Abstract
Sarcomas are uncommon malignant tumors and retroperitoneum is the less common site of origin accounting for approximately 10 % of soft tissue sarcomas. RPS present specific therapeutic challenges because of their location and frequent close association with several vital structures in the retroperitoneum. This proximity to major vessels, visceral organs, axial skeleton, and neural structures may significantly impair the ability to perform a margin-negative resection, which is the single potentially curative treatment approach in patients who have localized disease. Even in the setting of a complete resection, local recurrence is common. Because of the typically silent nature of these tumors, most RPS are large when diagnosed (>6 cm). Approximately two-thirds of cases are of high-grade histology, with liposarcomas and leiomyosarcomas representing the most common histologic findings. The initial diagnostic evaluation of patients who are suspected of having RPS should include a contrast enhanced CT of the abdomen and pelvis and MRI of the abdomen. The goal of therapy for most patients is surgical resection with negative margins. Rates of resection of visceral organs at the time of resection of RPS vary significantly by series from approximately 34–75 %. In most centers, radiotherapy is often reserved for patients who have high-grade lesions or in patients in whom a margin-positive resection is anticipated whereas the use of adjuvant chemotherapy in the management of sarcomas at any site to reduce the risk for distant disease failure is also controversial.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Retroperitoneal
- Sarcomas
- Multifocality
- Leiomyosarcomas
- Liposarcomas
- Multifocality
- Abdominoinguinal
- Reimplantation
- Pseudocapsule
- Reccurrent RPS
1 Diagnosis and Evaluation
RPS account for approximately one-third of all retroperitoneal masses. Differential diagnosis, including lymphoma, testicular neoplasm, germ cell tumor, desmoids, functioning and nonfunctioning adrenal masses, renal tumor, pancreatic tumor, and gastrointestinal stromal tumor, should be considered. If visceral invasion is present, the differential should include tumors of these organs and endoscopy with biopsy, if feasible, to evaluate for intraluminal evidence of involvement (e.g., stomach, duodenum, pancreas, colon), should be performed.
History and physical examination should be focused to narrow the differential diagnosis. Testicular examination for masses or/and testicular ultrasound should be considered.
The initial diagnostic evaluation of patients who are suspected of having RPS should include a contrast enhanced CT of the abdomen and pelvis to evaluate the size and extent of the lesion. MRI of the abdomen has been evaluated as a method of staging and may provide an alternative modality to assess local disease extent.
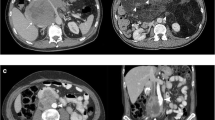
Voros et al. (1998) [1] published that in some cases, satellite tumours have been identified in the surrounding sarcoma’s fat, which may be quite far from the initial tumour and these may be responsible for local recurrence. Some of these are being seen in the preoperative CT scan and some are being revealed at the pathology report. These satellite tumours are small sarcomatous tumours in the surrounding of the main tumour fat, that could be even some centimetres away from it, and who if they remain could be responsible for local recurrence and so we must always search for them either preoperatively or intraoperatively. The MD Anderson study (2009) [2] defines the multifocality in STS as having more than one noncontiguous tumor and that has been considered a feature of more aggressive disease with worse outcome. They also found that a higher number of tumors (>7), is associated with worse overall survival. In one of our cases, a 64-year-old male presented with four different foci at the time of diagnosis. He complained of non typical mild discomfort and swelling of the left inguinal area. He underwent a CT scan which revealed: (a) left iliac fossa mass 11 × 10, 5 × 9 cm, (b) left inguinal mass 6 × 5, 5 × 2, 5 cm, (c) right iliac fossa mass 4.3 cm, and (d) during the operative exploration a big satellite mass was found (Fig. 14.1).
