Abstract
The Vishwamitri is one of the major rivers of central Gujarat and on its banks evolved the picturesque city of Vadodara. Like any other lotic ecosystem of the modern era, the Vishwamitri too is used as a dumping ground for domestic and industrial wastes. Nevertheless, the river inhabits a sizable population of microfauna and the notable among them is rotifer. The current study was aimed at understanding the factors influencing the structure and dynamics of rotifer community in the river Vishwamitri. Seasonal sampling was done during 2002–2004 from five selected sampling stations that were representing upstream, midstream, and downstream of Vishwamitri. These stations, therefore, varied in their pollution loads. The taxonomic analysis of rotifers revealed the presence of 59 species, belonging to 24 genera and 17 families. The Lecanidae family had the maximum representation with 18 species followed by Brachionidae with 15 species. However, species belonging to Brachionus genus are found as the predominant group, among rotifers, in Vishwamitri. Further, a definite periodicity in the rotifer community was noticed on a temporal scale at all the stations. The species diversity was observed to be highest during the post-monsoon, whereas the least diversity was observed during winter. Analysis for water chemistry followed by suitable statistical analysis revealed that the rotifer community responded differently to various physicochemical cues. Dissolved oxygen, normally a major rate-limiting parameter for aquatic life, was found to have no statistically significant influence in regulating rotifer diversity. The study further revealed that elevated levels of suspended solids and total reactive phosphate have a negative influence on the rotifer diversity. Pearson’s correlation between rotifer diversity and temperature as well as pH revealed that rotifers thrive well in warm alkaline part of the river. In addition nitrate nitrogen and chlorophyll-a levels had a significant positive influence on rotifer community composition. To sum it up in the current study, we observed that water chemistry does influence rotifer community in Vishwamitri River and the prominent among the chemical parameters that influence the rotifer community are pH and chlorophyll-a. The right blend of these abiotic factors together with the presence of aquatic macrophytes makes the upstream sampling stations of Vishwamitri richer in terms of rotifer diversity as compared to their more polluted downstream stations.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Extensive environmental variation is one of the most basic facts of life for any organism living in the tropical water bodies. Among the most notable contributors to this environmental variation are the temperature and chemistry of water. Chemical analysis measures an essential part of the environment, and when closely related with biological study, it greatly enhances its value. Hynes (1978) stated that when the chemist and the biologist both work on the assessment of pollution, they can discover much more than either can alone. Physicochemical analysis of the water is an important aspect from the point of view of aquatic biology. Tebutt (1992) observed that the physicochemical characteristics of water have a direct bearing on the faunal composition of ponds. Lougheed et al. (1998) stated that variability in abiotic factors contributes to seasonal and spatial variability in water quality characteristics and the amount of available habitat and aquatic invertebrates. Yoshinaga et al. (2001) also stated that animal populations live in a diversity of environments, and therefore their population dynamics are regulated by a complex mixture of environmental factors. Zooplankton species succession and spatial distribution is a function of their tolerance to various abiotic and biotic environmental parameters (Marneffe et al. 1998). Most of these factors follow a seasonal pattern of change within an annual cycle. Seasonal variation is clearly driven by climate (Green 2001). Rotifers, due to their high turnover rates, are particularly sensitive to changes in water quality (Sladecek 1983).
Changes in community structure can be explained numerically with diversity index (Kaushik and Saksena 1995). These indices are useful in assessing water quality. Diversity indices are used to characterize species abundance and their relationships in the communities. These mathematical expressions describe the components of community structure, namely, richness (number of species), evenness (uniformity in the distribution of individuals among species), and abundance (total number of organisms), that reveal the response of a community to the quality of its environment (Ludwig and Reynolds 1988). In addition to the changes in the physicochemical composition, interspecific and intraspecific composition, pollution level and the presence or absence of predators are some factors influencing rotifer species composition and structure (Kaushik and Saksena 1995). This chapter discusses the influence of various physicochemical parameters on the rotifer community structure in the various seasons in river Vishwamitri.
Study Area
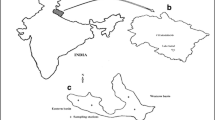
Location and Topography (Fig. 6.1)
Desai and Clarke (1923) in “The Gazette of Baroda” state that the River Vishwamitri takes its origin from the hills of Pavagadh, which is about 43 km away from the Northeast of Vadodara City. Of its total length of about 90 km, it flows for 58 km through Vadodara district. The entire stretch of the river was traced through a reconnaissance survey to select the suitable sampling stations. Considering the short length of the river, five sampling stations were selected in a manner such that two stations were in the clean zone of the river, two in the septic zone, and one in the recovery zone. Later, however, it was realized that no true recovery zone exists for this river. The five sampling stations and their location obtained with the help of a Geographical Positioning System (Garmin, GPS 12XL) are as follows:
Station I: Baska
Position: N – 22° 22.088′; E – 73° 27.079′; Altitude – 104 m.
This is the first upstream station near the foothills of Pavagadh. Here the water exists for most part of the year except in the month of May when the river dries up completely. By the end of June, the monsoon water begins to flow through the riverbed again. The river remains in the flowing condition for about 4 months. By the end of November, the water stops flowing, and slowly and steadily stagnation sets in and the water level begins to reduce till only small pools exist in the river bed till the end of April.
Station II: Haripura
Position: N – 22° 27.026′; E – 73° 19.361′; Altitude – 62 m.
This is the second station in the upstream part of the river. Water exists in here for a very short duration of time. With the onset of the monsoon by mid June, the water begins to flow through the river. This condition remains till the end of October. Later the farmers along the bank begin to use this water for cultivation, and all the water is pumped out of the river, thus causing the river to dry up by the month of January.
Station III: Sama
Position: N – 22° 20.260′; E – 73° 12.301′; Altitude – 46 m.
This station lies within the Vadodara city limits. Water exists at this site throughout the year on account of the sewage water that is released from the city into the river. For most of the year, the water remains flowing except during summer when the water level recedes. This site supports a fairly good population of aquatic vegetation.
Station IV: Munjmahuda
Position: N – 22° 17.093′; E – 73° 10.314′; Altitude – 43 m.
This sites lies at the point where the river begins to exit from the city limits. The water is in a flowing condition throughout the year. River at this point receives high amount of sewage that is evident from the black color and strong distasteful odor the water exudes. The aquatic vegetation is nonexistent at this station.
Station V: Karari
Position: N – 22° 10.755′; E – 73° 08.730′; Altitude – 40 m.
This is the last sampling station and is located outside the city limit, after the river passes through the industrial sector of the city. The water is in a flowing condition throughout the year. This would otherwise have been the recovery zone; however, right up to the very end of the river, wastes are dumped and the river gets no time for recovery.
