Abstract
There are two main indications for mapping of the motor cortex in patients eligible for surgery with rolandic tumors. First, mapping is indicated if the functional anatomy (i.e. the exact spatial relationship between the tumor and the presumed essential motor areas) remains unclear after anatomical imaging. The reasons for this can be the mass effect of the tumor or infiltrative growth. Second, mapping is indicated if there is a discrepancy between the imaging results and the clinical findings (for example, a large tumor within the primary motor cortex but no noticeable motor deficits). In such cases, the functional anatomy may have changed due to tumor-induced plasticity. In either of these two scenarios (which may occur separately or together), motor mapping provides elucidation of the functional anatomy and the state of the motor system. This chapter presents an overview of the possibilities and limitations of transcranial magnetic stimulation (TMS) and direct electrical stimulation (DES) for mapping of the cortical motor topography in the neurosurgical setting. The intriguing feature of TMS is that it is the only painless non-invasive method that allows for direct electrical stimulation of the brain. Findings in basic research have recently been backed up by current studies that TMS is a relevant tool for performing stimulation mapping procedures, which were previously only possible with direct electrical stimulation of the brain during surgery. All relevant studies comparing TMS to DES for mapping of the motor cortex are summarized and commented in this chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Transcranial Magnetic Stimulation
- Motor Cortex
- Tibialis Anterior
- Repetitive Transcranial Magnetic Stimulation
- Abductor Pollicis Brevis
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
When a patient has a brain tumor in or near the motor cortex, the neurosurgeon’s goal is to maximize the extent of tumor resection, without causing any new functional deficits. Achieving both of these goals simultaneously can be challenging, especially if the tumor is close to essential functional areas of the motor cortex. To achieve both these goals, the surgeon needs precise knowledge of which areas of the brain are functionally essential versus which areas are not essential and can be safely resected. Unfortunately, the functional relevance of tissue in an individual case cannot be predicted from standard anatomical landmarks, not only because of natural anatomical variation between all people, but even more importantly because the tumor mass can displace and/or obscure the familiar anatomical landmarks, and also because the tumor can induce plastic reorganization of the brain’s functional areas, especially in the case of slow-growing tumors. So in order to achieve maximal tumor removal without causing functional deficits, it is essential to have case-specific knowledge of the location of functionally essential areas. Intraoperative functional testing of the brain tissue surrounding the tumor is the most accurate and reliable way to obtain this knowledge, but there are many advantages to obtaining such functional maps also before the surgery starts.
In the past, intraoperative direct electrical stimulation (DES) of brain tissue was the only modality available for brain mapping. In recent decades, much effort has been spent on developing various technologies for non-invasive pre-operative brain mapping (Picht and Atalay 2012). One of the more promising modalities that has been developed is transcranial magnetic stimulation (TMS). Compared to all other modalities of pre-operative cortical mapping, TMS has the unique advantage that like DES it stimulates the brain and then records the motor output, rather than asking the patient to move, recording the brain activation, and then trying to interpret which cortical areas were essential for that movement. TMS works by holding a wire coil just above the patient’s head near the motor cortex and then sending a brief electric current through that wire coil. The electric current generates a corresponding magnetic field, as electricity always does, and this magnetic field passes through the patient’s skull. Inside the skull, this magnetic field then again creates an electric flow of ions which can depolarize the patient’s neurons and lead to nerve signals in that part of the brain. TMS has been available for more than 20 years already. In the early years of TMS, it was not really possible to accurately know the anatomical location of the stimulus, because the wire stimulation coil was held freehand according to anatomical landmarks, which vary between individuals (Krings et al. 1997). To overcome this problem, TMS has been refined by combining it with neuronavigation systems: “navigated TMS”, (nTMS) (Krings et al. 1997; Picht et al. 2009). This has made it possible to electrically stimulate precise areas of the brain with navigational targeting, thus achieving spatially accurate brain mapping pre-operatively (Picht et al. 2011a).
The main purpose of the present book chapter is to review previous reports assessing the spatial accuracy of nTMS by comparing it to the gold standard of DES, and also to summarize the advantages and disadvantages of nTMS. We begin with a general overview of the basic principles of DES and TMS. Next we summarize the literature on the safety and risks of TMS. Then we review the literature on the spatial accuracy of nTMS compared to DES, and we also discuss the limitations of such comparisons. Finally, we will discuss the role of nTMS in pre-operative mapping of motor areas.
Direct Electrical Stimulation: The Gold Standard of Cortical Mapping
For almost a century, applying an electrical current directly to the brain either by means of handheld electrodes or by implanted grids of electrodes has been the only reliable method for identification of brain areas carrying essential motor function. Clinically, direct electrical stimulation (DES) is still considered to be the “gold standard” for functional mapping of the primary motor cortex (Picht et al. 2011a), since it enables more extensive tumor resection at a lower rate of severe neurological sequela (De Witt Hamer et al. 2012). Current understanding of functional brain topography and connectivity is based on DES findings. And DES is still the only modality that enables cortical and subcortical localization of motor function intra-operatively with absolute spatial accuracy.
The basic principle of DES is to apply an electrical impulse to the brain cortex and record the muscle output. Technically, there are two different ways to do this: monopolar DES and bipolar DES (Kombos and Suss 2009). Comparing bipolar DES to monopolar DES neurophysiologically, it has been demonstrated that stimulation with a bipolar probe was very effective in producing localized current flows; whereas, a monopolar probe at the same stimulation level produced higher current densities and stimulated a larger region of the cortex. The stimulation parameters also differ significantly between the two methods. For monopolar stimulation, the frequency typically varies between 250 and 500 Hz, the pulse width is 0.2–0.7 ms, and the number of pulses in a stimulation train between two and seven, which leads to a stimulation time of 4–28 ms. For bipolar stimulation, the frequency is typically 50 Hz or 60 Hz, the pulse width is 0.2–0.7 ms, and the number of pulses in a train varies between 50 and 200, which leads to a total stimulation time of 1–4 s (Penfield and Boldrey 1937; Taniguchi et al. 1993). These variations lead to marked differences in the net amount of charge applied to the cortex. In addition to these differences of charge applied per time and net amount of charge, several other factors influence the results of the stimulation: the shape of the electrode tips, the type of stimulator used, and the way the electrodes are handled (e.g., pressured onto the cortex/light touch; lots of irrigation/dry field).
