Abstract
The circadian system in humans encompasses all organs, tissues and cells. Coordination of central and peripheral clocks and synchronization of cellular clocks within the brain regulate daily phases, neurophysiology and behavior. The mechanism of action of the circadian system is complex, but is centered on the paired structure of the suprachiasmatic nuclei (SCN) that serves as a pacemaker in humans. Light adjusts the phase of the SCN oscillator to the environmental light-dark cycle. Light therapy has been developed for clinical use and many apparatus and parameters have been extensively studied. Bright light therapy is the treatment of choice for seasonal affective disorder and circadian rhythm sleep disorders. Cumulative studies support the efficacy of light therapy for some clinical conditions which are characterized by seasonality or disrupted circadian rhythms. The benefit of light therapy is significant and warrants further clinical studies to optimize the treatment effect.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Bright light therapy
- Suprachiasmatic nucleus
- Circadian rhythm
- Seasonal affective disorder
- Depression
- Antidepressant effect
1 Introduction
Light therapy, or phototherapy, is used as monotherpay in some sleep disorders, especially in those related to disturbance of circadian rhythm, but has become increasingly popular as adjunctive treatment to pharmacotherapy. Light therapy includes the use of ultraviolet light to treat psoriasis and other skin disorders, and full-spectrum bright light to treat seasonal affective disorder (SAD). The latter, first introduced in the 1980s, is now a widely approved form of treatment for SAD [1, 2].
The effects of light therapy are comparable to those found in many antidepressant pharmacotherapy trials [3]. In the last two decades, many research groups have launched clinical trials which have resulted in light therapy being extended to other conditions, including non-seasonal mood disorders, Alzheimer’s disease, circadian-related sleep disorders, jet lag, eating disorders, shift-workers, and other behavioral syndromes [3–9]. Although a meta-analysis on the efficacy of light therapy in treating mood disorders [3] highlighted methodological limitations in some clinical trials which included brief treatment periods, small study groups, lack of replication, and study design issues (e.g., lux parameters for the active treatment, characteristics of placebo control, and random assignment to treatment conditions), it nonetheless concluded that bright light treatment and dawn stimulation for SAD, and bright light for non-seasonal depression are efficacious [10].
This chapter focuses on the application of bright light therapy in order to improve the understanding of its mechanisms as exploratory treatment for specific disorders. Light plays a major role in influencing the retino-hypothalamic tract to the suprachiasmatic nuclei (SCN), and in adjusting melatonin secretion. The SCN is intrinsically photosensitive, communicating with the dorsomedial hypothalamic nucleus (DMH) to regulate the circadian pattern of sleep-wakefulness, feeding, locomotor activity, and serum corticosteroid levels [11]. The DMH receives both direct and indirect SCN inputs and sends a predominantly GABAergic projection to the sleep-promoting ventrolateral preoptic nucleus, and a primarily glutamate-thyrotropin-releasing projection to the wake-promoting lateral hypothalamic area, including orexin (hypocretin) neurons [11]. In human, the 24-h circadian rhythm shifts through its daily phase mostly as a result of light exposure. The accumulated amount of light exposure alters the sleep-wake cycle and regulates the autonomic nervous system, the endocrine system, and internal homeostasis. Longer period is found for totally blind individuals, whose circadian pacemaker cannot be entrained by the environmental light-dark cycle. Pagani et al., hypothesized that circadian properties between blind and sighted groups differed for physiological reasons rather than genetic ones. Under certain circumstances these blind individuals can become entrained to the 24-h day via nonphotic cues such as exercise, food, and activity [12].
Light itself has a therapeutic effect on depression. The underlying mechanism has been associated with the phase shift [13–15] and more specifically, its effect on monoamine activity in the brain [16, 17]. Some studies manipulating these factors directly or indirectly suggest that light therapy attenuates depression [18–20]. Minimal adverse responses and promising treatment outcomes are encouraging for its clinical application. Light therapy has been applied to seasonal affective disorder, non-seasonal depression, bipolar depression, circadian rhythm sleep disorders, shift work, and specific conditions such as bulimia nervosa, late luteal phase dysphoric disorder (LLPDD), attention deficit hyperactivity disorder (ADHD), and dementia. However, its use for some of these conditions warrants further studies to demonstrate its validity and efficacy.
2 Mechanism of Action
Circadian rhythms include sleep-wake cycles that are endogenously driven by the SCN in the anterior hypothalamus. Light is the most potent external influence that can adjust the phasing of the SCN, and light therapy is mainly used to reset the circadian system. The SCN is functionally subdivided to generate a coordinated rhythm of neuronal and hormonal activities. Such activities incorporate photic and nonphotic time cues that reset the circadian phase of the SCN itself, and transmit circadian output signals to effector systems such as other brain regions and peripheral organs [21]. Outputs include neuronal connections, endocrine signals, body temperature rhythms, and indirect cues, provoked by oscillating behavior (e.g., feeding-fasting rhythms generated by rest-activity cycles). The SCN acts as a circadian pacemaker, and serves as a central station for processing top-down coordination. It is therefore associated with synchronizing circadian rhythms within other biological systems with daily changes in the environment (e.g., fluctuations in light intensity and temperature) [21]. This ever-regulating process entrains the nearly 24-h rhythm of internal activity in the SCN to the 24-h light-dark cycle given by the Earth’s rotation (“circa diem” means “approximately a day”).
2.1 Ocular Mechanism
The photoperiod is the most dominant environmental Zeitgeber (time giver) for the phase entrainment of circadian oscillators. Light enters the visual system and further alters the phase of ‘clock’ gene expression in a subset of SCN neurons (Fig. 8.1). The visual system consists of the eye (in particular the retina), optic nerve, optic chiasma, optic tract, lateral geniculate body, optic radiation, visual cortex, and visual association cortex, all of which enable people to see. By this system, information from visible light is interpreted to an assembled representation of the outside world.
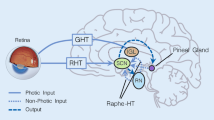
Mammals perceive light information mainly via the retina of the eye, where non-image- forming photoreceptors termed photosensitive retinal ganglion cells (pRGC), which express the photopigment melanopsin [22, 23]. Photic information reaches the SCN directly from the retino-hypothalamic tract (RHT) and indirectly through other retinorecipient structures, including the intergeniculate leaflet (IGL) in the thalamic lateral geniculate nucleus (LGN) [24].
2.2 Suprachiasmatic Nucleus (SCN)
The SCN is located at the base of the hypothalamus. SCN neurons display intrinsic circadian oscillations, orchestrated at the molecular level by translational and transcriptional loops in specific ‘clock’ genes [25, 26]. The rhythm in the SCN is conveyed to various endogenous neural systems governing physiologic and behavioral functions [27]. It remains unclear how SCN neurons actually communicate with their CNS target neurons. Understanding how these neurons are functionally connected will illuminate the mechanism of circadian rhythm and its regulation.
Based on differences in chemoarchitecture, peptide phenotype and afferent-efferent projection pattern, the mammalian SCN consists of ventrolateral (SCNvl) and dorsomedial (SCNdm) components [24]. There are three major afferent pathways to the SCN in rats: one from the outside photic input via the RHT, and two from nonphotic inputs of the IGL (via geniculo-hypothalamic tract, GHT), and serotonergic (5-HT) inputs from the dorsal and median raphe nucleus (DRN and MRN, Fig. 8.2). The monosynaptic RHT fibers terminate directly on neurons in the SCNvl that express vasoactive intestinal polypeptide (VIP). This photic signaling pathway of the RHT mainly expresses the excitatory neurotransmitter glutamate and the neuropeptide pituitary adenylate cyclase-activating protein (PACAP) [28]. The RHT projects to both the SCN and IGL, while the IGL projects to the SCN via the GHT. Thus, the GHT can indirectly involve processed light information by releasing neuropeptide Y (NPY) and gamma-aminobutyric acid (GABA) [29]. As such, the SCN receive two different triggers upon light stimulation of retina: one directly via the RHT and the other indirectly via the GHT. This suggests that the pathway via the IGL enables integration of photic and non-photic signals to entrain the SCN. In terms of the day-night switch, it is posited that the RHT relays photic information from the eyes to the SCN to adjust circadian timing via a glutamatergic pathway at night, and then adjusts the biological clock by a PACAP/cAMP- dependent mechanism during the daytime [28].
Main afferent pathways to the SCN in rats. 5-HT 5-hydroxytryptamine, DRN dorsal raphe nucleus, GABA gamma-aminobutyric acid, GHT geniculohypothalamic tract, Glu glutamate, IGL intergeniculate leaflet, MRN median raphe nucleus, NPY neuropeptide Y, PACAP pituitary adenylate cyclase-activating peptide, RHT retino-hypothalamic tract, SCN suprachiasmatic nuclei (Modified from Dibner et al. [21])
To function as a circadian pacemaker and master synchronizer, intrinsic time-keeping signals from the SCN must be transmitted to other brain and peripheral clocks. Efferent connections from the SCN have been identified by injecting antero- and retro-grade tracers. In the hypothalamus, efferent fibers from the SCN terminate most densely to the subparaventricular zone (SPZ or sPVZ), which is located immediately dorsal to the SCN and extends dorsocaudally to the paraventricular hypothalamic nucleus (PVH) [24, 30, 31]. Tracing studies suggest that the SCNvl projects predominantly to the lateral SPZ, whereas the SCNdm projects more densely to the medial SPZ [32]. Other SCN projections have been identified rostrally in the preoptic area (POA), bed nucleus of the stria terminalis (BNST), and the lateral septum (LS); and dorsally in the dorsomedial hypothalamic nucleus (DMH), and the arcuate nucleus (ARC).
It is postulated that the SPZ functions as an amplifier of circadian output from the SCN. In studies of fetal SCN grafts, transplanted SCN tissue can be implanted into many brain areas to restore rhythms [33, 34]. However, cell-specific lesion experiments show that ventral SPZ injury causes a profound reduction in the circadian rhythms of sleep and locomotor activity, while dorsal SPZ lesions caused greater reductions in the circadian index of body temperature [35]. Lesions of the PVH had no significant effect on any measured circadian rhythms. These findings suggest that the SPZ is part of the primary neuronal pathway mediating the output of SCN-generated circadian rhythms.
