Abstract
Hematopoietic stem cells represent the most studied and understood adult stem cell, and have consequently set the trends for the investigation of a wide array of stem cells, while their clinical use for over half a century and ever improving efficacy encourages the view that stem cell therapy will one day be useful in the treatment of a whole host of diseases that involve cellular loss. In this chapter we describe how hematopoietic stem cells can be identified, isolated and characterized, and how important it is to be able to conduct experiments on animal models as well as humans, especially as studies in animals can provide the best, sometimes only, way to test stem cell potential and new protocols for their therapeutic use. The increasing possibilities for bone marrow regenerative medicine raised by the rapid developments in our ability to derive pluripotent stem cells from any individual are discussed, in particular because these are likely to be a very effective source of hematopoietic stem cells for all people requiring them to be replaced, as well as the exciting prospect that they can provide a route for the correction of inherited diseases affecting the blood system.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The hematopoietic stem cell (HSC) represents a paradigm for much of present day stem cell biology and regenerative medicine, the first therapeutic application, predating any knowledge of its characterization or even of its actual existence, being the pioneering development by E. Donnall Thomas in 1957 of bone marrow transplantation (BMT) as a therapy to alleviate the consequences of radiation and chemotherapy (Thomas et al. 1957). This groundbreaking therapy formed the dogma that tissue stem cells held the future promise for regenerative medicine for numerous diseases. The strategies for characterization, purification and bioassay of HSCs have therefore been adapted for many other tissue-specific stem cells, while the drive to understand the cellular and molecular properties of HSCs has provided a framework for comparison to both embryonic and adult stem cell types. Studies on HSCs and comparison to the behaviour of leukemia cells was also highly influential in the origin of the concept of the cancer stem cell as the underlying component of many, perhaps all, tumors, and an exciting target for novel therapies that may succeed in achieving life-long remission where traditional treatments often fail.
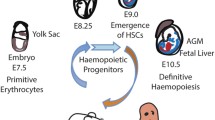
Given the extensive history in the use of HSCs in therapeutic practice, it would be easy to assume that these cells are well understood, however HSC research is a dynamic area, continually being revolutionized. Once believed to be a homogenous population, it has since emerged that the HSCs are actually not a single entity, but rather a collection of cell subtypes with largely pre-programmed differentiation and self-renewal behaviours, both of which will be discussed in detail below. The nature of HSCs also varies throughout development, distinct cell types arising to provide a transient source of hematopoietic cells. Since it is beyond the scope of this chapter, the reader is referred to one of the many excellent reviews that discuss the developmental aspects of HSC biology (Dzierzak et al. 1998; Mikkola and Orkin 2006; Cumano and Godin 2007; Dzierzak and Speck 2008), and here we will concentrate on those HSCs that have the most relevance to regenerative medicine, namely adult bone marrow derived cells and those HSCs that can be isolated from umbilical cord blood.
The necessity for continual regeneration of the various lymphoid (B-cells, T-cells, natural killer cells, dendritic cells) and myeloid (red cells, platelets, monocytes/macrophage, dendritic cells, granulocytes) cells that constitute the hematopoietic system is emphasized when we consider the vast number of cells, approximately 1012, arising in human bone marrow on a daily basis (Doulatov et al. 2012). These mature adult hematopoietic cells are generated through a succession of hierarchical steps initiating at the apex of the hematopoietic system with the HSC. The HSC gives rise to a series of transient amplifying progenitor cell populations with a gradual decrease in proliferative potential and an increase in cellular specialization, resulting ultimately in the supply of terminally differentiated functional blood cell types that make up the lymphoid and myeloid compartments. Although the hematopoietic system has been extensively studied for several decades, it is only recently that we have begun to understand some of the mechanisms by which HSCs are able to so proficiently play their role. These developments have been made with the help of improving technology, allowing complex cell sorting strategies to isolate rare HSCs to high purity and viability in order to further quantify and characterise them.
Parallel studies are now being done with human HSCs but the advancement of our knowledge of these cells is trailing behind that of the mouse. The primary indicator of stem cell activity being their ability to function in repopulation assays poses an obvious difficulty in human stem cell research. Secondly, a major obstacle in human HSC research is that the cells are incredibly rare, with only 1 in 106 cells in human bone marrow being a functional transplantable stem cell (Wang et al. 1997) and the availability of novel markers of these cells for purification from the bulk of differentiated cells hinders progress further.
In addition to increasingly refined definition of HSCs, especially those that have the greatest potential for application in a therapeutic context, key areas of investigation that will impinge heavily on the success or otherwise of advances in regenerative medicine include finding ways to expand HSCs in vitro without loss of any aspect of their functional potential, and improving upon the efficiency with which transplanted cells integrate into the hematopoietic system. However, perhaps the most exciting challenge, which could 1 day lead to an unlimited ability to provide replacement HSCs personalised for the patient, is their derivation from pluripotent stem cells, and this will be discussed with respect to advances that have been made using embryonic stem (ES) cells and more recently with the discovery of methods to produce so called induced pluripotent stem (iPS) cells from any cell in the body.
2 Derivation/Classification
Although the first application of bone marrow derived stem cells in a therapeutic context occurred over five decades ago, the vast majority of our understanding of the nature of HSCs has come from studies on mouse bone marrow. The single biggest hurdle in the identification and purification of HSCs from mouse bone marrow is their very low abundance; depending on the precise criteria applied this is only 0.05% or less of the nucleated cells, resulting in the isolation of around 5,000 HSCs per mouse. Modern day laboratories utilize two main methods for isolating HSCs from bone marrow. First is an enrichment method (MACS) employing magnetic beads conjugated to antibodies against a specific surface marker. The second, and notably more precise separation technique, utilizes fluorescence activated cell sorting (FACS), which is based on immunofluorescent labeling of surface antigens as an analytical tool to achieve cell sorting (Challen et al. 2009). Modern cell sorters are now equipped to analyse up to 18 fluorochrome-labeled antibodies directed against multiple markers (usually designated as ‘Cluster of Differentiation’ or CD markers) enabling prospective isolation of more infrequent cells, which can then be subjected to bioassay to assess their stem cell potency.
2.1 Bioassays of HSCs
In the strictest sense, the HSC is defined by its functional capacity to reconstitute the entire hematopoietic system for the lifetime of the individual or animal; however, a number of less stringent bioassays are also widely used, often as a preliminary guide because the definitive in vivo bone marrow transplantation assay is both time-consuming and costly.
The first assays of hematopoietic progenitor cell potential in vivo can be attributed to James Till and Ernest McCulloch, who famously demonstrated that colonies of myeloid cells developing in the spleen following transplantation of bone marrow into lethally irradiated mice were clonal (Till and McCulloch 1961). However, the existence of long-lived stem cells in the bone marrow was deduced from subsequent experiments involving clonal tracking of serial transplantations (Dick et al. 1985; Lemischka et al. 1986). Arising out of these studies, the current gold standard assay is generally accepted to be long-term repopulation of lethally irradiated mice in a situation in which the cells being tested are compared to a reference wild type population (Harrison et al. 1993), most often using test and reference strains that are congenic for allelic variants of CD45 (previously known as Ly5), which can easily be distinguished by immunofluorescent flow cytometry. ‘Long-term’ is taken to mean a sustained output from the graft of at least 1% of all circulating white blood cells for at least 4 months (Purton and Scadden 2007), but the most rigorous test of HSC potential involves assessment of their ability to be serially transplanted from the primary reconstituted recipient to a secondary irradiated host, thereby demonstrating that engrafting cells are undergoing self-renewal. Competitive repopulation assays performed this way are at best semi-quantitative, and a more refined method, involving limiting dilution, allows determination of the frequency of HSCs. In this assay, a series of dilutions of the test population are competed against reference wild type bone marrow cells. The number of mice negative for reconstitution in each cell dose is measured and the frequency of HSCs (‘competitive repopulating units’ or CRU) is estimated using Poisson statistics (Szilvassy et al. 1990). Purton and Scadden (2007) discuss the finer details of repopulation assays, how they are best interpreted, and their possible limitations.
Although in vivo assays are essential in order to fully define and quantify stem cell potential, they have some limitations that can be complemented by a range of assays that can be performed in vitro. First, and rather obviously, in vivo assays can take many months to complete and require extensive and costly facilities, so it is often useful to have a more simple assay that can be used to make an initial assessment of the likely HSC content, for example while developing a strategy for prospective cell sorting or following some experimental manipulation that is expected to have a significant effect on HSC function. Second, the output from an in vivo assay is the consequence of many biological events following transplantation, including homing, self-renewal, HSC commitment and the behaviour of downstream progenitors and differentiated cells, and it is often important to be able to determine cellular properties at a single cell level immediately following isolation of putative HSCs. Several distinct in vitro assays are used that measure the frequency of progenitors (colony-forming unit in culture; CFU-C), stem cells (long-term culture-initiating cell; LTC-IC), or both (cobblestone area-forming cell assay; CAFC), the latter two correlating at least to some extent with in vivo activity (van Os et al. 2004).
CFU-C assays, pioneered by the work of Don Metcalf and colleagues (Bradley and Metcalf 1966; Moore et al. 1973), allow detection and quantification of myeloid progenitors present in the population of cells being analyzed or that could have arisen in vitro from more immature cells, including the HSC. The culture conditions rely on the presence of growth factors and nutrients that will permit complete differentiation along one or more of the pathways of differentiation that a particular cell is expected to be capable of adopting. CFU-C assays have been essential for determining the specific growth factors necessary for HSC maintenance, proliferation and differentiation. They have also been crucial in the characterisation of leukemic stem cells (LSCs). The assessment of lymphoid CFU potential in vitro has in the past been more difficult, requiring co-culture systems such as that of OP9-DL1 cells, a mouse stromal cell line that ecotropically expresses the Notch ligand Delta-like 1 (DL1) for establishing T-cell differentiation (Whitlock and Witte 1982; Schmitt and Zúñiga-Pflücker 2002). However, recent demand for improved mouse B-cell differentiation has led to the development of media capable of supporting such specification, similar to that already used for myeloid lineages. The progress for human lymphoid cell differentiation is, however, still somewhat marred due to the insufficient knowledge of the cytokines responsible for this (Doulatov et al. 2012). Human B-cell differentiation is feasible when HSCs are co-cultured for 2–4 weeks upon the stromal cell lines MS-5 or S17 in the presence of SCF, TPO, IL-7 and IL-2.
