Abstract
Coral reefs of the eastern tropical Pacific (ETP) are unique in being the only reef region in the world that has experienced multiple episodes of mass coral bleaching while also being exposed to chronically depressed aragonite saturation states as a result of regional upwelling. These characteristics make them ideal case studies for the continued effects of climate change on coral reefs, and in particular for the responses of reef corals and their dinoflagellate algal symbionts (Symbiodinium spp.) to combined climate stressors. As a result, the diversity, distribution and stability of Symbiodinium in ETP corals have been studied since the mid-1990s, shortly after contemporary molecular methods to identify Symbiodinium were first developed. ETP reefs have been instrumental in the discovery that certain members of Symbiodinium in clade D impart bleaching resistance to their coral hosts. In the ETP, clade D is represented by a single symbiont type (D1, also referred to as S. glynni), which has been shown to be tolerant of both episodic El Niño-driven high temperature stress (e.g., 1997 in the Gulf of Chiriquí, Panama), and low temperature stress during an unusually cold winter (e.g., 2008 in Baja California, Mexico). Virtually all studies in the region have focused on corals in the genus Pocillopora, which is both the dominant reef-building genus and the most symbiotically diverse, being the only coral genus in the ETP that routinely hosts heat tolerant D1 symbionts at high abundance. There is debate over the mechanisms by which D1 becomes dominant on pocilloporid reefs, with evidence for both differential mortality of corals, and dynamic change in symbiont communities in response to thermal history and/or disturbance. The relative importance of these two mechanisms is likely to be critical in determining reef survival trajectories over the coming century, as is the degree to which non-pocilloporid corals in the region can associate with, or become dominated by, D1. Dynamic change in symbiont communities may allow corals to survive recurrent bleaching events if stepwise increases in the abundance of D1 following each bleaching event allow these thermotolerant symbionts to accumulate. However, controlled experiments and continued monitoring are required to resolve the debate over symbiont stability versus lability, and there may be significant differences in coral response across the region. Finally, understanding whether different Symbiodinium can alter coral response to high CO2 or depressed aragonite saturation state (Ωarag) remains a priority research area for the region, especially given the potential for strong interactions between Ωarag, coral growth, and symbiont community structure.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
13.1 Introduction
13.1.1 The ETP as a Case Study for Studying Coral-Algal Symbiosis Under Climate Change
Coral reefs of the eastern tropical Pacific (ETP) are unique case studies for the long-term ecological effects of thermal variability on coral reefs, and particularly for the impacts of repetitive high temperature stress on reef coral symbioses. Due to the influence of the El Niño-Southern Oscillation (ENSO) on these reefs, they have experienced multiple episodes of severe coral “bleaching ” (stress-induced expulsion of algal symbionts), which have led to coral mortality events followed by sustained periods of recovery. In addition, this region includes corals: (1) at the northern limit of their latitudinal distribution, with relatively cool winter temperatures and lower irradiance (Gulf of California, Mexico), (2) in nutrient-rich upwelling environments (Gulf of Panama, Panama), (3) in relatively warm, stable conditions (Gulf of Chiriquí, Panama), and (4) exposed to some of the lowest equatorial temperatures in the world (Galápagos, Ecuador). All of these circumstances result in a living laboratory for the investigation of temperature regime, and thermal extremes, on coral-algal symbioses, and represent unique systems for understanding the effects of climate change on coral reefs.
In addition, many coral reefs in the ETP, by virtue of their exposure to upwelling conditions, also experience chronically depressed aragonite saturation state (Ωarag). These conditions reduce calcification rates, resulting in slower coral growth and decreased reef formation. These conditions, combined with the thermal stress history of the ETP, make these reefs ideal “space-for-time” case studies for coral reefs under projected climate change scenarios (Manzello et al. 2008). Although the implications of depressed Ωarag for Symbiodinium diversity have not been explicitly investigated in the ETP, these conditions nevertheless underscore the relevance of these reefs for studying future climate change scenarios.
13.1.2 Marginal Environmental Conditions in the ETP
Prevailing environmental conditions in the eastern Pacific are marginal for reef development because of the: (1) narrow continental shelf on the western side of the Americas; (2) strong, permanent shallow thermocline; (3) relatively frequent occurrence of aerial exposure or mortality caused by extreme low tides, ENSO-related sea level drops and/or tectonic uplifts (Maté 2003); and (4) existence of dry and wet seasons (Dana 1975; Cortés 2003). During the wet season, many areas experience high cloud cover and intense rainfall, and the resulting terrestrial run-off leads to high turbidity, low light, and hyposaline conditions. In some areas, such as the Gulf of Panama (Panama), the Revillagigedo Islands, the Gulf of Tehuantepec (Mexico), and the northern coast of Costa Rica (Cortés and Jiménez 2003), high atmospheric pressure during the dry season pushes coastal surface waters offshore. This results in seasonal upwelling, which bathes associated reefs in relatively cold, nutrient-rich waters for part of the year. These conditions challenge coral reef growth and survival in several ways. First, temperatures may be relatively low compared to the average tolerance of coral-algal symbioses, sometimes resulting in cold-water bleaching events (Fig. 13.1). Second, coral animals are most competitive against filter feeders and macroalgae under low nutrient conditions; the nutrient-rich conditions that accompany upwelling can increase the competitive interactions experienced by corals on the benthos (Glynn 1996). Third, upwelled waters are relatively pCO2-enriched and low in pH and aragonite saturation state (Ωarag), making calcification more difficult on ETP reefs than in other areas, such as the Caribbean. The low Ωarag and high nutrient concentrations associated with upwelled waters limit early marine cementation of reef framework and/or stimulate bioerosion (Manzello et al. 2008). Consequently, ETP reefs can be viewed as proxies for reefs of the future under various ocean acidification (OA) scenarios predicted to occur in the world’s oceans due to increasing atmospheric pCO2 (IPCC 2007).
Diversity of algal symbionts (Symbiodinium spp.) in reef corals of the ETP and their importance in understanding coral reef ecology in the region. a Molecular phylogeny of Symbiodinium based on nuclear 28S (large subunit) ribosomal DNA (nr28S-rDNA). Shown are 9 clades (A-I), of which four have been found in scleractinian corals of the ETP. Members of clades C and D (yellow) are the most common symbionts in the region, with Symbiodinium D1 frequently occurring in Pocillopora. Members of clades A (green) and B (blue) have been found at low abundance in ETP scleractinians using high resolution molecular techniques, but B (blue) has also been found at high abundance in Pocillopora recovering from cold-water bleaching. Modified from Pochon and Gates (2010). b Spatial relationships in coral-algal symbiosis at the cellular, polyp, and reef-scale. Pocilloporid reef frameworks, shown here bleaching in response to cold temperatures, can show dramatic patterns of bleaching in response to temperature extremes. From McGinley et al. (2012)
Upwelling also affects sea surface temperatures (SSTs) across the ETP region. In areas that experience seasonal upwelling, temperature can drop by as much as 10 °C from the wet to the dry season (Manzello et al. 2008; see Chap. 8, Glynn et al.). In contrast, areas of the ETP that do not experience upwelling are more thermally stable throughout the year, although shoaling thermoclines and other effects may also result from seasonal weather patterns. In addition to this thermal variability over seasonal timescales, some regions of the ETP, including the west coast of the Baja California Peninsula (Mexico), Panama, and Colombia, are also subject to the El Niño-Southern Oscillation (ENSO). During intense ENSO events, SST temperatures can increase by 2–3 °C and upwelling is reduced, resulting in significant thermal stress and potentially catastrophic mass bleaching events (Glynn 1984). Mass bleaching in the ETP was associated with ENSO events occurring in 1982–83 (Glynn and Colgan 1992), and 1997–98, with coral mortality following the 1982-83 event being significantly higher than the 1997–98 event (Glynn et al. 2001a, b). This can likely be attributed to both differential survival and reproduction of temperature tolerant genotypes during the 1982–83 event, as well as symbiont community shifts within individual colonies in favor of thermally tolerant members of Symbiodinium clade D (Baker et al. 2004; LaJeunesse et al. 2010a, see also Sect. 13.4). Although ENSO events may ultimately lead to the collapse of ETP reefs under predicted climate scenarios for the current century (Toth et al. 2012), the unusual environmental and hydrodynamic settings that characterize this region provide a unique opportunity to examine the prevalence of thermally tolerant symbionts before, during, and after environmental stress events, and how these patterns are affected by differences in aragonite saturation state between reefs.
