Abstract
We have previously suggested that endogenous abscisic acid (ABA) may play a role of hormonal intermediate in the implementation of the salicylic acid (SA) induced protection of wheat plants against abiotic stress factors. With the use of an inhibitor of ABA biosynthesis fluridone there were obtained experimental arguments in favor of the key role of rapid reversible accumulation of ABA during the SA-treatment and maintaining elevated levels of ABA in SA-pretreated seedlings subjected to cadmium stress and salinity in the implementation of pre-adaptive and protective action of SA on wheat plants, respectively. Thus, it was detected that pretreatment of wheat seedlings with fluridone prevented SA-induced accumulation of ABA under normal conditions and maintenance under stress of increased ABA content in plants pre-treated with SA. This was manifested in inhibition of SA-induced effects: generation of ROS, activation of phenylalanine ammonia-lyase and antioxidant enzymes and deposition of lignin in the cell walls of roots, as well as the accumulation of wheat germ agglutinin, proline and enhanced transcription of TADHN gene coding for dehydrin that are making an important contribution to the development of plant resistance to oxidative stress and dehydration. In general, this is reflected in the prevention of SA-induced wheat resistance to the effects of toxic ions, as judged by the level of accumulation of MDA, release of electrolytes from the tissues and growth parameters of wheat seedlings. These data provide strong argument in favor of the likelihood of implementation of the endogenous ABA as a hormonal intermediate in triggering the defensive reactions under the influence of SA that form the basis for the development of SA-induced plant resistance to cadmium stress and sodium chloride salinity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Salicylic acid
- Abscisic acid
- Wheat germ agglutinin
- Dehydrin
- Proline
- Prooxidant-antioxidant balance
- Cadmium stress
- Salinity
- Triticum aestivum
1 Introduction
Salicylic acid (SA) being an endogenous regulator of growth and development of phenolic nature has gained great attention due to its practical importance for increasing plant resistance to stress and productivity. The great attention to SA was initiated by the discovery of its key role in induction of systemic acquired resistance (SAR), on the basis of which is the expression of SA-sensitive genes for PR- (pathogenesis-related) proteins (Metraux 2002; An and Mou 2011). The knowledge about SA signaling is still limited, which is due to the absence of information about its receptors although a range of SA binding proteins have been discovered: SABP2, having the greatest affinity to SA, chloroplast carbo-anhydrase, as well as catalase and cytoplasmic ascorbate peroxidase, which indicates the important role of H2O2 in the development of SA-induced SAR (Vlot et al. 2009). Information about other components particularly necessary for development of SAR is available due to the use of a set of mutants and transgenic plants (Bari and Jones 2009; An and Mou 2011). Transduction of SA-signal demands the presence of a regulatory protein NPR1 (non-expressor of PR genes1), also known as NIM1 or SAI1, which contains an ankyrin-repeat motif and a BTB/POZ domain, enabling protein–protein interactions (Vlot et al. 2009; An and Mou 2011). Interaction of nuclear localized NPR1 with the trans-factors of TGA family and structurally related NIMIN proteins that result in the transcription of genes of PR proteins sensitive to SA. The promoter region of the NPR1 gene also contains W-box sequences, which are binding sites of WRKY family protein, suggesting that WRKY transcription factors play an important role in mediating signaling between SA and NPR1 (An and Mou 2011). PR1 is the best studied gene containing in its promoter activation sequence-1 (as-1) motive necessary for binding of TGA-factors of transcription and gene expression and serving as a marker of SAR (An and Mou 2011).
At the same time SA participates in the regulation of different physiological processes in the course of plant ontogenesis under normal growth conditions including germination, flowering, leaf senescence, fruit ripening, thermogenesis, stomatal conductivity, ion transport, gravitropism (Raskin 1992; Shakirova 2001; Hayat et al. 2007, 2010; Vicente and Plasencia 2011). Moreover, a lot of information was obtained by now about participation of exogenous and endogenous SA in plant protection not only from biotic, but also from a wide range of abiotic stress factors (Shakirova and Bezrukova 1997; Yang et al. 2004; Liu et al. 2006; Janda et al. 2007; Shakirova 2007; Hayat et al. 2010; Gemes et al. 2011).
Previously it was shown by us that SA-treatment causes in wheat plants fast shifts in the state of hormonal system, associated with parallel reversible accumulation of abscisic acid (ABA) and indoleacetic acid (IAA) on the background of the absence of changes in cytokinin level. This allowed us to suggest that endogenous ABA may serve as a hormonal intermediate in the realization of SA-induced pre-adaptation of plant to the forthcoming stress (Shakirova et al. 2003; Shakirova 2007).
Increased synthesis and accumulation of ABA having frequently a transitory pattern may be characterized as the universal plant response to stressful impacts leading to disturbance of water relations (Xiong et al. 2002). ABA is known to play a key role in regulation of stomatal closure (Wilkinson and Davies 2010), resulting in a decline in transpiration and reduction of water loss. Stomatal closure is one of early plant responses to salinity caused by ABA-induced increase in Ca2+ concentration in cytoplasm, subsequent activation of ion channels in plasmalema and turgor losses by guard cells also linked with ABA-induced enhancement of H2O2 production serving as ABA signal intermediate in stomatal closure (Kim et al. 2010). At the same time ABA is involved in up-regulation of antioxidant enzyme genes and enhancement of corresponding enzyme activity (Xiong 2007) providing a protection against oxidative stress caused by conditions unfavourable for plant growth.
ABA is of pivotal importance for the induction of biosynthesis and accumulation of prolin, which functions as osmoprotectant participating in the stabilization of biopolymers and cell membranes and protection against injurious action of reactive oxygen species (ROS) (Yu et al. 2008; Szabados and Savoure 2009), as well as for production of many other ABA-induced components of plant protection (Rock et al. 2010). Among ABA-induced genes, important role belongs to those for dehydrins. Their massive accumulation is observed in plant seed embryos during their dehydration. However, sharp increase in expression of dehydrin genes and accumulation of their protein products is registered in vegetative plant tissues subjected to dehydration, dehydrins being the most abundant among stress proteins induced under these conditions (Close 1996; Hara 2010).
