Abstract
The kidney is a profusely vascularized organ which, unlike other organs, does not regulate renal blood flow (RBF) mainly by oxygen demand. Reflex (myogenic effect and tubular-glomerular feedback) and neurohormonal mechanisms modulate RBF and renal resistances by regulating the tone of afferent and efferent arterioles as well as that of the major resistance vessels in the kidneys. Arterial renal resistances are also influenced by a number of other pathophysiological factors, such as increased arterial stiffness, arterial atherosclerosis, renal parenchymal abnormalities, and intra-abdominal and central venous pressure. Finally, renal resistances can be permanently increased if a microvascular disease and vascular rarefaction occur as a result of vasoconstriction-related ischemia, endothelial dysfunction, and inflammatory cytokine activity.
In this setting, a parameter reflecting renal resistances could offer a useful tool to better characterize renal disease and the risk of progression. This chapter focuses on renal resistance index (RRI), a parameter obtained by pulsed Doppler which reflects renal arterial resistances. The possible clinical usefulness of RRI has been demonstrated in studies which show the pathophysiological correlates and prognostic role in predicting a greater risk of chronic kidney disease progression and cardiovascular events.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Renal resistance index
- Renal circulation
- Chronic kidney disease
- Cardiovascular diseases
- Heart failure
- Worsening of renal failure
Key Facts
Key Facts of Cardiorenal Syndrome (CRS)
-
Kidney and heart diseases share many pathophysiological mechanisms such as tissue hypoperfusion, venous congestion, inflammatory status, and neurohormonal activation.
-
Acute or chronic dysfunction of one of these two organs can lead to functional worsening of the other. Cardiorenal syndrome is the condition in which the heart and kidney can negatively affect each other.
-
The Consensus Conference of the Acute Dialysis Quality Group divided this syndrome into five subtypes.
-
Types 1 and 2 (cardiorenal syndrome): acute heart failure causing acute kidney injury (AKI) and chronic heart failure leading to a progressive renal dysfunction, respectively.
-
Types 3 and 4 (renocardial syndrome): acute kidney dysfunction causing acute heart failure and chronic kidney disease leading to a worsening of cardiac function, respectively.
-
Type 5: systemic diseases involving both the kidney and heart.
-
Therefore, in patients with heart failure, an accurate evaluation of renal function is important, and vice versa; therefore, close collaboration between nephrologists and cardiologists is necessary in order to improve patient prognosis.
Key Facts of Doppler Ultrasonography
-
Doppler ultrasonography is a noninvasive technique that uses high-frequency sound waves (ultrasound) to estimate blood flow through blood vessels.
-
During examination the operator uses a transducer that sends and receives ultrasounds. The sound waves are reflected by blood cells, and their frequency is modified (increase or decrease) by the movement of red cells (Doppler effect).
-
The Doppler shift is the difference in frequency between the reflected ultrasound and the initial ultrasound and is correlated with the velocity of the blood cells.
-
A computer receives and processes this information and gives a spectrum or an image that represents the blood flow.
-
Three main Doppler techniques are used: continuous wave, pulsed wave, and color Doppler.
-
Continuous wave Doppler: the transducer continuously emits trains of ultrasounds along a line and continuously receives reflected signals. Continuous wave Doppler ultrasound is unable to determine the specific location of velocities within the beam.
-
Pulsed wave Doppler: the transducer emits a train of ultrasound to a certain depth and then captures the reflected ultrasounds. The computer then calculates the flow velocity at that point.
-
Color Doppler: the principle is the same as for pulsed wave Doppler, but analyzes an area of small sample volumes. Based on the velocities calculated in that area, the computer builds a color image that represents the blood flow.
Key Facts of Heart Failure
-
Heart failure represents one of the main causes of morbidity and mortality in industrialized countries. The prevalence ranges from 0.4 % to 2.0 % in the European population. The incidence increases with age.
-
It is a condition in which the heart is not able to pump an adequate supply of blood to satisfy the body’s needs (oxygen and nutrients).
-
Heart dysfunction can involve the left or right ventricle or both. However, the left ventricle is usually affected first.
-
There are two different mechanisms underlying left heart dysfunction: the heart is not able to contract with enough force (systolic dysfunction), or the ventricle becomes stiff and develops resistance to filling.
-
Heart failure is defined as acute if signs and symptoms arise suddenly and require immediate medical treatment. It is defined as chronic if the patient is in a stable clinical condition but has, or has had, signs and symptoms of heart failure for some time.
-
Causes of heart failure are coronary artery disease; myocardial infarction; valvular, pericardium, endocardium, or heart muscle disease; congenital heart disease; severe lung disease; diabetes; abnormalities of heart rhythm; etc.
-
Possible symptoms are shortness of breath (dyspnea) during activity or at rest, tiredness, and palpitations. Possible signs are high jugular venous pressure, pulmonary crepitations and displaced apical impulse, and excess fluid in body tissues (edema) with swelling of feet, ankles, legs, or abdomen.
-
Heart failure treatment includes lifestyle changes, drugs (angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, beta-blockers, diuretics, vasodilators, antiplatelet agents, and anticoagulants), implantable defibrillators to prevent arrhythmic complications, left ventricular assist devices (a mechanical device that helps the heart to pump blood), and, ultimately, heart transplantation.
Definitions
Arteriolosclerosis
This is a disease of the small blood vessels characterized by a thickening and hardening of the arteriole wall. It can be due to concentric, smooth muscle wall hypertrophy or the buildup of hyaline material. It reduces blood flow and determines tissue ischemia.
Central venous pressure (CVP)
This is the pressure in the thoracic vena cava near the heart; therefore, it is an estimate of pressure in the right atrium. It can be measured invasively using a central venous catheter, but noninvasive evaluation is also possible. The most common method used is to evaluate the diameter of the vena cava and its changes during inspiration.