13.1.3 Isolation of ETP Reefs and Its Implications for the Study of Symbiodinium
In addition to the difficult environmental conditions encountered by corals in the ETP, they also face significant challenges colonizing the region from elsewhere. Coral reefs of the ETP are depauperate and have been isolated from the Caribbean Sea since the closure of the Isthmus of Panama about 3.7–3.0 Ma (Duque-Caro 1990; Coates and Obando 1996). Although occupying the same ocean basin as the center of global coral diversity (the ‘Coral Triangle’), migration from the Indo-west Pacific requires extreme larval dispersal (5000–8000 km, Dana 1975; Grigg and Hey 1992). The eastern Pacific ‘barrier’ is formed by strong currents running from east to west between the Coral Triangle and the ETP (see Chap. 3, Fiedler and Lavín) and, if not ‘impassable’ (Baums et al. 2012; see Chap. 16, Lessios and Baums), has long been considered the world’s most effective marine barrier to larval dispersal (Ekman 1953). This isolation has led to a distinct eastern Pacific reef-building coral fauna, with some endemism (e.g., Pavona chiriquiensis, Glynn et al. 2001a; see also Chap. 2, López-Pérez). Thirty-six stony coral species are found in the ETP (Glynn and Ault 2000; see Chap. 5, Glynn et al.), which contrasts starkly with the hundreds of species that can be found in the Indo-west Pacific. Pocillopora, the main reef-building genus of the region, typically dominates shallow-water ETP reefs. In some areas, reef framework development is minimal and only coral communities exist, often dominated by massive colonies in the genera Pavona and Porites. ETP coral species exist at the limits of their respective geographic ranges, which may subject these populations to reduced gene flow, and provide opportunities for speciation. The relatively depauperate nature of these reefs from both a coral host and an algal symbiont perspective also provides an opportunity to understand symbiont specificity and co-evolution (Pinzón and LaJeunesse 2011), and to study niche differentiation (Iglesias-Prieto et al. 2004), as well as ecological interactions and responses among corals and their Symbiodinium communities (Glynn et al. 2001a, b; Baker et al. 2004; LaJeunesse et al. 2008).
13.2 Symbiodinium Diversity in the ETP
13.2.1 Overview of Diversity in Symbiodinium
Modern investigations of diversity in Symbiodinium use molecular genetic methods to survey Symbiodinium and document its distribution and dynamics. Currently, the genus Symbiodinium consists of nine major clades (A-I, Pochon and Gates 2010), most of which are characterized by significant additional diversity at finer taxonomic scales (LaJeunesse 2005), leading to the description of a variety of new species (e.g., LaJeunesse et al. 2010a). Although no formal taxonomic revisions of the genus have yet been undertaken, the diversity present within this genus strongly supports its subdivision into multiple new genera.
Early investigations used variation in the small subunit ribosomal DNA (rDNA) to classify Symbiodinium into clades, and distinguish some variation within these clades (Rowan and Powers 1991a, b). These methods were then used to study the distribution and dynamics of Symbiodinium in Caribbean Montastraea (Orbicella) (Rowan and Knowlton 1995; Rowan et al. 1997). Shortly afterwards, using large subunit (LSU) rDNA, these methods were applied to ETP reefs to study symbiont diversity, distribution, and dynamics with a higher degree of taxonomic resolution (Baker and Rowan 1997; Baker 1999; Glynn et al. 2001a, b). Due to the relatively rapid adoption of these methods in the region, ETP reefs are represented by some of the longest running datasets on Symbiodinium in existence (dating to 1995 in Panama). Subsequent investigations of symbiont diversity applied even greater taxonomic resolution by analyzing the Internal Transcribed Spacer-2 (ITS-2) region of rDNA (LaJeunesse et al. 2008, 2010a, b), and more recent studies are beginning to reveal even finer-scale genetic variation among Symbiodinium types through the use of the non-coding region of the psbA minicircle in Symbiodinium chloroplasts (psbA ncr), which resolves more diversity than the ITS-2 (Pinzón and LaJeunesse 2011), while also reducing the problems associated with the latter marker’s high copy number and intragenomic variation (LaJeunesse and Thornhill 2011). These methods have greatly increased our understanding of Symbiodinium distributions in the ETP and elsewhere, but they do preclude comparisons with earlier datasets for all but the highest (typically clade-level) diagnoses. Additionally, microsatellite markers can further differentiate among Symbiodinium genotypes, with ~20–30 distinct Symbiodinium D1 genotypes identified at individual reefs in the Gulf of California (Pettay et al. 2011). Coral colonies can host single or multiple Symbiodinium genotypes simultaneously, and these associations can change over time. Particular Symbiodinium D1 genotypes may also be adapted to specific local environmental regimes (Pettay and LaJeunesse 2013). Parsing out this fine-scale genetic diversity is an important ongoing research objective in understanding Symbiodinium diversity and ecology.
Recently, higher detection resolution using quantitative PCR (qPCR) of both ITS-2 (LaJeunesse et al. 2010a) and the actin gene (Cunning and Baker 2013) has been applied to Symbiodinium communities in ETP hosts. Although these methods have greatly increased our ability to detect, distinguish, and quantify different Symbiodinium, they are also subject to constraints that can limit data interpretation (see Sect. 13.2.2). Next-generation sequencing may further advance these objectives; high-throughput ITS amplicon sequencing has been applied to resolve fungal community diversity (Jumpponen and Jones 2009), and is beginning to be applied to Symbiodinium (Kenkel et al. 2013; Green et al. 2014), although no studies to date have utilized these approaches to characterize Symbiodinium communities in the ETP.
Globally, scleractinian corals have been observed to host Symbiodinium in clades A, B, C, D, and less commonly, clades F and G (Baker 2003; van Oppen et al. 2009; Franklin et al. 2012). In the Pacific, most stony corals tend to host members of clades C and/or D (Baker and Rowan 1997; LaJeunesse et al. 2003), with clades A, B, F and G being only rarely found (e.g., Darius et al. 1998, 2000; Rodriguez-Lanetty et al. 2002; LaJeunesse et al. 2010b). Symbiodinium diversity in the ETP has mainly been assessed along the coast of Panama (Glynn et al. 2001a, b; Baker et al. 2004), in the Gulf of California, Mexico (LaJeunesse et al. 2008) and in the Galápagos Islands, Ecuador (Baker 1999; Pinzón and LaJeunesse 2011), as well as on Clipperton Atoll (Pinzón and LaJeunesse 2011, Pettay and LaJeunesse 2013). In these regions, Symbiodinium in clade C most commonly dominates scleractinian coral colonies, except in the genus Pocillopora, which commonly also hosts Symbiodinium in clade D, often at high abundance. Despite relatively higher diversity in clade D in the Indo-west Pacific, clade D in the ETP appears to be solely represented by a single “type” of Symbiodinium, D1, also referred to as S. glynni (LaJeunesse et al. 2010a). Symbiodinium B1 has also been observed in recovering branch tips of Pocillopora (LaJeunesse et al. 2010a). Using rt-PCR assays, Silverstein et al. (2012) detected members of clades A and B at low abundance in a variety of scleractinian corals from Panama and the Galápagos, but made no attempt to detect low abundance Symbiodinium in clades F and G, which have not yet been reported for the ETP. Although the dominant symbiont(s) within coral species are predictable to some extent, based on coral species, geographic location, and environment (see Chap. 14, Pinzón), the relative abundance of different symbionts can shift in response to seasonal cues or environmental disturbances, both acute and chronic (see Sect. 13.3.2). Since Symbiodinium genotypes can influence colony health and survival, these processes are key to understanding the potential trajectories of ETP reefs over the coming century.
13.2.2 Methodological Constraints in Detecting Symbiodinium
Symbiodinium diversity has been assessed from corals in the ETP using a variety of molecular methods including Restriction Fragment Length Polymorphisms (RFLP), Denaturing Gradient Gel Electrophoresis (DGGE), direct sequencing, and real-time PCR (rt-PCR) (Table 13.1). Markers that have been used in conjunction with these tools are multi-copy and include various regions of the ribosomal RNA gene, a non-coding region of the chloroplast psbA gene, as well as microsatellite loci, and the actin gene. These molecular approaches have been pivotal in understanding coral-algal symbioses because they revealed high genetic diversity within Symbiodinium (Stat et al. 2012), and previously unrecognized complexity in coral-algal associations. The sensitivity and taxonomic resolution of molecular assays applied to Symbiodinium communities have increased with time. Given this, comparisons of studies employing molecular assays of varied resolution (e.g., targeting clades, types, or sub-types) should be made conservatively and explicitly detail the approaches and markers involved. High-resolution approaches consistently report one or more symbiont variants present at low abundance within at least some surveyed host individuals, in addition to numerically dominant symbiont types (e.g., Mieog et al. 2007; Correa et al. 2009; McGinley et al. 2012; Silverstein et al. 2012; Cunning and Baker 2013; Thornhill et al. 2013). These detections must be interpreted carefully, given that Symbiodinium variants likely differ in terms of their impact (e.g., amount of photosynthates released, residence time) on the holobiont (Hill and Hill 2012), and that some symbionts detected at low abundance may represent ephemeral endosymbioses, transients in the coral gastrovascular cavity, or colony surface contaminants (Silverstein et al. 2012). Nevertheless, to obtain a complete understanding of coral-algal associations, both the numerically dominant symbionts, and those found at low abundance, should be characterized. Future research will confirm which (if any) low abundance symbionts impact holobiont physiology and ecology, and the time scales and contexts over which this may occur. Some of the major limitations in the detection of Symbiodinium using molecular techniques are briefly discussed below; for comprehensive discussion of technical details, the reader is referred to the individual papers cited.