The basic principle of such in vitro assays is to determine what a stem cell or progenitor is capable of giving rise to and their proliferative abilities following gene manipulation, as can be recognized after a number of days by the specific features of the differentiated cells (for example surface marker expression, cell morphology and the presence of characteristic cytoplasmic enzyme activities, etc). In the right conditions, a HSC can give rise to multiple cell lineages, whereas a more mature hematopoietic progenitor cell will have a more restricted capability. Since it would not be possible to discriminate from whence the individual differentiated cells originated if such assays were performed in a liquid culture of the whole sorted population, these assays are generally carried out in one of two ways so that the potential of individual cells can be observed. Most commonly, a cell population is seeded into the appropriate growth conditions in media that also contain a substance that is like a soft gel (usually methycellulose). This prevents the cells from moving around extensively, and if seeded at the correct density means that the differentiated derivatives from each cell are clearly separated and can eventually be collected for phenotypic analysis. Alternatively, sorted stem cells can be deposited as single cells into tiny individual wells in a plastic dish where they then can be allowed to grow and differentiate in liquid conditions (Ema et al. 2000; Takano et al. 2004).
The CAFC and LTC-IC assays, based on the original studies by Dexter and colleagues (Dexter et al. 1977), involve culture of stem cell populations with adherent cells that mimic the normal HSC microenvironment. The CAFC assay measures the frequency of cells that are capable of growing under the stromal layer, and by enumerating so-called ‘cobblestone’ areas at various times it is possible to assess mature progenitors back to repopulating HSCs (Ploemacher et al. 1989, 1991). The LTC-IC assay is similar to the CAFC assay except that the readout is the presence of progenitors that can themselves be assayed for CFU-C capability (Sutherland et al. 1989; Lemieux et al. 1995).
Just as many of the in vitro assays are adaptable for the measurement of both murine and human stem cells and progenitors, there is an equal and ever growing need in the context both of regenerative medicine and for therapeutic targeting of diseased cells to be able to test in vivo potential of human HSCs. Since this is clearly not feasible in humans a number of approaches have been developed over the last 30 years that rely on the generation of in vivo chimeras of human cells, or ‘xenografts’, in animals. Although not ideal from the perspective of logistics, cost, and time, several investigators have shown that human HSCs can be engrafted in sheep by direct introduction of cells into the early gestational age fetus. By in utero injection of human fetal liver HSCs, Esmail Zanjani and colleagues were able to demonstrate long-term (greater than 2 years) engraftment in sheep (Zanjani et al. 1992). Subsequently, this approach was used to prove for the first time that human adult bone marrow cells could elicit long-term chimerism and sustain human hematopoiesis (Srour et al. 1993), and has been further developed (Zanjani et al. 1995, 1998), enabling most recently the demonstration that engrafted human cells can be mobilized in the sheep model in the same way that they would normally be in human donors (Almeida-Porada et al. 2007).
By far the majority of xenograft experiments of human HSCs have been performed in mice, but unlike the experiments involving sheep, the human cells are injected into adults, thereby requiring that the hosts are immunologically deficient so that they will not bring about rejection of the xenogenic cells. Two strategies for human-into-mouse engraftment were developed originally by the work of McCune and Dick. In the first approach, human cells within a fragment of hematopoietic tissue are grafted under the kidney capsule, which provides a permissive environment for the donor cells (McCune et al. 1988; Namikawa et al. 1990; Chen et al. 1994). However, the much more widely utilized method involves adaptation of the usual protocol for transplantation of mouse HSCs into host animals, the main difference being that the mice are subjected to sub-lethal irradiation to pre-condition the bone marrow by increasing the opportunity for HSCs to occupy vacant niches (Kamel-Reid and Dick 1988).
To minimize the possibility of the human cells being rejected, several immunocompromised mouse models have been developed, in particular relying upon the Severe Combined Immunodeficiency (SCID) mutant strain (for reviews see Greiner et al. 1998; Pearson et al. 2008). As for mouse-into-mouse repopulation assays, xenografts of human cells can be made quantitative through a limiting dilution approach, the term SCID Repopulating Unit (SRU) usually being adopted to define the numbers of functional long-term HSCs. SCID mice are deficient in both B- and T-cell mediated immunity, and their usefulness has been enhanced in various ways through combination with other spontaneous or engineered mutations. The strain that has been most commonly used for xenografting is a combination of the Non-Obese Diabetic (NOD) mutation with SCID, usually referred to as NOD/SCID, which lacks not only functional B and T lymphocytes, but also has low levels of natural killer (NK) cell activity (Shultz et al. 1995). A reduced overall cellularity of the bone marrow in NOD/SCID mice may also facilitate engraftment of HSCs because of the availability of suitable niches for stem cells. The NOD/SCID mouse exhibits some features that partially limits its usefulness, especially for long-term xenograft models, such as shortened lifespan due to high incidence of thymic lymphoma, some spontaneous production of functional lymphocytes with aging, and residual innate immunity. Further incorporation of the β2 microglobulin knockout into the NOD/SCID background increased the efficiency of repopulation by umbilical cord blood cells by over tenfold (Kollet et al. 2000).
To circumvent the problems associated with NOD/SCID mice as models for xenografts, NOD/SCID mice with a truncation or a deletion of the IL-2R common γ chain, which is a critical component for IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21 signaling, were developed (the so-called ‘NSG’ strain, Ito et al. 2002). The deletion of the IL-2R common γ chain gene in mice results in a complete loss of B, T and NK cells. The NSG strain was shown to support a fivefold higher CD34+ cell engraftment compared with NOD/SCID mice (Goldman et al. 1998). The second benefit of these mice is that the deficiency in cytokine signaling prevented the formation of lymphomas, permitting long-term studies (Ito et al. 2002; Ishikawa et al. 2005; reviewed by Ito et al. 2008).
As hematopoietic research progresses and the importance of specific growth factors are determined, many investigators have found that the efficiency of engraftment of human HSCs can be enhanced by co-transplantation of accessory cells or treatment of the host with cytokines. For example, Bonnet et al. (1999) demonstrated that low numbers of purified cord blood-derived immature cells would engraft NOD/SCID mice effectively if co-transplanted with more mature cell populations that had been irradiated to prevent cell division or by short-term in vivo treatment with the growth factors and cytokines stem cell factor (SCF), interleukin 3 (IL3) and granulocyte macrophage colony stimulating factor (GM-CSF). Similarly, bone marrow chimerism in the SCID or Rag2/IL2Rγ double knockout models could be facilitated by administration of IL3, GM-CSF and erythropoietin (Lapidot et al. 1992) or IL3, GM-CSF and erythropoietin (Mazurier et al. 1999), respectively. Co-transplantation of stromal cells has also been shown to have some benefit in establishing HSC xenografts in mice. Hence, primary bone marrow stroma modified to express IL3 was able to enhance HSC engraftment (Nolta et al. 1994), while unmodified mesenchymal stem cells (MSCs) derived from the fetal lung or bone marrow increased HSC engraftment, but in the latter case it appeared that the effect might not to require homing of MSC to the bone marrow (Noort et al. 2002; in ‘t Anker et al. 2003; Bensidhoum et al. 2004). Advances in the last year in strains for xenografting have seen the generation of mice with cytokine-expressing transgene knock-ins, encoding for example TPO, IL-3, and GM-CSF, all of which have exhibited augmented engraftment of the human cells following transplantation (Rongvaux et al. 2011; Willinger et al. 2011).
This xenograft method has also been adopted to assess LSC behaviour from human patient samples and can hence act as a model for therapeutic approaches.
The protocols for engraftment of HSCs have undergone a number of modifications over the years, including additional preconditioning of mice by treatment with clodronate-containing liposomes in order to delete macrophage (Fraser et al. 1995; van Rijn et al. 2003) or with an antibody against the surface antigen CD122 in order to target NK cells and macrophage that act as a barrier to stem cell engraftment (McKenzie et al. 2005). Furthermore, to overcome the limitations of homing and cellular loss in the lungs that is inherent in intravenous injection of cells via the tail vein, a number of investigators have achieved much improved rates of engraftment by direct injection of HSCs into the bone marrow cavities of either the femur or tibia (Kushida et al. 2001; Wang et al. 2003; Yahata et al. 2003; McKenzie et al. 2006).
2.2 HSC Antigenic Phenotype and Purification Schemes
Over the last quarter of a century the combined power of flow cytometry and the availability of monoclonal antibodies raised against a vast array of hematopoietic cell surface molecules, together with the various bioassays described above, has enabled an incredibly detailed definition of the heterogenous population of cells with stem cell activity in the hematopoietic hierarchy. With perhaps one exception that will be described later, no single surface molecule has yet been found that enables identification of HSCs; however, a number of markers have been described that can be used in combination to very precisely define stem cells, the particular drive being to isolate the rare cells with the highest potential for long-term reconstitution. The advances in this area have been most successful in the case of mouse bone marrow, and the current state of play will be elaborated before considering what we know about the phenotype of human HSCs derived from the bone marrow or umbilical cord blood.
