Abstract
Coumarin derivates have been considered an ideal target of personalized medicine because of their pharmacological characteristics as vitamin K antagonists. Thus, many pharmacogenetic investigators have focused in this field, developing numerous warfarin algorithms based on CYP2C9 and VKORC1 genotypes. These pharmacogenetic algorithms include both genetic and nongenetic factors.
Coumarin derivates (warfarin in the UK and USA, acenocoumarol and phenprocoumon in European countries) are the most prescribed oral anticoagulants in the prevention and treatment of thromboembolic events associated with atrial fibrillation, prosthetic valves, venous thromboembolism, and orthopedic surgery.
Vitamin K antagonists have demonstrated efficacy in anticoagulation although they have a narrow therapeutic window. Patients need frequent monitoring by measuring the prothrombin time. The main challenge for physicians is to introduce patients as soon as possible within the therapeutic range (INR 2–3) in the beginning of therapy as the risk of undercoagulation, INR <2 (risk of thrombus), or overanticoagulation, INR> 4 (risk of bleeding), is higher in this period.
The adjustment of the dose to achieve effective and stable anticoagulation depends on several environmental factors including age, gender, weight, concomitant medications and interactions with foods containing vitamin K, smoking status and alcohol intake, and genetic factors.
Allelic variants in CYP2C9*2 (Arg144Cys, rs1799853), CYP2C9*3 Ile359Leu (rs1057910), and VKORC1 (rs9923231, rs9934438) gene polymorphisms have demonstrated an influence of about 40 % on coumarin-required dose.
Despite of knowledge that pharmacogenetic models can improve dosing recommendations according to CYP2C9 and VKORC1 polymorphisms and thus would decrease the risk of thrombus and bleeding events during initial phases of anticoagulation, the translation of pharmacogenetic algorithms into clinical practice is a challenge to pursue in the advancement of personalized medicine.
On the other hand, a new wave of oral anticoagulants has been developed to improve the efficiency and safety of anticoagulant therapy and avoid the several drawbacks of vitamin K antagonists. This new generation of anticoagulants is expected to be the alternative in prevention of stroke and systemic embolism in non-valvular atrial fibrillation patients.
In this chapter, we will review the most relevant pharmacogenetic studies performed with coumarin derivates, the application of pharmacogenetics in predicting the optimal coumarin derivates’ initial dose based on genetic and environmental factors, genetic tests available for determination of main gene polymorphisms associated to warfarin sensitivity, the development of new oral anticoagulants (dabigatran, rivaroxaban, apixaban), and comparison of different pharmacological therapies available to use in patients with non-valvular atrial fibrillation requiring long-term anticoagulation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
1.1 Main Indications for Anticoagulation Therapy
Since the 1950s, coumarin derivates (warfarin, acenocoumarol, and phenprocoumon) have been the cornerstone for prevention of stroke and systemic embolism in patients with atrial fibrillation (AF) and for prevention of recurrent venous thrombosis in patients with venous thromboembolism (VTE) and in patients with prosthetic heart valves (Hirsh 1992; Hirsh et al. 2001; Daly and King 2003; Davis et al. 2011; Dahl 2012).
1.2 Management of Antithrombotic Therapy
1.2.1 Oral Anticoagulation with Coumarin Derivates in AF Patients
Starting long-term oral anticoagulation therapy depends on stroke recurrence risk factors present in the patient. The AF Investigators and Stroke Prevention in Atrial Fibrillation (SPAF) group developed a scheme for assessing the risk of stroke through the CHADS2 (congestive heart failure, hypertension, age ≥ 65 years, diabetes mellitus, stroke) system (Camm et al. 2010; Furie et al. 2011; Skanes et al. 2012; You et al. 2012). This system assigned two points to a stroke antecedent and one point to other conditions (congestive heart failure, hypertension, age ≥ 65 years, diabetes mellitus). Risk assessment of thromboembolic and hemorrhagic events based on CHADS2 classification is described in Table 21.1.
Other clinically relevant nonmajor risk factors include female sex, age 65–74 years, and vascular disease (complex aortic plaque and peripheral arterial disease). The CHADS2 classification was modified including new parameters to improve the evaluation of the embolism risk, especially for patients classified with CHADS2 score < 2, creating the CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 [doubled], diabetes mellitus, stroke [doubled], vascular disease, age 65–74, and sex female). According to this new classification, the European Society of Cardiology (ESC) recommendations for vitamin K antagonists (AVKs) treatment are made in function of CHA2DS2-VASc scores (Camm et al. 2010):
-
CHA2DS2-VASc ≥ 2 (one major risk factor or ≥2 risk factors not clinically relevant) treatment with oral anticoagulant (OAC) with a dose adjusted to INR 2–3
-
CHA2DS2-VASc = 1 (1 risk factor for not clinically relevant) OAC treatment with a dose adjusted to INR 2–3 or aspirin 75–325 mg. OAC preferable to aspirin
-
CHA2DS2-VASc = 0 (no risk factors) aspirin 75–325 mg daily or no antithrombotic therapy. Preferably aspirin to antithrombotic therapy
This modification of CHADS2 is not considered in other countries like USA and Canada (Furie et al. 2011; Skanes et al. 2012; You et al. 2012).
1.2.2 Oral Anticoagulation with Coumarin Derivates in VTE
Anticoagulant therapy is effective in prevention of recurrent venous thrombosis in patients with VTE. This treatment begins with low molecular weight heparin (LMWH) or unfractionated heparin (UFH) and oral anticoagulant therapy one day after. Double anticoagulation LMWH + OAC is maintained until INR range 2–3 is reached on two consecutive days, which happens approximately in the first 4–5 days (Duran Parrondo et al. 2003).
Anticoagulant therapy is advisable to be maintained for 6 weeks to 3 months in patients with symptomatic distal or proximal thrombosis after surgery and at least 6 months in patients with idiopathic proximal thrombosis. OAC is recommended indefinitely in situations of idiopathic recurrent thrombosis or inherited or acquired thrombophilia (Duran Parrondo et al. 2003).
1.2.3 Oral Anticoagulation with Coumarin Derivates in Patients with Prosthetic Heart Valve
The risk of thromboembolism in the first 6 months after implantation of a prosthetic valve is particularly high (Eitz et al. 2008). There are three approaches for anticoagulant therapy in patients after valve surgery, depending on the bleeding risk score:
-
Subcutaneous UFH prophylactic dose + OAC started from the first postoperative day
-
Intravenous UFH + OAC from the second postoperative day. HNF to reach therapeutic INR
-
LMWH + OAC since the first postoperative day. LMWH to achieve therapeutic INR
After the first 6 months, the risk of bleeding is greatly reduced if the patient is regular and adherent with the treatment and complies with the drug dosage regimens; due to these, patients must take lifetime oral anticoagulation (Eitz et al. 2008).
1.3 Drugs Pharmacology and Pharmacokinetics
1.3.1 Vitamin K Antagonists
1.3.1.1 Warfarin
Warfarin is worldwide prescribed. This coumarin derivate is administered as a racemic mixture of R-warfarin and S-warfarin. The S-enantiomer has an anticoagulant activity of 3–5 times greater than the enantiomer R-warfarin (Choonara et al. 1986). S-warfarin is mainly metabolized by CYP2C9. Although R-warfarin is also metabolized through the CYP2C9 pathway, there are other minor isoenzymes involved in the metabolism of this enantiomer, such as CYP1A1, CYP1A2, CYP2C8, CYP2C18, and CYP2C19 (Rettie et al. 1992; Zhang et al. 1995; Wienkers et al. 1996; Ngui et al. 2001).
1.3.1.2 Acenocoumarol
Acenocoumarol is the main oral anticoagulant prescribed in many European countries (Beinema et al. 2008; Markatos et al. 2008; Spreafico et al. 2008; López-Parra et al. 2013). It is orally administered as a racemic mixture of enantiomers (R-) and (S-). Biotransformation is performed in the liver by Cytochrome P450. The most important metabolism enzyme of this drug is CYP2C9 isoenzyme, which performs a hydroxylation at position 6, 7, and 8 of both enantiomers. The S-enantiomer is rapidly metabolized (t 1/2 < 2 h); therefore, the anticoagulant activity depends on R-acenocoumarol enantiomer. Unlike S-acenocoumarol enantiomer, R-acenocoumarol enantiomer is also metabolized by CYP1A2, CYP3A5, and CYP2C19 isoenzymes (Thijssen et al. 2000).
1.3.1.3 Phenprocoumon
Phenprocoumon, like other coumarin derivates, is administered as a racemic mixture of the S- and R-enantiomers. Bioavailability is over 90 %, and it is stereoselectively metabolized by Cytochrome P-450 to inactive hydroxylated metabolites (Alberio 2003). Approximately 65 % of phenprocoumon dose is eliminated through the urinary tract and the remaining 35 % by the fecal via (Toon et al. 1985). CYP2C9 and CY3A4 are the main isoenzymes in the metabolism of phenprocoumon (Ufer 2005). The two enantiomers R- and S-phenprocoumon reach their half-life time around 110–130 h from the first-dose administration (Jähnchen et al. 1976).
1.3.2 Mechanism of Action of Vitamin K Antagonists
Vitamin K antagonists exert their effect by inhibiting vitamin K epoxide reductase complex 1 (VKORC1), decreasing vitamin K. Vitamin K is an essential cofactor in the carboxylation of glutamate residues at the N-terminal region of the vitamin K-dependent proteins, synthesis of vitamin K–dependent coagulation factors (factors II, VII, IX, and X), and fibrinolytic proteins, such as protein C and S (Fig. 21.1). These vitamin K–dependent proteins are inhibited by the action of coumarin derivates. When antagonizing these vitamin K dependent coagulation factors, no interaction between them and the calcium present in the vascular subendothelium occurs. The inhibition of coagulation factors causes low plasma levels of prothrombin, which generates the formation of fibrin required to trigger coagulation (DrugBank 2012b; The Pharmacogenomics Knowledge Base 2012).
Mechanism of action of coumarin derivates. CYP1A1 Cytochrome P450 family 1, subfamily A, polypeptide 1; CYP1A2 Cytochrome P450 family 1, subfamily A, polypeptide 2; CYP3A4 Cytochrome P450 family 3, subfamily A, polypeptide 4; CYP2C9 Cytochrome P450 family 2, subfamily C, polypeptide 9; CYP2C8 Cytochrome P450 family 2, subfamily C, polypeptide 8; CYP2C18 Cytochrome P450 family 2, subfamily C, polypeptide 18; CYP2C19 Cytochrome P450 family 2 subfamily C, polypeptide19; CYP2C3A5 Cytochrome P450 family 3, subfamily A, polypeptide 5; VKORC1 vitamin K epoxide reductase complex, subunit 1; R-W enantiomer R-warfarin; S-W enantiomer S-warfarin; R-A enantiomer R-acenocoumarol; S-A enantiomer S-acenocoumarol; R-P enantiomer R-phenprocoumon; S-P enantiomer S-phenprocoumon; inhibition VKE vitamin K epoxide; VKH vitamin K hydroquinone; CO 2 carbon dioxide; O 2 oxygen
1.3.3 Pharmacokinetic Differences Between AVKs
The differences between these molecules lie on their pharmacokinetics properties. Acenocoumarol presents a half-life time of 8–11 h, whereas warfarin half-life time is fourfold the acenocoumarol half-life, from 34 to 42 h, and reaches its maximum plasma concentration peak at 90 min after oral administration, and phenprocoumon has a half-life time of 110–130 h after initial oral administration (Shirolkar et al. 2010). Main differences in pharmacokinetics of vitamin K antagonists are shown in Table 21.2.
2 Pharmacogenetics of Vitamin K Antagonists
Although coumarin derivates are effective, the management of oral anticoagulation therapy is complicated. The pharmacokinetic and pharmacodynamic properties lead to a variable, unpredictable, and independent response in each individual treatment.
The anticoagulant activity is measured by a standardized laboratory test, the international normalized ratio (INR). The beginning of anticoagulation therapy is the most problematic period due to the risk of thrombi (associated to an INR < 2), and the risk of bleeding (associated to INR > 4) is higher in the first 3 months (Hylek et al. 2003; Ufer 2005). This risk is high because each patient requires individualized doses to achieve stability.
Currently, clinicians prescribe an initial dose of coumarin derivates based on clinical parameters, and dose adjustment is made by titration. The first INR monitoring is performed at day 3 or 4 after beginning anticoagulant therapy. Depending on the INR value obtained, the initial dose is increased, decreased, or maintained until three consecutive INR values are obtained within the therapeutic range with a dose variation lower than 10 %, which is considered as a stable anticoagulation status. The main goal of oral anticoagulation therapy is to keep the patient in INR therapeutic range. Patients treated with oral anticoagulants need periodic monitoring of anticoagulant doses to maintain a stable anticoagulation status.
There are several factors that affect the anticoagulant activity. Age, sex, weight, concomitant drugs (CYP450 inducers or inhibitors), other associated diseases, alcohol and smoking status, vitamin K intake, and genetic factors are responsible for interindividual dose–response variability.
For all these reasons, coumarin derivates have been considered as an ideal target for personalized medicine. Studies have focused on finding genetic markers that can predict a better response to treatment in terms of efficacy and safety.
2.1 Main Gene Polymorphisms Involved in Oral Anticoagulant Therapy
The most studied genetic variations correspond to genes encoding CYP2C9 and vitamin K epoxide reductase complex 1 (VKORC1). Genetic variations in these two genes are responsible for approximately 30 % of the variability in the variability dose required (Rieder et al. 2005; Li et al. 2009). Table 21.3 shows the main gene polymorphisms involved in anticoagulant response and the associated effect.
