Abstract
Breast cancer is undoubtedly a dreadful and life-threatening disease. It is fairly common in women and also the second deadliest cancer in the world. It is arguably the most frightening type of cancer because of its well-publicized nature and potential for lethality. If identified and properly treated in its early stage, the chance of cure increases. Different imaging techniques are there which plays a vital role in the detection of breast cancer. In recent days, mammography and thermography are the two main techniques accepted in the medical field to detect breast cancer followed by other screening methods. A literature survey is presented in this paper based on these two techniques followed by the analysis of their affordability, reliability, and outcomes.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
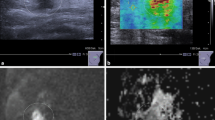
Breast cancer is a disease in which malignant (cancer) cells form in the tissue of the breast. It usually starts off in the inner lining of milk ducts or in the lobules that supply them with milk. A breast cancer that started off in the lobules is known as lobular carcinoma, while one that developed from the ducts is called ductal carcinoma. According to American Cancer Society’s 2002 report on Cancer Facts and Figures [1], breast cancer is the most commonly diagnosed cancer in women and accounts 30 % of all cancers in women. As a cause of cancer death, it is next to lung cancer and also one of the common cancers in India. On the other hand, research [2] has shown that the breast cancer patient has 85 % chance of cure if detected earlier (tumor size less than 10 mm) as opposed to 10 % if detected lately. Usuki et al. [3] have reported that the rate of the growth of tumor is proportionate to its temperature. In the present days, mammography and thermography are the two most widely used methods for the detection of the breast cancer. Mammography is a specific type of imaging that uses a low-dose X-ray system to examine breasts. A mammography exam, called a mammogram, is used to aid in the early detection and diagnosis of breast diseases in women. It is also very useful method for the detection of masses and microcalcifications.
Thermography is a physiologic test that manifests heat patterns and strongly indicates the breast abnormality. Thermal infrared (TIR) imaging is noninvasive, non-ionizing, risk-free, patient-friendly, and the cost is considerably low. These features, together with its early detection capability, have enabled TIR imaging a strong candidate for complementary diagnostic tool to traditional mammography. This paper presents the literature survey of the two techniques—mammography and thermography—and also provides the information of its affordability, reliability, and its outcomes.
The rest of the paper is organized as follows: Sects. 2 and 3 describe the techniques mammography and thermography. Section 4 provides information about the databases presently available and used by the researchers. Section 5 and 6 present the extensive survey of mammography and thermography. The comparative studies between these two techniques are carried out based on different conditions in Sect. 7. And lastly, in Sect. 8, conclusion and future work of this work is discussed.
2 Mammography
Mammography is a special type of X-ray imaging of the breast to find the tumor present in the breast. It uses very low dose of ionizing radiation (usually around 30 kVp) [4, 5]. Early diagnosis of the patient is the successful treatment of breast cancer. Mammography plays a vital role in early detection of breast cancers. According to the US Food and Drug Administration (FDA) [18], mammography can find 85–90 % of breast cancer in women those who are over 50 of age and also can detect a lump up to two years before it can be felt. Once a lump is detected, mammography can be a key in evaluating the lump to determine if it is cancerous or not. While screening, mammography can detect most breast cancer, but it can miss up to 15 % of cancers. These cancers may not be detected on a mammogram film, because of
-
Low differentiation between the appearances of the cancerous tissue compared against the normal parenchymal tissue.
-
Varied morphology of the findings, many of them not related to the cancer.
-
Similarities between the morphologies of the findings.
-
Possible deficiencies in the mammogram acquisition process.
-
Visual fatigue of the radiologist.