The gene coding for wheat germ agglutinin (WGA) also belongs to ABA-responsive genes (Skriver and Mundy 1990; Shakirova et al. 2001). WGA, being a typical representative of cereal lectins, is a constitutive wheat protein, whose presence in plant tissues increases significantly during ontogenesis. Thus, significant reversible increase in WGA content was observed in wheat plants in response to salinity, drought, osmotic stress and heat shock (Cammue et al. 1989; Shakirova et al. 1993, 1996; Singh et al. 2000; Shakirova and Bezrukova 2007). Data showing a decline in stress-induced oxidative damage in seedlings pretreated with WGA and an accelerated restoration of growth processes during the post-stress period in these plants confirm that WGA is an active participant in ABA-induced wheat resistance (Bezrukova et al. 2008). It is of interest that in the series of components of plant protection controlled by exogenous and endogenous ABA there are also those involved in the range of protective action of SA (Shakirova and Bezrukova 1997; Shakirova 2001, 2007; Shakirova et al. 2003; Fatkhutdinova et al. 2004; Rajjou et al. 2006; Hayat et al. 2010; Nazar et al. 2011). These data indicate in favor of possible implication of endogenous ABA as a hormonal intermediate in the regulation of realization of pre-adaptive and protective action of SA on plants.
2 Endogenous ABA in the Regulation of Pre-adaptive Effect of Salicylic Acid on Wheat Plants
In order to assess the regulatory role of ABA in SA-induced defense responses, we carried out experiments with the pre-treatment of wheat plants with ABA biosynthesis inhibitor fluridone effective in preventing stress-induced accumulation of ABA, when applied at concentration of 5 mg/l (Shakirova et al. 2009).
Figure 1 shows that pretreatment with fluridone in selected concentration completely prevented SA-induced accumulation of ABA in wheat seedlings. Consequently, ABA accumulated in SA-treated plants was new born indicating participation of SA in the control of de novo ABA synthesis in wheat plants.
In connection with this, it was of interest to estimate the importance of SA-induced accumulation of ABA in the regulation of protective reactions of wheat seedlings developing in plants in response to SA-treatment.
We have previously shown that SA-treatment itself leads to a significant accumulation of WGA and increased transcription of TADHN gene coding for dehydrin in wheat seedlings (Shakirova and Bezrukova 1997; Shakirova 2007), and in connection with this, it was of interest to conduct a comparative analysis of the impact of SA on the level of WGA and TADHN gene transcripts in wheat seedlings, untreated and pretreated with fluridone.
Figure 2 shows that treatment with SA causes an accumulation of WGA and TADHN dehydrin transcripts in seedlings, which is preceded by a rapid transient accumulation of ABA, whereas pretreatment of seedlings with fluridone completely prevents the stimulatory effect of SA on these processes. The results evidence in favor of an important role of SA-induced synthesis of ABA in triggering defense reactions, which may contribute to the development of the pre-adaptive effect of SA to further stresses and reducing their damaging effects on wheat plant.
Deposition of lignin in the root cell walls resulting in strengthening of their barrier functions is known to contribute significantly to the development of plant resistance to the stress factors leading to dehydration (Moura et al. 2010). Biosynthesis of this biopolymer is implemented with the help of H2O2, as well as enzymes phenylalanine ammonia-lyase (PAL) and peroxidase, ABA and SA being involved in the control of expression of their genes and enzyme activity (Thulke and Conrath 1998; Hiraga et al. 2001; Fatkhutdinova et al. 2004; Fernandes et al. 2006; Chen et al. 2006; Moura et al. 2010). In connection with this it was of interest to compare the effect of SA on dynamics of concentration of H2O2 and anionic peroxidase isoform (pI ~ 3.5), total activity of peroxidase and PAL as well as that of lignin deposition in the cell walls of the central cylinder of the basal part of roots of wheat seedlings and to estimate the role of ABA in the control of these processes.
The treatment with SA itself have been discovered by us earlier to result not only in the transitory accumulation of ABA, but in enhancement of O2 − and H2O2 in wheat seedlings, accompanied by activation of SOD and peroxidase, which was beneficial for plants as judged from the growth promoting effect of 50 μM SA (Fatkhutdinova et al. 2004; Shakirova 2007). In connection with this, it was important to carry out further a detailed analysis of the effect of SA on H2O2 and anionic isoperoxidase concentration and dynamics of total peroxidase and PAL enzyme activity in the seedlings untreated and pretreated with fluridone for 24 h under normal conditions for plant growth.
Figure 3 shows that pre-treatment of wheat seedlings with fluridone completely inhibited the enhancement of H2O2 production, anionic isoperoxidase accumulation, peroxidase and PAL activity exerted by SA alone. These results demonstrate that under normal conditions balanced increase of H2O2 and activation of peroxidase and PAL in wheat plants in response to SA-treatment is due to SA-induced de novo ABA production.
The obtained data indicate the key role of ABA in regulation of H2O2 production and confirm that under different external influences leading to ABA accumulation pretreatment of plants with inhibitors of ABA synthesis inhibits both H2O2 production and activation of the antioxidant defense system (Jiang and Zhang 2002; Ye et al. 2011).
We further carried out the analysis of dynamics of lignin deposition in the central cylinder of the basal part of the control and treated wheat plants (Table 1). It shows that treatment of seedlings with SA for 24 h contributes to acceleration of lignification in the cell walls of root xylem vessels as compared to the control: staining of cell walls with phloroglucinol was clearly revealed in the roots of 5-d-old seedlings, while in 6-d-old seedlings it was additionally enhanced. At the same time controlled lignin deposition in the cell walls was discovered later on, starting from the 6th day (Table 1).
Pretreatment of 3-day-old seedlings with fluridone for 24 h inhibited deposition of lignin in cell wall of roots not only in 5-day-old but also in 6-day-old seedlings (Table 1). Consequently, preventing SA-induced accumulation of endogenous ABA by the pretreatment with fluridone inhibited the increase in H2O2 and anionic peroxidase level and activation of PAL and peroxidase being the key enzymes in lignin biosynthesis. As a consequence, lignin deposition in the cell walls of the root central cylinder was inhibited. Those results convincingly indicate the regulatory role of endogenous ABA in acceleration of lignin deposition in the cell walls of roots of SA-treated seedlings as also evident from the data showing a complete recovery of this process during the subsequent action of exogenous ABA for 24 h on seedlings pretreated with fluridone and SA (Table 1).
