Abstract
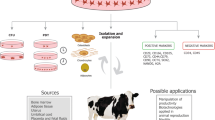
Fetal stem cells derived from amniotic fluid, amnion, umbilical cord, and fetal fibroblast have provided new insights regarding the nature and potential of these cells and can be an alternative source of stem cells in livestock, as scientifically validated embryonic stem (ES) cell lines are yet not available in these species. In this review we shall state the art, envision, and the prospects of fetal mesenchymal stem cells in livestock and their application in assisted reproduction and in veterinary medicine. Fetal stem cells express pluripotency markers and share similar growth kinetics providing strong support to the notion that these cells may be biologically closer to embryonic stem cells. The placenta and fetal adnexa, such as umbilical cord blood, umbilical cord matrix, amnion, amniotic fluid (AF) and fetal fibroblasts are immensely valuable and easily accessible sources of pluripotent and progenitor cells. These cells represent the intermediate between ES and adult stem cells regarding proliferation rates and plasticity features. These cells can be used in assisted reproduction and veterinary health applications. General applications include veterinary regenerative medicine and as donor cells for cloning and transgenic and iPS cell production. In equines, these cells can be used for orthopedic injuries, including repair of damaged ligaments and tendons and for laminitis. In domestic livestock, these can be used for producing environment-friendly transgenic animals for less methane or phosphorus production and in vitro meat production. Specific application of stem cells in canine can be in spinal injuries and diabetes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
In twenty-first century, the most exciting and emerging area of medicinal research is stem cell research. The fetal adnexa such as umbilical cord, amnion, and amniotic fluid have been proposed as an ideal source of stem cells in livestock due to their noninvasive isolation procedure, availability of large tissue mass for harvesting cells, ease of access without any ethical reservation, and multilineage differentiation potential. In view of technical and ethical concerns to establish ES (Yadav et al. 2005) cells and the restricted differentiation potential of adult stem cells, establishing the fetal stem cells could be a promising alternative approach. In recent years, fetal stem cells (FSCs) have emerged as a potential “half way house” between ES cells and adult stem cells (Abdulrazzak et al. 2010). The adnexal tissue cells preserve some of the characteristics of primitive embryonic layers from which they originate (Pappa and Anagnou 2009). Non-embryonic fetal-derived stem cells open new perspective for regenerative medicine in livestock species. Recent studies demonstrated the isolation of stem cells from sources such as umbilical cord blood, Wharton’s jelly, and amniotic fluid and membrane from various livestock and domestic species like equine, canine, bovine, buffalo, feline, caprine, ovine, and swine (Cremonesi et al. 2011; Yadav et al. 2012a, b). Histopaque and Ficoll density centrifugation were the most commonly used methods for isolation of mononuclear cells from umbilical cord blood and amniotic fluid. Mechanical chopping or enzymatic digestion of adnexal tissues or both were used for primary culture initiation in various studies. DMEM or MEM basal media supplemented with varying concentrations of FBS and other growth factors were used for cell culture by different workers. The cells cultured for prolonged periods were characterized for stemness properties through biochemical and molecular markers. High alkaline phosphatase activity and expression of key pluripotency transcription factors like Oct-4, Nanog, and Sox2 and immunostaining for expression of stage-specific embryonic antigens, tumor rejection antigen, etc., were employed in different studies for validation of stemness characteristics. Trans-differentiation of these cells into different lineages after induction was confirmed through lineage-specific staining or lineage-specific gene expression studies. Some reports demonstrated their ability to cryopreserve well and maintain their phenotypic and growth characteristics upon thawing which is of utmost importance for their therapeutic use.
2 Transplantation Studies
Porcine umbilical cord matrix cells engrafted and proliferated successfully in the rodent model of Parkinson’s disease without requiring immune suppression (Medicetty et al. 2004). Porcine amniotic fluid-derived stem cells injected in immunodeficient mice (Balb/c-Nu) developed no teratoma (Chen et al. 2011). Effect of transplanted mesenchymal stem cells from Wharton’s jelly (WJ) of caprine umbilical cord on wound healing revealed that reepithelialization was complete in 7 days in treated wounds whereas in control the wounds showed incomplete epithelization even after 12 days of wounding, elucidating the beneficial effect of caprine WJ cells on cutaneous wound healing (Azari et al. 2011). Canine umbilical cord blood (UCB)-derived MSCs transplanted in spinal cord-injured dogs resulted in recovery of nerve function without any inflammatory response even after direct transplantation of allogenic UCB-MSCs (Lim et al. 2007). Tendon injuries are common clinical problem in race horses. Lange-Consiligio et al. (2011) observed hyperintense population of equine umbilical cord MSCs in isolated tendons with artificial lesions indicating their possible role in equine orthopedics. These findings indicate that stem cell derived from fetal adnexa holds promise for therapeutic applications and may accelerate the field of veterinary medicine.