2.1.1 CYP2C9
CYP2C9 isoenzyme is important in the liver. It is responsible of metabolizing approximately 15 % of drugs that undergo phase I metabolism (Evans and Relling 1999). Two common polymorphisms in CYP2C9 have been described. These SNPs (single nucleotide polymorphisms) are CYP2C9*2 (Arg144Cys, rs1799853) and CYP2C9*3 (Ile359Leu; rs1057910). These allelic variants encode enzymes with reduced activity of 12% and 5% respectively compared to the wild-type allele (Rettie et al. 1994; Haining et al. 1996; Takanashi et al. 2000). Carriers of either allele variant CYP2C9*2 or CYP2C9*3 have more overanticoagulation risk (risk of bleeding), require lower doses, and need more time to achieve stable INR than wild-type patients for this gene (Aithal et al. 1999; Becquemont 2008).
Allelic variant frequencies differ between races, for example, CYP2C9*2 polymorphism is extremely rare in Asian population, the other allelic variant CYP2C9*3 was not found in this population (Rosemary and Adithan 2007), and in African Americans is around 6 % of the population (Limdi et al. 2008). However, 15–30 % of white individuals have any allelic variant for this gene (Jonas and McLeod 2009).
CYP2C9*8 (Arg150His, rs7900194) is manifested in 10–12 % of African American individuals (Scott et al. 2009; Perera et al. 2011). This allelic variant is responsible for a decrease of enzyme activity. Therefore, individuals carrying this minor allelic variant needed lower warfarin dose to achieve optimal levels of INR compared with wild-type individuals (Cavallari et al. 2010). These data have been corroborated by another study conducted with South African individuals with similar results (Mitchell et al. 2011).
2.1.2 VKORC1
Oral anticoagulants act by inhibiting vitamin K regeneration. This effect is performed through inhibition of vitamin K epoxide reductase. The vitamin K epoxide reductase complex 1 (VKORC1) gene encodes this protein and is located on the short arm of chromosome 16. It contains three exons and two introns and encodes a membrane protein of 163 amino acid residues (D’Andrea et al. 2005). It is located in the endoplasmic reticulum membrane (Cain et al. 1997). Several polymorphisms in the VKORC1 gene are associated to warfarin dose variability, mainly located in the noncoding region (Spreafico et al. 2008). Four haplotypes (VKORC1*1,*2,*3,*4) have been associated to variability in warfarin dose in Caucasian population (Osman et al. 2006). VKORC1*1 haplotype corresponds to absence of polymorphism; VKORC1*2 haplotype with C1173T allelic variation (rs9934438), which is in linkage disequilibrium with the G1639A polymorphism (rs9923231) in the 5′-upstream region and with the C2255T polymorphism (rs2359612); VKORC1*3 haplotype with the G9041A polymorphism (rs7294); and VKORC1*4 haplotype with C6009T (rs17708472) polymorphism (Loebstein et al. 2007; Stehle et al. 2008). According to Geisen et al. (2005), these haplotypes cover about 99 % of the genetic variability in European population. The VKORC1*2 haplotype is associated with coumarin derivate sensitivity, and haplotypes *3 and *4 are associated with an increase in warfarin dose requirements to achieve the same level of anticoagulation than wild-type patients for this gene (Rieder et al. 2005, 2007; Wadelius et al. 2005; Cini et al. 2012).
VKORC1 *2 is the most prevalent haplotype among Caucasian and Asian population. These haplotype alters the binding site of the transcription factor, which leads to decreased expression of the protein (Wang et al. 2008). According to HapMap Project (dbSNP Short Genetic Variation, 2012), VKORC1*2 haplotype frequency population observed in Caucasians is 39.8 % for wild-type, 40.7 % for heterozygous mutant, and 19.5 % homozygous mutant. It has been described that this VKORC1 polymorphism has an influence of 11–30 % of warfarin dose variability in Caucasian and Asian populations (Veenstra et al. 2005; Carlquist et al. 2006; Obayashi et al. 2006; Borgiani et al. 2007; Wadelius et al. 2007). However, the influence of this polymorphism in warfarin dose variability in North American blacks is 4–10 % (Takahashi et al. 2006; Momary et al. 2007; Schelleman et al. 2007).
2.2 Other Gene Polymorphisms Involved in Anticoagulant Response
2.2.1 CYP4F2
CYP4F2 catalyzes vitamin K1 hydroxylation in the liver. It is reported an allelic variant (rs2108622; V433M) in CYP4F2 gene that has influence on required dose of coumarin derivates. The allelic variant CYP4F2*3 is responsible for a reduced enzyme activity. Therefore, CYP4F2 V433M polymorphism has been associated with warfarin resistance (Caldwell et al. 2008). Although there is an association between the presence of CYP4F2*3 polymorphism and increased dose required, the influence of this polymorphism on total variability warfarin dose is of 1–2 % (Scott et al. 2010). However, several studies have shown that the presence of the allelic variant CYP4F2 V433M is associated to ischemic stroke and myocardial infarction (Fava et al. 2008; Fu et al. 2009).
2.2.2 CYP2C19
Acenocoumarol anticoagulant activity lies mainly on R-acenocoumarol, which is metabolized mainly by CYP2C9, but other CYP450 isoenzymes, including CYP1A2 and CYP2C19, are also involved. Although polymorphisms in these isoenzymes have not shown influence on the required dose, taking concomitant drugs metabolized by CYP450, such as proton pump inhibitors, mainly metabolized by CYP2C19, may increase the risk of overanticoagulation in long-term oral anticoagulation therapy (Teichert et al. 2011b)
2.2.3 GGCX
The gene encoding gamma-glutamyl carboxylase has been studied as a potential candidate gene that affects the pharmacodynamics of warfarin. The gamma-glutamyl carboxylase enzyme catalyzes biosynthesis of vitamin K–dependent coagulation factor. GGCX is an essential cofactor in the reduction of vitamin K epoxide 2–3 to biologically active vitamin K hydroquinone, responsible for synthesizing coagulation factors that trigger the coagulation cascade (Stafford 2005). A Swedish study examined the effect of a polymorphism in the GGCX (rs12714145) resulting in a small but significant association with the dose of warfarin (Wadelius et al. 2005). Another variant allele in the GGCX gene (rs11676382) is associated with a reduction in the required dose of warfarin and a total implication of 2 % in the dose variability (King et al. 2010).
2.2.4 CALU
Calumenin, encoded by CALU, binds to GGCX and inhibits the binding site of vitamin K epoxide. It produces an inhibition of the anticoagulant effect. An allelic variant (rs339097) in CALU gene has been described to predict higher warfarin doses in African-American and in a Egyptian population (Wajih et al. 2004; Shahin et al. 2011).
2.3 Influence of Gene Polymorphisms in Oral Anticoagulant Response: Drug Efficacy, Prognosis, and Adverse Drug Effects
Early research focused on the influence of CYP2C9 polymorphisms on the variability of the anticoagulant response. A small study conducted in England compared 36 patients with doses of ≤1.5 mg/day warfarin with 100 individuals from a random control group who had started as standard anticoagulation therapy (Aithal et al. 1999). The goals were to analyze the effect of CYP2C9*2 and CYP2C9*3 polymorphisms in the dose and the incidence of bleeding complications. In the low-dose group, 89 % (29/36) had at least one variant allele for CYP2C9. The incidence of major bleeding was higher in the low-dose group compared with the random controls (ratio 3.68 [1-43-9.5] p-value = 0.007).
An Italian study was one of the first to manifest that patients carrying CYP2C9*2 and/or CYP2C9*3 polymorphisms require lower doses of warfarin to achieve therapeutic INR range and had a higher rate of bleeding complications than wild-type patients for this gene (Margaglione et al. 2000). The study recruited 180 Caucasian patients and concluded that patients with CYP2C9*1 (wild-type) required higher dose than patients with a variant allele CYP2C9*2 or CYP2C9*3 (6.7, 5.2, and 3.8 mg, respectively). Fifty-nine bleeding episodes occurred (10 major and 49 minor events) in 36 patients; the incidence of bleeding episodes in patients with a variant allele CYP2C9*2 and/or CYP2C9*3 haplotypes was 27.9 % while only 12.8 % of wild-type patients showed some hemorrhagic event.
Despite the evidence that some patients carrying *2 or *3 polymorphisms in CYP2C9 metabolism isoenzyme showed warfarin sensitivity and increased risk of overanticoagulation, this could only explain 10 % of the warfarin dose variability (Gage et al. 2004). For this reason, research efforts focused on polymorphisms in the target of coumarin derivates (Li et al. 2004; Rost et al. 2004). It has been described that the presence of polymorphisms in the VKORC1 associated to the required dose of coumarin derivates. The identification of polymorphisms in the VKORC1 led researchers to study the influence of genes encoding VKORC1 and variability in the warfarin-required dose variability.
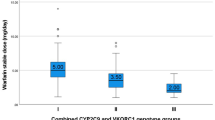
Several studies showed association between the presence of VKORC1 haplotypes and dosage requirements and with low required dose (sensitivity) warfarin (D’Andrea et al. 2005; Rieder et al. 2005; Wadelius et al. 2005; Yuan et al. 2005) . Rieder et al. (2005) identified 10 SNPs and two haplotypes related to variability in the dose required. Common haplotypes (H1, H2, H7, H8, and H9) were clustered forming two distinct evolutionarily distant groups designated A (H1 and H2) haplotype and B haplotype (H7, H8, and H9). The A haplotype was associated to warfarin sensitivity and B haplotype was associated to warfarin resistance. Patients were classified according to the haplotypes, being the A/A haplotype low dose, A/B haplotype intermediate dose, and B/B haplotype high dose. The maintenance dose of warfarin varied from 2.7 ± 0.2 mg/day for low dose (A/A) to 4.9 ± 0.2 mg/day for intermediate dose (A/B) and 6.2 ± 0.3 mg/day for high dose (B/B) (p-value < 0.001).
Aquilante et al. (2006) tested the impact of CYP2C9 and VKORC1 polymorphisms on warfarin response in a cohort of 350 patients with stable INR and dose. Patients with the AA genotype for VKORC1 3673 SNP received 22.5 mg/week less than GG carriers for this gene (p-value < 0.001). On the other hand, patients carrying one allelic variant CYP2C9*2 or CYP2C9*3 (*1/*2 or *1/*3) required 9.5 mg/week less than wild-type patients (*1/*1). In addition, patients carrying two allelic variants (CYP2C9*2/*2, CYP2C9*2/*3, or CYP2C9*3/*3) needed a dose of 14.1 mg/week lower than wild-type patients.
The influence of GGCX gene polymorphisms in addition to VKORC1 polymorphisms was analyzed in 201 patients, mostly Caucasian, treated with long oral anticoagulation therapy (Wadelius et al. 2005). They found five common SNPs, four of them in linkage disequilibrium (rs9923231, rs9934438, rs2359612, and rs7294) and a fifth SNP (rs11150606) with lower allele frequency (4 %). These SNPs were responsible for approximately 30 % of the variability in the required dose of warfarin. The GGCX gene polymorphism (rs12714145) showed a small but significant association with the dose of warfarin.
Allelic variants in CYP2C9 and VKORC1 and clinical factors can explain 54 % of the dose variability. An allelic variant (rs2108622) in CYP4F2 gene was identified in a small sample (Caldwell et al. 2008) which contributes to increase the prediction of the required dose of warfarin to 56 %. CC carriers (wild-type) require lower doses than CT or TT patients for this gene. This allelic variant is associated to coumarin derivates resistance.
The main objectives when a patient starts oral anticoagulation therapy with coumarin derivates are the time necessary to reach the INR therapeutic range and time within therapeutic range (TTR). Pharmacogenetics aims to improve the achievement of these goals through genotyping patients. Schwarz et al. 2008 evaluated the influence of CYP2C9 and VKORC1 polymorphisms on warfarin anticoagulation response in Caucasian populations. Patients with A/A haplotype for VKORC1 gene reached INR therapeutic range and overanticoagulation (INR > 4) before the remaining patients. Allelic variants in CYP2C9 were not decisive in achieving INR therapeutic range. However, patients carrying a variant allele CYP2C9*2 or CYP2C9*3 reached INR > 4 before wild-type patients for this gene. These findings are consistent with those found by Millican et al. (2007) in which those VKORC1 allelic variant carriers showed initial sensitivity to warfarin in a Caucasian cohort.
Personalized medicine and genetic algorithms should be useful for multiracial population; influence of genetic polymorphisms has been also evaluated in other populations different from Caucasian. Limdi et al. (2009) studied the influence of genetic polymorphisms in CYP2C9 and VKORC1 in a cohort of 521 patients that included African Americans and European Americans. They observed the influence of CYP2C9 and VKORC1 gene polymorphisms in TTR and risk of overanticoagulation (INR > 4). African Americans showed other allelic variants in addition to CYP2C9*2 and CYP2C9*3 allelic variants, CYP2C9*5, CYP2C9*6, and CYP2C9*11, while American European allelic variants showed only CYP2C9*2 and CYP2C9*3. Furthermore, the genotype frequency of VKORC1 G1639A polymorphism was higher among the European population than in the African population (60.4 % vs. 20.1; p-value < 0.001). Carriers of an allelic variant in CYP2C9 and VKORC1 reached the therapeutic INR range sooner than wild-type patients for these genes. On the other hand, VKORC1 polymorphism is more decisive than CYP2C9 polymorphisms in reaching the INR therapeutic range. Patients carrying CYP2C9 and/or VKORC1 allelic variant showed more overanticoagulation incidence (p-value < 0.0001) than patients without allelic variants for these genes. Overanticoagulation frequency was lower in African American patients (60 episodes in 46 patients) than European American patients (98 episodes in 78 patients, p-value = 0.017), so genetic polymorphisms in CYP2C9 and VKORC1 influence on the variability of warfarin dose in both populations.
