Abstract
The rapid development of the modern society has resulted in an increased demand for energy, and consequently an increased use of fossil fuel reserves. Burning fossil fuels is nowadays one of the main threats to the environment, especially due to the accumulation of greenhouse gases in the atmosphere, which are responsible for global warming. Furthermore, the continuous use of this non-renewable source of energy will lead to an energy crisis because fossil fuels are of limited availability. In response to this energy and environmental crisis, it is of extreme importance to search for different energy supplies that are renewable and more environmentally friendly. Microalgae are a promising sustainable resource that can reduce the dependence on fossil fuel. Biodiesel production through microalgae is actually highly studied. It includes several steps, such as cell cultivation and harvesting, oil extraction and biodiesel synthesis. Although several attempts have been made to improve biodiesel yields from microalgae, further studies are required to optimize production conditions and to reduce production costs.
This chapter reviews recent developments on oil extraction for biodiesel production. Two different processes are distinguished: (i) an indirect route, in which microalgal oil is recovered in an appropriate solvent and then converted into biodiesel through transesterification; and (ii) a direct route, in which the production of biodiesel is performed directly from the harvested biomass. Both routes, direct and indirect, should be preceded by cell wall disruption because this step facilitates the access of solvents to microalgal oil. The most advantageous disruption methods for lipid extraction are enzymatic and pulsed electric field disruption because enzymes present higher selectivity towards cell walls. In addition pulsed electric field requires less energy than other disruption methods.
For the indirect route, it is possible to use three different types of solvents to recover microalgal oil. Although extraction with supercritical fluids has higher extraction efficiencies and is safer for the environment, costs are very high. The use of ionic liquids is also safer for the environment, but their cost is also very high. An alternative is the use of organic solvents such as n-hexane because it is less harmful and has a higher selectivity for neutral oil fractions than other organic solvents. We conclude that the direct route, which involves production of biodiesel directly from the microalgal biomass, is more efficient. Indeed, the application of the direct route to the microalga Schizochytrium limacinum resulted in a biodiesel yield of 72.8 %, while the indirect route, in the same conditions, has resulted in a biodiesel yield of 63.7 %.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Biofuel
- Algae
- Microalgae
- Oil extraction
- Liquid
- Transesterification
- Chlorella vulgaris
- Schizochytrium
- Limacinum
- Cell wall disruption
- Pulse electric field
- Enzymatic disruption
1.1 Introduction
The depletion of fossil fuels reserves and the effect of exhaust gas emissions on global climate change have stimulated the search for sustainable sources of energy that are carbon neutral or renewable. As an alternative energy source, much attention has been paid to biodiesel production from vegetable oil crops, such as palm, rapeseed and soybean, and animal fats (Demirbas 2011; Ranjan et al. 2010). However, biodiesel production yields from oil crops and animal fats do not achieve the current demand for transport fuels (Chisti 2007; Demirbas 2011). Furthermore, producing biodiesel from vegetable crops is time consuming and requires great areas of arable land that would compete with the one used in food crops, leading to starvation in developing countries (Costa and de Morais 2011; Demirbas 2011; Demirbas and Demirbas 2011).
Avoiding the competition between energy and food production, attentions are now focused on evaluating the potential of microalgae as oil source for biodiesel production. Microalgae are eukaryotic photosynthetic microorganisms that can be found in aquatic or terrestrial ecosystems (Fig. 1.1). They present several advantages over oil crops, including: (i) higher oil contents; (ii) higher growth and biomass production rates; (iii) shorter maturity rates; and (iv) require far less land due to higher oil productivities (Chisti 2007; Mercer and Armenta 2011). As well as microalgae, there are other photosynthetic microorganisms with potential interest. These prokaryotic microorganisms, known as cyanobacteria, behave similarly to microalgae and present the same advantages. Despite the referred advantages, the production of biodiesel from microalgae is not economically viable. Technological improvements should be performed to reduce the associated costs, including: (i) improvement of photosynthetic efficiency by the study of photobioreactor design; (ii) reduction of water and carbon dioxide losses in microalgal cultures; (iii) improvement of energy balance for water pumping, CO2 transfer, biomass harvesting, oil extraction and biodiesel synthesis; and (iv) use of flue gas as CO2 source. This review focuses on the oil extraction and biodiesel synthesis, presenting the research advances in the associated processes.
Microalgae: (a) microscopic photograph of the microalga Chlorella vulgaris; (b) microscopic photograph of the microalga Pseudokirchneriella subcapitata; (c) microalgal culturing in small flasks; (d) microalgal culturing in horizontal tubular photobioreactors; (e) microalgal culturing in raceway ponds; and (f) harvesting of microalgae through sedimentation. (a), (b), (c), and (f) were obtained from our research group; (d) from http://badger.uvm.edu; and (e) from http://www.abc.net.au
1.2 Lipid Recovery
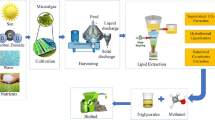
After cell cultivation, the downstream process towards the production of biodiesel includes: (i) harvesting of microalgae; (ii) drying or dewatering; (iii) cell disruption and oil extraction; and (iv) transesterification reaction (Amaro et al. 2011; Brennan and Owende 2010), as it is possible to see in Fig. 1.2.
Oil extraction from biomass requires a specific solvent with great affinity to microalgal oil. Extraction procedures involving organic solvents, supercritical fluids and ionic liquids are the most common applied methods to recover oil from microalgae (Amaro et al. 2011; Mercer and Armenta 2011; Taher et al. 2011; Kim et al. 2012). Although these procedures can be applied directly to the dewatered biomass, it is reported that their efficiency is very low because microalgae present cell walls that block the access of solvents to cytosol (Cravotto et al. 2008; Lee et al. 2010), the cell compartment where the majority of microalgal lipids accumulate (Chen et al. 2009). To overcome the low efficiencies associated to the application of solvent extraction methods, some authors have reported the application of cell wall disruption methods, such as (i) enzymatic disruption; (ii) pulsed electric field; (iii) ultrasound and microwave; and (iv) expeller pressing (Amaro et al. 2011; Cravotto et al. 2008; Lee et al. 2010; Mercer and Armenta 2011; Taher et al. 2011). Table 1.1 shows the most applied extraction methods and the achieved mass percentages of recovered oil and Table 1.2 summarizes the main advantages and disadvantages of the presented cell disruption methods and oil extraction procedures.
1.2.1 Cell Disruption Methods
Cell disruption methods aim to disintegrate cell walls to allow the release of intracellular components into an adequate solvent. The methods used in cell wall disruption can be classified into mechanical, where cell wall destruction is non-specific, and non-mechanical, where methods are more specific. Mechanical methods include bead mill, French press, ultrasound, microwaves and high pressure homogenizer, whereas non-mechanical methods comprise the physical methods thermolysis, decompression and osmotic shock, the chemical methods, where antibiotics, chelating agents, solvents and detergents are applied, and the enzymatic methods, which use lytic enzymes (Geciova et al. 2002; Chisti and Moo-Young 1986).
