Abstract
The divergent synthesis, properties and functions of dendrimers terminated by metallocenyl redox groups are briefly illustrated in this micro-review, with emphasis on molecular electronics, sensing with regenerable derivatized Pt electrodes and efficient catalysis with dendrimer-stabilized Pd nanoparticles.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
8.1 Introduction
Dendrimers have attracted considerable attention since their discovery three decades ago [1–43]. Potential applications involve supramolecular properties [11] in the fields of nanomedicine [29], materials science [4–13] and catalysis [16, 30, 38–43]. Since the late 1980s, we have focused our attention on metallodendrimers [14, 44] with the aim to develop knowledge concerning redox properties that are useful for redox sensing and catalysis as well as for the design of molecular batteries. In this microreview article, we will illustrate the design of our recent series of metallodendrimers and some of their properties and applications.
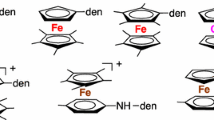
8.2 The Complexes [FeCp(η6-arene)][PF6] as a Source of Dendritic Core, Dendrons and Dendrimers
In the complexes, [FeCp(η6-arene)][PF6], the arene ligand undergoes reactions resulting from “Umpolung” of the arene reactivity [45], i.e. the benzylic groups are easily deprotonated [46], the chloride arene substituent is easily substituted by nucleophiles such as alkoxyde [45] and the aryl ethers are heterolytically cleaved by t-BuOK in THF below room temperature in the presence of an inorganic salt such as KBr [47]. Moreover, these organometallic cations can be reduced to 19-electron FeI complexes that have a specific radical- and electron-transfer reactivity [48]. The removal of the arene ligand from the complex can easily proceed either via the 19-electron complexes or using visible photolysis of the 18-electron cations [49].
Using these properties, dendritic cores are quantitatively synthesized under ambient conditions from the mesitylene complex, whereas a simple tripodal dendron is prepared in a one-pot eight-step synthesis from the p-ethoxytoluene complex (Scheme 8.1). With a nona-allyl arene core and the “phenoltriallyl” brick, dendrimers containing 3n+2 terminal allyl tethers (n = generation number) could be constructed [15] using the 1 →3 C connectivity pioneered by Newkome with arborols [50] by a series of hydrosilylation-Williamson reactions. The hydrosilylation was carried out using chloromethyldimethylsilane [51] and Karsted catalyst at 40°C whereas the Williamson step was performed between the chloromethyl-terminated dendrimers and phenoltriallyl using a catalytic amount of NaI and K2CO3 in DMF at 80°C. Each step was checked by 1H, 13C and 29Si NMR and gave virtually pure dendrimers at the accuracy of NMR. MALDI TOF mass spectra show, however, that if the molecular peak largely predominates for the second generation 81-allyl dendrimer, the defects predominate in the spectrum of the 3rd generation 243-allyl dendrimer and the molecular peak is not even seen in that of the 4th generation 729-allyl dendrimer which shows massifs near the molecular peak (Scheme 8.2).
Construction of giant dendrimers starting from ferrocene with 3n+2 terminal tethers (n = generation number) until G9 (theoretical number of 311 terminal tethers). Each dendrimer along the construction was characterized by 1H, 13C and 29Si NMR (till G9), MALDI TOF mass spectrometry (till G4), SEC (PI = 1.00 to 1.02 till G5), TEM and AFM (till G9) showing the steady size increase
The dendrimers were characterized by size exclusion chromatography until generation five showing a polydispersity between 1.00 and 1.02, atomic force microscopy showing the progression of the height of the monolayer from the first to the 9th generation and transmission electron microscopy of the polyiodo derivative of the last generation. Although the number of defects becomes larger and larger as the generation number increases, it may be estimated that the last generation reaches a number of terminal tethers of the order of 105. Beyond generation 5 (theoretical number: 37 = 2187 terminal tethers), it is compulsory that further dendritic construction reactions occur inside the dendrimers interior because the small termini must back fold toward the center in order to avoid the bulk at the periphery and fill the interior cavities. Thus the dendrimer construction becomes limited by the volume rather than by the surface. The reactions become slower and slower and the yields are lower as the generation number increases beyond generation 5.