Application of the stem cells in understanding fundamental events in embryonic development, therapeutic delivery system, and animal model testing of pharmaceutical research has made it the fastest growing area of research in biological sciences. With decoding of the genome sequences, stem cell promises to resolve many mysteries of the developmental biology. Stem cells provide efficient means to investigate the expression, regulation, and functions of genes involved in mammalian differentiation and development. What has really attracted the attention of general public as well as scientists is the potential use of stem cells in transplantation and cell replacement therapy. There is considerable interest in obtaining stem cell lines from the livestock species of economic importance. A stem cell is a special kind of cell that has a unique capacity to renew itself and to give rise to specialized cell types. A stem cell can also be defined as an uncommitted cell that remains uncommitted unless it receives signal(s) to develop into a specialized cell. There are two types of stem cells: adult stem cells and embryonic stem cells. While the adult stem cells are either unipotent or multipotent and can be available in any tissue, embryonic stem cells can only be derived from the inner cell mass of the blastocysts and are the pluripotent cells. Now from the last few years, a third type of cells from extraembryonic tissues named as fetal stem cells are gaining importance particularly in livestock species where true embryonic stem cells are yet not available. Here livestock fetal stem cells from amniotic fluid, amniotic membrane, umbilical cord blood and cord matrix, fetal fibroblast, and placental cells are discussed.
3 Amniotic Fluid (AF) Stem Cells
The AF, being a safe, reliable, and simple screening tool, is widely used for numerous basic and biomedical applications. The AF comprises of normal embryonic or fetal chipping cells derived from the three germ layers (ectoderm, endoderm, and mesoderm); it possesses the natural precursors of all differentiation lineages.
The isolation of AF cells with stem cell features opens many new venues including regeneration of tissues and ultimately even organs. AF stem cells of early gestation seem to express higher levels of endoderm- and mesoderm-specific markers compared with those of later gestation, while ectodermal markers show no difference. These are clonally expanded in mesenchymal stem cells that exhibit a series of stem cell-specific markers including Oct-4, Nanog, and SSEA-4 and have a wide range in differentiation potential. Recent reports suggest that AF stem cells might have additional benefits. The deployment of AF stem cells for tissue regeneration offers advantages over the use of ES or adult stem cells, namely, (1) AF represents a convenient and non-contested source for obtaining stem cells; (2) isolating them is relatively simpler and rapid; (3) no feeder layers are required for their culturing; (4) they display no spontaneous differentiation in culture; and (5) their stem cell phenotype is not affected by long-term storage. The AF cells in water buffaloes collected from young fetuses (50–70 days) maintained a characteristic round shape and then (80–100 days gestation) changed to cells with different morphologies including spherical cells with nucleus, spherical cells without nucleus, polygonal cells, and freely floating cells (Yadav et al. 2011). The AF cells were found to expand without feeder layer over a period of 100 days (up to passages 20) and overexpress the AP, Oct-4, Nanog and Sox2, GAPDH, and β-actin. These features are also noted in human AF cells. It has been noted that bubaline AF cells could be cultured and maintained in vitro for a prolonged period and offer a potential source of multipotent cells for applications like therapeutic assisted reproduction in animals (Yadav et al. 2011).