Methods
Water samples were collected and analyzed for the physicochemical parameters as per the treatise, “Standard Methods for the Examination of Water and Wastewater,” prepared and published jointly by the American Public Health Association (APHA), American Water Works Association (AWWA), and Water Environment Federation (WEF). Sampling was done on five consecutive days in each month during the years 2002–2004. Sample of each day was separately analyzed in the same day, and then the data were pooled to represent the monthly data. Five samples were collected from each site in clean and contamination-free polyethylene containers of two liters volume. They were maintained at 4 °C during transportation to the laboratory in order to reduce the growth of microorganisms. The water samples were collected from the middle of the stream at mid-depth. Stratified random sampling was not possible as the stations were having 1 m or less deep water during major part of the year. The containers were then labeled indicating the sample number, time, and weather conditions.
-
1.
Temperature: Temperature is basically important for its effect on the chemistry and biological reactions of the organisms in water. A rise in temperature of the water leads to the speeding up of the chemical reactions in water and reduces the solubility of gases (Sawyer et al. 1994). In the present study, the ambient as well as the water temperatures were measured at the site using calibrated good grade mercury-filled Celsius thermometer.
-
2.
pH: This is a measure of the intensity of acidity or alkalinity. pH of water gets drastically changed with time due to exposure to air, biological activities, and temperature changes. In natural waters, pH also changes diurnally and seasonally due to variation in photosynthetic activity (Sawyer et al. 1994). Therefore, pH was measured electrometrically using a handheld pH meter.
-
3.
Dissolved oxygen (DO): Dissolved oxygen is one of the most important parameters in water assessment. It reflects the physical and biological processes prevailing in the waters. Its presence is essential to maintain the higher forms of biological life in the water. Organisms have specific requirements of oxygen (APHA et al. 1998). Winkler’s modified method as described in APHA, AWWA (1998) was employed for determining the dissolved oxygen.
-
4.
Total suspended solids (TSS): Water samples were analyzed gravimetrically for TSS (APHA et al. 1998).
-
5.
Chlorophyll-a: The green pigment chlorophyll-a has been reported to be a reliable indicator of phytoplankton biomass (Trivedi and Goel 1986). Chlorophyll was, therefore, extracted in 90 % aqueous acetone and spectrophotometrically analyzed for its concentration as described in APHA, AWWA (1998).
-
6.
Nitrate nitrogen: In the present study, total oxidized nitrogen was estimated using the cadmium reduction method (APHA et al. 1998).
-
7.
Phosphorus: Phosphorous occurs in natural waters and in wastewater almost solely as phosphates. In the current study, the total reactive phosphorus of the water was estimated using the stannous chloride method (APHA et al. 1998).
-
8.
Biological oxygen demand (BOD): The BOD test is widely used to determine the pollution strength of domestic and industrial wastes and is one of the most important in stream pollution control activities. During the present study, the BOD was estimated employing the 5-day BOD test (APHA et al. 1998).
Biological Sampling
Separate samples were collected by filtering a volume of 10 L subsurface water through a plankton net made up of bolting silk cloth No. 20 (the inside width of the meshes was 74 μm). Extreme care was taken to keep water undisturbed at the time of sampling and also to avoid spilling of water from the net. The samples were immediately preserved by adding a few drops of 5 % formalin. The samples were then concentrated to 10 ml by centrifugation. For final analysis, 0.5 ml of sample was taken on Sedgwick-Rafter chambers and rotifers were enumerated and analyzed under a Leica DMRB research microscope.
Results
The rotifer fauna of River Vishwamitri is represented by a total of 59 species belonging to 24 genera and 17 families.
Species Numbers
Station III has the highest number of rotifer species. This station has 40 species (Table 6.1) out of a total of 59, thus harboring about 67.8 % of the total rotifer species. This was followed by station I which has 37 rotifer species (Table 6.2), representing 62.7 % of the rotifer fauna. Next was station II that had a total of 33 species (Table 6.3), thus having about 56 % of the total rotifer species. Station IV and station V had the least number of rotifer species, a total of 12 (Table 6.4) and 10 species (Table 6.5), respectively, thus harboring just about 20.3 % and 16.9 % of the total rotifer fauna of the river.
Exclusive Species
Rotifer species that occurred at a single station have been termed as “exclusive species” in the present study. Twenty rotifer species out of a total of 59 species have been found to occur exclusively at just one particular station. Thus, 33.9 % of the species can be termed as exclusive species or species occurring exclusively at a single station. From the data (Table 6.1), it is evident that station III supports the maximum number of such exclusive species. This station harbors a total of 12 such exclusive species accounting for 63 % of the total exclusive species. This is followed by station I having a total of five exclusive species (Table 6.2), thus accounting for 21 %; station II is next, harboring three exclusive species (Table 6.3) and accounting for 15.8 % of these exclusive species. Sites IV and V do not support any of the exclusive species.
Distribution Pattern of Species
As stated earlier, 20 species out of a total of 59 species are exclusive. Six species of rotifers in River Vishwamitri are common and found at all five stations. Thus, 10.2 % of the species are commonly found at all the stations. Four species are such that they occur at four stations, i.e., 6.8 % of the species can be found in at least four stations. Seven species are such that they occur at only three stations, i.e., 11 % species occur at three stations. Twenty-three species occur at only two stations, thus showing that 38.98 % of the species can be found at only two stations.
Similarities Between Regions
This involved calculating the numbers of species shared by each pair of stations. The general pattern was as expected, in that each site shares the greatest number of species with the closest other region and fewest species with the most remote region (Tables 6.6 and 6.7). For example, station I shares 24 species with the adjoining station II and only eight species with the remote station V. Similarly station II shares 20 species with the next station III but only eight with remote station V.
Unlike the raw figures of shared species quoted above, the similarity indices take account of the total number of species in the regions concerned. The Jaccard index that is used here incorporates total from both of the regions compared. Nonetheless, the index presents a broadly similar picture of faunal resemblance to the shared species and points that the greatest levels of species sharing and family sharing occur between regions that are geographically close together and the smallest levels between regions that are far apart. In other words, whether one uses the numbers of shared species or the Jaccard index, the conclusions on the rotifer faunal similarities are broadly similar (Table 6.7).
Species Diversity
Stations I, II, and III showed reasonably good rotifer species diversity, whereas stations IV and V had lower diversity of rotifers. On the whole, however, the post-monsoon season had the highest diversity of rotifers as indicated by the Shannon-Weiner and Margalef indices (Table 6.8) of species diversity, while lowest diversity was found during the winter months at all stations except IV and V (Table 6.8). Station I recorded the highest diversity in the month of September and the lowest in January. At station II also the months of August and September high rotifer species diversity was observed which, however, began to reduce by October and reached minimum levels in the month of December. Station III showed less variation in the rotifer diversity throughout the year; however, on the whole, the post-monsoon months had the highest levels of rotifer diversity, followed by the month of May. Stations IV and V had very low levels of rotifer diversity as compared to the other three stations.