2.2.1 Mouse HSCs
Most sorting strategies rely upon negative selection for markers of the differentiated hematopoietic lineages (Lin), which usually include B220, CD4 (sometimes CD3 or CD5 instead), CD8, Gr1, CD11b (Mac-1) and Ter119, in combination with positive selection for c-Kit (the receptor for SCF) and Sca-1 (stem cell antigen-1) (Okada et al. 1992), giving rise to the acronym LSK (or KSL, depending on laboratory preference). Historically, the Weissman group has been the driving force for the purification of HSCs and their favoured protocol incorporates staining for the Thy1.1 antigen and selection of cells that express only low levels together with an absence of lineage markers and the presence of Sca-1 (Thy1.1lo Lin- Sca-1+ or TLS cells; Spangrude et al. 1988), although this precise strategy has not been widely adopted because the Thy1.1 antigen is not expressed on many of the most commonly used laboratory strains of mice. Both LSK and TLS populations contain long-term repopulating cells (LT-HSCs), but these represent less than 10% of the LSK cells, the remains of which have only short-term activity (ST-HSCs) or are multipotent progenitors (MPPs) with no capacity for self-renewal. Following these early studies, there has been an ever-driving urge to discover a unique marker of the mouse LT-HSC that truly distinguishes it from the heterogeneous population of stem cells. A number of investigators have identified additional markers that can be used to resolve the stem cell and progenitor components within the LSK population, and to this day improvements are still being published on a fairly regular basis. The Nakauchi laboratory were first to show that the expression of CD34 could be used to discriminate LT-HSCs, in that single LSK CD34+ cells were able to bring about long-term reconstitution (Osawa et al. 1996). The subsequent addition of Flt-3 (also known as Flk-2 and CD135) into the mix allowed prospective purification of not only LT-HSCs (LSK CD34− Flt3−), but also ST-HSCs (LSK CD34+ Flt3−) and MPPs (LSK CD34+ Flt3+) (Christensen and Weissman 2001; Adolfsson et al. 2001; Yang et al. 2005). More recently, due to the adoption of more precise cell sorting strategies, it has become possible to fractionate these stem cell populations further into more discrete fractions with more specific properties. Hence, data suggests that the MPP initially differentiates into lymphoid-primed multipotential progenitors (LMPPs), which retain the potential to give rise to lymphoid and granulocyte-macrophage cells but which lack megakaryocyte-erythroid potential (Adolfsson et al. 2005; Lai and Kondo 2006), while more committed myeloid and lymphoid progenitors lie downstream of these LMPP cells (Akashi et al. 2000; Pronk et al. 2007). These latter publications led to a redrawing of the accepted hematopoietic hierarchy model, and as further work elucidates more and more discrete sub-populations within the HSC compartment, it is likely that the hematopoietic hierarchy as it is currently understood will undergo yet more restructuring in the future.
In addition to surface marker expression, there are other characteristics of HSCs that can be employed for their identification and isolation using flow cytometry, often most effective when used in combination with strategies such as those employing LSK or related staining protocols. The most widely used characteristic relies on the ability of HSCs to actively expel small molecules from their cytoplasm, a mechanism of cytotoxic evasion. A family of transmembrane proteins known as ABC transporters are involved in a wide variety of normal cells and stem cells with the purpose of removing diverse chemicals. One family member, ABC-G2, is often expressed by stem cells and has the ability to export certain chemical dyes that have entered the cytoplasm by passive diffusion. Empirically, it was found that one such DNA binding supravital dye, Hoechst 33342, is removed by ABC-G2 and that this can be visualized with a flow cytometer by measuring red and blue fluorescent light emissions upon stimulating with a UV laser. In the complex pattern of light emitted by a mixture of cells treated with Hoechst 33342, many stem cells appear as a population, usually called the ‘side population’, which exhibits low red and blue fluorescence because the dye has been largely removed by the transporter (Goodell et al. 1996). The drawbacks of the Hoechst 33342 exclusion method are that the staining method is highly sensitive to slight changes in protocol, producing inconsistencies between HSC isolations. Unfortunately, side population characteristics are not restricted to stem cells, with approximately 15% of whole bone marrow side population being negative for the stem cell markers c-Kit and Sca-1 (Challen et al. 2009). Also, not all stem cells exhibit the property, and therefore the technique is best utilized in combination with other methods, especially surface marker staining, as a means to refine stem cell identification and isolation (Challen et al. 2009). The other major flow cytometry method not involving specific antibodies makes use of the fluorescent vital dye rhodamine 123 (Rh-123), which preferentially accumulates in mitochondrial membranes and acts as an indicator of mitochondrial, and hence cellular, activity. Since the more immature HSCs tend to be quiescent, sorting for cells exhibiting a low degree of fluorescence in the presence of Rh-123 enriches for long-term repopulating cells (Spangrude and Johnson 1990).
The most recent and highly resolved strategies for the isolation of long-term repopulating HSCs have largely built upon the basis of one or more of the LSK, side population and Rh-123 staining methods. Chen et al. (2003) found that immunofluorescent staining for the ancillary TGFβ receptor endoglin (CD105) in combination with Sca-1 expression and low staining for Rh-123 defines a nearly homogenous population of LT-HSCs without the use of CD34, c-Kit or Lin markers. As discussed later in this chapter, another feature of immature HSCs is their tendency to be niche-associated and a marker linked to this property, namely the angiopoietin-2 receptor Tie2, has been used to select a subpopulation of LSK cells that are enriched in LT-HSCs (Arai et al. 2004). Two advances based on RNA microarray screening for genes expressed exclusively in subfractions of HSCs have probably made the most significant contribution to the robust isolation of highly enriched long-term repopulating cells. First, following initial identification from expression screening, antibodies against cell surface receptors of the SLAM family, including CD48, CD150 and CD244, were shown to discriminate HSCs (CD48− CD150+ CD244−), MPPs (CD48− CD150− CD244+) and the most restricted progenitors (CD48+ CD150− CD244+) (Kiel et al. 2005). This is the first family of receptors whose combinatorial expression can be used to precisely distinguish stem and progenitor cells in the mouse. Similarly, microarray technology led to the identification of murine endothelial protein C receptor (EPCR, CD201) as a marker to sort cells, especially when used in combination with positivity for the antigen Sca-1, as it is expressed at high levels in HSCs with a high reconstitution activity, and probably represents the first known marker that ‘explicitly’ identifies HSCs within murine bone marrow (Balazs et al. 2006). Most recently, the group of Conny Eaves has combined these two latter approaches and shown that LT-HSCs with the most durable self-renewal potential, as demonstrated following serial transplantation, are selectively and highly enriched in the CD150+ subset of the EPCR+ CD48− CD45+ fraction of bone marrow cells (Kent et al. 2009).
2.2.2 Human HSCs
The ability to identify and purify long-term reconstituting human HSCs are at present somewhat less sophisticated compared to the situation with the mouse due to the lack of adequate methods to segregate HSCs from MPPs. Similar to the mouse, purification of human HSCs requires simultaneous detection of several cell surface markers, and although informative, the specific strategies for isolating mouse HSCs cannot be duplicated for human HSC. This is due to differences in characteristic marker expression between the two species, the most prominent difference residing in their expression of CD34. The two principal sources of human HSCs for therapeutic application, namely bone marrow and umbilical cord blood, also demonstrate some differences in the precise pattern of markers, raising extra difficulties in determining the best strategies for cell purification in the clinic. Nevertheless, human HSCs capable of multilineage engraftment in animal models can now be resolved with a reasonably high degree of enrichment.
The majority of human HSCs are CD34+ in contrast to mouse, as was first demonstrated during the 1990s when human Lin−CD34+ fetal bone marrow cells were shown to be able to engraft in SCID mice (Baum et al. 1992). However, although capable of engrafting in SCID mice, most CD34+ cells were subsequently shown to be lineage-restricted progenitors and the true HSC remained elusive. Enrichment of human HSCs can be achieved further on the basis of expression of CD45RA (Mayani et al. 1993), Thy-1 (Baum et al. 1992; Craig et al. 1993; Majeti et al. 2007) and CD38 (Hao et al. 1995; Bhatia et al. 1997). The recognized pattern of expression that segregates human HSCs from MPPs is that of CD34+ CD38−CD45RA− and loss of Thy1 expression (Majeti et al. 2007).
In contrast to CD34+ subfractions, Lin− CD34− CD38− cells have low clonogenicity in short-and long-term in vitro assays. However, the number of CD34− SRUs increased in short-term suspension cultures in conditions that did not maintain SRU derived from CD34+ populations (Bhatia et al. 1998).
Based on its association with colony-forming potential and repopulation capacity, CD34 expression has, until recently, remained as a convenient marker for human HSCs. However, it has since been postulated that there is in fact a human CD34− HSC that is analogous to that of the mouse (Bhatia et al. 1998; Ando 2002; Engelhardt et al. 2002; Guo et al. 2003; Ishii et al. 2011), adding an increased complexity to the organisation of the human hematopoietic stem cell compartment.
Chimeras generated in either sheep or immunocompromised mice have shown that CD34− cells from cord blood, bone marrow, and granulocyte colony stimulating factor (G-CSF)-mobilized peripheral blood do have in vivo HSC activity in spite of failing to exhibit significant clonogenic activity in vitro. Further defining the phenotype of cord blood-derived CD34− SRUs, Kimura et al. (2007) proposed that the immunophenotype of very primitive long-term repopulating human HSCs is Lin− CD34− c-Kit− Flt3−. Paralleling studies on mouse HSCs, Goodell et al. (1997) showed that human bone marrow contains side population cells and, interestingly, that these too are CD34−. As in the mouse, Rh-123 staining can be employed in defining human HSCs, low dye retention being associated closely with the Lin− CD34+ CD38− population (McKenzie et al. 2007). However, taking all of this knowledge into account and using simple calculations of reported HSC frequencies, it can be established that more than 99% of human HSCs must be CD34+.
The differences between antigen expression on mouse and human HSCs is not unique to CD34, and other distinctive variations can be seen in the expression of the Flt-3 receptor, which is expressed on the surface of human HSCs but not on the mouse (Sitnicka et al. 2003), and the SLAM marker CD150, which unlike in the mouse is absent on human HSCs (Sintes et al. 2008; Larochelle et al. 2011).
Due to the discrepancies in the expression of CD34 and its relationship to stem cell activity, research continues to define better markers of human HSCs. A recent publication from the laboratory of John Dick revealed a novel human HSC marker, namely CD49f (α6 integrin). Single CD49f+ cells were shown to be capable of generating highly efficient long-term multilineage grafts and that loss of CD49f expression coincided with transient engrafting MPPs (Notta et al. 2011). Such markers could pave the way for the isolation of pure populations of human HSCs for therapeutic use and further research into HSC properties.