1.2.1.1 Enzymatic Disruption
Enzymes can be applied in oil extraction from microalgae, as they can mediate the hydrolysis of cell walls, enabling the release of their content into an appropriate solvent. Application of lytic enzymes with little volumes of organic solvent can improve oil recovery yields, as well as extraction times (Mercer and Armenta 2011). For cell wall degradation, cellulases are the most applied enzymes (Mercer and Armenta 2011; Sander and Murthy 2009). Although enzymatic extraction has not yet been applied to microalgae, it has been successfully used in oil extraction from Jatropha curcas L. seeds (Shah et al. 2004). Three phase partitioning (TPP) method and an enzyme-assisted TPP (EATPP) method were applied to recover oil from these seeds. The TPP consisted in the addition of three solvents to the seeds, in order to form a three phase system. The applied solvents were water, ammonium sulphate, and t-butanol. The three phases were separated by centrifugation and the phase containing the recovered oil was the one containing t-butanol, which was eliminated through evaporation. TPP and EATPP were performed at different pH conditions: 4.0, 7.0, and 9.0. Higher oil yields were obtained at pH 9.0: (i) 32.0 % (wt.) for TPP; and (ii) 36.8 % (wt.) for EATPP (Shah et al. 2004).
Despite being expensive, enzymes offer several advantages over other cell wall disruption methods. They present higher degradation selectivity than mechanical disruption methods. Furthermore, microalgal cell walls are more recalcitrant than the ones of other microorganisms, being very resistant to degradation. Thus, the use of mechanical disruption methods requires higher energy amounts (Sander and Murthy 2009).
1.2.1.2 Pulsed Electric Field
The pulsed electric field (PEF) technology seems to be a potential alternative for oil extraction from microalgae (Taher et al. 2011). This technique applies brief pulses of a strong electric field to cells, which induces non-thermal permeabilization of membranes (Guderjan et al. 2005; Taher et al. 2011). In determined conditions, PEF can also cause significant damage in microalgal cell walls (main barrier for oil extraction in most microalgae), membrane and it can led to complete disruption of cells into fragments. PEF is a relatively new method that has not yet been applied to extract microalgal oil. However, evidence of high extraction efficiencies in plant products, such as maize, olives (Guderjan et al. 2005) and Brassica napus cells (Guderjan et al. 2007), suggests that this can be a suitable method to improve the permeabilization of microalgal membranes and efficiently extract their oil (Mercer and Armenta 2011). Guderjan et al. (2005) used PEF to induce stress in plant cells and thus recover functional food ingredients, such as phytosterols and polyphenols. The authors applied 120 pulses with field strength of 0.6 and 7.3 kV.cm−1 to maize and they added a small amount of n-hexane to perform the oil extraction. On the other hand, olives were treated using the following conditions: 30 pulses with field strength of 0.7 kV.cm−1 and 100 pulses with field strength of 1.3 kV.cm−1. After application of PEF the membranes were completely disintegrated and the oil content was recovered by centrifugation. Oil recovery obtained for dried maize was 23.5 and 23.9 % (wt.) for pulses with 0.6 and 7.3 kV.cm−1, respectively. These results were obtained for maize treated with PEF and n-hexane and further drying for 24 h, at 38 °C. Application of electric pulses with field strength of 0.6 kV.cm−1, followed by n-hexane addition, an incubation period of 24 h and drying for 24 h at 38 °C, resulted in an oil yield of 43.7 %, which means that incubation of the mixture with the organic solvent allows higher oil recovery. Regarding olives, the application of PEF with strength of 0.7 and 1.3 kV.cm−1 followed by centrifugation resulted in an oil yield of 6.5 and 7.4 goil per gmash. Guderjan et al. (2007) applied 120 pulses with a field strength of 7.0 kV.cm−1 and a duration of 30 μs to hulled rapeseed, followed by drying at 50 °C for 10 h and an extraction step with n-hexane. With these extraction procedures, the authors obtained an oil yield of 32 % (wt.), against 23 % obtained without PEF application.
PEF requires less time and energy than other applied methods (Guderjan et al. 2005) and its use as a pre-treatment for organic solvent extraction requires far less organic solvents (usually presenting high toxicity) than the conventional organic solvent extraction methods, which reduces the energy needs in the extraction process (Guderjan et al. 2007).
1.2.1.3 Ultrasound and Microwave
Another method that can be used to promote cell wall disruption of microalgal cells is the application of ultrasounds and microwaves. In ultrasonic-assisted method, microalgal oil can be recovered by cavitation. This phenomenon occurs when vapour bubbles of the liquid are formed when the pressure is lower than its vapour pressure. As these bubbles grow when pressure is negative and compress under positive pressure, a violent collapse of the bubbles is promoted. When bubbles collapse near cell walls, damage can occur, leading to the release of cell contents (Mercer and Armenta 2011; Taher et al. 2011). Application of this ultrasound-assisted method to microalgal biomass can improve extraction efficiencies by reducing extraction times and increasing oil recovery yields. The experiments performed by Cravotto et al. (2008) with the microalga Crypthecodinium cohnii showed that cell disruption using ultrasounds increased oil extraction yields from 4.8 %, when applying Soxhlet extraction with n-hexane, to 25.9 % (wt.). Shen et al. (2009) used the microalgae Scenedesmus dimorphus and Chlorella protothecoides to evaluate the effect of sonication before solvent extraction using a mixture of ethanol:hexane in a ratio of 1:1 (v/v). Application of ultrasound-assisted disruption increased the oil yields from 6.3 % to 21.0 % (wt.) for S. dimorphus and from 5.6 % to 10.7 % (wt.) for C. protothecoides. Additionally, Ranjan et al. (2010) compared oil extraction yields from Scenedesmus sp. using the following methods: (i) Bligh and Dyer’s method (1959), organic solvent extraction using a solvent mixture of chloroform, methanol and water, where solvents were applied in a ratio of 3:1:0 (v/v); (ii) ultrasound-assisted extraction followed by the Bligh and Dyer’s method, using the same mixture of chloroform and methanol. Results obtained with these experiments showed an increase in oil yields from 2.0 % to 6.0 % (wt.), when applying method (i) and (ii), respectively. One possible reason for this increase in oil recovery is that when both methods are applied, oil extraction is a result of the interaction between two phenomena: oil diffusion across the cell wall and disruption of the cell wall with release of its contents to the solvent (Ranjan et al. 2010).
Microwave-assisted method is supported by the principle that microwaves directly affect polar solvents and materials. Even when they are applied to dried cells, trace amounts of moisture are affected: temperature increases due to microwaves, moisture is evaporated, and pressure in the cells increases, leading to a damage or rupture of the cell wall followed by the release of its contents. Microwave theory and the extraction principle are described in detail by Mandal et al. (2007). The use of microwaves followed by organic solvent extraction using small amounts of solvent contributes to an efficient and inexpensive extraction procedure, which does not require previous biomass dehydration. Cravotto et al. (2008) applied organic solvent extraction with n-hexane and microwave-assisted solvent extraction (using the same solvent) to the microalga C. cohnii, achieving oil recovery yields of 4.8 % and 17.8 % (wt.), respectively. Furthermore, Lee et al. (2010) used the Bligh and Dyer’s method (1959) with a mixture of chloroform:methanol in the ratio of 1:1 (v/v) preceded by the application of a microwaves treatment to the microalgae Botryococcus sp., Chlorella vulgaris, and Scenedesmus sp. With this microwave-assisted method, the oil extraction yields obtained for these organisms were 28.6, 9.9, and 10.4 % (wt.), respectively, against the 7.9, 4.9, and 1.9 % (wt.) obtained for the control method – Bligh and Dyer’s method (1959; Lee et al. 2010). Recently, Balasubramanian et al. (2011) promoted cell wall disruption of Scenedesmus obliquus using the microwave-assisted method and compared the achieved results with organic solvent extraction with n-hexane. Disruption using microwaves was performed for 30 min, while organic solvent extraction was performed by 10 h. Oil recovery yields obtained with solvent extraction and the microwave-assisted method were 46.9 % and 77.1 % (wt.), respectively (Balasubramanian et al. 2011).
