Abstract
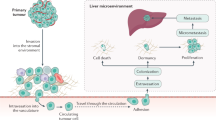
The liver is a common site of metastases that originate from diverse types of primary tumors, second only to lymph nodes as the most frequent metastatic site. As a vital organ with a number of varied functions, organ damage due to metastatic disease is associated with profound morbidity and mortality. The multiple functions, large blood volume as well as the unique anatomy (dual blood supply, fenestrated endothelium and sinusoids) of the liver play an important role in the frequency of liver metastasis. Yet, while a substantial knowledge base has been generated regarding the liver as a metastatic site, development of therapeutic options to treat liver metastases has not progressed as rapidly. Though metastasis is often studied in models that reduce the system to individual steps, a fully integrated understanding of the process, and the critical steps that may be targeted by therapy, remains incomplete and is likely necessary to develop suitable therapies. Towards this goal, in this chapter we describe the process of metastasis, liver anatomy and function, and models of liver metastasis and analysis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
- (CAM):
-
chorioallantoic membrane
- (HCC):
-
hepatocellular carcinoma
- (IVVM):
-
intravital videomicroscopy
- (MRI):
-
magnetic resonance imaging
- (PCR):
-
polymerase chain reaction
- (PET):
-
positron emission tomography
- (SPECT):
-
single photon emission computed tomography
7.1 Introduction—Metastasis and the liver
As with the detection of metastatic cancer in most organs, incidence of liver metastasis is correlated with short survival times [1–6] . This frequently poor outcome prevails despite an expansive and rapidly growing body of literature detailing the underlying causes and mechanisms that control cancer progression, and corresponding (though not as rapid) improvements in cancer therapies that have led to increased survival rates for multiple types of cancer. Yet many aspects of cancer progression, including cancer metastasis, remain incompletely understood. This is in part due to the complex, multistep nature of the metastatic process, during which a significant number of intrinsic variables play a role in progression. This includes the tissue of metastatic cell origin as well as destination, route of travel, normal host and metastatic cell genetic and protein expression variations [7–10]. As such, what may be considered a significant regulatory mechanism at one particular step in the metastatic process, which may allow or inhibit progression at that particular step, may be inconsequential if the cell is incapable of overcoming later steps in the metastatic cascade. Further, as metastatic spread has often occurred at the time of diagnosis and initial treatment, a focus on later (and highly inefficient) steps in the metastatic process is often necessary [11]. In addition to the expanding knowledge regarding cancer progression mechanisms, earlier detection and improved therapeutic interventions have been credited with increased survival, mainly due to treatment prior to the occurrence of overt metastatic cancer [12]. However, this is once again an incomplete explanation, as grade and size are correlated with metastatic disease but are not the sole determinant. This incomplete understanding of the metastatic process, and a corresponding lack of translation of known mechanisms to effective systemic treatment options specifically designed for metastatic disease, highlight the need for research specifically focused on further elucidation of the metastatic process and design of suitable, and more efficacious, treatment options.
While the individual steps that a cancer cell must take (detailed further below) in order to successfully form a metastatic tumor are generally understood, a comprehensive and integrated understanding of the entire process for all cells within a given population remains incomplete. The limited overall efficacy of a number of therapies that were expected to specifically inhibit specific steps in the metastatic process (e.g. matrix metalloproteinase inhibitors, angiogenesis inhibitors, etc.) highlight the need for improved understanding and better ability to translate experimental findings to the clinical setting [13]. Indeed, a number of basic principles remain far from understood, including the concepts of parallel and serial metastatic progression, the nature and roles of cancer stem cells or other metastatic cell subpopulations, the microenvironment etc. [14–18]. Further complicating the issue is the heterogeneous nature of metastatic progression among individual patients and even among cells originating from the same primary tumor [19]. Finding appropriate models to recapitulate such complexity, and understanding the data they yield, is a significant challenge. In order to facilitate such research, here we present an overview of basic concepts of metastasis, focusing on the liver as a secondary site, while also examining experimental models that have allowed a significant knowledge base to be generated.
The overall process of metastasis itself has been well documented and at its most basic can be described as consisting of cells leaving a primary tumor, invading local tissue, intravasation into blood or lymph vessels, circulation and survival in these vessels, arrest in a secondary tissue, extravasation, initiation and continuation of growth and angiogenesis [7–10, 20] . Indeed, breaking down the process into such steps has allowed for identification of factors contributing to, and mechanisms controlling, various steps of the process [21–26]. Yet despite this understanding of individual steps that control specific steps of the metastatic process, an integrated and complete understanding of the mechanisms controlling all steps in this process remains incomplete.
Metastasis in general remains a significant clinical problem. However, some organs are more prone to development of clinically apparent metastatic tumors than others [7, 20]. In particular, the liver is a frequent site of metastasis from multiple primary cancers including colorectal, pancreatic, breast, melanoma (skin and uveal), esophageal, gastric and liver cancer itself [27–29] . Considering the number of critical roles the liver plays, disruption of normal liver function can have significant effects on normal body function and ultimately on survival. Additionally, while the full role of the microenvironment on metastatic progression is still far from completely understood, the diverse range of functions performed by the cells of the liver may produce niche microenvironments that spatially and temporally affect disease progression. In order to provide a context for the nature of metastatic progression in the liver we provide a brief overview of the anatomy and function of the liver before describing models of liver metastasis . A number of anatomical and physiological factors including the unique vasculature, size, diverse functions and location all likely play an important role in not only metastatic progression, but in the design and analysis of in vivo models of metastasis. As with any model system, each has specific advantages, disadvantages and limitations and thus the model should be chosen carefully with full regard to the ability of answer the specific questions and hypotheses being addressed. This is of particular importance in models of a process as complicated as metastasis, as too broad a focus can easily be paid to a mechanism or step that may not ultimately be a significant rate limiting step in progression, or a suitable candidate for therapeutic intervention.
7.2 Anatomy of the Liver
The liver is located immediately below the diaphragm in the upper part of abdominal cavity. As both the largest internal organ and gland, it performs a number of vital functions including blood filtration, detoxification, bile production, glycogen storage, protein synthesis, hormone production and regulation of immune responses [28]. As a whole, the liver is traditionally described as divided into 4 lobes (left, right, caudate and quadrate), each divided by a number of ligaments and overlying the gallbladder. The lobes themselves are further subdivided into functional areas that are primarily defined as regions of tissue fed by a branch of the portal vein. At the histological level, the liver is divided into lobules that are hexagonal in structure and composed of branches of the terminal portal vein and artery, bile ducts, liver sinusoids and hepatic venules (Fig. 7.1). The primary structural organization of the lobules are the liver sinusoids, which themselves are composed of diverse types of cells including hepatocytes, endothelial cells, and macrophages/Kupffer cells, which are responsible for the equally diverse functions completed by the liver.