A number of additional discriminators of human HSC subpopulations have been investigated. Two features of human HSCs that have proven useful for the isolation of the most immature cells are worthy of mention. First, is the relative high expression of aldehyde dehydrogenase (ALDH) in hematopoietic progenitor cells (Kastan et al. 1990). Cord blood cells stained for ALDH activity using the substrate BODIPY-aminoacetaldehyde (‘Aldefluor’) and depleted for Lin+ cells are enriched for CD34+ CD38− cells (Storms et al. 1999). Second, and perhaps the more useful property of long-term repopulating human HSCs, is their expression of CD133, an antigen that characterizes several types of adult stem cells. For example, a rare population of cord blood cells expressing CD133 and negative for CD7 were found to be highly enriched for progenitor activity at a frequency equivalent to purified fractions of CD34+ stem cells, and they were the only subset among the Lin− CD34− CD38− population capable of giving rise to CD34+ cells in defined liquid cultures and of engrafting in NOD/SCID mice (Yin et al. 1997; Gallacher et al. 2000). Cell selection combining Lin antigen depletion together with staining for ALDH activity and CD133 expression provides a purification of HSCs with long-term repopulating function that has been considered to be an alternative to CD34 cell selection for stem cell therapies. Hence, limiting dilution analysis demonstrates a tenfold increase in the frequency of repopulating cells compared with Lin− CD133+ cells, with maintenance of immature hematopoietic phenotypes (CD34+ CD38−) and enhanced repopulating function in recipients of serial, secondary transplants (Hess et al. 2006).
3 Characteristics/Properties
Like other adult stem cells, HSCs are regulated and supported by the surrounding tissue microenvironment, generally referred to as the stem cell ‘niche’. As already discussed in detail in Chap. 3, the niche includes all cellular and non-cellular components that interact in order to control the adult stem cell, and the reader is also referred to a number of excellent recent reviews that specifically discuss the nature of these in relation to the HSC in the bone marrow (Taichman 2005; Wilson and Trumpp 2006; Li and Li 2006; Kiel and Morrison 2008; Raaijmakers and Scadden 2008; Mercier et al. 2011).
In brief, the current perception of HSCs in the bone marrow is that they reside at the interface of bone and the bone marrow (the endosteum), but it remains uncertain whether this interface itself is a niche, or whether endosteal cells secrete factors that diffuse to nearby niches. Indeed, recent work from the laboratory of David Scadden has shown that HSCs can reside in a niche that appears to involve a very close juxtaposition of both osteoblasts and microvessel endothelial cells (Lo Celso et al. 2009). Vascular or perivascular cells may also create niches as many HSCs are observed around sinusoidal blood vessels, and perivascular cells secrete factors that regulate HSC maintenance.
It is important that the bone marrow niche should not be viewed as a static environment, since both the hematopoietic and immune systems are required to respond rapidly and adapt to the needs of the individual, it should therefore be regarded as a fluid system that continually processes information from the organism as a whole (Mercier et al. 2011). Much of the knowledge that we have obtained from studies of the normal bone marrow microenvironment is leading us to a better understanding of the ways in which leukemic stem cells (LSCs) can manipulate the niche to enhance their survival and proliferation. Due to this adaptable nature of the bone marrow niche it is becoming clear that it represents a novel therapeutic target, its manipulation, for example by pharmacological enhancement of the number and function of osteoblasts, being a potential way to augment the effectiveness of stem cell therapies (Adams and Scadden 2008).
Much of the life of a HSC within its niche is one of inactivity in which it replicates only relatively infrequently. This state of ‘quiescence’ is thought to be an indispensable property for the maintenance of HSCs, protecting them from stress and hence the accumulation of DNA mutations and enabling them to sustain life-long hematopoiesis. The molecular mechanisms through which the niche controls the HSC cell cycle to establish quiescence are beginning to be elucidated. For example, it has been shown that the interaction of the Tie2 receptor tyrosine kinase with its ligand Angiopoietin-1 leads to tight adhesion of HSCs to stromal cells, and maintenance of their long-term repopulating activity (Arai et al. 2004). In spite of their generally quiescent state, the normal homeostatic balance in the hematopoietic system requires HSCs to be able to exit the niche and then achieve several transits through vascular endothelium to be able to migrate through the blood, enter different organs and then return back to the bone marrow. These processes of migration and specific homing need to be amplified during stress-induced recruitment of leukocytes from the bone marrow reservoir and during stem cell mobilization as part of defense and repair. Both HSC mobilization (reviewed by Pelus and Fukuda 2008) and homing (reviewed by Lapidot et al. 2005; Chute 2006) are also crucially important in the context of clinical stem cell transplantation.
HSCs induced to exit the bone marrow and mobilize to the peripheral blood following treatment with granulocyte-colony stimulating factor (G-CSF) have become the most widely used source of HSCs for engraftment and show significant superiority to cells obtained directly from the bone marrow. In addition to G-CSF, the growth factor SCF, adhesion molecules such as VLA-4 and P- and E-selectins, chemokines, proteolytic enzymes such as elastase and cathepsin G, and various matrix metalloproteinases (MMPs) have all been shown to have a role in stem cell mobilization. The chemokine stromal-derived factor 1 (SDF-1 or CXCL12) and its receptor CXCR4 are major players involved in the regulation of HSC mobilization and homing. During steady-state homeostasis, CXCR4 is expressed by HSCs and also by stromal cells, which are the main source for SDF-1 in the bone marrow. Stress-induced modulations in SDF-1 and CXCR4 levels participate in recruitment of immature and maturing leukocytes from the bone marrow reservoir to damaged organs as part of host defense and repair mechanisms. The recent finding that murine HSCs rapidly mobilized by the CXCR2 receptor agonist GROβ show superior repopulation kinetics and more competitive engraftment than the equivalent cells mobilized by G-CSF demonstrates that the chemokine/chemokine receptor axis has potentially superior therapeutic potential compared to the use of G-SCF (Fukuda et al. 2007).
In addition to the complex interplay with niche cells and diffusible mediators, it has emerged recently that there is an element of dynamic regulation via neurotransmitter signaling. Hence, ablation by genetic or chemical means of adrenergic neurotransmission or administration of a β2 adrenergic agonist results, respectively, in decreased or enhanced HSC mobilization indicating the involvement of norepinephrine signaling in the process (Katayama et al. 2006; Spiegel et al. 2008).
HSC homing involves rolling and firm adhesion to endothelial cells in small marrow sinusoids under blood flow, followed by trans-endothelial migration across the physical endothelium/extracellular matrix barrier, ultimately leading to access and anchorage to their specialized niches. Like mobilization, this coordinated, multistep process also involves signaling by SDF-1 and SCF, and activation of LFA-1, VLA-4/5 and CD44 and a role for MMPs.
Although HSCs and their niche clearly have to persist throughout life, a number of studies have shown that there are age-related changes in HSCs that have functional consequences for the hematopoietic system and are likely the result of a combination of cell-intrinsic and microenvironmental influences (for a review see Dykstra and de Haan 2008). Studies of X-chromosome inactivation in elderly females have suggested that the pool of HSCs normally diminishes with age, resulting in oligoclonal or even monoclonal hematopoiesis; however, by analyzing the pattern of allele-specific gene expression, Swierczek et al. (2008) have provided convincing evidence against this hypothesis and suggest that clonal hematopoiesis is not a normal consequence of aging. Nevertheless, consideration of HSC differentiation potential suggests that a degree of selection can operate. HSCs isolated from young and aged donors have been reported to differ in functional capacity, the complement of proteins on the cell surface, transcriptional activity, and genome integrity (reviewed by Woolthuis et al. 2011). In the mouse, several hallmark age-dependent changes in the HSC compartment have been identified, including an increase in HSC numbers and a decrease in homing efficiency. Increased proliferation and decreased function with age can be correlated with dramatic alterations in gene expression; one analysis of HSCs from mice aged 2–21 months identified approximately 1,500 genes that were age-induced and 1,600 that were age-repressed (Chambers and Goodell 2007). Genes associated with the stress response, inflammation, and protein aggregation dominated the up-regulated expression profile, while the down-regulated profile was marked by genes involved in the preservation of genomic integrity and chromatin remodeling. One gene in particular that has attracted attention in this context, and may have implications for treatment strategies, is the cyclin-dependent kinase inhibitor p16INK4a, the level of which accumulates and modulates specific age-associated HSC functions (Janzen et al. 2006). Notably, in the absence of p16INK4a, HSC repopulating defects and apoptosis were mitigated, improving the stress tolerance of cells and the survival of animals in successive transplants, suggesting that therapeutic inhibition of genes such as p16INK4a may ameliorate the physiological impact of ageing on stem cells. The differences in ‘aged’ behavior of HSCs were later explained by an accumulation of myeloid-biased HSCs with age in both mice (Challen et al. 2010) and humans (Pang et al. 2011) at the expense of lymphoid-biased cells (Cho et al. 2008). The fact that myeloid-biased HSCs from young and aged sources behave similarly in all aspects tested might suggest that aging does not change individual HSCs.
There has been a growing appreciation over the last 15 years of the role that reactive oxygen species (ROS) play in a variety of cellular processes. ROS are formed by the partial reduction of oxygen and include superoxide (O2 −), hydrogen peroxide (H2O2) and the hydroxyl radical (OH−) (Turrens 2003). ROS have been shown to regulate cell cycle progression, cell motility and growth factor signaling in a variety of normal cell types (Valko et al. 2007). ROS production and consequent oxidative stress has been linked to aging and degenerative disease (Sardina et al. 2012), although beneficial effects of moderate levels of ROS have been noted (Goldstone et al. 1996; Tatla et al. 1999). The importance of ROS in HSCs was made evident from studies on mouse models in which genes involved in the regulation of ROS levels were genetically reduced. For example, reduction of FOXO transcription factor function leads to loss of HSC quiescence and self-renewal capacity (Jang and Sharkis 2007). In the absence of external stimuli, FOXO proteins normally reside in the nucleus in an active state, promoting cell cycle arrest, resistance to stress, apoptosis and ROS detoxification (Coffer and Burgering 2007). Although it is evident that ROS levels are crucial to the function of HSCs, the precise mechanisms affected are not clear. There are actually distinct HSC niches in the bone marrow depending on oxygen availability and the consequent levels of ROS. Hence, ROSlow and ROShigh HSCs exhibit the same surface phenotype, but differ in that the population with lower ROS levels displays higher self-renewal (Jang and Sharkis 2007). The association between the oxidative state and HSC self-renewal capacity has led to interest in the manipulation of ROS levels as a way to enhance BMT and to delay the aging of HSCs.