Physicochemical Parameters
Temperature
The temperature of the river water varied with changes in the ambient temperature at all the stations. As expected the highest values were obtained in the summer months with April having the maximum temperature values (Table 6.9). Station V showed the highest mean summer temperature followed by station IV and then by station I and lastly by station III. The lowest temperature values were recorded during winter and ranged from 13.0 to 19.7 °C (Table 6.9). At all the stations, the month of January recorded the least temperature values. The temperature during the post-monsoon season was moderate and ranged between the summer and the winter values. Station I recorded the lowest mean post-monsoon value, while station IV recorded the highest mean post-monsoon temperature (Table 6.9).
pH
The mean pH values of the river varied between 7.51 and 9.01 during the study period. Station I did not show much variation in the pH levels, the maximum value of 7.89 was obtained during the month of April, while the lowest value was observed in August and September. Likewise at station II, the values varied only between 7.60 in August and September and 7.91 in December. At station III, slight increase in the variation was observed with values ranging from 7.49 to 8.05. The highest pH value of 9.01 was recorded from station IV in the month of May. Here the lowest recorded pH value was 7.59 in the month of September. The pH values at station V ranged from 7.67 in September to 8.98 during May. By and large, the pH values were lower in initial stations and gradually increased downstream. Also the summer values were in general highest and the post-monsoon values were the lowest (Table 6.10).
Dissolved Oxygen
The levels of dissolved oxygen fluctuated from season to season at all the sampling stations. During the sampling period, mean DO values, as low as 0 mg/L to as high as 7.8 mg/L, were obtained (Table 6.11). On the whole, however, stations I, II, and III showed fairly good amount of dissolved oxygen, while stations IV and V had very low levels of oxygen throughout the year (Table 6.11). As expected the dissolved oxygen levels were highest during the winter season at almost all the stations (Table 6.11) except at station V. Station III had the highest mean value (7.8 mg/L) of DO in the month of January. The lowest levels at all the stations were encountered during the hot summer season (Table 6.11). In fact during the month of April, stations IV and V had mean DO levels equal to 0.48 mg/L and 0 mg/L, respectively. Station II had dried up completely by the summer season. Station III showed fairly good amount (3.79 mg/L) of DO even in summer. Though station I had the highest dissolved oxygen values for the early summer season, it dried up by the end of April. Owing to the proper mixing of water during the post-monsoon period, most stations showed reasonably good amount of dissolved oxygen (Table 6.11). In fact highest mean value (1.44 mg/L) of dissolved oxygen for station V was recorded during the post-monsoon season in the month of August (Table 6.11). Similarly the other stations also showed good amount of dissolved oxygen during this period.
The DO values must also be seen in comparison with the BOD values. As the DO values fall, there is a concomitant rise in the BOD levels. This is clearly seen in (Table 6.16). Since biologically degradable organic matter constitutes 7 % of sewage, it has a direct influence on the dissolved oxygen content of the water (Hynes 1978) resulting in the “oxygen-sag curve.” As indicated in Table 6.11, the DO levels fall to such an extent that the river water nearly becomes devoid of any dissolved oxygen in the downstream direction at stations IV and V, causing anoxic conditions.
Total Suspended Solids
Much variation in the levels of TSS was recorded from all the stations (Table 6.12). Stations I and II had relatively low levels of TSS as compared to stations III, IV, and V. The values of TSS increased from station I to station V (Table 6.12). Thus, station I recorded the lowest values of TSS, while the highest values were met with at station V. The post-monsoon season recorded the highest values of suspended solids (Table 6.12) from all the stations. The highest mean value of 685.20 mg/L was recorded from station V in the month of August. The lowest value recorded at this station was in the month of December (Table 6.12). Overall the winter season had the least values of suspended solids (Table 6.12). The mean summer values were just a little higher than the winter values and thus ranged between the winter and the summer levels.
Chlorophyll-a
The chlorophyll-a content varied throughout the study period with the maximum being recorded during the post-monsoon period and the minimum in summer at all the stations (Table 6.13). Stations IV and V showed very low chlorophyll-a content throughout the year, while stations I, II, and II had a good level of chlorophyll-a (Table 6.13). The mean chlorophyll-a values at station IV ranged from 4.20 in April to 21.90 in October (Table 6.13). Similarly the mean chlorophyll-a content at station V ranged from 6.10 in April to 22.10 in October (Table 6.13). Stations I and III showed drastic fluctuations in the level of chlorophyll-a throughout the year (Table 6.13).
Total Reactive Phosphate
Stations I and II showed low levels of total reactive phosphate in comparison to station IV and V (Table 6.14). Station III showed moderate values of total reactive phosphate. Throughout the year, highest values were obtained in summer, while the lowest during the post-monsoon season at all the stations (Table 6.14).
Nitrate Nitrogen
Stations IV and V showed high levels of nitrate nitrogen in comparison to stations I and II. An increase in the level of nitrate nitrogen was observed from station I to station V (Table 6.15). By and large, the highest values were obtained during the post-monsoon season at all the stations (Table 6.15). The winter season showed the lowest values of nitrate nitrogen at all the stations.
Biological Oxygen Demand
Post-monsoon season recorded the lowest values of BOD at all the stations, while summer had the highest values (Table 6.16). The upstream stations I and II had lower BOD values as compared to the downstream stations (Table 6.16). Highest BOD value was recorded at station V in the month of May, while the lowest value was recorded at station I in August.
Discussion
Understanding rotifer community structure and the factors affecting its diversity, abundance, and richness is very complex. Contradictory reports exist on various factors that could be affecting it. Even the question of whether or not seasonality exists in the rotifer community is riddled with contradictions. Pennak (1955) from his observations concluded that there is no seasonal periodicity in North American Rotifers. Wesenberg-Lund (1908, 1930) has shown that these seasonal variations are not very marked in Danish waters. Mengestou et al. (1991) based on their study of rotifer dynamics in Ethiopia did not observe a consistent seasonal pattern or generalized scheme of succession in Rotifers. In a long-term study across four Polish lakes, Steinberg et al. (2009) observed a relatively stable species composition among these lakes within years and within the lakes between years, but they also report variation in the species abundance patterns that seem to be most affected by season. Nayar (1965) based on his study concluded that periodicity of occurrence cannot be assigned to a particular season. However, there are few reports that conclude that rotifers from India follow a marked periodicity. George (1961) attributed a summer periodicity to the rotifers in Delhi waters. Chacko and Rajagopal (1962) found that rotifers were dominant in the month of May and August. Michael (1968) observed different peaks in slightly different periods during his 2-year study period. Dhanapathi (1997) observed a bimodal curve of rotifer abundance from two ponds in Andhra Pradesh. In their study on the seasonal dynamics of rotifers of the river Yamuna in Delhi, Arora and Mehra (2003) reported to no seasonal variation in the species diversity. During the present study, a remarkable alteration in the rotifer community was observed with change in the various seasons. A less obvious change was observed on a monthly basis (Table 6.17), and hence only the seasonal data compiled by combining the monthly data has been discussed here. In the present study, rotifer diversity, richness, and equitability were found to be highest during the post-monsoon season. Similar results were obtained by Fernando and Rajapaksa (1983), who found rotifers in high numbers both during the dry and rainy seasons in tropical lakes. Green (1960) and Duncan and Gulati (1981) found high rotifer numbers during the flushing periods or flood cycle, while Robinson and Robinson (1971) and Burgis (1974) found that rotifer numbers were highest during warm dry months and lowest during the cold period. This is only partially in agreement with the results obtained in the present study wherein too the rotifer numbers in the winter season were low.
