Abstract
Pathological angiogenesis in the eye including exudative age-related macular degeneration (AMD), proliferative diabetic retinopathy, diabetic macular edema, neovascular glaucoma, and corneal neovascularization (trachoma) underlies the major causes of blindness in both developed and developing nations. Additionally, increased rates of angiogenesis are associated with several other disease states including cancer, psoriasis, rheumatoid arthritis and other vascular-associated disorders. Vascular endothelial growth factor (VEGF) and its receptors play an important role in the modulation of angiogenesis and have been implicated in the pathology of a number of conditions, including AMD, diabetic retinopathy, and cancer. AMD is a progressive disease of the macula and the third major cause of blindness worldwide. If not treated appropriately, AMD might progress to the second eye. Until recently, the treatment options for AMD were limited, with photodynamic therapy the mainstay treatment, which is effective at slowing disease progression but rarely results in improved vision. There are currently three approved anti-angiogenesis biologic therapies for ophthalmic diseases: an anti-VEGF aptamer (pegaptanib, Macugen®), a Fab fragment of a monoclonal antibody directed against VEGF-A (ranibizumab, Lucentis®), and VEGF trap (aflibercept, Eylea®). Several therapies have been and are now being developed for neovascular AMD, with the goal of inhibiting VEGF. At present, established therapies have met with great success in reducing the vision loss associated with neovascular AMD, whereas those still investigational in nature offer the potential for further advances. In AMD patients these therapies slow the rate of vision loss and in some cases increase visual acuity. Although these therapies are a milestone in the treatment of these disease states, several concerns need to be addressed before their impact can be fully understood.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Vascular Endothelial Growth Factor
- Vascular Endothelial Growth Factor Receptor
- Diabetic Macular Edema
- Intravitreal Bevacizumab
- Vascular Endothelial Growth Factor Inhibitor
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Angiogenesis is a term used to describe the formation of new blood vessels from the pre-existing vasculature. This process is critical for several normal physiological functions including the development of embryos, wound-healing, the female reproductive cycle and collateral vascular generation in the myocardium. However, aberrant angiogenesis has been implicated in the progression of several disease states including cancer, macular degeneration, diabetic retinopathy, rheumatoid arthritis and psoriasis.
Under normal physiological conditions, the process of angiogenesis is well controlled, and a perfect balance of endogenous pro-angiogenesis growth factors (positive regulator) and suppressors (negative regulator) exists. When angiogenic growth factors outnumber angiogenesis inhibitors, the balance shifts in favor of accelerated angiogenesis; this has been termed the “angiogenic switch” [1]. Rigorous research in the field of angiogenesis has led to the identification of many regulators involved in angiogenesis. Angiogenesis is driven by the production of pro-angiogenic growth factors including vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), interleukin 8 (IL-8), placental like growth factor (PlGF), transforming growth factor β (TGF-β), angiopoietin, platelet-derived endothelial growth factor (PDEGF), pleiotrophin, and several others [2]. In addition, angiogenesis can be caused by a deficiency in endogenous angiogenesis inhibitors including angiostatin, canstatin, endostatin, various glycosaminoglycan, interferon α, β, χ (INF α, β, χ), thrombospondin and others [3].
Although angiogenesis is not understood in its entirety, the roles of many of its regulators and the fundamental steps that result in angiogenesis have been well documented. Initially, vascular endothelial cells (ECs) are activated by pro-angiogenesis growth factors, which cause ECs to release proteases that degrade the basement membrane, allowing ECs to escape from the original vessel walls, proliferate, and extend toward the source of the angiogenic stimulus using integrin and extracellular matrix proteins to cause cell adhesion [1, 3].
Pathological Angiogenesis
Cancer research has shown that due to a lack of oxygen and other essential nutrients, tumor growth is limited to 1–2 mm, and in order to grow beyond this size tumor cells must promote angiogenesis by secreting various pro-angiogenesis factors [3, 4]. Tumor angiogenesis not only allows tumor growth, but also increases the rate of metastasis. Vessels formed by uncontrolled and unregulated angiogenesis supporting the tumor are drastically different from those of the normal vasculature and are characterized by unstructured blood vessels, hypoxia, and increased interstitial pressure. These irregularities may hinder the ability of chemotherapeutic agents to achieve the desired effective levels within tumor.
Age-related macular degeneration (AMD) is a disease with complex pathology, which could be presented in either the dry (geographic atrophy) or the wet (choroidal neovascularization) form, and in some cases the dry form leads to the wet form. The dry form represents the majority of the AMD cases as opposed to the wet form. However, the wet form leads to progressive vision loss associated with major social and economic impact for the patient [5]. The role of VEGF in the accelerated angiogenesis process in choroidal angiogenesis has been documented, leading the assumption for the use of various anti-VEGF strategies [6]. The main purpose of this chapter is to summarize VEGF’s physiological role (especially within the eye), the role in the development of AMD, and to understand and foresee both the benefits and potential side effects of the anti-VEGF-based therapy.
While the wet form of AMD can be managed using anti-angiogenesis strategies such as anti-VEGF [6], the dry form of advanced AMD results from atrophy of the retinal pigment epithelial layer and has no treatment option at this stage.
Research also shows that angiogenesis accompanies the progression of chronic inflammation. It has been demonstrated that VEGF is overexpressed in a number of pro-inflammatory conditions including psoriasis and rheumatoid arthritis [7, 8]. Thus, VEGF is an attractive target for the treatment of these diseases keeping in mind the redundancy in the pro-angiogenesis pathway and the potential for acquired resistance.
