Abstract
There have been many attempts worldwide to produce in captivity juveniles of Octopus vulgaris, one the most studied cephalopod species in the world because of its very strong market interest. This chapter reviews the different methods used to obtain and maintain broodstocks and the rearing technologies applied to the paralarvae. The main parameters and culture methods to rear the planktonic stage are discussed and a protocol for the rearing of the paralarvae is suggested. The main bottlenecks in the cultivation of this species are emphasized, and further research topics are suggested, including both technical and biological aspects.
In laboratory trials, the best growth and survival of the paralarval phase is currently achieved by feeding a mixed live diet composed of enriched Artemia and crustacean zoeae. However, this method is not transferable to a commercial scale as there is limited availability of live zoeae. In order to advance from a research to an industrial scale, it is essential to develop an inert diet with the appropriate nutritional composition to be supplied from an age of 1 month onwards. Another option would be to develop an appropriate enrichment protocol for Artemia so that its composition simulates more closely that of crustacean larvae or wild zooplankton.
A protocol for the first month of O. vulgaris paralarvae culture, which allows the production of good-quality individuals (in terms of dry weight and survival) to start the settlement process, is proposed. Relatively high survival rates and paralarvae dry weights of 1.3–1.8 mg can be attained after 1 month on a sole diet of Artemia. These weights are increased to 2.5–3.5 mg when that diet is supplemented with zoeae.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
1.1 Importance of this Species in the Market and Capture Methods
The common octopus, Octopus vulgaris, is a species with a substantial global market and consequent capture importance. It is widely distributed with a high market price due to its high demand as a food source. It is appreciated and used in food preparations in Asiatic, Mediterranean and Latin-American countries and can be found in the market as fresh, frozen, whole or sliced products. The edible part of octopus is very high (over 90 % of its body weight), resulting in a highly interesting product concerning yield.
It is a species that supports both industrial and artisanal fisheries. Local fishermen catch them using mainly hooks, lures and pots. In contrast, industrial fishermen catch large quantities in the oceanic sublittoral areas using trawls operated from large fishing boats. The declared annual world catches attributed to O. vulgaris declined from more than 100,000 t in the late 1970s to around 40,000–50,000 t during the period 2000–2010 (FAO 2012).
The common octopus is a highly valued species of great commercial interest in Spain. The octopus fishery has been overexploited in the past decades, which has forced the administration to regulate the fishery and, through research, to evaluate aquaculture techniques as an alternative source of supply.
Many of the species’ biological features, such as direct embryological development, short life cycle, rapid growth and elevated food conversion index (Vaz-Pires et al. 2004), make it an excellent candidate for aquaculture. In addition, its high fecundity, rapid growth and high food conversion index, together with its high market value, make the common octopus a promising species for diversifying the aquaculture industry.
1.2 State of the Art
Research on rearing O. vulgaris paralarvae started in Japan when Itami et al. (1963) obtained the first benthic juveniles after 33 days at a mean temperature of 24.7 ºC using shrimp (Palaemon serrifer) zoeae as prey. Later, Imamura (1990) and Hamasaki et al. (1991) reported successful rearing in 20 m3 tanks to which Artemia and Nannochloropsis sp. were added, suggesting for the first time the possibility of mass-producing octopus paralarvae. Subsequent research in Japan focused on the production of juveniles for enhancement programmes (Okumura et al. 2005; Kurihara et al. 2006; Arai et al. 2008), using Artemia supplemented with frozen slices of Pacific sandeel (Ammodytes personatus) as food for the paralarvae .
In Europe, the first successful rearing trials were carried out by Villanueva (1994, 1995). He obtained benthic juveniles after 47 days using decapod crab zoeae as prey and a rearing temperature of 21.2 ºC. Subsequently, Moxica et al. (2002) increased survival and dry weight of the paralarvae at 1 month by using larger prey (adult Artemia of up to 2 mm and spider crab zoeae). Settlement, however, was not attained. The complete culture cycle at an experimental level was first achieved in 2001 (Iglesias et al. 2004), using both Artemia and spider crab zoeae as live prey. Later, Carrasco et al. (2005) achieved similar results using the same prey, but different cultivation systems with regard to water circulation, volume, colour and shape of tanks (see Table 23.1). Recently, research on O. vulgaris paralarval rearing has spread to many regions of Spain including Andalusia, Asturias (Carrasco et al. 2006), Canary Islands (Hormiga et al. 2010; Feyjoo et al. 2011; Almansa et al. 2012), Catalonia (Estévez et al. 2009), Galicia (Seixas 2009; Seixas et al. 2010; Fuentes et al. 2011) and Valencia (Viciano et al. 2011). An Italian group, Maricoltura Di Rosignano Solvay (Livorno), has also researched this field (Lenzi et al. 2006; De Wolf et al. 2011). In 2007, they successfully reared paralarvae through to 160-day-old juveniles using enriched Artemia. More detailed information on growth and survival rates of trials cited above is given in Sects. 23.5.3 and 23.5.4.
In 2005, an international workshop on O. vulgaris reproduction and paralarvae rearing was held in Vigo (Spain) in order to discuss the different methods used, identify the causes of larval mortality and establish future research priorities (Iglesias et al. 2007). A review of the biology of the planktonic stages of benthic octopuses was later published (Villanueva and Norman 2008). An international workshop on Latin-American cephalopod culture was held during 2008 in Puerto Montt (Chile), the conclusions of which were published by Uriarte et al. (2011). Recently, another workshop on cephalopod culture, organised by CIAC 2012 (Cephalopod International Advisory Council), took place in Florianópolis, Brazil, with the aim of defining the current status and research priorities of four cultured cephalopod species (Sepia officinalis, Sepioteuthis lessoniana, Octopus maya and O. vulgaris; Vidal et al. manuscript in elaboration).
2 Broodstock Conditioning
High fecundity is one of the characteristics that encourages O. vulgaris to be considered as a serious candidate for diversification in aquaculture. According to Mangold (1983), wild females can lay up to 500,000 eggs and, in captivity, an output of approximately 100,000 eggs per kg has been achieved (Iglesias et al. 1997).
After several decades of work on the culture of this species, there is now little difficulty in capturing wild subadult and adult individuals, acclimatizing them to captive conditions and obtaining viable egg masses. When keeping wild males and females together under suitable environmental conditions and providing them with shelters, nearly 100 % of females can mature and lay egg strings (Iglesias et al. 2007).