In the clinical setting of neurosurgery today, intraoperative DES in patients with brain lesions in or near the motor cortex enables neurosurgeons to identify both cortical and subcortical motor pathways during surgeries (Sanai and Berger 2010). Although many neurosurgeons are aware that DES improves surgeries of brain lesions such as gliomas in or near the motor cortex, there are only a few studies that actually provide scientific evidence of this (De Witt Hamer et al. 2012; Duffau et al. 2005). In 2012, De Witt Hamer et al. reported a meta-analysis of observational studies with 8091 adults patients in an attempt to elucidate the usefulness of intraoperative DES for rolandic infiltrative glioma surgeries (De Witt Hamer et al. 2012). The percentage of gross total resections was higher with intraoperative DES (75 %) than without it (58 %). And the rate of severe neurologic deficits was lower with DES (3.4 %) than without it (8.2 %). That study provides level-one evidence that intraoperative DES make a substantial improvement in outcomes from resecting gliomas in or near the motor cortex, so DES should always be used for such surgeries.
The major drawback of DES is its invasiveness. This restricts its clinical application to the intraoperative situation and largely limits its research usage to mapping that is clinically necessary anyway. The limited understanding of spatial accuracy of the method and evoked current spread into the brain tissue can make interpretation of DES results difficult and in part dependent on the individual team’s experience. Induction of epileptic seizures is a possible problem, especially for bipolar DES (Kombos et al. 1999). Yet, the likelihood depends on the exact stimulation parameters and the susceptibility of the individual brain. It is reported that stimulation-associated seizures occur in 1.2 % of patients stimulated with the monopolar technique and in 9.5 % of patients with the bipolar technique (Szelenyi et al. 2007). Bipolar stimulation can also lead to activating several muscles and thus to movement of the patient’s extremities; it is therefore unsuitable for monitoring (Kombos et al. 1999). Thus even though DES is the gold standard, its usage is limited to what is clinically necessary during the restricted time period of the operation.
Basic Principles of Navigated Transcranial Magnetic Stimulation
Transcranial magnetic stimulation (TMS) is a technique for noninvasive and painless stimulation of the human brain. The stimulation of the brain is produced by passing a brief electric current through a wire coil held outside the skull. This electric current simultaneously creates a corresponding brief, high-intensity magnetic field, which passes through the skull. The induced electrical field then creates movements of electrically charged ions inside the brain tissue. Depending on the strength of this electrical current and local tissue factors, this can lead to depolarization of neurons, thus to neural signals. If the TMS stimulation coil is placed above the motor cortex, the stimulation can lead to muscle movements, which can be recorded with a standard EMG. The stimulation coil can have different shapes and the parameters of the electric current can also be varied.
TMS was first introduced into clinical practice in 1985 (Barker et al. 1985). But for many years, basic TMS was not much benefit for planning neurosurgery, because the locations stimulated could only be guessed from neuroanatomical landmarks, which was not sufficiently accurate for neurosurgical purposes. In recent years though, neuronavigational systems have been integrated together with TMS. This navigated TMS (nTMS) enables the examiner to see quite precisely on an uploaded MRI where the TMS stimulation is being applied, thus allowing us now to map the motor cortex of patients.
While the basic TMS technology used in neurosurgery is still the same, an important aspect of TMS must be understood to make the method useful: namely, the operator needs to accurately know the location of the maximum electric-field, induced by the magnetic impulse. The best assumption of the location of neuronal activation can be achieved when the electric field evoked by stimulation is displayed in the navigation system. It is important to point out that the primary magnetic field from the coil is not influenced by any tissue variations. In order to calculate the resulting electric field precisely for every intracranial location, several factors must be known: the exact specifications of the coil and the electrical characteristics of the stimulator, the size and shape of the extracranial and intracranial anatomy, and the exact location of the coil with respect to the head in all 6° of freedom (Ruohonen and Karhu 2010). Due to the spherical shape of the head, the absolute value of the electrical conductivity of the respective tissue is of secondary importance when calculating the e-field, when spherical head models are used (Ruohonen and Ilmoniemi 2005).
Yet knowing where the maximum electric field is acting does not necessarily mean that the neuronal activation also takes place at this point. The cortical neuronal structures are most sensitive to depolarization when the induced current is oriented longitudinally to the axons (Day et al. 1989). This means that the threshold for activation of the motor cortex is lowest when the coil is orientated perpendicular to the nearest underlying sulcus, due to the columnar structure of the cortical histological architecture. Thus the initiation of action potentials will most likely appear in the area where the e-field is optimally oriented to the cortex, which is not necessarily where the e-field is at its maximum.
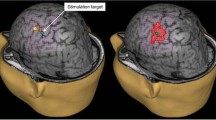
These improvements of the TMS technology in combination with standard EMG recordings have enabled accurate mapping of the motor cortex with delineation of individual muscle representations in healthy subjects (Hannula et al. 2005; Schmidt et al. 2009) and in patients with brain tumors and obscured anatomy (Krieg et al. 2012b; Picht et al. 2011a). Figure 23.1 shows a typical example of a TMS motor mapping in a case of a rolandic tumor and obscured anatomy of the central region. The 3D navigational view shows the results after TMS mapping have been performed. In the left panel of Fig. 23.1 all spots stimulated on the left hemisphere are displayed. The relevant area adjacent to the tumor has been stimulated in a dense raster. The premotor cortices have also been stimulated. In the right panel, the image displays only the spots where a muscle response was observed (MEP > 50 μV peak-to-peak amplitude). Three different hand muscles (abductor pollicis brevis, abductor digiti minimi, first dorsal interosseus) and one leg muscle (tibialis anterior) were recorded in this case. The color coding corresponds to the intensity of the response, whereby red indicates small responses (MEP 50–500 μV), yellow indicates medium responses (MEP 500–1,000 μV), and white indicates large responses (MEP > 1,000 μV). The responses close to the midline are from the leg (TA). This mapping makes it evident that the precentral gyrus has been displaced frontally.