The sum of obtained data indicate the important role of SA-induced synthesis of ABA in the regulation of SA-induced activation of the key components of lignin biosynthesis significantly contributing to the strengthening of barrier properties of root cell walls and pre-adaptation of plants to possible forthcoming action of environmental stress factors.
3 Endogenous ABA in SA-Induced Activation of Defensive Reactions in Wheat Seedlings Subjected to the Influence of Toxic Ions
Frequently changing environment demands plant adaptation to the conditions of their growth, which implies the development of a complex network of protective reactions aimed on the struggle for survival under the stressful environment. Based on this there is the integration of the effective systems of regulation of cell metabolic activity for switching genetic programs from norm to stress aimed on development of an adequate protection at the level of whole organism. Drought, disturbance of temperature regime and salinity are most important environmental factors, which are critical for survival of plants and lead to significant losses of crop yield. Responses to these stresses are known to be interconnected, employing common signaling pathways, which allow cell adaptation and lead to similar changes in plants on morphological, physiological, biochemical and molecular/or genetic levels (Verslues et al. 2006; Shinozaki and Yamaguchi-Shinozaki 2007; Potters et al. 2009; Arbona et al. 2010; Des Marais and Juenger 2010). Due to the increasing anthropogenic pollution of soils increasing attention is paid to the study of the effects of excess concentrations of heavy metals (HM) on the plants, which are also manifested in the disturbance of the processes of their growth and development (Pál et al. 2006; DalCorso et al. 2008; Yadav 2010).
Survival of plants themselves is known to be due to their ability to struggle against extreme environment, protecting their vital potential. However, realization of natural protective mechanisms taking place in plants, when conditions become worse, is well known to be accompanied by a decline in their productivity and quality. This raises important question about regulation of stress resistance. The problem of stress-resistance is most important for plant breeding and is under a steadfast attention of researchers all over the world. This is indeed the case, since information concerning the chain of reactions taking place in plants in response to extreme external conditions may really contribute to an increase in plant resistance and productivity. These goals are achieved not only by means of selection of stress tolerant cultivars, but also through the purposeful manipulation of adaptation with the help of natural plant growth regulators. To those, in particular, can salicylic acid be attributed.
3.1 Cadmium Stress
Due to the progressive contamination of soils with salts of toxic heavy metal (HM), causing not only a decrease in yield, but also the deterioration of soil quality (Pál et al. 2006; DalCorso et al. 2008). The investigation of the molecular mechanisms of plant resistance to HM becomes a serious goal. Cadmium can be attributed to the most toxic of HM, because it is not a necessary element for the normal functioning of plants and does not perform any physiological function in plants (DalCorso et al. 2008).
The reactions of plants in response to cadmium, mainly, are nonspecific and are similar to those of the effects of other HM. The most characteristic of them could be the accumulation of ABA, stomatal closure, the inhibition of uptake and transport of water, the inhibition of chlorophyll synthesis and photosynthesis, the imbalance of pro- and antioxidant components and disturbance of integrity of the cell membrane structure, which in general is reflected in a delay in plant growth and development and reduction of its productivity (Hsu and Kao 2005; Pál et al. 2006; DalCorso et al. 2008; Yadav 2010).
In recent years, special attention is paid to the study of molecular mechanisms of plant resistance to cadmium and other HM as well as of the ways of their regulation. Thus, transcriptomic and proteomic analysis allowed to identify a wide range of proteins involved in the responses of plants to cadmium. Among them an important place is occupied by the proteins associated with antioxidant protection and detoxification of ROS (Fusco et al. 2005; Ahsan et al. 2009; Wang et al. 2011). In addition, there has also been identified proteins involved in phytohormone signaling including salicylic acid (Ahsan et al. 2009), which indicates the involvement of this hormone in the regulation of plant resistance to HM. Since exposure to cadmium causes a disturbance of water relations, it is not surprising that under these conditions there has been detected the activation of the transcription of ABA sensitive genes (Fusco et al. 2005), playing, as is known, a key role in the regulation of plant defense responses during dehydration. Those, in particular, include genes coding for proteins dehydrins and lectin wheat germ agglutinin, whose synthesis and accumulation increases sharply under disturbance of the water regime, including the effects of HM (Hara 2010; Bezrukova et al. 2011).
To date, a lot of information accumulated, indicating the effectiveness of application of salicylic acid for reduction of the toxic effects of cadmium on different crops (Metwally et al. 2003; Janda et al. 2007; Meng et al. 2009; Hayat et al. 2010). Thus, it is shown that pretreatment with SA helps to prevent cadmium-induced shifts in the composition of fatty acids and in the integrity of membrane structures (Ivanova et al. 2008; Krantev et al. 2008), as well as to reduce the extent of the inhibitory effect of cadmium on the activity of key photosynthetic enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase and phosphoenol pyruvate carboxylase and on photosynthesis, in general (Krantev et al. 2008; Zhang and Chen 2011), and to reduce the level of reactive oxygen species and lipid peroxidation (Choudhury and Panda 2004; Zhang and Chen 2011). This, in general, is reflected in the maintenance of plant growth processes under these conditions at a level close the control (Hayat et al. 2010). In addition, there is evidence of the ability of SA to influence the lignin deposition in the cell walls of roots, making an important contribution to improve their barrier properties and to protection of plants from toxic effects of cadmium (Kováčik and Klejdus 2008).
Visual manifestation of the toxic effect of cadmium on plants is the inhibition of growth processes. Indeed, exposure to 1 mM cadmium acetate has a very pronounced inhibitory effect on wheat seedlings, as evidenced by the inhibition of mitotic activity of cells of root tips and root relative growth rate (RGR) (Fig. 4a, b). Shoots also experience negative effects of cadmium, but this is much less than in roots as suggested by the data on RGR (Fig. 5).
These results are in accordance with the observations that roots suffer from the toxic effects of cadmium to a greater extent, since it is in the roots it accumulates in larger quantities (Seregin and Ivanov 1997; Bezrukova et al. 2011). Comparison of the growth of SA-pretreated plants with/without cadmium showed that the pretreatment does not prevent, but significantly reduces the extent of the negative effects of stress on the seedlings, and helps to maintain the activity of the growth processes of these plants, at least at the level of control (Figs. 4, 5). Thus, pretreatment with SA has a clear protective effect on the growth of wheat plants under cadmium stress.
