Abstract
The tropical Pacific is a unique region to study marine algal phylogeographic patterns. The ancient age of the ocean basin, combined with the presence of numerous islands and archipelagos derived from a variety of geological and biological processes, has yielded several “cosmopolitan” algal species that likely achieved a broad distribution during the times of the Tethys Ocean. These cosmopolitan species consist, in almost all cases, of a series of lineages that can be interpreted as cryptic or pseudo-cryptic species. We review several example studies from the literature that examine phylogeographic patterns of marine algae from the tropical Pacific, and conclude (1) that in all cases the number of species discovered by molecular methods is large, (2) that the increase in diversity is correlated with sampling effort, and (3) that while morphological species are widespread in the tropics, cryptic or pseudo-cryptic species are often more localized, and even appear to have neighboring distribution patterns. These conclusions lead to a call for more large-scale collaborative studies to examine the phylogeographic trends of purportedly cosmopolitan species across the tropical Pacific.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Phylogeography examines the spatial arrangement of genetic lineages, with a focus on intraspecific patterns and those of closely related species (Avise 2009). Phylogeographic studies have greatly increased our understanding of the processes that have produced the great biological diversity found in the world. Understanding the factors that have influenced species and population diversity and distribution can help us predict how species and populations will change under scenarios of future environmental and climate change . The study of marine algal phylogeography has emerged over the last 10–15 years as the use of molecular tools for the investigation of genetic variation have become commonplace, and advances have been made in both phylogeographic methodologies and knowledge of patterns of other marine lineages (Knowles 2009). Marine algal phylogeographic patterns in the tropical Pacific are a result of many influences, including historical geography of the region, dispersal capabilities of individual species, tolerance to environmental conditions, and, more recently, the influence of anthropogenic transport. Macroalgae are starting to be studied more extensively, including in the tropics, and both the number of species examined and the analytical methodologies and sampling methods used will be summarized in this chapter.
2 Geology of the Tropical Oceans
The tropical oceans of the world have unique histories that have influenced and continue to influence species living in these environments. Tropical oceans span a latitudinal range from approximately the Tropic of Cancer in the north (23.5°N) to the Tropic of Capricorn (23.5°S) in the south, or the 20 °C winter isotherm. These boundaries also coincide with the distribution of hermatypic corals (Lüning 1990). The tropics, at present, span three ocean basins (Atlantic, Indian, and Pacific), which have had connections with each other in historical times and have had barriers, of various effectiveness, separating and joining them (Lomolino et al. 2009).
A historical geological feature linking all tropical oceans was the Tethys Ocean , which was a long-lived ocean basin (ca. 250–60 Ma) that separated the supercontinents of Laurasia and Gondwana (Lomolino et al. 2009). By the late Cretaceous (100–60 Ma) this ocean began to break apart with the splitting up of the supercontinents, followed by movement of the continents. The circum-equatorial Tethys Ocean (the Tethys Seaway) likely led to pan-tropical distributions of tropical marine species, which subsequently became isolated as individual ocean basins formed. Thus, the historical joining of oceanic basins in the Tethys Ocean was likely responsible for the evolution of widespread tropical algal species, or groups of related species (Lüning 1990). In the Eocene, the Tethys Seaway continued to close with the movement of Africa and India northward. This closure of the circum-equatorial tropical Tethys led to the semi-isolated ocean basins of today (Lomolino et al. 2009).
The Pacific Ocean originated following the breakup of Pangea. Its tropical regions were influenced by movements in the Tethys Seaway (Neall and Trewick 2008). While the Pacific is the largest ocean, and dispersal distances between land masses are enormous, it is also covered with a variety of islands. These islands break up distances between shallow marine habitats (strongly influencing the ecology of many marine species) and also give rise to the possibility of partial isolation of populations. The current islands of the tropical Pacific originated in a variety of ways, and include volcanically formed oceanic islands and seamounts (e.g., the Hawaiian Archipelago, the Line Islands), atolls that developed with coral growth resulting in reefs (e.g., Rarotonga, Aitutaki, and Palmerston Atoll of the Cook Islands), uplifted coral reefs and atolls (e.g., Rennell Island of the Solomon Islands), fragments of continental crust (e.g., New Zealand and New Caledonia), and the formation of island arcs on the Pacific margins (e.g., the Tonga-Kermadec Islands) (Neall and Trewick 2008). This extraordinarily rich range of mechanisms for the development of the islands of the tropical Pacific has enabled the evolution of a phenomenal level of biodiversity in both the marine and terrestrial realms.
3 Speciation in the Marine Tropical Pacific
Most marine invertebrates and many fishes disperse widely via planktonic larval stages (e.g., Grosberg and Levitan 1992), and this potential for dispersal for many marine animals has shaped assumptions about the degree of connectivity in the marine environment. Most marine phylogeographic research has been conducted under the premise that as population connectivity decreases through decreased planktonic-stage dispersal (e.g., at larger geographic distances from the larval source), genetic differentiation increases and populations become more isolated, which leads to speciation in the marine realm (Mayr 1963; Rocha and Bowen 2008).