Ejection fraction
This is the percentage of blood ejected by the heart with each beat. It is an indicator of heart contractility. Mathematically, it is the difference between the end-diastolic volume and the end-systolic volume divided by the end-diastolic volume. Normal values are 55–70 %.
Glomerular filtration rate (GFR)
This is considered the best index to assess renal function. It measures kidney filtration capacity using renal clearance of an exogenous marker (inulina or I-iothalamate) or more often an endogenous marker, i.e., serum creatinine. GFR is generally estimated on the basis of serum creatinine levels.
Glomerulosclerosis
This is a disease characterized by scarring and hardening of glomeruli. If only a part of glomeruli are affected, then it is defined as focal segmental glomerulosclerosis. It can be idiopathic or due to known causes (HIV, obesity, diabetes, drugs, lupus, etc).
Heart failure with preserved ejection fraction (HFpEF)
This term is used to define patients with signs and symptoms of heart failure but who have a normal or mildly reduced ejection fraction, no left ventricle dilatation, abnormalities of cardiac structure, and/or diastolic dysfunction. HFpEF has a higher prevalence among the elderly, females, and patients with hypertension.
Heart failure with reduced ejection fraction (HFrEF)
This term is used to define patients with signs and symptoms of heart failure but who have a reduced ejection fraction. The most frequent cause is coronary artery disease. Other possible causes are myocarditis, alcohol abuse, chemotherapy, idiopathic forms, etc.
Pulse arterial pressure
This is the difference between the systolic pressure and the diastolic pressure. It represents the maximal change in aortic pressure during systole. It depends on the compliance of the aorta and on cardiac output.
Pulse wave velocity
This is the best index of aortic stiffness. It is usually measured by determining the propagation time of the pulse pressure from the carotid to femoral artery. Different methods are used in order for measurement, among which the most common are mechanical methods requiring specific devices. However, it may also be easily measured using high reproducible, noninvasive ultrasound methods. A threshold of >10 m/s has been suggested by a recent expert consensus statement as an index of an altered arterial distensibility in hypertensive patients.
Renal blood flow (RBF)
This is the amount of blood that passes through the kidney in a specific time unit. Mathematically, it is the difference between the aortic pressure and renal venous pressure, divided by renal vascular resistance. The normal value is 1,200 ml/min.
Worsening renal function (WRF)
In patients affected by cardiovascular diseases, this is generally defined as an increase in serum creatinine of >0.3 mg/dl and/or >25 % between two time points. It has been shown to be associated with a worse prognosis, hospitalizations for heart failure and higher mortality.
Introduction
The kidney is a profusely vascularized organ, and, unlike other organs, renal blood flow (RBF) is not mainly influenced by oxygen demand but is determined by reflex (myogenic effect and tubular-glomerular feedback) and neurohormonal mechanisms that regulate the tone of afferent and efferent arterioles as well as of the major resistance vessels in the kidneys (Braam et al. 2012). Moreover, arterial renal resistances are also influenced by a number of other factors, such as an increased arterial stiffness, arterial atherosclerosis, renal parenchymal relevant pathophysiological abnormalities, and increased central venous pressure. These factors can cause a permanent increase in vascular resistance due to microvascular remodeling and capillary rarefaction caused by vasoconstriction-related ischemia, endothelial dysfunction, and the production of inflammatory cytokines and fibrosis (Chade 2013). Therefore, a parameter that provides information on functional and permanent changes in RBF by reflecting abnormalities in renal arterial resistances could offer an incremental value to better characterize renal function.
This potential use is even more obvious when considering the limitations of the parameters currently used to assess renal function. The estimation of renal function is generally based on the calculation of glomerular filtration rate (GFR) by serum creatinine levels (Stevens et al. 2006). While creatinine serum levels and estimated GFR represent the cornerstone in the evaluation of renal function and its worsening , they are limited by several factors, such as between-person and within-person variability, age, diet, gender and body mass, the active creatinine tubular secretion (Damman et al. 2012), drug interference, and the loss of muscle mass frequent in the end stages of systemic diseases (Smilde et al. 2006). Finally, it is worth noting that the kidneys use only part of their filtering capacity. A normal GFR does not exclude an impairment in filtration capacity; in other words, a normal GFR in kidneys with a reduced renal reserve which increases the filtration capacity of residual nephrons (Bosch et al. 1995) could be observed. Due to the limitations of creatinine, new biomarkers have been proposed to detect early glomerular dysfunction (cystatin C) or identify tubular damage (neutrophil gelatinase-associated lipocalin, N-acetyl-beta-glucosaminidase, and kidney injury molecule) preceding the drop in GFR.
In this clinical setting, a parameter reflecting RBF alterations could be useful because, by detecting abnormalities, patients at a higher risk of renal disease onset and/or its progression could be better identified (Fig. 1). This more accurate evaluation of renal function could be relevant not only for the treatment of chronic kidney disease (CKD) patients, but also for a better management of patients affected by cardiovascular diseases .
Changes in renal blood flow preceding the drop in glomerular filtration rate. The pathophysiological background of possible clinical usefulness of renal resistances (Modified by Damman et al. 2010). The glomerular filtration rate drops when the kidneys are no longer able to compensate for the loss of nephrons by enhancing the filtration of those remaining. Renal resistance index, as well as microalbuminuria and tubular dysfunction biomarkers, could provide early detection of functional and structural renal changes preceding the fall in GFR. GFR glomerular filtration rate, RBF renal blood flow, WRF worsening of renal function
Over the last few years, more attention has been focused on the link between the kidney and the heart. These two organs are characterized by many common pathophysiological mechanisms which can negatively affect each other, and, consequently, the term “cardiorenal syndrome” has recently been introduced. This term indicates a condition characterized by an acute or chronic dysfunction of one of the two organs which may induce the acute or chronic dysfunction of the other (Ronco et al. 2008).
