Abstract
With the discovery two decades ago that the adult brain contains neural stem cells (NSCs) capable of producing new neurons, a great deal of research has been undertaken to manipulate these cells to repair the damaged nervous system. Much progress has been made in understanding what regulates adult neural stem cell specification, proliferation and differentiation but much remains to be determined. Lessons can be learned from understanding how embryonic neural stem cells produce the exquisitely complicated organ that is the adult mammalian nervous system. This review will highlight the role of transcriptional regulation of mammalian neural stem cells during embryonic development and compare these to the adult neural stem cell/neural precursor cell (NPC) niches of the subventricular zone (SVZ) of the lateral ventricle and the subgranular zone (SGZ) of the hippocampal dentate gyrus. Normal physiological NSC/NPC regulation will be explored, as well as their regulation and responses following neural injury and disease. Finally, transcriptional regulation of the endogenous NSC/NPCs will be compared and contrasted with embryonic stem/induced pluripotent stem (ES/iPS) cell-derived NSC/NPCs. Recapitulation of the embryonic sequence of transcriptional events in neural stem cell development into specific neuronal or glial lineages improves directed differentiation of ES/iPS cells and may be useful for activation and specification of endogenous adult neural stem cells for therapeutic purposes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
With the discovery two decades ago that the adult brain contains neural stem cells (NSCs) capable of producing new neurons 1, 2, a great deal of research has been undertaken to manipulate these cells to repair the damaged nervous system. Much progress has been made in understanding what regulates adult neural stem cell specification, proliferation and differentiation but much remains to be determined. Lessons can be learned from understanding how embryonic neural stem cells produce the exquisitely complicated organ that is the adult mammalian nervous system.
The nervous system is derived from the embryonic neuroectoderm which generates a self renewing population of neural stem cells (NSCs) that eventually give rise to the majority of cells in the central and peripheral nervous systems. In the simplest pathway, neural specified ectoderm cells, which can be identified by their expression of neural specific markers, such as members of the Sox gene family 3 and Otx2 4, become the earliest neural stem cells, also known as neuroepithelial cells. These form the neural tube and eventually generate all central nervous system neurons and glial cells (astrocytes and oligodendrocytes, but not microglia, which are derived from the hematopoietic system and migrate into the CNS). Neuroepithelial cells give rise to radial glial cells in the Ventricular Zone (VZ), which are also self-renewing multipotent neural stem cells that can directly generate neurons and glia, as well as generate more restricted intermediate progenitor cells that produce cells of a neuronal or glial lineage, often after a small number of divisions. As the neural tissue expands with development, the ventricular zone shrinks and a new neurogenic site forms, the subventricular zone (SVZ). Stem cells in the SVZ continue to generate neurons, glia and intermediate precursor cells. This structure remains into adulthood, particularly lining the lateral ventricles, as one of two neurogenic niches in the adult brain, with the subgranular zone (SGZ) of the dentate gyrus of the hippocampus being the other. A general overview of different neural stem cell sources and locations is provided in Fig. 8.1.
Sources of neural stem cells. In vivo: Neural stem cells (NSCs)/neural progenitor cells (NPCs) are present throughout the nervous system during development, in the ventricular zone (VZ) and in the subventricular zone (SVZ), which contains more restricted intermediate progenitor cells (IPCs). In the adult brain, the SVZ remains as a remnant lining the lateral wall of the lateral ventricles, comprised of Type B neural stem cells, Type C transit amplifying cells (NPCs) and Type A neuroblasts that migrate along the rostral migratory stream (RMS) to differentiate primarily into interneurons in the olfactory bulb. A second adult neurogenic niche is found in the subgranular zone (SGZ) of the dentate gyrus in the hippocampus, which contains Type 1–3 NSC/NPCs that differentiate primarily into neurons in the adjacent granule cell layer (GCL). In vitro: NSC/NPCs are readily cultured, often in the form of neurospheres which, depending on the age and source of the NSC/NPCs, can usually differentiate into all neural cell lineages – neurons, astrocytes and oligodendrocytes. Neurospheres can be grown from embryonic neural tissue, as well as adult SVZ (and in a more restricted fashion from hippocampus). They can also be derived from embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) from a variety of adult tissues, such as skin fibroblasts
Neural stem cell maintenance and differentiation decisions are regulated, at least in part, by signal transduction pathways that culminate in transcription factor expression or repression. Expression of these transcriptional cascades is regulated temporally and spatially, with differences in relative expression levels and specific combinations of transcription factors leading to different outcomes. This starts with induction of NSC fate, followed by expansion of NSC numbers, neural cell fate decisions (neurons versus glia – astrocytes and oligodendrocytes) and regionalised specification of specific neuronal cell types. Many of the signals involved in development of the nervous system are recapitulated in some way in adult NSCs or in specification and differentiation of embryonic stem (ES) and induced pluripotent stem (iPS) cells into neural lineages.
This review will highlight the role of transcriptional regulation of mammalian neural stem cells during embryonic development and compare these to the adult neural stem cell/neural precursor cell (NPC) niches of the subventricular zone (SVZ) of the lateral ventricle and the subgranular zone (SGZ) of the hippocampal dentate gyrus. Normal physiological NSC/NPC regulation will be explored, as well as their regulation and responses following neural injury and disease. Finally, transcriptional regulation of endogenous NSC/NPCs will be compared and contrasted with ES/iPS cell-derived NSC/NPCs. Recapitulation of the embryonic sequence of transcriptional events in neural stem cell development into specific neuronal or glial lineages improves directed differentiation of ES/iPS cells and may be useful for activation and specification of endogenous adult neural stem cells for therapeutic purposes.
2 Developmental Regulation During Embryogenesis
2.1 Specification of Neuroectoderm Cells and the Neural Lineage
One of the first steps in neural development is the specification of ectodermal cells into neuroectoderm cells that comprise the earliest neural stem cells and are responsible for the formation of almost the entire nervous system. Of course, the nervous system is not a homogeneous organ and overlaid on the simple pathway of neural stem cell differentiation described above is a complex set of spatial regulatory cues that not only determine whether a neural stem cell or precursor cell will become a neuron or a glial cell but whether it will become a spinal cord cell or a brain cell and further, which specific sort of spinal cord or brain cell, e.g. a spinal motor neuron versus a hippocampal granule neuron versus a cortical interneuron. While specification of patterning of the nervous system will not be reviewed in detail, some of the signalling pathways and transcription factors involved in the process are also required for induction of neural stem cells and derivation of specific neural lineages from ES and iPS cells (see Sect. 8.4 and Fig. 8.1) and so will be covered briefly here. More extensive reviews on induction and patterning of the nervous system have been written recently 5– 7.
Neural induction of ectodermal cells is thought to be the default state and non-neural tissue is induced by bone morphogenetic proteins (BMPs). Therefore, for the cells to remain neural, BMP signalling needs to be inhibited; this is achieved by expression of BMP antagonists such as chordin and noggin. This induction is also supported by FGF signalling to maintain the neurally induced state. This early neural induction appears to specify anterior neural tissue (destined to become forebrain, midbrain and hindbrain) and involves transcription factors such as Otx2, Lim1 and FoxA2 4. Further refining of anterior/posterior patterning is regulated by gradients of Wnts, with reciprocal gradients of Wnt antagonists such as Dikkopf, Frzb and Cerberus 5. On top of this spatial patterning, the neural stem cells all undergo a similar sequence of events involving proliferation and subsequent differentiation, generally into neurons, followed by glial cells. These more general events, in specific contexts, will be the topic of the remainder of this review.
2.2 Regulation of NSC Proliferation Versus Differentiation in the Central Nervous System
The first decision a neural stem cell needs to make is whether to proliferate and self renew or whether to differentiate into more mature progeny. Maintaining the balance between total self-renewal, limited self-renewal and then differentiation, as cells progress from multipotent NSCs to multipotent or restricted intermediate neural progenitor cells (NPCs) to mature progeny is under tight transcriptional and temporal control. There are basically three somewhat inter-related functions transcription factors can perform to regulate expansion of NSC populations: (1) regulation of proliferation to expand numbers, (2) regulation of self-renewal i.e. maintenance of multipotent stem cell characteristics and (3) repression of differentiation. Different transcription factors can play multiple roles at different stages of development, depending on levels of expression and combinatorial interactions with other transcription factors and signalling pathways, therefore assigning specific roles for individual transcription factors can be rather complicated. Nonetheless, there has been a plethora of expression analyses, over-expression, deletion and mutation studies to indicate that a number of key transcription factors have a dominant effect on the decision to self-renew, proliferate or differentiate 8.
Notch signalling is one of the most widely studied pathways intimately linked to the balance between expansion of NSCs/NPCs and neural differentiation. The primary effectors of Notch signalling are the transcriptional repressors Hes1 and Hes5, which repress neuronal differentiation and maintain NSCs in an undifferentiated state 9, 10. While not required for development of neuroepithelial cells (the earliest NSCs), Hes repression of proneural genes is required to maintain neuroepithelial pluripotency as well as radial glial pluripotency and self renewal. This requires signalling through the Notch receptor via the Notch effector C-promoter binding factor 1 (CBF1, also known as RBP/J). Notch signalling is also involved in proliferation of the intermediate NPCs, which are no longer multipotent but largely neurogenic, due to downregulation of CBF1 in these cells 11.
In mammals, neuroepithelial cells are a pseudostratified epithelium forming the neural tube and they undergo symmetric cell division to produce more multipotent neuroepithelial cells (Fig. 8.2). In these cells, transcription factors such as Hes1 are equally shared between both daughter cells and both remain as neuroepithelial cells. In the absence of Notch activated Hes1 or Hes5, NSCs prematurely differentiate into neurons 9, 12, 13. Hes1 and Hes5 perform all three functions of factors that regulate NSC maintenance, with roles in promoting proliferation, inhibiting differentiation and maintaining multipotency. Later in development, when neuroepithelial cells become radial glial cells, Hes activity remains important for maintenance of radial glial NSC characteristics. Depending on the stage of development radial glial cells can undergo symmetric divisions like neuroepithelial cells or asymmetric divisions, whereby one daughter cell remains a radial glial cell and the other either becomes an intermediate NPC or generates a neuron 14. In invertebrates, the plane of cleavage during mitosis (vertical or horizontal) dictates segregation of Notch pathway regulatory factors and subsequent Notch pathway activity, leading to one daughter cell retaining activity and remaining a stem cell, with the other losing activity and becoming a more differentiated daughter cell. In mammals the radial glial cells undergo division largely in the vertical plane but such divisions can be symmetric or asymmetric 14 and may have more to do with whether or not cells maintain apical membrane or retain -catenin containing ventricular end feet 15 than segregation of Notch effectors, which also play a role in subsequent cell fate determination. Further, Notch pathway effectors do not act alone and interact with several other transcription factors that mediate more restricted functions in determining whether a NSC self-renews or differentiates.
