Abstract
An overview is presented on different catalytic routes for producing hydrogen from biomass via pyrolysis processes. Fundamentals of biomass pyrolysis along with general aspects related to the types of processes and catalysts are discussed. Processes that allow hydrogen production in this field have been divided into single-step and multi-step processes. These processes are reviewed in this chapter, showing the state of the art. In both strategies, a hydrogen-rich product gas is obtained which, conveniently conditioned and purified, may serve for various purposes.
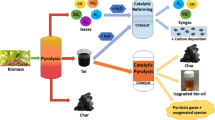
Catalytic pyrolysis of raw biomass feedstocks aiming at producing hydrogen can be carried out by directly contacting the raw material with a catalyst having a high selectivity towards hydrogen production inside the pyrolysis reactor, in a single-step process. Another possibility for producing hydrogen from biomass follows a strategy based on multiple steps. In the majority of multi-step processes, the biomass raw material is subjected to fast pyrolysis for producing a liquid product, denoted as biomass pyrolysis liquids or bio-oil, and afterwards processing the bio-oil or fractions of it in a catalytic steam reforming process with suitable catalysts.
Future trends of catalytic biomass pyrolysis process technologies are described in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Biomass is a renewable resource that can be used for hydrogen production. Some of the advantages of using biomass are the vast variety of materials and the high dispersion through the planet. This chapter is centered on lignocellulosic biomass since it is the most studied one.
Pyrolysis is a thermochemical process that consists in the thermal decomposition of carbon-containing resources (such as biomass) in a non-oxidizing atmosphere and in the absence of any other reactant agent. Solid, liquid, and gas product fractions are obtained with proportions that are much dependent on the process operating conditions, mainly process temperature, heating rate, and residence time of the vapors.
Pyrolysis is a thermochemical route of biomass conversion that has been explored for hydrogen production. There are a large number of research works focusing on hydrogen production by pyrolysis. These works have identified the catalyst as the key factor in the process. The catalyst increases hydrogen yield working at moderate temperatures, which is favorable from an energy point of view.
Catalytic biomass pyrolysis processes can be divided into one-step and multi-step processes. In one-step process, only one reactor is used. This reactor converts biomass into hydrogen. In a multi-step process, at least two reactors are required. In the first one, biomass pyrolysis takes place, and in the second reactor, pyrolysis products are converted to a high hydrogen yield. In most cases the biomass raw material is subjected to fast pyrolysis for producing a liquid product, bio-oil. Bio-oil is easily transported and different strategies can be employed for hydrogen production, for example, catalytic steam reforming of aqueous fraction of bio-oil. In the 1980s, several technologies of fast pyrolysis were developed to generate bio-oil. Bio-oil is the main intermediate in multi-step processes.
Fundamentals of biomass pyrolysis are presented to understand the main operating variables involved in the process. Some pyrolysis reactors for bio-oil production are included, given the relevance of this intermediate.
In the catalyst section, the main properties and performance of the catalyst in the biomass pyrolysis process are summarized. Also, many experimental works show the vast variety of catalysts used, being nickel-based catalysts preferred due to their price.
The main facts of the one-step processes are presented, analyzing hydrogen yield and the main findings of the literature works. Recent innovative processes such as biomass pyrolysis in molten alkali and microwave plasma are also included.
The multi-step processes section is mainly focused on catalytic steam reforming of bio-oil. This option presents some advantages that can be further implemented at an industrial scale based on economic studies.
2 Fundamentals of Biomass Pyrolysis
Pyrolysis is a thermochemical process that consists in the thermal decomposition of biomass in a non-oxidizing atmosphere and in the absence of any other reactant agent. Heating biomass in an inert atmosphere triggers a complex series of chemical reactions that include primary breakdown of biomass constituents, as well as secondary reactions involving the products of direct decomposition of biomass. All these chemical reactions occur coupled to mass and heat transfer processes, and solid, liquid, and gas product fractions are obtained. Usually, the term pyrolysis englobes all these simultaneous processes and not only the thermal decomposition of biomass. The solid fraction that can be obtained from pyrolysis is usually referred to as char, or, more recently, biochar. The liquid fraction, frequently named bio-oil, is a product of great interest for hydrogen production by means of catalytic processes, especially in multi-step processes. The gas fraction is maximized operating at high temperatures.
2.1 Composition and Characteristics of Lignocellulosic Biomass
Here, the main characteristics of lignocellulosic biomass will be presented. Note that other types of biomass materials with significant differences in their composition, such as animal residues, municipal solid waste, sewage sludge, and others, are not included in this description; however, they might also have potential for hydrogen production by means of pyrolysis. The three major constituents of lignocellulosic biomass are cellulose (40–50 %), hemicellulose (20–40 %), and lignin (5–30 %) [1]. The structure of cellulose, as well as the main monomers of hemicellulose and lignin, is shown in Fig. 5.1. Some authors have found that interactions between these components are negligible [2]; thus, the pyrolysis products could be considered as the summation of the individual contributions from the three main components.
Apart from the three main components, minor amounts of solvent-extractable compounds (extractives) can be found in lignocellulosic biomass: triglycerides, fatty acids, resin acids, steryl esters, sterols, and lignans [3]. Inorganic constituents can also be found in biomass, with total contents varying in a range from less than 1 % in softwoods to 15 % in herbaceous biomass [4]. Because of the catalytic effects of most of the main inorganic constituents (K, Ca, Na, P), biomass decomposition reactions and char formation can be altered by their presence, especially for cellulose [5].
The original water content of biomass also plays an important role during pyrolysis for various reasons. First, it influences heat requirements because it needs to be evaporated in the pyrolysis reactor and may render the process uneconomical. Second, an excessive water content impedes biomass particle size reduction, which is needed in several types of pyrolysis reactors. Third, most of the water ends up in the liquid fraction. Finally, the presence of water has been shown to produce higher yields of char [6, 7].
2.2 Reaction Pathways and Types of Pyrolysis
In most cases, the pyrolysis process takes place at moderate temperatures (300–600 °C) and is driven towards the production of a majority of solid or liquid fraction. If a major gaseous fraction is preferred, then gasification or combustion processes at higher temperatures, involving the presence of oxidizing agents such as air or steam, are more suitable. Bridgwater [8] classified pyrolysis processes and indicated that the main product obtained is gas when fast pyrolysis is carried out at temperatures higher than 700 °C.
Traditionally, a distinction between slow and fast pyrolysis, based on the process heating rate, has been made at the mentioned temperature interval to distinguish processes with major solid, liquid, or gas yields. However, this classification oversimplifies the complexity of the pyrolysis process [5]; the residence time of devolatilization products and the conditions in which this residence time goes by are equally relevant. These factors are crucial because primary decomposition condensable products (sometimes called primary tars) can undergo two competitive reaction pathways: cracking to non-condensable gaseous products or formation of secondary solid char depending on the reactor conditions (mainly pressure and gas flow rate) [7, 9] and inorganic content of biomass, as shown in Fig. 5.2. As a result of both competitive pathways, the final condensable yield is reduced; thus, they should be avoided if liquid yield has to be maximized. Therefore, the so-called fast pyrolysis conditions, i.e., high heating rates, careful temperature control, very low vapor residence times, and rapid cooling of the vapors, are required for maximum bio-oil production [10].
The thermodynamics of the pyrolysis process are also determined by these reaction pathways. For instance, secondary char formation from condensable compounds is an exothermal process [7] that might cause an overall exothermal heat of pyrolysis if operational conditions are directed towards maximum char formation, whereas if bio-oil (condensable products) is preferred, the overall process will be clearly endothermic.
2.3 Product Distribution and Characteristics
The proportions of the solid, liquid, and gaseous product fractions in non-catalyzed fast pyrolysis can range between 10–40 %, 20–75 %, and 10–30 %, respectively, and are greatly dependent on the process operating conditions, mainly process temperature and pressure, heating rate, and residence time of the vapors. The biomass feedstock composition and properties (moisture, particle size, or density) also play an important role in product distribution.
The liquid fraction or bio-oil is a complex mixture of water and diverse organic compounds. Water (15–30 %) comes from both the original biomass moisture and devolatilization reactions. A typical composition of the whole bio-oil is (average of different lignocellulosic biomasses, on a dry basis) [11] 56.7 % C, 6.3 % H, 36.8 % O, and 0.2 % N. The maximum amount of obtainable H2 is limited by both chemical composition and water content of the liquid fraction.
Bio-oil can be easily separated into two distinct phases either by fractionation (water addition) or centrifugation. The aqueous phase is a complex mixture consisting of carboxylic acids, aldehydes and ketones, alcohols, sugars, low molecular weight oligomers, and other more complex carbohydrates. The remaining fraction that is often referred to as pyrolytic lignin or organic phase contains a wide variety of high molecular mass lignin-derived compounds.
