Abstract
Precise and accurate measurement of traits plays an important role in the genetic improvement of crop plants. Therefore, a lot of development has taken place in the area of phenomics in the recent past. Both forward and reverse phenomics have been evolved, which can help in identification of either the best genotype having the desirable traits or mechanism and genes that make a genotype the best. This includes development of high throughput non-invasive imaging technologies including colour imaging for biomass, plant structure, phenology and leaf health (chlorosis, necrosis); near infrared imaging for measuring tissue and soil water contents; far infrared imaging for canopy/leaf temperature; fluorescence imaging for physiological state of photosynthetic machinery; and automated weighing and watering for water usage imposing drought/salinity. These phenomics tools and techniques are paving the way in harnessing the potentiality of genomic resources in genetic improvement of crop plants. These techniques have become much more advanced and have now entered the era of high throughput integrated phenotyping platforms to provide a solution to genomics-enabled improvement and address our need of precise and efficient phenotyping of crop plants.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Introduction
Worldwide demand for crops is increasing rapidly due to rising global population, rising demand for biofuel and feed stocks and changing food preferences. Meeting future demand of agricultural production poses the greatest challenge to agricultural scientists and policy makers (Bruinsma 2003) because demand for cereals, biofuels and feed stocks has already surpassed the current supply and is expected to rise further in the near future (Furbank et al. 2009; Sticklen 2007). Therefore, there is a competition among crops for arable land in order to increase their production. Rising global mean temperature by 0.8 °C since the 1850s, which is expected to increase further by 1.8–4.0 °C by the end of this century, will have further impact on agricultural production due to changing climate (Solomon et al. 2007) and prevalence of abiotic stresses with more intensity and frequencies (Tester and Langridge 2010). It has been estimated that in future average crop yields may decline across Africa and South Asia by 8 % by the 2050s (Knox et al. 2012). These declines in yields have been predicted about 17 % in wheat, 5–16 % in maize, 11–15 % in sorghum, and 10 % in millet across above regions under regimes of climate change (Wheeler and von Braun 2013). Therefore, development of ‘climate-smart’ germplasm would be a priority to tackle these future challenges of climate change (Ziska and Bunce 2007; Leakey et al. 2009).
The use of conventional plant breeding methods has made substantial gain in crop yield worldwide. However, researchers are now observing that current breeding methods will not be sufficient to meet the projected future demand of foods (Furbank et al. 2009; Tester and Langridge 2010; Sticklen 2007). Therefore, this has shifted our focus towards the use of genomics and gene technology advances for assisting the current breeding programs in order to increase grain yields. These developments are being utilized in trait discovery, genetic dissection of complex traits and discovery of associated genes and their deployment in varieties. This has resulted so far in more than 5,000 publications on mapping of quantitative trait loci (QTL) and their isolation during the past years (Zamir 2013). In spite of these efforts, the identified QTLs/genes could not be deployed in mainstream breeding programs because identification of most of these QTLs/genes was not based on the precise and accurate phenotyping data of targeted traits. Hence, association of these QTL/genes with the phenotype in a ‘real world’ environment remains elusive as many false positive QTL have been reported earlier.
Although a large collection of germplasm of different crop species are available worldwide, phenotypic descriptions of these genome wide knockout collections are still limited. As a result, it restricted the use of genomic resources for identifying the allelic variation for a promising candidate gene in natural germplasm collection (see Miyao et al. 2007). The poor utilization of genomic resources could also be due to the lack of analysis of invisible traits and sometimes complex phenotypic effects of genetic modification. Therefore, identification of a candidate germplasm that carries genes for targeted traits is only possible when we will have the precise and accurate phenotyping profile of the germplasm. Phenotyping of valuable agricultural traits such as grain yield, abiotic stress tolerance, and nutritional quality is widely recognized as the most laborious and technically challenging because replicated trials are necessary across multiple environments over a number of seasons. Some of the current phenotyping tools also require destructive harvesting at fixed time intervals or at a particular phenological stage and are slow and costly. These bottlenecks in field phenotyping have driven intense interest over the past decade and hence efforts have been made on development of new high throughput phenotyping tools and techniques such noninvasive imaging, spectroscopy, image analysis, robotics and high-performance computing for phenotyping. These tools can not only be used in laboratories but also in field leading to high-throughput analysis of phenotypes in natural conditions as well as under controlled-environment conditions. Now, field evaluation of plant performance is much faster, and facilitates a more dynamic, whole-of-lifecycle measurement less dependent on periodic destructive assays. The dedicated high throughput controlled-environment facilities have also improved the precision in recording the data and reduce the need for replication in the field. Thus these advances have revolutionized the field of the accurate and precise phenotyping for important traits and bring us to the age of ‘phenomics’ and overview of these developments have been presented in this chapter.