Factors regulating maintenance and differentiation of neural stem cells in vivo. During embryonic development NSCs initially undergo a rapid proliferative phase characterised by symmetric divisions to produce more stem cells. As development progresses NSC division becomes asymmetric, producing one NSC and a neural precursor cell (NPC) or neuron. Transcription factors that maintain the NSCs in a proliferative state include members of the Notch signalling pathway, such as Hes, as well as SoxB1 members (Sox1-3) and Pax6. As expression of these molecules decreases and expression of proneural factors such as neurogenins and Mash1/Ascl increase, NSCs commence differentiation into more mature cell fates. This also requires that the NSCs are able to detach from the basal and pial surfaces to undergo asymmetric division and subsequent differentiation and this requires expression of Forkhead transcription factors such as FoxP2/P4. In the adult SVZ the slowly proliferating NSCs undergo asymmetric division to produce rapidly dividing NPCs (transit amplifying cells). Expression of maintenance and proneural factors plays a similar role in the adult as during development. It is unclear whether FoxP2/P4 continues to play a role
The neuroepithelial attachments are maintained by adherens junctions and maintenance versus differentiation is regulated by the coordinated assembly and disassembly of these contacts. Some of the transcriptional regulators involved in this process have recently been identified and involve the progressive expression of two Forkhead transcription factors, Foxp2 and Foxp4. These repress expression of N-cadherin which is critical for maintenance of adherens junctions, leading to detachment of differentiating neurons from the neuroepithelium 16.
A generic overview of NSC proliferation and maintenance versus differentiation is provided in Fig. 8.2.
Members of the SoxB1 family of transcriptional activators (Sox1, Sox2 and Sox3) and in particular Sox2 are among the earliest markers of neural stem cell identity 17. They act in a partially redundant manner to maintain NSC self renewal capacity, both during development and in adult NSCs 18– 20. Sox2 acts at least in part through the Notch and Sonic hedgehog (Shh) pathways 21, 22 and its transcriptional activation was recently shown to be regulated by a new transcription factor, Ars2 23 which is also important for NSC self-renewal. SoxB1 family members that maintain self renewal are in balance with proneural basic helix-loop-helix (bHLH) transcription factors such as neurogenin2 (Ngn2) and Ascl1/Mash1, which promote neurogenesis and there is reciprocal antagonism and regulation of the two opposing roles 18, 24. Other transcription factors also play critical roles in NSC self renewal, including Gli2 and Gli3, which regulate expression of transcription factors such as Hes1, Hes5 and Sox2 25 and BMI-1, a transcriptional repressor that maintains NSC self renewal by repressing inhibitors of cyclin dependent kinases 26. Pax6 also plays a role in balancing NSC self renewal and neurogenesis, particularly in developing cortex 27 with the level of expression being critical in determining which way the balance is tipped 28. High levels of Pax6 lead to interactions with proneural transcription factors such as Ngn1 and Ascl1 and promotion of neurogenesis at the expense of self-renewal, while an absence of Pax6 leads to precocious neurogenesis as expression of key cell cycle regulators is decreased and neuronal differentiation is promoted. This highlights that it is not necessarily only the presence or absence of a transcription factor that is important but also the relative levels.
In addition to the transcription factors mentioned above, there are others that also promote NSC proliferation but are not necessarily important for maintenance of a multipotent state, including Olig2 29, Id4 30 and Gli1 31, while others actively repress differentiation, such as Hes-related bHLH transcription factors HesR1 and HesR2 8, 32.
2.3 Regulation of Neural Stem Cell Fate
2.3.1 Neural Precursor Cell Differentiation
As neural development progresses the symmetric division of radial glial cells decreases to be replaced by asymmetric divisions and production of intermediate progenitor cells (IPCs). During the neurogenic phase these cells largely generate neurons and a glial cell fate is inhibited, while at later embryonic stages an astrocyte fate is promoted at the expense of neuronal fate. The switch from radial glial cell to intermediate progenitor cell involves downregulation of factors important for self-renewal, such as CBF1, Emx2, Pax6 and Sox2 11, 33– 35, with upregulation of transcriptional regulators such as Tbr2, Svet1, Lmo4 and Cux1-2 33, 36, 37. Tbr2 expression is so specific to cortical intermediate progenitor cells and is switched off in their progeny, unlike many other markers, that it is a particularly good marker for this specific population of cells 33, 38, 39. However, Tbr2 is not just a marker, as mis-expression of Tbr2 in radial glial cells induces intermediate progenitor cell identity, indicating it is important for progenitor cell specification 40. In the absence of Tbr2 intermediate neuronal progenitor cells are depleted, stem cell numbers are increased and neurogenesis is decreased 41, 42, at least in part due to repression of Sox2 42. Radial glial and intermediate progenitor cells can also be distinguished by their differential responsiveness to Notch signalling: both cell types respond to Notch receptor activation but signalling via the Notch effector CBF1 is attenuated in the intermediate progenitor cells. Indeed, knockdown of CBF1 can convert stem cells to intermediate progenitor cells 11.
2.3.2 Neuronal Differentiation
As differentiation progresses, some transcription factors, such as Pax6, that are involved in regulation of neural stem/progenitor proliferation begin to regulate neuronal differentiation 43. In part they do this by inducing expression of other transcription factors, such as proneural basic helix-loop-helix (bHLH) transcription factors. During this neurogenic period a high level of proneural bHLH expression is required, not only to promote neuronal differentiation but also to inhibit premature astroglial differentiation 44. Proneural bHLH transcription factors are involved in specifying generic neuronal fate and, depending on the region of the nervous system and co-expression of other transcription factors, also lead to eventual production of specific different neuronal cell types.
Many of the signalling mechanisms involved in neural cell induction discussed above also play a role in neuronal specification, in conjunction with other signal transduction pathways, with the specific environment and developmental age promoting different cell fates. The Wnt signalling pathway is one such example. Activation of the canonical Wnt pathway by overexpression of stabilised -catenin in early cortical progenitor cells leads to excess proliferation and inhibition of neuronal differentiation 15, 45, 46, while its overexpression at later stages of development induces cell cycle arrest and neuronal differentiation 47. One of the mechanisms by which Wnt signalling can promote neuronal differentiation may be by inducing expression of the neurogenic bHLH transcription factors Neurogenin1 and Neurogenin2 (Ngn1/2). Conversely, other signalling pathways inhibit proneural gene expression and consequent neuronal differentiation. For example, FGF2 signalling increases Notch expression and promotes progenitor proliferation rather than neuronal differentiation 48, leading to increased activation of Notch signalling and induction of Hes family transcriptional repressors, which then inhibit expression of proneural genes such as Ngn1 and Ngn2 and Ascl1/Mash1 24. Other factors such as growth hormone (GH), also decrease Ngn expression and cortical progenitor neuronal differentiation, but during the neurogenic phase high levels of the intracellular regulator of cytokine signal transduction, suppressor of cytokine signalling-2 (SOCS2), blocks GH/STAT5 signalling and allows normal neurogenesis to proceed 49. Regulation of Ngn phosphorylation by GSK3 also regulates neurogenic activity. Wnt-mediated repression of GSK3 activity during the early neurogenic phase blocks Ngn phosphorylation, but GSK3 activity leads to phosphorylation and inactivation of Ngn during the late neurogenic/gliogenic phase 50.
Both Ngn1/2 and Ascl1/Mash1 induce broad but context-specific neuronal differentiation throughout the nervous system and their role in cortical neuron differentiation and subtype specification will be used here as an example, as cells in these locations will eventually form the hippocampus and SVZ of the adult lateral ventricle, the primary regions of neurogenesis in the adult. In the developing rodent forebrain excitatory (glutamatergic) cortical neurons are generated in columns above the dorsal telencephalic Ngn1/2-expressing VZ/SVZ progenitor cells. The VZ-derived progenitor cells give rise to the excitatory neurons in the lower regions of the cortex (layers 4–6) while intermediate progenitor cells in the SVZ give rise to upper cortical layers (2–4). Cortical interneurons (inhibitory GABAergic) are not generated in the same region as the excitatory neurons, instead they arise from VZ/SVZ of the ventral telencephalon (medial and caudal ganglionic eminences; MGE and CGE respectively) and migrate tangentially to integrate with excitatory neurons in the developing cortex 51. Ascl1/Mash1 expression is required in the ganglionic eminence progenitor cells to specify general cortical interneuron fate. More detail can be found in recent specific reviews on regulation of telencephalic cell fate 52, cortical projection neuron development 53 and cortical interneuron development 51.
Other regionally expressed transcription factors are required for production of specific neuronal subtype fates, some of which have different roles in different cortical progenitor cell populations and some of which are more specific. The homeobox transcription factors Cux1 and Cux2 are expressed by interneuron precursors in the MGE (and CGE for Cux1) and are redundantly required for specification of reelin-expressing cortical interneurons (which also express interneuron subtype markers such as calretinin, neuropeptide Y and somatostatin and thus are a heterogeneous population) 54. However, in the dorsal telencephalon, Cux2 is expressed in intermediate progenitors in the SVZ and plays a role in regulating their cell cycle exit so that appropriate numbers of upper layer cortical projection neurons are generated 55.
At the early stages of cortical neurogenesis, VZ-derived daughter cells generate the excitatory neurons of the lower cortical layers. These cells and the layer 5/6 neurons they generate express the zinc-finger transcription factor Fezf2, which is required for their fate specification as in its absence the cells become upper layer cortical neurons 56. Fezf2 induces the post-mitotic co-expression of another zinc-finger transcription factor, Ctip2, which is essential for further differentiation and regulates the axonal projections to subcortical targets 56– 58. Further specification of deep cortical layer subtypes arises depending on the combinatorial and relative levels of expression of Ctip2, Sox5 and Tbr1 53, 59, 60. Tbr1 promotes layer 6 neuron fate and represses layer 5 fate by reducing expression of Fezf2 and Ctip2 61. Ctip2 expression is also repressed in upper layer cortical projection neurons by SatB2, expression of which is required for their specification 62, 63, while FezF2 can inhibit SatB2 expression in lower cortical layers 53. Later in neurogenesis Pou domain transcription factors such as Brn1 and Brn2 are also required for generation of upper layer cortical projection neurons, with a particular effect in double mutants at layer 4, as well as some loss in higher layers 64.
Outside of the cortex different transcription factors are involved in specifying different neuronal types. For example, specification of midbrain dopaminergic neurons involves expression of Nurr1, which is regulated by PitX3 65 and FoxA1/A2 66, while raphe serotinergic neurons are specified by EAGLE 67, Pet1 68 and Lmx1b 69, which is also required for their maintenance 70.
2.3.3 Astrocyte Differentiation
Towards the end of the neurogenic period a gliogenic switch occurs, allowing production of oligodendrocytes (see below) and astrocytes. During the neurogenic phase, gliogenesis is inhibited and this is at least partly achieved by the high expression levels of bHLH transcription factors such as Ngns 71, which suppress gliogenesis by sequestering the gliogenic CBP/p300/Smad transcriptional complex and repressing the JAK/STAT pathway 71, 72. As development progresses, NPCs become more responsive to signals from gliogenic cytokines, such as BMPs and LIF/CNTF (reviewed in 52). This is at least in part due to demethylation of STAT3 binding sites in the promoters of astroglial genes such as GFAP and S10073– 75. However, compared to the large number of transcription factors and regulatory cascades that have been described for production of neurons and different neuron subtypes during the neurogenic phase, there is a relative paucity of data on transcriptional regulators of astrogliogenesis, and particularly on development of different astroglial types. Some of the transcription factors that have been identified are involved in a more general gliogenic switch (i.e. oligodendrocytes and astrocytes), rather than being specific for astrocytes per se, such as Sox9 76, Olig2 77 and serum response factor (SRF) 78. Sox9 is required for production of spinal cord grey matter astrocytes, while having little effect on white matter astrocytes. Nuclear factor-1A (NF1A) has been shown to regulate initiation of spinal cord gliogenesis 79 and expression of astrocyte-specific markers, such as glial fibrillary acidic protein (GFAP) 80. It has recently been shown that Sox9 induces expression of NF1A and together they form a transcriptional cascade that regulates expression of a range of genes involved in astroglial development and particularly those involved in metabolism and migration 81. In the ventral neural tube astrocyte specification is regulated by the bHLH transcription factor stem cell leukaemia (SCL) 82. In addition, although Pax6 regulates neurogenesis, as described above, it is also involved in astrocyte maturation by inhibiting precursor cell proliferation 83.