Liver morphology, as seen by scanning electron microscopy. Terminal portal veins (TPV) surround hepatic lobules. The hepatocytes (H) are arrayed in flat plates surrounding a centrilobular vein (CLV). The sinusoids (S) provide a microvasculature within the lobules. Blood flows through openings in the terminal portal veins (arrows) into and through the sinusoids, and is collected by centrilobular veins (CLV), and then to interlobular veins and to the suprahepatic vein (not shown). Also not shown, perilobular arteries, lymphatic vessels, nerve fibers and bile ducts, which also contribute to the portal tracts occupied by the TPV. Bar: 50 μm (reprinted with permission from [116])
Having a vital role in metabolism and maintenance of blood composition, and of particular significance in terms of haematogenous dissemination of metastatic cells, the liver is supplied by two major vascular branches; the hepatic artery and portal vein. Of the two, the portal vein is responsible for the majority (~70 %) of the blood delivered to the liver [30–32]. It should thus be of little surprise that a number of organs drained by the vessels feeding into the portal vein (including the colon, stomach, pancreas, esophagus and spleen) are common sites from which liver metastases originate. In contrast, the arterial blood delivered via the hepatic artery does not directly originate from upstream organs, but is instead oxygenated blood delivered from the heart via the abdominal aorta. As such, blood arriving via the hepatic artery is filtered through capillaries of several organs, including the lung, prior to arriving in the liver. Studies that have examined the growth of metastases from cells originating from the portal vein or hepatic artery have found that greater than 70 % of liver metastases likely arrive in the liver via the portal vein [33–36] (Table 7.1). However, the presence of breast, melanoma (skin and uveal), renal, ovarian and lung cancer metastases in the liver indicate that the frequency of metastatic growth in the liver is not only due to the sheer number of cells that arrive directly in the liver via the portal vein system, but also due to the relatively hospitable “soil” of the liver .
The exact role of a number of the unique anatomical features (e.g. sinusoids, fenestrated and phagocytic endothelium, etc.) and the complex and diverse metabolic functions of the liver on the progression of metastasis remain to be further explored. For example, how do the blood flow pattern, nutrient or toxin levels at various times post injection, specific cellular metabolic functions and the local cell and microenvironment heterogeneity, etc., influence metastatic cell fate and progression? Additionally, taking into account that the liver is large, highly dynamic and multifunctional organ, would specific factors both promote and inhibit metastases in the same organ at different times? In any case, because the liver is a vital organ, compromise of liver function by growth of metastases can lead to significant quality of life consequences for patients, and can results in patient death.
7.3 Experimental Models of Metastasis
The inefficiency of the metastatic process, by which few of the cells that leave a primary tumor successfully form a macroscopic metastasis, has been well validated by clinical data and experimental models [7, 37, 38]. The overall inefficiency of this stepwise process highlights the fact that individual steps within the process are regulated by a variety of cancer cell and micro-environment influenced mechanisms which the majority of cells shed from a primary tumor are not suitably equipped to overcome. In general, the methods used to understand and quantify metastasis reflect this multistep nature and examine only specific steps of the process. The use of mice with primary tumor formation and subsequent metastatic cell dissemination due to genetic modification, spontaneous growth or chemically induced tumors, allows for monitoring progression of cancer cells throughout the process [39]. However, limited availability, accessibility, significant variability and the often extended experimental time required to study spontaneous models makes them less attractive and less frequently used than experimental metastasis models. As such, both models of metastasis in general, and liver metastasis in particular, are most frequently performed using in vitro surrogate models or in vivo experimental models generally using mice, or in some cases rats or avian embryos. While any number of avian embryos and small or large mammals can be used as models, due to their common use (well characterized), reasonable cost and general availability we will primarily focus on mouse and chick embryo models .
7.3.1 In Vitro Models of Steps in the Metastatic Process
The multistep and multi-organ nature of metastasis essentially precludes the study of the entire process in vitro and thus necessitates that in vitro assays be used in a greatly minimized and focused manner in order to study specific steps in the process. Indeed, a number of assays have been developed which are meant to quantify and understand specific steps in the process including invasion, migration etc. [40–45]. However, as these models do not recapitulate the anatomical and functional complexity of the liver and are more general in nature, detailed description of these techniques and assays can be found in literature focused on that particular subject. In a general context, the effect of the microenvironment on cancer cell growth is applicable to all metastatic sites. Multiple in vivo studies using mice for experimental models of liver and lung metastasis have revealed that the fate of individual cells is diverse, with some remaining dormant, many undergoing apoptosis and only a small subset forming micrometastases and eventually large metastases [7, 46–49] . Along these lines, studies using 3D in vitro models in which cells are grown in basement membrane matrix gel (e.g. Matrigel™ and Cultrex®) have demonstrated that the growth of cells in 3D more closely replicates in vivo growth than 2D culture [40, 50–53]. This includes the observation that mixed cell populations in which extended periods of single cell dormancy, frequently observed in vivo but not in 2D culture, are observed in various proportions of the cells dependent on cell line . Co-culture methods and custom fabrication of tuneable (size, shape, modulus) microenvironments for 3D culture may present an opportunity to build increasingly complex microenvironments, however significant functional diversity observed in the liver would still be absent [42, 54–59] .
7.3.2 General Consideration of in Vivo Metastasis Models
In the same way that the majority of in vitro ‘metastasis assays’ essentially isolate individual steps of metastasis in order to examine the mechanisms of that specific step (often in a greatly simplified way), liver metastasis models can be modified in order to recapitulate only certain steps in the process . Spontaneous metastasis models are models in which cells naturally disseminate from a primary tumor to secondary site, regardless of the nature of the primary tumor (chemically induced, genetic, transplanted tissue, injected cells etc.) [39]. As such, cells complete all steps of metastasis, including primary tumor growth, migration from primary tumor intravasation into the circulation/lymphatics, delivery to secondary sites, extravasation and growth. In comparison, experimental metastasis models commence by injection of cells directly into the vasculature (blood or lymph) feeding a secondary tissue in order to introduce cells systemically or target cells to a particular site.
In the case of experimental liver metastasis models, a broad definition would include injection and arrival of cells via arterial blood flow, direct injection of the cells into the liver, or more commonly, via the portal vein system (either directly or injection via organs draining into the portal system such as the spleen) [39]. While spontaneous models may offer the benefit of recapitulating the entire process of metastasis, they are generally highly variable and require longer experimental times. Additionally, a number of experimental models have demonstrated that cell death leading to inefficiency of the metastatic process can occur after cell arrival in a secondary site [7, 46–49, 60]. As such, while experimental metastasis captures only the steps in the metastatic process following intravasation, steps following extravasation have been shown to be highly inefficient and thus elucidating the factors responsible for cell progression, or conversely loss of cells, may lead to development of logical therapeutics. Additionally, experimental models of liver metastasis offer a higher degree of control over the cell population being introduced into the liver, including the number and timing of cells injected, co-injection of other cells and/or particles, genetic or protein expression modification of the cells and pre-labelling with imaging contrast agents. As the vast majority of cells injected via the portal vein into the liver have been shown to arrest in the liver, the direct introduction of cells vs spontaneous dissemination from a primary tumor also allows for extended periods of observation that can be limited by primary tumor induced endpoints (size or morbidity) [46, 61, 62]. In the context of mimicking natural disease progression, introduction of cancer cells to the liver via the portal vein follows the natural path of many types of cancer, including colon, gastric and esophageal, for which embedding these cells in their natural site of origin would add significant technical complication. Loss of the influence of the organ of primary tumor growth may have an effect on growth in a secondary site, however most experimental animal models do not actually recapitulate normal primary tumor progression, largely negating this factor. While tumors originating from primary cancers such as breast and melanoma are not believed to arrive in the liver via the portal system, the hepatic artery and portal vein converge at the level of the liver sinusoid . This ensures that while the route of travel is not identical, the microenvironment of arrest is appropriate [29, 63].
