Abstract
In the laboratory we examined the effect of pH (5–10 with one interval) on survival, reproduction, egg viability and growth rate (intrinsic growth rate—r m and population growth rate—r) of five Brachionus rotifer species (B. calyciflorus, B. quadridentatus, B. urceolaris, B. patulus and B. angularis). The pH was shown to exert a major influence on egg viability and growth rate (r m and r) for each species. The age-specific survivorship curves within a species were not significantly different at pH 6–10. The optimal pH for each species is near-neutral pH (pH 6–8), and the fecundity decreased as the pH deviated from these values. For each Brachionus species, there was no significant difference between age-specific fecundity curves at pH 7 and 8. At acid pH (pH 5 or 6) higher egg mortality was observed for each species. The r m and population r of the five Brachionus species incubated at different pHs were significantly influenced by pH. The pH supporting the highest r m or r was obtained at pH 6–8, but varied due to species. In this study B. urceolaris and B. patulus could tolerate a broad range of pH, while the populations of B. calyciflorus, B. quadridentatus and B. angulari declined at acid conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Numerous studies have attempted to elucidate the responses of aquatic animals to different pH. Among these, results of field experiments and laboratory bioassays verified the lethal or sublethal effects of pH values of above 9 or below 6 on zooplankton species (O’Brien and deNoyelles 1972; Potts and Fryer 1979; Alibone and Fair 1981; Mitchell and Joubert 1986; Mitchell 1992; Vijverberg et al. 1996; Wang et al. 1997; Locke and Sprules 2000). In natural water bodies, due to the photosynthetic activity by algae and the respiration of aquatic animals, the pH usually fluctuates between 6 and 9. It is only under certain circumstances, such as biogeochemical activities of bacteria in acid mine drainage, poor acid neutralizing capacity in bogs and acid rain or well photosynthetic ability by specific algae species at high pH in eutrophic ponds, that the pH of these water bodies is more acid or more alkaline than usual levels (O’Brien and deNoyelles 1972; Wetzel 2001; Sigee 2005).
Compared to lethal effects of extreme pH, sublethal effects of pH on zooplankton species are more common. Sublethal influences of hydrogen or hydroxyl ion have been noted as impairing survival, growth, reproduction and feeding of zooplankton species (reviewed in Locke 1991; Mitchell 1992; Vijverberg et al. 1996). Considering the differential responses of zooplankton species to pH, the sublethal effects of pH indirectly alter the species interactions (e.g., competition, predator-prey) (Hessen et al. 1995; Fischer and Frost 1997; Locke and Sprules 2000), and result in changes of distribution and abundance of populations and species, which ultimately influence the community structure (Kiesecker 1996).
Rotifers are an important part of zooplankton communities. Many species in this phylum can tolerate a broad range of pH values with their peak occurrence ranging from pH 4.5 to 8.5 (Bērziņš and Pejler 1987). Interspecific differences in pH tolerance can be expected to alter biotic interactions among different rotifer species, shifting the competitive ability between species and regulating predation pressure (Mitchell and Joubert 1986; Frost et al. 1998). In response to long-term pH perturbations, rotifer communities exhibit changes and differences in species composition, distribution and abundance (Frost et al. 1998). Moreover, in closely related rotifer species, extensive niche overlap that results in strongest interspecific competition may occur (Schoener 1983). Apart from biotic interactions, abiotic factors such as pH can also determine the competitive outcome among different species (Hessen et al. 1995; Pehek 1995).
To assess how rotifer communities alter in response to changing pH conditions and how the pH modifies the competitive outcome among closely related species, it is necessary to quantify the direct effect of pH on life history characteristics and population growth rate of each species. In the present work, we examined the effects of moderate pH (pH 5–10) on survival, reproduction, egg viability and population growth rate of five common Brachionus rotifer species viz. B. calyciflorus Pallas, B. quadridentatus Hermann, B. urceolaris Müller, B. patulus Müller and B. angularis Gosse.
