Abstract
Retroperitoneal sarcomas (RPS) are a rare tumor type comprised of various histologies. For localized disease, surgical management is the mainstay of treatment. RPS cases should be referred to high-volume centers with multidisciplinary expertise in order to optimize outcomes. Adjunct treatments including chemotherapy and radiotherapy are areas of debate and under investigation, and enrollment in clinical trials is recommended. Preoperative radiotherapy is the recommended treatment strategy in cases in which radiotherapy is employed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction to Retroperitoneal Sarcoma
Retroperitoneal sarcomas (RPS) are relatively rare tumors, with an average incidence of 2.7 cases per million population [1]. Approximately 10–15% of adult soft tissue sarcomas (STS) arise in the retroperitoneum, the anatomic space in the abdominal cavity posterior to the peritoneal cavity and anterior to the paraspinous musculature. The majority of RPS present with large masses (median size of 15 cm) as they typically produce few symptoms until they are large enough to compress or invade surrounding structures [2]. The most common histologies of RPS in adults include well-differentiated and dedifferentiated liposarcoma and leiomyosarcoma, followed by undifferentiated/unclassified STS [3, 4]. The most common histologies of RPS in children are extraskeletal Ewing sarcoma/primitive neuroendocrine tumor, alveolar rhabdomyosarcoma, and fibrosarcoma [5]. Oncologic outcomes including patterns of spread differ based on the histologic subtype and grade of the tumor. In the future, these differences may impact treatment strategies including the role of neoadjuvant/adjuvant therapy and follow-up surveillance after definitive treatment.
2.2 Historical Outcomes
Studies have demonstrated that aggressive surgical management including a complete surgical resection is one of the most important prognostic factors in localized disease for RPS [6, 7]. In contrast to extremity sarcomas, even with a complete resection, locoregional recurrence is the majority of first recurrences in RPS with approximately 5% per year from time of initial operation [8]. Moreover, local recurrence is the site of first failure in 90% of cases even after complete resection. Distant metastases develop in 20–30% of patients, with an increased risk for those patients with high-grade tumors. Overall five-year survival rates for this disease range from 50 to 70% [6, 9,10,11]. Given these suboptimal outcomes, the role of neoadjuvant/adjuvant treatment including radiotherapy and chemotherapy is a current area of study.
2.3 Management Principles
A thorough workup with multidisciplinary review is necessary to guide treatment decisions (see Table 2.1). A key component to the evaluation of a patient with a retroperitoneal mass includes a complete radiographic evaluation. Preferred diagnostic studies include contrast-enhanced computed tomography (CT) of the chest, abdomen, and pelvis. A chest CT is included as the lung is the most common site of metastasis, and a CT of the abdomen and pelvis helps determine the anatomic relationship of the mass relative to other structures. Magnetic resonance imaging (MRI) of the abdomen and pelvis is helpful for assessing disease in the pelvis and to better assess involvement of the bone or muscle. MRI is better at defining the extent of the local tumor involvement and can be helpful in planning for radiation therapy. Other advanced images such as PET (positron emission tomography) can also be utilized to enhance detection of metastatic disease. Criteria for unresectability include radiographic evidence of peritoneal implants, distant metastases (not potentially resectable for cure), spinal cord involvement, and extensive vascular involvement that cannot be reconstructed. Kidney function workup is necessary in any patient who may receive ipsilateral nephrectomy as part of a surgical resection. Careful evaluation of liver function may also be necessary in selected cases where partial liver resection is recommended.
Example of target volumes for a 58-year-old woman with left retroperitoneal well-differentiated liposarcoma involving the left kidney, adrenal gland, ovary, and mesentery of the duodenum. iGTV in red using 4DCT imaging. CTV in green with a 1.0 cm expansion of iGTV. PTV in red with a 0.5 cm expansion of CTV. These volumes most closely resemble the NRG DT001 protocol
Tissue diagnosis recommendations with image-guided percutaneous core needle biopsy are recommended unless imaging is diagnostic and surgical resection planned first step in treatment. Risk of needle track seeding is minimal and therefore not a reason to avoid a core needle biopsy. If a retroperitoneal mass is found incidentally during surgical exploration for another procedure or it is thought to be an adnexal mass, biopsies should not be done at the time of surgery to avoid contamination of the peritoneal cavity. The patient should have appropriate imaging and then proceed with image-guided core biopsies [12]. Frozen biopsies for diagnostic purposes are not performed as management should be determined after final pathology and discussion at a multidisciplinary tumor board.
Defining the optimal treatment paradigm is difficult given the rarity of RPS and the complexity of treatment. A number of consensus groups comprised of sarcoma experts have recommended that RPS cases should be referred to high-volume centers with multidisciplinary expertise in order to optimize outcomes. However, even among clinical sarcoma experts, there remains equipoise as to the best treatment strategy (see Table 2.2, [12,13,14,15]). Thus, enrollment on clinical trials (to be discussed later) or prospective data registries is advised.
2.4 Surgery
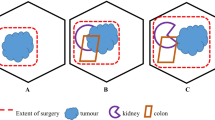
Surgical resection traditionally has been the mainstay curative treatment for localized RPS. The primary treatment for RPS is gross en bloc resection with the goal for a R0 (microscopically negative margins) surgery. Multiple studies have demonstrated that other than histology, the most important prognostic factor for local control and overall survival (OS) is the ability for a complete surgical resection R0 to be performed [3, 4]. While R0 resection may be the primary surgical goal, this is often difficult to achieve due to tumor size and anatomic constraints, and approximately 30–40% of RPS resections are R1 (microscopically positive margins). En bloc resection should include adherent organs to best achieve a negative microscopic resection with a goal of a negative rim of the tissue. This may not always be able to be achieved with critical neurovascular structures. The surgeon must determine the risk versus the benefit of the resection based on the individual patient and tumor characteristics, for instance, if the tumor abuts the liver and pancreas. A multidisciplinary team of surgeons may need to be assembled for their expertise including tumors involving major vascular resection and reconstruction, bone resection, and other visceral organs that may be involved. Once the specimen has been resected, the pathologist must be thoughtful to carefully select the samples of the tissue for pathologic evaluation given that it is generally not feasible to evaluate the entire specimen. Thus, determining true margin status can be challenging and prone to error.