1.2 Origin of Plant Phenotyping
Plant phenotyping has been a part of crop and variety selection since the time of human civilization when humans selected the best individuals of a crop species for domestication (Diamond 1997). Subsequently it has become common practice in plant breeding for selecting the best genotype after studying phenotypic expression in different environmental conditions and also using them in hybridization programs in order to develop new improved genotypes (Pearson et al. 2008; Fisher 1925; Annicchiarico 2002). Ecologists used phenotyping to study phenotypic plasticity of genotypes during the middle of the twentieth century and suggested the role of the genotype and environmental conditions in the expression of plant phenotypes under which it develops (Suzuki et al. 1981). Subsequently, developments in ecology in relation to phenotyping are the trait-based approaches, in which phenotypic characteristics of a wider range of different species are evaluated either in the field (Reich et al. 1992) or under laboratory conditions (Grime and Hunt 1975; Poorter et al. 1990). They were used to derive different strategies by which the ecological niche of species could be described (Grime 1979) and to analyze the interdependence of various traits (Wright et al. 2004).
1.3 Phenomics
The word ‘phenome’ refers to the phenotype as a whole (Soulé 1967) i.e., expression of genome for a trait in a given environment while in phenomics we get high-dimensional phenotypic data on an organism at large scale. Actually phenomics is used as analogy to genomics. However it differs from genomics. In genomics, complete characterization of a genome is possible while in phenomics, complete characterization of phenome is difficult due to the change in the phenotypic expression of traits over the environmental conditions (Houle et al. 2010).
1.4 Phenotype vs Phenomics
Phenotype of a plant can be described on the basis of morphological, biochemical, physiological and molecular characteristics. Different parameters are measured to describe these characteristics. Johannsen (1911) has coined the terms ‘genotype’ and ‘phenotype’. He demonstrated substantial variation in quantitative traits to which he called ‘phenotypical’ in genetically-identical material and thus proved that variation in a given observed traits is not controlled entirely by genetics. Therefore, use of statistical analysis has been suggested for identifying the differences among genotypes because phenotypic variation within a genotype can obscure phenotypic differences among genotypes. This leads to origin of pheno- word. After 1950, ‘phenotyping’ as a noun, ‘to phenotype’ as a verb and ‘phenome’ as the collective noun were introduced, which have been accepted scientifically and are being utilized commonly in literature.
1.5 Forward and Reverse Plant Phenomics
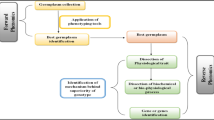
Plant phenomics is the study of plant growth, performance and composition. Figure 1.1 showed the use of forward and reverse phenomics in genetic improvement. Forward phenomics uses phenotyping tools to discriminate the useful germplasm having desirable traits among a collection of germplasm. This leads to identification of the ‘best of the best’ germplasm line or plant variety. Use of high-throughput, fully automated and low resolution followed by higher-resolution screening methods have accelerated plant breeding cycle by screening a large number of plants at seedling stage. Thus interesting traits can be identified rapidly at early stage and there is no need to grow plants up to the maturity stage in field. Now it is possible in forward phenomics to screen thousands of plants in pots running along a conveyor belt, and travelling through a room containing automated imaging systems such as infra-red or 3D cameras. The pots are labelled with barcodes or radio tags, so that the system can identify which pots contain plants with interesting traits. The selected plants can then be grown up to produce seed for further analysis and breeding.