The seasonality of rotifers can be ascribed to a number of climatological and biological factors (Mengestou et al. 1991). Herzig (1987) from an intensive study of the Rotifera from temperate lakes observed that some central factors such as physical, chemical limitations, food and mechanical interference, competition, predation, and parasitism regulate rotifer succession. Many studies have been conducted to find the causative factors for the seasonal variations. Studies conducted by Sarma et al. (2011) revealed that a wide range of physicochemical factors influence the seasonal variation in zooplankton abundances including rotifers. Various physicochemical factors have been studied to find the changes, if any, caused by these factors on the rotifer community.
Temperature is one such factor, which is often considered to be the most important, in determining the population dynamics of rotifers (Ruttner-Kolisko 1975; Hofmann 1977). In the present study, it was observed that rotifers were maximum in the post-monsoon season when the temperature was between 24.8 and 25.8 °C. However, when the water temperature increased in summer, in the range of 26.9–27.5 °C, a decrease in the rotifer population was observed. In winter and also when the water temperatures fell drastically, a subsequent decrease in the rotifer population was observed. It may be believed that the rotifers need an optimum temperature for survival, and when the temperature varies from the optimum, the rotifer population decreases drastically. Pejler (1977), Dumont (1983), and De Ridder (1984), however, stated that most species of planktonic rotifers have a global distribution and are characterized by wide temperature tolerances, most of them occurring from close to zero up to about 20 °C or more (Berzins and Pejler 1989). The effects of temperature on zooplankton populations are often linked with biotic effects such as increase in filamentous cyanophytes or predators (Threlkeld 1987). More direct mechanisms include temperature sensitivity of metabolism or life history characteristics (Hebert 1978; Taylor and Mahoney 1988). Temperature has also been positively correlated with zooplankton birth rates and mortality in laboratory experiments (Wolfinbarger 1999). Rotifers are able to reproduce over a wide temperature range, providing that other factors are not limiting. It is, however, difficult to determine the effect of temperature on an individual or population, as temperature influences other processes which in turn affect the rotifers. Additionally, the rate of biological processes is seldom influenced by temperature alone but also by a number of other factors too. It is nearly impossible to separate the direct and indirect effects of the environmental factor of temperature (Galkovskaja 1987). Berzins and Pejler (1989) designated some species, which peaked during the winter months as “winter species” and those that peaked in summer as “summer species.” However, they opined that the range of occurrence is often so wide that it is difficult to designate these as “warm-stenothermal species.” Pejler (1957) suggested that genetic differences could be suspected between populations and geographic areas, where Anuraeopsis fissa and Pompholyx sulcata, for instance, otherwise known as pronounced summer forms, were only found at comparatively low temperatures in northern Swedish Lapland. Berzins and Pejler (1989) found that many non-planktonic species had their peaks at comparatively high temperatures, and this could be because most of them could be periphytic and dependent on macrophytes and their epiphytic flora, which develops during summer. Persuad and Williamson (2005) have observed that changes in underwater UV and temperature can significantly influence the composition of the zooplankton community and ultimately food web dynamics. Thus, it can be that temperature does not solely decide when and where a species will occur. Its influence is mainly indirect, enhancing or retarding development and cooperating with other biotic and abiotic factors.
Another environmental factor that could affect the composition of rotifer community is the pH of water in which they live. According to Hofmann (1977), little is known about its influence on population dynamics of rotifers. However, according to Edmondson (1944) and Skadowsky (1923), pH plays a major role in the distribution of rotifers. In the present study, it was observed that the pH values ranged between 7.51 and 9.01, showing that the pH was alkaline. This observation is in agreement with the observations of Subramanian et al. (1987) who suggested that irrespective of the geology, climate, etc., the pH of Indian River waters is predominantly alkaline. Similar observations have also been made by Somashekar (1988), Venkateswarlu (1986), Bhargava (1985), and Mitra (1982). The pH values were the lowest during the post-monsoon season and ranged between 7.52 and 7.76 at all the stations. This was also the season when the rotifer diversity was at its maximum. During summer, the pH ranged between 7.74 and 8.80, while the rotifer diversity was moderate. Least diversity was seen in the winter months when the pH ranged between 7.70 and 8.35. When pH and rotifer diversity were correlated, a significant negative correlation (Table 6.17) was observed. Moreover, as evidenced from the elevated slope value (Table 6.17), even a slight alteration in the pH may lead to perceivable changes in the rotifer community. Contradictorily, Berzins and Pejler (1987) could not determine any correlation between peak rotifer abundance and pH and stated that rotifers as a group exhibit a very wide range of pH tolerance. They have been found in waters with pH values spanning at least 2.0 units, and many are found in waters, which defer by as much as 5.0 units (Berzins and Pejler 1987). Haque et al. (1988) from their study observed rotifers to be insensitive to pH. However, Green (1960) stated that there could be an optimum pH for the growth and development of a particular species. Supporting this statement, Yin and Nui (2008) demonstrated through their laboratory experiments that pH exerted a major influence on egg viability and growth rate of five closely related rotifer species of the genus Brachious. According to Berzins and Pejler (1987), several species have peak abundances in the acidic range (pH < 6) and thus may be adapted to these conditions. According to Brett (1989), rotifer genera found below pH 3 can also be found in less acidic soft waters. Deneke (2000) found species richness to be generally low in highly acidic environments of pH values 3. His studies also suggested that small littoral or benthic rotifers predominate over crustaceans under highly acidic environments. Wiszniewski (1936) suggested that the most important factor influencing psammic rotifer communities is pH of the lake water. Bielanska-Grajner (2001) observed larger number of rotifer species and their higher abundance in slightly acidic to neutral waters, and the lowest quantity and the number of rotifers were observed in waters with the lowest pH waters among psammic rotifers. On the contrary, Prabhavathy and Sreenivasan (1977), Sampath et al. (1979), and Mishra and Saksena (1998) have shown rotifers dominating in alkaline waters. Finally, it may be stated from the present study that even a slight alteration in pH value will significantly affect the rotifer diversity.