All of the molecular approaches that have been applied to coral-algal symbioses in the ETP (Table 13.1) are potentially susceptible to PCR bias. By preferentially amplifying some Symbiodinium genetic sequence variants over others, PCR bias can skew assessments of the relative abundance of different symbiont types within individual hosts. Additionally, if some Symbiodinium types (e.g., based on ITS-2) contain a high copy number for a given gene relative to other types, this could also produce a bias by artificially inflating detections of a given symbiont variant.
Community fingerprinting techniques, such as RFLP and DGGE, which utilize conventional PCR amplicons, require caution in assessing the diversity of coral-algal symbioses because they do not necessarily detect community members present at low relative abundances (e.g., <5–20 % of the total community, Lien et al. 2007; Loram et al. 2007). In a relatively extreme case, DGGE was shown to detect Symbiodinium ITS-2 type D1 only when it comprised at least 10–30 % of the total community (LaJeunesse et al. 2008). In scleractinian corals, which typically host 1–2 million Symbiodinium cells per cm2 of coral surface tissue (Drew 1972), diverse symbionts might therefore be present at densities well in excess of 100,000 cells per cm2, yet remain undetected using these approaches.
The problem of failed detections is further exemplified, for the ETP in particular, in comparisons of RFLP and rt-PCR data from colonies of Pocillopora. Correa (2009) showed that 96 % (n = 204 of 213) of samples contained both C and D symbionts based on rt-PCR, but these mixed communities were detected in only 12 % (n = 26 of 213) of samples analyzed using RFLPs. This problem occurred even in samples that contained relatively even abundances of C and D symbionts (e.g., each clade comprised ~30 % or more of the total community). The overall RFLP type II error rate (178 errors in 204 assays) suggests that earlier studies (Glynn et al. 2001a, b; Baker et al. 2004) identifying Symbiodinium based on LSU rDNA may have underestimated the presence of mixed symbiont clades by nearly 90 % (Correa 2009). This suggests that: (1) RFLP type II errors in published datasets are likely more pervasive than previously inferred (e.g., Baker and Romanski 2007); and (2) some abundant symbiont variants have likely been ‘hidden in plain sight’ by early RFLP analysis, at least in ETP Pocillopora.
Real-time PCR provides a 1000–10,000-fold increase in detection sensitivity over conventional PCR methods (Mieog et al. 2007). Such high-resolution techniques present their own caveats in assessing Symbiodinium diversity in hospite, however. For example, rt-PCR can potentially detect Symbiodinium cells present as surface contaminants on colonies or within the guts of sampled hosts, calling into question the biological relevance of high CT value amplifications. Silverstein et al. (2012) applied a Symbiodinium-specific rt-PCR assay (Correa et al. 2009) to the azooxanthellate coral Tubastraea coccinea; only 2 % (1 out of 52) of the analyzed samples harbored sufficient surface contaminants or recently ingested cells to produce a positive detection for Symbiodinium. Although this ‘biological negative control’ does not exactly estimate false positive Symbiodinium detections for zooxanthellate corals, it does suggest that the vast majority of positive Symbiodinium detections using rt-PCR represent Symbiodinium that are not surface or gut contaminants. Nevertheless, careful use of biological and technical negative controls is important to establish appropriate detection thresholds using rt-PCR. Another constraint of rt-PCR is that detection is limited to the specific symbiont taxa queried by user-developed primers and probes, which are based on known diversity and have, to date, targeted relatively coarse levels of taxonomic resolution (i.e., clades, Correa et al. 2009; Cunning and Baker 2013). Clade-level detection is unable to resolve sub-clade diversity, which may correlate with important functional variation (see Sect. 13.2.3). Fortunately, rt-PCR assays can be developed to detect and quantify any target sequence variant (e.g., Cunning and Baker 2013), facilitating its application at any scale of taxonomic resolution.
Another advantage of rt-PCR analysis is its ability to quantify the abundance and/or proportion of symbiont types in mixed communities, including those that contain low abundance symbionts, enabling more detailed studies of dynamism and shuffling in symbiont communities over time. Absolute quantitation of different Symbiodinium variants remains difficult due to the multi-copy nature of the markers currently in use (Mieog et al. 2007) and unsatisfactory parameters for standardizing symbiont numbers (e.g., per cm2 or ng DNA). These issues have been addressed via the development of markers with lower copy number (e.g., actin) normalized to host cell numbers (Mieog et al. 2009; Cunning and Baker 2013). However, the copy number of these markers may still vary among taxa, and must therefore be quantified for each Symbiodinium type studied. Normalizing symbionts to host cells (e.g., S/H cell ratio in Mieog et al. 2009) provides a metric of symbiont abundance (i.e., density) that relative ratios do not, but these metrics must still be interpreted carefully due to the dynamic nature of host cells (Cunning and Baker 2014).
The most informative snapshot of a Symbiodinium community may be obtained by utilizing a combination of these approaches to attain both high sensitivity detection and fine-scale taxonomic resolution, along with some quantitative information. Although this may be achieved by rt-PCR assays that target specific Symbiodinium types, next-generation sequencing is poised to provide another step forward in allowing investigators to comprehensively and quantitatively assess Symbiodinium community structure (Kenkel et al. 2013; Green et al. 2014), but this has yet to be applied to the ETP.
13.2.3 Functional Differences in Symbiodinium
Different Symbiodinium taxa can have distinct physiological traits (Iglesias-Prieto and Trench 1994, 1997; Warner et al. 1999; Savage et al. 2002; Goulet et al. 2005; Loram et al. 2007), causing them to be differentially suited to varied environmental conditions. Based on these differences, they are often predictably distributed along gradients of light and temperature across reefs (Fabricius et al. 2004; Iglesias-Prieto et al. 2004) and even within single coral colonies (Rowan et al. 1997; Kemp et al. 2008). This intraspecific (and intracolonial) symbiont diversity can influence holobiont physiology in a number of ways.
One of the clearest differences among symbionts, at least from the perspective of their coral hosts, is that some Symbiodinium, particularly members of clade D, are heat-tolerant (Rowan 2004) and confer increased resistance to thermal bleaching (Rowan et al. 1997; Glynn et al. 2001b; Berkelmans and van Oppen 2006; LaJeunesse et al. 2008). In the ETP, Pocillopora colonies dominated by D1 symbionts are resistant to heat-induced bleaching compared to those dominated by clade C (Glynn et al. 2001b). D1 symbionts also protect corals from bleaching events triggered by high irradiance (LaJeunesse et al. 2007) and cold stress (LaJeunesse et al. 2010a; McGinley et al. 2012). The higher incidence of bleaching in shallow water, and the higher irradiance at these depths, may thus explain the depth gradient in D1 characterizing Panamanian Pocillopora (Fig. 13.2, see also Sect. 13.3.2). These thermal differences may also occur at finer taxonomic scales. Sampayo et al. (2008) showed that Stylophora pistillata hosting C78 and C8/a on the southern Great Barrier Reef (GBR) was more thermally tolerant than those hosting C79 and C35/a, suggesting that fine-scale differences in symbiont type might similarly affect physiological outcomes in ETP corals. Different multilocus genotypes within Symbiodinium D1 also show distinct geographic partitioning in the ETP even though their hosts are widely distributed, suggesting that certain symbiont clones may be functionally distinct (Pettay and LaJeunesse 2013). Other more subtle aspects of coral physiology may also be impacted by symbiont type. DeSalvo et al. (2010) showed that gene expression profiles in the host are highly dependent on the genetic identity of the symbiont, suggesting that symbionts may modulate host physiology in complex ways.
