Abstract
-
1.
Strategies of aquatic organisms to cope with ambient environmental conditions involve avoidance reactions or more profound behavioural and physiological adjustments, collectively called “adaptations”.
-
2.
Modulative (irreversible) and modificative (reversible) adaptations are short-term compensatory changes (acclimations) in an individual in response to environmental change, which are made possible through phenotypic plasticity.
-
3.
Strong triggers for physiological adaptations that are more specific for the Baltic Sea than for most other water bodies are low salinity and low oxygen levels.
-
4.
Mechanisms for adaptation to the salinity of the Baltic Sea, as well as to salinity fluctuations in Baltic coastal regions due to freshwater discharge, involve ion regulation (through ion channels , ion exchange proteins or primary ion pumps ) and osmotic adaptation (e.g. through intracellular concentrations of osmotically active substances , such as low-molecular carbohydrates, amino acids and nucleic acids ).
-
5.
Low oxygen levels are dealt with by avoidance or a more effective energy metabolism.
-
6.
Stress proteins provide cellular and whole-body responses of organisms to a vast range of changes in environmental conditions, e.g. water temperature , salinity, acidification, light availability, chemical pollution and hypoxia.
-
7.
The photosynthetic apparatus of autotrophs is designed to cope with variability in irradiance ; it becomes more efficient at low irradiance and more protective against excess energy at high irradiance.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 General principles of adaptation
1.1 Homeostasis, tolerance and avoidance
In all natural habitats , organisms are affected by environmental variability and biotic interactions , the intensity of which is constantly changing. The time scales at which such changes act vary widely, from milliseconds in the case of solar radiation (Schubert et al. 2003) to thousands of years in the case of climate variability. All living organisms keep at least some components of their internal environment constant. In a stressed environment, maintaining this constancy (“homeostasis ”) is the main goal of an organism. Homeostasis is often achieved by simply avoiding a disturbance or by a behavioural change .
Most species can be broadly classified into two groups, conformers and regulators . Conformers may not need to exert themselves to maintain homeostasis as their plasticity (“conformity”) allows them to accommodate to environmental change . Regulators need to maintain their internal environment at a more or less constant level, irrespective of the extracellular conditions. However, the subdivision into conformers and regulators is not sharp and absolute because the two categories frequently overlap. A species may be a conformer with respect to one environmental driver but a regulator with respect to another one. For example, fish are temperature-conformers but ion-regulators.
Tolerance is achieved when an organism adapts physiologically and/or morphologically to the variability of environmental (abiotic and biotic) factors and therefore can persist in its habitat. When avoidance is employed, an organism eliminates the impact of the changing factor whenever its physiological tolerance limit is exceeded. Well-known examples of avoidance strategies are migrations of birds, resting stages of protists and dormancy (“diapause”) of zooplankton species.
1.2 The concept of adaptation
Adaptation is both a central and controversial concept in biology. The term means different things to scientists working in different fields of biology. In genetics and ecology, adaptation usually denotes a process involving heritable changes in the genome , which result in the emergence of functions that enable the organism to live in its ambient environment. Thus, adaptation is used for either a process of selection or a trait resulting from selection.
Evolutionary adaptation is a slow process, usually irreversible, which involves hundreds or thousands of generations (cf. Sect. 6.1). The end result of evolutionary adaptation is a habitat-specific genotype (an ecotype or a species), with a tolerance bandwidth adapted to the variability of the environment they live in. How fast a given set of habitat-specific characters evolves depends on the distance over which the respective trait pattern is shifted, the available gene pool, and the strength of the forcing factor. In physiology, the term “adaptation” is often used to describe the responses of an individual to environmental change . In this case, a geneticist or an ecologist would use the term “phenotypic plasticity”.
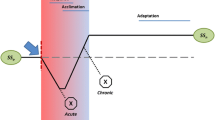
Modulative and modificative adaptations are the short-term compensatory changes of an individual in response to environmental change. Both are the outcome of phenotypic plasticity resulting from evolution. While modulative compensatory changes are irreversible, and can therefore be considered an optimisation of the evolutionarily achieved set of habitat-specific adaptations, modificative compensatory changes are fully reversible and adjust the organism’s performance to environmental changes within an individual’s life span . More appropriate, at least with respect to modificative compensatory changes, are the terms “acclimation ” or “acclimatisation ”. The term “acclimation” may be reserved for compensatory changes in physiological experiments according to the experiment set-up whereas “acclimatisation” is used to denote a natural process. However, in most cases the distinction between these two terms is not so sharp, and they are largely used as synonyms.
2 Environmental variability
2.1 Salinity and temperature
In addition to seasonal cycles and climatic gradients (e.g. latitudinal), which affect any large ecosystem, the dominant stress factor for all organisms living in the Baltic Sea is the wide salinity range. The Baltic Sea Area, including the Baltic Sea and the transition zone (Belt Sea and Kattegat), features a salinity gradient from 0 to 35 over a distance of >2,000 km (cf. Fig. 4.2). While water temperature varies during the year, salinity is relatively stable in most parts of the open Baltic Sea, compared to estuaries (Fig. 7.1). However, in estuaries, as well as in other coastal areas with freshwater discharges into the Baltic Sea, there are pronounced inshore-offshore salinity gradients between the freshwater discharge points and the open Baltic Sea water .
Comparison of seasonal salinity and temperature in a coastal lagoon connected to the Arkona Sea (the Darß-Zingster Boddenkette , DZBK, Germany), an estuarine area in the North Pacific Ocean (the Willapa River and Willapa Bay, USA), and two marine areas (the Irish Sea at Port Erin, Isle of Man, UK, and the Adriatic Sea at Rovinj, Croatia). The values for January are marked with a dot. Arrows indicate the direction of the seasonal cycle . Figure based on data in Hedgpeth (1951) for Willapa River, Willapa Bay, Port Erin and Rovinj and unpublished data for Zingst (H. Schubert, collected in 1995). Figure: © Hendrik Schubert
Benthic organisms in the Baltic Sea do not have the opportunity to just wait through a period of suboptimal salinity until conditions improve, as is the case for benthic organisms in the intertidal zone elsewhere. For example, molluscs and barnacles in tidal regions may close their shells and stop filter-feeding until salinity soon reaches optimum conditions again. During periods with low salinity , molluscs in the littoral of tidal seas may also apply avoidance mechanisms whereby the low temperature reduces their metabolic maintenance costs. In the virtually non-tidal Baltic Sea, such avoidance mechanisms are not applicable, and appropriate physiological adaptations of benthic organisms to temperature and salinity variations are of vital importance. The seasonal cycling of solar radiation regulates the heat balance at the water surface (Fig. 7.1), and thus the energetic input to the system, and triggers acclimation in the organisms. Both the energy flow and acclimation reactions have to be taken into account when analysing the biotic effects of the salinity regime.
Salinity adaptations depend on two major components: the ionic composition of the water and the osmotic pressure . No living cell is constantly “iso-ionic” with its environment. All extant organisms must perform ionic regulation , i.e. they need to maintain an active control over their intracellular ionic composition , which always differs from that outside the cell. In animals, the actively generated and maintained ion gradients over cell membranes are used for neural conduction, but they are also prerequisites for many cross-membrane transport processes channelling nutrients and other charged compounds into and out of the organism. For example, the intracellular K+/Na+ ratio is always kept at a level that is higher than that of the ratio in the marine environment. The reduced intracellular sodium level makes it possible for cells to use the ion gradient both in neural conduction and for substrate transport.
The species that have evolved in marine habitats are often “iso-osmotic ”. As the resultant intracellular concentration of ions exceeds the physiological demand, a species can stay iso-osmotic over a wide range of decreasing salinities by simply lowering its internal total ionic concentration. However, this so-called “poikilo-osmotic ” strategy cannot be maintained down to salinity zero because freshwater is too poor in ions and charged compounds to allow for a sufficient intracellular concentration of osmotically active substances (e.g. low-molecular carbohydrates , amino acids and nucleic acids ). In addition, the actively maintained ion gradients required for neural conduction cannot occur under these conditions. Thus, at a certain salinity, organisms must be capable of hypertonic regulation that allows them to maintain an osmotic gradient. This results in a gradient of the water potential (Ψ), which must be balanced either by active water pumping (e.g. by contractile vacuoles ) or by increasing the inner pressure potential (p). The latter requires either a rigid outer structure such as the cell walls of algae and plants or, in an animal cell, energy supply to make the cell functionally impermeable to sodium ions so that the cell volume can be maintained.
In addition to direct salinity effects, the success of organisms in brackish water may depend on indirect effects such as the influence of salinity on the availability of food items or how water density affects the buoyancy of organisms. This in turn impacts e.g. energy use and oxygen availability .