Both methods improve significantly oil extraction from microalgae, presenting higher efficiency, reduced extraction times and increased yields, as well as moderate costs and negligible added toxicity.
1.2.1.4 Expeller Pressing
Pressing techniques lie on the principle that when microalgal cells are submitted to high pressures, they start to crush, releasing their contents to an adequate solvent. As the methods described before, pressing techniques can be advantageous when using as a pre-treatment for organic solvent extraction. A pre-treatment using French press was applied by Shen et al. (2009) to the microalgae S. dimorphus and C. protothecoides. After pressing microalgae, the oil was recovered using a solvent system containing ethanol and n-hexane in a 1:1 (v/v) ratio. Comparing extracted oil yields with those obtained without pre-treatment, oil content achieved for S. dimorphus raised from 6.3 % to 21.2 % (wt.), while for C. protothecoides it raised from 5.6 % to 14.9 % (wt.).
Although this method is very simple and has reduced equipment costs, it presents some disadvantages when compared to other cell disruption methods, such as high power consumption and maintenance costs.
1.2.2 Lipid Extraction Methods
Extraction of microalgal oil can be performed directed to the harvested biomass or in addition to a cell wall disruption method. The second methodology generally presents higher lipid recoveries, as cell contents are released to the solvent applied. In the recovery of microalgal oil, it is very important to choose an appropriate solvent because this choice can improve lipid recovery yields and reduce process costs. Additionally, the majority of solvents applied are harmful and toxic, meaning that the selection of the solvents used should take into account their impact in the environment and in public health. The extraction procedures commonly applied to extract microalgal oil include the use of organic solvents, supercritical fluids and ionic liquids (Mercer and Armenta 2011; Kim et al. 2012).
1.2.2.1 Organic Solvent Extraction
The use of organic solvents to extract microalgal oil is the most applied extraction method. The main organic solvents applied include hexane, cyclohexane, benzene, ethanol, acetone, and chloroform (Brennan and Owende 2010; Mercer and Armenta 2011; Grima et al. 2003). These solvents have shown to be quite effective in oil extraction from microalgae. A good solvent for oil extraction may present the following characteristics: (i) to be insoluble in water; (ii) to have high affinity for oil, i.e. non-polar, to increase its permeability to cell membrane and also to solubilise the target compounds; (iii) to have a low boiling point to facilitate its removal after extraction; (iv) to have a considerably different density from that of water. Furthermore, the organic solvent applied should be inexpensive, non-toxic and reusable (Mercer and Armenta 2011).
Several studies have reported the use of a chloroform, methanol and water mixture, known as the Bligh and Dyer’s method (1959), to extract microalgal oil (Mercer and Armenta 2011). Lewis et al. (2000) studied the effect of applying the solvents chloroform, methanol and water in different sequences and proportions on an oil-producing strain of a marine microheterotroph, Thraustochytrid ACEM 6063. The authors used the following sequences and ratios: (i) water:methanol:chloroform (0.8:2:1, v/v/v); (ii) chloroform:methanol:water (1:2:0.8, v/v/v); (iii) chloroform:methanol:water (1:4:0.8, v/v/v). Total fatty acids extracted with these three solvent systems were 258.5, 350.0, and 326.5 mg.g−1 dry weight, respectively. With this study, the authors concluded that changing solvent sequence can have significant effects on extraction yields and that increasing the proportion of methanol did not significantly affect the extraction efficiency. Later, Lee et al. (2010) used a mixture of chloroform and methanol (1:1, v/v) to extract oil from the organisms Botryococcus sp., C. vulgaris, and Scenedesmus sp. Extraction yields obtained with this method were 12.0, 24.9, and 18.8 mg.g−1 dry weight for Botryococcus sp., C. vulgaris, and Scenedesmus sp., respectively.
Another common organic solvent applied to extract microalgal oil is n-hexane. Gouveia and Oliveira (2009) used n-hexane to determine oil contents of the microalgae Spirulina maxima, C. vulgaris, S. obliquus, Dunaliella tertiolecta, Nannochloropsis sp., and Neochloris oleoabundans. Oil yields obtained with this solvent ranged between 4.1 % (wt.) from S. maxima and 29.0 % (wt.) from N. oleoabundans (Gouveia and Oliveira 2009). Shen et al. (2009) applied a solvent system composed by a mixture of ethanol:n-hexane (1:1, v/v) to the microalgae S. dimorphus and C. protothecoides. Oil contents obtained with this method were 6.3 % and 5.6 % (wt.) for S. dimorphus and C. protothecoides, respectively (Shen et al. 2009).
Ranjan et al. (2010) compared oil extraction from the Scenedesmus sp. using two organic solvent methods: Soxhlet extraction with n-hexane, and the Bligh and Dyer’s (1959), using a chloroform and methanol mixture in a ratio of 3:1 (v/v). The achieved oil contents were 0.6 % (wt.) for extraction with n-hexane and 2.0 % (wt.) for extraction with the Bligh and Dyer’s method, showing that the last method is most efficient. This can be explained by the non-polar character of n-hexane, which results in a lower selectivity of microalgal oil, mainly composed by unsaturated fatty acids, toward n-hexane. On the other hand, chloroform has a polar nature, which allows a higher selectivity of microalgal oil toward this organic solvent (Ranjan et al. 2010).
Fajardo et al. (2007) used ethanol as an organic solvent for oil extraction from the microalga Phaeodactylum tricornutum. The authors applied an ethanol solution (96 % v/v) to freeze dried biomass, obtaining a oil yield of 6.4 % (wt.) (Fajardo et al. 2007).
Although n-hexane may be less efficient than chloroform, it is less toxic and it has an apparently higher selectivity for neutral oil fractions (Amaro et al. 2011). The application of this organic solvent coupled with an efficient cell wall disruption method could be a promising alternative to avoid the harmfulness of chloroform.
1.2.2.2 Supercritical Fluid Extraction
An alternative to the use of volatile and toxic organic solvents in microalgal oil extraction is the application of supercritical fluids as solvents (Amaro et al. 2011; Halim et al. 2011; Mercer and Armenta 2011). Supercritical fluids are compounds that behave both as a liquid or a gas when exposed to temperatures and pressures above their critical values. The most used supercritical fluid for oil extraction is CO2 (scCO2) because it presents low critical temperature (31.1 °C) and pressure (72.9 atm) (Mercer and Armenta 2011).
The scCO2 extraction presents several advantages over the traditional organic solvent extraction procedures, such as: (i) tuneable solvating power; (ii) low toxicity and flammability; (iii) favourable mass transfer rates; and (iv) production of solvent free extracts because at room temperature CO2 behaves as a gas (Amaro et al. 2011; Crampon et al. 2011; Halim et al. 2011; Macías-Sánchez et al. 2007). The main disadvantage is the associated cost, which is mainly due to the required infrastructure and operational conditions (Halim et al. 2011).