2.3.4 Oligodendrocyte Differentiation
In contrast, the oligodendrocyte lineage is striking in its expression of a well defined set of transcription factors including Olig1, Olig2, Sox10, Nkx2.2, Mash1/Ascl1 and, upon terminal differentiation, MyRF and Nkx6.2 84– 86. Many of these factors have indispensible roles during oligodendrocyte terminal differentiation/myelination, however there is a common theme with many of them also having more subtle roles in regulating oligodendrocyte lineage specification due to their involvement in neural patterning of the developing nervous system.
The process of specification to the oligodendrocyte lineage is strongly linked with the dorso-ventral patterning of the neural tube, where domains are established through gradients of factors such as Shh and BMP and defined through their expression of transcription factors. Within the spinal cord the oligodendrocyte lineage first arises from the pMN domain, which expresses the oligodendrocyte lineage marker Olig2 as well as Nkx6.1 and Nkx6.2. At later embryonic stages more dorsal regions of the spinal cord give rise to a second wave of oligodendrocyte progenitors which for the most part ultimately replace their earlier ventral counterparts (reviewed in 87). A similar phenomenon exists in the forebrain, where an earlier wave of oligodendrocyte progenitors from the MGE and enteropeduncular area are largely replaced by a later wave of progenitors that originate from the LGE and CGE 88.
A number of bHLH transcription factors have a role in oligodendrocyte specification, with the pan oligodendrocyte lineage marker Olig2 being the most notable. Olig2 expression in the pMN domain of the spinal cord inhibits factors that define neighbouring domains, such as Nkx2.2 and Irx3, thus ablation of the Olig2 gene is associated with an expansion of the p2 domain into what would otherwise be the pMN domain and a resulting loss of motor neuron and oligodendrocyte specification 89, 90. In contrast, oligodendrocyte specification in the brain is comparatively preserved in the absence of Olig2, most likely due to compensation by Olig1 89. This indicates that Olig2 is not an absolute requirement for specification of the lineage. Similarly, at least in chicken, some oligodendrocyte precursors arise from the Nkx2.2+, Olig2- P3 domain, though these oligodendrocyte progenitors subsequently express Olig2 91. Nevertheless, there is substantial evidence that Olig2 is important for both oligodendrocyte lineage specification and function in addition to its role in defining the pMN domain. Olig2 expressing cells of the pMN domain sequentially give rise to motor neurons and oligodendrocytes 89, 90; this fate decision is largely dictated by the phosphorylation state of the Olig2 protein 92. A continued role for Olig2 in maintenance of the lineage has also been recently demonstrated with conditional ablation of the Olig2 gene in committed oligodendrocyte progenitors diverting them to become astrocytes 93.
The bHLH transcription factor Ascl1/Mash1 also has a role in specification of a number of oligodendrocyte progenitor pools. Within the ventral telencephalon, Ascl1/Mash1 promotes oligodendrocyte specification by restricting the expression of Dlx1&2 which otherwise promote interneuron specification at the expense of the specification of Olig2+ oligodendrocyte progenitors 94, 95. Somewhat contrastingly, within the spinal cord Ascl1/Mash1 appears to mark a pool of neuronal/oligodendrocyte progenitors; ablation of Ascl1/Mash1 increases their commitment to the glial lineages 96. It should be noted that although Ascl1/Mash1 is not required for the generation of the oligodendrocyte lineage in totality, it is required for oligodendrocyte terminal differentiation 97.
Several Nkx factors have roles in the specification process. Nkx6.1 and Nkx6.2 have a strong role in promoting oligodendrogenesis in ventral regions via their inhibition of Nkx2.2 (thus allowing for the expression of Olig2 and definition of the pMN domain 98, 99). However, Nkx6.1 and Nkx6.2 are not required for the more dorsally derived oligodendrocytes in the spinal cord and within the hindbrain even act to limit specification to the oligodendrocyte lineage 99. Nkx2.2 also has a mixed role in oligodendrocyte specification; although within the ventral spinal cord it initially inhibits Olig2 expression and oligodendrocyte specification, ultimately Nkx2.2 and Olig2 are co-expressed in the lineage and Nkx2.2 has important roles in oligodendrocyte terminal differentiation 91, 99, 100.
In addition to the above factors, which are largely implicated in the patterning of the developing nervous system, roles for several other transcription factors have been identified in oligodendrocyte specification. In vitro, SoxE proteins Sox8, Sox9 and Sox10 can direct neural precursor cells towards the oligodendrocyte lineage, at least in part by regulation of Suppressor of Fused (Sufu) expression 101. The delta-notch system is also important in regulating oligodendrocyte differentiation 102 and also appears to promote specification to the lineage in the developing zebrafish nervous system 103. Although not strictly required for the initial specification of the oligodendrocyte lineage, REST has an important role in inhibiting neuronal gene expression once the lineage is specified, thus allowing the maintenance of oligodendrocyte identity 104.
3 Adult Neural Stem Cells
3.1 Endogenous Neural Stem Cells
Although the bulk of neurogenesis and gliogenesis occurs during embryonic and early postnatal development, NSCs/NPCs continue to produce neural cells in the adult brain. Interestingly, unlike during development, the vast majority of adult-derived cells are fated to a neuronal lineage, with a much smaller percent differentiating into astrocytes and oligodendrocytes in the normal adult brain. The two primary regions that contain adult NSCs/NPCs are the subventricular zone (SVZ) lining the lateral walls of the lateral ventricles and the subgranular zone (SGZ) of the hippocampal dentate gyrus (Fig. 8.2). The SVZ produces NPCs that form neuroblasts which migrate along the rostral migratory stream and become neurons in the olfactory bulb; while the NPCs in the SGZ become neurons of the granular cell layer of the dentate gyrus in the hippocampus. In addition, precursor cells (primarily oligodendrocyte precursor cells – OPCs) are scattered throughout the parenchyma and primarily generate cells of glial lineage 105, 106.
Both intrinsic and extrinsic factors regulate neurogenesis and, as in the embryo, transcription factors are involved in proliferation, migration and differentiation of new neurons and glial cells in the adult. As described below, some of the transcriptional regulation that defines embryonic NSC/NPC self-renewal versus differentiation are retained in the adult, either performing the same function as in the embryo or with a new/altered function in the adult (Fig. 8.3 and Table 8.1). However, in general, the diversity of cell types (and particularly neuronal subtypes) that can be spontaneously generated by adult NPCs is substantially limited compared to embryonic cells. This currently limits the ability of endogenous NSCs to replace specific neuronal types in different regions in the CNS. To induce appropriate neuronal specification of adult neural stem cells, a good understanding of the events that lead to appropriate specification during embryonic development is needed, so that NPCs can be manipulated in the adult to achieve the desired outcome.
Comparative expression and function of transcription factors from different sources in vivo and in vitro. A range of the more broadly characterised transcription factors known to play a role in NSC/NPC maintenance, differentiation and subsequent maturation are compared across the embryonic VZ/SVZ, adult SGZ, adult SVZ and neurospheres (embryonic or adult brain derived). Cells from each of these sources display a version of a general differentiation scheme which is summarised above, whereby a proliferative neural stem cell (NSC) produces a more proliferative neural progenitor cell, also known as a transit amplifying cell (TAC) or intermediate progenitor cell (IPC) depending on the source of cell. These then differentiate into neuroblasts or glioblasts which then further differentiate into mature neurons or astrocytes and oligodendrocytes respectively. Many of these factors play a similar role in the different types of brain derived stem cells, with some differences, particularly in the hippocampal SGZ cells. In addition, while adult SVZ cells primarily produce neurons under normal physiological conditions, they can also produce glial cells following neural injury or disease. While many factors are known that regulate brain-derived NSC/NPCs, this is sharply contrasted with the current state of knowledge regarding transcription factors regulating neural development of induced pluripotent stem cells (iPSCs) or induced neural stem cells (iNSCs). While the transcription factors that can induce a neural cell fate on these cells have been elucidated, knowledge of factors that regulate their subsequent differentiation is much more limited. Most attention has been focussed on production of dopaminergic neurons for replacement of cells lost in Parkinson’s disease, however specification of other neural fates, including glial cells, is currently limited to modification of culture conditions
3.1.1 Hippocampal Neurogenesis
There is a progression of development of neural progenitor cells in the hippocampus. Initially, there are radial and horizontal NPCs (type 1) that transition to intermediate progenitors (type-2a, 2b and 3) and on to immature granule neurons. Finally, the new neurons become dentate granular neurons and make large mossy fibre projections with CA3 pyramidal neurons 242. Within each of these transitions there are specific transcription factors that are expressed (reviewed in 243). Many of these recapitulate their function in embryonic neural development.
Multiple transcription factors are involved in proliferation and maintenance of the precursor pool within the SGZ. As in embryonic development, Sox2 is a marker of NSCs in the SVZ and SGZ and following Sox2 deletion there is a loss of neurogenesis 19, 244, 245. Thyroid hormone has recently been shown to act as a neurogenic switch in the SVZ by repressing expression of Sox2 218. Pax6 and the CCAAT/enhancer binding protein (C/EBP) are involved in the proliferation of type-1 NPCS along with Sox2, which is a mediator of Notch signalling also involved in maintaining the precursor pool via Shh in adult SGZ 22, 192, 205. The transcriptional repressor gene Hes1 is also activated by Notch signalling leading to repression of proneural gene expression and maintenance of NPCs 144 while expression of Hes5 distinguishes the cells as type-1 NPCs 152. The orphan nuclear receptor Tlx can activate the Wnt/-catenin pathway and is important for proliferation and maintenance of adult NPCs in both the SGZ and SVZ and has been shown to form a molecular network with SOX2 109. Recently, another factor, REST/NRSF (repressor element 1 silencing transcription/neuron restrictive silencer factor), has been shown to maintain NPC pools and direct stage-specific differentiation 246, while the forkhead transcription factors (FoxOs) have role in the long term maintenance of progenitors 133.
Neuronal fate specification occurs through the expression of NeuroD1, Sox3, Sox 4, Sox11 and Prox1 39, 200, 201, 221, 223. NeuroD1 is activated by the Wnt/-catenin pathway, which is necessary for survival and maturation of NPCs in both the SGZ and SVZ 108, 173. The bHLH transcription factors also control fate commitment. Ngn2, Tbr2 and Ascl1/Mash1 are expressed in Type 1/2a NPCs that will become glutamatergic neurons in the hippocampus 162, 178, 247, while over-expression of Ascl1/Mash1 produces oligodendrocytes 163. Synaptic integration of new born neurons is controlled by Kruppel like factor 9 (Klf9) and CREB. Furthermore, both transcription factors are involved in survival and late phase neuronal maturation 119, 120, 248.