Materials and methods
Collection and origin of rotifers
We obtained rotifer clones of five Brachionus species from resting eggs in dried sediment collected from an artificial freshwater pond (ca. 70 m2) in Beijing, China (39°57′ N; 116°21′ E). An amount of sediment (ca. 0.5 kg) was placed in a beaker and mixed with about 500 ml inorganic medium (pH ≈ 7.3) (see Gilbert 1963) containing green algae Chlorella pyrenoidosa that was cultured in SE medium (FACHB-Collection 2005). Every day all the water in the beaker was checked under a dissecting microscope and then returned to the beaker. B. calyciflorus, B. quadridentatus, B. urceolaris, B. patulus and B. angularis hatched out simultaneously (within 3–5 days) from the flooded sediment.
One newly hatched individual of each Brachionus species was isolated and allowed to reproduce parthenogenetically to obtain a culture of genetically identical individuals. The rotifers were cultured successively in the inorganic medium, and food alga C. pyrenoidosa was added to the culture medium at a density of 4 × 106 cells ml−1. The culture medium was renewed every 2 days. Before use in the treatments all the Brachionus rotifer clones were cultured in the laboratory for at least a month and were in exponential growth phase. All rotifer and algal cultures, experimental incubations and hatching of resting eggs in dried sediment were kept at 20 ± 1.0°C in a 14:10 (L:D) photoperiod (illuminance ≈ 50 μEin m−2 s−1) in a diurnal growth chamber.
The average body sizes of these five Brachionus species [body length (l) and width (w) in μm, mean ± standard deviation, n = 100] of B. calyciflorus, B. quadridentatus, B. urceolaris, B. patulus and B. angularis were: 196 ± 12 (l), 156 ± 8 (w); 188 ± 7 (l), 189 ± 9 (w); 183 ± 13 (l), 153 ± 16 (w); 145 ± 8 (l), 152 ± 7 (w) and 130 ± 7 (l), 115 ± 7 (w), respectively.
Variation of pH within 24 h
To asses the magnitude of possible pH variation within the interval the medium was renewed, and we monitored pH over a period of 24 h. We placed a 100-ml beaker, containing 80 ml culture medium (with no rotifer and alga), with required pH (pH 5–10 with one interval) in the diurnal growth chamber. All pH series consisted of three replicates. The pH of the culture medium, measured with pH meter (DELTA-320) at 20°C, was adjusted by adding NaOH (0.1 mol l−1) and HCl (0.1 mol l−1) into the medium following the method described in Mitchell (1992). Every 4 h, the pH of the medium was recorded. Fluctuations of pH within 24 h were relatively small in the pH range 5–8, but much larger for pH 9 and pH 10 (Fig. 1). Hence, we probably overestimated the value of pH 9 and 10 in our experiment.
Life table studies
To test the effect of pH (pH 5–10 with one interval) on the life table demography of the five Brachionus species, we designed experiments as follows. Prior to the treatments, a number of egg-bearing amictic females of each Brachionus species was isolated and incubated at a density of 5 ind. ml−1 in the culture medium with required pH (5, 6, 7, 8, 9 and 10) and food concentration (C. pyrenoidosa at 4 × 106 cells ml−1). After being acclimated to the required pH for 24 h, 36 newly hatched rotifers of each Brachionus species (old <8 h) were randomly caught and separately piped into culture plates, containing 0.2 ml fresh culture medium with appropriate pH-algal food combinations for treatment incubation. The experiment design consisted of a total 30 (= 6 pHs × 5 species) culture plates.
To minimize the pH fluctuations in the rotifer culture medium and the adverse influence to individuals by algal culture medium, C. pyrenoidosa, in the exponential growth phase, were separated from the growth medium by centrifuging for 10 min at 3,000 × rpm, counted, and suspended in the rotifer culture medium with required concentration. Concentrated algae solutions were stored in the dark at 4°C, and used within 10 days of harvest.
Everyday, surviving females with attached eggs were transferred to fresh medium with appropriate pH-algal food combinations, and the number of eggs and neonates was recorded and used to calculate the life history parameters described below. Furthermore, egg mortality of each individual was also noted. The experiment was terminated when all individuals died.
The intrinsic growth rate (r m) was calculated following the formulae described in Krebs (1994): ∑e −rx l x m x = 1, where x is the age interval in days, l x is the proportion of organisms surviving at the start of the age interval x and m x is the number of offspring produced per day per female aged x. The mean and variation of r m were based on 500 Jackknife samples of individual survivorship and fecundity schedules (Meyer et al. 1986). Since the egg viability of freshwater organism is greatly affected by pH (see Vijverberg et al. 1996), we computed the r m′, assuming all eggs are viable and will result in living neonates, with the method described above. During the experiments all individuals of B. angularis died without producing offspring in the first few days at pH 5. Thus, these data were removed from the results.