Prediction of the required warfarin dose before starting oral anticoagulation treatment with coumarin derivates is based on the determination of genetic polymorphisms that alter pharmacokinetics (CYP2C9), and pharmacodynamics (VKORC1) is more accurate than the dose prediction based on clinical parameters. The question is whether genetic polymorphisms keep their influence after the stable dose has been established for each patient. A recent study (Lund et al. 2012) tested the influence of VKORC1 G1639A CYP2C9*2 and CYP2C9*3 polymorphisms, during the first 3 months of anticoagulation therapy in 557 patients. The main goal was to assay the prevalence of INR > 5 (risk of bleeding), and the secondary aims were to quantify rate of bleeding events, time to reach the therapeutic range of warfarin stable dose, and time to achieve stable anticoagulation. Thirty-two percent of patients with genotype AA in VKORC1 had some INR > 5 in the first month, while patients with AG or GG genotype who showed INR > 5 were 12.4 and 5.7 %, respectively (p-value < 0.001). In the same analysis, repeated at the third month, VKORC1 polymorphisms had no influence in presenting INR > 5 (AA 2.9 %, AG 3.2 %, and GG 5.2 %). Patients with the AA genotype for VKORC1 presented more bleeding events than wild-type patients for this gene (4.9 % vs. 0.47 %, p-value < 0.009). Allelic variants in CYP2C9 showed no influence on INR > 5 or bleeding rate. VKORC1 and CYP2C9*2 polymorphisms also showed an influence on required stable doses between genotypes: (1) AA 2 mg, AG 4 mg, and GG 4.5 mg for VKORC1 genotype; (2) 4 mg for CYP2C9 wild-type; and (3) 3.6 mg for CYP2C9*2. According to these results, the determination of CYP2C9 and VKORC1 genotypes prior to therapy initiation is positive, but polymorphisms showed no influence after the first month.
In conclusion, polymorphisms in CYP2C9 and VKORC1 are responsible for approximately 40 % of the variability in dose (Bodin et al. 2005; Manolopoulos et al. 2010; Teichert et al. 2009, 2011a; Wadelius et al. 2009). Environmental factors together with genetic factors account for at least 50 % of the variability in the required dose of coumarin derivates (Cadamuro et al. 2010; D’Andrea et al. 2008; Hirsh et al. 2001; Limdi and Veenstra 2008; Schelleman et al. 2008). Individuals carrying CYP2C9 (*2,*3) and/or VKORC1 (*2) allelic variants need lower coumarin derivate dose than wild-type patients to achieve the same level of anticoagulation (Schalekamp et al. 2006). After CYP2C9*2 and CYP2C9*3 allelic variants, CYP2C9*5, CYP2C9*6, CYP2C9*11 allelic variants also show prominent prevalence in African Americans, compared to Caucasian population. The SNP G1639A in VKORC1 gene was higher among the European population than in the African population (Limdi et al. 2008). VKORC1 G1639A and CYP2C9*2 and CYP2C9*3 polymorphisms showed influence in the initial required warfarin dose (D’Andrea et al. 2005; Rieder et al. 2005; Lund et al. 2012).
2.4 Application of CYP2C9 and VKORC1 Gene Polymorphisms in the Management of Anticoagulant Therapy. Development of a Treatment Protocol: Pharmacogenetic Dosing Algorithms
Environmental factors together with genetic factors account for at least 50 % of the variability in the required dose of coumarin derivates (Cadamuro et al. 2010; D’Andrea et al. 2008; Hirsh et al. 2001; Limdi and Veenstra 2008; Schelleman et al. 2008). In 2007, the FDA (Food and Drug Administration) accepted that CYP2C9 and VKORC1 genotypes may be useful in recommending initial warfarin dosage (FDA News and Events 2012c). In 2010, the determination of polymorphisms CYP2C9*2 (rs1799853), CYP2C9*3 (rs1057910), and VKORC1 (rs9923231, rs9934438) was included in a table of recommended starting dose of warfarin (FDA. U.S. Food and Drug Administration 2012a).
Several dosing algorithms including demographic, clinical, and genetic variants (CYP2C9 and VKORC1) as predictors of the required dose of coumarin derivates have been proposed. The goals of these studies were improving efficiency and reducing adverse events (bleeding and thrombosis) that occur during initiation of oral anticoagulation therapy (Sconce et al. 2005; Gage et al. 2008; International Warfarin Pharmacogenetics Consortium et al. 2009; Wadelius et al. 2009; Lenzini et al. 2010; Markatos et al. 2008).
Studies conducted to predict warfarin, acenocoumarol, or phenprocoumon dose can be classified according to their particular objectives. Many pharmacogenetic studies have focused on searching a prediction of warfarin maintenance dose. These maintenance doses of warfarin include genetic factors (CYP2C9 and VKORC1) and also demographic and clinical parameters (age, sex, weight, amiodarone). These studies provided a basis for studies focused on developing algorithms that could be able to predict the initial required dose of warfarin before starting anticoagulation therapy with coumarin derivates. Other type of studies in the prediction of the required dose included dose adjustments depending on the INR values obtained in the first few days of therapy.
2.4.1 Pharmacogenetic Warfarin Dosing. Online Resources: Warfarin Dosing and IWPC Algorithms
Advances in pharmacogenetics and personalized medicine have encouraged researchers to conduct numerous investigations looking for warfarin pharmacogenetic dose algorithms, which include genetic and clinical factors in order to improve the management of oral anticoagulation therapy. Most of the developed algorithms are not powerful enough to recommend its use in a clinical setting. Two of the best-validated algorithms have been developed by Gage et al. and by the International Warfarin Pharmacogenetics Consortium (IWPC) (International Warfarin Pharmacogenetics Con-sortium (IWPC) Algorithm 2012) and are available online.
Gage et al. (2008) recruited a diverse multicentric cohort of 1,015 patients, 83 % Caucasian and 64 % women, to develop this algorithm. VKORC1 3673G > A was the most important factor in the prediction of warfarin initial dosing. CYP2C9*2 and CYP2C9*3 allele variants predicted 19 and 33 % of reductions in the therapeutic dose of warfarin, respectively. The derivation cohort was able to explain a 53–54 % of the variability in the stable therapeutic dose of warfarin.
IWPC algorithm was widely validated by several research groups that collected clinical and genetic data from more than 5,000 patients who were under warfarin treatment and with INR range of 2–3. This study classified patients into three ranges:
-
Low dose: ≤21 mg/week
-
High dose: ≥49 mg/week
-
Intermediate dose: 21–49 mg/week
The pharmacogenetic algorithm obtained a prediction of 35 and 38 % variability in warfarin dosing in the low and high dose, respectively. In both cases, low-dose (≤21 mg/week) and high-dose (≥49 mg/week) pharmacogenetic algorithm was more capable to predict the variability dose than clinical algorithm dose (35 % vs. 24 %, p < 0.001 and 32.8 % vs. 13.3 %, p-value < 0.001, respectively). Both groups (low dose and high dose) represented 46 % of the total cohort. This algorithm resulted to be more useful for patients who are in one of these groups. Thus, the pharmacogenetic algorithm is not recommendable for patients who are within the middle group.
Both algorithms are available online for free use and simple application, by inserting patient parameters in a calculation table. The algorithm developed by Gage and colleagues is available on the website www.warfarindosing.org, and IWPC algorithm is in the pharmgkb website. These pharmacogenetic algorithms have proved to be more accurate in predicting warfarin dose compared to other pharmacogenetic algorithms (Sagreiya et al. 2010; Shaw et al. 2010; Bazan et al. 2012). Table 21.4 shows the different algorithms and required parameters to calculate the individualized dose warfarin.
2.4.2 CoumaGen-II: A Randomized Trial and Clinical Effectiveness Comparing Two Pharmacogenetic Algorithms and Standard Care for Individualizing Warfarin Dosing
A total of 504 patients were randomized into three arms in the CoumaGen-II trial (Anderson et al. 2012). Recruitment period was 2 years and the follow-up was 3 months. The population was divided in three groups. Two pharmacogenetic (PG) algorithm groups and a control standard clinical group were designed. Nomenclature PG-1 and PG-2 was used to designate the pharmacogenetic groups. The algorithm of PG-1 group was based on the algorithm developed by the IWPC group with slight modifications. PG-2 algorithm incorporated two modifications; the first modification was not including CYP2C9 allelic variants in the PG algorithm. Considering that this isoenzyme exerts its effect on the elimination route of this molecule, i.e., after the third day (t ½: 15–42 h), they hypothesized that it should not affect the initial sensitivity to this drug (Schwarz et al. 2008). The second change was a reevaluation of the dose after 4 or 5 days (3 or 4 doses), based on INR values. They made two kinds of comparisons to evaluate clinical effectiveness between groups. The first comparison was blind, randomized comparison between groups using pharmacogenetic algorithm (PG-1 vs. PG-2) and the second comparison was between a combined algorithm-guided group (PG-1 + PG-2) vs. control standard clinical group. The main objectives were to calculate the percentage of “out of range” (OOR) time and the percentage of “in therapeutic range” (TTR) time at months one and three after initiation of oral anticoagulation therapy with warfarin. The groups PG-1 and PG-2 obtained similar results for both TTR as OOR (Table 21.5). However, there were differences between combined pharmacogenetic subgroups (PG-1 + PG-2) compared with the control group. Differences in these parameters were found at months one and three and are shown in Table 21.5.
Despite this study shows some limitations, as control group was not randomized and there were differences in baseline characteristics, it demonstrates that it is more accurate to adjust individualized dose using the pharmacogenetic algorithm rather than clinical dosage. This study constitutes an advance in personalized medicine and serves as contrast source to ongoing clinical trials.
2.4.3 Acenocoumarol Pharmacogenetic Algorithms
Many studies have been developed for warfarin pharmacogenetic algorithms. On the contrary, there are only three pharmacogenetic algorithms described in patients receiving acenocoumarol.
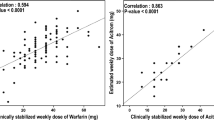
The first study included 193 Caucasian Spanish patients with atrial fibrillation, thromboembolic disease, valve replacement, and other conditions with stable anticoagulation dose adjusted to INR 3–4 for patients with prosthetic valves and INR 2–3 for the remaining patients (Verde et al. 2010). The aim of the study was to evaluate the influence of allelic variants of CYP2C9 (*2 and *3) and VKORC1 (G1639A, C497G, and C1173T) on the acenocoumarol dose to build an “acenocoumarol-dose genotype score” (AGS) in order to predict the dose required to achieve a stable and effective anticoagulation. Data from this study suggested that the AGS algorithm could be used to help in discriminating patients requiring high acenocoumarol doses to achieve stable anticoagulation. Particularly, the mean AGS was higher in the high-dose (>28 mg/week) compared with the low-dose (<7 mg/week) group. An AGS > 70 was associated with an increased odds ratio (OR) of requiring high acenocoumarol dosage (OR: 3.347; 95 % CI: 1.112–10.075; p = 0.032).
The second pharmacogenetic study includes phenprocoumon-treated patients in addition to acenocoumarol-treated patients. They collected data from over 1,000 patients with atrial fibrillation, thromboembolic disease, valve replacement, and other conditions in the Netherlands. They developed genotype-guided and non-genotype-guided algorithms to predict the maintenance dose by using genetic information (CYP2C9 and VKORC1) and clinical parameters. Their pharmacogenetic algorithm was validated externally in 229 patients treated with phenprocoumon and 168 with acenocoumarol explaining 59 and 49 % of the variability in maintenance dose of phenprocoumon and acenocoumarol, respectively (Van Schie et al. 2011).
The third published algorithm is a Spanish study that enrolled 147 patients with thromboembolic disease who had stable anticoagulation status or within INR 2–3 range. They developed an algorithm that included clinical parameters (age, weight, and concomitant medications) and allelic variations in VKORC1, CYP2C9, CYP4F2, and APOE genes. The goal was to evaluate the influence of clinical and genetic variables in acenocoumarol maintenance dose. The pharmacogenetic algorithm was able to explain 60.6 % of acenocoumarol variability dose. The two different allelic variants introduced were CYP4F2 and APOE that explain 3.6 and 1.3 % in the variability dose, respectively (Borobia et al. 2012).
Despite the small sample size of these studies, which may lead to controversial results and not enough statistic power, they have reached similar results, demonstrating that acenocoumarol pharmacogenetic algorithm is useful to predict dose requirement and explains variability in anticoagulant dose response. Currently there are three clinical trials ongoing that are expected to be powerful enough to resolve the question of whether it is really useful to genotype patients prior to coumarin derivative therapy (ClinicalTrials.gov identifier NCT00839657; ClinicalTrials.gov identifier NCT01006733; ClinicalTrials.gov identifier NCT01119300 (Warfarin), NCT01119261 (Aceno-coumarol), NCT01119274 (Phenprocoumon)).
