Abstract
Molecular imaging is an important tool in life sciences research and for clinical diagnosis and treatment. Among numerous imaging modalities, intravital microscopy (IVM) provides the best imaging spatial resolution in vivo and allows visualization of cellular and subcellular structures and functions. Because of its high resolution and the large number of available imaging agents, IVM has been used increasingly for the study of in vivo processes in many different fields. The application of IVM in cancer research and cancer treatment response assessment has been particularly fruitful. These IVM studies have disclosed that the cellular and subcellular dynamics during tumor progression and drug treatment in vivo are very different from those under in vitro conditions. Since the findings from IVM studies are obtained directly from intact living organisms, they may provide much more relevant information helpful to drug discovery and evaluation in clinics. In this chapter, we will briefly introduce the concepts of molecular imaging and the unique features of IVM. We will then highlight the most current IVM research in cancer biology and cancer drug response at the tissue, cellular and subcellular levels. We will end this chapter by outlining the future directions of IVM research.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Intravital imaging
- Molecular imaging
- Cancer
- Drug delivery
- Drug response
- Imaging agents
- Tumor microenvironment
- Tumor stroma
- Tumor circulation
- Hypoxia
- Tumor PO2
- Tumor pH
- Tumor extracellular matrix
- Tumor cell heterogeneity
- Cell tracking
12.1 Introduction to Molecular Imaging
12.1.1 Definition
Modern molecular imaging is defined as the noninvasive, real-time visualization of biochemical events at the tissue, cellular and molecular level in living organisms (James and Gambhir 2012). This makes molecular imaging fundamentally different from traditional clinical imaging in which mostly anatomic information is obtained. The rich information from modern molecular imaging is greatly improving the early detection, treatment selection, treatment management, and prognostication of many diseases. These achievements are mainly attributed to the rapid advancement of the two essential components of modern molecular imaging - imaging modalities and imaging agents.
12.1.2 Molecular Imaging Instrumentation
There are many imaging modalities. The classical ones include PET, SPECT, MRI, CT, ultrasound and optical imaging modalities. Many are often used in both clinical and preclinical settings. Other novel imaging modalities include fluorescence optical microscopy, bioluminescence optical imaging, photoacoustic imaging, and Raman spectroscopy. This latter group is used primarily for preclinical studies at the current time. Each of the above imaging modalities has its own strengths and limitations in terms of imaging depth, sensitivity, costs, and spatial and temporal resolution (James and Gambhir 2012). These properties largely determine the specific applications of each modality. For instance, intravital microscopy (IVM) gives the highest spatial resolution (1–10 μm or even sub-micrometer) and great flexibility for multiplexed imaging (monitoring multiple events simultaneously). The two properties are especially desired in studying cellular and subcellular events in living subjects. In the case of cancer, there exist large cellular and subcellular heterogeneities; each tumor and tumor cell can have very different pathological characteristics, activities and drug responses. Characterization of these heterogeneous elements and the underlying molecular mechanisms in vivo is the key to understanding the behavior of various cancers and designing effective treatments. These pressing needs demand high resolution imaging systems, such as IVM. In Part 2, we will discuss the current status of IVM in detail.
12.1.3 Molecular Imaging Agents
Molecular imaging agents (contrast agents or probes) are special classes of molecules and particles which bind or otherwise interact with their biological targets and enable non-invasive visualization of the targeted events. Every imaging agent needs to have sufficient specificity, sensitivity, and optimal pharmacokinetic properties for a specified in vivo imaging application. In order to comply with these requirements, these agents are designed to have at least two functional groups: one for the specific binding or reacting with the targets (e.g., small molecules, peptides, aptamers, antibodies or antibody fragments) and the other for providing signal(s) for its detection (e.g., radioisotopes, fluorophores, optical absorbers, inelastic light scattering materials). These imaging agents can be classified as non-targeted and targeted imaging agents (Fig. 12.1). Non-targeted imaging agents, such as fluorescent beads or particles, emit signal continuously independent of their binding states (Fig. 12.1a). The specificity of these agents is largely determined by their preferential accumulation into specific tissues. Targeted agents are designed to specifically bind to or interact with their targets. The targeting mechanism can involve specific antibodies for antigens, substrates for enzymes, ligands, agonists or antagonists for receptors, etc. (Fig. 12.1b) In particular, smart imaging agents can activate or switch signals exclusively in the presence of their intended target, which minimizes background signals and increases sensitivity (Fig. 12.1c). Reporter genes can be used to measure the location and levels of expression of specific genes of interest (Fig. 12.1d). Therefore, targeted imaging agents have increased specificity. Lastly, multimodality imaging agents are under rapid development (Fig. 12.1e). These agents contain in their backbone two or more of radioisotopes, fluorescent molecules, or nanoparticles that enable simultaneous PET, MRI, CT and/or optical imaging. Thus far, there are approximately 1,170 agents listed in the NCBI Molecular Imaging and Contrast Agents Database (MICAD). In this database, 41 % are PET imaging agents, 30 % are PET/CT imaging agents, 12 % are optical imaging agents, 9 % are MRI imaging agents, 3 % are multimodality imaging agents, 2 % are ultrasound imaging agents, and 1 % are x-ray/CT imaging agents. This variety of imaging agents makes it possible to visualize multiple biological targets and processes in vivo.
Major types of molecular imaging agents (a) Non-targeted imaging agents, such as fluorescent dyes, nanoparticles (b) Targeted imaging agents, such as fluorescent molecule conjugated antibodies; (c) smart imaging agents, such as fluorescence resonance energy transfer (FRET) caspase sensitive imaging agent. This caspase imaging agent is constructed by linking the cyan fluorescent protein (CFP) and yellow fluorescent protein (YFP) with a caspase-specific substrate (DEVD). Cleavage of DEVD by activated caspases results in the loss of fluorescence resonance energy transfer from CFP to YFP, thus reduced FRET signal. (d) Reporter genes, such as fusion reporter genes (top) and IRES-mediated bi-cistronic reporter genes (bottom). The fusion gene produces one single transcript and one polypeptide, whereas IRES-mediated bi-cistronic reporter gene produces one single transcript but two different polypeptides. (e) Multimodality imaging agents, such as a nanoparticle with an iron oxide core, a polymeric coating, and antibody and fluorescent dye conjugates for targeted MRI and optical imaging
12.2 Basics of Intravital Microscopy (IVM)
Intravital Microscopy (IVM) is a unique molecular imaging modality that enables live animal imaging at microscopic spatial resolution. In this section, we discuss why IVM is such a unique modality in molecular imaging, and the requirements for conducting IVM work.
12.2.1 Benefits of IVM in Molecular Imaging
The key strength of IVM over other modalities is its high spatial resolution. Imaging resolution critically affects early detection, diagnosis, and therapy monitoring. However, most molecular imaging modalities, such as CT, MRI, PET, SPECT and ultrasound, provide images with limited resolution (>1.0 mm). In contrast, IVM provides a spatial resolution of 1–10 μm which is critically required to resolve cellular and subcellular structures. Another key feature of IVM is that IVM studies focus on in vivo processes, which can sometimes be readily translated into the clinic. The other major advantage of IVM is the diversity of available imaging agents, which are mainly fluorescence imaging agents. This means that many cellular and subcellular processes and their molecular mechanisms can be studied in vivo with IVM. These benefits allow us to study critical biological questions, in a way that was previously impossible, to understand the development of many diseases. For example, in oncology, we can study the spatial and temporal relation between different tumor cells and stromal cells, their dynamic interactions, and the response of tumor cells to certain treatments. As much current cancer research efforts focus on cellular and subcellular structures and functions, IVM work can serve as an important tool for studying these processes within the context of the entire intact microenvironment. Therefore, IVM is a unique and essential molecular imaging modality. In summary, the key strengths of IVM are:
-
1.
Relatively high spatial resolution (1–10 μm)
-
2.
A wide array of imaging agents
-
3.
Multiplexed imaging capability
12.2.2 IVM Instrumentation
Using appropriate IVM instrumentation, imaging techniques, and agents are critical for successful IVM studies (Fig. 12.2). IVM instrumentation can include linear (e.g., single-photon confocal) and nonlinear microscopies (e.g., two-photon and other multiphoton systems), coherent anti-stokes raman scattering (CARS), fluorescence lifetime imaging microscopy (FLIM), optical frequency domain imaging (OFDI), etc. Among these, confocal microscopy and two-photon microscopy are the most commonly used IVM instrumentation types. Both systems enable imaging of cellular and subcellular events in living systems, but imaging principles and system setups are different between the two.
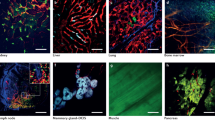
Typical workflow for IVM imaging: Step 1, set up imaging stations, such as two photon, and confocal microscopes with the appropriate objectives, light guides, heating pat and anesthesia system. Step 2, prepare animals for IVM imaging with the dorsal skin-fold window chamber model (left), skin flaps (middle), and ear model (right). In the dorsal skin-fold window chamber model and the ear model, special metal or glass supports are used to position the window chamber and the ear. Step 3, inject imaging agents into animals (e.g., intravenously, intradermally). Step 4, acquire images with the control software. An image acquired through a dorsal skin-fold window chamber with an IVM100 confocal system shows that RGD-Single walled nano-tubes bind to tumor blood vasculature (scale bar, 50 μm) (bottom left) (Smith et al. 2013) (Copyright 2013 Elsevier). The image acquired from an exposed mammary tumor with an IVM100 confocal system shows tumor angiogenesis (scale bar, 500 μm) (bottom middle). The image acquired through ears with an IVM100 confocal system shows RGD-Qdots bind to SKOV-3 tumor blood vessels (scale bar, 50 μm) (bottom right) (Smith et al. 2010) (Reprinted by permission from John Wiley & Sons)
Two-photon systems (2P) offer many benefits for in vivo imaging, including: (1) relatively deep tissue imaging due to decreased tissue absorption of longer wavelength light; and (2) less out-of-focus light due to the reduced two-photon excitation outside of the focal volume. 2P often requires objectives with high numerical aperture (NA) and high laser power to collect sufficient signal and obtain high Z-resolution. In practice, objectives with high NA have smaller working distances, which make imaging beyond 300 μm hard to achieve. Additionally, a high powered IR laser can overheat tissue and also saturate the detectors; consequently, infrared light blockers are often installed to protect systems from IR damage. This poses a significant problem considering the great interest and rapid progress in developing infrared and near infrared (IR/NIR) fluorophores for in vivo imaging. Compared with the 2P system, 1P systems offer comparable or better spatial resolutions but decreased imaging depth. Additional benefits with 1P are that there are many well-characterized imaging agents, options for lasers, objectives and built-in functionalities (Förster resonance energy transfer FRET, Fluorescence recovery after photobleaching FRAP). Therefore, the confocal microscope is a good choice for imaging thin tissues. The major differences between confocal and 2P systems are listed in Table 12.1.
