Abstract
Due to the variable severity of clinical manifestations of Behcet’s disease (BD), the therapy should be modulated in function of the specific clinical manifestations. New topical therapies such as pimecrolimus and amlexanox are useful for oral and genital ulcers. Old systemic therapies, especially colchicine, azathioprine, and cyclosporine represent the first-line therapy in most cases. In presence of BD serious manifestations, including uveitis, vascular, neurologic, and intestinal involvement, an aggressive therapy with high-dose corticosteroids plus an immunosuppressive drug such as cyclosporine, azathioprine, and cyclophosphamide is required. Recently, the efficacy of interferon alpha and anti-TNF for the treatment of refractory uveitis has been repeatedly reported. These therapies allowed the achievement of complete remission with improvement of visual acuity in 70–90 % of the cases of uveitis. Moreover, anti-TNF has been demonstrated as effective in all other BD manifestations. However, to date, these drugs should be used as second-line therapy in more severe forms of BD unresponsive to old immunosuppressive therapies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
19.1 Introduction
Behçet’s disease (BD) is a chronic, relapsing, systemic vasculitis of unknown cause involving veins and arteries of all sizes characterized by protean clinical manifestations. Due to the variable severity of clinical manifestations of BD, therapeutic intervention should be modulated in function of the specific clinical feature. Indeed, BD may occur with less serious clinical symptoms and signs including mucocutaneous lesions, and articular manifestations, and severe clinical features such as ocular, vascular, neurologic, gastrointestinal, urogenital, and pulmonary involvement [1].
19.2 Current Treatment Strategies
19.2.1 Topical Treatment
19.2.1.1 Old Topical Therapies
Local application of drugs is currently employed for the treatment of aphthous stomatitis including corticosteroids (CS), antimicrobials, sucralfate, anti-inflammatory agents (benzydamine, diclofenac), anesthetics and silver nitrate.
Topical CS are useful to treat the oral ulcers of BD. Topical CS such as triamcinolone acetonide (cream or spray), or dexamethasone elixir exert an anti-inflammatory effect, and seem to be more effective in pain reducing and healing shortening when employed in the early stage of ulcer formation. Topical CS eye drops may be also effective in mild forms of anterior uveitis of BD, and CS creams, usually in combined therapy with antimicrobials, have been successfully employed for the treatment of genital ulcers [2]. Local application of gel, drops, suspensions, or creams containing chlorhexidine, triclosan, tetracycline, minocycline, as well as sucralfate mouthwash four times/day are effective to improve the pain, to shorten the healing, and to reduce the frequency of ulcer recurrence [2].
19.2.1.2 New Topical Therapies
Pimecrolimus. Pimecrolimus, a calcineurin inhibitor widely used in dermatology for the treatment of several forms of atopic dermatitis, has been demonstrated effective in genital ulcers of BD with pain reduction and accelerated healing by recent trials [3, 4].
Amlexanox. This compound exerts an anti-allergic and anti-inflammatory agent by inhibiting the formation and release of histamine and leukotrienes from mast cells, neutrophils, and mononuclear cells. In a recent randomized controlled trial [5], amlexanox, in form of oral adhesive pellicles, resulted significantly superior to placebo in the treatment of common aphthous stomatitis and, although not specifically investigated, it has been proposed for BD [2].
19.2.2 Systemic Therapies
19.2.2.1 Old Systemic Therapies
Corticosteroids. Even in absence of controlled trials, there is a general agreement among clinicians on the efficacy of CS, at variable daily doses or in high-dose pulses, on all clinical features of BD. High-dose CS are usually employed combined with immunosuppressive drugs in the treatment of severe BD manifestations including uveitis, neuro-Behçet, angio-Behçet, and colitis, whereas at low dose CS are effective for mucocutaneous involvement such as oral aphthosis, papulo-pustolosa, and erythema nodosum. However, the well-known side effects and adverse events associated with the long-term use of CS represent an important limiting factor, and, possibly, their use would be limited to the role of symptomatic “bridge therapy” while waiting the effects of the combined immunosuppressive therapy which is usually slower.