2.4.4 Ongoing Clinical Trials for Coumarin Derivates
The COAG trial (Clarification of Optimal Anticoa-gulation through Genetics) aims to improve anticoagulation control for patients by using clinical plus genetic information to guide warfarin therapy initiation. This trial is intended to enroll 1,238 patients to evaluate time within therapeutic INR range and risk of presenting INR > 4. This study is currently recruiting participants diagnosed with stroke, venous thrombosis, atrial fibrillation, or atrial flutter. They are comparing two approaches (clinical vs. genetic guidance) in warfarin dosing to examine the utility of using genetic information for warfarin dosing; they have designed two different groups, a genotype-guided dosing algorithm warfarin group and a clinical-guided dosing algorithm warfarin group. Each study arm includes a baseline dose-initiation algorithm and a dose-revision algorithm applied over the first 4–5 doses of warfarin therapy. The first aim of efficacy is percentage of TTR and the second efficacy goal is occurrence of INR > 4 or serious clinical event. Outcomes are expected in 2013 (National Heart, Lung, and Blood Institute 2012).
The GIFT (Genetic Informatics Trial of Warfarin to Prevent DVT) plans to recruit 1,600 patients. They hypothesize that the use of genetics to guide warfarin therapy will reduce the risk of venous thromboembolism (VTE) postoperatively. They further hypothesize that using a target INR of 1.8 is non-inferior to using a target INR of 2.5 in thrombi prevention. The primary endpoint is to evaluate nonfatal thromboembolism, nonfatal major bleeding, or INR > 4; and the secondary endpoint is TTR. Results are expected in 2015 (Washington University School of Medicine 2012).
EU-PACT (European Pharmacogenetics of Anticoagulant Therapy) trial aims to enroll 970 patients with venous thromboembolism or atrial fibrillation. The main objective of this trial is to demonstrate that a dosing algorithm containing genetic information is able to increase the time within therapeutic INR range during anticoagulation therapy with each of warfarin, acenocoumarol, and phenprocoumon compared to a dosing regimen that does not contain this information. Secondary outcomes of the study include cost-effectiveness, number of thromboembolic and bleeding events, time to reach stable dose, and number of supratherapeutic INR peaks. The design is a two-armed, single-blinded, randomized controlled trial. One arm is constituted by a genotype-guided dosing algorithm group starting anticoagulation therapy with warfarin, acenocoumarol, or phenprocoumon and the other group of patients is being dosed according to a non-genotype-guided dosing. Primary outcome measures are to assay TTR and INR>4. Secondary outcome measures include supratherapeutic or undertherapeutic INR range; time to achieve a stable dose; quantify number of dose adjustments and number of major bleeding events or thromboembolic events followed for three months (Utrecht Institute for Pharmaceutical Sciences 2012). This trial is expected to be powerful enough to answer questions regarding clinical implementation of genotype dosing, if genetic dosing algorithm increases the TTR-INR range during anticoagulation therapy, cost-effectiveness, number of thromboembolic and bleeding events, time to reach stable dose, and number of supratherapeutic INR peaks. Outcomes are expected in November 2012 (Johnson et al. 2011).
2.5 Pharmacogenetic Tests
The challenge of pharmacogenetic tests is to improve efficacy and safety of anticoagulant treatment with coumarin derivates. Several genetic tests have been developed, based on performing determination of CYP2C9 and VKORC1 polymorphisms. Implementation of these tests aims to be useful at predicting the individualized warfarin-required dose.
Currently, there are several available pharmacogenetic tests approved by the FDA, such as Verigene Warfarin Metabolism, eSensor Warfarin Sensitivity, INFINITI Warfarin Assay, eQ-PCR LC Warfarin Genotyping kit, and ParagonDx Genetic Warfarin Assay, and other genetic tests that have been developed but are not authorized by the FDA yet, such as Luminex, Invader, and SimpleProbe Warfarin Assay (King et al. 2008; Babic et al. 2009; Langley et al. 2009; Lefferts et al. 2010; Maurice et al. 2010, Infiniti Warfarin Assay 2012). Table 21.6 shows the main characteristics of a selection of the most important pharmacogenetic tests available for determination of warfarin sensitivity. Test, company, genotyping methodology, gene polymorphisms, time after DNA isolation, FDA status, advantages, and limitations are detailed, and differences between different genetic tests are also described in Table 21.6.
Genetic tests are intended to be useful at multiracial populations; some genetic tests include allelic variants more prevalent in black and Asian populations. For example, Infinity performs the determination of CYP2C9*3, *4, *5, *6, and *11 allelic variants, and eSensor genetic test determines CYP2C9*5, *6,*11,*14, *15, and *16 allelic variants, which are more common in non-Caucasian populations, in addition to the common CYP2C9*2 and CYP2C9*3 allelic variants.
One of the most important variables in the choice of the genetic test is its cost-effectiveness. Maurice et al. (2010) compared the performance of a variety of commercial genotyping assays (Verigene, eSensor, Invader, and Luminex), to detect variants in the CYP2C9 and VKORC1 genes, used in genotype-based warfarin dosing algorithms (Maurice et al. 2010). According to their results, Verigene Warfarin Metabolism Test offers an acceptable cost per genotyped sample when determining from 1 to 24 samples, without a great increase in the price. Based on that study, Verigene is also the easiest system to operate and the least complex to perform, based on pipetting steps. Furthermore, it has the shortest turnaround time, due to the avoiding of the nucleic acid amplification step. Unlike the other systems, Verigene does not require separate external controls for each assay run; internal controls are preloaded into the test cartridges, and external controls are only run to verify the performance of each new batch of cartridges. On the other hand, eSensor, Luminex, and Invader have added costs and hands-on time because of their requirement for 2–3 external controls per run, even for single sample analysis. This results in a significant cost advantage of Verigene for single sample analysis that is reduced as the sample run size increases, so it has the same price for 1, 8, or 24 samples. It is the least expensive system for single and eight sample analysis and second, behind Invader test, in cost-effectiveness for 24 samples. Despite this advantages, Verigene is only available for warfarin metabolism, F5/F2/MTHFR, and CYP2C19 genetics test, so it does provide a broad panel of polymorphisms to study, unlike, for example, Luminex assay, which uses a method based on multiplex PCR, and allele-specific primer extension, which allows determination of more polymorphisms than the other systems. Verigene is therefore a closed system that cannot determine other polymorphisms outside its genotyping kit, while other genotyping tests offer the possibility of including gene polymorphism determinations chosen by the researchers. The versatility of the instrument is another factor to take into consideration in the cost-effectiveness of the selected genotyping platform.
The use of genetic testing for warfarin dosing may not be useful for the entire population that is susceptible of starting coumarin derivative treatment. A limitation of these genetic tests is that they are based on the identification of warfarin sensitivity, and therefore, allelic variants associated to coumarin derivate resistance cannot be determined. Furthermore, the use of closed genetic testing can significantly increase health costs.
3 New Oral Anticoagulant Drugs
Chronic treatment with VKAs (warfarin, acenocoumarol, and phenprocoumon) has been the treatment of choice for stroke prevention in atrial fibrillation patients and prevention and treatment of venous thromboembolism during the last 60 years (Dezee et al. 2006; Kamali and Pirmohamed 2006; Banerjee et al. 2011; Wartak and Bartholomew 2011). Because of several coumarin derivate drawbacks (Francis 2008), such as narrow therapeutic margin, delayed onset of action, many drugs and dietary interactions, the high interindividual variability in the anticoagulant response, and the necessity of periodic monitoring of anticoagulants doses, pharmaceutical development has focused on the search for new molecules that improve oral anticoagulation therapy.
The new anticoagulants can be classified in function of their mechanism of action into three groups (Douketis 2011):
-
1.
Oral direct thrombin inhibitor. Dabigatran etexilate
-
2.
Oral factor Xa inhibitor. Rivaroxaban, apixaban, betrixaban, edoxaban, and eribaxaban
-
3.
Parenteral factor Xa inhibitor. Idrabiotaparinux
Ximelagatran, the first oral direct thrombin inhibitor which was evaluated in a clinical trial compared to warfarin in prophylaxis and treatment of VTE and stroke prevention in atrial fibrillation patients was not approved by FDA because it showed hepatotoxicity (Francis et al. 2003; Schulman et al. 2003; Colwell et al. 2005).
Dabigatran etexilate and rivaroxaban are currently available on the market. In 2008, dabigatran etexilate was authorized for the prevention of VTE after major orthopedic surgery by the European Medicines Agency (EMA). Based on the RE-LY study results, the FDA (Food and Drugs Administration) approved dabigatran etexilate in the prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation (FDA News and Events 2012a). After the results shown in the ROCKET AF study, in 2010, the FDA approved the use of rivaroxaban in the prevention of stroke and embolism in adult patients with non-valvular atrial fibrillation (FDA News and Events 2012b).
3.1 Oral Direct Thrombin Inhibitors
3.1.1 Dabigatran Etexilate
Dabigatran etexilate is rapidly absorbed and is hydrolyzed by plasma and liver esterases to active dabigatran (Baetz and Spinler 2008). It is absorbed in the stomach and intestine and depends on an acid environment. Its absorption is diminished by 30 % when dabigatran etexilate is coadministered with proton pump inhibitors (Francis 2008). To facilitate the absorption of dabigatran etexilate, capsules contain tartaric acid. The peak plasma concentration of dabigatran is reached within 2–3 h after oral administration. The half-life time of dabigatran is around 12–17 h, and steady state is attained within 3 days of treatment with this new oral anticoagulant (Liesenfeld et al. 2011).
The oral bioavailability of dabigatran etexilate in capsules is 6.5 % and can be increased by up to 75 % when the active substance is separated from the capsule, so it should not be separated. It has a low plasma protein binding (35 %). Dabigatran is not metabolized by the CYP450 isoenzymes; microsomal esterases are responsible of its metabolism. Pharmacologically active acyl glucuronides are formed through conjugation metabolism. It is mostly eliminated unmodified (80–85 % of the dose) and partially in the form of glucuronides through glomerular filtration (Eriksson et al. 2009).
There are no known interactions with food, and direct interaction with alcohol intake in animal models has not been described (Liesenfeld et al. 2011). The prodrug dabigatran etexilate, but not the active form dabigatran, is a P-glycoprotein (p-pg) substrate (Liesenfeld et al. 2011). Dabigatran interaction may occur with p-pg inhibitors, such as amiodarone (increased 50–60 % dabigatran area under the curve), quinidine, verapamil, or ketoconazole (contraindicated), and p-pg inducers such as rifampicin, carbamazepine, and St. John’s wort (Eriksson et al. 2009; Schulman and Majeed 2012).
Plasma and liver esterases hydrolyze the biotransformation of dabigatran etexilate into dabigatran. Dabigatran binds to thrombin reversibly and competitively inhibiting its ability to convert fibrinogen to fibrin in the coagulation cascade. Since thrombin (serine protease) enables the conversion of fibrinogen to fibrin in the coagulation cascade, its inhibition prevents thrombi formation. Dabigatran also inhibits free thrombin, fibrin-bound thrombin, and thrombin-induced platelet aggregation (Brighton 2010).
3.1.1.1 RE-LY (Evaluation of Long-Term Anticoagulation Therapy)
Dabigatran etexilate was compared to warfarin in the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) study in 18,113 patients with atrial fibrillation (AF) recruited during two years. Patients were randomized to dabigatran 110 mg twice a day, dabigatran 150 mg twice a day, or warfarin INR dose adjusted. The main objective of this study was to evaluate the effectiveness in the prevention of thromboembolic events, stroke, and hemorrhage of dabigatran, compared with standard warfarin dosing. The dose of 110 mg twice daily showed similar efficacy to warfarin in preventing stroke and systemic embolism. The incidence of major, minor, and intracranial bleeding was significantly lower in dabigatran 110 mg compared with warfarin (2.87 % vs. 3.57 % dabigatran warfarin, p = 0.003). However, the incidence of myocardial infarction was higher with dabigatran 110 mg and 150 mg twice a day than with warfarin (0.82 %, 0.81 %, and 0.64 %, respectively) (Connolly et al. 2011). A meta-analysis published in January 2012 concluded that dabigatran was associated to 33 % of increased myocardial infarction or acute coronary syndrome risk (OR 1.33, 95 % CI 1.03–1.71, p = 0.03) compared to warfarin, enoxaparin, and placebo (Uchino and Hernandez 2012).
3.2 Oral Factor Xa Inhibitors
3.2.1 Rivaroxaban
Rivaroxaban is orally administered. It has a nearly 100 % bioavailability. It has 92–95 % protein binding (FDA. U.S. Food and Drug Administration 2012b). Rivaroxaban is a p-glycoprotein substrate and is transported through the membrane. About 2/3 of the dose is metabolized; metabolism is conducted through CYP3A4, CYP2J2, and CYP450-independent mechanisms (Perzborn et al. 2011). Approximately one third of the administered dose is excreted unmodified by the kidneys, another third is metabolized to inactive metabolites and eliminated by the kidneys, and the last third of the dose metabolized to inactive metabolites and eliminated via fecal. The half-life time in young subjects is 5–9 h and in older individuals is 11–13 h (FDA. U.S. Food and Drug Administration 2012b). Rivaroxaban has a low renal clearance (3–4 l/h). Toxicity can be reversed using activated charcoal and supportive measures such as mechanical compression and hemodynamic support. If the bleeding does not stop, activated prothrombin complex concentrate, prothrombin complex concentrate, and recombinant factor VIIa can be administrated.
Rivaroxaban is an oral anticoagulant that inhibits directly factor Xa. Factor Xa is required to perform the conversion of prothrombin (FII) into thrombin (FIIa). Thrombin is a serine protease required for the conversion of fibrinogen to fibrin, which is responsible for triggering the coagulation cascade.