Surgical specimens will often include en bloc removal of nearby organs suspected to be involved by tumor which commonly include the kidney, colon, small bowel, psoas muscle, and in selected cases spleen, pancreas, partial liver, gallbladder, adrenal gland, peritoneum, diaphragm, adnexae, bladder, and other structures. A 2009 retrospective analysis of 382 patients by Bovalot et al. suggested a potential benefit of more aggressive resection described as a systematic resection of noninvolved contiguous organs to ensure wide margins. They reported improved five-year OS rates of 86% versus 66% and 3.29-fold lower rate of abdominal recurrence compared to simple resection of tumor and a correlating three-year abdominal recurrence of ~10% versus 50%. R1 resections resulted in worse locoregional control (49% vs 79% at 3 years) and an OS detriment (54% vs 67% at 5 years) [3]. While other studies have concluded that local control is improved with an R0 resection, whether this translates into a survival benefit is less certain with survival being most strongly associated with grade and histology [16, 17]. Gronchi suggested a similar retrospective pattern of benefits in 288 patients after a shift in institutional surgical approach to systematically remove organs and tissues not clinically involved but located within 1–2 cm of tumor, resulting in an improved five-year local recurrence rate of 48% versus 28% and a statistically nonsignificant improvement of a five-year OS rate 51% versus 61% [18].
Despite the aforementioned results, the extent of surgery remains controversial given the retrospective nature of the above data and potential for patient selection and confounding bias, as well as neglecting rates of reoperation and postoperative complications. In addition, some retrospective data involving less aggressive approaches has demonstrated similar outcomes. In one large series of 675 patients with primary RPS treated at the Memorial Sloan Kettering, 73% of patients had 0–1 organs removed with an R0 rate of 50% and R1 rate of 35%. This translated to a five- and ten-year disease-specific survival of 69% and 55% and a five- and ten-year local recurrence rate of 39% and 45% which was similar to the above series [19]. Bremjit reported comparable outcomes in 132 patients, 30.3% of whom received preoperative RT, whose surgical approach involved only removing contiguous organs when they were grossly involved; 60.5% of patients had 0–1 organs removed. This resulted in 45.5% R0 and 44.7% R1 resections with two-year and five-year OS of 85% and 71% [20].
Appropriate recommendations for surgical extent may partly depend on tumor histology and grade. For example, well-differentiated liposarcoma (WD-LPS) has a high risk of local recurrence but rarely invades other organs and is widely thought to have virtually no capacity for metastasis [21]. Therefore, deferring an aggressive approach in this case may be prudent. MD Anderson retrospectively assessed 83 patients with retroperitoneal WD-LPS, 46% of whom received concomitant organ resections and 54% had no organs removed, collectively achieving a 92% R0/R1 resection rate. Fifteen percent of patients with organs removed showed organ invasion. However, in multivariate analysis, concomitant organ resection was not associated with improved OS or DFS (disease-free survival), and concomitant organ resection was associated with higher complication rates and longer hospital stays [22]. Thus, some experts emphasize the need for histology-guided approach to RPS surgical management [14, 15, 23]. Lastly, the role of debulking surgery (R2 resection) is typically reserved for palliation of large unresectable WD-LPS as gross residual disease and tumor rupture have been suggested as the worst indicators for OS [24].
2.5 Radiotherapy
Radiotherapy in the context of extremity STS is well established and based on multiple prospective randomized trials in the preoperative, intraoperative, and postoperative settings with significant improvement in local control allowing for limb-salvage therapy [25,26,27]. SEER (Epidemiology and End Results) and NCDB (National Cancer Database) analyses have suggested OS benefits in patients with high-grade extremity STS who received radiation therapy [28, 29]. Specifically in RPS, the primary treatment failure after resection is local, highlighting the potential importance of radiotherapy. However, due to lack of prospective randomized data to drive treatment decisions, the role of radiotherapy for RPS remains an area of debate. Currently, in most institutions, multidisciplinary teams with expertise in RPS recommend RT on a case-by-case basis.
There are a number of studies that have performed analyses of cancer registries to determine both practice trends in delivering adjunct radiotherapy and whether radiotherapy is a predictor for improved oncologic outcomes. Recently, Nussbaum and colleagues published the largest NCDB analysis of RPS sarcoma in which they performed a case control, propensity score-matched analysis of patients receiving preoperative or postoperative radiotherapy (PORT) versus surgery alone. Of 9068 patients, 563 patients received preoperative radiotherapy, 2215 received PORT, and 6290 received surgical resection alone. The authors demonstrated that both preoperative radiation therapy and PORT were significantly independent predictors for improved OS (preoperative radiotherapy vs no radiotherapy, 110 vs 66 months, p < 0·0001; and PORT vs no radiotherapy, 89 versus 64 months, p < 0·0001) [30]. Several limitations of the paper included the potential selection bias regarding those who received radiotherapy, lack of data for type of resection, and ability to analyze histologic subtypes separately. In a recent National Cancer Database (NCDB) analysis of a total 2264 patients, 727 (32.1%) of patients had perioperative radiotherapy. Of those who underwent radiotherapy, 27.9% received radiotherapy in the neoadjuvant context. Perioperative radiotherapy was independently associated with decreased mortality (HR 0.72). When stratified, radiotherapy was associated with an OS benefit for high-grade RPS, tumor less than 15 cm, and leiomyosarcoma histology [31]. An analysis of the Multi-Institutional Collaborative Retroperitoneal Sarcoma Working group demonstrated that radiotherapy was a significant independent predictor for local control but did not demonstrate an association with OS [21]. These analyses suggest that while radiotherapy confers a local control benefit, the effect of radiation on OS however is less certain.
Historically, radiotherapy in the postoperative setting has been employed in the setting of positive margin and/or high-risk histologies. The main advantages of this approach include proceeding immediately to surgical resection and the ability to better select patients who require adjuvant treatment due to having the full specimen available for pathologic review. Although many patients undergoing complete surgical resection have microscopically positive margins, there is no high-level evidence that postoperative radiation improves outcomes and retrospective data regarding the benefit of PORT is mixed [2, 32]. Most consensus groups do not favor postoperative RT for RPS for a number of reasons (Table 2.2). Most notably, once the tumor is removed, the bowel can “fall into” the previously occupied space, and postoperative adhesions are formed. This may result in significantly higher volume of fixed bowel (small and large bowel that do not move in and out of the radiation field) being irradiated. Additionally, the appropriate dose in postoperative setting (60–66 Gy) is not tolerable to large volumes in the abdomen and pelvis, and the postoperative target volume may be very difficult to delineate. Thus, risks for treatment-related toxicities are increased with postoperative treatment [33].