3.1.2 SVZ Neurogenesis
Similar to the SGZ, there is a progression of NPC development in the SVZ. Astrocytes in the SVZ (Type B cells) are the primary precursors of highly proliferative transit-amplifying Type C cells which will generate neuroblasts (Type A cells) destined for the olfactory bulb via migration along the rostral migratory stream (RMS) 249– 251. The zinc-finger protein ARS2 (arsenite-resistant protein 2) controls the multipotent progenitor state of NSCs through activation of SOX2 107. c-Myb is required for maintenance of the neural stem cell niche, promoting expression of Sox2 and Pax6 and subsequent proliferation 252.
New neurons migrating from the RMS to the olfactory bulb primarily become GABAergic granule neurons that provide lateral inhibition between mitral and tufted cells. A minority of the new neurons become periglomerular neurons that are involved in lateral inhibition between glomeruli, and a small number of these cells are dopaminergic.
Transcriptional regulation of transient amplifying cell fate is the result of Olig2 expression, and direction of neuronal fate is via Pax6 and Dlx2 126. These transcription factors also induce a dopaminergic periglomurular phenotype in adult mice 127, 182, 193. Recently, it was shown that the transition from amplifying cell to neuroblast requires the down-regulation of Sox9 by miR-124 253. In addition, bHLH transcription factors also control specific neuronal type commitment. Type C cells fated to become GABAergic interneurons in the olfactory bulb express Ascl1/Mash1 162. Ngn2 and Tbr2 are expressed in dorsal SVZ progenitors that become glutamatergic juxtaglomerular neurons 179, while Sp8 is required for parvalbumin-expressing interneurons in the olfactory bulb 226.
3.1.3 Transcriptional Regulation of NSCs/NPCs After Injury and Disease
Neurogenesis and gliogenesis are known to be initiated following brain injuries, such as ischemia, seizures, traumatic injury and neurodegenerative diseases 254– 256. However, these new neurons and glia do not usually effectively replenish those that were lost. Recent studies have begun to examine the fate and transcriptional regulation of NPCs following these insults with the aim of promoting cell replacement and functional repair. Table 8.1 provides a comparative summary of transcription factor expression in NPCs following injury and in the normal brain.
3.1.3.1 Ischemia
Focal ischemic stroke is the most common type of stroke, which results in a contained area of necrotic tissue and a surrounding area known as the penumbra. Focal ischemia promotes SVZ neural progenitor proliferation and neurogenesis 254, 257– 259. However, following cerebral ischemia, repressors to neurogenesis are expressed, such as Olig2 184. Subsequently, gliogenic cells are primarily induced from the adult SVZ 260. The majority of the SVZ neuroblasts in the damaged striatum express the transcription factor Sp8 and do not express the transcription factors of the primarily damaged medium spiny neurons 227, suggesting that after brain injury the NPCs do not change their intrinsic differentiation potential. However, following ischemia, pro-neuronal transcription factors are expressed in primate progenitors in the SGZ, including Emx2, Pax6 and Ngn2 130. Recently it has been shown that following 30 and 60 days after stroke, Ascl1/Mash1 expressing cells in the ischemic striatum gave rise to GABAergic neurons and mature oligodendrocytes 165.
3.1.3.2 Injury and Seizures
Both blunt and acute injuries to the brain and spinal cord trigger neurogenesis in both the SVZ and SGZ; however it is still unclear if the neurogenesis is stable and productive 261– 264. Following injury to the spinal cord Sox11b promotes neuronal determination of endogenous stem cells in adult zebrafish 225. However, following a stab wound to the brain in mice, Olig2 has been implicated in repressing neurogenesis. Interestingly, Olig2 is expressed in glial progenitors that precede the appearance of reactive astrocytes, suggesting that NPCs have a minor role in the repair process 184, 185. Conversely, following quinolinic acid induced striatal cell loss there is compensatory replacement of neurons from the SVZ, primarily from an increase in NPC proliferation and neuroblast formation induced by the expression of Dlx2 and Pax6 128. Similarly, neurogenesis is increased in the SGZ and SVZ after seizures 265– 267. However, the survival of the new born neurons is low as most undergo apoptotic cell death in proportion to the severity of the seizure 268. In the SGZ, proliferating NPCs show a transient expression of the transcription factor Ngn2 178.
3.1.3.3 Neurodegenerative Disorders
Alzheimer’s disease (AD) results in the degeneration of basal forebrain cholinergic neurons in the cortex and hippocampus from the deposition of neurofibrillary tangles and amyloid- plaques 269. The neuropathological hallmark of AD is the amyloid- plaques; however small oligomeric amyloid- appears to be the noxious component. Neurogenesis can be both increased and decreased in AD, depending on the transgenic model used (reviewed in 270. Early in the disease, oligomeric amyloid- may transiently promote the generation of immature neurons from NPCs. However, reduced concentrations of multiple neurotrophic factors and higher levels of FGF2 seem to induce a developmental arrest of newly generated neurons. Further, there is a down-regulation of Olig2 and over-expression of Ascl1/Mash1 caused by amyloid- that switches the cell fate to death 166, 167.
Parkinson’s disease (PD) is the outcome of the loss of dopaminergic neurons in the substantia nigra of the midbrain (reviewed in 271). In transgenic mouse models, there is a decrease in newly generated neurons in both the dentate gyrus and olfactory bulb 153, 272. Alterations in neurogenesis have been linked to a decrease in Notch1 and Hes5 expression 153. Neurogenesis research in PD has focused on generating replacement dopaminergic neurons, primarily with the use of transplanted ES/iPS cells (see below). Recent studies have elucidated the transcription factors necessary to produce dopaminergic neurons. The combination of Ascl1/Mash1, Nurr1 and Lmx1a result in the generation of functional dopaminergic neurons from mouse and human fibroblasts 159. Other studies have shown that Foxa2 in combination with Nurr1 can also induce the production of nigral (A9)-type midbrain neurons from NPCs 138.
Other neurodegenerative diseases such as Huntington’s disease have shown a decrease in neurogenesis. NPC proliferation is decreased in Huntington’s disease in both the SGZ and SVZ, with some reports of reduced numbers of newly born neurons (reviewed in 270. In a rat model of Huntington’s disease, SGZ progenitor cell proliferation is decreased due to an increase in Sox2-positive quiescent stem cells and a decrease in CREB signalling 124. Interestingly, during progressive striatal degeneration, new neurons are produced; however there is low survival and little replacement of lost striatal neurons. Furthermore, neither SVZ-derived nor intra-striatal generated neurons have the potential to differentiate into striatal projection neurons as they lack the transcription factors necessary for such specification 273.
Models of myelin injury have shown an increased production of oligodendrocytes from the SVZ. Oligodendrocyte production is increased following lysolecithin-induced focal demyelination 274, 275. In a model of inflammatory demyelination, experimental autoimmune encephalomyelitis (EAE), an increase in proliferation of cells in the SVZ, their migration to lesion sites and their expression of oligodendrocyte and astrocyte markers was reported 276, while upregulation of chordin in the SVZ following lysolecithin-induced demyelination changes the GAD65 and Dcx positive progenitors from neuronal to glial fates, producing more oligodendrocytes in the corpus callosum 193. In the cuprizone-induced demyelination model, infusion of noggin into the lateral ventricles inhibits BMP signalling and increases the numbers of oligodendroglia in the SVZ 277 and the number of oligodendrocytes in the corpus callosum 278. Also in the cuprizone model, overexpression of Zfp488, an oligodendrocyte-specific zinc finger transcription repressor, promotes oligodendrocyte production in the SVZ 279. This increased specification to the oligodendrocyte lineage following injury is associated with expression of Olig2 274, 279 and Sox10 279.
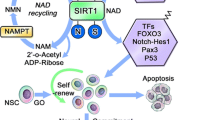
Other disease models show that exogenous factors have an influence on NPC intrinsic transcription that occurs following injury or pathology to the brain. Recently it was shown that the cytokine TWEAK which is induced by cerebral ischemia and other brain disorders activates NF-kappaB and reduces progenitor proliferation in the SVZ. Concurrently, TWEAK lowers the expression of Hes1, thereby inducing neuronal differentiation 147. Pathological brains can have an increase in oxidized redox state, which can alter NPC fate; oxidative conditions up-regulate the histone deacetylase Sirt1 (sirtuin 1). Sirt1 binds to a co-repressor complex of Hes1 and inhibits the pro-neuronal Ascl1/Mash1, in so doing, directing the NPCs toward glial differentiation 148, 149.
4 Derivation of Neural Stem/Precursor Cells from ES and iPS Cells
4.1 Transcriptional Networks Involved in the Differentiation or Reprogramming of Human Pluripotent and Somatic Cells Down Neural Stem/Precursor Cells Lineages
Human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs) express a cohort of transcription factors that maintain self-renewal and repress differentiation 280– 282. In order to induce differentiation in pluripotent stem cells, it first requires the down-regulation of the pluripotent transcriptional network followed by the up-regulation of lineage specific transcription factors. By mimicking the extrinsic signalling factors used during development hESCs and iPSCs can be pushed out of self-renewal and their differentiation biased towards a range of cell types including those of the nervous system 194, 283. The differentiation down a neuroectoderm lineage has been shown to utilise the extrinsic factor Noggin, which is found to be critical during neurogenesis across species 284, 285. The addition of the BMP antagonist Noggin biases human pluripotent stem cells towards a neuroectoderm cell lineage, resulting in early neural stem cells that no longer express the pluripotency-inducing transcription factor OCT4, but now express the transcription factor PAX6 194, 195. More recently the dual inhibition of BMP signalling by noggin and inhibition of Activin/Nodal signalling by the small molecule SB431542 was shown to be an efficient and rapid method for generating PAX6+ neural stem cells 196. Examination of human fetal development shows that PAX6 is expressed at the earliest stages of neuroectoderm commitment 197. Not only is it a marker of the human neural plate but forced expression of PAX6 in human embryonic stem cells drives their differentiation towards a neural fate, demonstrating that it is a determinant of neuroectoderm cell fate 197. Further to this, knockdown of PAX6 prevents neuroectoderm differentiation. Interestingly however, in mouse ES cells forced expression of PAX6 is more involved in the progression of neuroectoderm towards radial glia rather than specification of neural lineages and highlights a potential species difference between human and mouse 286.
4.2 Direct Specification of Neural Lineages
Over the last several years, through transgenic manipulation of cells, other transcriptional determinants of cell fate have been uncovered for the nervous system. Rapid progress in this field has been fuelled by the discovery that somatic cells can be reprogrammed back into a pluripotent state through the forced expression of a defined set of pluripotent transcription factors 282, 287, 288.