The reverse phenomics is used where the best of the best genotypes having desirable trait(s) is already known. Now through reverse phenomics, traits shown to be of value to reveal mechanistic understanding are dissected in details and subsequently the identified mechanisms are exploited in new approaches. Thus in reverse phenomics, we discover mechanisms which make ‘best’ varieties the best. This can involve reduction of a physiological trait to biochemical or biophysical processes and ultimately a gene or genes. For example, in case of drought tolerance, researchers try to work out the mechanisms underlying the drought tolerance and find out the gene or genes that are responsible for it. These genes are screened in germplasm or the gene can be bred into new varieties.
1.6 Genes and Phenes
To describe phenotype is more challenging than genotype because it changes over the environments. Therefore, the term ‘phenotype’ is not completely straight forward (Mahner and Kary 1997) and it varies among the various sub disciplines of biology. Ecologists traditionally define phenotype as trait when they refer to a phenotypic variable of a plant such as the specific leaf area (SLA). However, some ecologists also refer to traits in relation to characteristics of vegetation, such as the leaf area index (LAI). Like gene, ‘trait’ has been designated as ‘phene’. However it can be over simplification for a one-to-one relationship between gene and phene because one gene can have a range of pleiotropic effects and many genes can control a trait. The term ‘phenome’ is being utilized as a counterpart to ‘genome’. Thus as total constellation of all genes (alleles) present in an individual is known as genome. Therefore, similarly the phenome would be the aggregate of all the expressed traits of an individual. Actually, use of various terminology may overlap as they fulfill various and different needs for different niches of the scientific community. A clear and singular definition throughout the full domain of biology is desirable but probably unreachable (Mahner and Kary 1997).
1.7 Advances in Phenomics
Morphological, physiological and biochemical traits are important to breeders for making genetic improvement for yield, quality and tolerance to biotic and abiotic stresses. These traits have been discussed in details in Chap. 2. Conventionally, phenotyping data on these traits are recorded either visually or manually, which is time-consuming and required a lot of efforts. This also increases chance of errors in measurement of traits. As a result, it increases chance to identify the false positive alleles, which leads to slow gain in genetic improvement. Therefore during the past few years, focus has been shifted on precise, accurate and rapid phenotyping of traits on a large scale. High-throughput phenotyping using non-invasive imaging technologies is a rapidly advancing field (www.plantphenomics.org.au; Furbank et al. 2009; Finkel 2009; Jansen et al. 2009; Berger et al. 2010). These techniques are based on colour imaging for biomass, plant structure, phenology and leaf health (chlorosis, necrosis), near infrared imaging for measuring tissue water content and soil water content, far infrared imaging for canopy/leaf temperature, fluorescence imaging for physiological state of photosynthetic machinery and automated weighing and watering for water usage imposing drought/salinity conditions. These advanced phenotyping techniques have been discussed in details earlier in a number of reviews (see Furbank and Teste 2011; Walter et al. 2012). The genotypes capable of maintaining stomatal conductance under salt induced osmotic stress have been selected successfully at the young seedling stage in wheat and barley using infrared thermography (Sirault et al. 2009). This technique has also been suggested to use for high-throughput seedling screening for drought tolerance in the vegetative stages of crop development and has great potential for low-cost, high-throughput field phenotyping. The genotypes having better photosynthetic capability and higher water use efficiency in field can be screened by measuring the canopy temperature using handheld hermopile based infrared thermometers (i.e. canopy temperature ‘guns’). Chlorophyll fluorescence analysis has been used to test the maintenance of photosynthetic function under biotic and abiotic stresses leading to identification of resistance and susceptible genotypes. For this purpose, a commercial instrument namely pulse amplitude- modulated (PAM) or fluorometry has been developed which is based on fluorescence parameter measured in stress (Baker 2008). It can be used on whole leaves or small plants. It used successfully for abiotic stresses screening in Arabidopsis and tobacco (Nicotiana tabacum) or seedlings of dicots such as canola (Brassica napus) or cotton (Gossypium ssp.) (Baker 2008; Woo et al. 2008). It can also be used to determine projected leaf area and hence the growth rate if measurements are taken regularly over time (Barbagallo et al. 2003). The chlorophyll fluorescence images of the affected area of the leaf allow the early detection of disease symptoms caused by the pathogens. These infected areas can be quantified leading to identification of the susceptible and resistant response to pathogen attack, at least in the case of mildew on barley leaves (Swarbrick et al. 2006; Chaerle et al. 2009). Leaf spectroscopy or hyperspectral reflectance spectroscopy using radiometric or, more recently, imaging sensors are another established optical techniques related to chlorophyll fluorescence, which have been developed to study the stress related phenomics (Jones and Vaughan 2010). However, its use in plant breeding is limited due to difficulties in interpreting canopy temperature data.