2.2 Irradiance and temperature
Both osmotic regulation and ionic regulation in organisms requires energy. The major energy input to ecosystem processes is the photosynthesis -driving solar radiation , in addition to the dissolved and particulate matter from terrestrial runoff . The variability of the underwater irradiance climate is strikingly large (Fig. 7.2). The acclimation capabilities of photosynthetic organisms not only need to meet the challenges posed by seasonal and day/night irradiance cycles, but also challenges posed by mid- and short-term variability in Langmuir circulation (a series of shallow, counter-rotating vortices at the sea surface generated by steadily-blowing winds, Langmuir 1938; Thorpe 2004). Moreover, they also need to deal with the light-focusing effect of waves at the water surface (“wave-focusing” , Stramski and Legendre 1992). Photosynthetic organisms have to adapt to irradiance changes involving the quantitative variability in light intensity and the qualitative variability in spectral composition. Both light intensity and spectral composition vary at different frequencies.
Model of irradiance variability perceived by phytoplankton . (a) The seasonal cycle of irradiance , which at 60 °N ranges between 5 and 90 photons m−2 day−1 between the winter solstice (ws) and the summer solstice (ss), respectively. (b) Variability in day length and irradiance amplitude during the 365 day/night cycles of one year. The yellow bars indicate ws and ss. (c) Variability in one day/night cycle for a ws day and an ss day shown in blue and red, respectively. (d) Variability caused by wind-induced Langmuir circulation ; each of the day/night cycles consists, regardless of weather conditions (which, however, are an additional element of variability), of several vertical movements through the upper part of the water column. The yellow bar indicates the set of two cycles shown in more detail in (e). (e) An individual Langmuir cycle has been measured to take ~20–40 minutes for a ~2 m deep, mixed water layer. Shown here are two cycles of 20 minutes each. Irradiance fluctuates when the photic zone depth (Zeu) is shallower than or equals the mixing depth (Zm) (e, blue and red lines). However, when the turbidity is very high, the Zeu > Zm, irradiance may consist of a series of on/off light cycles (e, black line). (f) Superimposed on to the Langmuir circulation is wave focusing (the light-focusing effect of the water-surface waves), for which reason irradiance can increase up to five times the mean level measured by conventional radiometers for a couple of milliseconds. The black lines are flashes of light, resulting from wave focusing, on top of the red Langmuir-induced variability for Zeu = Zm. Figure modified from Schubert and Forster (1997)
As planktonic photoautotrophs are subjected to the full range of irradiance variability, they have to employ light-protection mechanisms and other functions that allow them to enhance their light-use efficiency. This is because, as opposed to e.g. nutrients, irradiance cannot be stored internally to dampen external variability. Active avoidance mechanisms in light protection are restricted to comparatively large organisms and calm weather. In contrast to lakes with a rather small wind fetch, the surface layer of the open Baltic Sea and the entire water column of shallow coastal areas are well-mixed most of the time, which prevents small unicellular organisms from actively controlling the depth of their occurrence in the water column.
Directly linked to irradiance are changes in the seawater temperature . Due to the high thermal capacity of water (so-called “thermal inertia ”), effects of temperature variation are delayed, compared to the faster changes in irradiance. Because enzyme kinetics is highly temperature-dependent , there is a difference in the degree to which temperature influences photosynthetic light harvesting and the purely enzyme-driven respiratory processes in primary producers . This may lead to a time lag between irradiance input and water temperature, which is manifested by e.g. high respiratory rates in the early night hours when water temperature is still high but primary production is limited by low irradiance .
2.3 Oxygen
Most organisms require oxygen to serve as the terminal electron acceptor in energy production (respiration ) and to drive many redox reactions . The oxygen concentration in the water provides key information about e.g. algal and cyanobacterial blooms and ecosystem health in general, and the oxygenation status of the environment (e.g. oxygen depletion ) can be estimated by measuring dissolved oxygen (DO). Changes in the shape of the DO depth curve (illustrating the vertical distribution of DO in the water column), as well as oxygen deficiency in near-bottom waters , are meaningful indices of eutrophication.
Oxygen availability changes with water depth. It is released during photosynthesis , which is restricted to the upper part of the water column (the photic zone , cf. Fig. 2.21). The salinity stratification of the water column hampers deep vertical mixing , and the organic material, accumulated below the halocline as a result of sedimentation , undergoes heterotrophic decomposition which requires oxygen to proceed. Consequently, oxygen is used up, and hypoxic (<2 mL O2 L−1), or even anoxic (0 mL O2 L−1), conditions ensue (cf. Sect. 3.6). Special adaptations are necessary for organisms if they are to survive such conditions. Hypoxia may occur not only in deep waters but also in shallow eutrophic bays when the amount of light is not adequate for photosynthesis. Under such conditions, hypoxia is usually intermittent, occurring during the night, whereas during the day the conditions may even be hyperoxic (i.e. the oxygen partial pressure in the water exceeds that of the air).
3 Ionic regulation
3.1 The ionic anomaly of brackish water
Generally , the major inorganic ions involved in metabolism can be classified into two groups: (1) metabolisable solutes, especially N- and P-containing compounds, which may exist in the inorganic form in osmotically significant quantities during nutrient sufficiency (luxury accumulation), but more frequently are assimilated into organic molecules, and (2) non-metabolisable ions, principally K+, Na+ and Cl−.
To sustain its interior ionic homeostasis , any cell in a liquid medium needs to employ active and selective ion transport. The ionic composition of the extracellular fluid of truly marine organisms, e.g. echinoderms , has been shown to be very similar to that of seawater. In truly marine organisms, almost all ion-regulation mechanisms are concentrated at the cell surface/body fluid border whereby the extracellular fluid buffers the ion-composition variability of the external medium. However, such a mechanism may fail in brackish water where the relative ionic composition exhibits anomalies (Fig. 7.3; Table 7.1).
The relative ion composition of water with different salinities. (a) Freshwater. (b) Baltic Sea water at Recknitz , Germany in the southern Belt Sea . (c) Marine water. (d) The Ca2+:HCO3 − and Na+:K+ ratios in relation to salinity on a logarithmic scale, showing the increase of the Ca2+:HCO3 − ratio with salinity and the ion anomaly of the Na+:K+ ratio in brackish water. Figure based on data in Nessim (1980). Figure © Hendrik Schubert
3.2 Effects of the ionic anomaly on biota
The physiological stress at salinities of 5–8 are most likely responsible for the restricted number of macrozoobenthos and macrophyte species in the unique permanent salinity gradient of the brackish Baltic Sea (cf. Sect. 4.5). Each species reaches its own physiological salinity limit (McLusky and Elliott 2004; Elliott and Whitfield 2011; Whitfield et al. 2012). Ionic composition and osmotic regulation and variability have been listed among the causes underlying this diversity minimum. For example, measurements of ion concentrations along the salinity gradient from Baltic Sea estuaries to the North Sea have shown that the Ca2+/Cl− ionic ratio is quite stable within the salinity range of 7–34. However, below salinity ~7 the relative proportion of Ca2+ ions increases, which also has consequences for the distribution of species (Khlebovich 1968, 1974).
Ionic composition and osmotic regulation and variability have been extensively studied along the large-scale Baltic Sea gradient. However, the physiological consequences of irregular salinity changes are poorly understood in the Baltic Sea. Without doubt, such variation induces stress because continuous responses of the organisms are required. In shallow coastal waters of the Baltic Sea, salinity may fluctuate erratically within a relatively broad range (Fig. 7.4). The extent to which such salinity changes hamper the adaptation of long-lived macrozoobenthos and macrophyte species, or increase the probability of coexistence (or even prevalence in pelagic biodiversity) of short-lived planktonic organisms – in the sense of the “intermediate disturbance hypothesis” (Grime 1973; Connell 1978) and the “protistan species maximum concept” for the horohalinicum in coastal waters (Telesh et al. 2011, 2013, 2015) – still needs further exploration.
Variability of salinity in the Darß-Zingster Boddenkette (Germany), a coastal lagoon connected to the Arkona Sea , on different time scales . Salinity was recorded every 15 min and was averaged to obtain daily means, which were used to calculate monthly means, annual means and the 17-year mean. (a) Monthly means, annual means and the 17-year mean for the time period 1983–1999. (b) Daily means and monthly means for the year 1987 and the 17-year mean. Figure modified from Sagert et al. (2008)
The rates of water and ion fluxes between organisms and their environment largely depend on the body wall permeability for water and ions. These rates are mainly determined by the structure and osmotic properties as well as by electrical charges in the extra- and intracellular compartments , including extracellular matrices or epithelial cell layers (Fig. 7.5). When animals are exposed to a diluted medium, specific loss rates of sodium (in µmol g−1 h−1) vary from >1,000 in fully marine crabs and 800–900 in marine intertidal crabs, to 100–200 in estuarine/freshwater crabs and ~5 in fully freshwater crayfish . These marked differences between marine and freshwater species can be generalised to a higher permeability of ions and water in marine organisms compared to freshwater ones.
