Abstract
The British Indian Ocean Territory consists of the Chagos archipelago, almost all of which was designated a no-take MPA in 2010. It covers 650,000 km2, with >60,000 km2 shallow limestone platform and reefs. This has doubled the global cover of such MPAs. It has strong biological affinities with the western Indian Ocean, and larval travel time to reefs to the west of it is 25–35 days. Genetic work is only recently commencing, but it is likely to be a cross-roads, or bridge, in this respect. A licensed fishery used to exist, but this too was closed in 2010, and the large diameter of the area may prove to be a significant reserve for pelagic fishes such as tuna also. The region probably contains about 300 sea mounts and knolls, which is about 10 % of all Indian Ocean seamounts and nearly half of all those protected worldwide, and so the area is regionally important for these features as well with their unexplored but probably diverse deep benthic and fish biota. The area is also well placed to fill a large gap in global monitoring systems; it is located in key region of climate variability, so programmes carried out there are particularly important to research into climate change effects also. The area has very high conservation value and is an important biological asset in an ocean where most reefs show significant and continuing decline in health.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Geographical and Historical Setting
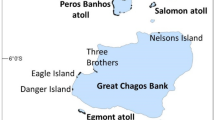
The British Indian Ocean Territory lies at the southern end of the Laccadive-Chagos ridge, in the centre of the tropical Indian Ocean. The area is geographically known as the Chagos archipelago, and has five islanded atolls and several other atolls and banks which are awash or completely submerged (Fig. 17.1 and Table 17.1). Its central feature is the 150 by 100 km Great Chagos Bank, the World’s largest atoll in terms of area. This is mostly submerged, but there are eight islands on its western and northern rim. This is surrounded by the smaller atolls: Peros Banhos and Salomon to the north, Egmont (on some charts the ‘Six Islands’) to the Southwest, and Diego Garcia to the south. Among these lie many submerged reefs, the whole complex covering about 250 by 400 km. The submerged structures are one of the most notable features of the central Indian Ocean.
There are no records to suggest whether the widespread seafaring civilisations of 500–2,000 years ago discovered Chagos, and in this respect it differs from the relatively nearby Maldives. The islands’ first documented discovery was by the Portuguese in the sixteenth Century but they were not inhabited for the next 200 years until, in the late eighteenth Century, the larger ones were farmed for coconuts or copra. The supply of copra, which exceeded about 0.5 million litres annually in the nineteenth Century (Moresby 1884), caused the atolls to be known as the Oil Islands. This lasted more than a century until the 1950s when the plantations on Egmont and the Great Chagos Bank were abandoned. The other plantations continued until the early 1970s, when all remaining atolls were evacuated, the archipelago became the British Indian Ocean Territory, and a military facility was built on Diego Garcia. Today all atolls except Diego Garcia are currently uninhabited, the latter supporting a communications, naval and air facility of a few thousand personnel.
The atolls and reefs attracted some distinguished early scientific attention. All its reefs were mapped in detail in 1837 by Moresby (1884), which permitted Darwin to incorporate them extensively in his exposition of coral reef formation, though Darwin did not land there. Bourne (1888) visited its islands and interpreted its rock strata as evidence against reef formation by subsidence, and Gardiner (1936) described several parts of Chagos which he had visited 30 years earlier. Then, few visits by scientists were undertaken until Stoddart and Taylor (1971) visited Diego Garcia and made observations of the atoll and collections of several groups of organisms from the island and reef flat. The first detailed, deeper reef ecology studies commenced in the 1970s when all the uninhabited atolls and several submerged banks were examined (Bellamy 1979; Sheppard and Wells 1988). No more scientific visits occurred until a visit in 1996 (Sheppard and Seaward 1999).
Chagos contains the largest expanse of totally unexploited reef in the Indian Ocean as well as some of the richest. With the exception of part of Diego Garcia, Chagos’ reefs have been almost completely undisturbed for at least 30 years.
Geological and Physical Background
Chagos is the southernmost part of the Chagos – Laccadive ridge, formed when the Indian tectonic plate migrated northwards towards Asia. The entire chain was created by hot-spot activity since the late Cretaceous, and the trace of this can be followed from the Deccan traps in western India and from there southwards down the chains of atolls to Chagos (Eisenhauer et al. 1999). The archipelago is a group of limestone caps a few hundred metres to a few kilometres thick, resting on volcanic rock (Francis and Shor 1966), and there are numerous pinnacles, seamounts and knolls on the western side of the Territory with an abyssal plain to the east (Fig. 17.2).
Most of the present islands are those of typical atolls, located on the atoll rim, with elevations of no more than 1 or 3 m. Even the Great Chagos Bank itself has the same atoll structure of lagoon area and passes, though it is mostly submerged. Only two areas in Chagos have raised reefs; these are found in southern Peros Banhos and the adjacent, north-western part of the Great Chagos Bank, both sites containing islands with small, uplifted and vertical cliffs about 6 m above high tide. One of the main features of the Chagos reefs is the number of submerged banks and ‘drowned’ atolls (see Table 17.1), the latter exhibiting typical atoll-like cross sections including ‘lagoons’ and passes cut through the submerged atoll rims. The atolls are classical islanded atolls, while Blenheim is an atoll of similar size but which is emerged at low tide only, though reports show that in the late 1700s it supported three vegetated islands. Other structures are atoll-shaped but submerged to at least 5 m.
All reefs visited have profusely growing corals, so the reason why some support islands, some are awash and others are drowned to 5 m or more is not known. Blenheim reef, for example, is a typical atoll in most respects except for its present lack of islands, and indeed its wave resistant algal ridges are the best developed in the Archipelago and possibly in the entire ocean. Other submerged reefs are crescent shaped (e.g. Benares, Colvocoresses) which may represent fragments of older atolls. On some large deeper reefs, such as Speakers Bank, corals are profuse, and thriving seagrasses have been found in parts of Speakers Bank and the Great Chagos Bank, indicating high benthic productivity. On the largest system of all, the Great Chagos Bank, complex patterns of reefs exist with several ring shaped structures within the main atoll rim, which attest to a complex past history of growth and erosion. Depths of over 1 km separate each atoll or reef.
Recent Research and the BIOT Marine Protected Area
Reasons behind the substantial recent research in Chagos are firstly, the understanding that unexploited reef systems like Chagos are increasingly scarce and so these reefs provide a valuable reference site, and secondly, increasing understanding that there is a need to afford full protection to more very large areas in the world. Coral reefs in particular are becoming increasingly affected by overfishing, pollution from agriculture and industry, shoreline construction and climate change (Wilkinson 2008; Burke et al. 2011). All coral reefs are highly vulnerable to these factors, which reduce their biomass and productivity, and consequences of reef deterioration may be greater than previously anticipated (Mora et al. 2011). In most of the world, conventional forms of marine management are failing to arrest the decline, so many marine science bodies, conservation organisations and international conventions have called for more and larger marine protected areas (MPAs) with effective levels of protection (e.g. Nelson and Bradner 2010; United Nations 2002; Wood et al. 2008; Veitch et al. 2012).
However, the term ‘protected’ varies widely, with most affording only partial protection, many allowing fishing (one of the most ecosystem distorting activities) while many, in reality, lack protection completely due to poor enforcement and implementation. The latter are commonly called ‘paper parks’ and, regrettably, these are the large majority given human pressures and poor in-country capacity in most places. For coral reefs, only 6% are effectively managed, 21% are ineffectively managed, and 73% lie outside any MPA (Burke et al. 2011). Reasons for MPA failures range from being declared to meet ‘targets’ which are inadequately resourced, to being simply overwhelmed by close proximity to human populations.
Because of this, the Pew Ocean Legacy Program recently included Chagos as one of five areas selected for protection, and promoted efforts to convince the UK Government to declare it a no-take MPA to the 200 NM boundary (Nelson and Bradner 2010). Part of this process was the creation of the Chagos Environment Network (CEN), a group of several leading UK science bodies and NGOs, whose aim was to ensure that Chagos’ globally important natural environment would be conserved as a unique and valuable resource for present and future generations. In 2010, CEN responded to the UK Government’s Consultation, saying that only designation as a no-take MPA “… guarantees full protection for the ecosystems and species of the Chagos Archipelago and its surrounding reefs, lagoons and waters. Only [this] provides the complete protection needed to underpin the Chagos Protected Area’s value as an important global reference site for a wide range of scientific ecological, oceanographic and climate studies, as well as its continued benefits to humans into the future” (CEN 2010).