3 Thermography
Thermography is a physiologic test that manifests heat patterns and strongly indicates the breast abnormality. The test can detect changes in the breast temperature that indicate breast diseases and abnormalities in the breast. In the last two decades, the breast thermography has achieved an average sensitivity and specificity around 90 % for breast tumor detection [6]. In addition, TIR imaging is a noninvasive, non-ionizing, risk-free, patient-friendly, and the cost is considerably low. These features together with its early detection capability have enabled TIR imaging a strong candidate for complementary diagnostic tool to traditional mammography. Thermography is very useful for detecting non-palpable breast cancer. It cannot be detected by other exams. Because of its non-radiation, non-contact, and low-cost, thermography has clearly manifested to be very valuable. In the field of medical, interest has increased in the application of thermography due to its low equipment cost and advance technologies in camera. As a breast cancer risk assessment tool in the USA, thermography has been approved by the FDA since 1982, as a screening tool for breast cancer. Thermography was first introduced in 1956 and was accepted widely by medical professionals at that time [7]. However, this acceptance rapidly ended in 1977 after a report written by Feig [8] tested the sensitivity of thermography compared to other methods of breast cancer detection. The main reason for the increase of temperature in breast cancer is due to nitric oxide (NO). The breast cancer cells produce this NO [9]. This NO interferes with the normal neuronal control of breast tissue blood vessel flow by causing regional vasodilatation in the early stages of cancerous cell growth and enhancing angiogenesis in later stages [10]. The subsequent increased blood flow in the area causes a temperature increase relative to the normal breast temperature, and even deep breast lesions seem to have the ability to induce changes in skin temperature [11]. Breast cancer metabolic processes may also contribute to the detectable increase in heat. These changes relate to physiological breast processes. It is believed that in healthy individuals, generally, the temperature is symmetrical across the midline of the body [12]. Subjective interpretation of many diagnostic imaging modalities, including infrared thermographic images, relies on the underlying philosophy that normal contralateral images are relatively symmetrical and that small asymmetries may indicate a suspicious lesion [13]. Therefore, in breast cancer, infrared thermography detects disease by identifying areas of asymmetric temperature distribution on the breasts’ surface.
4 Mammography and Thermography Databases
Several databases have commonly been used as test beds for the performance of the two imaging techniques—mammography and thermography—and to compare processing results with others for performance evaluations. Mammographic Image Analysis Society Digital Mammogram Database (MIAS) and Digital Database for Screening Mammography (DDSM) are examples of well-known and broadly used mammographic databases. MIAS database is in .png format with 8-bit images, DDSM database is in LJPEG format. Other examples of databases are computer-assisted library for mammography (CALMa) and Lawrence Livermore National Laboratory (LLNL)/UCSF database and LAPIMO. The images are in the TIFF default format with 12 bits of contrast images, and their spatial resolutions are either 0.085 or 0.150 mm.
The MIAS database contains 322 images with resolutions of 50 and 200 μm/pixel. Only 118 in the database contain some abnormality (66 are benign and 52 are malignant) and the other 204 are diagnosed as normal. The abnormalities found in these mammograms are microcalcifications circumscribed masses, speculated masses ill-defined masses, architectural distortions, and asymmetries. Since there are not many databases of thermography, only DMR is available online for accessing the database of thermogram of breast cancer. DMR or Mastology Research Database is an online platform that stores and manages mastological images for early detection of breast cancer (Table 1).
5 Previous Works on Mammography
Different approaches have been obtained by different researchers to detect breast cancer through mammography. Some of the approaches have been discussed here. One approach followed is based on wavelet transform. The author Laine et al. [14] exploited the orientation and selectivity of the frequency of multiscale wavelet transforms to make mammographic features more obvious through localized contrast gain. This paper is divided into two parts. First part contains a mathematical foundation for an approach to accomplish image contrast enhancement by multiresolution representations of the dyadic wavelet transform. In the second part, an arbitrary region of interest (ROI) of a digital mammogram by Deslauriers–Dubuc interval wavelets is extracted. Image used for the experiment was a digital mammogram of size 400 × 512 and size 512 × 512 containing stellate lesions. Another author Yu and Guan [15] proposed a CAD system consists of two main steps using wavelet transform. In the first step, segmentation of potential of microcalcification is done by using the mixed features, received from the wavelet transform and gray-level statistical analysis. The resultant is labeled into single potential microcalcification objects. In the next step, based on the 31 features, these single potential microcalcification objects are assorted as true or false single microcalcification objects. By using GRNN via SFS ad SBS, the distinguishing power of these mentioned features is also analyzed. This method is used on Nijmegen database of 40 mammograms. The images of this database are digitized at a size of 2,048 × 2,048 by a 12-bit CCD camera (Eikonix 1412). For evaluating the performance, free-response operating characteristics curve (FROC) is used. It is seen that the proposed system gives a satisfactory detection performance after getting the resultant. In the first step when the