Ion movements between a cell, the extracellular fluid (ECF, blood ) and the external medium in (a) marine fish and (b) freshwater fish. Green colour indicates that the transport is passive, blue that it is secondarily active and black that it is active. The ion transport behaviour of epithelium in brackish-water fish depends on the relationship between the salinity of the water and the animal. If the water salinity is higher than that of the animal, the marine model is followed, and if the salinity of water is lower than that of the animal, the freshwater model is followed. (a) In marine water, the animal must excrete salts (especially chloride) actively. The active step is the secondarily active uptake of chloride from the ECF into the epithelial cells via the Na-K-2Cl transporter that utilises the sodium gradient generated by the sodium pump (Na+/K+-ATPase ). Once in the cell, chloride can be excreted to the environment via passive channels. (b) In freshwater, the animal must take up salt from the water (especially sodium and chloride ) actively. For sodium, this takes place either via the secondarily active sodium/proton exchange or a passive sodium channel that is functionally coupled to a proton pump. The energetics of the chloride transport pathways , chloride/bicarbonate exchange and chloride channels, are not fully clarified (and therefore shown in grey); both active and secondarily active components have been suggested. Most of the carbon dioxide and ammonia are excreted as such, both in freshwater and seawater, but some carbon dioxide is converted to bicarbonate and some ammonia to ammonium in reactions catalysed by carbonic anhydrase (CA) and deamination (DA) reactions of amino acids . Bicarbonate serves as a counter ion for chloride, and ammonium can be transported via the sodium pump (or via sodium/proton exchange). Figure: © Mikko Nikinmaa
Osmoregulation characteristics of selected species. The external and internal water potentials are drawn inversely (1/Ψ) on the x- and y-axis, respectively. The equivalents of fully marine conditions (~25 bar at 5 °C) are indicated on both axes. The 45° black line marks iso-osmotic conditions and the red line marks the range of the organism. (a) A primary marine organism. (b) Littorina littorea in the marine intertidal zone and in brackish water. (c) Gammarus duebeni in the marine intertidal zone and in brackish water. (d) Orchestia sp. in the epilittoral zone . (e) Freshwater gastropods . (f) Freshwater vertebrates . (g) Marine teleost fish . The light-grey area indicates the range covered by osmolytes (urea ) and the dark-grey line that of the Selachimorpha (modern sharks ). (h) Fejervarya cancrivora (crab-eating frog). The light-grey area indicates the range covered by osmolytes (urea ). (i) Salt-lake organisms, with from top to bottom Palaemonetes sp. of marine origin , Ephydra sp. of terrestrial origin, Artemia sp. of freshwater origin . Figure modified from Remmert (1969a)
Evolutionary lines of osmoregulation and excretory strategies of nitrogen metabolism waste products in various groups of animals. Note that this figure does not cover all evolutionary pathways or all animal taxa. Figure modified from McShaffrey (2002)
The charophyte Chara canescens is one of the few species restricted to brackish-water habitats . In the Baltic Sea, only female plants that reproduce by ovoapogamy (apogamy by parthenogenetic formation of the oospores ) have been found. In the numerous “Lacken” (small temporary and permanent brackish-water ponds) in the Neusiedler See area (Austria/Hungaria) both ovoapogamic and bisexual lineages occur (Schaible et al. 2011). It is not known why only parthenogens seem to live in the Baltic Sea, but their success may be a result of a higher probability of reproduction . (a) Female Chara canescens from the Baltic Sea. (b) A bisexual Chara canescens population from the Neusiedler See area. (c) A ripe antheridium from the Neusiedler See area. (d) A male individual from the Neusiedler See area. Photo: (a), (b) © Hendrik Schubert, (c) © Anette Küster, (d) © Ralf Schaible
Summary of light acclimation strategies in photoautotrophs , including two directions of acclimation . Due to the wide variation in light conditions in the aquatic environment, photoautotrophs must be able to cope with periods of both overexcitation and light limitation . NPQ = non-photochemical quenching . Figure based on data in Schubert et al. (2004, 2006) and Marquardt et al. (2010)
The ion uptake and efflux can occur in three ways: by ion channels , ion exchange proteins or primary ion pumps (Box 7.1). Where substantial extracellular material (e.g. mucus ) is present, ion-binding effects complicate the ionic transport processes.
Box 7.1: Ion transport pathways
Ion transport
The permeability of a cell membrane to ions (or even to water) can be altered in the short term, within the lifespan of an individual, via acclimation mechanisms. At the level of cell membranes, acclimation involves either addition or removal of ion transporters or regulation of the transport properties of existing proteins . More than 300 types of ion transport pathways occur in living cells. They can be classified according to the ion species that pass through the gates (pores), the location of proteins, the number of gates, the requirement for counter-ions to make transport possible, or the active (with energy costs) or passive (without energy costs) nature of the transport. These transport pathways can be subdivided into ion channels, ion exchange proteins and active transport.
Ion channels
Ion channels (Box Fig. 7.1a) are pore-forming membrane proteins that allow the transport of ions along an electrochemical gradient made up by ion concentrations and membrane potential . They do not use metabolic energy. Thus, transport is by pure diffusion and the size of the channel determines which ion is transported through the channel. Ion channels control the flow of ions down their electrochemical gradient through the membranes that surround all biological cells. In many aquatic invertebrates, salts move relatively freely across the surfaces via such channels.
Examples of ion transport mechanisms across the cell membrane . (a) An ion channel through which Na+ is transported. (b) Ion exchange protein. An example is the anion exchanger by which most algae are able to take up carbon as bicabonate (HCO3 −) in exchange to chloride in an electroneutral manner. Anion exchangers are also used in the control of pH in most organisms. (c) The “Ca2+ pump” is a primary ion transport ATPase that serves to remove calcium ions from the cell. (d) The “Na+/K+ pump” is a primary ion transport ATPase that serves to pump sodium ions out of the cell and potassium into the cell (both ions move against their concentration gradient). This transport regulates the cell volume. Figure: © Irena Telesh
Ion exchange proteins
Ion exchange proteins carry out either passive (Box Fig. 7.1b) or secondarily active ion transport . In passive ion transport , ions on both sides of the membrane move down their electrochemical potential , i.e. transport is pure diffusion . The difference between ion channels and ion exchangers is that the latter require suitable ions for transport on both sides of the membrane whereas ion channels can transport ions without counter-ions. In secondarily active ion transport, the transport of one ion is coupled to the transport of another with a transmembrane gradient maintained actively by primary ion pumps . In this case, both transported ions can be displaced from their passive electrochemical gradient .
Active transport
Active transport is carried out with primary ion pumps (ATPases ), which use energy (ATP ) directly coupled to the function of the protein . An ATPase is an enzyme that catalyses the decomposition of ATP into ADP and a free phosphate ion, a reaction that releases energy. Some of the primary ion pumps do not need a counter-ion (Box Fig. 7.1c) whereas others do (Box Fig. 7.1d).
4 Osmotic adaptations
4.1 Osmotic variability and its effect on biota
Water is a vital constituent of any living cell, tissue, organ or organism (cf. Sect. 1.1). The cell membranes are highly permeable to water, which also acts as a solvent for substrates and products of metabolism and a reactant in several basic metabolic processes , including hydrolysis and photosynthesis . Moreover, water is required for regulation and adjustment of cell volume.
Cells of aquatic organisms contain a wide array of intracellular solutes. These have a diversity of functions, but a major role is also to regulate the osmotic pressure within a cell. The minimum intracellular osmotic pressure is ~0.1 MPa, which is consistent with the pressure resulting from the concentration of osmotically active substances required for normal metabolism. In the absence of other forces, immersion of a cell in freshwater would lead to water uptake and cell lysis . However, organisms can prevent this in either of two ways. One is the presence of a rigid cell wall (e.g. in plants, prokaryotes and some protists ), which allows a positive hydrostatic pressure (turgor ) to develop, whereby water entry and cell enlargement is prevented. The cell volume in multicellular animals is maintained by the so-called “double-Donnan equilibrium ” (Box 7.2). Regardless of the mechanism employed, the importance of maintaining the cell volume is that both condensing and dilution will affect the three-dimensional structure of functional proteins , which may negatively impact their functioning.
Box 7.2: Maintenance of steady state volume in animal cells
Osmotic pressure
Cells contain impermeable poly-ions and molecules that make the intracellular contents osmotically more active than the extracellular fluid, which generates an osmotic pressure difference between cells and their surroundings (Edwards and Marshall 2013). The presence of a higher number of osmotically active particles within the cell than outside the cell generates an influx of water into the cell for balancing the osmotic pressure difference (Box Fig. 7.2a). The influx of water generates a diffusive imbalance for small permeable ions, whereby they will diffuse into the cell, mostly through membrane protein pores. This again generates osmotic imbalance, and water will enter the cell. Unopposed, this situation would lead to a continuous inflow of water until the cells burst.
Explanation of the double-Donnan equilibrium . (a) A cell membrane with an intracellular fluid that is osmotically more active than the extracellular fluid. (b) An animal cell membrane with a sodium pump , fuelled by ATP. Images drawn with the help of templates in Smartdraw 7. Figure: © Mikko Nikinmaa
The double-Donnan equilibrium
In plant cells, continuous swelling is prevented by a rigid cell wall that counterbalances the osmotic pressure difference. However, animal cells do not possess rigid walls and, consequently, they need another mechanism to maintain the cell volume. This is achieved with the so-called “double-Donnan equilibrium ”, also known as the “the pump-leak model” (Macknight and Leaf 1977). This system requires that a permeable solute is removed as soon as it enters the cell. Thus, even though the solute is permeable, it is functionally impermeable. In animal cells, the functionally impermeable solute is sodium . As soon as sodium enters the cell via diffusive pathways , it is actively pumped out by the sodium pump (Box Fig. 7.2b). Owing to the functional impermeability of sodium by ATP -requiring extrusion, a steady state volume can be achieved.