Welfare considerations need to be taken into account when maintaining octopus in captivity. It has been argued that the European Union Directive 2010/63/EU on animal welfare should be applied to cephalopod breeding and experimentation in aquaculture research (Sykes et al. 2012). These authors suggested revisions to the animal welfare legislation, the definition of live cephalopods , stress, pain and suffering. Although some recent reports have been published on this topic, research on appropriate culture conditions, behaviour and stress responses must be carried out in the near future. For more information on this topic, see Chap. 6.
Iglesias et al. (2007) made a comparative analysis of the different broodstock conditioning systems used in the world and concluded that whereas methods of capture, transport, food supply and light intensity are similar among different research groups, male to female ratios and the holding temperatures (14–25 ºC) may differ widely. With regard to this subject, it is possible to establish the following recommendations.
2.1 Broodstock Capture and Transport
Trawl nets enable the capture of large numbers of octopus , but can both harm the individuals and cause a negative environmental impact. Consequently, selective fishing methods like creels or individual traps are recommended for the capture of spawners.
Individual mesh bags or separate containers can be used during and after transportation in order to avoid attacks between individuals, stress and subsequent mortality (Fuentes et al. 2005). Oxygen supply is recommended, particularly when stocking densities are high.
2.2 Food Supply
Broodstock diet can influence the biochemical composition and biometrical relationships of the newly hatched paralarvae (Quintana et al. 2007, 2009; Márquez et al. 2013). Frozen and fresh crustaceans and fish of low commercial value are usually used as food with optimal results (Iglesias et al. 2007; Quintana 2009; Estefanell 2012). Adult females refuse feed when spawning is imminent (see Chap. 1); in consequence, the quantity of food should be reduced during this period to maintain good water quality.
2.3 Sex Ratio
Some authors (Villanueva 1995; Okumura et al. 2005) capture only wild mature females during the natural spawning season to obtain eggs in captivity; these females are usually already fertilised from previous matings and can preserve viable sperm in their oviducts for long periods. In these cases, males are not needed.
Nevertheless, most authors obtain viable eggs by keeping males and females together in a ratio of 1:3 (Iglesias et al. 2000, 2007).
2.4 Physical and Chemical Parameters
Physical and chemical parameters in broodstock tanks are important determinants of successful egg laying. Density should not exceed 5 kg m−3 and tanks should be provided with filtered seawater and a minimal renewal rate of 400–800 % day−1 in order to maintain abiotic water parameters at optimal levels (Iglesias, personal communication). Dissolved oxygen levels should be kept around 100 % saturation. Temperature should follow that of the natural seawater temperature cycle as much as possible and should not drop below 14 °C nor exceed 25 °C (Iglesias et al. 2007). Similarly, water salinity should simulate local seawater values. Semi-dark conditions are commonly used, but natural photoperiod with shaded natural light is also utilized (De Wolf et al. 2011). Individual shelters (terracotta or polyvinyl chloride (PVC) tubes) should be provided to facilitate egg laying.
3 Spawning Process
3.1 Female Conditions
Each spawning female with its strings of eggs should be transferred to an individual tank to avoid being disturbed by conspecifics and to facilitate the counting of eggs laid and hatched paralarvae. Tank volume should be 200–500 L, and temperature must be the same as the broodstock tank .
Females take care of egg masses by oxygenating and cleaning them throughout the embryonic development process . They do not feed during this period and consequently reduce their weight; a 4-kg female, for example, can lose 30 % of its weight during this process (Fig. 23.1). Under suboptimal or stressful circumstances, females may leave the shelter and abandon the egg clusters. In this case, hatching percentage can fall to as little as 50 % due to egg detachment, fungal infection, etc. (Lenzi et al. 2002). Exceptionally, another different female from the broodstock may take over the egg-caring role (Iglesias, personal communication). A special incubator to maintain eggs without a female was patented by Rosas et al. (2010). It was originally developed for O. maya, a large-egg species, but is currently being tested for O. vulgaris in the Austral University of Chile. Simple PVC tube systems with continuous water flow and aeration have also been successfully tested at the Spanish Institute of Oceanography.
3.2 Egg Handling
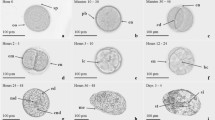
A weekly check of each egg cluster should be established to estimate the time in days necessary to hatch according to the incubation temperature. Using this method, the hatching date can be predetermined in order to estimate in advance the needs for larval rearing (phytoplankton and Artemia; Boletzky 1987). Figure 23.2 shows the evolution of the embryonic development of O. vulgaris at 18 ºC, which takes around 38 days to hatch; the different stages have been identified according to Naef (1928).
Embryonic development of the common octopus Octopus vulgaris at 18 ºC. a Prior to first inversion (Naef IV–VI stage). b Post first inversion (Naef VIII stage). c Embryo differentiation (Naef XI stage). d Naef XII–XIV stage. e Chromatophores and internal yolk (Naef XV stage). f Post second inversion (Naef XIX stage). (Photographs by M. Nande)
Transportation of egg strings should be performed together with the female in the original spawning shelter, and swinging movements that would cause damage to the egg mass should be avoided. In order to avoid premature hatching, this process must be performed before the second embryonic inversion occurs (Fig. 23.2f). Due to high oxygen consumption by eggs (Parra et al. 2000), additional oxygenation should be used during transportation. Drastic fluctuations in other parameters (e.g. temperature, salinity, pH, light intensity) should be avoided to prevent premature hatching.
In cases when egg strings need to be transported without the female, they should be placed in plastic containers filled with oxygen supersaturated seawater and a low temperature be maintained. Strings should be held in a vertical position by hanging them from the container cap.
4 Paralarvae Culture Conditions
Since the 1990s, many attempts have been made by several research groups to determine the feasibility of paralarval culture , and a wide range of rearing conditions have been tested (reviewed by Iglesias et al. 2007; De Wolf et al. 2011). Table 23.1 shows the culture conditions used, including the different feeding strategies, prey concentrations and larval densities.
The main problem that hampers the success of commercial culture of octopus is the high mortality rate observed during the first 2 months of paralarval rearing . The lack of a standardised culture system and the absence of appropriate food sources that fulfil nutritional requirements have been identified as two possible factors responsible for this mortality (Iglesias et al. 2007). Even if survival rates during the planktonic stage have increased considerably in the past decades, successful juvenile settlement is still very difficult to achieve.