A molecule of factor Xa can generate more than 1,000 molecules of thrombin. Therefore, rivaroxaban is useful in blocking the coagulation cascade; rivaroxaban exerts its effect irreversibly (DrugBank 2012a).
3.2.1.1 ROCKET AF (Rivaroxaban Once-Daily Oral Direct Factor Xa Inhibition Compared with the Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation)
Rivaroxaban, a factor Xa inhibitor, was studied in the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation. The ROCKET AF study compared the efficacy of rivaroxaban versus warfarin in 14,264 patients with non-valvular atrial fibrillation with moderate to high risk of stroke (Patel et al. 2011). Patients were randomized to rivaroxaban 20 mg or warfarin dose adjustment. The results of this study showed that rivaroxaban was not inferior to warfarin in preventing stroke and systemic embolism. The incidence of stroke or systemic embolism was 1.7 % in the rivaroxaban group and 2.2 % in the warfarin group. Moreover, the incidence of bleeding was 14.9 and 14.5 % in warfarin and rivaroxaban groups, respectively. The incidence of intracranial hemorrhage in a year was lower in the rivaroxaban group (0.5 % vs. 0.7 %). The incidence of gastrointestinal bleeding was higher in the rivaroxaban group compared to warfarin (3.2 % vs. 2.2 %).
3.2.2 Apixaban
Apixaban is the latest highly selective and reversible inhibitor oral direct factor Xa. It is orally administered twice daily, and like the other new oral anticoagulants (dabigatran and rivaroxaban), it does not require dose monitoring routine laboratory. It has a high oral bioavailability, reaching peak plasma concentration at 3 h after oral administration, with a half-life time of 9–14 h (Raghavan et al. 2009; Weitz 2010). It is metabolized by hepatic metabolism via CYP3A4, CYP3A5, and sulfotransferase 1A1 and by kidney and intestinal metabolism. It is eliminated through multiple pathways, renal and intestinal routes. It has a renal elimination of 25 % and the remaining 75 % through the hepatobiliary system (Deedwania and Huang 2012; Eriksson et al. 2009).
Two phase III randomized trials have been performed to evaluate the efficacy and safety of apixaban in prevention of stroke and systemic embolism in AF patients. The first trial was “Apixaban Versus Acetylsalicylic Acid to Prevent Stroke in Atrial Fibrillation Patients who have Failed or are Unsuitable for Vitamin K Antagonist Treatment” (AVERROES trial) and the second was “the Apixaban for Reduction in Stroke and Other Events in Atrial Thromboembolic Fibrillation” (ARISTOTLE trial) (Diener et al. 2012; Granger et al. 2011).
3.2.2.1 AVERROES Trial
AVERROES trial was a randomized study which included 5599 AF patients with increased risk of stroke and intolerance to therapy with vitamin K antagonists. Patients were randomized into two groups: the first group to apixaban 5 mg twice daily or 2.5 mg twice daily if the patient was ≥80 years, ≤60 kg body weight, or serum creatinine of ≥1.5 mg/dl plus aspirin placebo and the other group to aspirin (81–324 mg daily) plus placebo apixaban. The aims were efficacy (preventing stroke or systemic embolism) and safety (major bleeding). Major bleeding was defined as the overbleeding with one or more of the following clinical parameters: drop in hemoglobin level of ≥2 g/dl over 24-h period or transfusion of ≥2 units packed cells and critical bleeding or fatal bleeding. Patients treated with apixaban obtained lower incidence of stroke and embolic system compared to the aspirin group (1.6 % vs. 3.7 % per year, p-value < 0.001). Ischemic stroke was lower in apixaban group compared to aspirin (1.1 % vs. 3.0 % per year, p-value < 0.001), and hemorrhages were also lower in the apixaban group compared to aspirin (6 vs. 9). No significant differences in the rate of death per year were found (3.5% apixaban group vs 4.4% aspirin group, p-value < 0.07). This study concludes that apixaban is more effective than aspirin in preventing stroke and systemic embolism in patients who are not eligible for oral anticoagulation therapy with vitamin K antagonists (Eikelboom et al. 2010; Connolly et al. 2011).
3.2.2.2 ARISTOTLE Trial
ARISTOTLE trial was a double-blind, randomized study that included 18,201 patients with atrial fibrillation and at least one risk factor for stroke. Patients were randomized into two groups: apixaban group (5 mg daily or 5 mg twice daily if the patient was ≥80 years with body weight ≤60 kg or creatinine concentration ≥1.5 mg/dl) and warfarin group with dose adjusted to INR 2–3. The first efficacy objective was to evaluate the incidence of systemic embolism and stroke, and the first safety aim was bleeding and death from any cause. The result of the first efficacy endpoint was 1.7 % in the apixaban group vs. 1.60 % per year in the warfarin group (p < 0.001 for non-inferiority and p < 0.01 for superiority). Bleeding rate was lower in apixaban group compared with warfarin group in a year (2.13 % vs. 3.09 %, p < 0.001), and the rates of death from any cause were 3.52 % in the apixaban group compared to 3.94 % in the warfarin group (p = 0.047). ARISTOTLE trial concluded that apixaban was superior to warfarin in the prevention of stroke or systemic embolism results with less bleeding and death in both cases (Granger et al. 2011).
Apixaban has been approved in Europe for the prevention of venous thromboembolic events VTE in adults following a hip or knee replacement operation in 2011 (European Medicine Agency 2012a). Apixaban showed greater efficacy to warfarin in the prevention of stroke and systemic embolism in AF patients in the ARISTOTLE study. Apixaban is currently being evaluated by the FDA to obtain approval for the prevention of stroke and systemic embolism in AF patients.
3.3 Strengths and Limitations of the New Oral Anticoagulants
The new oral anticoagulants dabigatran, rivaroxaban (approved by the FDA for the prevention of stroke and systemic embolism in patients with AF), and apixaban (currently under review by the FDA for authorized for prevention of stroke and systemic embolism in AF patients) have showed to be not inferior in efficacy and safety compared to warfarin in preventing stroke and systemic embolism in AF patients in Phase III studies (Connolly et al. 2011; Diener et al. 2012; Granger et al. 2011; Patel et al. 2011). They are considered as the alternative to coumarin derivates in long-term oral anticoagulation therapy.
They have a rapid onset of action compared to coumarin derivates. This feature could allow avoiding bridge therapy with parenteral anticoagulants (UHF or LWMH). Figure 21.2 shows the comparison of action mechanisms of VKA vs. new oral anticoagulants.
One of the main limitations of VKA is that they present a high variability interindividual dose response. The new oral anticoagulants have a predictable anticoagulant response, and therefore a daily fixed dose can be given, and routine laboratory monitoring is not required in patients without severe renal impairment (Kubitza et al. 2005, 2008; Connolly et al. 2009; Schulman et al. 2009). No routine laboratory monitoring is a priori an advantage because it can increase the number of patients receiving long-term oral anticoagulation therapy. However, the daily fixed dose can cause a potential decrease of treatment adherence with consequent therapeutic failure. To avoid this potential poor adherence, education is needed in patients who start anticoagulation therapy with the new oral anticoagulants. In the case of dabigatran and apixaban, they are administrated in a twice-daily dose, while daily dose of rivaroxaban can be administrated once after the first weeks (European Medicine Agency 2012b).
New oral anticoagulants are less dependent of CYP450 metabolism compared to VKA; therefore, drug and dietary interactions are reduced. The presence of food increases the time to reach peak plasma concentration (≈4 h) and decreases 30 % in plasma levels of rivaroxaban (Kubitza et al. 2006).
Dabigatran is metabolized by CYP450-independent mechanisms. The prodrug dabigatran etexilate and rivaroxaban are substrates of p-glycoprotein and transported through the membrane. Pg-p inductor drugs (rifampicin, carbamazepine, or St. John’s wort) or Pg-p inhibitor drugs (ketoconazole, verapamil, amiodarone, or quinidine) may decrease or increase the anticoagulant response, respectively. Ketoconazole is contraindicated in patients taking dabigatran (Schulman and Majeed 2012). Factor Xa inhibitors (rivaroxaban and apixaban) are metabolized by CYP3A4, and therefore, concomitant drugs that are inhibitors (ketoconazole, ritonavir, erythromycin, or clarithromycin) or inducers (rifampicin) of this isoenzyme can influence the plasma drug concentration (Giorgi and Miguel 2012). Table 21.7 shows the molecular structures involved in pharmacodynamics and pharmacokinetics of new oral anticoagulants (dabigatran, rivaroxaban, and apixaban).
In the RE-LY study, dabigatran group showed a higher incidence of myocardial infarction compared to warfarin group. A recent analysis suggests that warfarin may be protective against myocardial infarction (Lip and Lane 2010). However, in the Phase III studies conducted with other new oral anticoagulants (rivaroxaban and apixaban), the rate of myocardial infarction was lower compared to warfarin group (Lip et al. 2010).
Although new oral anticoagulants have a shorter duration of the anticoagulant effect compared to VKA, there is no antidote available to reverse the anticoagulant effects if toxicity occurs (Schulman and Bijsterveld 2007; Wittkowsky 2010).
Because of the relevance of renal elimination in new anticoagulants (dabigatran and rivaroxaban), creatinine clearance (CLCR) of each patient has to be analyzed before starting anticoagulant therapy. Patients with moderate renal clearance (CL CR < 50 ml/min) or severe renal impairment (CL CR < 30 ml/min) can exhibit prolonged time excretion and increased drug concentrations in plasma (Stangier et al. 2010, (European Medicine Agency 2012b). Dabigatran is contraindicated in patients with severe renal impairment (CL CR < 30 ml/min) (European Medicines Agency 2012c)).
Dabigatran absorption is favored by an acidic environment, so the capsules contain tartaric acid. In the RE-LY study, dabigatran showed a higher rate of dyspepsia compared to warfarin patients, which contributed to a discontinuation in the dabigatran anticoagulation therapy in 2 % of patients (Connolly et al. 2009).
One of the most important limitations is the cost of the new oral anticoagulants, which is far superior to the known coumarin derivates. According to Freeman (AÑO), dabigatran may be cost-effective in patients with age ≥ 65 years with non-valvular atrial fibrillation and an increased risk of CHADS2 ≥ 1 (Reeman et al. 2011).
Although they are not ideal oral anticoagulants, dabigatran, rivaroxaban, and apixaban have suitable properties to become real alternatives to traditional anticoagulation therapy with coumarin derivates.
4 Future Perspectives
Prediction of the initial dose of coumarin derivates using CYP2C9 and VKORC1 polymorphisms increases efficacy and minimizes the toxicity of VKA in the first months of treatment. The inclusion of genotyping in the clinical setting reduces the risk of hospitalization for bleeding or thrombosis by approximately 30 % in outpatients treated with warfarin (Epstein et al. 2010). These results are consistent with the conclusions reached by Eckman et al. (2009), when evaluating the cost-effectiveness of genotype-guided dosing of warfarin versus standard induction therapy for non-valvular atrial fibrillation patients. The genotype-guided dosing resulted cost-effective in patients at high risk of bleeding but not for typical non-valvular atrial fibrillation patients, except for when the following conditions were complied: the genotyping was capable of reducing 32 % the rate of bleeding events, and it was available in 24 h and costs less than 200 dollars. Currently, PCR real-time techniques allow performing genotyping of CYP2C9 and VKORC1 within 24 h, and the cost of the genotyping method can be available for less than $40 per patient if it is performed “in-house,” at the hospital laboratory facilities. If genotyping-guided dose is implemented in clinical anticoagulant therapy, it can reach a 30 % reduction of thromboembolism or bleeding events in typical non-valvular atrial fibrillation patients.
Furthermore, VKAs still remain a recommended option for anticoagulation therapy in patients who are already on anticoagulant therapy with VKAs and with good INR control, for new patients with non-valvular atrial fibrillation except if there are any criteria that justify initiating treatment with the new oral anticoagulants, and also for atrial fibrillation patients with valvular involvement. Thereby, VKAs will keep being a real possibility for anticoagulant therapy despite of the increasing trend of using new oral anticoagulants in prevention of stroke and systemic embolism for patients with atrial fibrillation. However, there are some situations in which the new oral anticoagulants may have a benefit compared with the VKAs, such as patients with known hypersensitivity or contraindication to the use of VKA, patients with antecedents of intracranial hemorrhage or ischemic stroke patients with high-risk clinical and neuroimaging criteria for intracranial hemorrhage, and finally in patients treated with VKAs which have been presented thromboembolic and/or major bleeding episodes despite a good control of INR (Spanish Agency of Medicines and Health Products 2012).
Dabigatran has demonstrated to be cost effective compared to warfarin in preventing stroke and systemic embolism in patients with non-valvular atrial fibrillation (Davidson et al. 2013; González-Juanatey et al. 2012). Although the new molecules are presented as the future in the prevention of thrombotic events in patients requiring long-term oral anticoagulation, the high cost of these molecules is a limitation in the clinical implementation, especially for some countries. In those countries, if new molecules cannot be introduced in their hospitals, routine genotyping of patients with a high bleeding risk starting long-term oral anticoagulation with coumarin derivates or poor INR control remains a real possibility to be considered to improve the efficiency and safety of treatment.