Salinity may act as a driver of directional selection, and genetically determined variation in the salinity tolerances (osmo-adaptations ) of different species from the same group can be related to the salinity regimes of their habitats. In certain estuarine and marine populations of several species of red algae (e.g. Bangia atropurpurea and Vertebrata lanosa ), the salinity response can be interpreted in terms of intraspecific variation (Reed 1995).
In general, the Baltic Sea organisms are regarded as more euryhaline than organisms in marine waters, although this is not always the case. The lower turgor of estuarine organisms is likely to be an adaptive response, which may be a way to avoid problems associated with high turgor in response to hyposaline stress. Estuarine plants show a lower rate of intracellular solute loss in response to extreme hyposaline stress in Ca2+-deficient media (Reed 1995). For example, when placed in a hypersaline medium (150 % seawater), the red alga Delesseria sanguinea from the Baltic Sea showed evidence of damage, as opposed to Delesseria sanguinea from the North Sea (Reed 1995). In a survey of the salinity responses of several other algal species, including Ceramium tenuicorne and Rhodomela confervoides , a hyposaline shift in the halo-tolerance of the Baltic Sea algae compared with their North Atlantic conspecifics was found (Russell 1985).
4.2 Osmotic adaptation strategies
In an organism adapted to marine conditions, exposure to hypo-osmotic conditions elicits an immediate response in the form of swelling, resulting from inflow of freshwater (Remmert 1969b). If the organism is unable to release intracellular osmotically active substances and/or actively pump water out of its cells, it may even burst. Exposure to hyper-osmotic conditions, on the contrary, leads to shrinking up to the point when the external and internal water potentials are balanced, which may lead to plasmolysis . Organisms capable of acclimation by releasing osmotically active substances and/or by active pumping, and therefore able to adapt to changing salinity in brackish-water habitats , originate from different ancestral groups, e.g. marine, freshwater or terrestrial lineages.
The strategies of adapting to brackish-water conditions differ among organisms (Fig. 7.6). The first, but by no means the “simplest”, adaptation mechanism of marine-brackish organisms is to extend their iso-osmotic response compared to primary marine organisms (Fig. 7.6a). This requires complicated adaptations of biochemical pathway kinetics. An example of an organism with this strategy is the snail Littorina littorea (Fig. 7.6b).
Other mechanisms, shown by e.g. Carcinus sp. and Gammarus sp., which originate from the marine intertidal zone, involve hypertonic regulation at low extracellular salinities (Fig. 7.6c). In the epilittoral zone , both hypo- and hypertonic regulation has been found to occur in e.g. Orchestia sp. (Fig. 7.6d).
In freshwater, hypotonic regulation is not necessary and this function was therefore lost in most freshwater organisms , which are only able to perform hypertonic regulation, as in e.g. many freshwater gastropods (Fig. 7.6e). Some freshwater organisms are capable of a limited degree of both hypo- and hyper-osmotic regulation , e.g. freshwater arthropods and vertebrates (Fig. 7.6f).
Species that have returned to brackish and/or marine environments from freshwater or terrestrial habitats have re-adopted hypotonic regulation or re-extended their poikilo-osmotic range (Fig. 7.6g), often by means of osmolytes , which are substances that affect the water potential of a system. Osmolytes can be defined as physiologically compatible substances that increase the osmotic pressure at a low energetic cost, such as urea in sharks (Fig. 7.6g) and in the crab-eating frog Fejervarya cancrivora (Fig. 7.6h). Organisms invading brackish-water environments from salt lakes always employ both hypo- and hypertonic regulation, regardless of whether their evolutionary origin is marine, freshwater or terrestrial (Fig. 7.6i).
4.3 Physiological mechanisms of osmotic acclimation
In the brackish Baltic Sea water, the salinity range occupied by a species depends on the efficiency of the physiological mechanisms by which it is adapted to changes in ambient salinity. The ability to adapt evolutionarily depends on the generation length of the species. Generally, osmotic regulation is involved in the maintenance of a difference of ionic concentrations inside and outside the cells at appropriate physiological levels. In mobile animals the situation may be somewhat different than in sessile organisms, as they are capable of escaping from conditions of inappropriate salinity. However, numerous species migrate actively between saline water and freshwater (Box 7.3).
Box 7.3: Anadromous and catadromous fish
Salmon, lampreys and eels
Salmon and many of its relatives are anadromous fish . They feed in the marine environment and breed in freshwater where the young stay until they smoltify and migrate to sea. In addition to salmonids , lampreys often feed in marine environments and spawn in freshwater, where the young also grow for up to several years. These fish are called anadromous. Eels, in contrast, are catadromous fish : they breed in seawater, and migrate to freshwater to feed. A comprehensive account of the different aspects of anadromous and catadromous life cycles is given in McCormick et al. (2013).
Differences in osmoregulation between marine and fresh waters
In seawater, fish are hypo-osmotic regulators , while in freshwater they are hyper-osmotic regulators. The regulation is very effective and the osmolarity of body fluids changes little when the fish moves from the freshwater to the marine environment and vice versa. The development of hypo-osmotic regulation capacity has been studied by following changes accompanying the smoltification of young salmon and their migration from rivers to the marine environment. The development of hyper-osmotic regulation has been studied particularly intensively in eels migrating from the marine environment to the freshwater .
Hormones, drinking rates and urine production
In anadromous and catadromous fish , the development of both hypo- and hyper-osmotic regulation capacities is controlled by hormones . Cortisol and prolactin together enable successful acclimation to freshwater. Freshwater adaptation involves a marked reduction in the permeability of gills and skin to sodium and water, a reduction of the water drinking rate , and ultimately a cessation of drinking and an increase in the urine production rate. Alternatively, successful acclimation to seawater requires action by growth hormone, an insulin-like growth factor, cortisol, and in many cases, thyroid hormone. Important cues triggering hormonal changes include fish body size , photoperiod and temperature. When young salmon (parr ) move from freshwater to the sea (smoltification ), the direction of passive ion and water fluxes reverse. While the gradients in the freshwater favour water influx and salt efflux, the gradients in the seawater favour water efflux and salt influx. Consequently, animals increase their drinking rate, decrease their urine production , and modify their ion transport systems both in the gills and the intestine . The urine produced is markedly hypotonic compared to body fluids in freshwater, and it changes to virtually isotonic in the seawater. The intestinal uptake of sodium and chloride is facilitated in seawater; intestinal salt uptake is followed by osmotically obliged water enabling the water uptake by the animal to replace the amount lost by diffusion. The salt accumulated is secreted in the gills. Salt secretion occurs via the combined actions of sodium pump and Na-K-2Cl co-transporter. When smoltification occurs, the activity of Na/K ATPase (sodium pump) in the gills increases markedly because of an increase in the number of pump molecules. The number of Na-K-2Cl co-transporters increases as well.
Aquatic bacteria , including cyanobacteria (which lack nuclei, mitochondria and chromoplastids), as well as nucleus-bearing protists (algae with chromoplastids, fungi and protozoa with mitochondria), demonstrate high physiological adaptability to changes in salinity. These taxa show extensive adaptive radiation. Protists seem to have retained a considerable evolutionary euryhalinity and are widely distributed, the smallest planktonic representatives of them being particularly diverse under the conditions of the brackish Baltic Sea waters that are stressful for larger sessile organisms (Telesh et al. 2015). This is reflected in high bacterial and maximum protistan species richness in brackish water, especially at critical salinities 5–8 (Telesh et al. 2011).
Animals possess an excretory organ, which is often generically termed “kidney ”, and it ranges from subcellular and unicellular structures such as contractile vacuoles and flame cells to complex organs with several different constituent tissues. In unicellular organisms, the contractile vacuolar complex is necessarily the primary osmoregulatory system (Box 7.4). In practice, the primary function of these organelles and organs is almost always osmoregulation rather than excretion.
Box 7.4: The contractile vacuolar complex
The contractile vacuolar complex (CVC, Box Figs. 7.3 and 7.4) is a subcellular membrane-bound organelle used to eliminate the excess cytosolic water acquired by osmosis (Hausmann et al. 2003). Typically, the contractile vacuole (cv) fills slowly with a fluid from the narrow collecting channels (diastole), and the fluid is periodically expelled through the contractile vacuole pore (pvc) to the surrounding medium by contractions of the vacuole (systole). Depending on the species and the osmolarity of the environment, the amount of water expelled from the cell and the frequency of contraction may vary considerably. The CVC is found predominantly in freshwater and brackish-water protists that lack a cell wall (e.g. Amoeba , Paramecium ) and in several types of cells in sponges and fungi . Evolutionarily, the CVC was eliminated in multicellular organisms, but some of its molecular and cellular characteristics are used by multicellular organisms in their own osmoregulatory mechanisms.