It is known that plant growth is controlled by hormonal system, responsive to environmental changes (Wang et al. 2008; Shakirova et al. 2010), and a simultaneous analysis of different concentrations of phytohormones in the same plants provides a comprehensive picture of the stress-induced rearrangements in a state of hormonal system.
The results of analysis of changes in the balance of ABA, IAA and cytokinins in the wheat seedlings, pretreated and untreated with SA and exposed to cadmium acetate, are shown in Fig. 6. As can be seen, cadmium causes dramatic changes in the hormonal balance of seedlings, as can be evidenced by the decrease in the ratio of IAA and cytokinins to ABA associated with a sharp reversible accumulation of ABA and persistent fall in the concentration of cytokinins and IAA in particular, that in general is reflected in the inhibition of growth of these plants (Figs. 4, 5).
Pretreatment with SA did not prevent, but significantly reduced the amplitude of the cadmium induced changes in the concentration of ABA, auxin and cytokinins. Moreover, 5 h after the start of the experiment the ratios of IAA/ABA and cytokinins/ABA in SA-pretreated seedlings completely recovered (Fig. 6), which is reflected in the maintenance of the activity of growth processes in these plants at the control level (Figs. 4, 5).
Furthermore it was important to compare the effects of pre-treating the plants with SA and a mixture of SA and fluridone on ABA content in the seedlings exposed to cadmium stress.
Figure 7 shows that incubation of seedlings in cadmium led to a rapid reversible accumulation of ABA in wheat seedling untreated with SA, which is not surprising, since this response is a typical stress reaction (Shakirova et al. 2010). SA-pretreated seedlings were characterized by visibly lower level of cadmium-induced accumulation of ABA, which may be an indicator of lower degree of the damaging stress effect in these plants due to pre-adaptive action of SA in the course of pretreatment. At the same time pretreatment of seedlings initially with fluridone and then with a mixture of fluridone and SA during 24 h completely prevents cadmium-induced accumulation ABA.
Figure 8a and b shows that the effect of cadmium acetate leads to a transient increase in WGA content and level of transcription of TADHN gene in the seedlings, which is also not surprising, since the literature contains information on the involvement of wheat lectin and dehydrins in the responses of plants not only to drought, salinity, hypothermia, and also to HM (Shakirova et al. 1993, 2009; Hara 2010; Tamás et al. 2010; Bezrukova et al. 2011).
At the same time plants pretreated with SA within 24 h are characterized by a lower level of accumulation of the lectin and transcripts of TADHN gene coding for wheat dehydrin, indicating a less damaging effect of cadmium on them, in contrast to SA-untreated plants (Fig. 8). Co-pretreatment with fluridone and SA although did not prevent, but sharply reduced the stress-induced accumulation of WGA and the up-regulation of dehydrin gene transcription (Fig. 8a, b). These results are the indication in favor of the key role of endogenous ABA in the regulation of these protective components in the plants pretreated with SA. However, alongside with the ABA-dependent signaling pathway there was revealed the presence of ABA-independent pathways of regulation of protective reactions, which is in agreement with literature data (Verslues and Bray 2006; Shinozaki and Yamaguchi-Shinozaki 2007). Nevertheless, growth results showed (Figs. 4, 5) that these ABA-independent responses were not sufficient for the manifestation of defense related effects of SA in the presence of fluridone on cadmium-treated wheat plants.
The obtained results indicate in favor of an important role of maintaining increased concentration of endogenous ABA in SA-pretreated seedlings in the regulation of the content of WGA and the level of transcription of TADHN gene, contributing to the development of plant resistance to cadmium stress.
It is known that exposure to toxic cadmium ions leads to the overproduction of reactive oxygen species and increased lipid peroxidation, the intensity of which can be judged by the level of accumulation of malondialdehyde (MDA) (Hsu and Kao 2007; Wang et al. 2011). The data presented in Fig. 9 shows that cadmium causes a sharp increase in the concentration of MDA in comparison with the control, whereas in the plants pretreated with SA this characteristic is much lower. This is probably due to the ability of SA to induce, during its pretreatment, the transient activation of antioxidant enzymes in wheat seedlings (Fig. 3) involved in neutralization of oxidative stress-induced burst.
An additional argument in support of involvement of ABA in the regulation of SA-induced protective reactions reducing the damaging effect of cadmium stress on wheat plants is in the data showing significant decline in lignin accumulation in the cell walls of the basal part of seedling roots pretreated with the mixture of fluridone with SA (Table 2). This is likely to be due to prevention by fluridone of the SA-induced production of H2O2 and activation of the key enzymes of lignin biosynthesis (PAL and peroxidase) being under the control of ABA (Moura et al. 2010).
Since, as noted above, pretreatment with SA reduced the extent of the damaging effect of cadmium on the growth processes of wheat seedlings, one would expect that the manifestation of the protective effect of SA on the plants under cadmium stress is due to the increased barrier properties of the cell walls of roots bringing about the inhibition of the entry of toxic ions into the root tissues and their subsequent delivery to the shoot.
Thus, the histochemical analysis with the help of dithizone reagent (Seregin and Ivanov 1997) revealed the presence of cadmium in all tissues of the transverse sections of roots of 5 days-old wheat seedlings untreated with SA (Table 3). At the same time in the experiments with SA-pre-treatment cadmium was detected only in rhizoderm and outer layers of the primary cortex (Table 3). These data demonstrate the important contribution of SA-induced acceleration of lignin deposition in the basal part of seedlings roots and additional intensification of the process under stress to enhancement of the barrier properties of cell walls and consequent inhibition of the entry of toxic ions into the internal tissues of roots.
In a joint pretreatment of wheat seedlings with SA and fluridone there was observed an inhibition of cell wall lignification (Tables 1, 2), resulting in the prevention of SA-induced inhibition of cadmium entrance into root tissues (Table 3), which, in turn, indicates the involvement of endogenous ABA in the regulation of this protective mechanism by salicylic acid.
Therefore, for the first time inhibition analysis allowed to obtain experimental evidence indicating in favor of the key role of endogenous ABA in the regulation of SA-induced protective mechanisms making an important contribution to the development of resistance of wheat seedlings exposed to the toxic ions of cadmium.