A challenge is the one-pot synthesis of dendrimers using such a strategy [52]. This was shown to be possible if chlorodimethylsilane [53] is used instead of the chloromethyldimethylsilane in the construction scheme. Indeed, the terminal Si-Cl bonds formed at the periphery of the dendrimer subsequent to hydrosilylation are much more reactive in the Williamson reaction with phenolates than the chloromethylsilyl termini, which permits the one-pot synthesis of up to the 243-allyl G3 dendrimer. The Si-phenolate link is less robust than the Si-CH2-phenolate link, but stable enough for extensive characterization. Such fragile dendrimers might be useful for applications requiring the decomposition of the dendrimer interior after using it as a template, for instance in materials chemistry (Scheme 8.3) [52].
8.3 Ferrocenyl Dendrimers
The first ferrocenyl dendrimers designed for function were synthesized by reaction of amine-terminated dendrimers with ferrocenoyl chloride, which yielded amidoferrocenyl dendrimers that were redox exo-receptors of oxo-anions [54]. It was subsequently found that silylferrocenylation of polyolefin dendrimers yielded polysilylferrocenyl dendrimers (Scheme 8.4).
Likewise, the silylferrocenylation of the “phenoltriallyl” brick yielded triferrocenyl dendrons that could be condensed onto a polyhalogeno core to form polyferrocenyl dendrimers (Scheme 8.5) [55].
With gold-nanoparticle-cored dendrimers, it was found that the silyl group was an excellent alternative to the amido group when it was attached to the ferrocenyl termini for the recognition of oxo-anions including ATP [56]. The factors involved in the redox recognition are the electrostatic attraction between the anion and the ferrocenium cation upon anodic oxidation and the supramolecular bonding between the amido group (hydrogen bonding) of the silyl group (Si hypervalence). The amidoferrocenyl or silylferrocenyl monomers do not show any effect, however. Therefore, the dendrimer topology is important for recognition of oxo-anions. The appropriate encapsulation of the anionic host between the dendritic tethers is a key factor that very much increases the interaction between the functional ferrocenyl termini and the guest (Scheme 8.6).
8.4 Engineering the Dendrimer Family with Peripheral Ferrocenyltriazole Ligands: “Click” Dendrimers and Metallodendrimers for Oxo-Anion and Transition-Metal Cation Sensing
The 1,2,3-triazole is an ideal choice for the interaction with many substrates that have Brönsted or Lewis acid properties including transition metals and their complexes. Thus the encapsulation of such guests should prove feasible by introducing such triazole groups on the dendrimer tethers. The 1,2,3-triazole group is readily formed by “Click” chemistry recently reported by Sharpless to catalyze with CuI the regioselective Huisgens reaction between azido derivatives and terminal alkynes [57]. We used the dendrimer family that was constructed as indicated above and substituted the terminal halogeno group by azido upon reaction with NaN3. These azido-terminated dendrimers were engaged in reactions with ferrocenyl acetylene in order to locate the redox sensor directly on the triazole ring for adequate sensing of the interaction of guests with the triazole heterocycle by perturbation of the redox potential of the ferrocenyl system (Scheme 8.7).
Ferrocenyl terminated dendrimers are known as very good sensors of oxo-anions with positive dendritic effects, i.e. the magnitude of the recognition effect increases together with generation number, because the dendrimer topology of higher generations involves narrower channels for a better interaction with the dendritic site on the tethers. Thus oxo-anions including ATP, a DNA fragment, are well recognized by the “Click” ferrocenyltriazolyl dendrimers. The additional electron density brought by the oxo-anions makes the ferrocenyl oxidation easier, i.e. at less positive oxidations potentials. On the other hand, the interaction with acetonitrile complexes of several transition metals (CuI, CuII, PdII, PtII) withdraws electron density from the ferrocenyltriazolyl system, the ferrocenyl oxidation is rendered more difficult, and its wave is found at more positive potentials (Scheme 8.8) [58].