References
Aithal GP, Day CP, Kesteven PJ, Daly AK (1999) Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet 353:717–719
Alberio L (2003) Oral anticoagulation with vitamin K antagonists. Ther Umsch 60:5–9
Anderson JL, Horne BD, Stevens SM, Woller SC, Samuelson KM, Mansfield JW, Robinson M, Barton S, Brunisholz K, Mower CP, Huntinghouse JA, Rollo JS, Siler D, Bair TL, Knight S, Muhlestein JB, Carlquist JF (2012) A randomized and clinical effectiveness trial comparing two pharmacogenetic algorithms and standard care for individualizing warfarin dosing (CoumaGen-II). Circulation 125:1997–2005
Aquilante CL, Langaee TY, Lopez LM, Yarandi HN, Tromberg JS, Mohuczy D, Gaston KL, Waddell CD, Chirico MJ, Johnson JA (2006) Influence of coagulation factor, vitamin K epoxide reductase complex subunit 1, and cytochrome P450 2C9 gene polymorphisms on warfarin dose requirements. Clin Pharmacol Ther 79:291–302
Babic N, Haverfield EV, Burrus JA, Lozada A, Das S, Yeo KT (2009) Comparison of performance of three commercial platforms for warfarin sensitivity genotyping. Clin Chim Acta 406:143–147
Baetz BE, Spinler SA (2008) Dabigatran etexilate: an oral direct thrombin inhibitor for prophylaxis and treatment of thromboembolic diseases. Pharmacotherapy 28:1354–1373
Banerjee A, Marín F, Lip GY (2011) A new landscape for stroke prevention in atrial fibrillation: focus on new anticoagulants, antiarrhythmic drugs, and devices. Stroke 42(11):3316–3322
Bazan NS, Sabry NA, Rizk A, Mokhtar S, Badary O (2012) Validation of pharmacogenetic algorithms and warfarin dosing table in Egyptian patients. Int J Clin Pharm 34(6):837–844
Becquemont L (2008) Evidence for a pharmacogenetic adapted dose of oral anticoagulant in routine medical practice. Eur J Clin Pharmacol 64:953–960
Beinema M, Brouwers JR, Schalekamp T, Wilffert B (2008) Pharmacogenetic differences between warfarin, acenocoumarol and phenprocoumon. Thromb Haemost 100(6):1052–1057. Review
Bodin L, Verstuyft C, Tregouet DA, Robert A, Dubert L, Funck-Brentano C, Jaillon P, Beaune P, Laurent-Puig P, Becquemont L, Loriot MA (2005) Cytochrome P450 2C9 (CYP2C9) and vitamin K epoxide reductase (VKORC1) genotypes as determinants of acenocoumarol sensitivity. Blood 106(1):135–140
Borgiani P, Ciccacci C, Forte V, Romano S, Federici G, Novelli G (2007) Allelic variants in the CYP2C9 and VKORC1 loci and interindividual variability in the anticoagulant dose effect of warfarin in Italians. Pharmacogenomics 8:1545–1550
Borobia AM, Lubomirov R, Ramírez E, Lorenzo A, Campos A, Muñoz-Romo R, Fernández-Capitán C, Frías J, Carcas AJ (2012) An acenocoumarol dosing algorithm using clinical and pharmacogenetic data in spanish patients with thromboembolic disease. PLoS One 7:e41360
Brighton T (2010) Experimental and clinical pharmacology new oral anticoagulant drugs– mechanisms of action. Aust Prescr 33:38–41
Cadamuro J, Dieplinger B, Felder T, Kedenko I, Mueller T, Haltmayer M, Patsch W, Oberkofler H (2010) Genetic determinants of acenocoumarol and phenprocoumon maintenance dose requirements. Eur J Clin Pharmacol 66(3):253–260
Cain D, Hutson SM, Wallin R (1997) Assembly of the warfarin-sensitive vitamin K 2,3-epoxide reductase enzyme complex in the endoplasmic reticulum membrane. J Biol Chem 272:29068–29075
Caldwell MD, Awad T, Johnson JA, Gage BF, Falkowski M, Gardina P, Hubbard J, Turpaz Y, Langaee TY, Eby C, King CR, Brower A, Schmelzer JR, Glurich I, Vidaillet HJ, Yale SH, Qi Zhang K, Berg RL, Burmester JK (2008) CYP4F2 genetic variant alters required warfarin dose. Blood 111:4106–4112
Camm AJ et al (2010) Guías de práctica clínica para el manejo de la fibrilación auricular. Rev Esp Cardiol 63(12):1483.e1–1483.e83
Carlquist JF, Horne BD, Muhlestein JB, Lappé DL, Whiting BM, Kolek MJ, Clarke JL, James BC, Anderson JL (2006) Genotypes of the Cytochrome p450 isoform, CYP2C9, and the vitamin K epoxide reductase complex subunit 1 conjointly determine stable warfarin dose: a prospective study. J Thromb Thrombolysis 22:191–197
Cavallari LH, Langaee TY, Momary KM, Shapiro NL, Nutescu EA, Coty WA, Viana MA, Patel SR, Johnson JA (2010) Genetic and clinical predictors of warfarin dose requirements in African Americans. Clin Pharmacol Ther 87:459–464
Choonara IA, Haynes BP, Cholerton S, Breckenridge AM, Park BK (1986) Enantiomers of warfarin and vitamin K1 metabolism. Br J Clin Pharmacol 22:729–732
Cini M, Legnani C, Cosmi B, Guazzaloca G, Valdrè L, Frascaro M, Palareti G (2012) A new warfarin dosing algorithm including VKORC1 3730 G>A polymorphism: comparison with results obtained by other published algorithms. Eur J Clin Pharmacol 68:1167–1174
Colwell CW Jr, Berkowitz SD, Lieberman JR, Comp PC, Ginsberg JS, Paiement G, McElhattan J, Roth AW, Francis CW, EXULT B Study Group (2005) Oral direct thrombin inhibitor ximelagatran compared with warfarin for the prevention of venous thromboembolism after total knee arthroplasty. J Bone Joint Surg Am 87:2169–2177
Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L, RE-LY Steering Committee and Investigators (2009) Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 361:1139–1151
Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, Flaker G, Avezum A, Hohnloser SH, Diaz R, Talajic M, Zhu J, Pais P, Budaj A, Parkhomenko A, Jansky P, Commerford P, Tan RS, Sim KH, Lewis BS, Van Mieghem W, Lip GY, Kim JH, Lanas-Zanetti F, Gonzalez-Hermosillo A, Dans AL, Munawar M, O’Donnell M, LawrenceJ LG, Afzal R, Yusuf S, AVERROES Steering Committee and Investigators (2011) Apixaban in patients with atrial fibrillation. N Engl J Med 364:806–817
D’Andrea G, D’Ambrosio RL, Di Perna P, Chetta M, Santacroce R, Brancaccio V, Grandone E, Margaglione M (2005) A polymorphism in the VKORC1 gene is associated with an interindividual variability in the dose-anticoagulant effect of warfarin. Blood 105:645–649
D’Andrea G, D’Ambrosio R, Margaglione M (2008) Oral anticoagulants: pharmacogenetics relationship between genetic and non-genetic factors. Blood Rev 22:127–140
Dahl OE (2012) New oral antithrombotics: focus on dabigatran, an oral, reversible direct thrombin inhibitor for the prevention and treatment of venous and arterial thromboembolic disorders. Vasc Health Risk Manag 8:45–57
Daly AK, King BP (2003) Pharmacogenetics of oral anticoagulants. Pharmacogenetics 13:247–252
Davidson T, Husberg M, Janzon M, Oldgren J, Levin LÅ (2013) Cost-effectiveness of dabigatran compared with warfarin for patients with atrial fibrillation in Sweden. Eur Heart J 34(3):177–183
Davis EM, Packard KA, Knezevich JT, Campbell JA (2011) New and emerging anticoagulant therapy for atrial fibrillation and acute coronary syndrome. Pharmacotherapy 31(10):975–1016
Deedwania P, Huang GW (2012) An evidence-based review of apixaban and its potential in the prevention of stroke in patients with atrial fibrillation. Core Evid 7:49–59
Dezee KJ, Shimeall WT, Douglas KM, Shumway NM, O’malley PG (2006) Treatment of excessive anticoagulation with phytonadione (vitamin K): a meta-analysis. Arch Intern Med 166(4):391–397
Diener HC, Eikelboom J, Connolly SJ, Joyner CD, Hart RG, Lip GY, O’Donnell M, Hohnloser SH, Hankey GJ, Shestakovska O, Yusuf S, AVERROES Steering Committee and Investigators (2012) Apixaban versus aspirin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a predefined subgroup analysis from AVERROES, a randomised trial. Lancet Neurol 1:225–231
Douketis JD (2011) Dabigatran as anticoagulant therapy for atrial fibrillation. Which patients should receive it, which patients may not need it, and other practical aspects of patient management. Pol Arch Med Wewn 121(3):73–80
DrugBank (2012a) Rivaroxaban http://www.drugbank.ca/drugs/DB06228. Accessed 7 May 2012
DrugBank (2012b) Acenocoumarol (DB01418). http://www.drugbank.ca/drugs/DB01418. Accessed 7 May 2012
Duran Parrondo C et al (2003) Anticoagulación oral. An Med Interna 20:377–384
Eckman MH, Rosand J, Greenberg SM, Gage BF (2009) Cost-effectiveness of using pharmacogenetic information in warfarin dosing for patients with nonvalvular atrial fibrillation. Ann Intern Med 150:73–83
Eikelboom JW, O’Donnell M, Yusuf S, Diaz R, Flaker G, Hart R, Hohnloser S, Joyner C, Lawrence J, Pais P, Pogue J, Synhorst D, Connolly SJ (2010) Rationale and design of AVERROES: apixaban versus acetylsalicylic acid to prevent stroke in atrial fibrillation patients who have failed or are unsuitable for vitamin K antagonist treatment. Am Heart J 159:348–353
Eitz T et al (2008) International normalized ratio self-management lowers the risk of thromboembolic events after prosthetic heart valve replacement. Ann Thorac Surg 85:949–955
Epstein RS, Moyer TP, Aubert RE, O’Kane DJ, Xia F, Verbrugge RR, Gage BF, Teagarden JR (2010) Warfarin genotyping reduces hospitalization rates results from the MM-WES (Medco-Mayo Warfarin effectiveness study). J Am Coll Cardiol 55:2804–2812
Eriksson BI, Quinlan DJ, Weitz JI (2009) Comparative pharmacodynamics and pharmacokinetics of oral direct thrombin and factor xa inhibitors in development. Clin Pharmacokinet 48:1–22
European Medicine Agency (2012a) EPAR summary for the public. EMA/240842/2011 EMEA/H/C/48. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Summary_for_the_public/human/48/WC500107773.pdf. Accessed 10 Sept 2012
European Medicine Agency (2012b) Summary of Rivaroxaban characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-Product_Information/human/000944/WC500057108.pdf. Accessed 10 Sept 2012
European Medicines Agency (2012c) Updates on safety of Pradaxa http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2011/11/WC500117818.pdf. Accessed 10 Sept 2012
Evans WE, Relling MV (1999) Pharmacogenomics: translating functional genomics into rational therapeutics. Science 286:487–491
FDA News and Events (2012a) FDA approves Pradaxa to prevent stroke in people with atrial fibrillation. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2010/ucm230241.htm?utm_campaign=Google2&utm_source=fdaSearch&utm_medium=website&utm_term=dabigatran%20atrial%20fibrillation&utm_content=3. Accessed 7 May 2012
FDA News and Events (2012b) FDA approves Xarelto to prevent stroke in people with common type of abnormal heart rhythm. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm278646.htm?utm_campaign=Google2&utm_source=fdaSearch&utm_medium=website&utm_term=fda%20news%20and%20events%20rivaroxaban&utm_content=3. Accessed 7 May 2012
FDA News and Events (2012c) NEWS RELEASE. FDA clears genetic lab test for Warfarin sensitivity. http://www.fda.gov/newsevents/newsroom/pressannouncements/2007/ucm108984.htm. Accessed 10 Sept 2012
Fava C, Montagnana M, Almgren P, Rosberg L, Lippi G, Hedblad B, Engström G, Berglund G, Minuz P, Melander O (2008) The V433M variant of the CYP4F2 is associated with ischemic stroke in male Swedes beyond its effect on blood pressure. Hypertension 52:373–380
FDA. U.S. Food and Drug Administration (2012a) COUMADIN® TABLETS (Warfarin sodium Tablets, USP) Crystalline COUMADIN® FOR INJECTION (Warfarin Sodium for Injection, USP). http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/009218s108lbl.pdf. Accessed 7 May 2012
FDA. U.S. Food and Drug Administration (2012b) XARELTO (rivaroxaban) tablets, for oral use. http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202439s000lbl.pdf. Accessed 7 May 2012
Francis CW (2008) New issues in oral anticoagulants. Hematol Am Soc Hematol Educ Program 2008(1):259–265. doi:10.1182/asheducation-2008.1.259
Francis CW, Berkowitz SD, Comp PC, Lieberman JR, Ginsberg JS, Paiement G, Peters GR, Roth AW, McElhattan J, Colwell CW Jr, EXULT A Study Group (2003) Comparison of ximelagatran with warfarin for the prevention of venous thromboembolism after total knee replacement. N Engl J Med 349:1703–1712