The SCN also controls the autonomic nervous system (ANS); SCN neurons that project to PVH are important target areas of biological clock output. They also harbor the pre-autonomic neurons that control peripheral sympathetic and parasympathetic activities [36]. A direct projection from the SCN to the PVH mediates rhythmic control of melatonin secretion from the pineal gland, while an indirect projection via the DMH is critical for the circadian release of corticosteroids [37]. Metabolic activity and glucose uptake in the SCN are dependent on time [36] as well as on expression of clock genes [38]. Kalsbeek et al. have proposed that the SCN generates a daily rhythm in plasma melatonin concentration via the combination of a continuous (glutamatergic) stimulation of neurons in the PVH that are at the root of sympathetic innervation of the pineal gland, as well as a nocturnal withdrawal of the inhibitory (GABAergic) SCN inputs to these neurons. In Kalsbeek’s study, activation of PVH neuronal activity induced hyperglycemia. However, such physiological changes are time-dependent and can be caused by GABA-antagonists only when administered during the light period. Likewise, feeding-induced plasma glucose and insulin responses are suppressed by inhibiting PVH neuronal activity only during the dark period. These results suggest that the pre-autonomic neurons in the PVH are controlled by the interplay of inhibitory (GABAergic) and excitatory (glutamatergic) inputs. At the same time, the timing information of both sympathetic and parasympathetic pre-autonomic PVH neurons is provided mainly by the SCN GABAergic outputs [36].
The DMH projects to brain areas involved in the regulation of the sleep-wakefulness switch that includes a primary GABAergic projection to the ventrolateral preoptic nucleus (VLPO). The VLPO, located in the anterior hypothalamus, is thought to promote sleep via its inhibitory projections to the ascending monoaminergic arousal system [39]. The DMH sends a predominantly glutamatergic projection to the lateral hypothalamus (LHA) which harbors the wake- promoting population of orexin-expressing neurons [11]. Briefly, the DMH receives circadian input directly from the SCN and indirectly via the SPZ, and extends to the VLPO and LHA to regulate sleep-wakefulness cycles (Fig. 8.3). The transmission of SCN circadian signals in the SPZ and then the DMH may allow for alteration of circadian rhythms by other inputs, such as food availability, external temperature, or social cues.
Circadian regulation of sleep-wakefulness: Solid arrows indicate prominent neuronal projections and dashed arrows indicate relatively minor projections. 5-HT 5-hydroxytryptamine, DMH dorsomedial hypothalamic nucleus, DRN dorsal raphe nucleus, GABA gamma-aminobutyric acid, HA histamine, LC locus coeruleus, LHA lateral hypothalamic area, NE norepinephrine, PVH paraventricular hypothalamic nucleus, SCN suprachiasmatic nuclei, TMN tuberomammillary nucleus, TRH thyrotropin-releasing hormone, VLPO ventrolateral preoptic nucleus, vSPZ ventral subparaventricular zone (Modified from Gooley and Saper [24])
2.3 Light and Circadian Rhythms
Light synchronizes or entrains the internal clock to the 24-h period, with precision accomplished though daily phase shifts (a move to either an earlier or a later time of the day) resulting from light exposure [40, 41]. The effect of light on circadian rhythms follows a so-called phase-response curve (PRC) [42] that quantifies the phase dependence of light-induced phase shifts obtained by a PRC. The effect of light on the direction of a phase shift depends on the time of the day, and intensity and duration of exposure. Using this mechanism of a PRC, light can have two conflicting effects on circadian rhythm [43]. When light exposure occurs in the evening, it tends to phase delay with rising effects through the night. However, after core body temperature reaches nadir, light exposure tends to have the opposite effect of phase advance. Thus, applying bright light in the morning has an advancing effect on patients with delayed sleep phase syndrome.
The intensity and duration of light exposure follows a dose effect on the impact of phase shift. Bright light of 2,500 lux has been previously regarded as an intensity threshold to suppress nighttime melatonin production in humans. As such, brighter light and longer light exposure means greater suppression [44]. However, it is now suggested that low intensity (e.g. 180 lux as from an ordinary room light or even the light from a television set) has the potential to cause phase shifts [43, 45, 46]. This is clinically important for patients with delayed sleep phase syndrome, since even the dim light from a computer or a TV set may be a factor that impedes sleep onset. Given its phase-shifting properties, light is used for the management of different conditions, such as delayed sleep phase syndrome, advanced sleep phase syndrome, shift work, sleep maintenance insomnia, and seasonal affective disorder [47–50]. Light can be delivered using natural sources or light boxes with standard intensity of 10,000 lux for 30 min [51]. Their efficacy also depends on the distance from the light source to retina and duration of light exposure.
Melatonin is synthesized by the pineal gland, as well as in the retina and gastrointestinal tract. Control of melatonin secretion relies on neural input to pinealocytes in the pineal gland, which mainly contain adrenergic receptors, hence beta-blockers such as propranolol and atenolol can reduce the effects of melatonin. Melatonin secretion is similar in men and women, but gradually decreases in magnitude with aging after adolescence [52]. Melatonin has two main effects: a direct hypnotic effect on sleep and an effect on the circadian oscillator [53]. Light has a dose-dependent inhibitory influence on melatonin, and the SCN has a high density of melatonin receptors that modulates phase shifting via melatonin.
2.4 Antidepressant Effect of Light Therapy
Lewy proposed the phase-shift hypothesis (PSH), which posits that the depression seen in seasonal affective disorder (SAD) patients is as a result of a phase delay of the endogenous circadian oscillator relative the sleep-wake cycle [15]. Corroborating this hypothesis, the circadian rhythm of body temperature, melatonin, and cortisol secretion are known to be phase-delayed in SAD patients compared to controls [13, 14]. Administration of bright light in the morning, but not in the evening, is considered therapeutic because it corrects the abnormal delay in seasonal depression. In studies on morning versus evening light exposure for the antidepressant response of light therapy, 1-week phototherapy in early morning was superior than either in the evening (responding rate: 53% vs. 38%), dim light (11%), or brief light control [54]. In a another study, there was a positive association between lowered mood in winter and winter phase delay among a random community sample [55]. However, the degree of clinical improvement does not appear to correlate with baseline circadian phase or the degree of phase change associated with treatment [56–59]. These studies are therefore inconclusive as to whether circadian phase advance mediates the therapeutic mechanism in SAD patients. Lewy has since clarified the PSH and proposed that light treatment operates not by advancing circadian phase per se, but by normalizing the phase angle between sleep and circadian rhythms [60]. This revised hypothesis is supported by some studies [18, 61], but more sophisticated approaches that focus not only on phase shift in circadian rhythm, but also neurotransmitter activity are warranted.
The monoamine hypothesis of SAD has been another focus of interest in the past decade. Monoamine systems includes dopaminergic (DA), noradrenergic (NE) and serotonergic (5-HT) neurons. The treatment response to bright light was comparable to heterocyclic antidepressants and monoamine oxidase inhibitors (MAOI) [16]. Light deprivation for 6 weeks damaged monoamine neurons, in particular those located in the locus coeruleus (LC), and produced a depressive behavioral phenotype in rats. It was also found that the antidepressant desipramine decreased these neural and behavioral impacts of light deprivation [19]. These studies suggest an important link between the antidepressant effect of light therapy for SAD, and an underlying pathogenesis involving monoamine neurotransmitters.
Other studies suggest that the therapeutic effects of bright light in SAD may involve a serotonergic mechanism. As early as the 1980s, atypical symptoms in SAD patients, such as hyperphagia, carbohydrate craving or hypersomnia, were found to be associated with a dysfunctional serotonergic system [17]. Using the paradigm of tryptophan depletion (to deplete serotonin in humans), deficits in the brain serotonin system were found to play a key role in the pathogenesis of SAD, and it was hypothesized that light therapy may compensate for these underlying disturbances [62]. Serotonergic stimulation induces phase-shifting effects, and studies have demonstrated that tryptophan depletion did not worsen depression in untreated SAD patients, but by contrast, induced transient depressive relapses in stable patients who had received successful light therapy [20, 63, 64]. Sleep deprivation is a condition of reducing sleep time and not having enough sleep. Total sleep deprivation (TSD) and light therapy could enhance these 5-HT effects. In another study on bipolar depression, repeated TSD and morning light therapy were used as intervention therapy. In this 1-week treatment study with a before/after comparison of biological correlates, the responders (about two-thirds of the patients) showed an increase in daytime activity, reduced nighttime sleep and phase-advance of the activity-rest rhythm for 57 min compared to the pre-treatment baseline [18]. These results support the hypothesis that the antidepressant effects of light are partly mediated via serotonergic systems.
2.5 Side Effects of Light Therapy
The side effects of light therapy are small and usually temporary, and can be generally remedied by reducing exposure time. The appearance of side effects relates in part to the parameters of light exposure, including intensity, duration, spectral content, and methods of exposure (diffuse vs. focused, direct vs. indirect, as well as angle of incidence relative to the eyes). The most common side effects are headache and eye/vision problems (strain, excess glare, seeing spots, blurring and irritation) [65]. Other side effects include nausea, sedation, dizziness, irritability or tightness in the chest. Unless the patients are in severe discomfort, these side effects are generally well tolerated. Eye discomfort can be alleviated by sitting farther from the lights, taking a shorter period schedule, or by installing a humidifier. Bright light may worsen pre-existing eye problems or cause a skin rash in patients with a skin condition, and requires caution if glaucoma, cataracts, retinal detachment or retinopathy is reported by patients. The most profound side effect is a mood switch to hypomania with difficulty sleeping, becoming restless or irritable, and feeling speedy or “too high”. Nevertheless, such a change occurs quite infrequently [66]. Another rare complication of light therapy for SAD is suicidal tendencies; some cases have been reported [67].
3 Practical Aspects of Bright Light Therapy
Since the introduction of artificial bright light for the treatment of winter depression in 1984 [2], several protocols and apparatus for bright light therapy have been developed and adopted across a variety of studies and clinical settings. Conventionally, bright light therapy means the administration of visible light producing at least 2,500 lux at eye level [68]. However, dosing and timing regimens still need to be established for each clinical condition. Even for SAD, which has been the most extensively studied in clinical trials, the basic protocol requires revision to accommodate the introduction of many new devices [69]. Staring from the apparatus, important parameters for bright light therapy such as light intensity, length of session, treatment duration, timing, and wavelength are reviewed and discussed in the following section.