mixed features are used, 90 % mean true positive detection rate is achieved at a cost of 0.5 false positive per image. In the second step, 15 features are selected by the sequential backward selection method. Next, author Andrew et al. [16] described a method to make an approach for mammographic feature enhancement through wavelet transform. It consists of the application of local and global nonlinear operators within levels of a redundant multiresolution representation. The resultant accents the significant features in mammography which also improved the breast pathology visualization. On the basis of three over complete multiscale representations, this method is described: (1) the dyadic wavelet transform (separable), (2) the -transform (non-separable, non-orthogonal), and (3) the hexagonal wavelet transforms (non-separable). In this study, digitization of the film radiographs of the breast was done by using a sampling distance of 200 μm on a Kodak laser film digitizer, with 10-bit quantization (contrast resolution). Before processing, each digital image was cropped to a matrix size of 512 × 512. Wang and Karanyiannis [17] have also presented an approach to detect microcalcifications in digital mammograms by using wavelet-based sub-band image decomposition. The microcalcification represents the high-frequency components of the image spectrum. Microcalcification is detected by decomposing the mammograms into different frequency sub-band. Then, the low-frequency sub-band is suppressed, and finally, high-frequency containing mammograms from the sub-band are reconstructed. The original mammogram is decomposed into a set of orthogonal sub-band of different resolution and frequency content. The decomposition is based on wavelet analysis filtering and down sampling along the rows and columns of the image. By using statistical analysis alone and combining with Gaussian filter or only by using Gaussian, some work has also been done for the detection of microcalcification. Microcalcification plays an important role in breast cancer. The author Gurcan et al. [18] suggested a method in which the mammogram image is first processed by a sub-band decomposition filter bank. For the detection of microcalcification clusters, analysis of the resultant bandpass sub-image is done. Skewness and kurtosis have been used to locate the locations of microcalcification clusters. At first, the sub-images are divided into overlapping square regions in which the skewness and kurtosis are estimated. When any region has been seen having high positive skewness and kurtosis, then it is considered as a ROI. This method became successful in finding all the critical regions of the affected area. This method is applied over 40 different mammogram images of size 2,048 × 2,048, which constitute all the available images in the Nijmegen database. After using all these parameters in the experiments, all of the 105 microcalcification clusters in the database were detected. It gave an average of 3.3 false alarms for per mammogram image. Another author Hernandez-Cisneros et al. [19] also used the sequential difference of Gaussian filters (DoG) and three evolutionary artificial neural networks (EANNs) to classify the microcalcification clusters in mammograms. It is then compared against a feed-forward artificial neural network (ANN) trained with back propagation. The mammograms used in this project were provided by the Mammographic Image Analysis Society (MIAS). The MIAS database contains 322 images with resolutions of 50 and 200 μm/pixel. Only 118 in the database contain some abnormality (66 are benign and 52 are malignant) and the other 204 are diagnosed as normal. The abnormalities found in these mammograms are microcalcifications, circumscribed masses, speculated masses, ill-defined masses, architectural distortions (20 cases), and asymmetries (17 cases). Mudigonda et al. [20] proposed a method for the detection of masses in mammographic images that have used Gaussian smoothing and sub-sampling operations as preprocessing steps. Segmentation of the mass portion is done by grounding intensity links from the central portion of masses into the surrounding areas. This method is introduced to analyze oriented flow link textural information in mammogram. To classify the detected region as true mass region or false mass region, they proposed some features. These features are based on flow orientation in adaptive ribbons of pixels across the border of masses. The methods were tested with a total of 56 images (each of size 1,024 × 1,024 pixels at a resolution of 200 m) including 30 benign breast masses, 13 malignant, and 13 normal cases selected from the mini MIAS database. The overall detection accuracy is 74 % with a total of 43 cases. Some work has been on segmentation by using isocontour map and inclusion tree. The author Hong and Sohn [21] described a novel method for the segmentation of regions of interest in mammograms. The algorithm for this method describes the boundaries of the breast, the pectoral muscle, and dense regions that include candidate masses. The resulting representation constitutes an analysis of the global structure of the object in the mammogram. They proposed a topographic representation known as isocontour map, in which a salient region forms a dense quasi-concentric pattern of contours. The topological and geometrical structure of the image is analyzed by using an inclusion tree. The “saliency” of the region is measured topologically as the minimum nesting depth. Features at various scales are analyzed in multiscale isocontour maps and demonstrated the multiscale scheme, which provide an efficient way of achieving better delineations. Experimental results demonstrate that the proposed method has potential as the basis for a prompting system in mammogram mass detection. The segmentation algorithm has been applied to 400 mammograms with masses of varying size and subtlety obtained from the University of South Florida (USF) database. The algorithm achieved 100 % detection rate with 3.8 false positives per image and 90 % with 2.3, respectively (Tables 2 and 3).
