Abstract
Graphene-based nanostructures exhibit good mechanical strength, high porosity, outstanding electrical conductivity, and excellent thermal and chemical stability, which in addition to its low cost, versatile functionalization chemistry, and relative ease of large-scale preparation make it ideally suited to serve as a key component for the development of new electrode materials. Recently, a wide variety of methods have been developed for the formation of porous graphene architectures to further improve the performances. Porous graphene provides abundant pathways for rapid ion diffusion and high accessible surface area. In this chapter, the recent continued breakthroughs in the preparation of porous graphene-based nanoarchitectures as well as their applications as electrode materials for electrochemical energy storage devices are introduced.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Graphene
- Porous
- Electrochemistry
- Electrochemical energy storage
- Nanostructures
- Lithium-ion rechargeable batteries
- Supercapacitors
1 Introduction
Graphene is an atomic single layer of honeycomb carbon lattice. Recently, graphene and graphene-based nanomaterials have attracted increased attention because of their unique properties and great potential for numerous applications [3, 14, 21, 33, 36–38, 51, 63]. In particular, research on graphene-based nanomaterials for electrochemical energy storage has progressed rapidly during recent years due to the increasing demand for the development of these storage devices with improved performance including high energy, power density, and excellent cycle stability, while maintaining low production cost [15, 43, 44, 71, 76, 83, 92]. Among various carbon materials, graphene has awakened a tremendous interest because of its superior electronic conductivity, large theoretical specific surface area, and physicochemical stability [22, 30, 52]. These interesting properties make graphene and graphene-based nanomaterials promising electrode materials for various electrochemical energy storage devices (e.g., supercapacitors and batteries), which have a wide range of applications from microelectromechanical systems to portable electronic devices, and to electric vehicles.
Graphene-based nanomaterial from graphite oxide is being manufactured in large scale at relatively low cost [58]. During the past decade, various graphene-based nanomaterials (Figs. 1, 2, and 3) have been investigated as potential electrode materials with high specific capacity and long cycle stability [26, 29, 31, 40–42, 46, 48, 53, 62, 82]. However, in spite of the previous efforts to prepare better electrochemical energy storage devices by using graphene, market demand for higher performances of these devices are ever increasing. For lithium-ion batteries, since lithium ions cannot pass through the carbon atomic arrays in 2D sheets of graphene, therefore, lithium ions have to tortuously detour to reach the electrolyte. This results in a longer diffusion distance and slow charge–discharge rate in lithium-ion battery systems [35, 65]. Supercapacitors, also called ultracapacitors or electrochemical capacitors, store electrical charge on high-surface-area conducting materials. Their widespread use is limited by their low energy storage density and relatively high effective series resistance. As previously reported, the theoretical specific surface area of a single graphene sheet is extremely high (~2600 m2 g−1) [64]. However, the real accessible surface areas of graphene-based materials are far below this value, mainly due to the strong aggregation tendency of graphene sheets (Fig. 4).
a SEM image of Fe2O3; b SEM image of Fe2O3/graphene; c TEM image of Fe2O3/graphene; d TEM image of Fe2O3/graphene in high resolution. Reproduced from Ref. [82] with permission from Elsevier
FE-SEM photographs of a ZnO nanocrystals and different types of ZnO@GN hybrids, b ZnO@GN-1, c ZnO@GN-2, and d ZnO@GN-3. Reproduced from Ref. [26] with permission from Elsevier
TEM images of a GO before the one-step in-situ transformation reaction, and b Mn3O4/GNS composite after the one-step in-situ transformation reaction. c HRTEM image of individual Mn3O4 nanoparticle in Mn3O4/GNS composite; the inset shows the electron diffraction pattern of Mn3O4 nanoparticle in Mn3O4/GNS composite. d HAADF-STEM image of Mn3O4/GNS composite and EDS element mapping results for e Mn and f O species of Mn3O4/GNS composite. Reproduced from Ref. [48] with permission from Elsevier
TEM images of a and b LFP/CA-1, c and d LFP/CA-2, and e and f LFP/CA-3. And g HRTEM image and h SAED pattern of the LiFePO4 particle in LFP/CA-3. Reproduced from Ref. [79] with permission from Elsevier
To surmount these challenges, graphene sheets were further treated into porous structures which mean abundant pathways for rapid lithium-ion diffusion [79] and to achieve higher accessible surface area [90]. Due to its unique structural and electronic characteristics, a porous graphene opens up new opportunities for the development of electrode materials with novel nanoarchitectures in electrochemical energy storage devices. In this chapter, recent advances and novel strategies in the preparation of porous graphene architectures as well as their applications as electrode materials for electrochemical energy storage devices are introduced. Finally, the future prospects in the development of graphene-based nanocomposite materials with improved energy storage performances are discussed.
2 Strategies to Buildup Porous Graphene
A wide variety of strategies have been developed for the formation of porous graphene architectures as outlined in Table 1. These can be roughly divided into two categories. One is the in-plane generation of defective pores into the graphene sheets and the other is the out-of-plane generation of 3D graphene-based porous superstructures (Scheme 1) (Figs. 5 and 6).
Preparation pathway of the MC–GR and Pt/MC–GR composites. Reproduced from Ref. [2] with permission from Elsevier
a TEM image of GR. Inset shows SEM image of SiO2-GO after calcination. b TEM image of MC–GR. Inset shows SEM image of MC–GR. Reproduced from Ref. [2] with permission from Elsevier
2.1 Generation of Defective Pores into the Graphene Sheets
When etching of graphene is performed under appropriate acid/oxidizer solution, carbon erosion will occur. Accordingly, porous graphene is produced when graphene oxide dispersed in water is treated with acid/oxidizer solution under sonication [84] or microwave irradiation [18]. HNO3 and KMnO4 are typical examples of acid and oxidizer used.
Fan et al. [18] investigated the preparation of porous graphene using KMnO4 as oxidizer under microwave irradiation (Fig. 7). The obtained porous graphene revealed a pore size of approximately 3 nm and a specific surface area of 1374 m2 g−1.