The direct conversion of human and mouse fibroblasts into neurons has been achieved through use of various combinations of transcription factors. A screen of 19 neural tissue specific genes identified three critical factors, Ascl1/Mash1, Brn2 (also called Pou3f2), described above for their roles in neural stem cells during development, and Myt1l 168. Forced expression of these factors in mouse or human fibroblasts results in a rapid and efficient conversion into neurons in vitro 168, 169. NeuroD1 was further shown to enhance the maturation and functional characteristics in the reprogramming of human fibroblasts. However, a combination of 4 other transcription factors, Oct4, Sox2, Klf4, and cMyc have also been shown to directly convert mouse and human fibroblasts directly into NSCs 289, 290 and it has also been reported that Sox2 alone is sufficient to directly convert mouse and human fibroblasts into neural stem cells which were self renewing, multipotent and non-tumorigenic 220.
Further progress in this field of reprogramming has demonstrated that neurons with distinct functional neurotransmitter phenotypes can also be achieved. Most work has focussed on specification of dopaminergic neurons for replacement in Parkinson’s disease. The direct conversion of human fibroblasts into dopaminergic neurons has been obtained by using the same three transcription factors involved in neural specification Ascl1/Mash1, Brn2 and Myt1l, along with the addition of Lmx1a and FoxA2 to promote neurons with a dopaminergic phenotype 139. These two additional transcription factors had previously been demonstrated to be critical for mesencephalic dopaminergic differentiation from ES cells and present during embryonic development of these neurons 181. Interestingly an alternate set of transcription factors, Ascl1/Mash1, Nurr1 and Lmx1a was also shown to be capable of directly converting human and mouse fibroblasts into functional dopaminergic neurons without going through a progenitor cell stage 159.
Transcriptional determinants involved in the specification of neural progenitor cell types from hESCs have also been investigated. GLI1 has been shown to be a determinant of floor plate specification when expressed in PAX6 positive neural stem cells derived from hESC 291. Furthermore, neural differentiation of hESC under ventralising conditions, along with the forced expression of Lmx1a revealed it to be a determinant of mesencephlalic dopaminergic cell fate 160.
Overall, these studies highlight some of the transcriptional determinants that are critical during the development of the nervous system that can be capitalised upon to direct human cells along desired neural lineages. However, direct differentiation of other neural lineages from hESC/iPSCs, such as motor neurons and oligodendrocytes has not yet been achieved and still relies on manipulation of the extrinsic culture environment, with variable efficiency, such as use of retinoic acid (RA) and sonic hedgehog (Shh) to enhance differentiation along the motor neuron lineage (reviewed in 292.
References
Reynolds BA, Weiss S (1992) Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255(5052):1707–1710
Richards LJ, Kilpatrick TJ, Bartlett PF (1992) De novo generation of neuronal cells from the adult mouse brain. Proc Natl Acad Sci U S A 89(18):8591–8595
Mizuseki K et al (1998) Xenopus Zic-related-1 and Sox-2, two factors induced by chordin, have distinct activities in the initiation of neural induction. Development 125(4):579–587
Levine AJ, Brivanlou AH (2007) Proposal of a model of mammalian neural induction. Dev Biol 308(2):247–256
Grabel L (2012) Developmental origin of neural stem cells: the glial cell that could. Stem Cell Rev 8(2):577–585
Hoch RV, Rubenstein JL, Pleasure S (2009) Genes and signaling events that establish regional patterning of the mammalian forebrain. Semin Cell Dev Biol 20(4):378–386
Vieira C et al (2010) Molecular mechanisms controlling brain development: an overview of neuroepithelial secondary organizers. Int J Dev Biol 54(1):7–20
Ahmed S et al (2009) Transcription factors and neural stem cell self-renewal, growth and differentiation. Cell Adh Migr 3(4):412–424
Hatakeyama J, Kageyama R (2006) Notch1 Expression is spatiotemporally correlated with neurogenesis and negatively regulated by Notch1-independent Hes genes in the developing nervous system. Cereb Cortex 16(Suppl 1):i132–i137
Ohtsuka T et al (2011) Gene expression profiling of neural stem cells and identification of regulators of neural differentiation during cortical development. Stem Cells 29(11):1817–1828
Mizutani K et al (2007) Differential notch signalling distinguishes neural stem cells from intermediate progenitors. Nature 449(7160):351–355
Gaiano N, Nye JS, Fishell G (2000) Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron 26(2):395–404
Nakamura Y et al (2000) The bHLH gene hes1 as a repressor of the neuronal commitment of CNS stem cells. J Neurosci 20(1):283–293
Noctor SC, Martinez-Cerdeno V, Kriegstein AR (2008) Distinct behaviors of neural stem and progenitor cells underlie cortical neurogenesis. J Comp Neurol 508(1):28–44
Chenn A, Walsh CA (2003) Increased neuronal production, enlarged forebrains and cytoarchitectural distortions in beta-catenin overexpressing transgenic mice. Cereb Cortex 13(6):599–606
Rousso DL et al (2012) Foxp-mediated suppression of N-cadherin regulates neuroepithelial character and progenitor maintenance in the CNS. Neuron 74(2):314–330
Collignon J et al (1996) A comparison of the properties of Sox-3 with Sry and two related genes, Sox-1 and Sox-2. Development 122(2):509–520
Bylund M et al (2003) Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat Neurosci 6(11):1162–1168
Ferri AL et al (2004) Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development 131(15):3805–3819
Graham V et al (2003) SOX2 functions to maintain neural progenitor identity. Neuron 39(5):749–765
Bani-Yaghoub M et al (2006) Role of Sox2 in the development of the mouse neocortex. Dev Biol 295(1):52–66
Favaro R et al (2009) Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat Neurosci 12(10):1248–1256
Andreu-Agullo C, Maurin T (2012) Ars2, an essential player in neural stem cell identity. Med Sci (Paris) 28(5):459–462
Bertrand N, Castro DS, Guillemot F (2002) Proneural genes and the specification of neural cell types. Nat Rev Neurosci 3(7):517–530
Takanaga H et al (2009) Gli2 is a novel regulator of sox2 expression in telencephalic neuroepithelial cells. Stem Cells 27(1):165–174
Fasano CA et al (2007) shRNA knockdown of Bmi-1 reveals a critical role for p21-Rb pathway in NSC self-renewal during development. Cell Stem Cell 1(1):87–99
Gotz M, Stoykova A, Gruss P (1998) Pax6 controls radial glia differentiation in the cerebral cortex. Neuron 21(5):1031–1044
Sansom SN et al (2009) The level of the transcription factor Pax6 is essential for controlling the balance between neural stem cell self-renewal and neurogenesis. PLoS Genet 5(6):e1000511
Ligon KL et al (2007) Olig2-regulated lineage-restricted pathway controls replication competence in neural stem cells and malignant glioma. Neuron 53(4):503–517
Yun K et al (2004) Id4 regulates neural progenitor proliferation and differentiation in vivo. Development 131(21):5441–5448
Stecca B, Altaba ARi (2009) A GLI1-p53 inhibitory loop controls neural stem cell and tumour cell numbers. EMBO J 28(6):663–76
Sakamoto M et al (2003) The basic helix-loop-helix genes Hesr1/Hey1 and Hesr2/Hey2 regulate maintenance of neural precursor cells in the brain. J Biol Chem 278(45):44808–44815
Englund C et al (2005) Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci 25(1):247–251
Gangemi RM et al (2001) Emx2 in adult neural precursor cells. Mech Dev 109(2):323–329
Hutton SR, Pevny LH (2011) SOX2 expression levels distinguish between neural progenitor populations of the developing dorsal telencephalon. Dev Biol 352(1):40–47
Nieto M et al (2004) Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II-IV of the cerebral cortex. J Comp Neurol 479(2):168–180
Tarabykin V et al (2001) Cortical upper layer neurons derive from the subventricular zone as indicated by Svet1 gene expression. Development 128(11):1983–1993
Bulfone A et al (1999) Expression pattern of the Tbr2 (Eomesodermin) gene during mouse and chick brain development. Mech Dev 84(1–2):133–138
Hevner RF et al (2006) Transcription factors in glutamatergic neurogenesis: conserved programs in neocortex, cerebellum, and adult hippocampus. Neurosci Res 55(3):223–233
Sessa A et al (2008) Tbr2 directs conversion of radial glia into basal precursors and guides neuronal amplification by indirect neurogenesis in the developing neocortex. Neuron 60(1):56–69
Arnold SJ et al (2008) The T-box transcription factor Eomes/Tbr2 regulates neurogenesis in the cortical subventricular zone. Genes Dev 22(18):2479–2484
Hodge RD et al (2012) Tbr2 is essential for hippocampal lineage progression from neural stem cells to intermediate progenitors and neurons. J Neurosci 32(18):6275–6287
Osumi N et al (2008) Concise review: Pax6 transcription factor contributes to both embryonic and adult neurogenesis as a multifunctional regulator. Stem Cells 26(7):1663–1672
Nieto M et al (2001) Neural bHLH genes control the neuronal versus glial fate decision in cortical progenitors. Neuron 29(2):401–413
Hirabayashi Y, Gotoh Y (2005) Stage-dependent fate determination of neural precursor cells in mouse forebrain. Neurosci Res 51(4):331–336
Zechner D et al (2003) beta-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol 258(2):406–418
Hirabayashi Y et al (2004) The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development 131(12):2791–2801
Faux CH et al (2001) Interactions between fibroblast growth factors and Notch regulate neuronal differentiation. J Neurosci 21(15):5587–5596
Turnley AM et al (2002) Suppressor of cytokine signaling 2 regulates neuronal differentiation by inhibiting growth hormone signaling. Nat Neurosci 5(11):1155–1162
Li S et al (2012) GSK3 temporally regulates neurogenin 2 proneural activity in the neocortex. J Neurosci 32(23):7791–7805
Faux C et al (2012) Neurons on the move: migration and lamination of cortical interneurons. Neurosignals 20(3):168–189
Guillemot F (2007) Cell fate specification in the mammalian telencephalon. Prog Neurobiol 83(1):37–52
Leone DP et al (2008) The determination of projection neuron identity in the developing cerebral cortex. Curr Opin Neurobiol 18(1):28–35
Cubelos B et al (2008) Cux-1 and Cux-2 control the development of Reelin expressing cortical interneurons. Dev Neurobiol 68(7):917–925
Cubelos B et al (2008) Cux-2 controls the proliferation of neuronal intermediate precursors of the cortical subventricular zone. Cereb Cortex 18(8):1758–1770
Chen B et al (2008) The Fezf2-Ctip2 genetic pathway regulates the fate choice of subcortical projection neurons in the developing cerebral cortex. Proc Natl Acad Sci U S A 105(32):11382–11387
Arlotta P et al (2005) Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron 45(2):207–221
Molyneaux BJ, Arlotta P, Macklis JD (2007) Molecular development of corticospinal motor neuron circuitry. Novartis Found Symp 288:3–15, discussion 15–20, 96–8
Kwan KY et al (2008) SOX5 postmitotically regulates migration, postmigratory differentiation, and projections of subplate and deep-layer neocortical neurons. Proc Natl Acad Sci U S A 105(41):16021–16026