While the improved toxicity profile of preoperative radiotherapy in extremity STS has been confirmed in prospective and randomized controlled trial settings [34, 35], this question has not been explored prospectively in the setting of RPS. In contrast to postoperative radiotherapy, there are a number of practical and theoretical advantages in favor of neoadjuvant radiotherapy (see next section for further discussion). We await the final manuscript of STRASS EORTC 62092–22,092 trial to better define the role of preoperative radiotherapy in RPS (see Sect. 2.15 for further discussion).
2.5.1 Intraoperative Radiotherapy and Postoperative Brachytherapy
The delivery of intraoperative radiotherapy (IORT) allows for targeted delivery of radiotherapy boost to high-risk area of positive margins, most commonly delivered with MeV electrons (IOERT (intraoperative electron radiotherapy)). In 1993, the National Cancer Institute published their prospective study demonstrating higher locoregional control 60% versus 20% in patients who underwent a gross total resection for RPS followed by IOERT 20 Gy using 2–6 fields and EBRT (35–40 Gy) compared to postoperative RT (PORT) alone [36]. However, there was no benefit in OS, and 44% of patients who received IOERT developed radiation-related moderate-to-severe peripheral neuropathy compared to 0% in the arm that received PORT alone. More recent retrospective and prospective studies continue to suggest the potential improvement of local control with IOERT using median doses of 12–15 Gy (range 8.75–30), fewer treatment fields, and less field overlap with reduced grade 3–4 toxicities attributed to IOERT alone (see table below) [36,37,38,39,40]. Recently, a newly innovative unidirectional IORT technology (CivaSheet) is used to treat RPS [41]. A multicenter trial is currently planned to treat RPS using this IORT technology in combination with perioperative radiotherapy. Table 2.3 includes published experiences in utilization of IORT in RPS. However, technical challenges and limited availability have prevented the widespread use of IORT or postoperative brachytherapy. Thus, the consensus guidelines recommend IORT to be delivered only at experienced centers and/or on protocol.
Other centers have attempted delivery of additional dose to the high-risk margin with low-dose-rate or high-dose-rate brachytherapy. However, postoperative brachytherapy has been associated with more severe acute and late toxicities in the upper abdomen. In 2002, the Princess Margaret Hospital performed a prospective nonrandomized trial that studied the outcomes of patients treated with preoperative EBRT followed by surgery ± postoperative Ir-192 (iridium-192) brachytherapy. Forty-one patients with localized RPS were treated to a median preoperative dose that was 45 Gy (range 42–50 Gy). No patients required hospitalization and none terminated radiotherapy because of acute toxicity. Twenty-three patients then received postoperative brachytherapy (median dose 25 Gy, range 7.3–30 Gy). Of these, one patient was admitted for duodenitis/gastric outlet obstruction, another patient developed life-threatening small bowel obstruction, and two patients died during treatment due to perforation following NJ (nasojejunal) tube insertions for duodenal stricture, each following brachytherapy in the upper abdomen. The rate of fatal toxicity (2/41, 5%) prompted investigators to limit subsequent use of brachytherapy to the lower abdomen [42].
2.6 Systemic Therapy
To date, the role of systemic therapy in the treatment of localized RPS is very limited and largely extrapolated from retrospective and phase II extremity STS data in which neoadjuvant, adjuvant, or interdigitated chemotherapy with or without concurrent chemoradiotherapy has been utilized in patients with large, high-grade, or locally recurrent disease [43,44,45,46,47,48]. Due to conflicting results of these studies, the use of chemotherapy in localized sarcoma remains controversial and warrants further investigation. When delivered, chemotherapy is generally doxorubicin based with the most widely studied regimes including neoadjuvant mesna, adriamycin (doxorubicin), and ifosfamide (collectively known as MAI) or the above with added dacarbazine (MAID), neoadjuvant and concurrent with radiotherapy (RT) followed by surgery and additional adjuvant cycles.
In RPS, there have been small phase I and II studies assessing similar approaches in patients with histologies at high risk for distant progression such as leiomyosarcomas and undifferentiated pleomorphic sarcomas. Gronchi reported the results of a phase I/phase II trial of 83 patients with localized RPS demonstrating the feasibility of neoadjuvant concomitant chemoradiation with three cycles of high-dose long-infusion ifosfamide and 50.4 Gy RT with 72% of patients completing the protocol and no patients failing to obtain surgery due to toxicity [49]. As noted above, MD Anderson’s phase I trial demonstrated the feasibility of neoadjuvant EBRT up to 50.4 Gy with concurrent doxorubicin followed by definitive surgery and IOERT 12 Gy with high-grade III–IV acute GI toxicity (18%) and hematological toxicity (27%) [39].
A prospective phase II RTOG (Radiation Therapy Oncology Group) trial studying the role of sequential neoadjuvant MAI followed by radiation for intermediate- or high-grade primary or recurrent RPS commenced in 2003, however, was closed early due to lack of accrual (RTOG 0124). NRG-DT001 is an open phase IB trial of neoadjuvant AMG 232 concurrent with preoperative radiotherapy in wild-type p53 STS, discussed below [50]. Without more robust prospective data, routine systemic therapy has not been adopted and should only be performed in the context of a clinical trial or at experienced centers.
2.7 Radiotherapy Techniques and Planning
The large tumor size and complex anatomy of retroperitoneum create a therapeutic challenge in the management of RPS. Delivery of conventional radiotherapy is difficult as the dose required to effectively treat the tumor can exceed the tolerance of the adjacent organs at risk, both in the preoperative and postoperative settings. However, in contrast to postoperative radiotherapy, there are a number of practical and theoretical advantages in favor of preoperative radiotherapy. These include the following:
-
(a)
The gross tumor can be precisely identified and targeted.
-
(b)
The tumor displaces the adjacent abdominopelvic viscera from the high-dose treatment field improving plan dosimetry.
-
(c)
Potential to allow the delivery of higher RT doses.
-
(d)
The clinical target volume (CTG) may be smaller and typically will contain the less normal tissue in the treatment field.
-
(e)
Potential reduction of intraperitoneal tumor dissemination at time of operation.