Digital imaging is one of the least complicated but useful methods for quantitatively determining the stress tolerance. It is popular approach for in situ crop phenotyping in controlled environment facilities. It uses to take the digital images of growth over a period of plant development and measures quantitative changes in images caused by the sum of stress response mechanisms. In addition to this, taking digital images in visible wavelength regions also give opportunity to identify color of the plants. As a result, it enables to quantify senescence arising due to nutrient deficiencies or toxicities, or pathogen infections. It has been used successfully to quantify toxicity of germanium (as a toxic analogue of boron) in a mapping population of barley (Schnurbusch et al. 2010) and identified a QTL at the same locus as previously identified for boron tolerance using a visual score of symptoms (Jefferies et al. 1999). The attempt was also made to measure the water use efficiency in plants (Harris et al. 2010). Use of non-destructive imaging using fluorescence and hyperspectral reflectance offers great promise in quantitative scoring of such adult plant resistance phenotypes. However use these techniques for screening biotic stresses is still limited.
1.7.1 Development Towards the Phenotyping Machines
ring the past one decade, vast amount of genomic resources have been developed and rapid development in genome sequencing has increased the genomic data bases such as, e.g. GABI DB or TAIR DB in model plant species and crop plants (Meinke et al. 1998; Riano-Pachon et al. 2009; Huala et al. 2001). High throughput genotyping platforms have increased the speed of genotype selection in breeding programs (Langridge and Fleury 2011). However phenotyping for complex traits related to anatomy, morphology, physiology and development is still less advanced, although high-throughput phenotyping techniques have increased our detection ability substantially at subcellular level for protein interactions or metabolism (Houle 2010; Kolukisaoglu and Thurow 2010). For plant breeders, screening component traits contributing to yield under field conditions at large scale is more important for making genetic improvement, but it is still lacking (Furbank and Tester 2011). However significant efforts have been made towards the development of automated phenotyping platforms during the past years (Granier et al. 2006; Jansen et al. 2009; Furbank and Tester 2011; Delseny et al. 2010; see Chap. 18) by taking advantages of throughput phenotyping facilities developed in the field of drug discovery, development, and animal behavior (Mayr and Bojanic 2009; Noldus et al. 2001). In brief, these platforms are equipped with sensor or image based systems under the controlled growth leading to establishment and implementation of the non-destructive imaging approaches for phenotyping (Furbank and Tester 2011; Fiorani et al. 2012). In these platforms, we can measure the plant size and leaf area of large germplasm collections using 2D color images and dense canopy by using 3D image technology and magnetic resonance imaging (MRI) (Poorter et al. 1988; Dornbusch et al. 2012). The fluorescence and hyperspectral analysis allow evaluation of various plant traits in a fast and non-destructive manner to characterize the leaves and roots at physiological or biochemical level. However, only specific aspects of plant functioning can be evaluated in this way. An exciting new development is the robotised sensor-actor for destructive sampling of relevant plant parts has widen the phenotyping capabilities by automated measurement of cellular processes and/or gene expression at specific time points (Alenyà et al. 2012). Relevance of a laboratory and greenhouse phenotyping technique is actually tested in field because traits considered critical in the greenhouse may be less important in the field. For example, the canopy of a stand is more relevant than of a single plant under field conditions. Therefore, mobile platforms such as a tractor equipped with specific sensors enabled larger spatial flexibility have been developed for the mechanistic field phenotyping measurements with high accuracy and repeatability in given plots, while drones or airborne platforms can cover vast agricultural areas. Though multi- and hyperspectral technologies (Rascher and Pieruschka 2008; Comar et al. 2012) can be used to analyze physiological process, only few robust techniques such as the laser-induced fluorescence transient (LIFT) approach are available to estimate photosynthetic efficiency in the field (Pieruschka et al. 2010). Dedicated field sensors are already applied in precision agriculture for nutrient management (Scotford and Miller 2005) and may become important tools for sensing of plant disease in the near future (Mahlein et al. 2012). Establishment of wireless sensor networks enables continuous monitoring of the environment and crop properties and will provide valuable information for agricultural management (Ruiz-Garcia et al. 2009).