4.1 Paralarvae Collection and Transfer
Recently hatched O. vulgaris paralarvae (Young and Harman 1988) measure around 3 mm TL, have three suckers per arm and an individual dry weight of 0.20–0.35 mg (Villanueva and Norman 2008; Iglesias et al. 2007; Fig. 23.3).
The transfer of paralarvae to rearing tanks should be accomplished carefully in order to avoid stress or damage. When concentrations of paralarvae are high, they can be collected with 1–2 L plastic beakers, but when concentrations are low a 30 cm diameter PVC collector with 0.5 mm mesh can be used to collect them. Paralarvae can be counted either individually or by volumetric estimation (Iglesias et al. 2006; Fuentes et al. 2011).
4.2 Tank Colour and Volume
Dark tanks are used by most authors, even though observing paralarvae in these tanks is quite difficult. Other authors have also reported very good results for growth and survival using completely white tanks (Carrasco et al. 2005) or tanks with black walls and white bottom (De Wolf et al. 2011).
There is a general agreement that good growth and survival can be achieved in 500 and 1,000 L cylindro-conical tanks. Sánchez et al. (2010) concluded that growth is positively related to tank volume; paralarvae reared in 1,000 L tanks attained a dry weight of 1.73 ± 0.27 mg after 21 days, significantly greater than the 1.44 ± 0.33 mg obtained in 100 L tanks. De Wolf et al. (2011) also obtained better results using larger tanks attributing the differences to the less dramatic fluctuations in physical conditions in larger tanks. Seixas (2009) and Villanueva et al. (2002), using small rearing tanks of 25–50 L, obtained dry weights of 0.83 and 0.90 mg, respectively, for 1-month-old paralarvae. On the other hand, Moxica et al. (2006) and Viciano et al. (2011), working with 1,000 L tanks and using enriched Artemia (cultured with Isochrysis sp. and further enriched with Nannochloropsis sp.), obtained dry weights of 1.76 mg and 1.88 mg, respectively, for the same-aged paralarvae.
4.3 Physical and Chemical Culture Parameters
4.3.1 Water Circulation
Stagnant water is commonly used for the first week to maintain a green-water system; thereafter, some authors exchange water for 4 h day−1, corresponding to a 100 % day−1 renewal rate (Iglesias et al. 2007; Viciano et al. 2011), whereas others recommend maintaining a constant water flow to give an exchange rate of at least 150 % day−1 (De Wolf et al. 2011). The water inlet should produce a mild tangential circulation of the surface water. However, both lateral and centrally bottom-placed water outlets have been used by some investigators.
In order to obtain a homogeneous distribution of paralarvae and live prey in the culture tanks, gentle aeration can be supplied by air stones (Iglesias et al. 2004; Okumura et al. 2005) or open tube aeration can be done (De Wolf et al. 2011). Currents that result in the accumulation of paralarvae in very small areas should be avoided.
It is also important to prevent the production of small air bubbles that can be trapped in the paralarvae mantle. For this reason, special devices for the distribution of the incoming water, such as multiple superficial inlets (Villanueva 1995) or bottom water inlets combined with a superficial water outlet (Carrasco et al. 2005, 2006), have been used. Nevertheless, the use of a high water flow can increase the risk of skin damage and arm erosion, resulting in an increased mortality of the paralarvae (Vidal et al. 2002).
4.3.2 Light
A wide range of light conditions (natural light, incandescent bulbs, fluorescent tubes) has been used in paralarval culture. Optimal light conditions seem to depend on the type of tanks that are used. In general, higher light intensities and longer photoperiods are used in black tanks than in light-coloured tanks or white-bottom tanks. Surface light intensity in the rearing tank should be 500–700 Lx when using black tanks (Iglesias and Fuentes 2013), whereas other authors recommend using lower light intensities (60–250 Lx for a 14 h L (light):10 h D (dark) photoperiod) in black-walled and white-bottom tanks (De Wolf et al. 2011). Garrido et al. (2012) studied the effect of different types of lighting on the growth and survival of O. vulgaris paralarvae.
4.3.3 Temperature
The change of temperature between the broodstock tank and the paralarvae rearing tank must be gradual (an increase of approximately 1°C per day). Temperature is the most important determinant of development and growth of paralarvae (Mangold and Boletzky 1973). Paralarval rearing temperatures cited in the literature range between 19 and 25 ºC (Iglesias et al. 2007), but temperatures of 20–22 ºC are recommended to obtain optimal growth and survival. High daily temperature fluctuations should be avoided during paralarval culture .
4.3.4 Chemical Parameters
The most important water quality parameters in the larval rearing phase are salinity, pH and concentrations of dissolved oxygen, ammonia, nitrite and nitrate.
Octopuses show very low tolerance to low salinity; therefore, seawater of around 32–35 psu should be used. Salinity should be as constant as possible because sudden changes are not tolerated by paralarvae. Rearing places close to rivers and freshwater sources need to take into account this parameter.
Dissolved oxygen is crucial for gas exchanges and depends on temperature. Optimal levels in the paralarvae rearing process should be kept between 6 and 8 mg L−1, but should not be allowed to fall below 4 mg L−1. Cerezo-Valverde and García-García (2005) determined optimal oxygen saturation levels for subadult and adult individuals to be between 100 and 65 % and suboptimal saturation levels between 65 and 35 % (dangerous below 35 %) for temperatures between 17 and 20 ºC.
pH, nitrite and ammonia levels need to be monitored according to water renewal but at least once a week. Feyjoo et al. (2011) determined the acute toxicity of unionized ammonia and nitrite on newly hatched O. vulgaris paralarvae. The lethal concentration 50 (LC50) value after 24 h of exposure was 10.7 ppm for ammonia and 19.9 ppm for nitrite. This suggests that paralarvae are quite resistant to free ammonia compared to marine fish larvae, but much less resistant to nitrite. At concentrations much lower than the LC50 values, negative effects are observed on both prey intake and chromatophore activity (Feyjoo et al. 2011).
Gas supersaturation can explain the extensive mortality that sometimes happens during the early life stages in intensive production of marine species (Gunnarsli et al. 2008). Air bubbles can be formed in gills, fins, skin and blood of fishes (gas bubble trauma), whilst in the case of O. vulgaris paralarvae, bubbles usually appear inside the mantle cavity. This phenomenon can be related to water circulation, temperature and chemical parameters; for example, it can appear through the mechanical process of heating the water, long pipe runs, pump cavitation, etc. In order to prevent water supersaturation (mainly nitrogen gas), it is recommended to have a method of trickling water over a large surface area, as in a packed-column aerator (Hargreaves and Tucker 1999).