Colchicine. Colchicine acts inhibiting the chemotactic activity of neutrophils. In two placebo-controlled studies, the drug at the doses of 0.5–2 mg/day was effective on articular features, erythema nodosum, pseudofolliculitis, and in reducing the recurrences of genital ulcers of BD [6, 7]. An enhanced efficacy of colchicine in reducing BD disease activity has been recently reported if combined with benzathine penicillin administration [8]. Colchicine side effects, chiefly gastrointestinal complaints, may limit its use.
Rebamipide. This gastro-protective drug, acting on gastric mucosal prostaglandins release, was found to significantly improve the recurrence of oral aphthae of BD in a controlled trial of 6-month duration [9]. However, the drug is not licensed for clinical use in Western countries.
Dapsone. Dapsone inhibits the chemotactic activity of neutrophils, and in a short-term, controlled trial of 20 patients with BD, was demonstrated effective on mucocutaneous features, epididymitis, but not joint manifestations of BD [10]. Of note, dapsone employment is loaded by frequent hematological side effects with evident limits to its use.
Thalidomide. This compound exerts a weak anti-tumor necrosis factor (anti-TNF) activity. There is some evidence of efficacy on mucocutaneous manifestations of BD [2], but its clinical use is quite limited due to the frequent neurological side effects and the well-known teratogenicity.
Azathioprine (AZA). AZA is a purine analog prodrug which is converted to mercaptopurine and then metabolized to an active metabolite, thioguanine. This metabolite is incorporated into ribonucleotides, thereby exerting an anti-proliferative effect on mitotically active lymphocyte populations. AZA may also have direct anti-inflammatory properties by inhibiting cytotoxic T cell and natural killer cell function, and inducing apoptosis of T cells [11]. In early 1990s, a 24-month, randomized, placebo-controlled trial of 73 patients demonstrated the efficacy of AZA at the dose of 2.5 mg/kg/day on ocular manifestations of BD with significant reduction of uveitis flares and of the occurrence of new eye inflammatory involvement [12]. The drug was also effective on BD arthritis and oral ulcer healing, but not on papulopustular lesions and on the prevention of new ulcer recurrence. The drug has some limit in its use due to the rather low tolerability. As evidenced by a recent report [13], 17 % of 3,931 patients with Crohn’s disease receiving AZA had to discontinue the treatment for adverse events including myelotoxicity, nausea, gastrointestinal complaints, immunosuppression, opportunistic infections, and hepatotoxicity over a median follow up of 44 months.
Cyclophosphamide (CYC). CYC belongs to the drug class of alkylating agents and acts blocking the production of the deoxyribonucleic acid in cells. This prevents cells from dividing, leading to cell death. Some of the cells affected by CYC are immune cells, thus explaining its use in autoimmune diseases such as rheumatoid arthritis, lupus, scleroderma, or vasculitis. There are no published controlled trials demonstrating the efficacy of CYC in BD patients. In 24-month, head to head trial of comparison with cyclosporine, no significant reduction of ocular attacks and improvement of visual acuity was observed in CYC treatment arm [14]. However, the study was carried out on only 11 patients with an obvious limitation to its consequent evidence. Nevertheless, the drug is currently employed (as monthly 1 g intravenous boluses, or 1.5–2.5 mg/kg/day, orally) to treat the more severe manifestations of BD, such as eye and central nervous system involvement. The severe safety profile of CYC characterized by myelotoxicity frequently leading to leukopenia, pulmonary fibrosis, renal toxicity, hemorrhagic cystitis, infertility, malignancy, and alopecia, suggest that the drug should be considered for use in patients with severe disease who are refractory to other agents [2].
Chlorambucil. Chlorambucil, at the dose of 0.1–0.2 mg/kg/day orally, is another alkylating agent which has been demonstrated effective in patients with BD and severe eye involvement [15]. The frequency of chlorambucil-associated side effects and toxicity such as bone marrow suppression, seizures, tremors or shaking, severe vomiting, and diarrhea limit its use in selected BD patients not responding to other traditional or more recent therapies.
Cyclosporin A (CsA). CsA is a lipophilic cyclic peptide that binds with high affinity to its cytoplasmic receptor protein cyclophilin. This complex specifically and competitively binds to and inhibits calcineurin, a calcium and calmodulin dependent phosphatase. This prevents translocation of a family of transcription factors, nuclear factor activated T cells (NF-AT), which reduces activation of genes for interleukin (IL)-2, IL-3, IL-4, granulocyte macrophage colony-stimulating factor, tumor necrosis factor alpha and interferon gamma. T-cell transcription factors AP-1 and NF-kB are also inhibited. CsA acts predominantly on CD4 cells. Consequently, CsA diminishes cytokine production and exerts an anti-proliferative effect on lymphocytes [16]. The efficacy of CsA in the treatment of BD, with particular attention to uveitis, has been reported in one controlled trial [17]. CsA, at the high dose of 10 mg/Kg/day, was superior to colchicine in reducing the frequency of ocular attacks in 96 patients with BD, and was also effective on mucocutaneous features of the disease. Although with conflicting results, several open-label prospective trials have confirmed the usefulness of CsA in the treatment of different BD manifestations including hearing loss, thrombophlebitis, and joint symptoms [14, 18, 19]. CsA is not effective in neuro-Behçet, and a more frequent involvement of central nervous system has been reported in BD patients taking CsA compared to other therapies [20]. Important long-term adverse effects such as renal failure, hypertension, neurologic toxicity, and hirsutism suggest to use CsA only in more severe cases of BD, and in particular for the treatment of uveoretinitis.
Methotrexate (MTX). Although a few open-label trials reported the efficacy of MTX in neurological involvement [21], to date, the drug is prevalently employed in association with tumor necrosis factor inhibitors to treat the most severe cases of BD.
19.2.2.2 New Systemic Therapies
Mycophenolate mofetil (MMF). MMF is a prodrug of mycophenolic acid, which exerts its immunosuppressive action by inhibiting the inosine monophosphate dehydrogenase, which is the rate-limiting enzyme in de novo synthesis of guanosine nucleotides of T- and B-lymphocytes. MMF is a 5-fold more potent inhibitor of the type II isoform of inosine monophosphate dehydrogenase expressed in activated lymphocytes with consequent cytostatic effect [22]. The drug is usually employed at the dose of 1 g twice a day, orally. The efficacy of MMF in the treatment of non-infectious uveitis has been proven by several studies from ophthalmology settings. In a recently published cohort of 236 patients, 93 % of 94 patients with posterior uveitis achieved a complete remission after 6 and 12 months of MMF therapy [23]. Unfortunately, in the paper it is not reported whether BD patients were included in this group. Similarly, in a recent Italian study, cystoid macular edema secondary to non-infectious uveitis resolved in 18 out of 19 patients [24]. MMF has been also reported as effective in the long-term treatment of neuro-Behçet [25]. If the drug is effective on mucocutaneous manifestations of BD is still unclear. Some reserves to MMF use may be related to its safety profile and tolerability with a rate of drug discontinuation of around 15 % of the patients because of gastrointestinal complaints, bone marrow suppression, elevate liver enzymes, allergy [23]. However, MMF may represent an emergent therapy for ocular manifestation of BD, and controlled trials should be performed to confirm its use as a good therapeutic option in clinical practice.
Interferon-α (IFN)-α. IFN-α, in addition to its powerful antiviral activity, play a critical role in modulating the Th1 immune responses via up-regulation of the high-affinity interleukin-12 beta1/beta2, and represents a pivotal cytokine responsible for the amplification of the CD8 + T cell response [26]. Over the past 10 years, a consistent body of evidence of efficacy of (IFN)-α in the treatment of BD has been provided by several trials. A randomized, double-blind, placebo-controlled study demonstrated that IFN-α 2a is effective on the mucocutaneous lesions of BD, with a significant decrease of the frequency, duration and pain of oral and genital ulcers and pseudofolliculitis. A trend to reduction of erythema nodosum, thrombophlebitis and arthritis frequency also resulted from this trial [27]. Promising results were obtained by IFN-α use inBD sight-threatening refractory uveitis. In an open-label trial of 50 patients [28] IFN-α 2a, at a dose of 6 million IU (MIU) daily, a response rate of 92 % was observed at week 52, with a significant improvement of visual acuity and remission of extraocular manifestations, with the exception of oral ulcers. Similar findings were observed in an additional study of 44 BD patients with refractory uveitis [29]. Moreover, the long-term efficacy and safety of low-dose therapy of IFN-α 2a (3.0 MIU daily for 14 days, maintenance dose, 3 MIU three times per week for 24 months) was confirmed in 37 patients with BD and refractory pan-uveitis [30]. Multiple side effects are associated with IFN-α therapy including flu like symptoms, such as fever, chills, headache, fatigue, myalgia, that start a few hours after the initiation of the therapy and continue for at least 1 day. Acetaminophen (paracetamol) 1,000 mg orally, before injections and 500 mg after 6 h during the first weeks of the therapy, is of value to reduce these side effects. Psychiatric side effects and depression, nausea, vomiting, anorexia, diarrhea, loss of weight, hematologic changes, and transient raising of hepatic transaminases may constitute additional limiting factors for use of IFN-α. Efficacy data of IFN-α 2a on the other major clinical features of BD including neuro-Behçet, entero-Behçet, and angio-Behçet are not yet available. However, IFN-α 2a represents a valid therapeutic alternative in patients with severe uveitis of BD.
Anti-tumor necrosis factor-α agents (anti-TNF). In animal models, TNF-α plays a key role in the pathogenesis of ocular inflammation [31], and serum and intraocular-increased concentrations of TNF-α have been detected in patients with active BD [32]. Due to this evidence, some BD patients with refractory posterior uveitis have been treated with at least four infusions of infliximab (IFX), a chimeric monoclonal anti- TNF-α antibody at the dose of 5 mg/kg at weeks 0, 2, 6, and every 8 weeks afterwards [33–36]. All patients experienced a rapid remission of ocular inflammation over a few days. Over the following years, additional studies confirmed the efficacy of IFX in ocular manifestations of BD. Two consecutive Italian studies on 12 and 50 patients with refractory uveoretinitis, respectively, revealed a complete remission of ocular inflammatory involvement in 75 % of the cases, with a significant improvement of visual acuity [37, 38]. A similar response rate has been recently reported in a Japanese study of 50 patients [39]. In all previously quoted studies, IFX was also effective on the other manifestations of BD, and recently IFX efficacy was reported in 9 out of 10 patients with severe entero-Behçet [40], as well as in neuro-Behçet [41].
Recently, in keeping with previous case reports, adalimumab, a fully human anti-TNF-α antibody, given at the dose of 40 mg/every other week, has also been reported to be effective in all clinical features of BD in an open-label trial of 19 patients [42], and the soluble receptor anti-TNF etanercept (ETN) resulted effective for the treatment of mucocutaneous manifestations of BD in a double-blind, placebo-controlled study of 40 male patients [43]. However, the use of ETN in BD seems to be limited by its lack of efficacy on ocular inflammation as observed in anterior uveitis of ankylosing spondylitis [44]. In all studies, anti-TNF demonstrated a good safety profile.
In summary, there is an increasing evidence of the efficacy and safety of monoclonal anti-TNF for the treatment of all severe manifestations of BD, and for this reason IFX has obtained the approval for BD therapy by the Japanese Health Authorities. However, the high cost raises some concerns to promote anti-TNF as the first-line choice for the management of BD.
Rituximab (RTX). RTX, an anti-CD20 + monoclonal antibody, administered at the dose of 1 g/intravenously repeated at 2-week interval every 6 months has been recently found as an effective therapy in a pilot study of 20 BD patients with severe ocular involvement [45]. Over the last year, we also treated four patients with refractory BD uveitis and one with severe entero-Behçet. None of the patients with uveitis was responsive, while the patient with colitis achieved a complete remission (personal unpublished data). Hence, further trials on a larger number of patients are required to confirm these findings.
Tocilizumab (TCZ). TCZ is a recombinant, humanized, monoclonal, anti-interleukin (IL)-6 receptor antibody competing for both the membrane-bound and soluble forms of human IL-6 receptor with inhibition of the binding of IL-6 to its receptors and its pro-inflammatory activity [46]. IL-6 may play a pathogenic role in BD, and recently elevated cerebrospinal fluid IL-6 levels were detected in active neuro-Behçet [47]. To date, TCZ has been successfully used for the treatment of three patients with refractory BD, two of whom with neuro-Behçet [48–50]. Despite these encouraging reports, clinical trials on larger number of patients are required to confirm the efficacy of TCZ in BD.
19.3 Future Therapeutic Perspectives
Since new pathogenic pathways have been found to play an important role in BD, new biological agents targeted to IL-12/23 and IL-1β are currently under investigation. Recently, in a phase II study, all seven patients with BD and refractory ocular involvement receiving gevokizumab, a monoclonal antibody targeted to IL-1β, achieved a rapid complete remission [51]. These promising results should be confirmed in phase III trials on a larger number of subjects.
19.4 Treatment of Different Manifestations of BD in Clinical Practice
Based on the available evidence of efficacy [52], the therapeutic intervention of BD should be modulated by the different severity of the manifestations.
Mucocutaneous manifestations. Colchicine is preferable for the treatment of genital ulcers and erythema nodosum, and can be combined with benzathine penicillin [6–8]. Oral ulcers are usually more resistant, and often a short-term of combined CS therapy is required. In patients with particularly resistant mucocutaneous manifestations AZA [12], or dapsone [10] may be an effective choice, while thalidomide, due to its low tolerability profile, should be preserved as the last choice. An attempt can be also made with pentoxifylline [49]. In case of nonresponse, CsA may ensure a good result [17], otherwise anti-TNF [37, 39] or IFN-α 2a [28] should be used. Previously mentioned topical therapies should be added for oral or genital ulcers.
Articular manifestations. BD arthritis is usually mild and often self-limiting. Colchicine, preferably associated with non-steroidal anti-inflammatory drugs, and intra-articular CS represent the first choice [6]. Rarely, a low-dose, short-term CS course is needed.
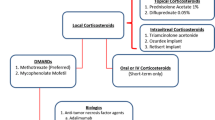
Ocular manifestations. Eye involvement in BD should be regarded as a serious manifestation and requires an aggressive therapy. Anterior uveitis usually responds to topical CS drops, mydriatics or cycloplegic agents, but in case of resistance systemic CS should be added until the remission. Posterior uveitis needs high dose CS (prednisone 1 mg/kg/day or equivalent) with weekly 5 mg tapering [2, 52]. If flare occurs, an immunosuppressive drug such as CsA or AZA should be started [12, 17]. If no response is obtained, IFN-α 2a or monoclonal anti-TNF allows to achieve the remission in most cases [28, 37, 39]. MMF, CyC, and eventually RTX, due to the scanty evidence of efficacy and the low tolerability should be postponed as the last choice in unresponsive patients [14, 23].
Vascular manifestations. In our experience, CsA 5 m/kg/day combined with CS at low dose (prednisone 12.5–25 mg/day with rapid tapering) is an effective therapy for deep vein thrombophlebitis, with a good resolution of venous occlusion over a few days and avoidance of post-phlebitic syndrome [19]. In alternative, AZA can be used [2]. CYC monthly boluses should be employed in patients with Budd-Chiari syndrome or thrombophlebitis of vena cava [2, 52]. The therapeutic role of anticoagulants or anti-platelets is still debated [53], but these drugs should be avoided in the case of pulmonary arterial aneurysm for the risk of bleeding. In arterial involvement, CS together with cyclophosphamide represents the first choice, followed by IFX in case of failure [2].
Neurologic manifestations. When central nervous system is involved, an aggressive therapy with high-dose CS (prednisone 1 mg/kg/day or methylprednisolone 1 g boluses for 3–5 consecutive days followed by oral CS) should be promptly initiated [2, 49]. In resistant cases, we suggest to employ IFX 5 mg/kg as second-line choice. Combined MTX in these cases may be helpful [21]. Due to its lower tolerability, CYC should be reserved as third-line option. Finally, in patients who are refractory to all previous therapies, TCZ may be tried [49, 50]. Anticoagulants are recommended in presence of deep sinus thrombosis [2, 52].
Gastrointestinal manifestations. CS combined with sulphasalazine is suggested as first-line choice [2, 49]. In unresponsive, IFX should be started.
References
Dalvi SR, Yildirim R, Yazici Y (2012) Behcet’s syndrome. Drugs 72(30):2223–2241
Alpsoy E (2012) New evidence-based treatment approach in Behçet’s disease. Patholog Res Int 2012:871019
Köse O, Dinç A, Simşek I (2009) Randomized trial of pimecrolimus cream plus colchicine tablets versus colchicine tablets in the treatment of genital ulcers in Behçet’s disease. Dermatology 218:140–145
Chams-Davatchi C, Barikbin B, Shahram F et al (2010) Pimecrolimus versus placebo in genital aphthous ulcers of Behcet’s disease: a randomized double-blind controlled trial. Int J Rheum Dis 13:253–258
Meng W, Dong Y, Liu J et al (2009) A clinical evaluation of amlexanox oral adhesive pellicles in the treatment of recurrent aphthous stomatitis and comparison with amlexanox oral tablets: a randomized, placebo controlled, blinded, multicenter clinical trial. Trials 6(10):30
Yurdakul S, Mat C, Tuzun Y et al (2001) A double-blind trial of colchicine in Behçet’s syndrome. Arthritis Rheum 44:2686–2692
Davatchi F, SadeghiAbdollahi B et al (2009) Colchicine versus placebo in Behçet’s disease: randomized, double-blind, controlled crossover trial. Mod Rheumatol 19:542–549
Al-Waiz MM, Sharquie KE, A-Qaissi MH et al (2005) Colchicine and benzathine penicillin in the treatment of Behçet disease: a case comparative study. Dermatol Online J 11:3
Matsuda T, Ohno S, Hirohata S et al (2003) Efficacy of rebamipide as adjunctive therapy in the treatment of recurrent oral aphthous ulcers in patients with Behçet’s disease: a randomised, double-blind, placebo-controlled study. Drugs R.D 4:19–28
Sharquie KE, Najim RA, Abu-Raghif AR (2002) Dapsone in Behçet’s disease: a double-blind, placebo-controlled, cross-over study. J Dermatol 29:267–279
Tiede I, Fritz G, Strand S et al (2003) CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4 + T lymphocytes. J Clin Invest 111:1133–1145
Yazici H, Pazarli H, Barnes CG et al (1990) A controlled trial of azathioprine in Behçet’s syndrome. N Engl J Med 322:281–285
Chaparro M, Ordás I, Cabré E et al (2013) Safety of thiopurine therapy in inflammatory bowel disease: long-term follow-up study of 3931 patients. Inflamm Bowel Dis 19:1404–1410
Ozyazgan Y, Yurdakul S, Yazici H et al (1992) Low dose cyclosporin a versus pulsed cyclophosphamide in Behçet’s syndrome: a single masked trial. Br J Ophthalmol 76:241–243
Mudun BA, Ergen A, Ipcioglu SU et al (2001) Short-term chlorambucil for refractory uveitis in Behcet’s disease. Ocul Immunol Inflamm 9:219–29
Aberra FN, Lichtenstein GR (2005) Monitoring of immunomodulators in inflammatory bowel disease. Aliment Pharmacol Ther 21:307–319
Masuda K, Nakajima A, Urayama A et al (1989) Double-masked trial of cyclosporin versus colchicine and long-term open study of cyclosporin in Behçet’s disease. Lancet 1(8647):1093–1096