Illustration of the formation of porous graphene material with pores on the surface of sheet. Reproduced from Ref. [18] with permission from Elsevier
The following equation shows the reaction of carbon with KMnO4:
Porous graphene could also be produced by using a strong base. Romanos et al. [57] presented nanospace engineering of KOH-activated carbon. It is reported that high specific surface areas, porosities, subnanometer (<1nm), and suprananometer (1–5 nm) pore volumes could be quantitatively controlled by a combination of KOH concentration and activation temperature. Recently, the chemical process was used to prepare chemically activated graphene. Typical examples of these bases are KOH and NaOH. After chemical activation, the specific surface area of the porous graphene is increased to become closer to the theoretical value [86, 90].
2.2 Out-of-Plane Generation of 3D Graphene-Based Porous Superstructures
Besides the in-plane generation of defective pores into the graphene sheets, 3D graphene-based out-of-plane porous superstructures could be built up. Using the hard template approaches, graphene layers were deposited on inorganic/organic particles larger than 50 nm or in situ grown on metallic porous frameworks followed by the elimination of template that can result into graphene-based materials with 3D porous structures.
Using uniform polymethyl methacrylate (PMMA) latex spheres as hard templates, Chen et al. [9] prepared a controllable 3D macroporous bubble graphene film with tailorable microstructure. Zhao et al. [27] developed a novel hydrophobic interaction-driven hard templating approach for the rational designed preparation of nanoporous graphene foams with controlled pore size, high surface area, and ultra-large pore volume. Monodisperse silica particles were used as the templates to prepare nanoporous graphene foams. The generated graphene foams show the highest total pore volume value in all the reported porous graphene materials. Additionally, they demonstrated that metal oxide nanoparticles can be easily decorated on the pore walls, due to the ultra-large open-porous feature and the homogeneous hydrophobic surface nature. Huh et al. [13] built a 3D macroporous structure that consists of chemically modified graphene by using polystyrene particles as a sacrificial template. Furthermore, for further capacitance boost, a thin layer of MnO2 was additionally deposited onto the embossed chemically modified graphene. The porous graphene nanostructure shows a large surface area facilitates fast ionic transport within the electrode while preserving decent electronic conductivity and thus endows the composite electrodes with excellent electrochemical properties. Using sulfonated polystyrene (SPS) sphere as hard template, Zhang et al. [68] also prepared porous graphene electrode by an in situ constructing strategy.
Chemical vapor deposition and electrochemical deposition were used to prepare graphene-based materials with 3D porous structures using Ni foam, porous MgO, etc., as templates. Zhang et al. [4] prepared a novel 3D porous graphene networks by the scalable ethanol-chemical vapor deposition method. They demonstrated that the 3D graphene network can be used as a good platform to construct graphene/metal oxide composites for surpercapacitor applications.
3D porous graphene-based composite materials were prepared by electrochemical deposition [10]. 3D graphene porous material is prepared electrochemically by reducing a concentrated graphene oxide dispersion. Subsequently, the second component is electrochemically deposited onto this 3D matrix, yielding graphene-based 3D porous composite material. The prepared graphene-based composite materials have a conductive graphene network as the matrix, onto which the second component is homogeneously coated.
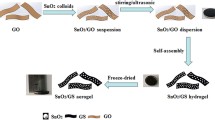
Sun et al. [67] reported a hydrothermal approach to prepare nitrogen-doped graphene in various forms, including a stable dispersion, a hydrogel and an aerogel of nitrogen-doped graphene. The stable dispersion mainly consists of single-sheet graphene and the hydrogel is physically cross-linked to be quite strong.
2.3 Other Methods
Ogale et al. reported a one-step, catalyst-free process for the preparation of single-layer-graphene-assembled porous carbon by a polymer pyrolysis route [77]. The surface area of the single-layer-graphene-assembled porous carbon was found to be 1720 m2 g−1. The nanomaterial was tested as a supercapacitor and showed a high capacitance value of ca. 154 F g−1 in an aqueous electrolyte in a typical electrochemical cell.
Zhu et al. reported a simple approach to transform the CVD graphene films through self-assembly into porous and continuous fibers with tunable diameter, pore distribution, and high electrical conductivity [39]. Graphene is first self-assembled from a 2D film to a 1D fiber-like structure in an organic solvent and then dried to give the porous and crumpled structure.
3 Applications in Lithium-Ion Rechargeable Batteries
Due to their attractive advantages over other types of batteries, lithium-ion batteries have been widely used as power sources for various portable electronic devices. More recently, they have attracted growing attention as power supplies for electric vehicles and hybrid electric vehicles. Intensive research has been performed to develop new electrode materials with improved performance for lithium-ion batteries. Accordingly, a great deal of effort has been made to find alternative electrode materials with improved electrochemical performance for lithium-ion batteries. To improve lithium storage capacity, the most promising carbon is disordered partially graphitic carbon from both a technological and scientific point of view, since defects provide large excess capacities [32]. Among the various novel nanostructured electrode candidate materials, graphene-based electrode materials (Fig. 8) are of particular interest due to their high surface area and good conductivity [54, 85].
a, b SEM images and c, d TEM images of M-NG composite (inset: HRTEM image of Mn3O4 nanoparticle on the graphene sheet). Reproduced from Ref. [54] with permission from Elsevier
3.1 Porous Graphene-Based Anode Materials
Porous graphene nanostructure could provide more space to accommodate the volume change of the active materials during the charge–discharge to enhance the electrochemical stability of the electrodes. Accordingly, porous graphene-based materials hold promise as novel electrode materials to further improve the performance of lithium-ion batteries.
Feng et al. presented a bottom-up approach to the large-scale production of 2D sandwich like graphene-based mesoporous carbon [80]. Their high surface area, thinness, and numerous mesopores are favorable for the accessibility of the electrolyte, rapid diffusion of lithium ions, and host uptake. Additionally, the graphene layers can act as mini-current collectors dispersed in the electrode, which facilitates the fast transport of electrons during the charge–discharge cycling due to its high electrical conductivity. When the porous nanocomposite material is used as an anode material for lithium-ion batteries, the nanostructured electrode material exhibits a first discharge capacity of 915 mA h g−1, which rapidly stabilizes and remains at 770 mA h g−1 even after 30 cycles, when cycled at a rate of C/5.