Schematic drawing of the contractile vacuolar complex (CVC), showing ampullae (amp), collecting canals (cca), the contractile vacuole (cv) and a pore (pvc). Collecting canals are connected with and surrounded by irregularly arranged spongiomal tubules (spo). Tubular aggregates (ta) are located at a larger distance. The entire CVC is stabilised by several microtubular ribbons (mtr). Figure reprinted from Hausmann et al. (2003) with permission from Schweizerbart’sche Verlagsbuchhandlung
The nitrogenous waste from the body that needs to be eliminated is added almost incidentally to the osmoregulated urine (or not added at all). Despite the large variation in origin, size and complexity of osmoregulatory organs, all of them operate under certain common structural and physiological principles. Nearly all consist of one or many tubular structures and most include an initial collecting area where the primary urine is formed, as well as one or more areas where it is modified by the addition or removal of particular solutes. Many of the organs include a distal area where the urine is more concentrated (in vertebrates, the hyper-osmotic urine can be produced only by mammals ) or more diluted (hypo-osmotic ) than the body fluids.
In terms of both evolution and contemporary life in brackish waters, osmoregulation is a major problem for which diverse mechanisms have been developed to regulate salt and water content in various groups of animals (Fig. 7.7). However, some marine species have achieved adaptation to low salinities without osmoregulation. They are poikilo-osmotic and allow their body fluids to be isotonic with the salinity of the external medium. This naturally requires that their cellular constituents are able to function in a wide salinity range. In contrast, the homoio-osmotic species, when exposed to minor changes in ambient salinity, tend to retain their initial internal osmotic concentration. The evolution of excretory strategies also involves the availability of water for excretion of nitrogenous wastes.
5 Osmotic and ionic adaptations in charophytes
5.1 Charophytes have adapted to all salinities
As shown above, ionic and osmotic homoeostasis are interlinked, and non-linear effects are expected because of the ionic anomaly of brackish water (Fig. 7.3). How this affects brackish-water organisms has been studied in detail in the charophytes , a group of green algae that have succeeded to adapt to all salinity ranges (Bisson and Kirst 1995).
Charophytes maintain an osmotic potential that is higher than the outer osmotic pressure , which results in a substantial turgor pressure (Winter and Kirst 1990, 1991, 1992). For example, in Chara vulgaris the turgor pressure adds up to ~340 mOsmol kg−1, which is equivalent to salinity 13 (Winter and Kirst 1990). When Chara species that are unable to acclimate to different osmotic potentials are grown at high salinity , cell elongation rather than the cell division rate is lowered because cell elongation depends on the turgor pressure (Winter and Kirst 1991). With respect to their abilities of osmotic adjustment, four groups of charophytes can be distinguished: freshwater, oligohaline , mesohaline and euryhaline species.
5.2 Freshwater and oligohaline charophytes
The first charophyte group consists of all the “purely freshwater species”, e.g. Chara corallina and Nitella spp., which are able to keep their osmotic potential constant mainly by a K+-regulation system, but which are incapable of adjusting it to ambient salinity changes. In freshwater, this regulation is sufficient to keep the turgor constant (Bisson and Kirst 1995).
The second group contains oligohaline or “halo-tolerant” freshwater species that are able to regulate their turgor via the accumulation of ions such as Na+ and Cl− as well as by accumulating osmolytes , especially sucrose . Examples of species in this group are Chara vulgaris (Winter et al. 1987; Winter and Kirst 1990) and Nitellopsis obtusa (Winter et al. 1999). This mechanism seems to be restricted to low salinities due to the toxic effect of Na+. The K+/Na+ ratio, which usually exceeds 1 in charophytes , decreases with increasing salinity. This results in a “reduced vitality” and competitive disadvantages of oligohaline charophyte species at salinities exceeding 5.
5.3 Mesohaline and euryhaline charophytes
The third group of charophytes consists of mesohaline brackish-water species, e.g. Chara aspera and Chara canescens (Fig. 7.8), which are successful competitors at salinities up to ~15 (Winter and Kirst 1991, 1992). These species exhibit a reduced spectrum of regulation capabilities found in euryhaline species. Chara canescens keeps its K+ concentration constant up to salinity ~4. When salinity increases, the species starts to regulate turgor pressure via K+ and Na+ (and Cl−) accumulation. However, Chara canescens seems to be unable to support its turgor regulation by sucrose accumulation, a mechanism observed in Chara aspera (Winter and Kirst 1992). At salinities of ~20, the K+/Na+ ratio in Chara canescens drops below 1, as the pronounced K+ import typical of euryhaline species seems to be missing. In contrast to Chara canescens, which starts turgor regulation only at salinities of ~4, Chara aspera regulates turgor pressure in freshwater as well, resulting in a perfect constancy of turgor pressure at salinities between 0.8 and 8 (Winter and Kirst 1991, 1992).
The fourth group of charophytes represents euryhaline species such as Lamprothamnium papulosum , Lamprothamnium succinctum and Chara buckellii , which are able to tolerate a very broad range of salinities. At low salinities (up to ~6), these species keep their K+ concentration constant and regulate their turgor mainly via the accumulation of Na+ and Cl−. At higher salinities (up to ~13), the turgor pressure is regulated by the uptake of both K+ and Na+ (and Cl−). At salinities >13, the turgor regulation is accomplished by the accumulation of mainly K+ and Cl− supported by accumulation of sucrose, whereas Na+ is kept constant (Beilby et al. 1999). The K+/Na+ ratio is thus kept at a high level, allowing these charophytes to survive at salinities of up to 70. In the field, these species seem to be poor competitors compared to other macrophytes, and they mainly occur in the salinity range 20–40 (Winter et al. 1996). This kind of salinity regulation also occurs in Tolypella glomerata and Tolypella nidifica , species which in their natural habitats are restricted to much lower salinity than the other species in this group. This is probably because they fail to develop oogonia at salinities >12, rather than because their growth is reduced (Winter et al. 1996).
6 Adaptation to ambient temperature
6.1 Temperature ranges in the sea
The overall upper limit of the temperature tolerance range of aquatic invertebrates is ~50 °C (Nguyen et al. 2011), while the lower limit is equal to the freezing point of −1.86 °C for fully marine seawater and about −0.4 °C for the brackish Baltic Sea water (cf. Fig. 2.17b). However, most aquatic organisms are seriously affected by temperature change outside their own temperature tolerance limits. With its specific heat capacity of ~3,000 times that of the air, water is a good heat conductor. Consequently, temperature differences in seas are highly buffered, and a considerable heat flux is required in order to modify the water temperature . Thus, even daily water temperature changes are rarely dramatic enough to cause functional changes in aquatic organisms in the sea, although they can be significant for the inhabitants of shallow coastal areas.
6.2 Enzymatic adaptations
All organisms use enzymes (proteins ) to adapt to changing thermal conditions. The performance of enzymes is affected by temperature in a variety of ways that may be adaptive and extend the thermal range tolerated by the organism. The nature and speed of such modifications vary with the time scale of the temperature change. Enzymatically regulated adaptations can be fast if only adjustments of the existing proteins are required, but they are much slower if de novo protein synthesis is needed.
An important mechanism involved in the responses of living organisms to thermal change is the synthesis of stress proteins , which are often referred to as “heat shock proteins” (HSPs, Box 7.5). HSPs increase the thermal tolerance and perform functions essential for cell survival under stressful conditions. These proteins are naturally present in a cell at constitutive levels under normal conditions, but they are expressed at a higher rate when a cell is exposed to a sudden thermal change, as well as to other sudden changes in the environment, e.g. salinity or pH (Durante and Colucci 2010; Roberts et al. 2010; Hartl et al. 2011).
Box 7.5: Stress proteins
Heat shock proteins (HSPs)
At the biochemical level, a basic and evolutionarily most conserved molecular defensive mechanism is the synthesis of stress proteins , often referred to as heat shock proteins (HSPs, Hartl et al. 2011). HSPs perform chaperone function, i.e. they assist in refolding proteins that were damaged by stress and stabilise new proteins by ensuring correct protein folding (Box Fig. 7.5). Thus, HSPs provide cellular and whole-body adaptation for all organisms studied so far in a vast range of extreme environmental conditions (Box Fig. 7.6). Their production can be triggered by many natural and human-induced stresses, e.g. fluctuations in seawater temperature , salinity, acidification , light availability and pollution levels, hypoxia or hyperoxia, etc. Once induced in response to a particular stress, the HSPs can make the organism more tolerant of other stresses.