3.1 Apparatus
The standard 60 by 120-cm fluorescent ceiling unit was used in many early studies [51]. This light box has a plastic prismatic diffusion screen and is placed vertically on the table about one meter from the user. It could provide approximately 2,500 lux illuminance to the eyes. Increasingly, more and more commercial products have been launched on the market, but not all of them have been tested through well-designed clinical trials. Some of these apparatus have not even been calibrated to demonstrate an equivalent output level to the standard light box. In fact, lamp type, filter, ballast frequency, radiating surface and heat emission differ among devices and could result in various outcomes.
Besides the light box, the light visor has been introduced for its convenience of use. With head-mounted ambulatory lighting units, the light visor provides bright light therapy without interfering with normal activities. An earlier multicenter clinical trial showed an antidepressant response to bright light therapy by using light visors [70]. In this 2-week randomized clinical trial, 105 patients with SAD received three intensities of light (60, 600, and 3,500 lux) delivered by the light visor. All three intensities produced a similar frequency of antidepressant response. Furthermore, Boulus and colleagues used a head-mounted light visor to demonstrate circadian phase shifting by bright light therapy [71]. They chose 20 individuals travelling from Zurich to New York (a westward flight across six time zones) and provided 3,000 lux bright white light or 10 lux dim red light for 3 h on the first two evenings after the flight. Results indicated a larger phase delay in the bright light group. However, the information regarding the clinical efficacy of the light visor remains scant. Thus, the use of light visors is not currently recommended [51, 68].
The light therapy room is another alternate configuration of bright light therapy and is usually applied in hospital settings. The light room setting provides full-spectrum light by fluorescent tubes in the ceiling and on the walls. There are white walls and light-colored furniture in the light room. Rastad and colleague studied the clinical efficacy in 50 patients with SAD randomized into light room therapy and waiting-list groups [72]. After 10 days of bright light treatment in a light therapy room, self-reported depression in subjects was reduced and the therapeutic effect was maintained after a 1-month follow-up. Still, more experimentally controlled studies are warranted to verify the clinical efficacy and safety of the light therapy room in other health conditions before recommendation. Currently, light boxes remain the mainstream device to deliver bright light therapy.
3.2 Intensity
In the early history of bright light therapy, 2,500 lux fluorescent illumination measured at eye level was the standard parameter for clinical protocols [2]. An earlier review by Terman and colleague indicated the clinical efficacy of 2,500 lux intensity light exposure for at least 2 h daily in the treatment of SAD by analyzing 332 patients in 25 studies [54]. Bright light therapy, with 2,500 lux illuminance, whether administrated in the early morning, evening, or midday, was shown to reduce depressive symptoms significantly compared to dim light controls.
Since a reciprocity between light intensity and duration has been found, a high-intensity fluorescent lighting system with 10,000 lux illuminance, having an irradiant dose estimated at 0.016 J/cm2, was developed and clinically tested. Studies have shown that the clinical efficacy of 30 min of bright light treatment with the 10,000 lux illuminance is comparable to that of 2 h treatment with 2,500 lux illuminance recommended in earlier studies [73]. Because the 10,000 lux intensity of light requires less time than the former 2,500 lux apparatus to achieve the same therapeutic effect, high-intensity light therapy has been recommended as the standard treatment dose for SAD. As regards to some other health conditions, devices yielding a maximum illuminance of 10,000 lux have been adopted and demonstrate clinical benefit [74–76]. In addition, bright light therapy with intensities between 2,500 and 10,000 lux are still applied in some clinical settings [77, 78]. Although 30 min of bright light treatment with the 10,000 lux illuminance is widely adopted nowadays, recommendations for light therapy dose levels should be cautious since the data is changing rapidly [79].
Safety concerns for high-intensity light therapy, in particular the potential impact on the eyes, have been raised. Indeed, side effects including headaches and eye or vision problems have been reported, but were mild and transient and did not interfere with treatment [65]. Gallin and colleagues performed ophthalmologic examinations in 50 patients with SAD before and after bright light therapy for 30 min at an illuminance level of 10,000 lux [80]. They did not detect any ocular changes after either short-term or long-term treatment. Still, patients with pre-existing ocular abnormalities or using photosensitizing drugs were suggested to undergo light therapy only with ophthalmologic monitoring.
3.3 Length of Session and Treatment Duration
As stated above, daily light exposure for 30 min at an illuminance level of 10,000 lux gives an equal effect to 2-h light exposure at 2,500 lux for the treatment of seasonal affective disorder. Thus, the length of treatment session varies across studies mainly based on the intensity of the lighting system, but also dependent on the targeted symptoms or diseases [79].
Early studies suggested that treatment response might occur within 2–4 days, and achieve remarkable improvement within 1–2 weeks [2]. Comparing treatment response in bright light therapy at the first and second week, Labbate and colleagues suggested the necessity of longer trials of bright light therapy [81]. In a recent study, treatment durations as long as 5 weeks of 60 min 10,000 lux lighting was applied to patients with non-seasonal depression [82]. Furthermore, long-term bright light therapy of up to 2 years with daily light exposure is suggested for patients with mild cognitive impairment and early dementia [83].
3.4 Timing
Researchers have found that the greatest therapeutic effects are achieved when light therapy is administered in the morning. An early meta-analysis by Terman and colleagues showed a greater remission rate when bright light therapy was administered for patients with SAD in the early morning [54]. Although some researchers claimed that bright light therapy in SAD is independent of time of day or circadian phase [59], morning light was found to produce phase advances in melatonin rhythm [84]. Based on their findings, the administration of light therapy is suggested to be optimal about 8.5 h after melatonin onset or 2.5 h after the sleep midpoint to potentiate therapeutic effects. Thus, patients with SAD might be given bright light sessions shortly after awakening. Although some of them would oversleep and need to wake up earlier to take their light treatment [85].
A similar strategy is applied to other health conditions, except for advanced sleep phase syndrome. However, to ensure the timing of light exposure is appropriate for an individual’s circadian rhythm, a simple solution is to apply the Horne-Östberg Morningness–Eveningness Questionnaire [86]. The score of this questionnaire correlates strongly with melatonin onset and can be used to estimate the optimal timing for bright light therapy [51, 69].
3.5 Wavelength
Initially, full-spectrum lights were used in bright light therapy. However, in addition to the intensity of light, ultraviolet and visible light could lead to ocular damage. Unfortunately, the potential hazard of ocular damage may still occur for unclear spectral emission of lamps. Reme and colleagues have reported irradiant doses for several bright light therapy regimens and analyzed the potential hazards [87]. They proposed screening out ultraviolet light and some low-wavelength visible light in a range up to 500 nm, which may potentially damage the eyes. Recently, a prismatic diffusion screen with ultraviolet filtering has become a standard component of bright light treatment devices. It has been demonstrated that ultraviolet light is not necessary for the clinical efficacy of bright light therapy in SAD [88].
Identifying optimal wavelengths for bright light therapy is important in optimizing clinical efficacy. Which spectral colors of light are the most efficient for treatment remains controversial, although shorter wavelength light has been shown to be more effective than longer wavelengths in suppressing nocturnal melatonin [89]. To balance the risk and benefit, filtering of wavelengths less than 450 nm might achieve clinical efficacy and lower the blue-light hazard, which is magnified in the range of 435–445 nm.
An earlier study by Oren and colleagues found that green light induced greater antidepressant effects than red light [90]. However, a later study on green light for elderly patients with depression did not show a significant antidepressant effect [91].
Recently, technological advancements in light emitting diodes (LEDs) allow for much narrower bandwidths of light, which enable more accurate and precise efficacy studies of different light wavelengths. Using blue LED units producing 468 nm light versus dimmer red light in a 3-week randomized controlled study, Glickman and colleagues demonstrated a significant therapeutic effect in a sample of 24 patients with seasonal depression [92]. A more recent study by Strong and colleagues verified the effectiveness of narrow-band blue light treatment in a 3-week, parallel, double-blind trial compared with red light [93]. They proposed that blue-light therapy could produce results similar to those of earlier 10,000 lux full-spectrum light studies and many medication studies.
However, although multiple blue-light devices have been developed under this concept, they require individual testing in clinical trials. In fact, there are still scant comparative dose-response studies to support the application of such blue-enriched device [94].
3.6 Other Considerations
Although more and more bright light therapy devices are available on the market, only some of them have been carefully evaluated in clinical trials. Besides the treatment protocols, comparing devices is difficult due to the problematic specification of lux, the dosing variable [51]. The power received from the light source could vary if the spectral composition of the light is different. Therefore, caution is required when choosing bright light therapy devices, and is preferable to make choices based on clinical evidence.
Another concern stems from patient’s compliance. A study by Michalak and colleagues identified a high drop-out rate of 31.6% among patients with SAD [95]. Among those completing treatment, compliance to the prescribed duration of exposure averaged 83.3% in the 4-week protocol. How to improve patient’s compliance in clinical practice by improving the convenience of the device and reducing adverse reactions, though not studied extensively, is indeed an important issue.
In summary, a prescription for bright light therapy of 30–120 min of daily exposure to a broad spectrum, ultraviolet-filtered 2,500–10,000 lux illuminance remains the typical for bright light therapy. New devices such as light visors, light therapy rooms and narrow-band blue light from LEDs require more evidence to support their clinical efficacy. At this moment, the dosing regimen and protocol are suggested to be tailored according to the needs of individual patient with various health conditions. In the next section, the application of bright light therapy in each clinical condition will be discussed individually.
4 Clinical Application of Bright Light Therapy
4.1 Seasonal Affective Disorder
Seasonal affective disorder (SAD) is a recurrent subtype of depression characterized by a predictable onset in the fall/winter months and spontaneous remission in the spring/summer period. Seasonal variations in affect, cognition, and drive occur in many individuals. Once these changes meet clinical severity and fulfill the diagnostic criteria for a major depressive episode, it can be determined whether the seasonal pattern is specified. Specified seasonal patterns can be regarded as patterns of Major Depressive Episode in Bipolar I Disorder, Bipolar II Disorder, or Major Depressive Disorder, Recurrent. Major depressive episodes that occur in a seasonal pattern are often characterized by prominent anergy, hypersomnia, over-eating, weight gain, and craving for carbohydrates [96]. The prevalence of SAD in the general population is 2–4% in temperate climates and increases with higher latitudes [97]. Around 15–20% of patients with mood disorders have a seasonal pattern [68].