In addition to bleaching susceptibility, other aspects of coral fitness may be dependent on the algal taxa they host, including the transfer of photosynthetically fixed carbon to the host (Loram et al. 2007; Cantin et al. 2009), and coral growth rates. Little et al. (2004) found that juvenile Acropora millepora and Acropora tenuis on the GBR that were infected by Symbiodinium in clade D grew 2–3 times more slowly than juveniles infected with members of clade C, as measured by the rate of addition of new polyps over the first 6 months (Little et al. 2004). This growth differential was also found in adult corals of A. millepora containing clade D, which grew 29 % more slowly than conspecifics with clade C in controlled experimental conditions, and 38 % more slowly in the field (Jones and Berkelmans 2010). However, studies of Pocillopora in the ETP found that these tradeoffs were temperature-dependent, with increasing temperatures lessening, and eventually eliminating, the growth reduction among corals hosting clade D (Fig. 13.3, Cunning et al. 2014). These findings suggest a more nuanced view in which different symbionts vary in their environmental optima, which in turn influences rates of carbon translocation and the eventual growth of coral hosts (Cunning et al. 2015).
Growth rates of Pocillopora damicornis fragments dominated by either Symbiodinium in clade C and D corals grown at different temperatures over 55 weeks. Sample sizes for 26, 27.5, and 29 °C treatments were n = 177, 164, and 62 for clade C fragments, and n = 72, 66, and 50 for clade D fragments. Error bars represent SEM. Group means that do not share a letter are significantly different (p < 0.05). From Cunning et al. (2014)
13.3 Distribution of Symbiodinium in the ETP
13.3.1 Host Systematic Distribution
Most coral species in the ETP are dominated by closely related Symbiodinium in clade C that include ITS-2 types C1c, C1b-c, C1f, C1d and C1ee (Table 13.2; LaJeunesse et al. 2008). In addition, some coral species can be found dominated by variants of C66 or C75 (Porites panamensis) or D1 (Pocillopora spp.). Only one non-pocilloporid has been found containing high abundance of D1, a single colony of Porites lobata collected shortly after the 1997–98 bleaching event at Uva Island (Baker 1999). Pocilloporid corals in the ETP can be dominated by a variety of Symbiodinium in clade C (including types C1b-c, C1d, and C1ee), D1 (LaJeunesse et al. 2008, 2010a, b; Cunning et al. 2013), and even B1, albeit temporarily (LaJeunesse et al. 2010a). Mixtures of D1 with clade C-types have been detected by real-time PCR in over half of examined Pocillopora colonies, but typically involve dominance of one symbiont type with low background levels of one or more additional types (Fig. 13.4; Correa 2009; McGinley et al. 2012; Cunning and Baker 2013). Although the proportions of Pocillopora dominated by members of clade C or D may vary among locations, both holobiont combinations are common throughout the region, and readily co-occur across reefscapes (Fig. 13.5; Glynn et al. 2001b; LaJeunesse et al. 2008). Although species boundaries within the genus Pocillopora are not clearly established (see Chap. 14, Pinzón), there may be some patterns in the distribution of Symbiodinium types among host taxa. Pinzón and LaJeunesse (2011) found that different lineages of Pocillopora hosted distinct types of Symbiodinium within clade C, with “type 1” Pocillopora hosting Symbiodinium C1b-c, “type 2” hosting C1ee, and “type 3” hosting C1d. In addition, they suggested that only type 1 Pocillopora was able to associate with the stress tolerant Symbiodinium D1 based on DGGE fingerprinting of the ITS-2 region. However, additional sampling in a subsequent study revealed that both type 1 and 3 Pocillopora in Panama and the Galápagos routinely associated with Symbiodinium in clade D (based on rt-PCR of the actin gene, Cunning et al. 2013), suggesting that certain host-symbiont combinations that were not previously identified may have restricted or patchy distributions. Branch tips of Pocillopora in the ETP have also been found to be dominated by Symbiodinium B1 (a common symbiont of the anemone Aiptasia) during the early stages of recovery from cold water bleaching. However, these symbionts were no longer detected in the corals once fully recovered (LaJeunesse et al. 2010a), suggesting these symbionts may have opportunistically colonized bleaching tissue. Nevertheless, these various results indicate that Pocillopora in the ETP can form associations with diverse symbionts in at least three clades of Symbiodinium.
Community structure of Symbiodinium in Pocillopora from Panama. Cell ratio densities (symbiont to host cell ratios) of clade C and D symbionts show a high incidence of mixed communities (64.1 % of colonies) that are heavily dominated by one clade, with background populations of a second clade. Open triangles represent colonies categorized as C-dominated (99.6–100 % clade C), and filled triangles are D-dominated (87.6–100 % clade D). Dashed line represents equal amounts of clades C and D in a sample. From Cunning and Baker (2013)
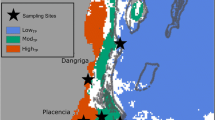
Reefscape distribution of Symbiodinium in Pocillopora at 2 m depth at Punta Galeras, Baja California, Mexico, and mosaic patterns of bleaching resulting from this distribution in response to spring bleaching in 2006. a Survey of 36 Pocillopora spp. colonies along a 25 m transect. Relative distances from each other, colony size, bleached or pigmented, and resident Symbiodinium taxon are given. Only those colonies surveyed are depicted. P. damicornis (Pd), P. verrucosa (Pv), P. meandrina (Pm); b a colony of P. damicornis is bleached while adjacent P. verrucosa exhibits “healthy” pigmentation. From LaJeunesse et al. (2007)
Other coral species in the ETP have not been studied as thoroughly as Pocillopora, but studies using high-resolution rt-PCR indicate that they also associate with multiple Symbiodinium clades (Table 13.2), although they are rarely (if ever) dominated by symbionts other than clade C. Surveys of symbiont diversity in other scleractinian species on Panamanian reefs have detected both clade C and clade D Symbiodinium in association with Gardineroseris planulata, Pavona clavus, Pavona gigantea, and Porites lobata, Porites panamensis, Pavona varians, and Psammocora superficialis (Silverstein et al. 2012; Cunning et al., unpub data). Members of clade A Symbiodinium have also been found in Panamanian P. gigantea, P. clavus, G. planulata, and P. panamensis, and clade B has been found in P. gigantea, P. clavus, and P. panamensis. Together, these findings suggest that the total diversity of Symbiodinium present in corals of the ETP (and elsewhere) extends beyond the dominant or most abundant symbionts typically characterized to date, and likely includes members of one or more additional clades present at low densities.
In addition to the symbionts of scleractinian corals on ETP reefs, additional zooxanthellate non-scleractinians also host a variety of Symbiodinium that add to the total pool of symbionts that occur in the region. As mentioned previously, the anemone Aiptasia hosts Symbiodinium B1, while anemones in the genus Isoaulactinia (=Bunodactis) and the zoanthid Zoanthus pacificus have both been found to host C66 (which is also found in the scleractinian Porites panamensis). Zoanthus pacificus has also been found hosting C29 and A12 (LaJeunesse et al. 2008). In contrast, zoanthids in the genera Palythoa and Protopalythoa host C1 and C1o, respectively (LaJeunesse et al. 2008), while the hydrozoan fire coral Millepora intricata hosts members of clade A (Baker 1999).
13.3.2 Environmental Control of Symbiodinium Distribution
ETP reefs show high variability in both temperature and water clarity, as well as large diurnal changes in irradiance due to extreme tidal ranges in some locations, such as Pacific Panama. While Pocillopora dominated by D1 or C1b-c co-occur throughout the ETP (with the exception of mainland Mexico, including Banderas Bay and Oaxaca, where only D1 has been reported, Baker 1999; LaJeunesse et al. 2010a), their relative dominance does appear to be related to environmental conditions at each location. High temperatures have been associated with clade D Symbiodinium (including D1 in the ETP), and areas that routinely experience high temperatures show high relative abundance of these symbionts (Baker 2003; Baker et al. 2004; Fabricius et al. 2004; Berkelmans and van Oppen 2006). Therefore, while some members of clade C may tolerate high seasonal temperature variability (e.g., C1b-c, LaJeunesse et al. 2010a), their sensitivity to extreme high temperatures, combined with the high thermal tolerance of D1, has resulted in dominance by D1 in areas that experienced severe thermal stress during El Niño bleaching events (LaJeunesse et al. 2010a; Cunning et al. 2013).
Depth-related trends in the distribution of Symbiodinium types in the Gulf of California (Iglesias-Prieto et al. 2004) suggest that stress-tolerant Symbiodinium D1 perform better in shallow, high light environments, whereas the more sensitive C1c performed better in deeper, low light environments. This niche differentiation between symbiont types was thought to drive the vertical distribution of two different host species at this site: Pocillopora verrucosa, which associated with clade D, was restricted to shallower habitats, while Pavona gigantea, which associated with clade C, was restricted to deeper habitats. Thus, host specificity may limit the vertical distribution of less flexible associations to the niche space occupied by the symbiont. However, D1 is also abundant in areas with relatively high turbidity and therefore lower light conditions (LaJeunesse et al. 2010a).