In patients affected by chronic cardiovascular diseases and especially in those with heart failure (HF) , CKD as well as worsening renal function (WRF) , i.e., type 2 cardiorenal syndrome, represents a clinical condition associated with a poor prognosis (Hillege et al. 2000; Smith et al. 2006; Damman et al. 2009).
The aim of this chapter is to focus on the possible clinical role of renal arterial resistances in patients with renal and cardiovascular diseases.
Renal Circulation and Renal Arterial Resistances
Twenty-two percent of cardiac output is directed to the kidney. It has a terminal-type circulation, without anastomosis (Fig. 2). At the level of the hilum branch, the renal arteries branch out into the segmental, arcuate, and interlobular arteries. The afferent arterioles derive from the interlobular arteries leading into the glomerular capillaries that coalesce to form the efferent arterioles. Efferent arterioles give rise to a second capillary network, i.e., peritubular capillaries that are followed by the venous system (Chade 2013).
In order to control arterial pressure and water and sodium reabsorption, reflex mechanisms (such as myogenic effect and tubular-glomerular feedback) and neurohormonal mechanisms (sympathetic system, renin-angiotensin-aldosterone system, endothelin, nitric oxide, prostaglandins, bradykinin, and natriuretic peptides) modulate the arteriolar tone, thus affecting renal resistance.
The myogenic effect is a fast mechanism consisting in the vasoconstriction of kidney small vessels when wall tension increases. The tubuloglomerular feedback is a slower mechanism of vasoconstriction of the afferent arteriole that occurs when an increase in GFR produces a rise in NaCl concentration, which is then sensed by the macula densa, and a vasoconstrictor mediator (probably adenosine) is secreted. Of the neurohormonal mediators, the most important are catecholamines and the renin-angiotensin-aldosterone system (RAAS) which determine vasoconstriction and an increased reabsorption of water and sodium (Braam et al. 2012).
The RBF self-regulation maintains the renal perfusion constant for arterial pressure changes between 70 and 180 mmHg. The abovementioned mechanisms regulate RBF and GFR in order to keep them constant when there are fluctuations in renal perfusion pressure both in physiological situations and pathological conditions.
A depletion or an overstimulation of these systems, for example, in heart failure, could determine an increase in intrarenal resistances and a dissociation between cardiac output and RBF. This is the consequence of a disproportional decrease in renal perfusion pressure and RBF in reduced cardiac output (Braam et al. 2012).
The arterial renal resistances are also influenced by a number of other relevant pathophysiological factors such as increased arterial stiffness, arterial atherosclerosis, renal parenchymal abnormalities, and increased central venous pressure (Fig. 3). Moreover, endothelin, nitric oxide (NO), prostaglandins, bradykinin, and natriuretic peptides are also involved in the regulation of arteriolar tone.
Factors influencing renal circulation . The factors influencing renal perfusion and renal resistances are summarized. The myogenic reflex and the tubular-glomerular feedback regulate the tone of afferent and efferent arterioles. However, arterial renal resistances are also influenced by a number of other pathophysiological factors, such as neurohormonal mechanisms, arterial stiffness, arterial atherosclerosis, renal parenchymal abnormalities, and intra-abdominal and central venous pressure. CVP central venous pressure, IAP intra-abdominal pressure, RAAS renin-angiotensin-aldosterone system, SNS sympathetic nervous system
Among these, the decreased availability of NO seems to play a pivotal role in determining abnormalities in renal circulation. A reduction, mediated by oxidative stress (reactive oxygen species, ROS), promotes vasoconstriction. Moreover, ROS-mediated inflammation further reduces NO availability thus perpetuating a vicious circle leading to permanent changes in renal vasculature.
In fact, the ischemia related to endothelial dysfunction and the inflammatory cytokines can cause fibrosis, vascular remodeling, and rarefaction (Chade 2013). This latter condition is generally due to changes in renal microcirculation, which are also defined as microvascular disease, and is a frequent feature of CKD. When it occurs, a permanent increase in renal resistances and a further decline in renal function can be observed.
Doppler Evaluation of Arterial Resistances
Arterial pulsed Doppler has been proposed as a useful tool to estimate the arterial resistance of an organ. The parameter generally used is the Doppler resistive index (RI), i.e., a measure obtained from pulsed wave Doppler velocity curves of the peripheral arteries according to the Pourcelot equation ([peak systolic velocity – end-diastolic velocity]/peak systolic velocity) (Pourcelot 1974).
The first studies on RI were aimed at evaluating its role in the assessment of the grade of stenosis of internal carotid artery lesions (Pourcelot 1974). Several studies have subsequently suggested the possible usefulness of RI also in the evaluation of other vascular beds, among which the kidney.
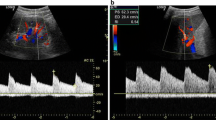
Assessment of renal resistance index. The examination of kidney and renal arteries is usually performed by an anterior approach by means of a convex ultrasound probe. The patient should be adequately prepared to avoid intestinal gases.
Nonetheless, it is possible to evaluate the renal resistance index (RRI) at the end of an echocardiogram also using the echocardiographic probe (Ciccone et al. 2014). With the patient in a lateral or sitting position, the scan images of the kidney are obtained from a posterior approach. The use of Color or Power Doppler helps to localize the vessels.
RRI slightly decreases from the hilum toward the renal cortex. Usually, pulsed Doppler volume sample is placed at the level of the segmental arteries. It is necessary to achieve the best alignment between the ultrasound beam and flow direction to record Doppler velocities maximizing waveforms gain and size, to obtain peak systolic velocity and end-diastolic velocity (Fig. 4). Three to five reproducible waveforms should be evaluated.
Renal resistance index calculation. Calculation of the renal resistance index. The renal arterial Doppler was performed using a 4 MHz probe with the patient in the sitting position and using a posterior approach to visualize the kidney. The course of the segmental arteries is visualized by color Doppler flow, and, at the middle tract level of the best one visualized, pulsed wave Doppler is performed. Peak systolic velocity and end-diastolic velocity are used to calculate the renal arterial resistance index according to Pourcelot formula
In healthy adult subjects, the RRI mean value is around 60 with nonsignificant differences between the two kidneys (Pontremoli et al. 1999). The index is affected by extreme bradycardia and tachycardia and pathologically increases with severe aortic valve insufficiency and decreases with severe aortic valve stenosis. It is less reliable during arrhythmias (Krumme et al. 2007).