5 Paralarvae Feeding
5.1 Prey Size
Paralarvae can start feeding from the first day of life, but usually a greater number of attacks are recorded 2 days after hatching at temperatures of 18–20 °C (Iglesias et al. 2006). These authors also reported that larger Artemia (1.4 ± 0.4 mm) were clearly preferred to smaller Artemia (0.8 ± 0.1 mm) at first feeding. Nevertheless, a wide range of prey sizes has been used in research on paralarvae rearing . Navarro and Villanueva (2003) used Artemia nauplii of 450–750 µm, in the first few weeks of culture, while others (Moxica et al. 2002; Iglesias et al. 2004; Carrasco et al. 2005; Estévez et al. 2009) used Artemia of 2 mm length or bigger.
In order to investigate the effect of prey size on growth, Fuentes et al. (2009) compared the use of small Artemia enriched for 24 h with Isochrysis galbana (0.7 mm TL) with that of larger Artemia grown for 4 days with the same microalga (1.5 mm TL) during a 30-day larval rearing experiment. There were no significant differences in growth during the first 15 days, but growth was significantly faster when larger Artemia was used as diet during the next 15 days of trial (Table 23.1). Considering these results, for experimental larval rearing, it is recommended that Artemia of 0.5–0.7 mm length are used for the first 15 days followed by Artemia of 1.5–2 mm length.
5.2 Paralarval Nutritional Requirements
Navarro and Villanueva (2000, 2003) considered that a lack of balance in lipid and fatty acid composition and a deficiency in polyunsaturated fatty acids (PUFAs) of the food supplied could be responsible for the high mortality and low growth during O. vulgaris paralarvae rearing . Okumura et al. (2005) used Artemia supplemented with flakes of A. personatus to increase the proportion of PUFAs in the diet . Seixas (2009) and Seixas et al. (2010) fed Artemia enriched with different microalgae species rich in PUFAs, whereas Bersano (2003) and Estévez et al. (2009) used live copepods and other zooplankton groups, respectively. Besides PUFAs, amino acids seem to be another important component in the diet . Villanueva et al. (2004) found that lysine, arginine and leucine represent about half of the total essential amino acids in cephalopod hatchlings. In addition, Villanueva and Bustamante (2006) studied the importance of essential elements in the diet and detected a higher content of copper in O. vulgaris hatchlings and wild juveniles than in Artemia-fed paralarvae. An approach to meeting their vitamin A and E requirements in culture has been published by Villanueva et al. (2009). Further information on nutritional requirements can be found in Chap. 5.
5.3 Artemia Enrichment
Even though Artemia is easily available and well accepted by O. vulgaris paralarvae, Navarro and Villanueva (2003) have reported that Artemia per se has an inadequate lipid composition, the docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) content being especially low.
The only experiment in which rearing to the adult stage has been reached on a sole diet of Artemia was carried out by Moxica et al. (2006). They obtained 67 % survival of paralarvae (dry weight of 1.89 mg) after 1 month of culture (Fig. 23.4), 0.9 % after 70 days, three individuals at 3 months and finally one adult octopus. In that experiment, a green-water system based on Nannochloropsis sp. was used, and the Artemia was cultured for 4 days with I. galbana and further enriched with Nannochloropsis sp. Fuentes et al. (2011) suggested that the combination of the DHA provided by I. galbana and the high EPA content present in Nannochloropsis sp. could cover the basic PUFA requirements of octopus paralarvae.
Growth of Octopus vulgaris during the first month obtained using Artemia cultured for 4 days with Isochrysis galbana and further enriched with Nannochloropsis sp. as food. (From Moxica et al. 2006)
Hamasaki and Takeuchi (2000) and Hamasaki and Morioka (2002) also reported successful results when adding Nannochloropsis sp. to the culture tank of planktonic larvae and as food for Artemia. Hamasaki and Takeuchi (2000), using Artemia biomass (1–2.7 mm) with or without Nannochloropsis sp. in the rearing tank, attained 88 % survival at day 24; Hamasaki and Morioka (2002) obtained 62.5 % survival rate at day 40, using Artemia biomass (1.5–2 mm) fed with Nannochloropsis sp. Another possible beneficial aspect of adding Nannochloropsis sp., previously cited for other marine species (Skiftesvik et al. 2003), is its inhibitory effect on harmful microflora in the culture tank. Besides the nutritional role, De Wolf et al. (2011) suggested that microalgae may generate a shading effect resulting in better prey visualisation and a more homogeneous distribution of the paralarvae in the water column.
A different Artemia enrichment process has been proposed by Seixas et al. (2010). They suggested that a microalgae combination of I. galbana and Rhodomonas lens was the best because it provides a high level of PUFAs (I. galbana) and a very high level of protein (R. lens).
The higher protein to lipid ratio present in Artemia can also positively affect paralarvae growth (Fuentes et al. 2011). Seixas et al. (2010) reached a similar conclusion and suggested that in order to sustain good paralarvae growth, a minimum dietary protein to lipid ratio should be maintained.
Other authors have used enrichments other than microalgae. Kurihara et al. (2006) enriched Artemia with fish egg powder (Plus Aquaran, BASF Japan), which was supplemented with frozen A. personatus flakes (improving DHA content), and obtained 10 % survival rate at day 42. Hamasaki and Takeuchi (2001) used Artemia biomass (2 mm), enriched or not with yeast or shark egg powder, with Nannochloropsis sp. in the tank and obtained 24 % survival rate at day 20.
5.4 Crustacean Zoeae as Optimal Prey
Very few studies of the natural prey of O. vulgaris paralarvae have been published because almost no early stages of this species have been found in nature. Roura et al. (2012) used a polymerase chain reaction (PCR)-based method with group-specific primers selected to identify prey consumed by O. vulgaris paralarvae in the wild; they identified 12 families of crustaceans (11 belonging to the order Decapoda and 1 to the order Euphausiacea) and two families of fishes (Gobiidae and Carangidae). Additionally, Couto (2012) found that a high number of attacks on cirripede larvae were registered when live wild zooplankton was provided to 2-day-old O. vulgaris paralarvae in first feeding laboratory experiences.