Second-generation “click” ferrocenyl dendrimer (81 terminal ferrocenyltriazolyl groups) that recognizes both oxo-anions including ATP and transition-metal dications (CuI, CuII, PdII, PtII) with positive dendritic effect (i.e. recognition, characterized by the shift of potential of the ferrocenyl CV wave, works all the better as the dendrimer generation is higher)
8.5 The Click Reaction as a Useful Iterative Method for Dendrimer Construction
In the preceding example, the “click” reaction was used for peripheral dendrimer functionalization. We then addressed the challenge of using the “click” reaction iteratively for divergent dendrimer construction. For this purpose, the “phenoltriallyl” brick used above was propargylated at the focal point before “click” reaction with an azido-terminated dendritic core as above. After the “click” reaction, the polyolefin dendrimer formed in which the number of terminal tethers has been multiplied by three is submitted to hydrosilylation with chloromethyldimethylsilane as in our classic dendrimer construction, then the terminal chloro groups are substituted by azido groups for further iteration of the “Click” reaction with the propargylated dendron (Scheme 8.9) [59].
8.6 Dendrimers Containing Triazole Ligands and Ferrocenyl Termini as Useful Templates for Transition-Metal Ions and Transition-Metal Nanoparticles
The triazole ligands were introduced in these dendrimers in order to bind transition-metal cations before their reduction to metal (0) to form nanoparticles that are either stabilized inside the dendrimer or, if the dendrimer is too small, that are stabilized by the dendrimer without encapsulation. The ferrocenyl groups located at the dendrimer periphery just near the triazole rings allow titrating the metal cations that interact herewith. Palladium (II) was coordinated to the triazole ligands in the dendrimer interior using Pd(OAc)2, then reduced to Pd(0) using NaBH4 or methanol. The coordination of Pd(OAc)2 onto the triazole ligands was monitored by cyclic voltammetry, showing the appearance of a new wave corresponding to the ferrocenyl groups attached to Pd(II)-coordinated triazoles.
The outcome was a one-to-one stoichiometry that allowed designing a given number of Pd atoms in the Pd nanoparticles if the dendrimer is large enough for nanoparticle encapsulation. This aspect is very important for applications (Scheme 8.10) [59].
Coordination of the triazole ligand by Pd(OAc)2 monitored by ferrocenyl redox sensing followed by Pd(II) reduction to dendrimer-encapsulated Pd (0) nanoparticles used further in catalysis. The variety of nanoparticle sizes obtained with this strategy is crucial for catalyst optimization and mechanistic investigation
8.7 Application in Catalysis of “Click” Dendrimers and Dendrimer-Stabilized Nanoparticles
Nanoparticles are attracting increasing attention as catalysts from both the homogeneous- and heterogeneous catalysis communities, because they are “ligandless” catalysts avoiding toxic phosphines, and they show remarkable activities and selectivities [60].
Nanoparticles can be stabilized by an extremely large variety of supports from organic to inorganic [61]. Polymers have been among the most popular supports for nanoparticle catalysts, [62] thus dendrimers also stabilize them, and dendrimer stabilization can proceed either by encapsulation [63] or, if the dendrimer is too small, by peripheral stabilization of the nanoparticle surrounded by a number of dendrimers [64]. Thus commercial polyamidoamine and polypropylene imine have been extensively used to stabilize nanoparticle catalysts [65].