Graphene-based 3D macroporous materials are favorable electrode candidates for lithium-ion batteries. Yan et al. reported a simple method for the preparation of 3D graphene/Fe3O4 architectures by a mild chemical reduction of graphene oxide in the presence of Fe3O4 nanoparticles [7]. The obtained superparamagnetic, porous, and lightweight material shows good electrochemical performance as anode material in lithium-ion battery.
Using porous MgO sheets as a template, Fan et al. reported a simple CVD approach for scalable preparation of porous graphene materials (Figs. 9, and 10) [19]. The resulting porous graphene networks exhibit a high reversible capacity of 1723 mA h g−1, and excellent high rate capability and cycling stability for Li-ion batteries.
TEM images of porous graphene (a) and (b), and hydrazine-reduced graphene oxide (c). XRD patterns of porous graphene and hydrazine-reduced graphene oxide (d). Reproduced from Ref. [19] with permission from Elsevier
a Pore size distribution and b Raman spectra of the porous graphene. c AFM image of the porous graphene, the arrows indicate the existence of pores in graphene sheet. Reproduced from Ref. [19] with permission from Elsevier
A 3D porous architecture of Si/graphene nanocomposite was rationally designed and constructed through an in situ magnesiothermic reduction of SiO2/graphene oxide composites series and spray-drying with additional graphene [73]. The porous nanoarchitectured composite has superior electrochemical stability and the 3D graphene network shows enhanced electrical conductivity as well as improves rate performance. Furthermore, the 3D nanoarchitechture can be cycled at extremely high Li+ extraction rates.
3D graphene foams cross-linked with Fe3O4 nanospheres were prepared by hydrothermal treatment [70]. The Fe3O4 nanospheres are wrapped by graphene sheets and further confined within continuous graphene networks. Such hierarchical Fe3O4/graphene hybrids provide double protection against the volume changes of Fe3O4 nanospheres during electrochemical processes. The graphene shells suppress the aggregation of Fe3O4 nanospheres and buffer the volume expansion, while the interconnected 3D graphene networks act to reinforce the core–shell structure of Fe3O4@graphene shell (Fe3O4@GS) and thus enhance the electrical conductivity of the overall electrode. As a result, 3D graphene foams cross-linked with Fe3O4 nanospheres (Fe3O4 NSs) encapsulated with graphene (Fe3O4@GS/GF) delivers a high reversible capacity of 1059 mA h g−1 over 150 cycles, and excellent rate capability, thus exhibiting great potential as an anode material for lithium storage.
Fan et al. reported a bottom-up strategy assisted by atomic layer deposition to graft bicontinuous mesoporous nanostructure Fe3O4 onto 3D graphene foams and directly use the composite as the lithium-ion battery anode [45]. This electrode exhibits high reversible capacity and fast charging and discharging capability. A high capacity of 785 mA h g−1 is achieved at 1 C rate and is maintained this high capacity up to 500 cycles. Moreover, the rate of up to 60 C is also demonstrated, rendering a fast discharge potential. For the first step, graphene foam was grown on Ni foam by CVD and then Ni was etched away by a mixture of FeCl3 and HCl solution. In the second step, a layer of ZnO was coated onto the graphene foam by atomic layer deposition.
A facile and general method was reported to prepare ordered porous binder-free 3D porous graphene–metal oxide@carbon electrodes at a large scale [91]. Viscous precursor paint was prepared by mixing graphene oxide slurry, polystyrene aqueous solution and metal salt. The ordered porous binder-free electrodes were obtained after heat treatment of the paint at 400 °C under Ar for 60 min. The overall framework is macroporous structure and made of metal oxides or a mixture of graphene and metal oxides. There are secondary pores in the walls of the porous electrode with size in the range of 5–10 nm. The macropores are derived from the duplication of sacrificing polystyrene spheres, while the mesopores are generated from the gas release during decomposition of precursors. The preparation process allows the adjustment of the selected components, the amount of graphene added, the thickness of the electrodes. Such ordered porous binder-free electrodes demonstrated superior Li storage properties. For example, graphene-Fe3O4@C binder-free electrode depicts high capacities of 1123.8 and 505 mAh g−1 at current densities of 0.5 and 10 A g−1, respectively. It shows that the surface Li storage mechanism contributes significantly to the total capacities in such 3D porous binder-free electrodes.
A novel composite, MoS2-coated 3D graphene network, is synthesized by a facile CVD method [5]. The 3D graphene network serves as a template for the deposition of MoS2 and provides good electrical contact between the current collector and deposited MoS2. As proof of concept, the nanocomposite shows excellent electrochemical performance as an anode material for lithium-ion batteries, which exhibits reversible capacities of 877 and 665 mA h g−1 during the 50th cycle at current densities of 100 and 500 mA g−1, respectively, indicating its good cycling performance. Furthermore, the nanocomposite also shows excellent high-current-density performance.
Ultrahigh rate capabilities of transition metal oxide-based electrodes were derived from the design of ordered hierarchically porous 3D electrodes with entrapped active nanoparticle configuration [28]. In contrast to previous reports on hierarchically porous electrodes from irregular self-assembly or post-incorporation of active nanoparticles, the strategy relies on in situ formation and entrapment of active nanoparticles inside the simultaneously formed ordered hierarchically 3D porous carbon, in which the periodic macroporous-mesoporous carbon was directly integrated with the open-porous Ni foam current collector without organic binder, and the electrode active nanoparticles were spatially entrapped inside the periodic porous carbon. Based on the unique electrode configuration, the as-prepared ordered hierarchically porous 3D electrodes show extraordinary rate capabilities.
Zhang et al. developed a simple method for the preparation of metal-oxide coated 3D graphene composites through a facile two-step annealing process [6]. The metal–organic frameworks that served as the precursors of the metal oxides were first synthesized on the 3D graphene networks. The desired nanocomposites were then obtained by a two-step annealing process. The method is expected to be used for synthesis of other metal oxide/graphene composites with 3D structures.