The most commonly accepted mechanism of speciation is allopatry (isolation of populations due to a barrier), and it has been largely accepted that speciation in the sea occurs via this mechanism (Rocha and Bowen 2008). However, allopatric speciation does not adequately explain all observed patterns of biodiversity in the oceans. Given the lack of obvious breaks in continuity in the marine realm, under a model of pure allopatric speciation one would expect to find relatively few unique species in the sea; it is clear, given levels of marine biodiversity, that this is not the case.
Other mechanisms of speciation have, therefore, been proposed for marine environments (Bowen et al. 2013). Ecological speciation can occur under parapatry and even sympatry in the oceans (Bowen et al. 2013) and has been proposed for a variety of coral reef taxa including fishes (Rocha et al. 2008), sponges (Rützler et al. 2007), corals (Bongaerts et al. 2010), limpets (Bird et al. 2011), and nudibranchs (Faucci et al. 2007). The rich diversity of available habitats in coral reefs ecosystems (i.e., high physical and ecological heterogeneity) , combined with an elevated intensity of competition among their occupants, is believed to drive these speciation processes. How the process of ecological speciation would function in macroalgae is less clear, however, given their lack of mobility.
Evidence is accumulating that speciation is high in macroalgae that mostly have poorly dispersing propagules . For example, up to 21 genetic species are recognized in the red alga Portieria in the Philippine archipelago alone (Payo et al. 2013). Whether these speciation events occurred allopatrically is unknown; perhaps more importantly, the scale at which allopatric speciation operates is not fully understood. Full or partial allopatric speciation may be possible even at small geographical distances for taxa such as macroalgae, which, on the whole, disperse poorly.
4 Dispersal of Seaweeds
Macroalgae lack “long-lived” propagules, which distinguishes them from many marine animals. Spores and gametes, as single cells, have very limited survival times (on the order of hours or days), and this is especially true for gametes that need to find companions before dilution increases distances from con-specifics (Coyer et al. 2003). Moreover, the locomotory powers of even the strongest algal swimming cells are simply ineffective against wave action and general oceanic water circulation, and algal gametes may be best suited to finding a nearby mate or settling in an appropriate location within the minute boundary layer, rather than influencing dispersal at larger geographical scales (Norton 1992). This problem is perhaps most profound for the red algae, which lack flagellated cells altogether, and instead rely on water motion for dispersal of gametes and spores. Local population genetic studies indicate that macroalgal populations are strongly structured (i.e., partially isolated) even at small scales (e.g., Valero et al. 2011; Kruger-Hadfield et al. 2013; Provan et al. 2013) and isolation by distance can explain some of this structure (e.g., Coyer et al. 2003; Provan et al. 2013). Of course, not all studies show isolation by distance , which indicates that stochastic processes (i.e., rare long distance dispersal events) can be significant (Zuccarello et al. 2011). One known method of frequent long distance dispersal is through floating, or rafting , of algal fronds, especially of large brown algae and their associated flora and fauna (Buchanan and Zuccarello 2012; Fraser et al. 2009).
Thus, both isolation, which drives population differentiation, ultimately leading to speciation if continued, and infrequent long distance dispersal , lead to the phylogeographic patterns of tropical Pacific macroalgae. The wide expanse of the tropical oceans and the many islands situated at various distances from each other result in the processes of speciation and differentiation being especially important in these marine environments.
5 General Patterns of Pacific Marine Tropical Biodiversity
The Coral Triangle (the region between Indonesia, New Guinea, and the Philippines) of the Indo-Pacific has documented high species richness for corals, reef fishes, and some gastropod and crustacean groups relative to other Pacific marine regions (Bellwood and Meyer 2009), and thus is widely recognized as the tropical marine biodiversity hotspot of the Pacific Ocean (e.g., Briggs 2005). The Coral Triangle has a long history of species accumulation, dating back 20–12 Ma (the Miocene) , and with exportation of biodiversity in the Pliocene , Pleistocene and Holocene (7 Ma until present) (Cowman and Bellwood 2013; Briggs and Bowen 2013). Three biogeographic explanations have been put forth for the observed pattern of increased biodiversity in the Coral Triangle marine biodiversity hotspot, termed the Center of Speciation , Center of Accumulation and Center of Overlap models (Bowen et al. 2013), which differ in interpretation of where rates of speciation are highest. Individual studies have provided evidence for all three models. The Center of Speciation model posits that the speciation process is most intense at biodiversity hotspots themselves (with evidence in support of this model from studies of sea turtles; Bowen et al. 1998). The Center of Accumulation model differs in that it assumes that new species arise peripherally to biodiversity hotspots and that these species are concentrated in the center of the range by prevailing oceanographic currents, and studies of fish have yielded support for this model (e.g., Bernardi et al. 2004). Finally, the Center of Overlap hypothesis promotes the idea of isolated distributions of taxa overlapping at their distributional edges, establishing a region of elevated biodiversity (with evidence provided, for example, from studies of fish; Barber and Bellwood 2005). More recently, some researchers have argued in favor of a Biodiversity Feedback model, which integrates elements of several or all of the above models to describe a more dynamic interchange of biodiversity among peripheral and central regions of tropical marine biodiversity (Bowen et al. 2013). Which, if any, of these models applies to the tropical marine algal diversity of the Pacific, has yet to be investigated in detail.