-
(f)
Increased biological effectiveness in the preoperative setting secondary to better oxygenation with an intact vasculature.
-
(g)
Improved resectability secondary to a “rind” formation of the acellular tissue following radiotherapy.
-
(h)
Potential to convert an initially unresectable tumor to resectable.
Smaller treatment volumes associated with preoperative treatment are thought to correlate with improved toxicity profiles compared to postoperative RT. This is well documented in extremity STS which resulted significantly lower rates of late fibrosis, edema, and joint stiffness resulting in improved long-term functioning though with higher wound complication rates [34, 35]. Multiple prospective and retrospective studies have demonstrated that preoperative radiotherapy in RPS is well tolerated and feasible [31, 39, 49, 51,52,53].
Without prospective data available, the decision to treat should be made on a case-by-case basis by a multidisciplinary team experienced in the treatment of RPS.
Preoperative EBRT is our recommended treatment strategy for patients in whom radiotherapy is recommended. Other techniques including intraoperative radiotherapy (often delivered with MeV electrons), postoperative brachytherapy, postoperative radiotherapy, and proton therapy may have selected roles within experienced institutions and in the context of clinical trials. These treatment options are less available, and their role established through multiple international consensus groups continues to evolve (see Table 2.2).
2.8 CT Simulation
Appropriate CT simulation in RPS (see Table 2.4) enables treatment planning that will maximize target coverage and minimize treatment of critical OARs (organs at risk) such as small bowel, spinal cord, cauda equina, and the contralateral kidney. Motion management with 4DCT (four-dimensional computed tomography) can be considered in any case but is highly recommended for any tumor arising above the pelvic brim. In the rare circumstance of >1 cm motion, respiratory control techniques are recommended as above. IV (intravenous) and PO contrasts at time of simulation are not critical but may facilitate tumor and bowel delineation, respectively. All available diagnostic images should be utilized for target delineation (Table 2.5). Gross tumor is most easily identified on MRI T1 post-contrast sequences. T2 sequences may be useful for identifying suspicious edema that may warrant inclusion in the CTV.
2.9 Target Volumes
There are no universally accepted guidelines to delineating target volumes in preoperative radiotherapy for RPS. Here, we have listed several reasonable approaches created by expert group consensus or defined by ongoing major prospective clinical trial protocols (Tables 2.6, 2.7, and 2.8).
They are all similar in defining GTV (gross target volume) (or iGTV (internal gross target volume)) by CT, MRI, and 4DCT if available. CTV expansion for microscopic disease ranges from 0.5 to 1.5 cm or more if four-dimensional imaging is not available. Two protocols recommend expanding GTV versus iGTV by 3 cm inferiorly when creating the CTV if the inguinal canal is involved. Careful CTV editing is required in each protocol to remove natural anatomic boundaries such as the uninvolved bone, organs, muscle compartments, and intact fascia not at risk for microscopic disease. With daily image-guided radiation therapy (IGRT), two protocols recommend adding 0.5 cm for PTV.
2.10 Prescription Dose
In the absence of strong, prospective, randomized data, appropriate prescription dose is adapted from expert consensus statements and ongoing treatment protocols as above.
Acceptable options include the following:
2.11 Boosts to High-Risk Margin and GTV
EBRT boost to the high-risk margin is under investigation in an attempt to improve local control (see Sect. 2.15). With limited prospective data, we do not recommend routine boosts to either GTV or high-risk margin off clinical protocol.
2.12 Target Coverage
-
At least 95% PTV receives over 95% dose.
-
At least 99–100% CTV receives over 95%.
-
No more than 10% of the PTV receives more than 107% of prescription dose.
2.13 Radiation Technique
Given the close proximity of radiosensitive organs including the small bowel, spinal cord, and cauda equina and the importance of sparing postoperative contralateral kidney function and/or compromised liver function, complex treatment planning is often needed to meet normal tissue constraints. IMRT (intensity-modulated radiation therapy) allows the planner to define the orientation and energies of all beams as in three-dimensional planning. Additionally, specific dose constraints for both normal structures and the target volume are achieved through a technique referred to as inverse planning, which uses specialized optimization algorithms that determine.
nonuniform intensities to tiny beamlets, or subdivisions of beams, resulting in increased control over radiation dose. This allows for the delivery of highly conformal dose to the grouse disease and high-risk subclinical disease regions while minimizing dose to the surrounding critical structures.
In 2003, Koshy and colleagues demonstrated that IMRT in the preoperative setting can be utilized in RPS and enhanced tumor coverage and better sparing of dose to critical normal structures such as the small bowel, liver, and kidney [55]. In RPS, Swanson and colleagues published their dosimetric analysis showing that IMRT (and three-dimensional conformal proton therapy) were more conformal and homogeneous than 3DCRT (three-dimensional conformal radiation therapy). Moreover, this resulted in improved dosimetric benefits [56]. Bossi published results of 16 3DCRT versus IMRT plans that showed superior sparing of high dose to small bowel and the contralateral kidney while maintaining target coverage and other critical constraints [57].
Not only is IMRT superior to 3DCRT in terms of normal structure sparing and improved conformality, but IMRT also can be utilized for dose escalation (or dose painting) to the region at high risk for a positive margin. In 2006, Tzeng and colleagues published their experience treating with preoperative radiation to a dose of 45 Gy in 25 fractions with a simultaneous integrated boost to 57.5 Gy to the margin at risk contoured in conjunction with the operating surgeon. This study demonstrated acceptable acute side effect profile and no severe postoperative morbidity or mortality. A two-year local control in the cohort of 16 patients was high at 80% [58]. In 2017, Washington University reviewed their institutional experience treating RPS with IMRT in perioperative setting. In their cohort of 30 patients, median RT dose to the high-risk area was 55 Gy and 60.4 Gy in the pre- and postoperative setting, respectively. Preoperative RT (compared to postoperative RT) was associated with improved LC. Despite the majority of patients treated in the postoperative setting (19/30) to high doses, there were low incidences of grade 3 toxicity and no grade 4 or 5 toxicity underlying the importance of IMRT treatment technique [59].
Often, delivery of IMRT plans for large RPS can take 20–30 min which can reduce the target uncertainty and OAR dose calculations secondary to intra-fraction motion. Volumetric-modulated arc therapy (VMAT) can overcome this secondary to fast delivery of the treatment. In a dosimetric analysis comparing VMAT to IMRT, Taggar and colleagues demonstrated that VMAT planning for large RPS demonstrated improved conformality index, reducing delivery time with comparable critical structure sparing [60].