To date, intraspecific depth-related trends in Symbiodinium diversity have not been investigated in detail in the ETP, particularly for coral taxa that routinely host multiple symbiont types (such as Pocillopora). To remedy this, we analyzed 44 samples of Pocillopora damicornis across a depth gradient at Uva Island, Panama, using real-time PCR (Cunning and Baker 2013) to quantify the abundance of clades C and D Symbiodinium. We found that P. damicornis was more commonly dominated by clade D in shallow water compared to deeper water (Fig. 13.3). These patterns confirm intraspecific depth zonation in coral-algal symbiosis for ETP coral hosts (Rowan and Knowlton 1995) and suggest that abiotic factors, especially light and temperature, are important axes for niche diversification between different symbionts, influencing their distribution across reefscapes. However, differences in disturbance history, such as higher rates of bleaching and mortality at shallower depths, likely also contribute to the depth zonation of symbionts in P. damicornis.
13.3.3 Biogeographic and Taxonomic Gaps in Our Understanding of Symbiodinium Distribution in the ETP
Due to tectonic and hydrodynamic factors, it was originally thought that coral reefs were not present in the ETP (Darwin 1842; Dana 1843; see Chap. 1, Glynn). The discovery of structural coral reefs in Panama (Glynn 1972; Glynn et al. 1972; Porter 1972) marked the beginning of a period of scientific exploration in the region. However, despite sustained efforts to document and characterize ETP reefs (many of which are documented in this volume; see Chap. 5, Glynn et al.; Chap. 6, Toth et al.), studies of Symbiodinium diversity in the ETP have largely focused on the Gulf of California (Mexico), the Galápagos (Ecuador), and two areas in Panama (the Gulfs of Chiriquí and Panama). Almost all knowledge of ETP Symbiodinium distributions is based on studies from these areas (but see Table 13.2). Yet, well-developed reefs also exist off the Pacific coasts of Ecuador, Costa Rica, southern Mexico and Colombia (Cortés 2003). The least known reefs in the ETP, however, are arguably those within the “Pacific central American faunal gap” (Springer 1958), which includes Nicaragua, El Salvador, and Guatemala. Although coral framework was thought to be largely absent from the gap (e.g., Ryan and Zapata 2003), coral communities and developed reefs built mainly by Pavona gigantea, Gardineroseris planulata, and Pocillopora elegans were recently reported from Nicaragua (Alvarado et al. 2010). Additional data on Symbiodinium diversity should be obtained for these areas, as they may play an important role in the connectivity of ETP coral meta-populations in coastal Mexico, offshore areas such as the Revillagigedo Islands, and Central and South America (i.e., Costa Rica to Ecuador; see Chap. 16, Lessios and Baums). Additionally, coral-algal associations in the faunal gap may provide insights regarding how, and to what extent, these symbioses cope with naturally marginal environmental conditions, such as high turbidity in central Mexico (LaJeunesse et al. 2010a). Such associations may be useful proxies for the potential responses of conspecifics to analogous anthropogenic disturbances, such as eutrophication.
We are not aware of any data on Symbiodinium diversity or distribution from Guatemala, El Salvador, Nicaragua, Costa Rica, or Colombia. Symbiodinium diversity is also relatively unknown from ETP oceanographic islands, particularly Cocos Island, and Salas y Gómez and Easter (Rapa Nui) Islands (Chile), although limited Symbiodinium surveys have been conducted on pocilloporid hosts of the Revillagigedo Islands (LaJeunesse et al. 2010a) and Clipperton Atoll (LaJeunesse et al. 2010a; Pinzón and LaJeunesse 2011). Rapid surveys of these coral-dominated ecosystems, including documentation of coral-algal associations, should be performed in these relatively undescribed regions to generate baseline data necessary for habitat management, valuation, and protection. As one of the first shallow platforms in the ETP encountered by the North Equatorial Countercurrent (Glynn 1996), a higher (albeit still modest) proportion of coral-algal associations at Cocos Island may represent long-distance colonizers (Cortés and Jiménez 2003). Thus, Symbiodinium surveys from Cocos Island reefs are of particular interest.
Taxonomic gaps also exist in our knowledge of ETP Symbiodinium distributions. By far, most information regarding symbiont distributions comes from the dominant reef-building stony coral genus in the region, Pocillopora. Symbiodinium diversity within a few other ETP branching corals (e.g., Psammocora) has been documented, and some data are available for massive and encrusting coral species common to Panama, the Galápagos, and the Gulf of California (e.g., Porites lobata, Pavona gigantea, Pavona clavus, Baker 1999; Iglesias-Prieto et al. 2004; LaJeunesse et al. 2008; Glynn et al. 2009; see Table 13.2). Under-sampled ETP sites, however, contain some host species that are absent or rare elsewhere. These host species constitute another Symbiodinium frontier in the ETP. Host sampling gaps include Leptoseris papyracea (Costa Rica, Colombia, Ecuador), Leptoseris scabra (Ecuador), Pavona maldivensis (Colombia, Ecuador), Porites paschalensis (Easter Island or Rapa Nui), and even, potentially, Porites rus (Costa Rica) and Acropora valida (Colombia) (Cortés and Jiménez 2003; Glynn 2003; Glynn et al. 2003; Zapata and Vargas-Ángel 2003).
13.4 Stability Versus Change in the Distribution of Symbiodinium in the ETP
Because ETP reefs have been exposed to repetitive heat stress and experience gradients in pH across the region, they are excellent case studies for understanding the mechanisms by which corals might adapt or acclimatize to combined temperature and acidification stressors. In particular, since laboratory experiments cannot capture the long-term timescales over which projected changes are likely to occur, these real-world changes are more appropriate for identifying potential compensatory mechanisms. Symbiont community changes have been proposed as one way by which corals might respond to changing environments, including those occurring as a result of climate change (Buddemeier and Fautin 1993). These changes might occur as a result of dynamic change in community structure within individual colonies in response to changing environmental conditions, or as a result of differential mortality of hosts containing unsuitable symbionts. Baker et al. (2004) documented shifts to favor symbionts in clade D following large scale bleaching of Pocillopora during the 1997–98 El Niño warming event at Uva Island (Gulf of Chiriquí, Panama), and suggested that both symbiont “shuffling” as well as differential mortality, might account for these changes.
In contrast, in a study of cold-water bleaching and mortality, LaJeunesse et al. (2010a) found that relatively few of the surviving colonies experienced directional change in their symbiont communities, suggesting that differential mortality, rather than dynamic change on the colony level, resulted in the observed Symbiodinium distributions across the ETP. This was also suggested by McGinley et al. (2012), who used high resolution rt-PCR to study changes in the abundance of background symbionts and found that changes in symbiont dominance occurred in only 3 % of colonies following the same cold water bleaching event, suggesting that changes in symbiont communities were rare. Given these different findings, it seems likely that both mechanisms—natural selection (differential mortality and reproduction) of coral colonies with different symbionts, and dynamic change in symbiont communities within colonies—are likely to be important in understanding the distribution and stability of symbionts in reef corals over time and space. Responses may be expected to vary depending on whether bleaching was caused by high or low temperatures, with different responses across the ETP as a result.
13.5 The Future of Coral-Algal Symbiosis in the ETP
13.5.1 Interactions Between High Temperature and High CO2
ETP reefs are model systems for the effects of periodic high temperature stress under conditions of high CO2. Chronically depressed aragonite saturation states at some sites in the ETP are expected to decrease calcification rates (Langdon and Atkinson 2005; Manzello et al. 2008), resulting in slower coral growth and lower reef resilience (Anthony et al. 2011). At the same time, symbiont shifts to favor thermotolerant symbionts (Symbiodinium D1) may also lead to slower growth as a result of tradeoffs (see Sect. 13.2.3), although these tradeoffs may not be present at higher temperatures (Cunning et al. 2014). Nevertheless, investigating whether there is an interaction between ocean acidification (OA), symbiont type, and coral growth rate should be a high research priority, especially in the ETP. These interactions have not yet been explored in detail.
Studies of isolated Symbiodinium in culture have revealed significant variation among different types in their growth rates and photosynthetic capacities (Brading et al. 2011), but studies of how corals of the same species respond to OA if they have different symbiont types have not yet been undertaken in a controlled experiment. At three natural CO2 seeps in Papua New Guinea, no difference in the dominant symbiont type was documented in six coral species at the seep sites versus nearby control sites (Noonan et al. 2013). This suggests that high CO2 does not select for particular symbionts, and that, consequently, corals are unlikely to adapt or acclimatize to high CO2 environments by shifts in the composition of their symbiont communities. However, it also implies that high CO2 will not impede ongoing shifts to Symbiodinium D1 (due to increased warming and recurrent bleaching events) if these shifts are neutral from the point of view of high CO2.