4 Differentiation Capacity and Their Precursors
What happens downstream of the HSC in the hematopoietic hierarchy is important in a therapeutic context when considering the specific requirements for progenitors and differentiated progeny to regenerate the normal homeostatic state. Work largely emanating from the laboratory of Irving Weissman has defined committed progenitors in the mouse that are immediately downstream of the most mature component of the LSK compartment. These cells mark the first distinction between the lymphoid and myeloid lineages. The existence of a common lymphoid progenitor (CLP) that can only give rise to T cells, B cells, and NK cells was first reported by Kondo et al. (1997), who described a bone marrow Lin− IL-7R+ Thy-1− Sca-1lo c-Kitlo population with these characteristics. A complementary clonogenic common myeloid progenitor (CMP) that gives rise to all myeloid lineages was similarly defined by Akashi et al. (2000) who also demonstrated that this cell can give rise to either megakaryocyte/erythrocyte progenitors (MEPs) or granulocyte/macrophage progenitors (GMPs). The resulting model, which proposes that the first lineage commitment step of HSCs results in a strict separation into CLPs and CMPs, has been challenged by the identification of a population of cells with lympho-myeloid differentiation potential, but that have lost the ability to adopt erythroid and megakaryocyte lineage fates (Adolfsson et al. 2005). Hence, LSK HSCs that co-express high levels of the tyrosine kinase receptor Flt3 were shown to sustain granulocyte, monocyte, and B and T cell potentials, but in contrast to LSK Flt3− HSCs failed to produce significant erythroid and megakaryocytic progeny. These cells were termed lymphoid-primed multipotent progenitor (LMPP) cells. The equivalent details of the hierarchy downstream of the HSC are yet to be fully elucidated in humans, and it cannot be assumed that these will be comparable between species. Using similar strategies for the identification of down-stream human progenitors, populations corresponding to the mouse LMPP were also defined in humans (MLPs) that sustained both lymphoid and myeloid lineages but excluded megakaryocytic/erythroid potential (Hoebeke et al. 2007; Six et al. 2007; Goardon et al. 2011).
Such details of the pathways of commitment and differentiation of HSCs as described above, and how these may differ during development, are becoming ever more important in attempts to optimize the production of replacement hematopoietic cells from ES cells. As for the definition of the HSC phenotype and functional testing of HSCs in vivo, attempts to elucidate protocols for the induction of hematopoietic differentiation from ES cells have been led by work in the mouse. Although conditions have been worked out to enable the derivation of most mature hematopoietic cell types from both mouse and human ES cells, it is important to be aware of the developmental stage that these cells represent and the extent to which it is possible to generate adult HSCs with repopulation potential (for reviews on the derivation of hematopoietic cells from human ES cells see Bhatia (2007), Tian and Kaufman (2008) and Moreno-Gimeno et al. (2010)). Following on from extensive studies on the differentiation of hematopoietic cells from murine ES cells (reviewed in Olsen et al. 2006), production from human ES cells was first described by Kaufman et al. (2001) who employed co-culture with murine bone marrow stromal or yolk sac endothelial cell lines. Amongst a number of subsequent modifications to this strategy, Vodyanik et al. (2005) were able to obtain large numbers of CD34+ cells at greater than 95% purity using co-culture with the mouse stromal line OP9. Although the latter ES cell-derived CD34+ cells contained ALDH+ Rh-123lo cells and were highly enriched in colony-forming cells, even after in vitro expansion, they displayed a phenotype of primitive hematopoietic progenitors. A potential solution to the problem of the stage of developmental maturity was found in the case of mouse ES cell differentiation in that expression of HoxB4 in primitive progenitors combined with culture on hematopoietic stroma induced a switch to the definitive HSC phenotype capable of engrafting primary and secondary recipients (Kyba et al. 2002).
Encouragingly, since the first successes with human ES cell differentiation into HSC-like cells, conditions have been improved considerably leading ultimately to the in vitro generation of HSCs with repopulation activity. For example, Narayan et al. (2006) were able to engraft sheep using Lin− CD34+ or CD34+ CD38− obtained by culturing human ES cells on stromal feeders, their long-term engrafting potential being confirmed by successful transplantation into secondary recipients. Similarly, using co-culture with stromal cells, this time derived from mouse aorta-gonad-mesonephros (AGM) and fetal liver, Ledran et al. (2008) obtained cells expressing CD34 at day 18–21 of differentiation that were capable of primary and secondary hematopoietic engraftment into immunocompromised mice at substantially higher levels than described previously.
5 Potential Applications for Therapies
The utilization of stem cells in the clinic has already met with great success and remains one of the most appealing prospects in regenerative medicine today. The therapeutic use of HSCs pioneered in the 1950s through the development of BMT, initially used matched siblings as donors but has subsequently come to involve the use of partially matched or mismatched donors that although deemed necessary in most situations can result in problems arising from immunogenic matching, resulting in rejection or graft-versus host disease (reviewed by Copelan 2006). A range of diseases have been successfully treated by BMT, including principally cancers of blood cells, but also other hematological disorders such as myeloproliferation, anemia and genetic defects that cause immunodeficiency. BMT is also an option for treatment of some inherited metabolic disorders that result from an enzyme deficiency affecting cells in addition to, or other than, blood cells, but which can be ameliorated through the production of the deficient protein from engrafted donor blood cells. The applications of BMT will be considered in much more detail in Chap. 26, and here the discussion will focus on factors that might improve the prospects for the therapeutic application of HSCs.
Possible improvements in HSC therapies can essentially be broken down into those that increase the availability of suitable, preferably autologous cells in large numbers, and those that maximize the efficiency of engraftment of the transplanted cells. The latter prospect relates to understanding of the mechanisms of homing and the factors that control niche occupancy as discussed above, and it is likely that this knowledge will have a significant impact in the years to come. To date, means to improve the availability of HSCs for BMT have received far more attention. Roughly 30% of patients requiring BMT have a matched sibling, while another 50% potentially have a good match to an individual amongst the nine million registered donors worldwide, although less than half of these will actually receive a donation. Although cord blood is a viable alternative source of HSCs it is not ideal because it only contains a limited number of HSCs, so that ex vivo expansion is almost certainly necessary. Ex vivo expansion of HSCs in combinations of cytokines and other soluble factors, designed to mimic the signals provided within the niche, has met with mixed success, although more recently quite significant degrees of amplification in the numbers of cells retaining engraftment potential have been achieved. For example, using a combination of SCF, Flt3 ligand (FL), thrombopoietin (Tpo) and IL6, two independent groups achieved significant expansion of CD34+ cord blood cells that retained the capacity to repopulate NOD/SCID mice (Kusadasi et al. 2000; Ueda et al. 2000). Direct manipulation of molecular mechanisms that are linked to proliferation and self-renewal is another potential way to expand stem cells and ectopic over-expression of the transcriptional regulator HoxB4 has proved to be effective at inducing rapid, extensive, and highly polyclonal expansions of murine HSCs that retained full lympho-myeloid repopulating potential and enhanced in vivo regenerative potential (Antonchuk et al. 2002). Other approaches that have been investigated include the use of fibroblast growth factors (FGFs), in particular FGF 1 and 2, which can maintain long-term repopulating activity of mouse bone marrow HSCs in vitro (de Haan et al. 2003; Yeoh et al. 2006), while the Notch ligand Delta 1 has a moderate effect in enhancing the expansion of SRUs in cultures of cord blood CD133+ cells employing the SCF, FL, Tpo, IL-6 cocktail of factors described above (Suzuki et al. 2006). Perhaps the greatest success has come from the laboratory of Harvey Lodish who identified angiopoietin-like 2 and angiopoietin-like 3 proteins as factors produced by HSC-supportive mouse fetal liver CD3+ cells (Zhang et al. 2006). These produced a roughly 30-fold expansion of long-term HSCs in culture, which has subsequently been applied to human cord blood cells by developing a serum-free culture containing SCF, TPO, FGF-1, angiopoietin-like 5, and IGFBP2 (Zhang et al. 2008).
Many believe that the solution to producing more cells for transplantation lies in the derivation of HSCs from alternative sources, and a number of options have been considered in order to achieve this goal. The prospect of in vitro production of HSCs as a futuristic potential supply for BMT derived from ES cells is an exciting opportunity for regenerative medicine as they represent a theoretically unlimited source of HSCs. Nevertheless as for cord blood-derived HSCs there are at present significant limitations to the number of appropriate cells that may be obtained.
As discussed already, ES cells can be differentiated into HSCs with repopulating capability and it may soon be possible to produce these in quantities that are sufficient for clinical application. However, the use of ES cell-derived HSCs is ultimately limited because they are unlikely to be perfectly matched to the donor and ethical consequences of the generation of human embryos for therapeutic applications must be considered. Alternatively, what if ES cells could be made that match every individual so that truly personalized stem cell transplantations would become a reality? Efforts have been made to do just this with ES-like cells being generated through processes such as nuclear transfer, involving either the fusion of ES cells with somatic cell or the transfer of somatic nuclear contents into an oocyte (Wilmut et al. 1997).
The real breakthrough came in 2006 when the Japanese researcher Shinya Yamanaka showed that it is possible to convert normal differentiated adult cells, firstly from the mouse (Takahashi and Yamanaka 2006; Okita et al. 2007) and then from humans (Takahashi et al. 2007), to become like ES cells by forced expression of specific pluripotent genes; namely OCT4, SOX2, KLF4 and c-MYC. These cells, which are usually referred to as induced pluripotent stem (iPS) cells, have the additional advantage that their generation does not involve the use of an embryo bypassing many ethical concerns. The subsequent demonstration that iPS cells can be, like ES cells, differentiated into HSCs (Hanna et al. 2007; Schenke-Layland et al. 2008; Niwa et al. 2009) means that they offer the real prospect of limitless autologous HSCs for all. Of course there are many details yet to be ironed out, but the progress in this area of stem cell science is nothing if not meteoric (see Hochedlinger and Plath (2009) and Robinton and Daley (2012) for reviews). Interestingly, a recent study has shown that immature hematopoietic cells derived from human iPS cells are more permissive to engraft the bone marrow of xenotransplantation recipients compared to phenotypically identical cells obtained from ES cells, although these HSCs failed to demonstrate multilineage differentiation unless they were removed from the animal, a phenomenon that could be attributed to their inability to down regulate key micro RNAs involved in hematopoiesis (Risueño et al. 2012).