Dhanapathi (2000) stated that dissolved oxygen (DO) plays an important role in determining the occurrence and abundance of rotifer communities. Arora (1966) has shown that dissolved oxygen can influence the survival of rotifers. Nayar (1964) suggested that dissolved oxygen could be an important factor influencing the growth and reproduction of Brachionus calyciflorus. In the present study, it was observed that the rotifer population was at its lowest during the winter season when DO levels were at its maximum. Similarly, Mishra and Saksena (1998) from their studies also found that rotifer numbers were inversely proportional to the dissolved oxygen. Prabhavathy and Sreenivasan (1977) suggested that rotifers are tolerant to low dissolved oxygen values. In the current study, when the dissolved oxygen levels were the lowest in the summer season, the rotifer population was not at its highest, in fact moderate rotifers counts were recorded in this season. Nevertheless, it was observed that River Vishwamitri supports the highest rotifer number during the post-monsoon season when the dissolved oxygen levels were moderate. The findings of the current work are in agreement with that of Zhou et al. (2007), who reported no relationship of dissolved oxygen with the vertical distribution of rotifers in Xiangxi Bay of China. This suggests that there is no direct correlation between the dissolved oxygen levels and rotifer population. However, Green (1956) has shown that dissolved oxygen plays an important role in controlling the growth of zooplankton. Berzins and Pejler (1989) suggested that though some species may be encountered in high abundance at low oxygen values, no true anoxybiosis ought to exist.
One of the effects of high suspended solid levels is increased turbidity. Increased turbidity has been shown to have a variety of influences on biota, affecting characteristics such as ecological conditions, resource availability, and species interaction (Hart 1990). Cottenie et al. (2001) from their study found that differences in zooplankton communities are strongly related to factors such as macroinvertebrate densities and turbidity. In the present study, it was observed that the post-monsoon season had the highest suspended solid levels throughout the river and the rotifers were also present in high. This is in complete agreement with Telesh (1995) who described rotifer diversity to be inversely proportional to transparency in highly turbid waters. Transparency in River Vishwamitri gets highly reduced in the post-monsoon season when the waters carry heavy loads of sediments from the surrounding areas. Telesh (1995) also observed that the contribution of rotifers to total zooplankton biomass was lower in less turbid waters. He described density of rotifers to be highest in the turbid section and low in regions with greater transparency. In the present study, the levels of crustaceans and copepods were low during the post-monsoon season (Suresh et al., unpublished). Thus, predation upon the rotifers is greatly reduced. Threlkeld (1979) also suggested that biotic mechanisms in the seasonal changes of zooplankton assemblages involve changes in predation. Increased turbidity altered predator efficiency, which might indirectly impact zooplankton community dynamics. In fact laboratory experiments illustrated asymmetrical exploitative competition between rotifers and Daphnia, leading to Daphnia dominance in zooplankton community (Gilbert 1985). Hart (1987) reported lower crustacean abundance in years of high turbidity. McCabe and O’ Brien (1983) found Daphnia pulex population growth rates were diminished in the presence of suspended silt. On the other hand, however, Kirk and Gilbert (1990) observed that inorganic turbidity inhibited the competitive abilities of Daphnia and this competitive inhibition may have lead to a decline of cladocerans, causing a competitive lease of rotifer population.
In all, however, it can be seen that stations I, II, and III, which have the highest rotifer diversity, have comparatively low suspended solids in comparison to stations IV and V. Thus, it would not be completely right to believe that the rotifer diversity is directly proportional to the suspended solids. Pollard et al. (1998) observed that turbidity had a minimum role in regulation of zooplankton population. They found that rotifer abundance patterns and species composition as well as rotifer population dynamics were similar at low and high turbidity sites. Contrary to all the above observations, Egborge (1981) observed highest rotifer numbers during periods of high water transparency.
Gulati et al. (1992) indicated that the important factors to be examined for changes in zooplankton composition and abundance are its food and predators. Threlkeld (1979) also suggested that biotic mechanisms in the seasonal changes of zooplankton assemblages involve changes in resource availability. Cecchine and Snell (1999) stated that food limitation may be an important factor in community structuring of rotifers. In oligotrophic systems, declines in cladoceran populations are often associated with decreased total phytoplankton biomass (Sommer et al. 1986). Restrictions associated with lack of optimal food (Pejler 1977) or diverse phytoplankton as food items (Burgis 1974) are known to be the reason for low rotifer diversity in low-latitude lakes (Lewis 1979; Fernando 1980). Rotifers feed on detritus, algae, etc., while some are predatory. In the present study, most of the recorded rotifers are herbivorous or detritivorous, suggesting that the phytoplankton constitute the major source of food. Any changes in the composition of these would lead to subsequent changes in the rotifer community. During the present study, a high positive correlation (Table 6.17) was observed between the chlorophyll-a content and the rotifer diversity. During the post-monsoon season, the chlorophyll-a levels were maximum, as was the rotifer diversity. And the lowest chlorophyll-a levels were encountered in the winter season. The summer months showed a moderate chlorophyll-a level and concomitantly moderate rotifer diversity (Table 6.13). Mishra and Saksena (1998) have also observed a high positive correlation between rotifer number and total phytoplankton population. It is evident from the results that stations I, II, and III have higher chlorophyll-a content as compared to stations IV and V; similarly the rotifer diversity at these stations is also low as compared to stations I, II, and III throughout the year. Yet another reason for low rotifer diversity downstream could be attributed to the fact that the cyanophytes are disproportionately high at these stations (Dhuru et al. 2003). It has been stated that blue-green algae are not edible as they are toxic to rotifers (Fulton and Pearl 1987). Threlkeld (1979, 1986) has attributed the decline in rotifer community in mesotrophic and eutrophic systems, to the replacement of palatable forms of phytoplankton with the less palatable filamentous cyanophytes. Moreover, filamentous cyanophytes, at high densities, are reported to affect the zooplankton adversely by mechanical interference with its filtering mechanism (Webster and Peters 1978; Porter and Orcutt 1980). Apart from food, availability of proper shelter is also an important factor determining the community structure of plankton.
Factors affecting the phytoplankton community would also indirectly affect the rotifer dynamics. In most freshwaters, phosphorous and nitrogen are limiting nutrient for phytoplankton growth (Plath and Boersma 2001). Even in marine waters, zooplankton substantially mediates the recycling of nutrients such as phosphorous and nitrogen that directly influences the phytoplankton therein (Trommer et al. 2012). Phosphate is an important nutrient, which controls plant growth (Hynes 1978). Tebutt (1992) and Dean and Lund (1981) mention that phosphorous occur in sewage effluents due partly to human excretion and partly due to their use in synthetic detergents. Consequently in Vishwamitri River, the values of phosphate increases as sewage gets dumped into the river from station III onwards. This can be seen clearly in Table 6.14, wherein the phosphate values are lowest at station I and gradually increase from there onwards. The highest values are found at station V. This trend is seen during all the seasons. The lowest total reactive phosphate levels were encountered during the post-monsoon season, while the highest values during summer. Thus, it would be expected that phytoplankton diversity and consequently rotifer diversity would be highest in the downstream stations in the summer season. This is, however, not the case. Both the phytoplankton levels (Suresh et al., unpublished) and the rotifer diversity in the downstream stations are low. This could probably be due to the very low dissolved oxygen content in this stretch of the river.