As a whole, bio-oil from lignocellulosic biomass is acidic in nature (with pH values between 2 and 4) and contains high amounts of oxygenated compounds. Both characteristics cause chemical instability over time, causing storage problems [12]. The high oxygen content confers bio-oil a low energy density compared to that of petroleum-based fuels. Indeed, it has a heating value of less than half of that of hydrocarbon fuels [13]. Finally, bio-oil might also have high contents of solid particles in suspension.
The origin of some of the individual components of bio-oil can be directly related to the main biomass constituents. For instance, levoglucosan, glycolaldehyde, and cellobiosan are products of cellulose pyrolysis [14], which has been extensively studied. Their amounts are significantly influenced by inorganic content, and they can further react to form gases or char depending on the operational conditions. Lignin pyrolysis has been much less investigated [5]; it produces mainly phenolic compounds such as guaiacols and syringols [15], and “pyrolytic lignin,” which is composed of relatively large fractions of the original lignin.
Biomass charcoal, also named char or biochar, is a carbonaceous solid that retains part of the original biomass structure and can have a carbon content as high as 90 % [7]. Lignin from biomass produces comparatively higher yields of char than cellulose and hemicellulose. Lignin also concentrates mostly in the original inorganic content of biomass. Its main uses are as activated carbon precursor and as a fuel. Recent research proposes its use as soil amendment with the additional benefit of long-term carbon capture [16].
The permanent gases from pyrolysis of biomass are mainly composed of CO and CO2, but also CH4, H2, and light hydrocarbons. Additionally, H2S or NH3 may be present if the amounts of sulfur or nitrogen in the original biomass are high. The gas mixture can be burned to provide part of the energy needed to drive the pyrolysis process or be subjected to catalytic treatment in a one-step process to improve H2 yield, as well as to convert the condensable fraction.
2.4 Pyrolysis Reactors
Because bio-oil is an important intermediate in hydrogen production by catalytic pyrolysis of biomass using multi-step processes, this subsection examines pyrolysis reactors as they pertain to bio-oil production.
At typical fast pyrolysis conditions (500 °C, hot vapor residence time of ~1 s), a high yield of up to 75 % bio-oil can be obtained. To achieve these operational conditions, a fluidized bed is the preferred choice. Other reactors will be briefly discussed. A more detailed description of the main features of fast pyrolysis reactors can be found in the literature [5, 17].
Fluidized bed reactors allow a very good temperature control and heat transfer to the biomass material, but require careful selection of feedstock particle size distribution. They can be scaled up to maximum throughputs of several tons per hour. Usually, a bed material is required for enhancing heat transfer and fluidization of biomass, if needed. The bed material can be kept bubbling or be transported (circulating fluidized beds) to a secondary reactor where char is burned and hot bed material is recycled to the fluidized bed. In both cases, an inert fluidizing agent (such as nitrogen or recycled combustion gases) is needed.
At temperatures between 400 and 650 °C, Fig. 5.3 shows a typical product distribution of non-catalyzed, fluidized bed pyrolysis of lignocellulosic biomass [18]. The yield of hydrogen is generally very low and increases with temperature [19] without reaching a maximum value within this temperature interval. The addition of catalysts significantly alters these product distributions and may trigger hydrogen formation at lower temperatures, as well as higher concentrations of the desired product.
Product distribution of poplar pyrolysis vs. temperature (Reprinted with permission from Bridgwater [17], Copyright © 2011 Elsevier)
In fluidized bed reactors, a catalyst of adequate physical properties and fluid dynamic behavior can be continuously added to the bed for hydrogen production. However, catalyst particles are subjected to high attrition rates in fluidized beds, and regeneration of the spent catalyst may require char separation. Additionally, char particles carry-over can cause volatile decomposition, decreasing bio-oil yield.
The rotating cone reactor, developed at University of Twente [20], uses centrifugal force to mix hot sand and biomass particles. Rapid heating and small vapor residence time are achieved. Combustion of char provides the necessary heat for the process, and no carrier gas is needed. It can be scaled up to several tons per day [5, 21].
In ablative pyrolysis reactors, biomass is pressed against a hot surface, either in motion (rotating cylinder, disk or blade) or static (vortex or cyclone reactors). The main advantages of these systems are the possibilities of processing big biomass particles, using cold gas carriers, and including a catalyst in the hot surface [5]. Its applicability to large-scale systems is limited because scaling is a linear function of heat transfer area [21].
Auger and screw reactors involve mechanically mixing biomass with hot sand and transporting the mixture inside a cylinder. No carrier gas is needed so that auger and screw reactors offer good potential for scale-up [21].
3 Catalysts
Like any other hydrogen production process, biomass pyrolysis requires the use of catalysts to produce a hydrogen-rich gas. In fact, the catalyst is considered as a critical factor in the hydrogen production process from biomass to obtain high selectivity and high hydrogen purity. Key properties for a catalyst to be used for hydrogen production are C-C and C-O bond cleavage activity, WGS activity, low coke formation, resistance to deactivation by poisoning, and thermal and mechanical stability. Properties of the catalyst support are also important: usually it also presents an intrinsic catalytic activity that can enhance the reforming or act as a deterrent to coke deposition, which is one of the main drawbacks of the process.
The performance of a catalyst depends on its intrinsic characteristics, such as active metal nature, metal dispersion, and surface area, but also on the chemical nature of the compounds to be reformed. Thus, the process is not only influenced by the biomass being treated, but also by the pyrolysis conditions, as explained in previous sections. Furthermore, the selected process, one-step or multi-step, and the reactors, fixed and fluidized beds, have also a significant influence on catalyst performance.
Regarding the nature of catalysts, one approach is the use of noble metals, which have a high activity towards hydrogen production and lower selectivity to coke formation. In Table 5.1, several noble metal catalysts used in pyrolysis-based hydrogen production are shown. As can be seen, platinum is the most widely used catalyst of this kind.
However, and due to the scarcity in nature and high prices of noble metals, the use of transition metals, especially nickel, has also been studied, in spite of their lower activity and higher tendency to coke deactivation.
Apart from noble metals, the use of several different catalysts such as Na2CO3, K2CO3, CaMgCO3, La/Al2O3, and Cr2O3, among others, was reported in a recent review [31]. ZnCl2- and Ni-based catalysts are mentioned as having more potential towards maximum gas production. The use of other metals is scarcer in literature, for instance, Shoja et al. [32] used iron fillings for tar cracking in a dual bed reactor, at 850 °C.
Ni-based catalysts constitute the most solid alternative approach to noble metals in the design of suitable catalysts for the steam reforming of bio-oil or its fractions. The advantages of Ni-based catalysts compared to those based on noble metals are principally high activity and selectivity towards H2 production at a much cheaper cost. However, Ni-based catalysts are more susceptible to carbon formation [33].
Table 5.2 shows both commercially prepared and laboratory-prepared Ni catalysts. Alumina is the preferred support, although many more materials can be found in the available literature. A previous reduction of the active metal is not always used, as the presence of a certain amount of hydrogen in the gas, or its in situ formation, is enough to reduce the Ni oxides at the reactor temperature.
As concluding remarks, and despite the wide range of materials, preparation methods, raw materials, and experimental conditions, it is clear that the presence of an active catalyst and a high temperature (over 1000 K) are needed in order to obtain a hydrogen yield that can reach around 100 g H2/kg of biomass. Still there is much work to do on the improvement of on-stream catalyst stability over long periods of time, on the grounds that the economy of the process will significantly rely on catalyst performance and durability.
4 One-Step Processes
In one-step processes of catalytic pyrolysis, the catalyst is located in the same vessel where pyrolysis occurs. Considering the schematic representation of catalytic pyrolysis proposed by Garcia et al. [48], both stages, being pyrolysis the first and the action of catalyst the second, occur in the same reactor.
One-step processes have the advantages of process integration and smaller equipment costs compared to multi-step processes. Process integration can achieve some energy savings, as endothermic reactions, such as pyrolysis, occur simultaneously with other exothermic reactions such as water-gas-shift reaction. Heating of pyrolysis products is not required, as it happens, for example, in catalytic steam reforming of bio-oil, one example of multi-step process. The equipment cost diminishes because only one reactor is required in the process.
The drawbacks of this alternative are more severe catalyst deactivation and lower hydrogen content in product gas compared to multi-step processes. Considering a one-step process with a continuously fed fluidized reactor, high liquid yield is produced at relatively low temperature and the catalyst must convert/transform it into gases; as a consequence, more “work” for the catalyst is required [48]. In contrast, considering a multi-step process with two reactors, where in the first pyrolysis is being carried out at high temperature, more cracking occurs and less liquid production is generated (also, liquid conversion by thermal cracking can occur in the piping between the first and the second step, where catalyst is placed); as a consequence, less “work” of the catalyst is needed and then less catalyst deactivation will be observed.