Click-dendrimer-stabilized nanoparticles are a new family of dendrimer-stabilized nanoparticles that is particularly suitable for catalytic studies [59, 66]. Different Pd nanoparticles were synthesized from the dendrimers of generations 0 (9 tethers) to 2 (81 tethers). Transmission electron microscopy shows that generations G1 and G2 form dendrimer-encapsulated nanoparticles whose sizes correspond to Pd nanoparticles that contain the same number of Pd0 atoms as that of PdII ions initially coordinated to the triazoles inside the dendrimer, whereas G0 is too small to encapsulate the nanoparticle formed. In this case, the nanoparticle is surrounded by a number of dendrimers that provide stabilization (Scheme 8.11).
The collection of different nanoparticles having different designed sizes is crucial to the study of the mechanisms in nanoparticle catalysis. These “click” dendrimer- stabilized nanoparticles are efficient catalysts for selective olefin hydrogenation under ambient conditions, and the turnover frequencies, turnover numbers and yields depend on the nanoparticle size. The smallest nanoparticles (from G1) are the most active ones, in agreement with a classic hydrogenation mechanism entirely proceeding at the nanoparticle surface [66]. On the other hand, the turnover numbers, turnover frequencies and yields are independent on the type of nanoparticle stabilization and sizes of the nanoparticles for the Suzuki cross coupling reaction between chlorobenzene or bromobenzene and PhB(OH)2. Moreover, the TON increases when the amount of nanoparticle catalyst is decreased or when the solution is diluted. The efficiency reaches 54% yield using 1 ppm Pd nanoparticles, i.e. the amount of nanoparticle catalyst is homeopathic. On the other hand, with high loading of catalyst, the yield is not quantitative, reaching only 70% at 1% Pd atom catalyst. These phenomena are taken into account by a leaching mechanism whereby one or two Pd atoms escape from the nanoparticle surface subsequent to the oxidative addition of the aryl halide onto the nanoparticle surface, then become extremely active in solution until it is quenched by the mother nanoparticle [66]. A similar mechanism had been proposed earlier by de Vries for the Heck reaction at high temperature (150–170°C) [67–69].
8.8 Conclusion and Outlook
The synthesis of high-generation dendrimers starting from organoiron activation provided suitable nanomaterials for molecular electronics, catalysis and sensing. In molecular electronics, the property of fast electron transfer (electrochemical reversibility) with metallocenyl-terminated dendrimers and the single wave of multi-ferrocenyl dendrimers in cyclic voltammetry leads to useful electrocatalytic and sensing properties. For sensing, the compared performances of the functional groups attached to the peripheral groups as exo-receptors offered flexibility of substrates using specific termini. Using the most recent “click” dendrimers with which the recognition can be achieved for both oxo-anions and transition-metal cations, redox recognition was very useful to determine the number of PdII ions coordinated into the dendrimer on the triazole ligands. The precise sizes of Pd nanoparticles designed in this way led to delineation of mechanistic experiments and catalyst optimization that significantly contribute to the knowledge and performances of Pd nanoparticle catalysis. This approach of dendrimer catalysis is complementary to the one introducing inorganic or organometallic catalysts at the core or periphery of dendrimers that was more classic and involved leaching and limited possibilities of catalyst recovery [70]. Studies are ongoing along this line to use suitable dendrimers for efficient “Green” catalysis [71].