Anti-angiogenesis Therapies
A wide range of therapies designed to inhibit pathological angiogenesis have been developed and many more are underway. Angiogenesis inhibitors have typically been divided into two categories, either a direct strategy targeting ECs or an indirect strategy targeting pro-angiogenesis growth factors or their receptors. Direct targeting of ECs versus the case of a single pro-angiogenesis factor such as VEGF was thought to be a better target for therapy because it is relatively more genetically stable than cancer cells. It is postulated that this stability reduces the likelihood of rapid mutation and acquired drug resistance [9]. Recent studies suggest, however, that genetic anomalies are present in tumor ECs and may be able to confer drug resistance [10]. Interestingly, it has also been suggested that traditional therapies, such as radiation therapy, may actually work in part by targeting the genomically stable ECs because these ECs are still proliferating at a higher than normal rate [11].
Indirect inhibition of angiogenesis can be further divided into two categories, either amplifying the effects of angiogenesis inhibitors and the activation of their pathways or by inhibiting the activation of pro-angiogenesis pathways. Currently, these therapies have employed a multitude of targets including many angiogenic regulators and their receptors. One example is a therapy designed to target TGF. A clinical trial investigating the use of the transforming growth factor (TGF)-β antisense vaccine belagenpumatucel-L (Lucanix®) in patients with non-small cell lung cancer (NSCLC) demonstrated favorable outcome as compared to historical control, with no observed adverse event [12, 13]. Another therapy being explored targets TGF and employs the use of a soluble TGF-ß receptor (sTGF-ßR) that specifically inhibits TGF-ß1 and TGF-ß3 [14].
VEGF
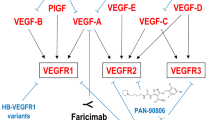
VEGF is a member of a family of dimeric glycoproteins that belong to the platelet-derived growth factor (PDGF) family of growth factors. While VEGF, also known as VEGF-A, is the most comprehensively studied member of the family, others include VEGF-B, VEGF-C, VEGF-D, and PlGF [15, 16]. VEGF-A has several isoforms (VEGF121, VEGF121b, VEGF145, VEGF165, VEGF189, VEGF206) resulting from alternative splicing of which VEGF145 is most the most abundant isoform [17]. All VEGF ligands bind to tyrosine kinase receptors, causing the receptors to dimerize and phosphorylate [18]. Upon binding to its receptor, VEGF initiates a cascade of signaling events that begins with auto-phosphorylation of both receptor kinases, followed by activation of numerous downstream proteins including phospholipase Cλ, PI3K, GAP, Ras, MAPK and others [19]. VEGF receptor-2 (VEGFR-2) has a higher affinity for VEGF, and one of its biological activities includes the potentiation of angiogenesis [19]. The function of VEGFR-1 is less well defined, but seems to include recruitment of monocyte [19]. In contrast, VEGF-C and VEGF-D bind to a different receptor,VEGFR-3, which mediates lymphangiogenesis [16]. The biological activities of VEGF have also been well documented, and because of its vascular permeability characteristic, it was also named as a vascular permeability factor [20] It has also been shown to promote the growth, migration, and proliferation of ECs [20, 21]. In addition it induces vasodilatation and enhances EC survival [20, 21]. These biological activities occur in few physiological processes outside wound-healing and ovulation, making VEGF an attractive target for therapy.
VEGF Role in AMD
VEGF expression and its regulation were studied in retinal pigment epithelial (RPE) cells [6, 22]. To understand VEGF expression, a recombinant adenovirus vector expressing rat VEGF164 was constructed and injected into the sub-retinal space. RPE cells increased their expression of VEGF messenger RNA (mRNA), and blood vessels became leaky 10 days post-injection. By 80 days post-injection, new blood vessels had originated from the choriocapillaris, which ultimately led to the formation of choroidal neovascular membranes and the death of photoreceptor cells. This study demonstrated that overexpression of VEGF in the RPE cells can induce vascular leakage, new choroidal blood vessel growth, the development of choroidal neovascularization (CNV), and neural retina degeneration [6]. This is the same process by which AMD has been shown to cause vision loss, suggestive that VEGF overexpression plays a key role in AMD.
In a retrospective study comparing the safety and efficacy of two anti-VEGF agents, bevacizumab and ranibizumab, in the treatment of patients with neovascular AMD, a comparable safety and efficacy in terms of gains in visual acuity and reduction in macular thickness was documented [23, 24]. It is likely that a randomized controlled trial, if it can be done, will show that bevacizumab is equivalent to ranibizumab in terms of efficacy and safety [24].
VEGF Inhibition
Currently, there are several approved therapeutic agents (and many more being studied) that employ several unique mechanisms of action to inhibit the VEGF pathway. One approach involves the use of monoclonal antibodies to target either VEGF itself or its receptors. Also, VEGF soluble receptors with high affinity for VEGF have been designed to prevent VEGF from binding to VEGF receptors on ECs. Furthermore, various small molecule tyrosine kinase inhibitors have been developed to inhibit VEGF tyrosine kinase receptors. Two unique classes of drugs are targeting the mRNA used to code for VEGF. One class is designed to target post-transcriptional modification of mRNA and actually prevent the protein translation of VEGF [25].
VEGF Inhibition in the Treatment of AMD
Pegaptanib (Macugen®), an aptamar that binds VEGF165 is approved by the FDA for the treatment of wet AMD. Its efficacy and safety analysis were reported in two randomized, sham-controlled clinical trials. These two combined trials are known as the VEGF Inhibition Study in Ocular Neovascularization (VISION), which enrolled 1,186 patients. The patients received either an intraocular injection pegaptanib or a similar sham injection every 6 weeks. Visual acuity (VA) was measured using Snellen eye charts in which patients are asked to identify specific-sized letters or lines at a set distance. Results from the VISION trials indicate that pegaptanib is effective at reducing vision loss compared to sham injection in patients with several types of AMD [26, 27].