BIOT has its own administration, located in the Foreign and Commonwealth Office in London. The senior UK official in the archipelago is the commanding military officer, who is also the British Representative of the Commissioner. The UK Foreign Secretary announced the creation of BIOT as a no-take MPA, instructing the Commissioner to declare it as such in April 2010. The Commissioner, in Proclamation Number 1 of 2010, designated it such “in the name of the Queen”. Existing environmental laws are currently being revised and consolidated to accommodate this status. Diego Garcia atoll is excluded from the MPA to its 3 NM boundary, and the area thus excluded is <1% of the total area though it has several pre-existing, strict environmental laws of its own, including a Ramsar site. Tuna fishing licences were discontinued as of October 2010, and the deficit of approximately $1 million per year from this was subsequently replaced from private sources. It is currently the largest no-take MPA in the world (Nelson and Bradner 2010) and is part of the ‘Big Ocean Network’, an information exchange network of managers and partners of existing and proposed large-scale marine managed areas (www.bigoceanmanagers.org/). Monitoring and enforcement are undertaken in large part by a patrol vessel which serves as a mobile base for both military purposes and civilian research expeditions. A Science Advisory Group provides advice to BIOT Administration, and a scientific review (Sheppard et al. 2012a) and the document ‘Conservation and management in the British Indian Ocean Territory’ which details scientific needs have also recently been released (Sheppard et al. 2012b).
Under the 2001 BIOT Environmental Charter, the UK Government facilitates the extension of the UKs ratification of multilateral environment agreements of benefit to the BIOT and which the BIOT has the capacity to implement. CITES and Convention on Migratory Species (CMS) have been extended to the territory, but the Convention on Biological Biodiversity (CBD) has not. The rationale is the current inability to fulfil all of the Convention requirements in Chagos, for practical reasons. But, as per the World Heritage Convention, the area is treated by the UK Government with no less strict regard, subject only to defence requirements, and in the case of CBD, the capacity to implement. Chagos harbours 76 threatened species (IUCN Red List) including Hawksbill turtle, Red foot booby, silky shark, Coconut crab, and Bigeye tuna, providing an internationally important refuge and reference site. This Ocean Legacy MPA will protect entire ecosystems rather than species in isolation, including deep-sea, pelagic, reef and small island systems including migratory species (cetaceans, sharks, turtles, birds) and those vulnerable to poaching and trade (sharks, turtles, sea cucumbers). While there is no international trade in CITES-listed species from Chagos, this emphasises its value as a reference site for comparison with exploited sites, particularly for corals, giant clams, cetaceans, marine turtles and sea cucumbers. This Convention is also relevant in Chagos for several bird species, notably boobies.
Island and Reef Areas
Figure 17.3 (left) shows the areas of islands and reefs. There are over 50 small islands (Fig. 17.4), the number varying to some degree with tidal height and shifts of sand banks. In contrast to this, sublittoral substrate in the photic zone is calculated to be approximately 60,000 km2 (Fig. 17.3, right) (Dumbraveanu and Sheppard 1999). The proportion of this which is actively growing reef remains uncertain because >95% of the territory still has never been studied, though some areas are apparently eroding and others support sand and/or large seagrass beds. There is enormous opportunity for new discoveries: in 2010 an expedition discovered many hectares of seagrass for example. Other parts of Chagos have been mapped using bathymetric or satellite data-based modelling (e.g. Purkis et al. 2008; Yesson et al. 2011, see Fig. 17.2).
(Left): Island areas in the Chagos Archipelago (scale in ha). The largest islands are named. Right: area of submerged substrate in the archipelago (scale in km2) (From Dumbraveanu and Sheppard 1999)
Three small islands on the Three Brothers group on the western Great Chagos Bank. These are, confusingly, three islands of the four that comprise the group. Note the huge reef surrounding the nearest; there are no reefs around the middle island (which has probably uplifted 2–3 m since being mapped as being only a shoal in the mid 1800s); while the furthest supports more typical reef flats all around (Photo Chris Davies)
This chapter summarises some of the recent scientific context of the British Indian Ocean Territory (BIOT) that is not covered in more detail in subsequent chapters. The Territory is, uniquely for the UK Territories, entirely built by coral reefs. Work carried out there over the past couple of decades has demonstrated the outstanding ecological value of the region, which has led to the creation of the world’s largest marine protected area of about 640,000 km2.
Biological Connections of Chagos in the Indian Ocean
Substantial values of Chagos are likely to be its role as a biological crossroad or stepping stone connecting different parts of the Indian Ocean, in possibly being a source of larvae to the west, and a reservoir of unexploited reef species for the western Indian Ocean. Currents and proximity of suitable substrata are key to these roles. To the east of Chagos, there is no shallow water until the Cocos-Keeling islands 2,750 km to the east, with Indonesia another 1,000 km further on. To the west, distances to shallow reefs are much less: 1,700 km to the Seychelles and only 1,050 km to the commonly overlooked Saya de Malha submerged banks at the northern end of the ‘Shoals of Capricorn’ between the Seychelles and the Mascarenes. Figure 17.5 shows these, along with estimates of travel times for inert particles, given existing currents. Currents passing across Chagos flow towards Southeast Asia from approximately January to April, and towards the western Indian Ocean for the rest of the year, with fluctuations (Couper 1987). At 0.5 m s−1 (Bonjean and Lagerloef 2002) planktonic larvae from reef species would need 65 days to reach shallow habitat in the east, but only 35 and 25 days to reach the Seychelles and Saya de Malha reef systems respectively, well within the pelagic larval duration of many reef organisms. Due to its location, Chagos may be an important ‘stepping-stone’ for marine organisms in the Indian Ocean.
Fifteen years ago, mapping methods in which geographical distances were replaced by similarities of coral presences, showed that Chagos does appear to function as an East–West stepping stone for corals (Sheppard 1999). Using more recent synonymies, Chagos shows 82% of corals in common with the west and 88% in common with the east, and higher values are found with reef fishes (Fig. 17.6). (Such figures are strongly influenced by total numbers of species in each site which are, of course, highest in the East.) Extensive sampling (Obura 2012) has shown the coral fauna of Chagos to be more similar to the western Indian Ocean and its islands, including northern Madagascar, than it is to the much nearer Maldive archipelago, Sri Lanka or India located to its north (Fig. 17.7). Obura (2012) has shown that the previously accepted biogeographic zones (Fig. 17.7 left) need to be adjusted to encompass this biological connectivity (Fig. 17.7 right). This potentially reflects patterns of connectivity as well as the influence of habitat area on species diversity. The coral fauna of the eastern Indian Ocean is also more affiliated with the West Pacific/Coral Triangle fauna than it is to the central and western Indian Ocean fauna (Obura 2012).
Left: Ecoregions and provinces of the Western Indo-Pacific Realm – previous definitions. Right: revised ecoregions according to coral distributions. The boundaries correspond to the EEZs of each location (For details see Obura 2012)
Preliminary examination of the coral Platygyra daedalea with five microsatellite loci, including samples from Chagos (Macdonald et al. submitted), revealed that, while the Chagos population had the lowest allelic diversity among the sites studied, it proved to be a source of genetic diversity for this species.
Another study has shown recent colonization of a fish species from the east, consistent with this stepping stone function, especially with reefs in the southern part of the group (Craig 2008).
Genetic programmes to examine connections between Chagos and other Indian Ocean reef sites have been initiated recently for numerous species, including about 24 reef fish species and several invertebrates including corals. For hawksbill turtles (Eretmochelys imbricata), genetic linkages were demonstrated for nesting females and foraging juveniles between Chagos and Seychelles, but no linkages were demonstrated with hawksbill rookeries of Western Australia (Mortimer and Broderick 1999; Mortimer et al. 2002). In the wider Indian Ocean, Vargas et al. (2010) identified nine genetic groupings, with hawksbill nesting in Chagos and Seychelles forming a single grouping distinct from those in the Arabian Gulf and from easterly sites including Western Australia, (Vargas et al. 2010 and Vargas et al. in prep). A similar pattern of connectivity was observed in foraging hawksbills in Chagos which derive mostly from rookeries in Chagos and Seychelles, though these rookeries were found to also contribute substantially to foraging aggregations in Cocos Keeling (FitzSimmons 2010 unpublished report; Hahn et al. in prep). While most mtDNA lineages found in the Chagos and Seychelles were not observed elsewhere, some uncommon lineages were identical to those found in Iran, Oman, and Australia, supporting the stepping stone model.
The crown-of-thorns starfish, an important coral predator, was previously believed to be a single species, Acanthaster planci, but Vogler et al. (2008) have shown that the species includes four highly differentiated lineages with restricted distributions, which together form a species complex. Two of these lineages are found in the Indian Ocean, and data indicate that crown-of-thorns starfish from Chagos belong to the Southern Indian Ocean lineage (Fig. 17.8a). A more detailed phylogeographic study (Vogler et al. 2012) revealed high gene flow among the geographically distant populations in the southern Indian Ocean lineage, including between Chagos and sites in the eastern and western Indian Ocean. Acanthaster larvae can extend their developmental period to 7 weeks in marginal food regimes (Lucas 1982). Although the occurrence of facultative teleplanic larva remains to be confirmed (Birkeland and Lucas 1990), the low productivity found over most of the Southern Indian Ocean (<130 gC.m−2 day−1; Reid et al. 2006) could result in extended larval durations there too, which would explain the observed high connectivity and low levels of genetic structure in the Southern Indian Ocean lineage, despite long geographic distances.