An RRI greater than 70 is generally considered abnormal in adults, while it could physiologically exceed this cutoff in the first years of life (Tublin et al. 2003).
According to a recent meta-analysis (Lubas et al. 2014), measures taken by expert staff showed an intraobserver variability ranging from 2.07 % to 5.1 % and an interobserver variability from 3.61 % to 6.2 %. Therefore, overall RRI has a good reproducibility and repeatability
RRI and renal resistances. The relationship between RRI and renal resistance is not actually linear. RRI changes have been evaluated in an ex vivo system in which vascular compliance and resistance could have been separately modified (Bude et al. 1999). The study authors concluded that RRI is influenced by a combination of compliance and resistance. For fixed compliance, the RRI increases with increasing resistance.
In vivo conditions are even more complicated than this because many factors play a role in perfusion pressure regulation. Also, considering that the majority of published studies have enrolled nonhomogeneous study populations, this preamble explains why the literature on RRI sometimes produced discordant results that have caused some skepticism about the routine use of this parameter in clinical practice.
Renal artery stenosis and RRI. Besides the exploration of the entire renal artery, also the examination of the intrarenal arteries is extremely important in the diagnosis of renal artery stenosis (RAS) (Fig. 5). The RRI, together with the acceleration time index, is in fact an indirect parameter of stenosis. In the presence of a significant RAS, reduction in blood flow and pulsatility occur leading to a tardus-parvus pattern Doppler waveform characterized by a longer time needed to achieve the peak systolic velocity. In addition, the vasodilatation of the distal capillary bed produces a reduced peak systolic velocity and a relatively increased end-diastolic velocity, reducing overall the poststenotic RRI.
Zeller and Schwerck have proved, in different studies, the accuracy of a side-to-side difference in an RRI >5 to detect RAS, while an AT >100 ms was found to be reliable for the diagnosis, with an 89 % sensitivity and a 91 % specificity (Zeller et al. 2008).
Factors Influencing RRI
In the absence of a significant renal artery stenosis, RRI is influenced by a number of physiological and pathophysiological factors able to modify arterial and venous RBF.
Aging and arterial stiffness. As previously mentioned, RRI could be influenced by changes in arterial compliance and resistance (Bude et al. 1999).
Consequently, RRI is influenced by all physiological and pathophysiological factors which change arterial compliance and resistance. It has been widely demonstrated that RRI positively correlates with age, pulse pressure, pulse wave velocity (Schwenger et al. 2006; Lubas et al. 2014; Ohta et al. 2005), and ambulatory arterial stiffness index (Ratto et al. 2006), which are all markers of reduced arterial compliance.
In a population of renal transplanted patients, RRI was correlated to the recipient’s ankle-brachial blood pressure index (Heine et al. 2005) and to subject’s age, rather than the age of the grafted kidney (Krumme et al. 1997).
All cardiovascular risk factors which can modify arterial stiffness, such as diabetes and essential hypertension, are also able to modify RRI. RRI has been found to be significantly correlated to daytime systolic blood pressure variability (Kawai et al. 2012). Similarly, RRI was also independently associated with blood glucose level (Otha et al. 2008) and insulin resistance (Afsar et al. 2010). These two risk factors affect the RRI not only by modifying arterial stiffness but also by determining parenchymal renal lesion and/or promoting oxidative stress.
Parenchymal renal diseases. Platt et al. (1990) first showed the utility of RRI to differentiate between isolated glomerular disease and vascular or interstitial disease as assessed by renal biopsies. Patients with glomerulopathies had normal RI values (mean value: 58), whereas the others had markedly elevated RI values (mean values are 87 in vascular alterations and 75 in interstitial fibrosis).
Numerous studies have subsequently confirmed the association of RRI with tubulointerstitial injury (Bigè et al. 2012; Sugiura et al. 2004; Boddi et al. 2005) and arteriolosclerosis. In particular, one found that RRI was correlated to renal involvement in patients with progressive sclerosis (Aikimbaev et al. 2001).
It is probable that parenchymal diseases, involving any parenchymal component, produce a scarring process that, in turn, leads to a reduction in the intrarenal vessels area and to an increase in interstitial pressure, which decreases vascular compliance and increases resistance to blood flow.
Endothelial dysfunction. As previously mentioned, endothelial dysfunction and the related NO imbalance play a key role in the vasomotor nephropathy. Intrarenal NO regulates glomerular hemodynamics, tubular transport, and tubuloglomerular feedback (Majid et al. 2001). The net result is increased renal and glomerular perfusion, natriuresis, and diuresis.
Changes in NO availability could influence RRI by modifying both afferent and efferent arteriole tone as well as renal medullary blood flow. Moreover, the ischemia related to reduced NO and the related increase in inflammatory cytokines can cause fibrosis, vascular remodeling, and rarefaction (Chade 2013).
Although these are potential effects, there are limited data about the relationship between RRI and endothelial dysfunction (Bruno et al. 2011).
Intra-abdominal and central venous pressure. Another important determinant of renal resistances is renal venous pressure. Its changes influence RBF more than changes in arterial blood pressure. It has been demonstrated experimentally that RBF decreases when renal venous pressure increases (Winton 1931).
The mechanisms influencing renal venous pressure include the obstruction of renal vein (thrombosis, neoplastic mass, inferior vena cava syndrome), an increase in abdominal pressure (ascites and hemorrhage), and a rise in central venous pressure (CVP) (HF and pulmonary hypertension) (Braam et al. 2012).