As an approach to determining the hypothetical optimal composition of prey for O. vulgaris paralarvae, Navarro and Villanueva (2000, 2003), Villanueva and Bustamante (2006) and Villanueva et al. (2004, 2009) studied the lipids , fatty acids , amino acids , essential and nonessential elements and vitamins A and E of mature ovaries, eggs, early stages and juveniles of wild O. vulgaris .
In general, the best growth and survival of paralarvae in culture conditions were attained using crustacean larvae alone or as a complement to enriched Artemia. Villanueva and Norman (2008) provided a detailed review of prey offered, survival and duration of the planktonic period. Itami et al. (1963), using shrimp (P. serrifer) zoeae of 2–4 mm body length as prey, obtained the first benthic juveniles (10–15 mm TL) after 33 days, with survival rates of 9 % at 40 days and 4 % after 3 months of culture. Villanueva (1994, 1995) obtained benthic juveniles with settlement starting after 47 days with a survival rate of 9 %. Wet weight after 60 days was 173.2 mg. They used crustacean zoeae (Liocarcinus depuratus and Pagurus prideaux) as prey. Afterwards, Moxica et al. (2002), using adult Artemia up to 2 mm and spider crab zoeae (Maja brachydactyla) as food, obtained 8.3 % survival rate after 1 month and 0.2 % after 56 days when the mean dry weight of the paralarvae was 9.21 ± 0.92 mg.
The complete culture cycle at an experimental level was attained for the first time in 2001 (Iglesias et al. 2004) using both Artemia and spider crab (Maja brachydactyla) zoeae as live prey, and obtaining a paralarvae dry weight of 9.5 ± 1.9 mg and a survival rate of 31.5 % after 40–45 days. Carrasco et al. (2006) using the same prey but different rearing systems (Fig. 23.5) achieved 13 % survival rate at day 40 (5.40 ± 0.20 mm dorsal mantle length and 6.10 mg dry weight). Settlement began from day 52 and survival at day 60 was 3.4 % when the paralarvae were 6.54 ± 0.13 mm long and weighed 22.02 ± 3.08 mg. Almansa et al. (2012) used a combination of Artemia juveniles and Grapsus adcensionis zoeae as live prey for first feeding and obtained one O. vulgaris juvenile of 7 months old (ventral mantle length 23 mm, 10.4 g).
Therefore, a mixed live diet , composed of enriched Artemia and crustacean zoeae, is currently the most balanced diet for achieving the best growth and survival results in the paralarval phase (Fig. 23.5). However, this methodology is not readily transferable to a commercial level since there is limited availability of live zoeae, and it is therefore difficult to expand the culture methodology to an industrial scale.
6 Paralarvae Rearing Protocol
In accordance with data reported in this chapter, a protocol to attain good-quality 30-day-old O. vulgaris paralarvae is proposed.
Use a minimum of three replicate 500–1,000 L cylindro-conical tanks. Use stagnant water for the first week, after which open the circuit for 4 h day−1 (100 % day−1 renewal rate). The number of replicates will ensure a suitable statistical treatment of the results. The recommended colour for tank walls and bottom is black, although a white (or clear) bottom can be used in order to improve observation of the paralarvae. Temperature should be kept at 20–22 ºC and salinity 32–35 psu. Tangential surface water input and drainage consisting of a central cylindrical pipe with 250 µm mesh are suggested. Another option is to use stagnant water during the day and open the flow during the night with a 500-µm outlet mesh in order to keep a more homogeneous enriched Artemia. Surface cleaners and moderate central aeration are recommended. Surface light intensity should be 500–700 Lx for 24 h photoperiod when using black wall and black bottom tanks, whereas light intensity can be 60–250 Lx for a 14 h:10 h (L:D) photoperiod when a clear bottom tank is used. A concentration of 1 × 106 cells mL−1 of Nannochloropsis sp. should be used in the culture medium (green-water system), and paralarvae concentration should be 10 individuals L−1. Paralarval prey should consist of 24 h Artemia (0.5 Artemia mL−1) enriched with I. galbana at a concentration of 0.75 × 106 cells mL−1 for the first 15 days, followed by larger Artemia (1.5–2 mm TL) cultured for 4–5 days with I. galbana and further enriched with Nannochloropsis sp., at a concentration of 1 × 107 cells mL−1, keeping a prey concentration of 0.3 Artemia mL−1. When zoeae of spider crab are used in co-feeding with Artemia, they should be added at a concentration of 0.05–0.1 individuals mL−1, at least 3–4 days per week but preferably every day.
For comparative purposes, total length and dry weight of 20 paralarvae should be recorded at the beginning of experiments and periodically (fortnightly is recommended) in each rearing trial.
7 Settlement Process
Around 65–75 days after hatching (at 20 ºC), when paralarvae have between 17 and 20 suckers per arm, they change their pelagic behaviour to a benthic life. At this stage, paralarvae start crawling on the walls and bottom of the tank, moving by attaching their arms to the substrate. This period, which lasts for 2 weeks before they fully adapt to benthic stage, is known as settlement.
During this phase, it is necessary to change feeding and habitat conditions. Artemia concentration should be gradually reduced while the benthic juveniles are increasingly fed with mussel, crab and sea urchin muscle and gonad (Iglesias et al. 2004) and/or frozen mysidaceans (Carrasco et al. 2005). In both cases, the authors placed small-angle or T-shaped PVC pieces on the bottom of the tanks as shelters and the bottom substrate was modified by the inclusion of pebbles (Fig. 23.6).
Octopus vulgaris 100-day-old juvenile obtained in captivity (Iglesias et al. 2004) in the artificial habitat (PVC tubes and pebbles) used during the settlement period
8 Conclusions
Reproduction in captivity has proved feasible on an experimental scale, but the high mortality observed during the paralarval and settlement stages are still the main constraints to the industrial cultivation of the common octopus.
A mixed live diet of enriched Artemia and crustacean zoeae is currently the most successful in terms of growth rate and survival during the paralarval phase. However, this feeding protocol is not transferable to a commercial level due to the limited availability of live zoeae.
A protocol for the first 30 days of O. vulgaris paralarvae culture is proposed that supports the development of individuals with good fitness (in terms of dry weight and survival) through to the settlement process. Using this method, relatively high survival rates and paralarvae dry weights between 1.3 and 1.8 mg can be attained after 1 month when enriched Artemia is used as the sole diet, but these values increase up to 2.5–3.5 mg when zoeae are used in a co-feeding regimen with Artemia.