The pathogenesis of SAD remains unclear. Light insensitivity [98, 99], reduced ambient light during fall/winter [100] and disturbed monoaminergic pathways [17, 63, 101] are proposed in the pathophysiology of SAD. One leading hypothesis for SAD is the phase shift hypothesis. Lewy et al. [102] have provided evidence that the prototypical SAD patient is phase delayed [102]. Phase delays in the 24-h rhythm in cortisol [13], core body temperature [13, 103] and melatonin secretion pattern [13, 104] are also detected in SAD patients. Abnormal circadian patterns of melatonin secretion are also considered to play a central role in the pathophysiology and rationale for phototherapy of SAD [105]. A phase delay of melatonin secretion takes place in SAD, as well as changes in the onset [14], duration [106, 107] and offset [107] of melatonin secretion. These observations might partially explain the effectiveness of bright light therapy, which suppresses extended melatonin level production and provides a corrective phase advance in SAD patients. However, it could be a summation of different mechanisms that underlie how light works.
Bright light therapy is the treatment of choice for SAD [104]. A work group organized by the American Psychiatric Association (APA) Council on Research has assessed evidence for the efficacy of light therapy in treating SAD. Their meta-analysis reveals that a significant reduction in depression severity is associated with bright light treatment in SAD (eight studies, with an effect size of 0.84 and 95% confidence interval [CI] of 0.60–1.08) [3]. The effect size is comparable to those of most antidepressant pharmacotherapy trials. In addition, a randomized controlled study has shown that light room therapy is also effective in reducing depressive symptoms in subjects with sub-clinical SAD [72].
There are treatment guidelines for bright light therapy in SAD patients. Some guidelines suggest scheduling 1–2 h of 2,500–10,000 lux exposure immediately upon awakening [102]. In contrast, others recommend conservatively in treatment duration, i.e. not more than 30 min or a maximum of 1 h [51]. The treatment response begins 2–4 days after the start of light therapy and it is usually completed within 2 weeks. If there is still no response, a trial of evening bright light (7–9 p.m.) may be necessary since a smaller subgroup of SAD patients may be phase advanced [108].
A further refinement of the timing of light relative to endogenous melatonin onset has also been proposed. Terman et al. have shown that within the favored morning interval, light administered 7.5–9.5 h after evening melatonin onset produces twice the remission rate (~80% vs. 40%) of light presented 9.5–11 h after melatonin onset [84].
In a randomized controlled trial, cognitive-behavioral therapy (CBT), light therapy and both in combination significantly improved depression severity of SAD patients relative to the control group, but only the combination treatment had a significantly higher remission rate than the control [109]. However, light therapy alone had a higher 1-year recurrence rate than CBT and the combination treatment. One substantial problem may be non-adherence. Long-term compliance of daily light therapy during the symptomatic months each year is questionable, and self-reported adherence is unreliable [110]. A pilot study suggests that adherence to light treatment in SAD patients has a similar order of magnitude to antidepressant medication. Therefore, evaluating adherence to light therapy and evidence-based techniques for maximizing treatment adherence are suggested in clinical practice [95].
The treatment efficacy of SAD using novel LED devices with 1,350 lux white light has been demonstrated in a randomized, double-blind, placebo-controlled trial [111]. An early meta-analysis suggests that light of short-to-medium wavelengths (blue/green/yellow) may be essential for the therapeutic effect of light on SAD [112]. Furthermore, recent evidence supports claims that wavelengths of ~470 nm account for the documented effectiveness of light therapy. Glickman et al. [92] report a placebo-controlled parallel trial of SAD comparing blue-light (blue LED units producing 468 nm light at 398 lux) with dim red-light (red LED units producing 654 nm light at 23 lux). This 3-week study showed that narrow bandwidth blue light outperforms dimmer red light in reversing the symptom severity of SAD [92].
In addition to better alleviation of depression symptoms, another recent randomized, double-blind, placebo-controlled trial also showed that treatment with narrow-band blue LED panels has a better response rate (60%) (Clinical Global Impression-Improvement ≤2) compared to treatment with red LED panels (13%) in SAD patients [93]. In the future, determining the potency of narrow-band short wavelength light relative to current standard treatment and other comparable conditions (e.g. narrow-band short wavelength light with equal photon density compared to broad-spectrum white light) is needed.
4.2 Non-seasonal Depression
The early hypothesis that light therapy specifically enables SAD patients to overcome long winter nights tends to overlook its use in non-seasonal depression [69]. Interestingly, in recent years, cumulative evidence tends to support the use of light treatment alone for non-seasonal depression [113–118] or combined with antidepressants [119–124]. Nonetheless, applications for other forms of non-seasonal depression, such as antepartum depression [125, 126], postpartum depression [127–130], and chronic depression [114], also show promise.
In fact, seasonality lies on a continuous spectrum rather than on distinct all-or-none categories [131]. In clinical observations, recurrent or chronic depression can occur at any time of the year but are usually exacerbated in winter. Therefore, non-seasonal depressions also manifests some seasonality, which may be the key to the treatment response from light therapy. In addition, depressive symptoms usually hinder normal social activities. Long-term absence of zeitgebers that entrain the internal clock to local time can further delay the circadian rhythm phase. Such delays may be causes of depression by themselves regardless of the season [69] and may explain the efficacy of light therapy for non-seasonal depression.
The APA work group has also examined the efficacy of light treatment for non-seasonal depression, both alone and as an adjuvant to antidepressants [3]. This meta-analysis of efficacy studies for light therapy alone in non-seasonal depression in the period 1975–2003 shows reduced significant reduction in depressive symptoms (effect size 0.53; 95% CI = 0.37–1.08; only three studies fulfilled the strict criteria for inclusion in the meta-analysis). In contrast, adjuvant light therapy in non-seasonal depression does not show significant efficacy (five studies; effect size = −0.01, 95% CI = −0.36 to 0.34). The heterogeneity of the methodology used in these studies, i.e. light intensity, duration of daily light, color of light and trial duration, is the major limitation in the general applicability of this negative finding [68]. After refining the inclusion criteria and recruiting up-to-date large studies [122, 123], a later systematic review has reversed this conclusion and offers evidence for the efficacy of light therapy as an adjuvant treatment to antidepressants [10]. However, most of the extracted studies poorly controlled the issue of blindness and are still limited by small sample sizes. Overall, light therapy alone or as adjuvant strategy for non-seasonal depression has vast potential. Future clinical trials should evaluate the differential efficacy for heterogeneous subgroups of patients with non-seasonal depressions.
4.3 Bipolar Depression
Bipolar depression is one of the most difficult psychiatric conditions to treat. A series of studies have been conducted to investigate the mechanisms and efficacy of chronotherapeutic interventions on bipolar depression [18, 132–135]. Neumeister et al. suggest bright light therapy may stabilize the antidepressant effect of partial sleep deprivation [136]. Subsequently, it was found that the combination of total sleep deprivation and light therapy in bipolar depression causes rapid antidepressant effects and its mechanism of action reportedly involves the phase advance of biological rhythm [18] and the enhancement of all monoaminergic systems targeted by antidepressant drugs [132, 135]. Benedetti et al. [133] combined 1-week administration of bright light therapy, three nights of total sleep deprivation, and concurrent antidepressants and lithium salts in the treatment of drug-resistant and non -resistant bipolar depression. Overall, 70% (23/33) of non-resistant versus 44% (12/27) of drug-resistant patients achieved response, with 57% (13/23) of non-resistant and 17% (2/12) of drug-resistant responders becoming euthymic after 9 months. These results suggest chronotherapeutic intervention with existing pharmacotherapy has remarkable success in managing bipolar depression even though drug-resistant responders are significantly more likely to relapse over the follow-up period [133]. Whether or not the maintenance regimen of light therapy helps prevent a relapse of depression in the long-term remains unknown.
Meanwhile, treatment parameters associated with light therapy for bipolar depression should be more carefully adjusted than those of SAD. The reported efficacy and does-range safety in nine women with long-standing non-seasonal bipolar I or II disorder in which mood stabilizers controlled the manic phase, but antidepressants did not relieve the depression phase, showed that three of four subjects treated with morning light developed mixed states [137]. To decrease the risk of inducing mixed episodes, the time of light exposure was changed to midday. Of the five women who received midday light therapy, two achieved full response and two exhibited early improvement but required a dose increase to sustain response. Obviously, this case series is a reminder of the substantial risk of inducing mixed states by light therapy in women with bipolar depression. Initiating treatment for a brief duration (15 min) of midday light for bipolar depression is advised [137].
4.4 Circadian Rhythm Sleep Disorders
Disorders of the circadian timing system are known as circadian rhythm sleep disorders (CRSD). According to the International Classification of Sleep Disorders, version II (ICSD-2), the essential feature of CRSD is a persistent or recurrent pattern of sleep disturbance due primarily to alternations in the circadian time-keeping system or a misalignment between the endogenous circadian rhythm and exogenous factors that affect the timing or duration of sleep. The etiology of this syndrome is multi-factorial such that biologic, psychosocial, and environmental factors all contribute to this syndrome [138]. In ICSD-2, CRSD is composed of six distinct disorders, namely: (1) delayed sleep phase type (DSPT), (2) advanced sleep phase type (ASPT), (3) irregular sleep-wake phase type, (4) free-running type, (5) jet lag type, and (6) shift work type.
The prevalence of CRSD is unknown, especially since there are few community-based epidemiologic studies. In Japan, a two-stage nationwide epidemiology survey estimated the prevalence of DSPT to be 0.13% (age, 15–59 years) [139]. Another study that combined formal diagnostic criteria with an epidemiologic sample suggests that the prevalence of shift work type CRSD is approximately 10% in the night and rotating shift work population [140]. More consistently, DSPT is the most common syndrome (71–85%) in patients with CRSD [141–143].