Normal protein molecules in living cells are naturally folded into specific configurations, requisite for their proper functioning, but they may unfold in response to various kinds of stress. Such unfolded proteins may then refold wrongly, and may be susceptible to interactions with other cellular components. Stress proteins as molecular chaperones serve to limit these interactions by binding temporarily to the unfolded proteins and thus stabilising their state. Thus, chaperones have important physiological roles through facilitating the synthesis, de novo folding, assembly, trafficking, and secretion of specific proteins in various cellular compartments as well as guarding the cellular proteome against misfolding and inappropriate aggregation. Figure modified from Hartl et al. (2011)
The epilittoral zone is an especially stressful habitat for both terrestrial and aquatic organisms. (a, b) Fucus vesiculosus in mixed stands with terrestrial plants on the virtually non-tidal coast of the island of Saaremaa (Estonia) in the Baltic Sea. (c) Fucus cottonii in mixed stands with terrestrial plants on the tidal coast of Ireland (Neiva et al. 2012). While changes on tidal coasts are fairly predictable, even in the epilittoral zone, erratic changes of temperature, water and irradiance on the virtually non-tidal Baltic Sea coast require fast and non-specific stress-protection mechanisms. Photo: © Hendrik Schubert
HSP families
Stress proteins are represented by a number of families, differing in molecular weight, the nucleotide sequences of the encoding genes, and functions (Hartl et al. 2011). HSPs include both relatively large (e.g. HSP60, HSP70, HSP90, HSP100) and small (e.g. HSP10, HSP27, ubiquitin) proteins. The structure of most HSP families is conserved even across kingdoms, and their action also seems to be highly conservative. Several HSPs may exist in both constitutive and stress-inducible forms (e.g. HSP70).
7 Adaptation to ambient light
7.1 Light and aquatic photosynthesis
Water bodies are variable photic environments due to variability in solar elevation and waves (Fig. 7.2) and, additionally, through complex, depth-dependent interactions between light and suspended particles and dissolved matter, which involve the absorption and scattering of light (cf. Sect. 15.2). Light is of utmost importance for primary production , as light availability directly affects the growth, survival and coexistence strategies of autotrophs . Changes in the depth distribution of phytoplankton, e.g. by vertical movement, may cause dramatic changes in light and nutrient availability over short time scales (seconds to days) and spatial scales (cm to m).
Autotrophs have evolved a broad variety of strategies to acclimate to the complex temporal and spatial variability of irradiance . Probably the best known evolutionary achievements include the construction of light-harvesting antenna systems that increase the energy supply to the photosynthetic reaction centres. There are three lineages of such antenna systems, which differ with respect to their absorbance characteristics: (1) the chlorophyll antenna system, (2) the xanthophyll antenna system, and (3) the phycobilin antenna system (van den Hoek et al. 1995).
Photosynthesis is non-linearly light-dependent inter alia because of photoinhibition occurring above a certain irradiance level (Fig. 7.9). With regard to the variability of the underwater light, all aquatic photoautotrophs need fast acclimation mechanisms, except for those living in a few habitats with permanent low irradiance conditions.
7.2 Surviving under low irradiance
Under low irradiance , the available photons can be efficiently used, either by increasing the amount of antenna pigments (a λ-neutral mechanism) or by spectral acclimation of the antenna system (Fig. 7.9). Without losing relative absorbance efficiency, organisms employing these strategies are able to acclimate, within a couple of days, to changes in the spectral composition of the underwater light caused by e.g. developing phytoplankton blooms (Schubert et al. 1997).
By increasing pigmentation , the absorbance efficiency of the pigments expressed as photons absorbed per unit time will decrease due to the packaging effect . Nevertheless, this kind of acclimation , which can result in rendering the algae almost optically black, is by far the most common strategy. Examples of this strategy in the Baltic Sea can be observed in dark-coloured individuals of red algae such as Furcellaria lumbricalis and Polyides rotundus . Alternatives to this mechanism are “chromatic acclimations ” whereby the absorbance characteristics of the antenna system are adjusted to the spectral composition of the prevailing irradiance. Probably the most sophisticated mechanism of this kind is the so-called “complementary chromatic adaptation ”, which occurs in some cyanobacteria. In this mechanism, the pigment phycoerythrin , which is absorbed in the green wavelength region and dominates the antenna under green light conditions , is replaced by phycocyanin , an orange region-absorbing light-harvesting pigment .
7.3 Dealing with high irradiance
At high irradiance , photoautotrophs are not just energetically “saturated”, but they need to be protected from damage by excess light energy (Fig. 7.9). An oversupply of energy to a reaction centre, especially to the reaction centre of photosystem II (PSII) , increases the probability that chlorophyll transfers excited electrons to oxygen instead of to plastoquinone. PSII is the main target because the rate-limiting step of oxygenic photosynthesis is the regeneration of the plastoquinone molecules at the cytochrome b6f-complex. Overexcitation of PSII therefore results in acceptor limitation, whereas photosystem I (PSI) lacks electrons (because of donor limitation) and cannot be excited anymore.
Plant cells can employ two main strategies to cope with situations of excessive energy supply. The “active” mechanisms allow excitation of the photosensitiser (chlorophyll), but protect biomolecules from the consequences of acceptor limitation. This can be performed by quenching the potentially harmful triplet excitation states of chlorophyll, i.e. before being transferred to oxygen or by quenching the already activated oxygen molecules. Once activated via electron transfer, oxygen soon forms radicals that must be quenched by specific reactions requiring energy input and the biosynthesis of specialised enzymes or alternative targets.
An alternative to this rather sophisticated and energy-demanding strategy of photoprotection is to prevent overexcitation of chlorophyll, which is most easily accomplished by shading pigments. However, this strategy is complicated as well because the irradiance is highly variable on short time scales (Fig. 7.2). Such a “sunscreen ” of shading pigments must be turned on and off very fast to be effective, otherwise it would make more sense to just reduce the chlorophyll content in order to solve the problem of the acceptor limitation of PSII. Therefore, it is not surprising that only little evidence for the existence of such a dynamic sunscreen mechanism has been found so far and the question of whether or not xanthophyll cycling can act as such a mechanism is still debated (Masojídek et al. 2004).
However, there are many mechanisms, including the xanthophyll cycle , with which the overall quantum efficiency of photosynthesis under the conditions of PSII acceptor limitation can be reduced. These processes, often collectively termed “non-photochemical quenching ” (NPQ), may either reduce the excitation energy transfer to the reaction centre by e.g. decoupling the light-harvesting complexes, transferring the already absorbed photon energy into alternative sinks or, alternatively, they may decrease the charge-separation efficiency itself and therefore reduce the extent of the acceptor limitation of PSII.
Another strategy under the conditions of photosynthesis overexcitation is the so-called “packaging effect ” in which chloroplasts are lined up in places along the cell wall receiving the lowest energy input. Yet another strategy is that employed by motile phytoplankton species, which can avoid overexcitation by vertical migration. This mechanism is also employed for “nutrient pumping”, allowing for uptake of nutrients in deeper strata of the water column.
8 Adaptation to low oxygen levels
8.1 Withstanding hypoxia and surviving anoxia
As organisms vary greatly in their oxygen requirements, low dissolved oxygen concentration may result in biodiversity loss (cf. Fig. 10.7). However, populations of most animal species living in estuaries and lagoons are able to tolerate short-term exposure to low dissolved oxygen concentrations without noticeable adverse effects. Extended exposure to dissolved oxygen concentrations below 60 % air saturation may cause behavioural modifications, reduced abundance and productivity , negative reproductive effects and mortality . Moreover, there is evidence that hypoxia (<2 mL O2 L−1) can inhibit immune responses , causing higher mortality than would otherwise occur when organisms are challenged with a pathogen (Burnett and Stickle 2002).
An early behavioural response to hypoxia can be to move toward better-oxygenated water , even when other conditions there might be unfavourable. Under hypoxic conditions an animal may also slow down its swimming and feeding activities, which reduces its need for energy and hence oxygen. However, while reduced activity may render the animal more hypoxia-tolerant for a short period, a lower swimming activity makes the animal more vulnerable to predation , and reduced feeding decreases its growth. If oxygen insufficiency persists, death will ultimately occur in animals using this strategy only.
Many aquatic animals respond to a short period of hypoxia by increasing their efficiency of oxygen transport to cells and mitochondria (Box 7.6). Because of the slow diffusion of oxygen in water relative to that in air, the movement of water across permeable membranes or tissue surfaces for respiratory needs is almost universal among aquatic animals. The pumping process places high energetic demands on the animals and additionally exposes cellular surfaces to osmotic gradients.
Box 7.6: Strategies of aquatic animals to cope with hypoxia
Reduction of energy use
When animals experience oxygen limitation, they may respond by reducing their energy use (Hochachka and Somero 2002). This is employed by virtually all hypoxia-tolerant species when they encounter low oxygen levels . For example, oxygen consumption of both the blue mussel Mytilus trossulus (Box Fig. 7.7) and the crucian carp Carassius carassius is directly dependent on the ambient oxygen tension .