6 Marine Algal Phylogeographic Patterns in the Tropical Pacific
Phylogeographic studies of seemingly cosmopolitan tropical marine algal species most often reveal what was formerly considered to be one species to actually be a species complex (e.g., Zuccarello et al. 2002; Andreakis et al. 2009; Sherwood et al. 2011; Payo et al. 2013). Some of these genetic species have restricted geographical distributions, while others are broader in distribution. These patterns clearly demonstrate that there are two differing processes affecting algal phylogeography: (1) differentiation due to drift, or selection, leading to isolated populations with the potential to produce locally adapted populations that could be considered species. These results contradict the assumption that marine species are cosmopolitan because of high dispersal capability in connected habitats (i.e., a lack of dispersal barriers in the sea); (2) long distance dispersal, which can lead to widely distributed species, and can be anthropogenic or natural. Observed phylogeographic distributions of macroalgae may in fact be a confluence of patterns from the above two forces.
Below we compare and contrast several studies of marine algal phylogeography in the tropical Pacific. Most marine algal phylogeographic research thus far has focused on the red algae, which perhaps reflects their species richness in these environments (e.g., the marine algal flora of the Hawaiian Islands comprises approximately 70 % red algal species; Abbott 1999; Abbott and Huisman 2004). The number of phylogeographic studies of tropical marine algae is still low, but some general patterns are beginning to emerge. For example, in one recent study, Kerswell (2006) suggested that richness of marine algae is actually lower in the tropics than at higher latitudes, which contrasts with the pattern found for many other marine organisms. We attempt to use the examples below to summarize phylogeographic patterns for marine algae in the tropical Pacific.
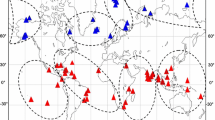
Amansia glomerata C. Agardh (Rhodophyta)—The tropical marine red alga Amansia glomerata was described from an unknown location on the Hawaiian Island of Oahu (Agardh 1822), but is recognized as being very broadly distributed throughout the tropical and subtropical Pacific and Indian Ocean (Fig. 8.1a, Guiry and Guiry 2015). Extreme morphological variability has been noted for this taxon (Phillips 2009). Sherwood et al. (2011) compared 61 specimens representing all eight Main Hawaiian Islands plus French Frigate Shoals of the Northwestern Hawaiian Islands using several molecular markers . Three distinct mitochondrial lineages were recognized, and the possibility of the specimens representing a species complex (either cryptic or incipient species) was discussed. Sampling of areas outside the Hawaiian Islands is needed to determine the full extent of molecular variation in this widespread “species” and to elucidate phylogeographical patterns beyond its eastern limit of distribution in the Hawaiian Islands.
Pacific basin distributions of case study taxa discussed in the current chapter (indicated with black circles). a Amansia glomerata b Asparagopsis taxiformis c Bostrychia moritziana d Bostrychia radicans. Distributional data from AlgaeBase (Guiry and Guiry 2015)
Asparagopsis taxiformis (Delile) Trevisan de Saint-Léon (Rhodophyta)—This red algal morphospecies was considered to be widely distributed and found in all tropical ocean basins (Fig. 8.1b). Initial phylogeographic analyses revealed it consisted of four cryptic lineages (Andreakis et al. 2009). Continued sampling has discovered a fifth cryptic lineage (Dijoux et al. 2014), indicating that cryptic diversity discovery is sensitive to sampling effort. These results support a presumed cosmopolitan species consisting of several genetic entities (designated as lineages) with each lineage often having a much more limited distribution. For example, A. taxiformis lineage 5 is found in the southern Pacific, indicating isolation in this area (area of origin) with subsequent limited dispersal. Lineage 4, on the other hand, is distributed widely (the Indian Ocean, both sides of the Pacific, and Hawaii). This lineage has only moderate levels of sequence divergence (3.59 % for the mitochondrial cox2-3 spacer), indicating that dispersal is a relatively recent historical event. Some haplotype distributions of A. taxiformis (e.g., a single cox2-3 spacer haplotype found in Hawaii, Taiwan and Costa Rica) also indicate that some of this dispersal is recent and possibly human-mediated (Sherwood 2008; Dijoux et al. 2014). The ability of A. taxiformis to disperse is potentially due to its heteromorphic alternation of generations in which the sporophytic stage (Falkenbergia-stage) is cryptic , epiphytic on other algae (acting as possible dispersal agents) and physiologically flexible (i.e., eurythermal; Chualáin et al. 2004). Recent research has established that the Falkenbergia-stages of the A. taxiformis lineages can in fact be distinguished morphologically, although the commonly encountered gametophytes remain cryptic (Zanolla et al. 2014). Another characteristic of Asparagopsis that may facilitate establishment in new environments is its chemical defense against herbivory and biofouling (e.g., in A. armata, see Vergés et al. 2008).
Bostrychia moritziana (Sonder ex Kützing) J. Agardh/B. radicans (Montagne) (Rhodophyta)—Another genus in which molecular evidence has shown that the morphological diversity underestimates that determined by molecular methods is the red alga Bostrychia . The two morphospecies Bostrychia moritziana and B. radicans have pan-tropical distributions (Fig. 8.1c, d), but consist of seven lineages that do not completely correspond to morphospecies designations (Zuccarello and West 2003). These seven lineages have distributions that range from fairly wide ranging to more localized. For example, lineage 1 is confined to the southern Pacific (Australia, Indonesia) and Indian Ocean (South Africa, Madagascar), while lineage 3 is found only in Atlantic South America, and other lineages have much wider distributions (e.g., lineage 6 in the Atlantic, Pacific and Indian Oceans) (Zuccarello and West 2003). Furthermore, these genetic lineages are known to be mostly reproductively isolated from each other (Zuccarello and West 1997; Zuccarello et al. 1999), demonstrating that the genetic species meet the criteria for recognition as distinct species under other species concepts (i.e., reproductive isolation under the Biological Species Concept).