12.2.3 IVM Imaging Techniques
Using the appropriate imaging technique is another key for successful IVM studies (Fig. 12.2). IVM requires the excitation and emission lights to be delivered and collected in a narrow optical path, which is very different from other whole body imaging modalities such as PET, CT and MRI. Furthermore, motions from heart-beat and respiration can have deleterious effects on high resolution IVM imaging. Therefore, tissues/organs to be imaged with IVM need to be effectively prepared to: (1) allow access of the optical components; (2) minimize motion artifacts; and (3) minimize perturbation of organ functions. To achieve these goals, three types of tissue preparations have been developed: window chambers, exposed tissue preparations, and in situ preparations (Fig. 12.2). In Table 12.2, we summarize these techniques and compare their strengths and limitations. For additional technique details, please refer to the review in (Jain et al. 2011) and other chapters (IVM: Principles and Technology).
12.2.4 IVM Imaging Agents
The third key requirement for IVM studies is the imaging agent (Figs. 12.1 and 12.2). In IVM studies, imaging agents critically help to increase the optical contrast or signal-to-noise ratio (SNR): the difference in intensity (or other measures) between the objects of interest and the adjacent background. Without sufficient optical contrast or SNR, it is difficult to obtain high resolution images, particularly under in vivo conditions where many endogenous molecules can give strong auto-fluorescence background (e.g., nicotinamide adenine dinucleotide NADH, flavin adenine dinucleotide FAD, collagen). Some endogenous contrast molecules can be used for IVM work. For examples, collagen produces a unique second harmonic generation (SHG) signal; lipids generate strong Raman signal, etc. But these applications are limited. For most applications, exogenous contrast agents are needed in order to image many different cell populations, cellular, and subcellular components. For IVM work, exogenous contrast agents can be either non-targeted or targeted optical imaging agents (Fig. 12.1). The targeted exogenous IVM imaging agents usually have one fluorescent functional component and another functional component for specific interaction with the target(s) of interest. Both functional components help to obtain high optical contrast. With higher molar extinction coefficients and quantum yields, the fluorescent components provide much higher fluorescence signal than the tissue autofluorescence background. (Molar extinction coefficient is a measurement of how strongly an imaging agent absorbs light at a given wavelength. Fluorescence quantum yield is the ratio of photons absorbed to photons emitted through fluorescence). Quantum dots, in particular, have extinction coefficients 10–50 times larger than fluorescent dyes and thus generate very high optical contrast for IVM work. IR/NIR fluorescent molecules (600–1,000 nm) are also very useful as the absorption and autofluorescence of endogenous biomolecules in the UV/Vis region are high (Weissleder and Ntziachristos 2003). IR/NIR fluorescent molecules are less prone to interfering absorption and fluorescence from tissues, have reduced scattering, and enable enhanced tissue penetration. These properties can greatly help to overcome some limitations of IVM. However there are relatively few NIR fluorescent agents currently available. Besides phthalocyanines, cyanine and squaraine dyes (Escobedo et al. 2010), there are only a few NIR fluorescent proteins with bacterial phytochrome-based NIR fluorescent proteins being only recently reported (Filonov et al. 2011). Additional functional components for targeting can be antibodies or antibody fragments, peptides or molecular substrates. These groups enable the imaging agents to preferentially localize to their targets rather than the background and therefore enhance the contrast. Other functional units (e.g., polyethylene glycol) that increase the circulation half-life and uptake of the agents can further improve the imaging contrast.
Many exogenous IVM imaging agents are available for high contrast IVM imaging of specific tissues, cellular, and subcellular events. New probes are continuously being developed. It is expected that IVM studies will be greatly empowered by future probes with: (1) IR and NIR spectrum; (2) photo-conversion capability; (3) smart detection; (4) self-amplification; (5) multimodality imaging and clinical translation potential. In the next sections, we will discuss specific applications of these IVM tools in cancer research.
12.3 IVM Applications
IVM has been applied in many research areas, including immunology, developmental biology, neuroscience, and cancer biology. These IVM studies have greatly improved our understanding of various human diseases and have helped build the IVM toolbox. In subsequent discussions, we will focus on IVM studies in the cancer field. We will highlight some novel imaging agents and techniques being developed, and elaborate on how they are applied in studying cancer biology and cancer drug response at the tissue level and at the cellular and subcellular levels.
12.3.1 Imaging Tumors at the Tissue Level
Tumors are abnormal organs with multiple cell populations co-evolving with their microenvironment (Hanahan and Weinberg 2011; Egeblad et al. 2010). This notion highlights the complicated composition, organization and development of many solid tumors. Indeed, tumors often have multiple tumor cell subpopulations and non-tumor stromal cell types, non-cellular components (e.g., soluble growth factors, cytokines; extracellular matrix ECM), and functional units (blood vessels and lymphatics). These components interact with each other, and together they create a tumor microenvironment of complicated circulatory systems, pH and oxygen profiles, ECM structure and other chemo-mechanical factors. Mapping and characterizing these factors is the first step in understanding the roles of the tumor microenvironment in tumor development and treatment response.
12.3.1.1 Circulatory System
Solid tumors often have abnormal circulation and this abnormal circulation is critical to tumor hypoxia, acidosis, high interstitial pressure, lymphangiogenesis, tumor progression and metastasis. IVM has become a major tool for high resolution analysis of the distribution, structure and functions of tumor circulation. Common imaging agents used for these assays are fluorescently labeled macromolecules (e.g., IgG, albumin, dextran, and fluorescent beads and polymers). These imaging agents are either intravenously injected to analyze the blood vessel functions or subcutaneously (or intradermally) injected to image the lymphatic functions (Padera et al. 2002; Hoshida et al. 2006; Isaka et al. 2004). For example, in IVM tail models (Padera et al. 2002), ear models (Hoshida et al. 2006), skinfold window chamber models (Isaka et al. 2004), it has been clearly shown that tumor vasculatures are tortuous and rich in fenestrations, vesicles and vesico-vacuolar channels. These tumor vessels lack the normal basement membrane and perivascular coverage. The inter-endothelial junctions are loose (100 nm–2 μm) whereas the leukocyte-endothelial interactions are strong. Therefore most tumor vessels are highly permeable (Fukumura et al. 2010). Similarly, tumor lymphatic vessels are often collapsed in the center of tumors but enlarged at the tumor periphery. This leads to reduced clearance of excess interstitial fluid from tumors (Padera et al. 2002; Leu et al. 2000). The defects in blood vessels and lymph systems together contribute to the high interstitial pressure, diffusion-dominant transport, and increased tumor lymphatic metastasis (Hoshida et al. 2006; Al-Rawi and Jiang 2011).
In the above work, fluorescently labeled macromolecules and particles with different sizes are particularly useful. In particular, dextran with size ranging from 2.36 to 27 nm, and fluorescent microspheres with sizes ranging from 20 nm to 5 μm, have been quite convenient to pinpoint the pore cut-off size in tumor vessels. Additionally, these imaging agents allow for the simulation of macromolecule transport in the tumor interstitial space. Combining IVM with FRAP and Fluorescence Correlation Spectroscopy (FCS) techniques allows for the quantification of the intratumoral diffusion, convection, and binding (Jain et al. 2011). These studies have assisted the rational design and selection of effective anti-tumor drugs based on their size, shape, charge and the diffusion distance in tumors. Besides these “inert” imaging agents, novel targeted imaging agents have been developed, such as αvβ3 integrin targeted imaging agents (Snoeks et al. 2010), VivoTag-680 conjugated αvβ3 integrin antagonist imaging agent (Kossodo et al. 2010), and Cy5.5 conjugated scVEGF (single-chain vascular endothelial growth factor) imaging agent (Backer et al. 2007). These novel imaging agents can provide further opportunities to image and study specific molecular events in tumor angiogenesis.