An increased renal venous pressure causes a decrease in arteriovenous gradient with a consequent reduction in RBF. Moreover, it determines a rise in efferent arterioles and end glomerular capillary pressures, thus inducing a decrease in net filtration pressure and in GFR and an increase in arterial renal resistances (Jessup and Costanzo 2009). Finally, besides the effects on gradients of renal vasculature, an increased renal venous pressure causes a rise in interstitial pressure. This is due to the low compliance of interstitium and to the tight capsule of the kidney (Braam et al. 2012).
As a consequence, in the clinical setting of heart failure, venous congestion is the other determinant mechanism responsible for the decrease in renal perfusion together with reduced cardiac output. This has recently been supported by a study evaluating a series of CHF outpatients in which a high CVP was independently associated with higher RRI values (Ciccone et al. 2014).
This is even more interesting considering that both in patients with acute and chronic heart failure (CHF) an increased CVP has been found to be associated with a WRF and a progression of cardiorenal syndrome (Damman et al. 2007; Winton 1931; Mullens et al. 2009; Iacoviello (a) et al. 2013).
Potential Applications to Prognosis
Arterial Hypertension
Several data show that a high RRI in hypertensive patients is also associated to organ-damage markers such as left ventricular hypertrophy and carotid intima-media thickness (Florczak et al. 2009; Kawai et al. 2012; Parolini et al. 2009; Otha et al. 2008; Doi et al. 2012).
Independently from the presence of overt nephropathy, hypertensive patients show higher vascular resistance and a higher RRI (Raff U et al. 2010).
In 426 hypertensive patients, Doi and coll. (2012) demonstrated that RRI >73 for males and >72 for females was predictive of a combined end point of cardiovascular and renal events (i.e., all-cause death, myocardial infarction, stroke, congestive heart failure requiring hospitalization, aortic dissection, and end-stage renal failure requiring regular hemodialysis).
Although RAAS inhibitors seem to decrease RRI values, showing a renoprotective effect, in some studies the stratification of the population on the basis of the use of these drugs did not alter the prognostic results (Sugiura and wada 2009; Doi et al. 2012).
Diabetes
As in arterial hypertension, diabetes is a systemic disease with a multiorgan target. Diabetic nephropathy is characterized by nodular and diffuse forms of intercapillary glomerulosclerosis, afferent and efferent glomerular arteriolar hyalinization, tubular atrophy, and interstitial fibrosis (Ohta et al. 2005), although glomerulosclerosis is the histological hallmark of the disease.
It has been demonstrated that in diabetic patients, high RRI values are significantly correlated to the observation of microvascular diabetic complications, including nephropathy, retinopathy, or sensory neuropathy (Liu et al. 2012). Moreover, in patients with renal diseases, RRI is higher in diabetic nephropathy than in chronic glomerulonephritis and/or nephrosclerosis (Ohta et al. 2005). Finally, higher RRI in diabetic patients is also associated with left ventricular diastolic dysfunction (MacIsaac et al. 2008), thus highlighting that type 2 diabetes leads to multiorgan damage (including simultaneous injury to the heart and kidneys).
In advanced CKD (eGFR <30 ml/min/1.73 m2), however, the RRI difference between diabetic and nondiabetic patients loses statistical significance (Kawai et al. 2011), thus suggesting the prevalent relevance of the advanced renal disease in affecting the value of the parameter.
Renal Diseases
More data are available for RRI in renal diseases.
Chronic kidney disease. In this clinical setting, several studies have shown that the RRI assessed at enrollment is predictive of WRF (Radermacher et al. 2002; Ikee et al. 2005; Splendiani et al. 2002; Sugiura et al. 2009; Parolini et al. 2009; Hanamura et al. 2012).
Splendiani et al. (2002) demonstrated a positive correlation between the initial value of RRI and the percentage of serum creatinine variation.
Radermacher and coll. (2002) found that RRI >80 can identify patients with a worse prognosis. In a multivariate regression analysis, only proteinuria and RRI were independent predictors of progressive renal dysfunction. In a study conducted by Hanamura and coll. in patients with CKD (Hanamura et al. 2012), a RRI >70 was an independent risk factor for WRF, and it was considered a marker of organ dysfunction, histological damage, and renal prognosis and a possible determinant for steroid therapy.
Bigè and coll. (2012) studied 58 patients affected by CKD, defined according to the KDOQI definition, and undergoing renal biopsy. In this series, a RRI >70 was predictive of a reduction in renal function and identified patients at higher risk of end-stage renal disease independently from GFR baseline values.
Also the studies conducted on renal transplanted patients showed very interesting conclusions. A RRI >80 was predictive of allograft failure. However, it did not permit identification of a specific cause of failure, but could raise the suspicion of vascular complications associated with transplantation, such as arteriovenous fistula or vein thrombosis. What is more important, however, is that the higher values of RRI were associated with patient deaths, also in cases of functioning grafts (Radermacher et al. 2003).
Acute kidney injury. Very recent studies suggest the usefulness of RRI in predicting acute kidney injury (AKI) in intensive care unit patients (namely, patients with polytrauma and sepsis or patients who underwent cardiac surgery) (Schnell et al. 2012; Bossard et al. 2011; Dewitte et al. 2012). Moreover, (Darmon et al. 2011) found even higher values of RRI in patients with persistent AKI.
Renal artery stenosis. Radermacher and coll. (2001) suggested RRI as a marker to predict survival after therapeutic intervention (angioplasty or surgery) for RAS. Patients with a RRI >80 showed no improvement in renal function, hypertension, or renal survival even after a successful procedure. Two subsequent studies (Yuksel et al. 2012; Cianci et al. 2010) confirmed similar results while another disproved them (Zeller et al. 2008).
Heart Failure
Renal impairment in CHF patients is very common and is associated with higher morbidity and mortality (Hillege et al. 2000; Smith et al. 2006; Damman et al. 2009). Therefore, its accurate characterization plays a key role in the management of these patients. The role of RRI in the clinical setting of CHF is a topic of interest since, reflecting kidney vascular and parenchymal abnormalities (Fig. 6), it adds information about renal impairment and the outcome of these patients (Ennezat et al. 2011; Ciccone et al. 2014).