References
Newkome GR, Yao Z, Baker GR, Gupta VK (1985) Micelles. J Org Chem 50:2003–2004
Tomalia DA, Naylor AM, Goddard WA III (1990) Angew Chem Int 29:138–175
Jansen JFGA, de Brabander-van den Berg EMM, Meijer EW (1999) Science 266:1226
(a) Newkome GR, Moorefield CN, Vögtle F (2001) Dendrimers and dendrons. Concepts, syntheses, applications. Wiley, Weinheim; (b) Newkome GR (ed) (1994, 1995, 1996, 1999, 2002) Advances in dendritic molecules, vols 1, 2, 3, 4, 5. JAI Press, Greenwich
Tomalia DA, Fréchet JMJ (eds) (2003) Dendrimers and other dendritic polymers. Wiley, Amsterdam
(a) Vögtle F, Richardt G, Werner N (2007) Dendritische Moleküle – Konzepte, Synthesen, Eigenschaften, Anwendungen. B. G. Teubner-Verlag, Stuttgart; (b) Vögtle F (ed) (1998, 2000, 2001) Dendrimer I, II and III. Springer, Berlin
(a) Newkome GR, Moorefield CN (1992) Aldrichim Acta 25:31; (b) Newkome GR (1998) Pure Appl Chem 70:2337
(a) Tomalia DA, Dupont Durst H (1993) In: Weber E (ed) Topics Curr Chem, Supramolecular chemistry, directed synthesis and molecular recognition. vol 165. Springer, Berlin, 193; (b) Tomalia DA (2005) Mater Today 34
Balzani V, Campagna S, Denti G, Juris A, Serroni S, Venturi M (1998) Acc Chem Res 31:26
Moore JS (1997) Acc Chem Res 30:402
Zeng F, Zimmermann SC (1997) Chem Rev 97:1681–1712
Bauer RE, Grimsdale AC, Müllen K (2005) Top Curr Chem 245:253–286
Percec V (1995) Pure Appl Chem 67:2031–2038
(a) Ardoin, N, Astruc, D (1995) Bull Soc Chim Fr 132:875–909; (b) Astruc D (1996) C. R. Acad. Sci 322: Sér. II b, 757–766
Matthews OA, Shipway AN, Stoddart JF (1998) Prog Polym Sci 23:1–56
Newkome GR, He E, Moorefield CN (1999) Chem Rev 99:1689–1746
Bosman AW, Janssen HM, Meijer EW (1999) Chem Rev 99:1665–1688
(a) Hawker C, Fréchet JMJ (1990) Chem Commun 1010–1011; (1990) J Am Chem Soc 112:7638–7643; (b) Miller TM, Neeman TX (1990) Mater Chem 2:346–350
Fréchet JMJ (1994) Science 263:1710–1715
Fréchet JMJ (1995) Science 269:1080–1082
Hecht S, Fréchet JMJ (2001) Angew Chem Int Ed Engl 40:74–77
Grayson SM, Fréchet JMJ (2001) Chem Rev 101:3819–3867
Fréchet JMJ (1999) Pure Appl Chem A33:1399–1407
Issberner J, Moors R, Vögtle F (1994) Angew Chem Int Ed 33:2413–2420; Fischer M, Vögtle F (1999) Angew Chem Int Ed 38:884–890
Friedhofen J, Vögtle F (2006) New J Chem 30:32–43
Chow H-F, Mong K-K, Nongrum MF, Wan C-W (1998) Tetrahedron 54:8543–8660
Gorman C (1998) Adv Mat 10:295–309
Reviews on phosphorus- and silicon-based dendrimers: (a) Gudat D (1997) Angew Chem Int Ed Engl 36:1951–1958; (b) Caminade A-M, Majoral J-P (1999) Chem Rev 99:845–863
Astruc D (2003) Pure Appl Chem 75:461–481
Astruc D (ed) (2003) Dendrimers and nanoscience C. R. Chimie 6
(a) Tomalia DA (1994) Adv Mat 6:529–539; (b) Tomalia DA, Dvornic PR (1994) Nature 372:617–618
Tomalia DA (2005) Mater Today 8:34–46