1.8 Harnessing the Potentiality of Genomics Through Phenomics
Vast amount of genomic resources are available in public domain but these could not be utilized with their potentially due to the lack of precise, accurate and high throughput phenotyping tools and techniques. Therefore, efforts have been made for the development of high throughput phenotyping tools and techniques for screening of morpho-physiological traits related to biotic and abiotic stresses. The genomic resources developed in a plant species can be linked with physiological and morphological data collected using current phenotyping approaches available at automated phenotyping platforms worldwide. These high throughput phenotyping tools collect the precise and accurate observations and allow analysis of data for understanding the whole phenome of the plant under a wide range of environmental conditions. Thus like genomic platforms, phenotyping platforms develop databases such as the plant meta-phenomics database (Poorter et al. 2010) or the Plant Trait database TRY (http://www.try-db.org, accessed September 2012) which bring together phenotypic responses to the environment for a wide range of plant traits and parameters. These phenotyping database along with available international genomic databases (TAIR, TIGR and NCBI, and with other ‘omics’ information such as metabolomic, proteomic and transcriptomic data) have now become important to understand the genetic architecture of complex traits. Phenomics has not only allowed to dissect the complex traits through genomics but also helped to use genomic resources in discovering new genes/QTL, identification of function of a gene sequence and helped to increase the genetic gain for traits having low heritability (see chap. 17 for details). This understanding will allow us to simulate and predict plant properties in particular of complex traits such as yield or biomass, the most important challenge to address future needs of a growing human population. Both forward and reverse phenomics approaches can be used to harness the potentiality of genomic resources. The accurate, cost-effective, high-throughput phenotyping is pivotal to fine mapping of traits, regardless of the genetic approach for producing allelic recombination or assessing variation by re-sequencing technologies. Phenomics can be used in reverse genetic studies and can help to identify the function of a particular gene(s) in growth and development of crop plants and can be used to identify the allelic variation to target the associated genes (Fig. 1.2).
1.9 Conclusion
For making successful genetic improvement in crop plants, plant breeders first identify the desirable genotypes having target traits by screening a collection of germplasm accessions. These target traits then are combined together through hybridization. This cycle of selection-hybridization-selection has been implementedon the basis of visual observation since domestication of crop plants. Though visual screening is easy and precise for qualitative and highly heritable traits, its use is less precise for quantitative traits and those traits, which are difficult to observe visually (physiological and biochemical traits). Moreover, vast amount of genomic resources have been developed in a number of crop species in the past. The available gene sequences and molecular markers could still not be associated with any traits due to the lack of phenotyping of germplasm collections. For utilizing these genomic resources and identification of desirable plants, the precise phenotyping of germplasm accessions for challenging traits is required in various crop species.