12.3.1.2 Hypoxia and pH
Many solid tumors have a hypoxic and acidic tumor microenvironment. Tumor hypoxia and acidosis can cause a landscape change in tumor genomics, proteomics, metabolic and signaling networks, and can also promote tumor invasion, metastasis and drug resistance (Parks et al. 2013; Harris 2002; Wilson and Hay 2011). It has been postulated that the loss of balance between tumor growth and poor oxygen delivery is the cause of tumor hypoxia and acidosis. This idea is supported by the facts that about 70 % of human cancers have a high uptake of 18F-FDG in clinical PET imaging (Parks et al. 2013) and that tumors have decreased blood and oxygen supply (Vaupel et al. 1989). However PET imaging and electrode based measurements do not have sufficient resolution for spatial correlation between the two. In order to fully understand the relation between tumor blood and oxygen delivery, and tumor hypoxia and acidosis, high resolution mapping of the spatial and temporal relationship of tumor pH, partial pressure of oxygen (PO2) and blood vessels is needed. IVM, together with other molecular imaging modalities, such as NMR, provides a fresh view of the causes of and relation between the changes in tumor PO2, pH, blood supply and metabolism. In such IVM studies, the PO2 profiles in tumors are derived from phosphorescence quenching imaging of oxygen sensitive porphyrine and the pH profiles are generated from fluorescence ratiometric imaging of pH sensitive seminaphthorhodafluors (SNARFs) (Martin and Jain 1994; Helmlinger et al. 1997; Dellian et al. 1996). It has been found that the pH and PO2 profiles in tumors are highly heterogeneous: hypoxic areas co-exist with oxygenated areas; acidic regions co-exist with relatively basic regions; highly glycolytic cancer cells (lower pH) can locate in oxygen-rich environments. Importantly, the pH and PO2 profiles in a tumor do not necessarily correlate with each other or blood supply; both pH and PO2 can independently control VEGF expression in tumors (Fukumura et al. 2001). These early imaging-based results have been confirmed and explained by recent biochemical analysis of tumor tissue: indeed, oncogene activation alone can cause tumor glycolysis and acidosis (Elstrom et al. 2004); and hypoxia can increase acidosis through hypoxia-inducible factor (HIF)-dependent pH-regulating systems (Wilson and Hay 2011). Furthermore, imaging mixed tumor populations expressing wild type HIF1α and HIF1α−/− mutant through a skinfold window chamber has shown that HIF1α−/− cells remain alive at regions distal to blood vessels (Brown et al. 2001). This stimulating result suggests HIF1α is necessary for some tumor cells to migrate but not to survive, which echoes recent research on the multiple functions of HIF1α in tumor pathology. Interestingly, recent IVM studies have also shown that the acidic peritumoral region is associated with the up-regulation of glucose transporter-1 (GLu-1) and this acidic extracellular pH is necessary for tumor cell migration and invasion (Estrella et al. 2013). It is not clear if HIF1α is involved in GLu-1 over-expression in this case. It will be very interesting to conduct imaging correlation work and mechanistic studies to see if these observations are related. These examples highlight the contributions of IVM to the research of tumor hypoxia and acidosis.
Specialized imaging agents are critical to the above IVM work. Accurate quantification of PO2 and pH in vivo is often difficult due to variability within tissues, cells, and the distribution and photo-bleaching effects of imaging agents. Because of these issues, great effort has been put into developing better imaging agents in recent years. Among those, ratiometric imaging agents, which include a reference dye or use emission wavelength shifts, allow better estimation of PO2 and pH in living subjects. For ratiometric imaging of oxygen, oxygen-sensitive fluorescent agents (e.g., Phosphor oxyphor G2, iridium complex BTP), or bioreductive fluorescent agents using O2 as a substrate (e.g., nitroimidazole and indolequinone based imaging agents), are linked with oxygen-insensitive reference fluorophores (e.g., NIR dyes Cy) (Apte et al. 2011). For ratiometric imaging of pH, small molecule-based imaging agents which can shift their emission spectrum under specific pH are commonly used. These imaging agents include fluorescein based imaging agents (e.g., BCECF), benzoxanthene dyes (e.g., SNARFs), BODIPY and cyanine-based pH indicators (Han and Burgess 2010). Conjugation of these pH sensitive molecules with nanoparticles, peptides and proteins, has shown improved signal, sensitivity, pharmacokinetics and tissue specificity. Furthermore, targeted molecular imaging agents have also been developed as the molecular mediators in hypoxia and acidosis are identified. Examples include HypoxiSense 680 [PerkinElmer] and fluorescence antibody targeting carbonic anhydrase IX (Bao et al. 2012), peptides with the oxygen-dependent degradation domain of HIF-1 α (Kuchimaru et al. 2010). These targeted imaging agents can help to dissect the molecular networks involved in tumor hypoxia and will likely eventually impact strategies for cancer diagnosis and therapy.
In summary, IVM studies have been able to provide high resolution mapping of tumor PO2 and pH in vivo. This imaging based research has demonstrated that tumor hypoxia and acidosis are spatially and temporally heterogeneous and are controlled by interrelated regulation networks.
12.3.1.3 Extracellular Matrix Composition and Remodeling
The extracellular matrix (ECM) is an important component of the tumor microenvironment. Tumor cells and stromal cells can deposit, degrade and dynamically remodel ECM at different tumor development stages. The tumor ECM, in turn, regulates a broad range of tumor cell activities from promoting tumor cell growth to building up metastatic foci. These roles of tumor ECM are closely related to their biochemical properties, and biophysical properties (Yu et al. 2011). IVM studies have greatly helped the characterization of ECM composition, structure and dynamic process and the understanding of ECM functions in tumor development.
IVM has been widely used to characterize type I collagen (Col I) in many tumor models due to the unique and robust SHG signals associated with Col I (Williams et al. 2005). These SHG signals arise from the highly noncentrosymmetric triple-helix structure of Col I. By combing Col I SHG and cell labeling techniques, it has been found that tumors have increased collagen density; the changes of stiffness, distribution and orientation of Col I are often associated with tumor progression (Yu et al. 2011; Provenzano et al. 2008; Provenzano et al. 2006; Levental et al. 2009). In particular, increased Col I density promotes mammary tumor initiation and progression. The reorganized Col I at the tumor-stromal interface facilitates local invasion. Most interestingly, increased collagen crosslinking and stiffening can force tumor malignant transformation (Provenzano et al. 2008; Provenzano et al. 2006; Levental et al. 2009). On the other hand, IVM at the single cell level has shown that both fibroblasts and macrophages can interact with and remodel collagen. Activated fibroblast can deposit Col I. Migrating fibroblasts can drag, push, and degrade collagen fibers in a β1 integrin and matrix metalloproteinase (MMPs) dependent manner (Perentes et al. 2009). Macrophages can also degrade collagen intra-cellularly or extra-cellularly (via MMPs). Thus, remodeled Col I, tumor cells and tumor stromal cells form an interactive network promoting tumor progression. Besides collagen, there are many other important ECM components, such as fibronectin, tenascin, decorin, fibromodulin, hyaluronic acid, SPARC, lumican, and osteopontin. The precise roles of these proteins in tumor progression are not yet clear (Frantz et al. 2010). Imaging these ECM components in vivo requires novel imaging agents for specific ECM proteins and specific function of interest. These imaging agents are still very limited and require further development and validation. For example, fibronectin FRET imaging agents have been used in cell culture work but not yet in vivo (Smith et al. 2007). Quantum dots-conjugated hyaluronic acid has been tried for in vivo real-time imaging but the imaging properties are to be improved (Bhang et al. 2009). It is expected that many new imaging agents for these ECM components will be developed in the future and imaging these ECM proteins in vivo will likely be possible.
Secreted proteases and enzymes play key roles in ECM remodeling and tumor progression. MMPs, cathepsins, urokinase-type plasminogen activator (uPA) are some of the well known proteases involved in tumor development. Because of their important roles, there has been great interest in developing optical imaging agents to visualize the distribution and activity of these proteases in tumors. Activity- based imaging agents are the most often used to detect these proteases as they give high signal-to-background ratio in vivo and enable differentiation of active and inactive proteases (Blum et al. 2005). For example, a fusion reporter of a collagen binding peptide and Renilla luciferase has been conjugated with a quencher dabcyl and MMP-2/9 substrate peptide to map MMP2/9 activity in tumors in vivo (Xia et al. 2011). The absorption spectrum of dabcyl overlaps with the emission spectrum of Renilla luciferase such that removing the quenchers by MMP-2 restores the bioluminescent emission. A similar imaging agent composed of Cy5.5, MMP substrate, a BHQ-3 fluorescence quencher and an RGD targeting sequence has been shown to have great tumor specificity and stability in vivo (Zhu et al. 2011). Additionally, nanoparticles are also used as quenchers to generate activatable fluorescent imaging agents for MMPs (Lee et al. 2009). Activity-based imaging agents are also available for the cathepsin families (Mahmood and Weissleder 2003), thrombin (Pinto and Schanze 2004), etc. Although these imaging agents can give high tumor-specific fluorescence signals in vivo, they still suffer from the non-specific cleavage by other enzymes and the low stability in serum. Currently, most of these imaging agents are used in whole body imaging, histology, or cell culture studies. It is expected that their applications in IVM will increase in the future.
12.3.1.4 Implications in Imaging Drug Distribution
Any drug has to reach its target tissues and cells before being effective. To reach its targets, a drug needs to leave the bloodstream, diffuse into the interstitial space and enter the target organs and tissues. It is known that the distribution of many anti-cancer drugs is insufficient and heterogeneous, but it is often unclear what causes this poor drug distribution and how to address it. IVM has been a powerful tool in providing insights into these questions. Careful studies with IVM have revealed that besides the size, charge, and hydrophobicity of the drugs, the tumor vasculature, interstitial pressure, pH, oxygenation and ECM all critically affect drug distribution (Goel et al. 2012; Amornphimoltham et al. 2010). The leaky and chaotic tumor circulation results in poor perfusion and reduced drug distribution in tumors. The high interstitial pressure and poor drainage, due to high solid stress and abnormal lymphatic function, further increase blood flow resistance and reduce the convective transport of drugs. Additionally, the dense ECM network with reduced pore size forms a barrier that limits drug diffusion in the interstitial space (Tufto et al. 2007; Erikson et al. 2008). Acidic and hypoxic conditions in tumors affect the physico-chemical properties of drugs (e.g., charge), the expression of drug transporters on cells, and drug activity (e.g., doxorubicin uses oxygen to generate free radicals and damage DNA) (Trédan et al. 2007). These findings suggest that drug distribution in tumors can be improved by increasing tumor blood flow, increasing vessel permeability, reducing interstitial fluid pressure (IFP), modifying tumor ECM and targeting tumor hypoxia and acidity. Indeed, therapeutic effects have been improved using these strategies (Fig. 12.3a) (Goel et al. 2012).