In case of nitrate nitrogen, the highest values are seen at the downstream stations, while low values in the upstream stations (Table 6.15). On basis of the seasons, the highest values are seen during the post-monsoon, while the lowest during the winter season (Table 6.15). Accordingly high rotifer diversity is seen during post-monsoon season and low during winter. However, as far as the stations are concerned where high nitrate nitrogen values are present (downstream stations), the rotifer diversity is not correspondingly high. This could again be attributed to low DO levels at these stations.
Water pollution also affects the rotifer community. Archibald (1972), Verma et al. (1984), and Kulshreshtra et al. (1989) observed that the species diversity is high in clean waters and low in polluted waters. Banerjea and Motwani (1960) reported an appreciable fall in the rotifer species just below the effluent outfall and further reduction in the septic zone of Suvaon stream. However, Prabhavathy and Sreenivasan (1977), Gannon and Stemberger (1978), Sampath et al. (1979), and Mishra and Saksena (1998) found that rotifer population was enhanced by increased load of pollution. Similarly Venkateswarlu and Jayanti (1968) recorded high counts of rotifers at polluted stations of Sabarmati River in comparison to clean stations. In River Vishwamitri, the sewage pollution begins from station III, and as is evident from the data, this station on the whole has a greater diversity of rotifers throughout the year. However, towards station IV and station V, the pollution load increases drastically as evidenced by the biological oxygen demand values (Table 6.16), and the dissolved oxygen levels are too low to support many organisms. At these sites, the suspended solid levels are also very high which greatly reduces the transparency. This would in turn affect the light penetration required by the primary producers. All these factors combined probably account for the low diversity at these stations.
Apart from the physicochemical factors, biotic factors might also play an important role in controlling the zooplankton community structure. The presence or absence of predators also affects the rotifer populations. The negative relation between the presence of Daphnia and rotifers has been well documented (Fussmann 1996). As already discussed earlier during the post-monsoon period, the cladoceran density is quite low, probably affected by the high levels of suspended solids, as the result of which the rotifers are found in high numbers.
Presence of macrophytes also affects the zooplankton diversity. Lougheed et al. (1998) stated that patchy distribution of aquatic vegetation contributes to seasonal variability in water quality characteristics and the amount of habitat available for aquatic invertebrates. Development of vegetation increases structural complexity, so providing more niches for rotifers. In a large body with a complex littoral zone, the numbers of rotifer species can reach over 200 (Segers and Dumont 1995; Dumont and Segers 1996). The macrophytes provide more diverse habitat (Van den Berg et al. 1997). This was well observed by Kuczyriska-Kippen (2007) that shallow lakes with a good macrophyte density offered a wide choice of habitat for the rotifer community, thus enhancing their diversity and density in such habitats.
In River Vishwamitri, macrophytes are present in highest numbers at station III followed by station II and station I. Station IV and station V have negligible macrophyte population (Dhuru et al., 2003). This could be yet another reason for higher diversity in the first three stations. Telesh (1995) also found rotifer diversity high in reed beds, the most common type of aquatic vegetation. Typha angustata beds seen at stations II and III of River Vishwamitri could be another factor contributing to the higher rotifer number often present in these stations. Telesh (1995) further describes that species like Brachionus calyciflorus, B. quadridentatus, and Filinia longiseta are commonly found in areas where macrophytic vegetations are plenty. All the above species were found at sampling stations II and III. Phytophilous species like Platyias quadricornis and Mytilina ventralis are abundant in macrophyte beds (Telesh 1995). Platyias quadricornis was found at station III, while Mytilina ventralis was located at station II of Vishwamitri.
Thus, it can be seen that by and large station III seems to provide a better habitat with diverse niche for the rotifer community. This station besides receiving domestic sewage has relatively good levels of dissolved oxygen throughout the year. Moreover, there is water throughout the year at this station. The reed beds provide more varied microhabitat which is needed for the survival of the periphytic rotifers. This could be the reason for a high number of exclusive species found at this station.
From the above discussion, it could be concluded that pH and chlorophyll-a play a major role in influencing the rotifer community structure. Additionally, both abiotic and biotic factors could be interacting with each other and their combined effect may be influencing the rotifer community structure.
References
APHA, AWWA, WEF (1998) Standard methods for the examination of water and wastewater. American Public Health Association, Washington, DC
Archibald M (1972) Diversity in some South African diatom associations and its relation to water quality. Water Res 6:1229–1238
Arora HC (1966) Studies on Indian Rotifera – Part III. On Brachionus calyciflorous and some varieties of the species. J Zool Soc India 16:1–6
Arora J, Mehra NK (2003) Seasonal dynamics of rotifers in relation to physical and chemical conditions of the river Yamuna (Delhi). India Hydrobiol 491:101–109
Banerjea S, Motwani MP (1960) Some observations on pollution of the Suvaon stream by the effluents of a sugar factory, Balrampur (UP). Ind J Fish 7:107–128
Berzins B, Pejler B (1987) Rotifer occurrence in relation to pH. Hydrobiologia 147:107–116
Berzins B, Pejler B (1989) Rotifer occurrence in relation to temperature. Hydrobiology 175:223–231
Bhargava DS (1985) Water quality variation and control technology of Yamuna river. Environ Pollut 37(series B):355–376
Bielanska-Grajner I (2001) The psammic rotifer structure in three Lobelian Polish lakes differing in pH. Hydrobiology 446/447:149–153
Brett MT (1989) Zooplankton communities and acidification process (a review). Wat Air Soil Pollut 44:387–414
Burgis MJ (1974) Revised estimates for the biomass and production of zooplankton in lake George, Uganda. Freshw Biol 4:535–541