The considerable recent effort in reprogramming cell phenotype towards a pluripotent state has also led to renewed interest in trans-differentiation directly from one cell type to another, without progressing through an iPS cell stage. This has been achieved, with varying degrees of success, for a number of cell types, including neural cells, cardiomyocytes and hepatocytes. One potentially exciting advantage of a trans-differentiation approach is that it may be possible more easily to produce mature cells with an adult rather than embryonic or fetal phenotype. To date, this approach has achieved only limited success with respect to hematopoietic cells, although the laboratory of Mickie Bhatia has been able to demonstrate direct conversion of human fibroblasts to multilineage blood progenitors through ectopic expression of Oct4 (Szabo et al. 2010).
The ability to produce iPS cells, and consequently patient-specific HSCs, also offers the exciting prospect that inherited blood-related disorders might be corrected. Proof-of-principle for this concept was first provided by the laboratory of Rudi Jaenisch who created a humanized sickle cell anemia mouse model, which was then rescued after transplantation with HSCs obtained in vitro from autologous iPS cells in which the mutant hemoglobin allele had been reverted to the normal sequence by gene-specific targeting (Hanna et al. 2007). A second important proof-of-principle, this time with human cells, has been the demonstration that somatic cells from Fanconi anemia patients can be used to generate iPS cells in which the Fanconi-anemia defect could be corrected by over expression of the normal version of the affected protein and then used to give rise to hematopoietic progenitors of the myeloid and erythroid lineages that are disease-free (Raya et al. 2009).
Although these exciting advances in iPS cell technology generate the prospect of patient-specific stem cell therapies, the transition from the bench to the patient bedside is still some distance into the future. Numerous obstacles must be overcome before these therapies are put into routine practice. Firstly, the original methods of iPS cell generation utilized retroviruses as the vectors to infect cells to initiate the expression of the pluripotent genes. This process would be entirely unacceptable in the clinic since retroviruses are known cancer-causing agents (Okita et al. 2007). New methods of generation of iPS cells have evolved with the means to remove the oncogenes after the induction of pluripotency reducing the risk of tumorigenesis (Yu et al. 2009).
Although it is some distance into the future before these techniques are put into clinical practice, the generation of HSCs from iPS cells from patients can be used in the present for phenotypic based drug screens in complex diseases for which the under-lying genetic mechanism is unknown.
6 Conclusions and Future Development in Research
Research into the nature and application of HSCs has come a long way since the earliest forays into transplantation of bone marrow into patients. Apart from the highly detailed understanding that we now have of the molecular and cellular characteristics of HSCs and ways in which they can be manipulated and used for clinical benefit, research in this area has provided the guiding light for the whole field of stem cell biology. The means of identifying and purifying HSCs and the sophisticated in vitro and in vivo tests that have been developed to assay their potential have been adopted and modified for the investigation of the now burgeoning array of stem cells that play roles in both development and the maintenance of adult tissues. The study of HSCs illustrates so well how investigations in animal models, in particular the mouse, can inform studies in man and how they can provide important pre-clinical information on the potency and behaviour of stem cells following ex vivo expansion or on ways in which to improve the efficiency of engraftment once cells are introduced into the recipient.
The improvements in the ability of clinicians to treat patients more effectively with transplantations as a result of the increasing knowledge of HSCs are likely to be given an even greater boost as a result of the astonishing developments in the ability to generate pluripotent stem cells and to then use these to produce HSCs with long-term engraftment potential. Apart from the chance to treat more people successfully, there has now opened up the real prospect that individuals born with genetic defects that affect blood cell production or function, as well as some other inherited disorders such as those affecting aspects of metabolism, can expect to have their deficiencies corrected by gene targeting in iPS cells generated from nothing more than a few skins cells.
For sure there are many hurdles yet to be overcome, but the future looks very exciting.
References
Adams GB, Scadden DT (2008) A niche opportunity for stem cell therapeutics. Gene Ther 15:96–99
Adolfsson J, Borge OJ, Bryder D et al (2001) Upregulation of Flt3 expression within the bone marrow Lin(−)Sca1(+)c-kit(+) stem cell compartment is accompanied by loss of self-renewal capacity. Immunity 15:659–669
Adolfsson J, Månsson R, Buza-Vidas N et al (2005) Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell 121:295–306
Akashi K, Traver D, Miyamoto T et al (2000) A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404:193–197
Almeida-Porada G, Porada C, Gupta N et al (2007) The human-sheep chimeras as a model for human stem cell mobilization and evaluation of hematopoietic grafts’ potential. Exp Hematol 35:1594–1600
Ando K (2002) Human CD34- hematopoietic stem cells: basic features and clinical relevance. Int J Hematol 75:370–375
in ‘t Anker PS, Noort WA, Kruisselbrink AB et al (2003) Nonexpanded primary lung and bone marrow-derived mesenchymal cells promote the engraftment of umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol 31:881–889
Antonchuk J, Sauvageau G, Humphries RK (2002) HOXB4-induced expansion of adult hematopoietic stem cells ex vivo. Cell 109:39–45
Arai F, Hirao A, Ohmura M et al (2004) Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 118:149–161
Balazs AB, Fabian AJ, Esmon CT et al (2006) Endothelial protein C receptor (CD201) explicitly identifies hematopoietic stem cells in murine bone marrow. Blood 107:2317–2321
Baum CM, Weissman IL, Tsukamoto AS et al (1992) Isolation of a candidate human hematopoietic stem-cell population. Proc Natl Acad Sci USA 89:2804–2808
Bensidhoum M, Chapel A, Francois S et al (2004) Homing of in vitro expanded Stro-1− or Stro-1+ mesenchymal stem cells into the NOD/SCID mouse and their role in supporting human CD34 cell engraftment. Blood 103:3313–3319
Bhatia M (2007) Hematopoietic development from human embryonic stem cells. Hematol Am Soc Hematol Educ Progr 2007:11–16
Bhatia M, Wang JC, Kapp U et al (1997) Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc Natl Acad Sci USA 94:5320–5325
Bhatia M, Bonnet D, Murdoch B et al (1998) A newly discovered class of human hematopoietic cells with SCID-repopulating activity. Nat Med 4:1038–1045
Bonnet D, Bhatia M, Wang JC et al (1999) Cytokine treatment or accessory cells are required to initiate engraftment of purified primitive hematopoietic cells transplanted at limiting doses into NOD/SCID mice. Bone Marrow Transplant 23:203–209
Bradley TR, Metcalf D (1966) The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci 44:287–299
Challen GA, Boles NC, Lin KK et al (2009) Mouse hematopoietic stem cell identification and analysis. Cytometry A 75:14–24
Challen GA, Boles NC, Chambers SM et al (2010) Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-beta1. Cell Stem Cell 6:265–278
Chambers SM, Goodell MA (2007) Hematopoietic stem cell aging: wrinkles in stem cell potential. Stem Cell Rev 3:201–211
Chen BP, Galy A, Kyoizumi S et al (1994) Engraftment of human hematopoietic precursor cells with secondary transfer potential in SCID-hu mice. Blood 84:2497–2505
Chen CZ, Li L, Li M et al (2003) The endoglin(positive) sca-1(positive) rhodamine(low) phenotype defines a near-homogeneous population of long-term repopulating hematopoietic stem cells. Immunity 19:525–533
Cho RH, Sieburg HB, Muller-Sieburg CE (2008) A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood 111:5553–5561
Christensen JL, Weissman IL (2001) Flk-2 is a marker in hematopoietic stem cell differentiation: a simple model to isolate long-term stem cells. Proc Natl Acad Sci USA 98:14541–14546
Chute JP (2006) Stem cell homing. Curr Opin Hematol 13:399–406
Coffer PJ, Burgering BM (2007) Stressed marrow: FoxOs stem tumour growth. Nat Cell Biol 9:251–253
Copelan EA (2006) Hematopoietic stem-cell transplantation. N Engl J Med 354:1813–1826
Craig W, Kay R, Cutler RL et al (1993) Expression of Thy-1 on human hematopoietic progenitor cells. J Exp Med 177:1331–1342
Cumano A, Godin I (2007) Ontogeny of the hematopoietic system. Annu Rev Immunol 25:745–785
de Haan G, Weersing E, Dontje B et al (2003) In vitro generation of long-term repopulating hematopoietic stem cells by fibroblast growth factor-1. Dev Cell 4:241–251
Dexter TM, Allen TD, Lajtha LG (1977) Conditions controlling the proliferation of hematopoietic stem cells in vitro. J Cell Physiol 91:335–344
Dick JE, Magli MC, Huszar D et al (1985) Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell 42:71–79
Doulatov S, Notta F, Laurenti E et al (2012) Hematopoiesis: a human perspective. Cell Stem Cell 10:120–136
Dykstra B, de Haan G (2008) Hematopoietic stem cell aging and self-renewal. Cell Tissue Res 331:91–101
Dzierzak E, Speck NA (2008) Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol 9:129–136
Dzierzak E, Medvinsky A, de Bruijn M (1998) Qualitative and quantitative aspects of haematopoietic cell development in the mammalian embryo. Immunol Today 19:228–236
Ema H, Takano H, Sudo K et al (2000) In vitro self-renewal division of hematopoietic stem cells. J Exp Med 192:1281–1288
Engelhardt M, Lübbert M, Guo Y (2002) CD34(+) or CD34(−): which is the more primitive? Leukemia 16:1603–1608