Examples of IVM imaging at the tissue level (a) Optical frequency domain images show the response to anti-angiogenic VEGFR-2 by mouse mammary tumor cells (MCaIV) grown in a dorsal skin-fold window chamber. Antiangiogenic VEGFR-2 treatment leads to reduced density, length and diameters of intratumor vessels compared to those in the control tumors. The lymphatic vascular network is in blue (scale bar, 500 μm) (Vakoc et al. 2009) (Reprinted by permission from Macmillan Publishers Ltd: Nature Medicine, copyright 2009). (b) Two-photon images show that hyaluronidase treatment increased the perfusion of tumor blood vessels (top) and the delivery of doxorubicin (bottom) in a pancreatic ductal adenocarcinoma of transgenic mice (scale bar, 50 μm). The autofluorescence of doxorubicin allows for the imaging of its distribution (left). ac acini, d duct. Asterisks highlight the large, functional lectin-positive vessels loaded with doxorubicin. Arrows in the top panels indicate well-perfused functional vessels. In the bottom panel, arrows in left image show thin-walled vessel and arrowheads point out the ductal epithelium; arrows in the middle and right images show the regions magnified in respective insert boxes; small arrows in the inserts show the distribution of doxorubicin in the nuclear (green) and extracellular space (Provenzano et al. 2012) (Reprinted by permission from Elsevier, copyright 2012)
The value of IVM for cancer treatment studies can be exemplified from research on anti-angiogenic therapy. Anti-angiogenic therapy in clinical cancer therapy remains a puzzle. It has been shown in the pre-clinical setting that anti-angiogenic drugs could cut off tumor blood supply and thus starve and shrink tumors. However, many anti-angiogenic agents showed modest effects in clinical trials and sometimes resulted in more aggressive tumors (Bergers and Hanahan 2008). Multiple mechanisms could contribute to this drug resistance but remain to be tested (Bergers and Hanahan 2008). Thus far, some IVM studies showed that the up-regulation of alternative pro-angiogenic signaling pathways (e.g., FGF) and reduction in VEGF signals are the major reasons for anti-VEGF drug resistance (Fukumura et al. 2010). On the other hand, other studies revealed that alternative factors can influence drug delivery (Tada et al. 2007; Smith et al. 2008). Reduced vascular permeability by anti-angiogenesis therapy can also hinder the extravasation of many drugs (Bhirde et al. 2009; Mikhail and Allen 2010; Pink et al. 2012) and lead to hypoxia and increase cancer stem cells and tumor metastasis (Gaustad et al. 2012; Rapisarda and Melillo 2012; Conley et al. 2012). Additionally, tumor vasculature is very heterogeneous; VEGF-dependent and -independent vascular zones coexist and interconvert dynamically (Manning et al. 2013). Tumor cells can also enter the blood stream when they are close to a structured tumor-associated vessel (Beerling et al. 2011). These diverse findings from IVM studies demonstrate that many factors can contribute to the lack of efficacy of anti-angiogenic therapy, and suggest that anti-angiogenic reagents should be applied to specific cancer types in a controlled manner (e.g., timing, location, and combination therapy). These results and potential therapeutic strategies need to be further tested for extrapolation to the clinical setting.
Tumor ECM can impact drug effects by direct influence on the diffusion and distribution of drugs in the tumor interstitium and by indirectly affecting the response of tumor cells to drugs. Two-photon FRAP work has shown that increased collagen content in tumors is associated with slow diffusion of molecules, and treating tumors with collagenase can improve the diffusion of drugs (Alexandrakis et al. 2004; Magzoub et al. 2008). Interestingly, hyaluronidase (HA) treatment does not improve molecular diffusion in tumors. But treating pancreatic ductal adenocarcinomas with HA reduced the interstitial fluid pressure, opened the microvasculature, and improved efficacy of chemotherapeutic drugs (Fig. 12.3b) (Eikenes et al. 2005; Provenzano et al. 2012). Indirectly targeting fibroblast-mediated ECM remodeling with TGF-β inhibitors also improved the distribution and efficacy of therapeutics in breast carcinoma (Liu et al. 2012; Salnikov et al. 2005).
These studies suggest that targeting ECM processing and remodeling might be a useful strategy to improve cancer therapy. However, improving drug distribution by targeting ECM is often not straightforward. Just like anti-angiogenesis therapy, inhibitors for MMP showed promising results in preclinical studies but failed in clinical trials. ECM proteins and their related proteases and factors have more complicated functions than expected (Yu et al. 2011; Mueller and Fusenig 2004). Some MMPs have ECM-independent functions and can have protective roles (Folgueras et al. 2004). Tumor cells can switch from mysenchymal, amoeboidal and collective modes of migration and invasion depending on tumor ECM, proteases and signaling. Thus, inhibitions of MMPs, TGF-β and other factors can often lead to adverse effects (Matise et al. 2012; Meulmeester and Ten Dijke 2011). Considering that some of these studies are based on a snap-shot of tumor progression and that ECM remodeling is a highly dynamic process, further real-time IVM imaging is necessary to better understand how to target ECM in cancer therapy.
In summary, IVM has been a useful tool in understanding drug delivery and distribution in tumors. The results from this research have helped in designing new drugs, developing novel therapeutic strategies and elucidating mechanisms of drug effects. However, drug efficacy does not only depend on drug distribution at the tissue level. Recent IVM imaging of single cell and subcellular pharmacokinetics showed that the proteomic heterogeneity in individual cells can be a major reason for limited drug action when tumor cells are exposed to sufficient drugs in vivo (Thurber et al. 2013). Therefore, imaging the structure and composition of tumors at the cellular and subcellular levels is another important direction leading to the understanding of drug resistance and response. In the next section, we will discuss IVM work at the cellular and subcellular levels and related work on drug response.
12.3.2 Imaging Tumors at the Cellular and Subcellular Levels
12.3.2.1 Cell Tracking
Tumors are heterogeneous and can have multiple tumor cell subpopulations and stromal cell populations within a single tumor (Marusyk et al. 2012; Pietras and Ostman 2010). These various populations can have different cellular states, spatial-temporal distributions, cell-cell interactions, as well as different functions during tumor development. Identifying the roles of each cell population in tumor development is always a critical task in cancer research and clinics. IVM, with its high resolution at the cellular level and diverse imaging agents, is an ideal tool for tracking cells and cell activities in their native in vivo environment.
Tracking cells requires specific labeling of cells of interest. There are three basic cell labeling methods: ex vivo labeling, direct in vivo labeling, and transgenic methods. For ex vivo labeling, specific cell populations are isolated and labeled with imaging agents in vitro and injected back into the hosts. Since cell specificity is achieved in the cell isolation and purification steps, a wide range of imaging agents can be used, such as fluorescent reporter genes, fluorescent molecules and particles. For short-term cell labeling and imaging experiments, CFSE (Carboxyfluorescein succinimidyl ester), Dil (1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate), quantum dots, etc. are often used. This transient cell labeling technique allows, for example, the visualization of T cell dynamics in lymph nodes, and tumor cell extravasation during metastasis (Stoll et al. 2002; Bajénoff et al. 2006; Voura et al. 2004). Because of the problems of dye retention, transfer, dilution and toxicity, reporter genes are preferred for long-term cell labeling (Hulit et al. 2012). By tracking cancer cells stably expressing GFP, CFP, RFP (green, cyan, red fluorescent proteins), etc., it has been found that tumor cells move much faster in vivo than in vitro (Condeelis and Segall 2003). Tumor cells are able to utilize collective, amoeboidal or mensenchymal migration strategies (Friedl and Alexander 2011), and take active or passive approaches for intravasation and extravasation (Kedrin et al. 2008). IVM in combination with whole body bioluminescence imaging has demonstrated that intravenously injected lymphoma cells home to spleen and bone marrow first and then disseminate to lymph nodes only a few days after (Ito et al. 2012). Furthermore, the reporter gene approach allows studying the molecular mechanisms involved in these processes. For example, by expressing mutant proteins or using inhibitors, it has been found that the migration speed and modes of tumor cells in vivo are regulated by signaling networks involved in actin polymerization, actomyosin contraction and cell adhesion, such as Arp2/3-cofilin-mena pathways, Rho family small GTPase, Integrin and focal adhesion kinase pathways (Philippar et al. 2008; Olson and Sahai 2009; McGhee et al. 2011; Timpson et al. 2011). Although simple and fast, the ex vivo labeling method has major drawbacks: the isolation and in vitro labeling can alter cell properties, and the implantation sites are often not the native environments.
In the direct in vivo labeling, imaging agents are systemically administered and taken up by a specific cell population. Systemic delivery of imaging agents is fast. Many imaging agents ranging from small molecules to antibody conjugated particles can be administrated in vivo to label cells for short-term imaging. But some major problems of these imaging agents include: high background signal, low specificity and stability in vivo. For example, dextran, DiR, etc. can be injected in vivo to label macrophages, but many other cells such as dendritic cells and cancer cells can also take up these dyes. Cell type-specific imaging agents, such as those based on unique surface ligands, receptors, antigens, or subcellular molecules, can have better specificity (Fig. 12.1b) (Kobayashi and Choyke 2011). However, creating such highly specific markers is not easy and remains an important research area in molecular imaging.