3.2 Sodium Chloride Salinity
Salinity caused by increased content of soluble salts in soil is one of the most widely spread abiotic stress factors resulting in significant inhibition of plant growth and decline in crop productivity, with sodium chloride being the most detrimental (Munns and Tester 2008; Cambrolle et al. 2011).
The decline in cell growth processes is due to dehydration resulting from osmotic effect of salts accumulating in the root zone and due to toxic effect of sodium and chloride accumulation in the plant tissues causing great damaging effect on the most important physiological processes and cell membrane integrity (Munns and Tester 2008; Nazar et al. 2011). It is necessary to emphasize that plants are able to develop a broad spectrum of protective reactions aimed on diminishing the detrimental effects of salinity (Flowers 2004; Munns and Tester 2008). Stress hormone ABA, whose fast and significant accumulation is a characteristic response to salinity, makes an important contribution to protective reactions (Rock et al. 2010; Shakirova et al. 2010). At the same time attention is drawn to the similarity in the several of the plant responses to sodium chloride salinity and cadmium stress.
We have previously reported that pretreatment with SA did not prevent, but significantly reduced the salinity-induced transient accumulation of ABA in wheat plants, suggesting that the maintenance of increased content of ABA in SA-pretreated plants plays an important regulatory role in the manifestation of the protective effect of SA (Shakirova 2007). To estimate the importance of maintaining increased level of endogenous ABA in the manifestation of the protective effect of SA on wheat seedlings under salt stress there was also used fluridone in the experiments. Like cadmium, salinity stress also caused a rapid reversible accumulation of ABA in untreated plants, whereas in pre-treated with SA seedlings increase of ABA level was much lower. This is reflected in the fact that SA-pretreated wheat seedlings are characterized by lower levels of stress-induced accumulation of WGA (Fig. 10a), as well as of osmoprotectant proline (Fig. 10b), which make an important contribution to the protection of plants against oxidative burst caused by salinity (Bezrukova et al. 2008; Hara 2010). The pretreatment of seedlings initially with fluridone and then with a mixture of fluridone and SA completely prevented the SA-induced increase in the level of ABA under salt-stress conditions.
At the same time, attention is drawn to the fact that the joint fluridone pretreatment with SA although greatly reduced, but not completely prevented the increase in WGA and proline content in seedlings subjected to salinity (Fig. 10). Similar results were obtained during the study of the activity of antioxidant enzymes. Salinity induces a fast increase initially in activity of SOD followed by peroxidase in wheat seedlings (Fig. 11), which is a typical plant response to stress-induced enhancement of ROS production (Jaspers and Kangasjarvi 2010). Stressed plants pretreated with SA are characterized with a significantly lower activity of antioxidant enzymes as compared to untreated plants (Fig. 11), which is likely to be due to the lower production of ROS in the plants (Shakirova 2007). This is associated with the fact that SA-treatment itself causes a balanced increase in production of ROS and activity of antioxidant enzymes (Fig. 3), sufficient for effective neutralization of salt-induced oxidative burst (Shakirova 2007).
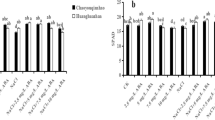
These results support the belief of a key role of endogenous ABA in the regulation of investigated protective components in the plants pretreated with SA. This notion is supported by 50 % decline in the level of MDA in comparison with the plants untreated with SA under salt stress (Fig. 12a).
Similar to cadmium stress the presence of fluridone in the medium for incubation of the seedlings did not prevent, but significantly reduced the SA-induced activation of antioxidant enzymes under conditions of sodium chloride salinity (Fig. 11). This was reflected in that in the variant of treatment with the mixture of fluridone and SA, the plants were characterized by the same level of MDA accumulation as in the salt-stressed plants untreated with SA (Fig. 12a).
Pretreatment with SA significantly decreased the degree of the negative stress effect on the seedlings, while the treatment with a mixture of SA with fluridone prevented the protective action of SA on growth (Fig. 12b) indicating the important role of endogenous ABA in realization of SA protective action in stressed plants. This suggests inhibition of ABA-mediated protective reactions and consequent prevention of protective action of SA on plants under salinity.
The obtained data illustrate the importance of ABA in realization of pre-adaptive effect of SA on wheat plants manifested in maintaining enzyme activity of SOD and peroxidase in SA-pretreated salt-stressed plants at the level sufficient for their protective effect and in significant decline in the level of the damaging effect of salinity on the integrity of cell membranes and their permeability in SA-pretreated plants under salinity.
It can be assumed that the SA-induced enhancement of the barrier properties of cell walls of root and the inhibition of the penetration of toxic ions into plant tissues play an important role in the manifestation of the protective effect of SA on wheat seedlings under sodium chloride salinity, as well as under cadmium stress.
Thus pretreatment with SA reduced the extent of the damaging effect of salinity on the growth of wheat seedlings, which is largely due to the ability of SA to cause reversible accumulation of ABA during the treatment with SA and to maintain the increased content of ABA in plants under salt-stress.
4 Conclusion
Based on our earlier data, we assigned an important role of SA-induced reversible accumulation of ABA in the development of SA pre-adaptive effect on plants and the importance of maintaining increased content of ABA in SA-pretreated plants in development of resistance to abiotic stresses, it was suggested that endogenous ABA may serve as an intermediate in the implementation of the protective action of SA on wheat plants.
Using an effective inhibitor of ABA biosynthesis fluridone we have obtained for the first time experimental evidence for the key role of SA-induced rapid transient accumulation of endogenous ABA in the regulation of SA pre-adaptive effect on wheat plants exposed to subsequent stressors of abiotic nature. It was found that, preventing ABA accumulation, by pretreatment with fluridone completely inhibits SA-induced accumulation of WGA, which is a typical representative of cereal lectins and a component of ABA-controlled responses of wheat to drought, salinity, hypo- and hyperthermia, cadmium ions, making an important contribution to the protection of plants from the damaging effect of osmotic and oxidative stress induced by these adverse environmental factors. Important role in protecting cellular structures from osmotic and oxidative stress is performed by dehydrins, belonging to the group of 2 LEA proteins, which are just as WGA responsive to abscisic acid (RAB) proteins, having also chelating properties. We found that the presence of fluridone in the medium for incubation of the seedling prevents SA-induced reversible enhancement of transcription TADHN dehydrin gene. It was shown that pretreatment with fluridone prevents the SA-induced rapid production of H2O2, activation of peroxidase and PAL, involved in the formation of lignin, which is reflected in the inhibition of SA-induced deposition of lignin in the cell walls of the central cylinder of the basal part of roots. Moreover, treatment of these plants with ABA restores completely the intensity of the deposition of lignin in the cell walls of roots.