The comparison of one-step process such as catalytic pyrolysis in a continuously fed fluidized reactor [48, 49] with a multi-step process such as catalytic steam reforming of bio-oil [50] indicates that lower hydrogen content in product gas is achieved in one-step catalytic pyrolysis than in catalytic steam reforming of bio-oil. For instance, the content of hydrogen in product gas was 67 vol.% (nitrogen and steam free) at 650 °C and 0.042 g catalyst h/g organics in the steam reforming of the aqueous fraction of bio-oil [50], a multi-step process, while in catalytic pyrolysis of biomass (pine sawdust), a one-step process, the content at the same temperature was 52 vol.% (nitrogen free) at 0.8 g catalyst h/g biomass [48].
In catalytic pyrolysis, one-step process, the moisture of biomass is involved and no more water is added; thus water-gas-shift reaction hardly takes place. In spite of the low content of hydrogen, this process can produce a gas with a H2/CO ratio useful as synthesis gas for processes such as methanol or Fischer-Tropsch, among others.
In catalytic steam reforming of bio-oil, multi-step process, water is added and water-gas-shift reaction converts CO into CO2 and H2, increasing the hydrogen content in product gas. If the purpose is the generation of hydrogen-rich gas in one-step process, the addition of water is required and then the suitable process is steam gasification [51].
One-step catalytic pyrolysis processes can be divided in two groups: conventional and innovative processes. Table 5.3 presents a summary of experimental works of conventional one-step catalytic pyrolysis processes, carried out at bench scale. In this table it can be observed that catalytic pyrolysis is carried out at atmospheric pressure and temperatures from 450 to 850 °C. There are two different types of experimental installations. In the first type, a batch of biomass is loaded in the reactor, being 90 g the highest amount of biomass reported [52]. The second type uses a continuously fed fluidized bed reactor with a biomass feeding rate lower than 32 g/h [49].
Chen et al. [52] recommended the use of 30 % weight of catalyst load to biomass. Also, they found Cr2O3 as the best catalyst with stronger catalytic role than other metal oxides. Cr2O3 showed the highest yields to total gas and hydrogen content in gas in their experiments.
In the work of Ansari et al. [46], the reactor contains two beds in a continuous downflow. This is considered as one-step process because only one reactor is used. In the first bed the pyrolysis of bagasse is carried out, and the second bed, below the first one, contains the catalyst and the catalytic cracking of tar is performed. They tested Ni-Fe/γ-Al2O3 catalysts prepared by two methods: co-impregnation and microemulsion. Using a microemulsion technique with a water to surfactant ratio of 1, the average metal particle size was decreased to 3.7 nm. Using this catalyst, the gas yield increased from 0.397 to 0.758 m3/kg and decreased the tar yield from 0.445 to 0.237 g/g biomass compared to the noncatalytic process, while the heating value of the product gas remained almost constant (10–11 MJ/m3). This last result is a consequence of the decrease in CnHm yield due to catalytic cracking.
Qinglan et al. [44] used a fluidized bed, where the primary decomposition and secondary reactions occurred simultaneously. The catalyst selected was a commercial NiMo/Al2O3 catalyst. In their study, four biomasses were tested: pine, Alaskan spruce, tropical lauan and rice husks. They found similar results in gas yields of the three woody biomass samples. They attributed this result to their similar compositions. Different results were obtained with rice husks compared to woody biomass. As an example, H2 yield of woody biomass was higher than that of rice husks under the same operating conditions.
Arauzo et al. employed nickel-based catalysts prepared by coprecipitation in the laboratory [49, 53]. Some catalysts were modified with magnesium or potassium. They concluded that the addition of magnesium in the catalyst did improve resistance to attrition but resulted in a minor loss in gasification activity and increased coke production. The addition of potassium had little effect.
Garcia et al. employed also a Ni-Al coprecipitated catalyst prepared in the laboratory [48, 54, 59]. They studied the influence of calcination and reduction conditions on catalyst performance. They concluded that carrying out the catalytic pyrolysis at 650 and 700 °C, the catalyst calcined at 750 °C can be reduced by H2 and CO generated during the pyrolysis reaction. The catalyst calcined at 850 °C required more severe reduction conditions to achieve the active phase of the catalyst. In pyrolysis at 650 °C, the most stable catalyst performance is achieved using the catalyst calcined at 850 °C and reduced during 1 h with a hydrogen flow rate of 3080 cm3 (STP)/min. At a reaction temperature of 700 °C, the highest H2 and CO yields are obtained using the catalyst calcined at 750 °C without previous reduction.
Garcia et al. [48] also studied the influence of catalyst weight/biomass flow rate (W/mb) ratio on gas production using the Ni-Al coprecipitated catalyst calcined at 750 °C without previous reduction. For W/mb ratios ≥0.4 h, no significant modifications were observed on the initial yields of different gases, with a gas composition similar to thermodynamic equilibrium. For W/mb ratios <0.4 h, a simple first-order kinetic equation has been suggested for H2 and CO formation. These authors analyzed gas yield evolution over time. Figure 5.4 shows the results for an experiment carried out at 700 °C with a W/mb ratio of 0.31 h. As can be observed, total gas and H2 and CO yields diminish with experimental time, while CO2, CH4, and C2 yields increase. This evolution is a consequence of the loss of catalyst activity. The deactivation of the catalyst is mainly caused by the formation of carbon deposits on the catalyst surface.
Gas yield evolution versus time for an experiment of catalytic pyrolysis. Temperature = 700 °C, W/mb = 0.31 h. Total gas ■; H2 ▲; CO □; CO2 Δ; CH4 ×; C2 ○ (Reprinted with permission from García et al. [48], Copyright © 1998 American Chemical Society)
In all the mentioned studies, the effect of the catalyst is the same: increasing gas yield and decreasing liquid yield. Hydrogen yield significantly increases with the presence of the catalyst. In Table 5.4 some results of gas yields and gas composition extracted from some experimental works are shown.
The comparison of these results is difficult. The temperatures of catalytic pyrolysis in the works of Qinglan et al. [44] and Garcia et al. [48] are different, although both studies were performed in an experimental installation with a fluidized bed reactor. Moreover, the catalysts are different. For both studies the maximum hydrogen content showed in Table 5.4 is around 52 vol.%. For a batch installation [58] a smaller hydrogen content was obtained, 28.7 vol.%.
It is worth mentioning that gas yield can be as high as 0.9 g gas/g biomass, for temperatures of 650 and 700 °C obtained in the work of Garcia et al. [48]. The time for these experiments was 49 and 181 min at 650 and 700 °C, respectively, which correspond to 20.4 and 50.6 g biomass fed in, respectively.
The use of a different approach to carry out the pyrolysis of biomass in innovative processes such as the use of a molten alkali reactor or a microwave plasma deserves also to be mentioned.
Jiang et al. [60] studied six biomass feedstocks (fir sawdust, birch sawdust, redwood sawdust, rice stalk, cole stalk, and rice husks) in a stainless steel reactor with about 700 g of molten alkali (NaOH) at temperatures from 350 to 550 °C. The product gas only contained hydrogen and methane. Redwood sawdust was the biomass with the highest H2 yield (65.4 g H2/kg biomass at 450 °C). The increase of temperature caused the increase in H2 yield with values from 30.7 to 66.5 g H2/kg biomass at 350 and 550 °C, respectively, using rice stalk as feed. The introduction of additives, especially NiCl2, led to increased H2 yields. H2 content in pyrolysis gas was higher than 80 % in most of the studied conditions.
Microwave plasma reactor was used in the study of Spirulina algae pyrolysis by Lin et al. [61]. The pyrolysis was carried out at temperatures of 790, 820, and 848 °C and at atmospheric pressure. 1 g of biomass was loaded in a quartz tube. Although no catalyst was used, significant content of H2 in product gas and high H2 yield were obtained, with values of 45 vol.% and 31.5 g H2/kg biomass, respectively, at 848 °C.
Figure 5.5 shows H2 yield generated in some one-step pyrolysis processes, both conventional and innovative. It is generated the highest yield of H2 in molten alkali (NaOH-NiCl2) with a value of 67 g H2/kg biomass [60].
5 Multi-step Processes
The present section aims at presenting an overview of the different multi-step approaches that have been proposed regarding hydrogen production from catalytic pyrolysis of biomass.
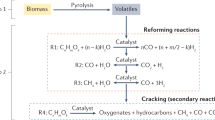
A scheme of the different multi-step routes that lead to hydrogen from catalytic pyrolysis of biomass is presented in Fig. 5.6.
As can be seen, after the pyrolysis stage, three main alternatives have been proposed by various research groups in the literature, which ultimately can be divided into two: catalytic reforming of bio-oil, fractions of it or from its resulting products after a preliminary thermal processing of the bio-oil (pre-reforming), and direct catalytic cracking of the bio-oil. In all these cases, different reactor configurations and designs have been proposed in addition to the significant efforts made by many researchers for developing suitable catalysts for these processes, as previously discussed in this chapter.