The light-dark cycle is the principle time cue for resetting human circadian rhythms. In healthy subjects, the most sensitive phase of PRC to light coincides with sleep, while the timing of the monophasic sleep-wake cycle is itself a major determinant of light input on the pacemaker [138]. Theoretically, precisely timed white light of suitable intensity and duration will both phase advance and phase delay circadian rhythms according to a PRC [144, 145]. The rationale of light therapy for CRSD is based on these patterns of human PRC to light, even though clinical application remains empirical. In general, bright light exposure before the core body temperature minimum causes phase delay, and bright light exposure after core body temperature minimum causes phase advance of the circadian rhythm. However, the minimum or optimal intensity or duration of light therapy for each CRSD remain unknown [79].
DSPT that occurs when the circadian timing system is altered relative to the external environment, is a representative syndrome of CRSD. Initiating insomnia accompanied by difficulty awakening in the morning is the typical manifestation. Although evidence is limited, a review by the American Academy of Sleep Medicine (AASM) suggests that light therapy may be a rational and effective intervention for DPST [146]. Practice parameters have been recommended by an earlier clinical guideline, which was reviewed and approved by the AASM in 1999. Bright light therapy with 2,000–2,500 lux from 6 to 9 a.m. is advised. Based on patient tolerance and preference, either a fixed regimen (i.e. based on targeted awakening timing) or a “nudging” technique (i.e. step-by-step phase advance of awakening timing) can be administered. Wearing dark goggles from 4 p.m. to dusk is an optional combination strategy [68, 79].
However, the sleep-wake cycle does not necessarily correlate with the circadian phase in severely afflicted individuals. Early morning light may be unintentionally given on the delayed part of the PRC and worsen the problem [147]. In addition, non-adherence also compromises the practicality of bright light therapy for DSPT because many individuals find it difficult to wake in time for the treatment [148]. Similarly, ASPT is a disorder wherein the patient’s sleep episode is advanced relative to the desired clock time. This syndrome is characterized by falling asleep in the evening and awakening earlier than desired. Effective approaches include 2,500 lux from 8 p.m. to midnight, or 4,000 lux from 8 or 9 p.m. to 11 p.m., which have been validated in the elderly [48, 149].
Another group of CRSD where the physical environment is altered relative to internal circadian timing is shift work and jet lag. Although rationale and simulation studies [150–155] for this group indicate bright light therapy as a potential treatment modality, practical application studies are limited. For shift workers, there are insufficient field studies that evaluate the long-term effectiveness of timed bright light therapy. Moreover, incorporating bright light treatment into the workplace seems difficult. As for jet lag, there is even less evidence [4, 79, 156] and fewer randomized controlled trials.
4.5 Bright Light Therapy in Specific Populations
Since bright light therapy is tuned into clinical conditions with symptom seasonality or desynchronized circadian rhythms, some specific populations may theoretically benefit. For example, bulimia nervosa and LLPDD patients are observed to have winter exacerbation of mood symptoms. Previous studies successfully demonstrated the effectiveness of bright light therapy for these two disorders [74, 157, 158].
In addition to a higher rate of winter depression, individuals with adult ADHD manifest later circadian preference [159, 160]. In the fall/winter period, a mood-independent delay in circadian phase is noted to contribute to the core pathology in many adults with ADHD [161]. Hence, effectiveness of light therapy for adult ADHD is hypothesized. Empirically, a 3-week open trial has shown that light therapy is a useful adjunct, with clinical improvement in core ADHD symptoms regardless of depression [162].
Bright light therapy is also a viable treatment for elderly patients with nonseasonal depression and dementia. As for nonseasonal major depressive episode, exposing to 1-h early-morning bright light (pale blue, approximately 7,500 lux) for 3 weeks, light therapy improved mood, enhanced sleep efficiency, and increased the upslope melatonin level gradient. Besides, continuing improvement in mood and an attenuation of cortisol hyperexcretion after discontinuation of treatment was observed [163]. With regard to dementia, changes in the SCN and environmental factors in nursing homes (i.e. noise and irregular light exposure) concomitantly contribute to a disrupted rest-activity pattern that accompanies dementia [164, 165]. In earlier studies, evidence of morning bright light therapy show inconsistent but promising effects for behavioral and psychological symptoms of dementia [166–169]. After prolonged duration of bright light exposure (whole day bright light with ±1,000 lux), a recent 5-year, randomized, controlled study presents a modest benefit from light therapy in attenuating cognitive deterioration, depressive symptoms, and function impairment [170]. The need for non-pharmacological management of many clinical conditions, e.g. elderly dementia, augurs well for bright light therapy.
5 Conclusions and Future Directions
Light theraphy has been developed for clinical use and many apparatus and parameters have been extensively studied. Bright light theraphy is the treatment of choice for SAD and CRSD. However, as for other clinical conditions, the benefit and dosing regimen of light theraphy warrants further studies to optimize the treatment effect. In addition, new devices such as light visors, light theraphy rooms and narrow-band blue light from LEDs require more investigations to examine their efficacy and saftey.
References
Lewy AJ, Kern HA, Rosenthal NE, Wehr TA (1982) Bright artificial light treatment of a manic-depressive patient with a seasonal mood cycle. Am J Psychiatry 139(11):1496–1498
Rosenthal NE, Sack DA, Gillin JC, Lewy AJ, Goodwin FK, Davenport Y, Wehr TA (1984) Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Arch Gen Psychiatry 41(1):72–80
Golden RN, Gaynes BN, Ekstrom RD, Hamer RM, Jacobsen FM, Suppes T, Nemeroff CB (2005) The efficacy of light therapy in the treatment of mood disorders: a review and meta-analysis of the evidence. Am J Psychiatry 162(4):656–662
Boulos Z, Campbell SS, Lewy AJ, Terman M, Dijk DJ, Eastman CI (1995) Light treatment for sleep disorders: consensus report. VII. Jet lag. J Biol Rhythms 10(2):167–176
Campbell SS, Eastman CI, Terman M, Lewy AJ, Boulos Z, Dijk DJ (1995) Light treatment for sleep disorders: consensus report. I. Chronology of seminal studies in humans. J Biol Rhythms 10(2):105–109
Dijk DJ, Boulos Z, Eastman CI, Lewy AJ, Campbell SS, Terman M (1995) Light treatment for sleep disorders: consensus report. II. Basic properties of circadian physiology and sleep regulation. J Biol Rhythms 10(2):113–125
Eastman CI, Boulos Z, Terman M, Campbell SS, Dijk DJ, Lewy AJ (1995) Light treatment for sleep disorders: consensus report. VI. Shift work. J Biol Rhythms 10(2):157–164
Parry BL, Mahan AM, Mostofi N, Klauber MR, Lew GS, Gillin JC (1993) Light therapy of late luteal phase dysphoric disorder: an extended study. Am J Psychiatry 150(9):1417–1419
Terman M, Lewy AJ, Dijk DJ, Boulos Z, Eastman CI, Campbell SS (1995) Light treatment for sleep disorders: consensus report. IV. Sleep phase and duration disturbances. J Biol Rhythms 10(2):135–147
Even C, Schroder CM, Friedman S, Rouillon F (2008) Efficacy of light therapy in nonseasonal depression: a systematic review. J Affect Disord 108(1–2):11–23
Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J (2003) Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J Neurosci 23(33):10691–10702. doi:23/33/10691 [pii]
Pagani L Semenova EA Moriggi E Revell VL Hack LM Lockley SW Arendt J Skene DJ Meier F Izakovic J Wirz-Justice A Cajochen C Sergeeva OJ Cheresiz SV Danilenko KV Eckert A Brown SA (2010) The physiological period length of the human circadian clock in vivo is directly proportional to period in human fibroblasts. PLos One 5: e13376
Avery DH, Dahl K, Savage MV, Brengelmann GL, Larsen LH, Kenny MA, Prinz PN (1997) Circadian temperature and cortisol rhythms during a constant routine are phase-delayed in hypersomnic winter depression. Biol Psychiatry 41(11):1109–1123
Dahl K, Avery DH, Lewy AJ, Savage MV, Brengelmann GL, Larsen LH, Prinz PN (1993) Dim light melatonin onset and circadian temperature during a constant routine in hypersomnic winter depression. Acta Psychiatr Scand 88(1):60–66
Lewy AJ, Sack RL, Singer CM, White DM, Hoban TM (1988) Winter depression and the phase-shift hypothesis for bright light’s therapeutic effects: history, theory, and experimental evidence. J Biol Rhythms 3(2):121–134
Dilsaver SC (1989) Neurobiologic effects of bright artificial light. Brain Res Brain Res Rev 14(4):311–333
Neumeister A, Konstantinidis A, Praschak-Rieder N, Willeit M, Hilger E, Stastny J, Kasper S (2001) Monoaminergic function in the pathogenesis of seasonal affective disorder. Int J Neuropsychopharmacol 4(4):409–420
Benedetti F, Dallaspezia S, Fulgosi MC, Barbini B, Colombo C, Smeraldi E (2007) Phase advance is an actimetric correlate of antidepressant response to sleep deprivation and light therapy in bipolar depression. Chronobiol Int 24(5):921–937
Gonzalez MM, Aston-Jones G (2008) Light deprivation damages monoamine neurons and produces a depressive behavioral phenotype in rats. Proc Natl Acad Sci USA 105(12):4898–4903
Lam RW, Zis AP, Grewal A, Delgado PL, Charney DS, Krystal JH (1996) Effects of rapid tryptophan depletion in patients with seasonal affective disorder in remission after light therapy. Arch Gen Psychiatry 53(1):41–44
Dibner C, Schibler U, Albrecht U (2010) The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol 72:517–549. doi:10.1146/annurev-physiol-021909-135821
Berson DM, Dunn FA, Takao M (2002) Phototransduction by retinal ganglion cells that set the circadian clock. Science 295(5557):1070–1073
Hattar S, Liao HW, Takao M, Berson DM, Yau KW (2002) Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 295(5557):1065–1070