Compared to photon radiotherapy, several theoretical advantages of proton beam radiotherapy (PBRT) exist secondary to the physical properties of the proton beam compared to photons. Protons’ energy loss per unit path length is relatively small and constant as it traverses the tissue until near the end of the proton range where the residual energy is lost over a short distance and the proton comes to rest, resulting in a distinctive sharp rise in the tissue absorbed dose known as the Bragg peak. Thus, PBRT offers additional advantages over IMRT and 3DCRT most notably almost no exit dose. Thus, PBRT reduces the radiation of adjacent normal organs and tissues by approximately 60% and allows delivery of the prescription dose to the tumor with greater sparing of adjacent organs and structures. Whether PBRT offers a clinical advantage for any given patient depends on the location of the tumor and the adjacent normal tissues. More recently, the advent of intensity-modulated proton therapy, a highly precise type of radiation therapy allowing intricate treatment planning and precise proton beam delivery, results in modulating the intensity of the beam in order to shape and match the contours of the tumor and minimizing exit dose. Dose escalation utilizing intensity-modulated proton radiotherapy (IMPT) or IMRT is currently the focus of phase I/phase II study (see Sect. 2.15).
2.13.1 Image-Guided Radiation Therapy (IGRT)
Image-guided radiation therapy (IGRT) is a broad term which involves the use of imaging modalities to augment target and normal tissue localization for radiotherapy planning and delivery, by providing opportunities for reviewing and adjusting the treatment delivery taken at the treatment console immediately prior to treatment. As a result of this improved certainty, planning treatment volume (PTV) margins can be reduced. An additional benefit is that the radiation treatment plan can be adapted to reflect anatomical/tumor changes during treatment. RTOG 0630 demonstrated significant reduction of late toxicities with extremity sarcoma with the use of IGRT (both 3DCRT and IMRT allowed) compared to historical cohorts without any marginal-field recurrences at a median follow-up of 3.6 years [35]. Image guidance is especially relevant for RPS as there can be significant setup area in irradiating the retroperitoneum as the immobilization device is not as rigid compared to other sites. Moreover, given the typical close proximity of retroperitoneal tumors to organs at risk for significant acute and long-term toxicity, daily image guidance can ensure that these organs are not falling into the treatment volume on a day-to-day basis. As such, most protocols now recommend daily image-guided radiation therapy for treatment of RPS (see Table 2.9).
2.14 Organs at Risk and Radiation Tolerance Doses
Radiotherapy for patients with RPS is complex secondary to the large treatment fields and proximity to critical anatomic structures. In addition to the potential toxicity to the bowel and liver, other structures at risk for late radiation-related injury include the ureters, kidneys, and spinal cord. Strict adherence to normal structure constraints is essential to reduce acute toxicity to an acceptable level and avoid long-term adverse radiotherapy effects.
2.14.1 DVH (Dose Volume Histogram) Considerations
Dose volume constraints in the setting of RPS have been mainly extrapolated from the gynecology and gastrointestinal (GI) literature. In a recent study quantifying GI toxicity during preoperative radiotherapy for RPS, Mak and colleagues reviewed 56 patient cases with RPS who underwent preoperative RT and found that acute gastrointestinal (GI) toxicity was very low (5% grade ≥ grade 3 toxicity) despite the bowel bag dose exceeding a number of established constraints taken from GI and gynecologic cancers. Tumor size and V25 ≥ 650 mL of bowel bag was significantly associated with grade ≥2 toxicity using RTOG criteria [51]. Further assessment of dose volume constraints specific for treatment of RPS is needed. In Table 2.10, a list of normal structure constraints is listed for treatment of RPS.
2.15 Current Trials
Given the lack of prospective data in RPS sarcoma, we recommend enrollment on clinical trials and/or cancer registries and referral to high-volume center. In this section, we identify three pivotal trials that will help to define the role of neoadjuvant radiotherapy in the treatment of RPS and when employed whether we can improve outcomes with dose escalation or concurrent systemic treatment.
2.15.1 STRASS EORTC 62092-22092 (ClinicalTrials.gov NCT01344018), Ref [54]
This EORTC trial is a multicenter and international phase III trial that enrolled patients with RPS and randomized them to preoperative RT followed by surgery or surgery alone. The studies’ primary endpoint is abdominal recurrence-free survival (ARFS), and secondary endpoints were recurrence-free survival, OS, acute toxicity of RT, perioperative and late complications, and quality of life. The abstract form was presented at ASCO (American Society of Clinical Oncology) in 2019 and were published in Lancet Oncology in 2020. The results failed to demonstrate a benefit in ARFS of preoperative RT for RPS for the entire cohort. However, there were twice as many local recurrences observed in the surgery group than in the radiotherapy plus surgery group. In the liposarcoma subgroup, an exploratory analysis demonstrated an improvement in a three-year ARFS 75.7% versus 65.2% in favor of preoperative radiotherapy [63].
2.15.2 Phase I/Phase II Trial of Preoperative IG-IMPT or IMRT with Simultaneous Integrated Boost (SIB) for Retroperitoneal Sarcomas (ClinicalTrials.gov NCT01659203), Ref [64]
Given the dosimetric benefits of IG-IMPT and IMRT, Delaney and colleagues have sought to determine the role of SIB to high-risk margin determined by the radiation oncologist and operating surgeon. In 2017, Delaney and colleagues published the phase I results of the IG-IMPT cohort in which they utilized preoperative dose of 50.4 Gy in 28 fractions to the CTV1 (gross tumor and adjacent tissues at risk for subclinical risk) with selective escalated radiation dose to tumor volume considered at high risk for positive margins with the aim to reduce local recurrence [65]. See Fig. 2.2 for an example treatment plan of a patient on protocol. Eleven patients showed increased IMPT dose levels from 60.2 to 63.0 GyRBE in 28 fractions utilizing SIB technique. The acute toxicity was mild with no radiation interruptions. There was one patient who developed hydroureter from treatment. At median 18-month follow-up, there were no local recurrences in this cohort, and the phase II study of IMPT is currently accruing patients to that dose. We await this data as well as the results from the phase I IMRT cohort.