Fraser CC, Chen BP, Webb S et al (1995) Circulation of human hematopoietic cells in severe combined immunodeficient mice after Cl2MDP-liposome-mediated macrophage depletion. Blood 86:183–192
Fukuda S, Bian H, King AG et al (2007) The chemokine GRObeta mobilizes early hematopoietic stem cells characterized by enhanced homing and engraftment. Blood 110:860–869
Gallacher L, Murdoch B, Wu DM et al (2000) Isolation and characterization of human CD34(−)Lin(−) and CD34(+)Lin(−) hematopoietic stem cells using cell surface markers AC133 and CD7. Blood 95:2813–2820
Goardon N, Marchi E, Atzberger A et al (2011) Coexistence of LMPP-like and GMP-like leukemia stem cells in acute myeloid leukemia. Cancer Cell 19:138–152
Goldman JP, Blundell MP, Lopes L et al (1998) Enhanced human cell engraftment in mice deficient in RAG2 and the common cytokine receptor gamma chain. Br J Haematol 103:335–342
Goldstone SD, Milligan AD, Hunt NH (1996) Oxidative signalling and gene expression during lymphocyte activation. Biochim Biophys Acta 1314:175–182
Goodell MA, Brose K, Paradis G et al (1996) Isolation and functional properties of murine hemopoietic stem cells that are replicating in vivo. J Exp Med 183:1797–1806
Goodell MA, Rosenzweig M, Kim H et al (1997) Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med 3:1337–1345
Greiner DL, Hesselton RA, Shultz LD (1998) SCID mouse models of human stem cell engraftment. Stem Cells 16:166–177
Guo Y, Lübbert M, Engelhardt M (2003) CD34- hematopoietic stem cells: current concepts and controversies. Stem Cells 21:15–20
Hanna J, Wernig M, Markoulaki S et al (2007) Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science 318:1920–1923
Hao QL, Shah AJ, Thiemann FT et al (1995) A functional comparison of CD34+ CD38− cells in cord blood and bone marrow. Blood 86:3745–3753
Harrison DE, Jordan CT, Zhong RK et al (1993) Primitive hemopoietic stem cells: direct assay of most productive populations by competitive repopulation with simple bionomial, correlation and covariance calculations. Exp Hematol 21:206–219
Hess DA, Wirthlin L, Craft TP et al (2006) Selection based on CD133 and high aldehyde dehydrogenase activity isolates long-term reconstituting human hematopoietic stem cells. Blood 107:2162–2169
Hochedlinger K, Plath K (2009) Epigenetic reprogramming and induced pluripotency. Development 136:509–523
Hoebeke I, De Smedt M, Stolz F et al (2007) T-, B- and NK-lymphoid, but not myeloid cells arise from human CD34(+)CD38(−)CD7(+) common lymphoid progenitors expressing lymphoid-specific genes. Leukemia 21:311–319
Ishii M, Matsuoka Y, Sasaki Y et al (2011) Development of a high-resolution purification method for precise functional characterization of primitive human cord blood-derived CD34-negative SCID-repopulating cells. Exp Hematol 39:203–213
Ishikawa F, Yasukawa M, Lyons B et al (2005) Development of functional human blood and immune systems in NOD/SCID/IL2 receptor gamma chain(null) mice. Blood 106:1565–1573
Ito M, Hiramatsu H, Kobayashi K et al (2002) NOD/SCIDgamma(c) (null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100:3175–3182
Ito M, Kobayashi K, Nakahata T (2008) NOD/Shi-scid IL2rgamma(null) (NOG) mice are more appropriate for humanized mouse models. Curr Top Microbiol Immunol 324:53–76
Jang YY, Sharkis SJ (2007) A low level of reactive oxygen species selects for primitive hematopoietic stem cells that may reside in the low-oxygenic niche. Blood 110:3056–3063
Janzen V, Forkert R, Fleming HE et al (2006) Stem cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature 443:42142–42146
Kamel-Reid S, Dick JE (1988) Engraftment of immune-deficient mice with human hematopoietic stem cells. Science 242:1706–1709
Kastan MB, Schlaffer E, Russo JE et al (1990) Direct demonstration of elevated aldehyde dehydrogenase in human hematopoietic progenitor cells. Blood 75:1947–1950
Katayama Y, Battista M, Kao WM et al (2006) Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 124:407–421
Kaufman DS, Hanson ET, Lewis RL et al (2001) Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci USA 98:10716–10721
Kent DG, Copley MR, Benz C et al (2009) Prospective isolation and molecular characterization of hematopoietic stem cells with durable self-renewal potential. Blood 113:6342–63450
Kiel MJ, Morrison SJ (2008) Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol 8:290–301
Kiel MJ, Yilmaz OH, Iwashita T et al (2005) SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121:1109–1121
Kimura T, Asada R, Wang J et al (2007) Identification of long-term repopulating potential of human cord blood-derived CD34-flt3- severe combined immunodeficiency-repopulating cells by intra-bone marrow injection. Stem Cells 25:1348–1355
Kollet O, Peled A, Byk TB et al (2000) Beta2 microglobulin-deficient (B2m(null)) NOD/SCID mice are excellent recipients for studying human stem cell function. Blood 95:3102–3105
Kondo M, Weissman IL, Akashi K (1997) Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell 91:661–672
Kusadasi N, van Soest PL, Mayen AE et al (2000) Successful short-term ex vivo expansion of NOD/SCID repopulating ability and CAFC week 6 from umbilical cord blood. Leukemia 14:1944–1953
Kushida T, Inaba M, Hisha H et al (2001) Intra-bone marrow injection of allogeneic bone marrow cells: a powerful new strategy for treatment of intractable autoimmune diseases in MRL/lpr mice. Blood 97:3292–3299
Kyba M, Perlingeiro RC, Daley GQ (2002) HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell 109:29–37
Lai AY, Kondo M (2006) Asymmetrical lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. J Exp Med 203:1867–1873
Lapidot T, Pflumio F, Doedens M et al (1992) Cytokine stimulation of multilineage hematopoiesis from immature human cells engrafted in SCID mice. Science 255:1137–1141
Lapidot T, Dar A, Kollet O (2005) How do stem cells find their way home? Blood 106:1901–1910
Larochelle A, Savora M, Wiggins M et al (2011) Human and rhesus macaque hematopoietic stem cells cannot be purified based only on SLAM family markers. Blood 117:1550–1554
Ledran MH, Krassowska A, Armstrong L et al (2008) Efficient hematopoietic differentiation of human embryonic stem cells on stromal cells derived from hematopoietic niches. Cell Stem Cell 3:85–98
Lemieux ME, Rebel VI, Lansdorp PM et al (1995) Characterization and purification of a primitive hematopoietic cell type in adult mouse marrow capable of lymphomyeloid differentiation in long-term marrow “switch” cultures. Blood 86:1339–1347
Lemischka IR, Raulet DH, Mulligan RC (1986) Developmental potential and dynamic behavior of hematopoietic stem cells. Cell 45:917–927
Li Z, Li L (2006) Understanding hematopoietic stem-cell microenvironments. Trends Biochem Sci 31:589–595
Lo Celso C, Fleming HE, Wu JW et al (2009) Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature 457:92–96
Majeti R, Park CY, Weissman IL (2007) Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell 1:635–645
Mayani H, Dragowska W, Lansdorp PM (1993) Cytokine-induced selective expansion and maturation of erythroid versus myeloid progenitors from purified cord blood precursor cells. Blood 81:3252–3258
Mazurier F, Fontanellas A, Salesse S et al (1999) A novel immunodeficient mouse model RAG2 x common cytokine receptor gamma chain double mutants requiring exogenous cytokine administration for human hematopoietic stem cell engraftment. J Interferon Cytokine Res 19:533–541
McCune JM, Namikawa R, Kaneshima H et al (1988) The SCID-hu mouse: murine model for the analysis of human hematoplymphoid differentiation and function. Science 241:1632–1639
McKenzie JL, Gan OI, Doedens M et al (2005) Human short-term repopulating stem cells are efficiently detected following intrafemoral transplantation into NOD/SCID recipients depleted of CD122+ cells. Blood 106:1259–1261
McKenzie JL, Gan OI, Doedens M et al (2006) Individual stem cells with highly variable proliferation and self-renewal properties comprise the human hematopoietic stem cell compartment. Nat Immunol 7:1225–1233
McKenzie JL, Takenaka K, Gan OI et al (2007) Low rhodamine 123 retention identifies long-term human hematopoietic stem cells within the Lin-CD34+ CD38− population. Blood 109:543–545
Mercier FE, Ragu C, Scadden DT (2011) The bone marrow at the crossroads of blood and immunity. Nat Rev Immunol 12:49–60
Mikkola HK, Orkin SH (2006) The journey of developing hematopoietic stem cells. Development 133:3733–3744
Moore MA, Williams N, Metcalf D (1973) In vitro colony formation by normal and leukemic human hematopoitic cells: characterization of the colony-forming cells. J Natl Cancer Inst 50:603–623
Moreno-Gimeno I, Ledran MH, Lako M (2010) Hematopoietic differentiation from human ESCs as a model for developmental studies and future clinical translations. Invited review following the FEBS anniversary prize received on 5 July 2009 at the 34th FEBS congress in Prague. FEBS J 277:5014–5025
Namikawa R, Weilbaecher KN, Kaneshima H et al (1990) Long-term human hematopoiesis in the SCID-hu mouse. J Exp Med 172:1055–1063
Narayan AD, Chase JL, Lewis RL et al (2006) Human embryonic stem cell-derived hematopoietic cells are capable of engrafting primary as well as secondary fetal sheep recipients. Blood 107:2180–2183
Niwa A, Umeda K, Chang H et al (2009) Orderly hematopoietic development of induced pluripotent stem cells via Flk-1(+) hemangiogenic progenitors. J Cell Physiol 221:367–377
Nolta JA, Hanley MB, Kohn DB (1994) Sustained human hematopoiesis in immunodeficient mice by cotransplantation of marrow stroma expressing human interleukin-3: analysis of gene transduction of long-lived progenitors. Blood 83:3041–3051
Noort WA, Kruisselbrink AB, in ‘t Anker PS et al (2002) Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol 30:870–878
Notta F, Doulatov S, Laurenti E et al (2011) Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science 333:218–221
Okada S, Nakauchi H, Nagayoshi K et al (1992) In vivo and in vitro stem cell function of c-kit and Sca-1-positive murine hematopoietic cells. Blood 80:3044–3050
Okita K, Ichisaka T, Yamanaka S (2007) Generation of germline-competent induced pluripotent stem cells. Nature 448:313–317