Population growth studies
To establish effects of pH (pH 5–10 with one interval) on the population growth rate (r) of five Brachionus species, we placed five neonates (old <8 h) of each Brachionus species in culture plates containing 5-ml culture medium with required pHs (5, 6, 7, 8, 9 and 10) and food concentration (C. pyrenoidosa at 4 × 106 cells ml−1). Every day all the living individuals in the container were transferred to fresh medium with appropriate pH-algal food combinations. After 5 days, the experiments were terminated and the total number of rotifers in the container was counted. Other procedures in acclimation and treatment incubations were the same as in the life table studies. All the experimental treatments consisted of four replicates. The experiment design consisted of a total 120 (= 6 pHs × 5 species × 4 replicates) culture plates.
We obtained population growth rate (r) with the equation: r = (lnN t −lnN 0)/t, where N 0 and N t are the initial and final population densities and t is the incubation time in days. During the experiments all individuals of B. angularis and B. calyciflorus died without producing offspring at pH 5. Therefore, we removed these data from the results.
Data analysis
We used non-parametric Mann-Whitney U-tests to compare pairwise the effect of pH on fecundity and age-specific survivorship. The effect of pH on egg mortality of each species was tested with the chi-square test. Differences in intrinsic growth rate (r m) and population growth rate (r) were analyzed with factorial ANOVA for pH effects. The differences between r m (actual intrinsic population growth rate) and r m′ (intrinsic population growth rate assuming all eggs are viable) were tested with the chi-square test. All statistical analyses were carried out using the statistical package SPSS version 12.0.
Results
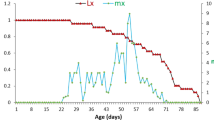
For each Brachionus species, pairwise comparisons showed that the age-specific survivorship curves were not significantly different at pH 6–10 (Table 1, Fig. 2). The survivorship curves showed heavy mortalities at pH 5 in B. quadridentatus and B. angularis, but not in B. calyciflorus, B. urceolaris and B. patulus (Table 1, Fig. 2).
Higher fecundity was obtained at pH 6–8 in B. calyciflorus, B. quadridentatus, B. urceolaris, B. patulus and at pH 7–10 in B. angularis (Fig. 2). At low pH (5), B. urceolaris had higher fecundity than the other Brachionus species, and at high pH (10), much higher fecundity was observed in B. angularis. For each Brachionus species, there was no significant difference between age-specific fecundity curves at pH 7 and 8 (Table 2, Fig. 2). The fecundity curves of B. calyciflorus and B. quadridentatus showed great variation between low pH (5) and circum-neutral pH (6–9); however, these were not the cases in B. urceolaris and B. patulus (Table 2).
The difference of egg mortality was not obvious at pH 6–10 in B. calyciflorus (χB.c. 2 = 6.636, df = 4, P = 0.156, Fig. 3), B. urceolaris (χ B.u. 2 = 5.707, df = 4, P = 0.222) and B. patulus (χ B.p. 2 = 4.50, df = 4, P = 0.343); however, when pH decreased to 5, a marked increment in egg mortality was observed in these three Brachionus species (χB.c. 2 = 146.83, χ B.u. 2 = 120.67, χ B.p. 2 = 58.57, df = 5, P < 0.001). For B. angularis and B. quadridentatus, egg mortality increased significantly as pH decreased from neutral or alkaline pH (7–10) to acid pH (5 and 6) (χB.a. 2 = 345.67, χ B.q. 2 = 260.67, df = 5, P < 0.001).
Data on egg mortality divided by total eggs (open bar) of five Brachionus species at each pH treatment. Shown are mean ± standard error values based on 36 individuals. Error bars that are not apparent are too small to be shown. See the caption in Fig. 2 for the meaning of abbreviations
The intrinsic growth rate (r m) and population growth rate (r) of five Brachionus species were greatly influenced by pH (two-way ANOVA, Frm = 277.01, F r = 487.23, df = 5, P < 0.001, Figs. 4 and 5). The pH supporting the highest r m or r was obtained at pH 6–8, but varied in values due to species (Fig. 4). B. calyciflorus and B. urceolaris had the highest r m and r at pH 6, while B. quadridentatus, B. patulus and B. angularis attained the highest r m or r at neutral pH (7 or 8). At neutral pH (7 and 8) B. quadridentatus had higher r m and r than other species, while B. urceolaris at more acid and alkaline pH (5 and 10) attained higher r m and r than other species (Figs. 4 and 5).
Data (mean ± standard error) on intrinsic growth rate (r m) of five Brachionus species at each pH treatment. Striped bar—r m based on life table data; Open bar—assuming all eggs are viable and result in living neonates (r ′m ). Error bars that are not apparent are too small to be shown. See the caption in Fig. 2 for the meaning of abbreviations
Data on population growth rate (r) of five Brachionus species at each pH treatment. Shown are mean ± standard error values based on four replicate recordings. Error bars that are not apparent are too small to be shown. See the caption in Fig. 2 for the meaning of abbreviations
The difference between r m and r m′ was not marked at pH 6–10 in B. calyciflorus (χ2 = 0.193, df = 4, P = 0.996), B. quadridentatus (χ2 = 3.674, df = 4, P = 0.452), B. urceolaris (χ2 = 0.743, df = 4, P = 0.946) and at pH 7–10 in B. angularis (χ2 = 0.057, df = 3, P = 0.996). However, this difference was obvious when we compared the r m and r m′ at all pH levels (pH 5–10 in B. calyciflorus, B. quadridentatus, B. urceolaris and pH 6–10 in B. angularis) (χB.c. 2 = 48.62, χ B.q. 2 = 51.422, χ B.u. 2 = 17.23, df = 5, P < 0.01; χB.a. 2 = 70.63, df = 4, P < 0.001). These data showed that the difference of r m and r m′ at low pH (5 in B. calyciflorus, B. quadridentatus, B. urceolaris and pH 6 in B. angularis) was significant in B. calyciflorus, B. quadridentatus, B. urceolaris and B. angularis. For B. patulus, no pronounced difference between r m and r m′ was detected at experimental pH treatments (χB.p. 2 = 1.043, df = 5, P = 0.959).
Discussion
The distribution and abundance of rotifers are confined by pH (see Wallace and Snell 2001). For the genus Brachionus, B. angularis, B. calyciflorus and B. quadridentatus are believed to be common alkaline species (see Sládeček 1983; Bērziņš and Pejler 1987), while B. patulus and B. urceolaris have been found in more acidic waters (Myers 1937; Parsons 1968; Horvath and Hummon 1980; McConathy and Stahl 1982; Bērziņš and Pejler 1987). Our results are consistent with these field investigations that B. patulus and B. urceolaris can tolerate more acid pH (5) than B. angularis, B. calyciflorus and B. quadridentatus (Figs. 2, 4, and 5).
The responses to pH (especially low pH) of rotifers seem to be consistent at a community level (Frost et al. 1998). Considering the differential responses of various taxa to pH, rotifer communities may vary due to the conditions of water bodies. The genus Brachionus is known for its tolerance to high pH (Ahlstrom 1940; Sládeček 1983; Mitchell and Joubert 1986); therefore, they dominate the rotifer community in eutrophic waters (see Sládeček 1983). However, in oligo-trophic or acid-stress waters, the dominant taxa will be those that can survive and reproduce at low pH conditions such as Cephalodella, Keratella and Trichocerca (Sládeček 1983; Brett 1989; Gonzalez and Frost 1994; Frost et al. 1998; Deneke 2000).
The distribution of the genus Brachionus is confined to waters with pH ≥ 6.6 (Ahlstrom 1940), although some species in this genus (e.g., B. urceolaris) can tolerate more acid conditions (Deneke 2000). At low pH conditions (pH < 6), feeding, respiration, and sodium balance of Brachionus may be impaired greatly (Kring and O’Brien 1976; Potts and Fryer 1979; Alibone and Fair 1981), which resulted in high egg mortality, low individual survival and negative population growth rate in some species of Brachionus (present work, Figs. 2–5). Although B. patulus and B. urceolaris had positive population growth rate (r m or r) at pH 5 (Figs. 4 and 5), their survival and reproduction decreased greatly at pH below 5 (no individual can survive to 12 h at pH 4, personal observations). These may be the main reasons to impede the distribution and abundance of this taxon in acidic waters.