Lai T et al (2008) SOX5 controls the sequential generation of distinct corticofugal neuron subtypes. Neuron 57(2):232–247
McKenna WL et al (2011) Tbr1 and Fezf2 regulate alternate corticofugal neuronal identities during neocortical development. J Neurosci 31(2):549–564
Alcamo EA et al (2008) Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron 57(3):364–377
Britanova O et al (2008) Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron 57(3):378–392
Sugitani Y et al (2002) Brn-1 and Brn-2 share crucial roles in the production and positioning of mouse neocortical neurons. Genes Dev 16(14):1760–1765
Jacobs FM et al (2009) Pitx3 potentiates Nurr1 in dopamine neuron terminal differentiation through release of SMRT-mediated repression. Development 136(4):531–540
Ferri AL et al (2007) Foxa1 and Foxa2 regulate multiple phases of midbrain dopaminergic neuron development in a dosage-dependent manner. Development 134(15):2761–2769
Couch JA et al (2004) robo2 and robo3 interact with eagle to regulate serotonergic neuron differentiation. Development 131(5):997–1006
Hendricks T et al (1999) The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. J Neurosci 19(23):10348–10356
Ding YQ et al (2003) Lmx1b is essential for the development of serotonergic neurons. Nat Neurosci 6(9):933–938
Zhao ZQ et al (2006) Lmx1b is required for maintenance of central serotonergic neurons and mice lacking central serotonergic system exhibit normal locomotor activity. J Neurosci 26(49):12781–12788
Sun Y et al (2001) Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell 104(3):365–376
He F et al (2005) A positive autoregulatory loop of Jak-STAT signaling controls the onset of astrogliogenesis. Nat Neurosci 8(5):616–625
Fan G et al (2005) DNA methylation controls the timing of astrogliogenesis through regulation of JAK-STAT signaling. Development 132(15):3345–3356
Namihira M, Nakashima K, Taga T (2004) Developmental stage dependent regulation of DNA methylation and chromatin modification in a immature astrocyte specific gene promoter. FEBS Lett 572(1–3):184–188
Takizawa T et al (2001) DNA methylation is a critical cell-intrinsic determinant of astrocyte differentiation in the fetal brain. Dev Cell 1(6):749–758
Stolt CC et al (2003) The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev 17(13):1677–1689
Cai J et al (2007) A crucial role for Olig2 in white matter astrocyte development. Development 134(10):1887–1899
Lu PPY, Ramanan N (2012) A critical cell-intrinsic role for serum response factor in glial specification in the CNS. J Neurosci 32(23):8012–8023
Deneen B et al (2006) The transcription factor NFIA controls the onset of gliogenesis in the developing spinal cord. Neuron 52(6):953–968
Cebolla B, Vallejo M (2006) Nuclear factor-I regulates glial fibrillary acidic protein gene expression in astrocytes differentiated from cortical precursor cells. J Neurochem 97(4):1057–1070
Kang P et al (2012) Sox9 and NFIA coordinate a transcriptional regulatory cascade during the initiation of gliogenesis. Neuron 74(1):79–94
Muroyama Y et al (2005) Specification of astrocytes by bHLH protein SCL in a restricted region of the neural tube. Nature 438(7066):360–363
Sakurai K, Osumi N (2008) The neurogenesis-controlling factor, Pax6, inhibits proliferation and promotes maturation in murine astrocytes. J Neurosci 28(18):4604–4612
Emery B (2010) Regulation of oligodendrocyte differentiation and myelination. Science 330(6005):779–782
Fancy SP et al (2011) Myelin regeneration: a recapitulation of development? Annu Rev Neurosci 34:21–43
Kessaris N, Pringle N, Richardson WD (2008) Specification of CNS glia from neural stem cells in the embryonic neuroepithelium. Philos Trans R Soc Lond B Biol Sci 363(1489):71–85
Richardson WD, Kessaris N, Pringle N (2006) Oligodendrocyte wars. Nat Rev Neurosci 7(1):11–18
Kessaris N et al (2006) Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci 9(2):173–179
Lu QR et al (2002) Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell 109(1):75–86
Zhou Q, Anderson DJ (2002) The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell 109(1):61–73
Fu H et al (2002) Dual origin of spinal oligodendrocyte progenitors and evidence for the cooperative role of Olig2 and Nkx2.2 in the control of oligodendrocyte differentiation. Development 129(3):681–693
Li H et al (2011) Phosphorylation regulates OLIG2 cofactor choice and the motor neuron-oligodendrocyte fate switch. Neuron 69(5):918–929
Zhu X et al (2012) Olig2-dependent developmental fate switch of NG2 cells. Development 139(13):2299–2307
Parras CM et al (2007) The proneural gene Mash1 specifies an early population of telencephalic oligodendrocytes. J Neurosci 27(16):4233–4242
Petryniak MA et al (2007) Dlx1 and Dlx2 control neuronal versus oligodendroglial cell fate acquisition in the developing forebrain. Neuron 55(3):417–433
Battiste J et al (2007) Ascl1 defines sequentially generated lineage-restricted neuronal and oligodendrocyte precursor cells in the spinal cord. Development 134(2):285–293
Sugimori M et al (2008) Ascl1 is required for oligodendrocyte development in the spinal cord. Development 135(7):1271–1281
Liu R et al (2003) Region-specific and stage-dependent regulation of Olig gene expression and oligodendrogenesis by Nkx6.1 homeodomain transcription factor. Development 130(25):6221–6231
Vallstedt A, Klos JM, Ericson J (2005) Multiple dorsoventral origins of oligodendrocyte generation in the spinal cord and hindbrain. Neuron 45(1):55–67
Qi Y et al (2001) Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development 128(14):2723–2733
Pozniak CD et al (2010) Sox10 directs neural stem cells toward the oligodendrocyte lineage by decreasing Suppressor of Fused expression. Proc Natl Acad Sci U S A 107(50):21795–21800
Wang S et al (1998) Notch receptor activation inhibits oligodendrocyte differentiation. Neuron 21(1):63–75
Park HC, Appel B (2003) Delta-Notch signaling regulates oligodendrocyte specification. Development 130(16):3747–3755
Dewald LE, Rodriguez JP, Levine JM (2011) The RE1 binding protein REST regulates oligodendrocyte differentiation. J Neurosci 31(9):3470–3483
Rivers LE et al (2008) PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci 11(12):1392–1401
Zhu X, Bergles DE, Nishiyama A (2008) NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development 135(1):145–157
Andreu-Agullo C et al (2012) Ars2 maintains neural stem-cell identity through direct transcriptional activation of Sox2. Nature 481(7380):195–198
Wexler EM et al (2009) Endogenous Wnt signaling maintains neural progenitor cell potency. Stem Cells 27(5):1130–1141
Qu Q et al (2010) Orphan nuclear receptor TLX activates Wnt/beta-catenin signalling to stimulate neural stem cell proliferation and self-renewal. Nat Cell Biol 12(1):31–40, sup pp 1–9
Zhang C et al (2010) The modulatory effects of bHLH transcription factors with the Wnt/beta-catenin pathway on differentiation of neural progenitor cells derived from neonatal mouse anterior subventricular zone. Brain Res 1315:1–10
Lei ZN et al (2012) Bcl-2 increases stroke-induced striatal neurogenesis in adult brains by inhibiting BMP-4 function via activation of beta-catenin signaling. Neurochem Int 61(1):34–42
Otero JJ et al (2004) Beta-catenin signaling is required for neural differentiation of embryonic stem cells. Development 131(15):3545–3557
He S et al (2009) Bmi-1 over-expression in neural stem/progenitor cells increases proliferation and neurogenesis in culture but has little effect on these functions in vivo. Dev Biol 328(2):257–272
Molofsky AV et al (2005) Bmi-1 promotes neural stem cell self-renewal and neural development but not mouse growth and survival by repressing the p16Ink4a and p19Arf senescence pathways. Genes Dev 19(12):1432–1437
Paquin A et al (2005) CCAAT/enhancer-binding protein phosphorylation biases cortical precursors to generate neurons rather than astrocytes in vivo. J Neurosci 25(46):10747–10758
Cortes-Canteli M et al (2011) Role of C/EBPbeta transcription factor in adult hippocampal neurogenesis. PLoS One 6(10):e24842
Menard C et al (2002) An essential role for a MEK-C/EBP pathway during growth factor-regulated cortical neurogenesis. Neuron 36(4):597–610
Dworkin S et al (2009) cAMP response element binding protein is required for mouse neural progenitor cell survival and expansion. Stem Cells 27(6):1347–1357
Jagasia R et al (2009) GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J Neurosci 29(25):7966–7977
Giachino C et al (2005) cAMP response element-binding protein regulates differentiation and survival of newborn neurons in the olfactory bulb. J Neurosci 25(44):10105–10118
Dworkin S et al (2007) CREB activity modulates neural cell proliferation, midbrain-hindbrain organization and patterning in zebrafish. Dev Biol 307(1):127–141
Dworkin S, Mantamadiotis T (2010) Targeting CREB signalling in neurogenesis. Expert Opin Ther Targets 14(8):869–879
Herold S et al (2011) CREB signalling regulates early survival, neuronal gene expression and morphological development in adult subventricular zone neurogenesis. Mol Cell Neurosci 46(1):79–88
Kandasamy M et al (2010) Stem cell quiescence in the hippocampal neurogenic niche is associated with elevated transforming growth factor-beta signaling in an animal model of Huntington disease. J Neuropathol Exp Neurol 69(7):717–728
Shan ZY et al (2008) pCREB is involved in neural induction of mouse embryonic stem cells by RA. Anat Rec (Hoboken) 291(5):519–526
Doetsch F et al (2002) EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron 36(6):1021–1034
Brill MS et al (2008) A dlx2- and pax6-dependent transcriptional code for periglomerular neuron specification in the adult olfactory bulb. J Neurosci 28(25):6439–6452
Jones KS, Connor B (2011) Proneural transcription factors Dlx2 and Pax6 are altered in adult SVZ neural precursor cells following striatal cell loss. Mol Cell Neurosci 47(1):53–60
Cooper-Kuhn CM et al (2002) Impaired adult neurogenesis in mice lacking the transcription factor E2F1. Mol Cell Neurosci 21(2):312–323
Tonchev AB, Yamashima T (2006) Differential neurogenic potential of progenitor cells in dentate gyrus and CA1 sector of the postischemic adult monkey hippocampus. Exp Neurol 198(1):101–113
Shimizu T et al (2010) Zinc finger genes Fezf1 and Fezf2 control neuronal differentiation by repressing Hes5 expression in the forebrain. Development 137(11):1875–1885
Berberoglu MA et al (2009) fezf2 expression delineates cells with proliferative potential and expressing markers of neural stem cells in the adult zebrafish brain. Gene Expr Patterns 9(6):411–422
Paik JH et al (2009) FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell 5(5):540–553
Aranha MM et al (2009) Caspases and p53 modulate FOXO3A/Id1 signaling during mouse neural stem cell differentiation. J Cell Biochem 107(4):748–758
Brancaccio M et al (2010) Emx2 and Foxg1 inhibit gliogenesis and promote neuronogenesis. Stem Cells 28(7):1206–1218
Jacquet BV et al (2011) Specification of a Foxj1-dependent lineage in the forebrain is required for embryonic-to-postnatal transition of neurogenesis in the olfactory bulb. J Neurosci 31(25):9368–9382
Renault VM et al (2009) FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell 5(5):527–539