(a) Crown of Thorns genetic groupings. (b): Peacock Hind (Cephalopholis argus). (c): Brown Surgeonfish (Acanthurus nigrofuscus). (d) Coconut crab (Birgus latro). Color coding for the Crown of Thorns (Vogler et al. 2008 and Vogler et al. 2012) and Peacock Hind (Gaither et al. 2011) indicate distinct genetic lineages. Dashed lines for the Brown Surgeonfish (Eble et al. 2011) indicate genetically independent populations (Photo credit: www.aquaportail.com. Image 12 b and 12c reprinted from Gaither et al. (2011) and Eble et al. (2011) with permission from the authors). For (d) solidity of arrow lines represents relative amounts of gene flow, so that for this terrestrial crab flow is mainly eastwards during the Equatorial Counter Current flow
Added support for Chagos as a bridge between eastern and western Indian Ocean can be found in reef fishes (Eble et al. 2011; Gaither et al. 2010). For the peacock hind (Cephalopholis argus, Fig. 17.8b) and brown surgeonfish (Acanthurus nigrofuscus) (Fig. 17.8c), patterns of genetic linkage are similar to those observed in the hawskbill turtle, with Chagos showing greater genetic affinity with sites in the western Indian Ocean than with the east. Though, for both fishes, differences in affinity are marginal and appear to be driven by the relatively recent introduction of Pacific lineages to the eastern Indian Ocean (Eble et al. 2011).
Despite being geographically part of the Indian Ocean, the eastern Indian Ocean locations at Cocos Keeling and Christmas Islands, and Western Australia are more closely affiliated with the Pacific ichthyofauna, with only 5% of species at Cocos-Keeling being exclusively of Indian Ocean origin (Allen and Smith-Vaniz 1994). The latter islands are considered to be a part of the Indo-Polynesian Province stretching from the eastern Indian Ocean to Easter Island (Briggs and Bowen 2012). However, Cocos-Keeling and Christmas islands are a known region of overlap between Indian and Pacific faunas (Gaither et al. 2011), as indicated by the presence of a hybrid zone with a westward limit at Cocos-Keeling (Hobbs et al. 2009). Exceptions to this pattern are found among species with highly dispersive larvae, including the bluestriped snapper (Lutjanus kasmira; Gaither et al. 2010), trumpetfish (Bowen et al. 2001) and two moray eels (Genus Gymnothorax, Reece et al. 2010) which freely intermix across all their Indo-Pacific range. For the majority of less dispersive reef fishes, Chagos may act as a bridge between western Indian Ocean and Pacific populations.
The coconut crab, Birgus latro, is terrestrial though females lay eggs in the sea. Mitochondrial genetic work has compared Chagos with sites in the Seychelles and East Africa, and showed the Chagos population was significantly differentiated (p < 0.05) from Seychelles and East African populations (Tables 17.2, 17.3, and 17.4). Asymmetric gene flow, favouring migration from East Africa to Seychelles, and Seychelles to Chagos, comes from estimates of direction and mean number of migrants per generation between regions. The rate of immigration to Chagos from the west was measured at about 5 effective females per generation (breeding commences after about 5 years), using a measured mean effective female population size in the study of about 3,000, or about 0.1–0.2% per generation (N.B. this is not the counted population of individuals which is orders of magnitude greater). Thus for this species, Chagos receives more larvae from the west than flow from Chagos to the west (Fig. 17.8d). This predominantly eastward dispersal may result from the timing of egg release which partly coincides with the period of eastward current flow, and there is a high level of genetic connectivity. Additionally, a strong genetic connectivity among three sites was also observed through population structure analysis.
The pattern is clearly complex: earlier fish surveys (Winterbottom and Anderson 1997) in Chagos found two distinct assemblages: a northern portion sharing affinities with the eastern Indian Ocean and the southern portion (including Diego Garcia) more closely aligned with faunal assemblages further west. Taken together, these results confirm that Chagos is part of the western Indian Ocean province as described by Briggs (1974), though with respect to fishes, Briggs and Bowen (2012) additionally acknowledge affinities with the Indo-Polynesian province. Interestingly, Chagos shows less connectivity with the much closer Maldives to the North in some groups, which may be a function of the predominantly East–West currents. While there are recent reports of localized larval recruitment in predominately small-range fishes, these are countered by studies that show high genetic connectivity across large oceanic distances (Eble et al. 2011).
Thus early results from the genetic and distribution data indicate that Chagos is an important biogeographic crossroad between the eastern and western Indian Ocean. The so-far limited molecular data are generally concordant with biogeographic patterns indicating greater connectivity between Chagos and sites to the west (Saya de Malha Banks, Seychelles, and East Africa) than with those to the east (Cocos Keeling, Christmas Island, and west Australia). However, patterns of differentiation and migration vary considerably between species, which may reflect species level differences in dispersal ability, reproductive strategy, competitive ability, or habitat requirements. In some species, large distances may be successfully bridged by the existence of so-called ‘teleplanic larvae’ which afford greatly expanded larval durations (Scheltema 1988). In other species, like some corals, larvae are competent for up to 105 days (Wilson and Harrison 1998). This contrasts with reef fishes which exhibit an average larval duration of about 1 month (though it varies enormously; Brothers and Thresher 1985; Sale 2002).
Although it is probable that Chagos is an important stepping stone in the western Indian Ocean, the rate at which this happens for most groups is still not known. But in fact, the number of migrants needed to maintain genetic coherence between populations is small (Slatkin 1977, 1982). As noted by Hellberg (2007), for management purposes we need to know whether or not connections are made en masse every several thousand generations, or whether connections occur at demographically relevant intervals.
Nonetheless, patterns of connectivity as they exist today highlight the importance of the Chagos as a biogeographic stepping stone between the eastern and western Indian Ocean, although many questions remain unresolved. If Chagos is mainly a net recipient of larvae then its rich and relatively undamaged state affords it a very high conservation value as refugia. If Chagos exports biological diversity to the over-exploited sites to the west and east, then the reefs of Chagos, and of the MPA, would have even greater value.
Pelagic Fishing and Fisheries
Fisheries provided most of the income for Chagos until the MPA was created, with the last licences expiring on 31st October 2010. The main fisheries were longline and purse seine for tuna, and a smaller Mauritian inshore fishery also existed. There is a recreational fishery in Diego Garcia, which operates within the 3 NM limit surrounding the atoll, which is relatively small, taking (in 2008) 25.2 tonnes of tuna and tuna-like species (76% of the catch) the remainder being reef-associated species (Mees et al. 2009 and see Chap. 19).
The longline fishery in Chagos was active year-round, mainly under Taiwanese and Japanese flagged vessels targeting large pelagic species, including yellowfin tuna (Thunnus albacares), bigeye tuna (Thunnus obesus), swordfish (Xiphias gladius), striped marlin (Tetrapturus audax) and Indo-Pacific sailfish (Istiophorus platypterus), with annual catches ranging from 371 to 1,366 tonnes between 2005 and 2010 (Koldewey et al. 2010). The purse-seine fishery targeted yellowfin- predominantly juveniles – and skipjack tuna (Katsuwonus pelamis) and was highly seasonal, operating between November and March with a peak usually in December and January (Mees et al. 2009). Log book records show that catches, mainly by Spanish and French flagged vessels, were highly variable, ranging from <100 to ∼24,000 tonnes annually (Koldewey et al. 2010).
The Mauritian inshore fishery targeted demersal species, principally snappers, emperors and groupers, and logbook records indicated that the catches were between 200 and 300 tonnes per year for the period 1991–1997, decreasing to between 100 and 150 tonnes from 2004 (Mees et al. 2008). There have been no fishing activities from Mauritius in the last few years prior to MPA designation.
Fisheries suffered from poor documentation of by-catch and from illegal fishing. By-catch was inadequately recorded through a logbook system supported by very limited observer coverage – mean coverage was 1.24% per season for longline fishing and 5.56% for purse-seine fishing (Koldewey et al. 2010). Even with this uncertainty, the by-catch in the Chagos was clearly substantial, particularly for sharks, rays and billfish (Pearce 1996; Roberts 2007; Graham et al. 2010; Koldewey et al. 2010). Shark and sea cucumber poaching (Fig. 17.9) are covered in later chapters.