The few studies undertaken to date suggest that OA effects on Symbiodinium distributions may not be dramatic, but controlled experiments are needed to properly test this idea. In addition, the interaction between high CO2 and high temperature in causing bleaching (Anthony et al. 2008) requires further research, especially in the ETP. It is possible that, instead of favoring particular symbiont types, high CO2 may increase the per-cell productivity of symbiont communities, resulting in higher symbiont productivity and lower overall skeletal density in the coral host. If this is the case, OA might actually increase the thermal tolerance of coral hosts because corals with relatively fewer symbionts may show less severe bleaching in response to heat stress (Cunning and Baker 2013). Similarly, the effect of high pCO2 on the rate of coral recovery from bleaching also represents a potential research question of particular relevance to the ETP.
13.5.2 Response of Reef Coral Symbioses to Climate Change : The Ratchet Hypothesis
Reef corals in the ETP represent an excellent “test bed” for how corals will respond to the combined effects of climate change, having already experienced multiple mass bleaching episodes as a result of recurring El Niño events, and also being subject to depressed aragonite saturation states as a result of regional upwelling conditions. Reefs throughout the region suffered severe mortality (52–97 %) following the 1982–83 El Niño event, but considerably less mortality (0–26.2 %) following the 1997–98 El Niño, despite the fact that the magnitude and duration of the two events were very similar (Enfield 2001; Glynn et al. 2001b). This suggests that the corals that survived and propagated on these reefs in the intervening ~15 years were considerably more thermotolerant than those that dominated reefs prior to 1982 (Baker 2002). This is probably a result of both differential mortality of thermally sensitive coral-symbiont combinations and increases in the abundance of thermotolerant symbionts within surviving colonies, with the relative importance of both of these mechanisms depending on site, species, stress exposure, and environmental history. Maynard et al. (2008) reached similar conclusions regarding the responses of Pocillopora damicornis, Acropora spp., and Porites spp. to two major thermal anomalies on the Great Barrier Reef (GBR). These corals experienced a major bleaching event in 1998, but during a severe thermal anomaly in 2002 bleached 30–100 % less than predicted. The mechanisms responsible for acclimatization/adaptation on the GBR were not directly observed, but Maynard et al. (2008) inferred that several mechanisms must have been acting in addition to differential mortality, because rates of mortality in 1998 were not well correlated with increases in thermal tolerance (measured as the difference between predicted and observed bleaching).
From a research perspective, what makes corals unusual is their ability to engage in mutualisms with diverse symbionts, and not the fact that they can experience differential mortality. Consequently, if dynamism in these symbiont communities is at least partially responsible for increased thermotolerance, then understanding how changes in symbiont communities occur, and the degree to which they are reversible, represent opportunities for insight into how corals might behave differently from other organisms, an element which is critical to projecting future survival trajectories for coral reefs. The apparent increase in the thermal tolerance of ETP corals between 1983 and 1997 suggests that, if mass bleaching resulted in corals that recovered with a higher abundance of thermotolerant symbionts, then these symbionts (Symbiodinium D1) must have remained in coral tissues at sufficient abundance over ~15 years to have had a measureable effect on the bleaching susceptibility and/or survivorship of corals in 1997–98. This suggests that, as bleaching events become more frequent and more severe, the abundance of Symbiodinium D1 may be subject to stepwise increases, with rapid increases in abundance following bleaching, and much slower declines when conditions return to normal. This “ratchet” mechanism may be one way in which coral communities might make the transition to thermotolerance without suffering the high levels of mortality which might be expected under scenarios of differential mortality and natural selection. Testing the ratchet hypothesis is a priority for further progress in this field, and additional studies are required that apply high-resolution methods to quantify change and persistence in symbiont communities before, during, and after a bleaching event. Furthermore, symbiont communities in the field should also be monitored over time using high-resolution methods, and any changes related to disturbance history and environmental conditions. Such studies are already underway in the ETP and continuing these efforts should be a priority research area for coral reef scientists interested in projecting reef futures for the coming century.
References
Alvarado JJ, Reyes-Bonilla H, Buitrago F, Aguirre-Rubí J, Álvarez del Castillo Cardenas PA (2010) Coral reefs of the Pacific coast of Nicaragua. Coral Reefs 29:201
Anthony KRN, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O (2008) Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc Natl Acad Sci USA 105:17442–17446
Anthony KRN, Maynard JA, Diaz-Pulido G, Mumby PJ, Marshall PA, Cao L, Hoegh-Guldberg O (2011) Ocean acidification and warming will lower coral reef resilience. Glob Change Biol 17(5):1798–1808
Baker AC (1999) Symbiosis ecology of reef-building corals. Ph.D. dissert, Univ Miami, Coral Gables, Miami, p 120
Baker AC (2003) Flexibility and specificity in coral-algal symbiosis: diversity, ecology, and biogeography of Symbiodinium. Annu Rev Ecol Evol Syst 34:661–689
Baker AC, Romanski AM (2007) Multiple symbiotic partnerships are common in scleractinian corals, but not in octocorals: comment on Goulet (2006). Mar Ecol Prog Ser 335:237–242
Baker AC, Rowan R (1997) Diversity of symbiotic dinoflagellates (zooxanthellae) in scleractinian corals of the Caribbean and eastern Pacific. In: Proceedings of 8th International Coral Reef Symposium, vol 1, Panama, pp 1301–1306
Baker AC (2002) Is bleaching really adaptive? Nature 415:601–602
Baker AC, Starger CJ, McClanahan TR, Glynn PW (2004) Corals’ adaptive response to climate change. Nature 430:741
Baums IB, Boulay JN, Polato NR, Hellberg ME (2012) No gene flow across the Eastern Pacific Barrier in the reef-building coral Porites lobata. Mol Ecol 21:5418–5433
Berkelmans R, van Oppen MJH (2006) The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proc R Soc B 273:2305–2312
Brading P, Warner ME, Davey P, Smith DJ, Achterberg EP et al (2011) Differential effects of ocean acidification on growth and photosynthesis among phylotypes of Symbiodinium (Dinophyceae). Limnol Oceanogr 56:927–938
Buddemeier RW, Fautin DG (1993) Coral bleaching as an adaptive mechanism. Bioscience 43:320–326
Cantin NE, van Oppen MJH, Willis BL, Mieog JC, Negri AP (2009) Juvenile corals can acquire more carbon from high-performance algal symbionts. Coral Reefs 28:405–414
Coates AG, Obando JA (1996) The geologic evolution of the Central American Isthmus. In: Jackson JBC, Budd AF, Coates AG (eds) Evolution and environment in tropical America. University of Chicago Press, Chicago, pp 21–56
Correa AMS (2009) Molecular ecology of endosymbiotic dinoflagellates (genus Symbiodinium) in scleractinian corals. Ph.D. dissert, Columbia University, New York, p 235
Correa AMS, McDonald MD, Baker AC (2009) Development of clade-specific Symbiodinium primers for quantitative PCR (qPCR) and their application to detecting clade D symbionts in Caribbean corals. Mar Biol 156:2403–2411
Cortés J (ed) (2003) Latin American coral reefs. Elsevier Science BV, Amsterdam p 497
Cortés J, Jiménez C (2003) Corals and coral reefs of the Pacific of Costa Rica: history, research and status. In: Cortes J (ed) Latin American coral reefs. Elsevier Science BV, Amsterdam, pp 361–385