Lee HS et al (2010) Foxa2 and Nurr1 synergistically yield A9 nigral dopamine neurons exhibiting improved differentiation, function, and cell survival. Stem Cells 28(3):501–512
Pfisterer U et al (2011) Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci U S A 108(25):10343–10348
Oh S et al (2009) Shh and Gli3 activities are required for timely generation of motor neuron progenitors. Dev Biol 331(2):261–269
Breunig JJ et al (2008) Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc Natl Acad Sci U S A 105(35):13127–13132
Wang H et al (2011) Gli3 is required for maintenance and fate specification of cortical progenitors. J Neurosci 31(17):6440–6448
Nat R et al (2012) Pharmacological modulation of the Hedgehog pathway differentially affects dorsal/ventral patterning in mouse and human embryonic stem cell models of telencephalic development. Stem Cells Dev 21(7):1016–1046
Imayoshi I et al (2010) Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J Neurosci 30(9):3489–3498
Veeraraghavalu K et al (2010) Presenilin 1 mutants impair the self-renewal and differentiation of adult murine subventricular zone-neuronal progenitors via cell-autonomous mechanisms involving notch signaling. J Neurosci 30(20):6903–6915
Wang X et al (2009) Involvement of Notch1 signaling in neurogenesis in the subventricular zone of normal and ischemic rat brain in vivo. J Cereb Blood Flow Metab 29(10):1644–1654
Scholzke MN et al (2011) TWEAK regulates proliferation and differentiation of adult neural progenitor cells. Mol Cell Neurosci 46(1):325–332
Prozorovski T et al (2008) Sirt1 contributes critically to the redox-dependent fate of neural progenitors. Nat Cell Biol 10(4):385–394
Teng FY, Hor CH, Tang BL (2009) Emerging cues mediating astroglia lineage restriction of progenitor cells in the injured/diseased adult CNS. Differentiation 77(2):121–127
Wang L et al (2009) The Notch pathway mediates expansion of a progenitor pool and neuronal differentiation in adult neural progenitor cells after stroke. Neuroscience 158(4):1356–1363
Kobayashi T, Kageyama R (2010) Hes1 regulates embryonic stem cell differentiation by suppressing Notch signaling. Genes Cells 15(7):689–698
Lugert S et al (2010) Quiescent and activehippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell 6(5):445–456
Crews L et al (2008) Alpha-synuclein alters Notch-1 expression and neurogenesis in mouse embryonic stem cells and in the hippocampus of transgenic mice. J Neurosci 28(16):4250–4260
Bai G et al (2007) Id sustains Hes1 expression to inhibit precocious neurogenesis by releasing negative autoregulation of Hes1. Dev Cell 13(2):283–297
Tzeng SF, de Vellis J (1998) Id1, Id2, and Id3 gene expression in neural cells during development. Glia 24(4):372–381
Bedford L et al (2005) Id4 is required for the correct timing of neural differentiation. Dev Biol 280(2):386–395
Havrda MC et al (2008) Id2 is required for specification of dopaminergic neurons during adult olfactory neurogenesis. J Neurosci 28(52):14074–14086
Deisseroth K et al (2004) Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron 42(4):535–552
Caiazzo M et al (2011) Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 476(7359):224–227
Friling S et al (2009) Efficient production of mesencephalic dopamine neurons by Lmx1a expression in embryonic stem cells. Proc Natl Acad Sci U S A 106(18):7613–7618
Dolmazon V et al (2011) Forced expression of LIM homeodomain transcription factor 1b enhances differentiation of mouse embryonic stem cells into serotonergic neurons. Stem Cells Dev 20(2):301–311
Kim EJ et al (2007) In vivo analysis of Ascl1 defined progenitors reveals distinct developmental dynamics during adult neurogenesis and gliogenesis. J Neurosci 27(47):12764–12774
Jessberger S et al (2008) Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nat Neurosci 11(8):888–893
Berninger B, Guillemot F, Gotz M (2007) Directing neurotransmitter identity of neurones derived from expanded adult neural stem cells. Eur J Neurosci 25(9):2581–2590
Zhang RL et al (2011) Ascl1 lineage cells contribute to ischemia-induced neurogenesis and oligodendrogenesis. J Cereb Blood Flow Metab 31(2):614–625
Uchida Y et al (2007) Differential regulation of basic helix-loop-helix factors Mash1 and Olig2 by beta-amyloid accelerates both differentiation and death of cultured neural stem/progenitor cells. J Biol Chem 282(27):19700–19709
Waldau B, Shetty AK (2008) Behavior of neural stem cells in the Alzheimer brain. Cell Mol Life Sci 65(15):2372–2384
Vierbuchen T et al (2010) Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463(7284):1035–1041
Pang ZP et al (2011) Induction of human neuronal cells by defined transcription factors. Nature 476(7359):220–223
Lim DA et al (2009) Chromatin remodelling factor Mll1 is essential for neurogenesis from postnatal neural stem cells. Nature 458(7237):529–533
Borgs L et al (2009) Period 2 regulates neural stem/progenitor cell proliferation in the adult hippocampus. BMC Neurosci 10:30
Cho JH, Tsai MJ (2004) The role of BETA2/NeuroD1 in the development of the nervous system. Mol Neurobiol 30(1):35–47
Gao Z et al (2009) Neurod1 is essential for the survival and maturation of adult-born neurons. Nat Neurosci 12(9):1090–1092
Kuwabara T et al (2009) Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci 12(9):1097–1105
Roybon L et al (2009) Neurogenin2 directs granule neuroblast production and amplification while NeuroD1 specifies neuronal fate during hippocampal neurogenesis. PLoS One 4(3):e4779
Roybon L et al (2009) Involvement of Ngn2, Tbr and NeuroD proteins during postnatal olfactory bulb neurogenesis. Eur J Neurosci 29(2):232–243
Fedele V et al (2011) Neurogenesis in the R6/2 mouse model of Huntington’s disease is impaired at the level of NeuroD1. Neuroscience 173:76–81
Ozen I et al (2007) Proliferating neuronal progenitors in the postnatal hippocampus transiently express the proneural gene Ngn2. Eur J Neurosci 25(9):2591–2603
Brill MS et al (2009) Adult generation of glutamatergic olfactory bulb interneurons. Nat Neurosci 12(12):1524–1533
Pieper AA et al (2005) The neuronal PAS domain protein 3 transcription factor controls FGF-mediated adult hippocampal neurogenesis in mice. Proc Natl Acad Sci U S A 102(39):14052–14057
Chung S et al (2009) Wnt1-lmx1a forms a novel autoregulatory loop and controls midbraindopaminergic differentiation synergistically with the SHH-FoxA2 pathway. Cell Stem Cell 5(6):646–658
Hack MA et al (2005) Neuronal fate determinants of adult olfactory bulb neurogenesis. Nat Neurosci 8(7):865–872
Hack MA et al (2004) Regionalization and fate specification in neurospheres: the role of Olig2 and Pax6. Mol Cell Neurosci 25(4):664–678
Buffo A et al (2005) Expression pattern of the transcription factor Olig2 in response to brain injuries: implications for neuronal repair. Proc Natl Acad Sci U S A 102(50):18183–18188
Magnus T et al (2007) Evidence that nucleocytoplasmic Olig2 translocation mediates brain-injury-induced differentiation of glial precursors to astrocytes. J Neurosci Res 85(10):2126–2137
Hernandez-Acosta NC et al (2011) Dynamic expression of the p53 family members p63 and p73 in the mouse and human telencephalon during development and in adulthood. Brain Res 1372:29–40
Holembowski L et al (2011) While p73 is essential, p63 is completely dispensable for the development of the central nervous system. Cell Cycle 10(4):680–689
Fletcher RB et al (2011) p63 regulates olfactory stem cell self-renewal and differentiation. Neuron 72(5):748–759
Agostini M et al (2010) p73 regulates maintenance of neural stem cell. Biochem Biophys Res Commun 403(1):13–17
Fujitani M et al (2010) TAp73 acts via the bHLH Hey2 to promote long-term maintenance of neural precursors. Curr Biol 20(22):2058–2065
Talos F et al (2010) p73 is an essential regulator of neural stem cell maintenance in embryonal and adult CNS neurogenesis. Cell Death Differ 17(12):1816–1829
Maekawa M et al (2005) Pax6 is required for production and maintenance of progenitor cells in postnatal hippocampal neurogenesis. Genes Cells 10(10):1001–1014
Jablonska B et al (2010) Chordin-induced lineage plasticity of adult SVZ neuroblasts after demyelination. Nat Neurosci 13(5):541–550
Pera MF et al (2004) Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J Cell Sci 117(Pt 7):1269–1280
Davidson KC et al (2007) Wnt3a regulates survival, expansion, and maintenance of neural progenitors derived from human embryonic stem cells. Mol Cell Neurosci 36(3):408–415
Chambers SM et al (2009) Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 27(3):275–280
Zhang X et al (2010) Pax6 is a human neuroectoderm cell fate determinant. Cell Stem Cell 7(1):90–100
Elkouris M et al (2011) Sox1 maintains the undifferentiated state of cortical neural progenitor cells via the suppression of Prox1-mediated cell cycle exit and neurogenesis. Stem Cells 29(1):89–98
Kaltezioti V et al (2010) Prox1 regulates the notch1-mediated inhibition of neurogenesis. PLoS Biol 8(12):e1000565
Karalay O et al (2011) Prospero-related homeobox 1 gene (Prox1) is regulated by canonical Wnt signaling and has a stage-specific role in adult hippocampal neurogenesis. Proc Natl Acad Sci U S A 108(14):5807–5812
Lavado A et al (2010) Prox1 is required for granule cell maturation and intermediate progenitor maintenance during brain neurogenesis. PLoS Biol 8(8):43–44. p ii: e1000460. doi: 10.1371/journal.pbio.1000460
Merson TD et al (2006) The transcriptional coactivator Querkopf controls adult neurogenesis. J Neurosci 26(44):11359–11370
Komine O et al (2011) RBP-J promotes the maturation of neuronal progenitors. Dev Biol 354(1):44–54
Gao F et al (2009) Transcription factor RBP-J-mediated signaling represses the differentiation of neural stem cells into intermediate neural progenitors. Mol Cell Neurosci 40(4):442–450
Ehm O et al (2010) RBPJkappa-dependent signaling is essential for long-term maintenance of neural stem cells in the adult hippocampus. J Neurosci 30(41):13794–13807
Fujimoto M et al (2009) RBP-J promotes neuronal differentiation and inhibits oligodendroglial development in adult neurogenesis. Dev Biol 332(2):339–350
Stipursky J, Francis D, Gomes FC (2012) Activation of MAPK/PI3K/SMAD pathways by TGF-beta(1) controls differentiation of radial glia into astrocytes in vitro. Dev Neurosci 34(1):68–81
Rajan P et al (2003) BMPs signal alternately through a SMAD or FRAP-STAT pathway to regulate fate choice in CNS stem cells. J Cell Biol 161(5):911–921
Nakashima K et al (1999) Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science 284(5413):479–482
Nakashima K et al (2001) BMP2-mediated alteration in the developmental pathway of fetal mouse brain cells from neurogenesis to astrocytogenesis. Proc Natl Acad Sci U S A 98(10):5868–5873
Colak D et al (2008) Adult neurogenesis requires Smad4-mediated bone morphogenic protein signaling in stem cells. J Neurosci 28(2):434–446
Fukuda S et al (2007) Potentiation of astrogliogenesis by STAT3-mediated activation of bone morphogenetic protein-Smad signaling in neural stem cells. Mol Cell Biol 27(13):4931–4937
Menendez L et al (2011) Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proc Natl Acad Sci U S A 108(48):19240–19245
Patani R et al (2009) Activin/Nodal inhibition alone accelerates highly efficient neural conversion from human embryonic stem cells and imposes a caudal positional identity. PLoS One 4(10):e7327