Pegaptanib was shown to be a cost-effective treatment for wet AMD in elderly patients as compared to the standard of care in the UK, and as compared to photodynamic therapy (PDT) (verteporfin), and as compared to the standard of care in Canada [28, 29].
Ranibizumab (Lucentis®) was approved for the treatment of wet AMD. Ranibizumab was studied in a 2-year, phase III, double-blind, randomized, sham-controlled study. Patients received either ranibizumab low dose (n = 238), ranibizumab high dose (n = 240), or a sham injection given intravitreally monthly for 2 years in one eye. The primary outcome of VA was assessed by measuring the number of patients who lost fewer than 15 letters from baseline. The mean VA improved by about seven letters in the ranibizumab group compared with a decline of 10 letters in the sham-injection group (p < 0.001). At the study conclusion, 26.1 and 33.3 % of patients in the low and high dose ranibizumab group, respectively, had a VA gain of 15 letters or more, compared with 3.8 % of patients in the sham-injection group (p < 0.001) [30, 31].
No trials have been conducted comparing ranibizumab to pegaptanib. However, overall data shows that ranibizumab actually produces an increase in VA from baseline.
Verteporfin PDT was also indicated for wet AMD, and previous to VEGF inhibiting therapy was the treatment of choice in wet AMD. A study compared ranibizumab to verteporfin PDT in a 2-year, multicenter, double-blind, randomized trial where patients received either low or high doses of ranibizumab or verteporfin PDT [32]. Patients receiving ranibizumab had significantly better VA as indicated by more patients losing fewer than 15 letters on Snellen charts, and patients in the ranibizumab group gained 15 or more letters in VA (35.7 % low dose and 40.3 % high dose) compared to the verteporfin group (5.6 %, p < 0.001). Severe loss of VA, indicated by a decline of 30 letters or more, occurred among 13.3 % of patients receiving verteporfin compared with none among patients receiving ranibizumab [32].
Ongoing clinical trials are currently investigating other therapies for AMD that target VEGF. A soluble VEGF receptor, VEGF trap, was studied in phase II and III trials as an intravitreal injection. Also, trials examining the systemic and intraocular administration of bevacizumab, a VEGF monoclonal antibody, to treat AMD showed that the maximum tolerated intravenous dose of VEGF trap in this study population was 1.0 mg/kg. This dose resulted in elimination of about 60 % of excess retinal thickness after either single or multiple administrations [33].
Bevasiranib, the first small interfering RNA agent developed for the treatment of neovascular AMD, has demonstrated clinical promise. Bevasiranib targets the production of VEGF protein. It does not affect existing VEGF protein, suggesting that it may offer a synergistic effect when given in combination with anti-VEGF treatments, such as ranibizumab. The safety of bevasiranib has been supported by preclinical and clinical research [34].
Comparison Among Different Pharmacotherapies
The effects of different treatments on serious pigment epithelium detachment in AMD were investigated. Results were significantly better in patients treated with bevacizumab and ranibizumab than in those treated with pegaptanib or with a combination of PDT and intravitreal triamcinolone acetonide. Even with treatment, tears of the RPE or partial flattening of the pigment epithelium detachment always indicated a worse prognosis in eyes with exudative AMD than in eyes with CNV [35].
Patients with AMD of any lesion type benefit from treatment with either pegaptanib or ranibizumab with regards to VA when compared with sham injection and/or PDT. When comparing pegaptanib and ranibizumab, the evidence was less clear due to the lack of a designed head-to-head comparison [36].
Aflibercept
A pivotal phase III VEGF trap-eye trial in patients with wet AMD showed that aflibercept was non-inferior to ranibizumab in preventing vision loss with comparable vision gains, safety, and perhaps at lower cost than ranibizumab [37]. Aflibercept gained FDA drug approval after two randomized, double-blind phase III trials were conducted: VIEW 1 and VIEW 2 (VEGF trap-eye: Investigation of Efficacy and Safety in Wet Age-related Macular Degeneration [37, 38]). These studies were conducted to measure the safety and efficacy of aflibercept compared to ranizumab, the standard of care for wet AMD [39]. VIEW 1 was the first study conducted, and VIEW 2 followed after strong evidence from VIEW 1 that aflibercept was comparable to the standard of care. Both studies had the same endpoints, treatment group population, and primary outcome measures. The only difference was that VIEW 1 was conducted in North America, whereas VIEW 2 was conducted internationally. Both trials had a set outcome goal at 52 weeks of treatment, and the primary outcome was identified as the percentage of patients who maintained vision at week 52. Maintaining vision was defined as patients who lost less than 15 letters based on the best-corrected visual acuity (BCVA) scale compared to baseline measurements [38]. The safety analysis in both VIEW trials displayed a well-tolerated drug in aflibercept [40]. When compared to ranibizumab, the safety profile was of approximately equivalent measurements [39–41]. The most common serious side effects presented were loss in VA, retinal hemorrhage, and endophtalmitis. These studies provided a foundation for aflibercept approval in the treatment of wet AMD.
Combined Therapies in AMD
The effect of combined PDT and intravitreal injection of bevacizumab in occult CNV with recent disease progression and in CNV due to AMD was investigated [42]. It was concluded that PDT combined with injection of intravitreal bevacizumab was well-tolerated and is effective along with stabilization of VA in 96 % of patients. Further studies are necessary to show the long-term effect of the PDT and anti-VEGF combination therapy [42].
Combination bevacizumab and low dose PDT significantly reduced the number of bevacizumab treatments required over 6 months. However, this study was powered to examine number of treatments, but not visual acuities, and further studies are required to explore visual outcomes [42, 43].