Illegal fishing remains a management issue following the implementation of the MPA and enforcement will be key to its effectiveness, as for most MPAs globally. The size and location of Chagos as an MPA is particularly important in these respects as the western Indian Ocean has some of the most exploited, poorly understood and badly protected and managed coastal and pelagic fisheries in the world (Kimani et al. 2009; van der Elst et al. 2005), while overall catches from them continue to dramatically increase (FAO 2010). The Regional Fisheries Management Organisation (RFMO) that oversees the region containing Chagos is included within the Indian Ocean Tuna Commission (IOTC). Unfortunately, the IOTC, as with many tuna RFMOs, is widely recognised as having numerous legal and technical weaknesses Anonymous 2009 Cullis-Suzuki and Pauly 2010). Tuna in the Indian Ocean are considered to be close to the maximum sustainable yield (bigeye) or overexploited (yellowfin) and even skipjack, which is generally considered a highly productive and resilient species, has been highlighted for close monitoring (IOTC 2010). This also needs to be considered in a global context where, in 2011, all species of tunas, bonitos, mackerels, swordfish and marlins were assessed for the IUCN Red List of Threatened Species, with 11% of the world’s 61 species documented to be at serious risk of extinction (Collette et al. 2011). Moreover, the IUCN classed a third of oceanic shark species, species that are regularly caught as by-catch in pelagic fisheries, in a threatened category (Camhi et al. 2009). Illegal, unreported and unregulated fishing is not a trivial component of the catch and adds substantial uncertainty into population assessments (Ahrens 2010).
There is increasing evidence that large MPAs like Chagos can benefit pelagic species that have the potential to exhibit highly mobile behaviours (reviewed in Game et al. 2009; Koldewey et al. 2010). In reality, the phrase ‘highly migratory’ which is frequently used to describe tuna and oceanic sharks, often has little biological meaning, with studies of tuna mobility demonstrating they would benefit from national-level closures (Sibert and Hampton 2003). Pelagic fish demonstrate considerable stability and persistence, and predictability of some habitat features does occur within the pelagic realm (Alpine 2005; Baum et al. 2003; Etnoyer et al. 2004; Hyrenbach et al. 2000; Worm et al. 2003). Migratory predators like tuna do not move randomly, but associate with certain environmental and/or physical features (Hughes et al. 2010; Itano and Holland 2000; Morato et al. 2010a; Schaefer and Fuller 2010) – indeed tuna fisheries have been shown to benefit from such aggregations (Morato et al. 2010b), meaning that positive, measurable reserve effects on pelagic populations exist (Baum et al. 2003; Hyrenbach et al. 2002; Jensen et al. 2010; Roberts and Sargant 2002; Worm et al. 2003, 2005). Several studies have shown that migratory species can benefit from no-take marine reserves (Beare et al. 2010; Jensen et al. 2010; Palumbi 2004; Polunin and Roberts 1993). Ranges of skipjack and yellowfin tuna have not been measured in the Indian Ocean, but if their ranges in a Pacific archipelago (Sibert and Hampton 2003) are superimposed onto the Indian Ocean, the BIOT MPA would encompas the median lifetime range of these two key species (Fig. 17.10). If these ranges do apply to the Indian Ocean for these species, it is likely that the Territory does provide considerable scope for their conservation. Studies are now underway to test this in Chagos using a combination of pelagic video monitoring and tagging (Zoological Society of London and University of Western Australia).
The median lifetime ranges of skipjack (red) and yellowfin tuna (yellow), superimposed on a map of the Chagos MPA (Ranges from Pacific: Sibert and Hampton 2003)
Pelagic MPAs such as Chagos are thus an important tool in marine conservation management (Game et al. 2009) and are rapidly becoming a reality (Pala 2009), although the challenges relating to their implementation may be both costly and logistically challenging (Kaplan et al. 2010). Large MPAs are considered essential to protect species such as large pelagic fish and marine mammals (Wood et al. 2008) as well as offsetting the concentration of fishing effort outside them (Walters 2000) and maintaining ecological value (Nelson and Bradner 2010). Their importance for top predators has been highlighted by the most comprehensive, decade-long, open ocean tagging study in the Pacific that clearly demonstrated that top predators – including whales, seals, tuna, sharks, seabirds, turtles – exploit their environment in predictable ways, providing the foundation for spatial management of large marine ecosystems (Block et al. 2011). Extending to 200 NM, the Chagos MPA offers an extremely valuable opportunity to understand the effects of large-scale protection on pelagic, migratory species, both within the MPA and within a regional context.
Deep-Water Ecosystems
Seamounts
Yesson et al. (2011) determined that 86 seamounts (conical topographic rises of >1,000 m elevation) and 243 knolls (conical topographic rises of elevation 200–999 m) are predicted to occur within the Chagos MPA (Fig. 17.11). Chagos thus could contain more than 10% of all Indian Ocean seamounts and so the area is regionally important for these features as well. Given that globally only 506 seamounts and 606 knolls lie in protected areas (Yesson et al. 2011, based on the world database of protected areas 2009), this means that the Chagos MPA increased the world’s protection of seamounts by 17% and knolls by 40%. Previous emphasis has been on shallow-water ecosystems, but protection of its seamounts is also important, especially considering their high biodiversity, often representing entirely unique ecosystems (Clark et al. 2006). Although the geology of some of the seamounts and ridges in the Indian Ocean has been explored, including the Chagos-Laccadives Ridge, seamount fauna is poorly known (Demopoulos et al. 2003; Rogers et al. 2007). Some data on fish exist, mainly resulting from exploratory or commercial fishing, but no specific information relates to the Chagos-Laccadive Ridge. Recent modelling studies based on 30-arc sec satellite bathymetry data indicate that the Indian Ocean hosts fewer seamounts than the Atlantic and Pacific Oceans (Yesson et al. 2011), and many are associated with ridges or originate at ridges.
Seamounts of the Chagos MPA as identified in Yesson et al. (2011). Bathymetry data from shuttle radar topography mission 30 arc-sec grid. (http://www2.jpl.nasa.gov/srtm/)
Deep Water Fishing
The Indian Ocean suffers increasing pressure from deep-sea fishing that threatens these seamounts and other benthic habitats. The fact that deep-water fishing or trawling has never been documented in Chagos makes it particularly important. Deep-sea fishing in the Indian Ocean was mostly undertaken by distant-water fleets, particularly from the USSR. These fisheries targeted redbait (Emmelichthys nitidus) and rubyfish (Plagiogeneion rubiginosus) with catches peaking about 1980 and then decreasing to the mid 1980s (Clark et al. 2007). Fishing then switched to alfonsino (Beryx splendens) in the 1990s as new seamounts were exploited. Some exploratory trawling was also carried out on the Madagascar Ridge and South West Indian Ridge by French vessels in the 1970s and 1980s, particularly targeting Walter’s Shoals and Sapmer Bank (Collette and Parin 1991). In the late 1990s, a new fishery developed on the South West Indian Ocean Ridge with trawlers targeting deep-water species such as orange roughy (Hoplostethus atlanticus), black cardinal fish (Epigonus telescopus), southern boarfish (Pseudopentaceros richardsoni), oreo (Oreosomatidae) and alfonsino (Clark et al. 2007). This fishery rapidly expanded, with estimated catches of orange roughy being approximately 10,000 t, until that fishery collapsed. Fishing then shifted to the Madagascar Plateau, Mozambique Ridge and Mid-Indian Ocean Ridge, targeting alfonsino and rubyfish (Clark et al. 2007). Most of these areas therefore have likely been significantly impacted by deep-sea bottom fisheries.
Deep-sea fishing in most of the Indian Ocean is continuing and showing signs of increasing its geographic spread, mainly targeting orange roughy and alfonsino. Recent fishing has also taken place on the Broken Ridge (eastern Indian Ocean), 90 East Ridge, possibly the Central Indian Ridge, the Mozambique Ridge and Plateau and Walter’s Shoal (western Indian Ocean), where a deep-water fishery for lobster (Palinurus barbarae) has developed (Bensch et al. 2008). The banks around Mauritius and high seas portions of the Saya da Malha Bank have been targeted by fisheries for Lutjanus spp., and lethrinid fish (SWIOFC 2009), and there are also reports of unregulated gillnet fishing in the Southern Indian Ocean such as at Walter’s Shoal, which target sharks (Shotton 2006). Currently, there is little or no information available on impacts of deep-sea fishing on high seas areas of the Indian Ocean on populations of target or by-catch species, or on seabed ecosystems. Reporting of data are complicated by issues of commercial confidentiality in fisheries where individual stocks may be located across a wide area (e.g. the South West Indian Ridge) and, up until June 2012, with the entering into force of the South Indian Ocean Fisheries Agreement (SIOFA), there was no adequate regional fisheries management organisation. At present, new fisheries are developing in the region with no apparent assessment of resource size or appropriate exploitation levels, and with no estimate of impacts on vulnerable marine ecosystems.