The Bostrychia moritziana /B. radicans species complex is also a good example of changes in recognized diversity and phylogeographic scenarios with increased sampling. Increased sampling of the southern USA as an expansion of the results presented in Zuccarello and West (2003) indicated a different level of diversity within populations in addition to altered phylogeographic interpretation of this diversity (Zuccarello et al. 2006).
Portieria hornemannii (Lyngbye) P.C. Silva (Rhodophyta)—Phylogeographic patterns within this red algal species (Fig. 8.2a) were studied from samples collected in the Philippines in one of the first algal studies to use formal species delimitation methodologies to determine the “species status” of distinct molecular lineages (Payo et al. 2013). These species delimitation methods (Leliaert et al. 2014) are becoming widely used to remove some of the subjective nature of species determination from phylogenetic trees (e.g., Vieira et al. 2014; Muangmai et al. 2014). Payo et al. (2013) discovered that collections of Portieria throughout the Philippines constituted up to 21 species based on these species delimitation methods. Most species were found to be highly restricted in distribution to single islands within the archipelago. They concluded that speciation in the marine realm can occur at spatial scales of less than 100 km; thus, this was a critical study clearly highlighting the potential of tropical marine algae with limited dispersal to speciate while maintaining close proximity. If other tropical marine algal species show similar patterns (speciation within tropical archipelagos), the tropics will be supported as one of the major regions of marine algal species generation.
Pacific basin distributions of case study taxa discussed in the current chapter (indicated with black circles). a Portieria hornemannii b Spyridia filamentosa c Colpomenia sinuosa d Lobophora variegata. Distributional data from AlgaeBase (Guiry and Guiry 2015)
Spyridia filamentosa (Wulfen) Harvey (Rhodophyta)—The red alga Spyridia filamentosa is well known due to its wide distribution (Fig. 8.2b), ease of identification and potential for being a nuisance species (Guiry and Guiry 2015). This “species” has received a considerable amount of attention throughout its distributional range (Zuccarello et al. 2002; Conklin and Sherwood 2012). Although this taxon was described from the Adriatic Sea, it is currently recognized as one of the most widely distributed marine algae in the world (although its distribution in the temperate regions of the North Atlantic may be due to misidentifications; Zuccarello et al. 2004).
Zuccarello et al. (2002) sequenced markers from the nuclear, plastid, and mitochondrial genomes of specimens of S. filamentosa from around the world and demonstrated that samples from different oceanic basins are usually well separated, and mostly reproductively isolated (with more complex patterns observed in the Mediterranean specimens explained as back-colonizations to that ocean after the Messinian salinity crisis ca. 5 Ma). Two main lineages were proposed—an Atlantic/Indian Ocean lineage and a Pacific lineage, consisting of multiple, cryptic species.
Subsequently, Conklin and Sherwood (2012) analyzed 124 specimens of Spyridia filamentosa from islands and atolls of the Hawaiian Islands in the subtropical Pacific, and demonstrated the presence of 5–6 mitochondrial lineages, again highlighting that increased sampling uncovers new diversity and can alter phylogeographic scenarios. Comparisons of these Hawaiian sequences with others for this “species” from other parts of its range illustrated that Spyridia filamentosa arrived in the Hawaiian Islands on at least six different occasions. One clade of specimens was recovered that appeared to be unique to the Hawaiian Islands (Clade D). It was also the most widespread clade in the archipelago, and likely represents the original clade in the islands (Conklin and Sherwood 2012).
The apparent ease of dispersal of Spyridia filamentosa could be due to its ability to fragment easily and produce large populations (West and Calumpong 1989). This dispersal ability could lead to the observed widespread distributions, but also obscure native lineages (or species) due to masking by more recent arrivals. These kinds of complicated phylogeographic patterns can be eventually unraveled, as they were for Hawaiian Spyridia, but this depends on intensive sampling throughout the “species” range.
Halimeda spp. (Chlorophyta)—Broad-scale phylogeographic patterns of species of Halimeda have been elucidated using molecular analyses and niche modeling (Verbruggen et al. 2005, 2009a). These data clearly showed that Halimeda has been a member of the tropical flora since its early evolution, with only a few range shifts to cooler waters. These analyses also demonstrated that the main separations between sister species, or closely related cryptic or pseudo-cryptic species, were between major ocean basins (Indo-Pacific vs. Atlantic). It is likely that these ocean basin phylogeographic separations are due to the limited dispersal since the closure of the Tethys Ocean as the diversification of genus commenced 150 Ma (Verbruggen et al. 2009b) and proceeded for several millions of years, with the sections of Halimeda (groups of related species that show an ocean basin separation within the genus) diversifying until approximately 90 Ma, the time interval when the Tethys Seaway was closing. Verbruggen et al. (2009a) also proposed that species have not dispersed between ocean basins since this time because of lack of suitable or available habitat (Waters et al. 2013).