Overall, PDT has been widely replaced by anti-angiogenesis agents (anti-VEGF) for the first-line therapy for exudative AMD. There is a strong basis for predicting that a combination of PDT and anti-VEGF drugs may address the relative disadvantages of each by improving the response rates and reducing the frequency with which intravitreal injections of anti-VEGF are required. Anti-VEGF drugs may augment the activity of PDT by inhibiting its counterproductive up-regulation of VEGF. Clinical studies of this combination are being advanced in both AMD and in the treatment of certain malignancies.
In a retrospective case series database study (registry), 1,196 patients with CNV due to AMD received one or more combination treatments of bevacizumab (1.25 mg) within 14 days of verteporfin. The use of verteporfin with bevacizumab resulted in vision benefit for most patients [44]. The efficacy and safety of triple therapy consisting of single-session PDT, intravitreal bevacizumab and intravitreal triamcinolone for treatment of neovascular AMD was evaluated in patients with subfoveal CNV secondary to AMD [45]. The study concluded that short-term results of single-session triple therapy suggested that it might be a useful treatment option for neovascular AMD based on its low re-treatment rates, sustainable CNV eradication result, and visual gain achievement. However, the risk and benefits of using intravitreal triamcinolone in addition to combined PDT and intravitreal bevacizumab warrant further evaluation [45].
Tyrosine Kinase Inhibitors
Currently in phase III clinical trials, VEGF trap is a receptor decoy that targets VEGF with higher affinity [32] than ranibizumab and other currently available anti-VEGF agents. Another promising therapeutic strategy is the blockade of VEGF effects by inhibition of the tyrosine kinase cascade downstream from the VEGF receptor; such therapies currently in development include vatalanib, TG100801, pazopanib, AG013958, and AL39324. Small interfering RNA technology-based therapies have been designed to down-regulate the production of VEGF (bevasiranib) or VEGF receptors (AGN211745) by degradation of specific mRNA. Other potential therapies include pigment epithelium-derived factor-based therapies, nicotinic acetylcholine receptor antagonists, integrin antagonists, and sirolimus.
An oral, multi-targeted receptor tyrosine kinase inhibitor, SU11248, that inhibits VEGFR-2, PDGF receptor, and Fms-like tyrosine kinase 3 (FLT3) demonstrated suppression of leakage in an experimental mouse model of CNV caused by AMD [46].
Additionally, inhibition of these tyrosine kinase receptors prevents tumor growth, pathologic angiogenesis, and metastatic progression of cancer [47]. Sunitinib is currently the only FDA-indicated drug for gastrointestinal stromal tumors. Compared with placebo, sunitinib improved time to tumor progression (TTP) and progression-free survival (PFS) time in patients with gastrointestinal stromal tumor who had previously not responded to imatinib [48, 49].
Another tyrosine kinase inhibitor, sorafenib (Nexavar®, BAY 43–9006) [50], also inhibits tumor angiogenesis by blocking the activation of several tyrosine kinase receptors involved in neovascularization and tumor progression, including VEGFR-2, VEGFR-3, PDGFR-β, FLT3, c-Kit and p38-alpha. In addition, sorafenib inhibits the activity of Raf-1 and B-Raf, which are involved in the regulation of endothelial apoptosis [51, 52]. Sorafenib is FDA-approved and has been studied in phase III trials for advanced renal cell carcinoma [51]. In phase III trials, oral sorafenib prolonged PFS compared with placebo in patients with advanced clear-cell renal cell carcinoma in whom first-line therapy had failed. Also, partial responses were significantly higher in the sorafenib group compared to placebo. Treatment was associated with increased adverse events including diarrhea, rash, fatigue, hand-foot skin reactions, hypertension, and cardiac ischemia. In addition, sorafenib significantly increased PFS in patients with advanced renal cell carcinoma in a phase II, placebo-controlled trial [52].
AEE788 is potent, combined inhibitor of both endothelial growth factor (EGF) receptor and VEGF receptor tyrosine kinase family members. In vitro, EGF receptor and VEGF receptor phosphorylation was efficiently inhibited, and AEE788 demonstrated anti-proliferative activity against a range of EGF receptor and ErbB2 overexpression cell lines and inhibited the proliferation of EGF- and VEGF-stimulated human umbilical vein ECs [53]. In vivo, AEE788 decreased tumor growth in a number of cancer cell lines that overexpress EGFR and/or ErbB2. Oral administration of AEE788 to tumor-bearing mice resulted in high and persistent compound levels in tumor tissue. In addition, AEE788 also inhibited VEGF-induced angiogenesis in a murine implant model [53]. Consequently, AEE788 is currently being studied in phase I clinical trials.
Axitinib (AG013736) is an oral selective inhibitor of VEGF receptors 1, 2, and 3. In a phase II clinical trial, axitinib was studied in patients diagnosed with metastatic renal cell cancer who had failed on previous cytokine-based treatment. The primary endpoint was objective response (based on RECIST criteria), and secondary endpoints were duration of response, TTP, overall survival, safety, pharmacokinetics, and patient-reported health-related quality of life. Results showed 2 out of 52 complete and 21 out of 52 partial responses, with an objective response rate of 44.2 %, and median response duration was 23.0 months [54]. Treatment-related adverse events included diarrhea, hypertension, fatigue, nausea, and hoarseness. Data suggest that axitinib might have clinical benefit in patients with cytokine-refractory metastatic renal cell cancer [54].
Cediranib (AZD2171) is a highly potent ATP-competitive inhibitor of recombinant KDR tyrosine kinase. In vitro experiments using human umbilical vein ECs showed AZD2171’s ability to inhibit VEGF-stimulated proliferation and KDR phosphorylation and inhibit vessel sprouting in fibroblast and EC models [55]. Additionally, AZD2171 inhibited tumor growth in a mouse xenograft model of colon, lung, prostate, breast, and ovarian cancer [56].