Example of an nTMS mapping, performed on a 63 year-old female patient with a left hemisphere brain tumor, suffering from a mild hemiparesis on her right side. In the left panel, all spots stimulated on the left hemisphere are displayed. In the right panel, the image displays only the spots where a muscle response was observed (MEP > 50 μV peak-to-peak amplitude). Three different hand muscles (abductor pollicis brevis, abductor digiti minimi, first dorsal interosseus) and one leg muscle (tibialis anterior) were recorded in this case. The color coding corresponds to the intensity of the response, whereby red indicates small responses (MEP 50–500 μV), yellow indicates medium responses (MEP 500–1,000 μV), and white indicates large responses (MEP > 1,000 μV)
Overview of the Safety and Risks of Transcranial Magnetic Stimulation
When thinking about the safety and risks of transcranial magnetic stimulation, we should recognize that there are different types of TMS: single-pulse TMS, paired-pulse TMS, and repetitive TMS (rTMS). The influence of the magnetic field from these three different types of TMS procedures differs greatly, and thus they have different safety profiles. The modality used for mapping the motor cortex in patients with brain tumors is single-pulse TMS, in which the electro-magnetic influence is the lowest of the three different types of TMS. Generally, single-pulse TMS is considered to have no significant risk from its more than 20 years of clinical experience (Rossi et al. 2009; Groppa et al. 2012). Nonetheless, it is recommended to use a short safety checklist such as a questionnaire developed by “The Safety of TMS Consensus Group” (Rossi et al. 2011) to identify patients with increased risk for performing TMS such as patients with a history of loss of consciousness due to seizures or syncope, brain diseases or medications associated with increased seizure risk, the presence of implanted metallic devices, and pregnancy (Groppa et al. 2012).
In general, TMS has some direct safety concerns such as heating and magnetic field exposure (Rossi et al. 2009). The heating effects to the brain induced by a single-pulse TMS is estimated to be less than 0.1° Celsius (Ruohonen and Ilmoniemi 2002). As for magnetic field exposure, its exposure to both patients and operators should be taken into account. Magnetic field exposure induced by single-pulse TMS to patients seems not to cause a significant risk, since the total time of exposure is so short, but the potential risk of long-term adverse consequences for TMS operators has not yet been adequately studied (Rossi et al. 2009).
TMS has also several known potential adverse events (Rossi et al. 2009). The adverse events can be divided into two subgroups: (1) adverse events reported both in single-pulse TMS and paired-pulse or rTMS and (2) adverse events reported only in paired-pulse and/or rTMS but not in single-pulse TMS. The former subgroup includes events such as seizure induction, syncope, transient headache, local pain, neck pain, toothache, paresthesia, and transient auditory threshold changes. The latter subgroup contains events such as transient cognitive/neuropsychological changes, induced currents in electrical circuits of medical devices, structural brain changes, histotoxicity, and other transient biological effects such as hormonal change (Rossi et al. 2009). The latter set of adverse events only from paired-pulse or rTMS will not be discussed further here in this chapter about single-pulse TMS.
Induction of seizures is the most severe acute adverse effect for TMS, but most TMS-associated seizures were induced during repetitive TMS (Rossi et al. 2009). Less than 5 % of all the reported TMS-related seizures occurred during single-pulse TMS (Groppa et al. 2012), but this only provides a rough approximation, since it remains unknown what percent of all TMS usage is repetitive TMS. The exact incidence of seizures after single-pulse TMS is not known. Of course this rate should not be underestimated, but it seems to be very low. The question of “what percentage of single-pulse TMS sessions result in a seizure”, is a very important issue for patient counseling and safety. Since the literature lacks firm answers, it should be investigated with large-scale multi-institutional studies in the near future. In most cases, single-pulse TMS-related seizures occurred in patients with known structural brain pathology or patients under medication such as amphetamines, lithium, and chlorpromazine, which can lower the seizure threshold (Groppa et al. 2012). Nonetheless, seizures can occur in patients without known risk factors. For example, Kratz et al. (2011) reported on a healthy subject who developed seizure after single-pulse TMS during motor threshold estimation. The risk and benefit balance must always be fully discussed with the patient before TMS is used.
Patients with rolandic tumors frequently suffer from symptomatic seizures and therefore may seem to be a patient group at high-risk of TMS induced seizures. Hufnagel et al. applied TMS to 13 patients with medically intractable complex partial seizures. They found that the epileptic focus was activated by TMS in 12 out of 13 patients, but clinical seizure was induced only in one patient (Hufnagel et al. 1990). Based on a literature review (Schrader et al. 2004), the risk of single-pulse TMS-induced seizure in patients with epilepsy ranges from 0.0 % to 2.8 %. In any case, the TMS examiner should prepare space and medications for managing a seizure, should it occur.
Although TMS-associated syncope is also a rare adverse event, it is more likely to occur than seizure. Because of the lack of systematic studies, the incidence of TMS-associated syncope remains unknown, but many laboratories have experienced it (Groppa et al. 2012). This is an area deserving more attention, and until better reviews have been published, TMS users should be aware of the risk of syncope and proceed with caution in this regards.
As for pain, single-pulse TMS seems to be generally well-tolerated and experienced by most participants as painless (Rossi et al. 2009), but the literature specifically addressing this point also remains scant. As mentioned above though, transient headache, local pain, neck pain, and toothache, as well as paresthesia have all been reported from single-pulse TMS (Rossi et al. 2009). We would suppose though that these pains were at least transient, if not also mild. As for transient auditory threshold changes, TMS produces a loud clicking sound from the coil, up to 120–130 dB, and all patients should be required to wear earplugs during the procedure to prevent transient auditory threshold changes, so called “noise induced temporary threshold shift” (Groppa et al. 2012).
Three reports have mentioned adverse events from nTMS in patients with rolandic tumors (Paiva et al. 2012; Krieg et al. 2012b; Forster et al. 2011). To summarize these reports, there were two adverse events (unpleasantness in one patient, and headache in one patient) out of 31 nTMS sessions in 30 patients. Although this is too small a sample size to support reliable estimates, provisionally it suggests that about 1 in 15 patients will experience such adverse events. Larger multi-institutional registries would help to better elucidate the frequency of these events, as well as monitor the occurrence of other possible rare adverse events.