7.3.3 Avian Embryo Models—Chick Chorioallantoic Membrane (CAM)
Avian embryo models, and in particular the chick chorioallantoic membrane (CAM), have been well characterized for their use as a model tumor progression, including metastasis and angiogenesis [64–67] . While used less frequently than some other animal models (i.e. mice and rats), chick embryo models offer a number of practical advantages including relatively simple housing and maintenance requirements and lower overall cost [64, 68–70]. From a technical perspective, avian models of tumor progression have additional advantages relative to both in vitro and other in vivo models. Compared to in vitro models, avian embryos (depending on their embryonic development when used experimentally) provide a complex in vivo environment (diverse cell types, vasculature, blood flow, filtering organs etc.) that allows multiple steps of metastasis to be studied sequentially. In comparison to larger mammalian animal models, chick embryos are easier to house and maintain, provide an accessible surface for imaging (the CAM generally or deeper tissue using near infrared (NIR) imaging techniques), are naturally immunodeficient until late in embryo development, and can be used in large number to facilitate higher throughput experiments . Some limitations of the embryo model include the sensitivity of the developing embryo to cytotoxic therapeutics, the closed system (i.e. no elimination administered chemicals) and shorter observation period that can reduce the ability to determine therapeutic efficacy in this model. As a model for metastatic progression however, the ability to image at the sub-cellular level is a distinct benefit.
Chicken embryos develop for 21 days before hatching. The embryo itself is protected and nourished by three membranes, the yolk sack membrane, the amnion and the chorioallantoic membrane, all of which are naturally enclosed by the egg shell. Experimental tumor models using the chicken embryo can be performed either in the egg shell (in ovo) or after removing the egg shell (ex ovo). Detailed protocols for both methods have been described in detail previously [64, 65, 67, 68, 71–73], with videos of the technique also available [73–75]. The general experiments that can be performed using either method are similar, with the most significant difference between the two methods being simply the presence or absence of the egg shell and the corresponding decreased viability and increased surface access (to the CAM) using the ex ovo method. This surface access allows for a large area on which multiple tumors can be implanted and imaged longitudinally at high resolution [67, 76]. While the CAM and its dense vasculature are most commonly used as the site of tumor cell growth and arrest, liver metastasis models can be performed using the chick embryo model via either spontaneous metastasis or injection of cells via the CAM vasculature [72] . Indeed, blood returning to the embryo form the CAM drains into the portal system and first passes through the liver of the embryo, arresting most of the cells and facilitating use as a liver metastasis model [77–78]. Analysis of liver metastasis has been performed by a number of methods including electron microscopy, polymerase chain reaction (PCR) based techniques to detect human cells within the liver, recovery of the cells from the embryo (via tissue dissociation and selective pressure) or by using fluorescent cell lines or histological techniques in order to visualize the metastases [64, 70, 73, 77–81]. The proximity of the embryo to the surface in the ex ovo model may also allow for direct observation of metastasis using tissue penetrating imaging techniques including near infrared (NIR) excitation and emission or multiphoton microscopy [82] . While the chick embryo has been used primarily to study progression and liver metastasis of solid tumors, this model has also been used to study progression of human leukemia cell lines that were found to form granulocytic sarcomas in the CAM and were detected in the liver and other organs by PCR. Interestingly, tumor engraftment was rapid and occurred in 100 % of eggs injected via the vasculature or amnion, but not the yolk sack or direct CAM implantation [81].
7.3.4 Rodent Models
Rodent models, and in particular mouse models, have been used extensively in order to study the process of liver metastasis [62, 83–88]. The method of introducing cancer cells into the liver however varies significantly with the most common injection routes including direct injection into one or more lobes of the liver, via the portal vein system or indirectly through injection of cells into the spleen [46, 63, 83, 84, 89–91] (Fig. 7.2). As with any experimental model, each has distinct advantages and disadvantages and should be chosen in the context of the experiment and hypothesis to be addressed. For instance, while direct injection into the liver is a simpler surgery, it recapitulates only the last steps of metastatic progression (from most primary tumors), sustained growth and angiogenesis . As such, this model would be limited to experiments examining the effect of treatment on large metastatic tumors. Additionally, while the size of cell clusters has been shown to influence metastatic progression, cell clusters that arrive in the liver via the blood vasculature would not be expected to aggregate as a dense population of millions of cells present following direct liver injections. The significant exception to this is that the most frequent site of metastasis for hepatocellular carcinoma (HCC) is the liver itself, making direct injection of cells into the liver a reasonable model of primary orthotopic HCC growth with subsequent local metastases [36]. Overall, a significant advantage of rodent, and in particular mouse models of liver metastasis, is the wealth of publications that detail the growth of a wide variety of cell lines, both mouse and human, in multiple strains (and with different immune status) of mice [62, 83–86, 88, 90] . As with any mouse experiments, the ability to genetically engineer mice in order to examine the influence of specific molecules and host cell types, or express reporter genes (e.g. fluorescence), allows for an expanded level of control over the microenvironment.
Establishment of single and disseminated liver metastases. The model of single liver macrometastasis is established by subcapsular injection of tumour cells or implantation of tumour tissue (A). The model of disseminated liver micrometastases is established by intrasplenic (B) or intraportal (C) injection of cancer cells
As the primary methods of introducing cells into the liver for metastasis models, the use of splenic injection and portal vein injections (Fig. 7.2) have a number of similarities and subtle differences that require discussion . The primary similarity between the two methods is arrival of the cells via the portal vein. Despite this, the rate and proportion (of injected population) of cancer cells, and accompanying cell types in the case of splenic injections, differ. Given the influence of the microenvironment on cancer progression and metastasis, this should not be overlooked. Indeed, studies examining liver metastases following splenic injections have found that cells from the mouse spleen are co-localized with the injected cancer cells in the liver and that in certain cell lines splenic injections are more efficient [92]. While the exact mechanism of this increased efficiency is unknown, it is likely similar to studies demonstrating that clusters of cells injected into the portal vein yield more metastases than individually injected cells [87]. Additionally, it has been suggested that cancer cells coated with red blood cells/platelets may exhibit increased metastatic efficiency [93]. As a method of cell injection, cannulation of the portal vein has also been reported, allowing for subsequent injection of systemic therapy directly to the liver as is sometimes done clinically [94].