4 Amniotic Membrane Cells
The amniotic membrane is a tissue of fetal origin and is composed of three major layers: a single epithelial layer, a thick basement membrane, and an avascular mesenchyme. There are no nerves, muscles, or lymphatics in the amnion. It can be easily separated from the underlying chorion, with which it never truly fuses at the cellular level. The amnion obtains its nutrition and oxygen from the surrounding chorionic fluid, the amniotic fluid, and the fetal surface vessels. One of the basic functions of the amniotic membrane is protection against desiccation to the developing embryo and provides an environment for suspension in which the embryo can grow without distortion by pressure from surrounding structures. The amnion also plays an important role during parturition through maintenance of uterine contraction and secretion of prostaglandins, especially prostaglandin E2. Amnion also expresses prostaglandin-biosynthesis enzymes such as phospholipase, prostaglandin synthase, and cyclooxygenase which are regulated by chorionic gonadotropin, and their receptors are found on the amniotic epithelium. Amniotic epithelium is metabolically highly active throughout gestation, and it is also responsible for regulating the pH of the amniotic fluid. Reports in human demonstrated that both amniotic epithelium cells and amniotic mesenchymal cells express stem cell markers such as OCT-4, which is specifically expressed in ES and germ cells; GATA-4, a marker of definitive embryonic and visceral endoderm; hepatocyte nuclear factor-3β, a marker of definitive endoderm; nestin, which is an intermediate protein and a neural stem cell-specific marker; and Nanog. These facts suggest that not only amniotic epithelium cells but also amniotic mesenchymal cells possess pluripotency.
The bubaline AM cells have been found to exhibit polygonal shape and completed 21 passages in 75 days of continuous culture and expressed Oct-4 (Yadav et al. 2011). The AM and cells derived from it are positive for AP, Nanog, Oct-4, and Sox2 overexpression (Mann et al. 2012). In equine, the differentiation induction for pancreatic and osteogenic cells yielded oil red O-positive cells for pancreatic and alizarin red positive for osteogenic cells. The technique for sampling, isolation, and expansion of equine AM stem cells forms a basis for establishment of a database for collecting and preserving stem cells used in the field of equine regenerative medicine. Human AM are used as healing accelerator and bone induction in dogs. The AM decreased fibrinoleukocytic exudates, inflammation, and suitable experimental cover for different injuries, and further acellular AM has the potential for rapid improvement and bone induction. The AM contains collagen, laminin, and fibronectin which provide an appropriate substrate for bone induction. This substrate has been reported to promote bone induction and might contribute to induction of progenitor cells and/or stem cells in the area where it had been undertaken and has also differentiated into bone.
The AM-derived cells have potential for osteogenic, adipogenic, chondrogenic, and myogenic differentiation in vitro and thus can have potential for use as engraftment material. As fetal tissue is usually discarded after birth, it involves no ethical concerns if it is used as source of MS stem cells. Equine amnion has further advantages for use in stem cell work as it is a rich and easily accessible source of MS cells, as equine amnion is easy to sample immediately after birth and is not attached with the rest of the placenta.
5 Fetal Fibroblasts and Stem Cell-Like Cells
Stem cell-like multipotent stem cells have been noted in the abattoir-derived fetuses of water buffaloes as reports indicate that the pluripotency genes expressed by ES cell are also expressed by fetal fibroblast. In this direction, the expression of AP, Oct-4, Sox2A and Sox2B, β-actin GAPDH, and Nanog has been detected in bubaline fetal fibroblasts (Yadav et al. 2011). The murine and porcine fetal explant cells have also been found to express the pluripotency genes. It was shown that fetal somatic explants contain a subpopulation of somatic stem cells, which can be induced to display features of lineage-uncommitted stem cells. After injection into blastocysts, fetal fibroblast cells differentiated into a variety of cell types including those of the mesodermal lineage; they even migrated into the genital ridge. In vitro, the fetal stem cells exhibit characteristics of ES cells, including extended self-renewal; expression of Oct-4, Stat3, and Akp2 (Tnap); and growth as multicellular aggregates (Kues et al. 2005). This indicates that fetal tissue contains stem cells with greater potency than previously thought, hence, might serve as a new source of animal stem cells.
6 Umbilical Cord Blood Stem Cells
The umbilical cord is a noncontroversial source of mesenchymal-like stem cells. The umbilical cord blood (UCB), which is normally discarded, can be easily collected at the time of delivery. Collection can be accomplished by venipuncture of the umbilical vein of the placenta still in utero or after the expulsion of the placenta itself. Advantages of UCB stem cells are their high proliferative capacity, low risk of viral contamination response to alloantigen, their availability, and donor safety. However, there are limited reports on the cord blood from livestock; main limitation in collection of cord blood in livestock including equines is breakage of the umbilical cord during parturition.