Global modelling studies are currently evaluating habitat suitability for deep-sea Scleractinia and Octocorallia, at 30-arc sec resolution (Davies and Guinotte 2011; Yesson et al. 2012) which indicate that suitable habitat for these organisms are likely to exist on deep slopes and seamounts within the Chagos MPA. Given the lack of a history of deep-sea fishing in the region around the Chagos Archipelago, it is likely that associated communities of deep invertebrates and fish are still largely intact, unlike on most other ridges in the Indian Ocean which have been subject to recent, continuing or expanding fisheries.
Considering the paucity of research on equatorial seamounts, the Chagos region is particularly important for deep-water ecosystem conservation too, both at a regional and global level. It also provides a unique opportunity to investigate the energetic links between production associated with shallow-water coral reefs and deep-water ecosystems.
Climate and Long Term Environmental Monitoring Programmes in BIOT
Work is increasingly needed on climate change projections, yet the Indian Ocean forms a very large gap in many global monitoring programmes. Numerous temperature loggers have been deployed on these reefs at 5, 15 and 25 m depth on several lagoonal and seaward facing reefs. These have shown upward movement of thermoclines with periods of 1–4 days, coinciding partly with times of the warmest seasonal temperatures (Sheppard 2009). This may have important consequences in terms of reducing stress on corals (next chapter). Furthermore, the temperature records report the possible intrusion of internal waves onto the platform, which is relevant to issues of nutrient cycling, though this key aspect is another which remains to be researched.
Climate observations are extremely sparse. Further, instrumental SST data are associated with errors that are as large as the SST anomalies across a range of time scales (Annamalai et al. 1999). There is currently one weather station at Diego Garcia, with much good data but with several gaps. ‘Conventional’ infrared satellite measurements of SST are not adequate to capture important SST perturbations that occur in the rainy season when convective activity and cloud cover is highest (Vialard et al. 2009), but from the late 1990s better quality SST measurement became possible to reveal the role of Chagos with respect to air-sea interactions. Therefore, a continuous monitoring programme of important climatic and oceanographic parameters at Chagos is needed for a better understanding of air-sea interactions that are crucial in global climate issues.
Chagos is situated in a key region of climate variability. It lies at the eastern margin of the ‘Seychelles-Chagos thermocline ridge’ (Hermes and Reason 2008), along which the thermocline rises close to the surface and upwelling of cold water (Vialard et al. 2009). In contrast to most upwelling regions, surface water of the Seychelles-Chagos ridge is extremely warm. In austral summer, the main rainy season, sea surface temperature (SST) varies only between 28.5°C and 30°C, a range in which the atmosphere is very sensitive to small oceanic changes (Timm et al. 2005). The high SST combined with the shallow thermocline makes this a region with very strong air-sea interactions.
The Seychelles-Chagos region has a distinct oceanic and atmospheric variability at multiple time scales, each with significant climatic consequences. It has the strongest intraseasonal SST variance in the Indo-Pacific warm pool, because the shallow thermocline is very responsive to atmospheric fluxes (Vialard et al. 2008, 2009). SST cooling of 1°C–1.5°C, lasting for 1–2 months, may occur during austral summer, followed by a short lag and sharp increase in atmospheric convective activity (Vialard et al. 2009) associated with the Madden-Julian-Oscillation (MJO) (Madden and Julian 1994). The MJO has a time scale of 30–80 days; it explains much of the variance of tropical convection, and modulates cyclonic activity.
On interannual time scales, the Seychelles-Chagos ridge is affected by the El Nino-Southern Oscillation (ENSO) and the Indian Ocean Dipole (IOD) (Webster et al. 1999; Saji et al. 1999). ENSO events lead to a displacement of the West Pacific warm pool and affect the Indian Ocean via the so-called atmospheric bridge. Anomalous subsidence over Indonesia during El Nino years leads to a cooling over the maritime continent and a basin-scale warming of the Indian Ocean, particularly the western part. The IOD is a coupled ocean–atmosphere instability centred in the tropical Indian Ocean that affects the climate of the countries that surround the Indian Ocean basin (Marchant et al. 2007). A positive IOD period is characterized by cooler than normal water and below average rainfall in the eastern Indian Ocean, Indonesia and over parts of Australia, while warmer water and increased rainfall is observed in the western Indian Ocean and equatorial East Africa. A negative IOD period is characterized by warmer than normal water and above average rainfall in the eastern Indian Ocean sector and cooler than normal water and below average rainfall in the western Indian Ocean. Some studies suggest that positive (negative) IOD events may be triggered by El Niño (La Niña), while others find that IOD events occur independently although they may overlap with El Niño/La Niña events, e.g. the strong 1998 ENSO (Saji and Yamagata 2003). The IOD has two ‘centres of action’: one off the coast of Sumatra, in the eastern Indian Ocean sector, and one at the Chagos Archipelago (Saji and Yamagata 2003).
Reliable instrumental records of the IOD are limited to the past 50 years. On decadal and longer time scales, information on climate variability at Chagos has been gained from geochemical proxies archived in long-lived corals (Pfeiffer et al. 2004, 2006, 2009; Timm et al. 2005). These data indicate that changes in the mean climate may influence the impact of large-scale climate phenomena at Chagos, possibly affecting climate in other countries surrounding the Indian Ocean. For example, a shift towards higher mean SSTs occurred in 1975 (Timm et al. 2005; Pfeiffer et al. 2006, 2009), after which small SST changes induced by ENSO caused much larger precipitation anomalies (Timm et al. 2005; Pfeiffer et al. 2006). Similar changes in rainfall variability have been found in South Africa (Richard et al. 2000), Sri Lanka and southern India (Zubair and Ropelewski 2006).
The long-term variability of the IOD has also been investigated with coral proxies from the Seychelles and Indonesia (Abram et al. 2008). The coral reconstruction suggests an increase in the strength and frequency of IOD events during the twentieth century, which may also influence the distribution of rainfall in the tropical Indian Ocean. The work highlights the importance of the Chagos Archipelago for climate variability research in the Indian Ocean sector and beyond, and emphasises the need for high-quality in-situ data recording of climatic parameters at Chagos.
Conclusions
Chagos is unquestionably a highly valuable biological asset in an ocean where most reefs show significant and continuing decline in health. The reefs are in exceptionally good condition (see next chapters on the corals and fishes) and are important in terms of biodiversity and productivity, and for their function as a biogeographic crossroads in the central Indian Ocean. Its protection is of great importance given global pressures from overfishing and other activities resulting from human population growth. As such, Chagos joins a small handful of large, protected sites in the world, and is the only one in the Indian Ocean. The importance of the BIOT MPA was reinforced by a recent report across marine science disciplines suggesting that the world’s ocean is at high risk of entering a phase of disturbance unprecedented in human history (Rogers and Laffoley 2011). Indeed, many more effective and very large MPAs need to be created if international goals for protecting the oceans are to be met, although the number of locations where this will be possible is diminishing.
The biological and conservation value of the Chagos therefore is evident. Nevertheless, the formation and maintenance of the MPA has been met with opposition from various sources, including the oceanic fishing industry, the government of Mauritius that claims the territory, and from some Chagossians who were removed approximately 40 years ago and some of their representatives. While the decision to remove the inhabitants at that time was based on politics and defence concerns rather than for conservation, the present excellent condition of this large area has been an unplanned consequence of the subsequent lack of exploitation. However, the MPA was created ‘without prejudice’ to any future resettlement, and if resettlement does occur then management must be adequate to avoid the problems evident from similar experience around the world.
The high value of relatively undisturbed areas encompassing a range of functionally linked ecosystems is becoming increasingly recognised at the same time that their number is diminishing world-wide ((http://www.globaloceanlegacy.org/)). While there are many ‘managed’ reef sites elsewhere in the tropical oceans, almost all are themselves in a poor condition compared to Chagos and a few Pacific sites. The ‘shifting baseline syndrome’ (Pauly 1995) applies to marine management and in many places has led to situations where decisions are taken that are highly disadvantageous to both the natural systems and to the people dependant on them. Most coral reefs remain unprotected or protected in name only (Burke et al. 2011). The huge scale of the interconnected network of atolls and banks in Chagos, and its effective governance, are likely to become increasingly important both directly and as a scientific reference site in the Indian Ocean. BIOT has, at present and the foreseeable future, a governance which will enable this protection to persist. Priorities for the region now are effective management, so that the benefits of a well protected MPA are likely to extend into the future including area far beyond the boundaries of the Chagos MPA.