Injection of cells via cardiac or intravenous injection can also be used to deliver cells systemically, including to the liver. These injection routes will however result in arrest of the vast majority of cells in the organ in which they the first encounter a capillary bed (e.g. lung for intravenous injections; multiple arterially supplied organs for intracardiac injections) [7, 95]. The ease of intravenous injection would make this method preferred for high throughput studies and indeed would be the logical method of injection for cells that pass through other organs prior to arriving in the liver (breast, lung, melanoma etc.). Indeed, some melanoma cell lines have been found to survive or grow primarily or exclusively in the liver following i.v. injections [85] . While a technically simpler method of introducing cells into the liver, cell lines that grow well in the lung (or any other site) will limit the observation period as most will arrest and grow in this site. Intracardiac injections via the right side of the heart would be similar to intravenous injections. However, injection into the left side of the heart would be expected to deliver cells to organs proportionally to blood flow patterns. In this case a significant proportion of cells would be expected to be delivered to the digestive system, including to the liver [7, 95]. Preferential growth in the liver would then be a function of the liver “soil” [20].
7.4 Imaging and Analysis Techniques
A significant challenge when trying to dissect the entire metastatic process is the simultaneous need to image a large number of metastatic cells in multiple diverse cell microenvironments at a molecular to organ or even whole animal level. These competing requirements often force the decision to monitor either small subpopulations of cells with high resolution (generally by optical microscopy of tissue sections or in vivo using intravital videomicroscopy (IVVM) or the entire population of cells via non-invasive imaging methods (e.g. magnetic resonance imaging (MRI), ultrasound, whole body fluorescence (FLU) or bioluminescence (BLU), radio labelling etc.) [67, 83, 86, 96] . The location of the liver provides additional challenges for imaging as the large size of the organ, its internal location and constant movement all pose technical hurdles. These challenges have in large part been overcome by advances in gating and surgical techniques, contrast agents and imaging hardware, but are not yet routine or simple. The decision to monitor smaller numbers of cells with high resolution, larger populations at lower resolution, at single time points (histology) or longitudinally must still be made in most cases. With continuing advances in imaging hardware and the availability of multi-purpose, multi-modal imaging contrast agents (and diagnostics and drug delivery vehicles), the technical capability to serially or simultaneously deliver therapeutics and image the same population of cells via multiple imagining methods exists, however is still technically challenging in vivo [97–99].
7.4.1 Optical Microscopy
Optical microscopy has the advantage of resolution at the sub-cellular (sub micrometer) level, but this is counter balanced by the currently limited depth of penetration, significant image degradation due to motion artifacts and limited field of view. Yet, as metastatic progression is controlled at the cellular (both intra and inter) level, microscopy has and continues to be indispensible in identifying many basic characteristics and mechanism of the metastatic process. For analysis of liver metastasis at a single time point, microscopy is often used to image and quantify cells or tumors in thin or thick sections (with various cell stains or fixation methods) of tissue. In order to image the metastatic process in vivo, IVVM has been well documented with detailed techniques described previously [65, 67, 71, 74, 95] .
The ability to section and stain liver tissue to be analyzed by microscopy has a number of advantages including technically simple (can reduce background signal and no motion) imaging procedures and a large number of stains that can be used to label different cells types in order to facilitate identification and quantification. However, limitations exist in the type of information that can be gathered from what is essentially a snapshot of a dynamic process. As a measure of quantification of the fate of cells, or the effect of treatment on progression and size of metastases, analysis of histological sections is still a preferred method. Indeed, significant data regarding metastatic cell fate following arrest in the liver has been generated via thin or thick section analysis of tissue [46, 62, 88]. These studies have revealed that the majority of cells arriving in the liver undergo apoptosis, while smaller proportions, dependent on cell type, remain in the liver as dormant cells (so-called dormant cancer cells, DCC) or begin proliferating to form metastases [46, 47, 62, 88] .
A wide variety of contrast agents (often fluorescent), labels and stains exist and are suitable for in vivo use. The choice of cell label is significant as it can considerably alter the type of information that can be obtained from the experiment. While somewhat more limited than options for staining histological samples, a number of methods can be used to highlight specific anatomy (commonly vascular dyes—variable “leakiness”) or label metastatic cells. This includes cell lines stably expressing fluorescent (FLU) or bioluminescent (BLU) proteins or labelled with inorganic particles or dyes of various excitation and emission properties, e.g. [83, 88, 100, 101]. Cells can be labelled transiently with fluorescent particles prior to injection in order to facilitate their tracking by optical microscopy or in combination with other modalities such as MRI [83, 88, 90, 100]. Advantages of nano or micron sized particles are their often increased optical properties including brightness, stability and defined excitation and emission spectra. Additionally, many novel nanoparticles are being designed to act as contrast agents for multiple imaging modalities simultaneously [97, 98, 102].
Though the maximum resolution for optical microscopy is currently higher than other imaging modalities (nano scale for super resolution techniques), in vivo imaging of the liver is complicated by its internal location and constant movement. However, a number of techniques for stabilization and tissue access have been published, including exposing and securing the liver to underlying cover glass or using a surgically implanted window to image micrometastases and even solitary cells [95, 103] . In the context of understanding and quantifying the fate of individual metastatic cells, portal vein injections have an advantage of delivering nearly all cells, and cell sized particles, directly to the liver to be arrested in the vasculature. This physical arrest of cells and particles by size has been used in order to account for cells by co-injection of fluorescent reference particles [46, 62, 95]. In this way the fate of cells in reference to particles can be quantified .
Overall, the greatest current advantage of optical microscopy analysis of liver metastasis is the ability to directly image intra- and inter-cellular events that may be obscured by imaging larger tumors or indirect cell indicators (e.g. iron oxide for MRI) . As much of the cell-microenvironment is different by location within the liver, it is probably ultimately necessary to use such high resolution imaging in order understand metastatic progression and the subcellular/intracellular events controlling progression mechanism. However, the addition of whole animal molecular imaging may capture large scale differences in growth, blood flow patterns, metabolic activity etc., which would not be apparent with the limited field of view possible using optical microscopy .
7.4.2 Whole Animal Imaging Techniques
Advances in the field of non-invasive whole animal imaging, with an emphasis on molecular imaging, have continued to improve the resolution of a number of imaging modalities to the point that they are able to detect smaller tumors, and in some cases individual cancer cells within an intact organ or animal [83, 90, 96, 100, 104–107]. It should be noted that while molecular or single cell imaging non-invasive imaging is indicative of the presence or activity of cells, it is generally not truly at the molecular level per cell but the average of many cells (e.g. PET, SPECT tracers), the minimum number and characteristics of which differ based on the contrast agent and sensitivity of the particular modality. Though not directly imaging the subcellular events of individual cells, a possibly significant benefit of non-invasive whole animal imaging is the lack of invasive surgery that could have effects on tumor progression. This is due to experimental and clinical studies in which results indicate invasive surgery may have an effect on shedding of cells from the primary tumor as well as subsequent growth [108–110].