In normal calving, after expulsion of the placenta, small quantity of cord blood (5–6 ml) can be collected. Morphology of buffalo umbilical cord blood, newborn calf blood, and adult buffalo blood revealed that cord blood parameters differed significantly from newborn calf and adult buffalo blood (Singh et al. 2012a, b).
In equine, UCB had significantly lower total erythrocyte (RBC) count, total leucocyte (WBC), lymphocyte, and granulocyte count than those of mare and foal. While RBC count was the highest in foals, WBC, lymphocyte, and granulocyte counts were highest in mares followed by foal and UCB, respectively. Hemoglobin, percent hematocrit values, and platelet count were the least in UCB, followed by mare and foal blood. Unlike buffalo, equine UCB is more intimate to mare blood, as there was significant difference in 8 parameters in UCB and foal blood out of 18 parameters. In man, horse, cattle, and dogs, umbilical cord blood-MS cells have multipotent abilities. UCB-derived stem cells in domestic animals are capable of differentiation in vitro, not only toward mesenchymal cell lineage (osteogenic, chondrogenic, myogenic, and adipogenic) but also toward endodermal (hepatogenic) and ectodermal (neurogenic) lineages under appropriate culturing conditions.
7 Wharton’s Jelly Stem Cells
The umbilical cord provides stem cells in two compartments: umbilical cord blood (UCB) and umbilical cord matrix, also known as Wharton’s jelly (WJ). Besides humans, the stromal cells with certain pluripotency markers have also been reported from WJ of pig and buffalo (Yadav et al. 2012a, b).
The WJ surrounding the two arteries and single vein of the umbilical cord has been observed in buffaloes. The salient features of buffalo cells from WJ include sticky jelly-type tissue, slow growing primary colony (8–10 days embedded cells, spikes formed but not as clear as fibroblasts, and passage time is 3–4 days) (Yadav et al. 2011). Bubaline cord matrix cells could be cultured for more than 100 days in continuous cell culture which expressed Oct-4, Nanog, and Sox (Yadav et al. 2008). The identification of WJ as an alternative source of MS cells provides significant clinical benefits, namely, harvesting, reduction of risks associated with transmitting infections, and acceptable level of HLA mismatch.
8 Placenta Stem Cells
In transition from morula to blastocyst, first differentiation takes place in the ICM cells and the trophectoderm. ICM forms the epiblast and the fetus in early development as well as the source of ES cells. The trophectoderm forms the placenta which supports and protects the developing fetus. The stem cell populations derived from human placenta tissues are chorionic mesenchymal stromal cells and the chorionic trophoblastic cells, both demonstrating variable plasticity. The cells from the placental tissues exhibit the markers of pluripotency (SSEA-4, Oct-4, Stro-1, and TRA 1–81) which are typical mesenchymal markers and have capacity of a wide range of differentiation. They are capable of in vivo differentiation into various types of cell lineages if seeded in scaffolds. The pretreated placenta-derived human MS cells with a hyaluronan mixed ester of butyric and retinoic acid could cure infracted pig hearts. The treated pigs in comparison to untreated infracted pigs had 40 % smaller infarct scar size and a significant improvement of end-systolic wall thickening and circumferential shortening of the infarct border zone. Scarce reports on animal placenta cells suggest that further work on this source is required.
9 Stem Cells in Poultry
Poultry production is a well-organized industry. The chicken eggs have been used in the manufacture of vaccines for more than three decades. One of the remarkable advantages of the avian embryo is its accessibility and availability in plenty. Since the avian embryo is self-contained in a calcified eggshell, it lends itself to direct manipulation. However, compared to other livestock species, the overall progress toward establishing avian stem cells is slow. Advances in culturing avian embryos have also led to developments in avian stem cells. A modern layer lays approximately 300 eggs a year, and the egg white alone contains 4 g of protein. The hen takes much less time to reach sexual maturity than any other livestock species (http://www.poulvet.com/poultry/articles/3.ph, Oct., 1, 2011). Nevertheless, only a few laboratories were involved in research and development in avian ES as well as EG cells (Petitte et al. 2004). The main impetus for the isolation and culture of avian stem cells is the hope that they could be used to generate transgenic birds.