Pathophysiology of renal resistance index in cardiorenal syndrome. The pathophysiological factors influencing the renal resistances and the consequent prognostic impact on cardiorenal syndrome are summarized. The combination of hemodynamic and neurohormonal factors leads to functional and structural changes responsible for the increase in renal resistance index. This further promotes the activation of neurohormonal systems as well as sodium and water retention, thus favoring the progression of cardiorenal syndrome
The first association between high RRI and worse outcome has been demonstrated in a series of patients affected by HF with preserved ejection fraction (HFpEF) (Ennezat et al. 2011). Subsequently, this association has been found in a series of CHF outpatients mainly affected by HF with reduced ejection fraction (HFrEF). In this series, RRI was an independent predictor of both a composite end point reflecting HF progression (i.e., death hospitalization due to HF worsening) (Ciccone et al. 2014) and mortality for all causes (Monitillo et al. 2014).
Also in CHF patients, the different pathophysiological conditions underlying the increase in renal vascular resistance and, as a consequence, in RRI can explain the relevant prognostic information carried by this parameter. This is confirmed by the independent association that has been found in CHF patients between RRI and the variables reflecting the presence of atherosclerosis and/or an increased arterial stiffness (i.e., age, diabetes, and pulse pressure) as well as an increased CVP.
This pathophysiological background underlying RRI values can also explain its incremental value when added to GFR. A high RRI could identify patients at higher risk of HF progression both in patients with GFR above and below 60 ml/min*1.73 m2. On the other hand, patients with low RRI were characterized by a similar risk of events also when dichotomized according to the presence or not of reduced GFR (Ciccone et al. 2014).
But it is also worth noting that RRI is not only associated with HF progression but also with WRF in CHF outpatients. In fact a RRI >70 is independently associated with a 1-year increase in creatinine >0.3 mg/dl (Citarelli et al. 2014).
Finally, in CHF outpatients, RRI was also demonstrated to be independently associated with high doses of loop diuretics as well as with their increase during a midterm follow-up (Iacoviello (b) et al. 2015). The increased intrarenal resistance could, in fact, cause a reduction in filtration pressure and lead to a reduced delivery of diuretic molecules at the level of Henle’s loop and a reduced response (Paul 2002). As a result, an increased RRI can allow the detection of patients with an altered diuretic dose-response curve who may develop diuretic resistance.
These data suggest that this parameter could be used together with GFR,in the clinical setting of CHF in order to obtain a better characterization of kidney function. This is further strengthened by the fact that RRI evaluation has been found to be easy, fast, and highly reproducible (Ciccone et al. 2014).
Summary Points
-
Renal resistances play a key role in renal function as well as in CKD onset and progression. It has been shown that RRI, a Doppler-derived parameter, reflects renal arterial resistances and is a noninvasive evaluation tool.
-
An altered RRI reflects the many factors which can modify renal resistances, such as increased arterial stiffness, arterial atherosclerosis, oxidative stress, endothelial dysfunction, renal parenchymal abnormalities, renal microvascular disease and vascular rarefaction, intra-abdominal and central venous pressure, and neurohormonal activation.
-
In patients with renal diseases, RRI is not a marker for a specific disease because it increases in different clinical conditions. However, it certainly could be an index to indicate renal disease progression as it reflects the abnormalities of both the systemic and local vascular bed.
-
In renal diseases, an increased RRI is associated with a higher risk of CKD progression and a worse outcome.
-
In cardiovascular diseases, RRI seems particularly relevant in patients with arterial hypertension and in those with CHF. In the latter group, an increased RRI has been found to be independently associated both with HF progression and mortality. Moreover, it offers incremental prognostic information when added to GFR and is able to detect patients prone to developing WRF.
Abbreviations
- AKI:
-
Acute kidney injury
- CHF:
-
Chronic heart failure
- CKD:
-
Chronic kidney disease
- CVP:
-
Central venous pressure
- GFR:
-
Glomerular filtration rate
- HF:
-
Heart failure
- HFPEF:
-
Heart failure with preserve ejection fraction
- HFREF:
-
Heart failure with reduced ejection fraction
- NO:
-
Nitric oxide
- RAAS:
-
Renin-angiotensin-aldosterone system
- RAS:
-
Renal artery stenosis
- RBF:
-
Renal blood flow
- RI:
-
Resistance index
- ROS:
-
Reactive oxygen species
- RRI:
-
Renal resistance index
- WRF:
-
Worsening of renal function
References
Afsar B, Elsurer R, Sezer S, Ozdemir FN. Insulin resistance is associated with increased renal resistive index independent of other factors in newly diagnosed type 2 diabetes mellitus and hypertensive patients. Metabolism. 2010;59:279–84.
Aikimbaev KS, Canataroglu A, Ozbek S, Usal A. Renal vascular resistance in progressive systemic sclerosis: evaluation with duplex Doppler ultrasound. Angiology. 2001;52:697–701.
Bigé N, Lévy PP, Callard P, et al. Renal arterial resistive index is associated with severe histological changes and poor renal outcome during chronic kidney disease. BMC Nephrol. 2012;13:139.
Boddi M, Cencioni I, Poggesi L, et al. Renal Resistive Index early detects chronic tubulointerstitial nephropathy in normo and hypertensive patients. Am J Kidney Dis. 2005;46:603–9.
Bosch JP. Renal reserve: a functional view of glomerular filtration rate. Semin Nephrol. 1995;15:381–5.
Bossard G, Bourgoin P, Corbeau JJ, Huntzinger J, Beydon L. Early detection of postoperative acute kidney injury by Doppler renal resistive index in cardiac surgery with cardiopulmonary bypass. Br J Anaesth. 2011;107:891–8.