Colpomenia sinuosa (Mertens ex Roth) Derbès & Solier—In one of the most geographically broad phylogeographic studies of a marine alga (Fig. 8.2c), mitochondrial and plastid DNA from specimens of the brown alga Colpomenia sinuosa from 19 countries were compared. Cryptic diversity was revealed within the taxon as three distinct genetic groups, with multiple lineages within two of the three groups (Lee et al. 2013); Group I was the largest, with pan-tropical representation, Group II was found in the Red Sea and the western Mediterranean, and Group III was found in the central and western Pacific, as well as the Indian Ocean and the western Atlantic. Colpomenia sinuosa was demonstrated to be a wide ranging yet morphologically variable taxon, and pairwise divergences among the recognized groups were high, suggesting a long evolutionary history . Additionally, the complex biogeographical patterns observed, especially for Group I, suggested that anthropogenic dispersal events have played a major role in shaping the distribution of this taxon. The authors noted that most lineages within Group I were distributed at relatively temperate latitudes, which contrasts with the distributions for the other Groups, and also with the idea of peripheral populations having reduced diversity (Lee et al. 2013).
Lobophora v ariegata (J.V. Lamouroux) Womersley ex E.C. Oliveira— Lobophora is a common tropical brown algal genus, with one cosmopolitan species reported from most tropical and temperate areas (L. variegata) (Fig. 8.2d). Early molecular diversity studies in the western Pacific indicated that several species existed within this taxon, and some of these morphologically distinguishable lineages (pseudo-cryptic species) were named (Sun et al. 2012), while other lineages were left as undescribed species. Recently, diversity studies of Lobophora focused on the southwestern Pacific islands of New Caledonia, and employed various species delimitation methods (see Leliaert et al. 2014) resulting in the recognition of 29 MOTUs (Molecular Operational Taxonomic Units) from this one, geographically limited, location (Vieira et al. 2014). Some of these MOTUs could not be delineated morphologically, but 10 new species of Lobophora were described from the specimens and had distinctive morphological features associated with them; thus, this study demonstrated that many genetic species segregate based on both morphological and ecological characters.
7 Summary of Tropical Marine Algal Phylogeographic Patterns
It is clear that morphology-based measures of biodiversity consistently underestimate that determined using molecular methods. Although this finding is hardly new, its implications for the study of algal phylogeography are profound; speciation in the marine tropical Pacific can occur at geographical scales much smaller than typically assumed (e.g., less than 100 km for Portieria in the Philippines; Payo et al. 2013), yielding presumed radiations of marine algal species. Additionally, it has become evident that while “morphological” algal species appear to be widespread in the tropics, many molecularly defined species have much more limited distributions.
Our understanding of seaweed diversity in the tropics has progressed a long way since the introduction of molecular data for examining phylogeographic trends. However, only a few species have been studied extensively (for further examples see: Padina , Win et al. 2011; Silberfeld et al. 2013 and Caloglossa , Kamiya and West 2014). Briefly, we see three main trends from the available data: (1) that in all studies examined the number of species discovered by molecular methods is large, (2) that the increase in estimates of diversity is correlated with sampling effort, and (3) that while morphological species are widespread in the tropics, cryptic or pseudo-cryptic species are often more localized, and even appear to have neighboring distribution patterns.
The tropics are well known as an area of high species diversity and richness (e.g., under the Latitudinal Diversity Gradient, e.g., Hillebrand 2004). It could be hypothesized that this pattern should also be found in the tropical Pacific for marine algae. Certainly, the Coral Triangle is recognized as a biodiversity hotspot for many marine species, with almost all supporting data stemming from studies of reef fishes and some invertebrate groups (Bowen et al. 2013). Some of the phylogeographic examples discussed in this chapter from or near the Coral Triangle (e.g., Portieria from the Philippines, Payo et al. 2013; Lobophora from New Caledonia, Vieira et al. 2014) support the idea of elevated speciation levels in this region. However, not all biogeographical studies based on species numbers support this idea. Kerswell (2006) suggested that richness of marine algae is actually lower in the tropics than at higher latitudes, but some questions remain as to the conclusiveness of this pattern given that the analysis was restricted to the genus level and was based on a limited number of literature records of mostly morphological identifications. Later, studies following up on this idea suggested that environmental tolerance influences species richness in the high latitudes, whereas biotic interactions are more important for species richness in the tropics (Keith et al. 2014). Schils et al. (2013) analyzed substantially larger numbers of algal records and included representation from many islands in the western and central Pacific Ocean, and concluded that marine algal richness was defined by local habitat diversity and availability; they did not uncover a latitudinal diversity gradient of marine algae in the region of study. However, all of these biogeographical studies are based on morphologically derived species lists that are almost certainly inaccurate, perhaps even more so in the tropics. The degree to which the phylogeographic studies discussed in previous sections of this chapter support the presence of numerous cryptic or pseudo-cryptic species within “cosmopolitan” marine taxa suggests that much work remains to be done defining and characterizing the marine algal species of the tropical Pacific before biogeographical patterns can be investigated with confidence.