Avian ES cells differentiate into embryoid bodies (EB) as well as various somatic cell lineages and can be used for a variety of applications including production of chimera or transgenic birds for biopharming. With adaptations to high-throughput in ovo vaccination technology, it could be possible to generate high-grade avian somatic chimeras, thereby shortening the time needed for conventional poultry breeding programs to generate superior stock. Therefore, transgenic chicken holds a tremendous potential to revolutionize the biotechnology industry and would contribute significantly to the national economy. At present, many private research companies have initiated research in the field of avian transgenics to harness this powerful emerging technology (http://www.poulvet.com/poultry/articles/3.ph). However, development of stem cell lines and robust methods for production of transgenic birds has been more of a challenge.
10 Fetal Stem Cells in Animal Health and Production
The last two decades has seen a surge of interest in the research and clinical availability of stem cell treatments. Innovative techniques of cell and tissue processing, based on tissue engineering, have been developed. Cell expansion and tissue reconstruction through ex vivo cultures are core processes used to produce engineered tissues with sufficient structural integrity and functionality.
Fetal stem cells expressing pluripotency markers provide strong support to the notion that these cells may be biologically homologous to pluripotent ES cells. There are some reports describing the derivation of putative fetal stem cells in domestic ungulates and pet animals with varying results. Commercial companies are emerging with strategies to enhance stem cell research and applications in veterinary health and livestock production. Formed in 2002, Vet-Stem Inc. (www.vet-stem.com) is the first company to offer regenerative medicine to veterinarians. In 2003, the company introduced the first veterinary stem cell service in the United States. Its technology has been used to treat tendon, ligament, and joint injuries in more than 3,000 horses and more than 1,000 dogs.
The use of fetal and adult stem cells in veterinary medicine is of great promise and is likely to show rapid uptake, as commercially available safe treatments with adipose and bone marrow-derived cells become more widespread. The features and the potential therapeutic properties of fetal stem cells from various sources described here are expected to be studied in more depth and then implemented at the clinical level following international guidelines.
Cell-based therapies with embryonic, fetal, adult, or iPS cells are thought to have great potential for augmenting assisted reproduction and treatment of several degenerative diseases, which currently are without effective therapy. For instance, bone fracture, as well as damaged cartilage, tendons, and ligaments, heal poorly in horses. Equine iPS cells bring new therapeutic potential to the veterinary field and open up the opportunity to validate stem cell-based therapies before clinical studies in humans. As well, stem cell-based studies using the horse as a model more closely replicate human illnesses, when compared with studies in mice. Therefore, the use of reprogrammed cells in these animals may help enhance long-term tissue repair.
11 Conclusions
Science and medicine place a lot of hopes in the development of stem cell research and regenerative medicine. Worldwide media reports about stem cell therapies are becoming common as stem cell applications are being pursued in diverse areas including cardiology, orthopedics, oncology, internal medicine, and assisted reproduction. Till date, significant progress has been made in stem cell research, and new strategies for somatic cell reprogramming have been developed. In view of the ethical problems in deriving ES cells, the researchers are interested in discovering alternative ways of deriving pluripotent stem cells. Non-embryonic fetal stem cells can be isolated during gestation from many different tissues as well as from a variety of extraembryonic tissues such as the AF and placenta. In domestic ungulates as well as poultry, the ES and EG cells and adult stem cell technology are still at initial stages of development. Cells isolated from livestock species might have important application in studies in developmental biology and especially in unraveling appropriate culture conditions and markers for stem cells. Several potential applications of transgenic chickens have been identified, and the two main areas include agriculture and health care. In addition, although many of the non-embryonic stem cells have demonstrated ES cell-like morphology and expression of some ES cell-specific markers, concerns should be raised over the lack of data regarding long-term culture and maintenance of pluripotency. Ethical concerns in use of animals for production of therapeutic concerns have to be followed strictly. Stem cells obtained from transgenic livestock having desired traits hold the key for harvesting the maximum potential from this promising technology.