The response of B. calyciflorus to pH received extensive studies (Mitchell and Joubert 1986; Mitchell 1992; Wang et al. 1997; Xi and Huang 1999). At moderate pH (5–10), although a typical bell-shaped curve was often observed in the response of B. calyciflorus to pH, optimum pH supporting the maximum population growth rate varied among these studies, ranging from 7.5 to 9.5 (Mitchell 1992; Wang et al. 1997; Xi and Huang 1999). Furthermore, in the present study B. calyciflorus attained the highest growth rate at a lower pH (6) (Figs. 4 and 5). The biotic and abiotic factors of the habitats where these B. calyciflorus clones were initially isolated may vary greatly. The different responses to pH of these clones are likely due to the intra-specific differences among zoogeographical populations, as some data revealed that the responses of ecotypes to pH would match their habitats of origin (Price and Swift 1985; Gonzalez and Frost 1994).
Within a particular community the interactions among different competitors or between predator and prey will be altered when species differ in their response to changing abiotic conditions (Chesson 1986). Many studies have been focused on the pH-mediated biotic interactions in aquatic ecosystems (Susan et al. 1993; Hessen et al. 1995; Pehek 1995; Kiesecker 1996; Locke and Sprules 2000). On one hand, due to anthropogenic factors (e.g., eutrophication or acid rain), the pH will be more acid or alkaline than usual levels (pH < 6 or > 9) in natural waters. The extreme increase or decrease of pH will decrease the abundance of those species that are more sensitive in both survival and reproduction to the change of pH, for example, B. angularis, B. calyciflorus and B. quadridentatus (present work), which will increase the relative competitive ability of less sensitive species (e.g., B. patulus or B. urceolaris in the present work). On the other hand, in our study the differentiation of Brachionus species in response to pH indicates that the pH may be an important abiotic factor in regulating the species composition of rotifers in a community, since some studies have revealed that when competing species differ in the sensitivity to pH, the compensatory dynamics (substitution among species) will occur between or among these species in relation to pH fluctuations (Klug et al. 2000).
References
Ahlstrom EH (1940) A revision of the rotatorian genera Brachionus and Platyias with description of one new species and two varieties. Bull Am Mus Nat Hist 77:143–184
Alibone MR, Fair P (1981) The effets of low pH on the respiration of Daphnia magna Straus. Hydrobiologia 85:185–188
Bērziņš B, Pejler B (1987) Rotifer occurrence in relation to pH. Hydrobiologia 147:107–116
Brett M (1989) The rotifer communities of acid-stressed lakes of maine. Hydrobiologia 186/187:181–189
Chesson PL (1986) Environmental variation and the coexistence of species. In: Diamond J, Case TJ (eds) Community ecology. Harper & Row, New York, pp 240–256
Deneke R (2000) Review of rotifers and crustaceans in highly acidic environments of pH values ≤3. Hydrobiologia 433:167–172
FACHB–Collection (2005) Institute of hydrobiology, Chinese academy of sciences. http://algae.ihb.ac.cn/search/data/SE%20medium.htm#SEmedium.htm Cited 17 Feb 2007
Fischer JM, Frost TM (1997) Indirect effects of lake acidification on Chaoborus population dynamics: the role of food limitation and predation. Can J Fish Aquat Sci 54:637–646
Frost TM, Montz PK, Gonzalez MJ, Sanderson BL, Arnott SE (1998) Rotifer responses to increased acidity: long-term patterns during the experimental manipulation of Little Rock Lake. Hydrobiologia 387/388:141–152
Gilbert JJ (1963) Mictic female production in the rotifer Brachionus calyciflorus. J Exp Zool 153:113–123
Gonzalez MJ, Frost TM (1994) Comparisons of laboratory bioassays and a whole-lake experiment: rotifer responses to experimental acidification. Ecol Applica 4:69–80
Hessen DO, Faafeng BA, Andersen T (1995) Competition or niche segregation between Holopedium and Daphnia: empirical light on abiotic key parameters. Hydrobiologia 307:253–261