Braam B, Cupples WA, Joles JA, Gaillard C. Systemic arterial and venous determinants of renal hemodynamics in congestive heart failure. Heart Fail Rev. 2012;17(2):161–75.
Bruno RM, Daghini E, Landini L, Versari D, Salvati A, Santini E, Di Paco I, Magagna A, Taddei S, Ghiadoni L, Solini A. Dynamic evaluation of renal resistive index in normoalbuminuric patients with newly diagnosed hypertension or type 2 diabetes. Diabetologia. 2011;54:2430–9. doi:10.1007/s00125-011-2148-y.
Bude RO, Rubin JM. Relationship between the resistive index and vascular compliance and resistance. Radiology. 1999;211:411–7.
Chade AR. Renal vascular structure and rarefaction. Compr Physiol. 2013;3:817–31.
Cianci R, Martina P, Cianci M, et al. Ischemic nephropathy: proteinuria and renal resistance index could suggest if revascularization is recommended. Ren Fail. 2010;32:1167–71.
Ciccone MM, Iacoviello M, Gesualdo L, et al. The renal arterial resistance index: a marker of renal function with an independent and incremental role in predicting heart failure progression. Eur J Heart Fail. 2014;16:210–6.
Citarelli G, Iacoviello M, Monitillo F, Leone M, et al. A high renal arterial resistance index is associated to one year worsening of renal function in heart failure outpatients. Eur J Heart Fail. 2014;16 Suppl 2:126.
Damman K, Navis G, Smilde TD, Voors AA, van der Bij W, vanVeldhuisen DJ, et al. Decreased cardiac output, venous congestion and the association with renal impairment in patients with cardiac dysfunction. Eur J Heart Fail. 2007;9:872–8.
Damman K, Jaarsma T, Voors AA, Navis G, Hillege HL, van Veldhuisen DJ, COACH Investigators. Both in- and out-hospital worsening of renal function predict outcome in patients with heart failure: results from the Coordinating Study Evaluating Outcome of Advising and Counseling in Heart Failure (COACH). Eur J Heart Fail. 2009;11:847–54.
Damman K, Kalra PR, Hillege H. Pathophysiological mechanisms contributing to renal dysfunction in chronic heart failure. J Ren Care. 2010;36:18–26. doi:10.1111/j.1755-6686.2010.00172.x.
Damman K, Voors AA, Navis G, van Veldhuisen DJ, Hillege HL. Current and novel renal biomarkers in heart failure. Heart Fail Rev. 2012;17:241–50.
Darmon M, Schortgen F, Vargas F, Liazydi A, Schlemmer B, Brun-Buisson C, Brochard L. Diagnostic accuracy of Doppler renal resistive index for reversibility of acute kidney injury in critically ill patients. Intensive Care Med. 2011;37:68–76.
Dewitte A, Coquin J, Meyssignac B, et al. Doppler resistive index to reflect regulation of renal vascular tone during sepsis and acute kidney injury. Crit Care. 2012;16:R165. doi:10.1186/cc11517.
Doi Y, Iwashima Y, Yoshihara F, et al. Renal resistive index and cardiovascular and renal outcomes in essential hypertension. Hypertension. 2012;60:770–7.
Ennezat PV, Maréchaux S, Six-Carpentier M, et al. Renal resistance index and its prognostic significance in patients with heart failure with preserved ejection fraction. Nephrol Dial Transplant. 2011;26:3908–13.
Florczak E, Januszewicz M, Januszewicz A, et al. Relationship between renal resistive index and early target organ damage in patients with never-treated essential hypertension. Blood Press. 2009;18:55–61.
Hanamura K, Tojo A, Kinugasa S, Asaba K, Fujita T. The resistive index is a marker of renal function, pathology, prognosis, and responsiveness to steroid therapy in chronic kidney disease patients. Int J Nephrol. 2012;2012:139565. doi:10.1155/2012/139565.
Heine GH, Gerhart MK, Ulrich C, Kohler H, Girndt M. Renal Doppler resistance indices are associated with systemic atherosclerosis in kidney transplant recipients. Kidney Int. 2005;68:878–85.
Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, Hampton JR, van Veldhuisen DJ. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000;102:203–10.
Iacoviello M (a), Puzzovivo A, Monitillo F, Saulle D, Lattarulo MS, Guida P, Forleo C, Gesualdo L, Favale S. Independent role of high central venous pressure in predicting worsening of renal function in chronic heart failure outpatients. Int J Cardiol. 2013;162:261–3.
Iacoviello M (b), Doronzo A, Paradies V et al. The independent association between altered renal arterial resistance and loop diuretic dose in chronic heart failure outpatients. IJC Heart & Vasculature 2015;7:119–123.
Ikee R, Kobayashi S, Hemmi N, et al. Correlation between the resistive index by Doppler ultrasound and kidney function and histology. Am J Kidney Dis. 2005;46:603–9.
Jessup M, Costanzo MR. The cardiorenal syndrome: do we need a change of strategy or a change of tactics? J Am Coll Cardiol. 2009;53:597–9.
Kawai T, Kamide K, Onishi M, et al. Usefulness of the resistive index in renal Doppler ultrasonography as an indicator of vascular damage in patients with risks of atherosclerosis. Nephrol Dial Transplant. 2011;26:3256–62.
Kawai T, Ohishi M, Kamide K, et al. The impact of visit-to-visit variability in blood pressure on renal function. Hypertens Res. 2012;35:239–43.
Krumme B, Hollenbeck M. Doppler sonography in renal artery stenosis—does the Resistive Index predict the success of intervention? Nephrol Dial Transplant. 2007;22:692–6.
Krumme B, Grotz W, Kirste G, Schollmeyer P, Rump LC. Determinants of intrarenal Doppler indices in stable renal allografts. J Am Soc Nephrol. 1997;8:813–6.