3.2 Porous Graphene-Based Cathode Materials
Porous graphene-based cathode materials were also studied. Yang et al. reported a composite of chemically activated carbon and LiFePO4 as a cathode active material. KOH activation was conducted to construct a 3D structure allowing for diffusion of lithium ions. The porous structure of chemically activated carbon is advantageous to lithium-ion diffusion due to its high rate capability [79]. A composite of chemically activated porous graphene and LiFePO4 was developed to improve the speed of charging–discharging and the cycling stability of lithium-ion batteries using LiFePO4 as a cathode material [23]. Chemically activated porous graphene was synthesized using KOH. Electrochemical properties have also been investigated after assembling coin cells with the porous graphene/LiFePO4 composite as an active material. The composite electrode exhibited better electrochemical properties than the conventional graphene/LiFePO4 composite as well as bare LiFePO4, including exceptional speed of charging–discharging and excellent cycle stability. The porous graphene in the electrode composite provides abundant porous channels for the diffusion of lithium ions. Moreover, it acts as a conducting network for easy charge transfer and as a divider, preventing the aggregation of LiFePO4 particles.
4 Applications in Supercapacitors
Electrochemical supercapacitors store energy using either ion adsorption or fast surface redox reactions [60]. Electrochemical capacitors are also promising energy storage devices due to the advantages of short charging times, a long cycle, and high power density [47, 49, 61]. However, current commercial electrochemical supercapacitors have much lower energy density than lithium-ion batteries. Design of a desirable, low-cost electrode material with a longer cycling lifetime and higher energy density is imperative for electrochemical capacitor. The performance of electrode materials for supercapacitors is dependent on the accessible specific surface area and the pore structure. The control over structure and morphology of carbon electrode materials is therefore an effective strategy to render them high surface area and efficient paths for ion diffusion. Accordingly, porous graphene-based materials have been proved favorable electrode candidates for supercapacitors due to their open-porous structure that allows electrolytes access more easily to the surface of frameworks.
4.1 Activation of Graphene for Supercapacitors
Defective pores could be generated on graphene by chemical methods to prepare activated graphene. Zhu et al. synthesized chemically activated graphene (CA-graphene) with a 3D morphology via KOH activation for application to supercapacitors. The extremely high energy and power density for supercapacitors were possible due to the large surface area from the abundant pore systems [33].
Hierarchical porous carbons are promising electrode materials in high-power supercapacitors. Kim et al. demonstrate the fabrication of highly porous graphene-derived carbons with hierarchical pore structures in which mesopores are integrated into macroporous scaffolds [34]. The macropores were introduced by assembling graphene-based hollow spheres, and the mesopores were derived from the chemical activation with KOH. The unique 3D pore structures in the graphene-based carbons give rise to a BET surface area value of up to 3290 m2 g−1 and provide an efficient pathway for electrolyte ions to diffuse into the interior surfaces of electrode particles. These carbons exhibit both high gravimetric (174 F g−1) and volumetric (∼100 F cm−3) specific capacitance in an ionic liquid electrolyte in acetonitrile. The energy density and power density of the cell assembled with this carbon electrode are also high, with gravimetric values of 74 Wh kg−1 and 338 kW kg−1 and volumetric values of 44 Wh L−1 and 199 kW L−1, respectively. The high supercapacitor performance achieved with these graphene-based carbons is attributed to their unique pore structure and makes them potentially promising for various energy storage devices.
Zheng et al. synthesized porous graphene/activated carbon composite by hydrothermal carbonization and subsequent two-step chemical activation with KOH (Fig. 11) [89]. The composite has a relatively high packing density and large specific surface area of 2106 m2 g−1, as well as containing plenty of mesopores (Fig. 12). As supercapacitor electrode material, it exhibits specific capacitance up to 210 F g−1 in an aqueous electrolyte and 103 F g−1 in organic electrolyte, respectively. The specific capacitance decreases by only 5.3 % after 5000 cycles. In this composite material, a layer of porous activated carbon coats on graphene improves dispersion of graphene sheets and increases its packing density. The graphene integrated into activated carbon matrix also increases conductivity. Additionally, the nanosheet-like electrode material has a short diffusion pathway, which facilitates rapid transport of the electrolyte ions. Three-dimensional graphene-based frameworks are also fabricated by hydrolysis of TEOS with graphene aerogel as support and CTAB as soft template [72]. The resulting hierarchical macro- and mesoporous structures exhibit narrow mesopore size distribution (2–3.5 nm), high surface area, and low mass density. Benefiting from the integration of meso- and macroporous structures, the material manifests outstanding specific capacitance (226 F g−1), high rate capability, and excellent cycling stability when it is applied in electrochemical capacitors.
Schematic illustration showing the experimental steps of preparing porous graphene/AC nanosheet composite. Reproduced from Ref. [89] with permission from Elsevier
a SEM image of char-like intermediate product, b SEM image of graphene/AC nanosheet composite, c, d TEM images of graphene/AC nanosheet composite. Reproduced from Ref. [89] with permission from Elsevier
4.2 3D Graphene-Based Porous Materials for Supercapacitors
Many research works have been published on 3D graphene-based porous nanostructures for supercapacitors. Here, some selected studies on this research field are reviewed.
Zhang et al. present a simple, green, and efficient approach using two standard and simple industry steps to make 3D graphene-based porous materials at bulk scale, with ultrahigh specific surface area (3523 m2 g−1) and excellent bulk conductivity [87]. The good properties of these materials are demonstrated by their superior supercapacitor performance in ionic liquid with specific capacitance and energy density of 231 F g−1 and 98 Wh kg−1, respectively.
A self-assembled macrostructured graphene architecture was prepared by a convenient one-step hydrothermal method [74]. The self-assembled graphene hydrogel is electrically conductive, mechanically strong, and thermally stable and exhibits a high specific capacitance. The self-assembled graphene hydrogel as a 3D supercapacitor electrode material exhibits high specific capacitance (175 F g−1) in an aqueous electrolyte.
Freestanding, lightweight, ultrathin, highly conductive, and flexible 3D graphene networks, loaded with MnO2 by electrodeposition, were prepared as the electrodes of a flexible supercapacitor [25]. The 3D graphene networks showed an ideal supporter for active materials and permitted a large MnO2 mass loading of 9.8 mg cm−2, leading to a high area capacitance of 1.42 F cm−2 at a scan rate of 2 mV s−1. The MnO2 content with respect to the entire electrode was further optimized and a maximum specific capacitance of 130 F g−1 was achieved. The excellent electrochemical performance of a symmetrical supercapacitor consisting of a sandwich structure of two pieces of 3D graphene/MnO2 composite network separated by a membrane and encapsulated in polyethylene terephthalate membranes was explored.