Transgenic reporter animal approaches allow for tracking of cells in their native environment for prolonged periods. This approach is extremely valuable when studying the tumor associated stromal cells, including immune cells, fibroblasts, mesenchymal cells, endothelial cells, and recruited bone marrow cells (Fig. 12.1d). Experiments with direct in vivo cell labeling and transgenic mice expressing GFP, YFP and RFP have shown that dendritic like cells, myeloid cells and carcinoma-associated fibroblasts migrate faster at the tumor periphery than within the tumor, whereas the regulatory T-cells (Tregs) mainly migrate near blood vessels (Egeblad et al. 2008). As these tumor stromal cells are involved in tumor lymphangiogenesis, immune surveillance, hypoxia response, ECM remodeling, tumor progression and metastasis (Mueller and Fusenig 2004; Tlsty and Coussens 2006; McMillin et al. 2013), these IVM studies can really help in understanding the dynamic interactions between stromal and tumor cells. The transgenic animal approach is also extremely useful in lineage tracing of heterogeneous tumor cells. As tumor cell heterogeneity can arise from genetic, epigenetic clonal evolution, environmental effects, or cancer stem cell differentiation (Magee et al. 2012), it is necessary to label a few tumor cells in their native environment and follow their fate for prolonged periods in vivo. It has not been until recently, that the technology breakthroughs in lineage tracing, transgenic mouse models and imaging techniques have made it possible to perform such long-term cell fate tracking experiments in vivo (Livet et al. 2007). With “Brainbow” mosaic expressing multicolor fluorescent proteins in individual cells and tracing their fates in the native environment (Fig. 12.4a), it has become evident that stem-cell-like cells that are long-lived and able to self-renew or divide to produce new progenitors do exist in papillomas, intestinal cancer, and glioblastoma models (Driessens et al. 2012; Schepers et al. 2012; Chen et al. 2012). These multipotent stem cells maintain human adenomas and the pattern of clonal expansion in tumor evolution (Humphries et al. 2013) and can also propagate glioblastoma growth after chemotherapy (Chen et al. 2012). These studies, for the first time, provide solid data supporting the idea that a small subset of cells drives tumor growth, and that it is necessary to target these cells in cancer therapy.
Reporter genes (a) an example of the brainbow CRE/loxP site-specific recombination systems. In this brainbow system, DNA transcriptional STOP cassette and fluorescent reporter genes (e.g., RFP, YFP, CFP) are flanked by pairs of mutually incompatible lox variants (three lox pairs here). The introduction of CRE results in random recombination between these loxP pairs. Each of these recombinations leads to the expression of a distinct XFP, such as RFP, YFP or CFP. This strategy is extremely useful in long-term cell-lineage tracing experiments (b) an example of the inducible reporter gene systems. In this tetracycline (Tet)-or doxycycline (Dox)-inducible system, the transcription of the gene of interest and the fluorescent protein genes is under the control of Tet response element (TRE). In the presence of doxycycline, the reversed tetracycline transactivator (rtTA) binds to TRE and turns on the transcription of TRE-controlled genes. In the absence of doxycycline, rtTA can not bind to TRE and thereby prevents the transcription of TRE-controlled genes
12.3.2.2 Subcellular Components and Processes
Microscopy has been an essential tool to study the dynamics of molecular localization, interaction and function of subcellular components during cell division, apoptosis, migration, metabolism, transcription and translation in vitro. Imaging subcellular processes in vivo is still very challenging due to the motion artifacts from live animals and the low signal to noise ratio in vivo. However with recent technical advancement in minimizing motion effects and the availability of better imaging agents, subcellular IVM imaging has become possible.
IVM has been used to image cell division in vivo. One of the classic methods for imaging cell division is to use GFP-tagged human histone H2B (H2B-GFP) to visualize chromatin structure and nuclear dynamics (Fig. 12.1d) (Hadjantonakis and Papaioannou 2004; Yamamoto et al. 2004; Orth et al. 2011; Janssen et al. 2013). This reporter gene can be further modified to include a tetracycline regulatory element such that H2B-GFP is only expressed when doxycycline is added (Fig. 12.4b). With this inducible H2B-GFP reporter, the cell cycling time of stem cells and their progenitors, the repopulation potential of stem cells, and the interactions between stem cells within their niches have been studied in vivo (Foudi et al. 2009; Wilson et al. 2008). One of the drawbacks of these H2B-GFP reporter systems is the requirement for high resolution images to analyze the morphology of H2B-GFP tagged chromatin for accurate cell cycle staging. This is a great challenge for in vitro and in vivo work. Therefore, a more elegant cell cycle reporter system is to directly color code each G1, S, or G2/M phase. For example, the Fucci reporter uses the cell-cycle-dependent proteolysis of Cdt1 and Geminin to control the expression of mKO2 and Azami green, such that the nuclei of cells appear in different color when cells cycle though G1/S/G2/M phase (Sakaue-Sawano et al. 2008). This reporter gene makes it very convenient to track the cell-cycle stages in real-time in vivo.
IVM and reporter genes have also been used to visualize the intracellular signaling pathways involved in cancer cell migration, invasion, and metastasis. In these studies, tumors cells are engineered to express fusion proteins composed of proteins of interest with fluorescent proteins, and injected into animals and tracked with IVM. Using these approaches, it has been found that ROCK regulates the phosphorylation and organization of myosin light chain and thus cancer cell motility in vivo (Wyckoff et al. 2006). Rac and Rho-kinase signaling control the switch between the mesenchymal and amoeboid movement of cancer cells in vivo (Sanz-Moreno et al. 2008) whereas TGFβ signaling is associated with single cell movement (Giampieri et al. 2009; Kardash et al. 2010). Similar work has revealed a series of important proteins regulating tumor invasion and migration in vivo, which is impossible to be studied in isolated cells.
Imaging caspase activity and apoptosis is another important area of IVM research. Some of this research takes advantage of genetically-encoded FRET imaging agents to report caspase activity under in vivo conditions. In these cases, the caspase sensor is constructed to have CFP and GFP linked by a caspase-3 cleavage sequence DEVD (Fig. 12.1c). Tumor cells are engineered to express this reporter gene construct and then injected into animals. The non-apoptotic cells give FRET signals, but the apoptotic cells lose FRET signals as activated caspase-3 cleaves DEVD (Keese et al. 2010; Breart et al. 2008). Other studies use systemically administered imaging agents, such as near-infrared FLIVO™ and caspase-triggered nanoaggregation imaging agent, to study apoptosis. The FLIVO™ imaging agent is a small molecule fluorescent inhibitor for active caspases and can give red fluorescence in apoptotic cells with little background (Tsai et al. 2007). Caspase-triggered nanoaggregation imaging agent has a DEVD caspase cleavage peptide linked with a polymeric fluorescence backbone which can aggregate and self-amplify signal upon caspase cleavage (Shen et al. 2013). These imaging agents can be a good alternative when the introduction of reporter genes into cells is not feasible, and when FRET signals are difficult to quantify in vivo.
FRAP, FLIM, photoswitching, etc. techniques have also been incorporated for IVM imaging of subcellular processes. For example, FRAP IVM imaging has shown that the immobile fraction of E-cadherin in cell-cell junctions is five times more in vivo than in vitro and E-cadherin mobility correlates with cell migration (Serrels et al. 2009). FLIM has also been combined with IVM to study the metabolic products in tumor cells in vivo. For examples, NADH and FAD have very different two-photon fluorescence life times depending on their bound states and can serve as reporters for mitochondrial activity. Mapping these endogenous NADH and FAD with FLIM-IVM within live cells has shown a stepwise change of intracellular metabolic states during cancer development (Skala et al. 2007; Provenzano et al. 2009).
12.3.2.3 Implications in Monitoring Drug Response
Currently, 70 % of oncology drugs that enter Phase 2 clinical trials fail to enter Phase 3. Among those drugs that do enter Phase 3 trials, 59 % fail (Kola and Landis 2004). This high failure rate is mainly due to the lack of drug efficacy in the clinic, the lack of predictive animal models and the lack of understanding of drug mechanisms in vivo. IVM might be a valuable tool to address these issues.
One area of IVM research is in cancer immunotherapy. These IVM studies have disclosed that the infiltration of cytotoxic T cells (CTL) in tumors is very heterogeneous and their tumor-elimination activities are limited by access to tumor cells (Boissonnas et al. 2007). Additionally, CD44 has been found to mediate cell migration and stable interactions between killer cells and tumor cells (Mrass et al. 2008). Such IVM based work greatly contributed to the discovery of novel anti-tumor immune therapies. Another important area of study is the stroma mediated drug sensitivity and resistance in chemotherapies (McMillin et al. 2013). Many drugs, such as dexamethasone, doxorubicin, vemurafenib, ruxolitinib, can be affected by the tumor stroma. But the mechanisms are not clear. Recently, Nakasone et al. addressed the question with IVM and transgenic mouse models for breast cancer (Nakasone et al. 2012). Their imaging results have shown that the sensitivity to doxorubicin changes with tumor stage. This stage dependent drug sensitivity was found to be related to the leakiness of blood vessels and the recruitment of CCR2+ myeloid cells but not the intrinsic properties of tumor cells (Fig. 12.5a). Further studies have shown that infiltrated CD11b + GR1+ myeloid precursors can also mediate the anti-VEGF resistance in colorectal cancer (Shojaei et al. 2007). However, the roles of myeloid cells in tumor drug response can be drug and tumor type specific (Germano et al. 2013). Therefore, more IVM studies are still needed in this area.