References
Abdulrazzak H, Moschidou D, Jones G, Guillot PV (2010) Biological characteristics of stem cells from foetal, cord blood and extraembryonic tissues. J R Soc Interface 6:689–706
Azari O, Babaei H, Derakshanfar A, Nematollahi-Mahani SN, Poursahebi R, Moshrerfi M (2011) Effect of transplanted mesenchymal stem cells isolated from Wharton’s jelly of caprine umbilical cord on cutaneous wound healing: histopathological evaluation. Vet Res Commun 35:211–222
Chen J, Lu Z, Cheng De, Peng S, Wang H (2011) Isolation and characterization of porcine amniotic fluid-derived multipotent stem cells. PLoS One 6(5):e19964. doi:10.1371//journal.pone.0019964
Cremonesi F, Corradetti B, Lange CA (2011) Fetal adnexa derived stem cells from domestic animal: progress and perspectives. Theriogenology 75:1400–1415
Kues WA, Petersen B, Mysegades W, Carnwath JW, Niemann H (2005) Isolation of murine and porcine fetal stem cells from somatic tissue. Biol Reprod 72:1020–1028
Lange-Consiligio A, Corradetii B, Rutigliano L, Cremonesi F, Bizzaro D (2011) In vitro studies of horse umbilical cord matrix-derived cells: from characterization to labeling for magnetic resonance imaging. Open Tissue Eng Regen Med J 4:120–133
Lim JH, Byeon YE, Ryu HH, Jeong YH, Lee YW, Kim WH, Kang KS, Kweon OK (2007) Transplantation of canine umbilical cord blood-derived mesenchymal stem cells in experimentally induced spinal cord injured dogs. J Vet Sci 8:275–282
Mann A, Yadav RP, Singh J, Kumar D, Singh B, Yadav PS (2012) Culture, characterization and differentiation of cells from buffalo amnion. Cytotechnology. doi:10.1007/s10616-012-9464-z
Medicetty S, Bledsoe AR, Fahrenholtz CB, Troyer D, Weiss ML (2004) Transplantation of pig stem cells into rat brain: proliferation during the first 8 weeks. Exp Neurol 1:32–41
Pappa KI, Anagnou NP (2009) Novel sources of fetal stem cells: where do they fit on the developmental continuum? Regen Med 4(3):423–433, http://www.ncbi.nlm.nih.gov/pubmed/19438317
Petitte JN, Liu G, Yang Z (2004) Avian pluripotent stem cells. Mech Dev 121:1159–1168
Singh J, Kumar P, Mann A, Singh V, Duhan JS, Yadav PS (2012a) Cell morphology and mucopolysaccharides in early gestation buffalo umbilical cord. Indian Vet J 89(11):36–38
Singh J, Singh V, Mann A, Singh JK, Jerome A, Sarkar SK, Duhan JS, Yadav PS (2012b) Collection of cord blood and comparison of its hematological parameters with newborn calf and adult buffalo. Indian J Anim Sci 82(8):865–867
Yadav PS, Kues WA, Herrmann D, Carnwath JW, Niemann H (2005) Bovine ICM derived cells express the OCT-4 ortholog. Mol Reprod Dev 72(2):182–190
Yadav PS, Jayanti Tokas, Sharma RK, Inderjeet Singh, Sethi RK (2008) Buffalo amniotic fluid, umbilical cord matrix and early fetal explants as possible source of adult stem cells. In: IX annual conference of Indian society of animal genetics and breeding, NASC complex Delhi, 3–4 July 2008, p 118
Yadav PS, Mann A, Singh V, Yashveer S, Sharma RK, Singh I (2011) Expression of pluripotency genes in buffalo (Bubalus bubalis) amniotic fluid cells. Reprod Domest Anim 46:705–711
Yadav PS, Mann A, Singh J, Kumar D, Sharma RK, Singh I (2012a) Buffalo (Bubalus bubalis) fetal skin derived fibroblast exhibited characteristics of stem cells. Agric Res. doi:10.1007/s 40003-012-0013
Yadav PS, Singh RK, Singh B (2012b) Fetal stem cells in farm animals: applications in health and production. Agric Res 1:67–77
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer India
About this chapter
Cite this chapter
Yadav, P.S., Gulati, B.R. (2013). Fetal Mesenchymal Stem Cells in Farm Animals: Applications in Health and Production. In: Salar, R., Gahlawat, S., Siwach, P., Duhan, J. (eds) Biotechnology: Prospects and Applications. Springer, New Delhi. https://doi.org/10.1007/978-81-322-1683-4_19
Download citation
DOI: https://doi.org/10.1007/978-81-322-1683-4_19
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-1682-7
Online ISBN: 978-81-322-1683-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)