Treatment plan of a 75-year-old woman with right retroperitoneal dedifferentiated liposarcoma (15.5 × 14.7 × 18.3 cm) displacing the natural right kidney anteriorly/medially. Patient treated on ongoing Delaney phase I/phase II protocol with preoperative photon IMRT 50.4 Gy/28 fractions with SIB to 61.6 Gy. Treatment delivered with helical tomotherapy system. Following radiation, patient underwent resection of primary tumor as well as the uninvolved right kidney, right adrenal gland, right hemicolon, and diaphragm showing ~50% necrosis and negative surgical margins. No adjuvant treatments. Patient remains disease free 2 years following treatment
Red = GTV
Green = CTV [GTV + 1.5 cm with editing]
Dark blue = PTV 50.4 Gy [includes ITV if available +5–10 mm]
Cyan = PTV SIB 61.6 Gy (area of high risk for + margins)
2.15.3 NRG-DT001 (ClinicalTrials.gov NCT03217266), Ref [50]
NRG-DT001 is a phase IB trial of neoadjuvant AMG 232 concurrent with preoperative radiotherapy in wild-type p53 STS. MDM2 is a selective small molecule inhibitor of MDM2 that blocks the protein-protein interaction between MDM2 and p53. This study was based on strong preclinical evidence suggesting that an MDM2 inhibitor and radiotherapy may have additive or synergistic antitumor activity in p53 WT STS. While this study’s primary objectives are to evaluate the safety and tolerability of this novel agent and to determine the maximum tolerated dose, its secondary objective is to observe and record antitumor activity as well as to determine percentage necrosis and pathologic complete response rate. Other exploratory objectives include determining tumor volume changes via advanced imaging such as MRI and characterize clinical outcomes by genomic biomarkers. This study highlights the potential for novel targeted agents that can improve the therapeutic ratio as well as the utilization of genomics to help prognosticate and eventually to better tailor treatment algorithms.
2.16 Future Directions
We await results from the currently ongoing trials to better help determine the best treatment paradigm for RPS and further improve the therapeutic ratio through novel systemic agents and technological advancements. We understand that retroperitoneal sarcoma represents a rare entity with diverse histologies. The need for histology-driven databases to be utilized to better determine the optimal treatment paradigms for each subtype of retroperitoneal sarcomas is needed. Moreover, the use of molecular profiling via next-generation sequencing may be useful in guiding treatment choices for patients with unresectable or recurrent/metastatic disease. The integration of genomics and radiomics (the process of extracting imaging biomarkers) may allow for outcome modeling and decision support for personalized treatment of RPS. Other EBRT techniques on the horizon including MRI-guided Linac may assist to continue to improve the precision of EBRT treatment delivery and reduce treatment planning target volume margins with the hope to improve outcomes in this difficult disease.
2.17 Treatment Algorithm
A brief treatment algorithm for the treatment of retroperitoneal sarcoma is shown in Fig. 2.3. As described above, enrollment in clinical trials is highly recommended.
2.18 Summary
-
The treatment of RPS is complex, and all patients should be treated in centers with multidisciplinary tumor boards and expertise in the treatment of sarcomas.
-
Surgical resection is the mainstay treatment for localized RPS. However, local recurrence remains the most common site of first failure even after complete resection.
-
The role of radiotherapy is unclear and is currently being investigated.
-
When recommended, radiotherapy is optimally delivered in preoperative setting using image-guided IMRT.
-
Dose escalation with dose painting to the region at high risk for margin positivity is not recommended as standard practice and is best used only as part of a protocol or at experienced centers.
-
IORT/IOERT is best delivered at experienced centers and/or on protocol.
-
The use of systemic therapy in localized setting should only be performed in the context of a clinical trial or at experienced centers.
-
Enrollment on clinical trials or prospective data registries is advised.
References
Porter GA, Baxter NN, Pisters PW. Retroperitoneal sarcoma: a population-based analysis of epidemiology, surgery, and radiotherapy. Cancer. 2006;106(7):1610–6.
Stoeckle E, Coindre JM, Bonvalot S, et al. Prognostic factors in retroperitoneal sarcoma: a multivariate analysis of a series of 165 patients of the French cancer center federation sarcoma group. Cancer. 2001;92(2):359–68.
Bonvalot S, Rivoire M, Castaing M, et al. Primary retroperitoneal sarcomas: a multivariate analysis of surgical factors associated with local control. J Clin Oncol. 2009;27(1):31–7.
Lewis JJ, Leung D, Woodruff JM, Brennan MF. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg. 1998;228(3):355–65.
Pham TH, Iqbal CW, Zarroug AE, Donohue JH, Moir C. Retroperitoneal sarcomas in children: outcomes from an institution. J Pediatr Surg. 2007;42(5):829–33.
Anaya DA, Lev DC, Pollock RE. The role of surgical margin status in retroperitoneal sarcoma. J Surg Oncol. 2008;98(8):607–10.
Kirane A, Crago AM. The importance of surgical margins in retroperitoneal sarcoma. J Surg Oncol. 2016;113(3):270–6.
Heslin MJ, Lewis JJ, Nadler E, et al. Prognostic factors associated with long-term survival for retroperitoneal sarcoma: implications for management. J Clin Oncol. 1997;15(8):2832–9.
Gronchi A, Casali PG, Fiore M, et al. Retroperitoneal soft tissue sarcomas: patterns of recurrence in 167 patients treated at a single institution. Cancer. 2004;100(11):2448–55.
Singer S, Antonescu CR, Riedel E, Brennan MF. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg. 2003;238(3):358–70.
Ferrario T, Karakousis CP. Retroperitoneal sarcomas: grade and survival. Arch Surg. 2003;138(3):248–51.
National Comprehensive Cancer Network (NCCN). NCCN Clinical practice guidelines in oncology. https://www.nccn.org/professionals/physician_gls/default.aspx Accessed 23 April 2019.
ESMO/European Sarcoma Network Working Group. Soft tissue and visceral sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii102–12.
Trans-Atlantic RPS Working Group. Management of primary retroperitoneal sarcoma (RPS) in the adult: a consensus approach from the trans-Atlantic RPS working group. Ann Surg Oncol. 2015;22(1):256–63.
Baldini EH, Wang D, Haas RL, et al. Treatment guidelines for preoperative radiation therapy for retroperitoneal sarcoma: preliminary consensus of an international expert panel. Int J Radiat Oncol Biol Phys. 2015;92(3):602–12.