Liu KH, Chu WC, Kong AP, et al. Intrarenal arterial resistance is associated with microvascular complications in Chinese type 2 diabetic patients. Nephrol Dial Transplant. 2012. doi:10. 1093/ndt/gfs471 59.
Lubas A, Kade G, Niemczyk S. Renal resistive index as a marker of vascular damage in cardiovascular diseases. Int Urol Nephrol. 2014;46:395–402. doi:10.1007/s11255-013-0528-6.
MacIsaac RJ, Thomas MC, Panagiotopoulos S, et al. Association between intrarenal arterial resistance and diastolic dysfunction in type 2 diabetes. Cardiovasc Diabetol. 2008. doi:10.1186/1475-2840-7-1560.
Majid DS, Navar LG. Nitric oxide in the control of renal hemodynamics and excretory function. Am J Hypertens. 2001;14:74S–82.
Monitillo F, Iacoviello M, Citarelli G, Leone M, et al. Independent and incremental role of renal resistance index in predicting mortality among heart failure outpatients. Eur J Heart Fail. 2014;16 Suppl 2:126.
Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, Young JB, Tang WH. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–96.
Ohta Y, Fujii K, Ibayashi S, et al. Renal and carotid vascular resistance assessed with Doppler sonography. J Clin Ultrasound. 2008;36:85–90.
Ohta Y, Fujii K, Hisatomi A. Increased renal resistive index in atherosclerosis and diabetic nephropathy assessed by Doppler sonography. J Hypertens. 2005;23:1905–11.
Parolini C, Noce A, Staffolani E, et al. Renal resistive index and long-term outcome in chronic nephropathies. Radiology. 2009;252:888–96.
Paul S. Balancing diuretic therapy in heart failure: loop diuretics, thiazides, and aldosterone antagonists. Congestive Heart Fail. 2002;8:307–12.
Platt J, Ellis J, Rubin J, et al. Intrarenal arterial Doppler sonography in patients with nonobstructive renal disease: correlation of resistive index with biopsy findings. AJR. 1990;154:1223–7.
Pontremoli R, Viazzi F, Martinoli C, et al. Increased renal resistive index in patients with essential hypertension: a marker of target organ damage. Nephrol Dial Transplant. 1999;14:360–5.
Pourcelot L. Velocimetrie ultrasonore Doppler. Seminaire INSERM. Paris: Edition INSERM; 1974. p. 213–40.
Radermacher J, Chavan A, Bleck J, et al. Use of Doppler ultrasonography to predict the outcome of therapy for renal artery stenosis. N Engl J Med. 2001;344:410–7.
Radermacher J, Ellis S, Haller H. Renal resistance index and progression of renal disease. Hypertension. 2002;39:699–703.
Radermacher J, Mengel M, Ellis S, et al. The renal arterial resistance index and renal allograft survival. N Engl J Med. 2003;349:115–24.
Raff U, Schmidt BM, Schwab J, Schwarz TK, Achenbach S, Bar I, Schmieder RE. Renal resistive index in addition to low-grade albuminuria complements screening for target organ damage in therapy-resistant hypertension. J Hypertens. 2010;28:608–14.
Ratto E, Leoncini G, Viazzi F, Vaccaro V, Falqui V, Parodi A, Conti N, Tomolillo C, Deferrari G, Pontremoli R. Ambulatory arterial stiffness index and renal abnormalities in primary hypertension. J Hypertens. 2006;24:2033–8.
Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–39.
Schnell D, Deruddre S, Harrois A, Pottecher J, Cosson C, Adoui N, Benhamou D, Vicaut E, Azoulay E, Duranteau J. Renal resistive index better predicts the occurrence of acute kidney injury than cystatin C. Shock. 2012;38:592–7.
Schwenger V, Keller T, Hofmann N, Hoffmann O, Sommerer C, Nahm AM, Morath C, Zeier M, Krumme B. Color Doppler indices of renal allografts depend on vascular stiffness of the transplant recipients. Am J Transplant. 2006;6:2721–4.
Smilde T, van Veldhuisen D, Navis G, Voors A, Hillege H. Drawbacks and prognostic value of formulas estimating renal function in patients with chronic heart failure and systolic dysfunction. Circulation. 2006;114:1572–80.
Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, DiCapua P, Krumholz M. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. 2006;47:1987–96.
Splendiani G, Parolini C, Fortunato L, et al. Resistive index in chronic nephropathies: predictive value of renal outcome. Clin Nephrol. 2002;57:45–50.
Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–83.
Sugiura T, Wada A. Resistive index predicts renal prognosis in chronic kidney disease. Nephrol Dial Transplant. 2009;24:2780–5.
Sugiura T, Nakamori A, Wada A, et al. Evaluation of tubulointerstitial injury by doppler ultrasonography in glomerular diseases. Clin Nephrol. 2004;61:119–26.
Tublin ME, Bude RO, Platt JF. The resistive index in renal Doppler sonography: where do we stand? AJR. 2003;180:885–92.
Winton FR. The influence of venous pressure on the isolated mammalian kidney. J Physiol. 1931;72:49–61.
Yuksel UC, Anabtawi AG, Cam A. Predictive value of renal resistive index in percutaneous renal interventions for atherosclerotic renal artery stenosis. J Invasive Cardiol. 2012;24:504–9.
Zeller T, Bonvini RF, Sixt S. Color-coded duplex ultrasound for diagnosis of renal artery stenosis and as follow-up examination after revascularization. Catheter Cardiovasc Interv. 2008;71:995–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Dordrecht
About this entry
Cite this entry
Iacoviello, M., Antoncecchi, V., Leone, M., Ciccone, M.M. (2016). Renal Arterial Resistance Index. In: Patel, V., Preedy, V. (eds) Biomarkers in Kidney Disease. Biomarkers in Disease: Methods, Discoveries and Applications. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7699-9_28
Download citation
DOI: https://doi.org/10.1007/978-94-007-7699-9_28
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7698-2
Online ISBN: 978-94-007-7699-9
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences