Abstract
The implementation of the European Water Framework Directive (WFD) requires the development of ecologically-based classification systems for anthropogenically-induced eutrophication in all types of water bodies. Due to the inherent high temporal and spatial variability of hydrological and geochemical parameters of the coastal waters of the southern Baltic Sea, discrimination between anthropogenic impact and natural variability is necessary. The development of statistical methods for this discrimination was the main aim of this study. These methods were used to derive indicative phytoplankton parameters for different stages of eutrophication for the investigation area. For this purpose, a long-term phytoplankton data series was analysed, which covered a broad salinity and eutrophication gradient. In order to detect eutrophication effects, the analysis was restricted to phytoplankton spring bloom events and to the salinity range between 5 and 10 psu, i.e. superimposing seasonal and hydrodynamic effects were eliminated. An artificial abiotic degradation vector was developed based on four typical water quality parameters. A total of 11 potentially indicative phytoplankton parameters on different taxonomical levels arose from a correlation analysis with this degradation vector. These indicators were then tested for their ability to discriminate between three eutrophication levels. Finally, seven phytoplankton indices could be proposed: total phytoplankton biovolume, the percentage of diatoms and the biovolume of different size ranges of diatoms and one indicative species (Woronichinia compacta).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The European Water Framework Directive (WFD) was released by the European Parliament and the Council of the European Union in October 2000 (2000/60/EC). This directive implies that the future water quality monitoring of coastal waters has to consider several ecological parameters, including the taxonomic structure, abundance and biomass as well as bloom frequency of the phytoplankton community. While phytoplankton biomass and bloom frequency are mostly assessed by means of a proxy (Chlorophyll a), taxonomic structure and abundance of species are, even if monitored, not yet taken into account. However, to fulfil the requirements of the WFD, an evaluation system based on taxonomic composition has to be developed (comp. 2000/60/EC, Annex V).
In the Baltic Sea, anthropogenically-induced eutrophication has been identified as the most important factor for degradation of the ecosystem, especially in the coastal areas (Rosenberg et al., 1990; Nehring, 1992; Wasmund & Uhlig, 2003). During the last decade, numerous indicators were developed for various marine and coastal areas to quantify the degree of eutrophication. Following historical freshwater approaches, various indices based on nutrient availability for aquatic primary producers were established (Nixon, 1995; Vollenweider et al., 1998; Cloern, 2001; Zurlini, 1996; Abdullah & Danielsen, 1992; Karydis, 1996; Aguilera et al., 2001). However, it became apparent that nutrient concentrations alone, even though being the causes of eutrophication, may not be applicable for all coastal regions (Dettmann, 2001; Cloern, 2001; Bricker et al., 2003, Rönnberg & Bonsdorff, 2004). Biological indices are more suitable since they integrate the effects of increased nutrient loads. Various ecological indices were developed for Aegean Sea based on diversity parameters of the phytoplankton community (Tsirtsis & Karydis, 1998 and Arhonditsis et al., 2003). On the other hand, Danilov & Ekelund (2001) showed that several biodiversity indices failed in coastal monitoring of Baltic Sea areas. Moreover, Wasmund et al. (2000) discussed that potential taxonomic group indicators like (Dino-) flagellates or Diatom biomass described in Radach et al. (1990), Conley et al. (1993) and Hajdu et al. (2000) were also not applicable. The lack of useful taxonomy-based evaluation systems for Baltic brackish coastal areas is probably caused by the high temporal and spatial variability of hydrological and geochemical parameters. Accordingly, phytoplankton eutrophication indices are also masked by their natural variability on a small and long term temporal scale (Wasmund & Kell, 1991).
This paper contributes to the development of a taxonomy-based phytoplankton indication system according to the requirement of the EU-WFD. A method was developed that discriminates between natural variability and eutrophication-induced changes in the phytoplankton community. For this purpose, long term phytoplankton data series of the national monitoring program and abiotic parameters of the coastal waters of the southern Baltic Sea were analysed with several statistical methods. Finally, phytoplankton parameters are presented, which are potentially indicative for three different stages of eutrophication.
Materials and methods
Sampling area
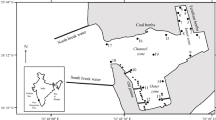
Altogether, 1,163 datasets from 15 oligo- to mesohaline sites along the coast of Mecklenburg-Vorpommern (Germany) were analysed. The sampling sites included freshwater-influenced off-shore sites as well as sites from semi-enclosed inner coastal waters (Fig. 1). However, all of these stations were hydrographically comparable, being generally well mixed throughout the year, but at least during spring (until July). The depth at the stations was between 5 and 11 m, except for one station of just 2-m depth (DB6) and three stations of 14 m (O5), 15 m (O9) and 22 m (O11), respectively.
Dataset
Phytoplankton composition and biovolume as well as abiotic water parameters were monitored within the National German Monitoring Program on the marine environment from 1986 to 1999. The dataset derived from the monitoring of the ‘Landesamt für Naturschutz und Geologie of Mecklenburg-Vorpommern (LUNG)’ and comprised, depending on sites, fortnightly and monthly samplings.
The phytoplankton was sampled at 0.5 m below surface with a Niskin bottle sampler, fixed in formaldehyde and counted according to Utermöhl (1958). Phytoplankton biovolume was calculated by approximating the cell shape to simple geometrical solids according to Rott (1981). As far as possible, taxonomic resolution was performed on the species level. In order to ensure a consistent database, all samplings with uncertain quantitative values or uncertain taxonomic determinations were eliminated. The abiotic parameters [salinity, temperature, pH, oxygen saturation, total nitrogen (TN) and total phosphorus (TP)] as well as Chlorophyll a (Chl a) concentration and Secchi depth were measured according to the COMBINE-manuals of HELCOM (2001).
In addition to the species level, indicative capability of higher taxonomic levels, functional groups and morphological groups were investigated. The assignment to taxonomical classes/groups (e.g. Cyanophyta, Chlorophyta, Cryptophyta, Bacillariophyceae and Dinophyceae) was performed according to the systematic classification of van den Hoek et al. (1993).
In order to exclude seasonal variability, the dataset was restricted to the spring bloom events, i.e. only samples taken during the spring maximum of Diatoms (relative biovolume of Diatoms exceed the annual mean by one standard deviation, see Rieling et al., 2003) and with a maximum in Chl a were used. Thus, the first combined Diatom-Chl a maximum of the period between February and June were analysed. The exact time period of the spring blooms depends on ice cover and temperature, both parameters with large interannual variability.
Statistical analyses
Spearman’s rank-order correlation, i.e. correlation coefficient r s for randomly distributed data, and dissimilarity matrices were calculated using the program NCSS for Windows (USA).
Environmental parameters were combined by principal component analysis (PCA). Canonical correspondence analysis (CCA) was chosen as a constrained unimodal method for the joint analysis of species and environmental parameter datasets. CCA was carried out to gain knowledge on the explanation of the variability of the structure of the phytoplankton community by environmental parameters. The artificial degradation vector, salinity and temperature were entered into a CCA. The calculated ordination scores were scaled according to the ‘biplot rule’ and focussed on ‘inter species distance’. CCA axes were tested for significance by Monte Carlo permutations. Cluster analysis (Bray–Curtis similarity, complete linkage) and dendrograms were prepared with the primer 5 software package (primer-e Ltd. 2000). PCA and CCA were performed with the Software package CANOCO for Windows Version 4.51 (ter Braak & Šmilauer, 2002).
Results
Standardisation of the data material
The German part of the South Arkona Sea is influenced by marine water bodies originating from the North Sea as well as by diffuse and riverine terrestrial run-off. Highly fluctuating salinities indicate that water bodies of different origin and composition are alternating at the individual stations (Fig. 2). In order to minimize this hydrographic variability, the analyses were restricted to samplings within a salinity range of 5–10 psu (Fig. 2). The resulting data matrix comprised 924 samples from 15 sampling sites. Despite being restricted to a small salinity range (5–10 psu), these sites cover a large bandwidth of abiotic (TN, TP) and biotic (Chl a concentration, Secchi depth) parameters (Fig. 3).
Characterisation of sampling sites by four water quality parameters: (A) total nitrogen (TN), (B) total phosphorus (TP), (C) Secchi depth and (D) Chlorophyll a (Chl a) concentration. The samples were restricted to salinities between 5 and 10 psu during spring bloom events. All samples outside of this range were neglected. The box-whiskers represent the medians, the 50%-, 75%- and 95%-percentiles of samples between 1986 and 1999. While TN and TP are represented as annual medians, the values of Secchi depth and Chl a are medians of the summer periods between April and September of each year. The order of sampling sites is determined by ascending median values of TN (A)
In addition to the hydrographic variability, large interannual variability of seasonal phytoplankton succession is typical for the Southern Baltic Sea with its irregular pattern of ice cover and weather conditions during vegetation period. Rieling et al. (2003) concluded that the spring bloom period was the least variable period throughout the year. As shown by these authors, focus on the spring bloom period not only results in decreased interannual variability for the investigated sampling sites but also ensures that phytoplankton growth is mainly limited by nutrient availability, while the biomass levels are not yet controlled by zooplankton grazing, as during the summer period (Peinert et al., 1982). However, spring bloom events defined as the combined maximum of diatoms and Chl a did not occur in all years. Finally, 89 phytoplankton samples from 15 sites covered the salinity range between 5 and 10 psu and contained a spring-bloom event and thus were included in the analysis.
Construction of an artificial abiotic degradation vector
In order to deduce changes of phytoplankton composition and biovolume in relation to changes of the eutrophication state by statistical analyses, it is necessary to parameterise the degree of eutrophication. This parameterisation is usually done by using single chemical (TN, TP) or biological (Chl a, Secchi depth) water quality parameters for subsequent correlation with biological parameters (e.g. phytoplankton biovolume). However, significant correlations between those single eutrophication factors and phytoplankton parameters were not observed in this dataset. Therefore, an artificial abiotic degradation vector was constructed by performing PCA using the four above-mentioned water quality parameters (Fig. 3) Almost 68% of the total variation of the abiotic dataset was explained by factor 1 (F1) (Table 1). F1 highly correlated with TN (0.953), Chl a concentration (0.651), and TP (0.561) of the water body (Table 1). Correlation of F1 to Secchi depth was lower (0.290). However, Secchi depth correlated best with factor 4. An additional influence on Secchi depths by other parameters than eutrophication-induced attenuation was possibly causing this result, e.g. resuspension processes as well as the input of particulate material, which depends mainly on the present hydrographical situation during the sampling than on phytoplankton abundance. Both processes are typical for the shallow coastal waters of the investigated area (Schubert et al., 2001). Since F1 based on TN, TP and Chl a concentration explained a large amount of the whole data variance, it was defined as the artificial degradation vector and used for further analyses.
Identifying indicative phytoplankton parameters on the basis of the degradation vector
In order to extract specific phytoplankton indices, 157 phytoplankton parameters were tested for their correlation with the degradation vector (not all shown). The correlation matrix included the following parameters and parameter groups of the phytoplankton community: species specific biovolume and abundance; total biovolume and abundance of the sample; abundance and biovolume as well as percentage of abundance and percentage of biovolume for taxonomic, morphological, functional groups (e.g. Dinophyta, Chlorophyta, Chrysophyta, Euglenophyta, Cryptophyta, Cyanophyta as well as selective taxonomical classes (such as Centrales, Pennales, Peridiniales, Gymnodinales) flagellates and N-fixing Cyanobacteria), and various size classes of taxonomic groups (e.g. Chlorophyceae < 10 μm); species number; diversity indices (Menhinick’s Shannon, Nygaard, Hurlbert’s, evenness and Margaleff). In order to test for remaining sensitivity for hydrological conditions, site depth was tested separately but did not exhibit any significant correlation to any of the parameters.
Of the 157 tested variables, 11 were significantly correlated (P < 0.05) with the degradation vector (Table 2). Total phytoplankton biomass as well as various genus biovolumes (Diatoms, Chlorophytes, Dinophytes and Cryptophytes) and the percentage of Chlorophytes showed positive correlation with the degradation vector, i.e. their values increased with eutrophication. The same was true for the biovolume of the species Woronichinia compacta (Lemmermann) Komárek & Hindák 1988. Only one negative correlation was found (percentage of Diatoms).
Test for remaining seasonal and hydrological dependencies
Although the phytoplankton parameters were standardised in terms of spring bloom events and the restriction to the salinity range between 5 and 10 psu, a remaining influence of temperature and salinity on the phytoplankton community could not be excluded a priori. During spring bloom events, the former ranged between 6.2 and 16.3°C (median = 11.2°C). A CCA was performed, which revealed remaining dependency of the potential indices on temperature and salinity (Fig. 4). In the species–environment biplot of the CCA, salinity and temperature vectors point almost rectangular to the degradation vector, indicating that they influenced different gradients of the species data (Fig. 4).
Biplot of the CCA based on the extracted degradation vector, temperature and salinity. The perpendicular distance gives the strength of correlation between the degradation vector and the several phytoplankton parameters. 1: percentage Diatoms, 2: biovolume Diatoms > 10 μm, 3: biovolume Diatoms < 10 μm, 4: biovolume Chlorophytes > 10 μm, 5: biovolume Dinophytes, 6: biovolume Cryptophytes, 7: biovolume Woronichinia compacta, 8: biovolume Chlorophytes < 10 μm, 9: percentage Chlorophytes, 10: biovolume Chroomonas sp., 11: total phytoplankton biovolume
Furthermore, the degradation vector was strongly positively correlated with the first environmental axis (0.983; Table 3). This is approximated with a high correlation with the first CCA-axis, since these are calculated from a linear combination of the original environmental predictors. The correlation of salinity and temperature with the second environmental axis was negative and considerably lower (−0.663 and −0.555, respectively). Since the first CCA axis explained already 15.4% of the total floristic variability (Table 3), the whole dataset was governed by a simple dominant gradient which was best represented by the degradation vector. All axes were significant in the Monte Carlo test (P = 0.002).
Since scaling of ordination scores in Fig. 4 was focussed on species distance, the perpendicular projection of species points on an arrow of an environmental variable approximates ordering of species optima with respect to that particular environmental variable. This type of presentation supports the results of the correlation analysis of the 11 phytoplankton parameters with the degradation vector. For instance, only ‘percentage Diatoms’ (1) had a preference for low values of the degradation vector. Optima of the other parameters sorted out along this gradient and increased in this order with the degradation vector. Accordingly, ‘biovolume Chlorophytes < 10 μm’ (8) had a preference for the highest values of the degradation vector.
Determination of eutrophication classes by phytoplankton indices
The 11 phytoplankton parameters potentially indicating eutrophication (Table 2), i.e. which significantly correlated with the degradation vector, were tested for their capability to differentiate three eutrophication classes. Therefore, the sampling sites with the phytoplankton data of the potential 11 phytoplankton indicators were clustered. The Cluster analysis formed three clusters, which showed more than 40% similarity (Fig. 5). Cluster I predominantly included off shore regions, whereas cluster III was dominated by inner coastal waters, already suggesting that the clusters are related to the eutrophication state.
Cluster analysis of the samples restricted to salinities between 5 and 10 psu during spring bloom events and based on the 11 phytoplankton parameters that were significantly correlated with the degradation vector (Table 2). Two sites (O5 and S23, right off the graphic) were excluded from further analysis because of extreme low phytoplankton biovolumes of 0.5 mm3 l−1
In Table 4, mean values of the water quality and environmental parameters as well as the potential phytoplankton parameters are summarised. While cluster III was significantly different from the others for all water quality parameters, only TP was different for clusters I and II. As the degradation vector is based on these water quality parameters, it showed the same relationship, i.e. was significantly higher in cluster III than in clusters I and II. In contrast, salinity and temperature were similar between the clusters, confirming independency from climatic and hydrological conditions (Table 4).
The median values of 7 out of the 11 potential phytoplankton indices were significantly different between the clusters (Table 4). ‘Total phytoplankton biovolume’ and ‘percentage of Diatoms’ discriminated between all clusters, whereas ‘biovolume Diatoms > 10 μm and <10 μm’, ‘biovolume Woronichinia compacta’, ‘percentage Chlorophytes’, and ‘biovolume Chlorophytes > 10 μm’ separated only two clusters. Within this last group, all potential indicators were able to differentiate the low eutrophic cluster I from high eutrophic cluster III. Although the parameters ‘biovolume Chlorophytes > 10 μm’, ‘biovolume Chroomonas sp.’, ‘biovolume Cryptophytes’ and ‘biovolume Dinophytes’ were significantly correlated with the degradation vector (Table 2), the three clusters were not significantly different with respect to these parameters (Table 4). This is presumably due to rare observations of these phytoplankton groups during the spring bloom event, which resulted in generally low and uniform mean values.
Discussion
Despite a long tradition of ecological studies of the coastal waters of the southern Baltic Sea and the existence of several national and international phytoplankton monitoring programmes, current approaches to assess eutrophication in the coastal areas only focus on phytoplankton sum parameters (e.g. Chl a concentration for biomass or productivity). The absence of taxonomy-based evaluation methods is probably attributed to the severe difficulties to take into account the high variability of hydrological and geochemical parameters in brackish coastal areas. In order to overcome this problem, the analysis of this approach was restricted to samples from a small salinity range (5–10 psu) and a comparable phytoplankton succession stage (spring bloom event).
Even though the eutrophication level of an aquatic system is defined by the primary production (e.g. Larsson et al., 1985; Smetacek et al., 1991; Gray, 1992), field data of primary production along the southern Baltic coast are restricted to short-term studies (Wasmund & Kell, 1991; Wasmund et al., 2001). Therefore, the eutrophication level is generally described by the concentrations of a couple of nutrients (TN, TP), believed to be the ‘limiting ones’ in the water. On the other hand, detailed studies of the limitation regime of coastal waters have shown that limiting factors change frequently (e.g. Wasmund & Schiewer, 1994). Thus, numerous resources and factors should be taken into account and the use of nutrient proxies might be problematic for shallow coastal waters, especially for partially enclosed bays (Schiewer, 1998). In particular, the biotic processes strongly influence the balance between nutrients and chlorophyll concentrations or Secchi depths, i.e. each of these parameters alone are unsuitable for an eutrophication index in shallow brackish waters. Four typical water quality parameters were therefore combined in this study to one single degradation vector.
Fluctuating salinities in the Southern Baltic are another natural source of phytoplankton variability in the coastal zones (e.g. Carstensen et al., 2004). Small scale salinity changes play an important role for the implementation process of the EU-WFD in the Baltic coastal waters as the typology system is mainly based on salinity (WFD 2000/60/EC). The inner coastal waters of the investigation area cover type B1a (0.5–3 psu, β-oligohaline), type B1b (3–5 psu, α -oligohaline), type B2a (5–10 psu, β-mesohaline) and type B2b (10–18 psu, α-mesohaline) (Schernewski & Wielgat, 2004; Reimers, 2005). The different water types are the basis for all other aspects of the application of the EU-WFD, including monitoring, assessment and reporting (Reimers 2005; Carstensen et al. 2004). In this study, a salinity range of more than 10 psu was observed at several sampling sites. Thus, these sampling sites exceed the salinity limits of the water types. This extremely complicates the definition of exact water types according to the requirements of the EU-WFD. The plankton community drifts with run-off or inflow events, i.e. it is solely determined by the abiotic conditions of water body itself, not by the sampling site. Thus, focussing on comparable water bodies instead of fixed sampling sites seems more appropriate for phytoplankton analyses. However, this sampling scheme is currently not under discussion. Therefore, the dataset of this study only comprises the β-mesohaline salinity range (5–10 psu), which excluded the most freshwater species (Wasmund & Kell, 1991), and therefore, minimises the ‘Fjord-effect’ (e.g. Braarud, 1974; De Jonge, 1988).
Due to short generation times, phytoplankton communities of temperate and boreal climates generally exhibit a high annual variability of biomass and taxonomic composition. In order to guarantee the analysis of comparable seasonal phytoplankton stages in a long-term dataset, it is important to restrict to temporal windows or specific seasonal stages (e.g. spring bloom, summer maximum, clear-water phase, Rieling et al., 2003).While for freshwater ecosystems a general model for the dependency of the shape of this seasonal phytoplankton succession on the eutrophication status exists (Sommer et al., 1986), no comparable concept has been developed for brackish systems. Nevertheless, specific seasonal succession stages, characterised by distinct biomass and taxonomical composition, were identified in the Baltic Sea as well (Wasmund et al., 1999, 2000; Rieling et al., 2003).
During summer and autumn, a great part of the primary production is grazed by the microzooplankton and mesozooplankton (e.g. Johansson et al., 2004). The indication of the eutrophication state by the phytoplankton community is therefore complicated at this time as the total phytoplankton biomass does not reflect the trophic state of the ecosystem alone. Grazing of the mesozooplankton reduces the phytoplankton biomass, while excretion and intense microbial decomposition processes dampens the effect of potential nutrient limitation. Thus, an evaluation based on phytoplankton sum parameters would lead to a classification of low trophy despite the fact that the water body might have a very high trophic potential due to the high nutrient concentrations. For this reason, an evaluation system indicating the trophic state of the water body should focus on a time period where the phytoplankton community is mainly bottom up controlled. In the coastal waters of the Southern Baltic Sea (and also in lakes and river), this is the case during spring time, i.e. the period between energetic limitation and onset of intense zooplankton grazing (Sommer et al., 1986; Wasmund & Schiewer, 1994).
Reduction of the dataset to the small salinity range (5–10 psu) and a narrow time window (spring bloom) as described above revealed 11 biomass-based phytoplankton parameters sensitive to eutrophication status (degradation index), but independent from climatic and hydrological variability. Cluster analysis resulted in a set of three clusters of stations, which are significantly different with respect to their ‘degradation status’. Not all of the 11 phytoplankton parameters were able to differentiate these clusters and four became excluded from further analysis. The seven parameters that are able to discriminate at least between two out of the three cluster were ‘total phytoplankton biovolume’, ‘percentage of Diatoms’, ‘biovolume Diatoms > 10 μm’, ‘biovolume Diatoms < 10 μm’, ‘biovolume Woronichinia compacta’, ‘percentage Chlorophytes’ and ‘biovolume Chlorophytes > 10 μm’).
In agreement with long-term studies in the Baltic (Wasmund et al., 1998; Wasmund & Uhlig, 2003) and other marine systems (summarised in Cloern, 2001), the percentage and biovolume of Diatoms decreased with increasing eutrophication level. Additionally, Wasmund & Uhlig, 2003 attributed increasing spring blooms of Dinoflagellates during the last 20 years to increasing eutrophication of the Southern Baltic, which would support our results of increasing biomasses of Cryptophytes and Dinophytes with increasing eutrophication parameter. Similar effects are described for various marine systems (Radach et al., 1990; Bodeanu 1993; Bricker et al. 2003).
Further potential indicators on the genus level are the percentage and the biomass of Chlorophytes. Especially smaller Chlorophytes (<10 μm) appear to be indicative at spring time, presumably caused by their fast growth rates. However, small Chlorococcales became typically abundant shortly after the Diatom spring bloom. Thus, their indicative value might be due to the wide sampling intervals, which did not allow an exact measure of the highest bloom event. On the other hand, changes in N:P:Si ratio during eutrophication processes promote ‘nondiatom taxa’ (Cloern, 2001; Sommer et al., 1993). Furthermore, small Chlorophytes grow faster than Diatoms and dominate the spring bloom only after winters without long ice cover (Wasmund & Schiewer, 1994).
Descriptions of sensitive indicator species are scarce for the Baltic phytoplankton. A relationship between nutrient state of coastal waters and Myrionecta rubra (Lohmann 1908) was postulated for Danish coastal waters (Sagert et al., 2005). Although Wasmund et al. (2005) described this species as dominant in the Baltic proper since 1999, M. rubra did not occur in the analysed dataset, presumably based on the exceptional taxonomic position of this species (photoautotroph ciliate) as well as on virtual absence during the investigated period. This present study suggests another indicator species (Woronichinia compacta). According to several international monitoring data along the coasts of the Baltic Sea (collected for the CHARM-project; EU, compare Gasiūnaitė et al., 2005), this colony forming cyanobacterium was monitored over a wide range of salinities (0.5–14.5 psu), which is in agreement with the result of the CCA-analysis. With respect to seasonal succession, Wasmund et al. (2004) reported that this species is most abundant in early and mid summer, which might explain the low abundances during the spring bloom period analysed here (mean values for all spring bloom data: 9% of total biovolume). However, its significantly increased abundance in eutrophicated areas (mean value 18%) suggests its indicative value.
A classification system requires clear distinction between the classes, and so borderlines between distinct levels of eutrophication must be drawn. In order to assure that the class limits reflect distinct levels, a cluster analysis based on the 11 potential phytoplankton parameters was performed. The analysis formed three clusters. Comparing the mean biovolume and the nutrient parameters of the three clusters with the values proposed by Wasmund et al. (2001), the three clusters can be assigned to mesotrophic (cluster I), eutrophic (cluster II) and polytrophic (cluster III) conditions of Baltic Sea water bodies. However, not all 11 potential indicators exhibit the same capability to discriminate these clusters. Especially, indicators with lower percentage on the total biovolume showed insignificant results between the eutrophication clusters, even though a significant correlation with the degradation vector exists. This discrepancy might be due to the small data basis, which is attributed to the restriction on spring bloom events and small salinity range. In order to validate the proposed seven phytoplankton indicators (Table 4), another independent dataset with similar taxonomic differentiation needs to be analysed. Furthermore, these classes must be calibrated against the reference conditions for the specific water type and finally a five-class system needs to be developed as asked for by the EU-WFD.
In conclusion, the statistical analysis of a long-term phytoplankton dataset allowed the identification of eutrophication indicators for highly variable brackish coastal waters. Prerequisites were (A) restriction to a small salinity range (5–10 psu), (B) grouping according to the sample salinity instead of station mean salinity and (C) restriction to a bottom-up controlled seasonal succession state (spring bloom).
References
Abdullah, M. I. & M. Danielsen, 1992. Chemical criteria for marine eutrophication with special reference to Oslofjord, Norway. Hydrobiologia 235–236: 711–722.
Aguilera, P. A., H. Castro, A. Rescia & M. F. Schmitz, 2001. Methodological development of an index of coastal water quality: application in a tourist area. Environmental Management 27: 295–301.
Arhonditsis, G., M. Karydis & G. Tsirtsis, 2003. Analysis of phytoplankton community structure using similarity indices: a new methodology for discriminating among eutrophication levels in coastal marine ecosystems. Environmental Management 31: 619–632.
Bodeanu, N., 1993. Microalgal blooms in the Romanian area of the Black Sea and contemporary eutrophication conditions. In Smayda, T. J. & Y. Shimizu (eds), Toxic Phytoplankton Blooms in the Sea. Elsevier, Amsterdam: 203–209.
Braarud, T., 1974. The natural history of the Hardangerfjord. 2. The fjord effect upon the phytoplankton in late autumn to early spring, 1955–56. Sarsia 55: 99–114.
Bricker, S. B., J. G. Ferreira & T. Simas, 2003. An integrated methodology for assessment of estuarine trophic status. Ecological Modelling 169: 39–60.
Carstensen, J., U. Helminen & A.-S. Heiskanen, 2004. Typology as a structuring mechanism for phytoplankton composition in the Baltic Sea. Coastline Reports 4: 55–64.
Cloern, J. E., 2001. Our evolving conceptual model of the coastal eutrophication problem. Marine Ecology Progress Series 210: 223–253.
Conley, D. J., C. L. Schelske & E. F. Stoermer, 1993. Modification of the biogeochemical cycle of silica with eutrophication. Marine Ecology Progress Series 101: 179–192.
Danilov, R. A. & N. G. A. Ekelund, 2001. Comparative studies on the usefulness of seven ecological indices for the marine coastal monitoring close to the shore on the Swedish East coast. Environmental Monitoring and Assessment 66: 265–279.
De Jonge, N., 1988. The abiotic environment. In Baretta, J. W. & P. Ruardij (eds), Tidal Flat Estuaries Simulation and Analyses of the Ems Estuary. Springer-Verlag, Berlin: 14–27.
Dettmann, E. H., 2001. Effect of water residence time on annual export and denitrification of nitrogen in estuaries: a model analysis. Estuaries 24: 481–490.
Gasiūnaitė, Z. R., A. C. Cardoso, A. S. Heiskanen, P. Henriksen, P. Kauppila, I. Olenina, R. Pilkaityte, I. Purina, A. Razinkovas, S. Sagert, H. Schubert & N. Wasmund, 2005. Seasonality of coastal phytoplankton in the Baltic Sea: influence of salinity and eutrophication. Estuarine Coastal and Shelf Science 65: 239–252.
Gray, J. S., 1992. Eutrophication in the sea. In Colombo, G., I. Frerrari, V. U. Ceccherelli & R. Rossi (eds), Marine Eutrophication and Population Dynamics. Olsen & Olsen, Fredensborg, Denmark: 3–15.
Hajdu, S., L. Edler, I. Olenina & B. Witek, 2000. Spreading and establishment of the potentially toxic dinoflagellate Prorocentrum minimum in the Baltic Sea. International Review of Hydrobiology 85: 561–575.
HELCOM, 2001. COMBINE-manuals of HELCOM; Part C: Programme of monitoring of eutrophication and its effects. http://sea.helcom.fi/Monas/CombineManual2/PartC/C_Content.htm
Johansson, M., E. Gorokhova & U. Larsson, 2004. Annual variability in ciliate community structure, potential prey and predators in the open northern Baltic Sea proper. Journal of Plankton Research 26: 67–80.
Karydis, M., 1996. Quantitative assessment of eutrophication: a scoring system for characterising water quality in coastal marine ecosystems. Environmental Monitoring and Assessment 41: 233–246.
Larsson, U., R. Elmgren & F. Wulff, 1985. Eutrophication and the Baltic Sea: causes and consequences. Ambio 14: 9–14.
Nehring, D., 1992. Inorganic phosphorus and nitrogen compounds as driving forces for eutrophicaqtion in semi-enclosed seas. ICES Marine Science Symposia 195: 507–514.
Nixon, S. W., 1995. Coastal marine eutrophication: a definition, social causes, and future concerns. Ophelia 41: 199–219.
Peinert, R., A. Saure, P. Stegmann, C. Stienen, H. Haardt & V. Smetacek, 1982. Dynamics of primary production and sedimentation in a coastal ecosystem (Kiel Bight). Netherlands Journal of Sea Research 16: 276–289.
Radach, G., J. Berg & E. Hagmeier, 1990. Long-term changes of the annual cycles of meteorological, hydrographic, nutrient and phytoplankton time series at Helgoland and at LV ELBE 1 in the German Bight. Continental Shelf Research 10: 305–328.
Reimers, H.-C., 2005. Typologie der Küstengewässer der Nord- und Ostsee. In Feld, C., S. Rödiger, M. Sommerhäuser & G. Friedrich (eds), Typologie, Bewertung und Management von Oberflächengewässern, Vol 11. Limnologie aktuell: 37–45.
Rieling, T., S. Sagert, M. Bahnwart, U. Selig & H. Schubert, 2003. Definition of seasonal phytoplankton events for analysis of long term data from coastal waters of the southern Baltic Sea with respect to the requirements of the European Water Framework Directive. In Brebbia, C. A., D. Almorza & D. Sales (eds), Water Pollution VII—Modelling, Measuring and Prediction. WIT Press, Boston: 103–114.
Rönnberg, C. & E. Bonsdorff, 2004. Baltic Sea eutrophication: area-specific ecological consequences. Hydrobiologia 514: 227–241.
Rosenberg, R., R. Elmgren, S. Fleischer, P. Jonssen, G. Persson & H. Dahlin, 1990. Marine eutrophication case studies in Sweden. Ambio 19: 102–108.
Rott, E., 1981. Primary productive and activity coefficients of the phytoplankton of a mesotrophic soft-water lake (Piburger See, Tirol, Australia). Internationale Revue der gesamten Hydrobiologie 66: 1–27.
Sagert, S., D. K. Jensen, P. Henriksen, T. Rieling & H. Schubert, 2005. Integrated ecological assessment of Danish Baltic Sea coastal areas by means of phytoplankton and macrophytobenthos. Estuarine Coastal and Shelf Science 63: 109–118.
Schernewski, G. & M. Wielgat, 2004. Towards a typology for the Baltic Sea. Coastline Reports 2: 35–52.
Schiewer, U., 1998. 30 years’ eutrophication in shallow brackish waters—lessons to be learned. Hydrobiologia 363: 73–79.
Schubert, H., S. Sagert & R. M. Forster, 2001. Evaluation of the different levels of variability in the underwater light field of a shallow estuary. Helgoland Marine Research 55: 12–22.
Smetacek, V., U. Bathmann, E. M. Nöthig & R. Scharek, 1991. Coastal eutrophication: causes and consequences. In Mantoura, R. C. F., J.-M. Martin & R. Wollast (eds), Ocean Margin Processes in Global Change. John Wiley & Sons, Chichester: 251–279.
Sommer, U., U. Gaedke & A. Schweizer, 1993. The first decade of oligotrophication of Lake Constance. II. The response of phytoplankton taxonomic composition. Oecologia 93: 276–284.
Sommer, U., Z. M. Gliwicz, W. Lampert & A. Duncan, 1986. The PEG-model of seasonal succession of planktonic events in freshwaters. Archiv für Hydrobiologie 106: 433–471.
ter Braak, C. J. F. & P. Šmilauer, 2002. CANOCO Reference Manual and CanoDraw for Windows user’s guide. Software for Canonical Community Ordination (version 4.5). Microcomputer Power, Ithaca NY, USA: 1–500.
Tsirtsis, G. & M. Karydis, 1998. Evaluation of phytoplankton community indices for detecting eutrophic trends in the marine environment. Environmental Monitoring and Assessment 50: 255–269.
Utermöhl, H., 1958. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. International Association of Theoretical and Applied Limnology Communications 9: 1–38.
van den Hoek, C., H. M. Jahns & D. G. Mann, 1993. Algen. 3., Thieme, Stuttgart: 411 pp.
Vollenweider, R. A., F. Giovanardi, G. Montanari & A. Rinaldi, 1998. Characterization of the trophic conditions of marine coastal waters with special reference to the NW-Adriatic Sea: proposal for a trophic scale, turbidity and generalized water qualitiy index. Environmetrics 9: 329–357.
Wasmund, N., A. Andrushaitis, E. Lysiak-Pastuszak, B. Müller-Karulis, G. Nausch, T. Neumann, H. Ojaveer, I. Olenina, L. Postel & Z. Witek, 2001. Trophic status of the south-eastern Baltic Sea: a comparison of coastal and open areas. Estuarine Coastal and Shelf Science 53: 849–864.
Wasmund, N. & V. Kell, 1991. Characterization of brackish coastal waters of different trophic levels by means of phytoplankton biomass and primary production. Internationale Revue der gesamten Hydrobiologie 76: 361–370.
Wasmund, N., G. Nausch & W. Matthaeus, 1998. Phytoplankton spring blooms in the southern Baltic Sea—spatio-temporal development and long-term trends. Journal of Plankton Research 20: 1099–1117.
Wasmund, N., G. Nausch, L. Postel, Z. Witek, M. Zalewski, S. Gromisz, E. Lysiak-Patuszak, I. Olenina, R. Kavolyte, A. Jasinskaite, B. Müller-Karulis, A. Ikauniece, A. Andrushaitis, H. Ojaveer, K. Kallaste & A. Jaanus, 2000. Tropic status of coastal and open areas of the south-eastern Baltic Sea based on nutrient phytoplankton data from 1993–1997. Marine Science Reports 38: 1–86.
Wasmund, N., F. Pollehne, L. Postel, H. Siegel & M. L. Zettler, 2004. Biologische Zustandseinschätzung der Ostsee im Jahre 2003. Marine Science Reports 60: 1–94.
Wasmund, N., F. Pollehne, L. Postel, H. Siegel & M. L. Zettler, 2005. Biologische Zustandseinschätzung der Ostsee im Jahre 2004. Marine Science Reports 64: 1–78.
Wasmund, N. & U. Schiewer, 1994. Overview on ecology and biological production of phytoplankton from the Darss-Zingst Bodden chain (southern Baltic). [Überblick zur Ökologie und Produktionsbiologie des Phytoplanktons der Darß-Zingster-Boddenkette]. Rostocker Meeresbiologische Beiträge 2: 41–60.
Wasmund, N. & S. Uhlig, 2003. Phytoplankton trends in the Baltic Sea. ICES Journal of Marine Science 60: 177–186.
Wasmund, N., M. Zalewski & S. Busch, 1999. Phytoplankton in large river plumes in the Baltic Sea. ICES Journal of Marine Science 56: 23–32.
WFD 2000/60/EC; Directive 2000/60/EC of the European parliament and of the council of 23 October 2000 establishing a framework for Community action in the field of water policy. Official Journal of the European Communities L 327/1: 1–72.
Zurlini, G., 1996. Multiparametric classification of trophic conditions. The OECD methodology extended: combined probabilities and uncertainties—application to the North Adriatic Sea. Science of the Total Environment 182: 169–185.
Acknowledgments
The authors are grateful for financial support by the BMBF (Bundesministerium für Bildung und Forschung, Germany, ELBO-Az 0330014), the LUNG (Landesamt für Umwelt, Naturschutz und Geologie Mecklenburg-Vorpommern) and the European Commission (CHARM-EVK3-CT-2001- 00065). Special thanks go to Mandy Bahnwart and Norbert Wasmund for their great contributions to compilation and quality assurance of the phytoplankton database.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: A. Razinkovas, Z. R. Gasiūnaitė, J. M. Zaldivar & P. Viaroli
European Lagoons and their Watersheds: Function and Biodiversity
Rights and permissions
About this article
Cite this article
Sagert, S., Rieling, T., Eggert, A. et al. Development of a phytoplankton indicator system for the ecological assessment of brackish coastal waters (German Baltic Sea coast). Hydrobiologia 611, 91–103 (2008). https://doi.org/10.1007/s10750-008-9456-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-008-9456-3