Zactima (ZD6474) is an orally bioavailable inhibitor of VEGFR-2 tyrosine kinase with additional activity against the EGF receptor tyrosine kinase. In preclinical studies, ZD6474 blocked in vivo phosphorylation of VEGF and EGF tyrosine kinase receptors and prevented the growth of human cancer cell lines in nude mice xenograft [57]. However, disappointing results from a phase II trial in patients with previously treated metastatic breast cancer were recently made available [58].
Vatalanib (PTK787/ZK 222584) is an orally bioavailable angiogenesis inhibitor targeting all known VEGF receptor tyrosine kinases, including VEGFR-1, VEGFR- 2, VEGFR-3, the PDGF receptor tyrosine kinase, and the c-Kit protein tyrosine kinase [59].
Pazopanib (GW786034) is a tyrosine kinase inhibitor that inhibits VEGFR-1, -2, -3, PDGF receptors, and c-Kit. A phase I study demonstrated activity in various types of advanced solid tumors [60]. In a phase II trial, pazopanib resulted in stable disease or partial response in 42 % (25/60) of patients at 12 weeks [61]. Adverse events included hypertension, fatigue, diarrhea, nausea, and proteinuria. Surprisingly, no cases of hand and foot syndrome were reported, and only one case of bleeding occurred. Results appear encouraging, and phase II/III trials are underway in CNV and cancer.
AV-951 (KRN951) is an orally bioavailable tyrosine kinase inhibitor that is specific for VEGF receptors 1, 2 and 3. AV-951 potently inhibited VEGF-induced VEGFR-2 phosphorylation in ECs but not VEGF-independent activation of mitogen-activated protein kinases and proliferation of ECs [62]. Following oral administration to rats, AV-951 decreased the microvessel density within tumor xenograft and decreased VEGFR-2 phosphorylation within tumor endothelium. It also inhibited tumor growth in a wide variety of human tumor xenografts, including lung, breast, colon, ovarian, pancreas, and prostate cancer [62]. In a phase I clinical trial consisting of 40 patients with advanced solid tumors, AV-951 showed promising results. Notably, of the 9 patients with refractory renal cell carcinoma, all achieved either a partial response or stable disease with one patient exhibiting a response lasting more than 30 months [63]. However, a phase II trial in renal cell carcinoma was disappointing, leading to discontinuation of AV-951 [64].
AMG 706 is an orally bioavailable inhibitor of the VEGFR-1, VEGFR-2, VEGFR-3, PDGF receptor, and Kit receptors in preclinical models. AMG 706 inhibited human endothelial cell proliferation induced by VEGF, but not by bFGF in vitro [65]. In addition, it inhibited vascular permeability induced by VEGF in mice, and its oral administration inhibited VEGF-induced angiogenesis in the rat corneal model and induced regression of established A431 xenografts [66]. In a phase I trial enrolling 71 patients, the most frequent adverse events were fatigue, diarrhea, nausea, and hypertension [66]. Thirty four patients (61 %) had stable disease (at least through 1 month). In this phase I study of patients with advanced refractory solid tumors, AMG 706 was well-tolerated and there is evidence of antitumor activity [67]. Additional studies of AMG 706 as monotherapy and in combination with various agents are ongoing.
Issues with VEGF Inhibitors
Although VEGF inhibitors represent the culmination of decades of research in the treatment of several disease states, numerous issues still need to be addressed before their true benefit can be known. Measuring the efficacy of VEGF inhibitors is difficult. Although tumor regression has occurred in some cases, angiogenesis inhibitors are not typically cytotoxic; rather they will most likely result in growth stasis. Some of the current criteria we use to define whether a therapy is efficacious may need to be modified. Tumor mass is likely to be a poor indicator of effective therapy with angiogenesis inhibitors.
Monoclonal antibodies have historically been considered the “magic bullet.” Consequently, utilizing monoclonal antibodies has become a cornerstone in cytokine-targeting therapies. However, cases have been reported where endogenous antibodies actually target these monoclonal antibodies, rendering them inactive [68, 69]. Therefore, as with any monoclonal antibody, it is likely that these types of reactions will occur with anti-VEGF antibodies. Additionally, pharmacoeconomic analysis for cost-effectiveness is still not well-developed enough to justify these expensive therapies.
In addition, blocking VEGF, or its receptors, may block or potentiate the effects of other ligands. It is likely that these receptors are not specific for VEGF. It is difficult to determine what the long-term effects of blocking VEGF and its receptors may be. In clinical trials, frequent adverse events of most VEGF inhibitors include a dramatic increase in the rate of thromboembolic events [70]. Cancer has been shown to increase the risk of these events alone, without anti-VEGF therapy, and thus it seems that concurrent anticoagulation therapy would be beneficial for many patients. However, other data reveal that bleeding is a common adverse event with these therapies as well. Hopefully, future research can enlighten practitioners as to which patient populations are at risk for an adverse event, and therapy can be tailored accordingly.
Beyond VEGF-targeted Therapies
VEGF inhibitors are a milestone in drug development; in spite of this, the issues discussed above make it unlikely that they will be useful in all patients. VEGF inhibitors do appear to be valuable in many types of cancer, nevertheless, not in all types. Alone or in combination with chemotherapy, VEGF inhibitors in trials have had mixed results. For this reason, it would be helpful to have diagnostic testing available to determine which patients would benefit from therapy. Perhaps certain patient populations will be identified that would benefit most by targeting a specific angiogenic growth factor or a specific drug class targeting that growth factor. Moreover, it seems necessary to identify potential antagonism/synergy between certain agents, thus allowing us to predict the most efficacious combinations and enabling practitioners to overcome redundancies that are built into the angiogenesis process. Other novel therapies with different targets may potentially have fewer adverse events and benefit certain populations that cannot receive anti-VEGF therapy.