9 Trends in Research and Industrial Level
The following research topics are recommended.
9.1 Wild Paralarvae and Natural Prey Studies
It is strongly recommended to study further the biochemical composition of wild paralarvae and their natural prey preferences and behaviour . It is also of interest to complete sequence information included in molecular databases (GenBank and Barcode of Life) of zooplankton species that are potential natural prey of O. vulgaris paralarvae in order to allow its molecular identification and traceability.
9.2 Nutrition
In order to move from the actual research situation to the next phase of industrial cultivation of the common octopus, it is essential to develop an inert diet to be supplied from 1 month of age onwards, with an appropriate nutritional composition that meets the requirements of the octopus paralarvae (PUFAs, lipids , proteins , protein to lipid ratio, amino acids, essential elements, vitamins, etc.), as well as a good level of acceptance, buoyancy, leaching, etc. Another issue to be addressed would be that of developing appropriate enrichment protocols for Artemia that would make its composition to more closely match that of crustacean zoea or natural wild zooplankton.
9.3 Zootechnical Improvements
Standardized methods should be established for the whole rearing process including the live prey period, weaning and settlement.
The settlement process needs to be further studied in order to better define requirements for food, shelter and type of substrate through a better understanding of juvenile behaviour.
9.4 Water Quality, Microbiology and Pathology
Further investigations of the chronic toxicity of nitrite compounds and other metabolites are strongly recommended in order to further improve water treatment systems. Gas supersaturation effects should be analysed in more detail. Specific pathogens and bacterial growth in the rearing systems should be also taken into account and identified. The effect of the application of probiotics in this context should also be evaluated in O. vulgaris paralarvae rearing .
9.5 Histology and Paralarvae Development
Studies of the anatomical changes of internal and external organs during the process of paralarval rearing are essential in defining welfare and fitness issues. Other aspects related to paralarval morphology, physiology, immunology, growth and internal rhythms are required to more fully understand paralarvae quality.
9.6 Welfare and Ethical Considerations
Although some recent reports have been published on this topic, research on appropriate culture conditions, behaviour and stress responses should be carried out in the near future.
References
Almansa E, Shcherbakova A, Jiménez P, Rodríguez C, Riera R, Felipe BC, Martín MV, Andrade JP, Sykes A (2012) Effects of different tank volumes and feeding regimes on growth, survival and lipid composition of Octopus vulgaris paralarvae. Book of abstracts, Aqua 2012. Czech Republic, Prague, p 52
Arai D, Kurihara A, Komi R, Iwamoto A, Takeuchi T (2008) Effect of feeding various amounts of Pacific sandeel flakes on growth survival and carcass fatty acid composition of common octopus Octopus vulgaris paralarvae. Aquac Sci 56(4):595–600
Bersano JGF (2003) Intensive cultivation of the calanoid copepod Acartia tonsa. A potential source of live food for aquaculture. In: Book of abstracts, proceedings: word aquaculture, p 95, Salvador, BA, Brazil, 19–23 May 2003
Boletzky SV (1987) Embryonic phase. In: Boyle PR (ed) Cephalopod life cycles, vol 2. Academic Press, London, pp 5–31
Carrasco JF, Rodríguez C, Rodríguez M (2005) Cultivo intensivo de paralarvas de pulpo (Octopus vulgaris, Cuvier) utilizando como base de la alimentación zoeas vivas de crustáceos. Libro de actas, IX Congreso Nacional de Acuicultura, Sevilla, pp 219–222
Carrasco JF, Arronte JC, Rodríguez C (2006) Paralarval rearing of the common octopus, Octopus vulgaris (Cuvier). Aquac Res 37:1601–1605
Cerezo Valverde J, García GB (2005) Suitable dissolved oxygen for common octopus (Octopus vulgaris, cuvier, 1797) at different weights and temperatures: analysis of respiratory behaviour. Aquac 244:303–314
Couto A (2012) Study on the wild diet of Octopus vulgaris paralarvae in Ría de Vigo (NW Spain). Dissertation application to the Master’s degree in marine sciences and marine resources submitted to Institute of Biomedical Sciences Abel Salazar, University of Porto. pp 1–90
De Wolf T, Lenzi S, Lenzi F (2011) Paralarval rearing of Octopus vulgaris (Cuvier) in Tuscany, Italy. Aquac Res 42:1406–1414
Estefanell JA (2012) Optimización de las condiciones de engorde y avances en el conocimiento de los requerimientos nutricionales del pulpo común Octopus vulgaris (Cuvier, 1797). Doctoral Thesis, Universidad de Las Palmas de Gran Canaria, Gran Canaria, p 292
Estévez A, Gairin I, Berger E (2009) Wild zooplancton for Octopus vulgaris larval rearing. In: Hendry CI, Van Stappen G, Wille M, Sorgeloos P (eds) LARVI 09, Fish & shellfish larviculture symposium, special publication No. 38. European Aquaculture Society, Oostende, pp 88–91
FAO (2012). FAO yearbook. Fishery and aquaculture statistics. 2010. Rome, pp 609
Feyjoo P, Riera R, Felipe CB, Skalli A, Almansa E (2011) Tolerance response to ammonia and nitrite in paralarvae of Octopus vulgaris and its toxic effects on prey consumption rate and chromatophores activity. Aquac Int 19(1):193–204
Fuentes L, Iglesias J, Sánchez FJ, Otero JJ, Moxica C, Lago MJ (2005) Técnicas de transporte de paralarvas y adultos de pulpo (Octopus vulgaris). Bol Inst Esp Oceanogr 21(1–4):155–162
Fuentes L, Sánchez FJ, Otero JJ, Lago MJ, Iglesias J (2009) Utilización de zooplancton natural y Artemia en el cultivo de paralarvas de pulpo Octopus vulgaris. Libro de resúmenes, Congreso Nacional de Acuicultura. Madrid, pp 122–123