In the recent past, various techniques and methodologies have been developed for screening biotic, abiotic, physiological and biochemical traits in crop plants. These technologies have become very advanced in the era of digital science. These plant phenomics developments are actually helping to make simply plant physiology in ‘new clothes’. Thus this trans-disciplinary approach promises significant new breakthroughs in plant science. Phenomics provides the opportunity to study previously unexplored areas of plant science, and it provides the opportunity to bring together genetics and physiology to reveal the molecular genetic basis of a wide range of previously intractable plant processes. The challenges ahead in plant-based agriculture will require the scale of quantum advances we have seen in information technology in the past 20 years and we need to build on these advances for security of global food, fiber and fuel.
References
Alenyà G, Dellen B, Foix S, Torras C (2012) Leaf segmentation from time-of-flight data for robotized plant probing. IEEE Robot Autom Mag 20:50–59
Annicchiarico P (2002) Genotype × environment interaction: challenges and opportunities for plant breeding and cultivar recommendations. FAO Plant Production and Protection Paper 74, FAO, Rome, pp 132
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113
Barbagallo RP, Oxborough K, Pallett KE, Baker NR (2003) ***Rapid, non-invasive screening for perturbations of metabolism and plant growth using chlorophyll fluorescence imaging. Plant Physiol 132:485–493, 37
Berger B, Parent B, Tester M (2010) High throughput shoot imaging to study drought responses. J Exp Bot 61:3519–3528
Bruinsma J (2003) World agriculture: towards 2015/2030: an FAO perspective. Earthscan, London
Chaerle L, Lenk S, Leinonen I, Jones HG, Van Der Straeten D, Buschmann C (2009) Multi-sensor plant imaging: towards the development of a stress-catalogue. Biotechnol J 4:1152–1167
Comar A, Burger PH, de Solan B, Baret F, Daumard F, Hanocq JF (2012) A semi-automatic system for high throughput phenotyping wheat cultivars in-field conditions: description and first results. Funct Plant Biol 39:914–924
Delseny M, Han B, Hsing YI (2010) High throughput DNA sequencing: the new sequencing revolution. Plant Sci 179:407–422
Diamond J (1997) Guns, germs, and steel: the fates of human societies. Norton and Company, New York
Dornbusch T, Lorrain S, Kuznetsov D, Fortier A, Liechti R, Xenarios I, Fiorani F, Rascher U, Jahnke S, Schurr U (2012) Imaging plants dynamics in heterogenic environments. Curr Opin Biotechnol 23:227–235
Finkel E (2009) With ‘phenomics’ plant scientists hope to shift breeding into overdrive. Science 325:380–381
Fiorani F, Rascher U, Jahnke S, Schurr U (2012) Imaging plants dynamics in heterogenic environments. Curr Opin Biotechnol 23:227–235
Fisher RA (1925) Statistical methods for research workers. Oliver & Boyd, Edinburgh
Furbank RT, Tester M (2011) Phenomics – technologies to relieve the phenotyping bottleneck. Trends Plant Sci 16:635–644
Furbank RT, von Caemmerer S, Sheehy J, Edwards G (2009) C4 rice: a challenge for plant phenomics. Funct Plant Biol 36:845–856
Granier C, Aguirrezabal L, Chenu K, Cookson SJ, Dauzat M, Hamard P, Thioux JJ, Rolland G, Bouchier-Combaud S, Lebaudy A, Muller B, Simonneau T, Tardieu F (2006) PHENOPSIS, an automated platform for reproducible phenotyping of plant responses to soil water deficit in Arabidopsis thaliana permitted the identification of an accession with low sensitivity to soil water deficit. New Phytol 169:623–635
Grime JP (1979) Plant strategies and vegetation processes. Wiley, Chichester
Grime JP, Hunt R (1975) Relative growth rate: its range and adaptive significance in a local flora. J Ecol 63:393–422