The performance of biopsy for RPS in the preoperative setting is controversial. Percutaneous biopsy is proposed to perform only when the diagnosis may change the preoperative therapy, such as using neoadjuvant for gastrointestinal stromal tumors or primary chemotherapy for lymphoma. If distant metastases are present and surgical therapy is not being considered for primary management, a biopsy of the primary tumor or a metastatic site may be required for alternative therapy to be administered. In the situation of an incidental finding of a RPS during abdominal surgery, a biopsy should be performed before resection to avoid a potentially morbid and unnecessary resection of a chemotherapy sensitive tumor. CT of the chest is typically performed to evaluate for the presence of lung metastases.
2 Management
Disease control for RPS is inferior to those obtained for sarcomas at other locations for a variety of reasons, including the difficulty to obtain wide negative surgical margins, higher rates of unresectability, higher rates of margin-positive resection, and difficulty in delivering adjuvant therapies (e.g., radiotherapy).
Patients who present with a RPS should ideally be evaluated by members of a multidisciplinary sarcoma team and managed at high-volume centers.
3 Surgical Therapy
The goal of therapy for most patients is surgical resection with negative margins. The possibility of a margin negative surgical resection depends on several factors, including invasion of adjacent visceral organs, vascular structures, and skeletal structures. Other operative findings that may impair the outcome of the surgical approach include the presence of peritoneal metastases or the presence of distant metastatic disease.
Complete resection rates vary by series but typically range from 54 to 88 %. Disease control outcomes are relatively close to the completeness of resection in surgical series, with inferior outcomes noted after incomplete resection or margin-positive resection [3].
The surgical approach is important to increase the chance of a margin-negative resection and may differ depending on the location and extent of the tumor. In general, a midline incision followed by medial visceral rotation is performed to provide adequate exposure of the tumor bed and surrounding structures. Ideally, dissection then proceeds outside the limits of the pseudocapsule in an effort to increase the likelihood of obtaining a margin-negative resection, although this may frequently require resection of surrounding vasculature or visceral organs. Other incisions that might help the exploration is the thoracoabdominal and abdominoinguinal incision [3] (Fig. 14.2).
Rates of resection of visceral organs at the time of resection of RPS vary significantly by series from approximately 34–75 %. Because disease control outcomes depend significantly on the adequacy of resection, this approach has also been extended to the setting of vascular involvement highlighting the importance of an aggressive surgical approach. Resection of the tumor and vasculature includes ligation, vessel repair, reimplantation, or bypass grafting in these patients. Morbidity of 36 % and operative mortality of 4 %, compares favorably with other surgical series. Completeness of resection is 60 % and margin-negative resection accomplished in 40 % of patients.
In one pelvic schwannoma of our series, difficult to resect at the initial laparotomy, we performed ischemic embolization and the tumor was completely removed after 1 week (Fig. 14.3). To our knowledge, there are no series in the literature, of ischemic embolization prior to resection for RPS. In the same case, a filter in the IVC was inserted preoperatively to minimize the possibility of pulmonary embolism as the patient already presented with deep vein thrombosis [4] (Fig. 14.4).
4 Multimodality Therapy
Because of the high rates of local recurrence after surgical resection of retroperitoneal sarcomas, especially with high-grade tumors or with margin-positive resection, the addition of radiotherapy has been evaluated to improve local control. Unlike extremity sarcomas, radiotherapy dose is often limited by the anatomic constraints of the abdominal compartment, primarily because of the proximity of several radiosensitive structures to the tumor bed, such as bowel, kidney, and neural structures as well as the extended area to be radiated.
In general, radiotherapy may be delivered before surgery, during surgery, or after surgery with a variety of techniques. These techniques include standard external beam radiation delivered before or after surgery, which typically targets the tumor or tumor bed with additional margin for suspected microscopic disease. In addition, such techniques as brachytherapy and intraoperative radiotherapy can be performed in conjunction with surgical resection and may be delivered in combination with external beam radiotherapy as a method to escalate radiation dose locally.
In most centers, radiotherapy is often reserved for patients who have high-grade lesions or in patients in whom a margin-positive resection is anticipated.