Horvath FJ, Hummon WD (1980) Influence of mine on planktonic rotifers. Ohio J Sci 80:104–107
Kiesecker J (1996) pH-Mediated predator-prey interactions between Ambystoma tigrinum and Pseudacris triseriata. Ecol Applica 6:1325–1331
Klug JL, Fischer JM, Ives AR, Dennis B (2000) Compensatory dynamics in planktonic community responses to pH perturbations. Ecology 81:387–398
Krebs CJ (1994) Ecology: the experimental analysis of distribution and abundance, 4th edn. Harper & Row, New York, pp 168–181
Kring RL, O’Brien WJ (1976) Accommodation of Daphnia pulex to altered pH conditions as measured by feeding rate. Limnol Oceanogr 21:313–315
Locke A (1991) Zooplankton responses to acidification: a review of laboratory bioassays. Wat Air Soil Pollut 60:135–148
Locke A, Sprules WG (2000) Effects of acidic pH and phytoplankton on survival and condition of Bosmina longirostris and Daphnia pulex. Hydrobiologia 437:187–196
McConathy JR, Stahl JB (1982) Rotifera in the plankton and among filamentous algal lumps in 16 acid strip-mine lakes. Trans Ill Acad Sci 75:85–90
Meyer JS, Ingersoll CG, McDonald LL, Boyce MS (1986) Estimating uncertainty in population growth rates: Jackknife versus Bootstrap techniques. Ecology 67:1156–1166
Mitchell SA (1992) The effect of pH on Brachionus calyciflorus Pallas (Rotifera). Hydrobiologia 245:87–93
Mitchell SA, Joubert JHB (1986) The effect of elevated pH on the survival and reproduction of Brachionus calyciflorus. Aquaculture 55:215–220
Myers FJ (1937) Rotifera from the Adirondack region of New York. Am Mus Novit 903:1–17
O’Brien WJ, deNoyelles F Jr (1972) Photosynthetically elevated pH as a factor in zooplankton mortality in nutrient enriched ponds. Ecology 53:605–614
Parsons JD (1968) The effects of acid strip-mine effluents on the ecology of a stream. Arch Hydrobiol 65:25–50
Pehek EL (1995) Competition, pH, and the ecology of larval Hyla andersonii. Ecology 76:1786–1793
Potts WTW, Fryer G (1979) The effects of pH and salt content on sodium balance in Daphnia magna and Acantholeberis curvirostris (Crustacea: Cladocera). J Comp Physiol 129:289–294
Price EE, Swift MC (1985) Intra- and inter-specific variability in the response of zooplankton to acid stress. Can J Fish Aquat Sci 42:1749–1754
Schoener TW (1983) Field experiments on interspecific competition. Am Nat 122:240–285
Sigee DC (2005) Freshwater microbiology: biodiversity and dynamic interactions of microorganisms in the aquatic environment. John Wiley & Sons, West Sussex, p 89
Sládeček V (1983) Rotifers as indicators of water quality. Hydrobiologia 100:169–201
Susan CW, Joseph T, William AD (1993) Effect of pH variation of interspecific competition between two species of Hylid tadpoles. Ecology 74:183–194
Vijverberg J, Kalf DF, Boersma M (1996) Decrease in Daphnia egg viability at elevated pH. Limnol Oceanogr 41:789–794
Wallace RL, Snell TW (2001) Rotifera. In: Thorp JH, Covich AP (eds) Ecology and classification of North American freshwater invertebrates, 2nd edn. Academic Press, New York, p 206
Wang JQ, Li DS, Luo YB, Wang F (1997) Influence of medium pH on population growth and reproduction of Brachionus calyciflorus. Chin J Appl Ecol 8:435–438
Wetzel RG (2001) Limnology: lake and river ecosystems, 3rd edn. Academic Press, New York
Xi YL, Huang XF (1999) Effect of pH on population dynamics and resting egg of Brachionus calyciflorus. J Fish Sci China 6:19–22
Acknowledgments
We thank four anonymous referees for constructive comments on the manuscript. We also thank X.X. Chen for technical assistance during the investigation. This study was supported by the National Natural Science Foundation of China (no. 30470309).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yin, X.W., Niu, C.J. Effect of pH on survival, reproduction, egg viability and growth rate of five closely related rotifer species. Aquat Ecol 42, 607–616 (2008). https://doi.org/10.1007/s10452-007-9136-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-007-9136-9