Fu Z, Nakayama T, Sato N, Izumi Y, Kasamaki Y, Shindo A, Ohta M, Soma M, Aoi N, Sato M, Ozawa Y, Ma Y, Matsumoto K, Doba N, Hinohara S (2009) A haplotype of the CYP4F2 gene associated with myocardial infarction in Japanese men. Mol Genet Metab 96:145–147
Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, Halperin JL, Johnston SC, Katzan I, Kernan WN, Mitchell PH, Ovbiagele B, Palesch YY, Sacco RL, Schwamm LH, Wassertheil-Smoller S, Turan TN, Wentworth D, American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, Interdisciplinary Council on Quality of Care and Outcomes Research (2011) Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 42(1):227–276
Gage BF, Eby C, Milligan PE, Banet GA, Duncan JR, McLeod HL (2004) Use of pharmacogenetics and clinical factors to predict the maintenance dose of warfarin. Thromb Haemost 91:87–94
Gage BF, Eby C, Johnson JA, Deych E, Rieder MJ, Ridker PM, Milligan PE, Grice G, Lenzini P, Rettie AE, Aquilante CL, Grosso L, Marsh S, Langaee T, Farnett LE, Voora D, Veenstra DL, Glynn RJ, Barrett A, McLeod HL (2008) Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clin Pharmacol Ther 84:326–331
Geisen C, Watzka M, Sittinger K et al (2005) VKORC1 haplotypes and their impact on the inter-individual and inter-ethnical variability of oral anticoagulation. Thromb Haemost 94:773–779
Giorgi MA, Miguel LS (2012) Rivaroxaban in atrial fibrillation. Vasc Health Risk Manag 8:525–531
González-Juanatey JR, Alvarez-Sabin J, Lobos JM, Martínez-Rubio A, Reverter JC, Oyagüez I, González-Rojas N, Becerra V (2012) Cost-effectiveness of Dabigatran for stroke prevention in non-valvular atrial fibrillation in Spain. Rev Esp Cardiol 65(10):901–910
Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L, ARISTOTLE Committees and Investigators (2011) Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 365:981–992
Haining RL, Hunter AP, Veronese ME, Trager WF, Rettie AE (1996) Allelic variants of human cytochrome P450 2C9: baculovirus-mediated expression, purification, structural characterization, substrate stereoselectivity, and prochiral selectivity of the wild-type and I359L mutant forms. Arch Biochem Biophys 333:447–458
Hirsh J (1992) Antithrombotic therapy in deep vein thrombosis and pulmonary embolism. Am Heart J 123(4 Pt 2):1115–1122
Hirsh J, Dalen J, Anderson DR, Poller L, Bussey H, Ansell J, Deykin D (2001) Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest 119(1 Suppl):8S–21S
Hylek EM, Go AS, Chang Y, Jensvold NG, Henault LE, Selby JV, Singer DE (2003) Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med 349:1019–1026
Infiniti Warfarin Assay (2012) Available from: http://www.autogenomics.com/pdf/EM-34004-D-Warfarin-Assay-Package-Insert.pdf. Accessed 10 Sept 2012
International Warfarin Pharmacogenetics Consortium (IWPC) Algorithm (2012) Available from: http://www.pharmgkb.org/do/serve?objId=PA162372936&objCls=Dataset#tabview=tabnull&subtab=. Accessed 7 May 2012
International Warfarin Pharmacogenetics Consortium, Klein TE, Altman RB, Eriksson N, Gage BF, Kimmel SE, Lee MT, Limdi NA, Page D, Roden DM, Wagner MJ, Caldwell MD, Johnson JA (2009) Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med 360:753–764
Jähnchen E, Meinertz T, Gilfrich HJ, Groth U, Martini A (1976) The enantiomers of phenprocoumon: pharmacodynamic and pharmacokinetic studies. Clin Pharmacol Ther 20:342–349
Johnson EG, Horne BD, Carlquist JF, Anderson JL (2011) Genotype-based dosing algorithms for warfarin therapy: data review and recommendations. Mol Diagn Ther 15:255–264
Jonas DE, McLeod HL (2009) Genetic and clinical factors relating to warfarin dosing. Trends Pharmacol Sci 30:375–386
Kamali F, Pirmohamed M (2006) The future prospects of pharmacogenetics in oral anticoagulation therapy. Br J Clin Pharmacol 61(6):746–751
King CR, Porche-Sorbet RM, Gage BF, Ridker PM, Renaud Y, Phillips MS, Eby C (2008) Performance of commercial platforms for rapid genotyping of polymorphisms affecting warfarin dose. Am J Clin Pathol 129:876–883
King CR, Deych E, Milligan P, Eby C, Lenzini P, Grice G, Porche-Sorbet RM, Ridker PM, Gage BF (2010) Gamma-glutamyl carboxylase and its influence on warfarin dose. Thromb Haemost 104:750–754
Kubitza D, Becka M, Wensing G, Voith B, Zuehlsdorf M (2005) Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939 an oral, direct Factor Xa inhibitor – after multiple dosing in healthy male subjects. Eur J Clin Pharmacol 61(12):873–880
Kubitza D, Becka M, Zuehlsdorf M, Mueck W (2006) Effect of food, an antacid, and the H2 antagonist ranitidine on the absorption of BAY 59–7939 (rivaroxaban), an oral, direct factor Xa inhibitor, in healthy subjects. J Clin Pharmacol 46:549–558
Kubitza D, Becka M, Roth A, Mueck W (2008) Dose-escalation study of the pharmacokinetics and pharmacodynamics of rivaroxaban in healthy elderly subjects. Curr Med Res Opin 24:2757–2765
Langley MR, Booker JK, Evans JP, McLeod HL, Weck KE (2009) Validation of clinical testing for warfarin sensitivity: comparison of CYP2C9-VKORC1 genotyping assays and warfarin-dosing algorithms. J Mol Diagn 11:216–225
Lefferts JA, Schwab MC, Dandamudi UB, Lee HK, Lewis LD, Tsongalis GJ (2010) Warfarin genotyping using three different platforms. Am J Transl Res 2:441–446
Lenzini P, Wadelius M, Kimmel S, Anderson JL, Jorgensen AL, Pirmohamed M, Caldwell MD, Limdi N, Burmester JK, Dowd MB, Angchaisuksiri P, Bass AR, Chen J, Eriksson N, Rane A, Lindh JD, Carlquist JF, Horne BD, Grice G, Milligan PE, Eby C, Shin J, Kim H, Kurnik D, Stein CM, McMillin G, Pendleton RC, Berg RL, Deloukas P, Gage BF (2010) Integration of genetic, clinical, and INR data to refine warfarin dosing. Clin Pharmacol Ther 87:572–578
Li T, Chang CY, Jin DY, Lin PJ, Khvorova A, Stafford DW (2004) Identification of the gene for vitamin K epoxide reductase. Nature 427:541–544
Li C, Schwarz UI, Ritchie MD, Roden DM, Stein CM, Kurnik D (2009) Relative contribution of CYP2C9 and VKORC1 genotypes and early INR response to the prediction of warfarin sensitivity during initiation of therapy. Blood 113:3925–3930
Liesenfeld KH, Lehr T, Dansirikul C, Reilly PA, Connolly SJ, Ezekowitz MD, Yusuf S, Wallentin L, Haertter S, Staab A (2011) Population pharmacokinetic analysis of the oral thrombin inhibitor dabigatran etexilate in patients with non-valvular atrial fibrillation from the RE-LY trial. J Thromb Haemost 9:2168–2175
Limdi NA, Veenstra DL (2008) Warfarin pharmacogenetics. Pharmacotherapy 28(9):1084–1097
Limdi NA, Arnett DK, Goldstein JA, Beasley TM, McGwin G, Adler BK, Acton RT (2008) Influence of CYP2C9 and VKORC1 on warfarin dose, anticoagulation attainment and maintenance among European-Americans and African-Americans. Pharmacogenomics 9:511–526
Limdi NA, Wiener H, Goldstein JA, Acton RT, Beasley TM (2009) Influence of CYP2C9 and VKORC1 on warfarin response during initiation of therapy. Blood Cells Mol Dis 43:119–128
Lip GY, Lane DA (2010) Does warfarin for stroke thromboprophylaxis protect against MI in atrial fibrillation patients? Am J Med 123:785–789
Lip GY, Huber K, Andreotti F, Arnesen H, Airaksinen KJ, Cuisset T, Kirchhof P, Marín F, European Society of Cardiology Working Group on Thrombosis (2010) Management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous coronary intervention/stenting. Thromb Haemost 103:13–28
Loebstein R, Dvoskin I, Halkin H, Vecsler M, Lubetsky A, Rechavi G, Amariglio N, Cohen Y, Ken-Dror G, Almog S, Gak E (2007) A coding VKORC1 Asp36Tyr polymorphism predisposes to warfarin resistance. Blood 109:2477–2480
López-Parra AM, Borobia AM, Baeza C, Arroyo-Pardo E, Carcas AJ (2013) A multiplex assay to detect variations in the CYP2C9, VKORC1, CYP4F2 and APOE genes involved in acenocoumarol metabolism. Clin Biochem 46(1–2):167–169
Lund K, Gaffney D, Spooner R, Etherington AM, Tansey P, Tait RC (2012) Polymorphisms in VKORC1 have more impact than CYP2C9 polymorphisms on early warfarin International Normalized Ratio control and bleeding rates. Br J Haematol 158(2):256–261
Manolopoulos VG, Ragia G, Tavridou A (2010) Pharmacogenetics of coumarinic oral anticoagulants. Pharmacogenomics 4:493–496
Margaglione M, Colaizzo D, D’Andrea G, Brancaccio V, Ciampa A, Grandone E, Di Minno G (2000) Genetic modulation of oral anticoagulation with warfarin. Thromb Haemost 84:775–778
Markatos CN, Grouzi E, Politou M, Gialeraki A, Merkouri E, Panagou I, Spiliotopoulou I, Travlou A (2008) VKORC1 and CYP2C9 allelic variants influence acenocoumarol dose requirements in Greek patients. Pharmacogenomics 9:1631–1638
Maurice CB, Barua PK, Simses D, Smith P, Howe JG, Stack G (2010) Comparison of assay systems for warfarin-related CYP2C9 and VKORC1 genotyping. Clin Chim Acta 411:947–954
Millican EA, Lenzini PA, Milligan PE, Grosso L, Eby C, Deych E, Grice G, Clohisy JC, Barrack RL, Burnett RS, Voora D, Gatchel S, Tiemeier A, Gage BF (2007) Genetic-based dosing in orthopedic patients beginning warfarin therapy. Blood 110:1511–1515
Mitchell C, Gregersen N, Krause A (2011) Novel CYP2C9 and VKORC1 gene variants associated with warfarin dosage variability in the South African black population. Pharmacogenomics 12:953–963
Momary KM, Shapiro NL, Viana MA, Nutescu EA, Helgason CM, Cavallari LH (2007) Factors influencing warfarin dose requirements in African-Americans. Pharmacogenomics 8:1535–1544
National Heart, Lung, and Blood Institute (2012) Clarification of Optimal Anticoagulation Through Genetics (COAG) [ClinicalTrials.gov identifier NCT00839657]. US National Institutes of Health, ClinicalTrials.gov. Available from: http://www.clinicaltrials.gov/show/NCT00839657. Accessed 20 Feb 2012
Ngui JS, Chen Q, Shou M, Wang RW, Stearns RA, Baillie TA, Tang W (2001) In vitro stimulation of warfarin metabolism by quinidine: increases in the formation of 4′- and 10-hydroxywarfarin. Drug Metab Dispos 29:877–886
Obayashi K, Nakamura K, Kawana J, Ogata H, Hanada K, Kurabayashi M, Hasegawa A, Yamamoto K, Horiuchi R (2006) VKORC1 gene variations are the major contributors of variation in warfarin dose in Japanese patients. Clin Pharmacol Ther 80:169–178
Osman A, Enström C, Arbring K, Söderkvist P, Lindahl TL (2006) Main haplotypes and mutational analysis of vitamin K epoxide reductase (VKORC1) in a Swedish population: a retrospective analysis of case records. J Thromb Haemost 4:1723–1729
Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM, Investigators ROCKETAF (2011) Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365:883–891
Perera MA, Gamazon E, Cavallari LH, Patel SR, Poindexter S, Kittles RA, Nicolae D, Cox NJ (2011) The missing association: sequencing-based discovery of novel SNPs in VKORC1 and CYP2C9 that affect warfarin dose in African Americans. Clin Pharmacol Ther 89:408–415
Perzborn E, Roehrig S, Straub A, Kubitza D, Misselwitz F (2011) The discovery and development of rivaroxaban, an oral, direct factor Xa inhibitor. Nat Rev Drug Discov 10(1):61–75
Raghavan N, Frost CE, Yu Z, He K, Zhang H, Humphreys WG, Pinto D, Chen S, Bonacorsi S, Wong PC, Zhang D (2009) Apixaban metabolism and pharmacokinetics after oral administration to humans. Drug Metab Dispos 37:74–81
Reeman JV, Zhu RP, Owens DK, Garber AM, Hutton DW, Go AS, Wang PJ, Turakhia MP (2011) Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med 154:1–11
Rettie AE, Korzekwa KR, Kunze KL, Lawrence RF, Eddy AC, Aoyama T, Gelboin HV, Gonzalez FJ, Trager WF (1992) Hydroxylation of warfarin by human cDNA-expressed Cytochrome P-450: a role for P-4502C9 in the etiology of (S)-warfarin-drug interactions. Chem Res Toxicol 5:54–59