Conclusions
AMD treatment before the year 2000 was limited to focal laser photocoagulation in an effort to limit the spread of CNV, although it is a destructive procedure and produces a permanent scar. It turned out to be only really viable for treating extra-foveal CNV, and even then it was not entirely effective. In the year 2000, PDT with verteporfin represented the first treatment proven to reduce the risk of vision loss in sub-foveal CNV. However, its efficacy was limited to classic or small CNV, and even though it is a relatively nondestructive form of therapy, it failed to improve vision in patients with AMD in clinical trials.
VEGF was shown to play an important role in promoting angiogenesis and vascular leakage, CNV infiltration, and fluid accumulation in neovascular AMD. Therefore, inhibiting VEGF held the promise of more effectively controlling neovascular AMD. Extensive pre-clinical and clinical research led to the FDA approval of pegaptanib in 2004 and ranibizumab in 2006. Off-label usage of bevacizumab has also become fairly standard. The VA gains recorded with ranibizumab proved particularly exciting, and ranibizumab has become the gold standard for AMD therapy. However, as with many new therapies, there are unresolved issues including safety, cost, and dosing frequency. Further preclinical and clinical investigations are in progress in the inhibition of VEGF-mediated effects at various levels and beyond VEGF.
References
Mousa SA (2000) Mechanisms of angiogenesis in vascular disorders: potential therapeutic targets. In: Mousa SA (ed) Angiogenesis inhibitors & stimulators: potential therapeutic implications. Landes Bioscience (Autsin, TX), pp 1–12, Chapter 1
Relf M, LeJeune S, Scott P et al (1997) Expression of the angiogenic factors vascular endothelial growth factor, acidic and basic fibroblast growth factor, tumor growth factor-β-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res 57:963–969
Ferrara N, Alitalo K (1999) Clinical applications of angiogenic growth factors and their inhibitors. Nat Med 5:1359–1364
Mousa SA, Mousa AS (2004) Angiogenesis inhibitors: current & future directions. Curr Pharm Des 10(1):1–9
De Jong PT (2006) Age-related macular degeneration. N Engl J Med 355(14):1474–1485
Spilsbury K, Garrett KL, Shen WY, Constable IJ, Rakoczy PE (2000) Overexpression of vascular endothelial growth factor (VEGF) in the retinal pigment epithelium leads to the development of choroidal neovascularization. Am J Pathol 157(1):135–144
Fink AM, Cauza E, Hassfeld W, Dunky A, Bayer PM, Jurecka W, Steiner A (2007) Vascular endothelial growth factor in patients with psoriatic arthritis. Clin Exp Rheumatol 25(2):305–308
Murakami M, Iwai S, Hiratsuka S, Yamauchi M, Nakamura K, Iwakura Y, Shibuya M (2006) Signaling of vascular endothelial growth factor receptor-1 tyrosine kinase promotes rheumatoid arthritis through activation of monocytes/macrophages. Blood 108(6):1849–1856
Kerbel R, Folkman J (2002) Clinical translation of angiogenesis inhibitors. Nat Rev Cancer 2:727–739
Marx J (2002) Cancer research: obstacle for promising cancer therapy. Science 295:1444
Casanovas O, Hicklin D, Bergers G, Hanahan D (2005) Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 4(8):299–309
Nemunaitis J, Dillman RO, Schwarzenberger PO et al (2006) Phase II study of belagenpumatucel-L, a transforming growth factor beta-2 antisense gene-modified allogeneic tumor cell vaccine in non–small-cell lung cancer. J Clin Oncol 24(29):4721–4730
Kelly RJ, Giaccone G (2011) Lung cancer vaccines. Cancer J 17(5):302–308
Suzuki E, Kapoor V, Cheung H, Ling LE, DeLong PA, Kaiser LR, Albelda SM (2004) Soluble type II transforming growth factor-ß receptor inhibits established murine malignant mesothelioma tumor growth by augmenting host antitumor immunity. Clin Cancer Res 10(17):5907–5918
Brown LF, Detmar M, Claffey K, Nagy JA, Feng D, Dvorak AM, Dvorak HF (1997) Vascular permeability factor/vascular endothelial growth factor: a multifunctional angiogenic cytokine. EXS 79:233–269
Joukov V, Kaipainen A, Jeltsch M et al (1997) Vascular endothelial growth factors VEGF-B and VEGF-C. J Cell Physiol 173(2):211–215
Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA (1991) The human gene for vascular endothelial growth factor: multiple protein forms are encoded through alternative exon splicing. J Biol Chem 266(18):11947–11954
Ferrara N (1999) Molecular and biological properties of vascular endothelial growth factor. J Mol Med 77(7):527–543
Dvorak HF, Nagy JA, Feng D et al (1999) Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. Curr Top Microbiol Immunol 237:97–132
Senger DR, Van De Water L, Brown LF et al (1993) Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev 12(3–4):303–324
Pettersson A, Nagy JA, Brown LF et al (2000) Heterogeneity of the angiogenic response induced in different normal adult tissues by vascular permeability factor/vascular endothelial growth factor. Lab Invest 80(1):99–115
Ford KM, D’Amore PA (2012) Molecular regulation of vascular endothelial growth factor expression in the retinal pigment epithelium. Mol Vis 18:519–527