Harris BN, Sadras VO, Tester M (2010) A water-centred framework to assess the effects of salinity on the growth and yield of wheat and barley. Plant Soil 336:377–389
Houle D (2010) Numbering the hairs on our heads: the shared challenge and promise of phenomics. PNAS USA 107:1793–1799
Houle D, Govindaraju DR, Omholt S (2010) Phenomics: the next challenge. Nat Rev Genet 11:855–866
Huala E, Dickerman AW, Garcia-Hernandez M, Weems D, Reiser L, LaFond F, Hanley D, Kiphart D, Zhuang M, Huang W, Mueller LA, Bhattacharyya D, Bhaya D, Sobral BW, Beavis W, Meinke DW, Town CD, Somerville C, Rhee SY (2001) The Arabidopsis Information Resource (TAIR): a comprehensive database and web-based information retrieval, analysis, and visualization system for a model plant. Nucleic Acids Res 29:102–105
Jansen M, Gilmer F, Biskup B, Nagel K, Rascher U, Fischbach A, Briem S, Dreissen G, Tittmann S, Braun S, De Jaeger I, Metzlaff M, Schurr U, Scharr H, Walter A (2009) Simultaneous phenotyping of leaf growth and chlorophyll fluorescence via GROWSCREEN FLUORO allows detection of stress tolerance in Arabidopsis thaliana and other rosette plants. Funct Plant Biol 36:902–914
Jefferies SP, Barr AR, Karakousis A, Kretschmer JM, Manning S, Chalmers KJ, Nelson JC, Islam AKMR, Langridge P (1999) Mapping of chromosome regions conferring boron toxicity tolerance in barley (Hordeum vulgare L.). Theor Appl Genet 98:1293–1303
Johannsen W (1911) The genotype conception of heredity. Am Nat 45(531):129–159
Jones HG, Vaughan RA (2010) Remote sensing of vegetation: principles, techniques and applications. Oxford University Press, Oxford
Knox J, Hess T, Daccache A, Wheeler T (2012) Climate change impacts on crop productivity in Africa and South Asia. Environ Res Lett 7:034032
Kolukisaoglu U, Thurow K (2010) Future and frontiers of automated screening in plant sciences. Plant Sci 178:476–484
Langridge P, Fleury D (2011) Making the most of ‘omics’ for crop breeding. Trends Biotechnol 29:33–40
Leakey ADB, Ainsworth EA, Bernacchi CJ, Rogers A, Long SP, Ort DR (2009) Elevated CO2 effects on plant carbon, nitrogen, relations: six important lessons from FACE. J Exp Bot 60:2859–2876
Mahlein AK, Oerke EC, Steiner U, Dehne HW (2012) Recent advances in sensing plant diseases for precision crop protection. Eur J Plant Pathol 133:197–209
Mahner M, Kary M (1997) What exactly are genomes, genotypes and phenotypes? And what about phenomes? J Theor Biol 186:55–63
Mayr LM, Bojanic D (2009) Novel trends in high-throughput screening. Curr Opin Pharmacol 9:580–588. doi:10.1016/j.coph.2009. 08.004
Meinke DW, Cherry JM, Dean C, Rounsley SD, Koornneef M (1998) Arabidopsis thaliana: a model plant for genome analysis. Science 282:662–682
Miyao A, Iwasaki Y, Kitano H, Itoh J, Maekawa M, Murata K, Yatou O, Nagato Y, Hirochika H (2007) A large-scale collection of phenotypic data describing an insertional mutant population to facilitate functional analysis of rice genes. Plant Mol Biol 63:625–635
Noldus LPJJ, Spink AJ, Tegelenbosch RAJ (2001) Etho vision: a versatile video tracking system for automation of behavioral experiments. Behav Res Methods 3:398–414
Pearson CH, Ernst SM, Barbarick KA, Hatfield JL, Peterson GA, Buxton DR (2008) Agronomy Journal turns one hundred. Agron J 100:1–8
Pieruschka R, Klimov D, Kolber Z, Berry JA (2010) Continuous measurements of the effects of cold stress on photochemical efficiency using laser induced fluorescence transient (LIFT) approach. Funct Plant Biol 37:395–402
Poorter H, Pot CS, Lambers H (1988) The effect of an elevated atmospheric CO2 concentration on growth, photosynthesis and respiration of Plantago major. Physiol Plant 73:553–559
Poorter H, Remkes C, Lambers H (1990) Carbon and nitrogen economy of 24 wild species differing in relative growth rate. Plant Physiol 94:621–627