Most common and clinically used imaging modalities are available and have specifically designed hardware for imaging of small animals including mice. This includes MRI, SPECT/CT, PET and ultrasound . The advantages and limitations of these common imaging modalities, including their cost, contrast agents and resolution have been reviewed thoroughly elsewhere [105]. It is worth noting however that in all cases, resolution using small animal equipment is now generally sufficient to detect the presence of micrometastases, if not single metastatic cells . This resolution can vary significantly though and requires substantial optimization of gating techniques, pulse sequences, image reconstruction algorithms and the choice/availability of contrast agents. An example of advances in the convergence of hardware, software and contrast agents for multimodality imaging is highlighted by the use of dual magnetite (for MR) and fluorescent (optical) particles that have facilitated quantification of solitary metastatic cells in multiple organs, including the liver, using both modalities [90, 100]. However, in this situation optical microscopy is still used to validate the presence of solitary cells as detected by signal voids (due to iron oxide) in MRI images. This is because the majority of contrast agents for non-invasive imaging are indicative of the presence of the contrast agent and not a metastatic cell. Retention of pre-loaded (in or on the cell) contrast agent, or preferential co-localized of post injected contrast agent are therefore assumed and must be validated .
As with the model of metastasis used, the choice of imaging modality should be chosen based on experimental questions to be answered. An illustration of the tradeoffs that must be made when choosing an imaging modality for analysis of liver metastasis growth is highlighted by a comparison of data obtained for B16F1 murine melanoma metastasis growth in the liver by MRI or ultrasound . Whole animal or organ imaging by MRI has been shown to be able to detect and quantify nearly the entire population of metastatic cells (single cells, micro and macrometastases), and a comparison of this technique vs traditional histological analysis is presented in Fig. 7.3 [83]. However, analysis by MRI requires expensive and customized hardware, pulse sequence optimization, use of contrast agent (for single cell imaging) and can require long image acquisition times. In comparison, imaging metastases by high frequency ultrasound (Fig. 7.4) is relatively inexpensive, requires no contrast agent and images can be acquired relatively quickly. Yet the ability to detect single cells and very small micrometastases, as well as image liver beneath the ribs, is currently limited [86] . While practical limitations generally result in the use of the imaging modality that is most readily available, the ability to image different populations (single cells, micrometastases and large metastases), surface antigens vs metabolic activity, blood flow etc., by various imaging modalities makes it realistic to image the entire level with cellular level resolution. Additionally, the use of multimodal contrast agents that also function as drugs or experimental cargo (e.g. shRNA etc.) delivery vehicles are beginning to allow for experimental manipulation and simultaneous imaging .
7.5 Conclusion
In most cases metastases, not local, non-invasive primary tumors, are responsible for cancer related deaths. The dual high volume blood supply to the liver, “unfiltered” in the case of the portal vein, in combination with what appears to be a relatively permissive tumor growth microenvironment make the liver a common site of lethal metastases. Advances in surgery, radiation therapy and systemic therapies have significantly increased survival following diagnosis of liver metastases, however prognosis is still generally poor. This is primarily due to the collateral damage/non-specific toxicity and technical complexity of treating multiple (spatially and temporally) metastases via surgery or radiation to a deep tissue vital organ. Considering the possibility of the presence of occult and perhaps dormant metastases that can emerge following removal of all clinically identifiable tumors (primary or metastatic at first treatment), it is likely that to make further significant advances in survival, advances in systemic therapy will be required. In order to achieve this, a better understanding of metastatic growth in liver is required. As a metastatic site for cells delivered via both arterial and portal vein blood, the liver provides a suitable microenvironment for determining the effect of site of growth based on preferred soil vs frequency of distribution. Additionally, multiple direct (portal vein system, directly into liver tissue) and indirect (intracardiac, intravenous, splenic injection) are available to recapitulate arrival via different routes as would be seen in patients.
Advances in systemic therapy including identification of surface ligands, molecular targeted therapy and nanoparticle drug delivery vehicles may offer a number of promising advantages for treatment of metastatic liver disease, including specificity and reduced toxicity [102, 111–113]. These include the ability to directly target surface receptors, deliver drug intracellular and exploit advances in non-cytotoxic therapies [114–115]. These combined properties may make molecular or cellular targeted therapies (vs treating the entire tumor as a single entity) capable of eliminating occult or dormant cells responsible for recurrent disease. Additionally, most therapeutics, including novel nanoparticles, normally accumulate in the liver, and elucidation of methods to take advantage of this effect, while avoiding Kupffer or endothelial cell uptake, could possibly provide a therapeutic opportunity. However, in order to develop novel systemic therapies for such applications, further knowledge regarding the fate of individual metastatic cells and population heterogeneity need to be understood. While acknowledging that the models and techniques outlined here are far from ideal and continue to evolve, when combined with other experimental advances a number of basic questions remain to be answered. The effect of the complex functional diversity of the liver, and its influence on the also heterogeneous metastatic cell population, is likely of particular importance. Corresponding to the diverse function of the liver, it has been well documented that even within a structure as small as the lobule, extensive variance with respect to specific molecular uptake, secretion and oxygen levels are observed. Would such niche environment provide a survival advantage for cells as has been documented in bone? It is hoped that the information presented here provide a basis to facilitate this required research and continue the necessary development on these models, integration of imaging techniques.
Analysis of liver metastases via MRI. MR images from a whole mouse liver can be used to quantify tumor numbers and volumes, as well as volumes of solitary (dormant) cells. Whole livers (A) were scanned by MRI to generate a series of two-dimensional images, from which areas representing metastatic B16F1 murine melanoma tumors as well as dormant solitary cells are calculated (B). These sections, from the full liver volume, were used to render three-dimensional images (C) and used to calculate tumor volume for the whole liver. In contrast, standard histology (D) samples only a small subset of the full liver volume. Image modified from Townson et al., Cancer Research 2009 [83].
Analysis of liver metastasis via ultrasound. Growth of an individual B16F1 murine melanoma metastasis in liver. Calculated metastasis volumes at three times after mesenteric vein injection of cells: day 10 (0.06 mm3); day 14 (0.61 mm3); day 18 (3.79 mm3). Bar, 1.0 mm. Image modified from Graham et al., Cancer Research 2005 [86].