Furthermore, we must keep in mind that some neurosurgical patients harbor metals such as titanium skull plates for craniotomy closure or DBS electrodes inside the cranium, both of which present risks in the presence of an electromagnetic field such as TMS. Titanium skull plates may be of greatest concern, since TMS is performed not only pre-operatively, but also post-operatively on these patient populations. Rotenberg et al. assessed the safety of applying rTMS (which has greater electromagnetic influence than single-pulse TMS) to patients with titanium skull plates. They found that small titanium skull plates are not likely to heat sufficiently to injure the surrounding brain tissue during conventional low-frequency rTMS protocols, and they concluded that low-frequency rTMS may be safe for patients with small titanium skull plates that are in the stimulation site (Rotenberg et al. 2007). Besides the problem of heating, one must consider the possibility that the induced currents might displace the titanium skull plates (“Lorentz interaction”). Yet only minimal displacement of loose titanium plates during simulated rTMS has been observed (Rotenberg et al. 2007). As for DBS electrodes, two ex vivo studies have shown that the current and voltages induced by TMS in the deep brain electrodes are smaller than those induced by DBS itself and seemed to be a safe level (Kumar et al. 1999; Kuhn et al. 2004). nTMS is still a quite new technology and further monitoring of its safety is needed from the broader community of clinical users. Clinical users should report any incidents of adverse events that they observe in clinical usage, ideally be sending a brief letter describing the incident to a scientific journal indexed by PubMed.
Review of Studies on the Accuracy of Navigated Transcranial Magnetic Stimulation
To establish the validity of any new method of non-invasive brain mapping, its accuracy must be assessed relative to the gold standard of DES. Several studies have assessed the ability of nTMS to identify the motor cortex and delineate the cortical representation of individual muscles, in order to evaluate its reliability and accuracy for motor mapping. We conducted a review of the literature up to June 2012. The search terms we used on PubMed were: “transcranial magnetic stimulation”, “TMS”, “direct cortical stimulation”, “direct electrical stimulation”, “DCS”, “DES”, “motor cortex”, “M1” and “brain tumo(u)r”. We reviewed the abstracts of those reports, and if they reported on evaluating patients with rolandic tumors with both nTMS and DES, then we extracted information from the report. A total of eight studies meeting these criteria were identified (Table 23.1).
The first study to compare nTMS to DES for evaluating the motor cortex was by Krings et al. (1997). A mechanical stereotactic arm was used for TMS navigation. They compared areas of motor responses identified by both nTMS and DES in two patients with rolandic tumors. The discrepancy between nTMS and DES maps was never more than 1 cm. The major limitations of this study are that it reported on only two cases, and it used a homemade system that is not commercially available.
The next study was published more than a decade later (Picht et al. 2009). In this study, the motor cortex of 10 patients with rolandic tumors were evaluated using a homemade nTMS system in which an electromagnetic navigation system was integrated with TMS for the purpose of positioning the TMS coil. The mean (SD) [range] distance between hotspots of the two modalities was 3.4 (3.0) mm [0–7] mm. The limitation of this study was that the preoperative and intraoperative mappings were performed in the same predefined 5-mm raster, so the resulting comparative data were semiquantitative (Picht et al. 2011a). This system is not commercially available, so the applicability of the findings is limited. The next study (Kantelhardt et al. 2010) evaluated hotspots determined by nTMS and DES in two patients with brain tumors. In one patient the distance between hotspots was estimated as less than 5 mm, but in the other case the comparison referred to post-op nTMS. This study is unreliable because of the very small sample size and inadequate reporting.
These first three studies were all semi-quantitative and/or had a sample size that was too small. From then on, all but one study (Paiva et al. 2012) have been using the same commercially available system (eXimia “Navigated Brain Stimulation”; Nexstim; Helsinki, Finland). The first and largest of these studies (Picht et al. 2011a) was on 20 patients with rolandic tumors, though only 17 had surgery and thus DES. In this study, DES locations were chosen independently of nTMS, and the distance between nTMS and DES hotspots was determined. The mean (SE) distance between the nTMS and DES hotspots was 7.83 (1.18) mm for the abductor pollicis brevis (APB) muscle (n = 15) and 7.07 (0.88) mm for tibialis anterior (TA) muscle (n = 8). Importantly, the mean (SE) distance decreased to 4.70 (1.09) mm for APB (n = 8), and 5.61 (0.47) mm for TA (n = 5) after exclusion of the patients in which possibly insufficient (<15 stimulations) DES mapping was performed for that muscle. This study also reported comparisons on three other muscles in subsets of the sample.
In the same year, Forster et al. reported their experience with nTMS in 10 patients with rolandic tumors when compared to DES and fMRI (Forster et al. 2011). This study has been of particular interest, despite the small sample size, because it provided a simultaneous comparison of fMRI to DES, thus enabling neurosurgeons to compare their options for pre-operative mapping. The mean (SD) [range] distance between the hotspots evaluated by nTMS and DES was 10.49 (5.67) [2.6–27.6] mm. One problem with this calculation though was that the pairs of nTMS and DES hotspots compared were from nine different muscles. Nonetheless, this result was smaller than the mean (SD) [range] distance between the hotspots of fMRI and DES: 15.03 (7.59) [3.4–22.2] mm. So this study advocated that nTMS is better correlated to DES than fMRI. One major limitation of this study however is that they did not compare responses from the same muscles: five hand/arm muscles, three leg muscles, and one facial muscle were recorded for TMS; whereas, activation areas from the first interosseous dorsal muscle or toe movement were obtained for fMRI. This use of different muscles may have accounted in part for the discrepancy between fMRI and nTMS. Nonetheless, one other interesting finding from this study was that the median [range] distance for the TA muscle relative to DES was larger for nTMS, 11.1 [5.9–15.9] mm, than for fMRI, 9.4 [5.7–19.1] mm. Thus nTMS may be less accurate for deeper lying cortical regions, such as the cortical region corresponding to leg muscles.