(a) Meltzer AD, Tirrel DA, Jones AA, Inglefield PT, Hedstrand DM, Tomalia DA (1992) Macromolecules 25:4541; (b) Mijovic J, Ristic S, Kenny J (2007) Macromolecules 40:5212
Chase PA, Gebbink RJ, Klein M, van Koten G (2004) J Organomet Chem 689:4016–4054
Schlüter AD, Rabe PJ (2000) Angew Chem Int Ed Engl 39:864
Flory PJ (1953) Principles of polymer chemistry. Cornell University Press, Ithaca
Voit BI (2003) C R Chimie 6:821–832
Oosterom GE, Reek JNH, Kamer PCJ, van Leeuwen PWNM (2001) Angew Chem Int Ed 40:1828–1849
Kreiter R, Kleij AW, Klein Gebbink RJM, van Koten G (2001) In: Vögtle F, Schalley CA (eds). Dendrimers IV: metal coordination, self assembly, catalysis, vol 217. Top Curr Chem, Springer, Berlin, 163
(a) Astruc D, Chardac F (2001) Chem Rev 101:2991–3024; (b) van Heerbeeck R, Kamer PCJ, van Leeuwen PWNM, Reek JNH (2002) Chem Rev 102:3717–3756
Méry D, Astruc D (2006) Coord Chem Rev 250:1965–1979
Moorefield CN, Newkome GR (2007) New J Chem 31:1192–1217
Crooks RM, Zhao M, Sun L, Chechik V, Yeung LK (2001) Acc Chem Res 34:181–190
Scott RWJ, Wilson OM, Crooks RM (2005) J Phys Chem B 109:692–704
Moulines F, Astruc D (1988) Angew Chem Int Ed Engl 27:1347–1349
(a) Astruc D (1983) Tetrahedron 39:4027–4095; (b) Trujillo HA, Casado C, Ruiz J, Astruc, D (1999) J Am Chem Soc 121:5674–5686
Astruc D, Hamon J-R, Román E, Michaud P (1981) J Am Chem Soc 103:7502–7514
Sartor V, Djakovitch L, Fillaut J-L, Moulines F, Neveu F, Marvaud V, Guittard J, Blais J-C, Astruc D (1999) J Am Chem Soc 121:2929–2930
(a) Hamon J-R, Astruc D, Michaud P (1981) J Am Chem Soc 103:758–766; (b) Desbois M-H, Astruc D, Guillin J, Varret F, Trautwein AX, Villeneuve G (1989) J Am Chem Soc 111: 5800–5809; (c) Lacoste M, Rabaa H, Astruc D, Le Beuze A, Saillard J-Y, Précigoux G, Courseille C, Ardoin N, Bowyer W (1989) Organometallics 8:2233–2242
(a) Catheline D, Astruc D (1983) J Organometal Chem 248:C9-C12; (b) Catheline D, Astruc D (1984) Organometallics 3:1094–1100; (d) Ruiz J, Astruc D (2008) Inorg Chim Acta 361:1–4
(a) Newkome GR, Yao Z, Baker GR, Gupta VK (1983) J Org Chem 50:2003; (b) Newkome GR (1998) Pure Appl Chem 70:2337; (c) Narayanan VV, Newkome GR (1998) Top Curr Chem 197:19
Krsda SW, Seyferth D (1998) J Am Chem Soc 120:3604
Ornelas C, Ruiz J, Astruc D (2006) Org Lett 8:2751
van der Made AW, van Leeuwen PWNM, Brandes RAC (1993) Adv Mater 5:466
(a) Valério C, Fillaut J-L, Ruiz J, Guittard J, Blais J-C, Astruc D (1997) J. Am Chem Soc 119:2588; (b) Ruiz J, Ruiz-Medel M-J, Daniel M-C, Blais J-C, Astruc D (2003) Chem Commun 464; (c) Daniel M-C, Ruiz J, Blais J-C, Daro N, Astruc D (2003) Chemistry. Eur J 9:4371; (d) Daniel M-C, Ruiz J, Astruc D (2003) J Am Chem Soc 125:1150; (e) Daniel M-C, Ba F, Ruiz J, Astruc D (2004) Inorg Chem 43:8649
(a) Nlate S, Ruiz J, Blais J-C, Astruc D (2000) Chem Commun 417; (b) Nlate S, Ruiz J, Sartor V, Navarro R, Blais J-C, Astruc D (2000) Chem Eur J 6:2544