6 Previous Works on Thermography
In thermography, also various approaches are followed to detect the breast cancer. Some of them are discussed here. An automated approach using asymmetry analysis is one of the methods followed for detection of breast cancer in thermography. Scales et al. [22] tried to outline the fully automated approach that is able to locate the breast regions in the analyzed images successfully. This consists of a sequence of canny edge detectors to determine the body boundaries and to isolate the most likely candidates for the bottom breast boundary. Three different strategies for identifying the bottom breast boundary are investigated: a variation of the Hough transform to identify the curved edges in the image, the algorithm used to detect the longest connected edges that are not part of the body boundary, and a third approach involving the density of detected edges in the breast region. The last two methods show great promise in successfully segmenting the breasts. Qi et al. [23] proposed an automatic approach to asymmetry analysis in thermograms including automatic segmentation and pattern classification. Hough transform is used to extract the four feature curves that can uniquely segment the left and right breasts. These feature curves include the left and the right body boundary curves and the two parabolic curves indicating the lower boundaries of the breasts. Upon segmentation, unsupervised learning technique is applied to classify each segmented pixel into a certain number of clusters. Asymmetric abnormalities have been identified based on pixel distribution within the same cluster. Another approach followed is based on fuzzy logic. Schaefer et al. [24] in their work have taken a different approach to arrive at a compact and effective rule base and applied a genetic algorithm that optimizes the features and parameters of the fuzzy rules. The resulting classification system is more compact and hence faster while maintaining the same good classification performance as is proved by experimental results on a set of nearly 150 cases where they achieved a correct classification rate of about 80 %, which is comparable to other imaging modalities such as mammography. Moghbel and Mashohor [25] in their paper compared different approaches to detect breast cancer. It was based on neural networks and fuzzy systems which have been implemented in different CAD designs. The greatest improvement in CAD systems was achieved with a combination of fuzzy logic and ANN in the form of FALCON-AART complementary learning fuzzy neural network (CLFNN). With a CAD design based on FALCON-AART, it was possible to achieve an overall accuracy of nearly 90 %. Some other approaches are also followed like Qi et al. [26] focused on the discussion of using TIR imaging in early detection of breast cancer. They provided a new method for analyzing a thermal system based on an analogy to electrical circuit theory, referred to as thermal–electric analog. They demonstrated the use of analog to estimate the depth of the heat source and furthermore help to understand the metabolic activities undergoing within the human body. The method has been used in early breast cancer detection and has achieved high sensitivity. Vreugdenburg et al. [27] aimed systematically to identify and evaluate all the available evidence of safety, effectiveness, and diagnostic accuracy for three emerging classes of technology promoted for breast cancer screening and diagnosis: Digital infrared thermal imaging (DITI), electrical impedance scanning (EIS), and elastography. The principal outcome measures were safety, effectiveness, and diagnostic accuracy. Data were extracted using a standardized form and validated for accuracy by the secondary authors. No effectiveness studies were identified. Only one EIS screening accuracy study was identified, while all other studies involved symptomatic populations. Sensitivity and specificity varied greatly for DITI (Sens 0.25–0.97, Spec 0.12–0.85), EIS (Sens 0.26–0.98, Spec 0.08–0.81), and ultrasound elastography (Sens 0.35–1.00, Spec 0.21–0.99). Acharya et al. [28] tried to evaluate the feasibility of using thermal imaging as a potential tool for detecting breast cancer. In this work, they have used 50 IR breast images (25 normal and 25 cancerous). Texture features were extracted from co-occurrence matrix and run length matrix. For automatic classification of normal and malignant breast conditions, these features were fed to the support vector machine (SVM) classifier (Table 4).