Cunning R, Baker AC (2013) Excess algal symbionts increase the susceptibility of reef corals to bleaching. Nat Clim Change 3:259–262
Cunning R, Baker AC (2014) Not just who, but how many: the importance of partner abundance in reef coral symbioses. Front Microbiol 5:400
Cunning R, Glynn PW, Baker AC (2013) Flexible associations between Pocillopora corals and Symbiodinium limit utility of symbiosis ecology in defining species. Coral Reefs. doi:10.1007/s00338-013-1036-y
Cunning R, Gillette P, Capo T, Galvez K, Baker AC (2014) Growth tradeoffs associated with thermotolerant symbionts in the coral Pocillopora damicornis are lost in warmer oceans. Coral Reefs. doi:10.1007/s00338-014-1216-4
Cunning R, Vaughan N, Gillette P, Capo T, Maté J, Baker AC (2015) Dynamic regulation of partner abundance mediates response of reef coral symbioses to environmental change. Ecology 96:1411–1420
Dana JD (1843) On the temperature limiting the distribution of corals. Am J Sci Arts 45:130–131
Dana T (1975) Development of contemporary eastern Pacific coral reefs. Mar Biol 33:355–374
Darius HT, Dauga C, Grimont PAD, Chungue E, Martin PMV (1998) Diversity in symbiotic dinoflagellates (Pyrrhophyta) from seven scleractinian coral species: restriction enzyme analysis of small subunit ribosomal RNA genes. J Eukaryot Microbiol 45:619–627
Darius HT, Martin PMV, Grimont PAD, Dauga C (2000) Small subunit rDNA sequence analysis of symbiotic dinoflagellates from seven scleractinian corals in a Tahitian lagoon. J Phycol 36:951–959
Darwin C (1842) The structure and distribution of coral reefs. Smith Elder & Co., London, p 214
DeSalvo MK, Sunagawa S, Fisher PL, Voolstra CR, Iglesias-Prieto R, Medina M (2010) Coral host transtriptomic states are correlated with Symbiodinium genotypes. Mol Ecol 19:1174–1186
Drew EA (1972) The biology and physiology of algal-invertebrate symbiosis. II. The density of algal cells in a number of hermatypic corals and alcyonarians from various depths. J Exp Mar Biol Ecol 9:71–75
Duque-Caro H (1990) Neogene stratigraphy, paleoceanography and paleobiogeography in northwest South America and evolution of the Panama Seaway. Palaeogeogr Palaeocl 77:203–234
Ekman S (1953) Zoogeography of the sea, 1967 edn. Sidgwick & Jackson Ltd., London 417
Enfield DB (2001) Evolution and historical perspective of the 1997–1998 El Niño-Southern oscillation event. Bull Mar Sci 69:7–25
Fabricius KE, Mieog JC, Colin PL, Idip D, van Oppen MJH (2004) Identity and diversity of coral endosymbionts (zooxanthellae) from three Palauan reefs with contrasting bleaching, temperature and shading histories. Mol Ecol 13:2445–2458
Franklin EC, Stat M, Pochon X, Putnam H, Gates RD (2012) GeoSymbio: a hybrid, cloud-based web application of global geospatial bioinformatics and ecoinformatics for Symbiodinium-host symbioses. Mol Ecol Resour 12:369–373
Glynn PW (1972) Observations on the ecology of the Caribbean and Pacific coasts of Panama. In: Jones ML (ed) The Panamic biota: some observations prior to a sea-level canal, vol 2, pp 13–30 (Bull Biol Soc Wash)
Glynn PW (1984) Widespread coral mortality and the 1982–83 El Niño warming event. Environ Conserv 11:133–146
Glynn PW (ed) (1996) Coral reefs of the eastern Pacific. Coral Reefs 15:69
Glynn PW (2003) Coral communities and coral reefs of Ecuador. In: Cortés J (ed) Latin American coral reefs. Elsevier Sci BV, Amsterdam, pp 449–472
Glynn PW, Ault JS (2000) A biogeographic analysis and review of the far eastern Pacific coral reef region. Coral Reefs 19:1–23
Glynn PW, Colgan MW (1992) Sporadic disturbances in fluctuating coral reef environments—El Niño and coral reef development in the eastern Pacific. Am Zool 32:707–718
Glynn PW, Stewart RH, McCosker JE (1972) Pacific coral reefs of Panama: structure, distribution and predators. Geol Rundsch 61:483–519
Glynn PW, Maté JL, Baker AC, Calderón MO (2001a) Coral bleaching and mortality in Panama and Ecuador during the 1997–1998 El Niño-Southern Oscillation event: spatial/temporal patterns and comparisons with the 1982–1983 event. Bull Mar Sci 69:79–109
Glynn PW, Maté JL, Stemann TA (2001b) Pavona chiriquiensis, a new species of zooxanthellate scleractinian coral (Cnidaria: Anthozoa: Agariciidae) from the eastern tropical Pacific. Bull Biol Soc Wash 10:210–225
Glynn PW, Wellington GM, Wieters EA, Navarrete SA (2003) Reef-building coral communities of Easter Island (Rapa Nui), Chile. In: Cortés J (ed) Latin American coral reefs. Elsevier Sci BV, Amsterdam, pp 473–494
Glynn PW, Riegl B, Correa AMS, Baums IB (2009) Rapid recovery of a coral reef at Darwin Island, Galápagos Islands. Galápagos Res 66:6–13
Goulet TL, Cook CB, Goulet D (2005) Effect of short-term exposure to elevated temperatures and light levels on photosynthesis of different host-symbiont combinations in the Aiptasia pallida/Symbiodinium symbiosis. Limnol Oceanogr 50:1490–1498
Green E, Davies SW, Matz MV, Medina M (2014) Next-generation sequencing reveals cryptic Symbiodinium diversity within Orbicella faveolata and Orbicella franksi at the Flower Garden Banks, Gulf of Mexico. PeerJ PrePrints 2:e246v1
Grigg RW, Hey R (1992) Paleoceanography of the tropical eastern Pacific Ocean. Science 255:172–178
Hill M, Hill A (2012) The magnesium inhibition and arrested phagosome hypotheses: new perspectives on the evolution and ecology of Symbiodinium symbioses. Biol Rev 87(804–821):804. doi:10.1111/j.1469-185X.2012.00223.x
Iglesias-Prieto R, Trench RK (1994) Acclimation and adaptation to irradiance in symbiotic dinoflagellates. I. Responses of the photosynthetic unit to changes in photon flux density. Mar Ecol Prog Ser 113:163–175
Iglesias-Prieto R, Trench RK (1997) Acclimation and adaptation to irradiance in symbiotic dinoflagellates. II. Response of chlorophyll-protein complexes to different photon-flux densities. Mar Biol 130:23–33
Iglesias-Prieto R, Beltrán VH, LaJeunesse TC, Reyes-Bonilla H, Thomé PE (2004) Different algal symbionts explain the vertical distribution of dominant reef corals in the eastern Pacific. Proc Roy Soc Lond Ser B 271:1757–1763
IPCC (2007) The physical science basis. In: Solomon S et al (eds) Contribution of working group 1 to the fourth assessment report of the Intergovernmental Panel on climate change. Cambridge University Press, Cambridge, UK, New York
Jones A, Berkelmans R (2010) Potential costs of acclimatization to a warmer climate: growth of a reef coral with heat tolerant vs. sensitive symbiont types. PLoS One 5:1–9
Jumpponen A, Jones KL (2009) Massively parallel 454 sequencing indicates hyperdiverse fungal communities in temperate Quercus macrocarpa phyllosphere. New Phytol 184:438–448
Kemp DW, Fitt WK, Schmidt GW (2008) A microsampling method for genotyping coral symbionts. Coral Reefs 27:289–293
Kenkel CD, Goodbody-Gringley G, Caillaud D, Davies SW, Bartels E, Matz MV (2013) Evidence for a host role in thermotolerance divergence between populations of the mustard hill coral (Porites astreoides) from different reef environments. Mol Ecol 22:4335–4348
LaJeunesse TC (2005) “Species” radiations of symbiotic dinoflagellates in the Atlantic and Indo-Pacific since the Miocene-Pliocene transition. Mol Biol Evol 22:570–581
LaJeunesse TC, Thornhill DJ (2011) Improved resolution of reef-coral endosymbiont (Symbiodinium) species diversity, ecology, and evolution through psbA non-coding region genotyping. PLoS One 6:e29013
LaJeunesse TC, Loh WKW, van Woesik R, Hoegh-Guldberg O, Schmidt GW, Fitt WK (2003) Low symbiont diversity in southern Great Barrier Reef corals relative to those of the Caribbean. Limnol Oceanogr 48:2046–2054
LaJeunesse TC, Reyes-Bonilla H, Warner ME (2007) Spring “bleaching” among Pocillopora in the Sea of Cortez, eastern Pacific. Coral Reefs 26:265–270
LaJeunesse TC, Reyes-Bonilla H, Warner ME, Wills M, Schmidt GW, Fitt WK (2008) Specificity and stability in high latitude eastern Pacific coral-algal symbioses. Limnol Oceanogr 53:719–727