(a) Daniel M-C, Ruiz J, Nlate S, Palumbo J, Blais J-C, Astruc D (2000) Chem Commun 2001; (b) Daniel M-C, Ruiz J, Nlate S, Blais J-C, Astruc D (2003) J Am Chem Soc 125:2617; (c) Daniel M-C, Astruc D (2004) Chem Rev 104:293; (d) Astruc D, Daniel M-C, Ruiz J (2004) Chem Commun 2637
(a) Kolb HC, Finn MG, Sharpless KB (2001) Angew Chem Int Ed 40:2004; (b) Bock VD, Hiemstra H, van Maarseveen JH (2006) Eur J Org Chem 51
Ornelas C, Ruiz J, Cloutet E, Alves S, Astruc D (2007) Angew Chem Int Ed Engl 46:872
(a) Ornelas C, Salmon L, Ruiz J, Astruc D (2007) Chem Commun 4946–4948; (b) Ornelas C, Salmon L, Ruiz J, Astruc D (2008) Chem Eur J 14:50–64
Bönnemann H, Nagabushana KS (2004) In: Nalwa HS (ed) Encyclopedia of nanoscience and nanotechnology, vol 1. ASP, Stevenson Ranch p 777
Toshima N, Yonezawa Y (1998) New J Chem 22:1179
(a) Astruc D, Lu F, Ruiz J (2005) Angew Chem Int Ed 44:7852; (b) Astruc D (2007) Inorg Chem 46:1884
(a) Zhao M, Sun L, Crooks RM (1998) J Am Chem Soc 120:4877; (b) Balogh L, Tomalia DA (1998) J Am Chem Soc 120:7355
Esumi K, Suzuki A, Aihara N, Usui K, Torigoe K (1998) Langmuir 14:3157
(a) Crooks RM, Zhao M, Sun L, Chechik V, Yeung LK (2001) Acc Chem Res 34:181; (b) Scott RWJ, Wilson OM, Crooks RMJ (2005) Phys Chem B 109:692; (c) Chandler BD, Gilbertson JD (2006) Top Organomet Chem 20:97
Diallo A, Ornelas C, Ruiz J, Salmon L, Astruc D (2007) Angew Chem Int Ed 46:8644–8648
de Vries AHM, Parlevliet FJ, Schmeder-van de Vondervoort L, Mommers JHM, Henderickx HJW, Walet MAN, de Vries AHM (2002) Adv Synth Catal 344:996
de Vries AHM, Mulders JMCA, Mommers JHM, Hendericks HJW, de Vries JG (2003) Org Lett 5:3285
de Vries JG (2006) Dalton Trans 421
(a) Plault L, Hauseler A, Nlate S, Astruc D, Gatard S, Neumann R (2004) Angew Chem Int Ed 43:2924–2928; (b) Heuze K, Méry D, Gauss D, Astruc D (2003) Chem Commun 2274–2275; (c) Heuze K, Méry D, Gauss D, Blais J-C, Astruc D (2004) Chem Eur J 10:3936–3944
Acknowledgements
The valuable efforts and contributions of students and colleagues cited in the references to the subject of this micro-review and financial assistance from the Institut Universitaire de France (IUF), the Université Bordeaux I, the Centre National de la Recherche Scientifique (CNRS) and the Agence Nationale de la Recherche (ANR) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media Dordrecht
About this paper
Cite this paper
Astruc, D., Ornelas, C., Ruiz, J. (2012). Organometallic Dendrimers: Design, Redox Properties and Catalytic Functions. In: Hill, C., Musaev, D.G. (eds) Complexity in Chemistry and Beyond: Interplay Theory and Experiment. NATO Science for Peace and Security Series B: Physics and Biophysics. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-5548-2_8
Download citation
DOI: https://doi.org/10.1007/978-94-007-5548-2_8
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-5547-5
Online ISBN: 978-94-007-5548-2
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)