Elidan J, Levi H, Cohen E et al (1991) Effect of cyclosporine a on the hearing loss in Behçet’s disease. Ann Otol Rhinol Laryngol 100:464–468
Cantini F, Salvarani C, Niccoli L et al (1999) Treatment of thrombophlebitis of Behçet’s disease with low dose cyclosporin A. Clin Exp Rheumatol 17:391–2
Kötter I, Günaydin I, Batra M et al (2006) CNS involvement occurs more frequently in patients with Behçet’s disease under cyclosporin A (CSA) than under other medications—results of a retrospective analysis of 117 cases. Clin Rheumatol 25:482–486
Kikuchi H, Aramaki K, Hirohata S (2003) Low dose MTX for progressive neuro-Behçet’s disease. A follow-up study for 4 years. Adv Exp Med Biol 528:575–578
Allison AC (2005) Mechanisms of action of mycophenolate mofetil. Lupus 14:S2–S8
Daniel E, Thorne JE, Newcomb CW et al (2010) Mycophenolate mofetil for ocular inflammation. Am J Ophthalmol 149:423–432
Neri P, Mariotti C, Cimino L et al (2009) Long-term control of cystoid macular oedema in non-infectious uveitis with Mycophenolate Mofetil. Int Ophthalmol 29:127–133
Shugaiv E, Tüzün E, Mutlu M et al (2011) Mycophenolate mofetil as a novel immunosuppressant in the treatment of neuro-Behçet’s disease with parenchymal involvement: presentation of four cases. Clin Exp Rheumatol 29(4):S64–S67, Suppl 67
Tompkins WA (1999) Immunomodulation and therapeutic effects of the oral use of interferon-alpha: mechanism of action. J Interferon Cytokine Res 19(8):817–828
Alpsoy E, Durusoy C, Yilmaz E et al (2002) Interferon alfa-2a in the treatment of Behçet disease: a randomized placebo-controlled and double-blind study. Arch Dermatol 138:467–471
Kötter I, Zierhut M, Eckstein AK et al (2003) Human recombinant interferon alpha-2a for the treatment of Behçet’s disease with sight threatening posterior or panuveitis. Br J Ophthalmol 87:423–431
Tugal-Tutkun I, Guney-Tefekli E, Urgancioglu M (2006) Results of interferon-alfa therapy in patients with Behçet uveitis. Graefe’s Arch Clin Exp Ophthalmol 244:1692–1695
Onal S, Kazokoglu H, Koc A et al (2011) Long-term efficacy and safety of low-dose and dose-escalating interferon alfa-2a therapy in refractory Behçet uveitis. Arch Ophthalmol 129:288–294
De Vos AF, van Haren MAC, Verhagen C et al (1994) Kinetics of intraocular tumor necrosis factor and interleukin 6 in endotoxin-induced uveitis. Invest Ophthalmol Vis Sci 35:1100–1106
Mege JL, Dilsen N, Sanguedolce V et al (1993) Overproduction of monocyte derived tumor necrosis factor alpha, interleukin (IL) 6, IL-8 and increased neutrophil superoxide generation in Behçet’s disease. A comparative study with familial Mediterranean fever and healthy subjects. J Rheumatol 20:1944–1949
Ohno S, Nakamnura S, Hori S et al (2004) Efficacy, safety, and pharmacokinetics of multiple administration of Infliximab in Behçet’disease with refractory uveitis. J Rheumatol 31:1362–1368
Sfikakis PP, Theodossiadis PG, Katsiari CG et al (2001) Effect of infliximab on sight-threatening panuveitis of Behçet’s disease. Lancet 358:295–296
Munoz-Fernandez S, Hidalgo V, Fernandez-Melon J et al (2001) Effect of infliximab on threatening panuveitis in Behçet’s disease. Lancet 358:1644
Triolo G, Vadalà M, Accardo-Palumbo A et al (2002) Anti-tumor necrosis factor monoclonal antibody treatment for ocular Behçet’s disease. Ann Rheum Dis 61:560–561
Niccoli L, Nannini C, Benucci M et al (2007) Long-term efficacy of infliximab in refractory posterior uveitis of Behcet’s disease: a 24-month follow-up study. Rheumatol (Oxford) 46:1161–1164
Cantini F, Niccoli L, Nannini C et al (2012) Efficacy of infliximab in refractory Behçet’s disease-associated and idiopathic posterior segment uveitis: a prospective, follow-up study of 50 patients. Biologics 6:5–12
Okada AA, Goto H, Ohno S et al (2012) Multicenter study of infliximab for refractory uveoretinitis in Behçet disease. Arch Ophthalmol 130:592–598
Iwata S, Saito K, Yamaoka K et al (2009) Effects of anti-TNF-alpha antibody infliximab in refractory entero-Behcet’s disease. Rheumatol (Oxford) 48:1012–1013
Giardina A, Ferrante A, Ciccia F et al (2011) One year study of efficacy and safety of infliximab in the treatment of patients with ocular and neurological Behçet’s disease refractory to standard immunosuppressive drugs. Rheumatol Int 31:33–37
Perra D, Alba MA, Callejas JL et al (2012) Adalimumab for the treatment of Behçet’s disease: experience in 19 patients. Rheumatol (Oxford) 51:1825–1831
Melikoglu M, Fresko I, Mat C et al (2005) Short-term trial of etanercept in Behçet’s disease: a double blind, placebo controlled study. J Rheumatol 32:98–105
van der Horst-Bruinsma IE, Nurmohamed MT (2012) Management and evaluation of extra-articular manifestations in spondyloarthritis. Ther Adv Musculoskelet Dis 4:413–422
Davatchi F, Shams H, Rezaipoor M et al (2010) Rituximab in intractable ocular lesions of Behcet’s disease; randomized single-blind control study (pilot study). Int J Rheum Dis 13:246–252
Schoels MM, van der Heijde D, Breedveld FC et al (2013) Blocking the effects of interleukin-6 in rheumatoid arthritis and other inflammatory rheumatic diseases: systematic literature review and meta-analysis informing a consensus statement. Ann Rheum Dis 72:583–589
Hirohata S, Kikuchi H, Sawada T et al (2012) Clinical characteristics of neuro-Behcet’s disease in Japan: a multicenter retrospective analysis. Mod Rheumatol 22:405–413
Hirano T, Ohguro N, Hohki S et al (2012) A case of Behçet’s disease treated with a humanized anti-interleukin-6 receptor antibody, tocilizumab. Mod Rheumatol 22:298–302
Shapiro LS, Farrell J, Haghighi AB (2012) Tocilizumab treatment for neuro-Behcet’s disease, the first report. Clin Neurol Neurosurg 114:297–298
Urbaniak P, Hasler P, Kretzschmar S (2012) Refractory neuro-Behçet treated by tocilizumab: a case report. Clin Exp Rheumatol 30(3):S73–S75, Suppl 72)
Gül A, Tugal-Tutkun I, Dinarello CA et al (2012) Interleukin-1β-regulating antibody XOMA 052 (gevokizumab) in the treatment of acute exacerbations of resistant uveitis of Behcet’s disease: an open-label pilot study. Ann Rheum Dis 71:563–566
Hatemi G, Silman A, Bang D et al (2008) EULAR recommendations for the management of Behçet’s disease: report of a task force of the European Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 67:1656–1662
Tayer-Shifman OE, Seyahi E, Nowatzky J et al (2012) Major vessel thrombosis in Behçet’s disease: the dilemma of anticoagulant therapy—the approach of rheumatologists from different countries. Clin Exp Rheumatol 30:735–740
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Italia
About this chapter
Cite this chapter
Cantini, F., Di Scala, G. (2014). Old and New Treatment for Behçet’s Disease. In: Emmi, L. (eds) Behçet's Syndrome. Rare Diseases of the Immune System. Springer, Milano. https://doi.org/10.1007/978-88-470-5477-6_19
Download citation
DOI: https://doi.org/10.1007/978-88-470-5477-6_19
Published:
Publisher Name: Springer, Milano
Print ISBN: 978-88-470-5476-9
Online ISBN: 978-88-470-5477-6
eBook Packages: MedicineMedicine (R0)