Krieg et al. (2012b) reported their experience on using nTMS pre-surgically for the resection of rolandic tumors. They performed preoperative nTMS on 14 patients with lesions located within or adjacent to the precentral gyrus and on 12 patients with lesions in the subcortical white mater motor tract. In the former patient group, they compared the borders between positive and negative stimulation points for nTMS and DES on axial slices by using recalibrated screenshots and BrainLAB iPlan Net Cranial 3.0.1. Although this method of comparing borders may have some advantage of accuracy over the usual hotspot method, it is complicated and idiosyncratic and renders comparisons to other studies problematic. Using this method, the mean (SD) [range] of the distance between borders for nTMS versus DES was 4.4 (3.4) [1.9–9.2] mm. They also evaluated the difference between borders delineating the primary motor cortex according to BOLD data of fMRI and mapping area identified by nTMS. The mean (SD) [range] deviation between nTMS and fMRI for this method was 9.8 (8.5) [5.3–39.7] mm for the upper extremity and 14.7 (12.4) [8.4–33.5] mm for the lower extremity. They mentioned that their data demonstrate that nTMS correlates well with intraoperative DES, while nTMS and fMRI differed significantly from each other. Regrettably, they did not make any comparison between preoperative fMRI and DES, so it remains difficult to say whether nTMS is more accurate than fMRI on the basis of this study. In particular, the fact that the discrepancy between nTMS and fMRI is greater for the lower extremity than the upper extremity may again, as in the study by Forster et al. (2011) reflect a lesser accuracy of nTMS for the deeper lying cortical representations of leg muscles.
Another study focused on patients with relatively homogeneous brain tumors (i.e. only patients with low grade gliomas with a maximum diameter or 4 cm were included), using an unspecified nTMS system (Paiva et al. 2012). In this study, they used the “center-of-gravity” approach to compare the difference between the two modalities. This method is more time consuming but also more accurate and reliable. They reported a mean [range] distance between nTMS and DES of 4.16 [2.56–5.27] mm. The limitations of this study were its small sample size and inadequate explanation of the statistical methods.
The most recent study identified by our review performed mapping on 24 patients but then made comparisons only in five, because DES revealed a positive motor site in only five patients with the tailored craniotomy they used (Tarapore et al. 2012). They calculated the difference between hotspots identified by nTMS and DES at eight points in five patients as a median (SE) of 2.13 (0.19) mm. An interesting point in this study is that they reported that negative nTMS mapping also correlates with negative DES mapping: in other words, DES mapping did not find any new motor sites where TMS had not. The study also included the result of a comparison between motor areas identified by nTMS and magnetoencepholography (MEG). The median (SE) distance between the two hotspots of 46 sites in 23 patients was reported as 4.71 (1.08) mm. Unfortunately, they did not report a comparison of MEG to DES. Although this study reports some interesting new information, it is otherwise limited by the small number of patients having DES data available.
In summary, all studies reviewed here concluded that nTMS correlated well with the gold standard of DES (Forster et al. 2011; Kantelhardt et al. 2010; Krieg et al. 2012b; Krings et al. 1997; Paiva et al. 2012; Picht et al. 2009, 2011a; Tarapore et al. 2012). A total of 97 attempts in 96 patients to identify the motor cortex using nTMS were described. In only one patient with an infiltrating glioma within the somatosensory cortex, could TMS not identify any motor site (Tarapore et al. 2012).
We have calculated the mean distance between motor cortex identified by nTMS and DES using the mean distance described in five quantitatively evaluated studies (Picht et al. 2011a; Forster et al. 2011; Krieg et al. 2012b; Paiva et al. 2012; Tarapore et al. 2012). We then weighted the mean from each study by the number of patients that mean was derived from. (In one study (Picht et al. 2011a), we used only the data for APB (n = 15) for simplicity.) With the method, we have calculated a weighted mean distance between nTMS and DES in 50 patients as 6.39 mm.
The Accuracy of Transcranial Magnetic Stimulation and Direct Electrical Stimulation
The basic mechanism of neuronal activation is the same for both TMS and DES. An electric field moves electric charges within the target tissue. Wherever the electric field is of adequate strength and direction in relation to the neuronal structures, neurons will be excited and action potentials triggered. Yet, the spread of the electric fields from the electrodes (DES) or the “virtual electrodes” (TMS) is difficult to predict since the electric current will follow the paths of least impedance in the tissue and is influenced by macroscopic factors (e.g. sulci, CSF) and microscopic factors (e.g. preferred orientation of cells). As a result, the exact extent of the stimulated cortical area remains unclear for both TMS and DES, so spatial discrepancies might reflect methodological differences rather than “inaccuracies” of either method.
For electrical stimulation, it has been established that neurons are excited at lower thresholds when the applied voltage induces currents that are oriented along the axon rather than across it (Day et al. 1989). It has been demonstrated that during bipolar cortical stimulation the current peaks in the region directly below the bipolar electrodes; whereas, current density decreases much less rapidly with depth during monopolar anodal stimulation (Nathan et al. 1993). Consequently, suprathreshold anodic stimulation of the motor cortex leads primarily to direct stimulation of the pyramidal cells. By contrast, single-pulse TMS is likely to involve both tangential cortical fibers and direct corticospinal axonal bundles (Di Lazzaro et al. 2004; Ruohonen and Ilmoniemi 2002). Depending on the e-field direction and the stimulation strength, TMS on the primary motor cortex will preferentially activate the pyramidal cells directly (D-waves) or indirectly (transsynaptically; I-waves) at their axon hillock. In the cerebral cortex, the threshold for TMS excitation is highly sensitive to orientation (Fox et al. 2006). In clinical practice, the coil orientation is adjusted for each stimulated position during the motor mapping, so that the induced electric field is set to be perpendicular to the bank of the gyrus with the help of MRI-based navigation. In sum, it can be hypothesized that suprathreshold anodal monopolar DES and nTMS at 110 % RMT perpendicular to the individual gyral anatomy elicit MEPs through direct axonal depolarization as well as through intracortical transverse connections. This implies that both methods stimulate preferentially the same population of neurons. Nevertheless, the exact stimulation path remains unknown in each individual case, especially around a tumor with possible conductivity changes. So TMS and DES can stimulate via somewhat different paths in any given patient.
In addition to these neurophysiological considerations, one should be aware that the comparison of spatial accuracy of TMS and DCS is also influenced by methodological factors concerning the hardware and study conception which may further inflate the discrepancy between the nTMS and DES results. There are four main reasons why these may cause discrepancy between the nTMS and DES results.
First, the mappings are conducted under different chemical influences and different states of alertness. DES is conducted under general anesthesia, while nTMS is not. Under general anesthesia, MEPs can only be evoked by using a train of stimuli, not by single pulses. This necessity of applying larger electrical charges to evoke muscle responses during DES mapping in comparison to TMS mapping can lead to different stimulation effects of the two methods even if exactly the same area is targeted. In addition, a significant proportion of patients with brain tumors have been using anti-epileptic medications, and we cannot rule out its possible influence.