All these routes necessarily require a downstream gas conditioning step if high-purity hydrogen is sought. Conventional hydrogen purification processes include further processing downstream of the hydrogen-rich product gas in catalytic water-gas-shift (WGS) reactors [62] and use of hydrogen-selective membranes [63] or pressure-swing adsorption (PSA) equipment [64, 65], among others.
5.1 Catalytic Steam Reforming of Bio-Oil
The first studies on hydrogen production from multi-step processes involving biomass pyrolysis were pioneered in 1993 by the National Renewable Energy Laboratory (Colorado, USA) [66, 67]. The strategy proposed by the research group led by Chornet is the catalytic steam reforming of the liquid pyrolysis products or its fractions. A separation of the bio-oil into two phases by water addition was used. The lignin-derived fraction would be devoted to the production of value-added chemicals, benefitting from a high content in phenolic compounds, whereas the carbohydrate-rich aqueous fraction would be subjected to catalytic steam reforming in a process similar to that employed in the production of hydrogen by catalytic steam reforming of natural gas and naphthas [35, 68].
The initial works of this group [34–36] preliminarily explored this process by means of thermodynamic analyses and also conducting experimental tests in a laboratory scale, using a dual fixed-bed quartz microreactor system coupled to a molecular beam mass spectrometer (MBMS). A detailed description of the setup can be found, for example, in the work of Wang and co-workers [34], where the possible reaction mechanisms for producing hydrogen were discussed in depth after conducting the steam reforming of bio-oil model compounds (acetic acid and hydroxyacetaldehyde). Further on, a catalytic fixed-bed reactor setup at bench scale was used in the catalytic steam reforming tests using the aqueous fraction of poplar bio-oil produced at NREL [36]. Screening of catalysts, feedstocks, and operating conditions was initially conducted with different oxygenates used as model compounds [35], representative of the complex composition of bio-oils, and subsequently extended to the aqueous fraction of bio-oil [36]. Both commercial and research Ni-based catalysts were tested, along with a commercial low-temperature shift conversion Cu-based catalyst [35].
In that work, two possible alternatives for hydrogen production from biomass pyrolysis oil were proposed: a regionalized system consisting of small- to medium-scale pyrolysis units coupled to a centralized large-scale catalytic reforming unit, in which the fractionation step would be carried out and subsequently the aqueous fraction of bio-oil would be processed for producing hydrogen. The other envisaged possibility was an integrated system consisting of larger processing plants in which biomass would be directly converted to hydrogen having all the necessary steps: pyrolysis reactor coupled to a catalytic reformer in which pyrolysis vapors would yield hydrogen via steam reforming. Given the intrinsic characteristics of biomass (low energy content, dispersed localization of the feedstock, etc.), from the feasibility analysis carried out, it was concluded that the first alternative was more adequate for producing hydrogen from biomass pyrolysis.
Later on, Czernik et al. [68] proposed a different configuration for the catalytic steam reforming reactor, as a result of the major problem encountered in their previous works, which is catalyst deactivation caused by the formation of carbonaceous deposits. Thus, it was proposed that a fluidized bed reactor could be a more appropriate configuration for the catalytic reformer. As a result, that work and the subsequent works of this group [69–71] focused on the development of suitable catalysts for steam reforming of oxygenates using both a microscale fixed-bed reactor configuration and a 2-in.-diameter fluidized bed reactor setup. A different approach for hydrogen production via distributed bio-oil reforming was further followed by this group, which will be discussed in depth later.
Following the approach of catalytic steam reforming of bio-oil or fractions of it, different research groups have also explored this route ever since. Universidad de Zaragoza (Zaragoza, Spain) worked on the development of suitable catalysts for the process using different process configurations as a result of collaborations with NREL [33].
Afterwards, the research conducted by Universidad de Zaragoza focused on the development of Ni-based catalysts using both a small bench-scale fixed-bed unit [50, 72–76] and a bench-scale fluidized bed setup [76–82]. The fluidized bed setup included a feeding system consisting of a quartz coaxial injection nozzle made of four concentric tubes which helped feeding the liquid feed in the form of spray while avoiding clogging as a result of the refrigeration supplied by the external cooling jacket formed by the outer tubes.
These studies aimed at the development of catalysts with good activity and selectivity to hydrogen, as well as resistant to deactivation by coke deposition and to attrition. The effect of the Ni content on the carbon conversion to gas and the product gas yields, especially H2, was studied. Furthermore, the incorporation of modifiers to the catalysts was also explored in various works in order to, on the one hand, decrease the formation of carbonaceous deposits on the catalyst surface and, on the other hand, increase the mechanical resistance of the catalysts, thus developing more stable catalysts that ultimately could be used in the fluidized bed reactor during longer operation times. Other works can be found in the literature aiming at the development of suitable catalysts using fixed-bed reactors, both with model compounds [22, 25, 26, 83–86] and with bio-oil or fractions of it [22, 25, 27, 87–91]. Li et al. [92] conducted the catalytic steam reforming of the aqueous fraction of bio-oil using various Ni-based and dolomite catalysts in a stainless steel lab-scale fluidized bed reactor. A similar approach was followed by Xu et al. [93] using sieved particles obtained from a milled commercial NiO/MgO catalyst for the steam reforming of bio-oil from rice husk. Panigrahi et al. at the University of Saskatchewan also conducted steam gasification of bio-oil aiming at hydrogen production in an Inconel tubular reactor containing a noncatalytic fixed bed [94]. The reaction bed was composed of quartz chips, but the possible catalytic wall effect provoked by the presence of active metals such as Ni, Cr, Fe, or Mn in the Inconel alloy was not discussed.
Other alternative configuration is that proposed by the University of Thessaloniki and the Centre for Research and Technology Hellas (CERTH) from Greece. The production of hydrogen was investigated in a spouted bed reactor at a pilot scale [95, 96]. The reactor was made of stainless steel and has two differentiated parts: an inverted conical base followed by a 0.05-m inner diameter cylindrical part. The main advantage of such reactor configuration compared to others is related to the efficient heat recirculation caused by the particular solids dynamics in the spouted bed reactor. On the negative side, spouted bed reactors, similarly to what occurs in fluidized bed configurations, present significant catalyst losses as a result of attrition, which compromises the scale-up and development of such processes at full scale.
These authors explored the steam reforming of model compounds and of the aqueous fraction of bio-oil at atmospheric pressure, varying the usual operating process parameters and also studying the addition of small amounts of oxygen (temperature, steam to carbon molar ratio, and oxygen to carbon ratio). Different catalysts were tested, both commercial and other Ni based prepared over various supports. The authors reported good results in terms of limiting the formation of carbonaceous deposits as a result of the type of reactor used, while particularly a Ni/olivine catalyst presented an adequate mechanical resistance to attrition and yielded the most promising results using model compounds. However, the results of the catalytic steam reforming tests using the aqueous fraction of pine bio-oil reflected a low reforming activity and, subsequently, low hydrogen yields.
The University of Twente (Netherlands) also developed an interesting proof of concept for hydrogen production from catalytic pyrolysis of biomass [97, 98]. Instead of bio-oil fractionation followed by catalytic steam reforming, the group led by Van Swaaij proposed a different approach, aiming at an industrial development of hydrogen production from bio-oil. A two-stage reactor concept consisting of a sand fluidized bed and a catalytic fixed bed was developed. The proposed alternative aimed at overcoming two main problems. On the one hand, large-scale fixed-bed reactors would require a high heat supply at the entrance of the reactor, which could result in a significantly lowered temperature at that point. On the other hand, decoupling of the evaporation, primary pyrolysis oil conversion, and gas conditioning enabled to diminish significantly the catalyst deactivation by deposition of coke on the catalyst surface, since the preliminary cracking of organics from the bio-oil upon heating took place in the inert bed of the fluidized reactor, and simpler molecules could be reformed in the catalytic fixed bed. This also made it unnecessary to develop catalysts with increased mechanical strength.
The two-stage reactor was also proposed by Wu et al. [99], though in this case, both primary and secondary reformers were two catalytic fixed-bed reactors made of stainless steel. The concept proposed by the authors aimed at preserving the steam reforming catalyst operating lifetime by including a low-cost primary reforming catalyst (olivine) that could prevent the Ni-based catalyst in the secondary reformer from rapid deactivation by coke formation. Kan et al. [100] also proposed a dual fixed-bed configuration with two reaction beds in a tubular quartz reactor at lab scale, though in this case the first reaction bed contained quartz sand, thus serving for the vaporization and cracking of the crude bio-oil, and in a second catalytic bed, containing a NiCuZnAl catalyst, steam reforming of the bio-oil vapors took place. More recently, researchers from the University of the Basque Country (Spain) have also explored this approach [101, 102], by using a two-reactor configuration composed of a fixed-bed pre-reformer (thermal step) containing an inert filler (e.g., glass spheres) operating at low temperature (between 200 and 400 °C) and a catalytic fluidized bed reactor for the steam reforming of the bio-oil vapors using Ni-based catalysts.
