Abstract
Although important efforts were carried out during the past decades in Brazil to understand and control snakebite envenomings, important gaps remain for the fulfillment of these goals, particularly in the Amazon region. Bothrops atrox is the most important venomous snake in the Brazilian Amazon, causing 80–90% of the snake envenomings in the region. In the Brazilian Amazon, Bothrops envenoming shows pain, swelling, regional lymphadenopathy, ecchymosis, blistering, and necrosis as the most common local clinical manifestations. Secondary bacterial infections were observed in around 40% of the Bothrops snakebites. Spontaneous systemic bleeding and acute renal failure are common systemic complications after Bothrops envenomings. It is difficult for riverine and indigenous populations to reach health centers for treatment of snakebites. As a result, the number of cases detected officially is probably underestimated. Current antivenoms (AVs) require conservation in adequate facilities, which are not always available in remote settings. In addition, training of multidisciplinary teams is not always appropriate for indigenous health services regarding AV administration, side effect management, and case monitoring and surveillance. Although clinical research related to venomous animal injuries has increased, most publications are based on case reports and lack methodological rigor. Moreover, outcome definitions, such as severity ranking criteria, were empirically established, making the results even less generalizable. Clinical research from hospital-based studies and community observational studies are needed. In addition to all the above recommendations, the importance of international cooperative efforts toward the control of these neglected health problems through international partnerships, namely, with other Amazonian countries, is highlighted.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
Keywords
Introduction
Snakebites impose a high burden worldwide and result in considerable social and economic impact. It is estimated that snakebite rates are as high as over 1.8 million cases per year, with associated deaths reaching more than 90,000 cases annually. However, snakebites are a neglected condition with no associated World Health Organization (WHO) programs for control and prevention. Countries most affected by snakebites are those located in the tropical zone with areas of high rates of field use for agriculture where the main affected populations are adult men working in agricultural activities. In Brazil, the Ministry of Health implemented the National Program for Snakebites Control in 1986, extended to other poisonous animals in 1988. Since then, antivenom (AV) production has been standardized, and all the AV production from the three national laboratories (Instituto Butantan, Fundação Ezequiel Dias, and Instituto Vital Brazil) has been acquired by the Ministry of Health for free-of-charge distribution to patients. Five types of snake AVs are currently available in Brazil: Bothrops AV (main one), Crotalus AV, Bothrops-Crotalus AV, Bothrops-Lachesis AV, and Micrurus AV.
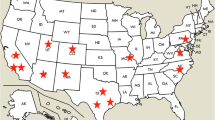
The Amazon rainforest covers several countries such as Bolivia, Peru, Ecuador, Colombia, Venezuela, Guyana, Suriname, and French Guiana. Studies conducted in these countries linking snakebites cases with species distribution have described similar epidemiological features. In the Brazilian Amazon, snakebites appear among the most important envenomations with the higher incidence (52.6/100,000 inhabitants). In 2013, the Brazilian Ministry of Health reported about 27,181 cases of snakebites (Saúde 2014). In the northern state of Roraima, eastern Pará, and Amapá, incidences higher than 100 cases per 100,000 inhabitants have been showed (Fig. 1). Bothrops atrox is largely responsible for bites in this region, with more than 80% of the reported cases, while Lachesis, Crotalus, and Micrurus species are secondary agents of envenomings (Feitosa et al. 2015). Spatial distribution of snakes is strongly influenced by Amazon ecosystem diversity. Surveys on species distribution and frequency, ecological structure, and other particular features about those venomous species must be carried out in order to improve health services (Fan et al. 2015). Incidence increase has been suggested to be associated with the rainy season. Since water volume is large, snakes use to seek for drier shelters, generally closer to human settlements in rural areas. In addition, urban sprawl along with deforestation causes major influence on the natural habitat of these animals, exerting pressure for animal migration and consequently leading to accidental snakebites (Bernarde and Gomes 2012).
Spatial distribution of snakebites in the Brazilian Amazon. Map were created using incidence per 100,000 inhabitants. Snakebites are largely distributed in the Amazonian states, with several counties presenting incidences higher than 100 cases per 100,000 inhabitants, especially in northern Roraima, eastern Pará, and Amapá and in unevenly distributed municipalities across all states
A few studies concerning this issue are available in the Amazon region; nevertheless, such results indicate the following profile of the affected population: victims of snakebite are predominantly men, in working age, rural residents, riverine and indigenous, linking a major cause as occupational hazard for many of these work in agriculture, hunting, and forest activities, as in the case of rubber tappers (Feitosa et al. 2015). A study conducted with indigenous and riverine populations showed that 13% of them had experienced snakebite during their lifetime (Pierini et al. 1996). Severity has been mostly classified from mild to moderate cases, although severe cases had been reported in around 8% of the cases (Feitosa et al. 2015). The most affected parts of the body are usually the lower limbs (Feitosa et al. 2015; Pierini et al. 1996). The Amazon region has a reduced coverage of highways and roads, with much of the human displacement happening through river transportation, leading to a delay in medical care. Time increasing to more than 6 h to care has been associated with undesirable outcomes such as severity and mortality. Other risk factors associated with poor outcomes are older age and bites related to work activities (Feitosa et al. 2015).
Injury outcomes are sometimes used to be influenced by cultural behaviors. Riverine, indigenous, and rural people often use such devices as tourniquet and even chemicals such as alcohol (ingested and applied to the bite) attempting to reduce the venom effects. Herbal extracts are also largely used in snakebite episodes, especially where AVs are scarce or not available. Puncture and suction of the injury in an attempt to remove the venom, although not recommended, are common practices among patients. Although AV availability is recommended for high-incidence areas, there are some drawbacks regarding this issue. The lack of healthcare facilities in remote rural areas impairs the access to AV because it requires low temperatures for conservation in addition to skilled health professionals. Such difficulties, besides those already mentioned, lead people to seek alternative therapies.
Bothrops Envenomings in the Brazilian Amazon
Bothrops Snakes in the Brazilian Amazon
In the Brazilian Amazon region and surrounding cerrado areas, there are 12 species of pit vipers, belonging to the Bothrops and Bothrocophias genera. Five of them (Bothrops lutzi, B. marmoratus, B. mattogrossensis, B. moojeni, and B. pauloensis) are present only in cerrado areas, while the others are characteristic of the Amazon rainforest environments (Bothrops atrox, B. bilineatus, B. brazili, B. marajoensis, B. taeniatus, Bothrocophias hyoprora, and B. microphthalmus). Bothrops atrox also occurs in deforested areas (pastures and crops) and in urban environments (Bernarde 2014).
Bothrops atrox is the most important venomous snake in the Brazilian Amazon, causing 80–90% of the snake envenomings in the region (Fan et al. 2015). This species is widely distributed in the Amazon and is the most abundant venomous snake in this region. The size of adult specimens ranges from 1 to 1.5 m, with a record of up to 1.72 m. This species is present both in forested areas as well as in disturbed areas, such as pastures, crops, and urban areas. Bothrops atrox is active especially during the night, when adults often occur on the ground for expected hunting, while juveniles hunt on vegetation (up to 1.5 m height). Regarding food, this snake is generalist, feeding on centipedes, fishes, amphibians, lizards, other snakes, rodents, marsupials, and birds. Juveniles prefer to prey on ectothermic animals (frogs, lizards, and centipedes), and adults prefer to prey on endothermic animals, namely, rodents. As a viviparous species, this snake may give birth between 11 and 43 offsprings of 28–35 cm, found between December and February (Martins and Oliveira 1998). This snake is commonly known by different common names according to the area (jararaca, surucucu, surucucu-do-barranco, boca-podre, and comboia). However, there is a possible confounding factor in snake identification by the local population since both Bothrops atrox and Lachesis muta receive the same popular name “surucucu” in certain Amazonian areas (Fan et al. 2015).
Popularly known as green pit viper or parrot’s beak jararaca, Bothrops bilineatus stands out for being relatively abundant in some regions and to present arboreal habits, which contributes to their bite that reaches the upper regions of the body of the victim. Two subspecies of the green pit viper are present in the Amazon: B. b. bilineatus is present in the states of Amazonas, Roraima, Amapá, Pará, south of Rondônia, and northern Mato Grosso; B. b. smaragdinus occurs in the states of Acre, Amazonas (Purus River basin), and Rondônia (northern state) (Bernarde et al. 2011a). Adult specimens are between 70 cm and 1 m and, same as the juveniles, have arboreal habits. This snake inhabits primary and secondary forests, especially near water courses, and is less common in anthropogenic environments (Bernarde 2014). Their prey consists mainly of rodents, amphibians, birds, snakes, and lizards. It is viviparous like Bothrops species, giving birth between 6 and 16 offsprings.