Rettie AE, Wienkers LC, Gonzalez FJ, Trager WF, Korzekwa KR (1994) Impaired (S)-warfarin metabolism catalysed by the R144C allelic variant of CYP2C9. Pharmacogenetics 4:39–42
Rieder MJ, Reiner AP, Gage BF, Nickerson DA, Eby CS, McLeod HL, Blough DK, Thummel KE, Veenstra DL, Rettie AE (2005) Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med 352:2285–2293
Rieder MJ, Reiner AP, Rettie AE (2007) Gamma-glutamyl carboxylase (GGCX) tag SNPs have limited utility for predicting warfarin maintenance dose. J Thromb Haemost 5:2227–2234
Rosemary J, Adithan C (2007) The pharmacogenetics of CYP2C9 and CYP2C19: ethnic variation and clinical significance. Curr Clin Pharmacol 2:93–109
Rost S, Fregin A, Ivaskevicius V, Conzelmann E, Hörtnagel K, Pelz HJ, Lappegard K, Seifried E, Scharrer I, Tuddenham EG, Müller CR, Strom TM, Oldenburg J (2004) Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature 427:537–541
Sagreiya H, Berube C, Wen A, Ramakrishnan R, Mir A, Hamilton A, Altman RB (2010) Extending and evaluating a warfarin dosing algorithm that includes CYP4F2 and pooled rare variants of CYP2C9. Pharmacogenet Genomics 20:407–413
Schalekamp T, Brassé BP, Roijers JF, Chahid Y, van Geest-Daalderop JH, de Vries-Goldschmeding H, van Wijk EM, Egberts AC, de Boer A (2006) VKORC1 and CYP2C9 genotypes and acenocoumarol anticoagulation status: interaction between both genotypes affects overanticoagulation. Clin Pharmacol Ther 80:13–22
Schelleman H, Chen Z, Kealey C, Whitehead AS, Christie J, Price M, Brensinger CM, Newcomb CW, Thorn CF, Samaha FF, Kimmel SE (2007) Warfarin response and vitamin K epoxide reductase complex 1 in African Americans and Caucasians. Clin Pharmacol Ther 81(5):742–747
Schelleman H, Limdi NA, Kimmel SE (2008) Ethnic differences in warfarin maintenance dose requirement and its relationship with genetics. Pharmacogenomics 9(9):1331–1346
Schulman S, Bijsterveld NR (2007) Anticoagulants and their reversal. Transfus Med Rev 21:37–48
Schulman S, Majeed A (2012) The oral thrombin inhibitor dabigatran: strengths and weaknesses. Semin Thromb Hemost 38:7–15
Schulman S, Wåhlander K, Lundström T, Clason SB, Eriksson H, Investigators THRIVEIII (2003) Secondary prevention of venous thromboembolism with the oral direct thrombin inhibitor ximelagatran. N Engl J Med 349(18):1713–1721
Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, Baanstra D, Schnee J, Goldhaber SZ, RE-COVER Study Group (2009) Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 361:2342–2352
Schwarz UI, Ritchie MD, Bradford Y, Li C, Dudek SM, Frye-Anderson A, Kim RB, Roden DM, Stein CM (2008) Genetic determinants of response to warfarin during initial anticoagulation. N Engl J Med 358:999–1008
Sconce EA, Khan TI, Wynne HA, Avery P, Monkhouse L, King BP, Wood P, Kesteven P, Daly AK, Kamali F (2005) The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: proposal for a new dosing regimen. Blood 106:2329–2333
Scott SA, Jaremko M, Lubitz SA, Kornreich R, Halperin JL, Desnick RJ (2009) CYP2C9*8 is prevalent among African-Americans: implications for pharmacogenetic dosing. Pharmacogenomics 10:1243–1255
Scott SA, Khasawneh R, Peter I, Kornreich R, Desnick RJ (2010) Combined CYP2C9, VKORC1 and CYP4F2 frequencies among racial and ethnic groups. Pharmacogenomics 11:781–791
Shahin MH, Khalifa SI, Gong Y, Hammad LN, Sallam MT, El Shafey M, Ali SS, Mohamed ME, Langaee T, Johnson JA (2011) Genetic and nongenetic factors associated with warfarin dose requirements in Egyptian patients. Pharmacogenet Genomics 21:130–135
Shaw PB, Donovan JL, Tran MT, Lemon SC, Burgwinkle P, Gore J (2010) Accuracy assessment of pharmacogenetically predictive warfarin dosing algorithms in patients of an academic medical center anticoagulation clinic. J Thromb Thrombolysis 30:220–225
Shirolkar SC, Fiuzat M, Becker RC (2010) Dronedarone and vitamin K antagonists: a review of drug-drug interactions. Am Heart J 160:577–582
Skanes AC, Healey JS, Cairns JA, Dorian P, Gillis AM, McMurtry MS, Mitchell LB, Verma A, Nattel S, Canadian Cardiovascular Society Atrial Fibrillation Guidelines Committee (2012) Focused 2012 update of the Canadian Cardiovascular Society atrial fibrillation guidelines: recommendations for stroke prevention and rate/rhythm control. Can J Cardiol 28(2):125–136
Spanish Agency of Medicines and Health Products (2012) Criteria and recommendations for the use of the new oral anticoagulants in the prevention of stroke and systemic embolism in patients with non-valvular atrial fibrillation. http://www.aemps.gob.es/medicamentosUsoHumano/informesPublicos/docs/criterios-aco-rev_05-09-12.pdf. Accessed Sept 2012
Spreafico M, Lodigiani C, van Leeuwen Y, Pizzotti D, Rota LL, Rosendaal F, Mannucci PM, Peyvandi F (2008) Effects of CYP2C9 and VKORC1 on INR variations and dose requirements during initial phase of anticoagulant therapy. Pharmacogenomics 9:1237–1250
Stafford DW (2005) The vitamin K cycle. J Thromb Haemost 3:1873–1878
Stangier J, Rathgen K, Stähle H, Mazur D (2010) Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet 49:259–268
Stehle S, Kirchheiner J, Lazar A, Fuhr U (2008) Pharmacogenetics of oral anticoagulants: a basis for dose individualization. Clin Pharmacokinet 47(9):565–594
Takahashi H, Wilkinson GR, Nutescu EA, Morita T, Ritchie MD, Scordo MG, Pengo V, Barban M, Padrini R, Ieiri I, Otsubo K, Kashima T, Kimura S, Kijima S, Echizen H (2006) Different contributions of polymorphisms in VKORC1 and CYP2C9 to intra- and inter-population differences in maintenance dose of warfarin in Japanese, Caucasians and African-Americans. Pharmacogenet Genomics 16:101–110
Takanashi K, Tainaka H, Kobayashi K, Yasumori T, Hosakawa M, Chiba K (2000) CYP2C9 Ile359 and Leu359 variants: enzyme kinetic study with seven substrates. Pharmacogenetics 10:95–104
Teichert M, Eijgelsheim M, Rivadeneira F, Uitterlinden AG, van Schaik RH, Hofman A, De Smet PA, van Gelder T, Visser LE, Stricker BH (2009) A genome-wide association study of acenocoumarol maintenance dosage. Hum Mol Genet 18(19):3758–3768
Teichert M, Eijgelsheim M, Uitterlinden AG, Buhre PN, Hofman A, De Smet PA, Visser LE, Stricker BH (2011a) Dependency of phenprocoumon dosage on polymorphisms in the VKORC1, CYP2C9, and CYP4F2 genes. Pharmacogenet Genomics 21(1):26–34
Teichert M, van Noord C, Uitterlinden AG, Hofman A, Buhre PN, De Smet PA, Straus S, Stricker BH, Visser LE (2011b) Proton pump inhibitors and the risk of overanticoagulation during acenocoumarol maintenance treatment. Br J Haematol 153:379–385
The Pharmacogenomics Knowledge Base (2012) Acenocoumarol. Available from: http://www.pharmgkb.org/drug/PA452632#tabview=tab3&subtab=31. Accessed May 2012
Thijssen HH, Flinois JP, Beaune PH (2000) Cytochrome P4502C9 is the principal catalyst of racemic acenocoumarol hydroxylation reactions in human liver microsomes. Drug Metab Dispos 28:1284–1290
Toon S, Heimark LD, Trager WF, O’Reilly RA (1985) Metabolic fate of phenprocoumon in humans. J Pharm Sci 74(10):1037–1040
Uchino K, Hernandez AV (2012) Dabigatran association with higher risk of acute coronary events: meta-analysis of noninferiority randomized controlled trials. Arch Intern Med 172:397–402
Ufer M (2005) Comparative pharmacokinetics of vitamin K antagonists: warfarin, phenprocoumon and acenocoumarol. Clin Pharmacokinet 44:1227–1246
Utrecht Institute for Pharmaceutical Sciences (2012). European Pharmacogenetics of Anticoagulant Therapy – Warfarin (EU-PACT) [ClinicalTrials.gov identifier NCT01119300]. US National Institutes of Health, ClinicalTrials.gov Available from: http://www.clinicaltrials.gov/show/NCT01119300. Accessed 20 Feb 2012
van Schie RM, Wessels JA, le Cessie S, de Boer A, Schalekamp T, van der Meer FJ, Verhoef TI, van Meegen E, Rosendaal FR, der Zee AH M-v, EU-PACT Study Group (2011) Loading and maintenance dose algorithms for phenprocoumon and acenocoumarol using patient characteristics and pharmacogenetic data. Eur Heart J 32:1909–1917
Veenstra DL, You JH, Rieder MJ, Farin FM, Wilkerson HW, Blough DK, Cheng G, Rettie AE (2005) Association of Vitamin K epoxide reductase complex 1 (VKORC1) variants with warfarin dose in a Hong Kong Chinese patient population. Pharmacogenet Genomics 15:687–691
Verde Z, Ruiz JR, Santiago C, Valle B, Bandrés F, Calvo E, Lucía A, Gómez Gallego F (2010) A novel, single algorithm approach to predict acenocoumarol dose based on CYP2C9 and VKORC1 allele variants. PLoS One 5:e11210
Wadelius M, Chen LY, Downes K, Ghori J, Hunt S, Eriksson N, Wallerman O, Melhus H, Wadelius C, Bentley D, Deloukas P (2005) Common VKORC1 and GGCX polymorphisms associated with warfarin dose. Pharmacogenomics J 5:262–270
Wadelius M, Chen LY, Eriksson N, Bumpstead S, Ghori J, Wadelius C, Bentley D, McGinnis R, Deloukas P (2007) Association of warfarin dose with genes involved in its action and metabolism. Hum Genet 121(1):23–34
Wadelius M, Chen LY, Lindh JD, Eriksson N, Ghori MJ, Bumpstead S, Holm L, McGinnis R, Rane A, Deloukas P (2009) The largest prospective warfarin-treated cohort supports genetic forecasting. Blood 113:784–792
Wajih N, Sane DC, Hutson SM, Wallin R (2004) The inhibitory effect of calumenin on the vitamin K-dependent gamma-carboxylation system. Characterization of the system in normal and warfarin-resistant rats. J Biol Chem 279:25276–25283
Wang D, Chen H, Momary KM, Cavallari LH, Johnson JA, Sadée W (2008) Regulatory polymorphism in vitamin K epoxide reductase complex subunit 1 (VKORC1) affects gene expression and warfarin dose requirement. Blood 112:1013–1021
Wartak SA, Bartholomew JR (2011) Dabigatran: will it change clinical practice? Cleve Clin J Med 78(10):657–664
Washington University School of Medicine (2012) Genetics Informatics Trial (GIFT) of warfarin to prevent DVT [ClinicalTrials.gov identifier NCT01006733]. US National Institutes of Health, ClinicalTrials.gov. Available from: http://www.clinicaltrials.gov/show/NCT01006733. Accessed 20 Feb 2012
Weitz JI (2010) New oral anticoagulants in development. Thromb Haemost 103:62–70
Wienkers LC, Wurden CJ, Storch E, Kunze KL, Rettie AE, Trager WF (1996) Formation of (R)-8-hydroxywarfarin in human liver microsomes. A new metabolic marker for the (S)-mephenytoin hydroxylase, P4502C19. Drug Metab Dispos 24:610–614
Wittkowsky AK (2010) New oral anticoagulants: a practical guide for clinicians. J Thromb Thrombolysis 29:182–191
You JJ, Singer DE, Howard PA, Lane DA, Eckman MH, Fang MC, Hylek EM, Schulman S, Go AS, Hughes M, Spencer FA, Manning WJ, Halperin JL, Lip GY, American College of Chest Physicians (2012) Antithrombotic therapy for atrial fibrillation: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141(2 Suppl):e531S–e575S
Yuan HY, Chen JJ, Lee MT, Wung JC, Chen YF, Charng MJ, Lu MJ, Hung CR, Wei CY, Chen CH, Wu JY, Chen YT (2005) A novel functional VKORC1 promoter polymorphism is associated with inter-individual and inter-ethnic differences in warfarin sensitivity. Hum Mol Genet 14:1745–1751
Zhang Z, Fasco MJ, Huang Z, Guengerich FP, Kaminsky LS (1995) Human Cytochromes P4501A1 and P4501A2: R-warfarin metabolism as a probe. Drug Metab Dispos 23:1339–1346
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer India
About this chapter
Cite this chapter
Jiménez-Varo, E., Cañadas-Garre, M., Aguilera, M., Callejas, D.G., Ramirez, C.P., Hernández, M.A.C. (2013). Pharmacogenetics of Oral Anticoagulants. In: Barh, D., Dhawan, D., Ganguly, N. (eds) Omics for Personalized Medicine. Springer, New Delhi. https://doi.org/10.1007/978-81-322-1184-6_21
Download citation
DOI: https://doi.org/10.1007/978-81-322-1184-6_21
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-1183-9
Online ISBN: 978-81-322-1184-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)