Another interesting possibility is the cyclic operation of the catalytic fixed-bed reactor by means of developing a chemical looping steam reforming process [103]. This cyclic approach is similar to that initially proposed by the group of Mirodatos for catalytic cracking of the crude bio-oil [41], which will be further discussed. The authors claimed that it is possible to use a Ni-based steam reforming catalyst as an oxygen transfer material operating in a series of cycles of reduction/oxidation. The bio-oil would be steam reformed during the reduction step, in which the catalyst could be reduced during the beginning of the step and afterwards the catalyst could be regenerated in the oxidation step by burning off the carbonaceous deposits formed on the catalyst surface.
Other reactor configurations include a Y-shaped dual catalytic reactor proposed by Hu and Lu that aimed at combining steam reforming of bio-oil and dry reforming of bio-oil using CO2 [104]. In one branch of the Y-shaped reactor, there is a catalytic fixed-bed reactor (catalytic bed I), in which steam reforming of bio-oil takes place (partial oxidation and oxidative steam reforming are suggested as alternative processes that could take place in this catalytic fixed bed with the pertinent selection of catalysts and reactants). In the second branch acting as inlet, another portion of the bio-oil would directly be fed into the reactor for conducting catalytic dry reforming using the CO2 from the product gas stream coming out of the catalytic bed I. The catalytic dry reforming takes place in the last section of the reactor, where a second catalytic fixed bed (catalytic bed II) is placed. Rather than focusing on the production of hydrogen, the goal is to produce syngas, though by varying the process conditions the syngas could have different H2/CO molar ratios.
From an industrial point of view, the most adequate configuration for the bio-oil steam reforming unit is probably that following a similar approach to what the University of Twente proposed. Thus, the industrial unit should have a pre-reformer plus a main catalytic steam reformer, since it would be more flexible in terms of admitting various feedstocks, would mitigate the catalyst deactivation by carbon formation, and could have a better energy efficiency, while benefiting at the same time from a smaller size of the main reformer [105]. This could enhance the economic viability of the process. Trane et al. [105] proposed a simple flow sheet of a two-reactor industrial plant for the steam reforming of bio-oil in which the production of hydrogen takes place in three steps: a low-temperature steam reforming reactor (pre-reformer), a high-temperature reformer, and a low-temperature shift reactor. An optional downstream desulfurization reactor was proposed in two possible locations: after the pre-reformer or right after the water-gas-shift reactor.
5.2 Catalytic Cracking of Bio-Oil
A different approach was proposed by the group of Mirodatos from the Catalysis Research Institute in France [41, 106, 107]. The group proposed an alternative strategy, which is to control the carbon deposition during a cracking step and to frequently regenerate the catalyst, in resemblance to the catalytic cracking of methane for hydrogen production. Therefore, the continuous hydrogen production process requires at least two (or more) parallel reactors. In one reactor the catalytic cracking reaction producing hydrogen is taking place, while on the other reactor, the coke is gasified or burned in order to restore the catalytic activity, periodically switching the cracking/regeneration cycle in each reactor. Thus, this approach is claimed to operate in autothermic regime, with the combustion of the coke during the regeneration step in one of the reactors supplying the necessary heat for the cracking hydrogen production step in the other reactor.
This group designed a double-walled stainless steel tubular reactor at laboratory bench scale that had an external cooling jacket using water as refrigerant in order to inject bio-oil at temperatures below 50 °C, thus avoiding undesired polymerization of bio-oil and clogging of the reactor. Bio-oil without any further addition of water was injected by means of a syringe pump through a capillary located on top of the catalytic fixed-bed reactor.
Recently, the research team led by Czernik at the National Renewable Energy Laboratory (USA) has proposed a different strategy, which is based on distributed bio-oil reforming aiming at developing an integrated system that will provide distributed production of hydrogen [108]. The project tackles the challenge of hydrogen production in a dispersed manner by converting bio-oil to H2 at a targeted total dispensed hydrogen cost between 2 and 4 US$/kg of H2 (produced, delivered, and dispensed, but untaxed). The rationale of the work lies on the much easier transportation of liquid bio-oil to scattered points in which H2 may be required (e.g., fueling stations), which could be delivered and stored in order to be processed into hydrogen in the vicinity of the demanding points.
The proof of concept aims at developing a compact, low-capital cost and little-to-none maintenance process. The proposed schematic flow diagram comprises a multi-step approach, in which bio-oil (blended with an alcohol, such as methanol, so as to control the physical and chemical properties of the liquid, namely, viscosity) is firstly volatilized using ultrasonic atomization in a nozzle evaporator operating at temperatures around 400 °C. The vapors would flow through a hot filter at operating temperatures between 400 and 600 °C in order to collect solid char formed during the volatilization process. The next step includes a catalytic partial oxidation/steam reforming packed-bed reactor operating at 800–850 °C in auto-thermal regime (by adding small amounts of air that will burn part of the feed and thus provide the necessary heat for maintaining the process in autothermal regime). The catalyst would either be a Ni-based or a Pt-based. The authors claim that the best results to date have been obtained in preliminary tests in a bench-scale reactor system using the latter (a Pt-based commercial catalyst supplied by BASF). The other elements from the system include a third step consisting of a catalytic WGS packed-bed reactor operating at 350 °C and further separation and purification of hydrogen from the product gas stream by means of an electrochemical separator.
5.3 Other Approaches
The production of hydrogen by means of aqueous phase catalytic reforming of bio-oil was explored by the University of Massachusetts [109]. The concept of aqueous phase reforming of biomass oxygenates derives from the preliminary studies on hydrogen production by biomass supercritical water gasification developed by the research group led by Antal [110]. Later on, the research group led by Dumesic at the University of Wisconsin extensively developed the concept of catalytic reforming of oxygenates in liquid phase [111–113].
The idea is to conduct the catalytic reforming in liquid medium by using moderate pressures, typically in the range of 1500–5000 kPa, and thus diminishing the reforming temperature to values around 500 K. The tubular reactor has an upflow packed catalytic bed configuration and is made of stainless steel due to the pressurized conditions. A gas-liquid separator is necessary in order to obtain the H2-rich product gas. In the work of the research group led by Huber [109], a diluted aqueous fraction of bio-oil (a concentration of around 4–5 % of bio-oil in the solution) was subjected to aqueous phase reforming for producing hydrogen over a 1 wt.% Pt/Al2O3 catalyst, with mixed results. The authors reported low conversions, but high hydrogen selectivity. A process flow diagram for the development of a bio-oil aqueous phase reforming process was proposed in the PhD thesis developed by Vispute [114].
Other alternative concepts deal with alternative means for supplying the necessary heat for the reforming reactions, namely, by using an annular Ni-Cr electric wire through which an alternative electric current passes [100, 115] or use of microwave power [47].
6 Concluding Remarks and Future Outlook
There are many research works that have studied hydrogen production from catalytic biomass pyrolysis.
Of the two approaches for producing hydrogen from pyrolysis of biomass, the multi-step can benefit from the economy of scale, as lignocellulosic biomass is a naturally dispersed resource, and transporting bio-oil to a central reforming unit, of bigger size, could be economically more favorable. Thus, bio-oil is the most important intermediate in the multi-step processes of catalytic biomass pyrolysis.
Catalysts are a key point in the process, and due to the heterogeneity of biomass available and the different processes, at this moment it has to be tailor-designed for each application. Research in new processes and new catalyst formulations is required to solve the inconveniences of catalyst deactivation, although studies are already being carried out in this direction.
There is lack of economy and energy analyses that can help to spur research and focus efforts on industrial application. Hydrogen is an important raw gas with application both in chemical synthesis and as a fuel in high efficiency systems such as fuel cells. The depletion of oil will increase even more the interest in hydrogen from renewable sources with pilot or demonstration scale plants probably becoming feasible as the price of oil rises.
References
McKendry P. Energy production from biomass (part 1): overview of biomass. Bioresour Technol. 2002;83:47–54.
Yang H, Yan R, Chen H, Zheng C, Lee DH, Liang DT. In-depth investigation of biomass pyrolysis based on three major components: hemicellulose, cellulose and lignin. Energy Fuel. 2006;20:388–93. doi:10.1021/ef0580117.
Kallioinen A, Vaari A, Rättö M, Konn J, Siika-aho M, Viikari L. Effects of bacterial treatments on wood extractives. J Biotechnol. 2003;103:67–76. doi:10.1016/S0168-1656(03)00051-8.
Agblevor FA, Besler S. Inorganic compounds in biomass feedstocks. 1. Effect on the quality of fast pyrolysis oils. Energy Fuel. 1996;10:293–8. doi:10.1021/ef950202u.
Lédé J. Biomass fast pyrolysis reactors: a review of a few scientific challenges and of related recommended research topics. Oil Gas Sci Technol Rev d’IFP Energ Nouvelles. 2013;68:801–14. doi:10.2516/ogst/2013108.