References
Tomlinson JS et al (2007) Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 25:4575–4580. doi:25/29/4575 [pii] 10.1200/JCO.2007.11.0833
Adam R et al (2009) Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol 27:1829–1835. doi:JCO.2008.19.9273 [pii] 10.1200/JCO.2008.19.9273
Peters S et al (2006) Intra-arterial hepatic fotemustine for the treatment of liver metastases from uveal melanoma: experience in 101 patients. Ann Oncol 17:578–583. doi:mdl009 [pii] 10.1093/annonc/mdl009
Chua TC, Saxena A, Liauw W, Chu F & Morris DL (2011) Hepatic resection for metastatic breast cancer: a systematic review. Eur J Cancer 47:2282–2290. doi:S0959-8049(11)00428-X [pii] 10.1016/j.ejca.2011.06.024
Kerkar SP, Kemp CD & Avital I (2010) Liver resections in metastatic gastric cancer. HPB (Oxford) 12:589–596. doi:10.1111/j.1477-2574.2010.00224.x
Nguyen KT, Gamblin TC & Geller DA (2009) World review of laparoscopic liver resection-2,804 patients. Ann Surg 250:831–841. doi:10.1097/SLA.0b013e3181b0c4df
Chambers AF, Groom AC. & MacDonald IC (2002) Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2:563–572. doi:10.1038/nrc865 nrc865 [pii]
Pantel K & Brakenhoff RH (2004) Dissecting the metastatic cascade. Nat Rev Cancer 4, 448–456. doi:10.1038/nrc1370 nrc1370 [pii]
Gupta GP & Massague, J (2006) Cancer metastasis: building a framework. Cell 127:679–695. doi:S0092–8674(06)01414–0 [pii] 10.1016/j.cell.2006.11.001
Steeg PS (2006) Tumor metastasis: mechanistic insights and clinical challenges. Nat Med 12:895–904. doi:nm1469 [pii] 10.1038/nm1469
Chambers AF, MacDonald IC, Schmidt EE, Morris VL & Groom AC (2000) Clinical targets for anti-metastasis therapy. Adv Cancer Res 79:91–121
Karim-Kos HE et al (2008) Recent trends of cancer in Europe: a combined approach of incidence, survival and mortality for 17 cancer sites since the 1990s. Eur J Cancer 44:1345–8049. doi:S0959–8049(07)01028–3 [pii] 10.1016/j.ejca.2007.12.015
Patricia S Steeg et al (2009) Preclinical drug development must consider the impact on metastasis. Clin Cancer Res 15:4529–4530
Visvader JE & Lindeman GJ (2008) Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 8:755–768. doi:nrc2499 [pii] 10.1038/nrc2499
Klein CA (2009) Parallel progression of primary tumours and metastases. Nat Rev Cancer 9:302–312. doi:nrc2627 [pii] 10.1038/nrc2627
Joyce JA & Pollard JW (2009) Microenvironmental regulation of metastasis. Nat Rev Cancer 9:239–252. doi:nrc2618 [pii] 10.1038/nrc2618
Uhr JW & Pantel K (2011) Controversies in clinical cancer dormancy. Proc Natl Acad Sci USA 108, 12396–12400. doi:1106613108 [pii] 10.1073/pnas.1106613108
Morgan SC & Parker CC (2011) Local treatment of metastatic cancer–killing the seed or disturbing the soil? Nat Rev Clin Oncol 8:504–506. doi:nrclinonc.2011.88 [pii] 10.1038/nrclinonc.2011.88
Rodenhiser DI, Andrews JD, Vandenberg TA & Chambers AF (2011) Gene signatures of breast cancer progression and metastasis. Breast Cancer Res 13:201.doi:bcr2791 [pii] 10.1186/bcr2791
Fidler IJ (2003) The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 3:453–458. doi:10.1038/nrc1098 nrc1098 [pii]
Chambers AF & Matrisian LM (1997) Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst 89:1260–1270
Deryugina EI & Quigley JP (2006) Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev 25:9–34.doi:10.1007/s10555–006-7886–9
Condeelis J & Pollard JW (2006) Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124:263–266.doi:S0092-8674(06)00055-9 [pii] 10.1016/j.cell.2006.01.007
Erler JT et al (2006) Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 440:1222–1226. doi:nature04695 [pii] 10.1038/nature04695
Chaudary N & Hill RP (2007) Hypoxia and metastasis. Clin Cancer Res 13:1947–1949. doi:13/7/1947 [pii] 10.1158/1078-0432.CCR-06-2971
Folkman J (2002) Role of angiogenesis in tumor growth and metastasis. Semin Oncol 29:15–18. doi:10.1053/sonc.2002.37263 S0093775402503353 [pii]
Tang ZY et al (2004) A decade’s studies on metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol 130:187–196. doi:10.1007/s00432-003-0511-1
Brodt P (2011) Liver metastasis: biology and clinical management. In Cancer Metastasis—Biology and Treatment 446 (Springer, 2011)
Burnier JV et al (2011) Type IV collagen-initiated signals provide survival and growth cues required for liver metastasis. Oncogene 30:3766–3783. doi:onc201189 [pii] 10.1038/onc.2011.89
Vollmar B & Menger MD (2009) The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev 89:1269–471. doi:89/4/1269 [pii] 10.1152/physrev.00027.2008
Schenk WG, Jr., Mc DJ, Mc DK & Drapanas T (1962) Direct measurement of hepatic blood flow in surgical patients: with related observations on hepatic flow dynamics in experimental animals. Ann Surg 156:463–471
Rappaport AM (1980) Hepatic blood flow: morphologic aspects and physiologic regulation. Int Rev Physiol 21:1–63
Bubendorf L et al (2000) Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol 31:578–583
Elias D et al (1998) Resection of liver metastases from a noncolorectal primary: indications and results based on 147 monocentric patients. J Am Coll Surg 187:487–493. doi:S1072-7515(98)00225-7 [pii]
Selzner M, Morse MA, Vredenburgh JJ, Meyers WC & Clavien PA (2000) Liver metastases from breast cancer: long-term survival after curative resection. Surgery 127:383–389. doi:S0039606000128053 [pii]
Hess KR et al (2006) Metastatic patterns in adenocarcinoma. Cancer 106:1624–1633. doi:10.1002/cncr.21778
Tarin D, Vass AC, Kettlewell MG & Price JE (1984) Absence of metastatic sequelae during long-term treatment of malignant ascites by peritoneo-venous shunting. A clinico-pathological report. Invasion Metastasis 4:1–12
Weiss L (1990) Metastatic inefficiency. Adv Cancer Res 54:159–211
Welch DR (1997) Technical considerations for studying cancer metastasis in vivo. Clin Exp Metastasis 15:272–306
Albini A et al (1987) A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res 47:3239–3245
Kumar S. & Weaver VM (2009) Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev 28:113–127. doi:10.1007/s10555-008-9173-4
Yates C et al (2007) Novel three-dimensional organotypic liver bioreactor to directly visualize early events in metastatic progression. Adv Cancer Res 97:225–246. doi:S0065-230X(06)97010-9 [pii] 10.1016/S0065-230X(06)97010-9
Kim JB, Stein R & O’Hare MJ (2004) Three-dimensional in vitro tissue culture models of breast cancer—a review. Breast Cancer Res Treat 85, 281–291. doi:10.1023/B:BREA.0000025418.88785.2b 5266910 [pii]
Auerbach R, Lewis R, Shinners B, Kubai L & Akhtar N (2003) Angiogenesis assays: a critical overview. Clin Chem 49:32–40