Why would the discoveries of cryptic species be higher in the tropics than other areas? We believe that there are several possibilities. First, the historically connected nature of the tropics in ancient oceans (i.e., Tethys Sea) may have produced widely distributed taxa in a tropical climate lacking the abiotic stressors that dominate in temperate and polar environments, and that these taxa became increasingly isolated as these regions became disconnected with the movements of land masses. Second, the combination of the wide expanse of the tropical oceans , the large number of isolated islands, and the poor dispersal ability of many macroalgae likely produced many opportunities for speciation under allopatry , sympatry or peripatry. Third, it is possible that biotic interactions have little influence on species morphology.
8 Perspectives for Future Research
Phylogeographical studies of marine algae have progressed substantially in the last several years, yet it is clear that many more example taxa need to be investigated in a high level of detail, and from a much broader geographical area than has been typically sampled in the past, in order to elucidate large-scale phylogeographical patterns for the tropical marine Pacific. As such, it would be beneficial to see a series of collaborative projects undertaken (in the spirit of the “ Portieria Evolution Consortium” spearheaded by O. De Clerck, F. Leliaert and colleagues, which aims to assess lineage diversity patterns within that genus throughout its global range) to investigate phylogeographic patterns of some of the most widespread tropical marine algae, including representatives from the red, green, and brown algae. The tropical Pacific is particularly difficult for any one research group to sample effectively because the area is dauntingly large and it is geopolitically divided into many nations, yet many “species” are widespread, making adequate sampling for phylogeographic studies a challenge. Combining strong sampling effort at fine geographical scales with large sample sizes and novel molecular analysis techniques (e.g., recent species delimitation methods), or even novel kinds of molecular data (e.g., analyses of single nucleotide polymorphisms with RAD sequencing) have the potential to take the field of tropical marine algal phylogeography forward at a fast pace in the near future.
References
Abbott IA. Marine red algae of the Hawaiian Islands. Honolulu: Bishop Museum Press; 1999. p. 477.
Abbott IA, Huisman JM. Marine green and brown algae of the Hawaiian Islands. Honolulu: Bishop Museum Press; 2004. p. 259.
Agardh CA. Species algarum. 1, part 2. Lund; 1822. pp viii + 169–531.
Andreakis N, Kooistra WHCF, Procaccini G. High genetic diversity and connectivity in the polyploid invasive seaweed Asparagopsis taxiformis (Bonnemaisoniales) in the Mediterranean, explored with microsatellite alleles and multilocus genotypes. Mol Ecol. 2009;18:212–26.
Avise JC. Phylogeography: retrospect and prospect. J Biogeogr. 2009;36:3–15.
Barber PH, Bellwood DR. Biodiversity hotspots: evolutionary origins of biodiversity in wrasses (Halichoeres: Labridae) in the Indo-Pacific and New World tropics. Mol Phylogenet Evol. 2005;35:235–53.
Bellwood DR, Meyer CP. Searching for heat in a marine biodiversity hotspot. J Biogeogr. 2009;36:569–76.
Bernardi G, Bucciarelli G, Costagliola D, Robertson DR, Heiser JB. Evolution of coral reef fish Thalassoma spp. (Labridae). 1. Molecular phylogeny and biogeography. Mar Biol. 2004;144:369–75.
Bird CE, Holland BS, Bowen BW, Toonen RJ. Diversification of endemic sympatric limpets (Cellana spp.) in the Hawaiian Archipelago. Mol Ecol. 2011;20:2128–41.
Bongaerts P, Riginos C, Ridgway T, Sampayo EM, van Oppen MJH, Englebert N, Vermeulen F, Hoegh-Guldberg O. Genetic divergence across habitats in the widespread coral Seriatopora hystrix and its associated Symbiodinium. PLoS ONE. 2010;5:e10871.
Bowen BW, Rocha LA, Toonen RJ, Karl SA, the ToBo Laboratory. The origins of tropical marine biodiversity. Trends Ecol Evol. 2013;28:359–366.
Bowen BW, Clark AM, Abreu-Grobois AF, Chaves A, Reichart HA, Ferl RJ. Global phylogeography of the ridley sea turtles (Lepidochelys spp.) as inferred from mitochondrial DNA sequences. Genetica. 1998;101:179–89.
Briggs JC. The marine East Indies: diversity and speciation. J Biogeogr. 2005;32:1517–22.
Briggs JC, Bowen BW. Evolutionary patterns: marine shelf habitat. J Biogeogr. 2013;40:1023–35.
Buchanan J, Zuccarello GC. Decoupling of short and long distance dispersal pathways in the endemic New Zealand seaweed Carpophyllum maschalocarpum (Phaeophyceae, Fucales). J Phycol. 2012;48:518–29.
Chualáin FN, Maggs CA, Saunders GW, Guiry MD. The invasive genus Asparagopsis (Bonnemaisoniaceae, Rhodophyta): molecular systematics, morphology, and ecophysiology of Falkenbergia isolates. J Phycol. 2004;40:1112–26.
Conklin KY, Sherwood AR. Molecular and morphological variation of the red alga Spyridia filamentosa (Ceramiales, Rhodophyta) in the Hawaiian Archipelago. Phycologia. 2012;51:347–57.
Cowman PF, Bellwood DR. The historical biogeography of coral reef fishes: global patterns of origination and dispersal. J Biogeogr. 2013;40:209–24.
Coyer JA, Peters AF, Stam WT, Olsen JL. Post-ice age recolonization and differentiation of Fucus serratus L. (Phaeophyceae; Fucaceae) populations in Northern Europe. Mol Ecol. 2003;12:1817–29.