A hybrid structure of ZnO on 3D graphene foam has been synthesized by CVD growth of graphene followed by a facial in situ precipitation of ZnO nanorods under hydrothermal conditions [16]. The results show that the ZnO nanorods have high crystallinity and cluster uniformly on graphene skeleton to form flower-like nanostructures. It is found that the graphene/ZnO hybrids display superior capacitive performance with high specific capacitance of ~400 F g−1 as well as excellent cycle life, making them suitable for high-performance energy storage applications.
3D graphene architectures in the macroworld can in principle maintain all the extraordinary nanoscale properties of individual graphene flakes. However, current 3D graphene products suffer from poor electrical conductivity, low surface area, and insufficient mechanical strength/elasticity; the interconnected self-supported reproducible 3D graphenes remain unavailable. A sugar-blowing approach based on a polymeric predecessor to synthesize a 3D graphene bubble network was reported [69]. The bubble network consists of mono- or few-layered graphitic membranes that are tightly glued, rigidly fixed, and spatially scaffolded by micrometer-scale graphitic struts. Such a topological configuration provides intimate structural interconnectivities, freeway for electron/phonon transports, huge accessible surface area, as well as robust mechanical properties. The graphene network thus overcomes the drawbacks of presently available 3D graphene products and opens up a wide horizon for diverse practical uses, for example, high-power high-energy electrochemical capacitors, as highlighted in this work.
Fan et al. demonstrated the fabrication of functionalized graphene nanosheets via low temperature thermal treatment of graphite oxide with a slow heating rate using Mg(OH)2 nanosheets as template [78]. Because of its dented sheet with high surface area, a certain amount of oxygen-containing groups, and low pore volume, the as-obtained graphene delivers both ultrahigh specific gravimetric and volumetric capacitances of 456 F g−1 and 470 F cm−3, almost 3.7 times and 3.3 times higher than hydrazine-reduced graphene, respectively. The assembled supercapacitor exhibits an ultrahigh volumetric energy density of 27.2 Wh L−1, which is among the highest values for carbon materials in aqueous electrolytes, as well as excellent cycling stability with 134 % of its initial capacitance after 10,000 cycles.
Mitlin et al. employed a microwave synthesis process of cobalt phthalocyanine molecules templated by acid-functionalized multiwalled carbon nanotubes to create 3D sponge-like graphene nanoarchitectures suited for ionic liquid-based electrochemical capacitor electrodes that operate at very high scan rates [75]. The 3D nanoarchitectures are able to deliver an energy density of 7.1 Wh kg−1 even at an extra high power density of 48 kW kg−1. In addition, the ionic liquid supercapacitor based on this material works very well at room temperature due to its fully opened structures, which is ideal for the high-power energy application requiring more tolerance to temperature variation. Moreover, the structures are stable in both ionic liquids and 1 M H2SO4, retaining 90 and 98 % capacitance after 10,000 cycles, respectively.
Porous yet densely packed carbon electrodes with high ion-accessible surface area and low ion-transport resistance are formed by capillary compression of adaptive graphene gel films in the presence of a nonvolatile liquid electrolyte [81]. This simple soft approach enables subnanometer-scale integration of graphene sheets with electrolytes to form highly compact carbon electrodes with a continuous ion-transport network. Electrochemical capacitors based on the resulting films can obtain volumetric energy densities approaching 60 Wh L−1.
4.3 Flexible Supercapacitors Using 3D Graphene-Based Porous Materials
There has been much research interest in the development of flexible supercapacitors over the past few years due to their high mechanical compliance. Li et al. reported that the combination of graphene chemistry with ice physics can lead to the formation of ultralight and superelastic graphene-based cellular monoliths [55]. Chi et al. reported the preparation of freestanding paper-like electrode materials have trigged significant research interest for their practical application in flexible and lightweight energy storage devices [12]. The utilization of 3D porous graphene scaffold to load nanostructured polyaniline dramatically enhances the electrical conductivity, the specific capacitance, and the cycle stability of the graphene–polyaniline nanocomposite. Shao et al. demonstrated a simple method for preparing high-performance flexible asymmetric supercapacitors based on 3D porous graphene/MnO2 nanorod and graphene/Ag hybrid thin-film electrodes [59]. These graphene hybrid films, which accelerate ion and electron transport by providing lower ion-transport resistances and shorter diffusion-distances, exhibit high specific capacitances and power performances, and excellent mechanical flexibility. These results suggest that such asymmetric graphene/MnO2 nanorod and graphene/Ag hybrid thin-film architectures are promising for next-generation high-performance flexible supercapacitors.
5 Future Perspectives
To overcome the limitations of conventional materials, numerous novel nanocomposite materials have been prepared by combining different types of nanomaterials with porous graphene for various electrochemical energy storage devices. The major challenges for the preparation of porous graphene-based nanocomposite materials include the preparation of nanostructures with precisely controlled of complex and hierarchical pore morphology as well as the designed fabrication of nanocomposite materials with novel compositions.
Porous graphene, as a new platform for nanocomposite materials, provides new possibilities to the nanodevices. Although there has been rapid growth in the development of porous graphene-based nanocomposite materials as promising candidate electrode materials for electrochemical energy storage application in the past few years, as evidenced by the sharp increase in research work published in this area, there remain several challenges needed to be overcome. In order for them to be used in real energy storage devices in the future, several issues including the production cost, scalable synthesis, and long-term mechanical stability of the nanocomposite materials need to be addressed. To study the performance of a porous material for electrochemical energy storage, more reliable parameter, such as the volumetric energy and power density against the whole electrochemical energy devices, should be measured. Nevertheless, we envisioned that numerous well-designed porous graphene-based novel nanocomposite materials with improved energy storage performances will be developed by using new preparation methods and novel compositions. These nanocomposite materials will provide many new chances for efficient electrochemical energy storage devices in the future.
References
Bi H, Yin K, Xie X, Zhou Y, Wan N, Xu F, Banhart F, Sun L, Ruoff RS (2012) Low temperature casting of graphene with high compressive strength. Adv Mater 24:5124
Bo X, Guo L (2013) Simple synthesis of macroporous carbon–graphene composites and their use as a support for Pt electrocatalysts. Electrochim Acta 90:283
Bolotin KI, Ghahari F, Shulman MD, Stormer HL, Kim P (2009) Observation of the fractional quantum hall effect in graphene. Nature 462:196
Cao X, Shi Y, Shi W, Lu G, Huang X, Yan Q, Zhang Q, Zhang H (2011) Preparation of novel 3D graphene networks for supercapacitor applications. Small 7:3163
Cao X, Shi Y, Shi W, Rui X, Yan Q, Kong J, Zhang H (2013) Preparation of MoS2-coated three-dimensional graphene networks for high-performance anode material in lithium-ion batteries. Small 9:3433
Cao X, Zheng B, Rui X, Shi W, Yan Q, Zhang H (2014) Metal oxide-coated three-dimensional graphene prepared by the use of metal-organic frameworks as precursors. Angew Chem Int Ed 53:1404
Chen W, Li S, Chen C, Yan L (2011) Self-assembly and embedding of nanoparticles by in situ reduced graphene for preparation of a 3D graphene/nanoparticle aerogel. Adv Mater 23:5679
Chen Z, Ren W, Gao L, Liu B, Pei S, Cheng HM (2011) Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat Mater 10:424
Chen CM, Zhang Q, Huang C-H, Zhao X-C, Zhang B-S, Kong Q-Q, Wang M-Z, Yang Y-G, Cai R, Su DS (2012) Macroporous ‘bubble’ graphene film via template-directed ordered-assembly for high rate supercapacitors. Chem Comm 48:7149
Chen K, Chen L, Chen Y, Bai H, Li L (2012) Three-dimensional porous graphene-based composite materials: electrochemical synthesis and application. J Mater Chem 22:20968
Chen S, Duan J, Tang Y, Qiao SZ (2013) Hybrid hydrogels of porous graphene and nickel hydroxide as advanced supercapacitor materials. Chem Eur J 19:7118
Chi K, Zhang Z, Xi J, Huang Y, Xiao F, Wang S, Liu Y (2014) Freestanding graphene paper supported three-dimensional porous graphene—polyaniline nanocomposite synthesized by inkjet printing and in flexible all-solid-state supercapacitor. ACS Appl Mater Interfaces 6:16312
Choi BG, Yang M, Hong WH, Choi JW, Huh YS (2012) 3D Macroporous graphene frameworks for supercapacitors with high energy and power densities. ACS Nano 6:4020
Dai L (2012) Functionalization of graphene for efficient energy conversion and storage. Acc Chem Res 46:31
Deng W, Ji X, Gómez-Mingot M, Lu F, Chen Q, Banks CE (2012) Graphene electrochemical supercapacitors: the influence of oxygen functional groups. Chem Commun 48:2770
Dong X, Cao Y, Wang J, Chan-Park MB, Wang L, Huang W, Chen P (2012) Hybrid structure of zinc oxide nanorods and three dimensional graphene foam for supercapacitor and electrochemical sensor applications. RSC Adv 2:4364
Estevez L, Kelarakis A, Gong Q, Da’as EH, Giannelis EP (2011) Multifunctional graphene/platinum/nafion hybrids via ice templating. J Am Chem Soc 133:6122
Fan Z, Zhao Q, Li T, Yan J, Ren Y, Feng J, Wei T (2012) Easy synthesis of porous graphene nanosheets and their use in supercapacitors. Carbon 50:1699
Fan Z, Yan J, Ning G, Wei T, Zhi L, Wei F (2013) Porous graphene networks as high performance anode materials for lithium ion batteries. Carbon 60:538
Fang Y, Lv Y, Che R, Wu H, Zhang X, Gu D, Zheng G, Zhao D (2013) Two-dimensional mesoporous carbon nanosheets and their derived graphene nanosheets: synthesis and efficient lithium ion storage. J Am Chem Soc 135:1524
Geim AK (2009) Graphene: status and prospects. Science 324:1530
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater 6:183
Ha J, Park S-K, Yu S-H, Jin A, Jang B, Bong S, Kim I, Sung Y-E, Piao Y (2013) A chemically activated graphene-encapsulated LiFePO4 composite for high-performance lithium ion batteries. Nanoscale 5:8647
Han TH, Huang YK, Tan ATL, Dravid VP, Huang J (2011) Steam etched porous graphene oxide network for chemical sensing. J Am Chem Soc 133:15264
He Y, Chen W, Li X, Zhang Z, Fu J, Zhao C, Xie E (2013) Freestanding three-dimensional graphene/MnO2 composite networks as ultralight and flexible supercapacitor electrodes. ACS Nano 7:174
Hsieh C-T, Lin C-Y, Chen Y-F, Lin J-S (2013) Synthesis of ZnO@graphene composites as anode materials for lithium ion batteries. Electrochim Acta 111:359
Huang X, Qian K, Yang J, Zhang J, Li L, Yu C, Zhao D (2012) Functional nanoporous graphene foams with controlled pore sizes. Adv Mater 24:4419
Huang X, Yu H, Chen J, Lu Z, Yazami R, Hng HH (2014) Ultrahigh rate capabilities of lithium-ion batteries from 3d ordered hierarchically porous electrodes with entrapped active nanoparticles configuration. Adv Mater 26:1296
Jang B, Park M, Chae OB, Park S, Kim Y, Oh SM, Piao Y, Hyeon T (2012) Direct synthesis of self-assembled ferrite/carbon hybrid nanosheets for high performance lithium-ion battery anodes. J Am Chem Soc 134:15010
Jang B, Choi E, Piao Y (2013) Preparation of well-dispersed Pt nanoparticles on solvothermal graphene and their enhanced electrochemical properties. Mater Res Bull 48:834
Jang B, Chae OB, Park S-K, Ha J, Oh SM, Na HB, Piao Y (2013) Solventless synthesis of an iron-oxide/graphene nanocomposite and its application as an anode in high-rate Li-ion batteries. J Mater Chem A 1:15442
Kaskhedikar NA, Maier J (2009) Lithium storage in carbon nanostructures. Adv Mater 21:2664