Olsen AL, Stachura DL, Weiss MJ (2006) Designer blood: creating hematopoietic lineages from embryonic stem cells. Blood 107:1265–1275
Osawa M, Hanada K, Hamada H et al (1996) Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science 273:242–245
Pang WW, Price EA, Sahoo D et al (2011) Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci USA 108:20012–20017
Pearson T, Greiner DL, Shultz LD (2008) Humanized SCID mouse models for biomedical research. Curr Top Microbiol Immunol 324:25–51
Pelus LM, Fukuda S (2008) Chemokine mobilized adult stem cells: defining a better hematopoietic graft. Leukemia 22:466–473
Ploemacher RE, van der Sluijs JP, Voerman JS et al (1989) An in vitro limiting-dilution assay of long-term repopulating hematopoietic stem cells in the mouse. Blood 74:2755–2763
Ploemacher RE, van der Sluijs JP, van Beurden CA et al (1991) Use of limiting-dilution type long-term marrow cultures in frequency analysis of marrow-repopulation and spleen colony-forming hematopoietic stem cells in the mouse. Blood 78:2527–2533
Pronk CJ, Rossi DJ, Mansson R et al (2007) Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor hierarchy. Cell Stem Cell 1:428–442
Purton LE, Scadden DT (2007) Limiting factors in murine hematopoietic stem cell assays. Cell Stem Cell 1:263–270
Raaijmakers MH, Scadden DT (2008) Evolving concepts on the microenvironmental niche for hematopoietic stem cells. Curr Opin Hematol 15:301–306
Raya A, Rodríguez-Pizà I, Guenechea G et al (2009) Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature 460:53–59
Risueño RM, Sachlos E, Lee JH et al (2012) Inability of human induced pluripotent stem cell-hematopoietic derivatives to downregulate micro RNAs in vivo reveals a block in xenograft hematopoietic regeneration. Stem Cells 30:131–139
Robinton DA, Daley GQ (2012) The promise of induced pluripotent stem cells in research and therapy. Nature 481:295–305
Rongvaux A, Willinger T, Takizawa H et al (2011) Human thrombopoietin knockin mice efficiently support human hematopoiesis in vivo. Proc Natl Acad Sci USA 108:2378–2383
Sardina JL, López-Ruano G, Sánchez-Sánchez B et al (2012) Reactive oxygen species: are they important for hematopoiesis? Crit Rev Oncol Hematol 81:257–274
Schenke-Layland K, Rhodes KE, Angelis E et al (2008) Reprogrammed mouse fibroblasts differentiate into cells of the cardiovascular and hematopoietic lineages. Stem Cells 26:1537–1546
Schmitt TM, Zúñiga-Pflücker JC (2002) Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity 17:749–756
Shultz LD, Schweitzer PA, Christianson SW et al (1995) Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol 154:180–191
Sintes J, Romero X, Marin P et al (2008) Differential expression of CD150 (SLAM) family receptors by human hematopoietic stem and progenitor cells. Exp Hematol 36:1199–1204
Sitnicka E, Buza-Vidas N, Larsson S et al (2003) Human CD34+ hematopoietic stem cells capable of multilineage engrafting NOD/SCID mice express flt3: distinct flt3 and c-kit expression and response patterns on mouse and candidate human hematopoietic stem cells. Blood 102:881–886
Six EM, Bonhomme D, Monteiro M et al (2007) A human postnatal lymphoid progenitor capable of circulating and seeding the thymus. J Exp Med 204:3085–3093
Spangrude GJ, Johnson GR (1990) Resting and activated subsets of mouse multipotent hematopoietic stem cells. Proc Natl Acad Sci USA 87:7433–7437
Spangrude GJ, Heimfeld S, Weissman IL (1988) Purification and characterization of mouse hematopoietic stem cells. Science 241:58–62
Spiegel A, Kalinkovich A, Shivtiel S et al (2008) Stem cell regulation via dynamic interactions of the nervous and immune systems with the microenvironment. Cell Stem Cell 3:484–492
Srour EF, Zanjani ED, Cornetta K et al (1993) Persistence of human multilineage, self-renewing lymphohematopoietic stem cells in chimeric sheep. Blood 82:3333–3342
Storms RW, Trujillo AP, Springer JB et al (1999) Isolation of primitive human hematopoietic progenitors on the basis of aldehyde dehydrogenase activity. Proc Natl Acad Sci USA 96:9118–9123
Sutherland HJ, Eaves CJ, Eaves AC et al (1989) Characterization and partial purification of human marrow cells capable of initiating long-term hematopoiesis in vitro. Blood 74:1563–1570
Suzuki T, Yokoyama Y, Kumano K et al (2006) Highly efficient ex vivo expansion of human hematopoietic stem cells using Delta1-Fc chimeric protein. Stem Cells 24:245624–245665
Swierczek SI, Agarwal N, Nussenzveig RH et al (2008) Hematopoiesis is not clonal in healthy elderly women. Blood 112:3186–3193
Szabo E, Rampalli S, Risueño RM et al (2010) Direct conversion of human fibroblasts to multilineage blood progenitors. Nature 468:521–526
Szilvassy SJ, Humphries RK, Lansdorp PM et al (1990) Quantitative assay for totipotent reconstituting hematopoietic stem cells by a competitive repopulation strategy. Proc Natl Acad Sci USA 87:8736–8740
Taichman RS (2005) Blood and bone: two tissues whose fates are intertwined to create the hematopoietic stem-cell niche. Blood 105:2631–2639
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676
Takahashi K, Tanabe K, Ohnuki M et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872
Takano H, Ema H, Sudo K et al (2004) Asymmetric division and lineage commitment at the level of hematopoietic stem cells: inference from differentiation in daughter cell and granddaughter cell pairs. J Exp Med 199:295–302
Tatla S, Woodhead F et al (1999) The role of reactive oxygen species in triggering proliferation and IL-2 secretion in T cells. Free Radic Biol Med 26:14–24
Thomas ED, Lochte HL Jr, Lu WC et al (1957) Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med 257:491–496
Tian X, Kaufman DS (2008) Differentiation of embryonic stem cells towards hematopoietic cells: progress and pitfalls. Curr Opin Hematol 15:312–318
Till JE, McCulloch EA (1961) A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res 14:213–222
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552:335–344
Ueda T, Tsuji K, Yoshino H et al (2000) Expansion of human NOD/SCID-repopulating cells by stem cell factor, Flk2/Flt3 ligand, thrombopoietin, IL-6, and soluble IL-6 receptor. J Clin Invest 105:1013–1021
Valko M, Leibfritz D, Moncol J et al (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84
van Os R, Kamminga LM, de Haan G (2004) Stem cell assays: something old, something new, something borrowed. Stem Cells 22:118111–118190
van Rijn RS, Simonetti ER, Hagenbeek A et al (2003) A new xenograft model for graft-versus-host disease by intravenous transfer of human peripheral blood mononuclear cells in RAG-/- gammac-/- double-mutant mice. Blood 102:2522–2531
Vodyanik MA, Bork JA, Thomson JA et al (2005) Human embryonic stem cell-derived CD34+ cells: efficient production in coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood 105:617–626
Wang JC, Doedens M, Dick JE (1997) Primitive human hematopoietic cells are enriched in cord blood compared with adult bone marrow or mobilized peripheral blood as measured by the quantitative in vivo SCID-repopulating assay. Blood 89:3919–3924
Wang J, Kimura T, Asada R et al (2003) SCID-repopulating cell activity of human cord blood-derived CD34- cells assured by intra-bone marrow injection. Blood 101:2924–2931
Whitlock CA, Witte ON (1982) Long-term culture of B lymphocytes and their precursors from murine bone marrow. Proc Natl Acad Sci USA 79:3608–3612
Willinger T, Rongvaux A, Stowig T et al (2011) Improving human hemato-lymphoid-system mice by cytokine knock-in gene replacement. Trends Immunol 32:321–327
Wilmut I, Schnieke AF, McWhir J et al (1997) Viable offspring derived from fetal and adult mammalian cells. Nature 385:810–813
Wilson A, Trumpp A (2006) Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol 6:93–106
Woolthuis CM, de Haan G, Huls G (2011) Aging of hematopoietic stem cells: Intrinsic changes or micro-environmental effects? Curr Opin Immunol 23:512–517
Yahata T, Ando K, Sato T et al (2003) A highly sensitive strategy for SCID-repopulating cell assay by direct injection of primitive human hematopoietic cells into NOD/SCID mice bone marrow. Blood 101:2905–2913
Yang L, Bryder D, Adolfsson J et al (2005) Identification of Lin(−)Sca1(+)kit(+)CD34(+)Flt3- short-term hematopoietic stem cells capable of rapidly reconstituting and rescuing myeloablated transplant recipients. Blood 105:2717–2723
Yeoh JS, van Os R, Weersing E et al (2006) Fibroblast growth factor-1 and -2 preserve long-term repopulating ability of hematopoietic stem cells in serum-free cultures. Stem Cells 24:1564–1572
Yin AH, Miraglia S, Zanjani ED et al (1997) AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood 90:5002–5012
Yu J, Hu K, Smugga-Otto K et al (2009) Human induced pluripotent stem cells free of vector and transgene sequences. Science 324:797–801
Zanjani ED, Pallavicini MG, Ascensao JL et al (1992) Engraftment and long-term expression of human fetal hematopoietic stem cells in sheep following transplantation in utero. J Clin Invest 89:1178–1188
Zanjani ED, Srour EF, Hoffman R (1995) Retention of long-term repopulating ability of xenogeneic transplanted purified adult human bone marrow hematopoietic stem cells in sheep. J Lab Clin Med 126:24–28
Zanjani ED, Almeida-Porada G, Livingston AG et al (1998) Human bone marrow CD34− cells engraft in vivo and undergo multilineage expression that includes giving rise to CD34+ cells. Exp Hematol 26:353–360
Zhang CC, Kaba M, Ge G et al (2006) Angiopoietin-like proteins stimulate ex vivo expansion of hematopoietic stem cells. Nat Med 12:240–245
Zhang CC, Kaba M, Iizuka S et al (2008) Angiopoietin-like 5 and IGFBP2 stimulate ex vivo expansion of human cord blood hematopoietic stem cells as assayed by NOD/SCID transplantation. Blood 111:3415–3423
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Clarke, M.L., Frampton, J. (2013). Hematopoietic Stem Cells. In: Steinhoff, G. (eds) Regenerative Medicine. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-5690-8_10
Download citation
DOI: https://doi.org/10.1007/978-94-007-5690-8_10
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-5689-2
Online ISBN: 978-94-007-5690-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)