Efficiencies of oil extraction using scCO2 depend on the following factors: (i) pressure; (ii) temperature; (iii) CO2 flow rate; and (iv) extraction time. Furthermore, the use of modifiers or co-solvents, such as ethanol can be adjusted to optimize extractions. When ethanol is applied as a co-solvent, polarity of CO2 increases and its viscosity is altered, increasing the fluid solvating power. In these conditions, lower temperature and pressure are required, improving the extraction efficiency. Another limiting factor of scCO2 extraction is the level of moisture in the sample. High moisture content can reduce contact time between the solvent and biomass, making difficult the diffusion of CO2 into the sample and the diffusion of oil out of the cell, because microalgal biomass tends to gain a thick consistency (Halim et al. 2011).
Studies performed by Mendes et al. (1995) showed that application of scCO2 with a gas flow rate of 21.4 kg.h−1 at 35.0 MPa and 55 °C to C. vulgaris cells resulted in an oil yield of 13.3 % (wt.). Application of organic solvents, such as acetone and n-hexane, resulted in an oil yield of 16.8 % and 18.5 % (wt.), respectively (Mendes et al. 1995). Andrich et al. (2005) applied different extraction conditions using scCO2 and also organic solvent extraction with n-hexane to the microalga Nannochloropsis sp. Extraction procedures allowed the achievement of 250 mg.g−1 dry weight (23.0 %) using scCO2 (gas flow rate of 10 kg.h−1, 70.0 MPa, and 55 °C) and 120 mg.g−1 dry weight (12.0 %) using n-hexane at both 52 °C and room temperature (Andrich et al. 2005; Crampon et al. 2011). Later, Andrich et al. (2006) used Spirulina platensis to verify the extraction efficiency of scCO2 technique using four different pressures (25.0, 40.0, 55, and 70.0 MPa) and two different temperatures (40 and 55 °C), with a gas flow rate of 10 kg.h−1. In addition to this method, the authors also tested solvent extraction with n-hexane. Results showed that after 45 min, the amount of oil extracted reached its maximum (78.2 mg.g−1 dry weight) for extraction performed at 55 °C and 70.0 MPa. The same amount of extracted oil was obtained after 2.5 h and after 3.5 h, for extraction performed at 55 °C and 40.0 MPa and at 40 °C and 40.0 MPa, respectively. For solvent extraction with n-hexane, the highest oil recovery (77.7 mg.g−1 dry weight) was achieved after 8 h of reaction (Andrich et al. 2006). Couto et al. (2010) performed supercritical fluid extraction from the microalga C. cohnii at temperatures of 40 and 50 °C and pressures of 20.0, 25.0 and 30.0 MPa. Gas flow rate was 0.6 kg.h−1 and extraction time was 3 h. Optimum extraction conditions were found to be 30.0 MPa and 50 °C (8.6 %), against the 19.9 % (wt.) achieved by application of Bligh and Dyer’s method (1959). With this work, it was possible to state that at the highest pressures (25.0 and 30.0 MPa) the extraction yield increases with the temperature, while at the lowest pressure (20.0 MPa), an increase in temperature leads to a decreased yield. Temperature influence on the extraction efficiency results from the combination of the following antagonic thermodynamic effects: (i) at constant pressure, an increase in temperature leads to a decrease in the density of the supercritical fluid and thus its solvatation capacity; (ii) vapour pressure of solutes increase with the temperature, resulting in an high solubility in the supercritical fluid (Couto et al. 2010). Using scCO2 to extract oil from the microalga Chlorococcum sp., Halim et al. (2011) achieved an oil recovery of 58 mg.g−1 dry weight (5.8 %) at a flow rate of 18.5 kg h−1, a pressure of 30.0 MPa and a temperature of 60 °C, during 80 min. By increasing temperature to 80 °C, oil yield was 48 mg.g−1 dry weight (4.8 %). The authors also applied organic solvent extraction (using n-hexane) obtaining a oil yield of 32 mg.g−1 dry weight (3.2 %) after a reaction time of 5.5 h (Halim et al. 2011). Bjornsson et al. (2012) used the microalga Nannochloropsis granulata to study the effect of pressure, time and temperature in oil extraction yields. Firstly, the authors evaluated the effect of pressure (35, 45, and 55 MPa; 50 °C; 6 kg h−1; 3 h), concluding that no significant differences in oil yields were obtained by varying this process variable. Later, different extraction times were applied (3, 4.5, and 6 h), maintaining pressure, temperature and gas flow rate constant (35 MPa; 50 °C; 6 kg.h−1). The increase of extraction time resulted in differences in oil yield that ranged from 8.67 mg.g−1 dry weight (over 180 min) to 15.56 mg.g−1 dry weight (over 270 min) and to 16.91 mg.g−1 dry weight (over 360 min). However, the differences in yields were not statistically significant. Finally, scCO2 extraction was performed by keeping pressure, gas flow rate, and time constant (35 MPa, 6 kg.h−1, and 4.5 h), and by varying temperature (50, 70, and 90 °C). The increase of extraction temperature resulted in a statistically significant increase in oil yield from 15.56 mg.g−1 dry weigh at 50 °C to 28.45 mg.g−1 dry weight at 70 °C and to 25.75 mg.g−1 dry weight at 90 °C. Although oil yield decreased by increasing temperature from 70 to 90 °C, this decrease was not statistically significant (Bjornsson et al. 2012).
Application of scCO2 to extract microalgal oil is very attractive, as it is a green technology and it allows a complete characterization of the extracted oil and the resulting biofuel. However, it still needs to be improved, because of its high cost-effectiveness and high energy-consuming drying step required before supercritical fluid extraction (Crampon et al. 2011).
1.2.2.3 Ionic Liquid Mediated Extraction
Ionic liquids (ILs) have been reported as an attractive alternative for volatile and toxic organic solvents because of their non-volatile character, thermal stability, and high solvatation capacity (Kim et al. 2012; Lateef et al. 2009). ILs are salts of relatively large asymmetric organic cations coupled with smaller organic or inorganic anions. These organic salts can be liquid at room temperatures or low melting point solids (<100 °C) (Lateef et al. 2009; Young et al. 2010; Khodadoust et al. 2006). Cations are generally composed of a ring structure containing nitrogen, such as imidazolium or pyrimidine. On the other hand, anions can vary from single ions, such as chloride, to larger complex ions, such as [N(SO2CF3)2]− (Young et al. 2010). By altering the nature of both cation and anion of the ionic liquid, either hydrophilic or hydrophobic ionic liquids can be prepared, in order to make them suitable for different applications (Lateef et al. 2009).
The use of ionic liquids for oil extraction has been reported by Young et al. (2010). The authors applied mixtures of 1-ethyl-3-methylimidazolium methyl sulphate [Ethyl-mim]MeSO4 and methanol to extract oil from microalgal biomass and different seeds. Ionic liquid extraction from the microalgae Dunaliella sp. and Chlorella sp. resulted in an oil yield of 8.6 % and 38.0 % (wt.), respectively (Young et al. 2010). A wide variety of ILs has also been used by Kim et al. (2012) for oil extraction from C. vulgaris. Oil was extracted from harvested biomass using systems of methanol and the following ILs: [Butyl-mim]CF3SO3, [Butyl-mim]MeSO4, [Butyl-mim]CH3SO3, [Butyl-mim]BF4, [Butyl-mim]PF6, [Butyl-mim]MeSO4, [Butyl-mim]Tf2N, [Butyl-mim]Cl, [Ethyl-mim]MeSO4, [Ethyl-mim]Cl, [Ethyl-mim]Br, and [Ethyl-mim]Ac. Bligh and Dyer’s method (1959) was also used in terms of comparison. Application of these methods resulted in a total fatty acid content of 106.2 (11.1 %), 125.4 (19.0 %), and 118.4 (17.4 %) mg.g−1 dry weight for Bligh and Dyer’s method, [Butyl-mim]CF3SO3, and [Butyl-mim]MeSO4, respectively. It was also shown that fatty acids profiles were very similar for the three extraction methods. The use of an IL-methanol system reduces high prices and aquatic toxicity of imidazolium-based ILs, providing an efficient and environmentally-friendly system for oil extraction from microalgal biomass (Kim et al. 2012).