References
Abram NJ, Gagan MK, Cole JE, Hantoro WS, Mudelsee M (2008) Recent intensification of tropical climate variability in the Indian Ocean. Nat Geosci 1:849–853
Ahrens RNM (2010) Global analysis of apparent trends in abundance and recruitment of large tunas and billfishes inferred from Japanese longline catch and effort data. Ph.D. thesis, University of British Columbia, 422pp
Allen GR, Smith-Vaniz WF (1994) Fishes of the Cocos (Keeling) Islands. Atoll Res Bull 412:1–21
Alpine J (2005) The application of closed area management in pelagic ecosystems: a viable strategy for longline fisheries? B.Sc. thesis, CSIRO Marine Research and School of Zoology, University of Tasmania, 68pp
Annamalai H, Slingo JM, Sperber KR, Hodges K (1999) The mean evolution and variability of the Asian summer monsoon: comparison of the ECMWF and NCEP/NCAR reanalysis. Mon Weather Rev 127:1157–1186
Anonymous (2009) Report of the IOTC performance review panel: January 2009. Indian Ocean Tuna Commission
Baum J, Myers R, Kehler D, Worm B, Harley S, Doherty P (2003) Collapse and conservation of shark populations in the Northwest Atlantic. Science 299:389–392
Beare D, Hölker F, Engelhard GH, McKenzie E, Reid DG (2010) An unintended experiment in fisheries science: a marine area protected by war results in Mexican waves in fish numbers-at-age. Naturwissenschaften 97:797–808
Bellamy DJ (1979) Half of paradise. Cassell, London, p 180
Bensch A, Gianni M, Grébroval D, Sanders JS, Hjort A (2008) Worldwide review of bottom fisheries in the high seas. FAO Fisheries and Aquaculture Technical Paper 522
Birkeland CE, Lucas JS (1990) Acanthaster planci: major management problem of coral reefs. CRC Press, Boca Raton
Block BA, Jonsen ID, Jorgensen SJ, Winship AJ, Shaffer SA, Bograd SJ, Hazen EL, Foley DG, Breed GA, Harrison AL, Ganong JE, Swithenbank A, Castleton M, Dewar H, Mate BR, Shillinger GL, Schaefer KM, Benson SR, Weise MJ, Henry RW, Costa DP (2011) Tracking apex marine predator movements in a dynamic ocean. Nature 475:86–90.doi:10.1038/nature10082
Bonjean F, Lagerloef GSE (2002) Diagnostic model and analysis of the surface currents in the tropical Pacific Ocean. J Phys Oceanogr 32:2938–2954
Bourne GC (1888) The atoll of Diego Garcia and the coral formations of the Indian Ocean. Proc Roy Soc 43:440–461
Bowen BW, Bass AL, Rocha LA, Grant WS, Robertson DR (2001) Phylogeography of the trumpetfishes (Aulostomus): ring species complex on a global scale. Evolution 55:1029–1039
Briggs JC (1974) Marine zoogeography. McGraw-Hill, New York
Briggs JC, Bowen BW (2012) A realignment of marine biogeographic provinces with particular reference to fish distributions. Journal of Biogeography 39:12–30
Brothers EB, Thresher RE (1985) Pelagic duration, dispersal and the distribution of Indo-Pacific coral reef fishes. In: Reaka ML (ed) The ecology of coral reefs, vol 3, No 1, Symposia series for undersea research. NOAA’s Undersea research program. US Department of Commerce, Washington, DC, pp 53–69
Burke L, Reytar K, Spalding M, Perry A (2011) Reefs at risk revisited. World Resources Institute, Washington, DC, p 130
Camhi DV, Valenti SV, Fordham SV, Fowler SL, Gibson C (2009) The conservation status of pelagic sharks and rays: report of the IUCN shark specialist group pelagic shark red list workshop. IUCN Species Survival Commission Shark Specialist Group, Newbury
Chagos Environment Network (2010) Consultation on whether to establish a Marine protected area in the British Indian Ocean Territory. protectchagos.org/wp-content/uploads/CEN_Submission-Final-FINAL.pdf
Clark MR, Tittensor D, Rogers AD, Brewin P, Schlacher T, Rowden A, Stocks K, Consalvey M (2006) Seamounts, deep-sea corals and fisheries: vulnerability of deep-sea corals to fishing on seamounts beyond areas of national jurisdiction. UNEP/WCMC, Cambridge, UK
Clark MR, Vinnichenko VI, Gordon JDM, Beck-Bulat GZ, Kukharev NN, Kakora AF (2007) Large-scale distant-water trawl fisheries on seamounts. In: Pitcher TJ, Morato T, Hart PJB, Clark MR, Haggan N, Santos RS (eds) Seamounts: ecology, fisheries & conservation, vol 12, Fish and Aquatic Resources Series. Blackwell, Oxford, UK, pp 361–399
Collette BB, et al (2011) High value and long life – double jeopardy for tunas and billfishes. Science 333:291–292
Collette BB, Parin NV (1991) Shallow-water fishes of Walters Shoals, Madagascar Ridge. Bull Mar Sci 48:1–22
Couper A (1987) The Times Atlas of the Oceans. Van Nostrand Reinhold, New York, p 256
Craig MT (2008) The goldrim surgeonfish (Acanthurus nigricans; Acanthuridae) from Diego Garcia, Chagos Archipelago: first record for the central Indian Ocean. Zootaxa 1850:65–68
Cullis-Suzuki S, Pauly D (2010) Failing the high seas: a global evaluation of regional fisheries management organizations. Mar Policy 34:1–141
Davies AJ, Guinotte JM (2011) Global habitat suitability for framework-forming cold-water corals. PLoS One 6(4):e18483
Demopoulos AWJ, Smith CR, Tyler PA (2003) Ecology of the deep Indian Ocean floor. In: Tyler PA (ed) Ecosystems of the world volume 28: ecosystems of the deep Ocean. Elsevier, Amsterdam, 569pp
Dumbraveanu D, Sheppard CRC (1999) Areas substrate at different depths in the Chagos Archipelago. In: Sheppard CRC, Seaward MRD (eds) Ecology of the Chagos Archipelago. Linnean Society/Westbury, London, p 44
Eble JA, Craig MT, Rocha L, Bowen BW (2011) Not all offspring stay close to home: trans-Pacific dispersal and gene flow in Indo-Pacific reef fishes. J of Mar Bio. Article ID 518516, 12 pages doi:10.1155/2011/518516
Eisenhauer A, Heiss G, Sheppard CRC, Dullo WC (1999) Reef and Island formation and late Holocene Sea-level Changes in the Chagos Islands. In: Sheppard CRC, Seaward MRD (eds) Ecology of the Chagos Archipelago, Special Issue of Linnean Society. Westbury for the Linnean Society of London, London, pp 21–33
Etnoyer P, Canny D, Mate B, Morgan L (2004) Persistent pelagic habitats in the Baja California to Bering Sea (BSB) ecoregion. Oceanography 17:90–101
FAO Fisheries and Aquaculture Department (2010) The State of World Fisheries and Aquaculture 2010 (SOFIA). Food and Agriculture Organisation of the United Nations, Rome, 218pp
FitzSimmons N (2010) Final report: population genetic studies in support of conservation and management of hawksbill turtles in the Indian Ocean. Marine turtle conservation fund award 98210-7-G126. Unpublished report to Multinational Species Conservation Fund, 24pp
Francis TJ, Shor GG (1966) Seismic refraction measurements in the northwest Indian Ocean. J Geophys Res 71:427–449
Gaither MR, Toonen RJ, Robertson DR, Plane S, Bowen BW (2010) Genetic evaluation of marine biogeographical barriers: perspectives from two widespread IndoPacific snappers (Lutjanus kasmira and Lutjanus fulvus). J Biogeogr 37:133–147
Gaither MR, Bowen BW, Bordenave TR, Rocha LA, Newman SJ, Gomez JA, van Herwerden L, Craig MT (2011) Phylogeography of the reef fish Cephalopholis argus (Epinephelidae) indicates Pleistocene isolation across the Indo-Pacific Barrier with contemporary overlap in the Coral Triangle. BMC Evol Biol 11(189):15
Game ET, Grantham HS, Hobday AJ, Pressey RL, Lombard AT, Beckley LE, Gjerde K, Bustamante R, Possingham HP, Richardson AJ (2009) Pelagic protected areas: the missing dimension in ocean conservation. Trends Ecol Evol 24:360–369
Gardiner JS (1936) The reefs of the western Indian Ocean. I. Chagos Archipelago. II The Mascarene region. Trans Linn Soc London 19(2):393–436
Graham NAJ, Spalding MD, Sheppard CRC (2010) Reef shark declines in remote atolls highlight the need for multi-faceted conservation action. Aquat Conserv Mar Freshw Ecosyst 20:543–548