Gray MR, Corcoran WH, Gavalas GR. Pyrolysis of a wood-derived material. Effects of moisture and ash content. Ind Eng Chem Process Des Dev. 1985;24:646–51. doi:10.1021/i200030a020.
Antal Jr MJ, Grønli M. The art, science, and technology of charcoal production. Ind Eng Chem Res. 2003;42:1619–40. doi:10.1021/ie0207919.
Bridgwater AV. Catalysis in thermal biomass conversion. Appl Catal A Genet. 1994;116:5–47. doi:10.1016/0926-860X(94)80278-5.
Di Blasi C. Modeling chemical and physical processes of wood and biomass pyrolysis. Prog Energ Combust Sci. 2008;34:47–90. doi:10.1016/j.pecs.2006.12.001.
Bridgwater AV, Meier D, Radlein D. Principles and practice of biomass fast pyrolysis processes for liquids. Org Geochem. 1999;51:3–22. doi:10.1016/S0146-6380(99)00120-5.
Sipilä K, Kuoppala E, Fagernäs L, Oasmaa A. Characterization of biomass-based flash pyrolysis oils. Biomass and Bioenergy. 1998;14:103–13. doi:10.1016/S0961-9534(97)10024-1.
Oasmaa A, Meier D. Norms and standards for fast pyrolysis liquids. J Anal Appl Pyrolysis. 2005;73:323–34. doi:10.1016/j.jaap.2005.03.003.
Czernik S, Bridgwater AV. Overview of applications of biomass fast pyrolysis oil. Energy Fuel. 2004;18:590–8. doi:10.1021/ef034067u.
Antal MJJ, Varhegyi G. Cellulose pyrolysis kinetics: the current state of knowledge. Ind Eng Chem Res. 1995;34:703–17. doi:10.1021/ie00042a001.
Nowakowski DJ, Bridgwater AV, Elliott DC, Meier D, de Wild P. Lignin fast pyrolysis: results from an international collaboration. J Anal Appl Pyrolysis. 2010;88:53–72. doi:10.1016/j.jaap.2010.02.009.
Woolf D, Amonette JE, Street-Perrott FA, Lehmann J, Joseph S. Sustainable biochar to mitigate global climate change. Nat Commun. 2010;1:56. doi:10.1038/ncomms1053.
Bridgwater AV. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy. 2012;38:68–94.
Meier D, Van De Beld B, Bridgwater AV, Elliott DC, Oasmaa A, Preto F. State-of-the-art of fast pyrolysis in IEA bioenergy member countries. Renew Sustain Energy Rev. 2013;20:619–41. doi:10.1016/j.rser.2012.11.061.
Garcia-Perez M, Wang XS, Shen J, Rhodes MJ, Tian F, Lee W-J, Wu H, Li CZ. Fast pyrolysis of oil Mallee woody biomass: effect of temperature on the yield and quality of pyrolysis products. Ind Eng Chem Res. 2008;47:1846–54. doi:10.1021/ie071497p.
Wagenaar BM, Kuipers JAM, Prins W, van Swaaij WPM. The rotating cone flash pyrolysis reactor. In: Bridgwater AV, editor. Advances thermochemical biomass conversion. Dordrecht: Springer; 1993. p. 1122–33.
Butler E, Devlin G, Meier D, McDonnell K. A review of recent laboratory research and commercial developments in fast pyrolysis and upgrading. Renew Sustain Energy Rev. 2011;15:4171–86. doi:10.1016/j.rser.2011.07.035.
Takanabe K, Aika K, Seshan K, Lefferts L. Sustainable hydrogen from bio-oil – steam reforming of acetic acid as a model oxygenate. J Catal. 2004;227:101–8.
Takanabe K, Aika K, Seshan K, Lefferts L. Catalyst deactivation during steam reforming of acetic acid over Pt/ZrO2. Chem Eng J. 2006;120:133–7.
Takanabe K, Aika K, Inazu K, Baba T. Steam reforming of acetic acid as a biomass derived oxygenate: bifunctional pathway for hydrogen formation over Pt/ZrO 2 catalysts. J Catal. 2006;263:263–9. doi:10.1016/j.jcat.2006.07.020.
Rioche C, Kulkarni S, Meunier FC, Breen JP, Burch R. Steam reforming of model compounds and fast pyrolysis bio-oil on supported noble metal catalysts. Appl Catal B Environ. 2005;61:130–9. doi:10.1016/j.apcatb.2005.04.015.
Basagiannis AC, Verykios XE. Catalytic steam reforming of acetic acid for hydrogen production. Int J Hydrog Energ. 2007;32:3343–55.
Basagiannis AC, Verykios XE. Steam reforming of the aqueous fraction of bio-oil over structured Ru/MgO/Al2O3 catalysts. Catal Today. 2007;127:256–64. doi:10.1016/j.cattod.2007.03.025.
Basile A, Gallucci F, Iulianelli A, Borgognoni F, Tosti S. Acetic acid steam reforming in a Pd-Ag membrane reactor: the effect of the catalytic bed pattern. J Memb Sci. 2008;311:46–52.
Iwasa N, Yamane T, Takei M, Ozaki J, Arai M. Hydrogen production by steam reforming of acetic acid: comparison of conventional supported metal catalysts and metal-incorporated mesoporous smectite-like catalysts. Int J Hydrog Energ. 2010;35:110–7. doi:10.1016/j.ijhydene.2009.10.053.
Dubey VR, Vaidya PD. Kinetics of steam reforming of acetol over a Pt/C catalyst. Chem Eng J. 2012;180:263–9. doi:10.1016/j.cej.2011.11.034.
Uddin MN, Daud WMAW, Abbas HF. Effects of pyrolysis parameters on hydrogen formations from biomass: a review. RSC Adv. 2014;4:10467–90. doi:10.1039/C3RA43972K.
Shoja M, Babatabar MA, Tavasoli A, Ataei A. Production of hydrogen and syngas via pyrolysis of bagasse in a dual bed reactor. J Energ Chem. 2013;22:639–44. doi:10.1016/S2095-4956(13)60084-4.
García L, French R, Czernik S, Chornet E. Catalytic steam reforming of bio-oils for the production of hydrogen: effects of catalyst composition. Appl Catal A Gen. 2000;201:225–39. doi:10.1016/S0926-860X(00)00440-3.
Wang D, Montane D, Chornet E. Catalytic steam reforming of biomass-derived oxygenates: acetic acid and hydroxyacetaldehyde. Appl Catal A Gen. 1996;143:245–70.
Wang D, Czernik S, Montane D, Mann M, Chornet E. Biomass to hydrogen via fast pyrolysis and catalytic steam reforming of the pyrolysis oil or its fractions. Ind Eng Chem Res. 1997;36:1507–18.
Wang D, Czernik S, Chornet E. Production of hydrogen from biomass by catalytic steam reforming of fast pyrolysis oils. Energy Fuel. 1998;12:19–24.
Marquevich M, Czernik S, Chornet E, Montané D. Hydrogen from biomass: steam reforming of model compounds of fast-pyrolysis oil. Energy Fuel. 1999;13:1160–6.
Marquevich M, Coll R, Montane D. Steam reforming of sunflower oil for hydrogen production. Ind Eng Chem Res. 2000;39:2140–7.
Marquevich M, Medina F, Montané D. Hydrogen production via steam reforming of sunflower oil over Ni/Al catalysts from hydrotalcite materials. Catal Commun. 2001;2:119–24.
Kechagiopoulos PN, Voutetakis SS, Lemonidou AA, Vasalos IA. Hydrogen production via steam reforming of the aqueous phase of bio-oil in a fixed bed reactor. Energy Fuel. 2006;20:2155–63.
Davidian T, Guilhaume N, Iojoiu E, Provendier H, Mirodatos C. Hydrogen production from crude pyrolysis oil by a sequential catalytic process. Appl Catal B Environ. 2007;73:116–27.
Waheed QMK, Williams PT. Hydrogen production from high temperature pyrolysis/steam reforming of waste biomass: rice husk, sugar cane bagasse, and wheat straw. Energy Fuel. 2013;27:6695–704. doi:10.1021/ef401145w.
Zhao B, Zhang X, Sun L, Meng G, Chen L, Xiaolu Y. Hydrogen production from biomass combining pyrolysis and the secondary decomposition. Int J Hydrog Energ. 2010;35:2606–11. doi:10.1016/j.ijhydene.2009.04.011.
Qinglan H, Chang W, Dingqiang L, Yao W, Dan L, Guiju L. Production of hydrogen-rich gas from plant biomass by catalytic pyrolysis at low temperature. Int J Hydrog Energ. 2010;35:8884–90. doi:10.1016/j.ijhydene.2010.06.039.
Zhang Y, Li W, Zhang S, Xu Q, Yan Y. Hydrogen production by the catalytic reforming of volatile from biomass pyrolysis over a bimetallic catalyst. Energ Sources A Recover Util Environ Eff. 2013;35:1975–82. doi:10.1080/15567036.2011.580327.