Gooley JJ, Saper CB (2005) Anatomy of Mammalian Circadian system. In: Kryger MH, Roth T, Dement WC (eds) Principles and practice of sleep medicine, 4th edn. Elsevier Saunders, Philadelphia, pp 335–350
Reppert SM, Weaver DR (2002) Coordination of circadian timing in mammals. Nature 418(6901):935–941
Zhang L, Kolaj M, Renaud LP (2006) Suprachiasmatic nucleus communicates with anterior thalamic paraventricular nucleus neurons via rapid glutamatergic and gabaergic neurotransmission: state-dependent response patterns observed in vitro. Neuroscience 141(4):2059–2066
Klein DC, Moore RY, Reppert SM (1991) Suprachiasmatic nucleus: the mind’s clock. Oxford University Press, New York
Hannibal J, Ding JM, Chen D, Fahrenkrug J, Larsen PJ, Gillette MU, Mikkelsen JD (1997) Pituitary adenylate cyclase-activating peptide (PACAP) in the retinohypothalamic tract: a potential daytime regulator of the biological clock. J Neurosci 17(7):2637–2644
Moore RY, Card JP (1994) Intergeniculate leaflet: an anatomically and functionally distinct subdivision of the lateral geniculate complex. J Comp Neurol 344(3):403–430. doi:10.1002/cne.903440306
Watts AG, Swanson LW (1987) Efferent projections of the suprachiasmatic nucleus: II. Studies using retrograde transport of fluorescent dyes and simultaneous peptide immunohistochemistry in the rat. J Comp Neurol 258(2):230–252. doi:10.1002/cne.902580205
Watts AG, Swanson LW, Sanchez-Watts G (1987) Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J Comp Neurol 258(2):204–229. doi:10.1002/cne.902580204
Leak RK, Moore RY (2001) Topographic organization of suprachiasmatic nucleus projection neurons. J Comp Neurol 433(3):312–334
LeSauter J, Lehman MN, Silver R (1996) Restoration of circadian rhythmicity by transplants of SCN “micropunches”. J Biol Rhythms 11(2):163–171
Silver R, LeSauter J, Tresco PA, Lehman MN (1996) A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature 382(6594):810–813. doi:10.1038/382810a0
Lu J, Zhang YH, Chou TC, Gaus SE, Elmquist JK, Shiromani P, Saper CB (2001) Contrasting effects of ibotenate lesions of the paraventricular nucleus and subparaventricular zone on sleep-wake cycle and temperature regulation. J Neurosci 21(13):4864–4874. doi:21/13/4864 [pii]
Kalsbeek A, Foppen E, Schalij I, Van Heijningen C, van der Vliet J, Fliers E, Buijs RM (2008) Circadian control of the daily plasma glucose rhythm: an interplay of GABA and glutamate. PLoS One 3(9):e3194. doi:10.1371/journal.pone.0003194
Kalsbeek A, Verhagen LA, Schalij I, Foppen E, Saboureau M, Bothorel B, Pevet P (2008) Opposite actions of hypothalamic vasopressin on circadian corticosterone rhythm in nocturnal versus diurnal species. Eur J Neurosci 27(4):818–827
Tei H, Okamura H, Shigeyoshi Y, Fukuhara C, Ozawa R, Hirose M, Sakaki Y (1997) Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature 389(6650):512–516. doi:10.1038/39086
Sherin JE, Shiromani PJ, McCarley RW, Saper CB (1996) Activation of ventrolateral preoptic neurons during sleep. Science 271(5246):216–219
Decoursey PJ (1964) Function of a light response rhythm in hamsters. J Cell Physiol 63:189–196
Lewy AJ, Sack RL (1986) Light therapy and psychiatry. Proc Soc Exp Biol Med 183(1):11–18
Khalsa SB, Jewett ME, Cajochen C, Czeisler CA (2003) A phase response curve to single bright light pulses in human subjects. J Physiol 549(Pt 3):945–952
Ambrogetti A (2006) Chronobiology: an introduction. In: Ambrogetti A, Hensley M, Olson L (eds) Sleep disorders: a clinical textbook. MA Healthcare Limited, London, pp 25–55
Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP (1980) Light suppresses melatonin secretion in humans. Science 210(4475):1267–1269
Boivin DB, Duffy JF, Kronauer RE, Czeisler CA (1996) Dose-response relationships for resetting of human circadian clock by light. Nature 379(6565):540–542. doi:10.1038/379540a0
Czeisler CA, Allan JS, Strogatz SH, Ronda JM, Sanchez R, Rios CD, Kronauer RE (1986) Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science 233(4764):667–671
Campbell SS (1995) Effects of timed bright-light exposure on shift-work adaptation in middle-aged subjects. Sleep 18(6):408–416
Campbell SS, Dawson D, Anderson MW (1993) Alleviation of sleep maintenance insomnia with timed exposure to bright light. J Am Geriatr Soc 41(8):829–836
Eastman CI (1992) High-intensity light for circadian adaptation to a 12-h shift of the sleep schedule. Am J Physiol 263(2 Pt 2):R428–R436
Zisapel N (2001) Circadian rhythm sleep disorders: pathophysiology and potential approaches to management. CNS Drugs 15(4):311–328
Terman M, Terman JS (2005) Light therapy for seasonal and nonseasonal depression: efficacy, protocol, safety, and side effects. CNS Spectr 10(8):647–663, quiz 672
Zhao ZY, Xie Y, Fu YR, Bogdan A, Touitou Y (2002) Aging and the circadian rhythm of melatonin: a cross-sectional study of Chinese subjects 30–110 yr of age. Chronobiol Int 19(6):1171–1182
Dijk DJ, Cajochen C (1997) Melatonin and the circadian regulation of sleep initiation, consolidation, structure, and the sleep EEG. J Biol Rhythms 12(6):627–635
Terman M, Terman JS, Quitkin FM, McGrath PJ, Stewart JW, Rafferty B (1989) Light therapy for seasonal affective disorder. A review of efficacy. Neuropsychopharmacology 2(1):1–22. doi:0893-133X(89)90002-X [pii]
Murray G, Allen NB, Trinder J (2003) Seasonality and circadian phase delay: prospective evidence that winter lowering of mood is associated with a shift towards Eveningness. J Affect Disord 76(1–3):15–22. doi:S0165032702000599 [pii]
Eastman CI, Gallo LC, Lahmeyer HW, Fogg LF (1993) The circadian rhythm of temperature during light treatment for winter depression. Biol Psychiatry 34(4):210–220. doi:0006-3223(93)90074-N [pii]
Murray G, Michalak EE, Levitt AJ, Levitan RD, Enns MW, Morehouse R, Lam RW (2005) Therapeutic mechanism in seasonal affective disorder: do fluoxetine and light operate through advancing circadian phase? Chronobiol Int 22(5):937–943
Thompson C, Childs PA, Martin NJ, Rodin I, Smythe PJ (1997) Effects of morning phototherapy on circadian markers in seasonal affective disorder. Br J Psychiatry 170:431–435
Wirz-Justice A, Graw P, Krauchi K, Gisin B, Jochum A, Arendt J, Poldinger W (1993) Light therapy in seasonal affective disorder is independent of time of day or circadian phase. Arch Gen Psychiatry 50(12):929–937
Lewy AJ, Emens J, Sack RL, Hasler BP, Bernert RA (2003) Zeitgeber hierarchy in humans: resetting the circadian phase positions of blind people using melatonin. Chronobiol Int 20(5):837–852
Burgess HJ, Fogg LF, Young MA, Eastman CI (2004) Bright light therapy for winter depression – is phase advancing beneficial? Chronobiol Int 21(4–5):759–775
Neumeister A, Praschak-Rieder N, Willeit M, Stastny J, Kasper S (1999) Monoamine depletion in non-pharmacological treatments for depression. Adv Exp Med Biol 467:29–33
Neumeister A, Praschak-Rieder N, Hesselmann B, Vitouch O, Rauh M, Barocka A, Kasper S (1998) Effects of tryptophan depletion in fully remitted patients with seasonal affective disorder during summer. Psychol Med 28(2):257–264
Neumeister A, Praschak-Rieder N, Hesselmann B, Vitouch O, Rauh M, Barocka A, Kasper S (1998) Effects of tryptophan depletion in drug-free depressed patients who responded to total sleep deprivation. Arch Gen Psychiatry 55(2):167–172
Kogan AO, Guilford PM (1998) Side effects of short-term 10,000-lux light therapy. Am J Psychiatry 155(2):293–294
Labbate LA, Lafer B, Thibault A, Sachs GS (1994) Side effects induced by bright light treatment for seasonal affective disorder. J Clin Psychiatry 55(5):189–191
Praschak-Rieder N, Neumeister A, Hesselmann B, Willeit M, Barnas C, Kasper S (1997) Suicidal tendencies as a complication of light therapy for seasonal affective disorder: a report of three cases. J Clin Psychiatry 58(9):389–392
Prasko J (2008) Bright light therapy. Neuro Endocrinol Lett 29(Suppl 1):33–64. doi:NEL290708A02 [pii]
Terman M (2007) Evolving applications of light therapy. Sleep Med Rev 11(6):497–507
Joffe RT, Moul DE, Lam RW, Levitt AJ, Teicher MH, Lebegue B et al (1993) Light visor treatment for seasonal affective disorder: a multicenter study. Psychiatry Res 46(1):29–39
Boulos Z, Macchi MM, Sturchler MP, Stewart KT, Brainard GC, Suhner A, Steffen R (2002) Light visor treatment for jet lag after westward travel across six time zones. Aviat Space Environ Med 73(10):953–963
Rastad C, Ulfberg J, Lindberg P (2008) Light room therapy effective in mild forms of seasonal affective disorder–a randomised controlled study. J Affect Disord 108(3):291–296
Terman JS, Terman M, Schlager D, Rafferty B, Rosofsky M, Link MJ, Quitkin FM (1990) Efficacy of brief, intense light exposure for treatment of winter depression. Psychopharmacol Bull 26(1):3–11
Lam RW, Goldner EM, Solyom L, Remick RA (1994) A controlled study of light therapy for bulimia nervosa. Am J Psychiatry 151(5):744–750
Magnusson A, Kristbjarnarson H (1991) Treatment of seasonal affective disorder with high-intensity light. A phototherapy study with an Icelandic group of patients. J Affect Disord 21(2):141–147
Stewart KT, Hayes BC, Eastman CI (1995) Light treatment for NASA shiftworkers. Chronobiol Int 12(2):141–151
Dunai A, Novak M, Chung SA, Kayumov L, Keszei A, Levitan R, Shapiro CM (2007) Moderate exercise and bright light treatment in overweight and obese individuals. Obesity (Silver Spring) 15(7):1749–1757
Levitt AJ, Lam RW, Levitan R (2002) A comparison of open treatment of seasonal major and minor depression with light therapy. J Affect Disord 71(1–3):243–248. doi:S0165032701003974 [pii]
Chesson AL Jr, Littner M, Davila D, Anderson WM, Grigg-Damberger M, Hartse K, Wise M (1999) Practice parameters for the use of light therapy in the treatment of sleep disorders. Standards of Practice Committee, American Academy of Sleep Medicine. Sleep 22(5):641–660
Gallin PF, Terman M, Reme CE, Rafferty B, Terman JS, Burde RM (1995) Ophthalmologic examination of patients with seasonal affective disorder, before and after bright light therapy. Am J Ophthalmol 119(2):202–210
Labbate LA, Lafer B, Thibault A, Rosenbaum JF, Sachs GS (1995) Influence of phototherapy treatment duration for seasonal affective disorder: outcome at one vs. two weeks. Biol Psychiatry 38(11):747–750