Bothrops brazili, the red pit viper, and B. taeniatus, the gray pit viper, occur less frequently in the Amazonian biome. Bothrocophias pit vipers are little frequent and have few records in the Brazilian Amazon. Bothrocophias hyoprora, the big-nose pit viper, has few records from the states of Acre, Amazonas, Rondônia, Mato Grosso, and Pará (Bernarde et al. 2011b), and B. microphthalmus has a single record for Brazil in the state of Rondônia (Bernarde 2012).
Main Bothrops species involved in biting humans in the Brazilian Amazon are shown in Fig. 2.
Main Bothrops species involved in biting humans in the Brazilian Amazon (a–f). Bothrops atrox (a and b) is implicated in most of the human snakebites registered in the Brazilian Amazon region (80–90 %). The species Bothrops bilineatus (c), Bothrops brazili (d), Bothrops taeniatus (e), and Bothrocophias hyoprora (f) present secondary medical importance in the region
Toxinology
Despite the wide geographic distribution in the Amazon, B. atrox venoms share the same family of toxins, such as PIII and PI snake venom metalloproteinase, phospholipase A2, serine proteinase, cysteine-rich secretory protein, L-amino acid oxidase, and C-type lectin-like (Calvete et al. 2011; López-Lozano et al. 2002). A bradykinin-potentiating peptide from B. atrox venom was also identified (Coutinho-Neto et al. 2013). The variability in venom composition of B. atrox from different geographical origins is mostly related to the expression level of each family of toxins than to the presence or absence of major families of toxins (Calvete et al. 2011; López-Lozano et al. 2002). B. atrox venoms from Colombia and Venezuela show an ontogenetic toxin profile, with PI metalloproteinases and phospholipases representing the most abundant toxins (Calvete et al. 2011). Venoms from Brazil, Ecuador, and Peru show a pedomorphic phenotype, with PIII metalloproteinases being the most abundant toxins (Núñez et al. 2009). Transcriptomic analysis of the B. atrox venom gland from Brazil indicates a predominance of transcripts encoding mainly metalloproteinases (Neiva et al. 2009). The biological activities of B. atrox venom can be correlated with geographical distribution and ontogenetic stage of the snake (newborn, juvenile, and adult snakes), as has been observed in venoms from Colombia and Brazil (López-Lozano et al. 2002). One speculates that differences in the expression level of each family of toxins and biological activities of B. atrox venom could explain the clinical manifestations observed in victims of bite caused by this species in different regions of the Amazon (Sousa et al. 2013).
The composition of venom from other Bothrops snakes in the Brazilian Amazon should be further elucidated. However, some components with coagulant activity have been described in the venom of B. brazili, B. marajoensis, and B. moojeni (Assakura et al. 1992).
Pathophysiology
Bothrops venom is characterized by three main pathophysiological activities: coagulant, hemorrhagic, and proteolytic or acute inflammatory effects. The coagulating activity of the B. atrox venom results from components of the venom with thrombin-like activity, which directly hydrolyzes fibrinogen in fibrin and procoagulant activity, which activate factors II and X of the coagulation, resulting in the formation of endogenous thrombin. Other clotting factors activated by components isolated of B. atrox venom are the factors XIII and V (Assakura et al. 1992; López-Lozano et al. 2002). B. marajoensis and B. hyoprora venoms have coagulant activity on plasma and fibrinogen (Assakura et al. 1992). Components with thrombin-like activity were isolated from the B. brazili venom. Components that act on platelets function were also isolated from the B. atrox venom (Freitas-De-Sousa et al. 2015).
Hemorrhagic activity has been observed in the B. atrox and B. marajoensis venoms (Assakura et al. 1992; Freitas-De-Sousa et al. 2015; Sousa et al. 2013). A PI metalloproteinase, called batroxase, isolated from B. atrox venom, has fibrinolytic and thrombolytic activities and induces weak bleeding through the digestion of the extracellular matrix components such as laminin, type IV collagen, and fibronectin (Jacob-Ferreira et al. 2016). The batroxrhagin, isolated from B. atrox venom, also induces bleeding (Freitas-De-Sousa et al. 2015), as well as atroxlysin-I, a PI metalloproteinase (Sanchez et al. 2010). Compounds with thrombolytic activity were found in the B. atrox venom (Jacob-Ferreira et al. 2016).
The proteolytic or acute inflammatory activity induced by B. atrox venom causes plasma extravasation; migration of leukocytes; vascular wall lesion, which results in bleeding; and musculoskeletal disruption (Moreira et al. 2012). Phospholipases A2, namely, BaPLA2I and BaPLA2III, which cause edema and myonecrosis, were isolated from B. atrox venom (Kanashiro et al. 2002). Besides these, a myotoxin isolated from B. atrox venom also induces edema and myonecrosis (Núñez et al. 2004). A genotoxic potential has been observed in B. atrox and B. brazili venoms (Marcussi et al. 2013). Nephrotoxic compounds were identified in the B. marajoensis venom (Dantas et al. 2015).
In victims of B. atrox, envenoming can be observed with coagulation disorders, such as hypofibrinogenemia, fibrinolytic system activation, and intravascular thrombin generation, resulting in blood incoagulability (Otero et al. 1996; Pardal et al. 2004). A study carried out in Belém, Pará State, Brazil, verified that approximately 10% of the victims have thrombocytopenia (Pardal et al. 2004). On the other hand, aggregating activity on rabbit’s washed platelet was not observed in B. atrox venom experimentally (Francischetti et al. 1998). The platelet function disorders in envenomings caused by B. atrox snake are not well described. Coagulation disorders, edema, and hemorrhage appear in envenomings as a result of the biological activities of B. atrox venom (Otero et al. 1996; Pardal et al. 2004).
Clinical Aspects
Bothrops envenomings cause local and, in a significant proportion, systemic manifestations, depending on the snake involved, characteristics of the victim, and circumstances of the injury. Snakebite diagnosis in general should consider epidemiological, clinical, and laboratorial aspects, which, when analyzed together, can lead clinicians to the probable perpetrating snake genus and to the correct interpretation of severity status and further therapeutic approach. Epidemiological diagnosis should consider the habitat, habits, and other information leading to snake identification as well as time, region of occurrence, and circumstances related to the accident. Importantly, clinical examination should be initiated by assessing the affected region to search for bite signs, especially fang marks that can be double or possibly only when only one prey is introduced. Bothrops snakebite may result in negligible or no envenoming, even if fang marks are visible (“dry bite”). This issue is important to be considered at the time of patient’s admission at the hospital to avoid giving antivenom when specific therapy may not be necessary. This may occur when a nonvenomous snake is implicated.
Local envenomation ranges from a painless reddened injury to intense pain and swelling at the site of bite, starting minutes after the event. Bleeding caused by traumatic injury due to fang introduction may be present. Local manifestations may increase progressively and may affect the whole limb. Enlargement of the regional lymph nodes draining the site of bite and bruising can also be observed some hours after bite, especially if patient delayed in reaching a health service (Pardal et al. 2004; Otero et al. 1996) (Fig. 3). In the first 24 h, blistering and tissue necrosis may be evident. Cellulitis or abscess occurs mostly in the moderate or severe cases, generally as a polymicrobial infection. Gram-negative bacteria have been implicated in secondary bacterial infection, which frequency may vary according to region. In Manaus, secondary bacterial infections were observed in around 40% of the Bothrops snakebites (Souza 2002) (Fig. 4). Necrosis of variable extension is more frequent when tourniquet is applied, associated with initial treatment with traditional healers, and delayed hospital admission resulting from problems with transportation. Although uncommon, compartment syndrome is a dangerous complication because of the potential ischemia, tissue necrosis, and neuropathy. Nonspecific symptoms such as headache, lethargy, weakness, nausea, and vomiting are often observed (Pardal et al. 2004; Souza 2002).