7 Comparative Studies of Mammography and Thermography
Some breasts conditions are there which are not visible to the mammography test. Breast thermography can detect it at an early stage. Mammography X-rays use a low energy form of ionizing radiation (around 30 kVp) during screening, but thermography does not use any radiation. Mammography is not capable of detecting breast cancer early enough. Thermography detects cancer in its earlier stages 8–10 years before a mammogram. Thermography detects the tumor at the size of 1.28 cm, whereas mammography can detect it at a size of 1.66 cm. Mammography is less effective than thermography due the density of breast tissue. Thermography is consistently much effective in the detection of active breast disease. Mammography screening is recommended after the age of 40, whereas thermography can be done after the age of 20. Table 5 shows the comparative studies of mammography and thermography.
8 Conclusion
This paper has overviewed the different techniques and comparative studies based on mammography and thermography for the detection of breast cancer. Digital mammography is very widely used for early detection of breast cancer, but due to its use of ionizing radiation, thermography is more acceptable in the field of medical science. Thermography does not use any ionizing radiation and is an excellent management tool for screening purpose. In the future, the next approach will be to have an extensive survey on these techniques and proposing a new method.
References
http://www.cancer.org/downloads/STT/CancerFacts&Figures2002TM.pdf (2002)
Ng, E.Y.K., Sudarshan, N.M.: Numerical computation as a tool to aid the thermographic interpretation. J. Med. Eng. Technol. 25(2), 53–60 (2001)
Usuki, H., Onoda, Y., Kawasaki, S., Misumi, T., Murakami, M., Komatsubara, S., Teramoto, S.: Relationship between thermographic observations of breast tumors and the DNA indices obtained by flow cytometry. Biomed. Thermol. 10(4), 282–285 (1990)
Ng, E.Y.K., Ung, L.N., Ng, F.C., Sim, L.S.J.: Statistical analysis of healthy and malignant breast thermography. J. Med. Eng. Technol. 25(6), 253–263 (2001)
Keyserlingk, J.R., Ahlgren, P.D., Yu, E., Belliveau, N., Yassa, M.: Functional infrared imaging of the breast. IEEE Eng. Med. Biol. 3M1 (2000)
Boyd, N.F., et al.: Mammographic density and breast cancer risk: current understanding and future prospects. Breast Cancer Res. 13(6), 223 (2011)
Feig, S.A.: Role and evaluation of mammography and other imaging methods for breast cancer detection, diagnosis, and staging. In: Seminars in nuclear medicine, vol. 29, no. 1. WB Saunders, Philadelphia (1999)
Kerr, J.: Review of the effectiveness of infrared thermal imaging (thermography) for population screening and diagnostic testing of breast cancer. New Zealand Health Technology Assessment, Department of Public Health and General Practice, Christchurch School of Medicine and Health Sciences (2004)
Anbar, M.: Clinical thermal imaging today. Eng. Med. Biol. Mag. IEEE 17(4), 25–33 (1998)
Snyder, R.L., et al.: Acute spiral ganglion lesions change the tuning and tonotopic organization of cat inferior colliculus neurons. Hear. Res. 147(1), 200–220 (2000)
Ng, E.Y.-K., et al.: Computerized detection of breast cancer with artificial intelligence and thermograms. J. Med. Eng. Technol. 26(4), 152–157 (2002)
Qi, H., Head, J.F.: Asymmetry analysis using automatic segmentation and classification for breast cancer detection in thermograms. Engineering in medicine and biology society, 2001. In: Proceedings of the 23rd Annual International Conference of the IEEE, vol. 3. IEEE (2001)
Laine, A., Jian, F., Wuhai, Y.: Wavelets for contrast enhancement of digital mammography. Eng. Med. Biol. Mag. IEEE 14(5), 536–550 (1995)