Second, there are a couple kinds of measurement errors related to the neuronavigation, such as registration error or measurement errors from brain shift (Suess et al. 2007), which could influence the measurement of DES stimulation locations. It can be assumed though that the impact of brain-shift is minimal, because the cortical mapping procedures are all performed before tumor resection begins. The error occurring during coregistration of the 3D MRI dataset and the patient’s head is stated to be below 2 mm for nTMS (Ruohonen and Karhu 2010).
Third, the kind of EMG electrodes used by the two methods differ: dermal surface electrodes for nTMS but intramuscular needle electrodes for DES. Cross-talk from adjacent muscles can be picked up by surface electrodes, while this is not the case when using needle electrodes. This also means that dermal electrodes can be more sensitive in picking up very small responses from different muscles as a summation of subliminal responses. Thus the use of different EMG electrodes may introduce a bias in terms of the recording sensitivity of the muscle output.
Fourth, after all the mapping is done, the most commonly used method for measuring the distance between the muscles representation of the nTMS and DES mappings is to compare the “hotspots”: the single point with the largest EMG response for that muscle. This hotspot method is likely to emphasize errors contained by a single response. The “wrong” DES hotspot may have been chosen, if there were multiple foci or a diffuse center for the motor cortex representation of the target muscle or if the true hotspot was never even stimulated. Similarly, the number of stimulation points during DES varies widely depending on the tumor location, craniotomy size, and other factors. Consequently the distance between nTMS and DES hotspots is much greater when there were fewer DES responses (Picht et al. 2011a). Also, in most studies the surgeon was not aware of the exact nTMS locations thus he could not deliberately stimulate them. So in cases where there was a limited number of DES stimulation spots, it was quite possible that the nTMS hotspot and/or the true cortical center of muscle control was never covered, thus leading to a wider discrepancy between nTMS and DES. Using a “center-of-gravity” approach or comparing mapping areas is more accurate, but it is usually restricted by time limitations of surgery, which usually prevent taking enough measurements for such an approach. Altogether, these considerations and findings suggest that DES may not really be a reliable gold standard when a low number of stimulations are performed. Of course DES does tell the surgical team when a spot on the brain is necessary for motor function, but unless extensive freehand mapping is performed, DES will not necessarily reveal the most essential center of cortical control for a muscle. Depending on the tumor location, extensive DES mapping may not be necessary, thus leaving its results unreliable for scientific comparisons to nTMS.
In summary TMS and DES are applying the same basic underlying methodology, namely the electrical stimulation of cortical neurons. In respect to identification of direct corticospinal motor connections differences in the specific neurophysiological details of neuronal activation are of minor relevance. Discrepancies between TMS and DES motor mappings are predominantly caused by system inherent errors (e.g. navigational error) or reflect inadequate surrogate parameters for evaluation of accuracy (e.g., comparison of “hot spots”).
The Clinical Role of Navigated Transcranial Magnetic Stimulation
The studies summarized above provide some evidence that nTMS has acceptably good accuracy for identifying cortical representations of individual muscles vis-à-vis the gold standard of DES. Yet preoperative nTMS is not therefore intended as a substitute for intraoperative DES. Instead, it provides complimentary information, derived from its unique features. The overarching strength of nTMS is that it is the only other mapping modality that is analogous to DES (stimulate the brain and record the output), but it can be performed pre-operatively and post-operatively; whereas, DES cannot be. So while DES is still used to guide the actual surgical resection of tumors, nTMS can be used to plan the surgery ahead of time, guide the DES, and assess postoperative or longitudinal changes in cortical motor representation.
Preoperative nTMS can be useful for planning surgeries, while there is still an opportunity to discuss it with the patient. The magnetic stimulation of a precise cortical spot enables the operator to identify cortical areas with direct cortico-spinal motor connections. The synthesis of the patient’s clinical status, MRI findings, and TMS mapping can improve the surgical team’s ability to better plan the surgical strategy. A prospective study has shown that in about one-fourth of the surgical cases of tumors in presumed motor eloquent location, nTMS brought objective benefit to the surgical team (nTMS changed the surgical indication or the planned extent of resection, or it modified the surgical approach), and in another one-fourth of cases it added critical awareness of high risk areas, which helped guide the intraoperative DES (Picht et al. 2012).
Several other imaging modalities – such as fMRI, PET, and MEG – have also been used to map the motor cortex preoperatively and plan the surgical resection. Yet nTMS has the advantage over other preoperative mapping methods that nTMS is analogous to DES: nTMS stimulates the brain and records the muscle output, rather than asking the patient to move, recording brain activation, and then trying to interpret which brain areas were essential for the movement versus which ones were merely co-activated. Also, nTMS can be used to evaluate responses from any muscles desired; whereas, functional imaging can only be used to evaluate responses from muscles that can still be moved voluntarily and, ideally, isolated from other muscles. For further comparisons to other preoperative mapping modalities, we refer the reader to a previous book chapter (Picht and Atalay 2012).
It has to be emphasized though that TMS has an entirely different role from DES and is not capable of being a substitute for DES. Surgical resection of brain tumors in eloquent location should be guided by intraoperative mapping and monitoring which nTMS cannot provide, so DES remains essential. Yet nTMS can be useful to plan and guide the DES, and the pre-operative nTMS maps can also serve as a back-up, if intraoperative technical errors or patient seizures make it impossible to continue with intraoperative DES. Also, if the resection will extend to subcortical levels, mapping needs to be carried out on these subcortical levels. nTMS cannot perform mapping of subcortical tracts, so DES remains essential for this function. nTMS can be beneficial by improving diffusion tensor imaging to visualize the subcortical fiber tracts; but the resulting information can only be used for surgical planning and intraoperative guidance of the stimulation probe and not for determining resection margins. In sum, TMS is performed pre-operatively and is used to plan the surgery; whereas DES is used intra-operatively to guide tumor resection. TMS does not have this capability to be performed intra-operatively. Both modalities should be used complementarily, drawing on their respective advantages, to maximize the quality of the surgery.