Kim KS, Zhao Y, Jang H, Lee SY, Kim JM, Kim KS, Ahn J-H, Kim P, Choi J-Y, Hong BH (2009) Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 457:706
Kim T, Jung G, Yoo S, Suh KS, Ruoff RS (2013) Activated graphene-based carbons as supercapacitor electrodes with macro- and mesopores. ACS Nano 7:6899
Kucinskis G, Bajars G, Kleperis J (2013) Graphene in lithium ion battery cathode materials: a review. J Power Sources 240:66
Li D, Kaner RB (2008) Graphene-based materials. Science 320:1170
Li X, Wang X, Zhang L, Lee S, Dai H (2008) Chemically derived, ultrasmooth graphene nanoribbon semiconductors. Science 319:1229
Li X, Cai W, An J, Kim S, Nah J, Yang D, Piner R, Velamakanni A, Jung I, Tutuc E, Banerjee SK, Colombo L, Ruoff RS (2009) Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 324:1312
Li X, Zhao T, Wang K, Yang Y, Wei J, Kang F, Wu D, Zhu H (2011) Directly drawing self-assembled, porous, and monolithic graphene fiber from chemical vapor deposition grown graphene film and its electrochemical properties. Langmuir 27:12164
Li L, Guo Z, Du A, Liu H (2012) Rapid microwave-assisted synthesis of Mn3O4-graphene nanocomposite and its lithium storage properties. J Mater Chem 22:3600
Li L, Seng KH, Chen Z, Liu H, Nevirkovets IP, Guo Z (2013) Synthesis of Mn3O4-anchored graphene sheet nanocomposites via a facile, fast microwave hydrothermal method and their supercapacitive behavior. Electrochim Acta 87:801
Liang MH, Zhi LJ (2009) Graphene-based electrode materials for rechargeable lithium batteries. J Mater Chem 19:5871
Liu C, Yu Z, Neff D, Zhamu A, Jang BZ (2010) Graphene-based supercapacitor with an ultrahigh energy density. Nano Lett 10:4863
Luo B, Liu S, Zhi L (2012) Chemical approaches toward graphene-based nanomaterials and their applications in energy-related areas. Small 8:630
Luo J, Liu J, Zeng Z, Ng CF, Ma L, Zhang H, Lin J, Shen Z, Fan HJ (2013) Three-dimensional graphene foam supported Fe3O4 lithium battery anodes with long cycle life and high rate capability. Nano Lett 13:6136
Mai YJ, Wang XL, Xiang JY, Qiao YQ, Zhang D, Gu CD, Tu JP (2011) CuO/graphene composite as anode materials for lithium-ion batteries. Electrochim Acta 56:2306
Miller JR, Simon P (2008) Electrochemical capacitors for energy management. Science 321:651
Nam I, Kim ND, Kim G-P, Park J, Yi J (2013) One step preparation of Mn3O4/graphene composites for use as an anode in Li ion batteries. J Power Sources 244:56–62
Naoi K, Naoi W, Aoyagi S, Miyamoto J, Kamino T (1075) New generation “nanohybrid supercapacitor”. Acc Chem Res 2012:46
Ning G, Fan Z, Wang G, Gao J, Qian W, Wei F (2011) Gram-scale synthesis of nanomesh graphene with high surface area and its application in supercapacitor electrodes. Chem Comm 47:5976
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666
Novoselov KS, Geim AK, Morozov SV, Jiang D, Katsnelson MI, Grigorieva IV, Dubonos SV, Firsov AA (2005) Two-dimensional gas of massless Dirac fermions in graphene. Nature 438:197
Park S-K, Yu S-H, Pinna N, Woo S, Jang B, Chung YH, Cho YH, Sung Y-E, Piao Y (2012) A facile hydrazine-assisted hydrothermal method for the deposition of monodisperse SnO2 nanoparticles onto graphene for lithium ion batteries. J Mater Chem 22:2520
Park S-K, Jin A, Yu S-H, Ha J, Jang B, Bong S, Woo S, Sung Y-E, Piao Y (2014) In situ hydrothermal synthesis of Mn3O4 nanoparticles onnitrogen-doped graphene as high-performance anode materials for lithium ion batteries. Electrochim Acta 120:452
Qiu L, Liu JZ, Chang SLY, Wu Y, Li D (2012) Biomimetic superelastic graphene-based cellular monoliths. Nat Commun 3:1241
Qiu H, Dong X, Sana B, Peng T, Paramelle D, Chen P, Lim S, Appl ACS (2013) Mater Inter 5:782
Romanos J, Beckner M, Rash T, Firlej L, Kuchta B, Yu P, Suppes G, Wexler C, Pfeifer P (2012) Nanospace engineering of KOH activated carbon. Nanotechnology 23:015401
Segal M (2009) Selling graphene by the ton. Nat Nanotechnol 4:612
Shao Y, Wang H, Zhang Q, Li Y (2013) High-performance flexible asymmetric supercapacitors based on 3D porous graphene/MnO2 nanorod and graphene/Ag hybrid thin-film electrodes. J Mater Chem C 1:1245
Simon P, Gogotsi Y (2008) Materials for electrochemical capacitors. Nat Mater 7:845
Simon P, Gogotsi Y, Dunn B (2014) Where do batteries end and supercapacitors begin. Science 343:1210
Song Z, Zhang Y, Liu W, Zhang S, Liu G, Chen H, Qiu J (2013) Hydrothermal synthesis and electrochemical performance of Co3O4/reduced graphene oxide nanosheet composites for supercapacitors. Electrochim Acta 112:120
Stankovich S, Dikin DA, Dommett GHB, Kohlhaas KM, Zimney EJ, Stach EA, Piner RD, Nguyen ST, Ruoff RS (2006) Graphene-based composite materials. Nature 442:282
Stoller MD, Park S, Zhu Y, An J, Ruoff RS (2008) Graphene-based ultracapacitors. Nano Lett 8:3498
Su F-Y, He Y-B, Li B, Chen X-C, You C-H, Wei W, Lv W, Yang Q-H, Kang F (2012) Could graphene construct an effective conducting network in a high-power lithium ion battery. Nano Energy 1:429
Vickery JL, Patil AJ, Mann S (2009) Fabrication of graphene-polymer nanocomposites with higher-order three-dimensional architectures. Adv Mater 21:2180
Wang G, Jia L-T, Zhu Y, Hou B, Li D-B, Sun Y-H (2012) Novel preparation of nitrogen-doped graphene in various forms with aqueous ammonia under mild conditions. RSC Adv 2:11249