Pretreatment with SA substantially reduces the damaging effects of cadmium stress and sodium chloride salinity on growth processes of wheat seedlings. This seems to be primarily due to the fact that the seedlings pretreated with SA and subjected to stress factors, are characterized by lower amplitude of imbalance of ABA, IAA and cytokinins, which could be due to the triggering in the course of pretreatment with SA (prior to stress) of the various protective mechanisms forming the basis of its pre-adaptive effect on plants, in particular, the activation of the systems osmoprotection and antioxidant defense, including the accumulation of WGA, and TADHN transcripts. In this regard, it is not surprising that under saline conditions or when exposed to toxic cadmium ions stress-induced activation of these important components of protection in the SA-pretreated seedlings occurs at notably lower level, which in general is reflected in a decrease in the levels of MDA and electrolyte leakage from plant tissues, as well as in maintaining the growth of these plants, at least at the level of control. It should be noted that the seedlings pre-treated with SA, in contrast to untreated with SA, are characterized by an additional acceleration of deposition of lignin in the cell walls of roots, contributing to the strengthening of their barrier properties, and inhibition of the entry of toxic ions into the internal tissues of roots, which is clearly demonstrated in particular for cadmium by histochemical methods using dithizone reagent.
The important role of maintaining high concentration of ABA in the development of the resistance of SA-pretreated wheat seedlings to cadmium and salinity was manifested in a significant inhibition of all studied defense responses in seedlings pretreated with SA in combination with fluridone. Despite the fact that fluridon completely prevents maintenance of elevated ABA content in plants pretreated with SA, attention is drawn to the fact of existence of alternative ABA-independent pathways of regulation of stress-induced activation of protective mechanisms, although, as can be seen in terms of growth, MDA level and release of electrolytes from the tissues, this was not sufficient for effective development of SA-induced wheat plant resistance.
Thus, the summary of the data presented in this chapter, obtained by using an inhibitor of ABA synthesis, fluridone, clearly demonstrates the key role of endogenous ABA in the implementation of pre-adaptation and protective action of SA on wheat plants and the likelihood of implementation of ABA as a hormonal intermediate in triggering the complex defensive reactions forming the basis for the development of SA-induced resistance of plants to abiotic stress factors.
References
Ahsan, N., Renaut, J., & Komatsu, S. (2009). Recent developments in the application of proteomics to the analysis of plant responses to heavy metals. Proteomics, 9, 2602–2621.
An, C., & Mou, Z. (2011). Salicylic acid and its function in plant immunity. Journal of Integrative Plant Biology, 53, 412–428.
Arbona, V., Argamasilla, R., & Gomez-Cadenas, A. (2010). Common and divergent physiological, hormonal and metabolic responses of Arabidopsis thaliana and Thellungiella halophila to water and salt stress. Journal of Plant Physiology, 167, 1342–1350.
Bari, R., & Jones, J. D. (2009). Role of plant hormones in plant defence responses. Plant Molecular Biology, 69, 473–488.
Bezrukova, M., Kildibekova, A., & Shakirova, F. (2008). WGA reduces the level of oxidative stress in wheat seedlings under salinity. Plant Growth Regulation, 54, 195–201.
Bezrukova, M. V., Fatkhutdinova, R. A., Lubyanova, A. R., Mursabaev, A. R., Fedyaev, V. V., & Shakirova, F. M. (2011). Lectin involvement in the development of wheat tolerance to cadmium toxicity. Russian Journal of Plant Physiology, 58, 1048–1054.
Cambrolle, J., Redondo-Gomez, S., Mateos-Naranjo, E., Luque, T., & Figueroa, M. E. (2011). Physiological responses to salinity in the yellow-horned poppy, Glaucium flavum. Plant Physiology and Biochemistry, 49, 186–194.
Cammue, B. P. A., Broekaert, W. F., Kellens, J. T. C., Raikhel, N. V., & Peumans, W. J. (1989). Stress-induced accumulation of wheat germ agglutinin and abscisic acid in roots of wheat seedlings. Plant Physiology, 91, 1432–1435.
Chen, J.-Y., Wen, P.-F., Kong, W.-F., Pan, Q.-H., Zhan, J.-C., Li, J.-M., et al. (2006). Effect of salicylic acid on phenylpropanoids and phenylalanine ammonia-lyase inharvested grape berries. Postharvest Biology and Technology, 40, 64–72.
Choudhury, S., & Panda, S. K. (2004). Role of salicylic acid in regulating cadmium induced oxidative stress in Oryza sativa L. roots. Bulgarian Journal of Plant Physiology, 30, 95–110.
Close, T. J. (1996). Dehydrins: emergence of a biochemical role of a family of plant dehydration proteins. Physiologia Plantarum, 96, 795–803.
DalCorso, G., Farinati, S., Maistri, S., & Furini, A. (2008). How plants cope with cadmium: staking all on metabolism and gene expression. Journal of Integrative Plant Biology, 50, 1268–1280.
Des Marais, D. L., & Juenger, T. E. (2010). Pleiotropy, plasticity, and the evolution of plant abiotic stress tolerance. Annals of the New York Academy of Sciences, 1206, 56–79.
Fatkhutdinova, D. R., Sakhabutdinova, A. R., Maksimov, I. V., Yarullina, L. G., & Shakirova, F. M. (2004). The effect of salicylic acid on antioxidant enzymes in wheat seedlings. Agrochimiya (in Russ), 8, 27–31.
Fernandes, C. F., Moraes, V. C. P., Vasconcelos, I. M., Silveira, J. A. G., & Oliveira, J. T. A. (2006). Induction of an anionic peroxidase in cowpea leaves by exogenous salicylic acid. Journal of Plant Physiology, 163, 1040–1048.
Flowers, T. J. (2004). Improving crop salt tolerance. Journal of Experimental Botany, 55, 307–319.
Fusco, N., Micheletto, L., Dal Corso, G., Borgato, L., & Furini, A. (2005). Identification of cadmium-regulated genes by cDNA-AFLP in the heavy metal accumulator Brassica juncea L. Journal of Experimental Botany, 56, 3017–3027.