Landa G, Amde W, Doshi V, Ali A, McGevna L, Gentile RC, Muldoon TO, Walsh JB, Rosen RB (2009) Comparative study of intravitreal bevacizumab (avastin) versus ranibizumab (lucentis) in the treatment of neovascular age-related macular degeneration. Ophthalmologica 223(6):370–375
Schouten JS, La Heij EC, Webers CA, Lundqvist IJ, Hendrikse F (2009) A systematic review on the effect of bevacizumab in exudative age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 247(1):1–11
Hirawat S, Elfring GL, Northcutt VJ, Paquette N (2007) Phase 1 studies assessing the safety, PK, and VEGF-modulating effects of PTC299, a novel VEGF. J Clin Oncol 25(18S):3562
Gragoudas ES, Adamis AP, Cunningham ET Jr, Feinsod M, Guyer DR (2004) VEGF inhibition study in ocular neovascularization clinical trial group. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 351(27):2805–2816
Chakravarthy U, Adamis AP, VEGF Inhibition Study in Ocular Neovascularization (V.I.S.I.O.N.) Clinical Trial Group et al (2006) Year 2 efficacy results of pegaptanib for neovascular age-related macular degeneration. Ophthalmology 113(9):1508–1525
Wolowacz SE, Roskell N, Kelly S, Maciver FM, Brand CS (2007) Cost effectiveness of pegaptanib for the treatment of age-related macular degeneration in the UK. Pharmacoeconomics 25(10):863–879
Earnshaw SR, Moride Y, Rochon S (2007) Cost-effectiveness of pegaptanib compared to photodynamic therapy with verteporfin and to standard care in the treatment of subfoveal wet age-related macular degeneration in Canada. Clin Ther 29(9):2096–2106
Rosenfeld PJ, Brown DM, Heier JS et al (2006) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355(14):1419–1431
Heier JS, Antoszyk AN, Pavan P et al (2006) Ranibizumab for treatment of neovascular age-related macular degeneration – a phase I/II multicenter, controlled, multidose study. Ophthalmology 113(N4):633–642
Brown DM, Kaiser PK, Michels M et al (2006) Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 355(14):1432–1444
Nguyen QD, Shah SM, Hafiz G, Quinlan E, Sung J, Chu K, Cedarbaum JM, Campochiaro PA, CLEAR-AMD 1 Study Group (2006) A phase I trial of an IV-administered vascular endothelial growth factor trap for treatment in patients with choroidal neovascularization due to age-related macular degeneration. Ophthalmology 113(9):1522.e1–1522.e14
Singerman L (2009) Combination therapy using the small interfering RNA bevasiranib. Retina 29(6 Suppl):S49–S50
Lommatzsch A, Heimes B, Gutfleisch M, Spital G, Zeimer M, Pauleikhoff D (2009) Serious pigment epithelial detachment in age-related macular degeneration: comparison of different treatments. Eye 23(12):2163–2168
Maier MM, Feucht N, Fiore B, Winkler von Mohrenfels C, Kook P, Fegert C, Lohmann C (2009) Photodynamic therapy with verteporfin combined with intravitreal injection of ranibizumab for occult and classic CNV in AMD. Klin Monatsbl Augenheilkd 226(6):496–502
Vascular Endothelial Growth Factor (VEGF) (2012) Trap-eye: investigation of efficacy and safety in wet Age-Related Macular Degeneration (AMD) (VIEW 2). http://clinicaltrials.gov/show/NCT00637377. Accessed 13 Mar 2013
Vascular Endothelial Growth Factor (2012) VEGF trap-eye: investigation of efficacy and safety in wet Age-Related Macular Degeneration (AMD) (VIEW1). http://clinicaltrials.gov/show/NCT00509795. Accessed 13 Mar 2013
Nguyen QD et al (2012) Evaluation of very high- and very low-dose intravitreal aflibercept in patients with neovascular age-related macular degeneration. J Ocul Pharmacol Ther 28:581–588
Frampton JE (2012) Aflibercept for intravitreal injection: in neovascular age-related macular degeneration. Drugs Aging 29(10):839–846
Xu D, Kaiser PK (2013) Intravitreal aflibercept for neovascular age-related macular degeneration. Immunotherapy 5(2):121–130
Potter MJ, Claudio CC, Szabo SM (2010) A randomised trial of bevacizumab and reduced light dose photodynamic therapy in age-related macular degeneration: the VIA study. Br J Ophthalmol 94:174–179
Busch T (2009) Approaches toward combining photodynamic therapy with pharmaceuticals that alter vascular microenvironment. Retina 29(6 Suppl):S36–S38
Kaiser PK, Registry of Visudyne AMD Therapy Writing Committee, Boyer DS, Garcia R, Hao Y, Hughes MS, Jabbour NM, Kaiser PK, Mieler W, Slakter JS, Samuel M, Tolentino MJ, Roth D, Sheidow T, Strong HA (2009) Verteporfin photodynamic therapy combined with intravitreal bevacizumab for neovascular age-related macular degeneration. Ophthalmology 116(4):747–755
Yip PP, Woo CF, Tang HH, Ho CK (2009) Triple therapy for neovascular age-related macular degeneration using single-session photodynamic therapy combined with intravitreal bevacizumab and triamcinolone. Br J Ophthalmol 93(6):754–758
Takahashi H, Obata R, Tamaki Y (2006) A novel vascular endothelial growth factor receptor 2 inhibitor, SU11248, suppresses choroidal neovascularization in vivo. J Ocul Pharmacol Ther 22(4):213–218
Faivre S, Delbaldo C, Vera K et al (2006) Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol 24(1):25–35
Demetri GD, VanOosterom AT, Garrett CR et al (2006) Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumor after failure of imatinib: a randomised controlled trial. Lancet 368:1329–1338
Strumberg D, Richly H, Hilger RA et al (2005) Phase I clinical and pharmacokinetic study of the novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43–9006 in patients with advanced refractory solid tumors. J Clin Oncol 23(5):965–972