Examples of IVM imaging at the cellular and subcellular levels (a) Spinning disk confocal images show doxorubicin-induced cell death in exposed mammary tumors in real-time. Reporter mice are generated by crossing MMTV-PyMT and ACTB-ECFP and c-fms-EGFP mouse strains such that tumor cells express ECFP and stromal macrophage express EGFP (left). The death of tumor cells (red arrow) and stromal cells induced by doxorubicin is visualized by propidium iodide (PI) administered intraperitoneally. Doxorubicin also induces the infiltration of stromal cells (white arrow) (scale bar, 10 μm) (Nakasone et al. 2012) (Reprinted by permission from Elsevier, copyright 2012). (b) IVM images show PARP inhibitors (PARPi) distribution in a HT-1080 tumor implanted in a dorsal skin-fold window chamber. Proliferating tumor cells are labeled with H2B-Apple, tumor associated macrophages (TAM) are labeled with Clio-680 nanoparticles, PARPi is synthesized with a fluorescent group attached to the PARP1 targeted group (left). The PARPi is accumulated in most tumor cells and some TAMs (Thurber et al. 2013) (Reprinted by permission from Macmillan Publishers Ltd, copyright 2013)
Subcellular mechanisms are also important in drug sensitivity and resistance of tumor. Janssen et al. set out to understand the mechanisms of docetaxel-induced tumor cell death in vivo (Janssen et al. 2013). They expressed H2B tagged photo-switchable Dendra2 and FRET caspase-3 biosensor simultaneously in tumor cells to track the mitotic progression and the onset of apoptosis within tumors. They showed, in contrast to the in vitro conditions, that docetaxel-induced apoptosis was independent of mitosis in vivo but rather dependent on the heterogeneous microenvironment. This hypothesis was tested in a related IVM study with Src-inhibitor applied to a subcutaneously grafted p53-mutant pancreatic tumor (Nobis et al. 2013). With a FLIM-FRET Src sensor, dasatinib-inhibition of Src activity was found to be limited within 50–100 μm from the vasculature. Cyclopamine was then administered to modify ECM structure for enhancing dasatinib effects. Although dasatinib efficacy was improved, it was mainly localized to the peri-vascular region (25–50 μm away from vessels) and the spatial limit remained similar as in the controls. This result suggests that tumor ECM may not be a limiting factor for dasatinib, which is in agreement with the recent finding from another group (Thurber et al. 2013). Imaging the intracellular kinetics of the PARP-1 inhibitor (PARPi) distribution in real time in live animals showed that the responses of tumor cells to PARPi are heterogeneous regardless of efficient drug delivery and sufficient nuclei accumulation of PARPi (Fig. 12.5b) (Thurber et al. 2013). This result suggests PARPi efficacy may be linked to both the intrinsic heterogeneity of individual cells and the stromal cells. These few cases together demonstrate that drug response in vivo is complicated and no single mechanism can explain all observations. In order to identify the exact mechanisms of drug response in vivo, more thorough IVM work will be required.
In summary, using different models, drugs, and methods, these IVM studies all demonstrate that drug sensitivity and responses are strongly affected by the in vivo environment and the cellular and subcellular heterogeneity. These initial studies have shown the great potential of IVM in these areas of investigation. With the advancement of imaging instrumentation, improved imaging techniques and imaging agents, IVM will undoubtedly impact the development of anti-cancer therapy and ultimately assist in clinical cancer management.
12.4 Future Directions
Although IVM has provided significant gains in our understanding of basic in vivo biology, there are still many potential advances in instrumentation and imaging probes that may allow further insight and the full realization of IVM strategies. Improving imaging depth and increasing multiplexing capability is a major goal in IVM work. Improvements in instrumentation are covered elsewhere in this book (See Chapter IVM: Principles and Technology). It will be important to expand our current library of IVM imaging agents, particularly IR and NIR fluorescent imaging agents, because of their apparent benefits in deep tissue imaging. The ability to use multiple imaging agents, each one specific for a given process of interest, will be highly important. This will require a team science approach in which biologists, chemists, molecular pharmacologists, and IVM specialists work hand-in-hand to develop a larger library of well validated agents. It will be important to show that such developed agents actually measure specific processes of interest through careful validation studies. In addition, it will be important to continue to make IVM more quantitative so that images obtained using specific imaging probes can be quantified to show the levels of underlying molecular targets or processes of interest.
Another important future direction is multimodality imaging. In multimodality imaging, a single imaging agent is often used for imaging with different imaging modalities to maximize the information from complementary methods (James and Gambhir 2012; Gambhir 2013). The limited field of view and imaging depth in IVM restrict its abilities to study the traffic of tumor cells and tumor metastasis. Combining IVM and other whole-body imaging modalities can often overcome such limitations. For these purposes, fusion reporter genes and reporter mice for luciferase-GFP and for PET-luciferase-RFP are available (Cao et al. 2004; Ray et al. 2004; Yan et al. 2013) and some multimodality imaging agents have also been developed (Tsourkas et al. 2005). It is expected that there will be many more important applications of multimodality imaging in the future (Cherry 2006; Culver et al. 2008).
The development of novel endomicroscopy (e.g., Raman, confocal), microcatheters, etc. instrumentation is another exciting direction. These types of instruments will likely allow new ways of imaging mouse models in which different tissue compartments (e.g., gastrointestinal tract) can be accessed. By allowing microscopes that go into the body one may be able to open up entirely new ways to study molecular and cellular events that are currently quite difficult to perform.
In summary, many important questions in cancer pathology and drug response remain to be answered. IVM based imaging research has already shown its power in addressing some of these questions. We can foresee that IVM will continue to make even more significant contributions in these research areas which hopefully will lead to a greater understanding of fundamental biology and for potential translational benefit.
Abbreviations
- 18F-FDG:
-
Fluorodeoxyglucose
- CARS:
-
Coherent anti-stokes raman scattering
- CCL1:
-
Chemokine (C-C motif) ligand 1
- CT:
-
Computed tomography
- DEVD:
-
Aspartic acid-glutamic acid-valine-aspartic acid
- ECM:
-
Extracellular matrix
- FAD:
-
Flavin adenine dinucleotide
- FCS:
-
Fluorescence Correlation Spectroscopy
- FLIM:
-
Fluorescence-lifetime imaging microscopy
- FRAP:
-
Fluorescence recovery after photobleaching
- FRET:
-
Förster resonance energy transfer
- GFP:
-
Green fluorescent protein
- IR/NIR:
-
Infrared/ Near-infrared
- IVM:
-
Intravital microscopy
- mKO2:
-
Monomeric Kusabira-Orange 2
- MMP:
-
Metalloproteinase
- MRI:
-
Magnetic resonance imaging
- NADH:
-
Nicotinamide adenine dinucleotide
- OFDI:
-
Optical frequency domain imaging
- PARP-1:
-
Poly(ADP-ribose) polymerase-1
- PARPi:
-
Poly(ADP-ribose) polymerase-1 inhibitor
- PET:
-
Positron emission tomography
- RFP:
-
Red fluorescent protein
- ROCK:
-
Rho-associated protein kinase
- scVEGF:
-
Single-chain vascular endothelial growth factor
- SERS:
-
Surface enhanced Raman scattering
- SHG:
-
Second harmonic generation
- SNR:
-
Signal-to-noise ratio
- SPECT:
-
Single-photon emission computed tomography
- TGF-β:
-
Transforming growth factor beta
- VEGF:
-
Vascular endothelial growth factor
- YFP:
-
Yellow fluorescent protein
References
Alexandrakis G, Brown EB, Tong RT et al (2004) Two-photon fluorescence correlation microscopy reveals the two-phase nature of transport in tumors. Nat Med 10:203–207
Al-Rawi AAM, Jiang WG (2011) Lymphangiogenesis and cancer metastasis. J Cell Mol Med 16:1405–1416
Amornphimoltham P, Masedunskas A, Weigert R (2010) Intravital microscopy as a tool to study drug delivery in preclinical studies. Adv Drug Deliv Rev 63:119–128
Apte S, Chin FT, Graves EE et al (2011) Molecular imaging of hypoxia: strategies for probe design and application. Curr Org Synth 8:593–603
Backer MV, Levashova Z, Patel V et al (2007) Molecular imaging of VEGF receptors in angiogenic vasculature with single-chain VEGF-based probes. Nat Med 13:504–509
Bajénoff M, Breart B, Huang AYC et al (2006) Natural killer cell behavior in lymph nodes revealed by static and real-time imaging. J Exp Med 203:619–631
Bao B, Groves K, Zhang J et al (2012) In vivo imaging and quantification of carbonic anhydrase IX expression as an endogenous biomarker of tumor hypoxia. PLoS One 7:e50860
Beerling E, Ritsma L, Vrisekoop N et al (2011) Intravital microscopy: new insights into metastasis of tumors. J Cell Sci 124:299–310
Bergers G, Hanahan D (2008) Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer 8:592–603
Bhang SH, Won N, Lee T-J et al (2009) Hyaluronic acid-quantum dot conjugates for in vivo lymphatic vessel imaging. ACS Nano 3:1389–1398
Bhirde AA, Patel V, Gavard J et al (2009) Targeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug delivery. ACS Nano 3:307–316
Blum G, Mullins SR, Keren K et al (2005) Dynamic imaging of protease activity with fluorescently quenched activity-based probes. Nat Chem Biol 1:203–209
Boissonnas A, Fetler L, Zeelenberg IS et al (2007) In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J Exp Med 204:345–356
Breart B, Lemaître F, Celli S, Bousso P (2008) Two-photon imaging of intratumoral CD8+ T cell cytotoxic activity during adoptive T cell therapy in mice. J Clin Invest 118:1390–1397