Martiny K, Lunde M, Unden M, Dam H, Bech P (2009) High cortisol awakening response is associated with an impairment of the effect of bright light therapy. Acta Psychiatr Scand 120(3):196–202
Most EI, Scheltens P, Van Someren EJ (2010) Prevention of depression and sleep disturbances in elderly with memory-problems by activation of the biological clock with light – a randomized clinical trial. Trials 11:19
Terman JS, Terman M, Lo ES, Cooper TB (2001) Circadian time of morning light administration and therapeutic response in winter depression. Arch Gen Psychiatry 58(1):69–75. doi:yoa20017 [pii]
Terman M, Terman JS (2010) In: Kryger MH, Roth T, Dement WC (eds) Principles and practice of sleep medicine, 5th edn. Elsevier/Saunders, St. Louis, pp 1682–1695
Horne JA, Ostberg O (1976) A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol 4(2):97–110
Reme CE, Rol P, Grothmann K, Kaase H, Terman M (1996) Bright light therapy in focus: lamp emission spectra and ocular safety. Technol Health Care 4(4):403–413
Lam RW, Buchanan A, Clark CM, Remick RA (1991) Ultraviolet versus non-ultraviolet light therapy for seasonal affective disorder. J Clin Psychiatry 52(5):213–216
Wright HR, Lack LC, Kennaway DJ (2004) Differential effects of light wavelength in phase advancing the melatonin rhythm. J Pineal Res 36(2):140–144. doi:10.1046/j.1600-079X.2003.00108.x
Oren DA, Brainard GC, Johnston SH, Joseph-Vanderpool JR, Sorek E, Rosenthal NE (1991) Treatment of seasonal affective disorder with green light and red light. Am J Psychiatry 148(4):509–511
Loving RT, Kripke DF, Knickerbocker NC, Grandner MA (2005) Bright green light treatment of depression for older adults [ISRCTN69400161]. BMC Psychiatry 5:42
Glickman G, Byrne B, Pineda C, Hauck WW, Brainard GC (2006) Light therapy for seasonal affective disorder with blue narrow-band light-emitting diodes (LEDs). Biol Psychiatry 59(6):502–507
Strong RE, Marchant BK, Reimherr FW, Williams E, Soni P, Mestas R (2009) Narrow-band blue-light treatment of seasonal affective disorder in adults and the influence of additional nonseasonal symptoms. Depress Anxiety 26(3):273–278
Terman M (2009) Blue in the face, Sleep Med 10: 277–278
Michalak EE, Murray G, Wilkinson C, Dowrick C, Lam RW (2007) A pilot study of adherence with light treatment for seasonal affective disorder. Psychiatry Res 149(1–3):315–320
American Psychiatric Association (APA) (1994) Diagnostic and statistical manual of mental disorders. 4th edn. American Pschiatric Association, Washington DC
Winkler D, Kasper S (2005) Seasonal affective disorder: from diagnosis to treatment. Medicographia 27:247–253
Lavoie M-P, Lam RW, Bouchard G, Sasseville A, Charron M-C, Gagne A-M, Hebert M (2009) Evidence of a biological effect of light therapy on the retina of patients with seasonal affective disorder. Biol Psychiatry 66(3):253–258
Provencio I (2005) Chronobiology. In: Sadock BJ, Sadock VA (eds) Comprehensive textbook of psychiatry, 8th edn. Lippincott Williams & Wilkins, Philadelphia, pp 161–171
Rosen LN, Targum SD, Terman M, Bryant MJ, Hoffman H, Kasper SF, Rosenthal NE (1990) Prevalence of seasonal affective disorder at four latitudes. Psychiatry Res 31(2):131–144
Neumeister A, Pirker W, Willeit M, Praschak-Rieder N, Asenbaum S, Brucke T, Kasper S (2000) Seasonal variation of availability of serotonin transporter binding sites in healthy female subjects as measured by [123I]-2 beta-carbomethoxy-3 beta-(4-iodophenyl)tropane and single photon emission computed tomography. Biol Psychiatry 47(2):158–160. doi:S0006-3223(99)00241-3 [pii]
Lewy AJ, Rough JN, Songer JB, Mishra N, Yuhas K, Emens JS (2007) The phase shift hypothesis for the circadian component of winter depression. Dialogues Clin Neurosci 9(3):291–300
Schwartz PJ, Rosenthal NE, Turner EH, Drake CL, Liberty V, Wehr TA (1997) Seasonal variation in core temperature regulation during sleep in patients with winter seasonal affective disorder. Biol Psychiatry 42(2):122–131
Monteleone P, Maj M (2008) The circadian basis of mood disorders: recent developments and treatment implications. Eur Neuropsychopharmacol 18(10):701–711
Srinivasan V, Smits M, Spence W, Lowe AD, Kayumov L, Pandi-Perumal SR, Cardinali DP (2006) Melatonin in mood disorders. World J Biol Psychiatry 7(3):138–151
Danilenko KV, Putilov AA, Russkikh GS, Duffy LK, Ebbesson SO (1994) Diurnal and seasonal variations of melatonin and serotonin in women with seasonal affective disorder. Arctic Med Res 53(3):137–145
Wehr TA, Duncan WC Jr, Sher L, Aeschbach D, Schwartz PJ, Turner EH, Rosenthal NE (2001) A circadian signal of change of season in patients with seasonal affective disorder. Arch Gen Psychiatry 58(12):1108–1114. doi:yoa20397 [pii]
Lewy AJ, Lefler BJ, Emens JS, Bauer VK (2006) The circadian basis of winter depression. Proc Natl Acad Sci USA 103(19):7414–7419
Rohan KJ, Roecklein KA, Tierney Lindsey K, Johnson LG, Lippy RD, Lacy TJ, Barton FB (2007) A randomized controlled trial of cognitive-behavioral therapy, light therapy, and their combination for seasonal affective disorder. J Consult Clin Psychol 75(3):489–500
Rohan KJ, Roecklein KA, Lacy TJ, Vacek PM (2009) Winter depression recurrence one year after cognitive-behavioral therapy, light therapy, or combination treatment. Behav Ther 40(3):225–238
Desan PH, Weinstein AJ, Michalak EE, Tam EM, Meesters Y, Ruiter MJ, Lam RW (2007) A controlled trial of the litebook light-emitting diode (LED) light therapy device for treatment of seasonal affective disorder (SAD). BMC Psychiatry 7:38
Lee TM, Chan CC, Paterson JG, Janzen HL, Blashko CA (1997) Spectral properties of phototherapy for seasonal affective disorder: a meta-analysis. Acta Psychiatr Scand 96(2):117–121
Deltito JA, Moline M, Pollak C, Martin LY, Maremmani I (1991) Effects of phototherapy on non-seasonal unipolar and bipolar depressive spectrum disorders. J Affect Disord 23(4):231–237
Goel N, Terman M, Terman JS, Macchi MM, Stewart JW (2005) Controlled trial of bright light and negative air ions for chronic depression. Psychol Med 35(7):945–955
Kripke DF, Mullaney DJ, Klauber MR, Risch SC, Gillin JC (1992) Controlled trial of bright light for nonseasonal major depressive disorders. Biol Psychiatry 31(2):119–134
Loving RT, Kripke DF, Elliott JA, Knickerbocker NC, Grandner MA (2005) Bright light treatment of depression for older adults [ISRCTN55452501]. BMC Psychiatry 5:41
Mackert A, Volz HP, Stieglitz RD, Muller-Oerlinghausen B (1991) Phototherapy in nonseasonal depression. Biol Psychiatry 30(3):257–268. doi:0006-3223(91)90110-8 [pii]
Yamada N, Martin-Iverson MT, Daimon K, Tsujimoto T, Takahashi S (1995) Clinical and chronobiological effects of light therapy on nonseasonal affective disorders. Biol Psychiatry 37(12):866–873
Beauchemin KM, Hays P (1997) Phototherapy is a useful adjunct in the treatment of depressed in-patients. Acta Psychiatr Scand 95(5):424–427
Holsboer-Trachsler E (1994) Monitoring the neurobiological and psychopathological course in therapy of depression. Trimipramine, sleep deprivation and light. Bibl Psychiatr 166:1–138
Loving RT, Kripke DF, Shuchter SR (2002) Bright light augments antidepressant effects of medication and wake therapy. Depress Anxiety 16(1):1–3. doi:10.1002/da.10036
Martiny K, Lunde M, Unden M, Dam H, Bech P (2005) Adjunctive bright light in non-seasonal major depression: results from clinician-rated depression scales. Acta Psychiatr Scand 112(2):117–125
Martiny K, Lunde M, Unden M, Dam H, Bech P (2005) Adjunctive bright light in non-seasonal major depression: results from patient-reported symptom and well-being scales. Acta Psychiatr Scand 111(6):453–459
Prasko J, Horacek J, Klaschka J, Kosova J, Ondrackova I, Sipek J (2002) Bright light therapy and/or imipramine for inpatients with recurrent non-seasonal depression. Neuro Endocrinol Lett 23(2):109–113. doi:NEL230202A04 [pii]
Epperson CN, Terman M, Terman JS, Hanusa BH, Oren DA, Peindl KS, Wisner KL (2004) Randomized clinical trial of bright light therapy for antepartum depression: preliminary findings. J Clin Psychiatry 65(3):421–425
Oren DA, Wisner KL, Spinelli M, Epperson CN, Peindl KS, Terman JS, Terman M (2002) An open trial of morning light therapy for treatment of antepartum depression. Am J Psychiatry 159(4):666–669
Corral M, Kuan A, Kostaras D (2000) Bright light therapy’s effect on postpartum depression. Am J Psychiatry 157(2):303–304
Corral M, Wardrop AA, Zhang H, Grewal AK, Patton S (2007) Morning light therapy for postpartum depression. Arch Womens Ment Health 10(5):221–224
Parry BL, Newton RP (2001) Chronobiological basis of female-specific mood disorders. Neuropsychopharmacology 25(5 Suppl):S102–S108
Parry BL, Curran ML, Stuenkel CA, Yokimozo M, Tam L, Powell KA, Gillin JC (2000) Can critically timed sleep deprivation be useful in pregnancy and postpartum depressions? J Affect Disord 60(3):201–212. doi:S0165032799001792 [pii]
Terman M (1988) On the question of mechanism in phototherapy for seasonal affective disorder: considerations of clinical efficacy and epidemiology. J Biol Rhythms 3(2):155–172
Benedetti F, Colombo C, Serretti A, Lorenzi C, Pontiggia A, Barbini B, Smeraldi E (2003) Antidepressant effects of light therapy combined with sleep deprivation are influenced by a functional polymorphism within the promoter of the serotonin transporter gene. Biol Psychiatry 54(7):687–692. doi:S0006322302018942 [pii]
Benedetti F, Barbini B, Fulgosi MC, Colombo C, Dallaspezia S, Pontiggia A, Smeraldi E (2005) Combined total sleep deprivation and light therapy in the treatment of drug-resistant bipolar depression: acute response and long-term remission rates. J Clin Psychiatry 66(12):1535–1540
Benedetti F, Calabrese G, Bernasconi A, Cadioli M, Colombo C, Dallaspezia S, Smeraldi E (2009) Spectroscopic correlates of antidepressant response to sleep deprivation and light therapy: a 3.0 Tesla study of bipolar depression. Psychiatry Res 173(3):238–242
Benedetti F, Barbini B, Bernasconi A, Fulgosi M, Dallaspezia S, Gavinelli C, Locatelli C, Lorenzi C, Pirovano A, Radaelli D, Smeraldi E, Colombo C (2010) Acute antidepressant response to sleep deprivation combined with light therapy is influenced by the catechol-O-methyltransferase Val(108/158)Met polymorphism. J Affect Disord 121(1–2):68–72
Neumeister A, Goessler R, Lucht T, Kapitany C (1996) Bright light therapy stabilizes the antidepressant effect of partial sleep deprivation. Biol Psychiatry 3:16–21.