The deep regions of the Baltic Sea are typically affected by hypoxia, but hypoxia may occur episodically in shallow-water habitats as well, e.g. within dense populations of filter feeders or in phytobenthic communities . (a) A Mytilus trossulus bed affected by hypoxia. (b) A Zostera marina -dominated community affected by hypoxia, overgrown by a Spirogyra mat. Photo: (a) © Hendrik Schubert, (b) © Sven Dahlke
Energy production efficiency
Animals may also respond to oxygen limitation by increasing the efficiency of their energy production. The animals try to keep their aerobic energy production active, as it is much more efficient than anaerobic energy production . Thus, the first response is to increase the water flow past the respiratory epithelium. The increase in the bioventilation rate and amplitude, however, demands an increased use of energy. The positive effect on the oxygen available for energy production vanishes when the increased ventilatory energy use exceeds the potential for aerobic energy production (Dejours 1975; Farrell and Richards 2009).
Respiratory pigments
The oxygen affinity of respiratory pigments is higher in hypoxia-tolerant species than in hypoxia-sensitive species (Weber and Jensen 1988). The amount of respiratory pigments increases under hypoxia in most species. For example, in aquatic vertebrates, erythrocytes are released from storage organs and the production of erythrocytes increases (Nikinmaa 1990; Gallaugher and Farrell 1998; Nilsson and Randall 2010).
Anaerobic energy production
If aerobic energy production cannot be maintained, the animals must resort to anaerobiosis . To extend the time during which anaerobiosis can be maintained, hypoxia-tolerant organisms have much larger supplies of appropriate substrates (e.g. glycogen ) than hypoxia-sensitive species. In addition, hypoxia-tolerant species use the so-called “alternative energy-producing pathways ” that produce more ATP per glucose molecule than traditional glycolysis . The end products include acetate , succinate or malate , depending on the invertebrate species (Grieshaber et al. 1994).
Changes in oxygen tension are sensed by haemoproteins . Altogether, 1–2 % of animal genes appear to be directly regulated by oxygen, although many of the molecular responses to hypoxia are still poorly known. In metazoans the major regulation of oxygen-dependent genes occurs via the function of the hypoxia-inducible factor (HIF, Rytkönen et al. 2011). In vertebrates , the most important regulatory hypoxia-inducible factor is HIF-1, which receives signals from the molecular oxygen sensor through redox reactions and/or phosphorylation, and regulates the transcription of a number of hypoxia-inducible genes, including those involved in erythropoiesis , angiogenesis and glycolysis (Wu 2002; Nikinmaa and Rees 2005). Multicellular species have evolved highly complex organs for oxygen uptake (lung), transport (blood ), and tissue distribution (cardiovascular system). Ingeniously, the main functional regulator of oxygen homeostasis is the local oxygen partial pressure itself rather than a genetically encoded developmental programme or a central oxygen-measuring regulator (Wenger 2002).
8.2 Consequences of hypoxia for biodiversity
In the Baltic Sea, the increasing prevalence of oxygen-depleted bottom water in deep areas has perhaps become the strongest factor influencing the biodiversity of zoobenthic communities (cf. Sect. 10.11). The Baltic Sea proper is permanently stratified , consisting of an brackish-water upper layer with a salinity of ~6–8 and lower layers of more saline waters with salinities of ~9–13 (cf. Fig. 2.15). A permanent halocline at depths of ~60–80 m (cf. Table 2.6) prevents vertical mixing of the water column and the transport of more oxygenated waters to the deeper parts of the basin. The separation between normoxic and moderately hypoxic water masses and hypoxic or anoxic waters creates a temporal and spatial mosaic of stress to benthic animals living at larger depths. Due to low oxygen levels , macrozoobenthic communities at larger depths differ from those living on shallower bottoms (Conley et al. 2009). Hypoxia often eliminates large deep-burrowing , actively bioturbating species because their long generation times prevent the development of viable populations (Solan et al. 2004).
At the ecosystem level, there is a general tendency for suspension feeders to be replaced by deposit feeders , demersal fish by pelagic fish and macrozoobenthos by meiobenthos due to hypoxia in the Baltic Sea. Nanoplankton also tend to dominate in the phytoplankton community in hypoxic environments. Even when species are not entirely lost, they may become functionally extinct due to low abundance. A reduction of bioturbation thus decreases the natural purification capacity and increases the internal nutrient loading of Baltic sediments (Karlson et al. 2007), which increases with the spatial extension of the anoxic/hypoxic zone in the Baltic Sea proper.
Intermittent hypoxia , which may occur in shallow eutrophic bays, will also affect the success of species. HIF-dependent regulation is only known to function in short-term hypoxic events (Rissanen et al. 2006), and this regulation may thus be important in habitats with intermittent hypoxia.
9 Review questions
-
1.
What are the major variable environmental drivers in the Baltic Sea that require physiological adaptations?
-
2.
Which osmo- and ion-regulation adaptations are typical of the brackish-water conditions in the Baltic Sea?
-
3.
What are the main strategies of osmoregulation in brackish water bodies?
-
4.
What are the main lines of irradiance acclimation?
-
5.
How do organisms deal with hypoxia?
10 Discussion questions
-
1.
Do phylogenetic relationships mirror the salinity-related distribution of organisms? How would you construct a “tree of life” when combining Fig. 7.7 and recent phylogeny?
-
2.
The obvious lack of macroalgae, except for green algae, in low-salinity and freshwater habitats are hypothesised as being a consequence of the lack of hard substrates. However, in the Baltic Sea there are plenty of low-salinity areas with hard substrates. Which macrophyte species live there? Are there alternative explanations for the absence of red and brown algae? What arguments definitely exclude any kind of osmotic or ionic regulation-based explanation for the absence of red and brown algae?
-
3.
How do organisms adapt to fast changes in environmental drivers? Which mechanism(s) shown in Fig. 7.9 back up the different time scales of irradiance variability shown in Fig. 7.2?
-
4.
The double-Donnan equilibrium contributes to maintaining homeostasis of animal cells lacking rigid cell walls . What problems do the unprotected surfaces (e.g. gills) of hypo-osmotic animals, such as marine teleost fish, face? How do they cope with the osmotic problems of their egg and sperm cells? Could this be a reason for anadromous behaviour? Is there a relationship between phylogeny and anadromous/catadromous behaviour? What alternative explanations for reproductive migration exist?
-
5.
Why does a rigid cell wall alleviate osmotic adjustment? What would Fig. 7.5 look like for a plant cell? What consequences can be expected from this for the ability of plants to invade brackish, freshwater and terrestrial environments, compared to animals?
References
Beilby MA, Cherry CA, Shepherd VA (1999) Dual turgor regulation response to hypotonic stress in Lamprothamnium papulosum. Plant, Cell and Environment 22:347–359
Bisson MA, Kirst GO (1995) Osmotic acclimation and turgor pressure regulation in algae. Naturwissenschaften 82:461–471
Burnett LE, Stickle WB (2002) Physiological responses to hypoxia. In: Rabalais NN, Turner RE (eds) Coastal hypoxia: consequences for living resources and ecosystems. American Geophysical Union, Washington, DC, Coastal and Estuarine Studies 58:101–114
Conley DJ, Björck S, Bonsdorff E, Carstensen J, Destouni G et al (2009) Hypoxia-related processes in the Baltic Sea. Environmental Science and Technology 43:3412–3420
Connell JH (1978) Diversity in tropical rain forests and coral reefs. Science 199:1302–1310
Dejours P (1975) Principles of comparative respiratory physiology. Elsevier, Amsterdam, 253 pp
Durante P, Colucci L (eds) (2010) Handbook of molecular chaperones: roles, structures and mechanisms. Nova Biomedical Books, New York NY, 567 pp
Edwards SL, Marshall WS (2013) Principles and patterns of osmoregulation and euryhalinity in fishes. In: McCormick SD, Farrell AP, Brauner CJ (eds) Euryhaline fishes. Elsevier – Academic Press, Amsterdam, Fish Physiology 32:1–44
Elliott M, Whitfield AK (2011) Challenging paradigms in estuarine ecology and management. Estuarine Coastal and Shelf Science 94:306–314
Farrell AP, Richards JG (2009) Defining hypoxia: an integrative synthesis of the responses of fish to hypoxia. In: Richards JG, Farrell AP, Brauner CJ (eds) Hypoxia. Elsevier – Academic Press, Amsterdam, Fish Physiology 27:487–503