Poorter H, Niinemets Ü, Walter A, Fiorani F, Schurr U (2010) A method to construct dose–response curves for a wide range of environmental factors and plant traits by means of a meta-analysis of phenotypic data. J Exp Bot 61:2043–2055
Rascher U, Pieruschka R (2008) Spatio-temporal variations of photosynthesis: the potential of optical remote sensing to better understand and scale light use efficiency and stresses of plant ecosystems. Precis Agric 9:355–366
Reich PB, Walters MB, Ellsworth DS (1992) Leaf life-span in relation to leaf, plant, and stand characteristics among diverse ecosystems. Ecol Monogr 62:365–392
Riano-Pachon DM, Nagel A, Neigenfind J, Wagner R, Basekow R, Weber E, Mueller-Roeber B, Diehl S, Kersten B (2009) GabiPD: the GABI primary database – plant integrative ‘omics’ database. Nucleic Acids Res 37:D954–D959
Ruiz-Garcia L, Lunadei L, Barreiro P, Robla JI (2009) A review of wireless sensor technologies and applications in agriculture and food industry: state of the art and current trends. Sensors (Basel, Switzerland) 9:4728–4750
Schnurbusch T, Hayes JE, Sutton TJ (2010) Boron toxicity tolerance in wheat and barley: Australian perspectives. Breed Sci 60:297–304
Scotford IM, Miller PCH (2005) Applications of spectral reflectance techniques in northern European cereal production: a review. Biosyst Eng 90:235–250
Sirault XRR, James RA, Furbank RT (2009) A new screening method for osmotic component of salinity tolerance in cereals using infrared thermography. Funct Plant Biol 36:970–977
Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (2007) Climate change 2007: the physical science basis: contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge Univ Press, New York, pp 1–8
Soulé M (1967) Phenetics of natural populations I. Phenetic relationships of insular populations of the side-blotched lizard. Evolution 21:584–591
Sticklen MB (2007) Feedstock crop genetic engineering for alcohol fuels. Crop Sci 47:2238–2248
Suzuki DT, Griffiths AJF, Lewontin RC (1981) An introduction to genetic analysis, 2nd edn. W H Freeman, New York
Swarbrick PJ, Schulze-Lefert P, Scholes JD (2006) The metabolic consequences of susceptibility and the activation of race specific or broad spectrum resistance pathways in barley leaves challenged with the powdery mildew fungus. Plant Cell Environ 29:1061–1076
Tester M, Langridge P (2010) Breeding technologies to increase crop production in a changing world. Science 327:818–822
Wheeler T, von Braun J (2013) Climate change impacts on global food security. Science 341:508–513
Walter A, Studer B, Kolliker R (2012) Advanced phenotyping offers opportunities for improved breeding of forage and turf species. Ann Bot 110:1271–1279
Woo N, Badger MR, Pogson BJ (2008) A rapid non-invasive procedure for quantitative assessment of drought survival using chlorophyll fluorescence. Plant Methods 4:27
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas ML, Niinemets Ü, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Zamir D (2013) Where Have All the Crop Phenotypes Gone? PLoS Biol 11(6): e1001595. doi:10.1371/journal.pbio.1001595
Ziska LH, Bunce JA (2007) Predicting the impact of changing CO2 on crop yields: some thoughts on food. New Phytol 175:607–618
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer India
About this chapter
Cite this chapter
Kumar, J., Pratap, A., Kumar, S. (2015). Plant Phenomics: An Overview. In: Kumar, J., Pratap, A., Kumar, S. (eds) Phenomics in Crop Plants: Trends, Options and Limitations. Springer, New Delhi. https://doi.org/10.1007/978-81-322-2226-2_1
Download citation
DOI: https://doi.org/10.1007/978-81-322-2226-2_1
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-2225-5
Online ISBN: 978-81-322-2226-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)