Hahn A, Jensen MP, Broderick D, FitzSimmons NN, Bell I, Mortimer JA, Whiting S, Limpus CJ, Trott S (In prep) Stock composition of hawksbill turtle (Eretmochelys imbricata) feeding grounds in the Indo-Pacific region
Hellberg ME (2007) Footprints on the water: the genetic wake of dispersal among reefs. Coral Reefs 26:463–473
Hermes JC, Reason CJC (2008) Annual cycle of the South Indian Ocean (Seychelles-Chagos) thermocline ridge in a regional ocean model. J Geophys Res 113:10. doi:10.1029/2007JC004363
Hobbs JA, Frisch AJ, Allen GR, Herwerden LV (2009) Marine hybrid hotspot at Indo-Pacific biogeographic border. Biol Lett 5:258–261
Hughes TP, Graham NAJ, Jackson JBC, Mumby PJ, Steneck RS (2010) Rising to the challenge of sustaining coral reef resilience. Trends Ecol Evol 25:633–642
Hyrenbach K, Forney K, Dayton P (2000) Marine protected areas and ocean basin management. Aquat Conserv 10:437–458
Hyrenbach K, Fernandez P, Anderson D (2002) Oceanographic habitats of two sympatric North Pacific albatrosses during breeding season. Mar Ecol Prog Ser 233:283–301
IOTC (2010) Report of the fourteenth session of the Indian Ocean tuna commission, Busan, Korea, 1–5 March 2010. IOTC-2010-S14-R[E]
Itano DG, Holland KN (2000) Movement and vulnerability of bigeye (Thunnus obesus) and yellowfin tuna (Thunnus albacares) in relation to FADs and natural aggregation points. Aquat Living Resour 13:213–223
Jensen OP, Ortega-Garcia S, Martell SJD, Ahrens RNM, Domeier ML, Walters CJ, Kitchell JF (2010) Local management of a “highly migratory species”: the effects of long-line closures and recreational catch-and-release for Baja California striped marlin fisheries. Prog Oceanogr 86:176–186
Kaplan DM, Chassot E, Gruss A, Fonteneau A (2010) Pelagic MPAs: the devil is in the details. Trends Ecol Evol 25:62–63
Kimani EN, Okemwa GM, Kazungu JM (2009) Fisheries in the Southwest Indian Ocean: trends and governance challenges. In: Laipson E, Pandya A (eds) The Indian Ocean: resource and governance challenges. The Henry L. Stimson Centre, Washington DC, p 107
Koldewey H, Curnick D, Harding S, Harrison L, Gollock M (2010) Potential benefits to fisheries and biodiversity of the Chagos Archipelago/British Indian Ocean Territory as a no-take marine reserve. Mar Pollut Bull 60:1906–1916
Lucas JS (1982) Quantitative studies of feeding and nutrition during larval development of the coral reef asteroid Acanthaster planci (L.). J Exp Mar Biol Ecol 65:173–193
Macdonald AHH, Lamb J, Schleyer MH (submitted) Population structure of Platygyra daedalea on the south-east African coastline
Madden RA, Julian PR (1994) Observations of the 40–50 day tropical oscillation—a review. Mon Weather Rev 122:814–837
Marchant R, Mumbi C, Behera S, Yamagata T (2007) The Indian Ocean dipole – the unsung driver of climatic variability in East Africa. Afr J Ecol 45:4–16
Mees C, Pearce J, Clarke J, Wilson O, Carroll A (2008) IOTC eleventh session of the scientific committee Mahé, Seychelles, 1–5 Dec 2008: IOTC-2008-SC-INF12
Mees C, Pearce J, Clarke J, Wilson O (2009) UK (Chagos/BIOT) national report. IOTC twelfth session of the scientific committee Mahé, Seychelles, 30 Nov-4 Dec 2009: IOTC-2009-SC-INF08
Mora C, Aburto-Oropeza O, Ayala Bocos A, Ayotte PM, Banks S et al (2011) Global human footprint on the linkage between biodiversity and ecosystem functioning in reef fishes. PLoS Biol 9(4):e1000606. doi:10.1371/journal.pbio.1000606
Morato T, Hoyle SD, Allain V, Nicol SJ (2010a) Tuna longline fishing around West and Central Pacific Seamounts. PLoS One 5(12):e14453. doi:10.1371/journal.pone.0014453
Morato T, Hoyle SD, Allain V, Nicol SJ (2010b) Seamounts are hotspots of pelagic biodiversity in the open ocean. Proc Natl Acad Sci USA 107:9707–9711
Moresby, Commander Indian Navy (1884) Untitled. Trans Bombay Geogr Soc 1:307–310
Mortimer JA, Broderick D (1999) Population genetic structure and developmental migrations of sea turtles in the Chagos Archipelago and adjacent regions inferred from mtDNA sequence variation. In: Sheppard CRC, Seaward MRD (eds) Ecology of the Chagos Archipelago. Westbury Publishing/Linnean Society, London, pp 184–194
Mortimer JA, Day M, Broderick D (2002) Sea turtle populations of the Chagos Archipelago, British Indian Ocean Territory. In: Mosier A, Foley A, Brost B (compilers). Proceedings of the twentieth annual symposium on Sea turtle biology and conservation. NOAA Technical Memorandum NMFSSEFSC-477. pp 47–49
Nelson J, Bradner H (2010) The case for establishing ecosystem-scale marine reserves. Mar Pollut Bull 60:635–637
Obura DO (2012) The diversity and biogeography of Western Indian Ocean reef-building corals. PLOS ONE. http://dx.plos.org/10.1371/journal.pone.0045013
Pala C (2009) Protecting the last great tuna stocks. Science 324:1133
Palumbi SR (2004) Marine reserves and ocean neighbourhoods: the spatial scale of marine populations and their management. Annu Rev Environ Resour 29:31–68
Pauly D (1995) Anecdotes and the shifting baseline syndrome of fisheries. Trends Ecol Evol 10:430
Pearce J (1996) A review of the British Indian Ocean Territory fisheries conservation and management zone tuna fishery 1991–1995. Marine Resources Assessment Group, London
Pfeiffer M, Dullo W-C, Eisenhauer A (2004) Variability of the intertropical convergence zone recorded in coral isotopic records from the central Indian Ocean (Chagos Archipelago). Quaternary Res 61:245–255. doi:10.1016/j.yqres.2004.02.009
Pfeiffer M, Timm O, Dullo W-C, Garbe-Schönberg D (2006) Paired Sr/Ca and delta18O records from the Chagos Archipelago: late 20th century warming affects rainfall variability in the tropical Indian Ocean. Geology 34:1069–1072
Pfeiffer M, Dullo W-C, Zinke J, Garbe-Schönberg D (2009) Three monthly coral Sr/Ca records from the Chagos Archipelago covering the period of 1950 to 1995 A.D.: reproducibility and implications for quantitative reconstructions of sea surface temperature variations. Int J Earth Sci 98:53–66
Polunin NVC, Roberts CM (1993) Greater biomass and value of target coral-reef fishes in two small Caribbean marine reserves. Mar Ecol Prog Ser 100:167–176
Purkis SJ, Graham NAJ, Riegl BM (2008) Predictability of reef fish diversity and abundance using remote sensing data in Diego Garcia (Chagos Archipelago). Coral Reefs 27:167–178
Reece JS, Bowen BW, Joshi K, Goz V, Larson L (2010) Phylogeography of two moray eels indicates high dispersal throughout the Indo-Pacific. J Hered 101:391–402
Reid D, Lal K, Mackenzie-Dodds J, Kaligis F, Littlewood D, Williams S (2006) Comparative phylogeography and species boundaries in Echinolittorina snails in the central Indo-West Pacific. J Biogeogr 33:990–1006
Richard Y, Trzaska S, Roucou P, Rouault P (2000) Modification of the southern African rainfall variability/ENSO relationship since the late 1960s. Clim Dynam 16:883–895
Roberts J (2007) Preliminary characterization of bycatch by tuna longliners operating in the Chagos/BIOT area. Report for MRAG, London
Roberts RC, Sargant H (2002) Fishery benefits of fully protected marine reserves: why habitat and behaviour are important. Nat Resoure Model 15:487–507
Rogers AD, Laffoley D d’A (2011) International Earth system expert workshop on ocean stresses and impacts. Summary report. IPSO Oxford, 18pp. http://www.stateoftheocean.org/pdfs/1906_IPSO-LONG.pdf
Rogers AD, Baco A, Griffiths HJ, Hart T, Hall-Spencer JM (2007) Corals on seamounts. In: Pitcher TJ, Morato T, Hart PJB, Clark MR, Haggan N, Santos RS (eds) Seamounts: ecology, fisheries and conservation. Blackwell, Oxford, pp 141–169