Dijoux L, Viard F, Payri C. The more we search, the more we find: discovery of a new lineage and a new species complex in the genus Asparagopsis. PLoS ONE. 2014;9:e103826.
Faucci A, Toonen RJ, Hadfield MG. Host shift and speciation in a coral-feeding nudibranch. Proc R Soc B. 2007;274:111–9.
Fraser C, Nikula R, Spencer H, Waters J. Kelp genes reveal effects of subantarctic sea ice during the last glacial maximum. Proc Nat Acad Sci USA. 2009;106:3249–53.
Grosberg RK, Levitan DR. For adults only? Supply-side ecology and the history of larval biology. Trends Ecol Evol. 1992;7:130–3.
Guiry MD, Guiry GM AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. 2015. http://www.algaebase.org; searched on 25 Mar 2015.
Hillebrand H. On the generality of the latitudinal diversity gradient. Am Nat. 2004;163:192–211.
Kamiya M, West JA. Cryptic diversity in the euryhaline red alga Caloglossa ogasawaraensis (Delesseriaceae, Ceramiales). Phycologia. 2014;53:374–82.
Keith SA, Kerswell AP, Connolly SR. Global diversity of marine macroalgae: environmental conditions explain less variation in the tropics. Glob Ecol Biogeogr. 2014;23:517–29.
Kerswell AP. Global biodiversity patterns of benthic marine algae. Ecology. 2006;87:2479–88.
Knowles L. Statistical phylogeography. Ann Rev Ecol Evol Syst. 2009;40:593–612.
Krueger-Hadfield SA, Roze D, Mauger S, Valero M. Intergametophytic selfing and microgeographic genetic structure shape populations of the intertidal red seaweed Chondrus crispus. Mol Ecol. 2013;22:3242–60.
Lee KM, Boo SM, Kain (Jones) JM, Sherwood AR. Cryptic diversity and biogeography of the widespread brown alga Colpomenia sinuosa (Ectocarpales, Phaeophyceae). Bot Mar. 2013;56:15–25.
Leliaert F, Verbruggen H, Vanormelingen P, Steen F, Lopez-Bautista JM, Zuccarello GC, De Clerck O. DNA-based species delimitation in algae. Eur J Phycol. 2014;49:179–96.
Lomolino MV, Riddle BR, Brown JH. Biogeography. 3rd ed. Sunderland: Sinauer Associates Inc; 2009. pp. 845.
Lüning K. Seaweeds: their environment, biogeography, and ecophysiology. New York: Wiley; 1990. pp 527.
Mayr E. Animal species and evolution. Cambridge: Harvard University Press; 1963. p. 797.
Muangmai N, West JA, Zuccarello GC. Evolution of four Southern Hemisphere Bostrychia (Rhodomelaceae, Rhodophyta) species: phylogeny, species delimitation and divergence times. Phycologia. 2014;53:593–601.
Neall VE, Trewick SA. The age and origin of the Pacific Islands: a geological overview. Philos Trans R Soc B. 2008;363:3293–308.
Norton TA. Dispersal by macroalgae. Br Phycol J. 1992;27:293–301.
Payo DA, Leliaert F, Verbruggen H, D’hondt S, Calumpong HP, De Clerck O. Extensive cryptic species diversity and fine-scale endemism in the marine red alga Portieria in the Philippines. Proc R Soc B. 2013;280:20122660.
Phillips L. The taxonomy of selected tribes and genera of the Rhodomelaceae: a molecular and anatomical appraisal of selected members of the red algal family Rhodomelacae (Ceramiales, Rhodophyta). Köln: Lambert Academic Publishing; 2009. p. 486.
Provan J, Glendinning K, Kelly R, Maggs CA. Levels and patterns of population genetic diversity in the red seaweed Chondrus crispus (Florideophyceae): a direct comparison of single nucleotide polymorphisms and microsatellites. Biol J Linn Soc. 2013;108:251–62.
Rocha LA, Bowen BW. Speciation in coral-reef fishes. J Fish Biol. 2008;72:1101–21.
Rocha LA, Lindeman KC, Rocha CR, Lessios HA. Historical biogeography and speciation in the reef fish genus Haemulon (Teleostei: Haemulidae). Mol Phylogenet Evol. 2008;48:918–28.
Rützler K, Duran S, Piantoni C. Adaptation of reef and mangrove sponges to stress: evidence for ecological speciation exemplified by Chondrilla caribensis new species (Demospongiae, Chondrosida). Mar Ecol. 2007;28:95–111.
Schils T, Vroom PS, Tribollet AD. Geographical partitioning of marine macrophyte assemblages in the tropical Pacific: a result of local and regional diversity processes. J Biogeogr. 2013;40:1266–77.
Sherwood AR. Phylogeography of Asparagopsis taxiformis (Bonnemaisoniales, Rhodophyta) in the Hawaiian Islands: two mtDNA markers support three separate introductions. Phycologia. 2008;47:79–88.
Sherwood AR, Kurihara A, Conklin KY. Molecular diversity of Amansieae (Ceramiales, Rhodophyta) from the Hawaiian Islands: a multi-marker assessment reveals unexpected diversity within Amansia glomerata. Phycol Res. 2011;59:16–23.