Fuentes L, Sánchez FJ, Lago MJ, Iglesias J, Pazos G, Linares F (2011) Growth and survival of Octopus vulgaris (Cuvier 1797) paralarvae fed on 3 Artemia-based diets complemented with frozen fish flakes, crushed zooplankton and marine microalgae. Sci Mar 75(4):771–777
Garrido D, Reis D, Orol D, Gonçalves R, Martín MV, Sykes AV, Rodríguez C, Felipe BC, Santamaría FJ, Zheng X, Almansa E (2012) Efecto de distintos tipos de iluminación en el cultivo de las paralarvas del pulpo común (Octopus vulgaris Cuvier, 1797). In: V Foro Iberoamericano de los Recursos Marinos y la Acuicultura, Cádiz, 26–29 Nov 2012
Gunnarsli K, Toften H, Mortensen A (2008) Effects of nitrogen gas supersaturation on growth and survival in juvenile Atlantic cod (Gadus morhua L.). Aquac 283:175–179. http://dx.doi.org/10.1016/j.Aquac. Accessed 29 June 2008
Hamasaki K, Morioka T (2002) Effects of temperature on egg incubation period, paralarval survival and growth of common octopus, Octopus vulgaris reared in the laboratory. Suisanzoshoku 50:407–413
Hamasaki K, Takeuchi T (2000) Effects of the addition of Nannochloropsis to the rearing water on survival and growth of planktonic larvae of Octopus vulgaris. Saibai- Giken 28:65–68
Hamasaki K, Takeuchi T (2001) Dietary value of Artemia enriched with ω-yeast or shark eggs as feed for planktonic larvae of Octopus vulgaris. Saibai-Giken 28:13–16
Hamasaki H, Fukunaga K, Yoshida Y, Maruyama K (1991) Effects of marine microalgae Nannochloropsis sp. on survival and growth on rearing pelagic paralarvae of Octopus vulgaris, and results of mass culture in the tank of 20 metric tons. Saibai-Giken 19:75–84
Hargreaves JA, Tucker CS (1999) Design and construction of degassing units for Catfish hatcheries. Southern Regional Aquaculture Center, publication No. 191. Mississippi State University
Hormiga JA, Almansa E, Sykes AV, Torres NV (2010) Model based optimization of feeding regimens in aquaculture: application to the improvement of Octopus vulgaris viability in captivity. J Biotechnol 149:209–214. doi: 10.1016/j.jbiotec.2009.12.008
Iglesias J, Fuentes L (2013) Cephalopods paralarval rearing with special reference to the common octopus (Octopus vulgaris, Cuvier 1797). In: Allan G, Burnell G (eds) Advances in aquaculture hatchery technology, Number 242. Woodhead Publishing Series in Food Science, Technology and Nutrition, Oxford, pp 374–403
Iglesias J, Sánchez FJ, Otero JJ (1997) Primeras experiencias sobre el cultivo integral del pulpo (Octopus vulgaris) en el Instituto Español de Oceanografía. In Costa J, Abellan E, García B, Ortega A, Zamara S (eds) Libro de actas, VII Congreso Nacional de Acuicultura. Cartagena, pp 221–226, ISBN: 84-491-0323
Iglesias J, Sánchez FJ, Otero JJ, Moxica C (2000) Culture of octopus (Octopus vulgaris, Cuvier). Present knowledge, problems and perspectives. Cah Options Méditer 47:313–321
Iglesias J, Otero JJ, Moxica C, Fuentes L, Sánchez FJ (2004) The completed life cycle of the octopus (Octopus vulgaris, Cuvier) under culture conditions: paralarval rearing using Artemia and zoeae, and first data on juvenile growth up to 8 months of age. Aquac Int 12:481–487
Iglesias J, Fuentes L, Sánchez FJ, Otero JJ, Moxica C, Lago MJ (2006) First feeding of Octopus vulgaris Cuvier, 1797 paralarvae using Artemia: effect of prey size, prey density and feeding frequency. Aquac 261(2):817–822
Iglesias J, Sánchez FJ, Bersano JGF, Carrasco JF, Dhont J, Fuentes L, Linares F, Muñoz JL, Okumura S, Roo J, van der Meeren T, Vidal EAG, Villanueva R (2007) Rearing of Octopus vulgaris paralarvae: Present status, bottlenecks and trends. Aquac 266:1–15
Imamura S (1990) Larval rearing of octopus (Octopus vulgaris Cuvier). The progress of technological development and some problems remained. Collect Breed 52:339–343
Itami K, Izawa Y, Maeda S, Nakai K (1963) Notes on the laboratory culture of the Octopus larvae. Bull Jap Soc Sci Fish 29(6):514–520
Kurihara A, Okumura S, Iwamoto A, Takeuchi T (2006) Feeding Pacific sandeel enhances DHA level in common octopus paralarvae. Aquac Sci 54:413–420
Lenzi F, Cittolin G, Ingle E, Tibaldi E (2002) Allevamento del polpo (Octopus vulgaris): riproduzione e allevamento larvale in avannotteria industriale. Ricerca per lo sviluppo dell’Aquacoltura Toscana, risultati conseguiti, pp 73–83
Lenzi F, Capiferri U, De Wolf T (2006) Paralarval rearing of the common Octopus Octopus vulgaris: state of the art in Italy. In: Book of abstracts Aqua, Firenze, Florence, Italy, p 523, 9–13 May 2006
Mangold K (1983) Octopus vulgaris. In: Boyle PR (ed) Cephalopod life cycles, vol I. Academic Press, London, pp 335–364
Mangold K, Boletzky S (1973) New data on reproductive biology and growth of Octopus vulgaris. Mar Biol 19:7–12
Márquez L, Quintana D, Lorenzo A, Almansa E (2013). Biometrical relationships in developing eggs and neonates of Octopus vulgaris in relation to parental diet. Helgoland Marine Research
Moxica C, Linares F, Otero JJ, Iglesias J, Sánchez FJ (2002) Cultivo intensivo de pararlarvas de pulpo, Octopus vulgaris Cuvier, 1797, en tanques de 9 m3. Bol Inst Esp Oceanogr 18(1–4):31–36
Moxica C, Fuentes L, Hernández J, Iglesias J, Lago MJ, Otero JJ, Sánchez FJ (2006) Efecto de Nannochloropsis sp. en la supervivencia y crecimiento de paralarvas de pulpo Octopus vulgaris. IX Foro dos Recursos Mariños e da Acuicultura das Rías Gallegas 9:255–261
Naef A (1928) Cephalopoda embryology, part I, vol II (final part of monograph no. 35). In: Fauna and flora of the Bay of Naples (translated by the Smithsonian Institution Libraries). Smithsonian Institution Libraries, Washington 35:1–461
Navarro JC, Villanueva R (2000) Lipid and fatty acid composition of early stages of cephalopods: an approach to their lipid requirements. Aquac 183:161–177