Gemes, K., Poor, P., Horvath, E., Kolbert, Z., Szopko, D., Szepesi, A., et al. (2011). Cross-talk between salicylic acid and NaCl-generated reactive oxygen species and nitric oxide in tomato during acclimation to high salinity. Physiologia Plantarum, 142, 179–192.
Hara, M. (2010). The multifunctionality of dehydrins. Plant Signaling & Behavior, 5, 503–508.
Hayat, S., Ali, B., & Ahmad, A. (2007). Salicylic acid: biosynthesis, metabolism and physiological role in plants. In S. Hayat & A. Ahmad (Eds.), Salicylic acid—a plant hormone. The Netherlands: Springer.
Hayat, Q., Hayat, S., Irfan, M., & Ahmad, A. (2010). Effect of exogenous salicylic acid under changing environment: a review. Environmental and Experimental Botany, 68, 14–25.
Hiraga, S., Sasaki, K., Ito, H., Ohashi, Y., & Matsui, H. (2001). A large family of class III plant peroxidases. Plant and Cell Physiology, 42, 462–468.
Hsu, Y. T., & Kao, C. H. (2005). Abscisic acid accumulation and cadmium tolerance in rice seedlings. Physiologia Plantarum, 124, 71–80.
Hsu, Y. T., & Kao, C. H. (2007). Toxicity in leaves of rice exposed to cadmium is due to hydrogen peroxide accumulation. Plant and Soil, 298, 231–241.
Ivanova, A., Krantev, A., Stoynova, Zh., & Popova, L. (2008). Cadmium-induced changes in maize leaves and the protective role of salicylic acid. General and Applied Plant Physiology, 34, 149–158.
Janda, T., Horváth, E., Szalai, G., & Páldi, E. (2007). Role of salicylic acid in the induction of abiotic stress tolerance. In S. Hayat & A. Ahmad (Eds.), Salicylic acid—a plant hormone. The Netherlands: Springer.
Jaspers, P., & Kangasjarvi, J. (2010). Reactive oxygen species in abiotic stress signaling. Physiologia Plantarum, 138, 405–413.
Jiang, M., & Zhang, J. (2002). Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. Journal of Experimental Botany, 53, 2401–2410.
Kim, T.-H., Bohmer, M., Hu, H., Nishimura, N., & Schroeder, J. I. (2010). Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annual Review of Plant Biology, 61, 561–591.
Kováčik, J., & Klejdus, B. (2008). Dynamics of phenolic acids and lignin accumulation in metal-treated Matricaria chamomilla roots. Plant Cell Reports, 27, 605–615.
Krantev, A., Yordanova, R., Janda, T., Szalai, G., & Popova, L. (2008). Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. Journal of Plant Physiology, 165, 920–931.
Liu, H.-T., Liu, Y.-Y., Pan, Q.-H., Yang, H.-R., Zhan, J.-C., & Huang, W.-D. (2006). Novel interrelationship between salicylic acid, abscisic acid, and PIP-specific phospholipase C in heat acclimation-induced thermotolerance in pea leaves. Journal of Experimental Botany, 57, 3337–3347.
Meng, H., Hua, S., Shamsi, I. H., Jilani, G., Li, Y., & Jiang, L. (2009). Cadmium-induced stress on the seed germination and seedling growth of Brassica napus L., and its alleviation through exogenous plant growth regulators. Plant Growth Regulation, 58, 47–59.
Metraux, J. P. (2002). Recent breakthroughs in study of salicylic acid biosynthesis. Trends in Plant Science, 7, 331–334.
Metwally, A., Finkemeier, I., Georgi, M., & Dietz, K.-J. (2003). Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiology, 132, 272–281.
Moura, J. C. M. S., Bonine, C. A. V., Viana, J. O. F., Dornelas, M. C., & Mazzafera, P. (2010). Abiotic and biotic stresses and changes in the lignin content and composition in plants. Journal of Integrative Plant Biology, 52, 360–376.
Munns, R., & Tester, M. (2008). Mechanisms of salinity tolerance. Annual Review of Plant Biology, 59, 651–681.
Nazar, R., Iqbal, N., Syeed, S., & Khan, N. A. (2011). Salicylic acid alleviates decreases in photosynthesis under salt stress by enhancing nitrogen and sulfur assimilation and antioxidant metabolism differentially in two mungbean cultivars. Journal of Plant Physiology, 168, 807–815.
Pál, M., Horváth, E., Janda, T., Páldi, E., & Szalai, G. (2006). Physiological changes and defense mechanisms induced by cadmium stress in maize. Journal of Plant Nutrition and Soil Science, 169, 239–246.
Potters, G., Pasternak, T., Guisez, Y., & Jansen, M. A. K. (2009). Different stresses, similar morphogenic responses: integrating a plethora of pathways. Plant, Cell and Environment, 32, 158–169.
Rajjou, L., Belghazi, M., Huguet, R., Robin, C., Moreau, A., Job, C., et al. (2006). Proteomic investigation of the effect of salicylic acid on Arabidopsis seed germination and establishment of early defense mechanisms. Plant Physiology, 141, 910–923.
Raskin, I. (1992). Role of salicylic acid in plants. Annual Review of Plant Physiology. Plant Molecular Biology, 43, 439–463.
Rock, C. D., Sakata, Y., & Quatrano, R. S. (2010). Stress signaling I: the role of abscisic acid (ABA). In: A. Pareek, S. A. Sopory, H. J. Bohner & Govindjee (Eds.) Abiotic stress adaptation in plants: physiological, molecular and genomic foundation. Dordrecht: Springer.
Seregin, I. V., & Ivanov, V. B. (1997). Histochemical investigation of cadmium and lead distribution in plants. Russian Journal of Plant Physiology, 44, 791–796.
Shakirova, F. M. (2001). Nonspecific resistance of plants to stress factors and its regulation. Ufa: Gilem.
Shakirova, F. M. (2007). Role of hormonal system in manifestation of growth promoting and antistress action of salicylic acid. In S. Hayat & A. Ahmad (Eds.), Salicylic acid—a plant hormone. The Netherlands: Springer.
Shakirova, F. M., Avalbaev, A. M., Bezrukova, M. V., & Gimalov, F. R. (2001). Induction of wheat germ agglutinin synthesis by abscisic and gibberellic acids in roots of wheat seedlings. Plant Growth Regulation, 33, 111–115.