Saji NH, Yamagata T (2003) Structure of SST and surface wind variability during Indian Ocean Dipole Mode events: COADS observations. J Climate 16:2735–2751. doi:10.1175/1520-0442(2003)016
Saji HH, Goswami BN, Vinayachandran PN, Yamagata T (1999) A dipole mode in the tropical Indian Ocean. Nature 401:360–363
Sale PF (ed) (2002) Coral reef fishes. Dynamics and diversity in a complex ecosystem. Academic, San Diego, 549pp
Schaefer KM, Fuller DW (2010) Vertical movements, behavior, and habitat of bigeye tuna (Thunnus obesus) in the equatorial eastern Pacific Ocean, ascertained from archival tag data. Mar Biol 157:2625–2642
Scheltema RS (1988) Initial evidence for the transport of teleplanic larvae of benthic invertebrates across the East Pacific Barrier. Biol Bull 174:145–152
Schubart CD, Diesel R, Hedges SB (1998) Rapid evolution to terrestrial life in Jamaican crabs. Nature 393:363–365
Sheppard CRC (1999) Corals of Chagos, and the role of Chagos reefs in the Indian Ocean. In: Sheppard CRC, Seaward MRD (eds) Ecology of the Chagos Archipelago. Westbury Publishing/Linnean Society, London, pp 53–66
Sheppard CRC (2009) Large temperature plunges recorded by data loggers at different depths on an Indian Ocean atoll: comparison with satellite data and relevance to coral refuges. Coral Reefs 28:399–403
Sheppard CRC, Seaward MRD (1999) Ecology of the Chagos Archipelago, Special Issue of Linnean Society. Westbury for the Linnean Society of London, London, 350pp
Sheppard CRC, Wells SM (1988) Coral reefs of the World. Volume 2: Indian Ocean, Red Sea and Gulf. UNEP Regional Seas directories and bibliographies. IUCN/UNEP, Gland/Nairobi, p 389
Sheppard CRC, and 40 others (2012a) Reefs and islands of the Chagos Archipelago, Indian Ocean: why it is the world’s largest no-take marine protected area. Aquatic Conservation: Mar Freshw Ecosyst 22:232–261
Sheppard CRC and 14 others (2012b) Conservation and management in British Indian Ocean Territory (Chagos Archipelago). Report for FCO, London, pp 28
Shotton R (2006) Managment of demersal fisheries resources of the Southern Indian Ocean, vol 1020, FAO Fisheries Circular. FAO, Rome, 90pp
Sibert J, Hampton J (2003) Mobility of tropical tunas and the implications for fisheries management. Mar Policy 27:87–95
Slatkin M (1977) Gene flow and genetic drift in a species subject to frequent local extinctions. Theor Popul Biol 12:253–262
Slatkin M (1982) Testing neutrality in subdivided populations. Genetics 100:533–545
Stoddart DR, Taylor DL (1971) Geology and ecology of Diego Garcia Atoll, Chagos Archipelago. Atoll Res Bull 149:237
SWIOFC (2009) South West Indian Ocean Fisheries Commission, report of the third session of the scientific committee. Mahe, Seychelles, 17–20 Dec 2007
Timm O, Pfeiffer M, Dullo W-C (2005) Nonstationary ENSO-precipitation teleconnection over the equatorial Indian Ocean documented in a coral from the Chagos Archipelago. Geophys Res Lett 32:4. doi:10.1029/2004GL021738, L02701
United Nations (2002) Report of the World summit on sustainable development, Johannesburg, South Africa, 26 Aug–4 Sept 2002. A/CONF.199/20. UN, NY, USA
van der Elst R, Everett B, Jiddawi N, Mwatha G, Afonso PS, Boulle D (2005) Fish, fishers and fisheries of the Western Indian Ocean: their diversity and status. A preliminary assessment. Philos Trans R Soc B 363:263–284
Vargas SM, Jensen MP, Broderick D, FitzSimmons NN, Mobaraki A, Mortimer JA, Prince R, Bell I, dos Santos FR (In prep) Phylogeography of the hawksbill turtle (Eretmochelys imbricata) in the Indo-Pacific region
Vargas SM, Jensen MP, Mobaraki A, Santos FR, Broderick D, Mortimer JA, Limpus C, Whiting S, FitzSimmons N (2010) Phylogeography of the Hawksbill turtles (Eretmochelys imbricata) from the Indo Pacific Region. Abstract, 29 April 2010. Symposium proceedings, (in press)
Veitch L, Dulvy NK, Koldewey H, Lieberman S, pauly D, Robberts CM, Rogers AD, Balie JE (2012) Avoiding empty ocean commitments at Rio + 20. Science 336:1383–1385
Vialard J, Foltz GR, McPhaden MJ, Duvel JP, de Boyer Montegut C (2008) Strong Indian Ocean sea surface temperature signals associated with the Madden-Julian Oscillation in late 2007 and early 2008. Geophys Res Lett 35:L19608. doi:10.1029/2008GL035238
Vialard J, Duvel JP, McPhaden MJ, Bouruet-Aubertot P, Ward B, Key E et al (2009) Cirene: air-sea Interactions in the Seychelles-Chagos thermocline ridge region. Bull Am Meteorol Soc 90:61. doi:10.1175/2008BAMS2499.1
Vogler C, Benzie J, Lessios H, Barber P, Wörheide G (2008) A threat to coral reefs multiplied? Four species of crown-of-thorns starfish. Biol Lett 4:696–699. doi:10.1098/rsbl.2008.0454
Vogler C, Benzie J, Barber P, Erdmann MV, Ambariyanto, Sheppard C, Tenggardjaja K, Gérard K, Wörheide G (2012) Phylogeography of the crown-of-thorns starfish in the Indian Ocean. PLoS ONE 7:e43499
Walters C (2000) Impacts of dispersal, ecological interactions, and fishing effort dynamics on efficacy of marine protected areas: how large should protected areas be? Bull Mar Sci 66:745–757
Ward JR, Rypien KL, Bruno JF, Harvell CD, Jordan-Dahlgren E, Mullen KM, Rodriguez-Martinez RE, Sanchez J, Smith G (2006) Coral diversity and disease in Mexico. Dis Aquat Organ 69:23–31
Webster PJ, Moore A, Loschnigg J, Leban M (1999) Coupled ocean–atmosphere dynamics in the Indian Ocean during 1997–98. Nature 401:356–360
Wilkinson C (2008) Executive summary. In: Wilkinson C (ed) Status of coral reefs of the World: 2008. Global Coral Reef Monitoring Network and Reef and Rainforest Research Center, Townsville, pp 5–19
Wilson JR, Harrison PL (1998) Settlement-competency periods of larvae of three species of scleractinian corals. Mar Biol 131:339–345
Winterbottom R, Anderson RC (1997) A revised checklist of the epipelagic and shore fishes of the Chagos Archipelago, Central Indian Ocean. JLB Smith institute of ichthyology. Ichthyol Bull 66:1–28
Wood LJ, Fish L, Laughren J, Pauly D (2008) Assessing progress towards global marine protection targets: shortfalls in information and action. Oryx 42:340–351
Worm B, Lotze K, Myers R (2003) Predator diversity hotspots in the blue ocean. Proc Natl Acad Sci 100:9884–9888
Worm B, Sandow M, Oschlies A, Lotze HK, Myers RA (2005) Global patterns of predator diversity in the open oceans. Science 309:1365–1369
Yesson C, Clark MR, Taylor M, Rogers AD (2011) The global distribution of seamounts based on 30-second bathymetry data. Deep Sea Res Part I Oceanogr Res Pap 58:442–453. doi:10.1016/j.dsr.2011.02.004
Yesson C, Taylor M, Tittensor DP, Davies A, Guinotte J, Baco-Taylor A, Black J, Hall-Spencer J, Rogers AD (2012) Global distribution and habitat preferences of deep-sea octocorals. J Biogeogr 39:1278–1292
Zubair L, Ropelewski CF (2006) The strengthening relationship between ENSO and northeast monsoon rainfall over Sri Lanka and southern India. J Climate 19:1567–1575
Acknowledgements
Some of this material was published previously in Sheppard et al. (2012) and is reproduced with permission of Wiley & Sons.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Sheppard, C.R.C. et al. (2013). British Indian Ocean Territory (the Chagos Archipelago): Setting, Connections and the Marine Protected Area. In: Sheppard, C. (eds) Coral Reefs of the United Kingdom Overseas Territories. Coral Reefs of the World, vol 4. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-5965-7_17
Download citation
DOI: https://doi.org/10.1007/978-94-007-5965-7_17
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-5964-0
Online ISBN: 978-94-007-5965-7
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)