Sit D, Wisner KL, Hanusa BH, Stull S, Terman M (2007) Light therapy for bipolar disorder: a case series in women. Bipolar Disord 9(8):918–927
Okawa M, Uchiyama M (2007) Circadian rhythm sleep disorders: characteristics and entrainment pathology in delayed sleep phase and non-24-h sleep-wake syndrome. Sleep Med Rev 11(6):485–496
Yazaki M, Shirakawa S, Okawa M, Takahashi K (1999) Demography of sleep disturbances associated with circadian rhythm disorders in Japan. Psychiatry Clin Neurosci 53(2):267–268
Drake CL, Roehrs T, Richardson G, Walsh JK, Roth T (2004) Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep 27(8):1453–1462
Dagan Y, Eisenstein M (1999) Circadian rhythm sleep disorders: toward a more precise definition and diagnosis. Chronobiol Int 16(2):213–222
Kamei Y, Urata J, Uchiyaya M, Hayakawa T, Ozaki S, Shibui K, Okawa M (1998) Clinical characteristics of circadian rhythm sleep disorders. Psychiatry Clin Neurosci 52(2):234–235
Yamadera H, Takahashi K, Okawa M (1996) A multicenter study of sleep-wake rhythm disorders: therapeutic effects of vitamin B12, bright light therapy, chronotherapy and hypnotics. Psychiatry Clin Neurosci 50(4):203–209
Boivin DB, Czeisler CA (1998) Resetting of circadian melatonin and cortisol rhythms in humans by ordinary room light. Neuroreport 9(5):779–782
Minors DS, Waterhouse JM, Wirz-Justice A (1991) A human phase-response curve to light. Neurosci Lett 133(1):36–40. doi:0304-3940(91)90051-T [pii]
Sack RL, Auckley D, Auger RR, Carskadon MA, Wright KP Jr, Vitiello MV, Zhdanova IV (2007) Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. An American Academy of Sleep Medicine review. Sleep 30(11):1484–1501
Regestein QR, Pavlova M (1995) Treatment of delayed sleep phase syndrome. Gen Hosp Psychiatry 17(5):335–345. doi:016383439500062V [pii]
Reid KJ, Zee PC (2005) Circadian disorders of the sleep-wake cycle. In: Kryger MH, Roth T, Dement WC (eds) Principle and pratice of sleep medicine, 4th edn. Elsevier Saunders, Philadelphia, p 696
Murphy PJ, Campbell SS (1996) Enhanced performance in elderly subjects following bright light treatment of sleep maintenance insomnia. J Sleep Res 5(3):165–172
Crowley SJ, Lee C, Tseng CY, Fogg LF, Eastman CI (2004) Complete or partial circadian re-entrainment improves performance, alertness, and mood during night-shift work. Sleep 27(6):1077–1087
Dawson D, Campbell SS (1991) Timed exposure to bright light improves sleep and alertness during simulated night shifts. Sleep 14(6):511–516
Eastman CI, Liu L, Fogg LF (1995) Circadian rhythm adaptation to simulated night shift work: effect of nocturnal bright-light duration. Sleep 18(6):399–407
Mitchell PJ, Hoese EK, Liu L, Fogg LF, Eastman CI (1997) Conflicting bright light exposure during night shifts impedes circadian adaptation. J Biol Rhythms 12(1):5–15
Reid K, Dawson D (2001) Comparing performance on a simulated 12 hour shift rotation in young and older subjects. Occup Environ Med 58(1):58–62
Wever RA (1985) Use of light to treat jet lag: differential effects of normal and bright artificial light on human circadian rhythms. Ann N Y Acad Sci 453:282–304
Deacon S, Arendt J (1996) Adapting to phase shifts. I. An experimental model for jet lag and shift work. Physiol Behav 59(4–5):665–673. doi:0031-9384(95)02147-7 [pii]
Lam RW, Carter D, Misri S, Kuan AJ, Yatham LN, Zis AP (1999) A controlled study of light therapy in women with late luteal phase dysphoric disorder. Psychiatry Res 86(3):185–192
Maskall DD, Lam RW, Misri S, Carter D, Kuan AJ, Yatham LN, Zis AP (1997) Seasonality of symptoms in women with late luteal phase dysphoric disorder. Am J Psychiatry 154(10):1436–1441
Levitan RD, Jain UR, Katzman MA (1999) Seasonal affective symptoms in adults with residual attention-deficit hyperactivity disorder. Compr Psychiatry 40(4):261–267. doi:S0010-440X(99)90125-6 [pii]
Rosenthal NE (1995) Syndrome triad in children and adolescents. Am J Psychiatry 152(9):1402
Rybak YE, McNeely HE, Mackenzie BE, Jain UR, Levitan RD (2007) Seasonality and circadian preference in adult attention-deficit/hyperactivity disorder: clinical and neuropsychological correlates. Compr Psychiatry 48(6):562–571
Rybak YE, McNeely HE, Mackenzie BE, Jain UR, Levitan RD (2006) An open trial of light therapy in adult attention-deficit/hyperactivity disorder. J Clin Psychiatry 67(10):1527–1535
Lieverse R, Van Someren EJ, Nielen MM, Uitdehaag BM, Smit JH, Hoogendijk WJ (2011) Bright light treatment in elderly patients with nonseasonal major depressive disorder: a randomized placebo-controlled trial. Arch Gen Psychiatry 68:61–70.
Ancoli-Israel S, Ayalon L (2006) Diagnosis and treatment of sleep disorders in older adults. Am J Geriatr Psychiatry 14(2):95–103
Pat-Horenczyk R, Klauber MR, Shochat T, Ancoli-Israel S (1998) Hourly profiles of sleep and wakefulness in severely versus mild-moderately demented nursing home patients. Aging 10(4):308–315
Ancoli-Israel S, Martin JL, Kripke DF, Marler M, Klauber MR (2002) Effect of light treatment on sleep and circadian rhythms in demented nursing home patients. J Am Geriatr Soc 50(2):282–289. doi:jgs50060 [pii]
Ancoli-Israel S, Gehrman P, Martin JL, Shochat T, Marler M, Corey-Bloom J, Levi L (2003) Increased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer’s disease patients. Behav Sleep Med 1(1):22–36
Dowling GA, Hubbard EM, Mastick J, Luxenberg JS, Burr RL, Van Someren EJ (2005) Effect of morning bright light treatment for rest-activity disruption in institutionalized patients with severe Alzheimer’s disease. Int Psychogeriatr 17(2):221–236
Dowling GA, Mastick J, Hubbard EM, Luxenberg JS, Burr RL (2005) Effect of timed bright light treatment for rest-activity disruption in institutionalized patients with Alzheimer’s disease. Int J Geriatr Psychiatry 20(8):738–743. doi:10.1002/gps.1352
Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJG, Van Someren EJW (2008) Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA 299(22):2642–2655
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Mao, WC., Lee, HC., Chen, HC. (2012). Management of Sleep Disorders: Light Therapy. In: Chiang, RY., Kang, SC. (eds) Introduction to Modern Sleep Technology. Intelligent Systems, Control and Automation: Science and Engineering, vol 64. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-5470-6_8
Download citation
DOI: https://doi.org/10.1007/978-94-007-5470-6_8
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-5469-0
Online ISBN: 978-94-007-5470-6
eBook Packages: Computer ScienceComputer Science (R0)