Although ILs are more expensive than the conventional organic solvents, application of these compounds as solvents for microalgal oil can be a promising alternative because of their higher affinities and non-toxic character.
1.3 Biodiesel Production
Biodiesel is a renewable fuel produced from vegetable oils, animal fats, microorganisms’ oils, or waste cooking oil (Wahlen et al. 2011). It constitutes the best candidate to substitute diesel fuel, as it can be used directly as a fuel requiring some engine modifications, or blended with petroleum diesel and used in diesel engines with few or no modifications (Leung et al. 2010). Chemically, biodiesel is a mixture of esters with long-chain fatty acids, such as lauric, palmitic, stearic, oleic, etc. (Demirbas and Demirbas 2010). Recently, this biofuel has become more attractive due to its environmental benefits: it is biodegradable and it has lower sulphur and aromatic content than diesel fuel, meaning that it will emit less toxic gases. Furthermore, it presents several advantages over conventional petroleum diesel, such as higher combustion efficiency and cetane number. The main disadvantages of biodiesel include the high production costs, its higher viscosity, lower energy content and higher nitrogen oxide (NOx) emissions (Demirbas and Demirbas 2010; Leung et al. 2010).
Biodiesel can be produced from extracted oil through four different methods: (i) direct use or blending of oils; (ii) microemulsification of oils; (iii) thermal cracking or pyrolysis; and (iv) transesterification, also known as alcoholysis (Balat and Balat 2010; Leung et al. 2010; Ma and Hanna 1999). Additional information of the referred methods can be obtained in Balat and Balat (2010) and Ma and Hanna (1999). Transesterification process, schematically represented in Fig. 1.3, constitutes the most applied method for biodiesel production, as it presents several advantages over the other methods. For example, blending and microemulsification may have some problems, such as carbon deposition and oil contamination, whereas pyrolysis is responsible for the production of low valuable products, as well as the production of gasoline instead of diesel (Sharma and Singh 2009). Therefore, transesterification, the chemical conversion of triglycerides in glycerol and esters in the presence of an alcohol, seems to be the most appropriate method for biodiesel production and it will be studied in detail in the next sections.
1.3.1 Transesterification Reaction
As shown in Eqs. 1.1, 1.2, and 1.3, the transesterification is a multi-step reaction where triglycerides are converted into diglycerides, monoglycerides and finally into glycerol during three reaction steps. These reactions are reversible and each one results in the formation of 1 mol of fatty acid ester (Leung et al. 2010; Ma and Hanna 1999).
R represents a small hydrocarbon chain, whereas R1, R2, and R3 represent long-chain hydrocarbons, also known as fatty acid chains.
Variables affecting biodiesel yields during transesterification include the alcohol and molar ratio employed, type of used catalyst, amount of free fatty acids (FFA), water content, and reaction temperature and time (Ehimen et al. 2010; Ma and Hanna 1999; Miao et al. 2009; Sharma and Singh 2009; Wahlen et al. 2011). Due to the reversibility of the above mentioned reactions, an excess of alcohol is used to shift the equilibrium towards fatty acid esters formation. In a study performed by Miao and Wu (2006), transesterification reaction was applied to oil recovered from the microalga C. protothecoides at 30 °C for 7 h, using 100 % (v/v) of catalyst (sulphuric acid) and the following molar ratios of methanol to oil: 25:1, 30:1, 45:1, 56:1, 70:1, and 84:1 (v/v). Results showed that the highest biodiesel yields (68.0 % and 63.0 %) were obtained using the molar ratios of alcohol to oil of 45:1 and 56:1, respectively (Miao and Wu 2006). In the transesterification process, short-chain alcohols such as methanol, ethanol, propanol, butanol, and amyl alcohol are used. However, the most used ones are methanol and ethanol. Methanol has been extensively applied in transesterification reactions because of its low cost (Gong and Jiang 2011; Leung et al. 2010; Ma and Hanna 1999) and physical and chemical properties, including its high polarity and small chain length (Ma and Hanna 1999). When methanol is used as alcohol the produced esters are known as fatty acid methyl esters (FAME). Wahlen et al. (2011) used five different alcohols (methanol, ethanol, butan-1-ol, 2-methylpropan-1-ol, and 3-methylbutan-1-ol) to produce biodiesel from the microalga Chaetoceros gracilis through transesterification. The assays were performed at 60 °C for 100 min, using sulphuric acid as a catalyst in a proportion of 1.8 % (v/v). The amount of fatty acid esters produced with the different alcohols was not significantly different, meaning that methanol, the cheapest one, is suitable for application in the transesterification process (Wahlen et al. 2011).
Three types of catalysts can be used in the transesterification reaction: alkalis, acids, and enzymes (Drapcho et al. 2008; Ma and Hanna 1999). Alkalis and acids are the most commonly used catalysts, both including homogeneous and heterogeneous catalysts (Drapcho et al. 2008; Leung et al. 2010). The alkalis used in this process include NaOH, KOH, carbonates and corresponding sodium and potassium alkoxides like sodium methoxide, sodium ethoxide, sodium propoxide, and sodium butoxide (Ma and Hanna 1999). The main acids used as catalysts include sulphuric acid, sulfonic acid, and hydrochloric acid. Normally, alkali catalysts are preferred over acid catalysts because reactions catalysed by alkali catalysts are faster than reactions catalysed by acids (Drapcho et al. 2008; Leung et al. 2010). However, transesterification reaction using an alkali catalyst results in the formation of small amounts of water (Sharma and Singh 2009). Water is undesirable in this process because it promotes the hydrolysis of the glycerides, forming FFA. FFA produce soap and water through a saponification reaction with the alkali catalyst (Leung et al. 2010). Soap formation must be avoided because it lowers the fatty acid esters yield and inhibits the separation of the esters from glycerol (Leung et al. 2010; Ma and Hanna 1999). Thus, when using an alkali catalyst, glycerides and the used alcohol must be substantially anhydrous (Ma and Hanna 1999) and FFA content of glycerides must be below 0.5 % (wt.) (Gong and Jiang 2011). When FFA contents in glycerides exceed 0.5 %, an acid catalyst should be employed (Drapcho et al. 2008; Leung et al. 2010; Ma and Hanna 1999). Acids catalyse the formation of water and fatty acid esters, i.e. biodiesel, from FFA and alcohol. The main disadvantage of acid catalyst application is the slow reaction rate and the high methanol to oil molar ratio that is required (Leung et al. 2010). Alternatively, triglycerides can be purified by saponification by alkali treatment before transesterification (Ma and Hanna 1999). The use of enzymes, e.g. lipases, as catalysts constitutes a promising alternative in biodiesel production. They avoid soap formation and facilitate the downstream process of purification, i.e. recovery of glycerol at the end of the reaction is easier when using an enzymatic catalyst. Furthermore, the reactions catalysed by lipases are not affected by FFA and water content (Gong and Jiang 2011; Leung et al. 2010). The main obstacles in using enzymes as catalysts are related with longer reaction times (Leung et al. 2010) and higher costs (Gong and Jiang 2011; Leung et al. 2010).