Kleinman HK & Jacob K (2001) Invasion assays. Curr Protoc Cell Biol Chapter 12, Unit 12 12.doi:10.1002/0471143030.cb1202s00
Luzzi KJ et al (1998) Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol 153:865–873. doi:S0002-9440(10)65628-3 [pii] 10.1016/S0002-9440(10)65628-3
Townson JL, Naumov GN & Chambers AF (2003) The role of apoptosis in tumor progression and metastasis. Curr Mol Med 3:631–642
Townson JL & Chambers AF (2006) Dormancy of solitary metastatic cells. Cell Cycle 5:1744–1750.doi:2864 [pii]
Cameron MD et al (2000) Temporal progression of metastasis in lung: cell survival, dormancy, and location dependence of metastatic inefficiency. Cancer Res 60:2541–2546
Bissell MJ et al (1999) Tissue structure, nuclear organization, and gene expression in normal and malignant breast. Cancer Res 59:1757–1763; discussion 1763–1764
Lee GY, Kenny PA, Lee EH & Bissell MJ (2007) Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods 4:359–365.doi:nmeth1015 [pii] 10.1038/nmeth1015
Barkan D et al (2008) Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res 68:6241-doi:68/15/6241 [pii] 10.1158/0008-5472.CAN-07–6849
Barkan D, Green JE & Chambers AF (2010) Extracellular matrix: a gatekeeper in the transition from dormancy to metastatic growth. Eur J Cancer 46:1181–1188. doi:S0959-8049(10)00154-1 [pii] 10.1016/j.ejca.2010.02.027
Kaehr B, Allen R, Javier DJ, Currie J & Shear JB (2004) Guiding neuronal development with in situ microfabrication. Proc Natl Acad Sci USA 101:16104–16108. doi:0407204101 [pii] 10.1073/pnas.0407204101
Kaehr B et al (2006) Direct-write fabrication of functional protein matrixes using a low-cost Q-switched laser. Anal Chem 78:3198–3202. doi:10.1021/ac052267s
Kaehr B & Shear JB (2007) Mask-directed multiphoton lithography. J Am Chem Soc 129:1904–1905. doi:10.1021/ja068390y
Kaehr B & Shear JB (2008) Multiphoton fabrication of chemically responsive protein hydrogels for microactuation. Proc Natl Acad Sci USA 105:8850–8854. doi:0709571105 [pii] 10.1073/pnas.0709571105
Nielson, R., Kaehr, B. & Shear, JB (2009) Microreplication and design of biological architectures using dynamic-mask multiphoton lithography. Small 5:120–125. doi:10.1002/smll.200801084
Knopeke MT et al (2011) Building on the foundation of daring hypotheses: Using the MKK4 metastasis suppressor to develop models of dormancy and metastatic colonization. FEBS Lett 585:3159–3165. doi:S0014-5793(11)00672-7 [pii] 10.1016/j.febslet.2011.09.007
Hedley BD & Chambers AF (2009) Tumor dormancy and metastasis. Adv Cancer Res 102:67–101. doi:S0065-230X(09)02003-X [pii] 10.1016/S0065-230X(09)02003-X
Chambers AF, Naumov GN, Vantyghem SA & Tuck AB (2000) Molecular biology of breast cancer metastasis. Clinical implications of experimental studies on metastatic inefficiency. Breast Cancer Res 2:400–407
Naumov GN et al (2003) Ineffectiveness of doxorubicin treatment on solitary dormant mammary carcinoma cells or late-developing metastases. Breast Cancer Res Treat 82:199–206. doi:10.1023/B:BREA.0000004377.12288.3c
Wu Y et al (2010) Insulin-like growth factor-I regulates the liver microenvironment in obese mice and promotes liver metastasis. Cancer Res 70:57–67. doi:0008-5472.CAN-09-2472 [pii] 10.1158/0008-5472.CAN-09-2472
Chambers AF, Shafir R & Ling V (1982) A model system for studying metastasis using the embryonic chick. Cancer Res 42:4018–4025
Chambers AF, Schmidt EE, MacDonald IC, Morris VL & Groom AC (1992) Early steps in hematogenous metastasis of B16F1 melanoma cells in chick embryos studied by high-resolution intravital videomicroscopy. J Natl Cancer Inst 84:797–803
Vargas A, Zeisser-Labouebe M, Lange N, Gurny R & Delie F (2007) The chick embryo and its chorioallantoic membrane (CAM) for the in vivo evaluation of drug delivery systems. Adv Drug Deliv Rev 59:1162–1176. doi:S0169-409X(07)00172-X [pii] 10.1016/j.addr.2007.04.019
Leong HS et al (2010) Intravital imaging of embryonic and tumor neovasculature using viral nanoparticles. Nat Protoc 5:1406–1417. doi:nprot.2010.103 [pii] 10.1038/nprot.2010.103
Chambers AF, Wilson SM, Tuck AB, Denhardt GH & Cairncross JG (1990) Comparison of metastatic properties of a variety of mouse, rat, and human cells in assays in nude mice and chick embryos. In Vivo 4:215–219
Subauste MC et al (2009) Evaluation of metastatic and angiogenic potentials of human colon carcinoma cells in chick embryo model systems. Clin Exp Metastasis 26:1033–1047. doi:10.1007/s10585-009-9293-4
Goulet B et al (2011) Nuclear localization of maspin is essential for its inhibition of tumor growth and metastasis. Lab Invest 91:1181–1187. doi:labinvest201166 [pii] 10.1038/labinvest.2011.66
MacDonald IC, Schmidt EE, Morris VL, Chambers AF & Groom AC (1992) Intravital videomicroscopy of the chorioallantoic microcirculation: a model system for studying metastasis. Microvasc Res 44:185–199. doi:0026-2862(92)90079-5 [pii]
Wilson SM & Chambers AF (2004) Experimental metastasis assays in the chick embryo. Curr Protoc Cell Biol Chapter 19, Unit 19 16.doi:10.1002/0471143030.cb1906s21
Palmer TD, Lewis J & Zijlstra A (2011) Quantitative analysis of cancer metastasis using an avian embryo model. J Vis Exp. doi:2815 [pii] 10.3791/2815
Cho CF, Ablack A, Leong HS, Zijlstra A & Lewis J (2011) Evaluation of nanoparticle uptake in tumors in real time using intravital imaging. J Vis Exp. doi:2808 [pii] 10.3791/2808
Dohle DS et al (2009) Chick ex ovo culture and ex ovo CAM assay: how it really works. J Vis Exp. doi:1620 [pii] 10.3791/1620
Lewis, JD. et al (2006) Viral nanoparticles as tools for intravital vascular imaging. Nat Med 12:354–360. doi:nm1368 [pii] 10.1038/nm1368
Kim J, Yu W, Kovalski K & Ossowski L (1998) Requirement for specific proteases in cancer cell intravasation as revealed by a novel semiquantitative PCR-based assay. Cell 94, 353–362.doi:S0092-8674(00)81478-6 [pii]
Locker J, Goldblatt PJ & Leighton J (1970) Ultrastructural features of invasion in chick embryo liver metastasis of Yoshida ascites hepatoma. Cancer Res 30:1632–1644
Endo Y, Sasaki T, Harada F & Noguchi M (1990) Specific detection of metastasized human tumor cells in embryonic chicks by the polymerase chain reaction. Jpn J Cancer Res 81:723–726
Zijlstra A et al (2002) A quantitative analysis of rate-limiting steps in the metastatic cascade using human-specific real-time polymerase chain reaction. Cancer Res 62:7083–7092
Taizi M, Deutsch VR, Leitner A, Ohana A & Goldstein RS (2006) A novel and rapid in vivo system for testing therapeutics on human leukemias. Exp Hematol 34:1698–1708. doi:S0301-472X(06)00443-7 [pii] 10.1016/j.exphem.2006.07.005