Cecchine G, Snell TW (1999) Toxicant exposure increases threshold food levels in freshwater rotifer populations. Environ Toxicol 14:523–530
Chacko PI, Rajagopal A (1962) Hydrobiology and fisheries of the Ennore river near Madras from April 1960 to March 1961. Madras J Fish 1:102–104
Cottenie K, Nuytten N, Michels E, Meester LD (2001) Zooplankton community structure and environmental conditions in a set of interconnected ponds. Hydrobiology 442:339–350
De Ridder M (1984) A review of rotifer fauna of Sudan. Hydrobiology 110:1113–1130
Dean RB, Lund E (1981) Water reuse: problems and solutions. Academic, London
den Berg V, Coops MSH, Noordhius R, Van Schie J, Simons J (1997) Macro invertebrate communities in relation to submerged vegetation in two Chara-dominated lakes. Hydrobiology 342/343:143–150
Deneke R (2000) Review of rotifers and crustaceans in highly acidic environments of pH values 3. Hydrobiology 433:167–172
Desai GH, Clarke AB (1923) Gazette of Baroda state, vol 1. General Information, Bombay
Dhanapathi MVSS (1997) Variations in some rotifers of the family Brachionidae. J Aquat Biol 12:35–38
Dhanapathi MVSS (2000) Taxonomic notes on the rotifers from India (1889–2000). Indian Association of Aquatic Biologists, Hyderabad
Dhuru S, Suresh B, Pilo B (2003) Additions to the rotifer fauna of Gujarat. J Aqua Biol 18(1):35–39
Dumont HJ (1983) Biogeography of rotifers. Hydrobiology 104:19–30
Dumont HJ, Segers H (1996) Estimating lacustrine zooplankton species richness and complementarity. Hydrobiology 341:125–132
Duncan AA, Gulati RD (1981) Parakrama Samudra (Sri Lanka) project, a study of a tropical lake ecosystem. III. Composition, density and distribution of the zooplankton in 1979. Verh Int Ver Limnol 21:1007–1014
Edmondson WT (1944) Ecological studies of the sessile Rotatoria. Part I. Factors affecting distribution. Ecol Monogr 14:31–66
Egborge ABM (1981) The composition, seasonal variation and distribution of zooplankton in Lake Asejire, Nigeria. Rev Zool Afr 95(1):136–180
Fernando CH (1980) The species and size composition of tropical freshwater zooplankton with special reference to the Oriental region (South East Asia). Int Revue Ges Hydrobiol 65:411–426
Fernando CH, Rajapaksa R (1983) Some remarks on long-term and seasonal changes in the zooplankton of Parakrama Samudra. In: Schiemer F (ed) Limnology of Parakrama Samudra – Sri Lanka. Dr. W. Junk, Hague
Fulton RS, Pearl HS (1987) Toxic and inhibitory effects of the blue green alga Microcystis aeruginosa on herbivorous zooplankton. J Plankton Res 9:837–856
Fussmann G (1996) The importance of crustacean zooplankton in structuring rotifer and phytoplankton communities: an enclosure study. J Plankton Res 10:1897–1915
Galkovskaja GA (1987) Planktonic rotifers and temperature. Hydrobiology 147:307–317
Gannon JE, Stemberger RS (1978) Zooplankton especially crustaceans and rotifers as indicators of water quality. Trans Am Micros Soc 77:16–35
George MG (1961) Observations on the rotifers from shallow ponds in Delhi. Curr Sci 30:268–269
Gilbert JJ (1985) Competition between rotifers and Daphnia. Ecology 66:1943–1950
Green J (1956) Growth, size and reproduction in Daphnia (Crustacea: Cladocera). Proc Zool Soc Lond 126:173–204
Green J (1960) Zooplankton of river Sokoto. The rotifers. Proc Zool Soc Lond 135:491–523
Green J (2001) Variability and instability of planktonic rotifer associations in Lesetho, Southern Africa. Hydrobiology 446/447:187–194
Gulati RD, Ooms-Wilms AL, Van Tongeren FR, Postema G, Siewetsen K (1992) The dynamics and role of limnetic zooplankton in the Loosdrecht (The Netherlands). Hydrobiology 233:69–86
Haque N, Khan AA, Fatima M, Barbhuyan SI (1988) Impact of some ecological parameters on rotifer population in a tropical perennial pond. Environ Ecol 6:998–1001
Hart RC (1987) Population dynamics and production of five crustaceans zooplankton in subtropical reservoir during years of contrasting turbidity. Freshw Biol 18:287–318
Hart RC (1990) Zooplankton distribution in relation to turbidity and related environmental gradient in a large subtropical reservoir: patterns and implications. Freshw Biol 24:241–263
Hebert PDN (1978) The population biology of Daphnia (Crustaceae: Daphnidae). Biol Rev 53:387–426
Herzig A (1987) The analysis of planktonic rotifer populations: a plea for long term investigations. Hydrobiology 147:163–180
Hofmann W (1977) The influence of abiotic environmental factors on population dynamics in planktonic rotifers. Arch Hydrobiol Beih Ergebn Limnol 8:77–83
Hynes HBN (1978) The ecology of running waters. Liverpool University Press, Liverpool
Kaushik S, Saksena DN (1995) Trophic status and rotifer fauna of certain water bodies in central India. J Environ Biol 16:283–291
Kirk KL, Gilbert JJ (1990) Suspended clay and population dynamics of planktonic rotifers and cladocerans. Ecology 71:1741–1755
Kuczyriska-Kippen N (2007) Habitat choice in rotifer communities of three shallow lakes: impacts of macrophyte substratum and season. Hydrobiology 593(1):27–37
Kulshreshtra SK, Adholia UN, Bhatnagar A, Khan AA, Saxena M, Bhagail M (1989) Studies on the pollution in river Kshipra: zooplankton in relation to water quality. Int J Ecol Environ Sci 15:27–36
Lewis WM Jr (1979) Zooplankton community analysis: studies on a tropical stream. Springer, New York/Berlin
Lougheed VL, Crosbie B, Chow-Fraser P (1998) Predictions on the effect of carp exclusion on water quality, zooplankton and submergent macrophytes in a Great Lakes wetland. Can J Fish Aquat Sci 55(5):1189–1197
Ludwig JA, Reynolds JE (1988) Diversity indices in statistical ecology. Wiley, New York
Marneffe Y, Comblin S, Thome J (1998) Ecological water quality assessment of the Butgenbach lake (Belgium) and its impact on the river Warche using rotifers as bioindicators. Hydrobiology 387/388:459–467
McCabe GD, O’ Brien WJ (1983) The effects of suspended silt on feeding and reproduction of Daphnia pulex. Am Midl Nat 110:324–337
Mengestou S, Green J, Fernando CH (1991) Species composition, distribution and seasonal dynamics of Rotifera in a Rift Valley lake in Ethiopia (Lake Awasa). Hydrobiology 209:203–214
Michael RG (1968) Studies on the zooplankton of a tropical fish pond, India. Hydrobiology 32:47–68
Mishra SR, Saksena DN (1998) Rotifers and their seasonal variation in a sewage collecting Morar (Kalpi) river, Gwalior, India. J Environ Biol 19:363–374
Mitra AK (1982) Chemical characteristics of surface water at selected gauging stations in the river Godavari, Krishna and Tungabhadra. Ind J Environ Health 24:165–179
Nayar CKG (1964) Morphometric studies on the rotifer, Brachionus calyciflorous Pallas. Curr Sci 33:469–470