Hojjat Ansari M, Jafarian S, Tavasoli A, Karimi A, Rashidi M. Hydrogen rich gas production via nano-catalytic pyrolysis of bagasse in a dual bed reactor. J Nat Gas Sci Eng. 2014;19:279–86. doi:10.1016/j.jngse.2014.05.018.
Liu S, Zhu J, Chen M, Xin W, Yang Z, Kong L. Hydrogen production via catalytic pyrolysis of biomass in a two-stage fixed bed reactor system. Int J Hydrog Energ. 2014;39:13128–35. doi:10.1016/j.ijhydene.2014.06.158.
García L, Salvador ML, Arauzo J, Bilbao R. Influence of catalyst weight/biomass flow rate ratio on gas production in the catalytic pyrolysis of pine sawdust at low temperatures. Ind Eng Chem Res. 1998;37:3812–9. doi:10.1021/ie9801960.
Arauzo J, Radlein D, Piskorz J, Scott DS. A new catalyst for the catalytic gasification of biomass. Energy Fuel. 1994;8:1192–6. doi:10.1021/ef00048a005.
Bimbela F, Oliva M, Ruiz J, García L, Arauzo J. Hydrogen production via catalytic steam reforming of the aqueous fraction of bio-oil using nickel-based coprecipitated catalysts. Int J Hydrog Energ. 2013;38:14476–87. doi:10.1016/j.ijhydene.2013.09.038.
García L, Salvador ML, Arauzo J, Bilbao R. Catalytic steam gasification of pine sawdust. Effect of catalyst weight/biomass flow rate and steam/biomass ratios on gas production and composition. Energy Fuel. 1999;13:851–9. doi:10.1021/ef980250p.
Chen G, Andries J, Spliethoff H. Catalytic pyrolysis of biomass for hydrogen rich fuel gas production. Energy Convers Manag. 2003;44:2289–96. doi:10.1016/S0196-8904(02)00188-7.
Arauzo J, Radlein D, Piskorz J, Scott DS. Catalytic pyrogasification of biomass. Evaluation of modified nickel catalysts. Ind Eng Chem Res. 1997;36:67–75. doi:10.1021/ie950271w.
Garcia L, Salvador ML, Bilbao R, Arauzo J. Influence of calcination and reduction conditions on the catalyst performance in the pyrolysis process of biomass. Energy Fuel. 1998;12:139–43. doi:10.1021/ef970097j.
Garcıa L, Salvador M, Arauzo J, Bilbao R. Catalytic pyrolysis of biomass: influence of the catalyst pretreatment on gas yields. J Anal Appl Pyrolysis. 2001;58–59:491–501. doi:10.1016/S0165-2370(00)00114-5.
Çağlar A, Demirbaş A. Hydrogen rich gas mixture from olive husk via pyrolysis. Energy Convers Manag. 2002;43:109–17. doi:10.1016/S0196-8904(01)00012-7.
Demirbaş A. Gaseous products from biomass by pyrolysis and gasification: effects of catalyst on hydrogen yield. Energy Convers Manag. 2002;43:897–909. doi:10.1016/S0196-8904(01)00080-2.
Bru K, Blin J, Julbe A, Volle G. Pyrolysis of metal impregnated biomass: an innovative catalytic way to produce gas fuel. J Anal Appl Pyrolysis. 2007;78:291–300. doi:10.1016/j.jaap.2006.08.006.
Garcia L, Salvador ML, Arauzo J, Bilbao R. CO2 as a gasifying agent for gas production from pine sawdust at low temperatures using a Ni/Al coprecipitated catalyst. Fuel Process Technol. 2001;69:157–74. doi:10.1016/S0378-3820(00)00138-7.
Jiang H, Wu Y, Fan H, Ji J. Hydrogen production from biomass pyrolysis in molten alkali. AASRI Procedia. 2012;3:217–23. doi:10.1016/j.aasri.2012.11.036.
Lin KC, Lin Y-C, Hsiao Y-H. Microwave plasma studies of Spirulina algae pyrolysis with relevance to hydrogen production. Energy. 2014;64:567–74. doi:10.1016/j.energy.2013.09.055.
Ratnasamy C, Wagner JP. Water gas shift catalysis. Catal Rev. 2009;51:325–440. doi:10.1080/01614940903048661.
Adhikari S, Fernando S. Hydrogen membrane separation techniques. Ind Eng Chem Res. 2006;45:875–81. doi:10.1021/ie050644l.
Sircar S, Golden TC. Purification of hydrogen by pressure swing adsorption. Sep Sci Technol. 2000;35:667–87.
Silva B, Solomon I, Ribeiro AM, Lee U-H, Hwang YK, Chang J-S, Loureiro JM, Rodrigues AE. H2 purification by pressure swing adsorption using CuBTC. Sep Purif Technol. 2013;118:744–56. doi:10.1016/j.seppur.2013.08.024.
Chornet E, Czernik S, Wang D, Gregoire C, Mann MK. Biomass to hydrogen via pyrolysis and reforming. In: Proc. 1994 U.S. DOE Hydrog. Progr. Rev. NREL/CP-470-6431, CONF-9404194. Livermore; 1994. p. 407–32.
Milne TA, Elam CC, Evans RJ. Hydrogen from biomass – state of the art research challenges. 2001 IEA Rep. IEA/H2/TR-02/001.
Czernik S, French R, Feik C, Chornet E. Hydrogen by catalytic steam reforming of liquid byproducts from biomass thermoconversion processes. Ind Eng Chem Res. 2002;41:4209–15.
Czernik S, French R, Magrini-Bair K, Chornet E. The production of hydrogen by steam reforming of trap grease progress in catalyst performance. Energy Fuel. 2004;18:1738–43.
Czernik S, Evans R, French R. Hydrogen from biomass-production by steam reforming of biomass pyrolysis oil. Catal Today. 2007;129:265–8. doi:10.1016/j.cattod.2006.08.071.
Magrini-Bair KA, Czernik S, French R, Parent YO, Chornet E, Dayton DC, Feik C, Bain R. Fluidizable reforming catalyst development for conditioning biomass-derived syngas. Appl Catal A Gen. 2007;318:199–206.
Bimbela F, Oliva M, Ruiz J, García L, Arauzo J. Hydrogen production by catalytic steam reforming of acetic acid, a model compound of biomass pyrolysis liquids. J Anal Appl Pyrolysis. 2007;79:112–20.
Bimbela F, Oliva M, Ruiz J, García L, Arauzo J. Catalytic steam reforming of model compounds of biomass pyrolysis liquids in fixed bed: acetol and n-butanol. J Anal Appl Pyrolysis. 2009;85:204–13. doi:10.1016/j.jaap.2008.11.025.
Bimbela F, Oliva M, Ruiz J, García L, Arauzo J. Steam reforming of bio-oil aqueous fractions for syngas production and energy. Environ Eng Sci. 2011;28:757–63. doi:10.1089/ees.2010.0367.
Bimbela F, Chen D, Ruiz J, García L, Arauzo J. Ni/Al coprecipitated catalysts modified with magnesium and copper for the catalytic steam reforming of model compounds from biomass pyrolysis liquids. Appl Catal B Environ. 2012;119–120:1–12. doi:10.1016/j.apcatb.2012.02.007.
Remón J, Medrano J, Bimbela F, García L, Arauzo J. Ni/Al–Mg–O solids modified with Co or Cu for the catalytic steam reforming of bio-oil. Appl Catal B Environ. 2013;132–133:433–44.
Galdámez JR, García L, Bilbao R. Hydrogen production by steam reforming of bio-oil using coprecipitated Ni−Al catalysts. Acetic acid as a model compound. Energy Fuel. 2005;19:1133–42. doi:10.1021/ef049718g.
Ramos MC, Navascués AI, García L, Bilbao R. Hydrogen production by catalytic steam reforming of acetol, a model compound of bio-oil. Ind Eng Chem Res. 2007;46:2399–406. doi:10.1021/ie060904e.
Medrano JA, Oliva M, Ruiz J, García L, Arauzo J. Catalytic steam reforming of acetic acid in a fluidized bed reactor with oxygen addition. Int J Hydrog Energ. 2008;33:4387–96. doi:10.1016/j.ijhydene.2008.05.023.
Medrano JA, Oliva M, Ruiz J, García L, Arauzo J. Catalytic steam reforming of model compounds of biomass pyrolysis liquids in fluidized bed reactor with modified Ni/Al catalysts. J Anal Appl Pyrolysis. 2009;85:214–25. doi:10.1016/j.jaap.2008.11.025.
Medrano JA, Oliva M, Ruiz J, García L, Arauzo J. Hydrogen from aqueous fraction of biomass pyrolysis liquids by catalytic steam reforming in fluidized bed. Energy. 2011;36:2215–24. doi:10.1016/j.energy.2010.03.059.