Schwartz PB, Vande walle K, Winslow ER, et al. Predictors of disease-free and overall survival in retroperitoneal sarcomas: A modern 16-year multi-institutional study from the United States sarcoma collaboration (USSC). Sarcoma. 2019;2019:5395131.
Malinka T, Nebrig M, Klein F, Pratschke J, Bahra M, Andreou A. Analysis of outcomes and predictors of long-term survival following resection for retroperitoneal sarcoma. BMC Surg. 2019;19(1):61.
Gronchi A, Lovullo S, Fiore M, et al. Aggressive surgical policies in a retrospectively reviewed single-institution case series of retroperitoneal soft tissue sarcoma patients. J Clin Oncol. 2009;27(1):24–30.
Tan MC, Brennan MF, Kuk D, et al. Histology-based classification predicts pattern of recurrence and improves risk stratification in primary retroperitoneal sarcoma. Ann Surg. 2016;263(3):593–600.
Bremjit PJ, Jones RL, Chai X, et al. A contemporary large single-institution evaluation of resected retroperitoneal sarcoma. Ann Surg Oncol. 2014;21(7):2150–8.
Gronchi A, Strauss DC, Miceli R, et al. Variability in patterns of recurrence after resection of primary retroperitoneal sarcoma (RPS): a report on 1007 patients from the multi-institutional collaborative RPS working group. Ann Surg. 2016;263(5):1002–9.
Ikoma N, Roland CL, Torres KE, et al. Concomitant organ resection does not improve outcomes in primary retroperitoneal well-differentiated liposarcoma: a retrospective cohort study at a major sarcoma center. J Surg Oncol. 2018;117(6):1188–94.
Fairweather M, Wang J, Jo VY, Baldini EH, Bertagnolli MM, Raut CP. Surgical management of primary retroperitoneal sarcomas: rationale for selective organ resection. Ann Surg Oncol. 2018;25(1):98–106.
Shibata D, Lewis JJ, Leung DH, Brennan MF. Is there a role for incomplete resection in the management of retroperitoneal liposarcomas? J Am Coll Surg. 2001;193(4):373–9.
Yang JC, Chang AE, Baker AR, et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol. 1998;16(1):197–203.
Pisters PW, Harrison LB, Leung DH, Woodruff JM, Casper ES, Brennan MF. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol. 1996;14(3):859–68.
Rosenberg SA, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg. 1982;196(3):305–15.
Koshy M, Rich SE, Mohiuddin MM. Improved survival with radiation therapy in high-grade soft tissue sarcomas of the extremities: a SEER analysis. Int J Radiat Oncol Biol Phys. 2010;77(1):203–9.
Ramey SJ, Yechieli R, Zhao W, et al. Limb-sparing surgery plus radiotherapy results in superior survival: an analysis of patients with high-grade, extremity soft-tissue sarcoma from the NCDB and SEER. Cancer Med. 2018;7(9):4228–39.
Nussbaum DP, Rushing CN, Lane WO, et al. Preoperative or postoperative radiotherapy versus surgery alone for retroperitoneal sarcoma: a case-control, propensity score-matched analysis of a nationwide clinical oncology database. Lancet Oncol. 2016;17(7):966–75.
Leiting JL, Bergquist JR, Hernandez MC, et al. Radiation therapy for retroperitoneal sarcomas: influences of histology, grade, and size. Sarcoma. 2018;2018:7972389.
Catton CN, O'sullivan B, Kotwall C, Cummings B, Hao Y, Fornasier V. Outcome and prognosis in retroperitoneal soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 1994;29(5):1005–10.
Gilbeau L, Kantor G, Stoeckle E, et al. Surgical resection and radiotherapy for primary retroperitoneal soft tissue sarcoma. Radiother Oncol. 2002;65(3):137–43.
O'Sullivan B, Griffin AM, Dickie CI, et al. Phase II study of preoperative image-guided intensity-modulated radiation therapy to reduce wound and combined modality morbidities in lower extremity soft tissue sarcoma. Cancer. 2013;119(10):1878–84.
Wang D, Zhang Q, Eisenberg BL, et al. Significant reduction of late toxicities in patients with extremity sarcoma treated with image-guided radiation therapy to a reduced target volume: results of radiation therapy oncology group RTOG-0630 trial. J Clin Oncol. 2015;33(20):2231–8.
Sindelar WF, Kinsella TJ, Chen PW, et al. Intraoperative radiotherapy in retroperitoneal sarcomas. Final results of a prospective, randomized, clinical trial. Arch Surg. 1993;128(4):402–10.
Gieschen HL, Spiro IJ, Suit HD, et al. Long-term results of intraoperative electron beam radiotherapy for primary and recurrent retroperitoneal soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 2001;50(1):127–31.
Petersen IA, Haddock MG, Donohue JH, et al. Use of intraoperative electron beam radiotherapy in the management of retroperitoneal soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 2002;52(2):469–75.
Pisters PW, Ballo MT, Fenstermacher MJ, et al. Phase I trial of preoperative concurrent doxorubicin and radiation therapy, surgical resection, and intraoperative electron-beam radiation therapy for patients with localized retroperitoneal sarcoma. J Clin Oncol. 2003;21(16):3092–7.
Roeder F, Ulrich A, Habl G, et al. Clinical phase I/II trial to investigate preoperative dose-escalated intensity-modulated radiation therapy (IMRT) and intraoperative radiation therapy (IORT) in patients with retroperitoneal soft tissue sarcoma: interim analysis. BMC Cancer. 2014;14:617.
Zhen H, Turian JV, Sen N, Luu MB, Abrams RA, Wang D. Initial clinical experience using a novel Pd-103 surface applicator for the treatment of retroperitoneal and abdominal wall malignancies. Adv Radiat Oncol. 2018;3(2):216–20.
Jones JJ, Catton CN, O’sullivan B, et al. Initial results of a trial of preoperative external-beam radiation therapy and postoperative brachytherapy for retroperitoneal sarcoma. Ann Surg Oncol. 2002;9(4):346–54.
Gortzak E, Azzarelli A, Buesa J, et al. A randomised phase II study on neo-adjuvant chemotherapy for ‘high-risk’ adult soft-tissue sarcoma. Eur J Cancer. 2001;37(9):1096–103.