Reaction temperature and time also influence biodiesel yields through transesterification. Increasing reaction time normally increases conversion rate. Alternatively, different optimum temperatures of transesterification can be determined, depending on the used oil (Ma and Hanna 1999). It is very common to study these variables together because an increase in temperature reaction allows the production of higher amounts of biodiesel in a shorter period of time. This conclusion is supported by a study of Ehimen et al. (2010), who studied the effect of reaction temperature and time on the production of biodiesel from Chlorella sp. Transesterification reaction was performed at four different temperatures (23, 30, 60, and 90 °C), the used alcohol was methanol and the catalyst was sulphuric acid. Results showed faster conversion rates at higher temperatures (60 and 90 °C). FAME conversion rates reached similar asymptotic values after 2 and 4 h of reaction for 60 and 90 °C (Ehimen et al. 2010).
At the end of the transesterification process, a mixture containing essentially esters and glycerol is obtained. As the phase containing glycerol has higher density than the one containing esters, the separation of the two phases becomes easier because glycerol phase tends to settle at the bottom. However, both glycerol and esters phase may contain residues of the alcohol, catalyst and oil that did not react during the transesterification, and also soap that has been formed (Leung et al. 2010; Ma and Hanna 1999). The presence of these contaminants indicates that crude biodiesel should be purified before its use in diesel engines. It is also important to refine the crude glycerol because it has a wide variety of industrial applications, such as soaps, cosmetics, medicines, and others (Leung et al. 2010). The procedures used in the purification of crude biodiesel and glycerol were described in detail by Leung et al. (2010).
There are two types of transesterification: transesterification applied to the extracted oil and transesterification directly applied to the oil source, without previous oil extraction, also known as transesterification in situ. The following sections compare current attempts in producing biodiesel from microalgae using these two types of transesterification. Table 1.3 shows a resume of the research studies about transesterification reaction, presenting the achieved biodiesel yields.
1.3.1.1 Transesterification from the Recovered Oil
Several studies have reported the use of transesterification to convert fatty acids extracted from microalgae into fatty acid esters. The applied reaction follows the above mentioned principles and can be influenced by the referred variables. Miao and Wu (2006) have applied acid-catalysed transesterification to oil extracted from the microalga C. protothecoides with n-hexane. The authors adopted an acid catalyst, e.g. sulphuric acid, because of the high acid value of microalgal oil due to high FFA content. Transesterification was performed at different conditions, to evaluate the effect of catalyst quantity, methanol to oil molar ratio, and reaction time and temperature on the yield and properties of biodiesel product. The reaction was then carried out using: (i) four levels of catalyst quantity – 25, 50, 60, and 100 % sulphuric acid based on oil weight; (ii) six different molar ratios of methanol to oil – 25:1, 30:1, 45:1, 56:1, 70:1, and 84:1 (v/v); and (iii) three different temperatures – 30, 50, and 90 °C. In each experiment, 9.12 g of microalgal oil was used. Results after oil extraction showed that the C. protothecoides produced 55.2 % (wt.) of oil under heterotrophic conditions. Transesterification of this oil resulted in maximum biodiesel yields of 68.0 and 63.0 % for a molar ratio of methanol to oil of 45:1 and 56:1 (v/v), respectively (Miao and Wu 2006). Later, in a study performed by Johnson and Wen (2009), oil extracted from Schizochytrium limacinum was submitted to transesterification reaction using methanol and sulphuric acid. Firstly, the authors applied the Bligh and Dyer’s method (1959) to extract oil from 1 g of microalgal biomass. Chloroform and methanol were added to biomass in the ratio of 1:2 (v/v). After the extraction step, oils were transesterified using a mixture of methanol, sulphuric acid and chloroform at 90 °C with the reaction time of 40 min. From the total fatty acids present in S. limacinum representing 40–50 % of dry biomass, transesterification reaction resulted in biodiesel yields of 63.7 % (Johnson and Wen 2009).
Transesterification of microalgal oil seems to be a promising alternative in biodiesel production, as conversion rates obtained with this method are very high. However, further improvements in operation conditions are needed, to reduce production costs and increase biodiesel yields. One possible alternative is the transesterification in situ, as it overtakes the high-costly extraction step.
1.3.1.2 Transesterification In Situ
Transesterification in situ is very similar to the previously referred method, but instead of being applied to oils, it is applied directly to the biomass containing the oils. As this process can produce biodiesel without the extraction step, it is thought that direct transesterification could lower the production costs of biodiesel fuel (Ma and Hanna 1999; Patil et al. 2011, 2012). Several authors have reported the use of transesterification in situ to produce biodiesel from micro and macroalgae. For instance, Ehimen et al. (2010) applied transesterification in situ to the microalga Chlorella sp. Different reaction conditions were applied to identify the main variables that affect biodiesel yields. Transesterification of 15 g of dried biomass was performed using: (i) 2.2 mL of sulphuric acid as a catalyst; (ii) different volumes of methanol – 20, 40, 60, 80, and 100 mL; (iii) different reaction temperatures – 23, 30, 60, and 90 °C; and (iv) different reaction times – 0.25, 0.5, 1, 1.5, 2, 4, 8, and 12 h. Conversion of microalgal biomass into biodiesel reached 92.0 % at 90 °C after 1 h of reaction, using 2.2 mL of catalyst and 60 mL of methanol (Ehimen et al. 2010). Carvalho Júnior et al. (2011) applied in situ methanolysis to Nannochloropsis oculata. The transesterification reaction of 2 g of biomass was carried out using a mixture of methanol:chlorydric acid:chloroform in a 10:1:1 (v/v/v) ratio, at 80 °C for 2 h under continuous stirring. These conditions allowed the production of 23.2 % of biodiesel in westers/wbiomass. Considering that microalgae has an oil content ranging from 20 % to 50 % (Chisti 2007), the performance obtained is quite satisfactory (Carvalho Júnior et al. 2011). Wahlen et al. (2011) used transesterification in situ to produce biodiesel from the microalgae C. gracilis, Tetraselmis suecica, and Chlorella sorokiniana, and from the cyanobacteria Synechocystis sp., and Synechococcus elongatus. Transesterification reaction was applied to 100 mg of biomass using 2 mL of methanol and sulphuric acid in a volume fraction of 1.8 %. Reaction temperature and time were 80 °C and 20 min. Levels of biodiesel per extractable oil were 82.0, 78.0, 77.0, 39.0 and 40.0 % for the organisms C. gracilis, T. suecica, C. sorokiniana, Synechocystis sp. and S. elongatus, respectively (Wahlen et al. 2011). To compare the two types of transesterification, Johnson and Wen (2009) also applied direct transesterification to cells of S. limacinum. Using a mixture of methanol, sulphuric acid, and chloroform at 90 °C and during 40 min, the authors obtained an ester yield of 72.8 %.
These studies show that biodiesel conversion yields are similar for both types of transesterification, meaning that transesterification in situ should be adopted instead of the double-step procedure of extraction and transesterification. However, these results also suggest that application of direct transesterification require higher volumes of alcohol and catalyst.