Nayar CKG (1965) Cyclomorphosis of B. calyciflorus. Hydrobiology 25:538–544
Pejler B (1957) Taxonomical and ecological studies on planktonic Rotatoria from northern Swedish Lapland. K. svenska Vetensk Akad Handl., Ser. 4, bd 6 no 68 pp
Pejler B (1977) On the global distribution of family Brachionidae (Rotatoria). Arch Hydrobiol (Suppl) 53:255–306
Pennak WR (1955) Comparative limnology of eight Colorado mountain lakes, University of Colorado studies, series of biology. University of Colorado Press, Boulder, 255 pp
Persuad AD, Williamson CE (2005) Ultraviolet and temperature effects on planktonic rotifers and crustaceans in northern temperature lakes. Freshw Biol 50(3):467–476
Plath K, Boersma M (2001) Mineral limitation of zooplankton: stoichiometric constraints and optima foraging. Ecology 82:1260–1269
Pollard AI, Gonzalez MJ, Vanni MJ, Headworth JL (1998) Effects of turbidity and biotic factors on the rotifer community in a Ohio reservoir. Hydrobiology 387/388:215–223
Porter KG, Orcutt JD (1980) Nutritional adequacy, manageability and toxicity as factors that determine the food quality of green and blue green algae for Daphnia. In: Kerfoot WC (ed) Evolution and ecology of zooplankton communities. University Press of New England, Hanover, pp 268–281
Prabhavathy G, Sreenivasan A (1977) Ecology of warm freshwater zooplankton of Tamil Nadu. In: Proceedings of the symposium on warm water zooplankton, Goa special publication. NIO, Goa, pp 319–329
Robinson AH, Robinson PK (1971) Robinson. seasonal distribution of zooplankton in northern basin of Lake Chad. J Zool (Lond) 163:25–61
Ruttner-Kolisko A (1975) The influence of fluctuating temperature on plankton rotifers. A graphical model based on life data of Hexarthra fennica from Neusiedlersee, Austria. Symp Biol Hung 15:197–204
Sampath V, Sreenivasan A, Ananthanarayanan R (1979) Rotifers as biological indicators of water quality in Cauvery river. Proc Symp Environ Biol 441–452
Sarma SSS, Osnaya-Espinosa LR, Aguilar-Acosta CR, Nandini S (2011) Seasonal variations in zooplankton abundances in the Iturbide reservoir (Isidro Fabela, State of Mexico, Mexico). J Environ Biol 32:473–480
Sawyer CN, McCarty PL, Parkin GF (1994) Chemistry of environmental engineering. McGraw – Hill International Education, New York, p 658
Segers H, Dumont HJ (1995) 102+ rotifer species (Rotifera: Monogononta) in Broa Reservoir (S.P. Brasil) on 26 August 1994, with a description of three new species. Hydrobiology 316:183–197
Skadowsky SN (1923) Hydrophysiologische und hydrobiologische Beobachtungen uber die Bedeutung der Reaktion des Mediums fur die Susswasserorganismen. Ver Int Ver Limnol 1:341–358
Sladecek V (1983) Rotifers as bioindicators of water quality. Hydrobiology 100:169–201
Somashekar RK (1988) Ecological studies on the two major rivers of Karnataka. In: Trivedy RK (ed) Ecology and pollution of Indian rivers. Ashish Publishing House, New Delhi
Sommer U, Gliwicz WL, Duncan A (1986) The PEG-model of seasonal succession of planktonic events in fresh waters. Arch Hydrobiol 106:433–471
Steinberg AJ, Ejsmont-Karabin J, Muirhead JR, Harvey CT, MacIssac HJ (2009) Consistent, long-term change in rotifer community composition across four Polish lakes. Hydrobiology 624:107–114
Subramanian V, Biksham G, Rames R (1987) Environmental geology of peninsular river basins of India. J Geol Soc Ind 30:393–401
Taylor BE, Mahoney DL (1988) Extinction and recolonization: processes regulating zooplankton dynamics in a cooling reservoir. Verh Int Ver Limnol 23:1536–1541
Tebutt THY (1992) Principles of water quality control, 4th edn. Pergamon Press, Oxford
Telesh IV (1995) Rotifer assemblages in the Neva Bay, Russia: principles of formation, present state and perspectives. Hydrobiology 313/314:57–62
Threlkeld ST (1979) The midsummer dynamics of two Daphnia species in Wintergreen lake, Michigan. Ecology 60:165–179
Threlkeld ST (1986) Resource mediated demographic variation during the midsummer succession of a cladoceran community. Freshw Biol 16:673–683
Threlkeld ST (1987) Daphnia population fluctuations: patterns and mechanisms. In: Peters RH, de Bernardi R (eds) Daphnia. Mem Ist Ital Idrobiol 45:367–388
Trivedi RK, Goel PK (1986) Chemical and biological methods for water pollution studies. Environmental Publications, Karad
Trommer G, Pondaven P, Siccha M, Stibor H (2012) Zooplankton-mediated nutrient limitation patterns in marine phytoplankton: an experimental approach with natural communities. Mar Ecol Prog Ser 449:83–94
Venkateswarlu V (1986) Ecological studies on the rivers of Andhra Pradesh with special reference to water quality and pollution. Proc Ind Sci Acad 96:495–508
Venkateswarlu T, Jayanti TV (1968) Hydrobiological studies of the river Sabarmati to evaluate water quality. Hydrobiology 31:442–448
Verma SR, Sharma P, Tyagi A, Rani S, Gupta AK, Dalela RC (1984) Pollution and saprobic status of eastern Kali nadi. Limnol (Berlin) 15:69–133
Webster KE, Peters RH (1978) Some size dependent inhibitions of larger cladoceran filterers in filamentous suspensions. Limnol Oceanogr 23:1238–1245
Wesenberg-Lund C (1908) Plankton investigations of the Danish lakes. Gyldendalske Boghandel, Copenhagen
Wesenberg-Lund C (1930) Contributions to the biology of Rotifera. Part II. The periodicity and sexual periods. Kgl Danske Vidensk Slesk Skifter Naturv Mathem 2:1–230
Wiszniewski J (1936) Notes sur le psammon III. Deux tourbieres aux environs de Varsovie. Arch Hydrobiol 10:173–187
Wolfinbarger WC (1999) Influences of biotic and abiotic actors on seasonal succession of zooplankton in Hugo Reservoir, Oklahoma, USA. Hydrobiology 400:13–31
Yin XW, Nui CJ (2008) Effect of pH on survival, reproduction, egg viability and growth rate of five closely related rotifer species. Aquat Ecol 42(4):607–616
Yoshinaga T, Atsushi H, Tsukamoto K (2001) Why do rotifer populations present a typical sigmoid curve? Hydrobiology 446/447:99–105
Zhou S, Huang X, Cai Q (2007) Vertical distribution and migration of planktonic rotifers in Xiangxi Bay of the three Gorgers reservoir. China J Freshw Ecol 22(3):441–449
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer India
About this chapter
Cite this chapter
Dhuru, S., Patankar, P., Desai, I., Suresh, B. (2015). Structure and Dynamics of Rotifer Community in a Lotic Ecosystem. In: Rawat, M., Dookia, S., Sivaperuman, C. (eds) Aquatic Ecosystem: Biodiversity, Ecology and Conservation. Springer, New Delhi. https://doi.org/10.1007/978-81-322-2178-4_6
Download citation
DOI: https://doi.org/10.1007/978-81-322-2178-4_6
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-2177-7
Online ISBN: 978-81-322-2178-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)