Local manifestations resulting from Bothrops snakebites. (a) Ulceration and local bleeding of the first finger of right foot with less than 12 h after envenoming. (b) Envenoming with blisters around the snakebite in the dorsal area of the right foot. (c) Envenoming on the hand; this patient arrived 12 h after the bite at the hospital, with swelling and serohemorrhagic blisters on left upper limb and incoagulable blood. (d) Envenoming in distal finger of the right ring finger with serohemorrhagic blisters 24 h after the bite. (e) Envenoming with intense swelling on right foot, local bleeding, and ecchymosis with purplish coloration on the first and second finger. (f) Severe snakebite with extensive swelling of the five segments of the left leg (from foot to thigh), less than 20 h after envenoming
Local complications resulting from Bothrops snakebites. (a) Envenoming on the left hand, patient with more than 48 h after the bite, with an extensive area of edema and necrosis in the left upper limb and gangrene of the fourth finger. (b) The same patient shown in A, after amputation of the fourth finger (in the healing phase). (c) Envenoming in the distal part of the little finger of the right hand with evolution to necrosis after 48 h after the bite. (d) Envenoming on dorsal region of the right hand spreading to hand palm, requiring surgical debridement of the necrosis area on 5 day after the bite. (e) Severe envenoming on the left hand; this patient arrived 24 h after the bite, presenting compartmental syndrome in the left upper limb, requiring fasciotomy. (f) Patient presenting abscess and cellulitis due to secondary bacterial infection, on the dorsal area of the left foot, 48 h after the bite
Symptoms and signs of systemic envenoming are mainly due to the incoagulable blood. Hemorrhage from venipunctures, other sites of trauma or healed wounds, gengivorrhagia, hemoptysis, macrohematuria, and hematemesis are observed in 16–18% of Bothrops snakes (Pardal et al. 2004; Souza 2002). In the Amazon, cases of hemorrhagic stroke have also been described (Machado et al. 2010). An important systemic complication of Bothrops snakebites is acute kidney injury (AKI), which has a great impact on morbidity and mortality. Oliguria or anuria may develop within the first 24 h of the bite. If patient is not treated, blood pressure rises within a few days of the onset of oliguria, and signs of uremia (drowsiness, irritability, vomiting, hiccups, convulsions) develop within 3–7 days after bite. AKI was observed in 10.9% of the patients in Manaus (Souza 2002) and in 20.5% of the cases in Colombia (Otero et al. 1996) (Fig. 5). Ischemia, hemorrhage, and direct nephrotoxic action of the venom may be implicated in the development of AKI. In the Brazilian Amazon, severe systemic complications were independently associated to age ≤15 years, age ≥65 years, and time to medical assistance >6 h. Lethality rates were 0.7% for Bothrops snakebites, associated with age ≥65 years and time to medical assistance >6 h (Feitosa et al. 2015). These features of victims of snakebite demand adequate management according to well-defined protocols, including prompt referral to tertiary centers when necessary, as well as an effective response from surveillance systems and policy makers for these vulnerable groups.
Systemic complications resulting from Bothrops snakebites. (a) Patient bitten on the dorsal part of the left forearm showing uremic face because of acute renal failure, 72 h after of the envenoming. (b) Patient presenting systemic hemorrhage, evidenced in the picture by bleeding in the lower lip 24 h after the bite
The blood is commonly incoagulable in patients with systemic envenoming. There is a variable hypofibrinogenemia associated with reduction of D-dimer. Levels of fibrin/fibrinogen degradation products are high. Thrombocytopenia is usual in severe cases. The total peripheral white blood cell count is usually elevated, and hematocrit may be increased initially as a result of hemoconcentration but falls subsequently depending on the occurrence of hemorrhage or liquid infusion. Coagulation tests are valuable in the initial investigation of snakebites, since incoagulable blood is present in about 50% of Bothrops-bitten patients in the Brazilian Amazon (Pardal et al. 2004; Souza 2002). In Colombia, incoagulable blood was found in 77% of Bothrops atrox envenomings (Otero et al. 1996).
Therapeutics
The specific treatment of Bothrops envenomations in the Brazilian Amazon follows the protocol established by the Ministry of Health according to the severity of the envenomation (Ministério da Saúde 2001). As soon as indications are fulfilled, antivenom should be administered. Delay in antivenom therapy is the main determinant for poor prognosis, where failure of antivenom in reversing clinical and laboratory effects are more likely to occur in patients admitted more than 6 h after bite. However, even a long delay between bite and admission to hospital should not exclude the indication of antivenom therapy if symptoms and signs of systemic envenoming are still evident. Antivenom should be administered by intravenous route, diluted in isotonic fluid, and infused over approximately 60 min. Preferably, patients should be hospitalized for antivenom therapy and be monitored in the first 24 h for early anaphylactic reactions. Clinical studies have shown that antivenoms are highly effective in reversing hematological disturbances and stopping local and systemic bleeding caused by Bothrops snake venoms (Pardal et al. 2004; Otero et al. 1996). Usually, coagulation disturbance is reversed in the first 24 h after antivenom therapy, and no relapse occurs if the recommended dose is given. On the other hand, the efficacy of antivenom in reducing local tissue damage shows to be limited, unless antivenom is given within a few hours of the bite.
The Bothrops, Bothrops-Lachesis, and Bothrops-Crotalus antivenoms, which are used in the treatment, are produced in horses immunized with a mixture of the snake venoms. The mixture of the Bothrops venom is composed for B. jararaca (50%), B. moojeni (12.5%), B. alternatus (12.5%), B. jararacussu (12.5%), and B. neuwiedi (12.5%). Thus, B. atrox venom, which is from a medically important snake in the Brazilian Amazon, is not part of the immunization pool used for the production of antivenoms used in the treatment of these envenomations (Fan et al. 2015). Experimentally, the Bothrops antivenom neutralizes the main biological activities, i.e., bleeding, lethality, and defibrinating, of B. atrox venom from Manaus, Amazonas State, and São Bento, Maranhão State, requiring, however, different doses of antivenoms for the distinct geographical areas (Furtado et al. 2010). On the other hand, a clinical study in Belém, Pará State, shows specific B. atrox-Lachesis, and standard Bothrops-Lachesis antivenoms were equally effective in reversing clinical manifestations and laboratories abnormalities observed in victims of Bothrops envenomations. Venom-induced hemostatic abnormalities were resolved with 24 h after the start of antivenom treatment (Pardal et al. 2004).
The victims of Bothrops envenomation in the Brazilian Amazon receive antivenom between 2 and 11.7 h on average after the snakebite (Pardal et al. 2004; Souza 2002). The delay between the elapsed time of the envenomation and the administration of antivenom in health services is the result of the vast territory and difficulty in access to health services, since often the transport used by the victims is by boat. Moreover, the practice of traditional treatments, such as the use of medicinal plants, could also delay the search for victims for specific treatment in the health services (Ministério da Saúde 2001). Indeed, various plant species are used to treat snakebites without any scientific validation. However, studies have shown the effectiveness of anti-snakebite plants to inhibit the local effects, i.e., edema, of B. atrox venom in the west of the state of Pará, Brazil (Moura et al. 2015).
Early adverse effects of antivenoms vary in frequency and severity, whose mechanisms can involve type I hypersensitivity, which seems not to be responsible in most cases, since acute reactions often occur in patients with no history of previous exposure to equine proteins; anti-complementary activity has been suggested; and human heterophilic antibodies toward equine immunoglobulins have been described. Independently of the mechanisms involved, clinical symptoms of early reaction are undistinguishable and have been reported in variable frequencies. Most of the early reactions are mild, including pruritus, urticaria, nausea, vomiting, and abdominal pain. Dyspnea and hypotension indicate severe reaction and demand interruption and the antivenom infusion and specific treatment. The risk of early reaction depends on the dose and speed of administration. There is a widespread practice in the antivenom therapy to recommend the slow infusion of antivenom, often achieved by diluting the antivenom in isotonic fluid, although clinical studies have not provided full support that the speed of antivenom infusion correlates with the frequency of acute reaction. Intradermal/conjunctival hypersensitivity tests have no longer been indicated since they do not predict antivenom reactions.