1.4 Conclusion
The extraction methods described in this review constitute promising alternatives to recover microalgal oil. Experiments conducted in the last decade have showed that organic solvent extraction is the most efficient method for microalgal oil extraction. The main drawback of applying organic solvents for oil extraction is related to the harmfulness of these compounds. The amount of organic solvent required can be reduced by previous disruption of cell walls using enzymatic, PEF, ultrasound, microwave and expeller pressing methods. These procedures are also responsible for an increase in oil extraction efficiencies. Enzymatic and PEF extraction could be promising techniques because enzymes present higher selectivity towards cell walls and PEF has reduced energetic costs compared to the other disruption methods presented in this chapter. Application of scCO2 extraction has shown to have high extraction efficiencies and to be safer to the environment, but the costs associated are extremely high. Another possibility to avoid the use of organic solvents is to use ionic liquids as solvents. Although extraction efficiencies are lower, these compounds are more environmentally friendly than organic solvents.
An important lack in the research of microalgal oil extraction is the process scale-up, including the analysis of the process cost and efficiency. The majority of studies concerning oil extraction and biodiesel production from microalgae have been performed for lab-scale. As a result, little is known about the feasibility of these processes in large scale. Further studies should be conducted using larger amounts of microalgal biomass to analyse the oil extraction efficiency and to compare with results already known for lab-scale.
In the transesterification reaction, the different variables affecting this reaction must be taken into account. As referred earlier in this review, the alcohol and its employed amount, the type of catalyst and its concentration, temperature and reaction time have a great influence in the alcoholysis reaction. As well as in the oil extraction procedures, the reaction scale is also a determining factor, as it has a great impact on the volumes of alcohol and catalyst employed and on reaction time, which reflects in the total costs of the reaction. Therefore, transesterification reaction should be considered using higher volumes of oil and also different combinations of operational conditions. Additionally, conditions that maximize oil extraction efficiencies should be applied together with those responsible for higher oil to biodiesel conversion rates, to produce biodiesel able to compete with current diesel fuel with reduced costs and higher productivities.
Finally, attention should also be paid to the transesterification in situ process, as it eliminates the oil extraction step. Future studies should focus on this method to verify if costs can be really reduced in the absence of oil extraction steps. Operational conditions at large scale should also be addressed in order to achieve higher productivities of fatty acids esters.
References
Amaro HM, Guedes AC, Malcata FX (2011) Advances and perspectives in using microalgae to produce biodiesel. Appl Energy 88(10):3402–3410. doi:10.1016/j.apenergy.2010.12.014
Andrich G, Nesti U, Venturi F, Zinnai A, Fiorentini R (2005) Supercritical fluid extraction of bioactive lipids from the microalga Nannochloropsis sp. Eur J Lipid Sci Technol 107(6):381–386. doi:10.1002/ejlt.200501130
Andrich G, Zinnai A, Nesti U, Venturi F (2006) Supercritical fluid extraction of oil from microalga Spirulina (Arthrospira) platensis. Acta Aliment 35(2):195–203
Balasubramanian S, Allen JD, Kanitkar A, Boldor D (2011) Oil extraction from Scenedesmus obliquus using a continuous microwave system – design, optimization, and quality characterization. Bioresour Technol 102(3):3396–3403. doi:10.1016/j.biortech.2010.09.119
Balat M, Balat H (2010) Progress in biodiesel processing. Appl Energy 87(6):1815–1835. doi:10.1016/j.apenergy.2010.01.012
Bjornsson WJ, MacDougall KM, Melanson JE, O’Leary SJB, McGinn PJ (2012) Pilot-scale supercritical carbon dioxide extractions for the recovery of triacylglycerols from microalgae: a practical tool for algal biofuels research. J Appl Phycol 24(3):547–555. doi:10.1007/s10811-011-9756-2
Bligh EG, Dyer WM (1959) A rapid method of lipid extraction and purification. Can J Biochem Physiol 37:911–917
Brennan L, Owende P (2010) Biofuels from microalgae – a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sust Energy Rev 14(2):557–577. doi:10.1016/j.rser.2009.10.009
Carvalho Júnior RM, Vargas JVC, Ramos LP, Marino CEB, Torres JCL (2011) Microalgae biodiesel via in situ methanolysis. J Chem Technol Biotechnol 86(11):1418–1427. doi:10.1002/jctb.2652
Chen W, Zhang C, Song L, Sommerfeld M, Hu Q (2009) A high throughput Nile red method for quantitative measurement of neutral lipids in microalgae. J Microbiol Methods 77(1):41–47. doi:10.1016/j.mimet.2009.01.001
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25(3):294–306. doi:10.1016/j.biotechadv.2007.02.001
Chisti Y, Moo-Young M (1986) Disruption of microbial cells for intracellular products. Enzyme Microb Technol 8(4):194–204. doi:10.1016/0141-0229(86)90087-6
Costa JAV, de Morais MG (2011) The role of biochemical engineering in the production of biofuels from microalgae. Bioresour Technol 102(1):2–9. doi:10.1016/j.biortech.2010.06.014
Couto RM, Simões PC, Reis A, da Silva TL, Martins VH, Sánchez-Vicente Y (2010) Supercritical fluid extraction of lipids from the heterotrophic microalga Crypthecodinium cohnii. Eng Life Sci 10(2):158–164. doi:10.1002/elsc.200900074
Crampon C, Boutin O, Badens E (2011) Supercritical carbon dioxide extraction of molecules of interest from microalgae and seaweeds. Ind Eng Chem Res 50(15):8941–8953. doi:10.1021/ie102297d
Cravotto G, Boffa L, Mantegna S, Perego P, Avogadro M, Cintas P (2008) Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason Sonochem 15(5):898–902. doi:10.1016/j.ultsonch.2007.10.009
Demirbas A (2011) Biodiesel from oilgae, biofixation of carbon dioxide by microalgae: a solution to pollution problems. Appl Energy 88(10):3541–3547. doi:10.1016/j.apenergy.2010.12.050
Demirbas A, Demirbas MF (2010) Biofuels. In: Demirbas A, Demirbas MF (eds) Algae energy: algae as a new source of biodiesel. Springer, New York, pp 56–59
Demirbas A, Demirbas MF (2011) Importance of algae oil as a source of biodiesel. Energy Convers Manage 52(1):163–170. doi:10.1016/j.enconman.2010.06.055
Drapcho CM, Nhuan NP, Walker TH (2008) Biodiesel. In: Drapcho CM, Nhuan NP, Walker TH (eds) Biofuels engineering process technology. McGraw Hill, New York, pp 201–208
Ehimen EA, Sun ZF, Carrington CG (2010) Variables affecting the in situ transesterification of microalgae lipids. Fuel 89(3):677–684. doi:10.1016/j.fuel.2009.10.011