Shakirova, F. M., Allagulova, Ch. R., Bezrukova, M. V., Avalbaev, A. M., & Gimalov, F. R. (2009). The role of endogenous ABA in cold-induced expression of the TADHN dehydrin gene in wheat seedlings. Russian Journal of Plant Physiology, 56, 720–723.
Shakirova, F. M., Avalbaev, A. M., Bezrukova, M. V., & Kudoyarova, G. R. (2010). Role of endogenous hormonal system in the realization of the antistress action of plant growth regulators on plants. Plant Stress, 4, 32–38.
Shakirova, F. M., & Bezrukova, M. V. (1997). Induction of wheat resistance against environmental salinization by salicylic acid. Biological Bulletin, 24, 109–112.
Shakirova, F. M., & Bezrukova, M. V. (2007). Current knowledge about presumable functions of plant lectins. J. General Biol., 68, 98–114.
Shakirova, F. M., Bezrukova, M. V., & Khayrullin, R. M. (1993). The increase in lectin level in wheat shoots under the action of salt stress. Biological Bulletin, 20, 142–145.
Shakirova, F. M., Bezrukova, M. V., & Shayakhmetov, I. F. (1996). Effect of heat shock on dynamics of ABA and WGA accumulation in wheat cell culture. Plant Growth Regulation, 19, 85–87.
Shakirova, F. M., Sakhabutdinova, A. R., Bezrukova, M. V., Fatkhutdinova, R. A., & Fatkhutdinova, D. R. (2003). Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Science, 164, 317–322.
Shinozaki, K., & Yamaguchi-Shinozaki, K. (2007). Gene networks involved in drought stress response and tolerance. Journal of Experimental Botany, 58, 221–227.
Singh, P., Bhaglal, P., & Bhullar, S. S. (2000). Wheat germ agglutinin (WGA) gene expression and ABA accumulation in developing embryos of wheat (Triticum aestivum) in response to drought. Plant Growth Regulation, 30, 145–150.
Skriver, K., & Mundy, J. (1990). Gene expression in response to abscisic acid and osmotic stress. Plant Cell, 2, 503–512.
Szabados, L., & Savoure, A. (2009). Proline: a multifunctional amino acid. Trends in Plant Science, 15, 89–97.
Tamás, L., Mistrík, I., Huttová, J., Halušková, L., Valentovičová, K., & Zelinová, V. (2010). Role of reactive oxygen species-generating enzymes and hydrogen peroxide during cadmium, mercury and osmotic stresses in barley root tip. Planta, 231, 221–231.
Thulke, O., & Conrath, U. (1998). Salicylic acid has dual role in activation of defence-related genes in parsley. Plant Journal, 14, 35–42.
Verslues, P. E., Agarwal, M., Katiyar-Agarwal, S., Zhu, J., & Zhu, J.-K. (2006). Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant Journal, 45, 523–539.
Verslues, P. E., & Bray, E. A. (2006). Role of abscisic acid (ABA) and Arabidopsis thaliana ABA-insensitive loci in low water potential-induced ABA and proline accumulation. Journal of Experimental Botany, 57, 201–212.
Vicente, M. R.-S., & Plasencia, J. (2011). Salicylic acid beyond defence: its role in plant growth and development. Journal of Experimental Botany, 62, 3321–3338.
Vlot, A. C., Dempsey, D. A., & Klessig, D. F. (2009). Salicylic acid, a multifaceted hormone to combat disease. Annual review of Phytopathology, 47, 177–206.
Wang, C., Yang, A., Yin, H., & Zhang, J. (2008). Influence of water stress on endogenous hormone contents and cell damage of maize seedlings. Journal of Integrative Plant Biology, 50, 427–434.
Wang, Y., Qian, Y., Hu, H., Xu, Y., & Zhang, H. (2011). Comparative proteomic analysis of Cd-responsive proteins in wheat roots. Acta Physiologiae Plantarum, 33, 349–357.
Wilkinson, S., & Davies, W. J. (2010). Drought, ozone, ABA and ethylene: new insights from cell to plant to community. Plant, Cell and Environment, 33, 510–525.
Xiong L. (2007). Abscisic acid in plant response and adaptation to drought and salt stress. In: M. A. Jenks, P. M. Hasegawa & S. M. Jain (Eds.). Advances in molecular breeding toward drought and salt tolerant crops. New York: Springer.
Xiong, L., Schumaker, K. S., & Zhu, J.-K. (2002). Cell signaling during cold, drought, and salt stress. Plant Cell, 14, 165–183.
Yadav, S. K. (2010). Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. South African Journal of Botany, 76, 167–179.
Yang, Y., Qi, M., & Mei, C. (2004). Endogenous salicylic acid protects rice plants from oxidative damage caused by aging as well as biotic and abiotic stress. Plant Journal, 40, 909–919.
Ye, N., Zhu, G., Liu, Y., Li, Y., & Zhang, J. (2011). ABA controls H2O2 accumulation through the induction of OsCATB in rice leaves under water stress. Plant and Cell Physiology, 52, 689–698.
Yu, H., Chen, X., Hong, Y. Y., Wang, Y., Xu, P., Ke, S. D., et al. (2008). Activated expression of an Arabidopsis HD-START protein confers drought tolerance with improved root system and reduced stomatal density. Plant Cell, 20, 1134–1151.
Zhang, W., & Chen, W. (2011). Role of salicylic acid in alleviating photochemical damage and autophagic cell death induction of cadmium stress in Arabidopsis thaliana. Photochemical & Photobiological Sciences, 10, 947–955.
Acknowledgments
This work was partially supported by the Russian Foundation for Basic Research, project nos. 11-04-01642 and 11-04-97051-povoljie.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Shakirova, F.M., Bezrukova, M.V., Maslennikova, D.R. (2013). Endogenous ABA as a Hormonal Intermediate in the Salicylic Acid Induced Protection of Wheat Plants Against Toxic Ions. In: Hayat, S., Ahmad, A., Alyemeni, M. (eds) SALICYLIC ACID. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6428-6_7
Download citation
DOI: https://doi.org/10.1007/978-94-007-6428-6_7
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-6427-9
Online ISBN: 978-94-007-6428-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)