Navarro JC, Villanueva R (2003) The fatty acid composition of Octopus vulgaris paralarvae reared with live and inert food: deviation from their natural fatty acid profile. Aquac 219:613–631
Okumura S, Kurihara A, Iwamoto A, Takeuchi T (2005) Improved survival and growth in Octopus vulgaris paralarvae by feeding large type Artemia and Pacific sandeel, A. personatus. Aquac 244:144–157
Parra G, Villanueva R, Yúfera M (2000) Respiration rates in late eggs and early hatchlings of the common octopus, Octopus vulgaris. J Mar Biol Ass UK 80:557–558
Quintana D (2009) Valoración de los requerimientos nutricionales de reproductores de pulpo común Octopus vulgaris. PhD Thesis, Universidad de La Laguna, Tenerife, p 225
Quintana D, Márquez L, Arévalo JR, Almansa E, Lorenzo A (2007) Aplicación de análisis multivariante al estudio de la influencia de la dieta de los reproductores de Octopus vulgaris sobre la composición lipídica de huevos y paralarvas y su relación con la calidad de puesta. Poster. XI Congreso Nacional de Acuicultura, Vigo, Sept 2007
Quintana D, Márquez L, Suárez H, Rodríguez E, Jerez S, Almansa E (2009) Efecto de la dieta de los reproductores de pulpo común (Octopus vulgaris) sobre la composición de aminoácidos de huevos y paralarvas: Relación con la calidad de puesta. XII Congreso Nacional de Acuicultura, Madrid
Rosas C, Caamal C, Cazares RJ (2010) Incubation process for octopuses and incubator. Universidad Autónoma de México. Organización Mundial de la Propiedad Intelectual. WO 2010/030155 A1
Roura A, González AF, Redd K, Guerra A (2012) Molecular prey identification in wild Octopus vulgaris paralarvae. Mar Biol 159(6):1335–1345
Sánchez FJ, Fuentes L, Otero JJ, Lago MJ, Linares F, Pazos G, Iglesias J (2010) Effect of tank volume on the growth and survival of reared Octopus vulgaris paralarvae. In: Aquaculture Europe, CD abstracts, Porto, pp 1170–1171
Seixas P (2009) Composición bioquímica y crecimiento de paralarvas de pulpo (Octopus vulgaris Cuvier, 1797), alimentadas con juveniles de Artemia enriquecidos con microalgas y otros suplementos nutricionales. PhD Thesis, University of Santiago de Compostela, p 279. ISBN: 978-84-9887-253–8
Seixas P, Rey-Méndez M, Valente LMP, Otero A (2010) High DHA content in Artemia is ineffective to improve Octopus vulgaris paralarvae rearing. Aquac 300:156–162
Skiftesvik AB, Browman HI, St-Pierre JF (2003) Life in green water: the effect of microalgae on the behaviour of Atlantic cod (Gadus morhua) larvae. In: 26th annual larval fish conference, Norway OS, 22–26 July 2002. In: Browman HI, Skiftesvik AB (eds) Big fish bang, pp 97–103
Sykes AV, Baptista FD, Gonçalves RA, Andrade JP (2012) Directive 2010/63/EU on animal welfare: a review on the existing scientific knowledge and implications in cephalopod aquaculture research. Rev Aquac 4:142–162
Uriarte I, Iglesias J, Domingues P, Rosas C, Viana MT, Navarro JC, Seixas P, Vidal E, Ausburger A, Pereda S, Godoy F, Paschke K, Farías A, Olivares A, Zúñiga O (2011) Current status and bottleneck of octopod aquaculture: the case of American species. J World Aquac Soc 42(6):735–752
Vaz-Pires P, Seixas P, Barbosa A (2004) Aquaculture potential of the common octopus (Octopus vulgaris Cuvier, 1797): a review. Aquac 238(1–4):221–238
Viciano E, Iglesias J, Lago MJ, Sánchez FJ, Otero JJ, Navarro JC (2011) Fatty acid composition of polar and neutral lipid fractions of Octopus vulgaris Cuvier, 1797 paralarvae reared with enriched on-grown Artemia. Aquac Res 42:704–709
Vidal EAG, DiMarco FP, Wormuth JH, Lee PG (2002) Optimizing rearing conditions of hatchling loliginid squid. Mar Biol 140:117–127
Vidal et al (manuscript in elaboration) In: Vidal E, Villanueva R (eds) Cephalopod culture: current status on main biological models and research priorities. Adv Mar Biol
Villanueva R (1994) Decapod crab zoeae as food for rearing cephalopod paralarvae. Aquac 128:143–152
Villanueva R (1995) Experimental rearing and growth of planktonic Octopus vulgaris from hatching to settlement. Can J Fish Aquat Sci 52:2639–2650
Villanueva R, Bustamante P (2006) Composition in essential and non-essential elements of early stages of cephalopods and dietary effects on the elemental profiles of Octopus vulgaris. Aquac 261:225–240
Villanueva R, Norman MD (2008) Biology of the planktonic stages of benthic octopuses. In: Gibson RN, Gordon JDM (eds) Oceanography and marine biology: an annual review, vol 46. CRC Press, pp 105–202
Villanueva R, Koueta N, Riba J, Boucaud-Camou E (2002) Growth and proteolytic activity of Octopus vulgaris paralarvae with different food rations during first-feeding, using Artemia nauplii and compound diets. Aquac 205:269–286
Villanueva R, Riba J, Ruíz-Capillas C, González AV, Baeta M (2004) Amino acid composition of early stages of cephalopods and effect of amino acid dietary treatments on Octopus vulgaris paralarvae. Aquac 242:455–478
Villanueva R, Escudero JM, Deulofeu R, Bozzano A, Casoliva C (2009) Vitamin A and E content in early stages of cephalopods and their dietary effects in Octopus vulgaris paralarvae. Aquac 286:277–282
Young RE, Harman RF (1988) Larva, paralarvae and subadult in cephalopod terminology. Malacologia 29:201–207
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Iglesias, J., Fuentes, L. (2014). Octopus vulgaris. Paralarval Culture. In: Iglesias, J., Fuentes, L., Villanueva, R. (eds) Cephalopod Culture. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-8648-5_23
Download citation
DOI: https://doi.org/10.1007/978-94-017-8648-5_23
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-8647-8
Online ISBN: 978-94-017-8648-5
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)