Medrano JA, Oliva M, Ruiz J, García L, Arauzo J. Catalytic steam reforming of butanol in a fluidized bed and comparison with other oxygenated compounds. Fuel Process Technol. 2014;124:123–33. doi:10.1016/j.fuproc.2014.02.022.
Hu X, Lu G. Investigation of steam reforming of acetic acid to hydrogen over Ni–Co metal catalyst. J Mol Catal A Chem. 2007;261:43–8. doi:10.1016/j.molcata.2006.07.066.
Vagia EC, Lemonidou AA. Hydrogen production via steam reforming of bio-oil components over calcium aluminate supported nickel and noble metal catalysts. Appl Catal A Gen. 2008;351:111–21. doi:10.1016/j.apcata.2008.09.007.
Hu X, Lu G. Investigation of the effects of molecular structure on oxygenated hydrocarbon steam re-forming. Energy Fuel. 2009;23:926–33. doi:10.1021/ef8008647.
Matas Güell B, Silva IMT, Seshan K, Lefferts L. Sustainable route to hydrogen – design of stable catalysts for the steam gasification of biomass related oxygenates. Appl Catal B Environ. 2009;88:59–65. doi:10.1016/j.apcatb.2008.09.018.
Wang Z, Pan Y, Dong T, Zhu X, Kan T, Yuan L. Production of hydrogen from catalytic steam reforming of bio-oil using C12A7-O- -based catalysts. Appl Catal A Gen. 2007;320:24–34. doi:10.1016/j.apcata.2006.12.003.
Yan C-F, Cheng F-F, Hu R-R. Hydrogen production from catalytic steam reforming of bio-oil aqueous fraction over Ni/CeO2–ZrO2 catalysts. Int J Hydrog Energ. 2010;35:11693–9.
Ortiz-Toral PJ, Satrio J, Brown RC, Shanks BH. Steam reforming of bio-oil fractions: effect of composition and stability. Energy Fuel. 2011;25:3289–97. doi:10.1021/ef200628q.
Seyedeyn-Azad F, Salehi E, Abedi J, Harding T. Biomass to hydrogen via catalytic steam reforming of bio-oil over Ni-supported alumina catalysts. Fuel Process Technol. 2011;92:563–9.
Chen T, Wu C, Liu R. Steam reforming of bio-oil from rice husks fast pyrolysis for hydrogen production. Bioresour Technol. 2011;102:9236–40.
Li H, Xu Q, Xue H, Yan Y. Catalytic reforming of the aqueous phase derived from fast-pyrolysis of biomass. Renew Energy. 2009;34:2872–7. doi:10.1016/j.renene.2009.04.007.
Xu Q, Lan P, Zhang B, Ren Z, Yan Y. Hydrogen production via catalytic steam reforming of fast pyrolysis bio-oil in a fluidized-bed reactor. Energy Fuel. 2010;24:6456–62. doi:10.1021/ef1010995.
Panigrahi S, Dalai AK, Chaudhari ST, Bakhshi NN. Synthesis gas production from steam gasification of biomass-derived oil. Energy Fuel. 2003;17:637–42. doi:10.1021/ef020073z.
Kechagiopoulos PN, Voutetakis SS, Lemonidou AA, Vasalos IA. Sustainable hydrogen production via reforming of ethylene glycol using a novel spouted bed reactor. Catal Today. 2007;127:246–55. doi:10.1016/j.cattod.2007.05.018.
Kechagiopoulos PN, Voutetakis SS, Lemonidou AA, Vasalos IA. Hydrogen production via reforming of the aqueous phase of bio-oil over Ni/olivine catalysts in a spouted bed reactor. Ind Eng Chem Res. 2009;48:1400–8. doi:10.1021/ie8013378.
Van Rossum G, Kersten SRA, Van Swaaij WPM. Catalytic and noncatalytic gasification of pyrolysis oil. Ind Eng Chem Res. 2007;46:3959–67.
Van Rossum G, Kersten SRA, Van Swaaij WPM. Staged catalytic gasification/steam reforming of pyrolysis oil. Ind Eng Chem Res. 2009;48:5857–66.
Wu C, Huang Q, Sui M, Yan Y, Wang F. Hydrogen production via catalytic steam reforming of fast pyrolysis bio-oil in a two-stage fixed bed reactor system. Fuel Process Technol. 2008;89:1306–16. doi:10.1016/j.fuproc.2008.05.018.
Kan T, Xiong J, Li X, Ye T, Yuan L, Torimoto Y, Yamamoto M, Li Q. High efficient production of hydrogen from crude bio-oil via an integrative process between gasification and current-enhanced catalytic steam reforming. Int J Hydrog Energ. 2010;35:518–32.
Valle B, Remiro A, Aguayo AT, Bilbao J, Gayubo AG. Catalysts of Ni/α-Al2O3 and Ni/La2O3-αAl2O3 for hydrogen production by steam reforming of bio-oil aqueous fraction with pyrolytic lignin retention. Int J Hydrog Energ. 2013;38:1307–18. doi:10.1016/j.ijhydene.2012.11.014.
Remiro A, Valle B, Oar-Arteta L, Aguayo AT, Bilbao J, Gayubo AG. Hydrogen production by steam reforming of bio-oil/bio-ethanol mixtures in a continuous thermal-catalytic process. Int J Hydrog Energ. 2014;39:6889–98. doi:10.1016/j.ijhydene.2014.02.137.
Lea-Langton A, Zin RM, Dupont V, Twigg MV. Biomass pyrolysis oils for hydrogen production using chemical looping reforming. Int J Hydrog Energ. 2012;37:2037–43. doi:10.1016/j.ijhydene.2011.05.083.
Hu X, Lu G. Bio-oil steam reforming, partial oxidation or oxidative steam reforming coupled with bio-oil dry reforming to eliminate CO2 emission. Int J Hydrog Energ. 2010;35:7169–76.
Trane R, Dahl S, Skjøth-Rasmussen MS, Jensen AD. Catalytic steam reforming of bio-oil. Int J Hydrog Energ. 2012;37:6447–72. doi:10.1016/j.ijhydene.2012.01.023.
Domine ME, Iojoiu EE, Davidian T, Guilhaume N, Mirodatos C. Hydrogen production from biomass-derived oil over monolithic Pt- and Rh-based catalysts using steam reforming and sequential cracking processes. Catal Today. 2008;133–135:565–73.
Davidian T, Guilhaume N, Daniel C, Mirodatos C. Continuous hydrogen production by sequential catalytic cracking of acetic acid: part I. Investigation of reaction conditions and application to two parallel reactors operated cyclically. Appl Catal A Genet. 2008;335:64–73.
Czernik S, French R, Penev M. Distributed bio-oil reforming. US DOE Hydrog Fuel Cells Progr – FY 2013. Annu Prog Rep. 2013;II:119–23.
Vispute TP, Huber GW. Production of hydrogen, alkanes and polyols by aqueous phase processing of wood-derived pyrolysis oils. Green Chem. 2009;11:1433–45. doi:10.1039/B912522C.
Yu D, Aihara M, Antal MJ. Hydrogen production by steam reforming glucose in supercritical water. Energy Fuel. 1993;7:574–7. doi:10.1021/ef00041a002.
Shabaker J, Davda RR, Huber GW, Cortright RD, Dumesic JA. Aqueous-phase reforming of methanol and ethylene glycol over alumina-supported platinum catalysts. J Catal. 2003;215:344–52. doi:10.1016/S0021-9517(03)00032-0.
Cortright RD, Davda RR, Dumesic JA. Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water. Nature. 2002;418:964–7. doi:10.1038/nature01009.
Huber GW, Shabaker JW, Dumesic JA. Raney Ni-Sn catalyst for H2 production from biomass-derived hydrocarbons. Science. 2003;300:2075–7. doi:10.1126/science.1085597.
Vispute T. Pyrolysis oils: characterization, stability analysis, and catalytic upgrading to fuels and chemicals. Dissertations. Paper 349. 2011. http://scholarworks.umass.edu/open_access_dissertations.
Hou T, Yuan L, Ye T, Gong L, Tu J, Yamamoto M, Torimoto Y, Li Q. Hydrogen production by low-temperature reforming of organic compounds in bio-oil over a CNT-promoting Ni catalyst. Int J Hydrog Energ. 2009;34:9095–107. doi:10.1016/j.ijhydene.2009.09.012.
Acknowledgments
The authors wish to express their gratitude to the Spanish MINECO (projects ENE2010-18985 and ENE2013-41523-R). It is also acknowledged the permission for reprinting figures from American Chemical Society and Elsevier.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
García, L., Ábrego, J., Bimbela, F., Sánchez, J.L. (2015). Hydrogen Production from Catalytic Biomass Pyrolysis. In: Fang, Z., Smith, Jr., R., Qi, X. (eds) Production of Hydrogen from Renewable Resources. Biofuels and Biorefineries, vol 5. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7330-0_5
Download citation
DOI: https://doi.org/10.1007/978-94-017-7330-0_5
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7329-4
Online ISBN: 978-94-017-7330-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)