nTMS has five unique capabilities that supplement the information provided by intraoperative DES: (1) nTMS provides an objective assessment of the possibility of recovery of motor function. For example, in patients who have become plegic, nTMS can show if motor function is still possible (Picht et al. 2011b). (2) nTMS provides pre-operative clarification of detailed cortical functional anatomy, which can resulting in smaller craniotomies and a modification of the surgical approach (Picht et al. 2012; Krieg et al. 2012b), also applicable in small kids (Coburger et al. 2012). (3) nTMS can be performed repeatedly across time, and this can enable visualization of plastic changes, which may influence the timing of surgical interventions (Takahashi et al. 2012). (4) nTMS enables an objective preoperative estimation of the extent of safe cortical tumor resection. In a prospective study, nTMS mapping changed the planned extent of resection in about 8 % of cases (Picht et al. 2012). (5) nTMS can be used to define the accurate “seed-points” for diffusion tensor imaging, to visualize the pathways of the pyramidal fiber tracts. This approach can improve the accuracy of diffusion tensor imaging for fiber tracking (Frey et al. 2012; Krieg et al. 2012a). Altogether, these five unique capabilities of nTMS enable neurosurgeons to improve tumor resection through better advanced planning of the surgery.
Conclusions
The recent addition of neuronavigation to transcranial magnetic stimulation has greatly improved the accuracy and usefulness of this cortical mapping technology. nTMS provides a valuable complement to the gold standard of DES for mapping the motor cortex. Because nTMS can be performed pre-operatively with little risk or discomfort to the patient, it provides the surgical team with important information about each individual patient’s functionally essential areas of the motor cortex. Having this information preoperatively is often quite useful in various ways for planning the surgery. nTMS has the advantage over all other preoperative methods of functional imaging that only nTMS stimulates the brain and records motor output – just like DES. All other forms of preoperative imaging ask the patient to move (if they can and will), record brain activation, and then attempt to interpret which areas of the brain were essential for the movement versus which ones were incidental.
Our literature review here supports the view that the accuracy of TMS is sufficiently high to rely upon its results for surgical planning. The overall weighted mean distance between nTMS from DES in 50 patients was calculated as 6.39 mm. Yet it must be emphasized that nTMS and DES have different roles and are not interchangeable: only nTMS can be used preoperatively and postoperatively, while only DES can be used intraoperatively and subcortically. nTMS also has many unique capacities that make it a promising new technology for better understanding the motor cortex. The neurosurgical community is still just beginning to explore the many capabilities of nTMS and further research is sure to yield many more exciting discoveries from this new technology.
References
Barker AT, Jalinous R, Freeston IL (1985) Non-invasive magnetic stimulation of human motor cortex. Lancet 1:1106–1107
Coburger J, Karhu J, Bittl M, Hopf NJ (2012) First preoperative functional mapping via navigated transcranial magnetic stimulation in a 3-year-old boy. J Neurosurg Pediatr 9:660–664
Day BL, Dressler D, Maertens de Noordhout A, Marsden CD, Nakashima K, Rothwell JC, Thompson PD (1989) Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J Physiol 412:449–473
De Witt Hamer PC, Robles SG, Zwinderman AH, Duffau H, Berger MS (2012) Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol 30:2559–2565
Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC (2004) The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol 115:255–266
Duffau H, Lopes M, Arthuis F, Bitar A, Sichez JP, Van Effenterre R, Capelle L (2005) Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: a comparative study between two series without (1985–96) and with (1996–2003) functional mapping in the same institution. J Neurol Neurosurg Psychiatry 76:845–851
Forster MT, Hattingen E, Senft C, Gasser T, Seifert V, Szelenyi A (2011) Navigated transcranial magnetic stimulation and functional magnetic resonance imaging: advanced adjuncts in preoperative planning for central region tumors. Neurosurgery 68:1317–1324, discussion 1324–1315
Fox PT, Narayana S, Tandon N, Fox SP, Sandoval H, Kochunov P, Capaday C, Lancaster JL (2006) Intensity modulation of TMS-induced cortical excitation: primary motor cortex. Hum Brain Mapp 27:478–487
Frey D, Strack V, Wiener E, Jussen D, Vajkoczy P, Picht T (2012) A new approach for corticospinal tract reconstruction based on navigated transcranial stimulation and standardized fractional anisotropy values. Neuroimage 62:1600–1609
Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen LG, Mall V, Kaelin-Lang A, Mima T, Rossi S, Thickbroom GW, Rossini PM, Ziemann U, Valls-Sole J, Siebner HR (2012) A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol 123:858–882
Hannula H, Ylioja S, Pertovaara A, Korvenoja A, Ruohonen J, Ilmoniemi RJ, Carlson S (2005) Somatotopic blocking of sensation with navigated transcranial magnetic stimulation of the primary somatosensory cortex. Hum Brain Mapp 26:100–109
Hufnagel A, Elger CE, Durwen HF, Boker DK, Entzian W (1990) Activation of the epileptic focus by transcranial magnetic stimulation of the human brain. Ann Neurol 27:49–60
Kantelhardt SR, Fadini T, Finke M, Kallenberg K, Siemerkus J, Bockermann V, Matthaeus L, Paulus W, Schweikard A, Rohde V, Giese A (2010) Robot-assisted image-guided transcranial magnetic stimulation for somatotopic mapping of the motor cortex: a clinical pilot study. Acta Neurochir (Wien) 152:333–343
Kombos T, Suss O (2009) Neurophysiological basis of direct cortical stimulation and applied neuroanatomy of the motor cortex: a review. Neurosurg Focus 27:E3
Kombos T, Suess O, Kern BC, Funk T, Hoell T, Kopetsch O, Brock M (1999) Comparison between monopolar and bipolar electrical stimulation of the motor cortex. Acta Neurochir (Wien) 141:1295–1301