Early hypersensitivity reactions to the use of antivenom may occur even after the use of premedication with corticosteroids and antihistamines, which are between 16% and 28%. (Pardal et al. 2004; Souza 2002). The frequency of delayed reactions (serum sickness) needs to be better known (Fan et al. 2015). Only one victim from 212 of Bothrops envenomation treated at a reference hospital in the Amazonas State had serum sickness (Souza 2002). In rural areas of the Brazilian Amazon without electricity to conserve the liquid antivenom in cold temperatures (2 °C to 8 °C), the use of lyophilized antivenom could be strategically used. Studies show that the frequency of adverse reactions observed in victims of Bothrops envenomations in the Amazonas State who received lyophilized Bothrops-Lachesis-Crotalus antivenom was not statistically different when standard Bothrops antivenom was used (Silva and Tavares 2012).
Regarding treatment of local complications such as necrosis and compartment syndrome, there have been described cases of amputation and fasciotomy, respectively (Fan et al. 2015). In the treatment of secondary infections, such as abscesses, it is necessary to use broad-spectrum antibiotics. Studies on the microorganisms present in these infections and sensitivity to antibiotics need to be performed. Moreover, it is important to get information on the vaccination status of the victim against infection with tetanus bacilli and proceed according to the guidance of the Ministry of Health. Thus, in the Brazilian Amazon, a region that shows peculiar characteristics, the frequent training of professionals in the management of envenomation is necessary, especially in small towns.
Lachesis Envenomings in the Brazilian Amazon
Envenomings caused by Lachesis snakes, the bushmasters, popularly known in Brazil as surucucu, surucucu-pico-de-jaca, and surucutinga, are unusual events due to their nonaggressive behavior. These snakes are found in dense, preserved, and rainy tropical forest environments, with high temperatures, in the countries of South and Central America (Souza et al. 2007). In the Amazon region, there are two main Lachesis species: L. acrochorda, present in the northwest of Colombia and Ecuador, and L. muta, found in Venezuela, Suriname, Guyana, French Guiana, Brazil, Ecuador, Peru, Bolivia, and the eastern Andes. L. muta is the bigger venomous snake in the Americas and may exceed 3 m in length (Fig. 6); it is a species of forest environment, with large body size (easier to be seen), no aggressive behavior, and low population density, thus contributing to the lower incidence of Lachesis bites in relation to Bothrops bites (Bernarde 2014). L. muta has nocturnal habits, hunting in the stalking ground and feeding on rodents and marsupials. It is the only oviparous species of viperid in Brazil, with records of up to 20 eggs. Female curls up next to the eggs to protect them.
In the Brazilian Amazon, Lachesis envenomings accounted for 6.6% of cases (Saúde 2014). In a study conducted in the city of Manaus, this genus was involved in 17% of cases (Bard et al. 1994). In Belém, state of Pará, through enzyme immunoassay or by examination of the dead snake, only one Lachesis envenoming was shown among 46 bitten patients (Pardal et al. 2004). In the state of Acre, from 45 previously bitten individuals, 14% tested positive for Lachesis antibodies using enzyme immunoassay (Pierini et al. 1996).
Although L. muta has a wide geographical distribution, Lachesis venoms from Brazil, Costa Rica, and Colombia share similar pathophysiological characteristics (Pla et al. 2013), with four major pathophysiological activities: coagulant, proteolytic, hemorrhagic, and neurotoxic (Torres et al. 1995). The coagulant action of the Lachesis venom is due to the presence of serine proteases, also called thrombin-like proteins, which act directly on fibrinogen-to-fibrin reaction transformed without thrombin participation (Pla et al. 2013), causing an acceleration in blood coagulation and consumption of clotting factors, resulting in blood incoagulability and prolongation of bleeding time (Torres et al. 1995). In the Amazonas State, Brazil, coagulant activity of the L. muta venom showed more intensity than the B. atrox (Bard et al. 1994). Other toxins found in Lachesis venom are the disintegrins, acting as platelet aggregation inhibitors, even though some components have been found that induce aggregation (Francischetti et al. 1998).
Regarding proteolytic activity, the venom has many enzymes that help in an acute inflammatory process in the first hours post-envenoming, namely, phospholipases and serine proteases (Jorge and Ribeiro 1997). The hemorrhagic activity of the venom occurs through metalloproteinase action, also called hemorrhagins, that compromise the vascular integrity and increase fibrinolytic activity, not only locally but systemically (Estêvão-Costa et al. 2000). The neurotoxic activity is characteristic of Lachesis envenomings caused by vagal stimulation by the action of phospholipases which act as potent neuromuscular presynaptic blockers (Jorge et al. 1997). The kininogenases also play an important role in the clinical picture with the release of kinins, which affect neuromuscular conduction process. Experimental models show that neurotoxic action of L. muta venom possesses presynaptic effects at low doses and postsynaptic in high doses (Damico et al. 2006). Besides these activities, L. muta venom has a minimum myotoxic action (Damico et al. 2006).
Clinical manifestations at the bite site are similar to those caused by Bothrops, with an intense tissue damage evidenced by pain, restricted edema or affecting the member, blisters, bleeding, and ecchymosis (Torres et al. 1995). Chronic ulcers are reported in patients bitten by Lachesis (Fig. 7). Systemic manifestations are characterized by coagulation disorders, nausea, frequent vomiting, intense sweating, and hypersalivation or oral dryness (Souza et al. 2007). Classic signs and symptoms of vagal stimulation are dizziness, blurred vision, diarrhea, abdominal cramps, sinus bradycardia, severe hypotension, and shock (Souza et al. 2007; Torres et al. 1995). Occasionally, divergent strabismus, dysarthria, and dysphagia may occur (Torres et al. 1995). Main local complications are secondary infection, functional impairment, and acute renal failure (Souza et al. 2003, 2007). Due to stimulation of the autonomic nervous system, Lachesis envenomings can still present with shock and death (Souza et al. 2007). Serological tests to distinguish between Bothrops and Lachesis in the absence of vagal manifestations are available only for research purposes (Pardal et al. 2004).
The Lachesis antivenom is the specific treatment for this type of envenoming, especially effective in the occurrence of inoculation of large amounts of venom. Despite the wide geographic distribution of Lachesis snakes, antivenom seems to give good coverage in different geographical areas of the Amazon (Theakston et al. 1995). Despite some similarities between Bothrops and Lachesis venom components, the Bothrops antivenom is not recommended for neutralization of the coagulant action of the Lachesis venom (Bard et al. 1994).
Crotalus Envenomings in the Brazilian Amazon
In the Brazilian Amazon, Crotalus durissus, the rattlesnake, is present in relictual cerrado spots in the states of Rondônia (Vilhena, Chupinguaia, Rolim de Moura, Alta Floresta d’Oeste, and Guajará-Mirim), Amazonas (Humaitá), Roraima, Amapá, and Pará (Serra do Cachimbo, Santarém, and Marajó Island) and is probably absent in the state of Acre (Bernarde 2014). Adults range between 1 and 1.5 m long (Fig. 8). This snake has nocturnal and terrestrial behavior, feeding on rodents. It is a viviparous species, giving birth between 11 and 33 offsprings.
In Brazil, the Crotalus bites accounted for 9.2% of cases in 2015. In the Brazilian Amazon, there were 341 recorded cases, representing 23.9% of notifications from the country (Saúde 2014). Epidemiological studies show that Crotalus cause 0.7% of accidents by snake envenomings in Amapá (Lima et al. 2009), 13.4% in Roraima (Nascimento 2000), and 0.5% in the Amazonas State (Feitosa et al. 2015). In the upper Juruá River, state of Acre, snakebites were classified as Crotalus bites in 2% of the patients, but it is believed that in this region, rattlesnakes are commonly named surucucu, a name also used for Bothrops and Lachesis (Bernarde 2014). In a case series from Rio Branco, also in the state of Acre, no Lachesis envenomings were recorded (Moreno et al. 2005).
Crotalus durissus venom has three main biological activities: neurotoxic, myotoxic, and coagulant (Azevedo-Marques et al. 2009). The venom of Crotalus durissus ruruima, a snake found in the northern state of Roraima in Brazil and southern Venezuela, has shown phospholipase, hemorrhagic, and edematogenic activities, with a notable intrapopulation variation (Dos-Santos et al. 2005). The venom of C. d. ruruima may vary in their composition and biological activity in accordance with the color yellow or white; white C. d. ruruima venom has activities similar to that of C. d. terrificus (Dos Santos et al. 1993). The major component of Crotalus venoms is the crotoxin, a neurotoxin with presynaptic activity that acts on motor nerve endings by inhibiting the release of acetylcholine. This inhibition may result in neuromuscular blockade and therefore motor and respiratory paralysis. The myotoxic activity produces injury of skeletal muscle fibers, resulting in rhabdomyolysis. The coagulant action is attributed to the presence of thrombin-like components in the venom, which can lead to hypofibrinogenemia and blood incoagulability (Azevedo-Marques et al. 2009). A potent platelet-aggregating protein, called convulxin, was also isolated from the venom of C. d. terrificus, but thrombocytopenia has not been observed in the Lachesis envenoming (Azevedo-Marques et al. 2009).
Clinical manifestations at the bite site generally are little evident, with fang marks, paresthesia, and discrete edema and erythema. Systemic manifestations include drowsiness, ptosis, ophthalmoplegia, sagging face muscles, blurred vision, diplopia, myalgia, arthralgia, and myoglobinuria. Swallowing difficulties and changes of smell and taste may occur in some patients. The major complications that can arise after Crotalus bites are acute renal failure and acute respiratory failure. Crotalus bites patients may present with increased serum levels of creatine kinase, lactate dehydrogenase, aspartate aminotransferase, and aldolase. Clotting time can be abnormal in some cases (Azevedo-Marques et al. 2009). In the Brazilian Amazon, few clinical descriptions of Crotalus envenomings have been reported in the state of Pará, evolving to acute renal failure (Pardal et al. 2003).
Treatment of Crotalus envenomings consists of the administration of the specific antivenom, which is produced in Brazil from the immunization of horses with C. d. terrificus and C. d. collilineatus venoms (Fan et al. 2015). In the presence of systemic complications, patient may require supportive treatment, using renal replacement therapy in case of acute renal failure, artificial ventilation in case of respiratory failure, and corticosteroids, antihistamines, and epinephrine in case of anaphylactic reactions following antivenom administration.
Micrurus Envenomings in the Brazilian Amazon
In Brazil, Elapidae snakes are called coral snakes because most species have colored rings along the body extension (black, red, or orange and white or yellow) (Bernarde 2014). However, there are exceptions of species presenting no colored rings (for instance, Micrurus albicinctus) (Fig. 9). Most coral species usually will not exceed 1 m in length, with no record of Micrurus spixii with 1.6 m. Since there are several species of false coral snakes, it is prudent only experts capture these animals. General population must treat all snakes with coral pattern as “possible true coral snakes,” thus avoiding accidents with these snakes. These snakes occur in primary forests or in disturbed areas of crops and pasture, including records of some species (e.g., M. lemniscatus and M. surinamensis) in urban areas. Most species have fossorial or terrestrial habits, but two species (M. lemniscatus and M. surinamensis) have aquatic habits. Micrurus mainly feed on elongated vertebrates (other snakes, amphisbaenians, lizards, and caecilians) but also on fishes (Callichthys, Gymnotus, and Synbranchus marmoratus, predated by Micrurus lemniscatus and Micrurus surinamensis) and velvet worms (recorded for M. hemprichii) (Bernarde 2014; Martins and Oliveira 1998). These snakes are oviparous, with a record of 2 to 15 eggs, which varies between species and the size of the snake (Martins and Oliveira 1998). In the Brazilian Amazon, it is estimated that 0.3% of the snakebites are caused by Micrurus. Clinical data from Micrurus envenomings are scarcely reported in this region, and M. hemprichii and M. lemniscatus are involved in such envenomings (Fan et al. 2015).
Micrurus venom primarily induces neurotoxic effects due to the presence of neurotoxins with pre- and postsynaptic activity. The neurotoxins can competitively bind with acetylcholine receptors causing postsynaptic blockage of neuromuscular transmission or act at the neuromuscular junction blocking the presynaptic release of acetylcholine (Ministério da Saúde 2001). M. surinamensis has a presynaptic neurotoxin (Dos-Santos 2009). Venoms of Micrurus spixii, M. averyi, M. lemniscatus, M. surinamensis, and M. hemprichii from the Brazilian Amazon region have no coagulant activity. However, with the exception of the M. surinamensis venom, the venoms of the other species have edematogenic and myotoxic activities (Terra et al. 2015). Myotoxic effect of Micrurus venom of the Brazilian and Colombian Amazon is evidenced experimentally by the increase in plasma levels of creatine kinase and acute muscle damage on histology (Gutiérrez et al. 1992). Toxin and crude venoms of M. spixii, an endemic species of South America and northern states of Brazil, show phospholipase activity. In a mouse phrenic nerve-diaphragm preparation, M. spixii venom and MsPLA2-I induced the blockage of both direct and indirect twitches (Terra et al. 2015).
Victims of Micrurus bites have ptosis; ophthalmoplegia; jaw, laryngeal muscles, and pharynx paralysis; drooling; and paralysis of the neck and limbs as a result of neurotoxic venom activity (Ministério da Saúde 2001). Acute respiratory failure has been observed in accidents caused by M. surinamensis that occurred in the state of Pará, as a result of paralysis of the respiratory muscles. In this state, Micrurus filiformis also causes envenomings with pain and mild edema at the bite site, epigastric pain, and vomiting (Pardal et al. 2010). In the Amazonian Ecuador, unusually, there was a case of M. lemniscatus helleri bite with severe local pain, slow evolution of neurological manifestations, thrombocytopenia, and mild coagulopathy (Manock et al. 2008).
Treatment of Micrurus bites is made with the use of specific antivenom. In Brazil, M. corallinus and M. frontalis venoms are used for the production of Micrurus antivenom. In cases of acute respiratory failure, intubation and mechanical ventilation are required (Manock et al. 2008; Pardal et al. 2010).
Prevention Measures
Snakebites are considered preventable injuries and most of the envenomings occur by lack of preventive habits, including individual protection equipment, especially for workers in rural activities. As lower and upper limbs are the most affected areas of the body, the use of jackboots, leggings, and gloves is supposed to be the major primary prevention measure. Some simple additional measures such as keeping clean household surroundings and closing garbage cans help to keep away small rodents, which are part of some snakes’ diet. In the Amazonian context, it should be noted that many Amerindian and traditional riverine individuals are habitually barefoot populations, representing a challenge for preventing snakebites. Education about safe habits for the most affected groups is essential. For instance, there is no systematic program of interventions for primary prevention of snakebites as an occupational hazard in the Amazon.
Secondary prevention of snakebites aims to reduce the impact of an already occurred envenoming, by detecting and treating patients as soon as possible to prevent severe complications such as local necrosis and secondary bacterial infections, systemic bleeding, and renal failure, commonly observed after Bothrops atrox snakebites (Souza 2002). In the Brazilian Amazon, more than 30% of patients took more than 6 h to receive medical assistance, and such delay was an independent risk factor for severe complications and associated mortality (Feitosa et al. 2015). Moreover, underdosing of antivenom in the region seems to be common. Improvement in the access to health facilities and systematic professional training on diagnosis, specific therapy, and clinical management of complications could have a significant impact in preventing poor outcomes, long-term disabilities, and lethality. The Brazilian Ministry of Health additionally recommends not doing tourniquets, cutting or sucking the bite site, or applying substances such as alcohol, coffee, kerosene, mud, and other traditional “medicines” (Ministério da Saúde 2001).
The burden of function loss associated to snakebites on vulnerable populations remains as a major research gap, both from the health system and society perspective. In the state of Acre, in the Western Brazilian Amazon, functional impairment of the bitten limb was recorded in 10% of the indigenous and riverine population surveyed, including permanent loss of function and sensibility, amputations, and permanent scarring (Pierini et al. 1996). In order to improve as much as possible their ability to function, their quality of life, and their life expectancy, public policies aiming to identify incapacitated victims and provide them socio-economical support and physical rehabilitation should be part of integrated national programs for chronic pathologies.
Conclusions and Future Directions
Despite important efforts carried out during the past decades in Brazil to understand and control the problem of snakebites, important gaps remain for the fulfillment of these goals, particularly in the Amazon region. A workshop was held in Manaus, Amazonas, in 2013 with representatives of Health Departments of Amazonian states, AV producers, universities, reference hospitals, and the Ministry of Health to identify research bottlenecks. A proposal to create the research network Snakebite and Scorpionism Network in the Amazon (Rede de Ofidismo e Escorpionismo da Amazônia (ROdA)) emerged from researchers at the Butantan Institute and the Tropical Medicine Foundation Dr. Heitor Vieira Dourado. The general aim of the network is to enhance implementation of collaborative work and multicenter studies resulting in integration of services, research institutions, and health professionals. Identified research gaps are listed below (Fan et al. 2015).
Burden on Vulnerable Populations
It is difficult for riverine and indigenous populations to reach health centers for treatment of snakebites. As a result, the number of cases detected officially is probably much lower than the real number. Current AVs require conservation in adequate facilities (2°–8 °C), which are not always available in these remote settings. In addition, training of multidisciplinary teams is not always appropriate for indigenous health services regarding AV administration, side effect management, and case monitoring and surveillance.
Recommendations:
-
1.
Assess disease burden through population- and hospital-based field studies in remote areas.
-
2.
Seek innovation in the network for efficient distribution of immunobiologicals, especially interaction with other networks such as those providing vaccines.
-
3.
Integrate different sectors (Health Surveillance Secretariat (SVS), Indigenous Health Special Secretariat (SESAI), National Agency of Sanitary Surveillance (ANVISA)) for articulation of common strategies to be pursued with other ministries (Agriculture, Environment, Science, and Technology).
-
4.
Review the skills of professionals assisting injured patients in areas without infrastructure and doctors according to international guidelines.
Venom Research and Revision of the AV Spectrum
Currently, AV immunoglobulins are the only treatment available for snake envenomings. The WHO List of Essential Medicines includes them in the basic package of healthcare in affected countries. There is an urgent need to ensure availability of effective AVs and to improve their manufacture regulation. However, the possible interspecific venom variation associated with the geographical distribution of snakes may affect the effectiveness of therapeutic AVs against the Amazon Bothrops venom.
Current AV production methods, based on studies conducted in the 1980s, need updating in light of new technologies. Antivenom recommendations are based on experimental studies of cross-neutralization between specific venoms and AVs. These excluded venom from Bothrops atrox, the main cause of snakebites in the Amazon. Efficacy of Brazilian AVs against venom from some Amazon Bothrops species has been investigated. Bothrops AV showed neutralization of B. atrox venom major toxins (Pardal et al. 2004). Thus, new studies are a needed investment in technological development to assess different AV candidate formulations.
A major concern relates to the failure in AV distribution. Antivenoms are usually available in the municipal hospitals, as opposed to being distributed to peripheral health clinics. The lack of adequate cold chain impairs AV distribution to rural areas. Also, inadequate storage and transportation may result in loss of material. Freeze-dried AVs are available, and one of the national producers (Butantan Institute) has been working to provide both liquid and freeze-dried products.
Recommendations:
-
1.
Revise toxicity of snake venoms, including proteomics, as well as the potential for AV neutralization against major venom activities.
-
2.
Study seroneutralization in experimental models to support the venom pool used to immunize animals for AV production, considering the absence of Bothrops atrox venoms in these pools. Experimental studies should indicate the need for inclusion of new venoms; the new product needs to be validated by clinical and epidemiological data.
-
3.
Perform stability studies of liquid AVs considering the Amazonian environmental conditions. Decisions on AV distribution, either liquid or freeze-dried products, should be based on careful and detailed analysis of the epidemiology of snakebites, the prevailing conditions, and health facilities available.
-
4.
Study the mechanisms of venom action for different populations of snakes in the Amazon (inter- and intraspecies variations).
-
5.
Study supporting action or herbal drugs with specific activity on certain venom components to enable complementary or alternative treatments.
Priorities in Clinical Research
Although clinical research related to venomous animal injuries has increased, most publications are based on case reports and lack methodological rigor. Moreover, outcome definitions, such as severity ranking criteria, were empirically established, making the results even less generalizable. Clinical research from hospital-based studies (patient follow-up for evaluation of the frequency of events related to envenoming and their risk factors) and community observational studies (verbal autopsy studies and seroepidemiological surveys, group population cohorts, and qualitative studies) is needed.
Delays in patient care, along with the use of substances that may aggravate the conditions at the bite site, lead to a high frequency of local complications resulting from Bothrops and Lachesis envenomings. However, severity is also possibly related to the composition of Amazon venoms. Medical management of secondary infection, abscess, necrosis, and compartmental syndrome has been the subject of controversy, partly because of the lack of standardization regarding concepts and management protocols. The possibility of reducing local effects by means of drugs with anti-inflammatory activity, early antibiotic therapy for secondary infection, cross-neutralization of AVs for different types of accidents, and new complementary treatments needs to be further investigated while observing good clinical practice and, preferably, in multicenter studies.
Systemic complications such as sepsis and acute kidney injury are less well known, and apparently less frequent, than local complications, but most frequent across the series from other regions in Brazil. The lack of patient follow-up, including laboratory tests, appears to be related to this observation.
Recommendations:
-
1.
Perform multicenter studies by standardization of clinical protocols for estimating independent risk factors for complications and also assessing AV efficacy (choice of outcomes) and defining objective criteria for recommending AV dosage.
-
2.
Identification of the species responsible for snakebites in the Amazon requires the establishment of a gold standard method and determination of levels of antigenemia, preferably by means of rapid diagnostic tests.
-
3.
Evaluate phase IV studies for adverse reactions (from three Brazilian manufacturers) under AV pharmacovigilance.
-
4.
Submit phase II/III protocols simultaneously to assess feasibility of comparative studies on efficacy and safety.
-
5.
Plan training in good clinical practices, as well as establish a link to the National Clinical Research Network (RNPC) from teaching hospitals.
-
6.
Inform the regulatory agencies about the limitations and peculiarities of research involving animal envenomings, paying clarification and consultation to the National Human Research Ethics Council and the National Regulatory Agency.
Adverse Reactions and Pharmacovigilance
Early adverse reactions (EAR) to AV therapy are expected events of varied frequency according to the type of AV used and individual hypersensitivity to heterologous proteins. Clinically, patients may present urticaria, itching, tachycardia, nausea, vomiting, abdominal colic, bronchospasm, hypotension, and angioedema. Over time, both the frequency and severity of early reactions have decreased due to the improvement of the AV purification process in Brazil. Frequency of delayed reactions (serum sickness) seems to be lower than EAR, but the true frequency is unknown. AV pharmacovigilance has been implemented, but reliable efficacy and safety data are still lacking.
There are no accurate predictive factors for side effect occurrence, and preventing them is not always possible, even with the use of premedication containing corticosteroids and/or antihistamines. Existing studies do not include controls for the intervening variables, and samples are of insufficient size, limiting the validity of the results.
Recommendations:
-
1.
Perform multicenter phase IV studies, identifying sentinel hospitals for monitoring cases for both early and late reactions.
Professional Training
Despite the high incidence of injuries from venomous animals, there is a lack of systematic professional training on diagnosis, specific therapy, and clinical management of complications. Thus, AV misuse is not infrequent, either in quantity (number of ampoules administered) or the specific AV. Current training programs seek to link medical knowledge with the snakes’ biology and surveillance. However, this approach often does not reflect the need for professional diagnosis algorithms and coherent and responsive medical management. Thus, adherence to medical training and courses in this area has been a major challenge. Furthermore, there is a high turnover of health professionals in small Amazon cities. Although communication technologies that greatly facilitate knowledge dissemination have proliferated in the area, these are still barely harnessed. The use of electronic media for training professionals in the management of envenomations is increasing and may be an alternative to classroom courses.
Recommendations:
-
1.
Investments in training should cover all health professionals, including nurses who are critical to initial management of the patient and follow-up of possible complications.
-
2.
Update systematically all relevant diagnosis and treatment guidelines.
-
3.
Encourage the use of technological resources for communication and other electronic media used in training programs and distance learning.
-
4.
Include the topic in the undergraduate curriculum of health professionals with regionalized approaches to issues involving venomous animals.
-
5.
Design new postgraduate and other courses, as well as interaction between graduate programs to increase the critical mass of professionals and researchers involved,
-
6.
Implement nonformal education activities for science communication, particularly aimed at school audiences.
Fauna Surveys and Capture of Animals for Venom Production
Traditionally, institutions producing AVs get animals caught from nature that are kept in vivariums and used to obtain venoms. However, environmental legislation restricts the collection and transport of wild animals. There is a requirement to establish specific policies to capture animals, and captive breeding is not done satisfactorily.
Field work is not limited to animal collection; it also includes studies of behavioral patterns such as diet, reproduction, and activities to establish phylogeny patterns for identifying risk factors of the envenomings. Increased knowledge on biodiversity of Amazon animals has applications on their use and species conservation. Zoological collections of invertebrates are also informative regarding their geographical distribution and diversity.
Recommendations:
-
1.
Establish partnerships for capture of Lachesis snakes and transport to vivariums of laboratories producing AVs.
-
2.
Guide Lachesis reproduction in captivity, which should be led by professionals familiar with their conditions both in nature and supportive maintenance environment.
-
3.
Follow shared standard operating procedures (SOPs) for the collections of venomous animals in order to facilitate exchange of information.
In addition to all the above recommendations, international cooperative efforts toward the control of this neglected health problem through international partnerships are needed, namely, with other Amazonian countries.
References
Assakura MT, Furtado MF, Mandelbaum FR. Biochemical and biological differentiation of the venoms of the lancehead vipers (Bothrops atrox, Bothrops asper, Bothrops marajoensis and Bothrops moojeni). Comp Biochem Physiol. 1992;102:727–32.
Azevedo-Marques MM, Hering SE, Cupo P. Acidente Crotálico. In Cardoso JLC, França FOS, Fan WH, Malaque CMS, Haddad Jr V, editors. Animais Peçonhentos no Brasil. Biologia, Clínica e Terapêutica dos Acidentes. São Paulo: Sarvier; 2009. p. 108–15.
Bard R, Lima JCR, Sá-Neto RP, Oliveira SG, Santos MC. Ineficácia do antivenneo botrópico na neutralização da atividade coagulante do veneno de Lachesis muta muta. Relato de caso e comprovação experimental. Rev Inst Med Trop Sao Paulo. 1994;36(1):77–81.
Bernarde PS. Anfíbios e répteis: introdução ao estudo da herpetofauna brasileira. Curitiba: Anolis Books; 2012.
Bernarde PS. Serpentes Peçonhentas e Acidentes Ofídicos no Brasil. São Paulo: Anolis Books; 2014.
Bernarde PS, Gomes JO. Serpentes peçonhentas e ofidismo em Cruzeiro do Sul, Alto Juruá, Estado do Acre, Brasil. Acta Amaz. 2012;42:65–72.
Bernarde P, Costa H, Machado R. Bothriopsis bilineata bilineata (Wied, 1821) (Serpentes: Viperidae): new records in the states of Amazonas, Mato Grosso and Rondônia, northern Brazil. Check List. 2011a;7:343–7.
Bernarde PS, Amaral ES, Vale MAD. Squamata, Serpentes, Viperidae, Bothrocophias hyoprora (Amaral, 1935): distribution extension in the state of Acre, northern Brazil. Checklist. 2011b;7(6):813–4.
Calvete JJ, Sanz L, Pérez A, Borges A, Vargas AM, Lomonte B, et al. Snake population venomics and antivenomics of Bothrops atrox: paedomorphism along its transamazonian dispersal and implications of geographic venom variability on snakebite management. J Proteomics. 2011;74:510–27.
Coutinho-Neto A, Caldeira CS, Souza GHMF, Zaqueo KD, Kayano AM, Silva RS, et al. ESI-MS/MS identification of a bradykinin-potentiating peptide from Amazon Bothrops atrox snake venom using a hybrid Qq-oaTOF mass spectrometer. Toxins (Basel). 2013;5:327–35.
da Saúde M. Manual de diagnóstico e tratamento de acidentes por animais peçonhentos. Brasília: Fundaçao Nacional de Saúde; 2001.
Damico DCS, Bueno LGF, Rodrigues-Simioni L, Maangoni S, Cruz-Hofling MA, Novello JC. Functional characterization of a basic D49 phospholipase A 2 (LmTX-I) from the venom of the snake Lachesis muta muta (bushmaster). Toxicon. 2006;47:759–65.
Dantas RT, Jorge ARC, Jorge RJB, Menezes RRPPB, Lima DB, Torres AFC, et al. L-amino acid oxidase from Bothrops marajoensis causes nephrotoxicity in isolated perfused kidney and cytotoxicity in MDCK renal cells. Toxicon. 2015;104:52–6.
Dos-Santos MC. Serpentes peçonhentas e ofidismo no Amazonas. In: Cardoso JLC, França FOS, Wen FH, Málaque CMS, Haddad Jr V, editors. Animais peçonhentos no Bras. Biol. clínica e Ter. dos Acid. 2nd ed. São Paulo: Sarvier; 2009. p. 132–42.
Dos-Santos MC, Borgesde E, Pinheiro J, Fortes-dias CL. Individual venom variability in Crotalus durissus ruruima snakes, a subspecies of Crotalus durissus from the Amazonian region. Toxicon. 2005;46:958–61.
Estêvão-Costa MI, Diniz CR, Magalhães A, Markland FS, Sanchez EF. Action of metalloproteinases mutalysin I and II on several components of the hemostatic and fibrinolytic systems. Thromb Res. 2000;99:363–76.
Fan WH, Monteiro WM, Moura da Silva AM, Tambourgi DDV, Mendonça da Silva I, Sampaio VS, et al. Snakebites and scorpion stings in the Brazilian Amazon: identifying research priorities for a largely neglected problem. PLoS Negl Trop Dis. 2015;9:e0003701.
Feitosa EL, Sampaio VS, Salinas JL, Queiroz AM, Silva IM, Gomes AA, et al. Older age and time to medical assistance are associated with severity and mortality of snakebites in the Brazilian Amazon: a case–control study. PLoS One. 2015;10:e0132237.
Francischetti IMB, Castro HC, Zingali RB, Carlini CR, Guimarães JA. Bothrops sp. snake venoms: comparison of some biochemical and physicochemical properties and interference in platelet functions. Comp Biochem Physiol. 1998;119:21–9.
Freitas-De-Sousa LA, Amazonas DR, Sousa LF, Sant’Anna SS, Nishiyama MY, Serrano SMT, et al. Comparison of venoms from wild and long-term captive Bothrops atrox snakes and characterization of Batroxrhagin, the predominant class PIII metalloproteinase from the venom of this species. Biochimie. 2015;118:60–70.
Furtado MDFD, Cardoso ST, Soares OE, Pietro PA, Fernandes DS, Tambourgi DV, et al. Antigenic cross-reactivity and immunogenicity of Bothrops venoms from snakes of the Amazon region. Toxicon. 2010;55:881–7.
Gutiérrez JM, Rojas G, Jorge NJS, Javier N. Experimental myonecrosis induced by the venoms of South American Micrurus (coral snakes). Toxicon. 1992;2:1299–302.
Jacob-Ferreira AL, Menaldo DL, Bernardes CP, Sartim MA, De Angelis CD, Tanus-Santos JE, et al. Evaluation of the in vivo thrombolytic activity of a metalloprotease from Bothrops atrox venom using a model of venous thrombosis. Toxicon. 2016;109:18–25.
Jorge MT, Ribeiro LA. Dose de soro (antiveneno) no tratamento do envenenamento por serpentes peçonhentas do gênero Bothrops. Rev Assoc Med Bras. 1997;43(1):74–6.
Jorge MT, Sano-Martins IS, Tomy SC, Castro SC, Ferrari RA, Ribeiro LA, et al. Snakebite by the bushmaster (Lachesis muta) in Brazil: case report and review of the literature. Toxicon. 1997;35:545–54.
Kanashiro MM, De Escocard RCM, Petretski JH, Prates MV, Alves EW, Machado OLT, et al. Biochemical and biological properties of phospholipases A2 from Bothrops atrox snake venom. Biochem Pharmacol. 2002;64:1179–86.
Lima ACSF, Campos CEC, Ribeiro JR. Perfil epidemiológico de acidentes ofídicos do Estado do Amapá. Rev Soc Bras Med Trop. 2009;42:329–35.