The goal of therapy is to allow a margin-negative resection, which would result in better local control and improved survival. Typically, if external radiotherapy is planned, a preoperative approach is preferred in order to decrease the risk for toxicity, to minimize the additional normal tissue that must be irradiated, and to improve the likelihood of a margin-negative resection.
In regard to chemotherapy, there is similar uncertainty regarding the benefit of chemotherapy delivered in the neoadjuvant setting with the intent of improving resectability and the use should be advocated only in the setting of a clinical trial.
The use of adjuvant chemotherapy in the management of sarcomas at any site to reduce the risk for distant disease failure is also controversial. Several prospective trials evaluating adjuvant chemotherapy in patients who have sarcomas have been completed but have not shown consistent evidence of disease-free survival or overall survival benefit. A final option for multimodality treatment of retroperitoneal sarcomas is the combination of radiation and chemotherapy but the efficacy of this approach has not been reported.
5 Recurrent Disease
Local recurrence is common for retroperitoneal sarcomas, a situation that presents therapeutic challenges. For lesions that are resectable, surgery remains the preferred treatment modality at the time of local recurrence. The likelihood of obtaining a margin-negative resection is significantly lower at the time of local recurrence and at subsequent recurrences. In general, primary resections result in complete resections in as many as 80 % of patients, whereas complete resection of recurrent disease occurs in 57 % of patients, with lower rates at subsequent recurrences [5].
As in the primary disease setting, complete resection of recurrent disease is associated with improved survival, and this should be the intended goal if surgical therapy is chosen.
If adjuvant therapy was not delivered at the time of the initial resection, this remains an option in the recurrent disease setting. Similar to the primary disease setting, options for additional therapy include neoadjuvant or adjuvant radiotherapy and chemotherapy. In the setting of unresectable local recurrence, palliative radiotherapy or chemotherapy may also be considered to relieve local symptoms or to gain other benefits.
6 Follow-Up
In general, patients who have high-grade tumors should undergo frequent clinical and imaging surveillance to be evaluated for recurrent disease.
Surveillance imaging should include CT of the chest, abdomen, and pelvis at intervals of every 3–6 months for the first 2 years for low- and highgrade lesions, followed by biannual evaluations in patients who have high-grade tumors and annual evaluations in patients who have low-grade tumors [6].
References
Voros D, Theodorou D, Ventouri K (1998) Retroperitoneal Tumors: Do the satellite tumors mean something? J Surg Oncol 68:30–33
Anaya D, Lahat G, Liu J (2009) Multifocality in retroperitoneal sarcoma. Aprognostic factor critical to surgical decision-making. Ann Surg 249:137–142
Karakousis C (2010) Refinements of surgical technique in soft tissue sarcomas. J Surg Oncol 101:730–738
Theodosopoulos T, Psychogiou V, Yiallourou A (2012) Management of retroperitoneal sarcomas: main prognostic factors for local recurrence and survival. J BUON 17:138–142
Gholami S, Jacobs CD, Kapp DS (2009) The value of surgery for retroperitoneal sarcoma. Sarcoma 605840. Epub 8
Sogaard AS, Laurberg JM, Sorensen M (2010) Intraabdominal and retroperitoneal soft-tissue sarcomas–outcome of surgical treatment in primary and recurrent tumors. World J Surg Oncol. 12(8):81
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer-Verlag Italia
About this chapter
Cite this chapter
Voros, D.C., Theodosopoulos, T.C. (2014). Clinical Implications of Retroperitoneal Sarcomas. In: Gouliamos, A., Andreou, J., Kosmidis, P. (eds) Imaging in Clinical Oncology. Springer, Milano. https://doi.org/10.1007/978-88-470-5385-4_14
Download citation
DOI: https://doi.org/10.1007/978-88-470-5385-4_14
Published:
Publisher Name: Springer, Milano
Print ISBN: 978-88-470-5384-7
Online ISBN: 978-88-470-5385-4
eBook Packages: MedicineMedicine (R0)