Kratz O, Studer P, Barth W, Wangler S, Hoegl T, Heinrich H, Moll GH (2011) Seizure in a nonpredisposed individual induced by single-pulse transcranial magnetic stimulation. J ECT 27:48–50
Krieg SM, Buchmann NH, Gempt J, Shiban E, Meyer B, Ringel F (2012a) Diffusion tensor imaging fiber tracking using navigated brain stimulation–a feasibility study. Acta Neurochir (Wien) 154:555–563
Krieg SM, Shiban E, Buchmann N, Gempt J, Foerschler A, Meyer B, Ringel F (2012b) Utility of presurgical navigated transcranial magnetic brain stimulation for the resection of tumors in eloquent motor areas. J Neurosurg 116:994–1001
Krings T, Buchbinder BR, Butler WE, Chiappa KH, Jiang HJ, Rosen BR, Cosgrove GR (1997) Stereotactic transcranial magnetic stimulation: correlation with direct electrical cortical stimulation. Neurosurgery 41:1319–1325, discussion 1325–1316
Kuhn AA, Brandt SA, Kupsch A, Trottenberg T, Brocke J, Irlbacher K, Schneider GH, Meyer BU (2004) Comparison of motor effects following subcortical electrical stimulation through electrodes in the globus pallidus internus and cortical transcranial magnetic stimulation. Exp Brain Res 155:48–55
Kumar R, Chen R, Ashby P (1999) Safety of transcranial magnetic stimulation in patients with implanted deep brain stimulators. Mov Disord 14:157–158
Nathan SS, Sinha SR, Gordon B, Lesser RP, Thakor NV (1993) Determination of current density distributions generated by electrical stimulation of the human cerebral cortex. Electroencephalogr Clin Neurophysiol 86:183–192
Paiva WS, Fonoff ET, Marcolin MA, Cabrera HN, Teixeira MJ (2012) Cortical mapping with navigated transcranial magnetic stimulation in low-grade glioma surgery. Neuropsychiatr Dis Treat 8:197–201
Penfield W, Boldrey E (1937) Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60:389–443
Picht T, Atalay A (2012) Preoperative motor mapping. In: Hayat MA (ed) Tumors of the central nervous system, vol 4. Springer, Netherlands, pp 289–300. doi:10.1007/978-94-007-1706-0_30
Picht T, Mularski S, Kuehn B, Vajkoczy P, Kombos T, Suess O (2009) Navigated transcranial magnetic stimulation for preoperative functional diagnostics in brain tumor surgery. Neurosurgery 65:93–98, discussion 98–99
Picht T, Schmidt S, Brandt S, Frey D, Hannula H, Neuvonen T, Karhu J, Vajkoczy P, Suess O (2011a) Preoperative functional mapping for rolandic brain tumor surgery: comparison of navigated transcranial magnetic stimulation to direct cortical stimulation. Neurosurgery 69:581–588, discussion 588
Picht T, Schmidt S, Woitzik J, Suess O (2011b) Navigated brain stimulation for preoperative cortical mapping in paretic patients: case report of a hemiplegic patient. Neurosurgery 68:E1475–E1480, discussion E1480
Picht T, Schulz J, Hanna M, Schmidt S, Suess O, Vajkoczy P (2012) Assessment of the influence of navigated transcranial magnetic stimulation on surgical planning for tumors in or near the motor cortex. Neurosurgery 70:1248–1256, discussion 1256–1247
Rossi S, Hallett M, Rossini PM, Pascual-Leone A (2009) Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120:2008–2039
Rossi S, Hallett M, Rossini PM, Pascual-Leone A (2011) Screening questionnaire before TMS: an update. Clin Neurophysiol 122:1686
Rotenberg A, Harrington MG, Birnbaum DS, Madsen JR, Glass IE, Jensen FE, Pascual-Leone A (2007) Minimal heating of titanium skull plates during 1Hz repetitive transcranial magnetic stimulation. Clin Neurophysiol 118:2536–2538
Ruohonen J, Ilmoniemi RJ (2002) Physical principles for transcranial magnetic stimulation. Handbook of transcranial magnetic stimulation. Oxford University Press, New York
Ruohonen J, Ilmoniemi RJ (2005) Basic physics and design of TMS devices and coils. In: Hallet M, Chokroverty S (eds) Magnetic stimulation in clinical neurophysiology. Butterworth, Boston, pp 17–30
Ruohonen J, Karhu J (2010) Navigated transcranial magnetic stimulation. Neurophysiol Clin 40:7–17
Sanai N, Berger MS (2010) Intraoperative stimulation techniques for functional pathway preservation and glioma resection. Neurosurg Focus 28:E1
Schmidt S, Cichy RM, Kraft A, Brocke J, Irlbacher K, Brandt SA (2009) An initial transient-state and reliable measures of corticospinal excitability in TMS studies. Clin Neurophysiol 120:987–993
Schrader LM, Stern JM, Koski L, Nuwer MR, Engel J Jr (2004) Seizure incidence during single- and paired-pulse transcranial magnetic stimulation (TMS) in individuals with epilepsy. Clin Neurophysiol 115:2728–2737
Suess O, Picht T, Kuehn B, Mularski S, Brock M, Kombos T (2007) Neuronavigation without rigid pin fixation of the head in left frontotemporal tumor surgery with intraoperative speech mapping. Neurosurgery 60:330–338, discussion 338
Szelenyi A, Joksimovic B, Seifert V (2007) Intraoperative risk of seizures associated with transient direct cortical stimulation in patients with symptomatic epilepsy. J Clin Neurophysiol 24:39–43
Takahashi S, Jussen D, Vajkoczy P, Picht T (2012) Plastic relocation of motor cortex in a patient with LGG (low grade glioma) confirmed by NBS (navigated brain stimulation). Acta Neurochir (Wien) 154(11):2003–8
Taniguchi M, Cedzich C, Schramm J (1993) Modification of cortical stimulation for motor evoked potentials under general anesthesia: technical description. Neurosurgery 32:219–226
Tarapore PE, Tate MC, Findlay AM, Honma SM, Mizuiri D, Berger MS, Nagarajan SS (2012) Preoperative multimodal motor mapping: a comparison of magnetoencephalography imaging, navigated transcranial magnetic stimulation, and direct cortical stimulation. J Neurosurg 117:354–362
Acknowledgments
We would like to thank Michael Hanna, Ph.D., (Mercury Medical Research & Writing) for reviewing and revising the manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Takahashi, S., Picht, T. (2014). Comparison of Navigated Transcranial Magnetic Stimulation to Direct Electrical Stimulation for Mapping the Motor Cortex Prior to Brain Tumor Resection. In: Hayat, M. (eds) Tumors of the Central Nervous System, Volume 12. Tumors of the Central Nervous System, vol 12. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7217-5_23
Download citation
DOI: https://doi.org/10.1007/978-94-007-7217-5_23
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7216-8
Online ISBN: 978-94-007-7217-5
eBook Packages: MedicineMedicine (R0)