Wang Z-L, Xu D, Wang H-G, Wu Z, Zhang X-B (2013) In situ fabrication of porous graphene electrodes for high-performance energy storage. ACS Nano 7:2422
Wang X, Zhang Y, Zhi C, Wang X, Tang D, Xu Y, Weng Q, Jiang X, Mitome M, Golberg D, Bando Y (2013) Three-dimensional strutted graphene grown by substrate-free sugar blowing for high-power-density supercapacitors. Nat Commun 4:2905
Wei W, Yang S, Zhou H, Lieberwirth I, Feng X, Müllen K (2013) 3D Graphene foams cross-linked with pre-encapsulated Fe3O4 nanospheres for enhanced lithium storage. Adv Mater 25:2909
Wu Z-S, Ren W, Wang D-W, Li F, Liu B, Cheng H-M (2010) High-energy MnO2 nanowire/graphene and graphene asymmetric electrochemical capacitors. ACS Nano 4:5835
Wu ZS, Sun Y, Tan YZ, Yang S, Feng X, Müllen K (2012) Three-dimensional graphene-based macro- and mesoporous frameworks for high-performance electrochemical capacitive energy storage. J Am Chem Soc 134:19532
Xin X, Zhou X, Wang F, Yao X, Xu X, Zhu Y, Liu Z (2012) A 3D porous architecture of Si/graphene nanocomposite as high-performance anode materials for Li-ion batteries. J Mater Chem 22:7724
Xu Y, Sheng K, Li C, Shi G (2010) Self-assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano 4:4324
Xu Z, Li Z, Holt CMB, Tan X, Wang H, Amirkhiz BS, Stephenson T, Mitlin D (2012) electrochemical supercapacitor electrodes from sponge-like graphene nanoarchitectures with ultrahigh power density. J Phys Chem Lett 3:2928
Xu C, Xu B, Gu Y, Xiong Z, Sun J, Zhao XS (2013) Graphene-based electrodes for electrochemical energy Storage. Energy Environ Sci 6:1388
Yadav P, Banerjee A, Unni S, Jog J, Kurungot S, Ogale S (2012) A 3D hexaporous carbon assembled from single-layer graphene as high performance supercapacitor. Chemsuschem 5:2159
Yan J, Wang Q, Wei T, Jiang L, Zhang M, Jing X, Fan Z (2014) Template-assisted low temperature synthesis of functionalized graphene for ultrahigh volumetric performance supercapacitors. ACS Nano 8:4720
Yang M, Gao Q (2011) LiFePO4/C composite cathode material with a continuous porous carbon network for high power lithium-ion battery. J Alloys Compd 509:3690
Yang SB, Feng XL, Wang L, Tang K, Maier J, Müllen K (2010) Graphene-based nanosheets with a sandwich structure. Angew Chem Int Ed 49:4795
Yang X, Cheng C, Wang Y, Qiu L, Li D (2013) Liquid-mediated dense integration of graphene materials for compact capacitive energy storage. Science 341:534
Ye J, Zhang J, Wang F, Su Q, Du G (2013) One-pot synthesis of Fe2O3/graphene and its lithium-storage performance. Electrochim Acta 113:212–217
Yoo E, Kim J, Hosono E, Zhou H, Kudo T, Honma I (2008) Large reversible Li storage of graphene nanosheet families for use in rechargeable lithium ion batteries. Nano Lett 8:2277
Yu D, Wei L, Jiang W, Wang H, Sun B, Zhang Q, Goh K, Si R, Chen Y (2013) Nitrogen doped holey graphene as an efficient metal-free multifunctional electrochemical catalyst for hydrazine oxidation and oxygen reduction. Nanoscale 5:3457
Yu S-H, Conte DE, Baek S, Lee D-C, Park S-K, Lee KJ, Piao Y, Sung Y-E, Pinna N (2013) Structure-properties relationship in iron oxide-reduced graphene oxide nanostructures for Li-ion batteries. Adv Funct Mater 23:4293
Zhang LL, Zhao X, Stoller MD, Zhu Y, Ji H, Murali S, Wu Y, Perales S, Clevenger B, Ruoff RS (1806) Highly conductive and porous activated reduced graphene oxide films for high-power supercapacitors. Nano Lett 2012:12
Zhang L, Zhang F, Yang X, Long G, Wu Y, Zhang T, Leng K, Huang Y, Ma Y, Yu A, Chen Y (2013) Porous 3D graphene-based bulk materials with exceptional high surface area and excellent conductivity for supercapacitors. Sci Rep 3:1408
Zhao X, Hayner CM, Kung MC, Kung HH (2011) Flexible holey graphene paper electrodes with enhanced rate capability for energy storage applications. ACS Nano 5:8739
Zheng C, Zhou X, Cao H, Wang G, Liu Z (2014) Synthesis of porous graphene/activated carbon composite with high packing density and large specific surface area for supercapacitor electrode material. J Power Sources 258:290
Zhu Y, Murali S, Stoller MD, Ganesh KJ, Cai W, Ferreira PJ, Pirkle A, Wallace RM, Cychosz KA, Thommes M, Su D, Stach EA, Ruoff RS (2011) Carbon-based supercapacitors produced by activation of graphene. Science 332:1537
Zhu J, Yang D, Rui X, Sim D, Yu H, Hng HH, Hoster HE, Ajayan PM, Yan Q (2013) Facile preparation of ordered porous graphene-metal oxide@C binder-free electrodes with high Li storage performance. Small 9:3390
Zhu J, Yang D, Yin Z, Yan Q, Zhang H (2014) Graphene and graphene-based materials for energy storage applications. Small 10:3480
Acknowledgments
This work was supported by the Center for Integrated Smart Sensors funded by the Ministry of Science, ICT and Future Planning, Republic of Korea, as a Global Frontier Project.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Piao, Y. (2016). Preparation of Porous Graphene-Based Nanomaterials for Electrochemical Energy Storage Devices. In: Kyung, CM. (eds) Nano Devices and Circuit Techniques for Low-Energy Applications and Energy Harvesting. KAIST Research Series. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9990-4_8
Download citation
DOI: https://doi.org/10.1007/978-94-017-9990-4_8
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-9989-8
Online ISBN: 978-94-017-9990-4
eBook Packages: EngineeringEngineering (R0)