Yu, S., Guan, L.: A CAD system for the automatic detection of clustered microcalcifications in digitized mammogram films. IEEE Trans. Med. Imaging 19(2), 115–126 (2000)
Andrew, L., Fan, J., Yang, W.: Wavelets for contrast enhancement of digital mammography. Eng. Med. Biol. Mag. IEEE 14(5), 536–550 (1995)
Wang, T.C., Karayiannis, N.B.: Detection of microcalcifications in digital mammograms using wavelets. IEEE Trans. Med. Imaging 17(4), 498–509 (1998)
Gurcan, M.N., et al.: Detection of microcalcifications in mammograms using higher order statistics. Sig. Process. Lett. IEEE 4(8), 213–216 (1997)
Hernández-Cisneros, R.R., Terashima-Marín, H., Conant-Pablos, S.E.: Detection and classification of microcalcification clusters in mammograms using evolutionary neural networks. In: Advanced Computational Intelligence Paradigms in Healthcare-3, pp. 151–180. Springer, Berlin (2008)
Mudigonda, N.R., Rangayyan, R.M., Leo Desautels, J.E.: Detection of breast masses in mammograms by density slicing and texture flow-field analysis. IEEE Trans. Med. Imaging 20(12), 1215–1227 (2001)
Hong, B.-W., Sohn, B.-S.: Segmentation of regions of interest in mammograms in a topographic approach. IEEE Trans. Inf. Technol. Biomed. 14(1), 129–139 (2010)
Scales, N., Herry, C., Frize, M.: Automated image segmentation for breast analysis using infrared images. In: Wang, T.C. (ed.) Proceedings of the 26th Annual International Conference of the IEEE EMBS, San Francisco
Qi, H., Kuruganti, P.T., Snyder, W.T.: Detecting breast cancer from thermal infrared images by asymmetry analysis. Med. Med. Res. 38 (1995)
Schaefer, G., Nakashima, T., Zavisek, M.: Analysis of breast thermograms based on statistical image features and hybrid fuzzy classification. In: Advances in visual computing, pp. 753–762. Springer, Berlin (2008)
Mehrdad, M., Mashohor, S.: A review of computer assisted detection/diagnosis (CAD) in breast thermography for breast cancer detection. Artif. Intell. Rev. 39(4), 305–313 (2013)
Qi, H., Kuruganti, P.T, Liu, Z.: Early detection of breast cancer using thermal texture maps. In: Proceedings of IEEE International Symposium on Biomedical Imaging 2002. IEEE (2002)
Vreugdenburg, T.D., et al.: A systematic review of elastography, electrical impedance scanning and digital infrared thermography for breast cancer screening and diagnosis. Breast Cancer Res. Treat. 137(3), 665–676 (2013)
Acharya, U.R., et al.: Thermography based breast cancer detection using texture features and support vector machine. J. Med. Syst. 36(3), 1503–1510 (2012)
Acknowledgments
The first author would like to thank Biometrics Laboratory and Biomedical IR Imaging Laboratory, Department of Computer Science and Engineering of Tripura University (A central university) for providing the necessary infrastructure facilities to carry out the work. The research is supported by the grant no. BT/533/NE/TBP/2013 dated 03/03/2014 from Department of Biotechnology (NER Division), Ministry of Science and Technology, Government of India.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer India
About this paper
Cite this paper
Saha, D., Bhowmik, M.K., De, B.K., Bhattacharjee, D. (2015). A Survey on Imaging-based Breast Cancer Detection. In: Das, K., Deep, K., Pant, M., Bansal, J., Nagar, A. (eds) Proceedings of Fourth International Conference on Soft Computing for Problem Solving. Advances in Intelligent Systems and Computing, vol 335. Springer, New Delhi. https://doi.org/10.1007/978-81-322-2217-0_22
Download citation
DOI: https://doi.org/10.1007/978-81-322-2217-0_22
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-2216-3
Online ISBN: 978-81-322-2217-0
eBook Packages: EngineeringEngineering (R0)