López-Lozano JL, Sousa MV, Ricart CAO, Chávez-Olortegui C, Sanchez EF, Muniz EG, et al. Ontogenetic variation of metalloproteinases and plasma coagulant activity in venoms of wild Bothrops atrox specimens from Amazonian rain forest. Toxicon. 2002;40:997–1006.
Machado AS, Barbosa FB, Mello GS, Pardal PPO. Acidente vascular cerebral hemorrágico associado à acidente ofídico por serpente do gênero Bothrops: relato de caso. Rev Soc Bras Med Trop. 2010;43:602–4.
Manock SR, Suarez G, Graham D, Avila-Aguero ML, Warrell DA. Neurotoxic envenoming by South American coral snake (Micrurus lemniscatus helleri): case report from eastern Ecuador and review. Trans R Soc Trop Med Hyg. 2008;102:1127–32.
Marcussi S, Stábeli RG, Santos-Filho NA, Menaldo DL, Silva Pereira LL, Zuliani JP, et al. Genotoxic effect of Bothrops snake venoms and isolated toxins on human lymphocyte DNA. Toxicon. 2013;65:9–14.
Martins M, Oliveira M. Natural history of snakes in forests of the Manaus region, Central Amazonia, Brazil. Herpetol Nat Hist. 1998;6:78–150.
Moreira V, Dos-Santos MC, Nascimento NG, Silva HB, Fernandes CM, Império-Lima MR, et al. Local inflammatory events induced by Bothrops atrox snake venom and the release of distinct classes of inflammatory mediators. Toxicon. 2012;60:12–20.
Moreno E, Queiroz-Andrade M, Lira-da-Silva RM, Tavares-Neto J. Características clínicoepidemiológicas dos acidentes ofídicos em Rio Branco, Acre. Rev Soc Bras Med Trop. 2005;38:15–21.
Moura VM, Freitas de Sousa LA, Dos-Santos MC, Raposo JD, Lima AE, Oliveira RB, et al. Plants used to treat snakebites in Santarém, western Pará, Brazil: an assessment of their effectiveness in inhibiting hemorrhagic activity induced by Bothrops jararaca venom. J Ethnopharmacol. 2015;161:224–32.
Nascimento SP. Aspectos epidemiológicos dos acidentes ofídicos ocorridos no Estado de Roraima, Brasil, entre 1992 e 1998. Cad Saude Publica. 2000;16:271–6.
Neiva M, Arraes FBM, Souza JV, Rádis-Baptista G, Prieto da Silva ÁRB, Walter MEMT, et al. Transcriptome analysis of the Amazonian viper Bothrops atrox venom gland using expressed sequence tags (ESTs). Toxicon. 2009;53:427–36.
Núñez V, Arce V, Gutiérrez JM, Lomonte B. Structural and functional characterization of myotoxin I, a Lys49 phospholipase A2 homologue from the venom of the snake Bothrops atrox. Toxicon. 2004;44:91–101.
Núñez V, Cid P, Sanz L, De La Torre P, Angulo Y, Lomonte B, et al. Snake venomics and antivenomics of Bothrops atrox venoms from Colombia and the Amazon regions of Brazil, Perú and Ecuador suggest the occurrence of geographic variation of venom phenotype by a trend towards paedomorphism. J Proteomics. 2009;73:57–78.
Otero R, Gutiérrez JM, Núñez V, Robles A, Estrada R, Segura E, et al. A randomized double-blind clinical trial of two antivenoms in patients bitten by Bothrops atrox in Colombia. Trans R Soc Trop Med Hyg. 1996;90:696–700.
Pardal PPO, Pardal JSDO, Castro LC, Cardoso BS, Sousa AMB, Wosny V. Acidentes por cascavel (Crotalus durissus) no estado do Pará. Resist Pará Médico. 2003;17:27–31.
Pardal PPO, Souza SM, Monteiro MRCC, Fan HW, Cardoso JLC, França FOS, et al. Clinical trial of two antivenoms for the treatment of Bothrops and Lachesis bites in the north eastern Amazon region of Brazil. Trans R Soc Trop Med Hyg. 2004;98:28–42.
Pardal PPO, Pardal JSO, Gadelha MAC, Rodrigues LS, Feitosa DT, Prudente ALC, et al. Envenomation by Micrurus coral snakes in the Brazilian Amazon region: report of two cases. Rev Inst Med Trop Sao Paulo. 2010;52:333–7. Instituto de Medicina Tropical de São Paulo.
Pierini SVV, Warrell DAA, Paulo A, Theakston RDGD. High incidence of bites and stings by snakes and other animals among rubber tappers and Amazonian Indians of the Juruá Valley, Acre State, Brazil. Toxicon. 1996;34:225–36.
Pla D, Sanz L, Molina-Sánchez P, Zorita V, Madrigal M, Flores-Díaz M, et al. Snake venomics of Lachesis muta rhombeata and genus-wide antivenomics assessment of the paraspecific immunoreactivity of two antivenoms evidence the high compositional and immunological conservation across Lachesis. J Proteomics. 2013;89:112–23.
Sanchez EF, Schneider FS, Yarleque A, Borges MH, Richardson M, Figueiredo SG, et al. The novel metalloproteinase atroxlysin-I from Peruvian Bothrops atrox (Jergón) snake venom acts both on blood vessel ECM and platelets. Arch Biochem Biophys. 2010;496:9–20.
Santos MC, Ferreira LCL, Silva WD, Furtado MDFD. Caracterizacion de las actividades biologicas de los venenos “amarillo” y “blanco” de Crotalus durissus ruruima comparados con el veneno de Crotalus durissus terrificus. Poder neutralizante de los antivenenos frente a los venenos de Crotalus durissus. Toxicon. 1993;31:1459–69.
Saúde M. Sistema de Informação de Agravos de Notificação. Acidentes por animais peçonhentos; 2014.
Silva IM, Tavares AM. Comparative evaluation of adverse effects in the use of powder trivalent antivenom and liquid antivenoms in Bothrops snake bites. Rev Soc Bras Med Trop. 2012;45:523–5.
Sousa LF, Nicolau CA, Peixoto PS, Bernardoni JL, Oliveira SS, Portes-Junior JA, et al. Comparison of phylogeny, venom composition and neutralization by antivenom in diverse species of Bothrops complex. PLoS Negl Trop Dis. 2013;7:e2442.
Souza ARB. Snakebite by Bothrops atrox (Lin. 1758) in the State of Amazonas – Brazil: study of 212 cases with identified snake. Rev Patol Trop. 2002;31:267–8.
Souza ARB, Muniz EG, López-Lozano JL, Ferreira LCL, Noronha MDN. Clinical manifestations of snakebite by the Bushmaster snake Lachesis muta muta in Manaus, Amazonas Brazil. J Venom Anim Toxins Incl Trop Dis. 2003;9:494.
Souza RCG, Nogueira APB, Lima T, Cardoso JLC. The enigma of the North Margin of the Amazon River: proven Lachesis bites in Brazil, report of two cases, general considerations about the genus and bibliographic review. Bull Chicago Herp Soc. 2007;42:105–15.
Terra ALC, Moreira-Dill LS, Simões-Silva R, Monteiro JRN, Cavalcante WLG, Gallacci M, et al. Biological characterization of the Amazon coral Micrurus spixii snake venom: isolation of a new neurotoxic phospholipase A. Toxicon. 2015;103:1–11.
Theakston RDG, Laing GDD, Fielding CMM, Lascano AFF, Touzet J-MM, Vallejo F, et al. Treatment of snake bites by Bothrops species and Lachesis muta in Ecuador: laboratory screening of candidate antivenoms. Trans R Soc Trop Med Hyg. 1995;89:550–4.
Torres JR, Torres MA, Arroyo-Parejo MA. Coagulation disorders in bushmaster envenomation. Lancet. 1995;346:449–50.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Science+Business Media B.V., part of Springer Nature
About this entry
Cite this entry
de Oliveira, S.S. et al. (2018). Snakebites in the Brazilian Amazon: Current Knowledge and Perspectives. In: Vogel, CW., Seifert, S., Tambourgi, D. (eds) Clinical Toxinology in Australia, Europe, and Americas. Toxinology. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7438-3_61
Download citation
DOI: https://doi.org/10.1007/978-94-017-7438-3_61
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7436-9
Online ISBN: 978-94-017-7438-3
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences