker K, Cavallin MA, Mast TG, Fadool DA (2009) Awake intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors. J Neurosci 29:6734–6751
Mielke JG, Taghibiglou C, Wang YT (2006) Endogenous insulin signaling protects cultured neurons from oxygen–glucose deprivation-induced cell death. Neuroscience 143:165–173
Militante JD, Lombardini JB (1998) Pharmacological characterization of the ef-fects of taurine on calcium uptake in the rat retina. Amino Acids 15(99):108
Petrik J, Arany E, McDonald TJ, Hill DJ (1998) Apoptosis in the pancreatic islet cells of the neonatal rat is associated with a reduced expression of insulin-like growth factor II that may act as a survival factor. Endocrinology 139:2994–3004
Plum L, Schubert M, Brüning JC (2005) The role of insulin receptor signaling in the brain. Trends Endocrinol Metab 16:59–65
Saransaari P, Oja SS (2000) Taurine and neuronal cell damage. Amino Acids 19:509–526
Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S (1997) Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology 138:1736–1741
Schaffer S, Takahashi K, Azuma J (2000) Role of osmoregulation in the actions of taurine. Amino Acids 19:527–546
Solis JM, Herranz AS, Erreras O, Lerma J, Martin del Rio R (1988) Does taurine act as an osmoregulatory substance in the rat brain. Neurosci Lett 91:53–58
Unger JW, Livingston JN, Moss AM (1991) Insulin receptors in the central nervous system: localization, signalling mechanisms and functional aspects. Prog Neurobiol 36:343–362
Zhang A, Shen CH, Ma SY, Ke Y, El Idrissi AE (2009) Altered expression of Autism-associated genes in the brain of Fragile X mouse model. Biochem Biophys Res Commun 379:920–923
Acknowledgements
This work was supported by PSC-CUNY and CSI.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media B.V.
About this paper
Cite this paper
El Idrissi, A., El Hilali, F., Rotondo, S., Sidime, F. (2017). Effects of Taurine Supplementation on Neuronal Excitability and Glucose Homeostasis. In: Lee, DH., Schaffer, S.W., Park, E., Kim, H.W. (eds) Taurine 10. Advances in Experimental Medicine and Biology, vol 975. Springer, Dordrecht. https://doi.org/10.1007/978-94-024-1079-2_24
Download citation
DOI: https://doi.org/10.1007/978-94-024-1079-2_24
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-024-1077-8
Online ISBN: 978-94-024-1079-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)