Gallaugher P, Farrell AP (1998) Hematocrit and blood oxygen carrying capacity. In: Perry SF, Tufts B (eds) Fish respiration. Academic Press, San Diego, Fish Physiology 17:185–217
Grieshaber MK, Hardewig I, Kreutzer U, Pörtner HO (1994) Physiological and metabolic responses to hypoxia in invertebrates. Reviews of Physiology Biochemistry and Pharmacology 125:43–147
Grime JP (1973) Competitive exclusion in herbaceous vegetation. Nature 242:344–347
Hartl FU, Bracher A, Hayer-Hartl M (2011) Molecular chaperones in protein folding and proteostasis. Nature 475:324–332
Hausmann K, Hülsmann N, Radek R (2003) Protistology, 3rd edn. E. Schweizerbart’sche Verlagsbuchhandlung, Stuttgart, 379 pp
Hedgpeth JW (1951) The classification of estuarine and brackish waters and the hydrographic climate. In: Ladd HS, Gunter G, Lohman KE, Revelle R (eds) National Research Council (Washington, DC) Report of the Committee on a Treatise on Marine Ecology and Paleoecology 11:49–56
Hochachka PW, Somero GN (2002) Biochemical adaptation: mechanism and process in physiological evolution. Oxford University Press, Oxford 466 pp
Karlson K, Bonsdorff E, Rosenberg R (2007) The impact of benthic macrofauna for nutrient fluxes from Baltic Sea sediments. AMBIO 36:161–167
Khlebovich VV (1968) Some peculiar features of the hydrochemical regime and the fauna of mesohaline waters. Marine Biology 2:47–49
Khlebovich VV (1974) The critical salinity of biological processes. Nauka, Leningrad, 236 pp [in Russian]
Langmuir I (1938) Surface motion of water induced by wind. Science 87(2250):119–123
Macknight ADC, Leaf A (1977) Regulation of cellular volume. Physiological Reviews 57:510–573
Marquardt R, Schubert H, Varela DA, Huovinen P, Henriquez L, Buschmann AH (2010) Light acclimation strategies of three commercially important red algal species. Aquaculture 299:140–148
Masojídek J, Kopecký J, Koblízek M, Torzillo G (2004) The xanthophyll cycle in green algae (chlorophyta): its role in the photosynthetic apparatus. Plant Biology 6:342–349
McCormick SD, Farrell AP, Brauner CJ (eds) (2013) Euryhaline fishes. Elsevier – Academic Press, Amsterdam, Fish Physiology 32:1–594
McLusky DS, Elliott M (2004) The estuarine ecosystem: ecology, threats and management, 3rd edn. Oxford University Press, Oxford 214 pp
McShaffrey D (2002) Nitrogen excretion and osmotic regulation. The Sextant [http://www.marietta.edu]
Neiva J, Hansen GI, Pearson GA, Van De Vliet MS, Maggs CA, Serrão EA (2012) Fucus cottonii (Fucales, Phaeophyceae) is not a single genetic entity but a convergent salt-marsh morphotype with multiple independent origins. European Journal of Phycology 47:461–468
Nessim RB (1980) Untersuchungen zur Verteilung der Hauptkomponenten des Salzgehaltes im Wasser und Sediment der Darß-Zingst-Boddenkette unter besonderer Berücksichtigung der Ionenanomalie. University of Rostock, Rostock, 149 pp [PhD Thesis, in German]
Nguyen KDT, Morley SA, Lai CH, Clark MS, Tan KS et al (2011) Upper temperature limits of tropical marine ectotherms: global warming implications. PLoS ONE 6(12):e29340
Nikinmaa M (1990) Vertebrate red blood cells. Springer, Berlin, 262 pp
Nikinmaa M, Rees BB (2005) Oxygen-dependent gene expression in fishes. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology 288:R1079–R1090
Nilsson GE, Randall DJ (2010) Adaptation to hypoxia in fishes. In: Nilsson GE (ed) Respiratory physiology of vertebrates: life with and without oxygen. Cambridge University Press, Cambridge, pp 131–173
Reed RH (1995) Solute accumulation and osmotic adjustment. In: Cole KM, Sheath RG (eds) Biology of the red algae. Cambridge University Press, Cambridge, pp 147–170
Remmert H (1969a) Der Wasserhaushalt der Tiere im Spiegel ihrer ökologischen Geschichte. Naturwissenschaften 56:120–124 [in German]
Remmert H (1969b) Über Poikilosmotie und Isoosmotie. Zeitschrift für vergleichende Physiologie 65:424–427 [in German]
Rissanen E, Tranberg HK, Sollid J, Nilsson GE, Nikinmaa M (2006) Temperature regulates hypoxia-inducible factor-1 (HIF-1) in a poikilothermic vertebrate, crucian carp (Carassius carassius). Journal of Experimental Biology 209:994–1003
Roberts RJ, Agius C, Saliba C, Bossier P, Sung YY (2010) Heat shock proteins (chaperones) in fish and shellfish and their potential role to fish health: a review. Journal of Fish Diseases 33:789–801
Russell G (1985) Recent evolutionary changes in the algae of the Baltic Sea. British Phycological Journal 20:87–104
Rytkönen KT, Williams TA, Renshaw GM, Primmer CR, Nikinmaa M (2011) Molecular evolution of the metazoan PHD-HIF oxygen-sensing system. Molecular Biology and Evolution 28:1913–1926
Sagert S, Rieling T, Eggert A, Schubert H (2008) Development of a phytoplankton indicator system for the ecological assessment of brackish coastal waters (German Baltic Sea coast). Hydrobiologia 611:91–103
Schaible R, Bergmann I, Schubert H (2011) Genetic structure of sympatric sexually and parthenogenetically reproducing population of Chara canescens (Charophyta). ISRN Ecology, 13 pp [doi:10.5402/2011/501838]
Schubert H, Andersson M, Snoeijs P (2006) Relationship between photosynthesis and non-photochemical quenching of chlorophyll fluorescence in two red algae with different carotenoid composition. Marine Biology 149:1003–1013
Schubert H, Forster RM (1997) Sources of variability in the factors used for modelling primary productivity in eutrophic waters. Hydrobiologia 349:75–85
Schubert H, Gerbersdorf S, Titlyanov E, Titlyanova T, Granbom M et al (2004) Circadian rhythm of photosynthesis in Kappaphycus alvarezii (Rhodophyta): independence of the cell cycle and possible photosynthetic clock targets. European Journal of Phycology 39:423–430
Schubert H, Matthijs HCP, Forster RM (1997) Occurrence of complementary chromatic adaptation in a eutrophic estuary. The Phycologist 46:28
Schubert H, Schlüter L, Feuerpfeil P (2003) The underwater light climate of a shallow Baltic estuary – ecophysiological consequences. ICES Research Report 257:29–37
Solan M, Cardinale BJ, Downing AL, Engelhardt KAM, Ruesink JL, Srivastava DS (2004) Extinction and ecosystem function in the marine benthos. Science 306:1177–1180
Stramski D, Legendre L (1992) Laboratory simulation of light-focusing by water-surface waves. Marine Biology 114:341–348
Telesh IV, Schubert H, Skarlato SO (2011) Revisiting Remane’s concept: evidence for high plankton diversity and a protistan species maximum in the horohalinicum of the Baltic Sea. Marine Ecology Progress Series 421:1–11
Telesh IV, Schubert H, Skarlato SO (2013) Life in the salinity gradient: discovering mechanisms behind a new biodiversity pattern. Estuarine Coastal Shelf Science 135:317–327
Telesh IV, Schubert H, Skarlato SO (2015) Size, seasonality, or salinity: what drives the protistan species maximum in the horohalinicum? Estuarine Coastal and Shelf Science 161:102–111
Thorpe SA (2004) Langmuir circulation. Annual Review of Fluid Mechanics 36:55–79
van den Hoek C, Mann DG, Jahns HM (1995) Algae – an introduction to phycology. Cambridge University Press, Cambridge, 640 pp
Weber RE, Jensen FB (1988) Functional adaptations in hemoglobins from ectothermic vertebrates. Annual Review of Physiology 50:161–179
Wenger RH (2002) Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J 16:1151–1162
Whitfield AK, Elliott M, Basset A, Blaber SJM, West RJ (2012) Paradigms in estuarine ecology: a review of Remane diagram with a suggested revised model for estuaries. Estuarine Coastal and Shelf Science 97:78–90
Winter U, Kirst GO (1990) Salinity response of a freshwater charophyte, Chara vulgaris. Plant, Cell and Environment 13:123–134
Winter U, Kirst GO (1991) Partial turgor pressure regulation in Chara canescens and its implications for a generalized hypothesis of salinity response in charophytes. Botanica Acta 104:37–46
Winter U, Kirst GO (1992) Turgor pressure regulation in Chara aspera (Charophyta): the role of sucrose accumulation in fertile and sterile plants. Phycologia 31:240–245
Winter U, Kirst GO, Grabowski V, Heinemann U, Plettner I, Wiese S (1999) Salinity tolerance in Nitellopsis obtusa. Australian Journal of Botany 47:337–346
Winter U, Kuhbier K, Kirst GO (1987) Characeen-Gesellschaften im oligohalinen Kuhgrabensee und benachbarten Gewässern. Abhandlungen der Naturwissenschaftlichen Vereine Bremen 40:381–394 [in German]
Winter U, Soulié-Märsche I, Kirst GO (1996) Effects of salinity on turgor pressure and fertility in Tolypella (Characeae). Plant, Cell and Environment 19:869–879
Wu RSS (2002) Hypoxia: from molecular responses to ecosystem responses. Marine Pollution Bulletin 45:35–45
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Schubert, H., Telesh, I., Nikinmaa, M., Skarlato, S. (2017). Physiological adaptations. In: Snoeijs-Leijonmalm, P., Schubert, H., Radziejewska, T. (eds) Biological Oceanography of the Baltic Sea. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-0668-2_7
Download citation
DOI: https://doi.org/10.1007/978-94-007-0668-2_7
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-0667-5
Online ISBN: 978-94-007-0668-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)