Silberfeld T, Bittner L, Fernández-García C, Cruaud C, Rousseau F, de Reviers B, Leliaert F, Payri CE, De Clerck O. Species diversity, phylogeny and large scale biogeographic patterns of the genus Padina (Phaeophyceae, Dictyotales). J Phycol. 2013;49:130–42.
Sun Z, Hanyuda T, Lim PE, Tanaka J, Gurgel CFD, Kawai H. Taxonomic revision of the genus Lobophora (Dictyotales, Phaeophyceae) based on morphological evidence and analyses rbcL and cox3 gene sequences. Phycologia. 2012;51:500–12.
Valero M, Destombe C, Mauger S, Ribout C, Engel C R, Daguin-Thiebaut C, Tellier F. Using genetic tools for sustainable management of kelps: a literature review and the example of Laminaria digitata. Cah Biol Mar. 2011;52:467–483.
Verbruggen H, DeClerck O, Schils T, Kooistra WHCF, Coppejans E. Evolution and phylogeography of Halimeda section Halimeda (Bryopsidales, Chlorophyta). Mol Phylogenet Evol. 2005;37:789–803.
Verbruggen H, Tyberghein L, Pauly K, Vlaeminck C, Van Nieuwenhuyze K, Kooistra WHCF, Leliaert F, De Clerck O. Macroecology meets macroevolution: evolutionary niche dynamics in the seaweed Halimeda. Glob Ecol Biogeogr. 2009a;18:393–405.
Verbruggen H, Ashworth M, LoDuca ST, Vlaeminck C, Cocquyt E, Sauvage T, Zechman FW, Littler DS, Littler MM, Leliaert F, De Clerck O. A multi-locus time-calibrated phylogeny of the siphonous green algae. Mol Phylogenet Evol. 2009b;50:642–53.
Vergés A, Paul NA, Steinberg PD. Sex and life-history stage alter herbivore responses to a chemically defended red alga. Ecology. 2008;89:1334–43.
Vieira C, D’hondt S, De Clerck O, Payri CE. Toward an inordinate fondness for stars, beetles and Lobophora? Species diversity of the genus Lobophora (Dictyotales, Phaeophyceae) in New Caledonia. J Phycol. 2014;50:1101–19.
Waters JM, Fraser CI, Hewitt GM. Founder takes all: density-dependent processes structure biodiversity. Trends Ecol Evol. 2013;28:78–85.
West JA, Calumpong HP. Reproductive biology of Spyridia filamentosa (Wulfen) Harvey (Rhodophyta) in culture. Bot Mar. 1989;32:379–387.
Win NN, Hanyuda T, Arai S, Uchimura M, Prathep A, Draisma SGA, Phang SM, Abbott IA, Millar AJK, Kawai H. A taxonomic study of the genus Padina (Dictyotales, Phaeophyceae) including the descriptions of four new species from Japan, Hawaii, and the Andaman Sea. J Phycol. 2011;47:193–191.
Zanolla M, Carmona R, De la Rosa J, Salvador N, Sherwood AR, Andreakis N, Altamirano M. Morphological differentiation of cryptic lineages within the invasive genus Asparagopsis (Bonnemaisoniales, Rhodophyta). Phycologia. 2014;53:233–42.
Zuccarello GC, Sandercock B, West JA. Diversity within red algal species: variation in world-wide samples of Spyridia filamentosa (Ceramiales) and Murrayella periclados (Rhodomelaceae) using DNA markers and breeding studies. Eur J Phycol. 2002;37:403–17.
Zuccarello GC, West JA. Multiple cryptic species: molecular diversity and reproductive isolation in the Bostrychia radicans / B. moritziana complex (Rhodomelaceae, Rhodophyta) with a focus on North American isolates. J Phycol. 2003;39:948–59.
Zuccarello GC, Prud’homme van Reine WF, Stegenga H. Recognition of Spyridia griffithsiana comb. nov. (Ceramiales, Rhodophyta): a taxon previously misidentified as Spyridia filamentosa from Europe. Bot Mar. 2004;47:481–489.
Zuccarello GC, Buchanan J, West JA, Pedroche FF. Genetic diversity of the mangrove-associated alga Bostrychia radicans/Bostrychia moritziana (Ceramiales, Rhodophyta) from southern Central America. Phycol Res. 2011;59:98–104.
Zuccarello G, West JA. Hybridization studies in Bostrychia: 2. Correlation of crossing data and plastid DNA sequence data within B. radicans and B. moritziana (Ceramiales, Rhodophyta). Phycologia. 1997;36:293–304.
Zuccarello G, West JA, King R. Evolutionary divergence in the Bostrychia moritziana/B. radicans complex (Rhodomelaceae, Rhodophyta): molecular and hybridization data. Phycologia. 1999;38:234–44.
Zuccarello GC, Buchanan J, West JA. Increased sampling for inferring phylogeographic patterns in Bostrychia radicans/Bostrychia moritziana in the eastern USA. J Phycol. 2006;42:1349–52.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Sherwood, A.R., Zuccarello, G.C. (2016). Phylogeography of Tropical Pacific Marine Algae. In: Hu, ZM., Fraser, C. (eds) Seaweed Phylogeography. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7534-2_8
Download citation
DOI: https://doi.org/10.1007/978-94-017-7534-2_8
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7532-8
Online ISBN: 978-94-017-7534-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)