Brown EB, Campbell RB, Tsuzuki Y et al (2001) In vivo measurement of gene expression, angiogenesis and physiological function in tumors using multiphoton laser scanning microscopy. Nat Med 7:864–868
Brown E, Munn LL, Fukumura D, Jain RK (2010) In vivo imaging of tumors. Cold Spring Harbor Protoc. 2010:pdb.prot5452, Vol 7
Cao Y-A, Wagers AJ, Beilhack A et al (2004) Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc Natl Acad Sci U S A 101:221–226
Chen J, Li Y, Yu T-S et al (2012) A restricted cell population propagates glioblastoma growth after chemotherapy. Nature 488:1–6
Cherry SR (2006) Multimodality in vivo imaging systems: twice the power or double the trouble? Annu Rev Biomed Eng 8:35–62
Condeelis J, Segall J (2003) Intravital imaging of cell movement in tumours. Nat Rev Cancer 3:921–930
Conley SJ, Gheordunescu E, Kakarala P et al (2012) Antiangiogenic agents increase breast cancer stem cells via the generation of tumor hypoxia. Proc Natl Acad Sci U S A 109:2784–2789
Coppieters K, Martinic MM, Kiosses WB et al (2010) A novel technique for the in vivo imaging of autoimmune diabetes development in the pancreas by two-photon microscopy. PLoS One 5:e15732
Culver J, Akers W, Achilefu S (2008) Multimodality molecular imaging with combined optical and SPECT/PET modalities. J Nucl Med 49:169–172
Dellian M, Helmlinger G, Yuan F, Jain RK (1996) Fluorescence ratio imaging of interstitial pH in solid tumours: effect of glucose on spatial and temporal gradients. Br J Cancer 74:1206–1215
Driessens G, Beck B, Caauwe A et al (2012) Defining the mode of tumour growth by clonal analysis. Nature 488:1–5
Egeblad M, Ewald AJ, Askautrud HA et al (2008) Visualizing stromal cell dynamics in different tumor microenvironments by spinning disk confocal microscopy. Dis Model Mech 1:155–167
Egeblad M, Nakasone ES, Werb Z (2010) Tumors as organs: complex tissues that interface with the entire organism. Dev Cell 18:884–901
Eikenes L, Tari M, Tufto I et al (2005) Hyaluronidase induces a transcapillary pressure gradient and improves the distribution and uptake of liposomal doxorubicin (Caelyx) in human osteosarcoma xenografts. Br J Cancer 93:81–88
Elstrom RL, Bauer DE, Buzzai M et al (2004) Akt stimulates aerobic glycolysis in cancer cells. Cancer Res 64:3892–3899
Erikson A, Tufto I, Bjønnum AB et al (2008) The impact of enzymatic degradation on the uptake of differently sized therapeutic molecules. Anticancer Res 28:3557–3566
Escobedo JO, Rusin O, Lim S, Strongin RM (2010) NIR dyes for bio imaging applications. Curr Opin Chem Biol 14:64–70
Estrella V, Chen T, Lloyd M et al (2013) Acidity generated by the tumor microenvironment drives local invasion. Cancer Res 73:1524–1535
Filonov GS, Piatkevich KD, Ting L-M et al (2011) Bright and stable near-infrared fluorescent protein for in vivo imaging. Nat Biotechnol 29:757–761
Folgueras AR, Pendás AM, Sánchez LM, López-Otín C (2004) Matrix metalloproteinases in cancer: from new functions to improved inhibition strategies. Int J Dev Biol 48:411–424
Foudi A, Hochedlinger K, Van Buren D et al (2009) Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat Biotechnol 27:84–90
Frantz C, Stewart KM, Weaver VM (2010) The extracellular matrix at a glance. J Cell Sci 123:4195–4200
Friedl P, Alexander S (2011) Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 147:992–1009
Fukumura D, Xu L, Chen Y et al (2001) Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Res 61:6020–6024
Fukumura D, Duda DG, Munn LL, Jain RK (2010) Tumor microvasculature and microenvironment: novel insights through intravital imaging in pre-clinical models. Microcirculation 17:206–225
Gambhir SS (2013) Molecular imaging primer, 1st edn. Available at the Apple bookstore for iPAD or Mac)
Gaustad J-V, Simonsen TG, Leinaas MN, Rofstad EK (2012) Sunitinib treatment does not improve blood supply but induces hypoxia in human melanoma xenografts. BMC Cancer 12:388
Germano G, Frapolli R, Belgiovine C et al (2013) Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell 23:249–262
Giampieri S, Manning C, Hooper S et al (2009) Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol 11:1287–1296
Goel S, Wong AH-K, Jain RK (2012) Vascular normalization as a therapeutic strategy for malignant and nonmalignant disease. Cold Spring Harb Perspect Med 2:a006486
Grinvald A, Shoham D, Shmuel A et al (1991) In-vivo optical imaging of cortical architecture and dynamics. Brain 34:1–91
Hadjantonakis A-K, Papaioannou VE (2004) Dynamic in vivo imaging and cell tracking using a histone fluorescent protein fusion in mice. BMC Biotechnol 4:33
Han J, Burgess K (2010) Fluorescent indicators for intracellular pH. Chem Rev 110:2709–2728
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Harris AL (2002) Hypoxia – a key regulatory factor in tumour growth. Nat Rev Cancer 2:38–47
Helmlinger G, Yuan F, Dellian M, Jain RK (1997) Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med 3:177–182
Hoshida T, Isaka N, Hagendoorn J et al (2006) Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res 66:8065–8075
Hulit J, Kedrin D, Gligorijevic B et al (2012) The use of fluorescent proteins for intravital imaging of cancer cell invasion. Methods Mol Biol 872:15–30
Humphries A, Cereser B, Gay LJ et al (2013) Lineage tracing reveals multipotent stem cells maintain human adenomas and the pattern of clonal expansion in tumor evolution. Proc Natl Acad Sci U S A 110:E2490–E2499
Imanishi Y, Batten ML, Piston DW et al (2004) Noninvasive two-photon imaging reveals retinyl ester storage structures in the eye. J Cell Biol 164:373–383
Isaka N, Padera TP, Hagendoorn J et al (2004) Peritumor lymphatics induced by vascular endothelial growth factor-C exhibit abnormal function. Cancer Res 64:4400–4404
Ito K, Smith BR, Parashurama N et al (2012) Unexpected dissemination patterns in lymphoma progression revealed by serial imaging within a murine lymph node. Cancer Res 72:6111–6118
Jain RK, Munn LL, Fukumura D (2011) Tumor models in cancer research. In: Teicher BA (ed) Cancer drug discovery and development. Springer, New York; pp 641–679
James ML, Gambhir SS (2012) A molecular imaging primer: modalities, imaging agents, and applications. Physiol Rev 92:897–965
Janssen A, Beerling E, Medema R, van Rheenen J (2013) Intravital FRET imaging of tumor cell viability and mitosis during chemotherapy. PLoS One 8:e64029
Kardash E, Reichman-Fried M, Maître J-L et al (2010) A role for Rho GTPases and cell-cell adhesion in single-cell motility in vivo. Nat Cell Biol 12:47–53
Kedrin D, Gligorijevic B, Wyckoff J et al (2008) Intravital imaging of metastatic behavior through a mammary imaging window. Nat Methods 5:1019–1021
Keese M, Yagublu V, Schwenke K et al (2010) Fluorescence lifetime imaging microscopy of chemotherapy-induced apoptosis resistance in a syngenic mouse tumor model. Int J Cancer 126:104–113
Kobayashi H, Choyke PL (2011) Target-cancer-cell-specific activatable fluorescence imaging probes: rational design and in vivo applications. Acc Chem Res 44:83–90
Kola I, Landis J (2004) Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov 3:711–715
Kossodo S, Pickarski M, Lin S-A et al (2010) Dual in vivo quantification of integrin-targeted and protease-activated agents in cancer using fluorescence molecular tomography (FMT). Mol Imaging Biol 12:488–499
Kotsuma M, Parashurama N, Smith BR et al (2012) Nondestructive, serial in vivo imaging of a tissue-flap using a tissue adhesion barrier Applications for IVM imaging in the mammary fat pad and lymph node. Intr Vital 1:69–76
Kuchimaru T, Kadonosono T, Tanaka S et al (2010) In vivo imaging of HIF-active tumors by an oxygen-dependent degradation protein probe with an interchangeable labeling system. PLoS One 5:e15736
Lee S, Ryu JH, Park K et al (2009) Polymeric nanoparticle-based activatable near-infrared nanosensor for protease determination in vivo. Nano Lett 9:4412–4416
Lee S, Vinegoni C, Feruglio PF et al (2012) Real-time in vivo imaging of the beating mouse heart at microscopic resolution. Nat Commun 3:1054
Lehr HA, Leunig M, Menger MD et al (1993) Dorsal skinfold chamber technique for intravital microscopy in nude mice. Am J Pathol 143:1055–1062
Leu AJ, Berk DA, Lymboussaki A et al (2000) Absence of functional lymphatics within a murine sarcoma: a molecular and functional evaluation. Cancer Res 60:4324–4327
Levental KR, Yu H, Kass L et al (2009) Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139:891–906
Li Z, Wilson KD, Smith B et al (2009) Functional and transcriptional characterization of human embryonic stem cell-derived endothelial cells for treatment of myocardial infarction. PLoS One 4:13
Liu J, Liao S, Diop-Frimpong B et al (2012) TGF-β blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proc Natl Acad Sci 109:16618–16623
Livet J, Weissman TA, Kang H et al (2007) Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450:56–62
Looney MR, Thornton EE, Sen D et al (2011) Stabilized imaging of immune surveillance in the mouse lung. Nat Methods 8:91–96
Magee JA, Piskounova E, Morrison SJ (2012) Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell 21:283–296
Magzoub M, Jin S, Verkman AS (2008) Enhanced macromolecule diffusion deep in tumors after enzymatic digestion of extracellular matrix collagen and its associated proteoglycan decorin. FASEB J 22:276–284
Mahmood U, Weissleder R (2003) Near-infrared optical imaging of proteases in cancer. Mol Cancer Ther 2:489–496
Manning CS, Jenkins R, Hooper S et al (2013) Intravital imaging reveals conversion between distinct tumor vascular morphologies and localized vascular response to Sunitinib. Intra Vital 2:1–12
Martin GR, Jain RK (1994) Noninvasive measurement of interstitial pH profiles in normal and neoplastic tissue using fluorescence ratio imaging microscopy. Cancer Res 54:5670–5674
Marusyk A, Almendro V, Polyak K (2012) Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer 12:323–334