Ying QL et al (2003) BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 115(3):281–292
Finley MF, Devata S, Huettner JE (1999) BMP-4 inhibits neural differentiation of murine embryonic stem cells. J Neurobiol 40(3):271–287
Gratsch TE, O’Shea KS (2002) Noggin and chordin have distinct activities in promoting lineage commitment of mouse embryonic stem (ES) cells. Dev Biol 245(1):83–94
Lopez-Juarez A et al (2012) Thyroid hormone signaling acts as a neurogenic switch by repressing sox2 in the adult neural stem cell niche. Cell Stem Cell 10(5):531–543
Brazel CY et al (2005) Sox2 expression defines a heterogeneous population of neurosphere-forming cells in the adult murine brain. Aging Cell 4(4):197–207
Ring KL et al (2012) Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell 11(1):100–109
Wang TW et al (2006) Sox3 expression identifies neural progenitors in persistent neonatal and adult mouse forebrain germinative zones. J Comp Neurol 497(1):88–100
Li Y et al (2012) Sox11 modulates neocortical development by regulating the proliferation and neuronal differentiation of cortical intermediate precursors. Acta Biochim Biophys Sin (Shanghai) 44(8):660–668
Haslinger A et al (2009) Expression of Sox11 in adult neurogenic niches suggests a stage-specific role in adult neurogenesis. Eur J Neurosci 29(11):2103–2114
Mu L et al (2012) SoxC transcription factors are required for neuronal differentiation in adult hippocampal neurogenesis. J Neurosci 32(9):3067–3080
Guo Y et al (2011) Transcription factor Sox11b is involved in spinal cord regeneration in adult zebrafish. Neuroscience 172:329–341
Li X et al (2011) The transcription factor Sp8 is required for the production of parvalbumin-expressing interneurons in the olfactory bulb. J Neurosci 31(23):8450–8455
Liu F et al (2009) Brain injury does not alter the intrinsic differentiation potential of adult neuroblasts. J Neurosci 29(16):5075–5087
Muller S et al (2009) Neurogenesis in the dentate gyrus depends on ciliary neurotrophic factor and signal transducer and activator of transcription 3 signaling. Stem Cells 27(2):431–441
Yu Y, Ren W, Ren B (2009) Expression of signal transducers and activator of transcription 3 (STAT3) determines differentiation of olfactory bulb cells. Mol Cell Biochem 320(1–2):101–108
Cao F et al (2010) Conditional deletion of Stat3 promotes neurogenesis and inhibits astrogliogenesis in neural stem cells. Biochem Biophys Res Commun 394(3):843–847
Gu F et al (2005) Suppression of Stat3 promotes neurogenesis in cultured neural stem cells. J Neurosci Res 81(2):163–171
Li W et al (2008) Nuclear receptor TLX regulates cell cycle progression in neural stem cells of the developing brain. Mol Endocrinol 22(1):56–64
Chavali PL et al (2011) Nuclear orphan receptor TLX induces Oct-3/4 for the survival and maintenance of adult hippocampal progenitors upon hypoxia. J Biol Chem 286(11):9393–9404
Elmi M et al (2010) TLX activates MASH1 for induction of neuronal lineage commitment of adult hippocampal neuroprogenitors. Mol Cell Neurosci 45(2):121–131
Shimozaki K et al (2012) SRY-box-containing gene 2 regulation of nuclear receptor tailless (Tlx) transcription in adult neural stem cells. J Biol Chem 287(8):5969–5978
Zhang CL et al (2008) A role for adult TLX-positive neural stem cells in learning and behaviour. Nature 451(7181):1004–1007
Liu HK et al (2008) The nuclear receptor tailless is required for neurogenesis in the adult subventricular zone. Genes Dev 22(18):2473–2478
Obernier K et al (2011) Expression of Tlx in both stem cells and transit amplifying progenitors regulates stem cell activation and differentiation in the neonatal lateral subependymal zone. Stem Cells 29(9):1415–1426
Shi Y et al (2004) Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature 427(6969):78–83
Zhang C et al (2011) Role of transcription factors in neurogenesis after cerebral ischemia. Rev Neurosci 22(4):457–465
Brown L, Brown S (2009) Zic2 is expressed in pluripotent cells in the blastocyst and adult brain expression overlaps with makers of neurogenesis. Gene Expr Patterns 9(1):43–49
Freund TF, Buzsaki G (1996) Interneurons of the hippocampus. Hippocampus 6(4):347–470
Hodge RD, Hevner RF (2011) Expression and actions of transcription factors in adult hippocampal neurogenesis. Dev Neurobiol 71(8):680–689
Ellis P et al (2004) SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci 26(2–4):148–165
Suh H et al (2007) In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell 1(5):515–528
Gao Z et al (2011) The master negative regulator REST/NRSF controls adult neurogenesis by restraining the neurogenic program in quiescent stem cells. J Neurosci 31(26):9772–9786
Kim EJ et al (2011) Ascl1 (Mash1) defines cells with long-term neurogenic potential in subgranular and subventricular zones in adult mouse brain. PLoS One 6(3):e18472
Scobie KN et al (2009) Kruppel-like factor 9 is necessary for late-phase neuronal maturation in the developing dentate gyrus and during adult hippocampal neurogenesis. J Neurosci 29(31):9875–9887
Luskin MB (1993) Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron 11(1):173–189
Lois C, Alvarez-Buylla A (1994) Long-distance neuronal migration in the adult mammalian brain. Science 264(5162):1145–1148
Doetsch F et al (1999) Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97(6):703–716
Malaterre J et al (2008) c-Myb is required for neural progenitor cell proliferation and maintenance of the neural stem cell niche in adult brain. Stem Cells 26(1):173–181
Cheng LC et al (2009) miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci 12(4):399–408
Arvidsson A et al (2002) Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 8(9):963–970
Rice AC et al (2003) Proliferation and neuronal differentiation of mitotically active cells following traumatic brain injury. Exp Neurol 183(2):406–417
Parent JM (2007) Adult neurogenesis in the intact and epileptic dentate gyrus. Prog Brain Res 163:529–540
Jin K et al (2001) Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A 98(8):4710–4715
Parent JM et al (2002) Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol 52(6):802–813
Zhang RL et al (2001) Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience 105(1):33–41
Li L et al (2010) Focal cerebral ischemia induces a multilineage cytogenic response from adult subventricular zone that is predominantly gliogenic. Glia 58(13):1610–1619
Richardson RM, Sun D, Bullock MR (2007) Neurogenesis after traumatic brain injury. Neurosurg Clin N Am 18(1):169–181, xi
Szele FG, Chesselet MF (1996) Cortical lesions induce an increase in cell number and PSA-NCAM expression in the subventricular zone of adult rats. J Comp Neurol 368(3):439–454
Blizzard CA et al (2011) Focal damage to the adult rat neocortex induces wound healing accompanied by axonal sprouting and dendritic structural plasticity. Cereb Cortex 21(2):281–291
Chirumamilla S et al (2002) Traumatic brain injury induced cell proliferation in the adult mammalian central nervous system. J Neurotrauma 19(6):693–703
Bengzon J et al (1997) Apoptosis and proliferation of dentate gyrus neurons after single and intermittent limbic seizures. Proc Natl Acad Sci U S A 94(19):10432–10437
Parent JM et al (1997) Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci 17(10):3727–3738
Parent JM, Valentin VV, Lowenstein DH (2002) Prolonged seizures increase proliferating neuroblasts in the adult rat subventricular zone-olfactory bulb pathway. J Neurosci 22(8):3174–3188
Ekdahl CT et al (2003) Death mechanisms in status epilepticus-generated neurons and effects of additional seizures on their survival. Neurobiol Dis 14(3):513–523
Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297(5580):353–356
Winner B, Kohl Z, Gage FH (2011) Neurodegenerative disease and adult neurogenesis. Eur J Neurosci 33(6):1139–1151
Goedert M (2001) Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci 2(7):492–501
Winner B et al (2004) Human wild-type alpha-synuclein impairs neurogenesis. J Neuropathol Exp Neurol 63(11):1155–1166
Luzzati F et al (2011) New striatal neurons in a mouse model of progressive striatal degeneration are generated in both the subventricular zone and the striatal parenchyma. PLoS One 6(9):e25088
Menn B et al (2006) Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci 26(30):7907–7918
Nait-Oumesmar B et al (1999) Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur J Neurosci 11(12):4357–4366
Picard-Riera N et al (2002) Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc Natl Acad Sci U S A 99(20):13211–13216
Cate HS et al (2010) Modulation of bone morphogenic protein signalling alters numbers of astrocytes and oligodendroglia in the subventricular zone during cuprizone-induced demyelination. J Neurochem 115(1):11–22
Sabo JK et al (2011) Remyelination is altered by bone morphogenic protein signaling in demyelinated lesions. J Neurosci 31(12):4504–4510
Soundarapandian MM et al (2011) Zfp488 promotes oligodendrocyte differentiation of neural progenitor cells in adult mice after demyelination. Sci Rep 1:2
Reubinoff BE et al (2000) Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol 18(4):399–404
Thomson JA et al (1998) Embryonic stem cell lines derived from human blastocysts. Science 282(5391):1145–1147
Yu J et al (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318(5858):1917–1920
Denham M, Dottori M (2011) Neural differentiation of induced pluripotent stem cells. Methods Mol Biol 793:99–110
Bachiller D et al (2000) The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature 403(6770):658–661
Smith WC, Harland RM (1992) Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell 70(5):829–840
Suter DM et al (2009) A Sox1 to Pax6 switch drives neuroectoderm to radial glia progression during differentiation of mouse embryonic stem cells. Stem Cells 27(1):49–58
Okita K, Ichisaka T, Yamanaka S (2007) Generation of germline-competent induced pluripotent stem cells. Nature 448(7151):313–317
Wernig M et al (2007) In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 448(7151):318–324
Matsui T et al (2012) Neural stem cells directly differentiated from partially reprogrammed fibroblasts rapidly acquire gliogenic competency. Stem Cells 30(6):1109–1119
Thier M et al (2012) Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell 10(4):473–479
Denham M et al (2010) Gli1 is an inducing factor in generating floor plate progenitor cells from human embryonic stem cells. Stem Cells 28(10):1805–1815
Lopez-Gonzalez R, Velasco I (2012) Therapeutic potential of motor neurons differentiated from embryonic stem cells and induced pluripotent stem cells. Arch Med Res 43(1):1–10
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Christie, K.J., Emery, B., Denham, M., Bujalka, H., Cate, H.S., Turnley, A.M. (2013). Transcriptional Regulation and Specification of Neural Stem Cells. In: Hime, G., Abud, H. (eds) Transcriptional and Translational Regulation of Stem Cells. Advances in Experimental Medicine and Biology, vol 786. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6621-1_8
Download citation
DOI: https://doi.org/10.1007/978-94-007-6621-1_8
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-6620-4
Online ISBN: 978-94-007-6621-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)