Wyckoff J, Gligorijevic B, Entenberg D, Segall J & Condeelis J (2011) High-resolution multiphoton imaging of tumors in vivo. Cold Spring Harb Protoc 2011 doi:2011/10/pdb.top065904 [pii] 10.1101/pdb.top065904)
Townson JL et al (2009) Three-dimensional imaging and quantification of both solitary cells and metastases in whole mouse liver by magnetic resonance imaging. Cancer Res 69:8326–8331. doi:0008-5472.CAN-09-1496 [pii] 10.1158/0008-5472.CAN-09-1496
Townson, J. L. et al (2011) The synthetic triterpenoid CDDO-Imidazolide suppresses experimental liver metastasis. Clin Exp Metastasis 28, 309-doi:10.1007/s10585-011-9374-z
Logan PT et al (2008) Single-cell tumor dormancy model of uveal melanoma. Clin Exp Metastasis 25:509-doi:10.1007/s10585-008-9158-2
Graham KC et al (2005) Three-dimensional high-frequency ultrasound imaging for longitudinal evaluation of liver metastases in preclinical models. Cancer Res 65, 5231–5237. doi:65/12/5231 [pii] 10.1158/0008-5472.CAN-05-0440
Enomoto, T. et al (2006) Consistent liver metastases in a rat model by portal injection of microencapsulated cancer cells. Cancer Res 66:11131–11139. doi:66/23/11131 [pii] 10.1158/0008-5472.CAN-06-0339
Naumov, GN. et al (2002) Persistence of solitary mammary carcinoma cells in a secondary site: a possible contributor to dormancy. Cancer Res 62:2162–2168
Rajendran S. et al (2010) Murine bioluminescent hepatic tumour model. J Vis Exp. doi:1977 [pii] 10.3791/1977
Shapiro EM, Sharer K, Skrtic S & Koretsky AP (2006) In vivo detection of single cells by MRI. Magn Reson Med 55:242–249. doi:10.1002/mrm.20718
Morris VL et al (1993) Early interactions of cancer cells with the microvasculature in mouse liver and muscle during hematogenous metastasis: videomicroscopic analysis. Clin Exp Metastasis 11:377–390
Bouvet M et al (2006) In vivo color-coded imaging of the interaction of colon cancer cells and splenocytes in the formation of liver metastases. Cancer Res 66:11293-doi:66/23/11293 [pii] 10.1158/0008-5472.CAN-06-2662
Kirstein JM et al (2009) Effect of anti-fibrinolytic therapy on experimental melanoma metastasis. Clin Exp Metastasis 26:121-doi:10.1007/s10585-008-9221-z
Vrancken Peeters MJ, Perkins AL & Kay MA (1996) Method for multiple portal vein infusions in mice: quantitation of adenovirus-mediated hepatic gene transfer. Biotechniques 20:278–285
MacDonald IC, Groom AC & Chambers AF (2002) Cancer spread and micrometastasis development: quantitative approaches for in vivo models. Bioessays 24:885–893. doi:10.1002/bies.10156
Heyn C et al (2006) In vivo MRI of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Magn Reson Med 56:1001–1010. doi:10.1002/mrm.21029
Kim J et al (2008) Multifunctional uniform nanoparticles composed of a magnetite nanocrystal core and a mesoporous silica shell for magnetic resonance and fluorescence imaging and for drug delivery. Angew Chem Int Ed Engl 47:8438–8441. doi:10.1002/anie.200802469
Lee JE, Lee N, Kim T, Kim J & Hyeon T (2011) Multifunctional mesoporous silica nanocomposite nanoparticles for theranostic applications. Acc Chem Res 44:893–902. doi:10.1021/ar2000259
Bardhan R, Lal S, Joshi A & Halas NJ (2011) Theranostic nanoshells: from probe design to imaging and treatment of cancer. Acc Chem Res 44:936–946. doi:10.1021/ar200023 ×
Heyn C et al (2006) In vivo magnetic resonance imaging of single cells in mouse brain with optical validation. Magn Reson Med 55:23–29. doi:10.1002/mrm.20747
Tsuji K et al (2006) Dual-color imaging of nuclear-cytoplasmic dynamics, viability, and proliferation of cancer cells in the portal vein area. Cancer Res 66:303–306. doi:66/1/303 [pii] 10.1158/0008-5472.CAN-05-2958
Ashley CE et al (2011) The targeted delivery of multicomponent cargos to cancer cells by nanoporous particle-supported lipid bilayers. Nat Mater 10:389–397. doi:nmat2992 [pii] 10.1038/nmat2992
Yang M et al (2002) Direct external imaging of nascent cancer, tumor progression, angiogenesis, and metastasis on internal organs in the fluorescent orthotopic model. Proc Natl Acad Sci USA 99:3824-doi:10.1073/pnas.052029099052029099 [pii]
Foster-Gareau P, Heyn C, Alejski A & Rutt BK (2003) Imaging single mammalian cells with a 1.5 T clinical MRI scanner. Magn Reson Med 49:968–971. doi:10.1002/mrm.10417
Weissleder R & Pittet MJ (2008) Imaging in the era of molecular oncology. Nature 452:580–589. doi:nature06917 [pii] 10.1038/nature06917
Condeelis J & Weissleder R (2010) In vivo imaging in cancer. Cold Spring Harb Perspect Biol 2, adoi:cshperspect.a003848 [pii] 10.1101/cshperspect.a003848
O’Neill K, Lyons SK, Gallagher WM, Curran KM & Byrne AT (2010) Bioluminescent imaging: a critical tool in pre-clinical oncology research. J Pathol 220:317–327. doi:10.1002/path.2656
Thaker PH et al (2006) Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med 12:939–944. doi:nm1447 [pii] 10.1038/nm1447
Hofer SO, Shrayer D, Reichner JS, Hoekstra HJ & Wanebo HJ (1998) Wound-induced tumor progression: a probable role in recurrence after tumor resection. Arch Surg 133:383–389
Demicheli R, Retsky MW, Hrushesky WJ, Baum M & Gukas ID (2008) The effects of surgery on tumor growth: a century of investigations. Ann Oncol 19:1821–1828. doi:mdn386 [pii] 10.1093/annonc/mdn386
Lo A, Lin CT & Wu HC (2008) Hepatocellular carcinoma cell-specific peptide ligand for targeted drug delivery. Mol Cancer Ther 7:579–589. doi:7/3/579 [pii] 10.1158/1535-7163.MCT-07-2359
Farokhzad OC et al (2006) Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci USA 103:6315–6320. doi:0601755103 [pii] 10.1073/pnas.0601755103
Peer D et al (2007) Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2:751–760. doi:nnano.2007.387 [pii] 10.1038/nnano.2007.387
Davis ME, Chen ZG & Shin DM (2008) Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov 7:771–782. doi:nrd2614 [pii] 10.1038/nrd2614
Byrne JD, Betancourt T & Brannon-Peppas L (2008) Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev 60:1615–1626. doi:S0169-409X(08)00225-1 [pii] 10.1016/j.addr.2008.08.005
Vidal-Vanaclocha F (2008) The prometastatic microenvironment of the liver. Cancer Microenviron 1:113–129. doi:10.1007/s12307-008-0011–6
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Chambers, A., Townson, J. (2013). Liver Metastases. In: Malek, A. (eds) Experimental Metastasis: Modeling and Analysis. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7835-1_7
Download citation
DOI: https://doi.org/10.1007/978-94-007-7835-1_7
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7834-4
Online ISBN: 978-94-007-7835-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)