Matise LA, Palmer TD, Ashby WJ et al (2012) Lack of transforming growth factor-beta signaling promotes collective cancer cell invasion through tumor-stromal crosstalk. Breast Cancer Res 14:R98
McGhee EJ, Morton JP, Von Kriegsheim A et al (2011) FLIM-FRET imaging in vivo reveals 3D-environment spatially regulates RhoGTPase activity during cancer cell invasion. Small GTPases 2:239–244
McMillin DW, Negri JM, Mitsiades CS (2013) The role of tumour-stromal interactions in modifying drug response: challenges and opportunities. Nat Rev Drug Discov 12:217–228
Meulmeester E, Ten Dijke P (2011) The dynamic roles of TGF-β in cancer. J Pathol 223:205–218
Mikhail AS, Allen C (2010) Block copolymer micelles for delivery of cancer therapy: transport at the whole body, tissue and cellular levels. J Control Release 138:214–223
Mrass P, Kinjyo I, Ng LG et al (2008) CD44 mediates successful interstitial navigation by killer T cells and enables efficient antitumor immunity. Immunity 29:971–985
Mueller MM, Fusenig NE (2004) Friends or foes – bipolar effects of the tumour stroma in cancer. Nat Rev Cancer 4:839–849
Nakasone ES, Askautrud HA, Kees T et al (2012) Imaging tumor-stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer Cell 21:488–503
Nobis M, McGhee EJ, Morton JP et al (2013) Intravital FLIM-FRET imaging reveals dasatinibinduced spatial control of Src in pancreatic cancer. Cancer Res 73:4674–4686
Olson MF, Sahai E (2009) The actin cytoskeleton in cancer cell motility. Clin Exp Metastasis 26:273–287
Orth JD, Kohler RH, Foijer F et al (2011) Analysis of mitosis and antimitotic drug responses in tumors by in vivo microscopy and single-cell pharmacodynamics. Cancer Res 71:4608–4616
Padera TP, Kadambi A, Di Tomaso E et al (2002) Lymphatic metastasis in the absence of functional intra tumor lymphatics. Science 296:1883–1886
Palmer GM, Fontanella AN, Shan S et al (2011) In vivo optical molecular imaging and analysis in mice using dorsal window chamber models applied to hypoxia, vasculature and fluorescent reporters. Nat Protoc 6:1355–1366
Parks SK, Mazure NM, Counillon L, Pouysségur J (2013) Hypoxia promotes tumor cell survival in acidic conditions by preserving ATP levels. J Cell Physiol 228:1854–1862
Perentes JY, Mckee TD, Ley CD et al (2009) In vivo imaging of extracellular matrix remodeling by tumor-associated fibroblasts. Nat Methods 6:2008–2010
Philippar U, Roussos ET, Oser M et al (2008) A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev Cell 15:813–828
Pietras K, Ostman A (2010) Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res 316:1324–1331
Pink DBS, Schulte W, Parseghian MH et al (2012) Real-time visualization and quantitation of vascular permeability in vivo: implications for drug delivery. PLoS One 7:e33760
Pinto MR, Schanze KS (2004) Amplified fluorescence sensing of protease activity with conjugated polyelectrolytes. Proc Natl Acad Sci U S A 101:7505–7510
Provenzano PP, Eliceiri KW, Campbell JM et al (2006) Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med 4:38
Provenzano PP, Inman DR, Eliceiri KW et al (2008) Collagen density promotes mammary tumor initiation and progression. BMC Med 6:11
Provenzano PP, Eliceiri KW, Keely PJ (2009) Multiphoton microscopy and fluorescence lifetime imaging microscopy (FLIM) to monitor metastasis and the tumor microenvironment. Clin Exp Metastasis 26:357–370
Provenzano P, Cuevas C, Chang A et al (2012) Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21:418–429
Rapisarda A, Melillo G (2012) Overcoming disappointing results with antiangiogenic therapy by targeting hypoxia. Nat Rev Clin Oncol 9:378–390
Ray P, De A, Min J-J et al (2004) Imaging tri-fusion multimodality reporter gene expression in living subjects. Cancer Res 64:1323–1330
Ritsma L, Steller EJA, Ellenbroek SIJ et al (2013) Surgical implantation of an abdominal imaging window for intravital microscopy. Nat Protoc 8:583–594
Sakaue-Sawano A, Ohtawa K, Hama H et al (2008) Tracing the silhouette of individual cells in S/G2/M phases with fluorescence. Chem Biol 15:1243–1248
Salnikov AV, Roswall P, Sundberg C et al (2005) Inhibition of TGF-beta modulates macrophages and vessel maturation in parallel to a lowering of interstitial fluid pressure in experimental carcinoma. Lab Invest 85:512–521
Sanz-Moreno V, Gadea G, Ahn J et al (2008) Rac activation and inactivation control plasticity of tumor cell movement. Cell 135:510–523
Schepers AG, Snippert HJ, Stange DE et al (2012) Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science 730:730–735
Serrels A, Timpson P, Canel M et al (2009) Real-time study of E-Cadherin and membrane dynamics in living animals: implications for disease modeling and drug development. Cancer Res 69:2714–2719
Shen B, Jeon J, Palner M et al (2013) Positron emission tomography imaging of drug-induced tumor apoptosis with a caspase-triggered nanoaggregation probe. Angewandte Chemie 52:10511–10514
Shojaei F, Wu X, Malik AK et al (2007) Tumor refractoriness to anti-VEGF treatment is mediated by CD11b + Gr1+ myeloid cells. Nat Biotechnol 25:911–920
Skala MC, Riching KM, Gendron-Fitzpatrick A et al (2007) In vivo multiphoton microscopy of NADH and FAD redox states, fluorescence lifetimes, and cellular morphology in precancerous epithelia. Proc Natl Acad Sci U S A 104:19494–19499
Smith ML, Gourdon D, Little WC et al (2007) Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLoS Biol 5:12
Smith BR, Cheng Z, De A et al (2008) Real-time intravital imaging of RGD-quantum dot binding to luminal endothelium in mouse tumor neovasculature. Nano Lett 8:2599–2606
Smith BR, Cheng Z, De A et al (2010) Dynamic visualization of RGD-quantum dot binding to tumor neovasculature and extravasation in multiple living mouse models using intravital microscopy. Small 6:2222–2229
Smith BR, Zavaleta C, Rosenberg J et al (2013) High-resolution, serial intravital microscopic imaging of nanoparticle delivery and targeting in a small animal tumor model. Nano Today 8:126–137
Snoeks TJA, Löwik CWGM, Kaijzel EL (2010) ‘In vivo’ optical approaches to angiogenesis imaging. Angiogenesis 13:135–147
Steven P, Bock F, Hüttmann G, Cursiefen C (2011) Intravital two-photon microscopy of immune cell dynamics in corneal lymphatic vessels. PLoS One 6:e26253
Stoll S, Delon J, Brotz TM, Germain RN (2002) Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science 296:1873–1876
Tada H, Higuchi H, Wanatabe TM, Ohuchi N (2007) In vivo real-time tracking of single quantum dots conjugated with monoclonal anti-HER2 antibody in tumors of mice. Cancer Res 67:1138–1144
Tanaka K, Morimoto Y, Toiyama Y et al (2012) In vivo time-course imaging of tumor angiogenesis in colorectal liver metastases in the same living mice using two-photon laser scanning microscopy. J Oncol 2012:265487
Thurber G, Yang K, Reiner T et al (2013) Single-cell and subcellular pharmacokinetic imaging allows insight into drug action in vivo. Nat Commun 4:1505
Timpson P, McGhee EJ, Morton JP et al (2011) Spatial regulation of RhoA activity during pancreatic cancer cell invasion driven by mutant p53. Cancer Res 71:747–757
Tlsty TD, Coussens LM (2006) Tumor stroma and regulation of cancer development. Annu Rev Pathol 1:119–150
Trédan O, Galmarini CM, Patel K, Tannock IF (2007) Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst 99:1441–1454
Tsai YC, Mendoza A, Mariano JM et al (2007) The ubiquitin ligase gp78 promotes sarcoma metastasis by targeting KAI1 for degradation. Nat Med 13:1504–1509
Tsourkas A, Shinde-Patil VR, Kelly KA et al (2005) In vivo imaging of activated endothelium using an anti-VCAM-1 magnetooptical probe. Bioconjug Chem 16:576–581
Tufto I, Hansen R, Byberg D et al (2007) The effect of collagenase and hyaluronidase on transient perfusion in human osteosarcoma xenografts grown orthotopically and in dorsal skinfold chambers. Anticancer Res 27:1475–1481
Vakoc BJ, Lanning RM, Tyrrell JA et al (2009) Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat Med 15:1219–1223
Vaupel P, Kallinowski F, Okunieff P (1989) Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res 49:6449–6465
Voura EB, Jaiswal JK, Mattoussi H, Simon SM (2004) Tracking metastatic tumor cell extravasation with quantum dot nanocrystals and fluorescence emission-scanning microscopy. Nat Med 10:993–998
Weissleder R, Ntziachristos V (2003) Shedding light onto live molecular targets. Nat Med 9:123–128
Williams RM, Zipfel WR, Webb WW (2005) Interpreting second-harmonic generation images of collagen I fibrils. Biophys J 88:1377–1386
Wilson WR, Hay MP (2011) Targeting hypoxia in cancer therapy. Nat Rev Cancer 11:393–410
Wilson A, Laurenti E, Oser G et al (2008) Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135:1118–1129
Wyckoff JB, Pinner SE, Gschmeissner S et al (2006) ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr Biol 16:1515–1523
Xia Z, Xing Y, Jeon J et al (2011) Immobilizing reporters for molecular imaging of the extracellular microenvironment in living animals. ACS Chem Biol 6:1117–1126
Yamamoto N, Jiang P, Yang M et al (2004) Cellular dynamics visualized in live cells in vitro and in vivo by differential dual-color nuclear-cytoplasmic fluorescent-protein expression. Cancer Res 64:4251–4256
Yan X, Ray P, Paulmurugan R et al (2013) A transgenic tri-modality reporter mouse. PLoS One 8:e73580
Yu H, Mouw JK, Weaver VM (2011) Forcing form and function: biomechanical regulation of tumor evolution. Trends Cell Biol 21:47–56
Zhu L, Xie J, Swierczewska M et al (2011) Dual-functional, receptor-targeted fluorogenic probe for in vivo imaging of extracellular protease expressions. Bioconjug Chem 22:1001–1005
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Yu, H., Gambhir, S.S. (2014). Intravital Microscopy for Molecular Imaging in Cancer Research. In: Weigert, R. (eds) Advances in Intravital Microscopy. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9361-2_12
Download citation
DOI: https://doi.org/10.1007/978-94-017-9361-2_12
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-9360-5
Online ISBN: 978-94-017-9361-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)