Fajardo AR, Cerdán LE, Medina AR, Fernández FGA, Moreno PAG, Grima EM (2007) Lipid extraction from the microalga Phaeodactylum tricornutum. Eur J Lipid Sci Technol 109(2):120–126. doi:10.1002/ejlt.200600216
Geciova J, Bury D, Jelen P (2002) Methods for disruption of microbial cells for potential use in the dairy industry – a review. Int Dairy J 12(6):541–553. doi:10.1016/s0958-6946(02)00038-9
Gong Y, Jiang M (2011) Biodiesel production with microalgae as feedstock: from strains to biodiesel. Biotechnol Lett 33(7):1269–1284. doi:10.1007/s10529-011-0574-z
Gouveia L, Oliveira AC (2009) Microalgae as a raw material for biofuels production. J Ind Microbiol Biotechnol 36:269–274
Grima EM, Belarbi EH, Fernández FGA, Medina AR, Chisti Y (2003) Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol Adv 20(7–8):491–515. doi:10.1016/s0734-9750(02)00050-2
Guderjan M, Töpfl S, Angersbach A, Knorr D (2005) Impact of pulsed electric field treatment on the recovery and quality of plant oils. J Food Eng 67(3):281–287. doi:10.1016/j.jfoodeng.2004.04.029
Guderjan M, Elez-Martínez P, Knorr D (2007) Application of pulsed electric fields at oil yield and content of functional food ingredients at the production of rapeseed oil. Innov Food Sci Emerg Technol 8(1):55–62. doi:10.1016/j.ifset.2006.07.001
Halim R, Gladman B, Danquah MK, Webley PA (2011) Oil extraction from microalgae for biodiesel production. Bioresour Technol 102(1):178–185. doi:10.1016/j.biortech.2010.06.136
Johnson MB, Wen Z (2009) Production of biodiesel fuel from the microalga Schizochytrium limacinum by direct transesterification of algal biomass. Energy Fuel 23(10):5179–5183. doi:10.1021/ef900704h
Khodadoust AP, Chandrasekaran S, Dionysiou DD (2006) Preliminary assessment of imidazolium-based room-temperature ionic liquids for extraction of organic contaminants from soils. Environ Sci Technol 40(7):2339–2345. doi:10.1021/es051563j
Kim Y-H, Choi Y-K, Park J, Lee S, Yang Y-H, Kim HJ, Park T-J, Hwan Kim Y, Lee SH (2012) Ionic liquid-mediated extraction of lipids from algal biomass. Bioresour Technol 109:312–315. doi:10.1016/j.biortech.2011.04.064
Lateef H, Grimes S, Kewcharoenwong P, Bailey E (2009) Ionic liquids in the selective recovery of fat from composite foodstuffs. J Chem Technol Biotechnol 84(11):1681–1687. doi:10.1002/jctb.2230
Lee J-Y, Yoo C, Jun S-Y, Ahn C-Y, Oh H-M (2010) Comparison of several methods for effective lipid extraction from microalgae. Bioresour Technol 101(1 Suppl):S75–S77. doi:10.1016/j.biortech.2009.03.058
Leung DYC, Wu X, Leung MKH (2010) A review on biodiesel production using catalyzed transesterification. Appl Energy 87(4):1083–1095. doi:10.1016/j.apenergy.2009.10.006
Lewis T, Nichols PD, McMeekin TA (2000) Evaluation of extraction methods for recovery of fatty acids from lipid-producing microheterotrophs. J Microbiol Methods 43(2):107–116. doi:10.1016/s0167-7012(00)00217-7
Ma F, Hanna MA (1999) Biodiesel production: a review. Bioresour Technol 70(1):1–15. doi:10.1016/s0960-8524(99)00025-5
Macías-Sánchez MD, Mantell C, Rodríguez M, de la Ossa EM, Lubián LM, Montero O (2007) Supercritical fluid extraction of carotenoids and chlorophyll a from Synechococcus sp. J Supercrit Fluids 39(3):323–329. doi:10.1016/j.supflu.2006.03.008
Mandal V, Mohan Y, Hemalatha S (2007) Microwave assisted extraction – an innovative and promising extraction tool for medicinal plant research. Pharmacog Rev 1(1):7–18
Mendes RL, Fernandes HL, Coelho J, Reis EC, Cabral JMS, Novais JM, Palavra AF (1995) Supercritical CO2 extraction of carotenoids and other lipids from Chlorella vulgaris. Food Chem 53(1):99–103. doi:10.1016/0308-8146(95)95794-7
Mercer P, Armenta RE (2011) Developments in oil extraction from microalgae. Eur J Lipid Sci Technol 113(5):539–547. doi:10.1002/ejlt.201000455
Miao X, Wu Q (2006) Biodiesel production from heterotrophic microalgal oil. Bioresour Technol 97(6):841–846. doi:10.1016/j.biortech.2005.04.008
Miao X, Li R, Yao H (2009) Effective acid-catalyzed transesterification for biodiesel production. Energy Convers Manage 50(10):2680–2684. doi:10.1016/j.enconman.2009.06.021
Patil PD, Gude VG, Mannarswamy A, Deng SG, Cooke P, Munson-McGee S, Rhodes I, Lammers P, Nirmalakhandan N (2011) Optimization of direct conversion of wet algae to biodiesel under supercritical methanol conditions. Bioresour Technol 102(1):118–122. doi:10.1016/j.biortech.2010.06.031
Patil PD, Reddy H, Muppaneni T, Mannarswamy A, Schuab T, Holguin FO, Lammers P, Nirmalakhandan N, Cooke P, Deng SG (2012) Power dissipation in microwave-enhanced in situ transesterification of algal biomass to biodiesel. Green Chem 14(3):809–818. doi:10.1039/C2gc16195h
Ranjan A, Patil C, Moholkar VS (2010) Mechanistic assessment of microalgal lipid extraction. Ind Eng Chem Res 49(6):2979–2985. doi:10.1021/Ie9016557
Sander KB, Murthy GS (2009) Enzymatic degradation of microalgal cell walls. Paper presented at the 2009 ASABE annual international meeting, Reno
Shah S, Sharma A, Gupta MN (2004) Extraction of oil from Jatropha curcas L. seed kernels by enzyme assisted three phase partitioning. Ind Crop Prod 20(3):275–279. doi: citeulike-article-id:4962402
Sharma YC, Singh B (2009) Development of biodiesel: current scenario. Renew Sust Energ Rev 13(6–7):1646–1651. doi:10.1016/j.rser.2008.08.009
Shen Y, Pei Z, Yuan W, Mao E (2009) Effect of nitrogen and extraction method on algae lipid yield. Int J Agric Biol Eng 2(1):51–57. doi:10.3965/j.issn.1934-6344.2009.01.051-057
Taher H, Al-Zuhair S, Al-Marzouqui AH, Haik Y, Farid MM (2011) A review of enzymatic transesterification of microalgal oil-based biodiesel using supercritical technology. Enzyme Res. doi:10.4061/2011/468292
Wahlen BD, Willis RM, Seefeldt LC (2011) Biodiesel production by simultaneous extraction and conversion of total lipids from microalgae, cyanobacteria, and wild mixed-cultures. Bioresour Technol 102(3):2724–2730. doi:10.1016/j.biortech.2010.11.026
Young G, Nippgen F, Titterbrandt S, Cooney MJ (2010) Lipid extraction from biomass using co-solvent mixtures of ionic liquids and polar covalent molecules. Sep Purif Technol 72(1):118–121. doi:10.1016/j.seppur.2010.01.009
Acknowledgements
Ana L. Gonçalves and José C.M. Pires are grateful to Foundation for Science and Technology (FCT), POPH-QREN and FSE for their fellowships SFRH/BD/88799/2012 and SFRH/BPD/66721/2009, respectively.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Gonçalves, A.L., Pires, J.C.M., Simões, M. (2013). Biodiesel from Microalgal Oil Extraction. In: Lichtfouse, E., Schwarzbauer, J., Robert, D. (eds) Green Materials for Energy, Products and Depollution. Environmental Chemistry for a Sustainable World, vol 3. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6836-9_1
Download citation
DOI: https://doi.org/10.1007/978-94-007-6836-9_1
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-6835-2
Online ISBN: 978-94-007-6836-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)