Grobmyer SR, Maki RG, Demetri GD, et al. Neo-adjuvant chemotherapy for primary high-grade extremity soft tissue sarcoma. Ann Oncol. 2004;15(11):1667–72.
Mullen JT, Kobayashi W, Wang JJ, et al. Long-term follow-up of patients treated with neoadjuvant chemotherapy and radiotherapy for large, extremity soft tissue sarcomas. Cancer. 2012;118(15):3758–65.
Kraybill WG, Harris J, Spiro IJ, et al. Long-term results of a phase II study of neoadjuvant chemotherapy and radiotherapy in the management of high-risk, high-grade, soft tissue sarcomas of the extremities and body wall: radiation therapy oncology group trial 9514. Cancer. 2010;116(19):4613–21.
Chowdhary M, Sen N, Jeans EB, et al. Neoadjuvant interdigitated chemoradiotherapy using mesna, doxorubicin, and ifosfamide for large, high-grade, soft tissue sarcomas of the extremity: improved efficacy and reduced toxicity. Am J Clin Oncol. 2019;42(1):1–5.
Chowdhary M, Chowdhary A, Sen N, Zaorsky NG, Patel KR, Wang D. Does the addition of chemotherapy to neoadjuvant radiotherapy impact survival in high-risk extremity/trunk soft-tissue sarcoma? Cancer. 2019;125(21):3801–9.
Gronchi A, De paoli A, Dani C, et al. Preoperative chemo-radiation therapy for localised retroperitoneal sarcoma: a phase I-II study from the Italian sarcoma group. Eur J Cancer. 2014;50(4):784–92.
Welliver MX, Wang D, et al. NRG oncology DT001: a phase 1B trial of neoadjuvant AMG 232 concurrent with preoperative radiotherapy in wild-type p53 soft tissue sarcoma. Protocol only. Accessed online ClinicalTrialsgov: NCT03217266.
Mak KS, Phillips JG, Barysauskas CM, et al. Acute gastrointestinal toxicity and bowel bag dose-volume parameters for preoperative radiation therapy for retroperitoneal sarcoma. Pract Radiat Oncol. 2016;6(5):360–6.
Albertsmeier M, Rauch A, Roeder F, et al. External beam radiation therapy for resectable soft tissue sarcoma: a systematic review and meta-analysis. Ann Surg Oncol. 2018;25(3):754–67.
Pawlik TM, Pisters PW, Mikula L, et al. Long-term results of two prospective trials of preoperative external beam radiotherapy for localized intermediate- or high-grade retroperitoneal soft tissue sarcoma. Ann Surg Oncol. 2006;13(4):508–17.
Bonvalot S, Gronchi A, et al. Preoperative radiotherapy plus surgery versus surgery alone for patients with primary retroperitoneal sarcoma (EORTC-62092: STRASS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncology 2020;21(10):1366–77.
Koshy M, Landry JC, Lawson JD, et al. Intensity modulated radiation therapy for retroperitoneal sarcoma: a case for dose escalation and organ at risk toxicity reduction. Sarcoma. 2003;7(3–4):137–48.
Swanson EL, Indelicato DJ, Louis D, et al. Comparison of three-dimensional (3D) conformal proton radiotherapy (RT), 3D conformal photon RT, and intensity-modulated RT for retroperitoneal and intra-abdominal sarcomas. Int J Radiat Oncol Biol Phys. 2012;83(5):1549–57.
Bossi A, De wever I, Van limbergen E, Vanstraelen B. Intensity modulated radiation-therapy for preoperative posterior abdominal wall irradiation of retroperitoneal liposarcomas. Int J Radiat Oncol Biol Phys. 2007;67(1):164–70.
Tzeng CW, Fiveash JB, Popple RA, et al. Preoperative radiation therapy with selective dose escalation to the margin at risk for retroperitoneal sarcoma. Cancer. 2006;107(2):371–9.
Cosper PF, Olsen J, Dewees T, et al. Intensity modulated radiation therapy and surgery for Management of Retroperitoneal Sarcomas: a single-institution experience. Radiat Oncol. 2017;12(1):198.
Taggar AS, Graham D, Kurien E, Gräfe JL. Volumetric-modulated arc therapy versus intensity-modulated radiotherapy for large volume retroperitoneal sarcomas: a comparative analysis of dosimetric and treatment delivery parameters. J Appl Clin Med Phys. 2018;19(1):276–81.
Banerjee R, Chakraborty S, Nygren I, Sinha R. Small bowel dose parameters predicting grade ≥3 acute toxicity in rectal cancer patients treated with neoadjuvant chemoradiation: an independent validation study comparing peritoneal space versus small bowel loop contouring techniques. Int J Radiat Oncol Biol Phys. 2013;85(5):1225–31.
Sanguineti G, Little M, Endres EJ, Sormani MP, Parker BC. Comparison of three strategies to delineate the bowel for whole pelvis IMRT of prostate cancer. Radiother Oncol. 2008;88(1):95–101.
Bonvalot S, Gronchi A, Le Pechoux C, et al. Abstract. STRASS (EORTC 62092): a phase III randomized study of preoperative radiotherapy plus surgery versus surgery alone for patients with retroperitoneal sarcoma. J Clin Oncol. 2019;37(15_suppl):11001.
Delaney TF, Chen YL, Baldini EH, et al. Phase I/II trial of pre-operative image guided intensity modulated proton radiation therapy (IMPT) or photon (IMRT) with simultaneously integrated boost to the high risk margin for retroperitoneal sarcomas. Protocol only Accessed online.
Delaney TF, Chen YL, Baldini EH, et al. Phase I trial of preoperative image guided intensity modulated proton radiation therapy with simultaneously integrated boost to the high risk margin for retroperitoneal sarcomas. Adv Radiat Oncol. 2017;2(1):85–93.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Blumenfeld, P.A., Camden, N.B., O’Donoghue, C., Batus, M., Wang, D. (2022). Retroperitoneal Sarcoma. In: Kim, E., Parvathaneni, U., Welliver, M.X. (eds) Radiation Therapy for Sarcomas and Skin Cancers. Practical Guides in Radiation Oncology. Springer, Cham. https://doi.org/10.1007/978-3-031-06706-8_2
Download citation
DOI: https://doi.org/10.1007/978-3-031-06706-8_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-06705-1
Online ISBN: 978-3-031-06706-8
eBook Packages: MedicineMedicine (R0)