Awada A, Hendlisz A, Gil T et al (2005) Phase I safety and pharmacokinetics of BAY 43–9006 administered for 21 days on/7 days off in patients with advanced, refractory solid tumors. Br J Cancer 92(10):1855–1861
Escudier B, Eisen T, Stadler WM et al (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356(2):125–134
Ratain MJ, Eisen T, Stadler WM et al (2005) Final findings from a phase II, placebo-controlled, randomized discontinuation trial (RDT) of sorafenib (BAY 43–9006) in patients with advanced renal cell carcinoma (RCC) (4544). J Clin Oncol 23(16 suppl):388s
Traxler P, Allegrini PR, Brandt R et al (2004) AEE788: a dual family epidermal growth factor receptor/ErbB2 and vascular endothelial growth factor receptor tyrosine kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res 64:4931–4941
Rixe O, Bukowski RM, Michaelson MD et al (2007) Axitinib treatment in patients with cytokine-refractory metastatic renal-cell cancer: a phase II study. Lancet Oncol 8(11):975–984
Wedge SR, Kendrew J, Hennequin LF, Valentine PJ (2005) AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res 65(10):4389–4395
Drevs J, Siegert P, Medinger M et al (2007) Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signaling inhibitor, in patients with advanced solid tumors. J Clin Oncol 25(21):3045–3054
Ciardiello F, Caputo R, Damiano V et al (2003) Antitumor effects of ZD6474, a small molecule vascular endothelial growth factor receptor tyrosine kinase inhibitor, with additional activity against epidermal growth factor receptor tyrosine kinase. Clin Cancer Res 9:1546–1556
Miller KD, Trigo J, Wheeler C et al (2005) A multicenter phase II trial of ZD6474, a vascular endothelial growth factor receptor-2 and epidermal growth factor receptor tyrosine kinase inhibitor, in patients with previously treated metastatic breast cancer. Clin Cancer Res 11(9):3369–3376
Thomas AL, Trarbach T, Bartel C et al (2007) A phase IB, open-label dose-escalating study of the oral angiogenesis inhibitor PTK787/ZK 222584 (PTK/ZK), in combination with FOLFOX4 chemotherapy in patients with advanced colorectal cancer. Ann Oncol 18(4):782–788
Hutson TE, Davis ID, Machiels JP et al (2007) Pazopanib (GW786034) is active in metastatic renal cell carcinoma (RCC): interim results of a phase II randomized discontinuation trial (RDT). J Clin Oncol 25(18S):5031
Suttle AB, Hurwitz H, Dowlati A et al (2004) Pharmacokinetics (PK) and tolerability of GW786034, a VEGFR tyrosine kinase inhibitor, after daily oral administration to patients with solid tumors. J Clin Oncol 22:3054
Nakamura K, Taguchi E, Miura T et al (2006) KRN951, a highly potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, has antitumor activities and affects functional vascular properties. Cancer Res 66(18):9134–9142
Eskens FA, Planting A, Van Doorn L et al (2006) An open-label phase I dose escalation study of KRN951, a tyrosine kinase inhibitor of vascular endothelial growth factor receptor 2 and 1 in a 4 week on, 2 week off schedule in patients with advanced solid tumors. J Clin Oncol [supplement ASCO Annual Meeting] 24(18S):2034
Nosov DA, Esteves B, Lipatov ON, Lyulko AA, Anischenko AA, Chacko RT, Doval DC, Strahs A, Slichenmyer WJ, Bhargava P (2012) Antitumor activity and safety of tivozanib (AV-951) in a phase II randomized discontinuation trial in patients with renal cell carcinoma. J Clin Oncol 30(14):1678–1685
Rosen LS, Kurzrock R, Mulay M et al (2007) Safety, pharmacokinetics, and efficacy of AMG 706, an oral multikinase inhibitor, in patients with advanced solid tumors. J Clin Oncol 25(17):2369–2376
Polverino A, Coxon A, Starnes C et al (2006) AMG 706, an oral, multikinase inhibitor that selectively targets vascular endothelial growth factor, platelet-derived growth factor, and kit receptors, potently inhibits angiogenesis and induces regression in tumor xenografts. Cancer Res 66(17):8715–8721
Rosen R, Kurzrock E, Jackson L et al (2005) Safety and pharmacokinetics of AMG 706 in patients with advanced solid tumors. J Clin Oncol 23(16S):3013
Carter P (2001) Improving the efficacy of antibody-based cancer therapies. Nat Rev Cancer 1:118–129
Vedula SS, Krzystolik MG (2008) Antiangiogenic therapy with anti-vascular endothelial growth factor modalities for neovascular age-related macular degeneration. Cochrane Database Syst Rev Apr 16(2):CD005139
Costagliola C, Agnifili L, Arcidiacono B, Duse S, Fasanella V, Mastropasqua R, Verolino M, Semeraro F (2012) Systemic thromboembolic adverse events in patients treated with intravitreal anti-VEGF drugs for neovascular age-related macular degeneration. Expert Opin Biol Ther 12(10):1299–1313
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Mousa, S.A. (2013). Anti-VEGF Strategies in Ocular Angiogenesis-mediated Disorders, with Special Emphasis on Age-related Macular Degeneration. In: Mousa, S., Davis, P. (eds) Angiogenesis Modulations in Health and Disease. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6467-5_13
Download citation
DOI: https://doi.org/10.1007/978-94-007-6467-5_13
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-6466-8
Online ISBN: 978-94-007-6467-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)