LaJeunesse TC, Pettay T, Phongsuwan N, Brown B, Obura D, Hoegh-Guldberg O, Fitt WK (2010a) Long-standing environmental conditions, geographic isolation and host-symbiont specificity influence the relative ecological dominance and genetic diversification of coral endosymbionts in the genus Symbiodinium. J Biogeogr 37:785–800
LaJeunesse TC, Smith R, Walther M, Pinzón J, Pettay DT, McGinley M et al (2010b) Host-symbiont recombination versus natural selection in the response of coral-dinoflagellate symbioses to environmental disturbance. Proc Roy Soc B-Biol Sci 277:2925–2934
Langdon C, Atkinson MJ (2005) Effects of elevated pCO2 on photosynthesis and calcification of corals and interactions with seasonal change in temperature/irradiance and nutrient enrichment. J Geophys Res 110:C09S07
Lien Y-T, Nakano Y, Plathong S, Fukami H, Wang J-T, Chen CA (2007) Occurrence of the putatively heat-tolerant Symbiodinium phylotype D in high-latitudinal outlying coral communities. Coral Reefs 26:35–44
Little A, van Oppen MJH, Willis BL (2004) Flexibility in algal endosymbioses shapes growth in reef corals. Science 304:1492
Loram JE, Boonham N, O’Toole P, Trapido-Rosenthal HG, Douglas AE (2007) Molecular quantification of symbiotic dinoflagellate algae of the genus Symbiodinium. Biol Bull 212:259–268
Manzello DP, Kleypas JA, Budd DA, Eakin CM, Glynn PW, Langdon C (2008) Poorly cemented coral reefs of the eastern tropical Pacific: possible insights into reef development in a high-CO2 world. Proc Nat Acad Sci USA 105:10450–10455
Maté JL (2003) Corals and coral reefs of the Pacific coast of Panama. In: Cortés J (ed) Latin American coral reefs. Elsevier Science BV, Amsterdam, pp 387–417
Maynard JA, Anthony KRN, Marshall PA, Masiri I (2008) Major bleaching events can lead to increased thermal tolerance in corals. Mar Biol 155:173–182
McGinley MP, Aschaffenburg MD, Pettay DT, Smith RT, LaJeunesse TC, Warner ME (2012) Symbiodinium spp. in colonies of eastern Pacific Pocillopora spp. are highly stable despite the prevalence of low-abundance background populations. Mar Ecol Prog Ser 462:1–7
Mieog JC, van Oppen MJH, Cantin NC, Stam WT, Olsen JL (2007) Real-time PCR reveals a high incidence of Symbiodinium clade D at low levels in four scleractinian corals across the Great Barrier Reef: implications for symbiont shuffling. Coral Reefs 26:449–457
Mieog JC, van Oppen MJH, Berkelmans R, Stam WT, Olsen JL (2009) Quantification of algal endosymbionts (Symbiodinium) in coral tissue using real-time PCR. Mol Ecol Resour 9:74–82
Noonan SHC, Fabricius KE, Humphrey C (2013) Symbiodinium community composition in scleractinian corals is not affected by life-long exposure to elevated carbon dioxide. PLoS One 8(5):e63985. doi:10.1371/journal.pone.0063985
Pettay DT, LaJeunesse TC (2013) Long-range dispersal and high-latitude environments influence the population structure of a ‘stress-tolerant’ dinoflagellate endosymbiont. PLoS One 8:e79208
Pettay DT, Wham DC, Pinzón JH, LaJeunesse TC (2011) Genotypic diversity and spatial-temporal distribution of Symbiodinium clones in an abundant reef coral. Mol Ecol 20:5197–5212
Pinzón JH, LaJeunesse TC (2011) Species delimitation of common reef corals in the genus Pocillopora using nucleotide sequence phylogenies, population genetics and symbiosis ecology. Mol Ecol 20:311–325
Pochon X, Gates RD (2010) A new Symbiodinium clade (Dinophyceae) from soritid foraminifera in Hawai’i. Mol Phylogenet Evol 56:492–497
Porter JW (1972) Ecology and species diversity of coral reefs on opposite sides of the Isthmus of Panamá. In Jones ML (ed) The Panamic biota: some observations prior to a sea level canal. Bull Biol Soc Wash 2:89–116
Rodriguez-Lanetty M, Cha H, Song JI (2002) Genetic diversity of symbiotic dinoflagellates associated with anthozoans from Korean waters. In: Proceedings of 9th International Coral Reef Symposium, vol 1, Bali, pp 163–166
Rowan R (2004) Coral bleaching: thermal adaptation in reef coral symbionts. Nature 430:742
Rowan R, Powers DA (1991a) A molecular genetic classification of zooxanthellae and the evolution of animal-algal symbiosis. Science 251:1348–1351
Rowan R, Powers DA (1991b) Molecular genetic identification of symbiotic dinoflagellates (zooxanthellae). Mar Ecol Prog Ser 71:65–73
Rowan R, Knowlton N (1995) Intraspecific diversity and ecological zonation in coral-algal symbiosis. Proc Nat Acad Sci USA 92:2850–2853
Rowan R, Knowlton N, Baker AC, Jara J (1997) Landscape ecology of algal symbiont communities explains variation in episodes of coral bleaching. Nature 388:265–269
Ryan J, Zapata Y (2003) Nicaragua’s coral reefs: status, health and management strategies. In: Cortés J (ed) Latin American coral reefs. Elsevier Sci BV, Amsterdam, pp 203–222
Sampayo EM, Ridgway T, Bongaerts P, Hoegh-Guldberg O (2008) Bleaching susceptibility and mortality of corals are determined by fine-scale differences in symbiont type. Proc Nat Acad Sci USA 105:10444–10449
Savage A, Trapido-Rosenthal HG, Douglas AE (2002) On the functional significance of molecular variation in Symbiodinium, the symbiotic algae of Cnidaria: photosynthetic response to irradiance. Mar Ecol Prog Ser 244:27–37
Silverstein RN, Correa AMS, Baker AC (2012) Specificity is rarely absolute in coral-algal symbiosis: implications for coral response to climate change. Proc Roy Soc B Biol Sci 279:2609–2618
Springer VG (1958) Systematics and zoogeography of the clinid fishes of the subtribe Labrisomini Hubbs. PhD dissert, University of Texas, Austin, Texas, p 226
Stat M, Baker AC, Bourne D, Correa AMS, Forsman Z, Huggett M, Pochon X, Skillings D, Toonen R, van Oppen MJH, Gates RD (2012) Molecular delineation of species in the coral holobiont. Adv Mar Biol 63:1–65
Thornhill DJ, Xiang Y, Pettay DT, Zhong M, Santos SR (2013) Population genetic data of a model symbiotic cnidarian system reveal remarkable symbiotic specificity and vectored introductions across ocean basins. Mol Ecol 22:4499–4515
Toth LT, Aronson RB, Vollmer SV, Hobbs JW, Urrego DH, Cheng H et al (2012) ENSO drove 2500-year collapse of eastern Pacific coral reefs. Science 337:81–84
van Oppen MJH, Baker AC, Coffroth MA, Willis BL (2009) Bleaching resistance and the role of algal endosymbionts. In: van Oppen MJH, Lough JM (eds) Coral bleaching. Springer, Berlin, Heidelberg, pp 83–102
Warner ME, Fitt WK, Schmidt GW (1999) Damage to photosystem II in symbiotic dinoflagellates: a determinant of coral bleaching. Proc Nat Acad Sci USA 96:8007–8012
Zapata FA, Vargas-Ángel B (2003) Corals and coral reefs of the Pacific coast of Colombia. In: Cortés J (ed) Latin American coral reefs. Elsevier Sci BV, Amsterdam, pp 419–447
Acknowledgements
We dedicate this chapter to Peter Glynn for first introducing us to coral reef ecosystems of the eastern tropical Pacific, and for his guidance and friendship over many years. A.B. also thanks N. Knowlton and R. Rowan for their long-lasting impact as mentors in Panama. We thank I. Enochs, P. Fong, J. Jara, D. Manzello, J. Maté, and T. Smith for field support, K. Dziedzic for laboratory assistance, and J. Maté for long-term permit and logistical support. This work was supported by grants from the National Science Foundation (BIO-OCE 0099301, 0526361, and 0547169), the Wildlife Conservation Society (Tiffany & Co. Foundation), and the Lenfest Ocean Program. It was also supported by a University of Miami Fellowship and NSF Graduate Research Fellowship (to R.C.), and a Columbia University Graduate Fellowship (to A.M.S.C.). No corals were harmed during the writing of this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Baker, A.C., Correa, A.M.S., Cunning, R. (2017). Diversity, Distribution and Stability of Symbiodinium in Reef Corals of the Eastern Tropical Pacific. In: Glynn, P., Manzello, D., Enochs, I. (eds) Coral Reefs of the Eastern Tropical Pacific. Coral Reefs of the World, vol 8. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7499-4_13
Download citation
DOI: https://doi.org/10.1007/978-94-017-7499-4_13
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7498-7
Online ISBN: 978-94-017-7499-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)